Abstract
Context
Severe forms of growth hormone insensitivity (GHI) are characterized by extreme short stature, dysmorphism, and metabolic anomalies.
Objective
This work aims to identify the genetic cause of growth failure in 3 “classical” GHI individuals.
Methods
A novel intronic growth hormone receptor gene (GHR) variant was identified, and in vitro splicing assays confirmed aberrant splicing. A 6Ω pseudoexon GHR vector and patient fibroblast analysis assessed the consequences of the novel pseudoexon inclusion and the impact on GHR function.
Results
We identified a novel homozygous intronic GHR variant (g.5:42700940T > G, c.618+836T > G), 44 bp downstream of the previously recognized intronic 6Ψ GHR pseudoexon mutation in the index patient. Two siblings also harbored the novel intronic 6Ω pseudoexon GHR variant in compound heterozygosity with the known GHR c.181C > T (R43X) mutation. In vitro splicing analysis confirmed inclusion of a 151-bp mutant 6Ω pseudoexon not identified in wild-type constructs. Inclusion of the 6Ω pseudoexon causes a frameshift resulting in a nonfunctional truncated GHR lacking the transmembrane and intracellular domains. The truncated 6Ω pseudoexon protein demonstrated extracellular accumulation and diminished activation of STAT5B signaling following GH stimulation.
Conclusion
Novel GHR 6Ω pseudoexon inclusion results in loss of GHR function consistent with a severe GHI phenotype. This represents a novel mechanism of Laron syndrome and is the first deep intronic variant identified causing severe postnatal growth failure. The 2 kindreds originate from the same town in Campania, Southern Italy, implying common ancestry. Our findings highlight the importance of studying variation in deep intronic regions as a cause of monogenic disorders.
Keywords: short stature, growth hormone insensitivity, GHR 6Ω pseudoexon, severe primary IGF-1 deficiency
Growth hormone insensitivity (GHI) presents in childhood with postnatal growth failure. The severe form, “classical GHI,” is associated with extreme short stature, dysmorphic facial features, and metabolic abnormalities. Biochemically, it is characterized by elevated circulating GH levels, severe insulin-like growth factor 1 (IGF-1) deficiency and subnormal IGF-binding protein 3 (IGFBP 3) and acid labile subunit (ALS) levels (1). Classical GHI was first described in 1966 (2), and termed “Laron syndrome” (OMIM 262500). This disorder was shown to be secondary to a defect in the growth hormone receptor gene (GHR) resulting in severe GH resistance (1). Since the first description, around 100 GHR mutations have been identified in more than 300 patients with significant phenotypic and biochemical variability (3).
During transcription, the entire sequence of a gene, including exons and introns, is copied to produce precursor messenger mRNA. To create a continuous coding sequence that can be translated into a protein, the introns are excised from the precursor mRNA by RNA splicing (4). The intron-exon boundaries are defined by short consensus sequences at the 5′ (donor) and 3′ (acceptor) splice sites that are recognized by the spliceosome. Aberrant splicing events are an established, frequent cause of monogenic human disease, but these genetic alterations are most frequently reported in the consensus sequences flanking the exons (5). Whole-genome sequencing approaches have resulted in the identification of an increasing number of pathogenic variants located deep within introns (6).
Introns frequently contain potential exonic sequences with canonical 5′ and 3′ sequences and flanking regions. These are termed pseudoexons because they are ignored by the cellular splicing machinery and not incorporated into mature mRNA (6). Most pathological pseudoexon inclusion events originate from mutations that create a novel donor splice site and activate a preexisting noncanonical acceptor splice site. Less frequently, the mutation creates a novel acceptor splice site or alternatively creates or disrupts splicing enhancer or silencer elements, respectively (6). Disease-causing pseudoexon inclusion was first reported in β-thalassemia (7, 8), but has subsequently been identified in patients affected by multiple disorders (6, 9).
In 2001, our group described the only known intronic pseudoexon mutation to cause a growth disorder. This point mutation in intron 6 of the GH receptor (GHR; c.618+792A > G), was identified in 4 siblings with mild or “nonclassical” GHI (10). This GHR “6Ψ” mutation results in the inclusion of a 108 base pair (bp) pseudoexon between exons 6 and 7 of the GHR (10), translating to an in-frame insertion of 36 amino acid residues in the extracellular domain of the GHR (10, 11). In 2007, a further 7 6Ψ patients were identified (12). Recently, an in-depth analysis of the spectrum of clinical and biochemical features was reported in 20 6Ψ individuals (13). Interestingly, only 50% of the 20 6Ψ patients had “classical” GHI facial features (13). This milder, very variable phenotype (even among affected members of the same family) may be explained by the efficiency of splicing events that result in GHR transcript variability, that is, the relative abundance of different GHR transcripts (10, 14). We report a novel GHR 6Ω pseudoexon resulting in severe postnatal growth failure/classical Laron syndrome.
Materials and Methods
Kindred 1
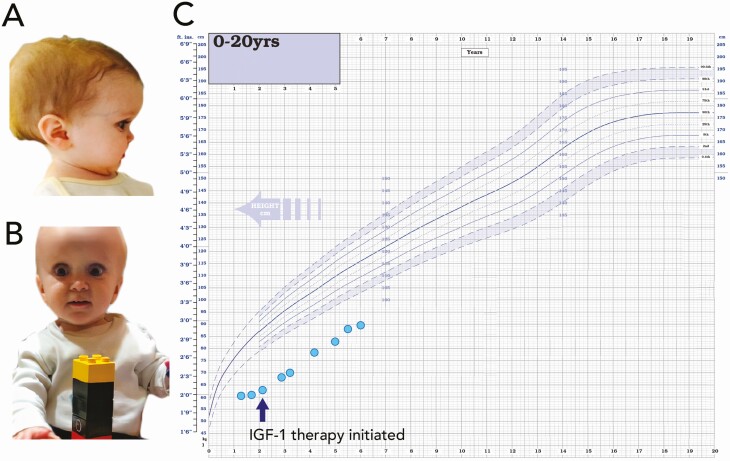
The index case (patient 1, Fig. 1A and 1B) was referred to our genetic sequencing service at age 2.9 years with classical biochemical and phenotypic features of GHI (Laron syndrome) (Table 1). He was the second child of unrelated nondysmorphic White parents. He was born at 37 weeks’ gestation with a normal birth weight (SD score [SDS] –0.4). Severe postnatal growth failure was first noted at age 5 months and by age 1.7 years his height was 61 cm (height SDS –7.4) (Fig. 1C), and he had a normal body mass index (BMI) of 16.5 (SDS –0.6) and relative macrocephaly (head circumference SDS –1.2). At presentation he had classical Laron facial features, delayed tooth eruption, small hands and feet, micropenis, bilateral undescended testes, and hypoplastic scrotum. Maternal and paternal heights were –2.0 and –1.5 SDS, respectively, and there was no family history of growth failure. Random serum GH was extremely elevated (38 µg/L; normal range [NR], 0-20 ng/mL). At diagnosis, he was noted to have severe deficiencies of IGF-1 (< 10 ng/mL; NR, 13-143 ng/mL) and IGFBP 3 (< 80 ng/mL; NR, 1612-4525 ng/mL), and ALS and growth hormone binding protein (GHBP) levels were undetectable (< 100 mU/mL and < 80 pM, respectively). IGF-1 levels during a 5-day IGF-1 generation test (IGFGT; GH 0.033 mg/kg/day, performed according to established protocols) demonstrated an IGF-1 level of less than 10 ng/mL at baseline and at 4 days following GH administration, indicating severe GH resistance (Table 2). He was diagnosed with severe GH resistance/primary IGF-1 deficiency and commenced recombinant human IGF-1 therapy (rhIGF-1; 120 µg/kg by subcutaneous injection twice daily) at age 2.1 years. He had many episodes of hypoglycemia that required continuous glucose monitoring for 6 months. He developed a mild, isolated but persistent elevation of thyrotropin (TSH) (maximum 7 µU/mL; NR, 0.3-4 µU/mL). Following commencement of rhIGF-1 therapy, his height velocity improved considerably from 2.2 cm/year to 8.1 cm/year and has remained consistently above baseline (5.0-8.5 cm/year), suggesting a good response to rhIGF-1 therapy (Fig. 2). At latest assessment at aged 6 years, his height was 89 cm (–5.0 SDS) (see Fig. 1C).
Figure 1.
Clinical images and growth chart of patient 1. A and B, Clinical images of patient 1 (P1; index case) aged 1.7 years with classical Laron features of midfacial hypoplasia, depressed nasal bridge, and frontal bossing. C, Growth chart showing severe postnatal growth failure and response to recombinant human insulin-like growth factor 1 therapy.
Table 1.
