Abstract
Context
Polycystic ovary syndrome (PCOS) is a common endocrine disorder associated with low-grade inflammation and increased incidence of pregnancy complications, but its influence on the maternal immune system in pregnancy is unknown. Longitudinal serum cytokine profiling is a sensitive measure of the complex immunological dynamics of pregnancy.
Objective
This work aimed to determine the immunological dynamics of serum cytokines throughout pregnancy in women with PCOS and compare it to pregnancy in women without PCOS.
Methods
A post hoc analysis was conducted of longitudinal serum samples from 2 randomized, placebo-controlled multicenter studies of pregnant women with PCOS and 2 studies of pregnant women without PCOS. Pregnant women with PCOS (n = 358) and without PCOS (n = 258, controls) provided 1752 serum samples from 4 time points in pregnancy (weeks 10, 19, 32, and 36). Main outcome measures included maternal serum levels of 22 cytokines and C-reactive protein (CRP) at 4 time points in pregnancy.
Results
Women with PCOS showed marked immunological changes in serum cytokines throughout pregnancy. Compared to controls, women with PCOS showed higher levels of 17 cytokines and CRP at week 10 of pregnancy and a distinct cytokine development throughout pregnancy. The immunological dynamics in women with PCOS was significantly affected by maternal body mass index, smoking, and fetal sex.
Conclusion
Pregnancy in women with PCOS was associated with a strong early mobilization of inflammatory and other serum cytokines persisting throughout pregnancy, indicating a more activated immune status. These findings provide a novel basis for further study of PCOS and pregnancy complications.
Keywords: PCOS, pregnancy, cytokine, chemokine, C-reactive protein, multivariate analysis
Polycystic ovary syndrome (PCOS) is a common endocrine disorder, affecting up to 17% of women of fertile age (1, 2). The pathogenesis of PCOS is multifactorial, involving both genetics and the intrauterine environment, while lifestyle factors can worsen the clinical presentations (3). The disorder is diagnosed based on the presence of hyperandrogenism, oligoovulation or anovulation, and polycystic ovarian morphology according to the Rotterdam criteria (4). Other features associated with PCOS are obesity, insulin resistance, and hypertensive disorders. PCOS is considered a low-grade inflammatory condition (5). The syndrome is associated with higher circulating levels of C-reactive protein (CRP) (6), interleukin (IL)-6 (7), IL-18 (8), tumor necrosis factor (TNF)-α (9) and monocyte chemotactic protein (MCP)-1 (10), and lower levels of IL-10 (11). Women with PCOS have a 15-fold increase in infertility, and fertility treatment is commonly used to achieve pregnancy (12). During pregnancy, women with PCOS have an increased risk of complications such as gestational diabetes mellitus (GDM), preeclampsia, and preterm birth regardless of body weight (13-15). A previous study has reported elevated levels of the inflammatory markers CRP, white blood cells, and ferritin during pregnancy in women with PCOS (16). However, studies in large cohorts are needed to determine how PCOS affects the immune system in pregnancy, and how these effects relate to the observed increase in pregnancy complications.
Pregnancy is a complex and dynamic immunological state, and the immune system plays a key role in orchestrating the stages of normal gestation from implantation to parturition. Women experiencing pregnancy complications show deviating immune activity, reflected in abnormal levels of signaling molecules such as cytokines and CRP, compared to women with normal pregnancies (17-20). Cytokines act in intertwined networks and investigating single cytokines does not fully reflect the complex biological processes of pregnancy. Multivariate analyses can model development of several cytokines simultaneously, while taking interactions between cytokines into account, as an overall measure of immunological status. We have previously shown that multivariate cytokine patterns sensitively mirror the immune activity during normal pregnancies and identify disease-related immunological changes (20, 21).
The deviant immune status of women with PCOS before pregnancy is likely to persist into pregnancy and affect the delicate dynamics of the maternal immune system during gestation. Whether such immunological changes contribute to the increased rate of pregnancy complications in women with PCOS needs to be explored. The aim of the present study was to determine the development of the serum cytokine pattern throughout pregnancy in women with PCOS and compare it to pregnancy in women without PCOS. We also assessed the influence of key maternal and fetal characteristics on the maternal serum cytokines in women with PCOS. Improved understanding of the immunological dynamics throughout pregnancy in women with PCOS may facilitate preventive measures, detection, and treatment of pregnancy complications in this group.
Materials and Methods
Study Population and Serum Samples
Serum samples from 2 cohorts of pregnant women with PCOS and 2 cohorts of women without PCOS and with normal pregnancies (hereafter referred to as controls) were included in this study. The PCOS group was included from the placebo groups of the 2 related studies PregMet and PregMet2 (22, 23). These were randomized, placebo-controlled, multicenter studies designed to determine whether metformin could reduce pregnancy complications in women diagnosed with PCOS according to the Rotterdam criteria (4). Women with no known inflammatory comorbidities such as rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease were selected and serum was sampled after an overnight fast at weeks 10, 19, 32, and 36 of pregnancy. Controls with normal pregnancies were selected from the Training in Pregnancy (TRIP) study (24) and the NormalFlow study (24). The TRIP study randomly assigned pregnant women to a 12-week exercise program or standard antenatal care. Women without hypertensive or inflammatory conditions or pregnancy complications from the standard antenatal care group were selected for the present study if they had provided serum samples after an overnight fast at weeks 18 to 22 and 32 to 36 of pregnancy. The NormalFlow study included healthy pregnant women and aimed to construct a reference curve for blood flow in the uterine artery during the first half of pregnancy. Women with nonfasting serum samples taken at weeks 10 and 19 of pregnancy were selected. The serum samples from all 4 studies were drawn from the antecubital vein in nonheparinized tubes and stored at –80 °C before analyses.
The studies were approved by the regional committee for medical and health research ethics (REC) (PregMet: REC No. 145.04; PregMet2: REC No. 2011/1434; TRIP: REC No. 4.2007.81; and NormalFlow: REC No. 4.2008.841). Further details about study-specific criteria and sampling are found in the respective original study reports (22-25).
Serum Measurements
All serum samples were analyzed for 27 cytokines (Bio-Plex Pro Human Cytokine 27-plex Assay, catalog No. M500KCAF0Y, RRID:AB_2893118) in single replicate using Luminex xMAP Technology on a Bio-Plex 200 System (Bio-Rad Laboratories). The serum samples were analyzed on a total of 59 plates in 3 separate batches: 1) PregMet and NormalFlow (lot No. 64075155), 2) TRIP (lot No. 64292035), and 3) PregMet2 (lot No. 64211339). The manufacturer’s protocol was followed with the recommended concentration of reagents and serum, but in reduced volume, either 1:2.5 (PregMet, PregMet2, and NormalFlow) or 1:2 (TRIP), as in other large-scale cytokine studies (20, 21). The serum cytokine data from the NormalFlow study have been included in a previous publication (21). All serum samples were analyzed for high-sensitivity CRP by enzyme-linked immunoassays (Human CRP Quantikine kit catalog No. DCRP000, RRID:AB_2893119, R&D Technologies) for the PregMet and NormalFlow studies, and by turbidimetric assay and measured at 571 nm by a Siemens Advia Chemistry XPT system at the Department of Clinical Chemistry at St. Olavs Hospital for the PregMet2 and TRIP studies. The comparability of the 2 methods has been validated at the Department of Clinical Chemistry at St. Olavs Hospital.
