Abstract
Context
Circulating adiponectin levels are decreased in pregnant women with obesity or gestational diabetes, and this is believed to contribute to the insulin resistance and increased risk of fetal overgrowth associated with these conditions. However, the molecular mechanisms regulating adiponectin secretion from maternal adipose tissues in pregnancy are poorly understood.
Objective
We tested the hypothesis that obesity in pregnancy is associated with adipose tissue insulin resistance and increased adiponectin ubiquitination and degradation, caused by inflammation and endoplasmic reticulum (ER) stress.
Methods
Visceral adipose tissues were collected from lean and obese pregnant humans and mice. Total and ubiquitinated adiponectin, and markers of inflammation, ER stress, and insulin resistance were examined in adipose tissues. The role of insulin, inflammation, and ER stress in mediating adiponectin ubiquitination and degradation was examined using 3T3L-1 adipocytes.
Results
Obesity in pregnancy is associated with adipose tissue inflammation, ER stress, insulin resistance, increased adiponectin ubiquitination, and decreased total abundance of adiponectin. Adiponectin ubiquitination was increased in visceral fat of obese pregnant women as compared to lean pregnant women. We further observed that insulin prevents, whereas ER stress and inflammation promote, adiponectin ubiquitination and degradation in differentiated 3T3-L1 adipocytes.
Conclusion
We have identified adiponectin ubiquitination as a key mechanism by which obesity diminishes adiponectin secretion in pregnancy. This information will help us better understand the mechanisms controlling maternal insulin resistance and fetal growth in pregnancy and may provide a foundation for the development of strategies aimed at improving adiponectin production in pregnant women with obesity or gestational diabetes.
Keywords: adipose tissue, human, insulin resistance
Almost two-thirds of American women now enter pregnancy overweight (body mass index, BMI = 25-29.9) or obese (BMI ≥ 30) (1, 2), and obese women are at high risk of developing a number of pregnancy complications, including gestational diabetes mellitus (GDM) (3). Obese women are also more likely to deliver an infant that is large for gestational age (> 90th percentile) (4-6), has increased adiposity (7, 8), and is insulin resistant at birth (9). In addition, babies of women with obesity or GDM are at risk for obesity, diabetes, and cardiovascular disease later in life (10-12).
Adipose tissue is a metabolically active organ producing adipokines that influence whole-body insulin sensitivity and energy homeostasis. The adipokine adiponectin enhances insulin sensitivity in the adipose tissues, liver, and skeletal muscle. Maternal circulating adiponectin levels decrease across gestation, which may contribute to the physiological state of insulin resistance associated with pregnancy (13). Moreover, circulating adiponectin levels are decreased in pregnant women with obesity and GDM (13-15), and low maternal serum adiponectin levels in women are associated with fetal overgrowth (13, 14). Studies in pregnant mice with deletion of the adiponectin gene have shown that low maternal adiponectin causes fetal overgrowth (16, 17), and normalization of maternal adiponectin levels in obese (OB) pregnant mice increases maternal insulin sensitivity and prevents fetal overgrowth by normalizing placental function (18). Moreover, administration of adiponectin to OB pregnant mice to increase maternal circulating adiponectin to levels observed in lean pregnant mice largely prevented the development of cardiac (19) and metabolic disease (20) in adult offspring. This suggests that low maternal adiponectin is mechanistically linked to the adverse placental and fetal outcomes associated with maternal obesity and GDM, and increasing maternal adiponectin levels may serve as an effective intervention strategy to prevent intrauterine transmission of obesity and metabolic disease. However, the molecular mechanisms governing adiponectin secretion from maternal adipose tissues in pregnancy are poorly understood.
Insulin stimulates the secretion of adiponectin in the murine-derived 3T3-L1 adipocytes, in nonpregnant animals, and in humans (21-23). Only 50% of the synthesized adiponectin protein is secreted (24, 25), suggesting that degradation plays a key role in regulating adiponectin release. Insulin prevents protein degradation in other tissues by inhibiting the ubiquitin-proteasome system (UPS) (26). The UPS is a highly regulated process controlling the degradation of specific proteins following ubiquitination. Previous reports in nonpregnant mice indicate that the UPS regulates adiponectin protein degradation, contributing to the decline in circulating adiponectin levels with obesity (27, 28). However, it is unknown if UPS regulates adiponectin degradation in humans. Moreover, the underlying mechanisms regulating adiponectin ubiquitination are unclear. In nonpregnant individuals, obesity causes inflammation and endoplasmic reticulum (ER) stress in the adipose tissue (29), conditions that impair insulin signaling, resulting in the dysregulation of total-body glucose and lipid homeostasis (30). However, the impact of obesity on adipose tissue insulin resistance in pregnancy is not known.
The aim of this study was to investigate adipose tissue inflammation, ER stress, insulin resistance, adiponectin ubiquitination, and total abundance of adiponectin in obese women and mice and to mechanistically link insulin, ER stress, and inflammation to adiponectin ubiquitination and degradation in cultured cells. Specifically, we tested the hypothesis that obesity in pregnancy is associated with adipose tissue insulin resistance and increased adiponectin ubiquitination and degradation. We tested this hypothesis using adipose tissues of obese pregnant women and a mouse model of obesity in pregnancy. Moreover, using the in vivo mouse model of obesity in pregnancy and in vitro adipocyte cultures, we determined whether adiponectin ubiquitination provides the mechanistic link between obesity-induced inflammation/ER stress and decreased adiponectin secretion.
Materials and Methods
We used our previously reported mouse model of obesity in pregnancy (18, 31) to determine if maternal obesity was associated with (i) altered ubiquitination and expression of adiponectin, (ii) inflammation and ER stress, and (iii) impaired insulin signaling, in adipose tissues (mouse protocols). We then determined if obese pregnant women exhibit similar changes in adipose tissue adiponectin ubiquitination and expression (human tissue protocols). Last, we explored the mechanistic relationship between inflammation, ER stress, and adiponectin ubiquitination regulated by insulin signaling in adipocytes in vitro (cultured adipocyte protocols).
Mouse Protocols
Mouse model of obesity in pregnancy and collection of adipose tissue
All experimental protocols were approved by the institutional animal care and use committees of the University of Texas Health Science Center San Antonio and University of Colorado Anschutz Medical Campus. C57BL/6J female mice (proven breeders) were fed a control (C) or a high-fat/high-sugar pelleted diet supplemented by ad libitum access to sucrose (20%) solution as previously reported (18, 31). Female mice were mated and studied at embryonic day E18. Gonadal (visceral) fat pads were collected; adipose tissue adiponectin levels, and insulin, inflammatory, and ER stress signaling were determined in protein extracts using immunoblotting. Adiponectin ubiquitination was assessed by immunoblotting following immunoprecipitation, as described in the following section.
Adiponectin and ubiquitin reciprocal immunoprecipitation
Reciprocal immunoprecipitation of adiponectin and ubiquitin was performed using the Pierce Co-Immunoprecipitation Kit (Thermo Fisher Scientific) with the following modifications. A total of 25 μg of antimonoclonal adiponectin antibody (Merck Millipore) was immobilized onto coupling resin by incubation overnight at 4 °C with end-over-end rotation. Immobilized antibodies were then incubated with 1 mg of precleared total protein lysate overnight at 4 °C on a rotator. Subsequently, immunoprecipitated products were eluted and denatured prior to immunoblotting analyses of ubiquitin as described above. To further confirm adiponectin-ubiquitin protein binding, reciprocal immunoprecipitations were performed using ubiquitin antibody followed by immunoblotting of adiponectin.
