Abstract
Context
In 2020, the terminology of metabolic dysfunction–associated fatty liver disease (MAFLD) was proposed to replace nonalcoholic fatty liver disease (NAFLD).
Objectives
This work aimed to investigate the prevalence and incidence of MAFLD and evaluate its effects on incident extrahepatic diseases.
Methods
A total of 6873 individuals, with a 4.6-year follow-up, were included in this study. Associations of MAFLD and NAFLD with diabetes, chronic kidney disease (CKD), and cardiovascular disease (CVD) were examined using logistic regression and Cox proportional hazards models.
Results
The prevalence of NAFLD and MAFLD was 40.3% (95% CI, 39.2%-41.5%) and 46.7% (95% CI, 45.6%-47.9%), respectively. Additionally, 321 (4.7%) and 156 (2.3%) participants had MAFLD with excessive alcohol consumption and hepatitis B virus (HBV) infection. During the follow-up period, the incidence of NAFLD and MAFLD was 22.7% (95% CI, 21.3%-24.0%) and 27.0% (95% CI, 25.5%-28.4%). MAFLD was associated with higher risks of incident diabetes (risk ratio [RR] 2.08; 95% CI, 1.72-2.52), CKD (RR 1.64; 95% CI, 1.39-1.94), and CVD (hazard ratio 1.44; 95% CI, 1.15-1.81). Similar associations for NAFLD were observed. Furthermore, the MAFLD subgroups with excessive alcohol consumption (RR 2.49; 95% CI, 1.64-3.78) and HBV infection (RR 1.98; 95% CI, 1.11-3.52) were associated with higher risks of incident diabetes.
Conclusion
The change from NAFLD to MAFLD did not greatly affect the associations with diabetes, CKD, and CVD. MAFLD further identified those patients of metabolically fatty liver combined with excessive alcohol consumption and HBV infection, who had increased risks of incident diabetes compared with those of non–fatty liver.
Keywords: metabolic dysfunction–associated fatty liver disease, nonalcoholic fatty liver disease, diabetes, chronic kidney disease, cardiovascular disease
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide, with a global prevalence of 25.24% (1) and 29.2% (2) in China. NAFLD often coexists with other liver conditions, such as alcoholic fatty liver disease and viral hepatitis, and these usually exert synergistic effects on the liver disease progression (3, 4). In addition, there is a lack of evidence supporting the currently recommended cutoff of alcohol consumption for NAFLD. Given the heterogeneous pathogenesis of metabolic dysfunction–associated fatty liver disease (MAFLD) and inaccuracies in the definition of NAFLD, experts suggested replacing the term NAFLD with MAFLD in 2020 (5).
MAFLD is more inclusive in the etiology of fatty liver diseases than NAFLD and is defined based on evidence of hepatic steatosis and simultaneously accompanied by the presence of at least one of the following conditions: overweight/obesity, diabetes, or metabolic dysregulation (5). However, the current data on whether MAFLD definition was more feasible than NAFLD in clinical practice were scarce and inconsistent. Three cross-sectional studies showed that, compared with NAFLD, the definition of MAFLD was more practical for identifying more patients at risk of liver disease progression (6, 7) and more patients at prevalent risk of chronic kidney disease (CKD) (8), whereas 2 studies (9, 10) reported that the MAFLD definition did not significantly affect the prevalence in contrast to NAFLD and a cohort study with a 7.5-year follow-up indicated that the presence of MAFLD did not increase mortality (11).
However, to our knowledge, the evidence of the associations of MAFLD with extrahepatic diseases based on large-scale, community-based cohort studies is limited. Therefore, we aimed to (i) investigate the prevalence and incidence rates of MAFLD and NAFLD among middle-aged and elderly Chinese individuals; and (ii) evaluate the associations of MAFLD and NAFLD with diabetes, CKD, and cardiovascular disease (CVD) using a retrospective cohort dataset.
Materials and Methods
Study Design and Participants
The Shanghai Nicheng Cohort Study, a community-based cohort study, was designed to prospectively investigate the prevalence, incidence, and related factors of cardiometabolic diseases (12, 13). The detailed introduction of this study was previously reported (12). Briefly, a total of 17 212 individuals aged 45 to 70 years completed the baseline survey between 2013 and 2014. Among them, 10 075 participants aged 55 to 70 years were invited to participate in the follow-up survey in 2018, and 7230 finally attended with a follow-up rate of 71.8%. We excluded 357 participants because of missing data for abdominal ultrasonography (N = 338) or lacking data for a diagnosis of MAFLD (N = 19). Finally, 6873 participants were included in this study (Fig. 1). The ethics committee of the Shanghai Sixth People’s Hospital approved this study (approval No. 2018-010). All participants provided written informed consent.