Clinical and auxological details for the patients with the novel c.618+836T > G growth hormone receptor 6Ω pseudoexon mutation
| Phenotypic details | P1 | P2 | P3 |
|---|---|---|---|
| Age, y | 1.7 | 9.6 | 3.4 |
| Height (SDS), cm | 61.0 (–7.4) | 83.2 (–9.3) | 67.0 (–6.9) |
| Height velocity (SDS), cm/y | 1.6 (–4.5) | 1.5 (–5.2) | 2.0 (–4.4) |
| Weight (BMI SDS), kg | 6.1 (–0.6) | 10.7 (–1.0) | 5.5 (–4.4) |
| Head circumference (SDS), cm | 45.9 (–1.2) | 45.0 (–5.7) | 43.0 (–5.6) |
| Bone age, y | NK | 4.0 | 1.5 |
| Birth weight (SDS) (gestation), kg | 2.8 (37/40) (–0.4) | 2.6 (40/40) (–2.3) | 2.1 (41/40) (–3.8) |
| Other phenotypic details | Small hands and feet | Small hands and feet | Small hands and feet |
| Undescended testes | Undescended testes | Undescended testes | |
| Hypoplastic scrotum | Micropenis | Micropenis | |
| Micropenis | Mild learning difficulties | Mild learning difficulties | |
| Delayed tooth eruption | Bilateral hearing loss | Recurrent hypoglycemia | |
| Pubertal delay | Mild papilledema | ||
| Necrotizing enterocolitis |
The bold numbers are those that lie outside of the normal range ie, SDS <-2 or >+2.
Abbreviations: BMI, body mass index; P, patient; NK, not known; SDS, SD scores.
Table 2.
Biochemical details of patients with the novel c.618+836T > G growth hormone receptor 6Ω pseudoexon mutation and their parents
| Kindred 1 | Kindred 2 | ||||||
|---|---|---|---|---|---|---|---|
| P1 | P1 mother | P1 father | P2 | P3 | P2/3 mother | P2/3 father | |
| Age at presentation, y | 1.7 | 42.5 | 42.1 | 9.6 | 3.4 | 42.5 | 42.9 |
| Height SDS | –7.4 | –2.0 | –1.5 | –9.3 | –6.9 | –3.2 | –0.7 |
| Basal GH, µg/L | 38.0 | ND | ND | 52.0 | 110.0 | 0.21 | 5.23 |
| IGF-1 (SDS), ng/mL | < 10 (–2.6) a | 165 (+0.9) | 244 (+2.7) | < 10 (–3.4) | < 10 (–2.7) | 70 (–1.7) | 45 (–2.8) |
| IGFGT: basal; peak IGF-1, ng/mL | < 10; < 10 | ND | ND | < 10; < 10 | < 10; < 10 | ND | ND |
| IGFBP 3 (SDS), ng/mL | < 80 (–4.1) a | 3333 (–1.1) | 3897 (–0.1) | < 80 (–4.6) | 274 (–3.8) | 1603 (–3.0) | 1700 (–2.9) |
| ALS (SDS), mU/mL | < 100 (–2.6) a | 620 (0.1) | 594 (–0.1) | < 100 (–4.3) | < 100 (–2.6) | 183 (–2.6) | 184 (–2.6) |
| GHBP (SDS), pM | < 80 a | 1345 (–1.0) | 701 (–1.8) | < 80 | < 80 | 247 (–2.4) | 111 (–2.6) |
| TSH (NR), µU/mL | 7.0 (0.3-4.0) | ND | ND | 6.8 (0.3-4.2) | 13.8 (0.3-4.2) | ND | ND |
SDS calculated based on normal ranges for age and sex. IGFGT follow established protocols using GH 0.033 mg/kg/day for 5 days (patient 1) and 7 days (patients 2 and 3). The bold numbers are those that lie outside of the normal range ie, SDS <-2 or >+2.
Abbreviations: ALS, acid labile subunit; GH, growth hormone; GHBP, growth hormone binding protein; IGFGT, insulin-like growth factor 1 generation tests; ND, not done; NR, normal range; P, patient; SDS, SD score; TSH, thyrotropin.
a Samples obtained at age 3.2 years.
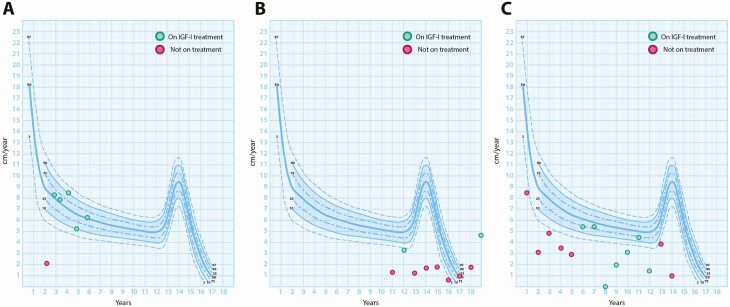
Figure 2.
Height velocity charts of the patients demonstrating the benefits of recombinant human insulin-like growth factor 1 (IGF-1) therapy. A, Patient 1; B, patient 2; and C, patient 3.
Kindred 2
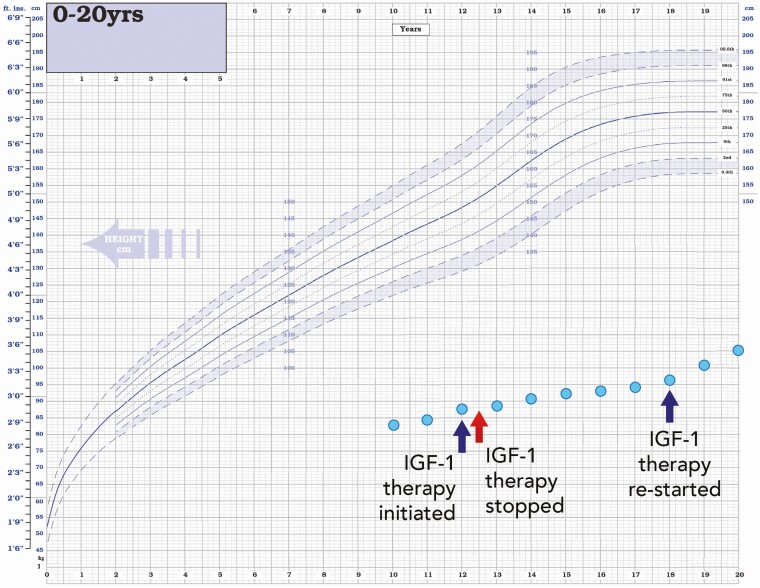
Patient 2 presented with severe growth failure at age 9.6 years with a height of 83.2 cm (–9.3 SDS) and height velocity of 1.5 cm/year (–5.2 SDS) (see Table 1 and Fig. 3). Head circumference was 45 cm (–5.7 SDS) and BMI within the normal range (–1.0 SDS). At a chronological age of 9.6 years, bone age was significantly delayed at 4.0 years. At presentation, he had small hands and feet, undescended testes, and micropenis. He did not have obvious “classical” Laron facial features (frontal bossing or midfacial hypoplasia) but had reduced facial height (nasion to menton; –4.9 SDS) compared to head width (maximal biparietal diameter; –1.2 SDS) (15). He also suffered from mild learning difficulties, bilateral hearing loss, and pubertal delay was later noted. At 40 weeks’ gestation, he was born small for gestational age (SGA) with a birth weight of 2.6 kg (–2.3 SDS). The end stages of the pregnancy were complicated by preeclampsia. At diagnosis the patient’s basal GH levels were very elevated at 52.0 µg/mL with undetectable IGF-1 and GHBP (< 10 ng/mL and < 80 pM, respectively) and severe deficiencies of IGFBP 3 and ALS (< 80 ng/mL and < 100 mU/mL, respectively). IGFGT (0.033 mg/kg/day for 7 days, as described earlier) showed no response to GH, with baseline and peak levels of IGF-1 less than 10 ng/mL (Table 2). His parents were nonconsanguineous with no dysmorphic features. His father had a normal height (0.7 SDS) and his mother had short stature (–3.2 SDS). TSH levels were slightly elevated (6.8 µU/mL; NR, 0.3-4.2 µU/mL) but free thyroxine (FT4) of 1.5 ng/dL (NR, 0.9-1.7 ng/dL) and free 3,5,3′-triiodothyronine (FT3) of 3.0 pg/mL (NR, 3.0-4.7 pg/mL) were consistently normal and levothyroxine therapy was never required. He commenced rhIGF-I at age 12 years (120 µg/kg by subcutaneous injection twice daily) but stopped after 6 months. He recommenced rhIGF-I therapy at age 18 years (height 96.2 cm, –11.8 SDS) and continued until age 21 years (height 109.1 cm, –9.9 SDS). His height velocity improved considerably during the periods of rhIGF-I therapy but unfortunately compliance was poor and the duration of treatment was suboptimal (see Fig. 2). His final adult height at age 23 years is 110 cm (–9.7 SDS) (see Fig. 3). He did not give consent for the clinical photographs at diagnosis to be included in this manuscript.
Figure 3.
Growth chart of patient 2. Growth chart showing severe postnatal growth failure. Periods of recombinant human insulin-like growth factor 1 (IGF-1) therapy are indicated.