Data Processing
The cytokine levels were adjusted before analyses in a 2-step process to account for possible variations induced by different study cohorts, batches, and plates (Supplementary data, Adjustment of batch and plate effects) (26). Briefly, the first step adjusted for cytokine assay inter-lot variation, and the second step adjusted for cytokine assay intra-lot variation. Cytokine values below the lower limit of detection were imputed using robust estimation by the zCompositions package in R (27) and values above the upper limit of detection (ULOD) were replaced by the ULOD value. Cytokines with more than 25% of values outside the limits of quantification were excluded to avoid biased results from a high fraction of imputed values (Supplementary Table 1) (26). The remaining cytokines were divided into 4 groups by main function: (1) inflammatory cytokines, (2) anti-inflammatory cytokines, (3) growth and colony-stimulating cytokines (hereafter called growth factors), and (4) chemokines. CRP was presented with the inflammatory cytokines. Women with outlier cytokine values were identified by visual inspection of score plots from principal component analyses of all included samples in each separate cohort. The samples were classified as first (< 98 days), second (98-196 days) or third trimester (> 196 days) based on gestational age at sampling.
Statistical Analyses
Study population characteristics and cytokine and CRP data were tested for normality using visual inspections of quantile-quantile-plots. Normally distributed data are reported as mean (± SD), nonnormal data as median (interquartile range) and categorical variables as numbers (percentages).
The development of each cytokine and CRP throughout pregnancy in women with PCOS was assessed in 3 ways. First, the continuous development of each cytokine and CRP was estimated with generalized additive mixed models (GAMMs) where gestational age in days and study cohort were used as fixed effects, and individual as random intercepts using the gamm4 package in R (28). The GAMM trajectories were displayed as relative change in concentration with increasing gestational age. Estimation of robustness of the GAMMs were performed by jackknifing where the trajectories were reestimated 50 times with 80% of the participants drawn at random each time. The correlation between the 50 trajectories was calculated using Spearman rank correlation, and the model was considered reliable if the mean correlation of the estimated trajectories gave a rho value (ρ) greater than or equal to 0.9. Second, linear mixed models (LMMs) were used to explore the development and significant changes in cytokine and CRP levels by trimester. LMMs were performed with log-transformed cytokine and CRP concentrations as response variables, trimester and study cohort as fixed effects, and individual specific random intercepts using the lme4 package in R (29). First trimester was used as reference level. The possible influence of body mass index (BMI), fetal sex, smoking status, parity, maternal age, and gestational weight gain on the cytokine development was tested in separate models with these factors as fixed variables, and the main effect of each clinical variable was evaluated. Gestational weight gain was categorized as small gain, normal gain, and large gain relative to early pregnancy BMI (30). Third, the recently developed multivariate statistical method called repeated-measures analysis of variance simultaneous component analysis (RM-ASCA+) was used to display the overall longitudinal cytokine pattern throughout pregnancy (31) using the ALASCA package in R (32). In brief, RM-ASCA+ is based on principal component analysis of effect matrices from the LMM analyses of individual variables. Normalization of cytokine values was performed before analysis by first subtracting the mean and then dividing by the SD for each cytokine and CRP within each study cohort (33). Again, the underlying LMM was performed with log-transformed cytokine and CRP concentrations as response variables, trimester and study cohort as fixed effects, and individual specific random intercepts. RM-ASCA+ can also visualize differences between groups, and the possible influence of BMI, fetal sex, smoking status, parity, maternal age, and gestational weight gain on the longitudinal cytokine pattern was explored. For the assessment of BMI, women were grouped as normal weight (≤ 24.9), overweight (25.0-29.9), and obese (≥ 30) (34). Estimations of robustness of the RM-ASCA+ results were performed by jackknifing with 7-fold random subsets cross-validation with 50 iterations. The 2.5th and 97.5th percentiles from the jackknifing estimations make out the error bars on both the loading and scores plots.
Spearman rank correlation coefficients between cytokines were calculated within trimesters using log-transformed cytokine values.
Comparison of cytokine levels at study inclusion between pregnant women with PCOS and controls from the same batch was performed with Mann-Whitney U test. The study cohorts with samples from late pregnancy were analyzed in different batches, making it impossible to separate batch effects from group differences at these time points. Therefore, the relative cytokine development in women with PCOS and controls were compared by assessing differences in log2 fold change in defined time intervals within the PCOS and control group before comparing the results by Mann-Whitney U test. Orthogonalized partial least squares discriminant analysis (PLS-DA) (35) based on the log2 fold-change values were used to explore differences in overall cytokine patterns between the 2 groups using the PLS_toolbox 8.9 (Eigenvector Research) in MATLAB 2020a (36). The log2 cytokine and CRP values were autoscaled before analysis. The resulting orthogonalized PLS-DA classification models were evaluated by double cross-validation by which a model was built on training data (90% of the included women) and used to predict independent test samples (the remaining 10%). The optimal number of latent variables included in the model was determined by cross-validation of the training data. Both the inner and the outer model were repeated 20 times and the median sensitivity, specificity, and accuracy of the classification were calculated. The statistical significance of the model was tested by permutation testing (n = 1000 permutations).
Correction for multiple testing was performed using the Benjamini-Hochberg procedure. P less than or equal to .05 was considered statistically significant. Except for the PLS-DA, all analyses were performed in R (37) and visualizations were prepared with the ggplot2 package (38).
Results
Characteristics of the Study Populations and Cytokine Measurements
Two women were excluded because of extreme cytokine outlier values (Supplementary Fig. 1) (26), leaving a total of 1275 serum samples from 358 pregnant women with PCOS and 477 serum samples from 258 pregnant control women without PCOS for analysis. The pregnant women with PCOS had significantly higher BMI and blood pressure at study inclusion and were more often nulliparous compared to controls (Table 1). A total of 157 (44%) of the women with PCOS experienced one or more pregnancy complications, the most common being GDM (see Table 1).
Table 1.
Characteristics of study populations and study visits
| Women with PCOS (n = 358) | Controls (n = 258) | P | |
|---|---|---|---|
| Age, y | 29.7 ± 4.3 | 29.3 ± 4.4 | .325 |
| BMI at inclusion | 26.8 (23.3-31.2) | 23.7 (22.0-26.0)22 | .001 |
| Diastolic BP, mm Hg | 72 ± 9 | 69 ± 821 | .001 |
| Systolic BP, mm Hg | 114 ± 12 | 111 ± 1021 | .001 |
| Nullipara, n (%) | 198 (55) | 143 (56)4 | .872 |
| Smoking, n (%) | 22 (6)1 | 12 (5)11 | .614 |
| Gestational age birth, d | 275 ± 212 | 280 ± 94 | .001 |
| Birth weight, g | 3495 ± 6751 | 3557 ± 47017 | .188 |
| Fetal sex male, n (%) | 180 (50)1 | 125 (50)8 | .985 |
| Women with pregnancy complications, n (%) | 157 (44) | – | |
| Preeclampsia | 21 (6) | ||
| Gestational diabetes | 128 (35) | ||
| Hypertension | 16 (4) | ||
| Preterm birth | 27 (7) | ||
| Late miscarriage | 5 (1) | ||
| Gestational age at study visit, d | |||
| Inclusion wk 10 | 75 ± 11 | 75 ± 6 | .646 |
| Wk 19 | 133 ± 6 | 135 ± 8 | .011 |
| Wk 32 | 225 ± 5 | 227 ± 7 | .067 |
| Wk 36 | 254 ± 5 | 251 ± 10 | .027 |
Data are reported as mean ±SDm, median (quartiles)m, and number (percentage)m, where m is the number of missing data points. Smoking status was registered at inclusion to the study. P values were calculated with independent-samples t test for normally distributed data, Mann-Whitney U test for nonparametric data, and chi-square test for categorical variables. Hypertension includes previous and gestational hypertension. Controls indicate women without PCOS with normal pregnancies. Significant P values are highlighted in bold.
Abbreviations: BMI, body mass index; BP, blood pressure; PCOS, polycystic ovary syndrome.
The majority of the cytokines were reliably measured in serum throughout pregnancy, but IL-5, IL-10, interferon-γ, vascular endothelial growth factor, and RANTES were excluded because of high incidence of nondetectable values (Supplementary Table 1) (26).
