Abstract
Context
Antenatal complications such as hypertensive disorders of pregnancy (HDP), fetal growth restriction (FGR), gestational diabetes (GDM), and preterm birth (PTB) are associated with placental dysfunction. Kisspeptin has emerged as a putative marker of placental function, but limited data exist describing circulating kisspeptin levels across all 3 trimesters in women with antenatal complications.
Objective
We aimed to assess whether kisspeptin levels are altered in women with antenatal complications.
Methods
Women with antenatal complications (n = 105) and those with uncomplicated pregnancies (n = 265) underwent serial ultrasound scans and blood sampling at the Early Pregnancy Assessment Unit at Hammersmith Hospital, UK, at least once during each trimester (March 2014 to March 2017). The women with antenatal complications (HDP [n = 32], FGR [n = 17], GDM [n = 35], PTB [n = 11], and multiple complications [n=10]) provided 373 blood samples and the controls provided 930 samples. Differences in circulating kisspeptin levels were assessed.
Results
Third-trimester kisspeptin levels were higher than controls in HDP but lower in FGR. The odds of HDP adjusted for gestational age, maternal age, ethnicity, BMI, smoking, and parity were increased by 30% (95% CI, 16%-47%; P < 0.0001), and of FGR were reduced by 28% (95% CI, 4-46%; P = 0.025), for every 1 nmol/L increase in plasma kisspeptin. Multiple of gestation-specific median values of kisspeptin were higher in pregnancies affected by PTB (P = 0.014) and lower in those with GDM (P = 0.020), but not significantly on multivariable analysis.
Conclusion
We delineate changes in circulating kisspeptin levels at different trimesters and evaluate the potential of kisspeptin as a biomarker for antenatal complications.
Keywords: fetal growth restriction (FGR), intrauterine growth restriction (IUGR), hypertensive diseases of pregnancy (HDP), gestational diabetes (GDM), preterm birth (PTB), kisspeptin
Antenatal complications are common: hypertensive disorders of pregnancy (HDP) affect ~5% (1), fetal growth restriction (FGR) ~4% (2), gestational diabetes (GDM) ~15% (3), and preterm birth (PTB) ~11% (4) of pregnancies. Such conditions present serious risks to maternal and fetal wellbeing (5). Despite intensive study, the precise mechanisms underlying many antenatal complications remain subject to conjecture (6). However, a shared abnormality in the temporo-spatial regulation of trophoblast invasion during the first trimester, resulting in defective spiral artery transformation has been proposed to underlie HDP, FGR, GDM, and PTB (7, 8).
The peptide kisspeptin, better known for its stimulatory role in hypothalamic gonadotropin-releasing hormone release (7, 8), has emerged as a putative regulator of trophoblast invasion (9, 10). Both kisspeptin and its receptor (KISS1R) are highly expressed in placental tissues; kisspeptin is expressed by syncytiotrophoblasts and its receptor by both cytotrophoblasts and syncytiotrophoblasts (11). Kisspeptin constrains trophoblast migration in vitro, and decreases expression of matrix metalloproteinases II and IX, which are essential for breakdown of the extracellular matrix during the migratory process (11). Thus, kisspeptin appears to be a key paracrine inhibitor of excessive trophoblast migration, and as such, safeguards physiological placentation.
Syncytiotrophoblasts secrete kisspeptin, along with other placental peptides, such as β-human chorionic gonadotropin (βhCG), into the circulation throughout pregnancy (12). However, whereas βhCG levels peak during the first trimester before subsequently declining (13), kisspeptin levels continue to rise with increasing gestation (14). Hitherto, kisspeptin has garnered interest as a biomarker for placental function; low circulating kisspeptin levels have been reported in patients with miscarriage, HDP (15-20), FGR (20-22), and GDM (15-23). Such abnormalities in kisspeptin levels in complicated pregnancies may be contingent on gestation, thus quantification of circulating kisspeptin levels in all 3 trimesters could provide insight into placental dysfunction in these conditions at different stages of pregnancy.
To date, there are limited data reporting alterations in kisspeptin levels during all 3 trimesters of complicated pregnancies. Therefore, we aimed to assess whether circulating kisspeptin levels differ in pregnancies complicated by HDP, FGR, GDM, and PTB as compared with healthy control pregnancies, and to identify the specific trimesters at which such perturbations become apparent.
Methods
Study Approval
This study was approved by the National Research Ethics Service (NRES) Riverside Committee London (14/LO/0199) and the North East, Newcastle and North Tyneside Two Research Ethics Committee (17/NE/0121). All participants provided written informed consent in accordance with the declaration of Helsinki.
Setting and Design
This was a nested case-control study of women with antenatal complications and healthy singleton control pregnancies recruited from March 2014 to March 2017. Women affected by antenatal complications (n = 105) and those with uncomplicated pregnancies (n = 265) underwent serial ultrasound scans and blood sampling at least once during each trimester. Women completed a detailed questionnaire regarding demographic details, past medical, gynecological, and obstetric history. Following recruitment, participants were invited to attend every 2 weeks during the first trimester for an ultrasound scan and blood test (plasma kisspeptin level). Participants were also assessed at the time of their anomaly scan (18 to 22 weeks of gestation) during the second trimester and once during the third trimester (31 to 36 weeks of gestation). Blood samples for kisspeptin were stored at −80 oC until the day of kisspeptin assay. Assays for plasma kisspeptin levels were conducted at the end of the study after completion of sample collection had taken place.
Study Participants
Participants were recruited via open advertisements (using posters) in local GP surgeries and hospitals. Pregnant women aged from 18 to 49 years old with an intrauterine pregnancy on ultrasound in the first trimester (<14 weeks) were invited to participate. Women with miscarriage, pregnancy of unknown location, or ectopic pregnancy were excluded.
A total of 1242 pregnant women were screened and 1045 were recruited to participate in the study. The commonest reasons for withdrawal/exclusion were booking antenatal care at a different hospital, personal choice, and being unable to attend future visits. Of the 1045 recruited patients, 122 were excluded from analysis due to pregnancy termination (n = 11), loss to follow-up (n = 11), withdrawal (n = 5), or miscarriage (n = 95). Of the remaining 923 women, 105 women had complicated pregnancies as detailed below and all samples from these women were included in the study. It is important to measure kisspeptin levels in all samples using a radioimmunoassay conducted on the same day to ensure consistent assay characteristics. Due to the large number of samples, it was not practically possible to assay samples from all 818 control women who were not affected by pregnancy complications, and therefore we assayed samples from 265 women, who had spontaneous singleton conception, did not suffer any pregnancy complications, nor any symptoms of possible pregnancy loss in early pregnancy (eg, vaginal bleeding), to act as the control group (24).
