Abstract
Background
Knowledge about the priorities and preferences of people living with dementia (PwD) might help to individualize treatment, care, and support, which could improve patient-related outcomes. This study aimed to summarize preferences of PwD or people with mild cognitive impairment (MCI), considering all relevant aspects of health care and everyday life.
Methods
We conducted a systematic literature review and included studies about patient preferences published in English between January 1, 1990 and October 28, 2019. The inclusion criteria were that preferences were elicited directly by PwD or patients with MCI. We used the International Consortium for Health Outcomes Management value set for dementia to categorize the preferences into the following topics: a) clinical status, b) symptoms, functioning, and quality of life, and c) sustainability of care.
Results
Of 578 initially identified studies, 45 met the inclusion criteria. Patients preferred to be informed about the diagnosis as early as possible, especially for anticipatory care planning. They ranked caregiver quality of life as their highest priority. They preferred not to be a burden to others more than their caregivers’ mood, their own functional status, or their own distressing behaviors. Furthermore, PwD are eager to participate in medical decisions, especially in those about creating an everyday life routine. PwD preferred their own quality of life, self-efficacy, and emotional well-being. Institutionalized PwD preferred individualized and person-centered care. According to the sustainability of care, PwD preferred to maintain close bonds with their family at the end of their life and wanted to be treated with empathy.
Conclusion
This systematic review provides essential insights into cognitively impaired patients’ preferences, which are rarely considered in treatment, care, and support services. Further studies should evaluate whether considering preferences in treatment and care or daily living can improve patient-reported outcomes.
Keywords: dementia, patient preference, patient outcome assessment, decision-making
Introduction
Dementia represents one of the most significant public health challenges. Worldwide, approximately 50 million people live with dementia.1,2 A diagnosis as early as possible is essential to initiate evidence-based treatment and care and to better cope with the disease. The progressive nature of the disease leads to declining self-responsibility levels, self-determination, and autonomy, which are associated with an increasing need for care. Decisional capacity steadily decreases, but persons with dementia (PwD) wish to be acknowledged in all disease stages and aspects of care, make individual decisions, and be involved in decisions about treatment and care, daily living, and support.3–7
Dementia care should promote patient autonomy and be person-centered and, thus, be preference-based, respecting the patient’s values and the need to improve the patient’s sense of self-efficiency.8,9 It is also important to offer patients a purpose in life, open up perspectives for them, support them, and transfer responsibility to them.10 Furthermore, informal caregivers need to be considered.11 In the progression of the diseases, many PwD experience emotional changes and difficulties with how to express and manage their feelings, which can lead to more intense emotional reactions, which can be a great challenge for caregivers.12 Additionally, it can be a challenge to meet the expectations of PwD when the share of responsibilities changes between the person with dementia and his or her caregiver.13
It is often assumed that informal caregivers know the preferences of PwD best. However, some studies found that more than one-third of caregivers could not predict PwD preferences correctly.14 Most caregivers try to compensate for the decreasing abilities to support the patient’s well-being but might sometimes project their own preferences instead of putting themselves into the position of the PwD.14,15 Furthermore, caregivers’ preference prediction for PwD can subconsciously be influenced by how the caregivers’ own life is affected by certain decisions.7 Such preference divergences have also been elicited between nursing care staff and patients. Dementia patients in long-term care appreciate autonomy, whereas staff assumed safety and a homey ambience were the most important, demonstrating the existence of discrepancies between professionals and patients.16
Knowing preferences for PwD could inform recommendations for dementia care practices, increase adherence to treatment, therapies, and care, and improve and individualize interventions and patient-related outcomes, including health-related quality of life (QoL).17–19 A systematic review that summarizes the existing qualitative and quantitative evidence about PwD preferences is currently lacking. Therefore, this systematic review aimed to summarize the preferences of PwD, including all relevant domains of treatment, care, support, and everyday life.
Methods
Search Strategy
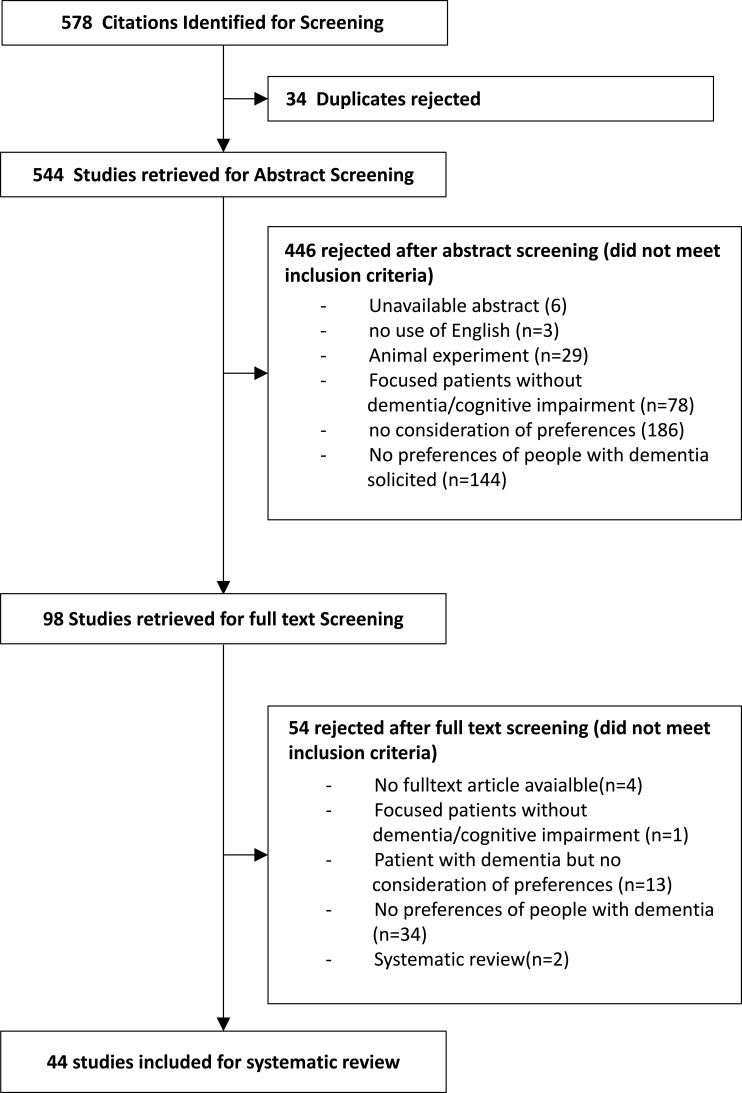
Studies published in English between January 1, 1990 and October 28, 2019 were identified through searches of the electronic databases PubMed, Science Direct, and Cochrane. Key phrases included “dementia”, “Alzheimer”, or “Alzheimer’s disease” in combination with “preferences” or “priority”. Titles and abstracts were reviewed for dementia terms, whereas only the title was considered for preference terms. In parallel, a search of MeSH terms was conducted in the PubMed database to find all articles that used the terms “mild cognitive impairment” or “dementia” in combination with “patient preference”. The systematic literature search process is presented in Figure 1.
Figure 1.
Study flow chart (CONSORT). Data from ICHOM standard set for Dementia. Available from: https://connect.ichom.org/standard-sets/dementia/.20
Data Extraction
Studies were included if i) patients were diagnosed with dementia, Alzheimer’s disease, or mild cognitive impairment or ii) priorities and preferences were directly elicited from PwD or patients with MCI. All studies were included, irrespective of the quantitative or qualitative methods used to elicit preferences. Studies were excluded if they reported animal experiments or if no abstract was available. Additionally, systematic reviews and studies eliciting preferences with hypothetical patients from the general population were excluded. A total of 578 studies were found. After removing 34 duplicates, 544 studies remained to be screened for titles and abstracts by two independent reviewers. A third reviewer was involved in discussing disagreements. Overall, 446 studies were excluded after title and abstract screening, and 98 studies remained for full text screening. After full text screening, an additional 54 studies were rejected, resulting in the inclusion of 44 studies. The study flow chart of the screening procedure is demonstrated in Figure 1.
