Abstract
Context
Maternal prepregnancy body mass index (BMI) has a strong influence on gestational metabolism, but detailed metabolic alterations are unknown.
Objective
First, to examine the associations of maternal prepregnancy BMI with maternal early-pregnancy metabolite alterations. Second, to identify an early-pregnancy metabolite profile associated with birthweight in women with a higher prepregnancy BMI that improved prediction of birthweight compared to glucose and lipid concentrations.
Design, Setting, and Participants
Prepregnancy BMI was obtained in a subgroup of 682 Dutch pregnant women from the Generation R prospective cohort study.
Main Outcome Measures
Maternal nonfasting targeted amino acids, nonesterified fatty acid, phospholipid, and carnitine concentrations measured in blood serum at mean gestational age of 12.8 weeks. Birthweight was obtained from medical records.
Results
A higher prepregnancy BMI was associated with 72 altered amino acids, nonesterified fatty acid, phospholipid and carnitine concentrations, and 6 metabolite ratios reflecting Krebs cycle, inflammatory, oxidative stress, and lipid metabolic processes (P-values < 0.05). Using penalized regression models, a metabolite profile was selected including 15 metabolites and 4 metabolite ratios based on its association with birthweight in addition to prepregnancy BMI. The adjusted R2 of birthweight was 6.1% for prepregnancy BMI alone, 6.2% after addition of glucose and lipid concentrations, and 12.9% after addition of the metabolite profile.
Conclusions
A higher maternal prepregnancy BMI was associated with altered maternal early-pregnancy amino acids, nonesterified fatty acids, phospholipids, and carnitines. Using these metabolites, we identified a maternal metabolite profile that improved prediction of birthweight in women with a higher prepregnancy BMI compared to glucose and lipid concentrations.
Keywords: pregnancy, obesity, metabolomics, birth complications
Overweight or obesity in women prior and during pregnancy is a major risk factor for birth complications, including delivering a large-for-gestational-age (LGA) newborn (1,2). These associations of a higher maternal prepregnancy body mass index (BMI) with a higher birthweight are not confined to the extremes but are already present across the full ranges of maternal prepregnancy BMI and birthweight (3). Although the association of a higher maternal prepregnancy BMI with a higher birthweight is well known, the underlying mechanisms are not understood.
Maternal prepregnancy BMI has a strong influence on maternal metabolism during pregnancy, leading to an increased and suboptimal composition of fetal nutrient supply (1,2). It has been proposed that these alterations in fetal nutrient supply cause increased fetal growth and stimulate adiposity development, leading to a higher birthweight (1,2). Higher maternal glucose and lipid concentrations during pregnancy have already been shown to be important factors leading to increased fetal growth but only partly explain the associations of a higher maternal prepregnancy BMI with a higher birthweight (4). Metabolomics techniques offer the opportunity to obtain a detailed characterization of maternal metabolism during pregnancy and may enable identification of novel metabolic pathways in the associations of a higher maternal prepregnancy BMI with macrosomia (5). Recent studies already reported that a higher maternal BMI during pregnancy was associated with altered maternal metabolite concentrations throughout pregnancy, in particular with alterations in branched chain amino acids (BCAA) and nonesterified fatty acid (NEFA) serum concentrations (6-9). Other studies observed associations of altered maternal amino acids (AA), phospholipids (PL), and carnitines (Carn) serum concentrations in the second half of pregnancy with higher birthweight (10,11). A previous study among 400 pregnant women showed that altered maternal acylcarnitine, lipid, carbohydrate, and organic acid-related metabolites associated with a higher maternal BMI in second half of pregnancy could improve prediction of birthweight and fat mass in the newborn (12). Identifying detailed maternal early-pregnancy metabolic profiles involved in the association of a higher maternal prepregnancy BMI with higher offspring birthweight may provide more insight into the mechanisms underlying this well-known association and offer novel biomarkers for early identification of pregnant women at increased risk of delivering a LGA newborn.
Therefore, in a subgroup of 682 Dutch women participating in a population-based prospective cohort study from early-pregnancy onward, we first examined the associations of maternal prepregnancy BMI across the full range with maternal nonfasting early-pregnancy serum concentrations of AA, NEFA, PL, and Carn. Second, we explored the predictive value of identified maternal early-pregnancy metabolites alterations on offspring birthweight in addition to maternal glucose and lipid concentrations.
Methods
Study Design
This study was embedded in the Generation R study, a population-based prospective cohort study from fetal life until adulthood in Rotterdam, the Netherlands (13). Study approval was obtained by the Medical Ethical Committee of the Erasmus Medical Center, University Medical Center, Rotterdam (MEC 198.782/2001/31). Written informed consent was obtained from all women participating in the study. In total, 8879 women were enrolled during pregnancy in the Generation R study. Metabolomics data were available in a preselected subsample of 1041 Dutch mother-child pairs, of whom 814 had early-pregnancy metabolomics data available (13). This subsample is a random group of mothers and their children of Dutch ethnicity selected for additional measurements within the Generation R study, already at the start of our cohort study (14). Dutch ethnicity of participants in this samples was defined as having both parents born in the Netherlands, according the classification of Statistics Netherlands (15). Prepregnancy BMI was available in 690 of these women. After exclusion fetal deaths (n = 7) and women without data on offspring birthweight available (n = 1), our population for analyses consisted of 682 women [all supplementary material and figures are located in a digital research materials repository (16)].
Maternal Prepregnancy BMI
Information on maternal prepregnancy weight was obtained through questionnaires at enrollment (17). At enrollment, height was obtained at research center without shoes, and prepregnancy BMI was calculated (correlation coefficient with BMI based on measured weight at enrollment 0.96). For analyses, we used prepregnancy BMI continuously and categorized into 4 categories: underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (>25 kg/m2), and obesity (≥30 kg/m2). To increase statistical power, we combined underweight (n = 18) with normal weight (n = 482) into 1 category and overweight (n = 132) with obesity (n = 50) into 1 category.