Diagnostic Criteria for Pregnancy Complications
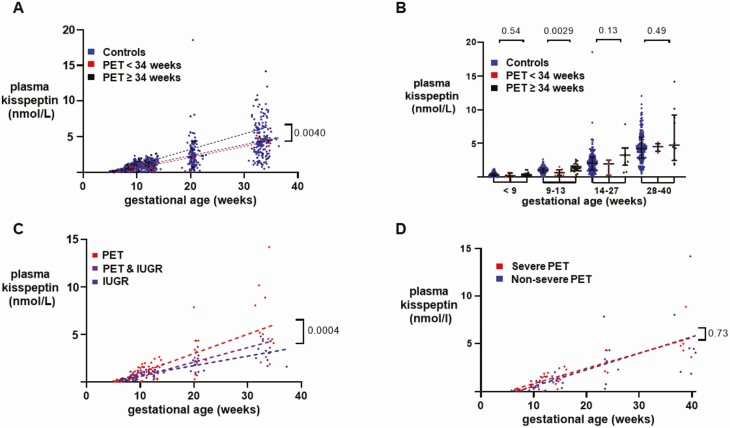
Hypertensive disorders of pregnancy (HDP)
Pregnancy-induced hypertension (PIH) was defined as raised blood pressure ≥140/90 mmHg on 2 occasions 4 hours apart at >20 weeks of gestation in a woman with previously normal blood pressure without proteinuria, growth restriction, or abnormal blood tests (25). Pre-eclampsia (PET) was defined as PIH with proteinuria (urine protein creatinine ratio >0.3 mg/dL or 24-hour urine collection >3 g/24 hours) (25, 26). In addition, a diagnosis of PET was applied if PIH occurred with either FGR or deranged blood tests (thrombocytopenia <100 × 109/L, serum creatinine concentration >1.1 mg/dL, or a doubling of this in the absence of renal disease, or elevated liver transaminases to twice normal concentration), or if they developed eclampsia (25, 27). Severe PET was diagnosed in the presence of one of blood pressure ≥160/110 mmHg, visual disturbance, chest pain, shortness of breath, flash pulmonary edema, seizures, or neonatal distress (25, 27). Analysis of subgroups of HDP in Fig. 2 are presented only as exploratory analyses and should be interpreted with caution as they may be subject to type 2 error.
Figure 2.
Variation in plasma kisspeptin levels according to severity, onset, and complications of pre-eclampsia. A, Scatterplot of plasma kisspeptin levels in healthy controls (blue) (n = 265 women providing 898 samples), women with early-onset pre-eclampsia (PET < 34 weeks) (red) (n = 8 women providing 27 samples) and women with late-onset PET (> 34 weeks) (black) (n = 16 women providing 65 samples) over weeks of gestational age estimated by crown– rump length (CRL) throughout pregnancy. Data were analyzed by simple linear regression (r2 = 0.55 in controls, 0.87 in early PET and 0.54 in late PET); P = 0.0040 by ANCOVA. Analysis of subgroups of HDP are presented only as exploratory analyses and should be interpreted with caution as they may be subject to type 2 error. B, Medians (IQR) of plasma kisspeptin levels in healthy controls (blue) (n = 265 women providing 898 samples), women with early-onset PET (< 34 weeks) (red) (n = 8 women providing 27 samples) and women with late-onset PET (> 34 weeks) (black) (n = 16 women providing 65 samples) over the early and late first trimester, and second and third trimesters. Data were analyzed by Kruskal–Wallis test. Analysis of subgroups of HDP are presented only as exploratory analyses and should be interpreted with caution as they may be subject to type 2 error. C, Scatterplot of plasma kisspeptin levels in women with PET (red) (n = 20 women providing 82 samples), women with intrauterine growth restriction (IUGR) (blue) (n = 17 women providing 56 samples) and women with both PET and IUGR (purple) (n = 3 women providing 8 samples) over weeks of gestational age estimated by CRL throughout pregnancy. Data were analyzed by simple linear regression (r2 = 0.55 in PET, 0.69 in IUGR, and 0.92 in PET& IUGR); P = 0.0004 by ANCOVA. Analysis of subgroups of HDP are presented only as exploratory analyses and should be interpreted with caution as they may be subject to type 2 error. D, Scatterplot of plasma kisspeptin levels in women with severe PET (red) (n = 13 women providing 39 samples) and women with nonsevere PET (blue) (n = 7 women providing 38 samples) over weeks of gestational age estimated by CRL throughout pregnancy. Data were analyzed by simple linear regression (r2 = 0.76 in severe PET and 0.47 in nonsevere PET); P = 0.73 by ANCOVA. Analysis of subgroups of HDP are presented only as exploratory analyses and should be interpreted with caution as they may be subject to type 2 error.
Gestational diabetes
GDM was diagnosed by an oral glucose tolerance test (OGTT) performed after 20 weeks of gestation. A fasting plasma glucose ≥ 5.6 mmol/L or a glucose level ≥ 7.8 mmol/L (2 hours following 75 g of glucose) confirmed GDM (28).
Preterm birth
PTB was diagnosed in women with delivery after 24 weeks and before 37 completed weeks of gestation (as dated by a routine dating scan), which included both iatrogenic and spontaneous preterm birth (29).
Fetal growth restriction
FGR was used to describe either intrauterine growth restriction (IUGR) or small for gestation age (SGA). IUGR was an ultrasound-based antenatal diagnosis where the estimated fetal weight was less than the tenth centile for gestational age with abnormal umbilical artery doppler results (pulsatility index >95th percentile with or without reverse or absent end diastolic flow) (30, 31). SGA was defined in accordance with the World Health Organization (WHO) criteria and WHO centiles, as delivery weight less than the tenth percentile for gestational age, where the final gestational age was estimated using the dating scan (performed at 11-14 weeks gestation) as a reference (32).
Assays
Plasma kisspeptin levels were measured using an in-house radioimmunoassay at Imperial College London (33). The assay has a 10.2% inter-assay and 8.2% intra-assay coefficient of variation. The antibody exhibits <0.01% crossreactivity with similarly structured RF-amide proteins, such as RF-amide-related peptides (RFRP): RFRP-1, RFRP-2, and RFRP-3. The assay measures all kisspeptin splicing variants, although kisspeptin-54 has been reported to be the dominant circulating form in human pregnancy (14).
Gestational Age Correction
The gestational age at the time of sample collection was determined to correct for changes in plasma kisspeptin levels with gestation. During the first trimester, pregnancies were dated using crown-rump length (CRL) on ultrasound scan (34). Multiple of median (MoM) values were derived from raw kisspeptin levels in order to correct for gestational age at the time of measurement; first, median hormone levels in healthy controls were determined at each week of gestation, and then each raw hormone level was expressed as a proportion of the median control value for the corresponding gestation. Thus, a MoM of 0.5 denotes a value that is half that of the corresponding median value in healthy controls for that gestation.
Statistical Analysis
Analysis was performed using Prism v8.4.3 (GraphPad) and Stata v13.0 (StataCorp) software packages. Normality was determined using the D’Agostino Pearson test. Parametrically distributed data are summarized as mean (± SD) and nonparametrically distributed data are summarized as median (interquartile range; IQR). Comparisons between 2 groups were made using unpaired t tests for parametrically distributed data or by Mann–Whitney U test for nonparametrically distributed data. Comparisons between multiple groups were made using 1-way ANOVA for parametrically distributed data and Kruskal–Wallis for nonparametrically distributed data. Categorical variables are presented in terms of frequencies and percentages. Proportions of categorical variables were compared between groups using chi-squared tests. Linear regression was used to assess associations between baseline characteristics and plasma kisspeptin levels. Logistic regression was used to assess associations between plasma kisspeptin levels and antenatal complication diagnoses. Multivariable logistic regression models were used to account for gestational age at the time of sampling and adjust for maternal age, ethnicity, body mass index (BMI), smoking status, and parity. A P value of < 0.05 was considered statistically significant.
