Abstract
MUC5AC has been indicated to be a marker for mucinous ovarian cancer (OC). We investigated the use of in situ proximity ligation assay (PLA) for blood group ABH expressing MUC5AC to differentiate between serous and mucinous OC, to validate preceding observations that also MUC5AC ABH expression is increased in mucinous OC. We developed PLA for anti-A, B, and H/anti-MUC5AC and a PLA using a combined lectin Ulex europaeus agglutinin I (UEA I)/anti-MUC5AC assay. The PLAs were verified with mass spectrometry, where mucinous OC secretor positive patients’ cysts fluids containing ABH O-linked oligosaccharides also showed positive OC tissue PLA staining. A nonsecretor mucinous OC cyst fluid was negative for ABH and displayed negative PLA staining of the matched tissue. Using the UEA I/MUC5AC PLA, we screened a tissue micro array of 410 ovarian tissue samples from patients with various stages of mucinous or serous OC, 32 samples with metastasis to the ovaries and 34 controls. The PLA allowed differentiating mucinous tumors with a sensitivity of 84% and a specificity of 97% both against serous cancer but also compared to tissues from controls. This sensitivity is close to the expected incidence of secretor individuals in a population. The recorded sensitivity was also found to be higher compared to mucinous type cancer with metastasis to the ovaries, where only 32% were positive. We conclude that UEA 1/MUC5AC PLA allows glycospecific differentiation between serous and mucinous OC in patients with positive secretor status and will not identify secretor negative individuals with mucinous OC.
Keywords: blood group H, mucin, O-linked glycosylation, ovarian cancer, PLA
Introduction
Ovarian cancer (OC) displays the most lethal gynecologic malignancy (Nash and Menon 2020), and is consisting of a number of subtypes with distinct precursor lesions, tissues of origin, molecular biology backgrounds, clinical presentations, chemosensitivities and patient outcomes (Lheureux et al. 2019). Pathological diagnosis of tumor tissue is essential to correctly define each histological OC subtype: high-grade serous, low-grade serous, clear-cell, endometrioid and mucinous OC.
We and others have identified MUC5AC as a biomarker candidate for mucinous OC, both in regards of mRNA expression and as mucin apoprotein (Albarracin et al. 2000; Wang and El-Bahrawy 2014, 2015; Vitiazeva et al. 2015). No difference in expression between the benign, borderline and malignant ovarian mucinous tumor for immunohistochemical detection of MUC5AC has been found (Hauptmann et al. 2017). In addition to mucinous OC, MUC5AC has also been shown to be dysregulated in cancer in tissues, where MUC5AC is naturally expressed, e.g., lung, cervix, gallbladder and gastric cancer but also in tissues where the MUC5AC background is low/absent, e.g., colorectal and pancreatic cancer (Krishn et al. 2018).
Using tissue-dependent glycosylation may provide further specificity of MUC5AC as a biomarker. Detailed glycomic analysis of the MUC5AC O-linked glycosylation associated with cancer tissue is scarce. The glycosylation profiles of O-linked oligosaccharides from a mixture of MUC5AC and MUC6 found in healthy gastric tissue adjacent to tumor (Kenny et al. 2012; Jin et al. 2017; Adamczyk et al. 2018) are similar to oligosaccharides identified from MUC5AC isolated from mucinous OC, mostly containing core 2 structures with blood group ABH epitopes (Vitiazeva et al. 2015). In contrast, gastric tumor MUC5AC glycosylation is found to be highly sialylated with less blood group antigens (Jin et al. 2017; Adamczyk et al. 2018). Pancreatic healthy tissue (with low MUC5AC expression) carries core 2 blood group containing structures similar to mucinous ovarian tissue (El Jellas et al. 2018), but pancreatic cancer with concomitant upregulation of MUC5AC appears to move towards sialyl Lex expression (Balmaña et al. 2018). Healthy lung mucins enriched in MUC5AC are dominated by sulfated and blood group containing structure on core 4 backbone (Schulz et al. 2004; Hayes et al. 2012), but in lung cancer, MUC5AC is found to express sialyl Lewis x, sialyl Lewis a and Tn/sialyl-Tn (Pinto et al. 2012).
In the current work, we expand on previous observation that blood group ABH expressing MUC5AC mucin is widely detected in mucinous OC cells and less so in the serous subtype. MUC5AC has been shown by tissue staining having a 95–100% incidence with primary mucinous ovarian tumors (Albarracin et al. 2000; Ji et al. 2002; Sugai et al. 2008; Wang and El-Bahrawy 2015), while the incidence of MUC5AC in the more prevalent serous OC subtype has only been reported to be 0–20% (Sugai et al. 2008; Vitiazeva et al. 2015). However, the difference of MUC5AC protein expression between serous and mucinous OC is not reflected in the mRNA expression, since it appears to be present in both types (Giuntoli 2nd et al. 1998). In contrast, in metastatic mucin type colon adenocarcinomas in the ovary, MUC5AC expression was only detected in 10–33% (Albarracin et al. 2000; Wang and El-Bahrawy 2014, 2015). To specifically monitor the existence of MUC5AC carrying blood group ABH determinants on ovarian tumor tissue micro arrays (TMA) we set up in situ proximity ligation assay (PLA; Blokzijl et al. 2014) (n = 427) using PLA probes against MUC5AC and blood group antigens. With this we wanted to evaluate the benefits and deficits monitoring an OC carcinoma associated mucin (single gene product) in combination with its glycosylation. The glycosylation is a consequence of multiple genes involved in its biosynthesis and is often found to be altered during cancer progression.
