Abstract
Background
The risk of developing gestational diabetes mellitus (GDM) is higher in women undergoing assisted reproductive treatment than in women conceiving spontaneously.
Objectives
To determine whether the GDM risk after day-3 embryo transfer differs from the GDM risk after day-5 blastocyst transfer.
Methods
Prospective observational study in women becoming pregnant after first fresh embryo or blastocyst transfer.
Results
A total of 1579 women got pregnant and had live birth; 1300 women got day-3 embryo transfer only, whereas 279 women received at least 1 blastocyst. Of 1579 women, 252 developed GDM. Age, body mass index, baseline estradiol, baseline high-density lipoprotein, and progesterone on the day of human chorionic gonadotropin injection were not different in women receiving day-3 embryos only vs women receiving at least 1 blastocyst. The number and quality of retrieved oocytes were not different in women receiving day-3 embryo transfer from those receiving blastocysts. Our study confirmed already established GDM risk factors such as age and body mass index, baseline estradiol, and high-density lipoprotein, as well as progesterone after ovarian stimulation. We furthermore demonstrate that the GDM incidence in women receiving day-5 blastocyst transfer was significantly higher than those who received day-3 embryo transfer (21.15% vs 14.85%; P = 0.009). Considering confounding factors, we likewise saw that blastocyst transfer was an independent procedure-related GDM risk factor [P = 0.009, Exp (B): 1.56, 95% CI: 1.12-2.18].
Conclusion
Blastocyst transfer after in vitro fertilization/intracytoplasmic sperm injection increases the risk of developing GDM.
Keywords: in vitro fertilization, gestational diabetes mellitus, embryo transfer, blastocyst transfer
Gestational diabetes mellitus (GDM) is defined as developing diabetes mellitus just during but not before pregnancy (1). The incidence of GDM varies substantially (1%-14%) between countries where studies have been done (2), presumably due to underlying socioeconomic and genetic differences. The glucose metabolism of most GDM patients usually normalizes after delivery (3). GDM is one of the most common pregnancy complications, which is associated with short- and long-term adverse maternal and fetal outcomes. For mothers, GDM increases the risk of miscarriage, gestational hypertension, preeclampsia, dystocia, cesarean section, infection, polyhydramnios, postpartum hemorrhage, and long-term complications such as a higher risk of developing type 2 diabetes and cardiovascular diseases later in life (4). For offspring of mothers with GDM, there are also several known adverse outcomes such as an increased risk of macrosomia, preterm birth, fetal malformations, respiratory distress syndrome, birth trauma, postnatal hypoglycemia, hyperbilirubinemia, and polycythemia (5). Additionally, babies born from GDM women have an increased risk of developing type 2 diabetes in their later life (6).
Well-known risk factors of GDM include maternal age, prepregnancy obesity, family history of diabetes, history of GDM, smoking, prepregnancy hypertension, and ethnicity (4,7,8). Noteworthy, the risk of developing GDM is substantially higher in women undergoing assisted reproductive technology (ART), compared with women who conceived spontaneously (9). ART was successfully developed in 1978 and has since helped many couples to have children. Normally, the ART procedure begins with controlled ovarian hyperstimulation. Depending on the characteristics of the patient, different protocols are used, such as protocols based on gonadotropin-releasing hormone (GnRH) agonists and GnRH antagonists. If ovarian stimulation is sufficient, human chorionic gonadotropin (hCG), GnRH agonists, or both will be injected to trigger the final maturation of the eggs. The oocytes are then retrieved from the ovary and, depending on sperm quality, undergo in vitro fertilization (IVF) (good sperm quality) or intracytoplasmic sperm injection (ICSI) (reduced sperm count or poor sperm quality). Zygotes are cultivated in vitro until the third day (cleavage stage) or until the fifth day as a blastocyst and 1 or 2 cleavage embryos or blastocysts can be transferred into the women’s uterus 3 or 5 days after IVF. The remaining embryos—if present—are usually subjected to cryopreservation (10).
The risk factors for GDM in women undergoing ART have not been well understood so far. Thus, we performed a prospective observational study to figure out the possible risk factors contributing to GDM in women undergoing fresh embryo transfer (ET).
Materials and Methods
Study Design, and Setting
We performed a prospective observational study in women undergoing ART to become pregnant. Participants were subdivided into 2 groups: (1) day-3 (D3) ET only (n = 1300) and blastocyst transfer (n = 279) and (2) non-GDM (n = 1327) and GDM groups (n = 252).
The current study was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya (approval number: LL-SC-2018-014) and written consent was obtained from all participating patients.
Participants
Young women who came to our hospital to undergo their first IVF/ICSI would be eligible for enrollment. Inclusion criteria were (1) age between 18 and 40 years and willing to participate in the study and (2) women undergoing their first IVF/ICSI treatment. Exclusion criteria were (1) abnormal uterine anatomy (single-horned uterus, double uterus, mediastinum ≥ 0.6 cm), (2) endometriosis, (3) intrauterine adhesion, (4) untreated hydrosalpinx, (5) uterine myoma (multiple, submucous, or intramural myoma > 3 cm), (6) women receiving oocyte donation, (7) preimplantation genetic test for aneuploid, (8) adult-onset adrenogenital syndrome, (9) Cushing syndrome, (10) infertility caused by hypothalamic or pituitary diseases, (11) preexisting diabetes mellitus type 1 or 2, and (12) prepregnancy hypertension.