Maternal Serum Metabolite Measurement
Maternal early-pregnancy nonfasting random venous blood samples were collected at study enrollment at a mean (SD) gestational age of 12.8 (1.7) weeks by research nurses at 1 of the dedicated research centers (18). A targeted metabolomics analysis was performed at LMU Munich to determine the serum concentrations (μmol/L) of AA, NEFA, and PL, including diacyl-phosphatidylcholines (PC.aa), acyl-alkyl-phosphatidylcholines (PC.ae), acyl-lysopho sphatidylcholines (Lyso.PC.a), alkyl-lysophosphatidylcho lines (Lyso.PC.e) and sphingomyelines (SM), and Carn including free Carn and acyl-carnitines (Carn.a), as described previously (14). IUPAC-IUB nomenclature was used for notation of AA (15). The following notation was used for NEFA, PL, and Carn.a: X:Y, where X denotes the length of the carbon chain, and Y the number of double bonds. The a denotes an acyl chain bound to the backbone of an ester bond (“acyl-”) and the e represents an ether bond (“alkyl-”). To assess the precision of the measurements, 6 quality control (QC) samples per batch were consistently measured between study samples. After exclusion of outliers, the coefficients of variation (CV; SD/mean) for each batch (intrabatch) and for all batches (interbatch) of the QC samples were calculated for each metabolite. In line with previous studies, for each metabolite we excluded batches with an intrabatch CV higher than 25% (6-8,19). Data on complete metabolites were excluded for metabolites with interbatch CV higher than 35% or if less than 50% of the batches passed the QC (ie., had an intrabatch CV lower than 25%). To correct for batch effects, the participant data at each time point were median corrected by dividing the metabolite concentration by the ratio of the intrabatch median and the interbatch median of the QC samples (7). Metabolites and participants with more than 50% of missing values were excluded. Missing metabolite values of the remaining metabolites and participants were imputed using the Random Forest algorithm (R package missForest) (7,20,21).
Individual metabolites were clustered in general metabolite groups, based on chemical structure (AA, NEFA, PC.aa, PC.ae, Lyso.PC.a, Lyso.PC.e, SM, free Carn, and Carn.a) (22). As we expected, fetal growth was mainly affected by Krebs cycle, inflammation, oxidative stress, and glucose and lipid metabolic processes due to a higher maternal prepregnancy BMI, we computed the following ratios: AA ratios, asparagine/aspartic acid (Asn/Asp), and glutamine/glutamic acid (Gln/Glu) as indicators for anaplerosis or replenishing of Krebs cycle metabolites; NEFA.18:1/NEFA.18:0 and NEFA.16:1/NEFA/16:0 ratios as markers of stearoyl-CoA desaturase-1 activity, which is associated with increased fat accumulation and reduced fatty acid oxidation; ΣPC.aa/ΣPC.ae, reflecting oxidative stress ΣLyso.PC.a/ΣPC.aa, as a lipid biomarker of inflammation; lyso.PC.a.C16:0 + lyso.PC.a.C18:0)/ΣPC.aa as a proinflammatory biomarker; (lyso.PC.a.C18:1 + lyso.PC.a.C18:2)/ΣPC.aa as an anti-inflammatory biomarker; Carn.a ratios (Carn.a.C:16:0/free Carn and Carn.a.C2:0/Carn.a.C16:0) as markers of Carn palmitoyl transferase-1 activity and fatty acid β-oxidation, respectively; and Val/PC.ae.C:32.2 as a marker of insulin resistance (13,23-28). To correct for right skewedness, individual metabolite concentrations and metabolite ratios were square-root transformed or log-transformed. To enable comparison of the effect estimates, SD scores (SDS) were calculated for individual metabolite concentrations.
Birthweight
Information about offspring sex, gestational age, and weight at birth was obtained from medical records (13). Gestational age– and sex-adjusted SDS for weight at birth were constructed using north European growth standards as the reference growth curve and represent the equivalent of z-scores (29). A LGA newborn was defined as the highest 10 percentiles of gestational age– and sex-adjusted birthweight in the study cohort.
Covariates
Information on maternal age, educational level, parity, folic acid supplementation, and smoking and alcohol consumption during pregnancy were obtained through questionnaires (13). Information on maternal daily dietary energy intake during pregnancy was obtained with a Food Frequency Questionnaire (30). Systolic blood pressure was measured at the research center prior to venous blood sampling at study enrollment (31). Triglycerides, high-density lipoprotein cholesterol (HDL-cholesterol) and glucose concentrations were analyzed in the same venous blood samples as used for metabolomics analyses (18). To enable comparison of effect estimates, SDS were calculated for triglycerides, HDL-cholesterol, and glucose, of which triglycerides was first log-transformed because of nonnormality.
Statistical Analysis
First, we performed a nonresponse analysis comparing characteristics of women with metabolomics data and information on prepregnancy BMI available to women with metabolomics data available but without information on prepregnancy BMI. Second, we assessed population characteristics according to maternal prepregnancy weight status. Third, we assessed the associations of maternal prepregnancy BMI across the full range and in clinical categories with maternal early-pregnancy individual metabolite concentrations, metabolite ratios, and metabolite groups. Analyses were adjusted for gestational age at blood sampling, maternal age, educational level, parity, smoking, alcohol use, folic acid supplementation, total dietary energy intake, early-pregnancy systolic blood pressure, and fetal sex. Confounder selection was based on a directed acyclic graph and association with exposure and outcomes in existing literature (16). Fourth, we used a penalized regression method (lasso regression) to select from the identified altered maternal early-pregnancy metabolites a combination of metabolites that were jointly associated with birthweight in addition to maternal prepregnancy BMI. Lasso regression is a highly useful method for developing a model with a high number of predictors, which are highly correlated, such as our metabolite data (32). We used maternal prepregnancy BMI and birthweight as continuous exposure and outcome, respectively, because of the continuous associations reported in previous studies and to maintain statistical power (7,10-12). A 10-fold cross-validation was performed, and the penalty parameter value yielding the smallest prediction error was used. Selection of maternal early-pregnancy metabolites was done in regression models including maternal prepregnancy BMI across the full range and all selected confounders, which could not be penalized. We compared the predictive performance for offspring birthweight of 3 linear regression models including (1) only maternal prepregnancy BMI and confounders (BMI model); (2) maternal prepregnancy BMI, confounders, maternal triglycerides, HDL-cholesterol, and glucose concentrations (conventional biomarker model); and (3) maternal prepregnancy BMI, confounders, and the set of selected maternal early-pregnancy metabolites associated with birthweight (metabolite model). Predictive performance per model was assessed by explained variance expressed as the adjusted R2, obtained from the 3 linear regression models. We also assessed the explained variance of the model including maternal prepregnancy BMI, conventional biomarkers, and selected maternal early-pregnancy metabolites. As an additional analysis, we assessed whether the maternal early-pregnancy metabolite profile selected on birthweight continuously could aid in the prediction of the risk of LGA at birth. We obtained the area under the receiving operator curve (AUC) with predicted probabilities obtained from logistic regression models for the risk of LGA at birth, for the maternal BMI model, conventional maternal biomarker model, and the maternal early-pregnancy metabolite model. Nominal and Benjamini-Hochberg false discovery rate (FDR) corrected P-values were obtained from regression models (33). Because of the explorative purpose of the study, we maintained a P-value < 0.05 in the analyses of maternal prepregnancy BMI with maternal early-pregnancy metabolites as a threshold for metabolites to be included in the penalized regression model. Missing values of covariates were imputed using multiple imputation, and we used pooled results from 5 imputed data sets. The analyses were performed using the Statistical Package for the Social Sciences version 24.0 (IBM Corp, Armonk, New York, USA) and R version 3.3.4 (R Foundation for Statistical Computing).