Results
Blood group ABH and MUC5AC in mucinous ovarian cancer tissue
To verify the presence of blood group ABH epitopes and MUC5AC in mucinous ovarian tumor, we used a benign mucinous ovarian adenoma tissue from a patient with blood group B, in which the epithelial goblet-like cells were be stained by both blood group B and H antibodies (Figure 1A and B). This suggested that the use of a more generic probe against the blood group H would allow detection of all A- B- and O(H)- individuals. This is because the H-antigen Fucα1–2Galβ1- is a precursor for the blood group A transferase (generating Fucα1–2(GalNAcα1–3)Galβ1-) and blood group B transferase (generating Fucα1–2(Galα1–3)Galβ1-). Incomplete biosynthetic conversion of blood group H antigens into blood group A or B, makes blood group A individuals A+H+ and blood group B individuals B+H+.
Fig. 1.
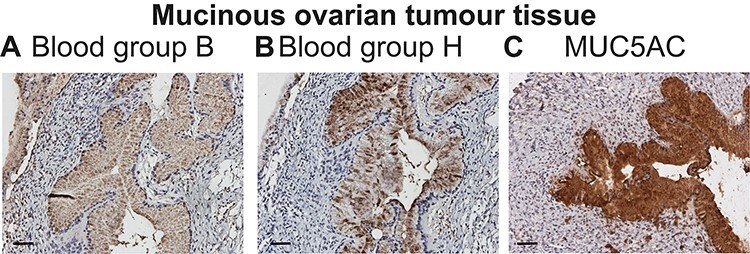
Blood group ABH and MUC5AC expression in mucinous ovarian cancer. Staining of blood group B Se+ mucinous benign tissue using antibodies against blood group B (A), blood group H (B) and MUC5AC (C). Inserted white scale bars represent 50 μm.
Furthermore, we found that the tissue was strongly positive for MUC5AC (Figure 1C). Hence, epithelial goblet-like cells were found to be positive for blood group B, H and MUC5AC, indicating that blood group antibodies in combination with antibody against the MUC5AC mucin could be used together to screen for glycosylation specific blood group ABH carrying MUC5AC in mucinous OC.
Blood group ABH antigens in mucinous ovarian tumor tissue are associated with MUC5AC secretor Se+
Using in situ PLA and liquid chromatography–mass spectrometry (LC–MS), we aimed to demonstrate that in patients diagnosed with mucinous OC, the cysts fluid expression of O-linked oligosaccharides containing ABH antigens is reflected in the same patient by a tissue expression of ABH antigens associated with the MUC5AC-mucin. In three patients confirmed as secretor positive Se+ (Vitiazeva et al. 2015) diagnosed as benign, borderline- and malignant mucinous ovarian tissue, respectively, we observed that the patients’ blood group coincided with the presence of blood group ABH O-linked oligosaccharides in the ovarian cyst fluids as well as with positive PLA signals using pairs of anti-MUC5AC/anti-A, B or H blood groups (Figure 2A–C). We further verified that the blood group A and B positive individuals also contained blood group H structures in the ovarian cyst fluids in addition to their O-linked group A or B determinants. All three selected Se+ ovarian tissues were positive for the MUC5AC/blood group H antibodies used as pair of probes in PLA, and the LC–MS analysis of the samples revealed the presence of blood H independent of the patients’ blood group status.
Fig. 2.
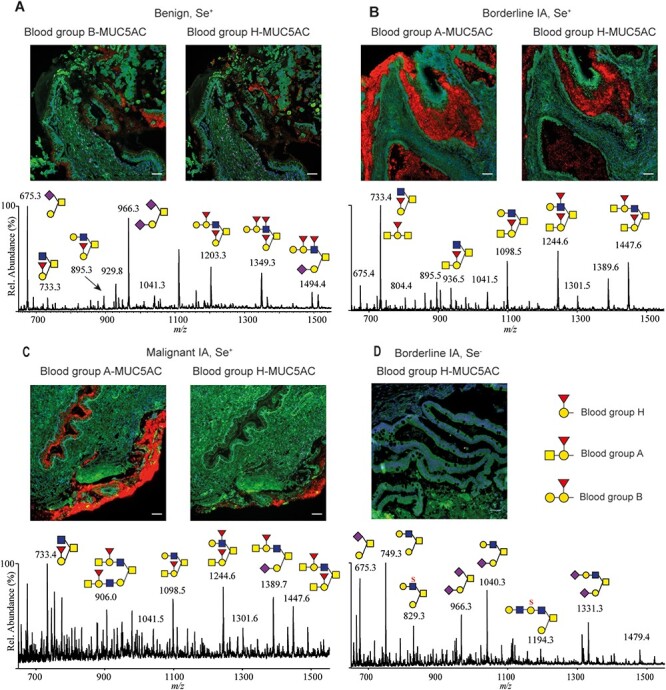
Ovarian cyst fluid ABH O-linked oligosaccharides glycans matched with in situ PLA-positive ABH/MUC5AC-staining. Positive staining from secretor individuals (Se+) from benign (A), borderline (B) and malignant (C) mucinous tumor tissue using PLA matching the patient blood group and MUC5AC as well as blood group H/MUC5AC PLA. The mass spectrometric profiles below images show the presence of oligosaccharides identified containing A, B or H determinants from the patients’ cysts fluid (data from (Vitiazeva et al. 2015). (D) A nonsecretor (Se−) mucinous OC patients’ tissue negative for blood group H/MUC5AC PLA without blood group ABH determinants in the patient’s cysts fluids O-linked oligosaccharides is displayed. Inserted white scale bars represent 50 μm.