Participating women received pituitary downregulation protocols exactly as described recently (11,12) (also see study protocol). The reproductive therapy of choice was a transfer of 2 cleavage embryos 3 days after fertilization. If the quality of the embryo was not optimal at that time or other factors were not optimal, blastocyst transfer 5 days after fertilization was applied. In other words, in the optimal cases, 2 D3 embryos were transferred; if only 1 embryo was transferred on D3, an additional blastocyst was transferred at day 5 (D5; if there were transferrable blastocysts), and if cleavage embryos were of poor quality, we took them for further blastocyst culture and transferred just blastocysts—if possible, 2—on D5.
Embryo Quality Evaluation
For D3 cleavage embryos, a numerical score was administrated according to the number of blastomeres [eg, 7 cells (7C)]. Then the embryo was graded as I to IV according to the following criteria: fragmentation, cell symmetry, nucleation, spatial orientation, compaction, and presence of vacuoles (13). Embryos scored 7C-II or above were defined as good-quality cleavage embryos in our current study (12).
For D5 blastocysts, we used a scoring system for human blastocysts established by Gardner and Schoolcraft (14). Blastocysts were initially given a numerical score from 1 to 6 based on their degree of expansion and hatching status. The inner cell mass was graded as A to C according to cell numbers and whether it was tightly packed or loosely grouped. The trophectoderm was also graded as A to C based on the number and size of cells forming a cohesive or loose epithelium (14). Blastocysts scored 4BB or above were defined as good-quality blastocysts in our current study (12).
The standard procedure was the transfer of 2 cleavage embryos. Blastocysts cultured and transferred only if
Slow cleavage embryo development/quality was present (≤6C; see previous discussion).
Women had scarred uterus, uterine mediastinum <0.6 cm, or uterine cavity effusion on D3 after oocyte retrieval.
Women had moderate ovarian hyperstimulation risk and refused to freeze embryos. Hyperstimulation risk was defined as follows: (1) estradiol (E2) level ≥ 4000 pg/mL on hCG day and ≥15 oocytes retrieved, (2) E2 level ≥ 5000 pg/mL on hCG day, or (3) oocytes retrieved ≥ 20).
Cervical incompetence was present.
Women were lightly physically discomfort, such as abdominal distension.
Poor sperm quality was present.
Patients’ preference was expressed.
The leading reason by far for blastocyst culture and transfer was poor D3 embryo development (59.50%) [see Supplementary Table 1 (15)].
Outcome Measurement
The count of live birth events was used to define live birth, while clinical pregnancy was defined as the existence of gestational sac(s) with fetal heart activity by ultrasound at week 4 after ET. Implantation rate was defined as the total number of embryos transferred divided by the number of sacs. Thereafter, miscarriage was defined as intrauterine pregnancy loss after confirmation of gestational sacs (16).
GDM screening was done in all participants by an oral glucose load. A 75-g oral glucose tolerance test (OGTT) was used according to guidelines on about 20 weeks of gestation or later (17,18). Pregnant women kept a normal diet for 3 consecutive days and fasted for at least 8 h before taking an OGTT test. During the examination, 300 mL of water containing 75 g of glucose was taken orally within 5 min, and venous blood of the pregnant woman before and 1 and 2 h (calculated from the start of drinking glucose water) after glucose intake was obtained using a test tube containing sodium fluoride. The glucose oxidase method was used to measure blood glucose levels. Standard diagnostic criteria of 75-g OGTT for GDM diagnosis: before glucose intake and 1 and 2 h after glucose intake were any blood glucose level meeting or exceeding 5.1, 10.0, or 8.5 mmol/L (92, 180, or 153 mg/dL), respectively (17,18).
Follow-up
We called every participant during their pregnancy and after delivery to follow up on pregnancy complications and prenatal outcomes. This follow-up information was obtained in other hospitals and provided by our participants since our center does not include an obstetrics department.
Data Analysis
Statistical Package for Social Sciences for Windows, version 25.0 (SPSS Inc, Chicago, IL, USA) was used to perform data analyses. Homogeneity of variance and normality of data was estimated using the Levene and Kolmogorov-Smirnov tests, respectively. Values were expressed as medians (interquartile ranges), means ± SD, or frequency (%). A comparison of quantitative variables between groups was performed using the Kruskal-Wallis test or t-test according to the normality. Qualitative variables were compared by the Chi-square test or Fisher’s exact test. Multivariate logistic regression analysis was performed to figure out the risk factors of GDM. Data were considered as statistically significant with a 2-sided P < 0.05.
Results
The study enrollment was done between January 2017 and December 2018 in the Reproductive and Genetic Hospital of CITIC-Xiangya (Changsha, China). Details of patient recruitment are shown in the flow chart [Supplementary Figure 1 (15)]. A total of 1579 women became pregnant after receiving IVF/ICSI treatment. Among them, 1300 women received D3 ET while 279 women received at least 1 blastocyst transfer. Blastocyst morphological characteristics were listed in Supplementary Table 2 (15). Out of the 1579 women, 252 developed GDM. The demographic and clinical characteristics of participants at baseline are given in Table 1 and Supplementary Table 3 (15). The overall ovarian hyperstimulation syndrome rate was 10.35% (266 cases out of 2569 study participants). No difference was observed between women who received D3 embryo and blastocyst transfer (Table 1). However, a comparison of women developing GDM with those who do not develop GDM showed that age [30.00 (28.00, 32.00) vs 29.00 (27.00, 31.00), P < 0.001], BMI [22.15 (20.40, 23.63) vs 21.14 (19.57, 22.89), P < 0.001], and waist-to-hip ratio [0.83 (0.79, 0.83) vs 0.81 (0.78, 0.84), P = 0.002] were significantly higher in women with GDM, while baseline E2 concentrations [31.50 (25.00, 41.00) vs 34.00 (27.00, 44.00), P = 0.001] and high-density lipoprotein (HDL) concentrations [1.39 (1.16, 1.63) vs 1.46 (1.23, 1.72), P = 0.003] were lower in these women [Supplementary Table 3 (15)].