Serum Cytokine and C-Reactive Protein Levels Throughout Pregnancy in Women with Polycystic Ovary Syndrome
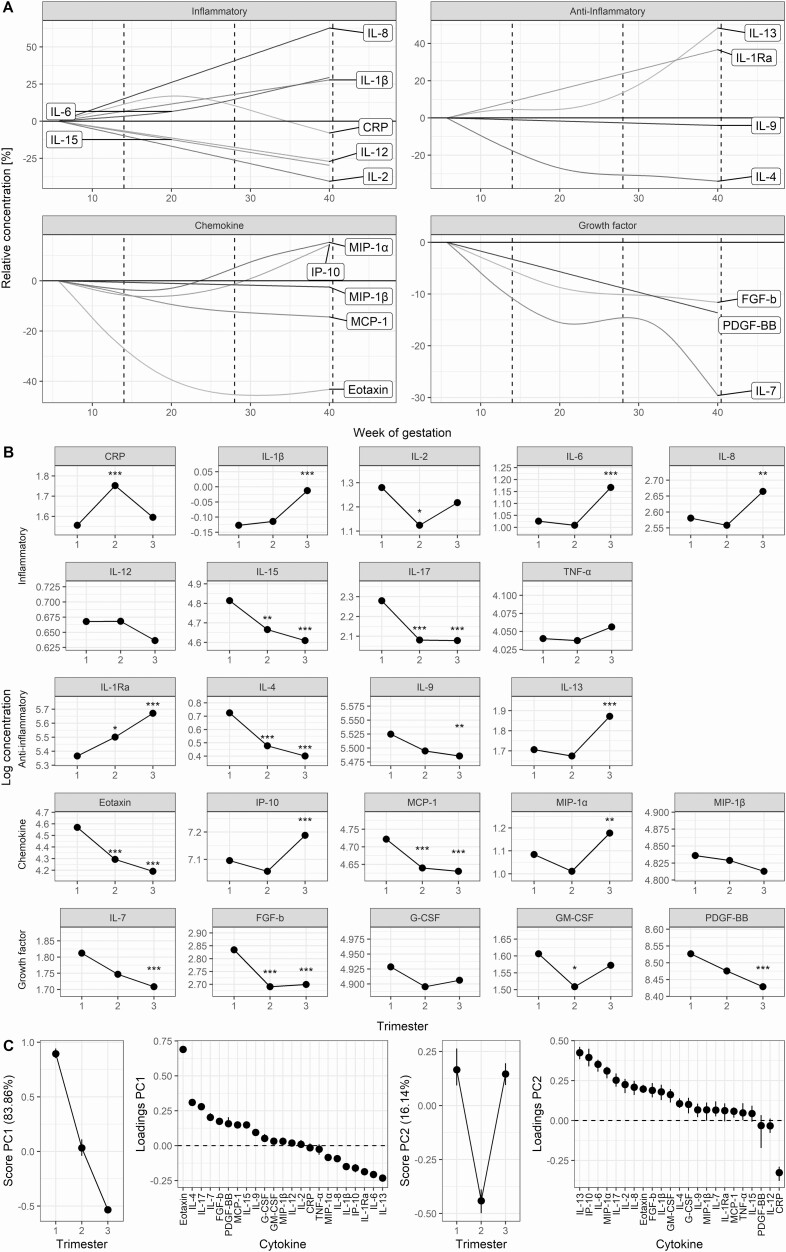
Of the 22 detectable serum cytokines in pregnancies of women with PCOS, 11 displayed the highest concentration in the first trimester and 7 in the third trimester, while only CRP showed highest levels in the second trimester (see Fig. 1). IL-8 showed the greatest relative increase and eotaxin the greatest relative decrease throughout pregnancy, while IL-12, TNF-α, granulocyte-colony stimulating factor (G-CSF), and macrophage-inflammatory protein (MIP)-1β showed no significant change during gestation (Fig. 1B). The inflammatory cytokines appeared with a dual development: The potent IL-1β, IL-6, and IL-8 steadily increased toward term, whereas IL-15 and IL-17 steadily decreased (Fig. 1A). A similar development for the anti-inflammatory cytokines showed increasing levels of IL-1 receptor antagonist (Ra) and IL-13 during gestation, whereas IL-4 and IL-9 decreased. Among the chemokines, eotaxin and MCP-1 decreased during gestation, while interferon-γ–induced protein (IP)-10 and MIP-1α displayed highest serum levels toward term. The growth factors generally displayed the highest concentration in the first trimester before decreasing toward term (see Fig. 1A). Fig. 1C displays the overall cytokine development, confirming the 2 main developmental trends throughout pregnancy in women with PCOS: 1) decreasing concentrations from implantation toward term, highly influenced by eotaxin; and 2) increased immune activity in the first and third trimester with the strongest impact from IL-13 and IP-10. Similar cytokine developments were seen when the women with PCOS with pregnancy complications were excluded (data not shown).
Figure 1.
Development of serum cytokine and CRP concentrations throughout pregnancy in women with PCOS. Cytokines are grouped according to main function. A, Trajectories from generalized additive mixed models displaying the relative development of cytokine and CRP concentrations in maternal serum throughout pregnancy in women with PCOS. Limits for the 3 trimesters are marked vertically with dotted lines. IL-17, G-CSF, GM-CSF, and TNFα are excluded from Fig. 1A because the gestational development could not be robustly modeled. B, Change in maternal log-transformed cytokine (pg/mL) and CRP (μg/mL) levels by trimester from univariate linear mixed models in pregnancies of women with PCOS. P values are adjusted using the Benjamini-Hochberg procedure. Asterisks indicate significant change from the first trimester. *P less than .05; **P less than .01; and ***P less than .001. C, Trajectories from multivariate RM-ASCA+ analyses compressing all cytokine developments by trimester into single variables called principal components (PCs). PC1 and PC2 explain most of the variation in cytokine development. A high PC score indicates higher concentrations of the cytokines with positive loadings and lower concentrations of the cytokines with negative loadings in the loadings score plot and vice versa. The vertical lines represent the error bars. CRP, C-reactive protein; FGF-b, basic fibroblastic growth factor; G-CSF, granulocyte colony-stimulating factor; GM, granulocyte macrophage; IL, interleukin; IP, interferon-γ–induced protein; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; PCOS, polycystic ovary syndrome; PDGF, platelet-derived growth factor; Ra, receptor antagonist; RM-ASCA+, repeated-measures analysis of variance simultaneous component analysis; TNF, tumor necrosis factor.
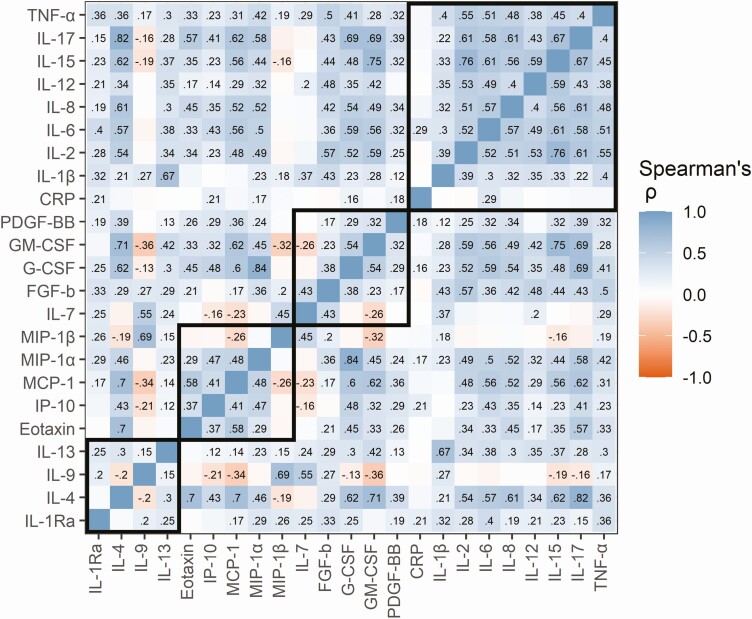
The correlations between the cytokines were largely consistent in all 3 trimesters (Fig. 2 and Supplementary Fig. 2) (26). The cytokines were mainly positively correlated, with the highest number of positive correlations in the first trimester (82%), and the highest number of negative correlations in the third trimester (6%). The inflammatory cytokines showed the highest within-group correlation (), while the within-group correlation was considerably lower for the anti-inflammatory cytokines (), growth factors (), and chemokines (). CRP levels were only weakly correlated with cytokine levels, and strongest with IL-1Ra in the first and second (ρ = 0.25 and 0.28, respectively) trimester, and IL-6 in the third (ρ = 0.29) trimester (all P values < .001) (see Fig. 2 and Supplementary Fig. 2) (26).