In order to demonstrate that not only the ABH glyco-phenotype of the patients was important for the glyco-specificity of the PLA detection, we also verified that the nonsecretors Se− mucinous ovarian borderline patient (Se−, i.e., no ABH present in secretion) identified earlier showed no signal (Figure 2D) (Vitiazeva et al. 2015).
The heterogeneity in staining of tumor tissues was apparent in regards of the ABH expression. This provided additional indications that the glyco-gene expression is important to consider when detecting glyco-specific mucins. Epithelial tissue with PLA positive MUC5AC/blood group A or B areas only partially overlapped the MUC5AC/blood group H positive areas (Figure 2A–C) in consecutive tissue slides. This indicated that the blood group A/B transferase converting blood group H to blood group A or B showed variable expression in different areas of the tissue, whereas blood group H was more widely distributed.
Blood group H/MUC5AC PLA differentiates mucinous and serous ovarian tumor tissue
In order to test if PLA targeting blood group ABH/MUC5AC was able to differentiate between mucinous and serous ovarian tumors, we extended our investigation using four patients with different blood groups diagnosed with either of the two ovarian tumor subtypes (Figure 3). We found that epithelial layer tissues surrounding the cysts from the mucinous subtype were positive for blood group ABH/MUC5AC PLA following the patients’ ABH blood group status as well as all were positive for the H/MUC5AC PLA, although the morphology was different between the benign and the malignant tissues. Whereas the former was observed to have a distinct goblet type morphology with continuous epithelial layers (Figure 3A, B, E and F), the malignant tumor had more patchy positive epithelial areas surrounded by negative areas consisting of tumorous epithelial cells (Figure 3C and G). Negative staining was noted in serous tumor OC cells and stroma from a patient with blood group A (Figure 3D and H).
Fig. 3.
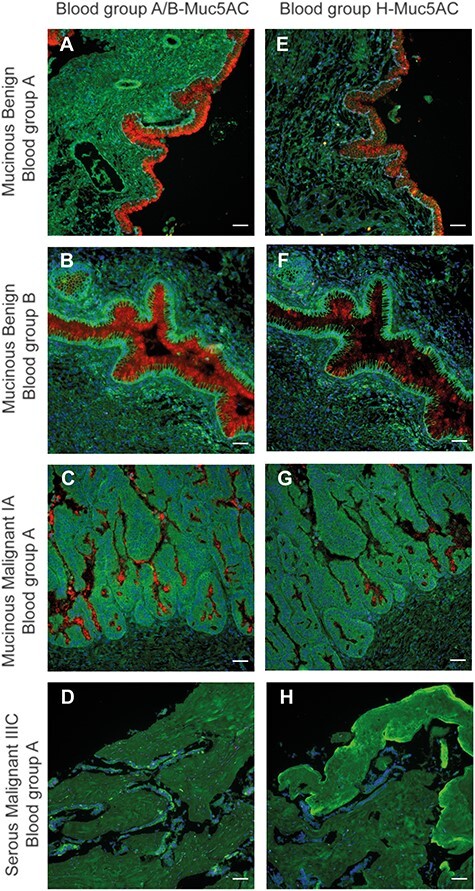
In situ PLA-positive ABH/MUC5AC is associated with mucinous and not serous ovarian tumors. Blood group ABH/MUC5AC PLA screening of benign and malignant tissue biopsies from secretor positive individuals diagnosed with mucinous ovarian tumors. (D) Tumor tissue biopsy from a Se+ individual diagnosed with serous OC. Tissues were probed with PLA blood group A or B together with MUC5AC according to the patients’ recorded blood group (A–D) as well as with blood group H/MUC5AC PLA (E–H). Inserted white scale bars represent 50 μm.
In situ PLA on tumor tissue biopsies
The results from the PLA/blood group validation studies in the previous sections inspired us to establish an in situ PLA to screen a larger number of ovarian tumor tissues laid out in a TMA format. This was to check the incidence of blood group expressing MUC5AC in a larger cohort of serous and mucinous OC tissue, and to check if other factors than the ABH and Se status influence the detection of blood group positive MUC5AC in mucinous OC. We aspired having a blood group independent array; hence, we aimed towards a PLA-based blood group H/MUC5AC type test. To avoid having a bias due to specificity of blood group H antibodies (e.g., O-linked core type (1, 2, 3 and 4) and N-acetyllactosamine type (type 1, 2 or 3)), we used the Ulex europaeus I lectin (UEA I) selectively targeting the Fucα1–2Galβ1- linkage present in all the blood group ABH antigens (Figure 4). The assay using the PLA probe pair of MUC5AC antibody and the lectin UEA I, provided similar results as the blood group H/MUC5AC PLA (compare Figure 3E–G with Figure 4A–C).
Fig. 4.