Table 1.
Demographic and baseline clinical characteristics of women transferring day-3 embryos and at least 1 blastocyst
| All (n = 1579) | D3 embryo transfer only (n = 1300) | At least 1 blastocyst transfered (n = 279) | P-value | |
|---|---|---|---|---|
| Age (years) | 29.00 (27.00, 31.00) | 29.00 (27.00, 31.00) | 29.00 (27.00, 31.00) | 0.70 |
| Menstrual cycle (days) | 30.00 (28.00, 30.00) | 30.00 (28.00, 30.00) | 30.00 (28.00, 30.00) | 0.54 |
| Infertility type | ||||
| Primary | 883 (56) | 719 (55) | 164 (59) | 0.29 |
| Secondary | 696 (44) | 581 (45) | 115 (41) | |
| Ethnic background | ||||
| Han Chinese | 1296 (82) | 1061 (82) | 235 (84) | 0.30 |
| Other | 283 (18) | 239 (18) | 44 (16) | |
| BMI (kg/m2) | 21.33 (19.63, 23.05) | 21.33 (19.72, 23.03) | 21.34 (19.48, 23.05) | 0.83 |
| Waist-to-hip ratio | 0.81 (0.78, 0.85) | 0.82 (0.78, 0.85) | 0.81 (0.78, 0.84) | 0.06 |
| Infertility duration (years) | 3.00 (2.00, 5.00) | 3.00 (2.00, 5.00) | 3.00 (2.00, 5.00) | 0.87 |
| Previous birtha | 46 (733/1579) | 46 (604/1300) | 46 (129/279) | 0.95 |
| Previous conceptiona | 46 (733/1579) | 46 (604/1300) | 46 (129/279) | 0.95 |
| AMH (ng/mL) | 5.69 (3.68, 9.18) | 5.62 (3.64, 9.09) | 5.96 (3.77, 9.37) | 0.45 |
| AFC | 23.00 (16.00, 30.00) | 23.00 (16.00, 30.00) | 23.00 (16.00, 30.00) | 0.99 |
| FSH (mIU/mL) | 5.65 (4.82, 6.57) | 5.65 (4.82, 6.56) | 5.61 (4.81, 6.61) | 0.86 |
| LH (mIU/mL) | 3.67 (2.67, 5.02) | 3.68 (2.66, 5.07) | 3.60 (2.70, 4.84) | 0.55 |
| E2 (pg/mL) | 34.00 (27.00, 44.00) | 34.00 (27.00, 44.00) | 33.00 (26.00, 42.00) | 0.15 |
| Progesterone (ng/mL) | 0.24 (0.18, 0.33) | 0.24 (0.17, 0.33) | 0.24 (0.18, 0.34) | 0.19 |
| Testosterone(ng/mL) | 0.28 (0.23, 0.36) | 0.28 (0.23, 0.36) | 0.29 (0.24, 0.37) | 0.13 |
| Total 25(OH)D (ng/mL) | 19.61 (16.61, 22.90) | 19.66 (16.61, 22.89) | 19.22 (16.58, 23.06) | 0.89 |
| Free 25(OH)D (pg/mL) | 4.72 (4.11, 5.34) | 4.72 (4.12, 5.32) | 4.76 (4.09, 5.36) | 0.80 |
| Systolic blood pressure (mm Hg) | 115.00 (108.00, 122.00) | 115.00 (109.00, 122.00) | 115.00 (108.00, 121.00) | 0.34 |
| Diastolic blood pressure (mm Hg) | 76.00 (70.00, 81.00) | 76.00 (70.00, 82.00) | 75.00 (69.00, 80.00) | 0.02 |
| Blood glucose (mmol/L) | 5.15 (4.93, 5.43) | 5.16 (4.94, 5.43) | 5.14 (4.88, 5.42) | 0.27 |
| Triglyceride (mmol/L) | 0.99 (0.72, 1.42) | 1.00 (0.72, 1.42) | 0.98 (0.71, 1.50) | 0.72 |
| Cholesterol (mmol/L) | 4.26 (3.81, 4.81) | 4.26 (3.80, 4.80) | 4.27 (3.87, 4.87) | 0.80 |
| LDL (mmol/L) | 2.72 (2.29, 3.20) | 2.72 (2.29, 3.22) | 2.69 (2.32, 3.18) | 0.76 |
| HDL (mmol/L) | 1.45 (1.22, 1.70) | 1.45 (1.22, 1.70) | 1.48 (1.24, 1.74) | 0.17 |
| Uric acid (μmol/L) | 282.00 (245.00, 327.00) | 282.00 (245.00, 328.00) | 283.00 (247.00, 327.00) | 0.92 |
Data are given as median (IQR) or n (%) unless otherwise noted. Three women got D3 embryo and D5 blastocyst transfer. 276 women got D5 blastocyst only. These cases were analyzed together as the “at least 1 blastocyst transferred” group (n = 279).