Figure 2.
Spearman rank correlation coefficients between cytokines within the third trimester in women with PCOS. Log-transformed cytokine values were used. Dark blue indicates a strong positive correlation, dark red indicates a strong negative correlation, and white indicates no correlation. Rho values are displayed in the figure tiles for correlations that remained significant after adjusting for multiple testing using the Benjamini-Hochberg procedure. The cytokines are grouped in bold squares by main function. CRP, C-reactive protein; FGF-b, basic fibroblastic growth factor; G-CSF, granulocyte colony-stimulating factor; GM, granulocyte macrophage; IL, interleukin; IP, interferon-γ–induced protein; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; PCOS, polycystic ovary syndrome; PDGF, platelet-derived growth factor; Ra, receptor antagonist; TNF, tumor necrosis factor.
Serum Cytokine Patterns in Pregnant Women With Polycystic Ovary Syndrome Compared to Pregnant Controls
The absolute cytokine levels were compared between women with PCOS and controls in week 10 of pregnancy (Table 2). Strikingly, 17 out of 22 cytokines and CRP were detected at significantly higher levels in serum samples of women with PCOS, including 7 out of 8 inflammatory cytokines. IL-1β, IL-2, IL-7, IL-12, IL-17, and basic fibroblastic growth factor (FGF-b) were more than 50% higher than in controls. IL-12 differed the most, with 4 times higher serum levels in PCOS as in controls. To control for the effect of pregnancy complications and BMI when comparing women with PCOS to controls, the comparisons were repeated with 2 subgroups of women with PCOS. For women with PCOS and without pregnancy complications, IL-6, IL-1Ra, MCP-1, and CRP did no longer differ, and for nonobese (BMI < 30) women with PCOS, IL-2, IL-6, IL-1Ra, MCP-1, and CRP no longer differed from controls during week 10 (see Table 2).
Table 2.
Comparison of absolute cytokine and C-reactive protein levels between pregnant women with polycystic ovary syndrome and controls in week 10 of pregnancy
| Cytokine | Women with PCOS (n = 125) | Controls (n = 110) | Levels in PCOS compared to controls | P |
|---|---|---|---|---|
| Inflammatory cytokines | ||||
| CRP | 4.8 (2.4-8.5) | 3.2 (1.4-7.0) | 1.48 | .026 a , b |
| IL-1β | 0.9 (0.8-1.2) | 0.6 (0.4-0.8) | 1.58 | < .001 |
| IL-2 | 4.6 (2.3-9.5) | 2.9 (0.6-7.5) | 1.55 | .026 b |
| IL-6 | 2.8 (2.2-3.4) | 2.4 (1.9-2.4) | 1.19 | .021 a , b |
| IL-8 | 13.9 (12.0-16.3) | 10.7 (9.1-13.2) | 1.30 | < .001 |
| IL-12 | 2.1 (0.9-3.8) | 0.5 (0.3-1.8) | 4.32 | < .001 |
| IL-15 | 127.1 (78.9-224.4) | 114.3 (67.0-191.2) | 1.11 | .395 |
| IL-17 | 9.1 (7.0-11.4) | 4.4 (2.7-6.6) | 2.08 | < .001 |
| TNF-α | 54.7 (47.7-63.5) | 43.0 (36.3-49.8) | 1.28 | < .001 |
| Anti-inflammatory cytokines | ||||
| IL-1Ra | 266.3 (168.5-388.1) | 196.7 (113.7-369.7) | 1.35 | .026 a , b |
| IL-4 | 1.7 (1.5-2.0) | 1.2 (0.8-1.6) | 1.44 | < .001 |
| IL-9 | 252.6 (217.9-290.5) | 203.2 (147.5-249.9) | 1.24 | < .001 |
| IL-13 | 5.2 (4.1-6.5) | 4.0 (3.3-5.3) | 1.29 | < .001 |
| Chemokines | ||||
| Eotaxin | 86.8 (73.6-113.4) | 91.1 (69.2-131.2) | 0.95 | .969 |
| IP-10 | 1243.6 (998.4-1538.3) | 1342.0 (1079.9-1686.0) | 0.93 | .151 |
| MCP-1 | 104.8 (92.2-121.2) | 98.7 (87.1-112.5) | 1.06 | .041 a , b |
| MIP-1α | 3.1 (2.7-3.7) | 2.2 (1.8-2.5) | 1.42 | < .001 |
| MIP-1β | 137.2 (108.6-167.6) | 130.7 (91.4-160.0) | 1.05 | .222 |
| Growth factors | ||||
| IL-7 | 5.9 (3.2-10.7) | 3.2 (1.3-6.1) | 1.85 | < .001 |
| FGF-b | 17.7 (12.4-25.0) | 8.1 (4.1-13.4) | 2.19 | < .001 |
| G-CSF | 136.6 (117.7-152.9) | 113.6 (95.9-131.2) | 1.20 | < .001 |
| GM-CSF | 5.0 (3.6-7.3) | 4.19 (2.8-7.6) | 1.19 | .159 |
| PDGF-BB | 5221.7 (4453.6-5851.8) | 3988.29 (2970.9-4645.0) | 1.31 | .001 |
Comparison of cytokine (pg/mL) and CRP (μg/mL) levels between women with PCOS and controls at week 10 (study inclusion visit) in pregnancy displayed as median (interquartile range) and levels in women with PCOS compared to controls. Comparison was performed with Mann-Whitney U test and the P values were adjusted using the Benjamini-Hochberg procedure. Significant P values are highlighted in bold. Controls indicate women without PCOS with normal pregnancies.
Abbreviations: CRP, C-reactive protein; FGF-b, basic fibroblastic growth factor; G-CSF, granulocyte colony-stimulating factor; GM, granulocyte macrophage; IL, interleukin; IP, interferon-γ–induced protein; MCP, monocyte chemotactic protein; PCOS, polycystic ovary syndrome; PDGF, platelet-derived growth factor; Ra, receptor antagonist; TNF, tumor necrosis factor.
a Comparisons no longer significantly different between women with PCOS and controls when the women with PCOS and pregnancy complications were excluded.
b Comparisons no longer significantly different between women with PCOS and controls when the obese women were excluded, making the mean body mass index in the PCOS and control group comparable.
The serum cytokines were further assessed for relative serum cytokine development throughout pregnancy in women with PCOS and pregnant controls (Table 3). Of the 17 cytokines with higher serum levels in pregnant women with PCOS in week 10 (see Table 2), IL-8, TNF-α, IL-1Ra, MCP-1, and platelet-derived growth factor (PDGF)-BB showed a strengthened association to PCOS throughout pregnancy either by stronger continued increase or less reduction over time compared to controls (see Table 3). In addition, IL-4, IL-7, G-CSF, FGF-b, eotaxin, and IP-10 showed different relative developments in pregnancy in women with PCOS compared to controls (see Table 3). The overall serum cytokine profile in women with PCOS could be separated from controls with a classification accuracy of 62% to 70% at all time intervals (all P values < .001) (see Table 3). The high classification accuracy indicates substantial and persistent differences in relative cytokine development in pregnancy between women with and without PCOS (see Table 3 and Supplementary Fig. 3) (26). Similar classification results were found for comparisons between controls and the women with PCOS and without pregnancy complications and the nonobese women with PCOS (data not shown). In the univariate cytokine development comparisons, IL-4, IL-7, G-CSF, FGF-b, eotaxin, and IP-10 no longer differed for the women with PCOS and without pregnancy complications, while only minor changes were shown for comparisons with the nonobese women with PCOS (see Table 3).
