Abstract
Context
Testicular adrenal rest tumors (TART) are a common complication in males with classic 21-hydroxylase deficiency (21OHD). TART are likely to contribute to the androgen excess in 21OHD patients, but a direct quantification of steroidogenesis from these tumors has not been yet done.
Objective
We aimed to define the production of 11-oxygenated 19-carbon (11oxC19) steroids by TART.
Methods
Using liquid chromatography-tandem mass spectrometry, steroids were measured in left (n = 7) and right (n = 4) spermatic vein and simultaneously drawn peripheral blood (n = 7) samples from 7 men with 21OHD and TART. For comparison, we also measured the peripheral steroid concentrations in 5 adrenalectomized patients and 12 age- and BMI-matched controls. Additionally, steroids were quantified in TART cell– and adrenal cell–conditioned medium, with and without adrenocorticotropic hormone (ACTH) stimulation.
Results
Compared with peripheral blood from 21OHD patients with TART, the spermatic vein samples displayed the highest gradient for 11β-hydroxytestosterone (11OHT; 96-fold) of the 11oxC19 steroids, followed by 11-ketotestosterone (47-fold) and 11β-hydroxyandrostenedione (11OHA4; 29-fold), suggesting production of these steroids in TART. TART cells produced higher levels of testosterone and lower levels of A4 and 11OHA4 after ACTH stimulation compared with adrenal cells, indicating ACTH-induced production of testosterone in TART.
Conclusion
In patients with 21OHD, TART produce 11oxC19 steroids, but in different proportions than the adrenals. The very high ratio of 11OHT in spermatic vs peripheral vein blood suggests the 11-hydroxylation of testosterone by TART, and the in vitro results indicate that this metabolism is ACTH-sensitive.
Keywords: CAH, TART, adrenal, testis, steroidogenesis, spermatic vein
Classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase (CYP21A2) deficiency (21OHD) is an autosomal recessive disorder affecting adrenal steroid biosynthesis and leading to impaired glucocorticoid production. Consequently, the pituitary adrenocorticotropin hormone (ACTH) production is increased. Precursor steroids upstream of the enzymatic defect, including progesterone and 17-hydroxyprogesterone (17OHP), accumulate (1-3) and shunt into the nonaffected adrenal androgen pathway, resulting in elevated adrenal androgen production. Clinical manifestations of adrenal androgen excess include virilization, hirsutism, irregular menses, and subfertility in girls and women (4), as well as precocious pubarche or puberty and advanced bone age in both sexes (5, 6). Both 17OHP and androstenedione (A4) serve as biomarkers for androgen excess in patients with 21OHD (7). The 11-oxygenated 19-carbon (11oxC19) steroids 11β-hydroxyandrostenedione (11OHA4), 11β-hydroxytestosterone (11OHT), 11-ketoandrostenedione (11KA4), and 11-ketotestosterone (11KT) are the main contributors to androgen excess in children and adults with 21OHD (8, 9) and might serve as better biomarkers for androgen excess in patients with 21OHD (9, 10), because—unlike 17OHP and A4—these steroids are solely produced via adrenal-specific 11β-hydroxylase (CYP11B1), and 11OHT and 11KT have androgenic activity (9).
Testicular adrenal rest tumors (TART) are a common complication in males with 21OHD. TART express adrenal-specific steroidogenic enzymes (CYP11B1; CYP11B2) and receptors (MC2R) (11, 12) and demonstrate adrenocortical enzyme (3β-hydroxysteroid dehydrogenase (3β-HSD) and CYP11B1) activity (13). However, little is known about the specific contribution of TART to the androgen excess in males with 21OHD. Patients with 21OHD and TART have higher levels of circulating 11oxC19 androgens compared to patients without TART (14). Furthermore, higher or similar levels of the steroid hormones 21-deoxycortisol (21dF), 11OHA4, 17OHP, progesterone, and A4 have been detected in spermatic vein samples compared with peripheral vein samples or adrenal vein samples in 21OHD patients, which indicates adrenal-specific steroid production by TART within the testes (11, 15, 16). Moreover, we reported ACTH-stimulated production of 21dF, 17OHP, progesterone, and 11-hydroxyprogesterone (11OHP) from in vitro cultured TART cells (3).
We previously reported increased levels of 21dF, 17OHP, and A4 in spermatic vein blood compared with peripheral blood samples in 7 men with 21OHD and TART (11). The goal of this study is to provide a more comprehensive steroidogenic profile of TART by measuring 11-oxygenated steroids in the spermatic vein and peripheral blood samples of males with 21OHD. In addition, we characterized 19-carbon steroids, including 11oxC19 steroids, in media of TART cells cultured in vitro (3). Knowledge on the contribution of TART to the production of 11oxC19 steroids as well as to other steroids is important to the understanding of the steroidogenic properties of TART in 21OHD patients.