Abbreviations: AFC, antral follicle count; AMH, anti-Müllerian hormone; BMI, body mass index; D3, day 3; D5, day 5; E2, estradiol; FSH, follicle-stimulating hormone; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; LH, luteinizing hormone.
a Data are given as % (n/N).
Patients’ characteristics during the controlled ovarian hyperstimulation and laboratory outcomes are presented in Table 2 and Supplementary Table 4 (15). Sex hormone concentrations, oocyte quality, and fertilization rate were not different between women who received D3 embryos vs women receiving at least 1 blastocyst transfer. Women who received D3 ET received more D3 transferable embryos [6.00 (4.00, 8.00) vs 5.00 (3.00, 8.00), P < 0.001], more embryos transferred (1.98 ± 0.12 vs 1.58 ± 0.50, P < 0.001), and even good-quality embryo transferred (1.85 ± 0.47 vs 0.32 ± 0.48, P < 0.001) (Table 2). Women later developing GDM had higher progesterone concentrations on hCG day [0.61 (0.45, 0.84) vs 0.57 (0.43, 0.78), P = 0.05], while women without GDM received more D3 embryos transferred (D3 ET 85.15% vs blastocyst transfer 76.59%, P = 0.009) and women developed GDM received more blastocyst transferred (D3 ET 14.85% vs blastocyst transfer 23.41%, P = 0.009) [Supplementary Table S4) (15)].
Table 2.
Controlled ovarian hyperstimulation characteristics and laboratory outcomes in women receiving embryos vs women receiving at least 1 blastocyst
| All (n = 1579) | D3 embryo transfer only (n = 1300) | At least 1 blastocyst transfered (n = 279) | P-value | |
|---|---|---|---|---|
| Protocol | ||||
| Long protocol | 1200 (76) | 982 (76) | 218 (78) | 0.36 |
| Ultra-long protocol | 379 (34) | 318 (24) | 61 (22) | |
| E2 level on hCG day (pg/mL)a | 3568 (2581, 4529) | 3576 (2615.25, 4529) | 3557.00 (2408, 4596) | 0.28 |
| Progesterone level on hCG day (ng/mL)a | 0.58 (0.43, 0.78) | 0.58 (0.43, 0.78) | 0.58 (0.44, 0.78) | 0.84 |
| LH level on hCG day (mIU/mL)a | 1.55 (1.20, 1.99) | 1.57 (1.22, 1.99) | 1.52 (1.15, 1.99) | 0.43 |
| EM thickness before ET (mm) | 13.30 (12.00, 14.70) | 13.30 (11.90, 14.70) | 13.40 (12.10, 14.60) | 0.87 |
| Gn dosage (IU) | 2100 (1538, 2850) | 2100 (1541, 2850) | 2100 (1538, 2888) | 0.89 |
| Gn duration (days) | 11 (10, 12) | 11 (10, 12) | 11 (10, 12) | 0.92 |
| The dosage of hCG for triggering (IU)a | 6500 (5000, 6500) | 6500 (5000, 6500) | 6500 (5000, 6500) | 0.92 |
| Oocytes retrievedb | 12 (9, 16) | 12 (9, 15) | 12 (9, 16) | 0.25 |
| Degenerated | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.69 |
| GV | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | 0.11 |
| MI | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0.73 |
| MII | 11 (8, 14) | 11 (8, 14) | 10 (7, 13) | 0.08 |
| Fertilization methods | ||||
| IVF | 1082 (69) | 895 (69) | 187 (67) | 0.28 |
| ICSI | 261 (17) | 219 (17) | 42 (15) | |
| IVF + ICSI | 236 (15) | 186 (14) | 50 (18) | |
| 2PN zygote | 7 (5, 9) | 7.00 (5, 9) | 6 (4, 9) | 0.10 |
| Fertilization rate | 67 (53, 80) | 67 (54, 80) | 67 (50, 83) | 0.96 |
| Day 3 transferrable embryo | 6 (3, 8) | 6 (4, 8) | 5 (3, 8) | <0.001 |
| Day 3 embryo quality | ||||
| Good quality | 3 (2, 6) | 4 (2, 6) | 4 (2, 6) | 0.93 |
| Fair | 3 (1, 5) | 1 (0, 3) | 1 (0, 2) | 0.06 |
| Embryos or blastocytes transferred | 1.91 ± 0.28 | 1.98 ± 0.12 | 1.58 ± 0.50 | <0.001 |
| Good-quality embryos transferred | 1.58 ± 0.75 | 1.85 ± 0.47 | 0.32 ± 0.48 | <0.001 |
Data are given as median (interquartile range), mean ± SD, or n (%). Three women got D3 embryo and D5 blastocyst transfer; 276 women got D5 blastocyst only. These cases were analyzed together as the “at least 1 blastocyst transferred” group (n = 279). Good-quality embryo, D3 embryo ≥7C-II, blastocyst ≥4BB; fair-quality embryo, D3 embryo <7C-II, blastocyst <4BB.