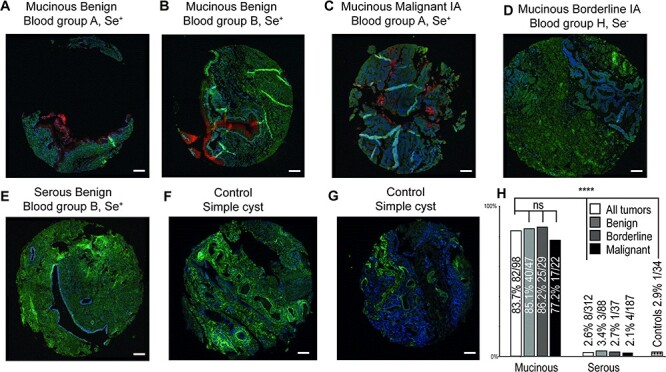
Incidence of in situ PLA-positive UEA I/MUC5AC in ovarian tumor tissue. TMA images using the UEA I/MUC5AC PLA pair on mucinous and serous tumor tissues showing positive staining of epithelial layers of mucinous cells from Se+ individuals (A, B, C), but negative staining of tumor tissue from a Se− individual diagnosed with mucinous OC (D) as well as a serous tumor tissue from a Se+ individual (E). (F) and (G) shows the negative staining of ovarian tissue from two control individuals diagnosed with benign noncancerous simple cysts. The bar graph (H) shows the incidence of positive staining of TMA from 410 patients diagnosed with mucinous (n = 98), serous ovarian (n = 312) tumors and benign noncancerous cyst tissue (34). Percentage and number of positive of the total (in brackets) is displayed over each bar divided up into all stages and subdivided into benign, borderline type and malignant. Contingency was calculated using Fisher’s exact test, where P > 0.05 was considered as not significant (ns). **** means P < 0.0001 significance comparing staining incidence both between mucinous and serous cancer and mucinous cancer against controls. Hematoxylin and eosin staining of the cancer samples are available in Supplementary Figure S1. Inserted white scale bars represent 100 μm.
Using MUC5AC antibody/UEA I as PLA probes, we screened a TMA with primary ovarian tumor tissues from 101 mucinous, 326 serous patients, 32 metastases to the ovary (mainly gastrointestinal-tract) and 34 healthy (functional ovarian cysts). Of these, 98 mucinous and 312 serous tumors could be evaluated for staining with PLA, while all the healthy cysts and metastases to the ovary could be evaluated. Unsuccessful cases included TMA cores where no tumor cells could be detected, or where all three cores were lost during sample processing. Overall, we found that mucinous tissues were stained more frequently (83.7% of the tissues were positive), compared to serous tumor tissues where only 2.6% were positive and 2.9% for healthy controls. The few positive serous tumors were displaying unspecific diffuse staining in stroma areas or staining of undetermined structures. Only one of the two control samples from patient diagnosed with a hemorrhagic cyst showed positive staining of epithelial cells, while other controls (mostly simple cysts) were negative (Figure 4E and F). The positive mucinous samples all showed positive staining of goblet-like cells and adjacent “mucin pools” of secreted material. Tumor stroma showed negative staining. As can be seen in Figure 4, the areas of the TMA cores (1.0 mm in diameter) were only covering limited sections of tumor cells. With TMA providing a binary signal (presence/absence), and limited sampling of the tumor due to the assay format, we restrained the evaluation of the TMA into incidence of positive or negative TMA, not considering % area of positivity and signal intensity. We did not observe any significant difference in the incidence of positive staining between benign/borderline and malignant stages, neither in the serous samples nor in the mucinous samples (Figure 4G). The slightly lower but not statistically significant incidence of blood group positive MUC5AC/UEA1 PLA in malignant mucinous tumors (77.2%) versus the benign (85.1%) and borderline tumors (86.2%) may suggest that nonblood group MUC5AC expressing mucinous ovarian tumors could be more aggressive (Figure 4F). However, the lower (nonsignificant) incidence of blood group antigens in malignant mucinous tumors may also be deferred to the low number of mucinous malignant OC patients in the TMA (n = 22) evaluated by the PLA screen. This low number of patients makes the measured incidence percentage less reliable. With the low number of advanced stage mucinous samples in the TMA (n = 4 of stage III, n = 0 of stage IV), statistically comparing incidence between stages was not feasible. Of the four stage-III samples present in the TMA, one was unsuccessful, two were positive and 1 negative.
Within the TMA, we also had 32 patients (Supplementary Table SI), where the ovary was the secondary tumor site. This allowed us to assess the existence of MUC5AC and/or blood group expression from other sites. We identified 19 patients displaying a mucinous phenotype. The primary origin of these were the gastrointestinal-tract (n = 15), pancreas (n = 1) and uterus (n = 2). Only six (32%) were found positive, indicating that blood group expressing MUC5AC is less frequent in secondary mucinous type cancers in the ovaries compared to primary mucinous OC.
Discussion
A human genome of only approximately 20,000 genes relies on expansion of its biological functionality using post-translational modifications. This puts a focus on proteoforms of expressed proteins to be pathologically relevant. The work in this report shows the potential in combining protein expression with post-translational modification, hence combining two independent biological entities to increase specificity for mucinous ovarian tumor diagnostics. It has been demonstrated in the more studied serous variant of ovarian tumors, that a PLA combination of CA125/MUC16 and MUC1 together with Tn and sialyl-Tn will increase the specificity of differentiating malignant/borderline tissue from benign ovarian tissue with up to 83% selectivity (Ricardo et al. 2015). Interestingly, the glycosylation changes of CA125/MUC16 on ovarian tumor tissue appear to be reflected in circulating CA125/MUC16. A glycospecific assay for serum CA125/MUC16 targeting Tn/sialyl-Tn has shown an area under curve value of 0.931, indicating that glycospecific mucin discovery may be translated into serological screening tests (Gidwani et al. 2020). Moreover, using microarray technique, measuring the sialyl-Tn and sialyl-T version of CA125/MUC16 together with sialyl-Tn form of MUC1 in serum, appears to differentiate benign versus severe OC (Chen et al. 2013).