Human Tissue Protocols
Serum and adipose tissue samples from pregnant women
Serum and adipose tissue samples were collected from our cohort of pregnant women with uncomplicated singleton pregnancies (32). In brief, healthy pregnant women undergoing planned cesarean delivery at term at Rikshospitalet, Oslo, Norway, were enrolled after written informed consent. Details on the clinical characteristics of this cohort have been reported previously (32). The study was approved by the institutional review board and the regional committee for medical and health research ethics, South East Norway (reference Nos. 2419/2011 and 13885). Women with GDM that was treated by diet only were included (N = 4). Exclusion criteria included known intrauterine growth restriction and preexisting morbidity. A further 4 women who were underweight (BMI < 18.5) in the original cohort were excluded from this study. Maternal blood samples were collected during cesarean delivery for serum analysis as previously described (32, 33). Clinical characteristics and serum results are provided in Table 1.
Table 1.
Clinical characteristics of study participants
| Normal weight group (n = 118) | Overweight (n = 32) |
Obese group (n = 11) |
P | ||||
|---|---|---|---|---|---|---|---|
| No. (%) | Mean (SD)/ Median (Q1-Q3) |
No. (%) | Mean (SD)/ Median (Q1-Q3) |
No. (%) | Mean (SD)/Median (Q1-Q3) |
||
| Age, y | 35.5 (3.7) | 36.3 (3.8) | 34.6 (3.8) | .40a | |||
| Prepregnancy BMI | 21.7 (1.7) | 26.7 (1.3) | 34.9 (5.1) | < .001a < .001b < .001c .001d |
|||
| Systolic blood pressure first trimester, mm Hg | 109.7 (12.3) | 113.2 (9.0) | 108.9 (8.9) | .30a | |||
| Diastolic blood pressure first trimester, mm Hg | 67.6 (9.0) | 68.5 (8.2) | 70.5 (7.0) | .56a | |||
| Birthweight, g | 3498 (415) | 3654 (489) | 3866 (550) | .01a .2b .02c .4d |
|||
| Placenta weight, g | 602 (131) | 650 (155) | 643 (110) | .2a | |||
| Higher education (> 12 y) |
107 (90.7) |
26 (81.3) |
8 (72.7) |
.2e | |||
| Infant sex male | 64 (54.8) | 19 (59.4) |
6 (54.5) |
.9e | |||
| Married or partner | 116 (98.3) |
30 (93.8) |
10 (90.9) |
.3e | |||
| Pregestational diabetes | 0 | 0 | 0 | ||||
| Gestational diabetes | 2 (1.7) |
1 (3.1) |
1 (9.1) |
.2e | |||
| Gestational age, wk | 39.3 (0.7) | 39.3 (0.4) | 39.4 (1.0) | .9a | |||
| HMW adiponectin | 3.0 (1.9-3.8) |
2.2 (1.8-2.7) |
2.5 (1.8-3.2) |
.02f .006g .3h .5i |
|||
| Total adiponectin | 5.3 (3.8-7.0) |
4.1 (3.2-5.2) |
4.5 (3.5-5.4) |
.02f .008g .3h .6i |
|||
| HMW/total adiponectin ratio | 0.55 (0.48-0.61) |
0.50 (0.46-0.59) |
0.52 (0.49-0.61) |
.2f | |||
| Glucose, mmol/L | 4.5 (0.4) | 4.7 (0.4) | 4.8 (0.7) | .04a .1b .1c .8d |
|||
| Cholesterol, mmol/L | 5.8 (1.0) | 5.8 (1.3) | 6.2 (1.0) | .4a | |||
| Insulin, pmol/L | 56.1 (35.5-75.9) |
61.7 (43.7-93.3) |
120.1 (92.0-137.8) |
< .001f .1g < .001h .004i |
Abbreviations: BMI, body mass index; HMW, high molecular weight.
a One-way analysis of variance with Dunnett T3 post hoc test for comparison of the following:
b normal weight vs overweight groups;
c normal weight vs obese groups;
d overweight vs obese groups.
e Pearson chi-square.
f Kruskal-Wallis test with Bonferroni post hoc test for comparison of the following:
g normal weight vs overweight groups;
h normal weight vs obese groups;
i overweight vs obese groups.
Adipose tissues were collected from a subcohort of women from the larger cohort. Prelabor cesarean delivery was performed under spinal anesthesia, and omental (visceral) adipose tissue was sampled from a subset of this cohort, immediately after delivery in women with normal prepregnancy BMI (N = 4, mean BMI = 22.35, SD = 1.15) and obese women (mean BMI = 32.78, SD = 2.21). Adipose tissue samples were snap frozen and stored at –80 °C before cryosectioning for proximity ligation assays (PLAs) and protein extraction for immunoblotting.
Proximity ligation assay and confocal microscopy
Adipose tissue collected from pregnant women was serially cryosectioned at 4 µm and fixed in 4% paraformaldehyde. Subsequently, sections were blocked using 5% newborn calf serum in phosphate-buffered saline for 1 hour, followed by incubation in antiadiponectin (antirabbit) and antiubiquitin (antimouse) antibodies for 2 hours at 37 °C. PLA probes antirabbit PLUS and antimouse MINUS were diluted in Duolink dilution buffer and incubated in a preheated humidity chamber for 30 minutes. This was followed by ligation, amplification, and detection according to the Duolink In Situ Orange kit (Sigma-Aldrich) manufacturer’s protocol. Confocal microscopy was performed using a Zeiss LSM 780 microscope at 63× magnification using oil immersion. Images were captured in the same laser settings with 4 Z-step of 0.4 um. The Image J (National Institutes of Health) was used for quantification of the detected PLA signals.
Cultured Adipocyte Protocols
3T3-L1 Cell culture and differentiation into adipocytes and treatments
3T3-L1 preadipocytes (American Tissue Culture Collection) were maintained in Pre-adipocyte Expansion Medium (PEM, 90% Dulbecco’s Modified Eagle Medium [DMEM low glucose], 10% newborn calf serum) and split at 70% cell confluency. Differentiation into adipocytes was performed according to standard protocols (34). In brief, 3T3-L1 cells were grown to confluence and maintained for an additional 48 hours in PEM (differentiation day 0). Cells were then grown in differentiation medium (90% DMEM low glucose, 10% fetal bovine serum [FBS], 1-µM dexamethasone, 0.5-mM methylisobutylxanthine, and 1-µg/mL insulin) for 3 days. Subsequently, culture media was replaced with adipocyte maintenance medium (90% DMEM low glucose, 10% FBS, 1-µg/mL insulin), which was replaced every 2 days until adipocytes had formed (differentiation day 7). Mature adipocytes were identified by lipid droplet formation and the accumulation of neutral lipid dye Oil Red O.
Following 3T3-L1 adipocyte differentiation, culture media was replaced with DMEM, 10% FBS without insulin for 21 hours. 3T3-L1 adipocytes were then incubated with tumor necrosis factor α (TNFα) or thapsigargin (Tg) for 24 hours with or without 1-nM insulin for 3 hours. The concentrations of glucose (1000 mg/L) and insulin correspond to normal postprandial levels in pregnant women (35, 36). Protein lysates were then collected for adiponectin immunoprecipitation and/or immunoblotting analyses as described in the following section.