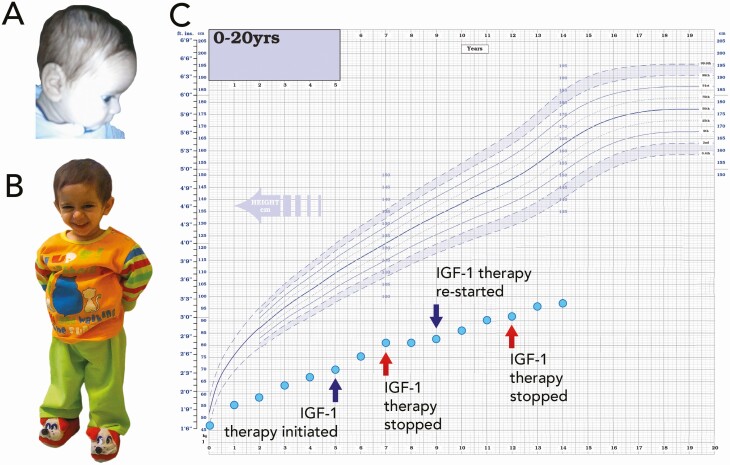
Patient 3 (Fig. 4A and 4B), the younger sibling of patient 2, was also born SGA (birth weight 2.1 kg; –3.8 SDS) at 41 weeks’ gestation. At age 3.4 years, he presented with a height of 67 cm (–6.9 SDS) and height velocity of 2.0 cm/year (–4.4 SDS) (see Table 1). His head circumference was 43.0 cm (–5.6 SDS) and BMI –4.4 SDS. At chronological age 3.4 years, his bone age was significantly delayed at 1.5 years. At presentation he had small hands and feet, undescended testes, micropenis, and mild papilledema. Images from early infancy showed frontal bossing (Fig. 4A). He also suffered from recurrent hypoglycemia, mild learning difficulties, and an episode of necrotizing enterocolitis. At diagnosis, baseline GH levels were elevated at 110.1 µg/L and IGFGT (0.033 mg/kg/day for 7 days, as described earlier) showed no response to GH, with baseline and peak IGF-1 levels of less than 10 ng/mL (see Table 2). He had deficiencies of IGFBP 3 and ALS (274 ng/mL and < 100 mU/mL, respectively). He was commenced on levothyroxine at age 2 months because of hyperthyrotropinemia with TSH of 13.8 µU/mL (NR, 0.3-4.2 µU/mL). FT4 and FT3 have remained within the normal range on treatment (latest FT3 level 3.1 pg/mL; NR, 3.0-4.70 pg/mL). He has undergone periods of rhIGF-1 therapy (120 µg/kg subcutaneous injection twice daily) with variable compliance (similar to his sibling), initially commencing treatment at age 5 years (height 70 cm, –8.1 SDS) until aged 7 years (81 cm, –7.2 SDS), restarting at age 9 years (height 83 cm, –8.1 SDS) until age 12 years (92 cm, –7.6 SDS). Subsequently he remained off treatment and his height at latest assessment at age 14 years is 97.0 cm (–7.7 SDS) (Fig. 4C). His height velocity improved during the initial period of rhIGF-1 therapy, but the treatment response and outcome was likely affected by the significant compliance issues (see Fig. 2).
Figure 4.
Clinical images and growth chart of patient 3. A and B, Clinical images showing patient 3 (P3; younger sibling of P2) aged A, 3.5 months, and B, 5.4 years, displaying classical Laron features of midfacial hypoplasia, depressed nasal bridge, and frontal bossing. C, Growth chart showing severe postnatal growth failure. Periods of insulin-like growth factor 1 (IGF-1) therapy are indicated.
Biochemical Assays
Biochemical assays were performed at the Endocrine Laboratory, LMU Klinikum, except for TSH, FT3, and FT4. For each assay, all samples from the same family were analyzed in the same analytical run.
Serum IGF-1, GH, and IGFBP 3 were measured using the IDS-iSYS platform (Immunodiagnostic Systems). The assays were calibrated against recombinant standards (98/574 for GH, 02/254 for IGF-1, and 93/560 for IGFBP 3). Intra-assay and interassay coefficients of variation (CVs) at various concentrations ranged from 4.0% to 8.7% (IGF-1), 1.3% to 5.4% (GH), and 5.5% to 12.4% (IGFBP 3). The limits of quantification are 10.0 ng/mL (IGF-1), 0.04 µg/L (GH), and 80.0 ng/mL, respectively (16-18). Serum ALS levels were measured in duplicate by sandwich immunometric assay using monoclonal antibodies directed against specific N- and C-terminal oligopeptides as previously described (19). A serum pool of healthy male volunteers was used for calibration and assigned 1000 mU/mL. Intra-assay and interassay CVs are less than 9%, the limit of quantification is 100 mU/mL, and the linear assay range is 100 to 5000 mU/mL (19). Serum GHBP concentrations were measured by an in-house, time-resolved fluorescence immunoassay based on monoclonal antibodies (20). The assay is standardized against recombinant nonglycosylated GHBP with concentration assigned by amino acid analysis (PRL). Within-assay CVs were 3.4% at 312 pM and 3.4% at 2034 pM. At the same concentrations, between-assay CVs were 16.0% and 11.7%, respectively. The lower limit of quantification was 80 pM and the linear range covered concentrations between 80 and 4880 pM.
Ethical Approval
Informed written consent for genetic research and publication of clinical details and images was obtained from patients and/or parents. This study was approved by the Health Research Authority, East of England–Cambridge East Research Ethics Committee (REC reference No. 17/EE/0178).
Variant Discovery
Genomic DNA was extracted from peripheral blood leukocytes from patient 1 and his parents using a Nucleon BACC2 Genomic DNA Extraction Kit (GE Healthcare) in accordance with the manufacturer’s instructions. Targeted whole-genome sequencing was conducted using our in-house custom short-stature next-generation sequencing gene panel covering the entire genomic sequence (including intronic regions, 2000 bases upstream and 500 downstream) of 64 genes of interest. These included all genes known to cause GHI and IGF-1 insensitivity (GHR, IGFI, PAPPA-2, STAT5B, IGF1R, IGFALS) and overlapping syndromes (3M, Noonan, and SRS). Probe design, preparation of libraries, capture, and sequencing was performed by Otogenetics Corporation. Sequencing was performed using an Illumina HiSeq 2500 platform (paired ends 100-125, designated average coverage of 100×). H sapiens GRCh37 (http://grch37.ensembl.org/index.html) was used as the reference genome for generating the coordinates of each region. Probes were designed to cover each genomic region of interest in as much detail as possible within the limitations of highly repetitive regions. For the GHR, coverage was approximately 94% (start and end coordinates 42421880 and 42722479, respectively; total size 300599 bp).
Otogenetics performed data mapping, duplicate removing, snv/Indel calling, vcf annotation, and generated VCF, BAM, and Bam.bai files for bioinformatic analysis using Ingenuity Variant Analysis (https://www.qiagenbioinformatics.com/products/ingenuity-variant-analysis; QIAGEN Inc). The following filter settings were applied: call quality 20 or greater, read depth 20 or greater, and only data outside 5% of most exonically variable 100 base windows in healthy public genomes (1000 genomes, ExAC) were included. Variants predicted as loss of function as well as very rare exonic and noncoding variants of uncertain significance were also included. Common variants were filtered by excluding those with an allele frequency of 0.05% or greater in the 1000 genomes, ExAC, gnomAD, and NHLBI ESP exomes.
As no exonic or canonical splice site variants were identified that could explain the phenotype, noncoding variants were explored. We identified all intronic homozygous variants with an allele frequency of 0.05% or less in the 1000 genomes, ExAC, gnomAD, and NHLBI ESP exomes. The list of variants generated was assessed using Human Splicing Finder (http://umd.be/HSF3/), which calculated the consensus values of potential splice sites, splice enhancer, and splice silencer sites. A very rare, homozygous variant in intron 6 of the GHR gene (42700940T > G, c.618+836T > G) was identified. This variant, altering the sequence from AGTT to AGGT, was predicted to activate an intronic cryptic donor splice site deep within intron 6 of the GHR (Fig. 5A). This is a novel variant not listed in the 1000 genomes, ExAC, gnomAD, and NHLBI ESP exomes. It was assigned a Combined Annotation Dependent Depletion score of less than 10, which is not unusual for a noncoding variant. The GHR sequence change was confirmed by polymerase chain reaction (PCR), followed by automated sequencing using primers designed to cover the affected region (GHR intron 6F [forward] and GHR intron 6R [reverse]; sequences provided in Supplementary Table 1) (21) in the patient and parents. Targeted Sanger sequencing of the coding, flanking intronic, and ‘6Ψ’ regions of the GHR was undertaken in patients 2 and 3 and their parents (primer sequences available on request). This identified the novel intronic GHR (42700940T > G, c.618+836T > G) and previously reported GHR mutation, c.181C > T, (R43X) (22).
Figure 5.
