Abstract
Background:
Respiratory syncytial virus (RSV) is one of the most important respiratory pathogens in young children. Infants <6 months of age and infants and young children with extreme pre-term birth, and cardiac and pulmonary co-morbidities experience the highest incidence of severe RSV disease. There are no licensed vaccines; immunoprophylaxis is recommended for the highest risk children. Extended half-life RSV monoclonal antibodies (EHL-mAbs) are under development intended for immunization of all infants and high-risk children <2 years of age. We modeled the anticipated public health benefits of RSV EHL-mAb immunization using the number needed to immunize (NNI).
Methods:
We combined RSV hospitalization, outpatient and outpatient lower respiratory tract infection (LRI) incidence estimates and a range of immunization efficacies to estimate the annual NNI. We calculated the absolute incidence rate reduction (ARR) by multiplying the incidence rates by immunization efficacy. NNI was calculated as the reciprocal of the ARR.
Results:
For an RSV EHL-mAb with 70% efficacy, 6–18 infants would need to be immunized to prevent one RSV-associated outpatient visit, and 13–33 infants would need to be immunized to prevent one RSV-associated LRI outpatient visit. To prevent one RSV-associated hospitalization, 37–85 infants 0–5 months of age, and 107–280 infants 6–11 months of age would need to be immunized.
Conclusions:
Public health benefits, such as disease cases averted due to immunization, are essential elements in consideration of candidate vaccines for a national immunization program. An RSV EHL-mAb of moderate efficacy could have high impact. These data provide an additional perspective for public health decision making.
Keywords: RSV monoclonal antibody, mAb, Immunization, Number needed to vaccinate
1. Introduction
Respiratory syncytial virus (RSV) is one of the most important respiratory pathogens in young children worldwide [1]. Infants less than six months of age and infants and young children with extreme pre-term birth, chronic lung disease, and congenital heart disease experience the highest incidence of severe RSV disease. In the US, approximately 20% of infants have an outpatient visit [2] and 2% of all infants are hospitalized due to an RSV infection [3-5]. More than half of all RSV hospitalizations in young children occur in infants <6 months of age [5]. Rates of RSV-associated hospitalization in children >1 year are also high, though they decline rapidly with age [6,7]. Severe disease in young infants may be mediated by a combination of lung/airway and immunological immaturity [8]. Despite the considerable burden, only symptomatic treatment is available and there are no licensed vaccines. Immunoprophylaxis is recommended for only the highest risk infants and children. Palivizumab, a short-acting monoclonal antibody administered monthly during the RSV season, was approved in 1998 but due to moderate efficacy [9] and high price, palivizumab is recommended for use in infants with extreme prematurity and infants and children with serious cardiac or pulmonary disease ≤24 months of age [10]. However, there are a growing number of RSV immunization candidates under development intended for RSV prevention in all infants with two main strategies in late stage: active immunization of pregnant women with transplacental transfer of maternal antibody to the infant [11-13], and passive immunization with high potency, long-acting EHL-mAbs [14-16] that require one dose for seasonal prophylaxis. RSV EHL-mAbs allow for flexibility of administration so that infants can be protected during their first RSV season when they are at highest risk of infection and severe disease. Infants born during the RSV season can receive the EHL-mAb at or shortly after birth. Infants born before the RSV season but entering their first RSV season can receive the EHL-mAb at the start of the season; administration may be synchronized with the 2, 4- or 6-month health maintenance visits or in ad hoc visits or seasonal clinics.
Public health programs consider candidate vaccines based on clinical efficacy and safety evaluated in the context of randomized clinical trials. Other important measures of vaccine value are the vaccine-attributable incidence rate reduction and cost-effectiveness. Several cost-effectiveness studies have modeled the anticipated benefits of RSV immunization with EHL-mAbs and maternal vaccines with a range of potential vaccine efficacies [17-19]; these strategies were cost-effective under certain ranges of assumptions. No published studies to date have modeled the anticipated benefits of RSV immunization using the number needed to vaccinate (NNV). The NNV estimates impact by expressing the benefit in terms of number of people needed to vaccinate to prevent one disease-related event each year. NNV is the reciprocal of the annual vaccine attributable incidence rate reduction [20]. We describe a corollary measure for the NNV, the number needed to immunize (NNI), which is more relevant to the passive EHL-mAb immunization strategy. The NNI is the number of infants that need to be immunized to prevent one RSV-associated outpatient visit, outpatient lower respiratory infection (LRI) or hospitalization. We calculated the NNI for outpatient LRI because RSV-associated LRI is a clinically significant outcome and this outcome is a primary endpoint in RSV EHL-mAb clinical trials (NCT03979313, NCT02624947) [21,22]. We combined RSV incidence estimates and a range of potential immunization efficacies to estimate the annual NNI for infants.