Abbreviations: 2PN, pronucleus; D3, day 3; E2, estradiol; EM, endometrium; ET, embryo transfer; Gn, gonadotropin; GV, germinal vesicle; hCG, human chorionic gonadotropin; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; LH, luteinizing hormone; MI, metaphase I; MII, metaphase II.
a The last day of ovarian stimulation, hCG was injected to trigger the final maturation of oocytes.
b Reflects oocytes quality; only MII oocyte can be fertilized.
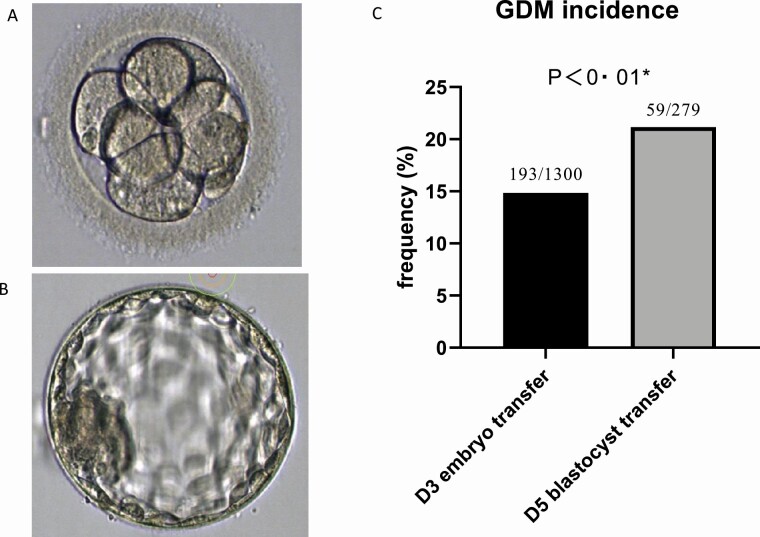
Table 3 and Supplementary Table 5 (15) show the results of pregnancy complications and prenatal outcomes. Although more cases of twin pregnancy (D3 ET 36.63% vs blastocyst transfer 25.72%, P = 0.001), preterm birth (D3 ET18.86% vs blastocyst transfer11.59%, P = 0.004), and low birth weight (D3 ET 27.27% vs blastocyst transfer 17.58%, P < 0.001) were observed in women who received only ET, the incidence of GDM was significantly higher in women who received blastocyst transfer (D3 ET 14.85% vs blastocyst transfer 21.15%, P = 0.009) (Fig. 1 and Table 3).
Table 3.
Pregnancy outcomes and prenatal outcomes of embryo transfer only and blastocyst transfer women
| All (n = 1579) | D3 embryo transfer only (n = 1300) | At least one blastocyst transfered (n = 279) | P-value | |
|---|---|---|---|---|
| Gestational sacs | 1.40 ± 0.52 | 1.43 ± 0.52 | 1.29 ± 0.48 | <0.001 |
| Live birth rate | 99 (1559/1579) | 99 (1283/1300) | 99 (276/279) | 0.40 |
| Singleton | 64 (1018/1579) | 63 (813/1283) | 74 (205/276) | 0.001 |
| Twins | 34 (541/1579) | 37 (470/1283) | 26 (71/276) | |
| Boys | 52 (1097/2100) | 52 (913/1753) | 53 (184/347) | 0.75 |
| Girls | 48 (1003/2100) | 48 (840/1753) | 47 (163/347) | |
| Birthweight (all live birth) | ||||
| <2500 g | 26 (539/2100) | 27 (478/1753) | 18 (61/347) | <0.001 |
| 2500-4000 g | 71 (1506/2100) | 70 (1233/1753) | 79(273/347) | |
| ≥4000 g | 3 (55/2100) | 2 (42/1753) | 4 (13/347) | |
| Birthweight (full-term live birth) | ||||
| <2500 g | 13 (218/1619) | 14 (188/1324) | 10 (30/295) | 0.12 |
| 2500-4000 g | 83 (1346/1619) | 83 (1094/1324) | 85 (252/295) | |
| ≥4000 g | 3 (55/1619) | 3 (42/1324) | 4 (13/295) | |
| Gestation weeks | ||||
| <37 weeks | 178(274/1559) | 19 (242/1283) | 12 (32/276) | 0.004 |
| ≥37 weeks | 82 (1285/1559) | 81 (1041/1283) | 88 (244/276) | |
| Delivery methods | ||||
| Natural labor | 28 (439/1559) | 27 (349/1283) | 33 (90/276) | 0.07 |
| Cesarean section | 72 (1120/1559) | 73 (934/1283) | 67 (186/276) | |
| Prenatal complications | ||||
| GDM | 16 (252/1579) | 15 (193/1300) | 21 (59/279) | 0.009 |
| Anemia | 13 (203/1579) | 13 (164/1300) | 14 (39/279) | 0.54 |
| Edema | 15 (233/1579) | 15 (194/1300) | 14 (39/279) | 0.69 |
| Polyhydramnios | 0 (3/1579) | 0 (2/1300) | 0 (1/279) | 0.44 |
| Oligohydramnios | 1 (17/1579) | 1 (13/1300) | 1 (4/279) | 0.52 |
| Placenta previa | 1 (13/1579) | 1 (11/1300) | 1 (2/279) | >0.99 |
| Placenta abruption | 0 (3/1579) | 0 (3/1300) | 0 | >0.99 |
| Intrauterine hypoxia | 1 (8/1579) | 1 (7/1300) | 0 (1/279) | >0.99 |
Data were given as median (interquartile range) or n (%). The number of embryo transferred were presented as mean ± SD. Three women got D3 embryo and D5 blastocyst transfer. 276 women got D5 blastocyst only. These cases were analyzed together as the “at least 1 blastocyst transferred” group (n = 279).