Results
Study Population
Among 105 women with complicated pregnancies there was a total of 117 diagnoses of pregnancy complications (24 PET, 14 PIH, 24 FGR, 41 GDM, and 14 PTB). This larger number of diagnoses was because 10 of these 105 women with pregnancy complications had more than one pregnancy complication diagnosed (eg, both PET and FGR) and these 10 women were excluded from analyses of the specific pregnancy complication (eg, PET alone). Thus, 95 women were included in analyses of individual pregnancy complications: PET (n = 20), PIH (n = 12), FGR (n = 17), GDM (n = 35), and PTB (n = 11). In total, 370 patients provided 1273 plasma kisspeptin samples (24). No significant differences in baseline characteristics of maternal age, BMI, smoking status, gravidity, or parity were observed between control and antenatal complication group (Table 1).
Table 1.
Baseline characteristics and pregnancy outcomes of the total sample (N) and pregnancies grouped by antenatal complications
| Controls N = 265 | PET N = 20 | Severe PET N = 13 | Nonsevere PET N = 7 | PIH N = 12 | FGR N = 17 | GDM N = 35 | PTB N = 11 | Pts with > 1 complication N = 10 | All pregnancies N = 370 | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal age (years) | 32.2 ± 5.3 | 33.2 ± 5.5 | 33.7 ± 6.0 | 32.0 ± 5.2 | 32.5 ± 6.1 | 28.7 ± 6.8 | 33.8 ± 4.8 | 30.0 ± 5.7 | 33.8 ± 8.4 | 32.2 ± 5.5 | 0.217 |
| BMI (kg/m 2 ) | 24.3 ± 4.8 | 27.7 ± 5.1 | 28.6 ± 7.3 | 29.1 ± 4.7 | 27.7 ± 7.3 | 26.0 ± 4.8 | 26.9 ± 4.1 | 24.2 ± 4.2 | 29.6 ± 6.9 | 25.1 ± 5.1 | 0.143 |
| Gravida | 3.0 ± 1.9 | 3.0 ± 2.1 | 2.6 ± 2.1 | 3.0 ± 2.2 | 3.0 ± 1.9 | 2.3 ± 1.5 | 2.5 ± 1.7 | 3.4 ± 1.4 | 3.0 ± 2.4 | 2.9 ± 1.9 | 0.528 |
| Parity | 0.7 ± 1.0 | 0.6 ± 0.9 | 0.5 ± 1.1 | 0.6 ± 0.8 | 0.8 ± 1.3 | 0.5 ± 0.9 | 0.5 ± 0.7 | 0.7 ± 0.9 | 0.5 ± 1.0 | 0.7 ± 0.9 | 0.268 |
| Maternal ethnicity | |||||||||||
| White | 184 (69.4%) | 11 (55.0%) | 6 (46.2%) | 3 (42.9%) | 8 (66.7%) | 7 (41.2%) | 13 (37.1%) | 9 (81.8%) | 3 (30.0%) | 235 (63.5%) | <0.0005 |
| Black | 31 (11.7%) | 5 (25.0%) | 5 (38.5%) | 2 (28.6%) | 2 (16.7%) | 8 (47.1%) | 6 (17.1%) | 0 (0.0%) | 4 (40.0%) | 56 (15.1%) | |
| Asian | 27 (10.2%) | 3 (15.0%) | 1 (7.7%) | 1 (14.3%) | 1 (8.3%) | 1 (5.9%) | 12 (34.3%) | 1 (9.1%) | 1 (10.0%) | 46 (12.4%) | |
| Other | 23 (8.7%) | 1 (5.0%) | 1 (7.7%) | 1 (14.3%) | 1 (8.3%) | 1 (5.9%) | 4 (11.4%) | 1 (9.1%) | 2 (20.0%) | 33 (8.9%) | |
| Smoking | 30 (11.3%) | 2 (10.0%) | 0 (0.0%) | 1 (14.3%) | 1 (8.3%) | 3 (17.6%) | 2 (5.7%) | 1 (9.1%) | 1 (10.0%) | 40 (10.8%) | 0.929 |
| Alcohol consumption | 165 (62.3%) | 12 (60.0%) | 7 (53.8%) | 5 (71.4%) | 9 (75.0%) | 9 (52.9%) | 22 (62.9%) | 3 (27.3%) | 7 (70%) | 227 (61.4%) | 0.007 |
| Baby weight | 3398 ± 441 | 3126 ± 560 | 2892 ± 763 | 3272 ± 560 | 3212 ± 353 | 2091 ± 511 | 3119 ± 596 | 2621 ± 589 | 2007 ± 813 | 3229 ± 605 | <0.0005 |
| Baby weight centile | 60.6 ± 27.6 | 62.6 ± 30.8 | 41.9 ± 35.7 | 68.6 ± 28.5 | 56.6 ± 24.0 | 5.2 ± 7.5 | 58.4 ± 31.6 | 68.5 ± 16.1 | 28.9 ± 34.5 | 57.0 ± 30.2 | 0.750 |
| Baby gender | |||||||||||
| Male | 109 (41.1%) | 9 (45.0%) | 9 (69.2%) | 3 (42.9%) | 7 (58.3%) | 9 (52.9%) | 19 (54.3%) | 5 (45.5%) | 4 (40.0%) | 162 (43.8%) | |
| Female | 145 (54.7%) | 11 (55.0%) | 4 (30.7%) | 4 (57.1%) | 5 (41.7%) | 8 (47.1%) | 15 (42.9%) | 6 (54.5%) | 6 (60.0%) | 196 (53.0%) |
Mean ± SD for continuous, normally distributed variables, and numbers of patients (percentages) for categorical variables are presented. differences in mean and proportion were tested with ANOVA and Chi2, respectively. A P value of < 0.05 was classified as statistically significant. Severe and nonsevere PET were presented as subgroups and were not compared statistically. Alcohol consumption indicates maternal alcohol consumption at all during pregnancy. Smoking indicates maternal smoking during pregnancy.
Abbreviations: BMI, body mass index; FGR, fetal growth restriction; GDM, gestational diabetes mellitus; PET, pre-eclamptic toxemia; PIH, pregnancy-induced hypertension; PTB, preterm birth; pts, patients.
Plasma Kisspeptin Levels in Healthy Control Pregnancies
Multivariable linear regression was used to determine baseline factors that contributed to the variability in plasma kisspeptin levels in healthy pregnancies. Gestational age was positively associated with plasma kisspeptin levels, whereas maternal BMI and Afro-Caribbean ethnicity were negatively associated with plasma kisspeptin levels (24).