Data Description
To categorize the heterogeneous studies with respect to their evaluated domains, we used the Standard Set of Dementia Criteria of the International Consortium for Health Outcomes Management (ICHOM).20 Experts and patient representatives developed this standardized set of outcomes to focus on what is most important for PwD. As this systematic review was focused on preferences of PwD and not the disease in general, the Standard Set was modified and adapted. Specifically, the category “disease and progression” was replaced by the categories “treatment and care” and “diagnosis”. Given that some studies used modern assistive technology, we added the category “technology”. Caregiver QoL was removed, as preferences of PwD and not of their caregivers were the focus of this study. In cases in which study outcomes overlapped across two or more categories, three reviewers discussed the categorization. The final set categorized the elicited preferences of PwD into the following three main domains and subcategories: (A) clinical status including i) treatment and care and ii) diagnosis, (B) symptoms, functioning, and QoL, including i) cognitive, ii) social, iii) daily living, and iv) overall QoL, and (C) sustainability of care, including i) time to full-time care, ii) end-of-life care, and iii) assistive technology.
Results
Characteristics of the Included Studies
Most of the studies were published in Europe (n = 21) and the USA (n = 14). The ages of the study participants ranged from 57 to 87 years. Sample sizes differed substantially, ranging from two to 2028. An overview, including summaries of the included studies, is presented in Table 1.
Table 1.
Study Characteristics and Results Preference Studies of People with Dementia (PwD)
| Area | Author/Year | Population/Country | Aim of Study | Mean Age | Study Description | n | Preferences |
| Symptoms, Functioning and Quality of Life | |||||||
| Cognitive | Raetz et al 201327 | PwD under a 24-hour care by FM or staff, USA | To identify stimuli to ↑ engagement and to ↓ negative effects and problem behavior | 82 years | Paired-stimulus preference assessments | 8 PwD | • The use of a single-array presentation is as effective as the use of a three-array presentation to identify preferences • For some PwD, preferences remain stable |
| Wijk et al 199721 | PwD recruited from a psychiatric clinic, Sweden | To examine the ability to detect color naming/discrimination | 80 years | Descriptive preference assessments | 50 PwD | • Cognitive decline influenced the naming of mixed colors • preferences for colors are stable despite the disease |
|
| McMurtray et al 200974 | Japanese PwD who presented at a memory disorder clinic, Hawaii | To determine the influence of dementia on the native speech of bilingual PwD | 76 years | Language assessments | 2 PwD | • A regression to the primary language of bilingual PwD may help clinicians to identify dementia | |
| Lombrozo et al 200724 | HA from Boston, PwD from a general hospital, USA | To examine explanatory judgments in PwD | 84 years | Explanation choice task, causal-belief task | 24 HA, 17 PwD | • Unlike HA, PwD prefer teleological explanations | |
| Halpern et al 200723 | PwD living at home, HA from a senior citizen center, USA | To explore the stability of art preferences | 78 years | Comprehensive evaluation and neuropsychological tests | 16 PwD, 27 HA | • Aesthetic preferences can be preserved in PwD and are not dependent on explicit memory | |
| Stanzani Maserati et al 201922 | PwD were recruited from to a cognitive disorder center of Bologna, Italy | To explore the emotional state of PwD through color choice preference | 77 years | Lüscher colour test | 121 PwD, 68 HA | • With a higher severity of the disease, auxiliary colors like violet and brown are preferred, whereas black and gray are rejected | |
| Motzek et al 201753 | PwD at a general acute care hospital in Dresden, Germany | To identify picture categories that are liked and easily remembered by PwD | 78 years | PwD votes of preferences and the ability to recall pictures were tested | 37 PwD | • A main effect of familiarity on positive votes and recall pictures was seen | |
| Social | Feliciano et al 200925 | PwD living in 6 different long-term care settings, USA | To examine the use of stimuli in behavioral management | 86 years | Preference assessment | 11 PwD | • Using preferred items in behavioral management: ↓ agitated behaviors and partially ↓ depressive symptoms |
| Van de Ven et al 201728 | PwD living in 23 different care networks in the Netherlands | To describe the decision-making process of PwD, shared decision-making | 80 years | Qualitative study, a secondary analysis of 117 interviews | 23 PwD, 44 CG, 45 Prof. | • Collaborative deliberation model is extended by engaging the network, recognizing the need for a decision, defining/ evaluating decisions, trying out alternatives … | |
| Lopis et al 201754 | PwD were recruited from a local memory center, France | To determine if eye contact influences the likeability/memory relating to faces in HA/PwD | 81 years | Identifying faces they had previously seen during a surprise recognition test | 19 PwD, 40 HA | • PwD show a positive correlation between ratings of likeability and recognition scores | |
| Daily living | Cohen-Mansfield et al 201030 | PwD out of 7 nursing homes in Maryland, USA | To study whether activities/stimuli based on PwD interests result in more engagement than other activities | 86 years | The expanded version of the self-identify question-naire75 was used to identify PwD interests | 193 PwD | • PwD with current interests in art, pets, and music were more engaged with stimuli that were related to these interests than PwD without these interests |
| Cohen-Mansfield et al 200955 | PwD at four nursing homes in Maryland, USA | To examine the influence of stimulus attributes on the engagement of PwD | 86 years | 1-on-1 interview, systematic observations via OME | 69 PwD | • Preference for the work-related rather than the manipulative stimuli, preference for small rather than large blocks | |
| Chong et al 201229 | Australian patients | To show PwD attitudes toward physical activity | 76 years | Analysis of focus groups, individual interviews | 36 PwD, 14 HA | • Preferred activity program: simple/light/safe activities, ac- cessible group setting, adjusted to individual income/interest | |
| Mungas et al 199076 | PwD at the UC Davis Medical Center, Sacramento, USA | To compare the sweet food cravings of PwD to HA | 76 years | A food preference survey | 45 PwD, 43 HA | • Compared to normal controls, PwD show an increased preference for sugar and sweet foods | |
| Milte et al 201732 | PwD recruited from rest homes, Australia | To describe the food experience of PwD in rest homes | 78 years | 1-on-1 interview, focus groups with PwD and FM | 19 PwD and FM | • Maintaining choice and individual preferences around food is important to PwD | |
| Ortega et al 201277 | PwD living at home, USA | To find the most preferred item in preference assessments | 84 years | Paired-stimulus procedure to assess preferences of 4 edible and 4 leisure items | 14 PwD | • A leisure activity was the most preferred in the preference assessment | |
| Van’t Leven et al 201931 | Dutch patients | To test how activating dyadic interventions fit the needs and preferences of PwD and CG | 78 years | Qualitative design with semistructured interviews | 27 PwD, 34 CG, 19 Prof. | • Five factors influenced dyad “fit”: timing, need for an activity, lifestyle, apart-or-together, and meaning of (lost) activity | |
| Overall quality of life | Bohn et al 201859 | PwD living in long-term care facilities, Edmonton area, Canada | To study if PwD prefer emotional gratification, despite their cognitive decline | 84 years | Social activity preference card-sort task | 75 HA, 23 PwD | • PwD prefer emotionally meaningful ends and retain high levels of well-being |
| Barrios et al 201618 | Couples of the summer 2014 HABIT session at Mayo Clinic, Rochester, USA | Ranking of behavioral outcomes of PwD, pre and post ranking after participation | 77 years | Ranking task | 16 dyads (PwD+HA) | • Preferences of PwD in descending order: PwDs QoL, self- efficacy, CGs QoL, depression of CG, CG burden, daily functioning, anxiety of CG | |