Figure 1.
(A) typical human day-3 embryo. (B) Typical human day-5 blastocyst. (C) Bar graphs showing a comparison of gestational diabetes mellitus (GDM) incidence in women receiving just embryos or women receiving at least one blastocyst. *Groups were compared by chi-square test. The morphology and size of the day-3 embryo and the day-5 blastocyst differed substantially indicating a fast development and differentiation during these 2 days of early human life. Blastocyst transfer is an independent GDM risk factor. Yet unknown factors that influence embryo/blastocyst development are potentially also factors that increase the GDM risk later during pregnancy. The blastocyst transferred at day 5 after fertilization might differ substantially from normal blastocysts after natural conception and may later release or stimulate the maternal release of transmitters/hormones promoting GDM development. Abbreviations: D3, day 3; D5, day 5.
Next, multivariate logistic regression analysis was performed to investigate whether this finding was independent of confounding factors. We first included all factors that were different between groups in women with and without GDM [age, BMI, waist-to-hip ratio, HDL, baseline E2, progesterone concentration on hCG day, D3 embryos/blastocysts transferred; for details, see Supplementary Table S3 and S4) (15)]. This analysis revealed that age [P < 0.001, Exp (B): 1.12, 95% CI: 1.07-1.16], BMI [P = 0.001, Exp (B): 1.11, 95% CI: 1.04-1.17], progesterone concentration on hCG day [P = 0.02, Exp (B): 1.74, 95% CI: 1.09-2.78], and blastocyst transfer [P = 0.009, Exp (B): 1.56, 95% CI: 1.12-2.18] were positively associated with GDM risk, while baseline E2 [P = 0.02, Exp (B): 0.99, 95% CI: 0.98-1.00] and HDL concentrations [P = 0.02, Exp (B): 0.60, 95% CI: 0.40-0.91] were negatively associated with GDM risk (Table 4).
Table 4.
Multivariate regression analysis of confounding factors according to present analysis (forward elimination model)
| B | P | Exp(B) | 95% CI of Exp(B) | ||
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Age | 0.11 | <0.001 | 1.12 | 1.07 | 1.16 |
| BMI | 0.10 | 0.001 | 1.11 | 1.04 | 1.17 |
| HDL | −0.51 | 0.02 | 0.60 | 0.40 | 0.91 |
| Baseline E2 | −0.01 | 0.02 | 0.99 | 0.98 | 1.00 |
| Progesterone on hCG day | 0.55 | 0.02 | 1.74 | 1.09 | 2.78 |
| Blastocyst transferred (yes or no) | 0.45 | 0.009 | 1.56 | 1.12 | 2.18 |
| Constant | −6.43 | <0.001 | 0.002 |
We added factors that were significantly different in the univariate analysis of baseline characteristics and characteristics after stimulation: age, BMI, waist-to-hip ratio, HDL, baseline E2, progesterone level on hCG day, and whether blastocysts transferred into this kick-out model. The waist-to-hip ratio was ruled out from the final regression model.
Abbreviations: BMI: body mass index; E2: estradiol; hCG: human chorionic gonadotropin; HDL: high-density lipoprotein.
We next built a logistic regression model by forwarding elimination based on some potential ART-related GDM risk factors: age, BMI, fasting glucose, ethnic background, infertility type (primary vs secondary), polycystic ovarian syndrome (yes/no), ovarian stimulation protocol, gonadotropin dosage, endometrium thickness, E2 on hCG day, progesterone on hCG day, luteinizing hormone on hCG day, and IVF/ICSI procedure. These factors were included based on the current literature (7,9,17). We also tested blastocyst transfer (yes/no). Again, blastocyst transfer was identified as an independent procedure-related GDM risk factor [P = 0.01, Exp (B): 1.54, 95% CI: 1.11-2.15]. Also, age [P < 0.001, Exp (B): 1.12, 95% CI: 1.07-1.16], BMI [P < 0.001, Exp (B): 1.12, 95% CI: 1.06-1.19], and progesterone concentrations on hCG day [P = 0.03, Exp (B): 1.67, 95% CI: 1.04-2.66] were identified as GDM risk factors [Supplementary Table 6 (15)].
We also analyzed the rate of GDM in those patients who did not get pregnant after the first fresh ET but had enough embryos to freeze them for later transfer of frozen embryos. Overall the number of these cases was low, but we saw a similar picture. Numerically, the GDM rate was higher in those women with frozen embryo transfer getting at least 1 D5 ET (12.95%) vs 7.41% in those women getting D3 ET. Due to the small sample size, however, the difference was statically not significantly different.
Discussion
The present study showed that well-known risk factors (age and BMI) for GDM in the general population are also risk factors for GDM in women undergoing fresh ET after IVF/ICSI. The study identified in addition ART-specific risk factors such as baseline E2, baseline HDL, and progesterone on the day of hCG injection, confirming earlier studies on this topic. We analyzed for the first time the potential impact of blastocyst transfer as a GDM risk factor and showed that blastocyst transfer is independent of other confounding factors associated with the risk of developing GDM in women undergoing first fresh embryo or blastocyst transfer.