Figure 1.
Flowchart of study population. MAFLD, metabolic dysfunction-associated fatty liver disease.
Clinical Data Collection and Measurements
At baseline and follow-up surveys, a standardized questionnaire was used to collect data concerning demographics, educational background, smoking status, alcohol consumption, leisure-time exercise, medical history, and medication use. Excessive alcohol consumption was defined as more than 140 g weekly of alcohol consumption in men and more than 70 g weekly in women. Current smoking was defined as having smoked at least 1 cigarette per day over the past year. Leisure-time exercise was categorized into less than 30 minutes/day and 30 minutes/day or more. The measurements of height, weight, waist circumference, and blood pressure were performed using the established standard methods (14). Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters).
Overnight fasting (at least 10 hours) venous blood samples and random urine samples were collected. Fasting plasma glucose (FPG) was assessed by the glucose oxidase method. Glycated hemoglobin A1c (HbA1c) was assessed by high-performance liquid chromatography. Triglycerides were assessed by an enzymatic colorimetric method. High-density lipoprotein cholesterol was assessed by a direct method. Fasting insulin was assessed by an electrochemiluminescence immunoassay. Urine creatinine, urine albumin, and high-sensitivity C-reactive protein (hs-CRP) were assessed by the rate of nephelometry assay. Serum creatinine was assessed by the sarcosine oxidase–PAP (phenol-aminophenazone peroxidase) method. Hepatitis B surface antigen (HBsAg) and hepatitis C virus antibody (HCVAb) were assessed by an enzyme-linked immunosorbent assay using the Hepatitis B Virus Surface Antigen (ELISA) Diagnostic Kit (Cat# 30811010101, RRID: AB_2892704) and the Hepatitis C Virus (ELISA) Diagnostic Kit (Cat# 30811060102, RRID: AB_2892705), respectively. Insulin resistance was quantified through the homeostasis model assessment of insulin resistance (HOMA-IR), calculated as FPG (mmol/L) × FINS (μU/mL)/22.5 (15).
Abdominal ultrasonography was performed using an ultrasound system (Z.One Ultra, Zonare Medical Systems Inc) by experienced ultrasonographists.
Definitions
Fatty liver was diagnosed according to the Asia-Pacific Guidelines (16).
MAFLD was diagnosed based on ultrasound evidence of fatty liver in addition to the presence of at least 1 of the following 3 criteria, namely overweight/obesity (defined as BMI ≥ 23.0 in Asia), diabetes, or metabolic dysregulation (5). Metabolic dysregulation was defined as the presence of at least 2 of the following metabolic abnormalities in those with lean/normal weight (defined as BMI < 23.0 in Asia): (1) waist circumference greater than or equal to 90 cm in Asian men and greater than or equal to 80 cm in Asian women; (2) blood pressure greater than or equal to 130/85 mm Hg or specific drug treatment; (3) triglycerides greater than or equal to 1.70 mmol/L or specific drug treatment; (4) high-density lipoprotein cholesterol less than 1.0 mmol/L for men and less than 1.3 mmol/L for women or specific drug treatment; (5) prediabetes (FPG = 5.6-6.9 mmol/L and/or HbA1c = 5.7%-6.4% in participants without a prior diabetes diagnosis); (6) HOMA-IR greater than or equal to 2.5; and (7) hs-CRP level greater than 2 mg/L (5). MAFLD was further categorized into 2 subgroups: MAFLD with excessive alcohol consumption and HBV infection.
NAFLD was based on ultrasound evidence of fatty liver, in the absence of excessive alcohol consumption and other concomitant liver diseases (viral hepatitis, total parenteral nutrition, hepatolenticular degeneration, drug-induced hepatitis, autoimmune hepatitis, etc) (16).
Diabetes was defined as having a self-reported history of diabetes, and/or FPG greater than or equal to 7.0 mmol/L, and/or HbA1c greater than or equal to 6.5% (17). Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (18). CKD was defined as estimated glomerular filtration rate less than 60 mL/min/1.73 m2 or urinary albumin-to-creatine ratio greater than or equal to 30 μg/mg (19). According to participants’ self-reports, nonfatal CVD included coronary heart disease and stroke. Coronary heart disease was determined as having a history of angina pectoris, myocardial infarction, a surgical history of coronary angiography, coronary stent implantation, and/or coronary artery bypass, and stroke included a history of cerebral hemorrhage and/or cerebral infarction.