Results
Subject Characteristics
Table 1 shows that the median (95% range) maternal prepregnancy BMI was 22.6 (18.5; 33.3) kg/m2. Mean (SD) birthweight was 3538 (511) g. Birthweight was slightly lower among normal-weight pregnant women as compared to overweight or obese pregnant women. Nonresponse analyses showed that women with metabolomics data and information on prepregnancy BMI available had a similar offspring birthweight as compared those without information on prepregnancy BMI available (16).
Table 1.
Population characteristics
| Total group (N = 682) | Prepregnancy underweight or normal weight (n = 500) | Prepregnancy overweight or obesity (n = 182) | |
|---|---|---|---|
| Maternal characteristics | |||
| Maternal age at enrollment, years | 31.4 (4.2) | 31.4 (4.2) | 31.4 (4.0) |
| Gestational age at enrollment, weeks | 13.1 (1.7) | 13.3 (1.7) | 12.8 (1.7) |
| Prepregnancy BMI | 22.6 (18.4; 33.3) | 21.6 (18.3; 24.7) | 27.7 (25.1; 37.4) |
| Parity, nulliparous | 418 (61) | 317 (63) | 101 (55) |
| Higher education completed, yes | 436 (64) | 348 (70) | 88 (49) |
| Folic acid supplement use, yes | 546 (91) | 402 (92) | 144 (90) |
| Alcohol use during pregnancy, yes | 442 (69) | 336 (72) | 106 (61) |
| Smoking during pregnancy, yes | 154 (24) | 111 (24) | 43 (25) |
| Total energy intake, kcal/d | 2125 (490) | 2147 (489) | 2046 (491) |
| Systolic blood pressure, mmHg | 118 (97; 146) | 116 (95; 139) | 124 (104; 156) |
| Glucose concentrations, mmol/L | 4.4 (0.8) | 4.3 (0.8) | 4.6 (0.9) |
| Triglycerides,mmol/L | 1.2 (0.7; 2.6) | 1.3 (0.6; 2.4) | 1.4 (0.8; 2.6) |
| HDL-cholesterol, mmol/L | 1.8 (0.3) | 1.8 (0.3) | 1.7 (0.3) |
| Birth characteristics | |||
| Sex, female | 315 (46) | 226 (45) | 89 (49) |
| Gestational age at birth, weeks | 40.3 (36.6; 42.4) | 40.3 (36.6; 42.4) | 40.4 (36.7; 42.4) |
| Birthweight, g | 3538 (511) | 3523 (491) | 3578 (560) |
| Large-for-gestational-age infants | 69 (10) | 45 (9) | 23 (13) |
Values represent mean (SD), median (95% range) or number of participants (valid %).
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein.
Maternal Prepregnancy BMI and Early-pregnancy Metabolite Concentrations
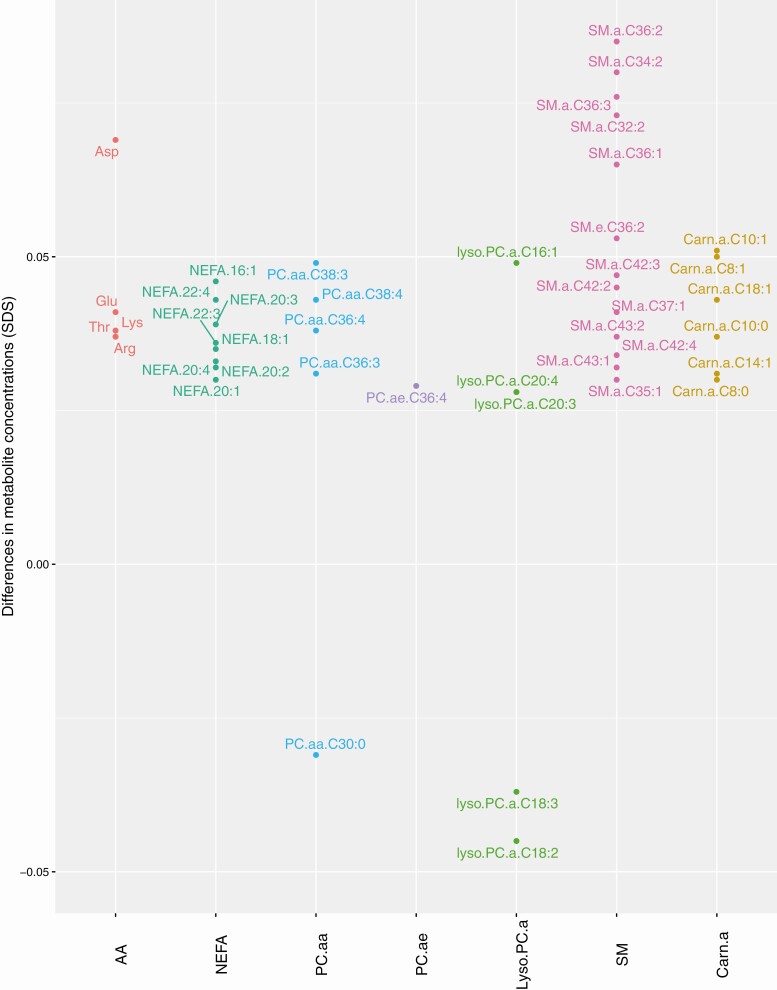
Of the overall metabolite groups, a higher maternal prepregnancy BMI was associated with higher NEFA and SM concentrations only, which remained after multiple testing correction (16). A higher maternal prepregnancy BMI was associated with alterations of 72 individual early-pregnancy AA, NEFA, PC.aa, PC.ae, lyso.PC.a, SM, and Carn.a metabolite concentrations (all P-values < 0.05) but not with alterations in lyso.PC.e and free Carn metabolite concentrations (Fig. 1). After multiple testing correction, 43 associations of maternal prepregnancy BMI with individual metabolites from the AA, NEFA, PC.aa, lyso.PC.a, SM, and Carn.a groups remained significant (FDR-corrected P-values < 0.05). The strongest associations were present for SM.a.C34:2, SM.a.C36:2, and SM.a.C36:3 (difference in maternal metabolite concentrations for SM.a.C34:2 0.08, 95% CI 0.06; 0.10 SDS; for SM.a.C36:2 0.09, 95% CI 0.06; 0.11; and for SM.a.C36:3 0.08, 95% CI 0.06; 0.10 SDS per kg/m2 increase in maternal prepregnancy BMI). A higher maternal prepregnancy BMI was associated with higher NEFA 16:1/16:0, NEFA 18:1/18:0, and ΣPC.aa/ΣPC.ae ratios and lower Asn/Asp, glutamine/glutamic acid, and lyso.PC.a.C:18.1 + C:18.2/ΣPC.aa ratios (P-values < 0.05), all remaining significant after multiple testing correction (FDR-corrected P-values < 0.05) (Table 2).