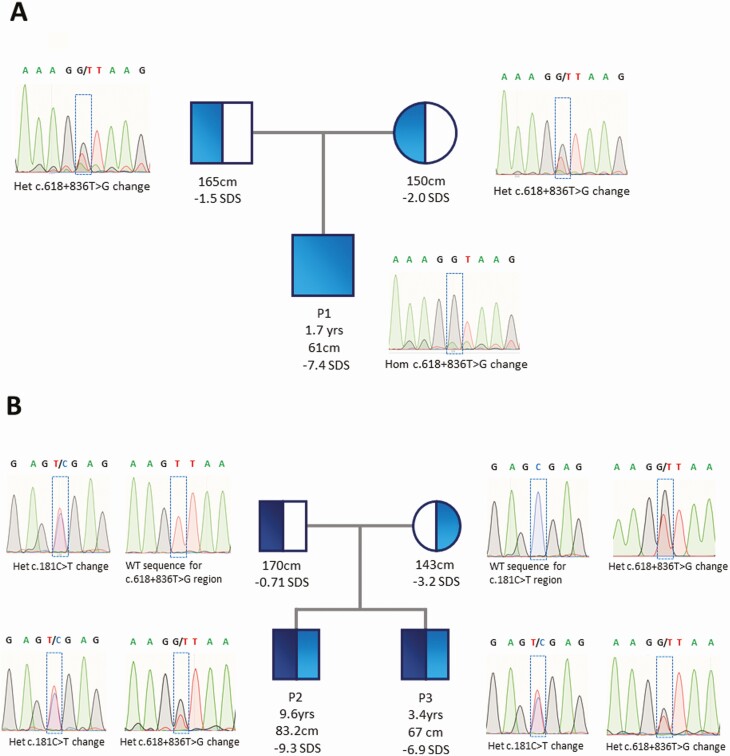
Pedigrees and electropherograms for kindreds 1 and 2. Electropherograms and pedigrees showing the segregation of the c.618+836T > G growth hormone receptor (GHR) variant in affected families. A, Homozygous and heterozygous c.618+836T > G GHR variants in patient 1 (P1) and both parents, respectively. B, Patients 2 and 3 (P2 and 3) harbored compound heterozygous c.618+836T > G (inherited from the mother) and c.181C > T (R43X) (inherited from the father) GHR mutations.
In Vitro Splicing Assay to Assess the Effect of the Patient Variant
An in vitro splicing assay was performed using the Exontrap cloning vector pET01 (MoBiTec GmbH) containing an intronic sequence interrupted by a multiple cloning site. DNA fragments of interest from patient 1, a wild-type control (WT), and a patient with the original GHR pseudoexon variant (GHR-6Ψ; c.618+792A > G) were amplified using PCR with primers incorporating an XbaI restriction enzyme target site (GHRXbaI F [forward] and GHRXbal R [reverse]). PCR products were assessed by Sanger sequencing, column purified using the QIAquick PCR Purification Kit according to the manufacturer’s protocol, and cloned into the Exontrap vector pET01. Recombinant vector sequences were verified by Sanger sequencing using pET01 primers (ET PRIM 06 [forward] and 07 [reverse]). WT or mutant (patient 1 and GHR-6Ψ) vectors were transfected into HEK293T cells using Lipofectamine 2000 (Invitrogen). RNA was extracted (QIAGEN RNeasy Plus Mini kit) 24 hours after transfection and cDNA generated using a High-Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific). cDNA fragments were amplified using pET01 primers ET PRIM 02 (forward) and 03 (reverse) and visualized on a 2% agarose gel. Primer sequences are provided in Supplementary Table 1 (21). A detailed protocol of the minigene assay has been published previously (23).
Fibroblast Culture
Primary fibroblast cultures were established from skin biopsies of patients 2 and 3, performed at the Department of Translational Medical Sciences, University of Naples Federico II. Cells were subcultured in 75-cm2 flasks at a ratio of 1:5 in Dulbecco’s modified Eagle’s medium (DMEM) high glucose (Sigma D5648) supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin at 37 °C in 5% CO2. Primary dermal fibroblasts of normal human neonatal origin, (ATCC PCS201010) were used as controls.
RNA Extraction, Complementary DNA Synthesis, and Reverse Transcriptase–Polymerase Chain Reaction
RNA was extracted from control- and patient-derived dermal fibroblasts using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions. Genomic DNA removal was achieved using an RNase-Free DNase Set (Qiagen, 79254). For cDNA synthesis, 1 µg of RNA (with 10-mM random hexamer and nuclease-free water to a volume of 15 µL) was incubated at 70 °C for 5 minutes. MuMLV reverse transcriptase (RT) enzyme (20 U) and 5X buffer, RNase Inhibitor (25 U), and deoxynucleoside triphosphates (2.5 mM each) were then added to the reaction and placed on a thermocycler at 25 °C for 10 minutes, 42 °C for 90 minutes, and 70 °C for 15 minutes.
RT-PCR was performed using GHR cDNA Exon 4F (forward) and GHR cDNA Exon 8R (reverse) primers to amplify both WT GHR constructs and those containing the 6Ω pseudoexon insertion. RT-PCR was also performed to amplify only constructs containing the 6Ω pseudoexon insertion using GHR cDNA pseudo F1 (forward) primer, designed at the junction of the insertion of the 6Ω pseudoexon insertion, and GHR cDNA Exon 8R (reverse). Primer sequences are provided in Supplementary Table 1 (21). Thermal cycling conditions were as follows: 95 °C for 5 minutes, 13 × (95 °C for 20 seconds, 70 °C for 30 seconds (–1 °C per cycle), 72 °C for 60 seconds), 30 × (95 °C for 20 seconds, 57 °C for 30 seconds, 72 °C for 60 seconds) and 72 °C for 5 minutes. The annealing temperature was progressively lowered from 70 °C to 57 °C. PCR products were run on a 2% agarose gel, visualized with LI-COR Image Studio software (LI-COR Corp), and confirmed by Sanger sequencing.
Creation of a 6Ω Pseudoexon Growth Hormone Receptor Vector
Gibson assembly was used to recreate the novel 6Ω pseudoexon GHR using a pcDNA1 expression vector (generous gift from Prof Richard Ross) including the entire coding sequence of GHR. Primers were designed using Benchling assembly wizard (Benchling Biology Software 2020, https://benchling.com) and are listed in Supplementary Table 1 (21). The 6Ω target sequence was amplified using a Phusion High-Fidelity PCR Kit (New England Biolabs). PCR products were visualized by gel electrophoresis to verify sizes and DpnI-treated to remove methylated DNA (the original WT vector template). A total of 1 µL of DpnI (concentration of 10 units/µL) was added to each PCR tube and the sample incubated at 37 °C for 3 hours. PCR product cleanup was then performed (Macherey-Nagel NucleoSpin Gel and PCR Clean-up Kit) and DNA quality/concentration was assessed using a NanoDrop spectrophotometer. NEBiocalculator calculated the volume of each product needed for optimum annealing (https://nebiocalculator.neb.com/#!/ligation). The fragments were combined in an equimolar ratio to a total of 0.2 pmol with 2 times the volume of NEBuilder HiFi DNA Assembly Master Mix (New England Biolabs) and incubated at 50 °C for 60 minutes to anneal the 2 fragments into a circular vector (Supplementary Figure 1) (21). This construct was then transformed into NEB 5-α competent Escherichia coli. Single colonies were selected for minipreparation and DNA products verified by Sanger sequencing.
Expression of Constructs in Mammalian Cell Line and Growth Hormone Stimulation
Human embryonic kidney 293T (HEK293T) cells were seeded into 6-well plates and transfected (in duplicate) with empty vector pcDNA3.1, WT (pcDNA1-GHR) and mutant 6Ω pseudoexon constructs using Lipofectamine 3000 reagent (Thermo Fisher Scientific). Cells were maintained in DMEM high glucose supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (2 mL/well) at 37 °C in 5% CO2 for 24 hours. Media were then discarded and cells serum-starved for a further 24 hours by the addition of reduced volume (1-mL) serum-free DMEM containing 0.1% bovine serum albumin. Cell lysates and supernatants (conditioned media) were harvested at baseline and following treatment with recombinant human GH (500 ng, 0.5 µg/mL) (Life Technology) for 20 minutes.