| Smith et al 201833 | HABIT program completers (2008–2014), USA | Outcome and treatment preferences of PwD | 73 years | Ranking task | 29 PwD, 54 spouses | • Preferences of PwD in descending order: QoL, self- efficacy, depression, CG QoL, PwDs anxiety/, CG burden | |
| Shelton et al 201634 | American patients recruited from the northern Ohio region, USA | To understand how actual and perceived incongruence of care preferences affects the social well-being of PwD/CG | 73 years | In-depth interviews | 128 dyads (PwD+CG) | • Actual incongruence for socioemotional care preferences was a predictor of greater relationship strain and worse mood for PwD, whereas perceived incongruence for socioemotional care preferences was related to lower QoL | |
| Reamy et al 201135 | Dyads from research/service organizations, USA | To find discrepancies in care preferences of PwD and CG | 76 years | Interviews | 266 dyads (PwD+CG) | • CG underestimated the PwDs values for autonomy, burden, control, family, and safety | |
| Clinical status | |||||||
| Treatment | Harrison Dening et al 201626 | Dyads were identified by psychiatrists in the UK | To explore if CG and PwD have the same end-of-life care preference | 79 years | Cross-sectional study, LSPQ | 60 dyads (PwD+CG) | • CG had low to moderate agreement with PwD on preferences for end-of-life care treatment |
| Dickinson et al 201343 | PwD identified by health-care prof. in the UK | To investigate views of PwD on ACP | Qualitative study with semistructured interviews | 17 PwD, 29 CG | • 5 barriers to ACP: lack of knowledge, finding the right timing, lack of support, preference for informal plans vs written documentation, lack of choice around future care | ||
| Cohen et al 201945 | PwD recruited from 64 Boston-area facilities, USA | To examine concordance between advance directives and proxy care preferences among PwD | 87 years | A cluster randomized clinical trial | 328 dyads (PwD+HA) | • The most prevalent directive: DNR, least common: antibiotics • Concordance between directives and each level of care preference: comfort, 7%; basic, 49%; and intensive, 58% |
|
| Area | Author/Year | Population/Country | Aim of Study | Mean Age | Study Description | N | Main Outcome |
| Diagnosis | Jung et al 201737 | PwD visiting the psychiatric outpatient clinic of Seoul University Hospital, Korea | To identify preferences regarding the disclosure of dementia and the awareness of ACP | 75 years | Structured questionnaire | 98 PwD, 62 FM | • A disclosure of the dementia diagnosis was favored • The majority of PwD also agreed on the necessity of ACP |
| Mahieux et al 201839 | PwD attending a memory clinic in Paris, France | To report disclosure preferences of PwD | 78 years | Prospective single-center study | 737 PwD, 268 HA | • 85% wished to be informed of a dementia diagnosis, 7% did not want to know, and 7% were not sure | |
| Hellström et al 201242 | Pwd recruited from two memory clinics in Sweden | To report the understanding and experiences of PwD | 71 years | Qualitative interviews | 28PwD, 20 HA, 9 CG | • Regarding couples, the PwD are the ones deciding about the disclosure of their disease to others | |
| Elson et al 200538 | Patients over 65, UK | To report the disclosure preference of PwD | 76 years | Qualitative interviews | 32 PwD, 4 HA | • 2/3 did not know the cause of their memory complaint • 70% of the PwD wanted to know the diagnosis |
|
| Bamford et al 201640 | PwD living in the UK | To assess preferences for diagnostic processes (PET and SPECT), comparison with HA | 76 years | Card sorting exercise relating to importance (ranking) | 68 PwD, 59 CG, 30 HA | • PwD prioritized accuracy over other scan characteristics, followed by helpful staff, comfort, and companion | |
| Walker et al 201772 | PwD recruited from the Alz-heimer’s association, Australia | To show PwDs experience with dementia assessment services | 80 years | Qualitative, semistructured in-depth interviews | 9 PwD, 7 CG | • An important contact during a dementia assessment is the GP | |
| Mate et al 201236 | PwD recruited by 169 GPs in Australia | To examine the predictors of QoL of PwD | 84 years | Cross-sectional study | 167 PwD, 1861 others | • Satisfaction with GP communication is associated with higher QoL | |
| Sustainability of Care | |||||||
| Technology | LaMonica et al 201751 | PwD recruited from Healthy Brain Aging Clinic, Sydney Australia | To show PwD internet use and interest in eHealth technology | 68 years | HBA E-Health questionnaire78 | 160 PwD, 61 HA | • PwD preferred e-health intervention: memory strategy training |
| O’Philibin et al 201852 | PwD were recruited through the Join Dementia research database, UK | To explore preferences of PwD in relation to digital life story work (DLSW) | 62 years | CG responded to DCE and PwD completed an online survey | 67 CGs, 17 PwD | • Most preferred setting: individual one-to-one • Most preferred use of the DLSW was to share memories |
|
| De Sant` Anna et al 201079 | PwD recruited from the Broca Geriatric Day Care Hospital in Paris, France | To compare the use of keyboard, mouse pad, and a computer screen of PwD with HA | Pilot study, qualitative and quantitative analyses | 8 PwD, 10 HA | • Basic computers can be accessible to PwD under certain conditions | ||
| T-t-F-t Care | Dickins et al 201810 | PwD living in Australia | To understand how CG/FM conceptualize the issue of risk | 66 years | 83 semistructured interviews | 53 prof., 20 HA, 7 PwD | • There is no single approach to risks that can be applied to all PwD |
| Groenewoud et al 201550 | PwD living in the Netherlands | Influences on PwD health-care decisions | 57 years | DCE, 11 attributes | 421 PwD, 984 others | • Expertise was the most valued attribute when choosing a health-care provider | |
| Smebye et al 20167 | PwD living at home, Norway | To explore ethical dilemmas concerning PwD autonomy | 83 years | Qualitative, hermeneutic design | 9 PwD, 9 FM, 9 Prof. | • Three ethical dilemmas: PwD autonomy vs CG need to prevent harm, beneficence of FM/CG, autonomy of FM | |
| EoL care | Ayalon et al 201247 | Couples recruited at two psychogeriatric clinics in Israel | To evaluate concordance in end-of-life preferences between PwD and their spouses | 76 years | Face-to-face interviews | 53 couples (PwD+HA) | • Moderate agreement regarding end-of-life care between PwD and their spouses |
| Dening et al 201348 | PwD and CG attending memory assessment service, UK | To explore PwD preferences for end-of-life care | 83 years | Nominal group technique | 12 PwD, 11 CG | • Moderate agreement between PwD and their CG • Preferences of PwD in descending order: maintain family links and independence, feel safe, not be a burden |
|
| Hill et at 201714 | PwD were recruited through internet databases like Join Dementia research, UK | To identify the aspects of end-of-life care for PwD | 73 years | Q-methodology qualitative and quantitative techniques | 14 PwD, 43 CG | • There is no universal opinion on what is important about end-of-life care for PwD | |
| Goodman et al 201344 | PwD living in 6 care homes in the UK | To explore how PwD discuss their priorities and preferences for end-of-life care | 85 years | Exploratory, qualitative study, interviews | 18 PwD | • For PwD, the experiences of living and dying in a care home are inextricably linked • End-of-life care can be improved by documenting the preoccupations, key relationships, and wishes about everyday care of PwD |
|
| Mulqueen et al 201749 | PwD living in a residential care facility, Ireland | To explore preferences of PwD for end-of-life care | 83 years | Nominal group technique | 6 PwD, 6 CG | • Preferences of PwD in descending order: comfort, family presence, communication, familiar staff and surroundings | |
Abbreviations: ↑, increase; ↓, decrease; CG, caregiver; PwD, people with dementia; HA, healthy adults; Prof., professionals; OME, observational measurement of engagement; LSPQ, Life Support Preference Questionnaire; T-t-F-T care, time-to-full-time care.