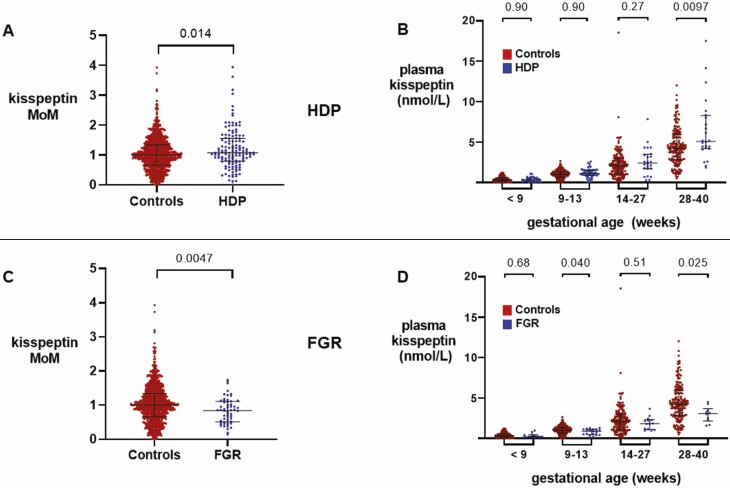
Plasma Kisspeptin Levels in Patients With Hypertensive Disorders of Pregnancy
MoM kisspeptin levels were higher in HDP-affected pregnancies than in healthy control pregnancies (P = 0.014) (Fig. 1A). The rate of rise in plasma kisspeptin with gestation throughout pregnancy was higher in pregnancies affected by HDP than in control pregnancies (P < 0.0001) (24).
Figure 1.
Kisspeptin levels throughout pregnancy in women with hypertensive disorders of pregnancy (HDP) compared with control pregnancies and in women with fetal growth restriction (FGR) compared with control pregnancies. A, Scatterplot of medians (IQR) of multiples of gestation-specific median kisspeptin levels in healthy controls (red) (n = 265 women providing 898 samples) and women with HDP (blue) (n = 32 women providing 133 samples); groups were compared by Mann–Whitney U Test. B, Medians (IQR) of plasma kisspeptin levels in healthy controls (red) (n = 265 women providing 898 samples) and women with HDP (blue) (n = 32 women providing 133 samples) over the early and late first trimester, and second and third trimesters. Data were analyzed by Mann–Whitney U Test. C, Scatterplot of medians (IQR) of multiples of gestation-specific median kisspeptin levels in healthy controls (red) (n = 265 women providing 898 samples) and women with FGR (blue) (n = 17 women providing 56 samples); groups were compared by Mann–Whitney U Test. D, Medians (IQR) of plasma kisspeptin levels in healthy controls (red) (n = 265 women providing 898 samples) and women with FGR (blue) (n = 17 women providing 56 samples) over the early and late first trimester, and second and third trimesters. Data were analyzed by Mann–Whitney U Test.
We compared unadjusted kisspeptin levels stratified by trimester at the time of measurement; unadjusted plasma kisspeptin levels were higher in HDP pregnancies in the third trimester (28-40 weeks) than in control pregnancies (P = 0.0097), whereas levels in earlier gestations did not differ (Fig. 1B). The odds of HDP, adjusted for maternal age, ethnicity, BMI, smoking status, and parity, were increased by 30% (95% CI, 16%-47%) for every 1 nmol/L increase in plasma kisspeptin (P < 0.0001) (Table 2).
Table 2.
Association between plasma kisspeptin and hypertensive disorders of pregnancy
| Crude | Adjusted | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Plasma kisspeptin (nmol/L) | 1.12 (1.04-1.20) | 0.004 | 1.30 (1.16-1.47) | <0.0001 |
| Gestational age (weeks) | 1.00 (0.98-1.02) | 0.972 | 0.95 (0.92-0.98) | 0.003 |
| Maternal age (years) | 1.04 (1.01-1.8) | 0.018 | 1.03 (0.99-1.08) | 0.089 |
|
Maternal ethnicity
(vs Caucasian) |
||||
| Afro-Caribbean | 1.70 (1.06-2.71) | 0.027 | 1.28 (0.75-2.18) | 0.374 |
| Asian | 1.23 (0.72-2.12) | 0.452 | 0.82 (0.46-1.45) | 0.493 |
| Other | 0.59 (0.25-1.40) | 0.232 | 0.51 (0.21-1.24) | 0.139 |
| Maternal BMI (kg/m 2 ) | 1.11 (0.08-1.15) | <0.0001 | 1.14 (1.10-1.18) | <0.0001 |
| Cigarette smoker | 1.00 (0.99-1.00) | 0.215 | 0.99 (0.99-1.00) | 0.121 |
| Parity | 0.96 (0.79-1.18) | 0.721 | 0.78 (0.63-0.97) | 0.025 |
Logistic regression was used to assess the association between (1) kisspeptin with HDP diagnosis in univariable analysis and (2) after adjustment for gestational age (estimated using CRL), maternal age, ethnicity, BMI, smoking status, and parity. Odds ratios denote odds of HDP diagnosis for every 1 nmol/L increase in plasma kisspeptin. A P value of < 0.05 was classified as significant. Bold numbers indicate statistically significant predictors of HDP after adjustment.
Abbreviations: BMI; body mass index; CRL, crown-rump length; HDP, hypertensive disorders of pregnancy; OR, odds ratio.
Interestingly, the rate of increase in kisspeptin levels with gestation was higher in women with late-onset PET (ie, onset of PET at 34 weeks of gestation or later) compared with controls and women with early-onset PET (P = 0.0040) (Fig. 2A). Late first trimester kisspeptin levels (9-13 weeks) in women with late-onset PET were higher than in controls, whereas early-onset PET levels were lower than in controls (P = 0.0029) (Fig. 2B). In an exploratory analysis, women with PET and IUGR had lower kisspeptin levels than those who had PET alone, but higher levels than women with IUGR alone (P = 0.0004) (Fig. 2C). Moreover, the rate of increase in kisspeptin levels with gestation did not significantly differ by the severity of PET (Fig. 2D).
Plasma Kisspeptin Levels in Patients With Fetal Growth Restriction
MoM kisspeptin was lower in FGR-affected pregnancies than in healthy pregnancies (P = 0.0047) (Fig. 1C). The rate of increase in plasma kisspeptin with gestation throughout pregnancy was lower in pregnancies affected by FGR than in control pregnancies (P = 0.0040) (24). Plasma kisspeptin levels in the late first trimester (9-13 weeks) and third trimester (28-40 weeks) were lower in FGR than in control pregnancies (P = 0.040 and P = 0.025, respectively) (Fig. 1D). The odds of FGR adjusted for maternal age, ethnicity, BMI, smoking status, and parity were decreased by 28% (95% CI, 4%-46%) for every 1 nmol/L increase in plasma kisspeptin (P = 0.025) (Table 3). There was no significant association between birthweight and plasma kisspeptin in women with healthy pregnancies (data not presented).
Table 3.