Table 3.
Comparison of relative cytokine development in pregnant women with polycystic ovary syndrome and pregnant controls
| Univariate analyses | Multivariate analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time interval, wk | PCOS, No. | Controls, No. | Cytokine differences (PCOS/controls) | P | Classification accuracy, % | Sensitivity/Specificity | LVs | Top 4 cytokines contributing to separation between groups | P |
| 10 → 19 | 324 | 89 | IL-7 (↓/↑) | .013 a | 70 | 0.61/ 0.81 | 3 | IL-7 | < .001 |
| IL-4 (↓↓/↓) | .048 a | IL-4 | |||||||
| G-CSF (↔/↑) | .013 a | G-CSF | |||||||
| FGF-b (↓↓/↓) | .049 a | FGF-b | |||||||
| 19 → 32 | 293 | 75 | MCP-1 (↓/↓↓) | < .001 | 62 | 0.55/ 0.7 | 5 | MCP-1 | < .001 |
| Eotaxin (↓/↓↓) | < .001 a | Eotaxin | |||||||
| IL-8 (↑/↓) | < .001 | IL-8 | |||||||
| IL-7 (↔/↓) | .010 | IL-7 | |||||||
| IP-10 (↑/↔) | .017 a , b | ||||||||
| PDGF-BB (↔/↓) | .028 | ||||||||
| TNF-α (↑/↓) | .028 | ||||||||
| 19 → 36 | 286 | 55 | IL-1Ra (↑/↓) | < .001 | 68 | 0.67/ 0.6 | 2 | IL-1Ra | < .001 |
| MCP-1 (↓/↓↓) | < .001 | MCP-1 | |||||||
| Eotaxin (↓/↓↓) | .021 a , b | Eotaxin IP-10 | |||||||
Results from univariate and multivariate comparison of log2 fold-change values of 22 cytokines and CRP in defined time intervals in pregnant women with PCOS and pregnant controls. Univariate cytokine differences were analyzed by Mann-Whitney U test showing only the cytokines with significantly different development in women with PCOS compared to controls. Arrows before the slash indicate development in women with PCOS and arrows after the slash indicate development in controls. Double arrows indicate a greater change in cytokine concentration compared to the other group. P values are adjusted according to Benjamini-Hochberg procedure, and significant P values are highlighted in bold. Multivariate classification analyses are performed with orthogonalized PLS-DA. The classification accuracy describes the percentage of serum samples that are correctly classified according to PCOS status based on the cytokine and CRP profile. LVs indicate number of latent variables in the PLS-DA model. The PLS-DA scores and loadings plots can be found in Supplementary Fig. 4. Controls indicate women without PCOS with normal pregnancies.
Abbreviations: CRP, C-reactive protein; FGF-b, basic fibroblastic growth factor; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; IP, interferon-γ–induced protein; LV, latent variable; MCP, monocyte chemotactic protein; PCOS, polycystic ovary syndrome; PLS-DA, partial least squares-discriminant analyses; PDGF, platelet-derived growth factor; Ra, receptor antagonist; TNF, tumor necrosis factor.
a Comparisons no longer significantly different between women with PCOS and controls when the women with PCOS and pregnancy complications were excluded.
b Comparisons no longer significantly different between women with PCOS and controls when the obese women were excluded, making the mean body mass index in the PCOS and control group comparable.
The Influence of Key Clinical Parameters on the Maternal Immune System in Pregnancy in Women with Polycystic Ovary Syndrome
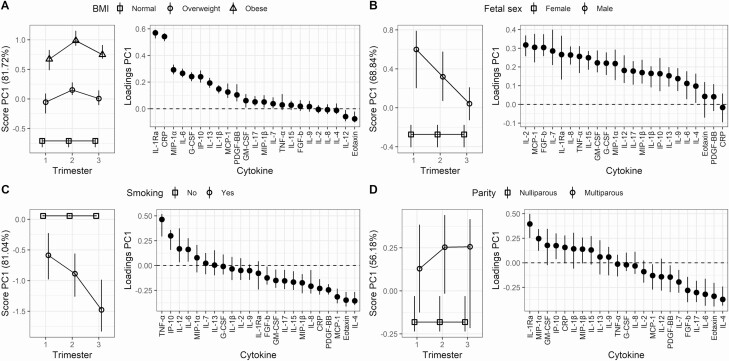
Parameters considered important for development and outcome of pregnancy were investigated for impact on the longitudinal cytokine pattern of pregnant women with PCOS by RM-ASCA+ analyses (Fig. 3). The trajectories for the defined reference groups can be found elsewhere (Supplementary Fig. 4) (26). Both obesity and overweight showed a pronounced effect on the serum cytokine patterns (Fig. 3A). Obese women presented with a distinct serum cytokine pattern characterized by higher levels of IL-1Ra, CRP, MIP-1α, IL-6, and G-CSF compared to overweight women, who in turn had higher levels of the same cytokines compared to normal-weight women. The influence of BMI on the maternal immune system was further supported by univariate LMM models showing significantly increased levels of CRP (P < .001), IL-6 (P = .017), IL-1Ra (P < .001), MIP-1α (P < .005), and IP-10 (P = .037) with increasing BMI in pregnancy (Supplementary Fig. 5A) (26). Importantly, although BMI markedly affected these specific serum cytokines, the differences in cytokine levels between women with PCOS and controls at week 10 persisted when the obese women with PCOS were excluded (data not shown). Women with PCOS with a male fetus displayed a distinct serum cytokine pattern characterized by higher levels of IL-2, MCP-1, FGF-b, IL-7, and IL-1Ra compared to women with female fetuses in the first and second trimester (Fig. 3B). Additionally, smoking showed a prominent influence on the serum cytokine patterns in pregnant women with PCOS (Fig. 3C). Maternal smoking was reflected by lower levels of the inflammatory cytokines TNF-α, IL-12, and IL-6 and the chemokine IP-10, and higher levels of anti-inflammatory IL-4 and the chemokines eotaxin and MCP-1. These smoking-associated changes were apparent from the first trimester and increased throughout the pregnancy. A similar assessment with RM-ASCA+ of whether parity (nullipara or multipara) (Fig. 3D), maternal age (< 25 years, 25-35 years, or > 35 years) or gestational weight gain during pregnancy (data not shown) influenced the serum cytokine patterns in pregnancy in women with PCOS showed no apparent effects. Univariate LMM analyses did not detect any significant differences related to fetal sex, smoking, parity, maternal age or gestational weight gain (Supplementary Fig. 5B-5G) (26). Assessment of the impact of clinical parameters on the cytokine patterns in the subgroup of women with PCOS without pregnancy complications showed similar results, except for a less prominent influence from fetal sex, and BMI was no longer associated with the changes in IL-6, MIP-1α, or IP-10 in Supplementary Fig. 5A (data not shown) (26).
Figure 3.
