Abstract
Context
Novel metrics of high-density lipoprotein (HDL) (subclasses, lipid content, and function) may improve characterization of the anti-atherogenic features of HDL. In midlife women, changes in these metrics vary by time relative to the final menstrual period (FMP), supporting a contribution of estradiol (E2) and follicle-stimulating hormone (FSH).
Objective
We tested associations of endogenous E2 and FSH with novel HDL metrics and assessed whether these associations varied by time relative to FMP.
Methods
This study was a longitudinal analysis from the Study of Women’s Health Across the Nation (SWAN) HDL study, using a community-based cohort of 463 women, baseline mean age 50.2 (2.7) years. The main outcome measures were HDL cholesterol efflux capacity (HDL-CEC), HDL phospholipids (HDL-PL), HDL triglycerides (HDL-Tg), HDL particles (HDL-P), HDL size, and HDL cholesterol (HDL-C).
Results
In multivariable analyses, E2 was positively associated with HDL size, large HDL-P, HDL-CEC, and HDL-Tg, but negatively with medium HDL-P (P values < 0.05). The positive association between E2 and HDL-Tg was stronger 2 years post-FMP than before, (interaction P = 0.031). FSH was positively related to total and medium HDL-P, but negatively to HDL size, large HDL-P, and HDL-CEC per particle (P values < 0.05). Associations of higher FSH with greater total HDL-P and smaller HDL size were only evident at/after menopause (interaction P values < 0.05).
Conclusion
Some of the associations linking E2 and FSH with novel HDL metrics were vulnerable to time relative to menopause onset. Whether a late initiation of hormone therapy relative to menopause could have a detrimental effect on lipid content of HDL particles should be tested in the future.
Keywords: sex hormones, follicle-stimulating hormone, subclasses, climacteric, high-density lipoprotein
The Mendelian randomization approach has provided the strongest evidence to date against a causal cardioprotective effect of high level of high-density lipoprotein cholesterol (HDL-C) (1). Despite findings from epidemiological studies showing that higher HDL-C is cardioprotective (2, 3), clinical trials designed to raise HDL-C in statin-treated individuals do not show similar effects (4-6). This puzzle is especially relevant to midlife women who experience accelerated increase in cardiovascular disease (CVD) risk as they transition through menopause (7).
An evolving body of literature suggests that the cardioprotective association of higher HDL-C in midlife women (8-10) varies by time relative to menopause. Among women transitioning through menopause, greater increases in HDL-C were associated with lower risk of atherosclerosis before menopause, but with higher risk after menopause (11). In another study, higher HDL-C was associated with higher risk of carotid plaque presence mainly in women who were >10 years postmenopausal (12). The consistent observation of a moderation effect of time relative to menopause on association between HDL-C and CVD risk suggests a critical role of the cardinal hormonal markers of the menopause transition: estradiol (E2) and follicle-stimulating hormone (FSH) (7). Interestingly, level of E2 modified associations between HDL-C and subclinical measures of vascular health. In midlife women at different stages of the menopause transition, the association between HDL-C and % change in brachial artery flow mediated dilation (%FMD), a measure of endothelial function (13), and aortic coronary calcification presence varied (14) by levels of E2. A protective association between HDL-C and %FMD (13) or aortic coronary calcification presence (14) was only evident among women in the highest E2 tertile. The reported findings suggest a contribution of E2 in the cardioprotective features of HDL that may not be adequately captured by HDL-C. Novel metrics of HDL including HDL subclasses, lipid content (phospholipid [HDL-PL] and triglyceride [HDL-Tg]), and function (HDL cholesterol efflux capacity [HDL-CEC]) have shown independent associations with CVD risk beyond HDL-C, promising a better understanding of the clinical utility of HDL (15-19).
Changes in these novel metrics over the menopause transition have been recently characterized (20). As women traversed menopause, although HDL-C increased, HDL subclasses and lipid content showed adverse changes. Within the 1 to 2 years bracketing the menopause onset, large HDL particles (HDL-P) and HDL size declined while small HDL-P and HDL-Tg increased. Moreover, while HDL-CEC increased, HDL-CEC per HDL particle (HDL-P) declined, consistent with reduced function per particle. Whether these changes are related to the dynamic changes in E2 and FSH that accompanies the menopause transition remains to be assessed.
Limited studies have tested the associations of E2 and/or FSH with metrics of HDL other than HDL-C in women (21-24). These studies were cross-sectional in nature, very limited in sample size, and/or did not comprehensively assess other HDL metrics in midlife women. Given the growing line of evidence that associations between HDL-C and CVD risk vary by time since menopause, it is possible that associations between hormonal markers of menopause and novel metrics of HDL vary by time relative to menopause. However, the previous literature has not tested this possibility.
The Study of Women’s Health Across the Nation (SWAN) HDL provides an opportunity to enhance our understanding of the hormonal contributions to changes in HDL metrics during midlife. The main objective of the current analysis was to test associations of endogenous E2 and FSH with HDL subclasses, lipid content, and function in midlife women. A second exploratory objective was to test whether these associations vary by time relative to menopause. We hypothesized that (1) lower levels of E2 and higher levels of FSH over the menopause transition would be associated with an adverse HDL metric profile (characterized by lower HDL-CEC, lower concentrations of HDL-PL and large HDL-P, smaller overall HDL size, and increases in levels of HDL-Tg and small HDL-P (17, 25-27)); and (2) associations of E2 and FSH with HDL metrics would vary by time relative to the final menstrual period (FMP).
Methods
Study Subjects
SWAN is an ongoing, multi-site, multi-ethnic, community-based longitudinal study that aims to assess the physiological and psychological changes accompany the menopause transition. Details on the SWAN study design have been described before (28). Briefly, 3302 women aged 42 to 52 years were recruited between 1996 and 1997 at 7 different sites across the United States (Boston, MA; Pittsburgh PA; Chicago, IL; Newark, NJ; Detroit, MI; Los Angeles, CA; Oakland, CA). Women were eligible for SWAN if (i) they had an intact uterus and at least one ovary, (ii) had at least one menstrual bleed within the last 3 months prior to recruitment, (iii) were not pregnant or lactating at time of recruitment, (iv) were not on hormone therapy, and (v) self-identified as White, Black, Hispanic, Chinese, or Japanese.
SWAN HDL is an ancillary study to SWAN that aims to characterize the changes in HDL subclasses, lipid content, and function measures that accompany ovarian aging, and to understand how these changes interact to affect the athero-protective features of HDL in women as they progress through the menopause transition. A cohort of 558 women from SWAN were included in SWAN HDL based on having at least 1 visit before and 2 visits after the FMP with available stored blood specimens (a total of 1461 samples). HDL metrics were measured on stored samples 2 to 5 times over the menopause transition for each participant (coincident with SWAN visit 1, follow-up visits 3 to 9, and visit 12).