2. Materials and methods
We assumed that the RSV EHL-mAb was administered at the start of the RSV season and effectiveness was maintained at a steady state for the duration of the season. Our approach for calculation of NNV was adapted from a pediatric influenza NNV study conducted by Lewis et al. [23] We selected incidence studies for which we could ascertain the rates per 1000 children of RSV outpatient visits and hospitalizations in children 0–5 and 6–11 months of age [2,4-5,7,24]. Data for incidence rates of outpatient lower respiratory infection (LRI) were extrapolated from two of the outpatient studies [25-26]. In order to explore a range of values for NNI, we selected studies that represented a range of incidence rates [2,4-5,7,24-26]. We varied immunization efficacy (IE) from a low of 50% to a high of 90%. We calculated the absolute incidence rate reduction (ARR) due to immunization by multiplying the incidence rate by IE. We calculated 95% Wilson confidence intervals (CIs) for the ARR [27]. NNI was calculated as the reciprocal of the ARR (1/ARR) [20]. NNI 95% CIs were calculated as the reciprocal of the ARR 95% CI ((Lower Limit (NNI) = 1/Upper Limit (ARR) and Upper Limit (NNI) = 1/Lower Limit (ARR)).
2.1. Incidence source data
2.1.1. Outpatient visits
We selected three outpatient incidence studies with a range of incidence rates (Table 1). Two of these were multi-site studies conducted in the Nashville, Tennessee, Rochester New York, and Cincinnati, Ohio, using the same surveillance methods but different years [2,7]. The third study was conducted in Nashville, Tennessee, with different methods in different years [24] (Table 1).
Table 1.
RSV-Associated Pediatric Practice Visit and Hospitalization Rates and Rate Reduction by Month of Age and Immunization Efficacy.
| Incidence Source Data |
IncidenceRate/ 1000 |
ARR (95% CI) |
NNI (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| 50% Immunization Efficacy |
70% Immunization Efficacy |
90% Immunization Efficacy |
50% Immunization Efficacy |
70% Immunization Efficacy |
90% Immunization Efficacy |
||
| Outpatient Studies | |||||||
| Fisher [24] | |||||||
| Age 0–5 months | 79.8 | 39.9 (27.1–56.4) | 55.9 (40.4–74.8) | 71.8 (54.2–92.8) | 25 (18–37) | 18 (13–25) | 14 (11–18) |
| Age 6–11 months | 89.8 | 44.9 (29.9–66.1) | 62.9 (44.4–86.4) | 80.8 (59.2–106.2) | 22 (15–33) | 16 (12–23) | 12 (9–17) |
| Hall [7] | |||||||
| Age 0–5 months | 132.0 | 66.0 (49.43–87.3) | 92.4 (73.1–117.2) | 118.8 (95.9–145.0) | 15 (11–20) | 11 (9–14) | 8 (7–10) |
| Age 6–11 months | 177.0 | 88.5 (67.19–118.5) | 123.9 (98.4–157.6) | 159.3 (130.3–195.9) | 11 (8–15) | 8 (6–10) | 6 (5–8) |
| Lively [2] | |||||||
| Age 0–5 months | 215.7 | 108.0 (80.3–140.0) | 151.0 (118.9–187.8) | 194.1 (158.5–234.6) | 9 (7–12) | 7 (5–8) | 5 (4–6) |
| Age 6–11 months | 246.1 | 123.1 (93.7–157.2) | 172.3 (137.4–210.2) | 221.5 (182.2–262.1) | 8 (6–11) | 6 (5–7) | 5 (4–6) |
| Outpatient LRI Studies | |||||||
| Fisher (Lee) [25] | |||||||
| Age 0–5 months | 45.0 | 22.5 (13.23–35.6) | 31.5 (20.1–46.0) | 40.5 (27.1–56.4) | 44 (28–76) | 32 (22–50) | 25 (18–37) |
| Age 6–11 months | 42.7 | 21.4 (12.0–37.8) | 29.9 (17.7–47.5) | 38.5 (23.7–56.9) | 47 (26–84) | 33 (21–56) | 26 (18–42) |
| Hall [26] | |||||||
| Age 0–5 months | 81.8 | 40.9 (29.2–59.9) | 57.3 (43.0–78.8) | 73.7 (57.2–97.3) | 24 (17–34) | 17 (13–23) | 14 (10–17) |
| Age 6–11 months | 109.7 | 54.9 (37.2–78.2) | 76.8 (56.4–104.4) | 98.8 (78.1–132.4) | 18 (13–27) | 13 (10–18) | 10 (8–13) |
| Hospitalization Studies | |||||||
| Hall [7] | |||||||
| Age 0–5 months | 16.9 | 8.5 (7.3–9.8) | 11.8 (10.4–13.5) | 15.2 (13.6–17.0) | 118 (102–138) | 85 (74–96) | 66 (59–74) |
| Age 6–12 months | 5.1 | 2.6 (1.9–3.3) | 3.6 (2.8–4.5) | 4.6 (3.7–5.6) | 392 (299–526) | 280 (224–360) | 218 (177–270) |
| Paramore [4] | |||||||
| Age 0–5 months | 25.7 | 12.9 (12.5–13.2) | 18.0 (17.6–18.4) | 23.1 (22.7–23.6) | 78 (76–80) | 56 (54–57) | 43 (42–44) |
| Age 6–12 months | 9.0 | 4.5 (4.3–4.7) | 6.3 (6.0–6.7) | 8.1 (7.7–8.4) | 222 (212–233) | 159 (150–167) | 123 (119–130) |
| Stockman [5] | |||||||
| Age 0–5 months | 38.6 | 19.3 (14.8–25.3) | 27.0 (21.4–33.7) | 34.7 (28.4–42.3) | 52 (39–68) | 37 (30–47) | 29 (24–35) |
| Age 6–12 months | 13.4 | 6.7 (4.5–10.6) | 9.4 (6.3–13.7) | 12.1 (8.5–16.8) | 149 (95–235) | 107 (73–158) | 83 (60–118) |
ARR = Absolute Rate Reduction, CI = Confidence Interval, NNI = Number Needed to Immunize.