Western Blotting
Whole-cell lysates were prepared by lysis in radioimmunoprecipitation assay buffer (Sigma Aldrich) supplemented with protease and phosphatase inhibitor tablets (Roche). Protein concentrations of cell lysates were quantified using a Bradford protein assay (Bio-Rad). Cell lysates were denatured in SDS sample buffer 4X (Sigma Aldrich, MERCK) and boiled for 5 minutes at 95 °C. Equal concentrations of protein for whole-cell lysates and standard volumes of conditioned media were loaded into wells of a NuPAGE 4-12% Bis-Tris gel (Thermo Fisher) prior to electrophoretic separation using MOPS buffer. Protein transfer to nitrocellulose membrane was achieved by electroblotting at 15 V for 50 minutes. The membrane was blocked with either 5% fat-free milk in Tris-buffered saline (TBS)/0.1% Tween-20 (GHBP) or 5% bovine serum albumin in TBS/0.1% Tween-20 (STAT5, phosphorylated STAT5) and left to gently agitate for 1 hour. Individual primary antibodies were added at specific concentrations (GHBP; BioVision, catalog No. 6660, RRID:AB_2892616; 1:1000 dilution, STAT5B; Boster Biological Technology, catalog No. PA1841, RRID:AB_2892617; 1:1000 dilution, Phospho-Stat5 (Tyr694); Cell Signaling Technology catalog No. 4322, RRID:AB_10544692; 1:750 dilution), and β-actin (Proteintech catalog No. 66009-1-Ig, RRID:AB_2687938; 1:5000 dilution) used as a housekeeping control. The membrane was incubated with primary antibody overnight at 4 °C. The membrane was washed for 5 minutes (×3) with Tris-buffered saline-Tween20 0.1%. Secondary goat antirabbit (GHBP, STAT5, phosphorylated STAT5) and goat antimouse (β-actin) antibodies were added at concentrations of 1:10 000 to blocking buffer and the membrane incubated at room temperature for 60 minutes. The membrane was subsequently washed 3 times (5 minutes each) with Tris-buffered saline-Tween20 0.1% and visualized with LI-COR Image Studio software for immunofluorescent detection.
Results
Characterization of the Novel Growth Hormone Receptor 6Ω Pseudoexon Variant
The next-generation short-stature gene panel identified a novel homozygous variant deep within intron 6 of GHR (42700940T > G, c.618+836T > G) in patient 1. PCR amplification of the region of interest in patient 1 and his parents verified the homozygous variant in the proband and confirmed both parents were heterozygous for this genetic variant (Fig. 5A). This variant altered the genetic sequence from AGTT to AGGT and was predicted to create an intronic cryptic donor site. This variant was novel and not listed in the ExAC or GnomAD databases. It was assigned a Combined Annotation Dependent Depletion score of less than 10, but this is not unusual for noncoding variants. Targeted Sanger sequencing of the coding and flanking intronic regions of the GHR gene in patients 2 and 3 identified compound heterozygous GHR mutations. Patients 2 and 3 both inherited the previously published heterozygous c.181C > T (R43X) (22, 24) GHR variant from their father and the heterozygous c.618+836T > G novel GHR (6Ω) variant from their mother (Fig. 5B).
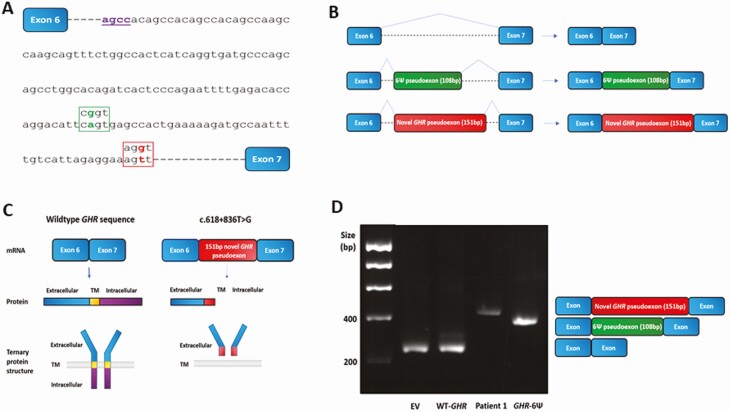
Interestingly, the novel intronic c.618+836T > G variant is 44 bp downstream of the original GHR pseudoexon variant (6Ψ; c.618+792A > G) (Fig. 6A) (10). The inclusion of this novel 151-bp GHR 6Ω pseudoexon is predicted to lead to a frameshift and introduction of a premature stop codon after 245 amino acids (Fig. 6B and Supplementary Figs. 2 and 3) (21). The resultant truncated protein is expected to be nonfunctional given it would lack both the transmembrane (encoded by exon 8; residues 265-288) and intracellular (encoded by exons 9 and 10; residues 289-638) domains of the GHR (Fig. 6C and Supplementary Fig. 3) (21).
Figure 6.
Effect of novel 6Ω growth hormone receptor (GHR) pseudoexon c.618+836T > G variant. A, The novel 6Ω GHR pseudoexon c.618+836T > G variant creates an AGGT splice donor site (red) downstream of the original GHR 6Ψ pseudoexon variant (c.618+792A > G) (green) that produces a CGGT splice donor site. The dormant intronic AGCC acceptor splice site involved in missplicing and inclusion of both pseudoexons is shown in purple. Dashed lines indicate interrupted intronic sequence. B, Schematic of the 6Ψ and novel 6Ω GHR pseudoexons inclusion events into the messenger RNA (mRNA). C, Schematic of the novel 6Ω GHR pseudoexon inclusion event and predicted GHR protein compared to wild-type (WT) sequence. The 6Ω pseudoexon inclusion is predicted to cause a frameshift and result in premature truncation of the GHR lacking both transmembrane (TM) and intracellular domains. D, Gel electrophoresis of complementary DNA splicing products following the splicing assay using an exon trap vector (MoBiTec-Exontrap cloning vector pET01). EV, empty vector, pET01 alone; WT-GHR, pET01 with 600 base pair (bp) of wild-type GHR intron 6 sequence inserted; patient 1, pET01 with 600 bp of patient 1 intron 6 sequence inserted (including the c.618+836T > G variant). GHR-6Ψ, pET01 with 600-bp sequence from a patient with the original GHR pseudoexon (6Ψ) c.618+792A > G variant. The spliced products were amplified by polymerase chain reaction and visualized on a 2% agarose gel. Lanes 1 and 2: A 250-bp band is seen in EV and WT sequence, as expected, representing the 2 exons of the exon trap vector and confirming normal splicing with WT sequence (lane 2). Lane 3: A 401-bp band is seen in the proband and sequencing revealed a 151-bp insert between the 2 exons of the exon trap vector (250 bp), confirming novel 6Ω pseudoexon inclusion. Lane 4: A 358-bp band is seen in the GHR 6Ψ patient sample and sequencing revealed a 108-bp insert between the 2 exons (250 bp) of the exon trap vector, confirming the original pseudoexon inclusion, as expected.
An in vitro splicing assay revealed the inclusion of 151 bp in addition to the 2 exons of the exon trap vector confirming 6Ω pseudoexon inclusion (Fig. 6D). Sanger sequencing of the spliced product verified this prediction, confirming that the novel variant activates an intronic cryptic donor site deep within intron 6 of GHR. The close proximity of a dormant splice acceptor site leads to misrecognition of this region as an exon by the spliceosome and its retention during the splicing process. Interestingly, the same dormant acceptor site is involved in the missplicing and inclusion of the original GHR 6Ψ pseudoexon (see Fig. 6A).
Patients 2 and 3 of the second kindred were compound heterozygous for the GHR 6Ω pseudoexon variant (c.618+836T > G) and another previously published nonsense point mutation in exon 4 of GHR (c.181C > T; R43X) (22, 24). The latter mutation was inherited from their father and is predicted to lead to frameshift and introduction of an early stop codon at residue 43 of the GHR.
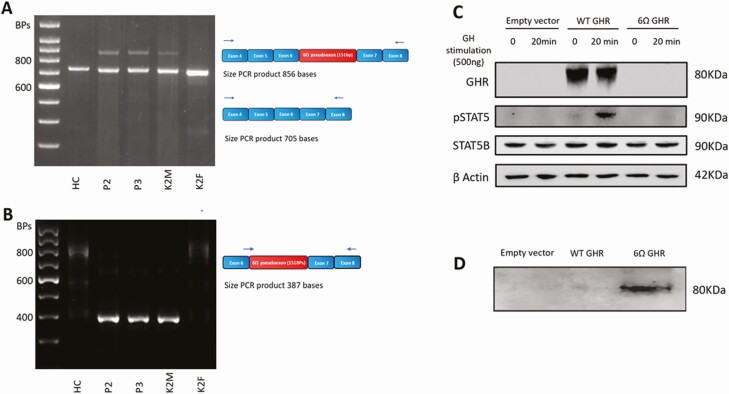
RNA samples derived from healthy control, patients 2 and 3, and their parents’ dermal fibroblasts were used to generate cDNA. RT-PCR was performed using primers using GHR cDNA Exon 4F (forward) and GHR cDNA Exon 8R (reverse) primers to amplify both the WT GHR sequence and the 6Ω pseudoexon insertion. A “normal” band of expected size (705 bp) was seen in all the samples, and a larger (856-bp) band was seen in patients 2 and 3 and their mother, who were all heterozygous for the GHR 6Ω variant (c.618+836T > G) (Fig. 7A). This larger band corresponds to the retention of the additional 151 bases 6Ω pseudoexon.
Figure 7.