For the current study, visits at which women had missing HDL metric (n = 2 observations) or hormone measures (n = 5 observations) were excluded. We additionally excluded women for whom FMP were not observed (n = 87 women [168 observations]). Finally, visits at which women used hormone therapy or had unknown menopausal status were excluded (n = 8 women [42 observations]). The final analysis included 463 women (1244 observations). Compared with women included in this analysis, at the first available visit the excluded 87 women were older, more likely to be White and postmenopausal, had higher ApoA-I, total HDL-P, small HDL-P, HDL-PL, and HDL-Tg and lower HDL-CEC/total HDL-P, P values < 0.05.
Written informed consent was provided by all participants before enrollment in SWAN, and study protocols were approved by the institution review board at each site.
Blood Assays
Phlebotomy was performed after a minimum of 10-hour overnight fast. This was scheduled 2 to 5 days after a spontaneous menstrual bleed when possible, or randomly within 90 days of the annual SWAN visit when menstrual cycles were less predictable. Cycle day of blood draw was reported as either within days 2 to 5 of the menstrual cycle or unknown. Stored serum samples that have been frozen at −80 °C and never been thawed were used for SWAN HDL assays to guarantee the validity of results.
Cholesterol efflux capacity and HDL lipid content
HDL cholesterol efflux capacity (HDL-CEC), HDL phospholipids (HDL-PL) and HDL triglycerides (HDL-Tg) were measured in a Centers for Disease Control and Prevention (CDC)-certified lipid lab the University of Pennsylvania. HDL-CEC assessment was similar to the protocol reported by Khera et al (17). In brief, J774 mouse macrophage cells were plated and labeled with 2 μCi/mL of 3H-cholesterol overnight. The cells were then incubated for 4 hours in the presence of 0.3 mM 8-(4-chlorophenylthio)-cyclic AMP, which is an upregulator of ATP-binding cassette transporter-1 (ABCA1). Lipoproteins containing apolipoprotein B (ApoB) were removed from plasma by polyethylene glycol precipitation generating ApoB-depleted plasma. Then, the cells were incubated for 2 hours with the equivalent of 1% ApoB-depleted serum or plasma at 37 °C. Cells incubated with media alone were used as a baseline control. Media was collected and passed through a 0.22-µM filter to remove cell debris. Once media was removed, isopropanol was used to extract lipids from the cells. 3H-cholesterol was quantitated in media and cells by scintillation counting. Each medium was then collected and passed through a 0.22-μM filter to remove cell debris and radioactivity determined by liquid scintillation counting. Then, isopropanol extraction was utilized to quantify radioactive cholesterol that has been incorporated into cellular lipids, and quantity of radioactive cellular cholesterol was determined afterwards. Percent efflux was calculated by the following formula: [([cpm of 3H cholesterol in the media − cpm of 3H cholesterol in serum free media] / [cpm of 3H cholesterol in the cells + cpm of 3H cholesterol in the media]) × 100]. The intra- and inter-assay coefficients of variation for cholesterol efflux capacity were 3.7% and 10.1%, respectively.
For HDL-PL and HDL-Tg quantification, HDL was isolated from serum by phosphotungstic acid precipitation (FujiFilm Wako Pure Chemical Corporation). HDL-PL and HDL-Tg were then measured according to manufacturer’s protocol (Wako: 433-36201 and Roche: 20767107322, respectively) using the Roche Cobas C311 clinical analyzer. The inter-assay coefficients of variation were 3.5% for HDL-PL and 3.9% for HDL-Tg.
Nuclear magnetic resonance spectroscopy
HDL subclasses and overall size were analyzed at LabCorp (Morrisville, NC, USA) by the Nuclear Magnetic Resonance (NMR) Spectroscopy LipoProfile-3 algorithm (29), by the automated 400 MHz NMR spectroscopy Vantera Clinical Analyzer. NMR lipoprotein particle quantification employs composite signal envelopes at 0.8 ppm, which contain the signals emitted by terminal methyl group protons of the phospholipids, unesterified cholesterol, cholesteryl ester, and triglycerides that are carried within each HDL lipoprotein particle. Signal amplitudes that contribute to the composite plasma signal were produced as a result of the deconvolution of the composite signal. Each lipoprotein subclass produces unique NMR signals that are specific in frequency and shape to the subclass. The amplitude of the signal is proportional to the number of particles releasing the signal.
To obtain the amplitude of each subpopulation of subclasses, the line shape of the signal envelope was modeled as a sum of all lipoprotein signals. The areas of different subpopulations were multiplied by conversion factors to quantify the concentrations, which were then grouped into small (7.3-8.2 nm), medium (8.2-9.4 nm), or large (9.4-14 nm) HDL subclasses. The total HDL particle concentration was obtained by summing the concentrations of all subclasses. The overall size of the HDL particles was calculated by adding the diameter of each subclass multiplied by its relative mass percentage from the NMR signal amplitude. Due to the magnetic property of lipoproteins which produces signals of different shapes and frequencies for different lipoproteins, NMR spectroscopy does not require the separation of lipoprotein subclasses as is required by other methods such as electrophoresis or ultracentrifugation. The intra- and inter-assay coefficients of variation for HDL-P concentrations and size ranged from 0.6% to 3.7% (intra-assay) and 1.5% to 4.0% (inter-assay).
HDL cholesterol and ApoA-I
Lipid fractions were determined from EDTA-treated plasma (30, 31). Fasting HDL-C was separated with heparin 2M manganese chloride (at the Medical Research Laboratory (MRL), Lexington, KY between baseline SWAN visit and follow-up visit 7; or at University of Michigan Pathology, Ann Arbor, MI for SWAN follow-up visits 9 and 12) (30, 31). ApoA-I was determined by immunonephelometry on the Behring Nephelometer II at the MRL between baseline SWAN visit and follow-up visit 7; and by reagents from Beckman-Coulter (Brea, CA) at the University of Pittsburgh Heinz laboratory at SWAN visits 9 and 12. To ensure that the results across labs are comparable, HDL-C and ApoA-I were calibrated by converting the results from the University of Michigan or University of Pittsburgh, respectively, to equivalent MRL results. The equations formulas were created using linear regression analysis, and both the slope and intercept were adjusted to achieve the most accurate calibration.