Association between plasma kisspeptin and fetal growth restriction
| Crude | Adjusted | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Plasma kisspeptin (nmol/L) | 0.89 (0.74- 1.06) | 0.188 | 0.72 (0.54-0.96) | 0.025 |
| Gestational age (weeks) | 1.00 (0.98-1.03) | 0.757 | 1.04 (1.00-1.09) | 0.053 |
| Maternal age (years) | 0.86 (0.82-0.90) | <0.0001 | 0.90 (0.85-0.95) | <0.0001 |
| Maternal Ethnicity (vs Caucasian) | ||||
| Afro-Caribbean | 5.50 (3.05-9.95) | <0.0001 | 4.74 (2.39-9.41) | <0.0001 |
| Asian | 0.46 (0.11-1.95) | 0.289 | 0.55 (0.13-2.40) | 0.427 |
| Other | 1.44 (0.49-4.25) | 0.506 | 1.59 (0.52-4.85) | 0.416 |
| Maternal BMI (kg/m 2 ) | 1.02 (0.96-1.06) | 0.562 | 0.98 (0.92-1.04) | 0.461 |
| Cigarette smoker | 1.01 (1.00-1.02) | 0.006 | 1.01 (1.00-1.01) | 0.154 |
| Parity | 0.77 (0.54-1.10) | 0.157 | 0.75 (0.51-1.11) | 0.155 |
Logistic regression was used to assess the association between (1) kisspeptin with FGR diagnosis in univariable analysis and (2) after adjustment for gestational age (estimated using CRL), maternal age, ethnicity, BMI, smoking status, and parity. Odds ratios denote odds of FGR diagnosis for every 1 nmol/L increase in plasma kisspeptin. A P value of < 0.05 was classified as significant. Bold numbers indicate statistically significant predictors of FGR after adjustment.
Abbreviations: BMI; body mass index; CRL, crown-rump length; FGR, fetal growth restriction; HDP, hypertensive disorders of pregnancy; OR, odds ratio.
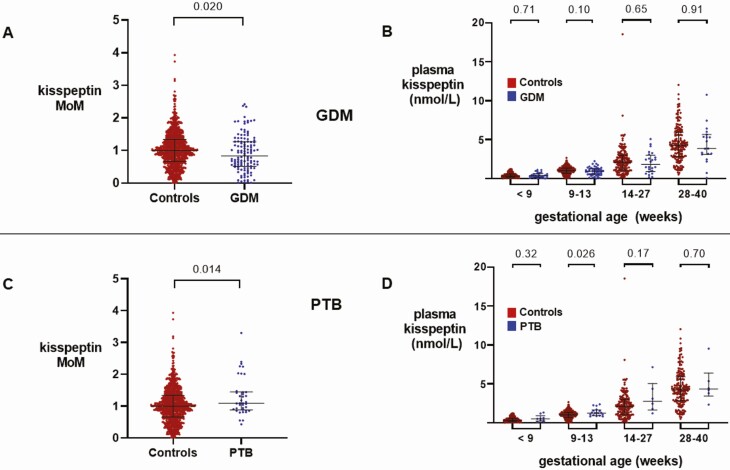
Plasma Kisspeptin Levels in Patients With Gestational Diabetes Mellitus
Overall, MoM kisspeptin was lower in GDM-affected pregnancies than in healthy control pregnancies (P = 0.020) (Fig. 3A). However, there was no difference in the rate of increase in plasma kisspeptin with gestation throughout pregnancy between GDM and healthy control pregnancies (P = 0.90) (24). When unadjusted kisspeptin levels were analyzed by each trimester, there were no differences between plasma kisspeptin levels between GDM and healthy control pregnancies in any trimester (Fig. 3B). Plasma kisspeptin levels were not significantly altered in GDM pregnancies, both in univariable analysis and after adjustment for maternal age, ethnicity, BMI, smoking status, and parity (24).
Figure 3.
Kisspeptin levels throughout pregnancy in women with gestational diabetes mellitus (GDM) compared with control pregnancies and in women with preterm birth (PTB) compared with control pregnancies. A, Scatterplot of medians (IQR) of multiples of gestation-specific median kisspeptin levels in healthy controls (red) (n = 265 women providing 898 samples) and women with GDM (blue) (n = 35 women providing 122 samples); groups were compared by Mann–Whitney U Test. B, Medians (IQR) of plasma kisspeptin levels in healthy controls (red) (n = 265 women providing 898 samples) and women with GDM (blue) (n = 35 women providing 133 samples) over the early and late first trimester, and second and third trimesters. Data were analyzed by Mann–Whitney U Test. C, Scatterplot of medians (IQR) of multiples of gestation-specific median kisspeptin levels in healthy controls (red) (n = 265 women providing 898 samples) and women with PTB (blue) (n = 11 women providing 38 samples); groups were compared by Mann–Whitney U Test. D, Medians (IQR) of plasma kisspeptin levels in healthy controls (red) (n = 265 women providing 898 samples) and women with PTB (blue) (n = 11 women providing 38 samples) over the early and late first trimester, and second and third trimesters. Data were analyzed by Mann–Whitney U Test.
Plasma Kisspeptin Levels in Women With Preterm Birth
Of the 11 women with PTB, only 1 had an emergency cesarean section for fetal distress, whereas the remainder delivered by spontaneous vaginal delivery. MoM kisspeptin at all gestations was higher in PTB-affected pregnancies than in control pregnancies (P = 0.014) (Fig. 3C). However, there was no difference in the rate of increase in plasma kisspeptin levels with gestation between PTB and control pregnancies (P = 0.39) (24). When unadjusted kisspeptin levels were analyzed per trimester, plasma kisspeptin levels in the late first trimester (9-13 weeks) were higher in PTB than in control pregnancies (P = 0.026) (Fig. 3D). The odds of PTB adjusted for maternal age, ethnicity, BMI, smoking status, and parity were increased by 20% (95% CI, 1%-42%) for every 1 nmol/L increase in plasma kisspeptin (P = 0.036) (24).
Discussion
In the present study, we investigated whether circulating kisspeptin levels are altered in women affected by antenatal complications and report the specific trimester at which changes in plasma kisspeptin levels occur. We found that unadjusted plasma kisspeptin levels were principally abnormal in the third trimester of pregnancies affected by HDP and FGR. Multiple of gestation-specific median values of kisspeptin were higher in pregnancies affected by PTB (P = 0.014), and lower in those affected by GDM (P = 0.020). These data are consistent with the suggestion that changes in plasma kisspeptin levels could reflect placental dysfunction manifested clinically as pregnancy complications.
Plasma kisspeptin levels were increased during the third trimester in pregnancies affected by HDP. Several studies have reported increased placental KISS1 expression in the third trimester of pregnancies affected by HDP in comparison with uncomplicated pregnancies (19, 35-38). However, most studies have reported lower circulating kisspeptin levels in women with PET than in normotensive controls during the first (1.554 vs 1.995 nmol/L) (18), second (4.46 vs 10.3 nmol/L) (15), (1109 vs 1188 pg/mL) (20), and third trimesters (0.58 vs 1.66 ng/mL) (19). The constraint of plasma kisspeptin alterations to occurring only during later trimesters in our study is not consistent with the hypothesis that these complications are routed in abnormalities of placentation, which occurs during the first trimester. However, it is noteworthy that circulating kisspeptin levels do not necessarily proportionately reflect placental KISS1 expression; ie, while circulating kisspeptin levels rise throughout pregnancy (14), placental KISS1 expression peaks in the first trimester and subsequently declines (11, 39). In keeping with this, it has been noted that circulating kisspeptin levels increase in multiple pregnancy in proportion to the number of chorions (13). Consequently, circulating kisspeptin levels are likely to reflect changes in both KISS1 expression and placental mass.