Methods
Patients and Controls
Spermatic vein- and peripheral vein blood samples were previously obtained from 7 men with 21OHD (mean age 31 years; range: 23-51) who underwent TART-removing surgery, as described earlier (11) (Table 1). All patients had high-stage TART, containing strands of fibrous tissue (17). Diagnosis of CAH due to 21OHD was confirmed by mutation analysis in all patients. The mean BMI was 28.2 kg/m2 and ranged from 23.7 to 38.2 kg/m2. Six patients had salt-wasting 21OHD and 1 patient had simple virilizing 21OHD. Luteinizing hormone (LH), follicle-stimulating hormone (FSH), 17OHP, A4, and ACTH levels were previously measured (11) 3 days prior to surgery before the morning dose of glucocorticoid treatment (Table 1). Written informed consent was obtained from all patients. The study was approved by the institutional review board (CMO Radboudumc #2016–2977 and CMO-nr 2004/007). Peripheral blood was collected from 12 body mass index (BMI)- and age-matched healthy men and from 5 men who underwent bilateral adrenalectomy. The mean age and BMI of the matched male controls were 36 years (range: 23-68) and 28.2 kg/m2 (range: 22.9-36.5), respectively.
Table 1.
Clinical characteristics of 21OHD patients (n = 7) with bilateral TART
| TART weight | 17OHP | A4 | ACTH | Total GC dose | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | Age (y) | BMI (kg/m2) | Left (g) | Right (g) | FSH (U/L) | LH (U/L) | (nmol/L) | (nmol/L) | (pmol/L) | (mg/m2)b |
| 1 | 29 | 25.7 | 13 | 11 | 0.6 | <0.2a | 480 | 86 | 180 | 16.0 (HC) |
| 2 | 23 | 25.6 | 4.3 | 5.5 | 8.6 | 5.2 | 26 | 1.5 | 9.1 | 8.2 (HC and DXM) |
| 3 | 32 | 28.3 | 27.4 | 18.9 | 15.9 | 2.9 | 367 | 14 | 42.2 | 16.9 (HC) |
| 4 | 51 | 29.0 | 16.2 | 22.9 | 55.2 | 44.9 | 5.1 | 0.9 | 33.0 | 16.2 (HC) |
| 5 | 26 | 38.2 | 1.6 | 0.9 | <0.2a | <0.2a | 865 | 50 | 270.0 | 10.8 (HC) |
| 6 | 26 | 23.7 | 1.9 | 1.5 | 6.3 | 5.6 | 10 | 2.2 | 5.8 | 12.1 (DXM) |
| 7 | 31 | 27.0 | 1.3 | 0.5 | 39.3 | 12.3 | 4.3 | 1.2 | 0.5 | 30.1 (HC) |
From Claahsen-van der Grinten et al. (2007) (11).
Normal values of our laboratory: FSH 1.5 – 11 U/L; LH 1.4–8.5 U/L; 17OHP 2.0–10.8 nmol/L; A4 1.4–9.7 nmol/L; ACTH 2.2–13.2 pmol/L.
Abbreviations: 17OHP, 17-hydroxyprogesterone; A4, androstenedione; ACTH, adrenocorticotropic hormone; BMI, body mass index; DXM, dexamethasone; FSH, follicle-stimulating hormone; GC, glucocorticoid; HC, hydrocortisone; LH, luteinizing hormone; TART, testicular adrenal rest tumor.
aBelow lower limit of quantification.
bGC Dose: DXM doses were converted to HC equivalents (1mg DXM = 40 mg HC)
Spermatic and Peripheral Vein Sampling
The procedure of spermatic and peripheral vein sampling was described previously (11). All 21OHD patients received 2.5 mg dexamethasone as stress medication, immediately before surgery. Via an inguinal incision and opening of the inguinal canal, the spermatic cord was exposed. The left spermatic vein was cannulated first in 3 patients and the right spermatic vein was cannulated first in the remaining 4 cases. Peripheral blood was collected simultaneously from a cubital vein. The second spermatic vein was cannulated after the surgical excision from the opposite side. All patients had bilateral TART, but for 3 patients (2, 3, and 7) no material was longer available from the right spermatic vein. Sera were stored at −20 °C until laboratory analysis.
Steroid Quantification in TART Cell– and Adrenal Cell–Conditioned Medium
Steroids were measured in previously collected media (3) of in vitro cultured TART cells and adrenal cells, that were treated for 24 or 48 hours with ACTH (10 nmol/L), LH (25 ng/mL), or basal medium. Experiments were performed in 3 replicates using cells obtained from a 7 cm TART, which had been surgically removed from a 46-year-old man with classic 21OHD. Written informed consent was obtained from this patient to use excess tissue for research purposes. Adrenal cells had been obtained from cadaveric kidney donors. Single cell suspensions were obtained, and cells were cultured to 60% confluence in 10% Cosmic calf serum with antibiotics. Before treatment, cells were starved with 1% Cosmic calf serum for 18 hours. Steroid concentrations were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
LC-MS/MS
From the samples, 11 steroids, including 11oxC19 steroids (11OHA4, 11KA4, 11OHT, 11KT) were extracted and quantified as described elsewhere (18), with minor modifications in sample processing to minimize potential decomposition of cortisol and cortisone into 11OHA4 and 11KA4, respectively (19). Target steroid analytes were chromatographically separated using a Kinetex 2.1 × 150 mm 2.6-μm particle size resolving column in a 20-minute cycle. After electrospray ionization, the analytes were quantitated on an Agilent 6495 triple quadrupole tandem mass spectrometer using multiple reaction monitoring.