Endogenous hormones
Estradiol (E2) and follicle-stimulating hormone (FSH) were analyzed by the Bayer Diagnostics ACS:180 instrument between baseline SWAN visit and follow-up visit 9 or on the ADVIA Centaur for SWAN visit 12. Calibration equations were developed to convert E2 and FSH results obtained from the ADVIA Centaur to equivalent ACS:180 values. The R-squared values from the calibration equations were 92.4% for E2 and 98.5% for FSH. E2 was measured twice and the average of the 2 measures was used in the analysis. The lower limit of detection (LLD) of E2 ranged from 1.0 pg/mL to <12.38 pg/mL unit and of FSH ranged from 0.4 mIU/mL to 2.4 mIU/mL over the study visits. Visits at which estradiol levels were less than the LLD (n = 44 observations) were randomly assigned a number less than the LLD. No FSH observations were below LLD.
Other blood assays
C-reactive protein (CRP) was assessed by immunonephelometry using Behring reagents in the Behring Nephlometer II at the MRL, Lexington, KY between baseline SWAN visit and follow-up visit 7; or by the Alfa Wasserman ACE analyzer at CLASS laboratory at the University of Michigan Pathology, Ann Arbor, MI for SWAN follow-up visits 9 and 12. A calibration equation was developed and applied to convert the MRL and CLASS assays to a high-sensitivity enzyme-linked immunosorbent assay (ELISA) to obtain high-sensitivity CRP (hsCRP).
Study Covariates
Age was determined at each SWAN visit as the difference between date of study visit and date of birth. Race/ethnicity was self-reported by the women at the baseline SWAN visit. Body mass index (BMI in kg/m2) was calculated as measured weight (in kg) divided by the square of measured height (in meters2). The date of the final menstrual period (FMP) was determined as the date of last menstrual period reported in the visit immediately prior to the first visit when the woman was classified as postmenopausal. Time since FMP was calculated as the difference between the SWAN study visit date and the FMP date.
Menopause status was determined by self-reported questionnaires at every visit based on bleeding patterns over the past 12 months. Menopause status was categorized as either pre/early perimenopausal (no changes in patterns of the menstrual bleeds over the past 12 months or at least one bleed within the last 3 months with perceived changes in menstrual cycle intervals), late perimenopause (no bleed within the past 3 months but at least one menstrual cycle within the past 12 months), or postmenopausal (lack of menstrual bleeding for over the last 12 consecutive months, either naturally or surgically due to bilateral salpingo-oophorectomy, or due to unknown menopausal status post-hysterectomy).
Alcohol use and smoking status were determined by self-administered questionnaires at every visit. Frequency of alcohol use was categorized into either <once/month or ≥once/month. Current smoking status was categorized as either yes or no. Physical activity score was obtained by the Modified Kaiser Permanente Health Plan Activity Survey (32). Anti-lipid medication use was self-reported. Any medication use was created as the self-reported use of any cardiovascular medication (antidiabetics, lipid-lowering medications, and/or antihypertensives).
Statistical Analysis
Due to the small number of Hispanics, Chinese, and Japanese women in this analysis, race was recoded as White, Black, or other. Study variables were summarized at the first available visit using mean (SD), median (Q1, Q3) or frequencies (%), as appropriate. E2, FSH, HDL-Tg, and hsCRP were log-transformed to reduce skewness. Linear mixed-effect models with random intercept were used to assess associations of repeated measures of E2 and FSH with repeated measures of HDL metrics (separately). All models were adjusted for time-varying cycle day of blood draw to account for variability in hormonal level during menstrual cycle, race, and time-varying age, log-hsCRP (33, 34), and BMI (35, 36). Since increases in BMI and inflammation have been linked to menopause, it is plausible that they could impact HDL remodeling and function, justifying including them in final models (37).
Two sets of analysis were conducted; one with E2 and FSH being log-transformed and one using tertiles of E2 and FSH. HDL-Tg was analyzed with and without log-transformation in these analyses. Results were very comparable. Results from analysis of tertiles of E2 and FSH were included in this manuscript for simple clinical presentation. Results from analysis of E2 and FSH with log-transformation were included as Supplemental Tables (38).
To determine whether the associations of E2 and FSH with each HDL metric vary by time relative to the FMP, time relative to the FMP was categorized into time periods based on inflection points identified for each HDL metric from a previous work assessing changes in HDL metrics over time relative to the FMP (20), Supplemental Table 1 (38). Interaction term of each of these indicator variables with each hormone measure was included in final models to test effect modification by time relative to the FMP. For this analysis, E2 and FSH were analyzed as continuous log-transformed variables, Supplemental Tables (38). For significant interactions, stratified linear mixed-effect models by time relative to FMP time period while treating E2 and FSH as tertiles were conducted. Adjusted means of HDL metrics by E2 and FSH tertiles were estimated from these models and used for illustration. All analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC).
Results
Table 1 presents the characteristics of the women included in this analysis at the first available visit. On average, women were 50.2 (2.7) years old; 51.6% were White and 81.2% were pre-/early perimenopause.
Table 1.
Characteristics of women included in analysis at the first available visit
| Variable | n = 463 |
|---|---|
| Age, years, mean (SD) | 50.2 (2.7) |
| Race/ethnicity, n (%) | |
| White | 239 (51.6%) |
| Black | 127 (27.4%) |
| Other | 97 (21.0%) |
| Education level, n (%) | |
| ≤High School | 82 (17.8%) |
| Some college | 140 (30.4%) |
| College/post-graduate | 239 (51.8%) |
| Menopausal status | |
| Premenopause/early perimenopause | 376 (81.2%) |
| Late perimenopause | 39 (8.4%) |
| Postmenopause (BSO/natural/hysterectomy) | 48 (10.4%) |
| BMI, kg/m2, median (Q1, Q3) | 26.6 (23.0, 31.7) |
| Alcohol use, n (%) | |
| ≤1 drink/month | 234 (51.5%) |
| >1 drink/month | 220 (48.5%) |
| Lipid-lowering medication use, yes, n (%) | 13 (2.8%) |
| Any medication use, yes (n%) | 71 (15.3%) |
| Current smoker, n (%) | 48 (10.7%) |
| Physical activity score, mean (SD) | 7.8 (1.7) |
| LDL-C, mg/dL, mean (SD) | 112.6 (31.1) |
| Total cholesterol, mg/dL, mean (SD) | 194.1 (34.1) |
| Triglycerides, mg/dL, median (Q1, Q3) | 93.0 (70.0, 129.5) |
| Time since FMP, years, median (Q1, Q3) | -2.1 (-3.6, -0.8) |
| E2, pg/dL, median (Q1, Q3) | 37.7 (20.2, 99.1) |
| FSH, mIU/mL, median (Q1, Q3) | 26.3 (14.8, 59.3) |
| hsCRP, mg/dL, median (Q1, Q3) | 1.6 (0.6, 5.4) |
| HDL-C, mg/dL, mean (SD) | 59.3 (14.2) |
| ApoA-I, mg/dL, mean (SD) | 163.2 (25.8) |
| Total HDL-P, umol/L, mean (SD) | 34.6 (5.8) |
| Large HDL-P, umol/L, mean (SD) | 8.5 (3.6) |
| Medium HDL-P, umol/L, mean (SD) | 11.2 (6.2) |
| Small HDL-P, umol/L, mean (SD) | 14.8 (7.0) |
| HDL size, nm, mean (SD) | 9.5 (0.54) |
| HDL-PL, mg/dL, mean (SD) | 54.1 (10.1) |
| HDL-Tg, mg/dL, median (Q1, Q3) | 17.0 (14.0, 21.0) |
| HDL-CEC, %, mean (SD) | 3.9 (0.7) |
| HDL-CEC (%)/ Total HDL-P, mean (SD) | 0.1(0.02) |
LDL-C, total cholesterol, and HDL-C: 1 mg/dL = 0.0259 mmol/L; Triglycerides: 1 mg/dL = 0.0113 mmol/L; E2: 1 pg/dL = 0.0367 pmol/L; FSH: 1mIU/mL = 1 IU/L
Abbreviations: ApoA-I, apolipoprotein A-I; BMI, body mass index; FSH, follicle-stimulating hormone; HDL-C, high-density lipoprotein cholesterol; HDL-CEC, HDL cholesterol efflux capacity; HDL-P, high-density lipoprotein particles; HDL-PL, HDL phospholipids; HDL-Tg, HDL triglycerides; LDL-C, low-density lipoprotein cholesterol.