Using PLA, it has been demonstrated that CA125/MUC16 and MUC1 are the main carrier of sialyl-Lea and sialyl-Lex in malignant/borderline serous ovarian tumors but not in benign cases (Ricardo et al. 2016). A study looking at a larger panel of mucin- and carcinoma-associated carbohydrates has also shown that PLA can be used as a powerful tool to identify new mucin glyco-forms associated with different types of cancers (Pinto et al. 2012). All these studies have relied on using PLA with two different antibodies, one against the mucin, while the other targets the carbohydrate epitopes. With the introduction of lectins in the PLA format (Oliveira et al. 2018), the probing for mucin glyco-forms can be further extended. In this article, we used the U. europaeus I lectin against the fucosylated H-antigen to screen an OC TMA. It is plausible that the transition into blood group ABH MUC5AC in mucinous ovarian tumors is not only a bystander transformation, but also has a functional role in the cancer development. The expression of blood group antigens on cancer tissue may hold a clue to cancer tumorigenesis and metastasis, where the ectopically expression of a MUC5AC mucus layer in the ovaries surrounding itself with carbohydrate blood group ABH self-antigens on MUC5AC would make the tumor area difficult to penetrate and avoid being detected by the immune system. A knock-down of MUC5AC of pancreatic cancer cells in a xenograft model indicated that MUC5AC allows the cell to escape from immunosurveillance (Hoshi et al. 2011).
In this report, the MUC5AC/UEA I PLA on the TMA demonstrated a sensitivity of 84% and a specificity of 97% in correctly identifying mucinous ovarian tumor from serous ovarian tumor and healthy ovarian cyst tissue. The incidence in mucinous cancer tissue is close to the predicted incidence of secretors (80% in European population), indicating that most of the negative tissues is due to the Se− status. We confirmed the existence of Se− mucinous cancer patients in our cohort (Vitiazeva et al. 2015), which were all found to be negative with the MUC5AC/UEA I PLA as exemplified in Figure 3. In saliva, we have shown that the nonsecretor status is associated with expression of sialyl-Lea, also known as CA19-9. Hence, including a probe for MUC5AC/sialyl Lea could be a next step to improve sensitivity of the assays including also the Se− individuals. In order to improve the selectivity, the unspecific staining of stroma and of the assay of some serous TMA samples, suggesting that careful evaluation of the morphology of the staining can exclude additional false positive staining of un-identified structures within the tissue, since none of the false positive in the TMA showed staining of areas identified as epithelial tissue. However, pathological assessment of the mucinous OC is routinely and consistently performed on hematoxylin/eosin-stained tissue. Since neither MUC5AC nor blood-group specific MUC5AC provide additional specificity and selectivity in relation to serous OC, MUC5AC type staining are likely only to be used as complementary or confirmatory. For this application, blood-group specific MUC5AC will suffer by missing out on the Se− population. In addition, our data suggest that Se− individuals are not overrepresented in our malignant ovarian mucinous TMA cohort. However, survival and aggressiveness in relation to Se status needs to be addressed in a more targeted and larger study cohort. Also, we can currently not address if Se status needs to be included in order to improve stratification of patients to improve precision cancer treatment. With glycans being a modulator of the immune system, efficient targeted immunotherapy may have to include knowledge about the patients’ glyco-status.
Only limited information is available for the existence of circulating MUC5AC expressing blood group ABH oligosaccharides for diagnosing the mucinous subtype of ovarian tumors. We found that primary mucinous type cancer from other origin with metastasis to the ovary had a lower incidence (32%) compared to primary mucinous ovarian carcinoma tissue (84%). Since MUC5AC staining could be used for confirmation of primary mucinous ovarian carcinoma versus metastatic carcinoma of the ovaries (Ji et al. 2002), glyco-specific MUC5AC staining may provide additional specificity. The sporadic expression of MUC5AC mucins in mucinous carcinoma originating from pancreas, pseudomyxoma peritonei (O'Connell et al. 2002) and the gastrointestinal tract, suggest that negative signal in the PLA in tumors originating from these sites was a combination of absence of MUC5AC and/or blood group ABH expression.
Using glyco-specific MUC5AC probes to trace the primary origin of circulating MUC5AC may improve serological cancer diagnosis, compared to only measuring the circulating MUC5AC level. Other examples where this type of detection has proven to be successful include diagnosing pancreatic malignancy, where a combination of the total serum CA19-9/sialyl-Lex together with measuring the serum level of sialyl -Lex carried by MUC5AC and CA125/MUC16 have been demonstrated to improve both sensitivity and selectivity (Yue et al. 2011).
Based on the previous reports and our results presented herein, it can be suggested that blood group expression of MUC5AC is present in both late- and early-stage mucinous OC. Hence, circulating glyco-specific MUC5AC could be an approach for cancer diagnostics and tracing the primary origin that express MUC5AC during cancer progression. Our data point out specifically that a glyco-oriented serological test needs to include glyco-probes capable of detecting MUC5AC from ovarian mucinous cancer from Se− individuals. By extrapolating this observation, it becomes obvious that in order to develop an assay for organ-specific cancer using mucins and carbohydrates, there is a requirement to identify and include multiple glyco-probes that reflect the tissue and patient specific glycosylation variation. Using the multitude of available lectins in combination with protein specific antibodies is a way to increase this repertoire for glyco-specific detection using PLA.