Statistical Analyses
Data are reported as median with ranges. Steroid concentrations below the lower limit of quantification (LLOQ) were set at 0.5*LLOQ for statistical analysis. To test if steroid concentrations were significantly increased in spermatic vein samples compared to peripheral blood samples, Wilcoxon signed-rank tests (one-sided; greater) were done. To correct for multiple testing, P values were adjusted using the Bonferroni method. Differences in peripheral steroid concentrations between 21OHD patients (n = 7), adrenalectomized patients (n = 5), and healthy individuals (n = 12) were tested using the Kruskal-Wallis test, followed by Dunn’s post hoc test for pairwise comparisons. Adjusted P values < 0.05 were considered significant. A 2-way analysis of variance (ANOVA) was conducted to examine the interaction between cell type (TART vs adrenal) and effect of ACTH treatment on steroid levels, followed by pairwise comparison using Tukey post hoc testing in case of a significant interaction effect. Student’s t tests were performed to study simple main effects.
Results
11-Oxygenated Steroid Concentrations Are Significantly Elevated in Spermatic Vein Compared With Peripheral Vein Concentrations
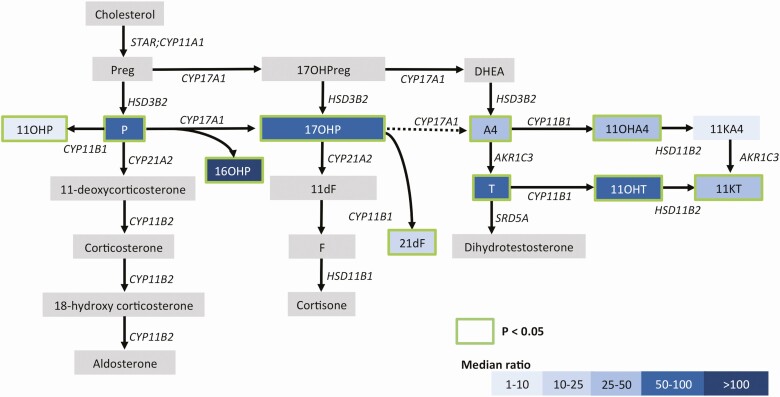
To study the steroid production by TART, the left and right spermatic vein steroid concentrations were compared with peripheral vein concentrations (Supplementary Table 1 (20)). Overall, 10 of the 11 quantified steroid hormones were significantly elevated in the spermatic vein compared with peripheral vein samples (summarized in Table 2; Fig. 1). Of the 4 11oxC19 steroids, 11OHT, 11KT, and 11OHA4 were significantly elevated in spermatic vein samples, with median ratios of 96, 47, and 29 (all P < 0.01), respectively. In all men with 21OHD, the spermatic-to-peripheral vein gradient was disproportionately higher for 11OHT as compared to 11OHA4 (Wilcoxon signed rank test; P < 0.01; Supplementary Table 1 (20)). The 11-oxygenated 21-carbon (11oxC21) steroids 21dF (11-fold; P = 0.04) and 11OHP (2-fold; P = 0.02) were both higher in the spermatic vein vs periphery, as were testosterone (T; median ratio = 66; P < 0.01), 16α-hydroxyprogesterone (16OHP; median ratio = 110; P = 0.01), 17OHP (median ratio = 98), progesterone (median ratio = 51; P = 0.01) and A4 (median ratio = 29; P = 0.01).
Table 2.
Median steroid hormone concentrations (nmol/L) of bilateral spermatic vein samples (n = 11) and corresponding peripheral vein samples (n = 7) of 21OHD patients with TART, with interquartile ranges and corresponding median ratios
| Spermatic | Peripheral | Fold | P adj | |
|---|---|---|---|---|
| 16OHP | 23.9 (20.8–44) | 0.3 (0.2–0.9) | 110 (24–181) | 0.01 |
| 17OHP | 276.0 (155–472.9) | 2.9 (1.8–9.5) | 98 (19–149) | <0.01 |
| 11OHT | 30.3 (21.7–69.6) | 0.4 (0.2–0.8) | 96 (51–224) | <0.01 |
| T | 462.9 (145.7–829.6) | 4.2 (3.0–7.5) | 66 (41–99) | <0.01 |
| Prog | 7.7 (5.2–15.3) | 0.2 (0.1–0.8) | 51 (10–61) | 0.01 |
| 11KT | 15.3 (12–15.9) | 0.6 (0.2–1.2) | 47 (18–115) | <0.01 |
| A4 | 18.8 (14.6–43.4) | 0.7 (0.5–2.5) | 29 (6–81) | 0.01 |
| 11OHA4 | 72.4 (28.8–129.8) | 1.7 (0.6–5.8) | 29 (15–110) | <0.01 |
| 21dF | 34.2 (16.3–65.5) | 2.0 (0.3–6.1) | 10 (3–90) | 0.04 |
| 11OHP | 0.8 (0.6–1.2) | 0.5 (0.2–0.6) | 2 (2–2) | 0.02 |
| 11KA4 | 1.6 (0.4–2.7) | 0.3 (0.2–1.1) | 2 (2–6) | 0.46 |
Abbreviations: 11KA4, 11-ketoandrostenedione; 11KT, 11-ketotestosterone; 11OHA4, 11β-hydroxyandrostenedione; 11OHT, 11β-hydroxytestosterone; 16OHP, 16α-hydroxyprogesterone; 17OHP, 17-hydroxyprogesterone; 21dF, 21-deoxycortisol; 21OHD, 21-hydroxylase deficiency; A4, androstenedione; Prog, progesterone; T, testosterone.
Figure 1.