Fisher et al. enrolled a birth cohort of 1113 healthy full-term infants (for a total of 3615 child-years of observation) in the vaccine treatment and evaluation unit (VTEU) over a 20-year period from 1973 to 1993. The median age was 13.4 months (range of 2 weeks to 5 years). Parents were instructed to bring their child to the clinic if they developed runny nose, cough, fever, or symptoms suggesting ear infection. Patients were seen if ill. Nasal wash cultures for virus isolation were obtained for any of the following indications: URI accompanied by fever of ≥38.4 °C, signs or symptoms suggesting LRI, and acute otitis media. Specimens were tested by viral culture during RSV season defined as November 1 to April 30. During the 20-year period the VTEU conducted two RSV vaccine trials and trial participants were excluded [24]. Incidence rates of RSV-associated LRI from this study were published in a later paper [25] (Table 1).
Hall et al. conducted active, prospective outpatient surveillance in the New Vaccine Surveillance Network (NVSN) from November until April over 2 years from 2002 to 2004 in children 0–59 months who visited a pediatric practice with acute respiratory infection in three US sites (Nashville, Rochester and Cincinnati (2003–2004 only)). Nasal and throat swabs were collected from each enrolled patient. RSV was detected by a reverse-transcriptase polymerase chain reaction (RT-PCR) assay performed at each study site. ARI/fever outpatient visits were estimated between November and April in 2002 to 2004 using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9), codes from the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital Ambulatory Medical Care Survey NHAMCS) for primary care practices. Rates of ARI/fever visits by month of age were multiplied by the proportion of RSV positive cases in NAMCS and NHAMCS according to month of age to estimate outpatient RSV rates [7]. The proportion of outpatient infants with LRI were published in a later paper by Hall et al. [26] Incidence rates of outpatient LRI were estimated by multiplying the proportion of infants with LRI by the outpatient incidence rates in each age group (Table 1).
Lively et al. performed a recent analysis of NVSN outpatient data collected between November and April in the 2004–2009 RSV seasons in children aged 0–23 months [2]. Case ascertainment methods for this study were the same as those described in the Hall et al. study [7] (Table 1).
2.1.2. Hospitalization
We selected three studies for comparison of hospitalization rates (Table 1). Two studies were nationally representative medical encounter database studies and one study was an active prospective surveillance study. The studies used different methods; study periods overlapped for some years.
Hall et al. conducted active, prospective inpatient surveillance in the NVSN over 4 years from October 2000 until September 2004 in children 0–59 months in counties surrounding Rochester and Nashville and from 2003 until 2004 in Cincinnati. Eligible children were those who received a diagnosis of acute respiratory infection. Children were enrolled within 48 h after admission from Sunday through Thursday. During the study period, surveillance hospitals cared for more than 95% of each county’s children. Nasal and throat swabs were collected from each enrolled patient. RSV was detected by a RT-PCR assay. The weighted number of hospitalizations for RSV infection per 1000 children were expressed as the weighted number of RSV hospitalizations divided by the number of children in the county population determined by the 2000 US Census, multiplied by 1000. Rates were calculated by weighting the observed number of enrolled RSV hospitalizations to account for sampling 4 days per week and eligible patients who were not enrolled, by age < 1 year (<12 months), 1 year (12–23 months), and 2 to <5 years (24–59 months), with sites as sampling strata [7] (Table 1).
Paramore et al. retrospectively analyzed cross-sectional data from year 2000 from the Healthcare Cost & Utilisation Project’s Nationwide Inpatient Sample, a nationally representative federally funded medical encounter database, for children <5 years. Identification of RSV infection-associated hospitalization was based on at least one of the following ICD-9 diagnosis codes 079.6 (RSV); 466.11 (acute bronchiolitis due to RSV infection); 466.19 (acute bronchiolitis due organisms other than RSV; and 480.1 (pneumonia due to RSV infection) [4]. Sampling weights were assigned to each record for the calculation of rates [4] (Table 1).