Statistical Analysis
Continuous variables were presented as medians (interquartile ranges), and categorical variables were presented as frequencies (proportions). To address potential confounding, logistic regression models were used to estimate the odds ratio (OR) and 95% CI for prevalent diabetes, and to estimate risk ratio (RR) and 95% CI for incident diabetes and CKD; and Cox proportional hazards models were used to estimate the hazard ratio and 95% CI for incident CVD. Model 1 was adjusted for age and sex, and model 2 was additionally adjusted for educational background, smoking status, and leisure-time exercise. Missing data were not imputed, and participants with missing data for a variable were not included in the analysis involving that particular variable. All statistical analyses were conducted using SPSS, version 22.0 (SPSS Inc). A 2-tailed P value of less than .05 was considered to be statistically significant.
Results
General characteristics of the 6873 participants at baseline are presented in Table 1. Among those individuals with a median age of 61.6 years (interquartile range, 58.7-65.2 years), 57.6% were female; 10.1% excessively consumed alcohol, and 20.4% were current smokers; 47.2% were diagnosed with fatty liver; 72.6%, 20.4%, and 17.4% were overweight/obesity, diabetes, and metabolic dysregulation, respectively; and 5.3% had tested positive for HBV.
Table 1.
Baseline sociodemographic and clinical characteristics of participants
| Variables | Total N = 6873 | Men N = 2915 (42.4%) | Women N = 3958 (57.6%) |
|---|---|---|---|
| Age, ya | 61.6 (58.7-65.2) | 61.7 (58.8-65.1) | 61.6 (58.7-65.2) |
| BMIa | 24.9 (22.8-27.0) | 24.9 (22.8-26.9) | 24.9 (22.8-27.1) |
| Waist circumference, cma | 85.0 (79.0-91.0) | 87.0 (80.0-92.0) | 84.0 (78.0-90.0) |
| SBP, mm Hga | 132.0 (124.0-143.0) | 132.0 (124.0-143.0) | 132.5 (124.0-143.5) |
| DBP, mm Hga | 82.0 (79.5-89.0) | 83.0 (80.0-90.0) | 82.0 (79.0-88.0) |
| FPG, mmol/La | 5.9 (5.5-6.5) | 5.9 (5.5-6.5) | 5.9 (5.5-6.5) |
| HbA1c, %a | 5.7 (5.4-6.0) | 5.6 (5.4-6.0) | 5.7 (5.5-6.1) |
| TGs, mmol/La | 1.3 (0.9-2.0) | 1.3 (0.9-1.8) | 1.4 (1.0-2.0) |
| TC, mmol/La | 5.1 (4.5-5.8) | 4.9 (4.4-5.5) | 5.3 (4.7-6.0) |
| HDL-C, mmol/La | 1.3 (1.1-1.5) | 1.2 (1.0-1.5) | 1.4 (1.1-1.6) |
| LDL-C, mmol/La | 3.1 (2.6-3.6) | 2.9 (2.5-3.4) | 3.2 (2.7-3.7) |
| ALT, U/La | 17.0 (13.0-22.0) | 18.0 (14.0-24.0) | 16.0 (12.0-21.0) |
| AST, U/La | 22.0 (19.0-26.0) | 23.0 (19.0-27.0) | 22.0 (19.0-26.0) |
| GGT, U/La | 23.0 (17.0-36.0) | 28.0 (20.0-43.0) | 20.0 (15.0-30.0) |
| HOMA-IRa | 1.9 (1.3-2.8) | 1.6 (1.1-2.4) | 2.0 (1.4-3.0) |
| Hs-CRP, mg/La | 0.9 (0.5-1.9) | 0.9 (0.5-1.9) | 1.0 (0.5-1.9) |
| Overweight/obesity, n (%)b | 4976 (72.6) | 2104 (72.4) | 2872 (72.8) |
| Central obesity, No. (%)b | 3869 (56.5) | 1114 (38.4) | 2755 (69.8) |
| Hypertension, No. (%)b | 5048 (73.5) | 2123 (72.9) | 2925 (74.0) |
| Diabetes, No. (%)b | 1396 (20.4) | 535 (18.4) | 861 (21.8) |
| Prediabetes, No. (%)b | 4125 (60.2) | 1754 (60.3) | 2371 (60.1) |
| Elevated TGs, No. (%)b | 2310 (33.7) | 889 (30.5) | 1421 (36.0) |
| Reduced HDL-C, No. (%)b | 2360 (34.3) | 604 (20.7) | 1756 (44.4) |
| Elevated HOMA-IR, No. (%)b | 2114 (30.9) | 689 (23.7) | 1425 (36.1) |
| Elevated hs-CRP, No. (%)b | 1553 (23.9) | 633 (23.2) | 920 (24.4) |
| Metabolic dysregulation, No. (%) | 1171 (17.4) | 427 (15.0) | 744 (19.1) |
| Fatty liver, No. (%) | |||
| No | 3632 (52.8) | 1652 (56.7) | 1980 (50.0) |
| Yes | 3241 (47.2) | 1263 (43.3) | 1978 (50.0) |
| Excessive alcohol consumption, No. (%)b | 695 (10.1) | 690 (23.7) | 5 (0.1) |
| HBV infection, No. (%)b | 366 (5.3) | 172 (5.9) | 194 (4.9) |
| Middle school or higher level of education, No. (%) | 2070 (30.1) | 1239 (42.5) | 831 (21.0) |
| Current smoking, No. (%) | 1405 (20.4) | 1401 (48.1) | 4 (0.1) |
| Leisure-time exercise ≥ 30 min/d, No. (%) | 249 (3.6) | 106 (3.6) | 143 (3.6) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GGT, gamma-glutamyl transpeptidase; HbA1c, glycated hemoglobin A1c; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; MAFLD, metabolic dysfunction–associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; SBP, systolic blood pressure; TC, total cholesterol; TGs, triglycerides.