Overview of the steroid-producing pathways and median ratios of the 11 quantified steroids in spermatic vein blood vs peripheral vein blood as an indicator for TART- and testis-specific production. Blue color indicates the median ratio of the quantified steroids and a green border indicates significant increase in spermatic vein samples vs peripheral vein samples (1-sided Wilcoxon-signed rank test followed by Bonferroni correction for multiple testing). Abbreviations: 11dF, 11-deoxycortisol; 11KA4, 11-ketoandrostenedione; 11KT, 11-ketotestosterone; 11OHA4, 11β-hydroxyandrostenedione; 11OHP, 11-hydroxyprogesterone; 11OHT, 11β-hydroxytestosterone; 16OHP, 16-hydroxyprogesterone; 17OHP, 17-hydroxyprogesterone; 17OHPreg, 17-hydroxypregnenolone, 21dF, 21-deoxycortisol; A4, androstenedione; AKR1C3, Aldo-keto reductase family 1 member C3; DHEA, dehydroepinandrosterone; CYP11A1, cholesterol side-chain cleavage; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; CYP17A1, 17α-hydroxylase/17,20-lyase; CYP21A2, 21-hydroxylase; F, cortisol; HSD11B1, hydroxysteroid 11β-dehydrogenase 1; HSD11B2, Hydroxysteroid 11β-dehydrogenase 2; HSD3B2, 3β-hydroxysteroid dehydrogenase type 2; P, progesterone; Preg, pregnenolone; SRD5A, 5α-reductase; STAR, steroidogenic acute regulatory protein; T, testosterone.
TART Cell–Conditioned Medium Has Higher Levels of T and Lower Levels of A4
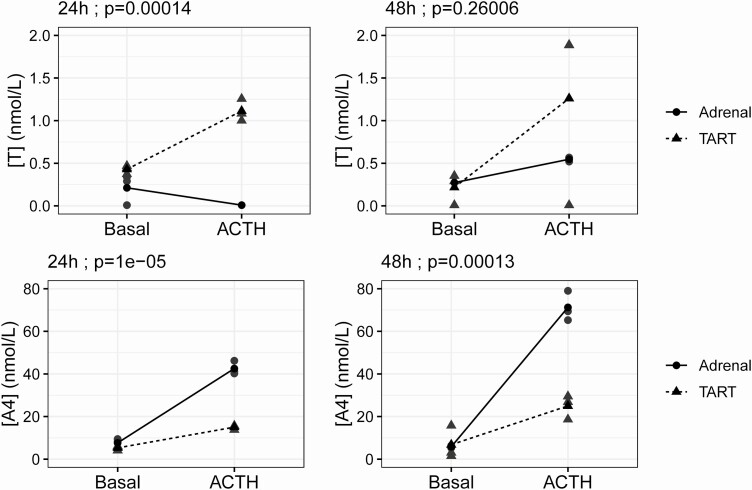
To study the steroid production by TART cells in vitro, steroids were measured in culture media of TART cells and normal adrenal cells under basal conditions and after 24 (Supplementary Table 2 (20)) or 48 hours (Supplementary Table 3 (20)) of ACTH treatment. Under basal conditions, A4, 11OHA4, T, and 11OHT levels were similar between TART and normal adrenal cells (Supplementary Tables 2 and 3 (20)). Interestingly, significant interaction effects were observed between cell type (TART vs adrenal) and ACTH treatment (vs basal treatment) for T (24 hours), A4 (24 and 48 hours) (Fig. 2), and 11OHA4 (24 hours) concentrations (2-way ANOVA; all P < 0.01), which indicates that the effect of ACTH treatment on the production of these steroid hormones differed between the 2 cell types; Twenty-four hours and 48 hours of ACTH treatment resulted in lower concentrations of A4 and 11OHA4 (24 hours only) in medium of TART cells compared to medium of adrenal cells (Tukey post hoc testing; all P < 0.01). In contrast, 24 hours of ACTH treatment resulted in higher levels of T in the medium of TART cells than adrenal cells (Tukey post hoc testing; P < 0.01). The interaction effect between 48-hour ACTH treatment and cell type on 11OHA4 production was not significant (P = 0.09). No interaction was detected between 48-hour ACTH treatment and cell type on T production (P = 0.26) (Fig. 2). No significant differences were observed in T concentrations between TART- and adrenal cell–conditioned media after 48-hour treatment with either basal media or ACTH (Supplementary Table 3 (20)); however, no T was detected in medium for 1 of 3 TART samples after 48 hours of ACTH treatment, despite comparable T production in all 3 samples at 24 hours.
Figure 2.
Interaction effects (2-way ANOVA) between cell type (adrenal vs TART) and 24-hour (left) or 48-hour (right) treatment (basal vs ACTH) on testosterone (T) and androstenedione (A4) concentrations (nmol/L) in cell culture media. The 3 replicates (gray) and their mean concentrations (black) are displayed.
Twenty-four hours of ACTH treatment vs basal media treatment resulted in elevated levels of T in TART-cell culture media (ratio = 2.6; P < 0.01), suggesting that the production of T is ACTH-responsive.
The concentrations of 21dF, progesterone, 11OHP, 16OHP, and 17OHP were significantly higher in TART cells compared with normal adrenal cells, in all conditions, as previously reported (3). The concentrations of 11OHT, 11KA4, and 11KT were below the LLOQ in most cases; however, 11OHT was detected in 2 out of 3 TART samples after 48 hours of ACTH treatment. LH treatment did not substantially affect steroid production in TART or adrenal cells.