Materials and methods
Patient information
Ovarian cyst fluids (n = 19) and ovarian biopsies (n = 476) were selected from a prior collected biobank at Sahlgrenska University Hospital, division for Gynecologic Oncology Surgery, Gothenburg, Sweden, as previously described (Kristjansdottir et al. 2012). The tumor biopsies comprised of samples from patients diagnosed with benign, borderline type and malignant serous and mucinous ovarian tumors, metastasis to the ovaries with primary origin elsewhere and healthy ovaries with functional cysts (Table I). Gothenburg local ethics committee approved the study (Dnr; S324-00 T079-06 and 201-15). Only women with signed written informed consent were included.
Table I.
Clinicopathological characteristics of ovarian tissue evaluated for staining with PLA
| Malignancy n (%) | Stage n (%) | Grade n (%) | Age span (mean age) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Total | Benign | Borderline | Malign | I–II | III–IV | Low | High | Benign | Borderline | Malign |
| Primary | |||||||||||
| Mucinous | 98 | 47 (47.9) | 29 (29.5) | 22 (22.4) | 47 (92.1) | 4 (7.8) | NA | NA | 16–88 (56.8) | 16–85 (50.2) | 36–86 (61.9) |
| Serous | 312 | 88 (28.2) | 37 (11.8) | 187 (59.9) | 71 (31.6) | 153 (68.3) | 18 (9.6) | 129 (68.9) | 23–86 (65.1) | 23–85 (50.1) | 28–88 (62) |
| Metastasis | 32 | See separate Supplementary Table SI | Age 36–91 (60.3) | ||||||||
| Healthy | 34a | NA | NA | NA | NA | NA | NA | NA | Age 16–88 (60.0) | ||
NA: Not applicable.
aSimple cyst (n = 29), follicle cyst (n = 2), hemorrhagic cyst (n = 2), torsio (n = 1).
Immunohistochemistry
Ovarian tumor tissues were processed for immunohistochemical staining according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA, Thermo Scientific). Paraformaldehyde-fixed paraffin-embedded 4-μm sections were deparaffinized, rehydrated and subjected to an antigen retrieval procedure using antigen unmasking solution (Vector Laboratories). The tissue sections were incubated with mouse monoclonal antibody: anti-MUC5AC 1:400 (clone 45 M1; Abcam), mouse anti-blood group anti-blood group B (HEB-29) 1:40 (GeneTex/Abcam), anti-blood group H Antibody 1:40 (clone A70-A/A9; ThermoFisher Scientific). After incubation with secondary antibodies, immunodetection was performed using UltraVision Quanto Detection System HRP (ThermoFisher Scientific).
The TMA was constructed with three cylindrical core biopsies of 1.0-mm diameter from each whole tumor biopsy, which were re-embedded into a new paraffin block, sectioned and mounted onto slides as previously described (Levan et al. 2017). TMAs were designed by study authors (KS and CM) and constructed in house. The whole section was digitally scanned with Leica SCN400 (Leica Microsystems, Milton Keynes, UK) and analyzed using SlidePath Gateway Client LAN software. Diagnostic confirmation was based on morphologic review by gynecological pathologist (CM) of original hematoxylin and eosin slides and any accompanying IHC stains using criteria based on the 2014 World Health Organization Classification of Gynecologic Tumors.
In situ PLA
In situ PLA was performed with paraffin-embedded sections from human ovarian tissues and TMAs for the detection of coexpression in proximity of blood type antigens (ABH) and MUC5AC. Duolink II kit (Olink Bioscience, Uppsala, Sweden) was used according to the manufacturer’s instructions. The paraffin-embedded sections were dewaxed and rehydrated. Heat-induced antigen retrieval was performed using 10 mM Tris, pH 9.0, 1 mM EDTA and 0.05% Tween-20. The sections were incubated with blocking solution (Olink Bioscience, Uppsala, Sweden) for 1 h at 37°C. Primary antibodies against blood type H, A, B (anti-blood group A (1:80; HE193; ThermoFisher Scientific), anti-blood group B (1:40; HEB-29; GeneTex/Abcam), anti-blood group H antibody (1:40; clone A70-A/A9; ThermoFisher Scientific), biotinylated UEA I (1:200; B-1065; Vector Laboratories) and anti-MUC5AC, oligomeric mucus/gel-forming (MUC5AC; N-Term, AA 552–567; 1:100; antibodies-online GmbH)) were used and incubated at 4°C overnight. Secondary antibodies (Olink Bioscience) or streptavidin conjugated with oligonucleotides (Avidomics, CA, USA) were added and incubate for 1 h at 37°C. After washing, ligation and amplification were performed at 37°C for 30 and 90 min, respectively. The cell nuclei were visualized by DAPI. Sections were examined under a Zeiss Imager Z2 Axio fluorescence microscope (Zeiss, Oberkochen, Germany). The proximity ligation resulted in bright red fluorescent dots. Images were acquired using a Plan-Apochromat 20x/0.8, a Hamamatsu C11440 Orca Flash 4.0 v2 sCMOS camera and Zen Blue 2.5 software.
Evaluation of UEA1/MUC5AC PLA staining of TMA
PLA tissue staining of the ovarian tumor TMA was evaluated by two independent researchers (VV and JÖ). Positive ovarian serous tumors and negative ovarian mucinous tumors were further evaluated by pathologist (CM) to verify the cell/tissue type of positive PLA staining or presence or absence of epithelial cells in the negative mucinous section cores in the sectioned TMA. Consistent staining of epithelial layers and/or stroma was considered as positive if it was detected in one of the three TMA tissue cores. The occurrence of sparse occasional dots within the tissue was considered as background.