Expression of wild-type (WT) and mutant transcripts in affected family members with the heterozygous c.618+836T > G growth hormone receptor (GHR) 6Ω variant. The GHR 6Ω pseudoexon also diminishes GH-dependent STAT5B activation and accumulates extracellularly. A, Complementary DNA (cDNA) was prepared from dermal fibroblasts derived from a healthy control (HC) and patients 2 (P2) and P3 and both parents. Schematic showing the locations of the primers (GHR cDNA exon 4F [forward] and GHR cDNA exon 8R [reverse] [blue arrows]) used to amplify the region encompassing WT GHR, the GHR 6Ω pseudoexon insertion. A “normal” 705–base pair (bp) polymerase chain reaction (PCR) product was seen in all samples. An additional larger (856-bp) PCR product was seen in P2, P3, and their mother, who were heterozygous for the c.618+836T > G GHR 6Ω variant, indicating the additional 151-bp 6Ω pseudoexon insertion. B, Schematic showing the locations of the primers (GHR cDNA pseudo F1 [forward] and GHR cDNA exon 8R [reverse] [blue arrows]). The forward primer at the junction of the 6Ω pseudoexon insertion means only sequences containing the GHR 6Ω pseudoexon insertion are amplified. The expected 387-bp PCR product is seen in patient 2, patient 3, and their mother, all of whom are heterozygous for the c.618+836T > G GHR variant. C, Whole-cell lysates from untreated or GH-stimulated (20 minutes) HEK293 cells transfected with pcDNA3.1 empty vector, WT GHR or 6Ω GHR mutant constructs. Representative immunoblots of 3 experiments are shown. D, Immunoblot analysis of conditioned media with anti-GH binding protein (BP) antibody from HEK293 cells transfected with the 6Ω GHR mutant construct showing extracellular accumulation of the truncated mutant 6Ω GHR protein. B actin, β actin; K2M, kindred 2 mother; K2F kindred 2 father.
RT-PCR was also performed using primers designed to amplify only sequences containing the 6Ω pseudoexon inclusion (GHR cDNA pseudo F1 [forward] and GHR cDNA Exon 8R [reverse]). A 387-bp band was seen in patients 2 and 3 and their mother, all of whom are heterozygous for the GHR 6Ω variant (c.618+836T > G) (Fig. 7B). Sanger sequencing confirmed the inclusion of the 151 bases GHR 6Ω pseudoexon in keeping with the in vitro findings of the MoBiTec-Exontrap splicing assay. Primer sequences are provided in Supplementary Table 1 (21).
The impact of the GHR 6Ω pseudoexon on GHR signaling was assessed following GH stimulation (500 ng) both of the WT and 6Ω pseudoexon GHR constructs expressed in HEK293T cells. Tyrosine phosphorylation of STAT5B was used as a marker of intact GHR signaling. When compared to WT GHR, the 6Ω pseudoexon construct exhibited reduced phosphorylated-STAT5B following GH stimulation (Fig. 7C). As the truncated 6Ω pseudoexon GHR lacks both transmembrane and intracellular domains, it is unlikely to be able to anchor onto the cell surface or dimerize, significantly abrogating the activation of STAT5B and the downstream effects of GH stimulation.
Forty-eight hours following transfection of the GHR 6Ω pseudoexon construct into HEK293T cells, the serum-free conditioned media was probed using a GHBP antibody. This revealed extracellular accumulation of mutant (truncated) GHR in the GHR 6Ω pseudoexon–transfected cells that was not present in the WT GHR–transfected cells (Fig. 7D). The GHR 6Ω pseudoexon protein lacks both transmembrane and intracellular domains and would result in defective anchoring to the plasma membrane. The truncated protein is secreted extracellularly and recognized by the polyclonal GHBP antibody. Interestingly, biochemical assays using serum from all 3 patients revealed undetectable GHBP (see Table 1). The GHBP assay relies on highly specific monoclonal antibodies, and the GHR 6Ω pseudoexon protein lacks an epitope crucial for one of these monoclonal antibodies.
Biochemical Analysis of Kindreds 1 and 2
Biochemical analysis of patient 1 and the siblings (patients 2 and 3) revealed classical GH insensitivity with elevated basal GH levels associated with severe deficiencies of IGF-1, IGFBP 3, and ALS in keeping with their significant postnatal growth failure (see Table 2). IGF-1 levels did not increase even after 5 and 7 days of GH stimulation (respectively) in IGFGTs. Both parents of patient 1 and the mother of patients 2 and 3 (all carriers of the novel GHR 6Ω pseudoexon variant) had normal IGF-1 levels. The father of kindred 2 (a carrier of the known GHR R43X mutation) had low IGF-1 levels, suggesting this mutation in heterozygosity has a greater impact on IGF-1 secretion than the GHR 6Ω pseudoexon variant. Patient 1’s parents also had normal IGFBP 3 and ALS, whereas both parents of patients 2 and 3 had insufficient IGFBP 3 and ALS consistent with the notion that IGFBP 3 and ALS levels can be regulated independently of IGF-1 and have stronger GH dependency, respectively (25). GHBP levels were normal in the parents of patient 1 and low in both parents of patients 2 and 3. The cause of this variability is unclear.
Discussion
Here we report a novel homozygous variant c.618+836T > G in intron 6 of GHR, 44 bp downstream of the previously recognized pseudoexon mutation, detected by custom-targeted, whole-gene sequencing. A minigene assay revealed inclusion of a 151-bp pseudoexon due to activation of the same dormant acceptor site involved in the missplicing and inclusion of the original 6Ψ GHR pseudoexon. In contrast to the original 6Ψ GHR pseudoexon, incorporation of the 6Ω pseudoexon into the mature mRNA transcript leads to a frameshift and introduction of a premature termination codon after 245 amino acids resulting in a 45-KDa mutant 6Ω GHR protein lacking both transmembrane and intracellular domains required to anchor the receptor in the cell membrane and intracellular signaling, respectively. We also demonstrated that the mutant 6Ω GHR leads to diminished STAT5 signaling in vitro. The predicted deleterious impact of the 6Ω pseudoexon inclusion is in keeping with the severe postnatal growth failure seen in all 3 patients.
Our center previously described the first GHR pseudoexon (6Ψ) mutation in 2001 in 4 siblings from a highly consanguineous Pakistani family with mild GHI (10). This homozygous point mutation (c.618+792A > G) altered the intronic sequence activating a cryptic donor splice site. Owing to the presence of a nearby dormant cryptic acceptor site, this region is recognized as an exon (a “pseudoexon”) by the spliceosome and is retained during GHR splicing. The inclusion of this pseudoexon caused an in-frame insertion of 36 amino acid residues (lacking a stop codon) between exons 6 and 7 in the dimerization domain of the GHR. This resulted in defective trafficking (and concomitant reduced cell surface expression) rather than impaired signaling, causing a partial loss of function (11). As such, moderate postnatal growth failure was observed (Height SDS -3.3 to -6.0) (14). The intronic 6Ψ GHR mutation, 792 bases into the intron, was identified using homozygosity mapping of several polymorphic markers surrounding the GHR (10). It would not be detected by conventional exonic or whole-exome sequencing, which covers only exons and intron-exon boundaries.
Mutations resulting in aberrant pseudoexon inclusion have been found to be disease-causing in more than 50 genes (9). In comparison to genuine exons, pseudoexons tend to have less splicing enhancer and more splicing silencer motifs (26-29). The inclusion of pseudoexons can have significant effects on the resulting protein, particularly if their inclusion leads to a frameshift.
Classically, patients with severe GHI exhibit distinctive facies characterized by frontal bossing, midfacial hypoplasia, and acromicria; however, marked phenotypic variability exists even within families harboring identical mutations (30). More than 90 GHR gene mutations have been described to date (Human Gene Mutation Database), of which 21 are splice-site mutations. The heterozygous nonsense GHR mutation c.181C > T (R43X) identified in kindred 2 has been reported in Ecuadorian, Mediterranean, and Russian populations (22, 31, 32). It is thought to have arisen independently in these diverse populations. The c.181C > T mutation occurs at a highly mutable CpG dinucleotide “hot spot” and has been detected in patients with a variety of GHR haplotypes.
Consistent with the severe IGF-1 deficiency, patients 1 and 3 also had “classical” Laron syndrome facial features. Although patient 2 did not have the typical frontal bossing and depressed nasal bridge, he did have had reduced facial height, suggesting some phenotypic variability despite comparable biochemical abnormalities. This is in contrast to the mild to moderate growth failure seen in patients harboring the original 6Ψ pseudoexon variant that results in a less deleterious molecular defect with in-frame insertion of 36 amino acids. Interestingly, 6Ψ pseudoexon patients also have variable clinical features with lack of dysmorphic facial features in about 50% patients (13). Our previous observation similarly indicated little correlation between facial features and the degree of short stature (13) and that linear growth may be more consistently affected by the degree of IGF-1 deficiency. Patients 2 and 3 had head circumferences (–5.7 and 5.6 SDS, respectively) lower than expected for classical GHI. It is established that untreated Laron syndrome patients have reduced head circumference (mean –3.3 SDS; range, –1.8 to –5.2 SDS) that do not correlate with the severity of growth failure (33). However, the mean head circumference deficit is typically less than the mean height deficit (33). This was observed in patients 1 (–1.2 vs –7.4 SDS) and 2 (–5.2 vs –9.3 SDS) but was less apparent in patient 3 (–5.6 vs -6.9 SDS). Patients 2 and 3 were born SGA and coexisting prenatal growth restriction may have further affected their head size. Interestingly, patient 3 had more severe intrauterine growth restriction (birth weight SDS –3.8) than patient 2 (birth weight –2.3 SDS), and this may explain the differences in head circumference between the siblings. We did not undertake more extensive genetic testing, for example, whole-exome sequencing in patients 2 and 3, therefore we cannot definitively rule out another underlying genetic cause for their reduced head circumferences.