11-Oxygenated Steroids Are Elevated in 21OHD Patients With TART and Low in Adrenalectomized Controls
Compared to age- and BMI-matched healthy men, patients with 21OHD and TART had higher peripheral concentrations of the 11oxC21 steroids 21dF (median ratio = 27.6; P < 0.01) and 11OHP (median ratio = 7.7; P < 0.01) (Table 3). The levels of 21dF and 11OHP were below LLOQ in all healthy controls but detected in 5 of the 7 men with 21OHD. The median concentrations of the 11oxC19 steroids (11OHA4, 11OHT, 11KA4, and 11KT) did not significantly differ between 21OHD patients and controls, while T was significantly lower (median ratio = 0.3; P < 0.01) compared with controls. Compared with an additional cohort of 10 men with 21OHD (mean age 31.5 years (19-51)) during conventional therapy (3, 9), the current cohort of 21OHD men at the time of spermatic vein sampling displayed lower peripheral steroid levels (Supplementary Figure 1 (20)).
Table 3.
Median peripheral steroid concentrations in 21OHD male patients with TART (n = 7); male individuals who underwent bilateral adrenalectomy (ADX; n = 5); and age-, sex-, and BMI-matched controls (MC; n = 12) with interquartile ranges and pairwise comparisons
| 21OHD | ADX | MC | 21OHD vs MC | ADX vs MC | 21OHD vs ADX | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| nmol/L | nmol/L | nmol/L | p a | ratio | p b | ratio | p b | ratio | p b | |
| 21dF | 2.0 (0.3–6.1) | <0.1 (<0.1-<0.1)* | <0.1 (<0.1-<0.1)* | <0.01 | 27.6 | <0.01 | 1.00 | 1.00 | 27.6 | <0.01 |
| 17OHP | 2.9 (1.8–9.5) | 1.5 (0.9–1.7) | 1.8 (1.6–2.4) | 0.11 | 1.5 | 0.36 | 0.8 | 0.21 | 1.8 | 0.11 |
| A4 | 0.7 (0.5–2.5) | 0.7 (0.7–0.8) | 1.7 (1.1–2.3) | 0.04 | 0.4 | 0.07 | 0.4 | 0.07 | 0.9 | 0.67 |
| T | 4.2 (3.0–7.5) | 8.9 (7–12.4) | 14.2 (10.3–18.1) | <0.01 | 0.3 | <0.01 | 0.6 | 0.20 | 0.5 | 0.20 |
| 11OHA4 | 1.7 (0.6–5.8) | 0.2 (0.1–0.3) | 3.7 (3.0–5.3) | <0.01 | 0.5 | 0.24 | 0.1 | <0.01 | 8.2 | 0.06 |
| 11OHT | 0.4 (0.2–0.8) | <0.2 (<0.2-<0.2)* | 0.4 (0.3–0.6) | <0.01 | 0.9 | 0.73 | 0.2 | <0.01 | 4.4 | 0.02 |
| 11KA4 | 0.3 (0.2–1.1) | <0.1 (<0.1-<0.1) | 0.5 (0.4–0.6) | <0.01 | 0.5 | 0.39 | 0.2 | <0.01 | 3.1 | 0.05 |
| 11KT | 0.6 (0.2–1.2) | <0.2 (<0.2-<0.2)* | 0.7 (0.6–0.9) | <0.01 | 0.8 | 0.53 | 0.1 | <0.01 | 6.8 | 0.03 |
| 16OHP | 0.3 (0.2–0.9) | 0.1 (0.1–0.2) | 0.3 (0.2–0.5) | 0.02 | 1.0 | 0.77 | 0.5 | 0.02 | 2.1 | 0.02 |
| 11OHP | 0.5 (0.2–0.6) | <0.1 (<0.1-<0.1)* | <0.1 (<0.1-<0.1)* | <0.01 | 7.7 | <0.01 | 1.0 | 1.00 | 7.7 | <0.01 |
| Prog | 0.2 (0.1–0.8) | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | 0.04 | 1.9 | 0.16 | 0.8 | 0.16 | 2.2 | 0.03 |
Abbreviations: 11KA4, 11-ketoandrostenedione; 11KT, 11-ketotestosterone; 11OHA4, 11β-hydroxyandrostenedione; 11OHT, 11β-hydroxytestosterone; 16OHP, 16α-hydroxyprogesterone; 17OHP, 17-hydroxyprogesterone; 21dF, 21-deoxycortisol; 21OHD, 21-hydroxylase deficiency; A4, androstenedione; ADX, adrenalectomy; BMI, body mass index; MC, matched controls; Prog, progesterone; T, testosterone; TART, testicular adrenal rest tumor.
*All samples below lower limit of quantification (LLOQ)
aPeripheral steroid concentrations were compared using Kruskal-Wallis test (n = 24), followed by
b Dunn’s post hoc test for pairwise comparisons with P value adjustment for multiple testing using the Benjamin-Hochberg method. Steroid concentrations below the LLOQ were set at 0.5*LLOQ for statistical analysis.
In all 5 control men who underwent bilateral adrenalectomy, the CYP11B1-metabolized steroids 21dF, 11OHP, 11OHT, and 11KT were all below the LLOQ (Table 3). Adrenalectomized controls had lower levels of the 11-oxygenated steroids 11OHA4 (0.06-fold; P < 0.01) and 11KA4 (0.18-fold; P < 0.01) than the healthy individuals (Table 3).