Cycloheximide-chase assay of adiponectin degradation
Adiponectin protein half-life was assessed in differentiated 3T3-L1 adipocytes by cycloheximide (CHX)-chase assay as described previously (37). The CHX-chase assay allows for the analysis of protein degradation over time. In brief, 3T3-L1 adipocytes were treated with CHX (an inhibitor of protein synthesis). Protein lysates were collected immediately and subsequently at specific time points indicated in the figures following the addition of the compound. Protein degradation was assessed by immunoblotting analyses as described in the following section.
Immunoblotting analyses
3T3-L1 adipocytes were harvested, and mouse and human adipose tissue were homogenized in radioimmunoprecipitation buffer (50-mM Tris HCl, pH = 7.4; 150-mM NaCl; 0.1% sodium dodecyl sulfate; 0.5% Na-deoxycholate, and 1% Triton X100) containing protease inhibitors and phosphatase inhibitor cocktail 1 and 2 (1:100, Sigma). Immunoblotting was then carried out as described (38). The expression of β-actin was used to control for any differences in loading and transfer. Details of antibodies and Research Resource Identifier numbers are provided in Table 2.
Table 2.
Key resources: antibodies and enzyme-linked immunosorbent assays
| Antibody or ELISA | Supplier | Catalog No. | RRID |
|---|---|---|---|
| Adiponectin Antibody, clone MADI 14 | Merck/Millipore | MAB3832 | RRID:AB_179634 |
| Duolink In Situ PLA Probe Anti-Rabbit PLUS | Sigma | DUO92002 | RRID:AB_2810940 |
| Duolink In Situ PLA Probe Anti-Mouse MINUS | Sigma | DUO92004 | RRID:AB_2713942 |
| Beta-actin | Sigma | A2228 | RRID:AB_476697 |
| Ubiquitin | AbCam | ab411 | RRID:AB_308708 |
| Phospho-IRS-1 (S307) | Cell Signaling | 2381 | RRID:AB_330342 |
| IRS1 | Cell Signaling | 3407 | RRID:AB_2127860 |
| Phospho-AKT (Thr308) | Cell Signaling | 4056 | RRID:AB_331163 |
| AKT | Cell Signaling | 9272 | RRID:AB_329827 |
| IκBα | Cell Signaling | 9242 | RRID:AB_331623 |
| Phospho-SAPK/JNK (Thr183/Tyr185) | Cell Signaling | 4668 | RRID:AB_823588 |
| SAPK/JNK | Cell Signaling | 9252 | RRID:AB_2250373 |
| Phospho-IRE1alpha (Ser724) | Novus Bio | NB100-2323 | RRID:AB_10145203 |
| IRE1alpha | Cell Signaling | 3294 | RRID:AB_823545 |
| Phospho-PERK (Ser 713) | Cell Signaling | 649401 | RRID:AB_10642966 |
| PERK | Cell Signaling | 3192 | RRID:AB_2095847 |
| Phospho eIF2alpha (ser51) | Cell Signaling | 3398 | RRID:AB_2096481 |
| eIF2alpha | Cell Signaling | 5324 | RRID:AB_10692650 |
| XBP1s | Cell Signaling | 40435 | RRID:AB_2891025 |
| BiP | Cell Signaling | 3177 | RRID:AB_2119845 |
| High-molecular-weight and total adiponectin ELISA | Alpco Diagnostics | 80-ADPHU-E01 | RRID:AB_2892778 |
Abbreviations: AKT, protein kinase B; ELISA, enzyme-linked immunosorbent assay; IRS-1, insulin-receptor substrate 1; PLA, proximity ligation assay.
Statistical Analyses
Unless otherwise stated, data are presented as means ± SEM. Data were analyzed with GraphPad Prism software (GraphPad Software) and Statistical Package for the Social Sciences (IBM Corp, released 2016. IBM SPSS Statistics for Windows, version 24.0). Statistical differences between groups were assessed using one-way analysis of variance and Dunnett T3 post hoc test for normally distributed data, or Kruskal-Wallis test and post hoc test with Bonferroni correction for multiple tests for nonnormally distributed data. A P value of less than .05 was considered statistically significant.
Results
Decreased Total Adiponectin and Increased Adiponectin Ubiquitination, Insulin Resistance, Inflammation, and Endoplasmic Reticulum Stress in Adipose Tissue of Obese Pregnant Mice
We previously demonstrated that the obesogenic diet used in this study increases maternal fat mass by 2.2-fold and results in glucose intolerance with normal fasting glucose in pregnancy (31). Maternal circulating insulin, leptin, and cholesterol are increased in OB dams, whereas total and high-molecular-weight (HMW) adiponectin are decreased (31). Thus, this animal model is associated with maternal metabolic alterations similar to that observed in pregnant women with increased BMI and without GDM. Importantly, this model of obesity in pregnancy results in increased fetal weight (+18%), replicating fetal overgrowth, which is common in obese pregnant women (4-6, 8).
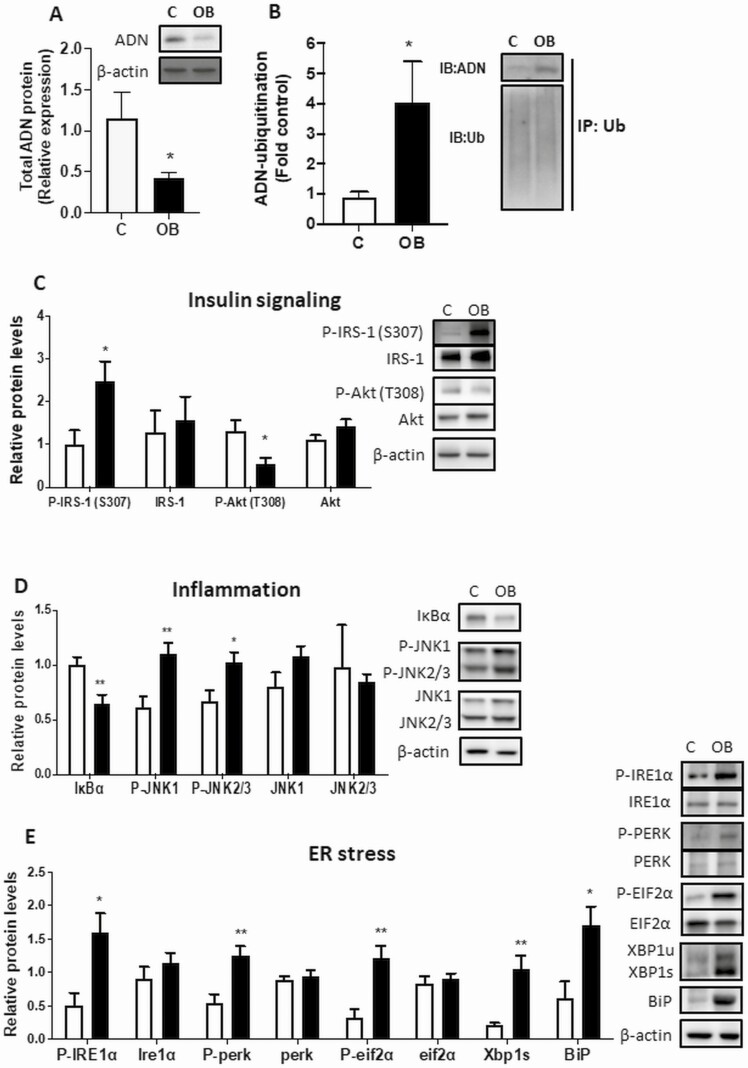
Consistent with our previous reports, total adiponectin protein was decreased (Fig. 1A), and adiponectin ubiquitination was increased (Fig. 1B) in gonadal adipose tissue of OB pregnant (E18) mice compared to normal-weight pregnant mice (C). These findings show that the previously reported decrease in circulating adiponectin in OB pregnant mice (18, 31) is associated with increased adiponectin ubiquitination in adipose tissue. Moreover, we explored whether maternal obesity is associated with adipose inflammation and ER stress, which are known mediators of adipose insulin resistance. OB dams exhibited adipose tissue insulin resistance, as indicated by increased inhibitory serine phosphorylation of insulin-receptor substrate 1 (IRS-1; S307) and decreased protein kinase B (Akt; T308) phosphorylation (Fig. 1C). Obesity was also associated with increased adipose tissue inflammation, as evidenced by decreased IκBα (inhibitor of nuclear factor–κB) expression and increased phosphorylation of JNK (see Fig. 1C). Moreover, the adipose tissue ER stress pathways IRE1α and PERK were activated in OB pregnant mice, as demonstrated by their phosphorylation. Activation of ER stress was further supported by increased phosphorylation of eIF2α, a key downstream target of IRE1α and PERK, and increased expression of spliced XBP1 (XBP1s) and BiP (see Fig. 1C).