The univariate associations between each hormone and study covariates are presented in Table 2. Older age was associated with lower E2 and higher FSH, whereas higher BMI and hsCRP were associated with higher E2 and lower FSH, respectively. Higher physical activity score was associated with lower FSH. Compared with White women, those of other racial/ethnic groups (Hispanic, Chinese, and Japanese) had lower E2 and higher FSH; Black women had lower FSH compared with Whites. Current smokers had higher levels of E2.
Table 2.
Univariate linear mixed-effect models of E2 and FSH with study covariatesa
| E2b,c | FSHb,c | |||
|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | |
| Age | −0.06 (0.007) | <0.0001 | 0.05 (0.005) | <0.0001 |
| BMI | 0.01 (0.004) | 0.002 | −0.03 (0.003) | <0.0001 |
| Race | 0.004 | <0.001 | ||
| White | Ref | Ref | Ref | Ref |
| Black | −0.001 (0.07) | 0.98 | −0.15 (0.06) | 0.006 |
| Other | −0.22 (0.07) | 0.002 | 0.15 (0.06) | 0.013 |
| hsCRPb | 0.04 (0.02) | 0.021 | −0.10 (0.02) | <0.0001 |
| Physical activity score | 0.009 (0.02) | 0.57 | 0.03 (0.01) | 0.030 |
| Alcohol use | 0.28 | |||
| No | Ref | Ref | Ref | Ref |
| Yes | −0.06 (0.05) | 0.28 | 0.06 (0.04) | 0.20 |
| Current smoker | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.26 (0.09) | 0.014 | -0.06 (0.07) | 0.41 |
| Any medication use | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.02 (0.07) | 0.79 | −0.06 (0.05) | 0.31 |
Abbreviations: BMI, body mass index; E2, estradiol; FSH, follicle-stimulating hormone.
a Cycle day of blood draw included in models
b Log-Transformed
c For continuous variables, data presented per 1-unit increase.
Associations of E2 and FSH With HDL Metrics
Adjusting for race/ethnicity, time-varying age, cycle day of the blood draw, log-hsCRP, and BMI, compared to lower E2 tertile, higher E2 tertile was associated with higher concentrations of HDL-C, large HDL-P, HDL-Tg, and larger overall HDL size. Furthermore, higher E2 tertile was associated with higher HDL-CEC and HDL-CEC per particle (HDL-CEC/total HDL-P), and with lower concentrations of medium HDL-P than lower E2 tertile (Table 3).
Table 3.
Adjusted means of HDL metrics by E2 and FSH tertiles in women traversing menopause
| Study outcomes | E2 tertilesa | FSH tertilesa | ||
|---|---|---|---|---|
| Adjusted mean (95% CI)b | Trend P value | Adjusted mean (95% CI)b | Trend P value | |
| HDL-C, mg/dL | 0.017 | 0.37 | ||
| Low | 59.9 (58.4, 61.4) | 60.6 (59.1, 62.1) | ||
| Medium | 60.4 (60.0, 62.9) | 60.2 (58.7, 61.6) | ||
| High | 61.4 (60.0, 62.9)c | 61.3 (59.6, 62.9) | ||
| ApoA-I, mg/dL | 0.57 | 0.046 | ||
| Low | 163.8 (160.7, 166.9) | 161.9 (158.8, 165.1) | ||
| Medium | 162.3 (159.3, 165.2) | 162.0 (158.9, 165.1) | ||
| High | 162.9 (159.9, 165.9) | 166.0 (162.4, 169.6) | ||
| Total HDL-P, μmol/L | 0.16 | 0.0005 | ||
| Low | 35.3 (34.7, 36.0) | 34.6 (33.9, 35.3) | ||
| Medium | 35.1 (34.5, 35.7) | 35.0 (34.3, 35.6) | ||
| High | 34.9 (34.2, 35.5) | 36.1 (35.3, 36.9)c | ||
| Large HDL-P, μmol/L | <0.0001 | 0.017 | ||
| Low | 8.3 (8.0, 8.7) | 9.2 (8.8, 9.5) | ||
| Medium | 8.6 (8.3, 9.0) | 8.4 (8.0, 8.8)c | ||
| High | 9.3 (8.9, 9.7)c | 8.6 (8.1, 9.0)c | ||
| Medium HDL-P, μmol/L | 0.034 | 0.0097 | ||
| Low | 11.4 (10.8, 12.1) | 10.2 (9.5, 10.9) | ||
| Medium | 10.9 (10.3, 11.6) | 11.5 (10.8, 12.1)c | ||
| High | 10.6 (9.9, 11.3)c | 11.6 (10.8, 12.4)c | ||
| Small HDL-P, μmol/L | 0.23 | 0.50 | ||
| Low | 15.5 (14.7, 16.3) | 15.3 (14.6, 16.1) | ||
| Medium | 15.6 (14.8, 16.3) | 15.1 (14.4, 15.9) | ||
| High | 15.0 (14.3, 15.8) | 15.7 (14.8, 16.5) | ||
| HDL size, nm | <0.0001 | 0.0003 | ||
| Low | 9.48 (9.42, 9.53) | 9.59 (9.53, 9.64) | ||
| Medium | 9.50 (9.45, 9.56) | 9.51 (9.45, 9.57)c | ||
| High | 9.62 (9.56, 9.67)c | 9.47 (9.41, 9.53)c | ||
| HDL-PL, mg/dL | 0.41 | 0.20 | ||
| Low | 54.4 (53.3, 55.5) | 54.4 (53.3, 55.5) | ||
| Medium | 54.2 (53.1, 55.2) | 54.1 (53.0, 55.2) | ||
| High | 54.8 (53.8, 55.9) | 55.2 (53.9, 56.4) | ||
| HDL-Tg, mg/dL | <0.0001 | 0.078 | ||
| Low | 17.7 (17.1, 18.4) | 18.9 (18.2, 19.5) | ||
| Medium | 18.3 (17.7, 18.9) | 18.2 (17.5, 18.8) | ||
| High | 19.2 (18.5, 19.8)c | 18.0 (17.3, 18.8) | ||
| HDL-CEC, % | 0.010 | 0.39 | ||
| Low | 3.91 (3.85, 3.98) | 3.95 (3.88, 4.02) | ||
| Medium | 3.95 (3.88, 4.02) | 3.94 (3.87, 4.01) | ||
| High | 4.00 (3.93, 4.07)c | 3.99 (3.91, 4.07) | ||
| HDL-CEC/Total HDL-P | 0.001 | 0.016 | ||
| Low | 0.113 (0.110, 0.115) | 0.116 (0.114, 0.119) | ||
| Medium | 0.115 (0.113, 0.117) | 0.115 (0.113, 0.117) | ||
| High | 0.117 (0.115, 0.119)c | 0.113 (0.110, 0.115)c | ||
Abbreviations: ApoB, apolipoprotein B; E2, estradiol; FSH, follicle-stimulating hormone; HDL-C, high-density lipoprotein cholesterol; HDL-CEC, HDL cholesterol efflux capacity; HDL-P, HDL particles; HDL-PL, HDL-phospholipid; HDL-Tg, HDL-triglyceride.