Stockman et al. analyzed data from the National Hospital Discharge Survey from 1997 to 2006 for children 0–59 months. RSV-associated hospitalizations were classified using the ICD-9-CM codes: 466.11 (RSV bronchiolitis), 480.1 (RSV pneumonia), 079.6 (RSV, other), and bronchiolitis (466.1), acute bronchiolitis due to an infectious organism (466.19), pneumonia (480–486); for the second group of ICD-9 codes, Stockman estimated proportions of bronchiolitis (30%) and pneumonia (20%) hospitalizations likely to be associated with RSV infection from NVSN data [7]. Rates of hospitalization were based on bridged race census estimates from 1997 to 2006 (Table 1) [5].
3. Results
3.1. Outpatient visits
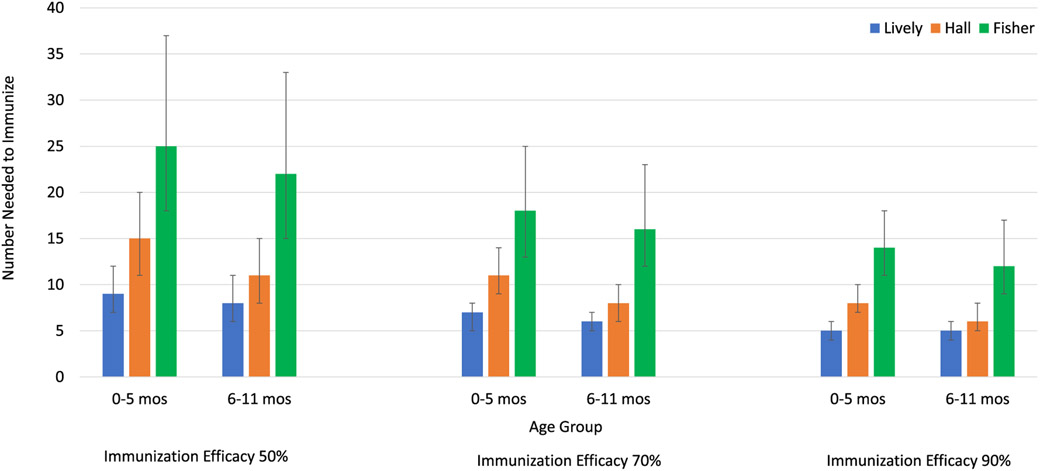
Among 0–5-month-old infants, 9–25 (95% CI 7–37) need to be immunized at 50% IE, 7–18 (95% CI 5–25) at 70% IE, and 5–14 (95% CI 4–18) at 90% IE to prevent 1 RSV-associated outpatient visit (Table 1, Fig. 1). Among 6–11-month-old infants, 8–22 (95% CI 6–33) would need to be immunized at 50% IE, 6–16 (95% CI 5–23) at 70% IE, and 5–12 (95% CI 4–17) at 90% IE to prevent 1 RSV-associated outpatient visit (Table 1, Fig. 1). These results indicate for an EHL-mAb with 70% efficacy 6–18 infants would need to be immunized to prevent 1 RSV-associated outpatient visit in the first year of life.
Fig. 1.
Number Needed to Immunize to Avert One RSV-Associated Pediatric Outpatient Visit in Infants by Age Group and Immunization Efficacy.
3.2. Outpatient LRI
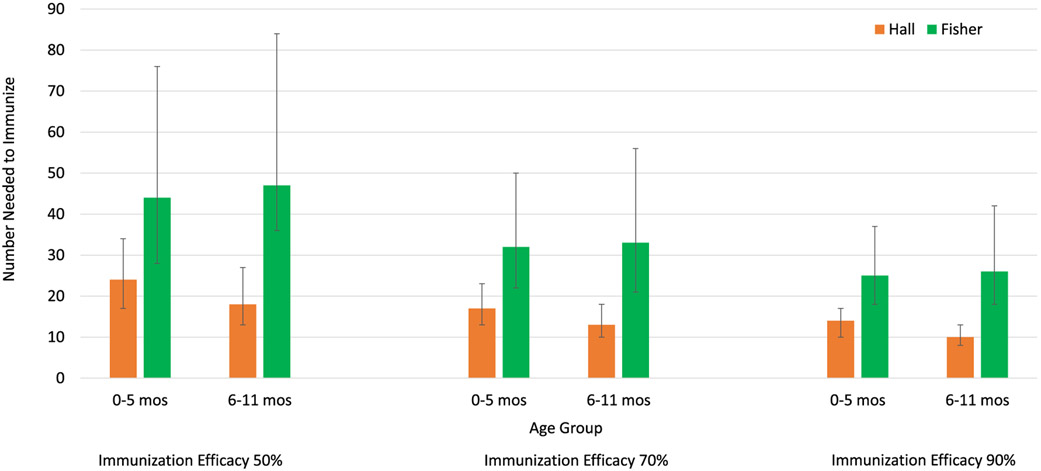
Among 0–5-month-old infant, 24–44 (95% CI 17–76) would need to be immunized at 50% IE, 17–32 (95% CI 13–50) at 70% IE, and 14–25 (95% CI 10–37) at 90% IE to prevent 1 RSV-associated LRI outpatient visit (Table 1, Fig. 2). Among 6–11-month-old infants, 18–47 (95% CI 13–84) would need to be immunized at 50% IE, 13–33 (95% CI 10–56) at 70% IE, and 10–26 (95% CI 8–42) at 90% IE to prevent 1 RSV-associated LRI outpatient visit (Table 1, Fig. 2). These results indicate for an EHL-mAb with 70% efficacy 13–33 infants would need to be immunized to prevent 1 RSV-associated LRI outpatient visit in the first year of life.