Figure 1.
Significant associations of maternal prepregnancy BMI with maternal early-pregnancy metabolites.
Table 2.
Associations of maternal prepregnancy BMI with maternal early-pregnancy metabolite ratios
| Maternal prepregnancy BMI (kg/m2) | Maternal prepregnancy overweight or obesity | ||||||
|---|---|---|---|---|---|---|---|
| Early-pregnancy maternal metabolite ratios | Difference in metabolite concentration (SDS) (95% CI) per kg/m2 increase in BMI) | P-value | FDR corrected P-value | Maternal normal prepregnancy weight | Difference in metabolite concentration (SDS) (95% CI)) | P-value | FDR corrected P-value |
| Asn/Asp | −0.08 (−0.10; −0.06) | 0.000 | 0.000 | Reference | −0.51 (−0.69; −0.33) | 0.000 | 0.000 |
| Gln/Glu | −0.04 (−0.06; 0.01) | 0.002 | 0.004 | Reference | −0.16 (−0.35; 0.03) | 0.090 | 0.165 |
| NEFA.18:1/18:0 | 0.04 (0.02; 0.06) | 0.000 | 0.001 | Reference | 0.23 (0.04; 0.42) | 0.016 | 0.035 |
| NEFA.16:1/16:0 | 0.05 (0.03; 0.07) | 0.000 | 0.000 | Reference | 0.25 (0.06; 0.43) | 0.010 | 0.035 |
| Carn.a.16.0/free carnitine | 0.01 (−0.01; 0.03) | 0.413 | 0.504 | Reference | 0.06 (−0.12; 0.25) | 0.505 | 0.694 |
| Carn.a.C:2/C:16 | −0.03 (−0.03; 0.02) | 0.784 | 0.863 | Reference | −0.02 (−0.21; 0.17) | 0.822 | 0.849 |
| ΣLyso.PC.a/ΣPC.aa | −0.02 (−0.04; 0.00) | 0.109 | 0.171 | Reference | −0.13 (−0.31; 0.05) | 0.165 | 0.260 |
| (Lyso.PC.a.C:18.1 + C:18.2)/ΣPC.aa | −0.06 (−0.08; −0.04) | 0.000 | 0.000 | Reference | −0.32 (−0.50; −0.14) | 0.001 | 0.003 |
| (Lyso.PC.a.C:16:0 + C18:0)/ΣPC.aa | 0.00 (−0.02; 0.02) | 0.929 | 0.929 | Reference | −0.05 (−0.23; 0.13) | 0.570 | 0.697 |
| Val/PC.ae.C:32.2 | 0.01 (−0.01; 0.04) | 0.237 | 0.326 | Reference | 0.02 (−0.17; 0.20) | 0.849 | 0.849 |
| ΣPC.aa/ΣPC.ae | 0.03 (0.01; 0.05) | 0.016 | 0.029 | Reference | 0.23 (0.05; 0.40) | 0.014 | 0.035 |
Values represent regression coefficients (95% CI) and corresponding P-values and false discovery rate corrected P-values from linear regression models that reflect the difference in maternal early-pregnancy metabolite ratios in SD score per kg/m2 increase in BMI and for overweight or obese women as compared to normal weight women. Models were adjusted for gestational age at blood sampling, age, educational level, parity, smoking, alcohol consumption, folic acid supplementation, daily total energy intake, systolic blood pressure, and fetal sex.
Abbreviations: Asn/Asp, asparagine/aspartic acid; BMI, body mass index; FDR, false discovery rate; Gln/Glu, glutamine/glutamic acid.
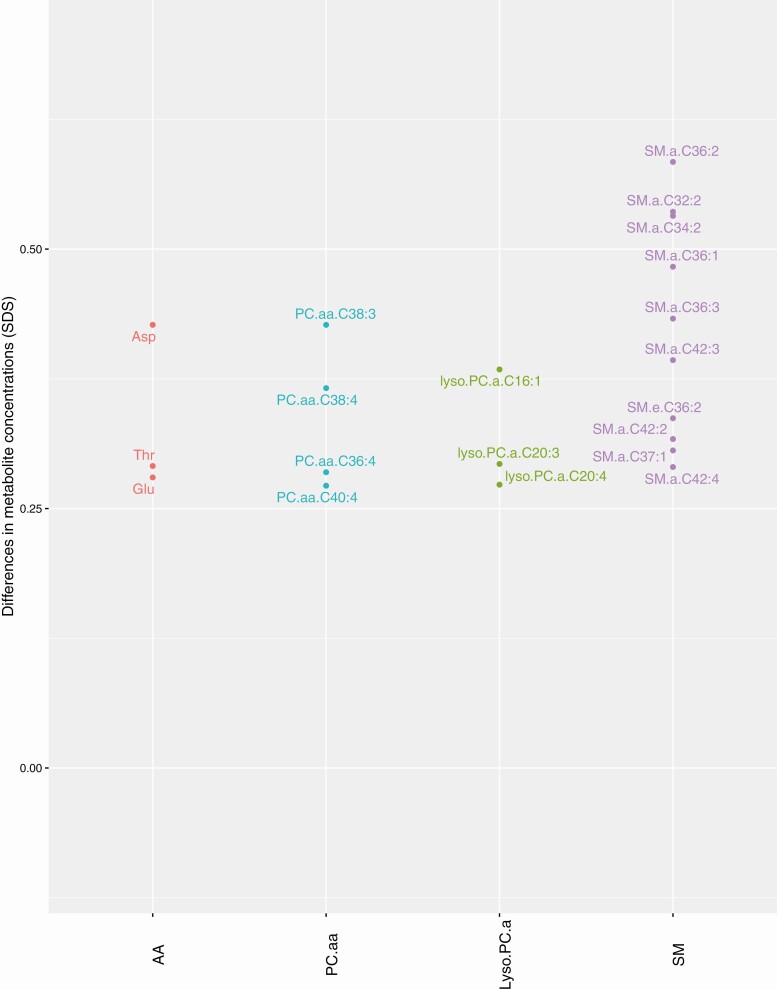
Partly in line with the associations of maternal prepregnancy BMI across the full range, 46 maternal early-pregnancy AA, NEFA, PC.aa, PC.ae, lyso.PC.a, SM, and Carn.a metabolite concentrations were higher in overweight and obese pregnant women, as compared to normal weight pregnant women (P-values < 0.05) (Fig. 2). After multiple testing correction, the associations of maternal prepregnancy BMI with 20 individual AA, PC.aa, lyso.PC.a, and SM metabolite concentrations remained significant (FDR-corrected P-values < 0.05). Overweight and obese women had higher NEFA 16:1/16:0, NEFA 18:1/18:0, and ΣPC.aa/ΣPC.ae ratios but lower Asn/Asp and lyso.PC.a.C:18/PC.aa ratios as compared to normal weight women, remaining significant after multiple testing correction (FDR-corrected P-values < 0.05) (Table 2). Effect estimates for associations of maternal prepregnancy BMI continuously and in categories with all individual maternal early-pregnancy metabolite concentrations are shown in Supplementary Figure 3 and Supplementary Table 3 (16).