Diagnosis
PwD and caregivers emphasized the importance of receiving a formal dementia diagnosis. Several studies found that more than 86% of PwD preferred to be informed about the dementia diagnosis.37–39 Those PwD who receive a formal diagnosis show a higher QoL related to social life and the environment than PwD who have not received a formal diagnosis.36 Another study highlighted that PwD would rather receive a false-positive diagnosis than staying undiagnosed.40 Approximately 40% of PwD prefer that their family is present while receiving the diagnosis, and 30% of PwD prefer to be alone.39 However, some PwD also express a feeling of “falling into a black hole” after they receive the diagnosis and explain that it takes time until they receive access to support services.41
In many relationships, PwD decide on the disclosure of the diagnosis to relatives and friends. Concerning this disclosure, preferences are divergent. Some people want no one to know, others only tell their spouses, some only want their family to know about the disease, and some feel fine with everybody knowing about their diagnosis. PwD express various reasons concerning their wish not to disclose: some never try to talk about it, whereas others feel regret after informing others about their medical condition. Others presume a change in others’ perceptions after disclosure and want to avoid being treated differently.42
Treatment and Care
Studies have shown that it is difficult to identify the right moment to start advanced care planning (ACP). Most PwD have reported that right after the diagnosis is not good timing for ACP. It is often hard for both PwD and their relatives to cope with the new diagnosis. Sometimes this process of developing a coping strategy takes years.43 Generally, willingness to plan is present, but most PwD prefer informal plans over written documentation and trust their family members to make the right decisions. PwD are not aware of all components in ACP. They described a lack of knowledge concerning all the opportunities, including the range of available services. Most PwD are not aware that the progression of dementia in the future might impede them from expressing preferences, eg, the preferred place of care. However, some PwD, for example, plan their funeral, ie, choosing songs and paying for a funeral plan because they do not want their relatives to spend too much money.43,44 When PwDs were asked about future scenarios, receiving invasive life-prolonging treatments, eg, cardiopulmonary resuscitation and tube feeding, was less preferred. Concordance between each level of care preference and directives that PwD want to withhold were as follows: comfort care 7%, primary care 49%, and intensive care 58%. Proxies preferred comfort care the most, followed by primary care and intensive cars, concluding that better alignment between preferences for comfort-focused care and advance directives is needed in advanced dementia.26,45
Daily Living, Activities and Social Life
Studies have revealed that PwD have a positive attitude toward physical activity, as evidenced by their endorsing statements that described physical activity as being “important for health”, “good”, “enjoyable”, and “social”. The most preferred ones were “simple/light/safe” and “affordable” exercises with “accessible” settings, preferably in a group. In general, leisure activities were the most preferred. PwD agitation was found to be reduced by including their preferences for leisure activities in behavioral management.25 The use of different kinds of stimuli that reflect past and present interests of PwD helps to increase engagement and responsiveness as well as to support positive behavior.30 Most PwD prefer activities that are suggested by their general practitioner. Exercises should be adapted to individual needs and expectations. “A lack of company” and “memory” prevented PwD from doing exercises.29 Joint-activating interventions to fit both PwD and their informal caregivers were influenced by factors, such as “timing”, “need for activity”, “lifestyle”, “apart or together (with their spouse)”, and “meaning of life”. Many dyads share similar values, such as “keeping active”, “getting out of the house everyday”, and “staying mobile”. Programs helping dyads organize activities and adapt lifestyles are highly appreciated.31
In addition to daily living activities, the food preferences of PwD are very heterogeneous and individual. However, PwD want to be involved in related daily life routines and decisions on mealtimes, meal sizes, and food options.32 Halpern et al23 also found that PwD can state their aesthetic preferences and that these preferences did not change over time and with cognitive decline.23 According to the design of the living environment, auxiliary colors, such as brown and violet, are most preferred, whereas black and gray are least preferred.21,22 Additionally, pictures that patients are able to relate to in terms of familiarity and urban and natural characteristics seem to be suitable for use as environmental cues. Pictures can further enhance the ambiance or serve as prompts for communication and interaction.53
Social interaction has been found to slow down the progression of the disease in PwD.25 It may be challenging to understand what PwD want to express because their verbal expression does not necessarily reflect what they mean and act upon. Observing PwD behaviors and emotions can yield essential indications. In shared decision-making, PwD sometimes prefer to rely on their caregiver.26 Some claim to be dependent on their caregiver because they cannot make decisions on their own. Additionally, it should be considered that PwD often need a great deal of time for their decisions, which may change over time.27 Researchers have suggested adopting the Model of Collaborative Deliberation for the context of care for PwD by drawing particular attention to recognize and define decision-making.28
According to the method of communication, PwD prefer teleological explanations over mechanical ones. Lombrozo et al24 asked study participants questions, such as “Why are there eyes?”. Participants chose the (teleological) answer “so people and animals can see.”. The mechanistic explanation is “because bodies have special cells that combine to produce eyes”. McMurtray et al74 additionally revealed that bilingual PwD relapse more and often return to their primary language during the progression of dementia.