O-linked oligosaccharides from ovarian cancer cysts fluid
Mucin type molecules from ovarian cyst fluids were enriched by anion exchange chromatography as described previously (Vitiazeva et al. 2015). Selected datasets from previous of released and analyzed O-linked oligosaccharide were used to identify tissue samples for PLA analysis to verify MUC5AC.
Statistical analysis
Significance of the contingency table containing ovarian tumor mucinous and serous positive PLA was tested using Fisher’s exact test in Prism 8.4.2 (Graphpad Softwares Inc., San Diego, CA, USA).
Supplementary Material
Acknowledgment
In situ PLA was performed by the PLA and Single Cell Proteomics Facility, Uppsala University, Science for Life Laboratory.
Contributor Information
Constantina Mateoiu, Department of Clinical Pathology, Sahlgrenska University Hostpital Gotenburg, 41345, Sweden.
Varvara Vitiazeva, Department of Medical Biochemistry, Sahlgrenska Academy, Institute of Biomedicine, University of Gothenburg, 405 30 Gothenburg, Sweden.
Björg Kristjansdottir, Department of Obstetrics and Gynecology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, 405 30 Gothenburg, Sweden; Region Västra Götaland, Sahlgrenska University Hospital, Department of Gynecology, 413 45 Gothenburg, Sweden.
Birgitta Weijdegård, Department of Obstetrics and Gynecology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, 405 30 Gothenburg, Sweden.
Jessica Örnros, Department of Medical Biochemistry, Sahlgrenska Academy, Institute of Biomedicine, University of Gothenburg, 405 30 Gothenburg, Sweden.
Radiosa Gallini, Department of Immunology, Genetics and Pathology, Science for Life Laboratory, 751 08 Uppsala, Sweden.
Masood Kamali-Moghaddam, Department of Immunology, Genetics and Pathology, Science for Life Laboratory, 751 08 Uppsala, Sweden.
Karin Sundfeldt, Department of Obstetrics and Gynecology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, 405 30 Gothenburg, Sweden; Region Västra Götaland, Sahlgrenska University Hospital, Department of Gynecology, 413 45 Gothenburg, Sweden.
Niclas G Karlsson, Department of Medical Biochemistry, Sahlgrenska Academy, Institute of Biomedicine, University of Gothenburg, 405 30 Gothenburg, Sweden; Department of Life Sciences and Health, Faculty of Health Sciences, Oslo Metropolitan University, 0167 Oslo, Norway.
Funding
Swedish state under the agreement between the Swedish government and the county council, the ALF-agreement (ALFGBG-72239 to N.G.K. and ALFGBG-70950 to K.S.); the Swedish Research Council (621-2013-5895 to N.G.K.); Swedish Cancer Foundation (CAN 2018/384 to K.S.); Assar Gabrielsson Foundation (C.M.); Kung Gustav V:s 80-års Foundation (N.G.K.) and Petrus and Augusta Hedlund’s Foundation (M-2016-0353 to N.G.K.).
Conflict of interest statement
The authors declare that they have no conflict of interest in the performed research.
References
- Adamczyk B, Jin C, Polom K, Muñoz P, Rojas- Macias MA, Zeeberg D, Borén M, Roviello F, Karlsson NG. 2018. Sample handling of gastric tissue and O-glycan alterations in paired gastric cancer and non-tumorigenic tissues. Sci Rep. 8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarracin CT, Jafri J, Montag AG, Hart J, Wan S-F. 2000. Differential expression of MUC2 and MUC5AC mutin genes in primary ovarian and metastatic colonic carcinoma. Hum Pathol. 31:672–677. [DOI] [PubMed] [Google Scholar]
- Balmaña M, Duran A, Gomes C, Llop E, López-Martos R, Ortiz MR, Barrabés S, Reis CA, Peracaula R. 2018. Analysis of sialyl-Lewis x on MUC5AC and MUC1 mucins in pancreatic cancer tissues. Int J Biol Macromol. 112:33–45. [DOI] [PubMed] [Google Scholar]
- Blokzijl A, Nong R, Darmanis S, Hertz E, Landegren U, Kamali-Moghaddam M. 2014. Protein biomarker validation via proximity ligation assays. Biochim Biophys Acta. 1844:933–939. [DOI] [PubMed] [Google Scholar]
- Chen K, Gentry-Maharaj A, Burnell M, Steentoft C, Marcos-Silva L, Mandel U, Jacobs I, Dawnay A, Menon U, Blixt O. 2013. Microarray glycoprofiling of CA125 improves differential diagnosis of ovarian cancer. J Proteome Res. 12:1408–1418. [DOI] [PubMed] [Google Scholar]
- El Jellas K, Johansson BB, Fjeld K, Antonopoulos A, Immervoll H, Choi MH, Hoem D, Lowe ME, Lombardo D, Njølstad PR et al. 2018. The mucinous domain of pancreatic carboxyl-ester lipase (CEL) contains core 1/core 2 O-glycans that can be modified by ABO blood group determinants. J Biol Chem. 293:19476–19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidwani K, Kekki H, Terävä J, Soukka T, Sundfeldt K, Pettersson K. 2020. Nanoparticle-aided glycovariant assays to bridge biomarker performance and ctDNA results. Mol Aspects Med. 72:100831. [DOI] [PubMed] [Google Scholar]
- Giuntoli RL 2nd, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. 1998. Mucin gene expression in ovarian cancers. Cancer Res. 58:5546–5550. [PubMed] [Google Scholar]