Several large studies have reported subset-specific associations between PET and placental mass (40-42). Early-onset (<34 weeks) PET, arising due to defective trophoblast invasion, incomplete spiral artery remodeling, and subsequent placental insufficiency, is associated with low placental mass (40, 41). Late-onset (≥ 34 weeks) PET is believed to arise from hypoxic stress in term placentae as they outgrow their circulation, restricting intervillous perfusion, and is associated with increased placental mass (42, 43). However, Qiao et al reported that third-trimester placental KISS1 overexpression was limited to early-onset PET (38). Furthermore, low placental mass is associated with HDP severity (44), ie, placental mass decreases from PIH, through late-onset PET, to early-onset PET. In the present study, late-onset PET was associated with an increased trajectory of plasma kisspeptin rise with gestation than in early-onset PET or controls, predominantly driven by higher levels in the second trimester.
Previous reports have identified a negative association between circulating kisspeptin levels and severity of HDP; Cetokovic observed lower kisspeptin levels in PET than in controls, but not in PIH (15). Likewise, Ziyaraa reported lower kisspeptin levels in severe PET (1.59 ± 0.26 ng/mL), but not in mild PET (2.18 ± 0.76 ng/mL), in comparison with normotensive controls (2.30 ± 0.51 ng/mL) during the second trimester (although not during the third trimester) (16). However, Adali reported a trend toward even lower third-trimester kisspeptin levels in severe PET (1.17 ± 0.24 ng/mL) than in mild PET (2.61 ± 0.40 ng/mL), as compared with controls (9.69 ± 1.35 ng/mL) (17). Thus, an increasing severity of PET may be associated with reductions in circulating kisspeptin levels. However, we did not find significant alterations in plasma kisspeptin with severity of PET in the present study. Furthermore, we were careful to distinguish participants with more than 1 pregnancy complication that could have disparate effects on plasma kisspeptin levels; which, to our knowledge, was only done in 1 previous study (19). For example, we observed that PET complicated by IUGR was associated with an attenuation of the increase in kisspeptin levels observed in women with PET alone.
Another potential confounder relevant to HDP is BMI; we observed a negative association between BMI and kisspeptin levels in healthy control pregnancies. Notably, obesity is also a known risk factor for PET (45). Logie et al reported lower kisspeptin levels during the second trimester in obese normotensive women than in lean normotensive controls, and even lower levels in obese women who subsequently developed PET (46). Such complexities in the categorization, severity, and onset of PET, and correction for possible confounders such as BMI and gestational age, could explain differences between kisspeptin levels observed in the present study and in other reports.
In FGR in the absence of PET, we observed lower third-trimester plasma kisspeptin levels than in controls. This is consistent with the existing literature; 3 studies have previously reported reduced circulating kisspeptin levels in FGR compared with controls, during the first (1376 ± 1317 vs 2035 ± 1260 pmol/L) (21), second (1164 vs 1188 pg/mL) (20), and third (1.6 ± 0.3 vs 2.9 ± 0.6 ng/mL) trimesters (22). FGR is hypothesized to arise from abnormal trophoblast invasion and spiral artery conversion that limits oxygen supply to the placenta (47, 48). The resulting ischemic injury generates reactive oxygen species (49), leading to apoptosis and restriction of placental and fetal growth (50-52). Thus, low circulating kisspeptin levels could reflect low placental mass (53, 54) in pregnancies affected by FGR (20, 21, 55).
We observed no significant differences in gestation-specific kisspeptin levels in pregnancies affected by GDM; however, the gestation adjusted kisspeptin levels from all trimesters were lower than in control pregnancies. Previous data thus far are inconclusive, with 2 studies reporting lower second- and third-trimester plasma kisspeptin levels in women with GDM than in controls (15, 23), while another reported no differences (56). Nonetheless, kisspeptin has been proposed to play a significant role in beta-cell adaptation to the pregnant state (23). Bowe et al reported impaired insulin response to glucose in pregnant mice following pharmacological kisspeptin blockade (23), and a glucose intolerant phenotype in beta-cell Kiss1R knockout pregnant mice (23).
Recently, a putative role for kisspeptin in the initiation of labor has emerged (57). Intracerebroventricular administration of kisspeptin to late trimester pregnant rats has been demonstrated to increase firing of oxytocin neurons (57). Reverse-transcriptase polymerase chain reaction studies have also identified higher placental KISS1 mRNA expression in preterm placentae than in those of term cesarean sections, which, in turn, demonstrated higher levels of KISS1 mRNA expression than placentas delivered vaginally at term (58). However, no alterations in circulating kisspeptin have been observed during the third trimester in those studies (58), in keeping with the results of the present study.
Kisspeptin exists in several physiological isoforms, produced through splicing of a 154-amino acid pre-propeptide encoded by the KISS1 gene (14). While only kisspeptin -13, -14, and -54 isoforms have been isolated from placental trophoblasts (59), matrix-assisted laser desorption/ionization–time of flight analysis (MALDI-TOF; a form of mass spectrometry) of trophoblast culture medium has yielded kisspeptin-10, -13, -14, and -54 (11). The major circulating kisspeptin isoform in pregnancy is believed to be kisspeptin-54 (14). However, only kisspeptin-10 has been shown to increase intracellular Ca2+ levels (via Kiss1R binding) in trophoblast cells expressing physiological levels of Kiss1R in vitro (11, 60). It is possible that kisspeptin-10 is spliced from kisspeptin-54 to act at a cellular level, before reaching the circulation (61). Thus, further data assaying specific kisspeptin isoforms would be of interest (62).
In conclusion, this is the first study to directly compare circulating kisspeptin levels between healthy pregnancies and those complicated by HDP, FGR, GDM, and PTB throughout all 3 trimesters. We identified higher circulating levels of kisspeptin in HDP and lower kisspeptin levels in FGR-affected pregnancies than in uncomplicated control pregnancies. Our data on circulating kisspeptin levels in HDP are in keeping with published data reporting increased expression of kisspeptin in women affected by HDP (19, 35-38) and with data reporting reduced circulating kisspeptin levels in pregnancies complicated by FGR. Although differences were observed in plasma kisspeptin levels of women with pregnancy complications compared to healthy controls, there was sufficient heterogeneity in the perturbations, to limit clinical application of plasma kisspeptin for diagnosis of these complications. However, these data provide insight into kisspeptin’s potential to reflect placental dysfunction associated with these placental complications throughout pregnancy and highlight the potential of circulating kisspeptin to reflect the risk of pregnancy complications.
Acknowledgments
This paper presents independent research supported by the National Institute for Health Research (NIHR) Clinical Research Facility and the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The Section of Endocrinology and Investigative Medicine is funded by grants from the MRC and NIHR. A.A. is supported by an NIHR Clinician Scientist award (CS-2018-18-ST2-002). M.A.M. is funded by the Tommy’s National Centre for Miscarriage Research. M.P. is supported by an NIHR Academic Clinical Lectureship. C.I.-E. is supported by an Imperial College-BRC IPPRF Fellowship. S.A.C. is supported by an NIHR Academic Clinical Lectureship. E.G.M. is supported by an MRC clinical training fellowship (MR/T006242/1). L.Y. is supported by an MRC clinical training fellowship (MR/R000484/1). A.N.C. is supported by the NHS and BRC. T.B. is supported by the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust. W.S.D. is supported by an NIHR Research Professorship (RP-2014-05-001).