Quality of Life
PwD and caregivers state that PwD QoL and self-efficacy are most important. Functional status, patient and caregiver mood, caregiver burden, and PwD memory performance have been ranked as the most essential aspects of QoL.18,33 Incongruence between socioemotional care preferences of both the caregiver and the PwD was found to be associated with lower QoL of PwD. Additionally, perceived incongruence of care preferences was found to negatively influence the mood of PwD and worsen the relationship between caregivers and PwD. Following this, a lack of correlation of preferences can predict a decrease in the QoL of PwD and adverse social and psychological outcomes.34 Many caregivers underestimate preferences of PwD regarding autonomy, being a burden to others, control, family, and safety.35 Generally, both PwD and their spouses have altruistic preferences (ie, they put the other’s needs before their own).18,33
Technology
More than 80% of PwD regularly use a computer. Nearly everyone has a phone, and many of them know how to send text messages. More than 90% have access to the Internet at home, and every fourth person reported facing issues with using it. Only a few PwD use social media, but the use of e-mail is widespread. The majority expressed that a website designed for health issues in old age (eg, tracking physical and cognitive health conditions) would be beneficial.51 A “digital life story book” to share memories with others is preferred by PwD.52
Time to Full-Time Care
One of the major concerns for PwD is becoming less independent over time in areas, such as mobility (ie, driving a car), dealing with finances and work, and self-care. PwD try to upkeep as many activities for as long as possible. PwD understand that safety must be ensured either by someone helping them or by task modification. However, they want to have a purpose in life, and meaningful activity is crucial to them. PwD state that they can better cope with the disease when they are more active.10 Another study focused on the preferences for the selection of health-care services and providers, revealing that expertise is most important for PwD.50
End-of-Life Care
More than 50% of PwD did not state their opinion on end-of-life decisions to their family caregivers. However, those PwD who stated their preferences usually had a different opinion than their relatives. The incongruence of preferences occurs, for example, in 48% of cases concerning CPR and tube feeding and in 60% of cases of artificial ventilation.46,47 At the end of life, PwD prefer to maintain family links, maintain independence, feel safe, not be a burden, be treated with respect and dignity, have a choice in their place of care, engage in pleasurable activities, experience person-centered care, be in touch with the world, and have comfortable care. However, most important for caregivers was to ensure good quality care at all times.48 Hill et al14 identified four main preferences that are most important for PwD at the end of life: family involvement, living in the present, autonomy, and individuality. If PwD are unable to express their wishes, they prefer their family to make decisions. They highly value having their family and friends close during that time. PwD want to be cared for with compassion and want to be seen as individuals who can maintain hobbies and interests. Preserving their independence in self-care, such as eating and taking medications, as long as possible is of great importance for many PwD.14
PwD self-esteem and confidence related to expressing opinions are affected by their disease awareness and the related memory loss, which makes many PwD feel as if they have nothing to say that would be worth listening to. The feeling of being understood and well cared for is important for PwD to entrust themselves to the staff. PwD in care homes at the end of life have stated that it is an excellent experience to live in a care home and values it as “his home” now. Furthermore, losses have a significant impact on whether PwD see a purpose in life or not. Frequently, PwD cannot think about their own care needs because their thoughts are overshadowed by past experiences (eg, a loss of a family member or spouse). Others prefer to return home to their family but know that it is not possible because they would be a burden.44 Mulqueen et al49 found that nurses do not always precisely predict what patients consider most important. PwD value comfort, family presence, familiar staff, and surroundings most, whereas nurses thought they most preferred good communication, pain management, ACP, and care by professional staff.49
Cognitive Ability to State Preferences
Some studies have evaluated whether patients with cognitive decline are able to state their preferences, whether preferences are stable over time instead of associated with cognitive decline, and how cognitive decline affects the communication of preferences. Wijk et al21 evaluated the ability of color naming, color discrimination, and color preference in Alzheimer’s disease, revealing that the ability to discriminate and name colors was affected but that preferences for color are stable over time, despite cognitive decline.21
Discussion
This systematic literature review summarizes the preferences of PwD from 44 publications with very heterogeneous results, capturing preferences for the diagnosis and disclosure of the disease, aspects that have to be considered in the medical decision-making process physical and everyday life activities, QoL and self-efficiency as well as concordance on care preference and end-of-life care. The review revealed that PwD are able to state their preferences according to these domains and that these preferences are stable over time, even though the cognition of PwD declines and the disease progresses. Additionally, proxy preference ratings (ie, statement of preferences of caregivers for the PwD) have been found to provide useful aspects of PwD preferences, but they are not perfect substitutes for patients’ preferences. As a result, possible differences should be taken into account within decision-making processes.
The majority of PwD wants to be informed about the diagnosis as early as possible.37–39 In 2000, 40% of patients received a formal diagnosis.60 A few years later, less than 50% received a formal dementia diagnosis.61,62 In the last 10 years, there has been increasing evidence that the prevalence of dementia is stagnating or even decreasing in some countries.63–65 Röhr et al65 and Wolters et al66 confirmed that there is evidence of decreasing age-specific incidence rates in industrialized nations. In Germany, the prevalence (incidence) of dementia decreased from 2.2% (0.4%) in 2015 to 2.0% (0.3%) in 2019, causing a decrease in the number of PwD, despite continued demographic changes.80 Many physicians are somewhat skeptical of the ascribed effects of evidence-based and dementia-specific treatment opportunities, such as anti-dementia drugs.67 For this reason and because of potential side effects, some experts have advised against the use of anti-dementia medication.68 Some health authorities stopped covering the costs of such drugs. Therapeutic nihilism that the GPs are forming, is a disincentive for the state of that diagnostic workup. Therefore, practitioners’ current diagnostic behavior is not in line with patients’ preferences for an early and uncovered dementia diagnosis.
In previous times, it was believed that PwD do not need to know much about the disease due to missing curative treatments, but this perception has changed over the years.69,70 One reason was that PwD might not cope well with the diagnosis, which can deteriorate the relationship between the doctor and the patient. On the other hand, advantages were seen in reducing uncertainty and having sufficient time to organize social support services, appropriate treatments, and plans for the future when symptoms start to worsen.71 Furthermore, a dementia diagnosis can be a relief for older people who perceive memory loss without knowing the cause. It is assumed that coping with the disease is easier in early stages of dementia. PwD in these stages can still be meaningfully involved in conversations about ACP.36,61,62 Additionally, QoL was found to be positively associated with an early diagnosis, as it improves freedom and self-determination.19,36 Hence, clinicians and practitioners should not avoid discussing the disease with their patients.36 Future research should evaluate the best ways to deliver the diagnosis and minimize negative emotional and psychological impacts, such as fear, as well as whether an early diagnosis is associated with better patient-reported outcomes later on.37
Concerning postdiagnostic treatment and care, PwD prefer to focus on the present, and it is difficult to discuss and decide on treatment and care in the future, considering end-of-life care options.48 Family members’ and caregivers’ support plays a significant role in current and future decisions around treatment and care, even though PwD should not ultimately leave important decisions regarding their own future in their relatives’ hands. Uncertainty about the future can be reduced by including joint anticipatory planning between PwD and their families. Some PwD make informal flexible plans, but usually only in areas they perceive as necessary (eg, to decrease stress for their family). Additionally, families have to be prepared for potential changes in future care realities.26,43 This process should be initiated as early as possible to comply with PwD preferences as closely as possible for the current and future treatment and care situations.45 Concerning the choice between behavioral treatment options, memory compensation training was found to be the most popular among PwD. Following this, it seems that many PwD are always aware of their memory problems and try to work against the disease progression.18,34
Preferences could also play a crucial role in the daily life of PwD. Therefore, it would be beneficial to observe the emotions of PwD during daily life routines because they can agree or disagree with the current living situation, allowing PwD to be engaged in certain decisions without being a burden to others.28 An introduction of behavior plans that used PwD-preferred items (such as personal photos, books, or music) or activities can reduce behavioral and neuropsychiatric symptoms and, thus, relieve the burden of caregivers and health professionals. However, not only is it essential for patients’ behaviors to be considered in daily life, but specific behaviors of other people in the surroundings could also affect PwD. A majority of cognitive processes can be positively influenced by direct gaze because mere perception improves the likability of faces and helps with remembering them.54
PwD want to be active and engaged and need a purpose to conclude a task. Identifying and carrying out activities that satisfy PwD might keep them physically and cognitively active for longer, which, in turn, could improve the living and caring situation.25 The implementation of such activities in the daily routine could be beneficial. Such activity programs should use work-related stimuli to improve engagement duration and attention.55 We identified various ways to retain high levels of well-being for PwD. Meaningful activities, including social interaction, provide satisfaction by giving patients a purpose in life.59 Dickins et al10 found that PwD in early stages look for activities that match their cognitive abilities, especially everyday activities.
Within their daily life routine, PwD want to be surrounded by a social environment. Being alone with no company and having memory problems are some of the main aspects that hindered them from being active. Simple, light, and safe exercises are preferred, such as walking.29,55 PwD are always more engaged in activities that suit their main interests.30 Therefore, they prefer leisure activities over edible items. Furthermore, the method of communication should be considered because PwD prefer teleological explanations over mechanistic ones.24 Such aspects should be considered within decision-making processes with PwD as well as within daily living and communication. Regarding preferences for food, PwD do not differ from nondemented controls. However, a systematic “one-size-fits-all” approach, as is often used in nursing homes, is not appropriate for either nonimpaired adults or PwD. As one aspect of person-centered care, PwD have to be able to keep control and be engaged in various aspects of food. A range of food choices and individual mealtimes should always be offered. Additionally, in cases where PwD are diagnosed with dysphagia, meals should still be taste-modified regularly and aligned with individual tastes.32 Concerning the living environment, different studies21,22 have revealed that pictures with familiar content or positive emotions are easier to recall for PwD and could be used as cues for the design and setup of the environment and ambience of PwD homes as well as within conversations and interactions with PwD.