In patient 1, rhIGF-1 therapy significantly improved the height velocity from 2.2 to 8.1 cm/year during the first year of treatment. This response is in keeping with the published data in which the mean height velocity among 21 children with severe primary IGF-1 deficiency increased from 3.1 cm/year prior to treatment to 7.4 cm/year during the first year of IGF-1 therapy (34). Subsequent height velocities on treatment were not as high as the initial year of IGF-1 therapy but remained above baseline for up to 12 years (34). Patients 2 and 3 had some improvement in their height velocities on rhIGF-1 therapy, but the significant issues with compliance meant their treatment responses and outcomes were suboptimal.
Heterozygous GHR mutations may have a variable effect on carriers. The site of the mutation within the gene and the corresponding modification of the protein may influence the observed phenotype of heterozygous individuals. The genetic background of the individual may also contribute to the phenotypic diversity (35, 36). The relatives of severe GHI patients exhibit a range of heights, from reduced height SDS to normal stature. The parental heights of both our pedigrees are consistent with several studies in which family members carrying heterozygous GHR mutations have a modest reduction in height SDS (37-39). This has been most extensively studied in the large Ecuadorian cohort carrying the E180 (c.594A > G) GHR defect in which heterozygosity accounted for a mean height reduction of 0.55 SDS (37).
The mothers of both kindreds who carried the 6Ω pseudoexon had short stature (–2.0 and –3.2 SDS). It has been recognized that some mothers and sisters who are heterozygous for deleterious GHR mutations have more significant growth failure (height < –2 SDS) compared to male carriers (39). Heterozygosity of the functionally null E180 mutation was not associated with a reduction in circulating GHBP, IGF-1, IGF-2, IGFBP 2, or IGFBP 3 levels (37, 40). The parents of patient 1 and the mother of patients 2 and 3 who all carried the novel GHR 6Ω pseudoexon variant had normal IGF-1 levels. Patient 1’s parents (novel GHR 6Ω pseudoexon variant carriers) also had normal IGFBP 3 and ALS levels. In contrast, the father of kindred 2, who carried the known GHR R43X mutation, had low IGF-1 levels suggesting this mutation in heterozygosity has a greater impact on IGF-1 secretion than either of the GHR pseudoexon variants.
It is notable that all 3 patients had elevated TSH. Most patients with GHI have thyroid function within the normal range (41). Furthermore, exogenous administration of IGF-1 in individuals with Laron syndrome did not negatively affect thyroid function (42). However, the relationship between the GH–IGF-1 system and the hypothalamic-pituitary-thyroid axis is complex and incompletely understood. GH therapy in children and adults with GH deficiency can induce a fall in serum T4 (43). This is thought to be due to the GH effect on deiodination of T4 to T3, leading to higher serum T3 levels (44). It could be hypothesized that the supraphysiological levels of GH seen in patients 2 and 3 are responsible for their raised TSH levels due to reduced T4 feedback, but the mechanisms are not fully understood.
Both sets of parents from the 2 kindreds originate from Frattamaggiore, a town in the Campania region of Southern Italy, suggesting they share common ancestry. Interestingly, the majority of reported patients with GHR 6Ψ mutations are of Pakistani origin, and previous work by our group suggests the presence of a common ancestor (12). The E180 GHR splice mutation is the most common mutation identified in patients with classic GHI, comprising approximately one-third of the known population with GHR deficiency. This mutation is concentrated in a large population of individuals with Laron syndrome in Southern Ecuador and is thought to have also originated from a single common ancestor (24, 45).
The presence of the secreted truncated GHR protein seen following 6Ω pseudoexon inclusion in vitro suggests that the novel 6Ω pseudoexon leads to a GHR protein unable to anchor to the cell membrane. This truncated GHR lacking the transmembrane and intracellular domains is recognized by the polyclonal GHBP antibody. Similarly, characterization of the truncating p.W267*GHR mutation, which resides early in the transmembrane domain, demonstrated elevated extracellular GHBP due to defective anchoring of the mutant protein (3). The GHBP assays performed in our patients use highly specific monoclonal GHBP bind antibodies. One of these recognizes a critical epitope that resides within a large proportion of the 6Ω pseudoexon inclusion region. This explains the undetectable GHBP levels in the serum samples of all 3 patients harboring the novel 6Ω pseudoexon. We know from previous analyses in Laron patients that this particular antibody is also unable to bind to GHBP in patients carrying missense R161C and R211G GHR mutations, the nonsense R217X mutation, and the E180 and G223 splicing mutations that also modify this region.
Missplicing events of the GHR gene may be due to its large intronic regions. In vertebrates, splicing of genes with large introns (> 250 bp) may be error prone with activation of cryptic splice sites compounded by an inefficient 5′ splice site in the preceding exon leading to intron inclusion. Both the 6Ψ and 6Ω GHR pseudoexon inclusion events occur in the same intronic region, suggesting that intron 6 may harbor a number of cryptic splice sites. Alternatively, this cryptic acceptor position may be particularly attractive, predisposing this region to be recognized as an exon.
In summary, we have identified a novel intronic GHR 6Ω pseudoexon inclusion event that results in a functionally null GHR. Three individuals from 2 kindreds harboring this mutation exhibit a severe GHI phenotype and originate from the Campania region of Southern Italy, suggesting common ancestry. It is very likely that pseudoexons are an underrecognized cause of disease. In the new genomic era, our findings highlight the importance of studying variation in deep intronic regions as a cause of monogenic disorders.
Acknowledgments
Financial Support: This work was supported by a Barts Charity Large Project Grant (reference No. MRC0161 to H.L.S.), the 2018 European Society for Paediatric Endocrinology Research Fellowship (to E.C.), the Sandoz Limited UK research grant (No. 1010180 to H.L.S. and E.C.), the National Institute for Health Research (No. NIHR300098 to H.L.S.).
Glossary
Abbreviations
- ALS
acid labile subunit
- BMI
body mass index
- cDNA
complementary DNA
- CV
coefficient of variation
- DMEM
Dulbecco’s modified Eagle’s medium
- FT3
free 3,5,3′-triiodothyronine
- FT4
free thyroxine
- GH
growth hormone
- GHBP
growth hormone binding protein
- GHI
growth hormone insensitivity
- GHR
growth hormone receptor
- IGF-1
insulin-like growth factor 1
- IGFBP
insulin-like growth factor binding protein
- IGFGT
insulin-like growth factor 1 generation test
- mRNA
messenger RNA
- NR
normal range
- PCR
polymerase chain reaction
- rhIGF-1
recombinant human insulin-like growth factor 1
- RT-PCR
reverse transcriptase–polymerase chain reaction
- SGA
small for gestational age
- TBS
Tris-buffered saline
- TSH
thyroid-stimulating hormone
- WT
wild-type
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The data sets generated and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Eshet R, Laron Z, Pertzelan A, Arnon R, Dintzman M. Defect of human growth hormone receptors in the liver of two patients with Laron-type dwarfism. Isr J Med Sci. 1984;20(1):8-11. [PubMed] [Google Scholar]
- 2. Laron Z, Pertzelan A, Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone—a new inborn error of metabolism? Isr J Med Sci. 1966;2(2):152-155. [PubMed] [Google Scholar]
- 3. Rughani A, Zhang D, Vairamani K, Dauber A, Hwa V, Krishnan S. Severe growth failure associated with a novel heterozygous nonsense mutation in the GHR transmembrane domain leading to elevated growth hormone binding protein. Clin Endocrinol (Oxf). 2020;92(4):331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berget SM. Exon recognition in vertebrate splicing. J Biol Chem. 1995;270(6):2411-2414. [DOI] [PubMed] [Google Scholar]
- 5. Nakai K, Sakamoto H. Construction of a novel database containing aberrant splicing mutations of mammalian genes. Gene. 1994;141(2):171-177. [DOI] [PubMed] [Google Scholar]
- 6. Vaz-Drago R, Custódio N, Carmo-Fonseca M. Deep intronic mutations and human disease. Hum Genet. 2017;136(9):1093-1111. [DOI] [PubMed] [Google Scholar]