It is well known that maternal age and prepregnancy BMI were associated with GDM in women who became pregnant naturally or in women who received ART (1,5–8). Carolan et al analyzed the GDM rate in women of different age groups and showed that there was an age-related trend of GDM regardless of ethnicity (19). A meta-analysis of 70 studies involving 671 945 women showed that the risk of GDM was positively associated with the BMI before pregnancy (20). Our study also showed that HDL concentrations are inversely associated with GDM risk, as likewise described by another study (21). Numerous studies have shown that the GDM risk after IVF/ICSI treatment is higher than the GDM risk in women after natural conception (7,9,22). Women having higher progesterone concentrations on hCG day do have an increased GDM risk. This fits very well with a preclinical study showing that progesterone increases insulin resistance by reducing the expression of glucose transporter 4, thus contributing to GDM (23).
In our study, we investigated ART-related risk factors in women who received their first fresh ET and became successfully pregnant after ART. We showed that the blastocyst transfer was a significant GDM risk factor. This statement is based on independent analyses [Fig. 1, Table 4; also see Supplementary Table 6 (15)]. The transfer approach of choice was the transfer of 2 D3 embryos. If this was not possible or blastocyst transfer was chosen by the mother, blastocysts were transferred [Supplementary Table 2 provides the reasons for blastocyst transfer (15)].
For analysis of an association with GDM, we included all baseline parameters that were different between the non-GDM and GDM groups into a regression model and could show that blastocyst transfer was positively associated with GDM risk. We then built another model that included all potential ART-related GDM risk factors based on published studies and got the same results. The main reason for blastocyst transfer was a delayed development of the embryo on D3 after fertilization [Supplementary Table 1 (15)]. This would fit very well with a recent study from Israel. Doron-Lalehzari et al performed a small pilot study in 85 patients undergoing IVF. They showed that the time interval from the fading of the pronuclei to the beginning of blastocyst formation was positively associated with GDM risk (24). Our findings might be likewise explained by the following hypotheses: the blastocysts transferred at D5 after fertilization differ substantially—due to differences regarding the conditions in the culture media vs natural environment in the human uterus—from normal blastocysts after natural conception and thus later may itself release or stimulate the release of transmitters/hormones promoting GDM development. We favor this hypothesis because blastocyst transfer increased the risk of GDM regardless of the underlying reason for the later transfer timing. Age, BMI, baseline E2, baseline HDL, and oocyte number and quality as well as progesterone on the day of hCG injection were not different in women receiving D3 ET only vs women receiving at least 1 blastocyst. This again indicates that the observed differences of blastocyst transfer vs ET concerning the GDM risk are due to blastocyst-related factors as previously discussed but not to the women or their number and quality of the oocytes.
Our study also revealed that baseline E2 concentrations were lower in women developing GDM later in pregnancy, and it was negatively associated with GDM risk, which is in agreement with Shiqiao et al (25). E2 is secreted by granulosa cells and could reflect the quality of oocytes. Shiqiao et al reported an inverse relationship between baseline E2 concentrations and GDM risk. E2-related quality of the oocyte and probably later blastocyst quality might be 2 independent factors influencing maternal glucose metabolism during pregnancy (25).
The ovarian hyperstimulation syndrome (OHSS) rate was high in our study. However, most of the cases (>75%) were mild and moderate. The OHSS rate reported in our study was within the range reported also by other well-established IVF centers (26). In addition, it needs to be considered that the criteria for OHSS in our center were even more strict than suggested in the guideline of Practice Committee of the American Society for Reproductive Medicine (27). We additionally considered the decreased albumin and elevated D-dimer levels as a diagnosis criteria. These stricter criteria in our center contribute to the overall higher OHSS rate in our study. In cases with high E2 concentrations such as 4000 to 5000 pg/mL or even higher after ovarian stimulation, the hCG trigger is usually substituted with GnRH agonists and/or freeze all policy. We suggested this to women at potential risk for OHSS and suggested the freeze-all strategy as our favorite option nowadays for these cases, but some patients sometimes refused and insisted on the classical ET protocol and signed related written consent.
To the best of our knowledge, our study showed for the first time that blastocyst transfer is a risk factor for GDM in women undergoing fresh first embryo/blastocyte transfer. Although the sample size in our study was considerably large and we analyzed different models to adjust the confounding factors, limitations existed. First, some potential risk factors such as a family history of diabetes and a history of GDM were not included in our data set. Moreover, participants in our study only received fresh ET; whether the results could apply to frozen ET is unclear yet and needs further investigation. Finally, our study is a prospective observational study. Thus, yet unknown confounding factors could influence the primary study outcome GDM. A randomized prospective controlled clinical trial would be the appropriate method to control for unknown confounding factors. Our study provides the rationale to initiate such a randomized controlled trial that is needed to finally prove or disprove our hypothesis that blastocyst transfer is an independent GDM risk factor. However, our study is the first observational study indicating that blastocyte transfer is an independent GDM risk factor. Given our data, a study with a random embryo or blastocyte transfer procedures would be justified and will control yet unknown confounding factors.
In conclusion, our study clearly showed that blastocyst transfer was positively associated with GDM in women undergoing first fresh ET after IVF/ICSI.