Financial Support: NIHR Clinical Scientist Award (CS-2018-18-ST2-002), Tommy’s National Centre for Miscarriage Research, NIHR Academic Clinical Lectureship, Imperial College-BRC Imperial Post-Doctoral, Post-CCT Research Fellowship Fellowship, Medical Research Council clinical training fellowship (MR/T006242/1), MRC clinical training fellowship (MR/R000484/1), NIHR Biomedical Research Centre, NIHR Professorship (RP-2014-05-001).
Author Contributions: A.A., M.A.M., M.P., E.D., B.P., P.C.E., R.N., C.I.-E., S.A.C., E.G.M., T.H., E.P., L.Y., P.B., A.N.C., T.W.K., C.K., H.F., T.B., and W.S.D. designed the study, analyzed the data, prepared the manuscript, and designed the figures and tables. A.A., M.A.M., M.P., P.C.E., C.K., and H.F. conducted data collection. A.A., M.A.M., M.P., E.D., B.P., P.C.E., R.N., C.K., and T.W.K. performed statistical analysis. T.B. and W.D. were the project supervisors, reviewed and edited the manuscript, and are the guarantors of this research project. All authors have made a substantial, direct, and intellectual contribution to the work and approved the manuscript prior to its submission.
Glossary
Abbreviations
- BMI
body mass index
- CRL
crown-rump length
- FGR
fetal growth restriction
- GDM
gestational diabetes mellitus
- hCG
human chorionic gonadotropin
- HDP
hypertensive disorders of pregnancy
- IQR
interquartile range
- IUGR
intrauterine growth restriction
- KISS1R
kisspeptin receptor
- MoM
multiple of median
- PET
pre-eclampsia
- PIH
pregnancy-induced hypertension
- PTB
preterm birth
- SGA
small for gestational age
- WHO
World Health Organization
Additional Information
Disclosures: The research was conducted in the absence of any personal, professional, commercial, or financial relationships that could be construed as a potential conflict of interest. The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1-7. [DOI] [PubMed] [Google Scholar]
- 2. Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009; 6 Suppl 3: 332-336. [PubMed] [Google Scholar]
- 3. Saeedi P, Petersohn I, Salpea P, et al. ; IDF Diabetes Atlas Committee . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 4. Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3-12. [DOI] [PubMed] [Google Scholar]
- 5. Bakketeig LS, Bergsjø P. Perinatal epidemiology. In: International Encyclopedia of Public Health. Elsevier Inc.; 2008:45-53. [Google Scholar]
- 6. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. [DOI] [PubMed] [Google Scholar]
- 7. Sõber S, Reiman M, Kikas T, et al. . Extensive shift in placental transcriptome profile in preeclampsia and placental origin of adverse pregnancy outcomes. Sci Rep. 2015;5:13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13(4):591-599. [DOI] [PubMed] [Google Scholar]
- 9. Hiden U, Bilban M, Knöfler M, Desoye G. Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord. 2007;8(1):31-39. [DOI] [PubMed] [Google Scholar]
- 10. Romero-Ruiz A, Avendaño MS, Dominguez F, et al. . Deregulation of miR-324/KISS1/kisspeptin in early ectopic pregnancy: mechanistic findings with clinical and diagnostic implications. Am J Obstet Gynecol. 2019;220(5):480.e1-480.e17. [DOI] [PubMed] [Google Scholar]
- 11. Bilban M, Ghaffari-Tabrizi N, Hintermann E, et al. . Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117(Pt 8):1319-1328. [DOI] [PubMed] [Google Scholar]
- 12. Costa MA. The endocrine function of human placenta: an overview. Reprod Biomed Online. 2016; 32(1): 14-43. [DOI] [PubMed] [Google Scholar]
- 13. Jayasena CN, Abbara A, Izzi-Engbeaya C, et al. . Reduced levels of plasma kisspeptin during the antenatal booking visit are associated with increased risk of miscarriage. J Clin Endocrinol Metab. 2014;99(12):E2652-E2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horikoshi Y, Matsumoto H, Takatsu Y, et al. . Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88(2):914-919. [DOI] [PubMed] [Google Scholar]
- 15. Cetković A, Miljic D, Ljubić A, et al. . Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr Res. 2012;37(2):78-88. [DOI] [PubMed] [Google Scholar]
- 16. Ziyaraa MA, Hamdan FB, Mousa LR. Correlation of Kisspeptin-10 level and fetal well-being in preeclamptic patients. Taiwan J Obstet Gynecol. 2016;55(6):840-846. [DOI] [PubMed] [Google Scholar]
- 17. Adali E, Kurdoglu Z, Kurdoglu M, Kamaci M, Kolusari A, Yildizhan R. Metastin levels in pregnancies complicated by pre-eclampsia and their relation with disease severity. J Matern Fetal Neonatal Med. 2012;25(12):2671-2675. [DOI] [PubMed] [Google Scholar]
- 18. Madazli R, Bulut B, Tuten A, Aydin B, Demirayak G, Kucur M. First-trimester maternal serum metastin, placental growth factor and chitotriosidase levels in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2012;164(2):146-149. [DOI] [PubMed] [Google Scholar]
- 19. Matjila M, Millar R, van der Spuy Z, Katz A. Elevated placental expression at the maternal-fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens. 2016;6(1):79-87. [DOI] [PubMed] [Google Scholar]
- 20. Armstrong RA, Reynolds RM, Leask R, Shearing CH, Calder AA, Riley SC. Decreased serum levels of kisspeptin in early pregnancy are associated with intra-uterine growth restriction and pre-eclampsia. Prenat Diagn. 2009;29(10):982-985. [DOI] [PubMed] [Google Scholar]
- 21. Smets EM, Deurloo KL, Go AT, van Vugt JM, Blankenstein MA, Oudejans CB. Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates. Prenat Diagn. 2008;28(4):299-303. [DOI] [PubMed] [Google Scholar]
- 22. Khalil SS, Abulfadle KA, Elnagar WM. Serum kisspeptin-10 levels in pregnant women complicated with intrauterine growth restriction with or without preeclampsia. Med J Cairo Univ. 2018; 86(4): 1975-1982. [Google Scholar]
- 23. Bowe JE, Hill TG, Hunt KF, et al. . A role for placental kisspeptin in β cell adaptation to pregnancy. JCI Insight. 2019;4(20):e124540. doi:10.1172/jci.insight.124540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abbara A, Al-Memar M, Phylactou M, et al. . Changes in circulating kisspeptin levels during each trimester in women with antenatal complications—Supplemental Material. Imperial College London Research Data Repository. Deposited July 22, 2021. https://data.hpc.imperial.ac.uk/resolve/?doi=8611&access=. doi: 10.14469/hpc/8611 [DOI]
- 25. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237-e260. doi: 10.1097/AOG.0000000000003891 [DOI] [PubMed] [Google Scholar]
- 26. Williams D, Craft N. Pre-eclampsia. BMJ. 2012;345:e4437. [DOI] [PubMed] [Google Scholar]
- 27. National Institute for Care and Excellence. Hypertension in pregnancy: diagnosis and management. NICE guideline [NG133]. June 25, 2019. https://www.nice.org.uk/guidance/ng133 [PubMed]