- 7. Dobkin C, Pergolizzi RG, Bahre P, Bank A. Abnormal splice in a mutant human beta-globin gene not at the site of a mutation. Proc Natl Acad Sci U S A. 1983;80(5):1184-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treisman R, Orkin SH, Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983;302(5909):591-596. [DOI] [PubMed] [Google Scholar]
- 9. Dhir A, Buratti E. Alternative splicing: role of pseudoexons in human disease and potential therapeutic strategies. FEBS J. 2010;277(4):841-855. [DOI] [PubMed] [Google Scholar]
- 10. Metherell LA, Akker SA, Munroe PB, et al. Pseudoexon activation as a novel mechanism for disease resulting in atypical growth-hormone insensitivity. Am J Hum Genet. 2001;69(3):641-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maamra M, Milward A, Esfahani HZ, et al. A 36 residues insertion in the dimerization domain of the growth hormone receptor results in defective trafficking rather than impaired signaling. J Endocrinol. 2006;188(2):251-261. [DOI] [PubMed] [Google Scholar]
- 12. David A, Camacho-Hübner C, Bhangoo A, et al. An intronic growth hormone receptor mutation causing activation of a pseudoexon is associated with a broad spectrum of growth hormone insensitivity phenotypes. J Clin Endocrinol Metab. 2007;92(2):655-659. [DOI] [PubMed] [Google Scholar]
- 13. Chatterjee S, Shapiro L, Rose SJ, et al. Phenotypic spectrum and responses to recombinant human IGF1 (rhIGF1) therapy in patients with homozygous intronic pseudoexon growth hormone receptor mutation. Eur J Endocrinol. 2018;178(5):481-489. [DOI] [PubMed] [Google Scholar]
- 14. Chatterjee S, Cottrell E, Rose SJ, et al. GHR gene transcript heterogeneity may explain phenotypic variability in GHR pseudoexon (6Ψ) patients. Endocr Connect. 2020;9(3):211-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gripp KW. Handbook of Physical Measurements. 3rd ed. Oxford University Press; 2013;101-111. [Google Scholar]
- 16. Manolopoulou J, Alami Y, Petersenn S, et al. Automated 22-kD growth hormone-specific assay without interference from Pegvisomant. Clin Chem. 2012;58(10):1446-1456. [DOI] [PubMed] [Google Scholar]
- 17. Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for insulin-like growth factor-1 (IGF-I) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712-1721. [DOI] [PubMed] [Google Scholar]
- 18. Friedrich N, Wolthers OD, Arafat AM, et al. Age- and sex-specific reference intervals across life span for insulin-like growth factor binding protein 3 (IGFBP-3) and the IGF-I to IGFBP-3 ratio measured by new automated chemiluminescence assays. J Clin Endocrinol Metab. 2014;99(5):1675-1686. [DOI] [PubMed] [Google Scholar]
- 19. Stadler S, Wu Z, Dressendörfer RA, et al. Monoclonal anti-acid-labile subunit oligopeptide antibodies and their use in a two-site immunoassay for ALS measurement in humans. J Immunol Methods. 2001;252(1-2):73-82. [DOI] [PubMed] [Google Scholar]
- 20. Rowlinson SW, Behncken SN, Rowland JE, et al. Activation of chimeric and full-length growth hormone receptors by growth hormone receptor monoclonal antibodies. A specific conformational change may be required for full-length receptor signaling. J Biol Chem. 1998;273(9):5307-5314. [DOI] [PubMed] [Google Scholar]
- 21. Cottrell E, Maharaj A, Williams J, et al. Data for: Growth hormone receptor (GHR) 6Ω pseudoexon activation: a novel cause of severe growth hormone insensitivity (GHI). Figshare digital repository. Deposited March 11, 2021. 10.6084/m9.figshare.14199779 [DOI] [PubMed]
- 22. Amselem S, Sobrier ML, Duquesnoy P, et al. Recurrent nonsense mutations in the growth hormone receptor from patients with Laron dwarfism. J Clin Invest. 1991;87(3):1098-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maharaj A, Buonocore F, Meimaridou E, et al. Predicted benign and synonymous variants in CYP11A1 cause primary adrenal insufficiency through missplicing. J Endocr Soc. 2019;3(1):201-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berg MA, Guevara-Aguirre J, Rosenbloom AL, Rosenfeld RG, Francke U. Mutation creating a new splice site in the growth hormone receptor genes of 37 Ecuadorean patients with Laron syndrome. Hum Mutat. 1992;1(1):24-32. [DOI] [PubMed] [Google Scholar]
- 25. Storr HL, Chatterjee S, Metherell LA, et al. Nonclassical GH insensitivity: characterization of mild abnormalities of GH action. Endocr Rev. 2019;40(2):476-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang XH, Chasin LA. Computational definition of sequence motifs governing constitutive exon splicing. Genes Dev. 2004;18(11):1241-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Z, Rolish ME, Yeo G, Tung V, Mawson M, Burge CB. Systematic identification and analysis of exonic splicing silencers. Cell. 2004;119(6):831-845. [DOI] [PubMed] [Google Scholar]
- 28. Sironi M, Menozzi G, Riva L, et al. Silencer elements as possible inhibitors of pseudoexon splicing. Nucleic Acids Res. 2004;32(5):1783-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corvelo A, Eyras E. Exon creation and establishment in human genes. Genome Biol. 2008;9(9):R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. David A, Hwa V, Metherell LA, et al. Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. Endocr Rev. 2011;32(4):472-497. [DOI] [PubMed] [Google Scholar]
- 31. Berg MA, Argente J, Chernausek S, et al. Diverse growth hormone receptor gene mutations in Laron syndrome. Am J Hum Genet. 1993;52(5):998-1005. [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenbloom AL, Berg MA, Kasatkina EP, et al. Severe growth hormone insensitivity (Laron syndrome) due to nonsense mutation of the GH receptor in brothers from Russia. J Pediatr Endocrinol Metab. 1995;8(3):159-165. [DOI] [PubMed] [Google Scholar]
- 33. Laron Z, Iluz M, Kauli R. Head circumference in untreated and IGF-I treated patients with Laron syndrome: comparison with untreated and hGH-treated children with isolated growth hormone deficiency. Growth Horm IGF Res. 2012;22(2):49-52. [DOI] [PubMed] [Google Scholar]
- 34. Backeljauw PF, Kuntze J, Frane J, Calikoglu AS, Chernausek SD. Adult and near-adult height in patients with severe insulin-like growth factor-I deficiency after long-term therapy with recombinant human insulin-like growth factor-I. Horm Res Paediatr. 2013;80(1):47-56. [DOI] [PubMed] [Google Scholar]
- 35. Sjoberg M, Salazar T, Espinosa C, et al. Study of GH sensitivity in chilean patients with idiopathic short stature. J Clin Endocrinol Metab. 2001;86(9):4375-4381. [DOI] [PubMed] [Google Scholar]
- 36. Goddard AD, Dowd P, Chernausek S, et al. Partial growth-hormone insensitivity: the role of growth-hormone receptor mutations in idiopathic short stature. J Pediatr. 1997;131(1 Pt 2):S51-S55. [DOI] [PubMed] [Google Scholar]
- 37. Guevara-Aguirre J, Rosenbloom AL, Guevara-Aguirre M, et al. Effects of heterozygosity for the E180 splice mutation causing growth hormone receptor deficiency in Ecuador on IGF-I, IGFBP-3, and stature. Growth Horm IGF Res. 2007;17(3):261-264. [DOI] [PubMed] [Google Scholar]
- 38. Woods KA, Dastot F, Preece MA, et al. Phenotype: genotype relationships in growth hormone insensitivity syndrome. J Clin Endocrinol Metab. 1997;82(11):3529-3535. [DOI] [PubMed] [Google Scholar]
- 39. Laron Z, Klinger B, Erster B, Silbergeld A. Serum GH binding protein activities identifies the heterozygous carriers for Laron type dwarfism. Acta Endocrinol (Copenh). 1989;121(4):603-608. [DOI] [PubMed] [Google Scholar]
- 40. Rosenbloom AL, Guevara-Aguirre J, Rosenfeld RG, Fielder PJ. Is there heterozygote expression of growth hormone receptor deficiency? Acta Paediatr Suppl. 1994;399:125-127. [DOI] [PubMed] [Google Scholar]
- 41. Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958-2003. J Clin Endocrinol Metab. 2004;89(3):1031-1044. [DOI] [PubMed] [Google Scholar]
- 42. Klinger B, Ionesco A, Anin S, Laron Z. Effect of insulin-like growth factor I on the thyroid axis in patients with Laron-type dwarfism and healthy subjects. Acta Endocrinol (Copenh). 1992;127(6):515-519. [DOI] [PubMed] [Google Scholar]
- 43. Moayeri H, Hemati A, Bidad K, Dalili H. Effects of growth hormone replacement therapy on thyroid function tests in growth hormone deficient children. Acta Med Iran. 2008;46(6):473-476. [Google Scholar]
- 44. Yamauchi I, Sakane Y, Yamashita T, et al. Effects of growth hormone on thyroid function are mediated by type 2 iodothyronine deiodinase in humans. Endocrine. 2018;59(2):353-363. [DOI] [PubMed] [Google Scholar]
- 45. Gonçalves FT, Fridman C, Pinto EM, et al. The E180splice mutation in the GHR gene causing Laron syndrome: witness of a Sephardic Jewish exodus from the Iberian Peninsula to the New World? Am J Med Genet A. 2014;164A(5):1204-1208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.