- Hauptmann S, Friedrich K, Redline R, Avril S. 2017. Ovarian borderline tumors in the 2014 WHO classification: evolving concepts and diagnostic criteria. Virchows Arch. 470:125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CA, Nemes S, Karlsson NG. 2012. Statistical analysis of glycosylation profiles to compare tissue type and inflammatory disease state. Bioinformatics. 28:1669–1676. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Sawada T, Uchida M, Saito H, Iijima H, Toda-Agetsuma M, Wada T, Yamazoe S, Tanaka H, Kimura K et al. 2011. Tumor-associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int J Oncol. 38:619–627. [DOI] [PubMed] [Google Scholar]
- Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. 2002. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 21:391–400. [DOI] [PubMed] [Google Scholar]
- Jin C, Kenny DT, Skoog EC, Padra M, Adamczyk B, Vitizeva V, Thorell A, Venkatakrishnan V, Linden SK, Karlsson NG. 2017. Structural diversity of human gastric mucin glycans. Mol Cell Proteomics. 16:743–758. [DOI] [PubMed] [Google Scholar]
- Kenny DT, Skoog EC, Lindén SK, Struwe WB, Rudd PM, Karlsson NG. 2012. Presence of terminal N-acetylgalactosamineβ1-4N-acetylglucosamine residues on O-linked oligosaccharides from gastric MUC5AC: involvement in Helicobacter pylori colonization? Glycobiology. 22:1077–1085. [DOI] [PubMed] [Google Scholar]
- Krishn SR, Ganguly K, Kaur S, Batra SK. 2018. Ramifications of secreted mucin MUC5AC in malignant journey: a holistic view. Carcinogenesis. 39:633–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansdottir B, Partheen K, Fung ET, Marcickiewicz J, Yip C, Brännström M, Sundfeldt K. 2012. Ovarian cyst fluid is a rich proteome resource for detection of new tumor biomarkers. Clin Proteomics. 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan K, Mehryar M, Mateoiu C, Albertsson P, Bäck T, Sundfeldt K. 2017. Immunohistochemical evaluation of epithelial ovarian carcinomas identifies three different expression patterns of the MX35 antigen, NaPi2b. BMC Cancer. 17:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lheureux S, Gourley C, Vergote I, Oza AM. 2019. Epithelial ovarian cancer. Lancet. 393:1240–1253. [DOI] [PubMed] [Google Scholar]
- Nash Z, Menon U. 2020. Ovarian cancer screening: current status and future directions. Best Pract Res Clin Obstet Gynaecol. 65:32–45. [DOI] [PubMed] [Google Scholar]
- O'Connell JT, Hacker CM, Barsky SH. 2002. MUC2 is a molecular marker for pseudomyxoma peritonei. Mod Pathol. 15:958–972. [DOI] [PubMed] [Google Scholar]
- Oliveira FMS, Mereiter S, Lonn P, Siart B, Shen Q, Heldin J, Raykova D, Karlsson NG, Polom K, Roviello F et al. 2018. Detection of post-translational modifications using solid-phase proximity ligation assay. Nat Biotechnol. 45:51–59. [DOI] [PubMed] [Google Scholar]
- Pinto R, Carvalho AS, Conze T, Magalhaes A, Picco G, Burchell JM, Taylor-Papadimitriou J, Reis CA, Almeida R, Mandel U et al. 2012. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J Cell Mol Med. 16:1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo S, Marcos-Silva L, Pereira D, Pinto R, Almeida R, Soderberg O, Mandel U, Clausen H, Felix A, Lunet N et al. 2015. Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol Oncol. 9:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo S, Marcos-Silva L, Valente C, Coelho R, Gomes R, David L. 2016. Mucins MUC16 and MUC1 are major carriers of SLe(a) and SLe(x) in borderline and malignant serous ovarian tumors. Virchows Arch. 468:715–722. [DOI] [PubMed] [Google Scholar]
- Schulz BL, Sloane AJ, Robinson LJ, Sebastian LT, Glanville AR, Song Y, Verkman AS, Harry JL, Packer NH, Karlsson NG. 2004. Mucin glycosylation changes in cystic fibrosis lung disease are not manifest in submucosal gland secretions. Biochem J. 387:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai M, Umezu H, Yamamoto T, Jiang S, Iwanari H, Tanaka T, Hamakubo T, Kodama T, Naito M. 2008. Expression of hepatocyte nuclear factor 4 alpha in primary ovarian mucinous tumors. Pathol Int. 58:681–686. [DOI] [PubMed] [Google Scholar]
- Vitiazeva V, Kattla JJ, Flowers SA, Linden SK, Premaratne P, Weijdegard B, Sundfeldt K, Karlsson NG. 2015. The O-linked glycome and blood group antigens ABO on mucin-type glycoproteins in mucinous and serous epithelial ovarian tumors. PLoS One. 10:e0130197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, El-Bahrawy M. 2015. Expression profile of mucins (MUC1, MUC2, MUC5AC, and MUC6) in ovarian mucinous tumours: changes in expression from benign to malignant tumours. Histopathology. 66:529–535. [DOI] [PubMed] [Google Scholar]
- Wang J, El-Bahrawy MA. 2014. Expression profile of mucins in ovarian mucinous tumors: distinguishing primary ovarian from metastatic tumors. Int J Gynecol Pathol. 33:166–175. [DOI] [PubMed] [Google Scholar]
- Yue T, Maupin KA, Fallon B, Li L, Partyka K, Anderson MA, Brenner DE, Kaul K, Zeh H, Moser AJ et al. 2011. Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19-9 antigen on specific protein carriers. PLoS One. 6:e29180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