Figure 1.
Impact of maternal obesity in mice on adipose tissue (AT) adiponectin expression and ubiquitination, insulin signaling, inflammation, and endoplasmic reticulum (ER) stress. A, Gonadal AT was collected at embryonic day (E)18 in pregnant obese (OB) and control (C) mice, and total adiponectin protein expression measured. B, Adiponectin ubiquitination was determined in gonadal AT by immunoprecipitation (IP) of ubiquitin followed by immunoblotting of adiponectin and ubiquitin in OB pregnant mice. C, OB dams exhibited AT insulin resistance as indicated by increased inhibitory serine phosphorylation of insulin-receptor substrate 1 (IRS-1; S307) and decreased protein kinase B (Akt; T308) phosphorylation. Obesity was associated with increased AT inflammation and ER stress. AT inflammation was evidenced by decreased IκBα (inhibitor of nuclear factor–κB) expression and increased phosphorylation of JNK. Activation of ER stress pathways was indicated by phosphorylation of IRE1α and PERK, and their downstream target EIF2α, and increased expression of spliced XBP1 (XBP1s) and BiP. Asterisks adjacent to blots indicate significant differences between C and OB. Mean + SEM, unpaired t test. Figures are representative of n = 7 C, n = 10 OB. *P less than .05, **P less than .01.
Increased Adipose Adiponectin Ubiquitination Is Correlated With Reduced Circulating Total Adiponectin in Obese Pregnant Women
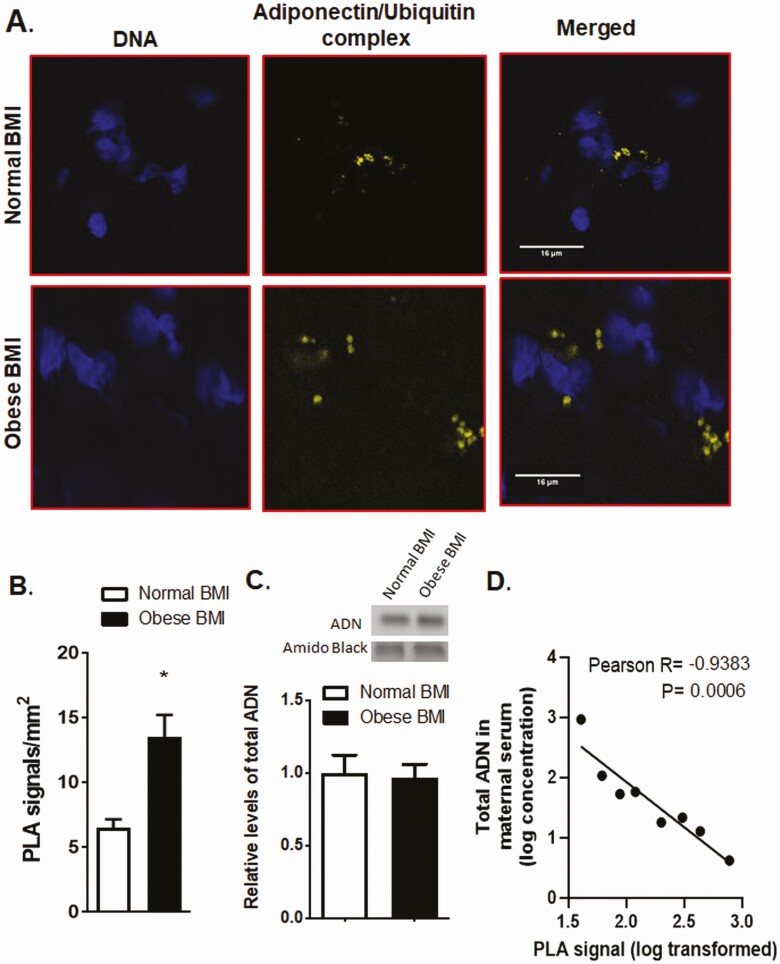
Using PLA in omental tissue samples from pregnant women with normal BMI (range, 21.0-23.4) or obese BMI (range, 30.4-35.2), we observed an increase in adiponectin ubiquitination in obese pregnant women (Fig. 2A and 2B), but no changes in total adiponectin in maternal adipose tissues (Fig. 2C). Similarly, compared to normal weight pregnant women, the high molecular weight adiponectin and total adiponectin levels in the serum were not significantly altered in obese women, possibly because of the small sample size, but was significantly reduced in overweight women (see Table 1). However, adiponectin ubiquitination in maternal adipose tissues was inversely associated with maternal serum levels of total adiponectin (Fig. 2D).
Figure 2.
Obesity is associated with increased adipose tissue adiponectin ubiquitination in women. A, Proximity ligation assay (PLA) and confocal microscopy were used to determine the association between adiponectin and ubiquitin in the adipose tissue of obese and normal–body mass index (BMI) pregnant women. In normal BMI, adipose tissue (A, top) adiponectin and ubiquitin associations were limited as evidenced by a low number of PLA signals (yellow dots), suggesting low adiponectin ubiquitination. In contrast, in obese pregnant women (A, bottom), the number of PLA signals was greater, suggesting increased adiponectin ubiquitination as compared to adipose tissue in normal-BMI women. Blue, nuclei; Scale bar, 16 µm. B, Bar graph summarizes the PLA data of adipose tissue adiponectin-ubiquitin interactions. In each section, at least 10 randomly selected microscopic fields were used to calculate the number of adiponectin-ubiquitin association positive sites (yellow dots) per millimeters squared. Data were averaged to represent a single adipose tissue. Values are given as mean + SEM; *P less than .05 vs control; unpaired t test; n = 4 adipose tissue/each group. C, Total adiponectin abundance in the adipose tissue of obese and normal-BMI pregnant women as determined by immunoblotting. The bar graph summarizes the Western blot data. Equal protein loading was assessed by amido black staining. After normalization to amido black, the mean density of control samples was assigned an arbitrary value of 1. Means + SEM, unpaired t test. D, Inverse correlation between maternal serum total adiponectin and adipose tissue adiponectin ubiquitination. Linear regression analyses were performed on log-transformed maternal serum concentrations of total adiponectin and adipose tissue PLA signals.