a E2 Tertiles: Low: E2 ≤ 17.47 pg/dL; Medium: 17.47 pg/dL < E2 ≤ 33.35 pg/dL; High: E2 > 33.35 pg/dL; FSH Tertiles: Low: FSH ≤ 44.7 mIU/mL; Medium:44.7 mIU/mL < FSH ≤ 93.9 mIU/mL; High: FSH > 93.9 mIU/mL
b Models adjusted for race, time-varying age and cycle day of blood draw + time-varying log-hsCRP, and BMI
c Significantly different from Low
Compared with lower FSH tertile, higher FSH tertile was associated with higher total and medium HDL-P, and with lower large HDL-P, overall HDL size, and HDL-CEC per particle (Table 3). Similar results were obtained when E2 and FSH were analyzed as continuous log-transformed variables, Supplemental Table 2 (38).
Adding any medication use to final models did not change the analysis (data not shown).
Effect Modification of Time Since FMP on the Associations of E2 and FSH With HDL Metrics
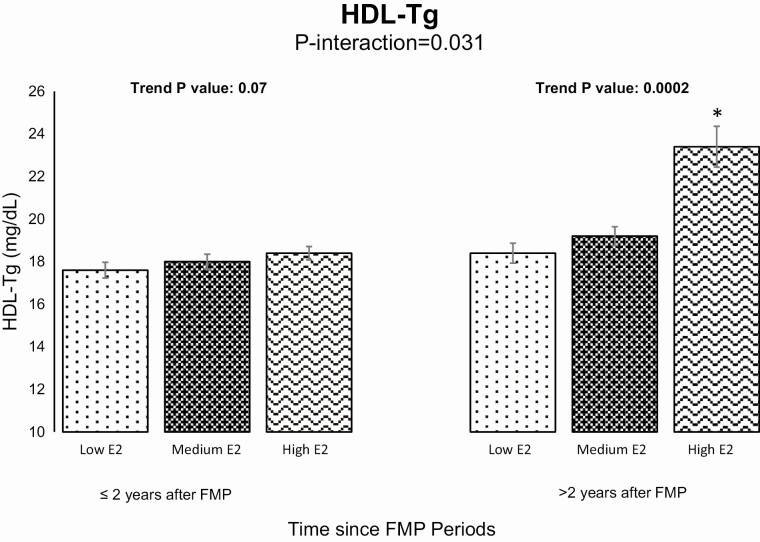
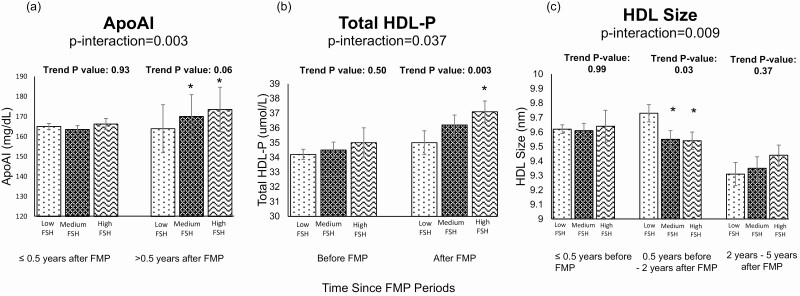
Time since FMP modified the association between E2 and HDL-Tg, such that the positive association between E2 and HDL-Tg was significantly stronger after 2 years from the FMP compared with the time before, as shown in Supplemental Table 3 (38) and Fig. 1. Time since FMP also modified the associations between FSH and ApoA-I, total HDL-P and HDL size (Fig. 2). Higher FSH was associated with higher ApoA-I and higher total HDL-P after 0.5 year since FMP (Time Period 2). Compared with before 0.5 year prior the FMP (Time Period 1), higher FSH was associated with a smaller overall HDL size between 0.5 year prior the FMP and 2 years after FMP (Time Period 2), as well as after more than 5 years post-FMP (Time Period 4). Interestingly, associations between FSH and total HDL-P and HDL size were only statistically significant close to menopause or after menopause, shown in Supplemental Table 3 (38). Adding any medication use to final models did not change the analysis (data not shown).
Figure 1.
Adjusted means of HDL-Tg by E2 tertiles stratified by time relative to the FMP. Models adjusted for race/ethnicity, time-varying age, cycle day of blood draw, log-transformed hsCRP, and BMI. *Significantly different from Low E2.
Figure 2.
Adjusted means of (a) ApoA-I, (b) Total HDL-P, (c) HDL size by FSH tertiles stratified by time relative to the FMP. Models adjusted for race/ethnicity, time-varying age, cycle day of blood draw, log-transformed hsCRP, and BMI. *Significantly different from Low FSH.