a Data are presented as median (interquartile range).
b Overweight/obesity: BMI of 23.0 or greater; central obesity: waist circumference greater than or equal to 90/80 cm in men and women; hypertension: blood pressure greater than or equal to 130/85 mm Hg or specific drug treatment; diabetes: FPG greater than or equal to 7.0 mmol/L, or HbA1c greater than or equal to 6.5% or a history of diabetes; prediabetes: FPG 5.6 to 6.9 mmol/L or HbA1c 5.7% to 6.4% in participants without a prior diabetes diagnosis; elevated TGs: TGs greater than or equal to 1.70 mmol/L or specific drug treatment; reduced HDL-C: HDL-C less than 1.0 mmol/L for men and less than 1.3 mmol/L for women or specific drug treatment; elevated HOMA-IR: HOMA-IR greater than or equal to 2.5; elevated hs-CRP: hs-CRP greater than 2 mg/L; excessive alcohol consumption was defined as more than 140 g weekly of alcohol consumption in men and more than 70 g weekly in women; HBV infection was defined as positive HBsAg or a history of HBV infection.
Prevalence and Incidence Rates of Metabolic Dysfunction–Associated Fatty Liver Disease and Nonalcoholic Fatty Liver Disease
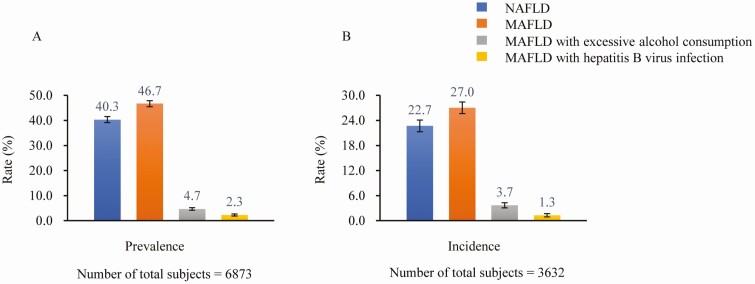
Among 6873 participants, 2771 (40.3%) and 3212 (46.7%) were diagnosed with NAFLD and MAFLD, respectively; and 321 (4.7%) and 156 (2.3%) had MAFLD with excessive alcohol consumption and HBV infection, respectively (Fig. 2A). After an average 4.6-year follow-up, among 3632 individuals with non–fatty liver (non-FL) at baseline, the incidence rates of NAFLD and MAFLD were 22.7% (95% CI, 21.3%-24.0%) and 27.0% (95% CI, 25.5%-28.4%), respectively (Fig. 2B).
Figure 2.
A, Prevalence, and B, incidence, of nonalcoholic fatty liver disease (NAFLD) and metabolic dysfunction–associated fatty liver disease (MAFLD).
Associations of Metabolic Dysfunction–Associated Fatty Liver Disease and Nonalcoholic Fatty Liver Disease With Diabetes
The prevalence of diabetes at baseline was 12.4% (95% CI, 11.3%-13.5%), 29.6% (95% CI, 28.0%-31.2%), and 29.5% (95% CI, 27.8%-31.2%) among those with non-FL, MAFLD, and NAFLD, respectively; and was 31.4% (95% CI, 26.3%-36.4%) and 23.7% (95% CI, 17.0%-30.4%) in the MAFLD subgroups with excessive alcohol consumption and HBV infection, respectively. Compared with those with non-FL, patients with MAFLD and NAFLD had significantly higher risks of prevalent diabetes both in model 1 and model 2. Furthermore, MAFLD with excessive alcohol consumption and HBV infection was also associated with increased risks of prevalent diabetes (Fig. 3).