Fig. 2.
Number Needed to Immunize to Avert One RSV-Associated Outpatient Lower Respiratory Infection in Infants by Age Group and Immunization.
3.3. Hospitalization
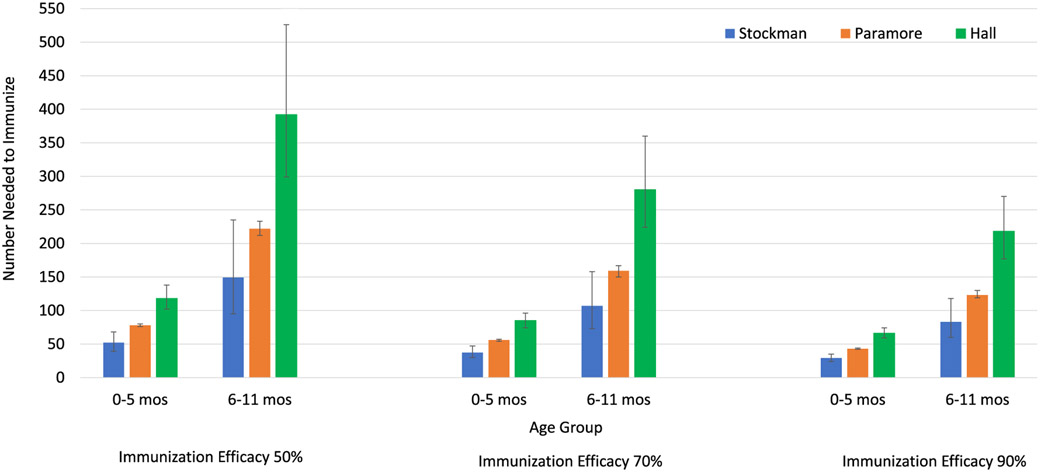
Rates of RSV-associated hospitalization were much higher in 0–5-month-old infant compared to those 6–11 months of age, thus NNI to prevent hospitalization was lower among infants 0–5 months of age. Among 0–5-month-old infants, 52–118 (95% CI 39–138) would need to be immunized at 50% IE, 37–85 (95% CI 30–96) at 70% IE, and 29–66 (95% CI 24–74) at 90% IE to prevent 1 RSV-associated hospitalization (Table 1, Fig. 3). Among 6–11-month-old infants, 149–392 (95% CI 95–526) would need to be immunized at 50% IE, 107–280 (95% CI 73–360) at 70% IE, and 83–218 (95% CI 60–270) for 90% to prevent 1 RSV-associated hospitalization (Table 1, Fig. 3). These results indicate for an EHL-mAb with 70% efficacy 37–280 infants would need to be immunized to prevent 1 RSV-associated hospitalization in the first year of life. Approximately three times as many infants 6–11 months of age would need to be immunized to prevent 1 RSV-attributable hospitalization compared those 0–5 months of age.
Fig. 3.
Number Needed to Immunize to Avert One RSV-Associated Hospitalization in Infants by Age Group and Immunization Efficacy.
4. Discussion
This analysis demonstrates that infant immunization with an RSV EHL-mAb of even moderate efficacy could have a high impact. The estimated NNI for the RSV EHL-mAb was lower than the NNVs for several vaccines against other pathogens included in the routine childhood immunization schedule in the United States. For an influenza vaccine with 50% efficacy, 1000–3000 children 6–23 months of age would need to be vaccinated to prevent 1 influenza-attributable hospitalization, and 12–42 children vaccinated to prevent 1 influenza-attributable outpatient visit [23]. For a EHL-mAb with 50% efficacy, an estimated 149–392 infants 6–12 months of age would need to be immunized to prevent 1 RSV-attributable hospitalization and 8–22 infants to prevent 1 RSV-attributable outpatient visit. For live pentavalent rotavirus vaccine at 85% efficacy, an estimated 200 infants would need to be immunized to prevent 1 rotavirus-associated hospitalization [28]. For an RSV EHL-mAb with 70% efficacy, an estimated 37–280 infants would need to be immunized to prevent 1 RSV-attributable hospitalization. The NNV for pneumococcal disease is substantially higher than the NNI for the RSV EHL-mAb. An estimated 671 infants would need to be immunized with pneumococcal conjugate vaccine to prevent one case of invasive pneumococcal disease [29] and an estimated 448 children <36 months of age would need to be immunized to prevent 1 case of consolidated community acquired pneumonia [28].