Discussion
The goal of this study was to extend prior studies of the steroidogenic profile of TART by the quantification of 11-oxygenated steroids in effluent of spermatic vein samples and corresponding peripheral blood samples of 7 21OHD patients harboring TART, as well as in medium of in vitro cultured TART cells. The peripheral levels of 11oxC19 steroids in 21OHD patients with TART were found to be higher compared to those without TART (14); however, this previous study could not assess whether this association reflected the contribution of TART to the circulating concentrations of 11oxC19 steroids. This study demonstrates that TART contributes to the production of the 11oxC19 steroids 11OHA4 and 11OHT. Despite the required preoperative glucocorticoid dosing, spermatic-to-peripheral vein gradients were large, which are considered to reflect the TART rather than testicular steroidogenesis. Normally, 11-oxygenated steroids derive primarily, if not exclusively, from the adrenal cortex. Indeed, we showed undetectable, or very low (11OHA4), levels of CYP11B1-catalyzed steroids in adrenalectomized individuals, underscoring the dominance of adrenal (or adrenal-like) tissue in the production of 11oxC19 steroids. Although CYP11B1 has also been reported to be expressed in rat Leydig cells (21) or human testicular tissue (22), the expression levels are negligible compared to adrenal or TART tissue (23, 24), which are in line with our transcriptome dataset including human testis, adrenal, and TART tissues (25).
The presence of elevated 11-oxygenated steroid concentrations in spermatic vein blood and in TART cell–conditioned media stresses the adrenocortical steroidogenic profile of TART; however, we observed an important difference between the steroidogenic profile of TART and previously reported steroidogenic profile of adrenal tissue (9). Whereas 11OHA4 was found to be the most highly elevated 11oxC19 steroid in adrenal vein blood (9, 26), 11OHT was by far the most highly elevated 11oxC19 steroid in spermatic vein samples (Fig. 1). Given that 11OHA4 is minimally converted to 11OHT by aldo-keto reductase 1C3 (AKR1C3) or 17β-hydroxysteroid dehydrogenase type 3 (HSD17B3) in vitro (27), the elevated levels of 11OHT are presumably a result of T 11-hydroxylation by CYP11B1. Although it could be speculated that the conversion of 11OHA4 into 11OHT might occur in states of substantially elevated 11OHA4 levels (similar to the atypical conversion of 17OHP into A4), the relatively high levels of 11OHT in spermatic vein samples stresses a likely dominant metabolism of T in TART tissue. As the T measured in the spermatic vein of 21OHD patients with TART could originate from both Leydig cells and TART, it is difficult to determine the origin of T production in these patients. We previously proposed that when T is derived predominantly from the testicular Leydig cells, the peripheral A4/T ratio is below 0.2 (little A4 is produced by the testis) and that when a significant fraction of circulating T is produced by adrenal tissue, the A4/T ratio was increased to >0.5 (28). The peripheral A4/T ratios below 0.2 in patients 3 to 7 might suggest a predominant Leydig cell origin of T, while the A4/T ratios of 3.2 and 2.5 for patient 1 and 2, respectively, suggest predominant adrenal(-like) origin of T. Nonetheless, the suppressed gonadotropin levels in 2 patients suggests that the elevated spermatic vein T levels are derived primarily from TART rather than from Leydig cells. To study the production of T by TART exclusively, we have also quantified T production by in vitro cultured TART cells and found that in vitro cultured TART cells produce greater amounts of T than cultured adrenal cells after ACTH stimulation, yet lower amounts of A4 and 11OHA4. The expression of the ACTH receptor MC2R and the Leydig cell enzyme HSD17B3 in TART cells (25) explains the ACTH-induced T production in TART, and the time course of T generation at 24 hours but 11OHT following at 48 hours is consistent with the pathway A4 to T to 11OHT. Thus, the relatively high elevation of 11OHT in the spermatic vein of patients with 21OHD and TART presumably indicates higher production and 11-oxygenation of T by TART. In addition, TART might also metabolize T that percolates from the surrounding gonadal tissue, which might explain why the 11OHT production relative to T by TART cells was somewhat less pronounced and delayed in vitro. The differences in steroidogenic profile between TART and adrenal tissue suggests, as do previous studies (29), that these tissues are not identical.
In addition to 11OHA4 and 11OHT, 11KT was also elevated in the spermatic vein of patients with 21OHD and TART compared to peripheral vein blood. Cultured TART cells did not produce detectable levels of 11KT, suggesting that 11KT might be produced by other tissue in the testicular region. The 11oxC19 steroids 11KT and 11KA4 are, in contrast to 11OHA4 and 11OHT, considered to be mainly produced from their respective 11-hydroxysteroids in peripheral tissues, and to a lesser extent in the adrenal gland/adrenal-like tissue (9, 30). In addition, our data suggest that TART produces the precursor steroids progesterone and 17OHP upstream of the CYP21A2 enzymatic block (Fig. 1). In the absence of CYP21A2, CYP11B1 atypically converts progesterone and 17OHP into 11OHP and 21dF, respectively (27). Both 11OHP and 21dF were significantly elevated in spermatic vein effluent.
Reduced fertility in 21OHD men with TART is considered to be associated with mechanical obstruction of the seminiferous tubules (31). In addition to the mechanical effects, adrenal-derived androgens likely contribute to the suppression of the hypothalamic-pituitary-gonadal axis, as suggested by the suppressed gonadotropins in men with poorly controlled 21OHD, and by the inverse correlation of 11KT with T in reproductive age men (as opposed to the direct correlation of these 2 steroids in females and pre-pubertal boys) (9, 14). Hypothetically, the local (adrenal-like) steroid production by TART might have paracrine effects on healthy testicular tissue, impairing the normal gonadal function (31).