Thus, individual preferences of PwD alongside their available resources have to be determined individually. As the disease progresses, isolation and the loss of commonplace occupations are some of the main challenges PwD face. Over time, autonomy declines, but most PwD still wish to remain in their homes. There is no general approach defining at which point in time PwD should leave their homes. To be dependent on others can be accepted by PwD to maintain activities that determine their daily life at home, which could still improve or maintain their well-being.10,38 Preferences for end-of-life care depend on the characteristics, individual ideas, and personal needs of PwD. Due to their cognitive decline, PwD sometimes feel unhelpful and not worth listening to. In such cases, caregivers need to show empathy and sensitivity to PwD to support their feelings of safety and trust.14,44 Home is considered a familiar place where PwD feel safe, surrounded by people who care and know them very well.44,73 Communication is also a vital part of end-of-life care, as PwD want to be kept informed about what is happening around them and their health condition. As PwD cannot comprehend the future very well, it is necessary to make them aware of their influence on a variety of aspects in present and future care. Evidence-based guidelines need to be adapted individually by caregivers and clinicians to allow a person-centered approach and to reassure the excellent quality of care at all times.14,44
This systematic review has shown a variety of heterogeneous preferences, that patient preferences are present in all aspects of care and daily living, and that PwD can state their preferences. Even though the disease progresses, preferences remain stable over time, irrespective of cognitive decline. Therefore, PwD preferences should always be taken into account. However, there is a lack of quantitative preference studies which identify the most and least preferred aspects of diagnosis, treatment, and care, and PwD daily life routines, as well as quantitative differences between these aspects. Until now, there has been a lack of studies evaluating whether a strict consideration of PwD preferences could improve patient-related outcomes, such as PwD QoL. Therefore, further research is needed. Such studies should be conducted to create a fundamental basis to extend existing evidence-based guidelines based on the clinical efficacy and effectiveness of interventions.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Deuschl G, Maier W. S3-Leitlinie Demenzen. Leitlinien für Diagnostik und Therapie in der Neurologie. (25.1. 2016). Deutsche Gesellschaft für Neurologie, Hrsg. Im Internet. [S3-guidelines Dementia. Guidelines for Diagnostic and Therapy in Neurology. (25.01.2016). German Society for Neurology]. Available from: www.dgn.org/leitlinien. Accessed November 7, 2020.
- 2.World Health Organisation. Dementia; 2020. Available from: www.who.int/news-room/fact-sheets/detail/dementia. Accessed November 7, 2020.
- 3.Samsi K, Manthorpe J. Everyday decision-making in dementia: findings from a longitudinal interview study of people with dementia and family carers. Int Psychogeriatr. 2013;25(6):949–961. doi: 10.1017/S1041610213000306 [DOI] [PubMed] [Google Scholar]
- 4.Fetherstonhaugh D, Tarzia L, Nay R. Being central to decision making means I am still here!: the essence of decision making for people with dementia. J Aging Stud. 2013;27(2):143–150. doi: 10.1016/j.jaging.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 5.Hamann J, Bronner K, Margull J, et al. Patient participation in medical and social decisions in Alzheimer’s disease. J Am Geriatr Soc. 2011;59(11):2045–2052. doi: 10.1111/j.1532-5415.2011.03661.x [DOI] [PubMed] [Google Scholar]
- 6.Hirschman KB, Joyce CM, James BD, et al. Do Alzheimer’s disease patients want to participate in a treatment decision, and would their caregivers let them? Gerontologist. 2005;45(3):381–388. doi: 10.1093/geront/45.3.381 [DOI] [PubMed] [Google Scholar]
- 7.Smebye KL, Kirkevold M, Engedal K. How do persons with dementia participate in decision making related to health and daily care? A multi-case study. BMC Health Serv Res. 2012;12(1):241. doi: 10.1186/1472-6963-12-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edvardsson D, Winblad B, Sandman P-O. Person-centred care of people with severe Alzheimer’s disease: current status and ways forward. Lancet Neurol. 2008;7(4):362–367. doi: 10.1016/S1474-4422(08)70063-2 [DOI] [PubMed] [Google Scholar]
- 9.Dworkin R. Autonomy and the Demented Self. The Milbank Quarterly; 1986:4–16. [PubMed] [Google Scholar]
- 10.Dickins M, Goeman D, O’Keefe F, et al. Understanding the conceptualisation of risk in the context of community dementia care. Soc Sci Med. 2018;208:72–79. doi: 10.1016/j.socscimed.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 11.Aneshensel CS, Pearlin LI, Mullan JT, Zarit SH, Whitlatch CJ. Profiles in Caregiving: The Unexpected Career. Elsevier; 1995. [Google Scholar]
- 12.Alzheimer ´s Society. The psychological and emotional impact of dementia. 2020. Available from: https://www.alzheimers.org.uk/get-support/help-dementia-care/understanding-supporting-person-dementia-psychological-emotional-impact. Accessed November 7,2020.
- 13.Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. J Am Acad Nurse Pract. 2008;20(8):423–428. doi: 10.1111/j.1745-7599.2008.00342.x [DOI] [PubMed] [Google Scholar]
- 14.Hill SR, Mason H, Poole M, et al. What is important at the end of life for people with dementia? The views of people with dementia and their carers. Int J Geriatr Psychiatry. 2017;32(9):1037–1045. doi: 10.1002/gps.4564 [DOI] [PubMed] [Google Scholar]
- 15.Shalowitz DI, Garrett-Mayer E, Wendler D. The accuracy of surrogate decision makers: a systematic review. Arch Intern Med. 2006;166(5):493–497. doi: 10.1001/archinte.166.5.493 [DOI] [PubMed] [Google Scholar]
- 16.Popham C, Orrell M. What matters for people with dementia in care homes? Aging Ment Health. 2012;16(2):181–188. doi: 10.1080/13607863.2011.628972 [DOI] [PubMed] [Google Scholar]
- 17.Okonkwo O, Griffith HR, Belue K, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology. 2007;69(15):1528–1535. doi: 10.1212/01.wnl.0000277639.90611.d9 [DOI] [PubMed] [Google Scholar]
- 18.Barrios PG, González RP, Hanna SM, et al. Priority of treatment outcomes for caregivers and patients with mild cognitive impairment: preliminary analyses. Neurol Ther. 2016;5(2):183–192. doi: 10.1007/s40120-016-0049-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dröes R-M, Boelens-van Der Knoop ECC, Bos J, et al. Quality of life in dementia in perspective: an explorative study of variations in opinions among people with dementia and their professional caregivers, and in literature. Dementia. 2006;5(4):533–558. doi: 10.1177/1471301206069929 [DOI] [Google Scholar]
- 20.ICHOM. Standard Set for Dementia. Available from: https://connect.ichom.org/standard-sets/dementia/. Accessed November 8, 2020.