We took a conservative approach to the estimation of NNI. We used a wide range of immunization efficacies given that the efficacy of the RSV EHL-mAb is not yet determined. We used 70% as the mid-range reference point given similar recent Phase 2 efficacy results for a EHL-mAb in development [30]. We varied incidence estimates as seasonal epidemics of RSV vary from year to year and the incidence of medically attended visits may vary by geography due to health care utilization and access. In addition, we estimated the NNI for outpatient LRI. LRI is clinically meaningful and the NNI to prevent outpatient LRI may resonate globally with policymakers whose incidence of outpatient visits and hospitalization may differ by geography but for whom outpatient LRI may be a more constant measure.
Our analysis has some limitations. One of the primary criticisms of NNV is that it does not account for potentially significant indirect or dynamic effects of vaccines on the broader population [31]. The RSV EHL-mAb is targeted at infants and high-risk children <2 years of age (<2% of population) and is projected to confer immunity in this population for six months or less. Household genomic studies indicate that infants are not a primary source of transmission in the household but instead likely to get infected from older children [32,33]. Evidence from a modeling study that evaluated the maternal immunizations strategy, indicated that an immunization strategy in a narrow age group with a limited duration of protection is unlikely to provide indirect protection [34]. Thus, the dynamic effect of immunization strategies on the broader population is expected to be limited. If the mAb conferred indirect protection, that would make our estimates even more conservative.
We selected incidence studies that had included population rates per 1000 infants/children, that represented a two to threefold range of incidence and that used different methods of incidence estimation to reduce the bias associated with a given method. However, all methods of RSV incidence estimation are imperfect, and underestimation of incidence has been described in studies using population-based active surveillance [35] and in studies using ICD coding for identification of cases [36]. Underestimation of incidence would decrease the NNI, thus the true RSV EHL-mAb NNI may be even lower than estimated. Overall, we believe our estimates are conservative.
RSV infection may be severe in infants and young children born with severe prematurity, chronic lung disease other respiratory conditions [3], and hemodynamically significant congenital heart disease, leading to high incidence of hospitalization and outpatient visits [37]. Incidence of RSV hospitalization in this high-risk population is 2–10 times that of the healthy pre-term and full-term population [38]. Children with conditions that place them at high risk of severe disease are included in overall incidence estimates. Given the high incidence of medically attended visits in high-risk children, we would expect the NNI to be very low within these risk groups. It would be worthwhile to explore the specific benefits of the EHL-mAb in children with high-risk conditions, including children ≥1 year of age for which the rates of severe RSV-associated outcomes are high [3].
Most children are infected with RSV in the first year of life [39] and the greatest burden of severe RSV infection occurs in infants, perhaps due to a combination of lung and immunological immaturity [8]. Because RSV infection does not induce durable immunity [40], repeated infections occur during childhood and adulthood [39]. Reinfections are generally milder than primary infections evidenced by milder symptoms and lower levels of viral shedding [41-42] and by lower rates of RSV-associated hospitalization after one year of age. EHL-mAbs have a limited duration of protection and infants immunized in the first season may be susceptible to primary infection in the season after immunization. An increase in clinically significant RSV disease in the year following immunization would decrease the overall impact of the EHL-mAb. However, follow-up studies of infants and children prophylaxed with palivizumab or motavizumab monoclonal antibody have demonstrated that children were not at risk for increased rates of severe RSV disease in the subsequent seasons [43-45]. Follow-up for 510 days after immunization is planned in an ongoing RSV EHL-mAb clinical trial [21] and may provide information about the risk of clinically significant RSV disease in the year after immunization.
5. Conclusion
Population health benefits such as disease cases averted due to immunization, are of prime importance when considering the introduction of a new immunization into a national immunization program. The NNI is useful as it expresses the vaccine-mediated reduction in the underlying disease burden in an unvaccinated population [46]. The NNI can also provide a conceptual measure for the cost of preventing one disease event as the product of the number of doses needed and the immunization cost per dose [20,47]. The NNI is easy to understand and communicate and may help express the value of immunization to a variety of different stakeholders for whom complex output derived from economic or mathematical models may be more difficult to digest. The RSV EHL-mAb NNI compares favorably with other childhood vaccines included in the routine vaccination schedule. Given the competition for and scarcity of public health resources, these data provide an additional insight for resource allocation and public health decision making.
Acknowledgements:
The authors would like to acknowledge Marc Lipsitch, D.Phil for his thoughtful contribution to the manuscript. Dr. Goldstein’s work was supported by Award Number U54GM088558 from the National Institute of General Medical Sciences. Drs. Finelli and Choi are employees of Merck, Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and their work was supported by Merck Sharp & Dohme Corp.