Discussion
Using multiple data points over the menopause transition, the current study provides one of the most comprehensive assessment of associations between 2 cardinal hormonal markers of the menopause transition and multiple metrics of HDL including subclasses, lipid content, and function. The study showed that levels of E2 and FSH were not only related to HDL subclasses but also to HDL lipid content and function. Moreover, the current findings demonstrated that some of the associations linking E2 and FSH with novel metrics of HDL were vulnerable to time relative to the menopause onset. In midlife women, higher level of E2 was associated with both anti-atherogenic (larger particle size, greater concentrations of large HDL-P, and higher HDL-CEC and HDL-CEC per particles—features that have been linked to a lower CVD risk) (17, 25) and pro-atherogenic HDL metric profiles (lower concentrations of total and medium HDL-Ps, and greater HDL-Tg—features that have been linked to a higher CVD risk) (27, 39). Interestingly, the atherogenic association between higher E2 and higher HDL-Tg was stronger after 2 years post-FMP than before. Similarly, higher FSH level was related to both a pro-atherogenic HDL metric profile (smaller particle size, lower concentrations of large HDL-P, and lower HDL-CEC per particle) and anti-atherogenic profile (higher concentrations of total and medium HDL-P and lower concentrations of HDL-Tg). The associations of higher FSH level with higher concentrations of total HDL-P with smaller HDL size were only evident either close to the menopause timing or after menopause.
Associations between endogenous hormone levels and HDL metrics other than HDL-C have been evaluated in a limited number of studies, with the majority of these studies focusing on E2. Among 120 women from the Pittsburgh Healthy Women Study who were 1-year amenorrheic, a positive association between E2 level and larger HDL subfractions (HDL 2-C), as measured by precipitation procedure, was reported (21). In line with these findings were results from an earlier cross-sectional analysis from the SWAN study among 120 pre-/early peri- or late peri-/postmenopausal women where higher E2 level was associated with larger HDL size (23) as measured by NMR (29). In that SWAN analysis, the reported association for E2 and HDL size did not vary by menopause stage. Both the Pittsburgh Healthy Women Study and SWAN analyses did not assess similar associations with FSH (21, 23). A more recent work from SWAN among a smaller sample of 46 midlife women with E2, FSH, and HDL metrics (HDL subclasses as measured by calibrated ion mobility method and HDL-CEC) available on 2 time points before and after menopause, showed that a greater decline in E2 was significantly correlated with a larger decline in large HDL-P and a larger increase in small HDL-P (24). In this pre/post analysis from SWAN, a greater increase in FSH was significantly correlated with a larger decline in the size of medium HDL-P but not with any other HDL subclasses. Change in HDL-CEC did not correlate with change in E2 or FSH from pre- to postmenopause (24).
The findings from the current study were in line with findings from previous studies showing higher E2 to be related to larger HDL subclasses and/or larger HDL particle size, irrespective of the method used to quantify HDL subclasses. However, unlike previous pre/post menopause analysis from SWAN (24), the current study showed a significant positive association of E2 with HDL-CEC and HDL-CEC per particle, and extended findings on FSH to be related to HDL-Tg and HDL-CEC per particle in addition to specific HDL subclasses. Our ability to detect additional associations of E2 and FSH with metrics of HDL could be related to having higher power due to larger sample size and including multiple data points over the menopause transition. The current findings support the hypothesis that E2 and FSH play roles in HDL subclass remodeling and HDL’s major function of promoting cholesterol efflux from macrophages in women traversing menopause.
The mechanisms through which menopause-related hormones might impact HDL metrics are not completely understood. Interestingly, estrogens and their receptors (specifically ERα) are involved in the process of hepatic lipid metabolism. In animal studies, it was shown that HDLs undergo structural and functional remodeling that depends on ERα, which is dependent on ovarian E2 production (40). A reduction in plasma estrogens as a result of the menopause transition enhances the activity of hepatic triglyceride lipase, which converts large HDL particles to smaller ones (41, 42). In our study, we found that lower E2 was associated with smaller HDL size and a lower concentration of large HDL-P. E2 might also contribute to HDL lipid content and function. E2 positively correlates with cholesteryl ester transfer proteins (CETPs) (43), which promote cholesteryl ester transfer from HDL to triglycerides-rich low-density lipoproteins allowing the formation of triglycerides-rich HDL particles (44). In line with that, our study showed a positive association between E2 and HDL-Tg content in midlife women. By contributing to HDL subclasses and HDL lipid content, E2 may indirectly contribute to HDL-CEC as reported in our study. E2 promotes the cholesterol efflux capacity from vascular smooth muscle cells (45) and reduces cholesteryl ester accumulation in human monocyte–derived macrophages obtained from patients with CHD (46).
The exact mechanism linking FSH and HDL needs more research. Among postmenopausal women aged 53 to 73 years not using hormone therapy (HT), FSH was positively associated with HDL-C and this association was stronger in older women (47). FSH might be related to HDL through stimulating lipid biosynthesis in animal adipose tissue (48) and the formation of lipid droplets in human adipose tissue (49).
A unique contribution of the current study is the moderation effect of time relative to menopause on associations of E2 and FSH with HDL metrics. We reported that the pro-atherogenic associations of higher E2 with greater HDL-Tg and higher FSH with higher concentrations of total HDL-P with smaller size were more evident at or after menopause. Accumulating evidence suggest a critical impact of timing of initiating HT use on CVD risk in women (50); such that initiating HT use in older women who have been postmenopausal for a long time could result in a greater risk of CVD than initiating HT in recently menopausal women (7). Whether the use of HT as related to time relative to menopause could have a detrimental effect on the lipid content of HDL particles that may contribute to the increased CVD risk linked to use HT in older women will require additional research.
The strengths of this study included the comprehensive assessment of the associations of 2 cardinal hormonal markers of the menopause transition and novel metrics of HDL in a well-characterized sample of women traversing menopause, the longitudinal design of the analysis with the repeated measures of HDL and hormones over the menopause transition, as well as the accurate collection of dates of the FMP. Some limitations for this study exist, such as the use of a single functional metric of HDL, which is the cholesterol efflux capacity. The cholesterol efflux capacity is a central step of the reverse cholesterol transport which is thought to be one of the major anti-atherogenic functions of HDL (51); however, other functions could be impacted by hormonal levels of the menopause transitions such as the anti-inflammatory or anti-oxidative abilities; these were not collected in the current study. It is worth noting that the women who were excluded due to missing data had higher HDL-Tg, total HDL-PL, and ApoA-I. This could have biased our results; however, given that they were older and postmenopausal, excluding these women most likely weakened our observed associations. We measured HDL-CEC by the J774 mouse macrophages and not human macrophages. However, mouse cells are the most commonly used donor cells for CEC, and it has been shown that the results of ABCA1-mediated HDL efflux are comparable to results from human macrophage cell line (52, 53). Studies have also shown that HDL-CEC measured in J744 cells is inversely associated with coronary artery disease in humans. We used an immunoassay to measure sex steroid hormone levels in our population rather than direct methods such as mass spectrometry, as is more currently used in clinical practice. Future studies using more advanced method of quantifying hormones should replicate our work.