Author Contributions
Conceptualization: B.H. Data curation: H.C., J.L., S.C, S.T., S.Z., and C.C. Formal analysis: H.C. and B.H. Methodology: H.C. and B.H. Project administration: S.C. and S.T. Software: H.C. Supervision: L.H., G.L., F.G., and B.H. Validation: H.C., S.T., and B.H. Writing (original draft): H.C., C-F.H., and B.H. Writing (review and editing): H.C., J.L., B.K.K., B.R., L.H., and G.L. B.H. is the guarantor of this study and, as such, takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Financial Support: None.
Additional Information
Disclosures: The authors declare that there were no conflicts of interest in this study.
Data Availability
The data sets are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. Br J Nutr. 2010;104(6):775-787. [DOI] [PubMed] [Google Scholar]
- 2. Soheilykhah S, Mojibian M, Rahimi SS, Rashidi M, Soheylikhah S, Pirouz M. Incidence of gestational diabetes mellitus in pregnant women. Int J Reprod BioMed 2010;8:24. [Google Scholar]
- 3. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773-1779. [DOI] [PubMed] [Google Scholar]
- 4. Yang H, Wei Y, Gao X, et al. ; China National GDM Survey Working Group . Risk factors for gestational diabetes mellitus in Chinese women: a prospective study of 16 286 pregnant women in China. Diabet Med. 2009;26(11):1099-1104. [DOI] [PubMed] [Google Scholar]
- 5. Metzger BE, Contreras M, Sacks D, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. [DOI] [PubMed] [Google Scholar]
- 6. Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohammadi M, Morasae EK, Maroufizadeh S, et al. Assisted reproductive technology and the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Middle East Fertil Soc J. 2020;25(1):6. [Google Scholar]
- 8. Kiani F, Naz MSG, Sayehmiri F, Sayehmiri K, Zali H. The risk factors of gestational diabetes mellitus: a systematic review and meta-analysis study. Diabetes. 2017;10:17. [Google Scholar]
- 9. Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur J Obstet Gynecol Reprod Biol. 2014;176:149-152. [DOI] [PubMed] [Google Scholar]
- 10. Niederberger C, Pellicer A, Cohen J, et al. Forty years of IVF. Fertil Steril. 2018;110(2):185-324.e5. [DOI] [PubMed] [Google Scholar]
- 11. Chen H-J, Li Y, Li X-F, Lin G, Lu G-X, Gong F. A modified ultra-long downregulation protocol improves pregnancy outcomes in high body mass index patients undergoing in vitro fertilization/intracytoplasmic sperm injection treatment. Rep Dev Med. 2020;4(3):156. [Google Scholar]
- 12. Cai S, Li J, Zeng S, et al. Impact of vitamin D on human embryo implantation-a prospective cohort study in women undergoing fresh embryo transfer. Fertil Steril. 2021;115(3):655-664. [DOI] [PubMed] [Google Scholar]
- 13. Prados FJ, Debrock S, Lemmen JG, Agerholm I. The cleavage stage embryo. Hum Reprod. 2012;27(suppl 1):i50-i71. [DOI] [PubMed] [Google Scholar]
- 14. Gardner DK. In-vitro culture of human blastocyst. In: Jansen R and Mortimer D, eds. Toward Reproductive Certainty: Infertility and Genetics Beyond 1999. Parthenon Press; 1999:378-388.
- 15. Hocher B, Gong F.Supplementary data for: Blastocyst transfer: a risk factor for gestational diabetes mellitus in women undergoing in vitro fertilization. Figshare. Posted June 18,2021. doi: 10.6084/m9.figshare.14812062.v3 [DOI]
- 16. Wilkinson J, Roberts SA, Showell M, Brison DR, Vail A. No common denominator: a review of outcome measures in IVF RCTs. Hum Reprod. 2016;31(12):2714-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. International Association of Diabetes Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang H, Xu X, Wang Z, et al. Guidelines for the diagnosis and treatment of gestational diabetes mellitus (2014). Diabetes World. 2014;11:489-498. [Google Scholar]
- 19. Carolan M, Davey MA, Biro MA, Kealy M. Maternal age, ethnicity and gestational diabetes mellitus. Midwifery. 2012;28(6):778-783. [DOI] [PubMed] [Google Scholar]
- 20. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203. [DOI] [PubMed] [Google Scholar]
- 21. Jin WY, Lin SL, Hou RL, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. 2016;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bosdou JK, Anagnostis P, Goulis DG, et al. Risk of gestational diabetes mellitus in women achieving singleton pregnancy spontaneously or after ART: a systematic review and meta-analysis. Hum Reprod Update. 2020;26(4):514-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brănişteanu DD, Mathieu C. Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab. 2003;14(2):54-56. [DOI] [PubMed] [Google Scholar]
- 24. Doron-Lalehzari A, Wainstock T, Szaingurten-Solodkin I, et al. Are morphokinetic parameters of embryo development associated with adverse perinatal outcomes following fresh blastocyst transfer? Reprod Biomed Online. 2021;42(1):207-216. [DOI] [PubMed] [Google Scholar]
- 25. Shiqiao H, Bei X, Yini Z, Lei J. Risk factors of gestational diabetes mellitus during assisted reproductive technology procedures. Gynecol Endocrinol. 2020;36(4):318-321. [DOI] [PubMed] [Google Scholar]
- 26. Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8(6):559-577. [DOI] [PubMed] [Google Scholar]
- 27. Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril 2016;106(7):1634-1647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets are not publicly available but are available from the corresponding author on reasonable request.