- 21.Wijk H, Berg S, Sivik L, et al. Colour discrimination, colour naming and colour preferences among individuals with Alzheimer’s disease. Int J Geriatr Psychiatry. 1999;14(12):1000–1005. doi: [DOI] [PubMed] [Google Scholar]
- 22.Stanzani Maserati M, Mitolo M, Medici F, et al. Color choice preference in cognitively impaired patients: a look inside Alzheimer’s disease through the use of Lüscher Color Diagnostic. Front Psychol. 2019;10:1951. doi: 10.3389/fpsyg.2019.01951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halpern AR, Ly J, Elkin-Frankston S, et al. “I know what I like”: stability of aesthetic preference in Alzheimer’s patients. Brain Cogn. 2008;66(1):65–72. doi: 10.1016/j.bandc.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 24.Lombrozo T, Kelemen D, Zaitchik D. Inferring design: evidence of a preference for teleological explanations in patients with Alzheimer’s disease. Psychol Sci. 2007;18(11):999–1006. doi: 10.1111/j.1467-9280.2007.02015.x [DOI] [PubMed] [Google Scholar]
- 25.Feliciano L, Steers ME, Elite-Marcandonatou A, et al. Applications of preference assessment procedures in depression and agitation management in elders with dementia. Clin Gerontol. 2009;32(3):239–259. doi: 10.1080/07317110902895226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison Dening K, King M, Jones L, et al. Advance care planning in dementia: do family carers know the treatment preferences of people with early dementia? PLoS One. 2016;11(7):e0159056. doi: 10.1371/journal.pone.0159056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raetz PB, LeBlanc LA, Baker JC, et al. Utility of the multiple‐stimulus without replacement procedure and stability of preferences of older adults with dementia. J Appl Behav Anal. 2013;46(4):765–780. doi: 10.1002/jaba.88 [DOI] [PubMed] [Google Scholar]
- 28.van de Ven LG, Smits C, Elwyn G, et al. Recognizing decision needs: first step for collaborative deliberation in dementia care networks. Patient Educ Couns. 2017;100(7):1329–1337. doi: 10.1016/j.pec.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 29.Chong TW, Doyle CJ, Cyarto EV, et al. Physical activity program preferences and perspectives of older adults with and without cognitive impairment. Asia Pac Psychiatry. 2014;6(2):179–190. doi: 10.1111/appy.12015 [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Mansfield J, Marx MS, Thein K, et al. The impact of past and present preferences on stimulus engagement in nursing home residents with dementia. Aging Ment Health. 2010;14(1):67–73. doi: 10.1080/13607860902845574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van’t Leven N, de Lange J, Prick A-E, et al. How do activating interventions fit the personal needs, characteristics and preferences of people with dementia living in the community and their informal caregivers? Dementia. 2019;18(1):157–177. doi: 10.1177/1471301216662378 [DOI] [PubMed] [Google Scholar]
- 32.Milte R, Shulver W, Killington M, et al. Struggling to maintain individuality–describing the experience of food in nursing homes for people with dementia. Arch Gerontol Geriatr. 2017;72:52–58. doi: 10.1016/j.archger.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 33.Smith GE, Chandler M, Fields JA, et al. A survey of patient and partner outcome and treatment preferences in mild cognitive impairment. J Alzheimers Dis. 2018;63(4):1459–1468. doi: 10.3233/JAD-171161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelton EG, Orsulic-Jeras S, Whitlatch CJ, Szabo SM. Does it matter if we disagree? The impact of incongruent care preferences on persons with dementia and their care partners. Gerontologist. 2018;58(3):556–566. [DOI] [PubMed] [Google Scholar]
- 35.Reamy AM, Kim K, Zarit SH, et al. Understanding discrepancy in perceptions of values: individuals with mild to moderate dementia and their family caregivers. Gerontologist. 2011;51(4):473–483. doi: 10.1093/geront/gnr010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mate KE, Pond CD, Magin PJ, et al. Diagnosis and disclosure of a memory problem is associated with quality of life in community based older Australians with dementia. Int Psychogeriatr. 2012;24(12):1962–1971. doi: 10.1017/S1041610212001111 [DOI] [PubMed] [Google Scholar]
- 37.Jung JH, Kim MJ, Choi S-H, et al. Do patients want to listen to a diagnosis of Dementia in Korea? Preferences on disclosing a diagnosis of dementia and discussing advance care planning in elderly patients with memory concerns and their families. Psychiatry Investig. 2017;14(6):779–785. doi: 10.4306/pi.2017.14.6.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elson P. Do older adults presenting with memory complaints wish to be told if later diagnosed with Alzheimer’s disease? Int J Geriatr Psychiatry. 2006;21(5):419–425. doi: 10.1002/gps.1485 [DOI] [PubMed] [Google Scholar]
- 39.Mahieux F, Herr M, Ankri J. What are the preferences of patients attending a memory clinic for disclosure of Alzheimer’s disease? Rev Neurol (Paris). 2018;174(7–8):564–570. doi: 10.1016/j.neurol.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 40.Bamford C, Olsen K, Davison C, et al. Is there a preference for PET or SPECT brain imaging in diagnosing dementia? The views of people with dementia, carers, and healthy controls. Int Psychogeriatr. 2016;28(1):123. doi: 10.1017/S1041610215001039 [DOI] [PubMed] [Google Scholar]
- 41.Byszewski AM, Molnar FJ, Aminzadeh F, et al. Dementia diagnosis disclosure: a study of patient and caregiver perspectives. Alzheimer Dis Assoc Disord. 2007;21(2):107–114. doi: 10.1097/WAD.0b013e318065c481 [DOI] [PubMed] [Google Scholar]
- 42.Hellström I, Torres S. A wish to know but not always tell–couples living with dementia talk about disclosure preferences. Aging Ment Health. 2013;17(2):157–167. doi: 10.1080/13607863.2012.742491 [DOI] [PubMed] [Google Scholar]
- 43.Dickinson C, Bamford C, Exley C, et al. Planning for tomorrow whilst living for today: the views of people with dementia and their families on advance care planning. Int Psychogeriatr. 2013;25(12):2011–2021. doi: 10.1017/S1041610213001531 [DOI] [PubMed] [Google Scholar]
- 44.Goodman C, Amador S, Elmore N, et al. Preferences and priorities for ongoing and end-of-life care: a qualitative study of older people with dementia resident in care homes. Int J Nurs Stud. 2013;50(12):1639–1647. doi: 10.1016/j.ijnurstu.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 45.Cohen SM, Volandes AE, Shaffer ML, et al. Concordance between proxy level of care preference and advance directives among nursing home residents with advanced dementia: a cluster randomized clinical trial. J Pain Symptom Manage. 2019;57(1):37–46. e1. doi: 10.1016/j.jpainsymman.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai C-F, Lee Y-T, Lee W-J, et al. Depression of family caregivers is associated with disagreements on life-sustaining preferences for treating patients with dementia. PLoS One. 2015;10(7):e0133711. doi: 10.1371/journal.pone.0133711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayalon L, Bachner YG, Dwolatzky T, et al. Preferences for end-of-life treatment: concordance between older adults with dementia or mild cognitive impairment and their spouses. Int Psychogeriatr. 2012;24(11):1798. doi: 10.1017/S1041610212000877 [DOI] [PubMed] [Google Scholar]
- 48.Dening KH, Jones L, Sampson EL. Preferences for end-of-life care: a nominal group study of people with dementia and their family carers. Palliat Med. 2013;27(5):409–417. doi: 10.1177/0269216312464094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulqueen K, Coffey A. Preferences of residents with dementia for end of life care. Nurs Older People. 2017;29(2):26–30. doi: 10.7748/nop.2017.e862 [DOI] [PubMed] [Google Scholar]