Abbreviations:
- ARR
Absolute Rate Reduction
- CI
Confidence Interval
- EHL-mAbs
Extended half-life RSV monoclonal antibodies
- IE
Immunization efficacy
- ICD-9
International Classification of Diseases, Ninth Revision, Clinical Modification
- LL
Lower Limit
- LRI
Lower respiratory infection
- NAMCS
National Ambulatory Medical Care Survey
- NHAMCS
National Hospital Ambulatory Medical Care Survey
- NVSN
New Vaccine Surveillance Network
- NNI
Number needed to immunize
- NNV
Number needed to vaccinate
- RT-PCR
Real-time polymerase chain reaction
- RSV
Respiratory syncytial virus
- UL
Upper Limit.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Piedra PA. Long term healthcare costs associated with respiratory syncytial virus infection in children: the domino effect. J Infect Dis 2019:jiz161. 10.1093/infdis/jiz161 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [2].Lively JY, Curns AT, Weinberg GA, et al. Respiratory syncytial virus associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc 2019;8:284–6. [DOI] [PubMed] [Google Scholar]
- [3].Goldstein E, Finelli L, O’Halloran A, et al. Hospitalizations associated with respiratory syncytial virus (RSV) and influenza in children, including children diagnosed with asthma. Epidemiology 2019;30:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22:275–84. [DOI] [PubMed] [Google Scholar]
- [5].Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr Infect Dis J 2012;31:5–9. [DOI] [PubMed] [Google Scholar]
- [6].Arriola C, Kim L, Langley G, et al. Estimated burden of community-onset respiratory syncytial virus-associated hospitalizations among children aged <2 years in the United States, 2014–15. J Pediatr Infect Dis Soc 2019. 10.1093/jpids/piz087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatr 1998;102:531–7. [PubMed] [Google Scholar]
- [10].American Academy of Pediatrics, Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. Technical report: updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatr 2014;134:415–20. [DOI] [PubMed] [Google Scholar]
- [11].Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018;18(10):e295–311. [DOI] [PubMed] [Google Scholar]
- [12].Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development – a global agenda. Vaccine 2016;34:2870–5. [DOI] [PubMed] [Google Scholar]
- [13].August A, Glenn GM, Kpamegan E, et al. A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine 2017;35:3749–59. [DOI] [PubMed] [Google Scholar]
- [14].Domachowske JB, Khan AA, Esser MT, et al. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J 2018;37:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tang A, Chen Z, Cox KS, et al. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat Commun 2019;10:4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maas B, Wolford D, Fayad G, et al. RSV monoclonal antibody (MK-1654) phase 1 pharmacokinetics (PK) in healthy adults and population PK modeling to support pediatric development. Open Forum Infect Dis 2018;5(Suppl 1):S424–5. [Google Scholar]
- [17].Acedo L, Díez-Domingo J, Moraño JA, Villanueva RJ. Mathematical modelling of respiratory syncytial virus (RSV): vaccination strategies and budget applications. Epidemiol Infect 2010;138:853–60. [DOI] [PubMed] [Google Scholar]
- [18].Cromer D, van Hoek AJ, Newall AT, Pollard AJ, Jit M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health 2017;2:e367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meijboom MJ, Rozenbaum MH, Benedictus A, et al. Cost-effectiveness of potential infant vaccination against respiratory syncytial virus infection in The Netherlands. Vaccine 2012;30:4691–700. [DOI] [PubMed] [Google Scholar]
- [20].Kelly H, Attia J, Andrews R, Heller RF. The number needed to vaccinate (NNV) and population extensions of the NNV: comparison of influenza and pneumococcal vaccine programmes for people aged 65 years and over. Vaccine 2004;22:2192–8. [DOI] [PubMed] [Google Scholar]
- [21].ClinicalTrials.gov. Bethesda (MD): US National Library of Medicine (US). Identifier NCT03979313, a study to evaluate the safety and efficacy of MEDI8897 for the prevention of medically attended RSV LRTI in healthy late preterm and term infants (MELODY); 2019. June 7 [cited 2020 Jan 10]; Available from: https://clinicaltrials.gov/ct2/show/NCT03979313?term=NCT03979313&draw=2&rank=1. [Google Scholar]
- [22].ClinicalTrials.gov [Internet]. Bethesda (MD): US National Library of Medicine (US). Identifier NCT02624947, A Study to Determine the Safety and Efficacy of the RSV F Vaccine to Protect Infants Via Maternal; 2015. December 9 [cited 2020 Jan 10]; Available from: https://clinicaltrials.gov/ct2/show/NCT02624947?term=NCT02624947&draw=2&rank=1. [Google Scholar]
- [23].Lewis EN, Griffin MR, Szilagyi PG, Zhu Y, Edwards KM, Poehling KA. Child-hood influenza: number needed to vaccinate to prevent 1 hospitalization or outpatient visit. Pediatr 2007;120:467–72. [DOI] [PubMed] [Google Scholar]