In conclusion, the current study demonstrated a clear link between 2 cardinal hormones of the menopause transition and multiple metrics of HDL. Both E2 and FSH were related to HDL subclasses, lipid content, and function in midlife women. Interestingly, E2 and FSH each showed anti- and pro-atherogenic associations with specific metrics of HDL, and the pro-atherogenic associations were found to be stronger at or after menopause. The findings support the evolving literature of the timing hypothesis suggesting different effect of HT on CVD risk based on time relative to menopause onset. Our study calls for a future evaluation of how initiation timing of HT might impact HDL metrics in midlife women and thus may contribute to CVD risk linked to HT use in older women.
Acknowledgments
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011-present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999-present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009-present; Lynda Powell, PI 1994-2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011-present, Rachel Wildman, PI 2010-2011; Nanette Santoro, PI 2004-2010; University of Medicine and Dentistry, New Jersey Medical School, Newark—Gerson Weiss, PI 1994-2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016- present; Winifred Rossi 2012-2016; Sherry Sherman 1994-2012; Marcia Ory 1994-2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor—Siobán Harlow 2013-Present; Dan McConnell 2011-2013; MaryFran Sowers 2000-2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012-present; Kim Sutton-Tyrrell, PI 2001-2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995-2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Glossary
Abbreviations
- ABCA1
ATP-binding cassette transporter-1
- ApoA-I
apolipoprotein A-I
- ApoB
apolipoprotein B
- BMI
body mass index
- CRP
C-reactive protein
- CVD
cardiovascular disease
- E2
estradiol
- %FMD
% change in brachial artery flow mediated dilation
- FMP
final menstrual period
- FSH
follicle-stimulating hormone
- HDL-C
high-density lipoprotein cholesterol
- HDL-CEC
HDL cholesterol efflux capacity
- HDL-P
HDL particles
- HDL-PL
HDL-phospholipid
- HDL-Tg
HDL-triglyceride
- hsCRP
high-sensitivity C-reactive protein
- HT
hormone therapy
- LLD
lower limit of detection
- MRL
Medical Research Laboratory, Lexington, KY
- NMR
nuclear magnetic resonance
- SWAN
Study of Women’s Health Across the Nation
Financial Support
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS (US Department of Health and Human Services), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The SWAN Repository (U01AG017719).
The Study of Women’s Health Across the Nation (SWAN) HDL ancillary study has grant support from National Institute on Aging (NIA) AG058690.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Additional Information
Disclosures: S.R.E., A.N., J.B., M.M.B., D.M., S.C., T.J.O., and K.A.M. have nothing to disclose. D.J.R. is the founder of Vascular Strategies.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. SWAN provides access to public use datasets that include data from SWAN screening, baseline, and follow-up visits (https://agingresearchbiobank.nia.nih.gov/ and http://www.swanstudy.org/swan-research/data-access/). Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center (swanaccess@edc.pitt.edu).
The authors declare that all supporting data are available within the article (and its Data Supplement).
References
- 1. Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380(9841):572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256(20):2835-2838. [PubMed] [Google Scholar]
- 3. Sharrett AR, Ballantyne CM, Coady SA, et al. ; Atherosclerosis Risk in Communities Study Group . Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104(10):1108-1113. [DOI] [PubMed] [Google Scholar]
- 4. Barter PJ, Caulfield M, Eriksson M, et al. ; ILLUMINATE Investigators . Effects of torcetrapib in patients at high risk for coronary events. n Engl j Med. 2007;357(21):2109-2122. [DOI] [PubMed] [Google Scholar]
- 5. Nissen SE, Tardif JC, Nicholls SJ, et al. ; ILLUSTRATE Investigators . Effect of torcetrapib on the progression of coronary atherosclerosis. n Engl j Med. 2007;356(13):1304-1316. [DOI] [PubMed] [Google Scholar]
- 6. Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation. 2008;118(24):2506-2514. [DOI] [PubMed] [Google Scholar]
- 7. El Khoudary SR, Aggarwal B, Beckie TM, et al. ; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing . Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142(25):e506-e532. [DOI] [PubMed] [Google Scholar]
- 8. Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles Atherosclerosis Study. Atherosclerosis. 2007;195(1):e191-e196. [DOI] [PubMed] [Google Scholar]
- 9. Keidar S, Bogner I, Gamliel-Lazarovich A, Leiba R, Fuhrman B, Kouperberg E. High plasma high-density lipoprotein levels, very low cardiovascular risk profile, and subclinical carotid atherosclerosis in postmenopausal women. j Clin Lipidol. 2009; 3(5):345-350. [DOI] [PubMed] [Google Scholar]
- 10. Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women’s Health Across the Nation Heart women. Menopause. 2011;18(4):376-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby CA, Matthews KA. Increase HDL-C level over the menopausal transition is associated with greater atherosclerotic progression. j Clin Lipidol. 2016;10(4):962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El Khoudary SR, Ceponiene I, Samargandy S, et al. HDL (high-density lipoprotein) metrics and atherosclerotic risk in women. Arterioscler Thromb Vasc Biol. 2018;38(9):2236-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Khoudary SR, Chen X, Brooks M, Janssen I, Hollenberg SM, Thurston RC, Matthews K.Abstract AP118: Cardioprotective association between high density lipoprotein cholesterol and endothelial function attenuated at lower levels of estradiol in women at midlife. The SWAN Heart Study. Circulation. 2017;135:AP118. doi.org:10.1161/circ.135.suppl_1.p118 [Google Scholar]
- 14. Swabe G, Matthews K, Brooks M, Janssen I, Wang N, El Khoudary SR. High-density lipoprotein cholesterol and arterial calcification in midlife women: the contribution of estradiol and C-reactive protein. Menopause. 2020;28(3):237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mackey RH, Greenland P, Goff DC Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). j Am Coll Cardiol. 2012;60(6):508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128(11):1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. n Engl j Med. 2011;364(2):127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. n Engl j Med. 2014;371(25):2383-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agarwala AP, Rodrigues A, Risman M, et al. High-density lipoprotein (HDL) phospholipid content and cholesterol efflux capacity are reduced in patients with very high HDL cholesterol and coronary disease. Arterioscler Thromb Vasc Biol. 2015;35(6):1515-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El Khoudary SR, Chen X, Nasr A, et al. HDL (high-density lipoprotein) subclasses, lipid content, and function trajectories across the menopause transition: SWAN-HDL study. Arterioscler Thromb Vasc Biol. 2021;41(2):951-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuller LH, Gutai JP, Meilahn E, Matthews KA, Plantinga P. Relationship of endogenous sex steroid hormones to lipids and apoproteins in postmenopausal women. Arteriosclerosis. 1990;10(6):1058-1066. [DOI] [PubMed] [Google Scholar]