Influence of important clinical parameters on the serum cytokine pattern in pregnant women with PCOS by RM-ASCA+ for A, overweight and obese women relative to normal-weight women; B, women with a male compared to female fetus; C, smoking compared to nonsmoking women; and D, multiparous compared to nulliparous women. The first principal component (PC1) from the RM-ASCA+ analysis explains most of the variation of the comparisons, thus only PC1 is shown. Cytokine development in the clinical groups is shown relative to the indicated reference group and must be interpreted in relation to the corresponding reference trajectories in Supplementary Fig. 4. Samples with a high PC1 score have higher concentrations of the cytokines with positive loadings and lower concentrations of the cytokines with negative loadings in the loadings score plot compared to samples with low score values. The vertical lines represent the error bars. CRP, C-reactive protein; FGF-b, basic fibroblastic growth factor; G-CSF, granulocyte colony-stimulating factor; GM, granulocyte macrophage; IL, interleukin; IP, interferon-γ–induced protein; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; PC, principal component; PCOS, polycystic ovary syndrome; PDGF, platelet-derived growth factor; Ra, receptor antagonist; RM-ASCA+, repeated-measures analysis of variance simultaneous component analysis; TNF, tumor necrosis factor.
Discussion
This study provides the first extensive characterization of serum cytokine dynamics throughout pregnancy in women with PCOS. Women with PCOS showed marked changes in serum cytokines throughout pregnancy, with high immunological activation in the first and third trimesters. Compared to controls, women with PCOS showed higher levels of most cytokines in the first trimester, with a strong contribution from inflammatory cytokines. The relative cytokine development was also different in women with PCOS compared to controls, particularly influenced by IL-1Ra, IL-8, TNF-α, MCP-1, and PDGF-BB. Maternal BMI, smoking, and fetal sex induced distinct changes in the serum cytokine pattern in pregnant women with PCOS, whereas no apparent changes were induced by parity, maternal age, or gestational weight gain.
To our knowledge, no prior study has compared absolute cytokine levels or relative cytokine development in pregnancy in women with PCOS and controls. The strong increase in baseline levels of a broad selection of serum cytokines compared to controls indicate markedly higher systemic immunological activity already in early pregnancy in women with PCOS. A prominent inflammatory character was apparent by coordinated involvement of IL-1β, IL-2, IL-6, IL-12, and CRP in early pregnancy and IL-8 and TNF-α in all trimesters in women with PCOS. Interestingly, the additional strong association with other multifunctional cytokines of anti-inflammatory, chemotactic, and growth factor characteristics points to a complex immune regulation in women with PCOS. Combined assessment of the cytokines allowed for sensitive separation of women with PCOS from controls in all trimesters, demonstrating the value of a multicytokine approach. Importantly, the cytokine pattern in women with PCOS was shown to be independent of pregnancy complications in these women, confirming the existence of a baseline state of elevated immune mobilization unique to pregnant women with PCOS. Still, the important immunological milestones of normal pregnancy could be recognized (39, 40). The first trimester was characterized by high concentrations of growth factors, chemokines, and inflammatory cytokines, reflecting the critical immunological processes of implantation and placental development. Most cytokines decreased or remained stable in concentration from the first to the second trimester, in agreement with the less activated immunological state of the second trimester, a period facilitating rapid fetal growth. The immune activity markedly increased toward delivery with increased levels of both potent inflammatory cytokines and modulating anti-inflammatory cytokines.
The distinct serum cytokine profile of pregnant women with PCOS includes cytokines that may affect the development of pregnancy. As revealed in early pregnancy, IL-12 is a strong inflammatory cytokine previously associated with preeclampsia (18), while FGF-b is regarded important for adequate vascular and embryonic development (41, 42). The increased serum levels of IL-6, MCP-1, and TNF-α are also found in nonpregnant women with PCOS (7, 9, 10). The continued increase of IL-8 and TNF-α levels in the second and third trimester may indicate prolonged inflammatory development throughout pregnancy in women with PCOS. The simultaneous increase in the anti-inflammatory IL-1Ra may point to compensatory immunological regulation. Combined mobilization of inflammatory and anti-inflammatory cytokines is regarded as necessary to balance and fine-tune the increased inflammatory activation, as is also observed in association with pregnancy complications such as preeclampsia and preterm birth (43-45). Elevated levels of CRP have been reported both in nonpregnant women with PCOS and in pregnant women with PCOS during all trimesters compared to controls (6, 16). We found that CRP showed the highest levels in the second trimester in women with PCOS, even though the first and third trimesters have been regarded as more inflammatory (40). This finding is supported by a study of CRP in women without PCOS (21). The development of CRP differed from the trajectory of related inflammatory cytokines such as IL-1β and IL-8. CRP levels correlated significantly with levels of IL-6 during all trimesters, although less than found in a previous study (46). This opposite development of CRP compared to several inflammatory cytokines and the expected immunological milestones in pregnancy demonstrates the need for caution when interpreting serum CRP levels in pregnancy.
Some of the serum cytokines seemed to be linked to pregnancy complications or obesity in the women with PCOS; for IL-6 and CRP this is in line with previous findings of their association with preeclampsia, preterm birth, and GDM in women without PCOS (17, 19, 47). IL-6 and CRP are also reported higher in obese compared to normal-weight pregnant women without PCOS (46). A relation between IP-10 and pregnancy complications is supported by studies associating IP-10 with obesity (48) and preeclampsia (18, 49) in women without PCOS. Eotaxin in pregnancy has scarcely been studied, but has been reported as predictive of hypertensive disorders in pregnancy in women without PCOS (50). Elevated baseline levels of MCP-1 related to pregnancy complications in women with PCOS are supported by a study linking elevated levels of MCP-1 to GDM in non-PCOS women (51). However, our results of altered MCP-1 levels later in pregnancy in women with PCOS independent of GDM may indicate that increased insulin resistance without GDM affects the MCP-1 development in pregnancy (52). Still, more targeted studies on pregnancy complications in pregnant women with PCOS are needed to elucidate the role of serum cytokines in this context.
Both maternal and fetal characteristics had significant effects on the maternal immune status throughout pregnancy in women with PCOS. The elevated levels of numerous multifunctional cytokines in overweight and obese women compared to normal-weight women demonstrate that body weight is an important modulator of the immune system in PCOS. Several of the cytokines most influenced by BMI were also shown to be associated with pregnancy complications in PCOS and therefore seem to reflect a worsening of the PCOS-associated immune profile. High BMI is regarded as a key feature of PCOS, and androgen excess and visceral adipocyte hypertrophy are linked as PCOS symptoms often worsen with increased BMI (53). An increase in single cytokines related to increased BMI both in pregnant and nonpregnant women without PCOS has been previous reported (46, 48, 54, 55), but this is the first report of an extensive list of longitudinally altered cytokines related to BMI in PCOS.
An interesting finding in our study was the influence of fetal sex on the maternal immunological status in early pregnancy, identified as higher serum levels of IL-2, MCP-1, FGF-b, IL-7, and IL-1Ra in pregnancies with male fetuses. Studies of women without PCOS carrying male fetuses have shown increased levels of IL-12, FGF-b, and IL-1β and reduced IL-13 in plasma (56, 57), whereas others have found no such differences (58). Previous research on fetal sex-specific differences in relation to pregnancy complications in women without PCOS show diverging results but may point to an increased risk of preterm birth and GDM with male fetuses (59-62). This may be due to increased susceptibility to maternal inflammation in women carrying male compared to female fetuses (61, 63, 64), which is in line with our results. The sex-specific differences in serum cytokines were less pronounced in women with PCOS without pregnancy complications, supporting that the increased immune activation in pregnancy disorders induces greater cytokine differences between fetal sexes.
Smoking had a distinct influence on the maternal immunological status, marked by decreased levels of several inflammatory cytokines in pregnant smokers with PCOS. This may represent a pregnancy-specific immunologic feature of PCOS, as previous studies on nonpregnant women with PCOS associate smoking with unbeneficial metabolic features such as increased lipids and insulin resistance (65, 66). Our findings in pregnancy are supported by reports of decreased levels of IL-12 in nonpregnant smokers without PCOS (67, 68), while contrasted by a study showing unaffected TNF-α levels in smoking pregnant women (69). It may be speculated that reduced IP-10 in smoking women with PCOS is related to the inverse association of smoking and risk of preeclampsia found by others, as increased IP-10 levels are linked to preeclampsia (18, 49). However, the subgroup of smokers in this study was small and the connections between cytokine patterns and smoking status in women with PCOS warrant analyses of larger study populations.