Figure 2.
Significant associations of maternal prepregnancy overweight or obesity with maternal early-pregnancy metabolites. Regression coefficients were obtained from linear regression models that reflect the difference in maternal early-pregnancy metabolite concentrations in SD score (SDS) per kg/m2 increase in maternal prepregnancy body mass index (Fig. 1) and difference in maternal early-pregnancy metabolite concentrations in SDS for overweight or obese women as compared to normal weight women (Fig. 2) of associations with false discovery rate corrected P-values < 0.05. Models were adjusted for gestational age at blood sampling, age, educational level, parity, smoking, alcohol consumption, folic acid supplementation, daily total energy intake, systolic blood pressure, and fetal sex. Abbreviations: AA, amino acid; NEFA, nonesterified fatty acid; PC.aa diacyl-phosphatidylcholines, PC.ae, acyl-alkyl-phosphatidylcholines; Lyso.PC.a, acyl-lysophosphatidylcholines; SM, sphingomyelines Carn.a, acylcarnitines.
Maternal Prepregnancy BMI, Early-pregnancy Metabolites, and Birthweight
A higher maternal prepregnancy BMI was significantly associated with a higher birthweight (0.03, 95% CI 0.01; 0.05 SDS per kg/m2 increase in maternal prepregnancy BMI) (Table 3). Based on their joint association with birthweight, a combination of 15 individual maternal early-pregnancy metabolites and 4 metabolite ratios were selected using lasso regression models retaining maternal prepregnancy BMI in the model (Table 3). Adding these selected individual maternal early-pregnancy metabolites and metabolite ratios to the model resulted in a higher effect estimate for the association of maternal prepregnancy BMI with birthweight (0.05, 95% CI 0.03; 0.07 SDS per kg/m2 increase in maternal prepregnancy BMI). The explained variance of the model for offspring birthweight including maternal prepregnancy BMI and the individual maternal early-pregnancy metabolites and metabolites ratios was 12.9% (SD of the residuals 0.90), which was higher than the explained variance of the model only including maternal prepregnancy BMI (6.1%, SD of the residuals 0.93) and the model including maternal prepregnancy BMI and conventional maternal biomarkers (6.2%, SD of the residuals 0.93). For presentation purposes, the predicted values versus observed values for all models are shown in Supplementary Figure 3 (16) and show more accurate predictions of the model including maternal early-pregnancy metabolites and metabolite ratios as compared to the model only including maternal prepregnancy BMI and the model including maternal prepregnancy BMI and conventional maternal biomarkers. A model including maternal prepregnancy BMI, conventional biomarkers, and the maternal early-pregnancy metabolites and metabolite ratios had an explained variance of 13.0%.
Table 3.
Selected models for the prediction of birthweight
| Models | Difference in birthweight (SDS) (95% CI) | Adjusted R2 (%) | SD of the residuals |
|---|---|---|---|
| BMI modela | |||
| Prepregnancy BMI | 0.03 (0.00; 0.05) | 6.1 | 0.93 |
| Biomarker modelb | |||
| Prepregnancy BMI | 0.02 (0.00; 0.04) | 6.2 | 0.93 |
| Glucose | 0.06 (-0.01; 0.14) | ||
| Triglycerides | 0.01 (-0.07; 0.05) | ||
| HDL-cholesterol | -0.03 (-0.11; 0.05) | ||
| Metabolite modelc | |||
| Prepregnancy BMI | 0.05 (0.03; 0.07) | 12.9 | 0.90 |
| Gln | 0.03 (-0.05; 0.13) | ||
| Lys | -0.04 (-0.12; 0.04) | ||
| NEFA.18:2 | 0.08 (-0.04; 0.19) | ||
| NEFA.20:3 | 0.06 (-0.06; 0.18) | ||
| NEFA.22:3 | -0.15 (-0.26; -0.05) | ||
| PC.aa.C30:0 | 0.05 (-0.04; 0.15) | ||
| PC.aa.C38:3 | 0.14 (0.00; 0.27) | ||
| PC.ae.C34:4 | -0.14 (-0.24; -0.05) | ||
| Lyso.PC.a.C16:1 | -0.23 (-0.33; -0.12) | ||
| Lyso.PC.a.C20:4 | 0.04 (-0.07; 0.14) | ||
| SM.a.C36:1 | -0.05 (-0.22; 0.12) | ||
| SM.a.C36:2 | -0.15 (-0.34; 0.04) | ||
| SM.a.C40:2 | 0.13 (0.01; 0.25) | ||
| SM.a.C42:4 | 0.12 (-0.01; 0.26) | ||
| Carn.a.C10:1 | -0.09 (-0.18; 0.00) | ||
| Asn/Asp ratio | 0.03 (-0.05; 0.11) | ||
| Gln/Glu ratio | 0.03 (-0.06; 0.12) | ||
| NEFA.16:1/16:0 ratio | -0.06 (-0.13; 0.01) | ||
| ΣPC.aa/ΣPC.ae ratio | -0.01 (-0.10; 0.07) |
Values represent regression coefficients (95% CI) and adjusted R2 obtained from linear regression models.
Abbreviations: Asn/Asp, asparagine/aspartic acid; BMI, body mass index; Gln/Glu, glutamine/glutamic acid; HDL, high-density lipoprotein; SDS, SD score.
aBMI model includes maternal prepregnancy BMI, gestational age at blood sampling, age, educational level, parity, smoking, alcohol consumption, folic acid supplementation, daily total energy intake, systolic blood pressure, and fetal sex.
bBiomarker model includes the BMI model with additional adjustment for maternal glucose, triglycideride, and HDL-cholesterol concentrations
cMetabolite model includes the BMI model with additional adjustment for selected maternal early-pregnancy metabolites.
Predictive performance for the risk of LGA at birth improved after addition of the maternal early-pregnancy metabolite profile (AUC 0.76, 95% CI 0.70; 0.82), when compared to the performance of models only including maternal prepregnancy BMI (AUC 0.67, 95% CI 0.61; 0.73) and the model including maternal prepregnancy BMI and conventional maternal biomarkers (AUC 0.69, 95% CI 0.62; 0.76) (16).