- [24].Fisher RG, Gruber WC, Edwards KM, et al. Twenty years of outpatient respiratory syncytial virus infection: a framework for vaccine efficacy trials. Pediatr. 1997;99(2):e7. [DOI] [PubMed] [Google Scholar]
- [25].Lee M, Walker RE, Mendelmen PM. Medical burden of RSV and parainfluenza virus type 3 among US children. Human Vac 2005;1:6–11. [DOI] [PubMed] [Google Scholar]
- [26].Hall CB, Simoes EAF, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol 2013;372:39–57. [DOI] [PubMed] [Google Scholar]
- [27].Bender R Calculating confidence intervals for the number needed to treat. Control Clin Trials 2001;22:102–10. [DOI] [PubMed] [Google Scholar]
- [28].Gessner BD, Wilder-Smith A. Estimating the public health importance of the CYD-tetravalent dengue vaccine: vaccine preventable disease incidence and numbers needed to vaccinate. Vaccine 2016;34:2397–401. [DOI] [PubMed] [Google Scholar]
- [29].Palmu AA, Jokinen J, Nieminen H, et al. Vaccine-preventable disease incidence of pneumococcal conjugate vaccine in the Finnish invasive pneumococcal disease vaccine trial. Vaccine 2018;36:1816–22. [DOI] [PubMed] [Google Scholar]
- [30].Griffin MP, Yuan Y, Takas T, et al. MEDI8897 prevents serious RSV disease in healthy preterm infants (Abstract #901). In: Proceedings of IDWeek 2019, October 3-7, 2019. Washington DC. [Google Scholar]
- [31].Tuite AR, Fisman DN. Number-needed-to-vaccinate calculations: Fallacies associated with exclusion of transmission. Vaccine 2013;31:973–8. [DOI] [PubMed] [Google Scholar]
- [32].Agoti CN, Phan MVT, Munywoki PK, Githinji G, Medley GF, Cane PA, Kellam P, Cotten M, Nokes DJ. Genomic analysis of respiratory syncytial virus infections in households and utility in inferring who infects the infant. Sci Rep 2019;9(1). 10.1038/s41598-019-46509-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Munywoki PK, Koech DC, Agoti CN, et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis 2014;209:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pan-Ngum W, Kinyanjui T, Kiti M, et al. Predicting the relative impacts of maternal and neonatal respiratory syncytial virus (RSV) vaccine target product profiles: a consensus modelling approach. Vaccine 2017;35:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Grijalva CG, Weinberg GA, Bennett NM, et al. Estimating the undetected burden of influenza hospitalizations in children. Epidemiol Infect 2007;135:951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cai W, Tolksdorf K, Hirve S, et al. Evaluation of using ICD-10 code data for respiratory syncytial virus surveillance. Influenza Other Respi Viruses. 2019;00:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Manzoni P, Figueras-Aloy J, Simoes EAF, et al. Defining the incidence and associated morbidity and mortality of severe respiratory syncytial virus infection among children with chronic diseases. Infect Dis Ther 2017;6:383–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boyce TG, Mellen BG, Mitchel EF, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr 2000;137:865–70. [DOI] [PubMed] [Google Scholar]
- [39].Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543–6. [DOI] [PubMed] [Google Scholar]
- [40].Sande CJ, Cane PA, Nokes DJ. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine 2014;32:4726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of Age. Pediatr 2013;132:e341–8. [DOI] [PubMed] [Google Scholar]
- [42].Openshaw PJM, Chiu C, Culley FJ, Johansson C. Protective and harmful immunity to RSV infection. Annu Rev Immunol 2017;35:501–32. [DOI] [PubMed] [Google Scholar]
- [43].Butt ML, Elliot L, Paes B. Respiratory syncytial virus hospitalization and incurred morbidities the season after prophylaxis. Paediatrics Child Health 2018;23:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Campbell AL, Pollack PF, Fredrick LM, Groothuis JR. RSV hospitalization rates in preterm children in the season following palivizumab prophylaxis compared to children without palivizumab prophylaxis. Arch Dis Child. 2008;93(Suppl II):339–46. [Google Scholar]
- [45].Driscoll A, Hammitt L, Gern J, et al. Does prevention of RSV disease by monoclonal antibody result in rebound RSV disease? [Abstract 108] In: 11th International respiratory syncytial virus symposium, October 31–November 4, 2018. Ashville NC: International Society for Influenza and other Respiratory Virus Diseases; 2018. [Google Scholar]
- [46].Gessner BD, Kaslow D, Louis J, et al. Estimating the full public health value of vaccination. Vaccine 2017;35:6255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Heikkinen T, Valkonen H, Lehtonen L, Vainionpaa R, Ruuskanen O. Hospital admission of high-risk infants for respiratory syncytial virus infection: implications for palivizumab prophylaxis. Arch Dis Child Fetal Neonatal Ed 2005;90:F64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]