An important strength of our study is the longitudinal design and the large number of participants with PCOS providing serum samples during all trimesters of pregnancy. Broad serum cytokine profiling of this scale is so far unique in PCOS research. Cytokines act together to modulate immune responses but are often analyzed individually in small sample sizes, causing contradictory results unable to mirror the actual immune status. The use of advanced multivariate statistical methods can mirror the complex dynamics of the immune system more accurately and is another important strength of our study. A possible limitation of the study were lot-to-lot differences in multiplex kits, making comparison of absolute cytokine concentrations between different batches challenging. To account for this, plate and batch effects were adjusted for and study cohort was included in all regression models to make up for baseline differences. Further, comparisons of absolute cytokine levels were performed only on samples analyzed in the same batch.
To conclude, pregnant women with PCOS present a distinct serum cytokine profile throughout pregnancy, characterized by increased cytokine mobilization compared to controls. The broad mobilization of inflammatory and other multifunctional cytokines associated with pregnancy in women with PCOS underlines the complexity of the immunological dynamics in these women. Still, the link between specific cytokines, pregnancy complications, and unfavorable gestational influences such as high BMI or smoking may prove useful for targeted identification of immune deviation and pregnancy complications in women with PCOS. This study forms a unique and important basis for further research on pregnancy and pregnancy complications in women with PCOS.
Acknowledgments
We thank all the participants of the PregMet study, the PregMet2 study, the TRIP study, and the NormalFlow study.
Financial Support: This work was supported by The Research Council of Norway (project No. 213497) and its Center of Excellence funding scheme (project No. 223255; CEMIR), The Liaison Committee for education, research and innovation in Central Norway (project No. 16/29034), The Norwegian Fund for Postgraduate Training in Physiotherapy (project No. 7/370-00/05), the Norwegian University of Science and Technology (project No. 2006/9264-96) and The Joint Research Committee.
Clinical Trial Information: The original studies are registered at http://www.clinicaltrials.gov with the following ClinicalTrials.gov identifier numbers: NCT00159536 (The PregMet study), NCT01587378 (The PregMet2 study) and NCT00476567 (The Training in Pregnancy study).
Glossary
Abbreviations
- BMI
body mass index
- BP
blood pressure
- CRP
C-reactive protein
- FGF-b
basic fibroblastic growth factor
- G-CSF
granulocyte colony-stimulating factor
- GAMM
generalized additive mixed models
- GDM
gestational diabetes mellitus
- GM
granulocyte macrophage
- IL
interleukin
- IP
interferon-γ–induced protein
- LMM
linear mixed models
- LV
latent variable
- MIP
macrophage inflammatory protein
- MCP
monocyte chemotactic protein
- PC
principal component
- PCOS
polycystic ovary syndrome
- PLS-DA
partial least squares-discriminant analyses
- PDGF
platelet-derived growth factor
- Ra
receptor antagonist
- REC
Regional Committee for Medical and Health Research Ethics
- RM-ASCA+
repeated-measures analysis of variance simultaneous component analysis
- TNF
tumor necrosis factor
- TRIP
the Training in Pregnancy study
- ULOD
upper limit of detection
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The data sets generated and/or analyzed during the present study are not publicly available, but can be made available from the corresponding author on reasonable request.
References
- 1. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855. [DOI] [PubMed] [Google Scholar]
- 2. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544-551. [DOI] [PubMed] [Google Scholar]
- 3. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Rotterdam ESHRE/ASRM‐sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47. [DOI] [PubMed] [Google Scholar]
- 5. Rocha AL, Oliveira FR, Azevedo RC, et al. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Res. 2019;8:F1000 Faculty Rev-565. [Google Scholar]
- 6. Escobar-Morreale HF, Luque-Ramírez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95(3):1048-1058.e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng Z, Sun Y, Lv X, Zhang H, Liu C, Dai S. Interleukin-6 levels in women with polycystic ovary syndrome: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0148531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaya C, Pabuccu R, Berker B, Satiroglu H. Plasma interleukin-18 levels are increased in the polycystic ovary syndrome: relationship of carotid intima-media wall thickness and cardiovascular risk factors. Fertil Steril. 2010;93(4):1200-1207. [DOI] [PubMed] [Google Scholar]
- 9. Gao L, Gu Y, Yin X. High serum tumor necrosis factor-alpha levels in women with polycystic ovary syndrome: a meta-analysis. PLoS One. 2016;11(10):e0164021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu W, Qiao J, Yang Y, Wang L, Li R. Elevated C-reactive protein and monocyte chemoattractant protein-1 in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):53-56. [DOI] [PubMed] [Google Scholar]
- 11. Talaat RM, Mohamed YA, Mohamad EH, Elsharkawy M, Guirgis AA. Interleukin 10 (–1082 G/A) and (–819 C/T) gene polymorphisms in Egyptian women with polycystic ovary syndrome (PCOS). Meta Gene. 2016;9:254-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joham AE, Teede HJ, Ranasinha S, Zoungas S, Boyle J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community-based cohort study. J Womens Health (Larchmt). 2015;24(4):299-307. [DOI] [PubMed] [Google Scholar]
- 13. Bahri Khomami M, Joham AE, Boyle JA, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—a systematic review, meta-analysis, and meta-regression. Obes Rev. 2019;20(5):659-674. [DOI] [PubMed] [Google Scholar]
- 14. Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. 2011;204(6):558.e1-. e6. [DOI] [PubMed] [Google Scholar]
- 15. Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2016;95(51):e4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palomba S, Falbo A, Chiossi G, et al. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. J Clin Endocrinol Metab. 2014;99(8):2942-2951. [DOI] [PubMed] [Google Scholar]
- 17. Kalagiri RR, Carder T, Choudhury S, et al. Inflammation in complicated pregnancy and its outcome. Am J Perinatol. 2016;33(14):1337-1356. [DOI] [PubMed] [Google Scholar]
- 18. Szarka A, Rigó J Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lucaroni F, Morciano L, Rizzo G, et al. Biomarkers for predicting spontaneous preterm birth: an umbrella systematic review. J Matern Fetal Neonatal Med. 2018;31(6):726-734. [DOI] [PubMed] [Google Scholar]
- 20. Tangerås LH, Austdal M, Skråstad RB, et al. Distinct first trimester cytokine profiles for gestational hypertension and preeclampsia. Arterioscler Thromb Vasc Biol. 2015;35(11):2478-2485. [DOI] [PubMed] [Google Scholar]
- 21. Stokkeland LMT, Giskeødegård GF, Stridsklev S, et al. Serum cytokine patterns in first half of pregnancy. Cytokine. 2019;119:188-196. [DOI] [PubMed] [Google Scholar]
- 22. Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010;95(12):E448-E455. [DOI] [PubMed] [Google Scholar]
- 23. Løvvik TS, Carlsen SM, Salvesen Ø, et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(4):256-266. [DOI] [PubMed] [Google Scholar]
- 24. Stafne SN, Salvesen KÅ, Romundstad PR, Eggebø TM, Carlsen SM, Mørkved S. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstet Gynecol. 2012;119(1):29-36. [DOI] [PubMed] [Google Scholar]
- 25. Stridsklev S, Salvesen Ø, Salvesen KÅ, Carlsen SM, Husøy MA, Vanky E. Uterine artery Doppler measurements during first and second trimesters of normal pregnancy. Acta Obstet Gynecol Scand. 2017;96(3):366-371. [DOI] [PubMed] [Google Scholar]