Discussion
A higher maternal prepregnancy BMI was associated with alterations in individual maternal early-pregnancy metabolite concentrations from the AA, NEFA, PC.aa, PC.ae, lyso.PC.a, SM, and Carn.a groups and alterations in metabolite ratios marking processes of the Krebs cycle, inflammation, oxidative stress, and lipid metabolism. Using these altered maternal early-pregnancy metabolites and metabolite ratios, we identified an early-pregnancy maternal metabolite profile consisting of 15 metabolites and 4 metabolite ratios, which was associated with a higher birthweight in addition to maternal prepregnancy BMI. Use of this identified maternal metabolite profile together with maternal prepregnancy BMI resulted in a better prediction of birthweight than a model with maternal prepregnancy BMI alone or maternal prepregnancy BMI and maternal early-pregnancy glucose and lipid concentrations.
Interpretation of Main Findings
Overweight and obesity are well-known to be associated with major alterations in metabolism, especially glucose and lipid metabolism. However, detailed underlying metabolic processes in these associations remain to be elucidated (9). Previous studies among nonpregnant populations not only suggested mainly associations of a higher BMI with higher NEFA and BCAA concentrations but also reported less consistent associations with alterations in other AA and PL (9). Among pregnant populations, only a few studies have assessed the associations of maternal BMI with detailed metabolite profiles in pregnancy, and these studies showed inconsistent results (6,7,12). A study from the United States among 167 pregnant women using a targeted metabolomics approach observed that a higher maternal prepregnancy BMI was associated with alterations in second-trimester maternal AA, first- and second-trimester NEFAs, first- and third-trimester PL but not with Carn.a throughout pregnancy (6). A multinational study among 400 pregnant women observed a cross-sectional association of a higher maternal BMI at 28 weeks gestation with altered AA, Carn.a, carbohydrates, and fatty acids concentrations using a targeted and nontargeted metabolomics approach (12). A Spanish study among 200 pregnant women reported associations of a higher prepregnancy BMI with mainly higher maternal BCAA concentrations at delivery and weaker associations with alterations in maternal NEFA.22:4, PC.aa.C38:4, SM.C32:2, SM.C34.2, and Carn.a.C4:0 concentrations (7).
Largely in line with previous studies, we observed that a higher maternal prepregnancy BMI was associated with alterations in maternal early-pregnancy AA, NEFA, and PL, including PC.aa, PC.ae, lyso.PC.a, lyso.PC.e and SM, and Carn.a concentrations. We observed the strongest associations for higher SM.a.36:2, SM.a.C34:2, SM.a.C36:3, and SM.a.C32:2 concentrations, which may be involved in the development of insulin resistance (34). In contrast to these previous studies among nonpregnant and pregnant populations, we did not find associations of a higher maternal prepregnancy BMI with higher concentrations of the BCAA, valin, leucin, and isoleucin. These metabolites are markers of insulin resistance (34). The lack of associations in the current study with BCAA could be due to our relatively healthy population, with low mean glucose concentrations. It could also be due to our nonfasting samples and the timing in early pregnancy, as insulin resistance may be more pronounced in the fasting state and later in pregnancy (6,35). In line with previous studies, we focused on metabolite ratios that are markers for processes in the Krebs cycle, inflammation, oxidative stress, lipid metabolism, and insulin resistance (7,13,23-28). We observed associations of a higher maternal prepregnancy BMI with metabolite ratios reflecting reduced anaplerosis or replenishing of the Krebs cycle metabolites, stearoyl-CoA desaturase-1 activity, anti-inflammatory biomarkers, and oxidative stress. Thus, our results suggest that a maternal higher prepregnancy BMI is associated with alterations in maternal early-pregnancy AA, NEFA, PC.aa, PC.ae, lyso.PC.a, SM, and Carn.a metabolite concentrations, with the strongest effect on SM metabolites and with metabolite ratios marking processes in the Krebs cycle, inflammation, oxidative stress, and lipid metabolism.
Hyperglycemia and dyslipidemia during pregnancy in response to a higher prepregnancy BMI only partly explain the associations of a higher maternal prepregnancy BMI with a higher birthweight (1,2,4). The role of altered metabolites during early pregnancy in the associations of a higher maternal prepregnancy BMI with a higher birthweight is largely unknown. Recently, a study among 400 pregnant women used targeted and nontargeted metabolomics approaches to obtain fasting and 1-h metabolites (12). This study showed associations of a higher maternal BMI in second half of pregnancy with alterations in AA, NEFA, Carn, and sugars/alcohols metabolite concentrations. These metabolites improved the prediction of birthweight by increasing the explained variance for birthweight from 4.4% to 6.5% after addition of maternal fasting metabolites to a model with maternal prepregnancy BMI and from 7.1% to 9.2% after addition of 1-h metabolites. A study among 8212 women obtained nuclear magnetic resonance-derived metabolite concentrations in mid-pregnancy (36). They observed that addition of 66 metabolites, including AA, fatty acids, phospholipids, apolipoproteins, cholesterol, and very low density lipoprotein, to a risk factor model with maternal age, pregnancy BMI, ethnicity, and parity improved prediction of LGA newborns. The AUC was 0.71 (95% CI 0.66; 0.75) for the risk factor model alone and increased to 0.75 (95% CI 0.70; 0.79) after addition of the 66 metabolites. Largely in line with the metabolites identified in these previous studies, we observed that a maternal early-pregnancy metabolite profile including altered AA, NEFA, and Carn.a and PL concentrations and metabolite ratios marking processes in the Krebs cycle, inflammation, and lipid metabolism was associated with a higher birthweight in addition to maternal prepregnancy BMI. The explained variation improved from 6.1% for the model only including maternal prepregnancy BMI and confounders to 12.9% for the model additionally including the maternal early-pregnancy metabolite profile.
Maternal metabolic disturbances due to a higher maternal prepregnancy BMI can affect fetal growth and development not only directly through altering fetal exposure to an adverse maternal metabolite profile but also by affecting placental development and leading to an altered regulation of maternal nutrient transfer to the fetus (1,37). In the identified maternal metabolite profile, we observed an effect of AA, glutamine, and lysin, which are important for protein synthesis, essential for fetal growth (19,38,39). The highest number of metabolites selected in the maternal early-pregnancy metabolite profile were from the NEFA and PL groups, which underlines the importance of lipid metabolism in affected fetal growth in women with a higher prepregnancy BMI. Animal studies and studies among women with gestational diabetes have suggested that increased NEFA are transported to the fetus, leading to increased fetal growth and adiposity development (40,41). Higher maternal NEFA metabolite concentrations may also have an effect on placental development, increasing transfer of triglycerides to the fetus and altering fetal lipid metabolism and adipose tissue development (42). Increase of maternal PL, especially SM, is a normal physiological process during pregnancy to preserve fetal nutrient supply, but the fetus does not depend on maternal PL (19). While SM inhibits cholesterol absorption, an excess increase in maternal SM can cause increased insulin resistance, leading to higher maternal and fetal glucose concentrations and subsequent accelerated fetal growth (34). Metabolite ratios selected in the maternal early-pregnancy metabolite profile were markers of reduced anaplerosis and replenishing processes of metabolites in the Krebs cycle and altered lipid metabolism and oxidative stress. These processes are well known to be influenced by a higher BMI and also affect placental development and fetal growth (43). Thus, our findings suggest that an altered maternal early-pregnancy metabolite profile are involved in pathways underlying the associations of a higher maternal prepregnancy BMI with a higher birthweight. Further studies among larger multiethnic populations with higher variability in metabolite concentrations and birthweight are needed to replicate our findings.