- 22. Badeau RM, Metso J, Kovanen PT, Lee-Rueckert M, Tikkanen MJ, Jauhiainen M. The impact of gender and serum estradiol levels on HDL-mediated reverse cholesterol transport. Eur j Clin Invest. 2013;43(4):317-323. [DOI] [PubMed] [Google Scholar]
- 23. El Khoudary SR, Brooks MM, Thurston RC, Matthews KA. Lipoprotein subclasses and endogenous sex hormones in women at midlife. j Lipid Res. 2014;55(7):1498-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El Khoudary SR, Hutchins PM, Matthews KA, et al. Cholesterol efflux capacity and subclasses of HDL particles in healthy women transitioning through menopause. j Clin Endocrinol Metab. 2016;101(9):3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piperi C, Kalofoutis C, Papaevaggeliou D, Papapanagiotou A, Lekakis J, Kalofoutis A. The significance of serum HDL phospholipid levels in angiographically defined coronary artery disease. Clin Biochem. 2004;37(5):377-381. [DOI] [PubMed] [Google Scholar]
- 27. Holmes MV, Millwood IY, Kartsonaki C, et al. ; China Kadoorie Biobank Collaborative Group . Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. j Am Coll Cardiol. 2018;71(6):620-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sowers MF, Crawford S, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, eds. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000:175-180. [Google Scholar]
- 29. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847-870. [DOI] [PubMed] [Google Scholar]
- 30. Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. j Lipid Res. 1978;19(1):65-76. [PubMed] [Google Scholar]
- 31. Steiner PFJ, Bremner W, Stein E. Standardization of micro-methods for plasma cholesterol, triglyceride and HDL-cholesterol with the Lipid Research Clinics’ methodology. J Clin Chem Clin Biochem. 1981;19(8):850. [Google Scholar]
- 32. Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the kaiser physical activity survey in women. Med Sci Sports Exerc. 2000;32(7):1327-1338. [DOI] [PubMed] [Google Scholar]
- 33. Sowers MR, Matthews KA, Jannausch M, et al. Hemostatic factors and estrogen during the menopausal transition. j Clin Endocrinol Metab. 2005;90(11):5942-5948. [DOI] [PubMed] [Google Scholar]
- 34. Hartz J, Krauss RM, Göttsater M, Melander O, Nilsson P, Mietus-Snyder M. Lipoprotein particle predictors of arterial stiffness after 17 years of follow up: the malmö diet and cancer study. Int j Vasc Med. 2020;2020:4219180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tepper PG, Randolph JF Jr, McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). j Clin Endocrinol Metab. 2012;97(8):2872-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zaid M, Miura K, Okayama A, et al. ; INTERLIPID and INTERMAP Research Groups . Associations of high-density lipoprotein particle and high-density lipoprotein cholesterol with alcohol intake, smoking, and body mass index- The INTERLIPID Study. Circ j. 2018;82(10):2557-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mascarenhas-Melo F, Sereno J, Teixeira-Lemos E, et al. Markers of increased cardiovascular risk in postmenopausal women: focus on oxidized-LDL and HDL subpopulations. Dis Markers. 2013;35(2):85-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. El Khoudary SR. Supplemental Material-Associations of endogenous hormones with HDL novel metrics across the menopause transition: The SWAN HDL Study . Ann Arbor, MI: Inter-university consortium for political and social research (openICPSR). Posted July 14, 2021. doi: 10.3886/E145103V1 [DOI] [Google Scholar]
- 39. Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113(12):1556-1563. [DOI] [PubMed] [Google Scholar]
- 40. Della Torre S, Mitro N, Fontana R, et al. An essential role for liver ERα in coupling hepatic metabolism to the reproductive cycle. Cell Rep. 2016;15(2):360-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wakatsuki A, Sagara Y. Lipoprotein metabolism in postmenopausal and oophorectomized women. Obstet Gynecol. 1995;85(4):523-528. [DOI] [PubMed] [Google Scholar]
- 42. Berg GA, Siseles N, González AI, Ortiz OC, Tempone A, Wikinski RW. Higher values of hepatic lipase activity in postmenopause: relationship with atherogenic intermediate density and low density lipoproteins. Menopause. 2001;8(1):51-57. [DOI] [PubMed] [Google Scholar]
- 43. Zhang C, Zhuang Y, Qiang H, Liu X, Xu R, Wu Y. Relationship between endogenous estrogen concentrations and serum cholesteryl ester transfer protein concentrations in Chinese women. Clin Chim Acta. 2001;314(1-2):77-83. [DOI] [PubMed] [Google Scholar]
- 44. Kunitake ST, Mendel CM, Hennessy LK. Interconversion between apolipoprotein A-I-containing lipoproteins of pre-beta and alpha electrophoretic mobilities. j Lipid Res. 1992;33(12):1807-1816. [PubMed] [Google Scholar]
- 45. Wang H, Liu Y, Zhu L, et al. 17β-estradiol promotes cholesterol efflux from vascular smooth muscle cells through a liver X receptor α-dependent pathway. Int j Mol Med. 2014;33(3):550-558. [DOI] [PubMed] [Google Scholar]
- 46. Corcoran MP, Lichtenstein AH, Meydani M, Dillard A, Schaefer EJ, Lamon-Fava S. The effect of 17β-estradiol on cholesterol content in human macrophages is influenced by the lipoprotein milieu. j Mol Endocrinol. 2011;47(1):109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Serviente C, Tuomainen TP, Virtanen J, Witkowski S, Niskanen L, Bertone-Johnson E. Follicle-stimulating hormone is associated with lipids in postmenopausal women. Menopause. 2019;26(5):540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cui H, Zhao G, Liu R, Zheng M, Chen J, Wen J. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. j Lipid Res. 2012;53(5):909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu XM, Chan HC, Ding GL, et al. FSH regulates fat accumulation and redistribution in aging through the Gαi/Ca(2+)/CREB pathway. Aging Cell. 2015;14(3):409-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clarkson TB, Meléndez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20(3):342-353. [DOI] [PubMed] [Google Scholar]
- 51. von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2001;21(1):13-27. [DOI] [PubMed] [Google Scholar]
- 52. Du XM, Kim MJ, Hou L, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116(7):1133-1142. [DOI] [PubMed] [Google Scholar]
- 53. Favari E, Calabresi L, Adorni MP, et al. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 2009;48(46):11067-11074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. SWAN provides access to public use datasets that include data from SWAN screening, baseline, and follow-up visits (https://agingresearchbiobank.nia.nih.gov/ and http://www.swanstudy.org/swan-research/data-access/). Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center (swanaccess@edc.pitt.edu).
The authors declare that all supporting data are available within the article (and its Data Supplement).