Insulin Increases Cellular Adiponectin Protein Abundance by Decreasing Adiponectin Ubiquitination in Cultured Adipocytes
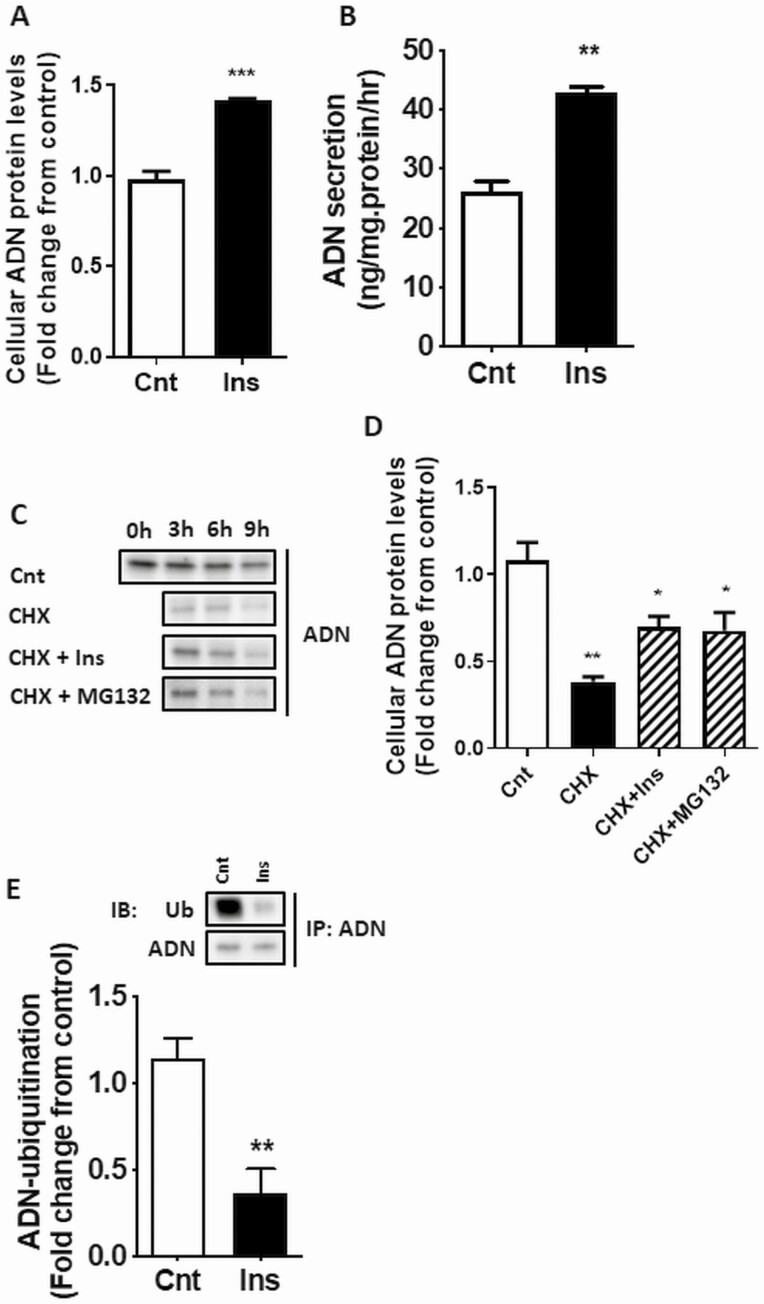
Using differentiated 3T3-L1 adipocytes as our adipocyte model, we demonstrate that insulin increased cellular adiponectin protein abundance (Fig. 3A) and secretion to the media (Fig. 3B). 3T3-L1 adipocytes were treated with insulin followed by CHX-chase assay. Because treatment with CHX markedly reduced cellular adiponectin protein after 3 hours (Fig. 3C), demonstrating effective inhibition of protein synthesis, the 3-hour time point was used in subsequent studies. CHX treatment decreased cellular adiponectin protein by more than 50% after 3 hours (Fig. 3D), consistent with previous reports estimating adiponectin protein half-life (24). The degradation of adiponectin was prevented, in part, by stimulation of cells with insulin before CHX treatment (Fig. 3D). Similarly, treatment with a proteasome inhibitor (MG132) counteracted the decline in adiponectin levels, suggesting that proteasome degradation accounts for much of the loss of cellular adiponectin in normally functioning adipocytes. Because UPS is a major mechanism regulating protein degradation, we determined whether adiponectin protein was ubiquitinated and the role of insulin in this process. As shown in Fig. 3E, ubiquitination of adiponectin was significantly reduced following insulin treatment.
Figure 3.
Insulin increases adiponectin (ADN) secretion and inhibits ubiquitin-proteasome system (UPS)-mediated degradation of ADN. 3T3-L1 cells were differentiated into mature adipocytes using standard protocols. 3T3-L1 adipocytes were cultured for 3 hours with and without CHX (10 μg/mL) or MG132 (10 μM) before insulin stimulation (10 nM). Cellular ADN protein A, expression, and B, secretion, into culture media were determined by immunoblotting (IB) analysis and enzyme-linked immunosorbent assay, respectively. C, IB showing the time course of ADN protein degradation following CHX treatment with or without insulin or the proteasome inhibitor (MG132), and D, the quantification of the results from the 3-hour time point. E, Ubiquitination of ADN after 3-hour insulin stimulation was determined by immunoprecipitation (IP) of ADN followed by IB analysis of ADN and ubiquitin in the IP protein lysates. Values are mean + SEM (n = 6). Unpaired t test or one-way analysis of variance *P less than .05, **P less than .01, ***P less than .001. Cnt, control; CHX, cycloheximide; Ins, insulin.
Inflammation and Endoplasmic Reticulum Stress Inhibits the Effects of Insulin on Adiponectin Abundance, Degradation, and Ubiquitination in Cultured Adipocytes
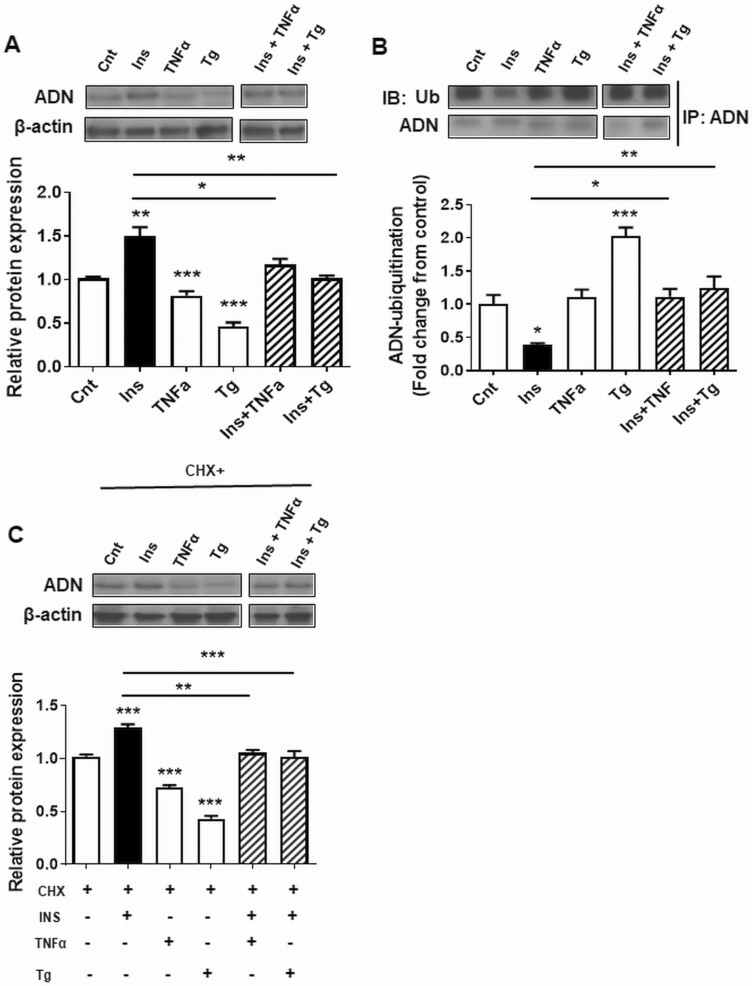
Both TNFα (proinflammatory cytokine) and Tg (ER stress inducer) decreased cellular adiponectin protein levels in 3T3-L1 adipocytes and prevented insulin-stimulated adiponectin production (Fig. 4A). Furthermore, Tg increased the ubiquitination of adiponectin, whereas both TNFα and Tg prevented the insulin-mediated decrease in adiponectin ubiquitination (Fig. 4B). CHX-chase assays indicated that TNFα and Tg increased adiponectin protein degradation and prevented the effect of insulin on adiponectin degradation (Fig. 4C).