To study whether steroid production by TART translated into elevated circulating concentrations in 21OHD patients, the peripheral blood steroid levels from 21OHD patients were compared with matched controls. Whereas the levels of all 4 11oxC19 androgens were previously found to be increased in peripheral plasma of 21OHD patients (9), these 11oxC19 androgens were not elevated in the peripheral blood samples of 21OHD patients in this study cohort. This discrepancy might be explained by the smaller sample size of this study or, more likely, the partially suppressive effect of dexamethasone, which was required as stress medication in this study. As this dose was given immediately prior to surgery, the suppressive effect of dexamethasone was delayed, which is confirmed by the elevation of peripheral steroid concentrations in men with 21OHD vs those with history of adrenalectomy (only testicular contribution) and by the trend of slightly lower steroid concentrations in the spermatic veins cannulated last vs those cannulated first (described in Claahsen-van der Grinten et al (11)). Although this suggests a very small effect of dexamethasone on the negative feedback-loop, this trend demonstrates the existence of kinetics and ACTH-responsiveness of steroid production, confirming that adrenal-like ACTH-sensitive steroid production was not completely suppressed. We could not measure plasma ACTH in the peripheral serum samples obtained during spermatic vein sampling to demonstrate the degree of ACTH suppression at that time. The peripheral steroid hormone concentrations in these men with 21OHD acutely treated with dexamethasone were lower compared to a previously described cohort of men with 21OHD during conventional therapy. This finding is most likely due to the clinically required dexamethasone dose just prior to spermatic vein sampling, given the generally poor hormonal control of both cohorts (Table 1). The preoperative dexamethasone in this study is a limitation but suggests that the spermatic vein gradients might have been even greater if dexamethasone was not administered. In agreement with previous findings (3), the peripheral levels of the upstream 11oxC21 steroids, 21dF, and 11OHP, were higher in patients with 21OHD than in unaffected individuals. The comparison of peripheral serum steroid concentrations in 21OHD men to healthy control men is, however, limited because of the dexamethasone stress medication given only to the men with 21OHD. Thus, although it is difficult to quantitate the proportions of peripheral steroids coming from TART vs adrenals, our findings suggest that TART contribute to the production of 11-oxygenated steroids.
In conclusion, TART produce 11-oxygenated steroids and contribute to the peripheral concentrations of 11oxC19 androgens in male patients with 21OHD. Compared to the adrenal steroid profile, 11OHT was disproportionately elevated in spermatic vein samples, suggesting that 11-hydroxylation of T is a unique feature of TART in 21OHD.
Glossary
Abbreviations
- 11KA4
11-ketoandrostenedione
- 11KT
11-ketotestosterone
- 11OHA4
11β-hydroxyandrostenedione
- 11OHT
11β-hydroxytestosterone
- 11oxC19
11-oxygenated 19-carbon
- 11oxC21
11-oxygenated 21-carbon
- 16OHP
16α-hydroxyprogesterone
- 17OHP
17-hydroxyprogesterone
- 21dF
21-deoxycortisol
- 21OHD
21-hydroxylase deficiency
- A4
androstenedione
- ACTH
adrenocorticotropic hormone
- ANOVA
analysis of variance
- BMI
body mass index
- CAH
congenital adrenal hyperplasia
- CYP11B1
11β-hydroxylase
- CYP21A2
21-hydroxylase
- FSH
follicle-stimulating hormone
- HSD
hydroxysteroid dehydrogenase
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- LH
luteinizing hormone
- LLOQ
lower limit of quantitation
- T
testosterone
- TART
testicular adrenal rest tumors
Acknowledgments
Financial Support: This work was partially supported by a grant (K08DK109116) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to A.F.T.