- 28. Brian Jacklin P, Maresh MJ, Patterson CC, et al. . A cost-effectiveness comparison of the NICE 2015 and WHO 2013 diagnostic criteria for women with gestational diabetes with and without risk factors. BMJ Open 2017;7:16621. doi:10.1136/bmjopen-2017-016621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tucker J, McGuire W. Epidemiology of preterm birth. BMJ. 2004;329(7467):675-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Unterscheider J, Daly S, Geary MP, et al. . Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol. 2013;208(4):290.e1-290.e6. [DOI] [PubMed] [Google Scholar]
- 31. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol. 1985;151(3):333-337. [DOI] [PubMed] [Google Scholar]
- 32. McCowan LM, Roberts CT, Dekker GA, et al. ; SCOPE consortium . Risk factors for small-for-gestational-age infants by customised birthweight centiles: data from an international prospective cohort study. BJOG. 2010;117(13):1599-1607. [DOI] [PubMed] [Google Scholar]
- 33. Dhillo WS, Chaudhri OB, Patterson M, et al. . Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609-6615. [DOI] [PubMed] [Google Scholar]
- 34. Napolitano R, Dhami J, Ohuma EO, et al. . Pregnancy dating by fetal crown-rump length: a systematic review of charts. BJOG. 2014;121(5):556-565. [DOI] [PubMed] [Google Scholar]
- 35. Kapustin RV, Drobintseva AO, Alekseenkova EN, et al. . Placental protein expression of kisspeptin-1 (KISS1) and the kisspeptin-1 receptor (KISS1R) in pregnancy complicated by diabetes mellitus or preeclampsia. Arch Gynecol Obstet. 2020;301(2):437-445. [DOI] [PubMed] [Google Scholar]
- 36. Zhang H, Long Q, Ling L, Gao A, Li H, Lin Q. Elevated expression of KiSS-1 in placenta of preeclampsia and its effect on trophoblast. Reprod Biol. 2011;11(2):99-115. [DOI] [PubMed] [Google Scholar]
- 37. Vazquez-Alaniz F, Galaviz-Hernandez C, Marchat LA, et al. . Comparative expression profiles for KiSS-1 and REN genes in preeclamptic and healthy placental tissues. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):67-71. [DOI] [PubMed] [Google Scholar]
- 38. Qiao C, Wang C, Zhao J, Liu C, Shang T. Elevated expression of KiSS-1 in placenta of Chinese women with early-onset preeclampsia. Plos One. 2012;7(11):e48937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cartwright JE, Williams PJ. Altered placental expression of kisspeptin and its receptor in pre-eclampsia. J Endocrinol. 2012;214(1):79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eskild A, Vatten LJ. Do pregnancies with pre-eclampsia have smaller placentas? A population study of 317 688 pregnancies with and without growth restriction in the offspring. BJOG. 2010; 117(12): 1521-1526. [DOI] [PubMed] [Google Scholar]
- 41. Dahlstrøm B, Romundstad P, Øian P, Vatten LJ, Eskild A. Placenta weight in pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87(6):608-611. [DOI] [PubMed] [Google Scholar]
- 42. Nelson DB, Ziadie MS, McIntire DD, Rogers BB, Leveno KJ. Placental pathology suggesting that preeclampsia is more than one disease. Am J Obstet Gynecol. 2014;210(1):66.e1-66.e7. [DOI] [PubMed] [Google Scholar]
- 43. Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol. 2019;134-135:1-10. [DOI] [PubMed] [Google Scholar]
- 44. Krielessi V, Papantoniou N, Papageorgiou I, et al. . Placental pathology and blood pressure’s level in women with hypertensive disorders in pregnancy. Obstet Gynecol Int. 2012;2012:684083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bodnar LM, Ness RB, Markovic N, Roberts JM. The risk of preeclampsia rises with increasing prepregnancy body mass index. Ann Epidemiol. 2005;15(7):475-482. [DOI] [PubMed] [Google Scholar]
- 46. Logie JJ, Denison FC, Riley SC, et al. . Evaluation of kisspeptin levels in obese pregnancy as a biomarker for pre-eclampsia. Clin Endocrinol (Oxf). 2012;76(6):887-893. [DOI] [PubMed] [Google Scholar]
- 47. Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr. 2010;2010:401323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yinon Y, Nevo O, Xu J, et al. . Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3. Am J Pathol. 2008;172(1):77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takagi Y, Nikaido T, Toki T, et al. . Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004;444(1):49-55. [DOI] [PubMed] [Google Scholar]
- 50. Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004;58(6): 894-900. [DOI] [PubMed] [Google Scholar]
- 51. Hafner E, Metzenbauer M, Höfinger D, et al. . Placental growth from the first to the second trimester of pregnancy in SGA-foetuses and pre-eclamptic pregnancies compared to normal foetuses. Placenta. 2003;24(4):336-342. [DOI] [PubMed] [Google Scholar]
- 52. Gabbe S, Niebyl J, Simpson J, et al. Obstetrics: Normal and Problem Pregnancies. 7th edn. Elsevier; 2016. [Google Scholar]
- 53. Molteni RA, Stys SJ, Battaglia FC. Relationship of fetal and placental weight in human beings: fetal/placental weight ratios at various gestational ages and birth weight distributions. J Reprod Med. 1978;21(5):327-334. [PubMed] [Google Scholar]
- 54. Heinonen S, Taipale P, Saarikoski S. Weights of placentae from small-for-gestational age infants revisited. Placenta. 2001;22(5):399-404. [DOI] [PubMed] [Google Scholar]
- 55. Possomato-Vieira JS, Khalil RA. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. In: Advances in Pharmacology. Academic Press Inc.; 2016:361-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Arslan E, Gorkem U, Togrul C. Is there an association between kisspeptin levels and gestational diabetes mellitus? Gynecol Obstet Reprod Med. 2020;26(3):179. [Google Scholar]
- 57. Seymour AJ, Scott V, Augustine RA, Bouwer GT, Campbell RE, Brown CH. Development of an excitatory kisspeptin projection to the oxytocin system in late pregnancy. J Physiol. 2017;595(3):825-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Torricelli M, Galleri L, Voltolini C, et al. . Changes of placental Kiss-1 mRNA expression and maternal/cord kisspeptin levels at preterm delivery. Reprod Sci. 2008;15(8):779-784. [DOI] [PubMed] [Google Scholar]
- 59. Kotani M, Detheux M, Vandenbogaerde A, et al. . The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276(37):34631-34636. [DOI] [PubMed] [Google Scholar]
- 60. Francis VA, Abera AB, Matjila M, Millar RP, Katz AA. Kisspeptin regulation of genes involved in cell invasion and angiogenesis in first trimester human trophoblast cells. Plos One. 2014;9(6):e99680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jayasena CN, Nijher GM, Comninos AN, et al. . The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96(12):E1963-E1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hu KL, Chang HM, Zhao HC, Yu Y, Li R, Qiao J. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum Reprod Update. 2019;25(3):326-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.