Figure 4.
The effects of inflammation and endoplasmic reticulum (ER) stress on insulin-dependent adiponectin (ADN) protein expression, ubiquitination, and degradation. 3T3-L1 adipocytes were treated with the proinflammatory cytokine tumor necrosis factor α (TNFα; 20 ng/mL) or ER stress-inducing agent thapsigargin (Tg, 1 μM) for 24 hours before stimulation with insulin and/or cycloheximide (CHX) for 3 hours. A, ADN protein expression; B, CHX-mediated ADN degradation; and C, ubiquitination of ADN. Values are mean + SEM, n = 6. One-way analysis of variance *P less than .05, **P less than .01.
Discussion
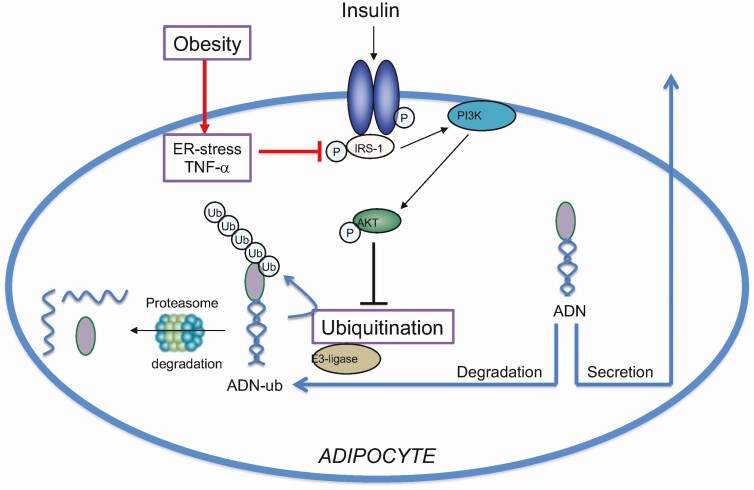
We have identified a molecular mechanism regulating adiponectin secretion that involves insulin signaling and ubiquitination. This new information will help us better understand the mechanisms underlying the decrease in circulating maternal adiponectin in obesity in pregnancy. Specifically, using visceral adipose tissue from lean and OB pregnant mice, we show that obesity in pregnancy is associated with adipose tissue inflammation, ER stress, insulin resistance, increased adiponectin ubiquitination, and decreased total abundance of adiponectin. In addition, as compared to lean pregnant women, adiponectin ubiquitination was increased in visceral fat of obese pregnant women. Moreover, we report that insulin prevents, whereas ER stress and inflammation promote, adiponectin ubiquitination and degradation in cultured adipocytes. This may explain the underlying mechanisms by which obesity mediated adiponectin ubiquitination and degradation contributes to the decline in circulating adiponectin levels in pregnant women (Fig. 5).
Figure 5.
Model for regulation of adiponectin (ADN) secretion in pregnancy. We propose that insulin stimulates ADN secretion in pregnancy mediated by inhibition of ADN ubiquitination and degradation, and that obesity is associated with adipose inflammation, endoplasmic reticulum (ER) stress, and insulin resistance resulting in increased ADN ubiquitination and degradation.
Circulating adiponectin levels are decreased in pregnant women with obesity (15, 39-41) and GDM (16, 41), that is, pregnancy complications associated with glucose intolerance. Adiponectin is well established to promote glucose tolerance. Therefore, these observations are consistent with the possibility that low adiponectin levels directly contribute to glucose intolerance (42, 43). Consistent with this hypothesis, elegant studies in genetically modified mice demonstrate that maternal adiponectin is required to expand β-cell mass in pregnancy (17). Moreover, we have shown that low maternal adiponectin levels in OB pregnant mice are mechanistically linked to enhanced placental function and fetal overgrowth (18), as well as the development of metabolic (20) and cardiovascular disease (19) in the offspring. Thus, understanding the molecular mechanisms governing adipose adiponectin secretion in pregnancy may help us understand the cause of impaired glucose metabolism in pregnancies among women with obesity and GDM. Furthermore, it potentially provides critical insights into the molecular mechanism linking the endocrine function of the maternal adipose tissue with placental function, fetal growth, and programming of adult disease.
We explored the effect of obesity in pregnancy on adipose tissue adiponectin levels and ubiquitination, insulin signaling, and ER stress using a mouse model. Specifically, in this model, obesity is induced in female mice before mating by feeding them a diet high in saturated fat, cholesterol, and simple sugars (18, 31), resembling a diet common in Western societies (44). This mouse model results in fetal overgrowth associated with maternal metabolic alterations similar to those observed in obese pregnant women (14, 18, 31, 45). Using this mouse model, we report for the first time that maternal obesity in pregnancy is associated with decreased adipose tissue adiponectin levels and increased adiponectin ubiquitination, consistent with the decreased circulating adiponectin concentrations (18, 31). Moreover, our data on IRS-1 (Ser307) and Akt (Thr308) phosphorylation, together with elevated circulating insulin levels (18, 31), suggest that adipose tissue in OB dams is insulin resistant. Moreover, inflammation signaling through JNK (46) and ER stress (30) is known to phosphorylate IRS-1 at Ser307. Thus, we propose that the observed adipose inflammation, particularly JNK activation and ER stress in adipose tissue in OB mice, represents the mechanistic underpinnings of adipose tissue insulin resistance in OB pregnant mice (see Fig. 5).
To confirm the clinical relevance of our findings, we collected omental (visceral) adipose tissue at cesarean delivery from term-normal BMI and obese women. We observed an increase in adiponectin ubiquitination in obese pregnant women, in agreement with our observations in our mouse model of obesity in pregnancy. This suggests that the fraction of total adiponectin that is ubiquitinated is higher in adipose tissue of obese pregnant women as compared to adipose tissue of pregnant women with normal BMI. However, total adipose adiponectin was not altered in obese women as compared to pregnant women with normal BMI. Nevertheless, a significant association between maternal prepregnant BMI and maternal adiponectin levels was observed in this cohort (32). Furthermore, in the larger cohort (n = 161) (32), circulating HMW and total adiponectin levels among overweight (prepregnant BMI = 25-30) but not obese pregnant women (prepregnant BMI ≥ 30) were significantly lower than in normal-weight pregnant women (prepregnant BMI 18.5 < 25) (see Table 1). However, the between-group comparison must be interpreted with caution because of the low number of obese women. Determining the differences in adiponectin concentrations between normal and obese pregnant women was not the primary purpose of the original study (32). Hence, we were underpowered to detect statistically significant differences in circulating adiponectin. Nevertheless, the demographic of the cohort is consistent with the prevalence of obesity in Norwegian women of reproductive age (47). Furthermore, previous studies in countries with high obesity rates have demonstrated reduced circulating total and HMW adiponectin with increasing maternal BMI or obesity (15, 48). Nevertheless, adiponectin ubiquitination was inversely correlated with circulating maternal adiponectin levels in the subset of these women with available adipose tissues. These findings suggest that although adiponectin ubiquitination plays a crucial role in maternal adiponectin secretion, additional factors may contribute to maternal circulating adiponectin levels during pregnancy.