Additional Information
Disclosures: All authors declare that there is no conflict of interest.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Tonetto-Fernandes V, Lemos-Marini SH, Kuperman H, Ribeiro-Neto LM, Verreschi IT, Kater CE. Serum 21-Deoxycortisol, 17-Hydroxyprogesterone, and 11-deoxycortisol in classic congenital adrenal hyperplasia: clinical and hormonal correlations and identification of patients with 11beta-hydroxylase deficiency among a large group with alleged 21-hydroxylase deficiency. j Clin Endocrinol Metab. 2006;91(6):2179-2184. [DOI] [PubMed] [Google Scholar]
- 2. Speiser PW, Dupont J, Zhu D, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. j Clin Invest. 1992;90(2):584-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turcu AF, Rege J, Chomic R, et al. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. j Clin Endocrinol Metab. 2015;100(6):2283-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194-2210. [DOI] [PubMed] [Google Scholar]
- 5. Pescovitz OH, Comite F, Cassorla F, et al. True precocious puberty complicating congenital adrenal hyperplasia: treatment with a luteinizing hormone-releasing hormone analog. j Clin Endocrinol Metab. 1984;58(5):857-861. [DOI] [PubMed] [Google Scholar]
- 6. Seth A. Optimizing stature in congenital adrenal hyperplasia: challenges and solutions. Indian j Pediatr. 2019;86(6):489-491. [DOI] [PubMed] [Google Scholar]
- 7. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. j Clin Endocrinol Metab. 2018;103(11):4043-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamrath C, Wettstaedt L, Boettcher C, Hartmann MF, Wudy SA. Androgen excess is due to elevated 11-oxygenated androgens in treated children with congenital adrenal hyperplasia. j Steroid Biochem Mol Biol. 2018;178:221-228. [DOI] [PubMed] [Google Scholar]
- 9. Turcu AF, Nanba AT, Chomic R, et al. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur j Endocrinol. 2016;174(5):601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turcu AF, El-Maouche D, Zhao L, et al. Androgen excess and diagnostic steroid biomarkers for nonclassic 21-hydroxylase deficiency without cosyntropin stimulation. Eur j Endocrinol. 2020;183(1):63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Claahsen-van der Grinten HL, Otten BJ, Sweep FC, et al. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. j Clin Endocrinol Metab. 2007;92(9):3674-3680. [DOI] [PubMed] [Google Scholar]
- 12. Smeets EE, Span PN, van Herwaarden AE, et al. Molecular characterization of testicular adrenal rest tumors in congenital adrenal hyperplasia: lesions with both adrenocortical and Leydig cell features. j Clin Endocrinol Metab. 2015;100(3):E524-E530. [DOI] [PubMed] [Google Scholar]
- 13. Clark RV, Albertson BD, Munabi A, et al. Steroidogenic enzyme activities, morphology, and receptor studies of a testicular adrenal rest in a patient with congenital adrenal hyperplasia. j Clin Endocrinol Metab. 1990;70(5):1408-1413. [DOI] [PubMed] [Google Scholar]
- 14. Turcu AF, Mallappa A, Elman MS, et al. 11-Oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. j Clin Endocrinol Metab. 2017;102(8):2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bercovici JP, Fiet J, Gibault L, et al. Testicular adrenal rest tumours in salt wasting congenital adrenal hyperplasia (in vivo and in vitro studies). j Steroid Biochem Mol Biol. 2005;93(1):67-72. [DOI] [PubMed] [Google Scholar]
- 16. Combes-Moukhovsky ME, Kottler ML, Valensi P, Boudou P, Sibony M, Attali JR. Gonadal and adrenal catheterization during adrenal suppression and gonadal stimulation in a patient with bilateral testicular tumors and congenital adrenal hyperplasia. j Clin Endocrinol Metab. 1994;79(5):1390-1394. [DOI] [PubMed] [Google Scholar]
- 17. Claahsen-van der Grinten HL, Otten BJ, Hermus AR, Sweep FC, Hulsbergen-van de Kaa CA. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89(3):597-601. [DOI] [PubMed] [Google Scholar]
- 18. Wright C, O’Day P, Alyamani M, Sharifi N, Auchus RJ. Abiraterone acetate treatment lowers 11-oxygenated androgens. Eur j Endocrinol. 2020;182(4):413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davio A, Woolcock H, Nanba AT, et al. Sex differences in 11-oxygenated androgen patterns across adulthood. J Clin Endocrinol Metab. 2020;105(8):e2921-e2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schröder MAM, Turcu AF, O’Day P, et al. Data from: production of 11-oxygenated androgens by testicular adrenal rest tumors. DANS. Deposited June 2021. 10.17026/dans-xqw-cxxw [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang GM, Ge RS, Latif SA, Morris DJ, Hardy MP. Expression of 11beta-hydroxylase in rat Leydig cells. Endocrinology. 2002;143(2):621-626. [DOI] [PubMed] [Google Scholar]
- 22. Imamichi Y, Yuhki KI, Orisaka M, et al. 11-Ketotestosterone is a major androgen produced in human gonads. j Clin Endocrinol Metab. 2016;101(10):3582-3591. [DOI] [PubMed] [Google Scholar]
- 23. Lottrup G, Nielsen JE, Skakkebæk NE, Juul A, Rajpert-De Meyts E. Abundance of DLK1, differential expression of CYP11B1, CYP21A2 and MC2R, and lack of INSL3 distinguish testicular adrenal rest tumours from Leydig cell tumours. Eur j Endocrinol. 2015;172(4):491-499. [DOI] [PubMed] [Google Scholar]
- 24. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 25.Schröder MAM, Sweep FCGJ, van Herwaarden AE, et al. Transcriptional comparison of Testicular Adrenal Rest Tumors with fetal and adult tissues. bioRxiv. 2020. doi: 10.1101/2020.05.07.082313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. j Clin Endocrinol Metab. 2013;98(3):1182-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013;377(1-2):135-146. [DOI] [PubMed] [Google Scholar]
- 28. Auchus RJ. Management considerations for the adult with congenital adrenal hyperplasia. Mol Cell Endocrinol. 2015;408:190-197. [DOI] [PubMed] [Google Scholar]
- 29. Engels M, Span PN, van Herwaarden AE, Sweep FCGJ, Stikkelbroeck NMML, Claahsen-van der Grinten HL. Testicular adrenal rest tumors: current insights on prevalence, characteristics, origin, and treatment. Endocr Rev. 2019;40(4):973-987. [DOI] [PubMed] [Google Scholar]
- 30. Turcu AF, Auchus RJ. Clinical significance of 11-oxygenated androgens. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Claahsen-van der Grinten HL, Stikkelbroeck N, Falhammar H, Reisch N. Management of endocrine disease: gonadal dysfunction in congenital adrenal hyperplasia. Eur j Endocrinol. 2021;184(3):R85-R97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.