- 26. Stokkeland LMT, Giskeodegard GF, Ryssdal M, et al. Supplementary data for “Changes in serum cytokines throughout pregnancy in women with polycystic ovary syndrome.” Figshare. Deposited June 15, 2021. 10.6084/m9.figshare.14775972.v2 [DOI]
- 27. Palarea-Albaladejo J, Martín-Fernández JA. zCompositions—R package for multivariate imputation of left-censored data under a compositional approach. Chemom Intell Lab Syst. 2015;143:85-96. [Google Scholar]
- 28. Wood F. SF. Gamm4: generalized additive mixed models using “Mcgv” and Lme4’. R package version 0.2-6; 2020. Accessed November 9, 2020. https://cran.r-project.org/web/packages/gamm4/index.html
- 29. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. ArXiv Prepr ArXiv14065823. Published online 2014. [Google Scholar]
- 30. National Research Council. Weight gain during pregnancy: reexamining the guidelines. National Academies Press (US); 2010. https://www.ncbi.nlm.nih.gov/books/NBK32813/. [PubMed]
- 31. Madssen TS, Giskeødegård GF, Smilde AK, Westerhuis JA. Repeated measures ASCA+ for analysis of longitudinal intervention studies with multivariate outcome data. medRxiv. Published online January 1, 2020, preprint: not peer reviewed. doi: 10.1101/2020.12.03.20243097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jarmund AH. Andjar/ALASCA. 2021. Accessed March 2, 2021. https://github.com/andjar/ALASCA
- 33. Timmerman ME, Hoefsloot HC, Smilde AK, Ceulemans E. Scaling in ANOVA-simultaneous component analysis. Metabolomics. 2015;11(5):1265-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization. Obesity and overweight. Obesity and overweight. Published January 19, 2021. Accessed January 19, 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 35. Barker M, Rayens W. Partial least squares for discrimination. J Chemom. 2003;17(3):166-173. [Google Scholar]
- 36. MATLAB (R2020a). The MathWorks Inc; 2020. Accessed September 1, 2021. [Google Scholar]
- 37. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistcial Computing. 2021. Accessed June 9, 2021. https://www.R-project.org/ [Google Scholar]
- 38. Wickham H. Ggplot2: elegant graphics for data analysis. Springer Verlag; 2016. Accessed March 21, 2021. https://ggplot2.tidyverse.org
- 39. Olmos-Ortiz A, Flores-Espinosa P, Mancilla-Herrera I, Vega-Sánchez R, Díaz L, Zaga-Clavellina V. Innate immune cells and Toll-like receptor-dependent responses at the maternal-fetal interface. Int J Mol Sci. 2019;20(15):3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S10-S18. [DOI] [PubMed] [Google Scholar]
- 42. Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287(2):390-402. [DOI] [PubMed] [Google Scholar]
- 43. Equils O, Kellogg C, McGregor J, Gravett M, Neal-Perry G, Gabay C. The role of the IL-1 system in pregnancy and the use of IL-1 system markers to identify women at risk for pregnancy complications. Biol Reprod. 2020;103(4):684-694. [DOI] [PubMed] [Google Scholar]
- 44. Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shao Y, Cheng Z, Li X, Chernaya V, Wang H, Yang XF. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction—a novel mechanism for maintaining vascular function. J Hematol Oncol. 2014;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine. 2014;70(2):134-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao X, Liu J, Shen L, Wang A, Wang R. Correlation between inflammatory markers (hs-CRP, TNF-α, IL-1 β, IL-6, IL-18), glucose intolerance, and gestational diabetes mellitus in pregnant women. Int J Clin Exp Med. 2018;11(8):8310-8316. [Google Scholar]
- 48. Hueso L, Ortega R, Selles F, et al. Upregulation of angiostatic chemokines IP-10/CXCL10 and I-TAC/CXCL11 in human obesity and their implication for adipose tissue angiogenesis. Int J Obes (Lond). 2018;42(8):1406-1417. [DOI] [PubMed] [Google Scholar]
- 49. Gotsch F, Romero R, Friel L, et al. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J Matern Fetal Neonatal Med. 2007;20(11):777-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adela R, Borkar RM, Mishra N, et al. Lower serum vitamin D metabolite levels in relation to circulating cytokines/chemokines and metabolic hormones in pregnant women with hypertensive disorders. Front Immunol. 2017;8:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kapustin RV, Chepanov SV, Babakov VN, et al. Maternal serum leptin, adiponectin, resistin and monocyte chemoattractant protein-1 levels in different types of diabetes mellitus. Eur J Obstet Gynecol Reprod Biol. 2020;254:284-291. [DOI] [PubMed] [Google Scholar]
- 52. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38(9):1165-1174. [DOI] [PubMed] [Google Scholar]
- 53. Delitala AP, Capobianco G, Delitala G, Cherchi PL, Dessole S. Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Arch Gynecol Obstet. 2017;296(3):405-419. [DOI] [PubMed] [Google Scholar]
- 54. Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond). 2006;30(9):1347-1355. [DOI] [PubMed] [Google Scholar]
- 55. Friis CM, Frøslie KF, Røislien J, et al. The interleukins IL-6 and IL-1Ra: a mediating role in the associations between BMI and birth weight? J Dev Orig Health Dis. 2010;1(5):310-318. [DOI] [PubMed] [Google Scholar]
- 56. Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. 2015;73(3):251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ramiro-Cortijo D, de la Calle M, Böger R, et al. Male fetal sex is associated with low maternal plasma anti-inflammatory cytokine profile in the first trimester of healthy pregnancies. Cytokine. 2020;136:155290. [DOI] [PubMed] [Google Scholar]
- 58. Mitchell AM, Palettas M, Christian LM. Fetal sex is associated with maternal stimulated cytokine production, but not serum cytokine levels, in human pregnancy. Brain Behav Immun. 2017;60:32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Melamed N, Yogev Y, Glezerman M. Fetal gender and pregnancy outcome. J Matern Fetal Neonatal Med. 2010;23(4):338-344. [DOI] [PubMed] [Google Scholar]
- 60. Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19-30. [DOI] [PubMed] [Google Scholar]
- 61. Al-Qaraghouli M, Fang YMV. Effect of fetal sex on maternal and obstetric outcomes. Front Pediatr. 2017;5:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Broere-Brown ZA, Adank MC, Benschop L, et al. Fetal sex and maternal pregnancy outcomes: a systematic review and meta-analysis. Biol Sex Differ. 2020;11(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155(7):2635-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hunter S, Hoffman M, D’Alessandro A, et al. Male fetus susceptibility to maternal inflammation: C-reactive protein and brain development. Psychol Med. 2021;51(3):450-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Glintborg D, Mumm H, Hougaard DM, Ravn P, Andersen M. Smoking is associated with increased adrenal responsiveness, decreased prolactin levels and a more adverse lipid profile in 650 white patients with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28(3):170-174. [DOI] [PubMed] [Google Scholar]
- 66. Cupisti S, Häberle L, Dittrich R, et al. Smoking is associated with increased free testosterone and fasting insulin levels in women with polycystic ovary syndrome, resulting in aggravated insulin resistance. Fertil Steril. 2010;94(2):673-677. [DOI] [PubMed] [Google Scholar]
- 67. Opstad TB, Arnesen H, Pettersen AÅ, Seljeflot I.. Combined elevated levels of the proinflammatory cytokines IL-18 and IL-12 are associated with clinical events in patients with coronary artery disease: an observational study. Metab Syndr Relat Disord. 2016;14(5):242-248. [DOI] [PubMed] [Google Scholar]
- 68. Sun X, Xiang C, Wu J, et al. Relationship between serum inflammatory cytokines and lifestyle factors in gastric cancer. Mol Clin Oncol. 2019;10(3):401-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hasan S, Alshaikh B, Yusuf K. Serum levels of soluble Fas and Fas ligand in pregnant women who smoke. Am J Reprod Immunol. 2021;85(6):e13382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the present study are not publicly available, but can be made available from the corresponding author on reasonable request.