We provide compelling evidence that insulin increases cellular adiponectin protein abundance by decreasing adiponectin ubiquitination, resulting in reduced UPS-mediated degradation using differentiated 3T3-L1 adipocytes, a widely used in vitro model of white adipocyte biology (34, 49). These findings are in general agreement with literature reports showing that insulin stimulates the secretion of adiponectin in 3T3-L1 adipocytes and in nonpregnant animals and humans (21-23). Posttranslational regulation of adiponectin protein abundance and secretion is established (50, 51), whereas the role of the UPS in regulating adiponectin secretion is only beginning to be explored. Wang and colleagues (28) reported that exposure of primary mouse adipocytes to 4-hydroxynonenal, a lipid peroxidation end product inducing oxidative stress, caused a decrease in adiponectin secretion by accelerating ubiquitin-proteasome degradation of the adipokine. Moreover, inhibition of MEK/extracellularly regulated kinase 1/2 signaling accelerated UPS-mediated adiponectin degradation in cultured mouse adipocytes (27).
Obesity is associated with inflammation and ER stress in adipose tissue (29), and these inhibit insulin signaling (30). These factors have previously been reported to regulate adiponectin messenger RNA (mRNA). For example, inflammation and ER stress decrease adiponectin mRNA (52-55), while physiological concentrations of insulin do not alter adiponectin mRNA, although supraphysiological concentrations decrease it (56). However, the effects of inflammation and ER stress on adiponectin ubiquitination have not previously been reported. Inflammation and ER stress with maternal obesity may also induce leptin resistance leading to hyperleptinemia (57, 58). However, the role of leptin in regulating adiponectin secretion is unclear, since leptin administration in normal-weight or obese men and women does not alter adiponectin levels (59), and leptin’s effects on adiponectin in rodents may be dependent on the genetic background (60, 61).
We found that inflammation and ER stress inhibit the effects of insulin on adiponectin abundance, ubiquitination, and degradation in differentiated 3T3-L1 adipocytes. These results suggest a role for UPS in mediating the decline in adiponectin production associated with adipose tissue inflammation and ER stress. The findings that insulin signaling inhibits adiponectin degradation support insulin’s role in regulating proteostasis (62), in part through the inhibition of the UPS (63). For example, activation of the UPS caused by insulin resistance is linked to protein degradation and skeletal muscle wasting (64). Therefore, impaired adiponectin secretion due to insulin resistance may be one of several phenotypes associated with the regulation of the UPS by insulin signaling. While it is clear that adiponectin sensitizes insulin’s actions, the role of insulin in regulating adiponectin secretion is only now being recognized. Our findings suggest that regulation of adiponectin secretion and insulin signaling are interrelated and may explain why individuals with insulin resistance exhibit low adiponectin levels.
In conclusion, we show that adiponectin protein is regulated by the UPS and that insulin inhibits the UPS leading to elevated adiponectin abundance whereas inflammation and ER stress, associated with maternal obesity, accelerate adiponectin ubiquitination and degradation. This information will help us better understand the mechanisms controlling maternal insulin resistance and fetal growth in normal and complicated pregnancies and may provide a foundation for the development of strategies aimed at improving adiponectin production in pregnant women with obesity or GDM.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (grant No. HD065007). The imaging experiments were performed in the Advanced Light Microscopy Core part of NeuroTechnology Center at University of Colorado Anschutz Medical Campus, supported in part by a Rocky Mountain Neurological Disorders Core Grant (No. P30 NS048154) and by the Diabetes Research Center (grant No. P30 DK116073).
Author Contributions: I.A., T.J., and T.P. designed the research; I.A., F.R., O.K., and A.K. analyzed the data; I.A., F.R., O.K., A.K., and T.M. conducted the research. I.A., F.R., T.M., T.J., and T.P. wrote the paper. T.J. is the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviations
- Akt
protein kinase B
- BMI
body mass index
- C
control
- CHX
cycloheximide
- DMEM
Dulbecco’s Modified Eagle Medium
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- GDM
gestational diabetes mellitus
- HMW
high-molecular-weight
- IRS-1
insulin-receptor substrate 1
- mRNA
messenger RNA
- OB
obese
- Tg
thapsigargin
- TNFα
tumor necrosis factor α
- UPS
ubiquitin-proteasome system
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief. 2013;( 131):1-8. PMID:24152742. [PubMed] [Google Scholar]
- 3. Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126-1133. [DOI] [PubMed] [Google Scholar]
- 4. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91(3):436-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sebire NJ, Jolly M, Harris JP, et al. . Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25(8):1175-1182. [DOI] [PubMed] [Google Scholar]
- 6. Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964-968. [DOI] [PubMed] [Google Scholar]
- 7. Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195(4):1100-1103. [DOI] [PubMed] [Google Scholar]
- 8. HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG. 2010;117(5):575-584. [DOI] [PubMed] [Google Scholar]
- 9. Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3): e290-e296. [DOI] [PubMed] [Google Scholar]
- 11. Reynolds RM, Allan KM, Raja EA, et al. . Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity—a vicious circle across generations. Int J Obes (Lond). 2012;36(10):1320-1324. [DOI] [PubMed] [Google Scholar]
- 13. Lekva T, Roland MCP, Michelsen AE, et al. . Large reduction in adiponectin during pregnancy is associated with large-for-gestational-age newborns. J Clin Endocrinol Metab. 2017;102(7):2552-2559. [DOI] [PubMed] [Google Scholar]
- 14. Jansson N, Nilsfelt A, Gellerstedt M, et al. . Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87(6):1743-1749. [DOI] [PubMed] [Google Scholar]
- 15. Luo ZC, Nuyt AM, Delvin E, et al. . Maternal and fetal leptin, adiponectin levels and associations with fetal insulin sensitivity. Obesity. 2013;21(1):210-216. [DOI] [PubMed] [Google Scholar]
- 16. Qiao L, Wattez JS, Lee S, et al. . Knockout maternal adiponectin increases fetal growth in mice: potential role for trophoblast IGFBP-1. Diabetologia. 2016;59(11):2417-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiao L, Wattez JS, Lee S, et al. . Adiponectin deficiency impairs maternal metabolic adaptation to pregnancy in mice. Diabetes. 2017;66(5):1126-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aye IL, Rosario FJ, Powell TL, Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci U S A. 2015;112(41):12858-12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaughan OR, Rosario FJ, Powell TL, Jansson T. Normalisation of circulating adiponectin levels in obese pregnant mice prevents cardiac dysfunction in adult offspring. Int J Obes (Lond). 2020;44(2):488-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paulsen ME, Rosario FJ, Wesolowski SR, Powell TL, Jansson T. Normalizing adiponectin levels in obese pregnant mice prevents adverse metabolic outcomes in offspring. FASEB J. 2019;33(2):2899-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blümer RM, van Roomen CP, Meijer AJ, Houben-Weerts JH, Sauerwein HP, Dubbelhuis PF. Regulation of adiponectin secretion by insulin and amino acids in 3T3-L1 adipocytes. Metabolism. 2008;57(12):1655-1662. [DOI] [PubMed] [Google Scholar]