- 50.Groenewoud S, Van Exel NJA, Bobinac A, et al. What influences patients’ decisions when choosing a health care provider? Measuring preferences of patients with knee arthrosis, chronic depression, or Alzheimer’s disease, using discrete choice experiments. Health Serv Res. 2015;50(6):1941–1972. doi: 10.1111/1475-6773.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaMonica HM, English A, Hickie IB, et al. Examining internet and eHealth practices and preferences: survey study of Australian older adults with subjective memory complaints, mild cognitive impairment, or dementia. J Med Internet Res. 2017;19(10):e358. doi: 10.2196/jmir.7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Philbin L, Woods B, Holmes E. People with dementia and caregiver preferences for digital life story work service interventions. A discrete choice experiment and digital survey. Aging Ment Health. 2020;24(2):353–361. doi: 10.1080/13607863.2018.1525606 [DOI] [PubMed] [Google Scholar]
- 53.Motzek T, Bueter K, Marquardt G. Investigation of eligible picture categories for use as environmental cues in dementia-sensitive environments. HERD. 2017;10(4):64–73. doi: 10.1177/1937586716679403 [DOI] [PubMed] [Google Scholar]
- 54.Lopis D, Baltazar M, Geronikola N, et al. Eye contact effects on social preference and face recognition in normal ageing and in Alzheimer’s disease. Psychol Res. 2019;83(6):1292–1303. doi: 10.1007/s00426-017-0955-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen-Mansfield J, Dakheel-Ali M, Thein K, et al. The impact of stimulus attributes on engagement of nursing home residents with dementia. Arch Gerontol Geriatr. 2009;49(1):1–6. doi: 10.1016/j.archger.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeLeon IG, Iwata BA. Evaluation of a multiple‐stimulus presentation format for assessing reinforcer preferences. J Appl Behav Anal. 1996;29(4):519–533. doi: 10.1901/jaba.1996.29-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deleon IG, Iwata BA, Roscoe EM. Displacement of leisure reinforcers by food during preference assessments. J Appl Behav Anal. 1997;30(3):475–484. doi: 10.1901/jaba.1997.30-475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bojak SL, Carr JE. On the displacement of leisure items by food during multiple‐stimulus preference assessments. J Appl Behav Anal. 1999;32(4):515–518. doi: 10.1901/jaba.1999.32-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bohn L, Kwong See ST, Fung HH. Preference for emotionally meaningful activity in Alzheimer’s disease. Aging Ment Health. 2019;23(11):1578–1585. doi: 10.1080/13607863.2018.1506750 [DOI] [PubMed] [Google Scholar]
- 60.Johnson H, Bouman WP, Pinner G. On telling the truth in Alzheimer’s disease: a pilot study of current practice and attitudes. Int Psychogeriatr. 2000;12(2):221–229. doi: 10.1017/S1041610200006347 [DOI] [PubMed] [Google Scholar]
- 61.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–384. doi: 10.1016/j.jalz.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 62.Van Hout H, Vernooij-Dassen MJ, Jansen DA, et al. Do general practitioners disclose correct information to their patients suspected of dementia and their caregivers? A prospective observational study. Aging Ment Health. 2006;10(2):151–155. doi: 10.1080/13607860500310468 [DOI] [PubMed] [Google Scholar]
- 63.Prince M, Ali G-C, Guerchet M, et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stephan BCM, Birdi R, Tang EYH, et al. Secular trends in dementia prevalence and incidence worldwide: a systematic review. J Alzheimers Dis. 2018;66(2):653–680. doi: 10.3233/JAD-180375 [DOI] [PubMed] [Google Scholar]
- 65.Roehr S, Pabst A, Luck T, et al. Is dementia incidence declining in high-income countries? A systematic review and meta-analysis. Clin Epidemiol. 2018;10:1233–1247. doi: 10.2147/CLEP.S163649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolters FJ, Chibnik LB, Waziry R, et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the Alzheimer cohorts consortium. Neurology. 2020;95(5):e519–e531. doi: 10.1212/WNL.0000000000010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glinz D, Gloy VL, Monsch AU, et al. Acetylcholinesterase inhibitors combined with memantine for moderate to severe Alzheimer’s disease: a meta-analysis. Swiss Med Wkly. 2019;149:w20093. [DOI] [PubMed] [Google Scholar]
- 68.Fink HA, Linskens EJ, MacDonald R, et al. Benefits and harms of prescription drugs and supplements for treatment of clinical Alzheimer-type dementia. Ann Intern Med. 2020;172(10):656–668. doi: 10.7326/M19-3887 [DOI] [PubMed] [Google Scholar]
- 69.Woods R. Discovering the person with Alzheimer’s disease: cognitive, emotional and behavioural aspects. Aging Ment Health. 2001;5(sup1):7–16. doi: 10.1080/713650008 [DOI] [PubMed] [Google Scholar]
- 70.Bamford C, Lamont S, Eccles M, et al. Disclosing a diagnosis of dementia: a systematic review. Int J Geriatr Psychiatry. 2004;19(2):151–169. doi: 10.1002/gps.1050 [DOI] [PubMed] [Google Scholar]
- 71.Phillips J, Pond CD, Paterson NE, et al. Difficulties in disclosing the diagnosis of dementia: a qualitative study in general practice. Br J Gen Pract. 2012;62(601):e546–e553. doi: 10.3399/bjgp12X653598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker R, Ratcliffe J, White A, et al. Dementia assessment services: what are the perceptions of older people? Australas J Ageing. 2018;37(1):43–47. doi: 10.1111/ajag.12455 [DOI] [PubMed] [Google Scholar]
- 73.Badrakalimuthu V, Barclay S. Do people with dementia die at their preferred location of death? A systematic literature review and narrative synthesis. Age Ageing. 2014;43(1):13–19. doi: 10.1093/ageing/aft151 [DOI] [PubMed] [Google Scholar]
- 74.McMurtray A, Saito E, Nakamoto B. Language preference and development of dementia among bilingual individuals. Hawaii Med J. 2009;68(9):223. [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen-Mansfield J, Golander H, Arnheim G. Self-identity in older persons suffering from dementia: preliminary results. Soc Sci Med. 2000;51(3):381–394. doi: 10.1016/S0277-9536(99)00471-2 [DOI] [PubMed] [Google Scholar]
- 76.Mungas D, Cooper JK, Weiler PG, et al. Dietary preference for sweet foods in patients with dementia. J Am Geriatr Soc. 1990;38(9):999–1007. doi: 10.1111/j.1532-5415.1990.tb04423.x [DOI] [PubMed] [Google Scholar]
- 77.Ortega JV, Iwata BA, Nogales-González C, et al. Assessment of preference for edible and leisure items in individuals with dementia. J Appl Behav Anal. 2012;45(4):839–844. doi: 10.1901/jaba.2012.45-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kremer HP, Roos RAC, Dingjan GM, et al. The hypothalamic lateral tuberal nucleus and the characteristics of neuronal loss in Huntington’s disease. Neurosci Lett. 1991;132(1):101–104. doi: 10.1016/0304-3940(91)90443-W [DOI] [PubMed] [Google Scholar]
- 79.de Sant’Anna M, Vallet C, Kadouche R, et al. Computer accessibility for individuals suffering from mild to moderate Alzheimer’s disease. Eur Geriatr Med. 2010;1(3):186–192. doi: 10.1016/j.eurger.2010.04.003 [DOI] [Google Scholar]
- 80.Bohlken J, Riedel-Heller S, Steininger G, Kostev K, Michalowsky B. Trends in dementia and mild cognitive impairment prevalence and incidence in German general and specialist practices between 2015 and 2019. J Alzheimers Dis. 2021;79:1683–1690. doi: 10.3233/JAD-201385 [DOI] [PubMed] [Google Scholar]