The identification of a maternal early-pregnancy metabolite profile that relates to the associations of a higher maternal prepregnancy BMI with a higher birthweight is important from an etiological and public health perspective. Our findings may contribute to the understanding of mechanisms underlying the associations of a higher maternal prepregnancy BMI with a higher birthweight. Associations for maternal prepregnancy overweight or obesity with maternal early-pregnancy metabolites and metabolite ratios were largely similar as for maternal prepregnancy BMI across the full range. These findings suggest that effects of a higher maternal prepregnancy BMI on early-pregnancy metabolites are not limited to thresholds of disease but are already present within a healthy range. Importantly, the identified maternal early-pregnancy metabolite profile improved prediction of birthweight in addition to maternal prepregnancy BMI and had a better predictive performance than conventional maternal biomarkers including glucose and lipid concentrations. Although the variance explained of birthweight strongly improved after addition of the maternal metabolite profile to the model, the overall variance explained remained relatively low, and this prediction model cannot be directly translated to clinical practice. However, we consider this identified maternal early-pregnancy metabolite profile important as it provides novel insight into potential novel markers for more accurate prediction of birthweight after replication and validation. Further studies are needed focused on the development of more advanced prediction models and identification of novel markers before and during pregnancy to further improve the prediction of birthweight. After replication of our findings and incorporation in more advanced prediction models, determination of the maternal metabolite profile in early pregnancy may enable more accurate identification of women with a higher prepregnancy BMI at increased risk of delivering a LGA newborn and provide novel targets for interventions.
Methodological Considerations
We obtained metabolomics data in a subgroup of our multiethnic cohort, which consists of Dutch participants only (13). Ethnicity is likely to have a major influence on the metabolome, via both genetic and environmental factors (44). By performing our study within an ethnic homogenous population, we reduced the risks of potential residual confounding or effect modification by ethnicity. This is especially important since little is known about the influence of maternal prepregnancy BMI on early-pregnancy metabolite adaptations. However, our selected study population may affect the generalizability of our findings. Further studies are needed to replicate our findings among multiethnic populations and to explore whether ethnic-specific effects are present. As we used self-reported prepregnancy weight, misclassification bias may be an issue. However, we observed a high correlation between self-reported prepregnancy weight and early-pregnancy weight measured at enrollment at the research center. We used nonfasting blood samples for metabolomics analyses; consequently, dietary intake may have influenced the metabolomics data. Although metabolomics research is generally performed using fasting samples, a large cohort study among 6671 adults observed a better biological reproducibility of nonfasting samples compared to fasting samples and suggested that nonfasting samples may be more useful for the prediction of subsequent disease, as the human physical state is nonfasting the majority of the day (45). We used a targeted metabolomics approach, allowing us to optimize the quantification of the metabolites of interest, but relevant biological pathways might be missed. Further studies using both untargeted and targeted metabolomics in fasting and nonfasting serum samples are needed to replicate our findings and to identify further novel pathways. Finally, we adjusted our analyses for many potential confounders. However, due to the observational nature of the study, residual confounding cannot be excluded.
Conclusion
A higher maternal prepregnancy BMI is associated with alterations in maternal early-pregnancy AA, NEFA, PC.aa, PC.ae, lyso.PC.a, SM, and Carn.a metabolite concentrations. Using these altered metabolites, we identified a maternal early-pregnancy metabolite profile, which improves the prediction of birthweight in addition to maternal prepregnancy BMI and has a better performance for the prediction of birthweight than conventional maternal biomarkers. These findings are important from an etiological perspective and, after further replication, may enable early identification of pregnant women at increased risk of delivering a LGA newborn.
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam; the Municipal Health Service Rotterdam Area, Rotterdam; the Rotterdam Homecare Foundation, Rotterdam; and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
Author Contributions : R.W. and R.G. designed and constructed the research, wrote the paper and had primary responsibility for the final content. O.U. was responsible for maternal serum metabolite measurements. R.W., E.V., and R.G. carried out the statistical analysis. V.J., G.R., B.K., and J.F. coordinated data acquisition and critically reviewed and revised the manuscript. All authors approved the final manuscript and agree to be accountable for all aspects of the work.
Funding : The Generation R Study is financially supported by the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. R.G. received funding from the Dutch Heart Foundation (Grant No. 2017T013), the Dutch Diabetes Foundation (Grant No. 2017.81.002), and the Netherlands Organization for Health Research and Development (ZonMW, Grant No. 543003109). V.W.V.J. received a European Research Council Consolidator Grant (ERC-2014-CoG-648916). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant Agreement No. 633595 (DynaHEALTH) and from the European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL, NutriPROGRAM project, ZonMw the Netherlands No. 529051022) and from the European Union’s Horizon 2020 research and innovation programme under the ERA-NET Cofund action (no 727565), European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL, EndObesity, ZonMW the Netherlands, (no. 529051026). The metabolomic analyses were financially supported in part by the European Research Council Advanced Grant META-GROWTH ERC-2012-AdG–no.322605, the European Joint Programming Initiative Project NutriPROGRAM, the German Ministry of Education and Research, Berlin (Grant Nr. 01 GI 0825), and the German Research Council (INST 409/224-1 FUGG).