- 22. Hajri T, Tao H, Wattacheril J, Marks-Shulman P, Abumrad NN. Regulation of adiponectin production by insulin: interactions with tumor necrosis factor-α and interleukin-6. Am J Physiol Endocrinol Metab. 2011;300(2):E350-E360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746-26749. [DOI] [PubMed] [Google Scholar]
- 24. Wang ZV, Schraw TD, Kim JY, et al. . Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27(10):3716-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Combs TP, Pajvani UB, Berg AH, et al. . A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145(1):367-383. [DOI] [PubMed] [Google Scholar]
- 26. Grizard J, Dardevet D, Balage M, et al. . Insulin action on skeletal muscle protein metabolism during catabolic states. Reprod Nutr Dev. 1999;39(1):61-74. [DOI] [PubMed] [Google Scholar]
- 27. Gu D, Wang Z, Dou X, et al. . Inhibition of ERK1/2 pathway suppresses adiponectin secretion via accelerating protein degradation by ubiquitin-proteasome system: relevance to obesity-related adiponectin decline. Metabolism. 2013;62(8):1137-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Z, Dou X, Gu D, et al. . 4-Hydroxynonenal differentially regulates adiponectin gene expression and secretion via activating PPARγ and accelerating ubiquitin-proteasome degradation. Mol Cell Endocrinol. 2012;349(2):222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boden G, Duan X, Homko C, et al. . Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57(9):2438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ozcan U, Cao Q, Yilmaz E, et al. . Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457-461. [DOI] [PubMed] [Google Scholar]
- 31. Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring). 2015;23(8):1663-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kristiansen O, Zucknick M, Reine TM, et al. . Mediators linking maternal weight to birthweight and neonatal fat mass in healthy pregnancies. J Clin Endocrinol Metab. 2021;106(7):1977-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holme AM, Holm MB, Roland MCP, et al. . The 4-vessel sampling approach to integrative studies of human placental physiology in vivo. J Vis Exp. 2017;2017(126):55847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang X, Heckmann BL, Liu J. Studying lipolysis in adipocytes by combining siRNA knockdown and adenovirus-mediated overexpression approaches. Methods Cell Biol. 2013;116:83-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phelps RL, Metzger BE, Freinkel N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids in late normal pregnancy. Am J Obstet Gynecol. 1981;140(7):730-736. [PubMed] [Google Scholar]
- 36. Harmon KA, Gerard L, Jensen DR, et al. . Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care. 2011;34(10):2198-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buchanan BW, Lloyd ME, Engle SM, Rubenstein EM. Cycloheximide chase analysis of protein degradation in Saccharomyces cerevisiae. J Vis Exp. 2016;2016(110):53975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosario FJ, Dimasuay KG, Kanai Y, Powell TL, Jansson T. Regulation of amino acid transporter trafficking by mTORC1 in primary human trophoblast cells is mediated by the ubiquitin ligase Nedd4-2. Clin Sci. 2016;130(7):499-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hendler I, Blackwell SC, Mehta SH, et al. . The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol. 2005;193(3 Pt 2):979-983. [DOI] [PubMed] [Google Scholar]
- 40. Nien JK, Mazaki-Tovi S, Romero R, et al. . Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J Perinat Med. 2007;35(6):522-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soheilykhah S, Mohammadi M, Mojibian M, et al. . Maternal serum adiponectin concentration in gestational diabetes. Gynecol Endocrinol. 2009;25(9):593-596. [DOI] [PubMed] [Google Scholar]
- 42. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903-916. [DOI] [PubMed] [Google Scholar]
- 43. Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140(3):365-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gilbert PA, Khokhar S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr Rev. 2008;66(4):203-215. [DOI] [PubMed] [Google Scholar]
- 45. Jansson N, Rosario FJ, Gaccioli F, et al. . Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275(12):9047-9054. [DOI] [PubMed] [Google Scholar]
- 47. Jacobsen BK, Aars NA. Changes in body mass index and the prevalence of obesity during 1994-2008: repeated cross-sectional surveys and longitudinal analyses. The Tromsø Study. BMJ Open. 2015;5(6):e007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haghiac M, Basu S, Presley L, Serre D, Catalano PM, Hauguel-de Mouzon S. Patterns of adiponectin expression in term pregnancy: impact of obesity. J Clin Endocrinol Metab. 2014;99(9):3427-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med. 2010;235(10):1185-1193. [DOI] [PubMed] [Google Scholar]
- 50. Clasen R, Schupp M, Foryst-Ludwig A, et al. . PPARgamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46(1):137-143. [DOI] [PubMed] [Google Scholar]
- 51. Combs TP, Wagner JA, Berger J, et al. . Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143(3):998-1007. [DOI] [PubMed] [Google Scholar]
- 52. Fasshauer M, Kralisch S, Klier M, et al. . Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301(4):1045-1050. [DOI] [PubMed] [Google Scholar]
- 53. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52(7):1779-1785. [DOI] [PubMed] [Google Scholar]
- 54. Chang E, Choi JM, Kim WJ, et al. . Restoration of adiponectin expression via the ERK pathway in TNFα-treated 3T3-L1 adipocytes. Mol Med Rep. 2014;10(2):905-910. [DOI] [PubMed] [Google Scholar]
- 55. Mondal AK, Das SK, Varma V, et al. . Effect of endoplasmic reticulum stress on inflammation and adiponectin regulation in human adipocytes. Metab Syndr Relat Disord. 2012;10(4):297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290(3):1084-1089. [DOI] [PubMed] [Google Scholar]
- 57. Misra VK, Trudeau S. The influence of overweight and obesity on longitudinal trends in maternal serum leptin levels during pregnancy. Obesity. 2011;19(2):416-421. [DOI] [PubMed] [Google Scholar]
- 58. Aye IL, Lager S, Ramirez VI, et al. . Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90(6):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gavrila A, Chan JL, Yiannakouris N, et al. . Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88(10):4823-4831. [DOI] [PubMed] [Google Scholar]
- 60. Frühbeck G, Catalán V, Rodríguez A, et al. . Normalization of adiponectin concentrations by leptin replacement in ob/ob mice is accompanied by reductions in systemic oxidative stress and inflammation. Sci Rep. 2017;7(1):2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cummings BP, Bettaieb A, Graham JL, et al. . Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc Natl Acad Sci U S A. 2011;108(35):14670-14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. James HA, O’Neill BT, Nair KS. Insulin regulation of proteostasis and clinical implications. Cell Metab. 2017;26(2):310-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bennett RG, Hamel FG, Duckworth WC. Insulin inhibits the ubiquitin-dependent degrading activity of the 26S proteasome. Endocrinology. 2000;141(7):2508-2517. [DOI] [PubMed] [Google Scholar]
- 64. Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147(9):4160-4168. [DOI] [PubMed] [Google Scholar]
- 65. Li N, Liu E, Guo J, et al. . Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One. 2013;8(12):e82310. doi: 10.1371/journal.pone.0082310. PMID:24376527; PMCID:PMC3869661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pretz D, Le Foll C, Rizwan MZ, Lutz TA, Tups A. Hyperleptinemia as a contributing factor for the impairment of glucose intolerance in obesity. FASEB J. 2021;35(2):e21216. doi: 10.1096/fj.202001147R. Epub 2020 Nov 23. PMID:33230896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”