Additional Information
Disclosure Summary : The authors have no conflicts of interest relevant to this article to disclose.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update. 2010;16(3):255-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PloS One. 2013;8(4):e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geurtsen ML, van Soest EEL, Voerman E, Steegers EAP, Jaddoe VWV, Gaillard R. High maternal early-pregnancy blood glucose levels are associated with altered fetal growth and increased risk of adverse birth outcomes. Diabetologia. 2019;62(10):1880-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tzoulaki I, Ebbels TM, Valdes A, Elliott P, Ioannidis JP. Design and analysis of metabolomics studies in epidemiologic research: a primer on -omic technologies. Am J Epidemiol. 2014;180(2):129-139. [DOI] [PubMed] [Google Scholar]
- 6. Hellmuth C, Lindsay KL, Uhl O, et al. Association of maternal prepregnancy BMI with metabolomic profile across gestation. Int J Obes (Lond). 2017;41(1):159-169. [DOI] [PubMed] [Google Scholar]
- 7. Shokry E, Marchioro L, Uhl O, et al. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: results from the PREOBE cohort study. Acta Diabetol. 2019;56(4):421-430. [DOI] [PubMed] [Google Scholar]
- 8. Rauschert S, Kirchberg FF, Marchioro L, Koletzko B, Hellmuth C, Uhl O. Early programming of obesity throughout the life course: a metabolomics perspective. Ann Nutr Metab. 2017;70(3):201-209. [DOI] [PubMed] [Google Scholar]
- 9. Rangel-Huerta OD, Pastor-Villaescusa B, Gil A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics. 2019;15(6):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ciborowski M, Zbucka-Kretowska M, Bomba-Opon D, et al. Potential first trimester metabolomic biomarkers of abnormal birth weight in healthy pregnancies. Prenat Diagn. 2014;34(9):870-877. [DOI] [PubMed] [Google Scholar]
- 11. Kadakia R, Nodzenski M, Talbot O, et al. ; HAPO Study Cooperative Research Group. Maternal metabolites during pregnancy are associated with newborn outcomes and hyperinsulinaemia across ancestries. Diabetologia. 2019;62(3):473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandler V, Reisetter AC, Bain JR, et al. ; HAPO Study Cooperative Research Group. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia. 2017;60(3):518-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kooijman MN, Kruithof CJ, van Duijn CM, et al. The generation R study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaddoe VW, van Duijn CM, van der Heijden AJ, et al. The generation R study: design and cohort update until the age of 4 years. Eur J Epidemiol. 2008;23(12):801-811. [DOI] [PubMed] [Google Scholar]
- 15. Statisics Netherlands. Allochtonen in Nederland. CBS; 2004. [Google Scholar]
- 16. Wahab RJ, Jaddoe VWV, Voerman E, et al. Supplementary file for “Maternal body mass index, early-pregnancy metabolite profile and birth weight” Accessed July 6, 2021. https://figshare.com/s/8483ce2f4d4e9746b390
- 17. Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The generation R study. Int J Obes (Lond). 2015;39(4):677-685. [DOI] [PubMed] [Google Scholar]
- 18. Kruithof CJ, Kooijman MN, van Duijn CM, et al. The generation R study: biobank update 2015. Eur J Epidemiol. 2014;29(12):911-927. [DOI] [PubMed] [Google Scholar]
- 19. Lindsay KL, Hellmuth C, Uhl O, et al. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PloS One. 2015;10(12):e0145794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellmuth C, Weber M, Koletzko B, Peissner W. Nonesterified fatty acid determination for functional lipidomics: comprehensive ultrahigh performance liquid chromatography-tandem mass spectrometry quantitation, qualification, and parameter prediction. Anal Chem. 2012;84(3):1483-1490. [DOI] [PubMed] [Google Scholar]
- 21. Wei R, Wang J, Su M, et al. Missing value imputation approach for mass spectrometry-based metabolomics data. Sci Rep. 2018;8(1):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voerman E, Jaddoe VWV, Uhl O, et al. A population-based resource for intergenerational metabolomics analyses in pregnant women and their children: the Generation R Study. Metabolomics. 2020;16(4):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molnos S, Wahl S, Haid M, et al. Metabolite ratios as potential biomarkers for type 2 diabetes: a DIRECT study. Diabetologia. 2018;61(1):117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Squeri A. Coronary Artery Disease: New Insights and Novel Approaches. Books on Demand; 2012. [Google Scholar]
- 25. Zhang W, Sun G, Aitken D, et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology (Oxford). 2016;55(9):1566-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pickens CA, Vazquez AI, Jones AD, Fenton JI. Obesity, adipokines, and C-peptide are associated with distinct plasma phospholipid profiles in adult males, an untargeted lipidomic approach. Sci Rep. 2017;7(1):6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirchberg FF, Brandt S, Moß A, et al. Metabolomics reveals an entanglement of fasting leptin concentrations with fatty acid oxidation and gluconeogenesis in healthy children. PloS One. 2017;12(8):e0183185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sampath H, Ntambi J. Role of stearoyl-CoA desaturase in human metabolic disease. Future Lipidol. 2008;3(2):163-173. [Google Scholar]
- 29. Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981). Acta Paediatr Scand. 1991;80(8-9):756-762. [DOI] [PubMed] [Google Scholar]
- 30. Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, et al. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998;52(8):588-596. [DOI] [PubMed] [Google Scholar]
- 31. Gaillard R, Bakker R, Willemsen SP, Hofman A, Steegers EA, Jaddoe VW. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: the generation R study. Eur Heart J. 2011;32(24):3088-3097. [DOI] [PubMed] [Google Scholar]
- 32. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B (Methodological). 1996;58(1):267-288. [Google Scholar]
- 33. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological). 1995;57(1):289-300. [Google Scholar]
- 34. Li Z, Zhang H, Liu J, et al. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol Cell Biol. 2011;31(20):4205-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Catalano P, deMouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond). 2015;39(4):642-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McBride N, Yousefi P, White SL, et al. Do nuclear magnetic resonance (NMR)-based metabolomics improve the prediction of pregnancy-related disorders? Findings from a UK birth cohort with independent validation. BMC Med. 2020;18(1):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duggleby SL, Jackson AA. Higher weight at birth is related to decreased maternal amino acid oxidation during pregnancy. Am J Clin Nutr. 2002;76(4):852-857. [DOI] [PubMed] [Google Scholar]
- 39. Kalhan SC. Protein metabolism in pregnancy. Am J Clin Nutr. 2000;71(5 Suppl):1249S-1255S. [DOI] [PubMed] [Google Scholar]
- 40. Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31(9):1858-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balogh O, Bruckmaier R, Keller S, Reichler IM. Effect of maternal metabolism on fetal supply: glucose, non-esterified fatty acids and beta-hydroxybutyrate concentrations in canine maternal serum and fetal fluids at term pregnancy. Anim Reprod Sci. 2018;193:209-216. [DOI] [PubMed] [Google Scholar]
- 42. Fattuoni C, Mandò C, Palmas F, et al. Preliminary metabolomics analysis of placenta in maternal obesity. Placenta. 2018;61:89-95. [DOI] [PubMed] [Google Scholar]
- 43. Myatt L, Maloyan A. Obesity and placental function. Semin Reprod Med. 2016;34(1):42-49. [DOI] [PubMed] [Google Scholar]
- 44. Hellmuth C, Lindsay KL, Uhl O, et al. Maternal metabolomic profile and fetal programming of offspring adiposity: identification of potentially protective lipid metabolites. Mol Nutr Food Res. 2019;63(1):e1700889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li-Gao R, Hughes DA, le Cessie S, et al. Assessment of reproducibility and biological variability of fasting and postprandial plasma metabolite concentrations using 1H NMR spectroscopy. PloS One. 2019;14(6):e0218549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.