Figure 3.
Associations of metabolic dysfunction–associated fatty liver disease (MAFLD) and nonalcoholic fatty liver disease (NAFLD) with prevalent diabetes. *Metabolic dysfunction was defined as the presence of at least 1 of 3 criteria: overweight/obesity, diabetes, or metabolic dysregulation. †Model 1 was adjusted for age and sex. ‡Model 2: model 1 plus adjustment for educational background, smoking status, and leisure-time exercise at baseline. HBV, hepatitis B virus; non-FL, non–fatty liver; OR, odds ratio.
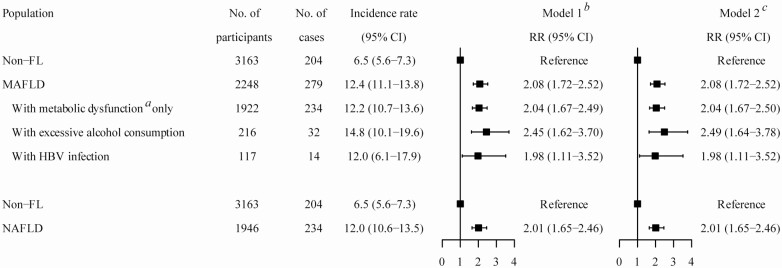
After an average 4.6-year follow-up, of 5440 individuals without diabetes at baseline, the incidence rates of diabetes were 6.5% (95% CI, 5.6%-7.3%), 12.4% (95% CI, 11.1%-13.8%), and 12.0% (95% CI, 10.6%-13.5%) among those with non-FL, MAFLD, and NAFLD, respectively; and were 14.8% (95% CI, 10.1%-19.6%) and 12.0% (95% CI, 6.1%-17.9%) in the MAFLD subgroups with excessive alcohol consumption and HBV infection, respectively. Compared with those with non-FL, patients with MAFLD and NAFLD had increased risks of incident diabetes (RR 2.08; 95% CI, 1.72-2.52; RR 2.01; 95% CI, 1.65-2.46, respectively) after adjusting for age, sex, educational background, smoking status, and leisure-time exercise. Positive associations of MAFLD with excessive alcohol consumption (RR 2.49; 95% CI, 1.64-3.78) and HBV infection (RR 1.98; 95% CI, 1.11-3.52) with incident diabetes were observed (Fig. 4).
Figure 4.
Associations of metabolic dysfunction–associated fatty liver disease (MAFLD) and nonalcoholic fatty liver disease (NAFLD) with incident diabetes. *Metabolic dysfunction was defined as the presence of at least 1 of 3 criteria: overweight/obesity, diabetes, or metabolic dysregulation. †Model 1 was adjusted for age and sex. ‡Model 2: model 1 plus adjustment for educational background, smoking status, and leisure-time exercise at baseline. HBV, hepatitis B virus; non-FL, non–fatty liver; RR, risk ratio.
Associations of Metabolic Dysfunction–Associated Fatty Liver Disease and Nonalcoholic Fatty Liver Disease With Chronic Kidney Disease and Cardiovascular Disease
After an average 4.6-year follow-up, of 6176 participants without CKD at baseline, the incidence rates of CKD among those with non-FL, MAFLD, and NAFLD were 8.2% (95% CI, 7.3%-9.2%), 12.9% (95% CI, 11.7-14.1), and 13.4% (95% CI, 12.0%-14.7%), respectively; and were 8.1% (95% CI, 4.9%-11.2%) and 11.0% (95% CI, 5.8%-16.3%) in the MAFLD subgroups with excessive alcohol consumption and HBV infection, respectively (Table 2). Table 3 shows that, of 6395 individuals without CVD at baseline, the incidence rates of CVD (per 1000 person-years follow-up) among those with non-FL, MAFLD, and NAFLD were 8.7 (95% CI, 7.4-10.3), 12.3 (95% CI, 10.6-14.4), and 12.6 (95% CI, 10.7-14.9), respectively; and were 9.0 (95% CI, 5.1-15.8) and 12.8 (95% CI, 6.4-25.7) in the MAFLD subgroups with excessive alcohol consumption and HBV infection, respectively. Compared with those with non-FL, increased risks of incident CKD and CVD were observed among patients with MAFLD and NAFLD, but not observed in the patients with MAFLD subgroups with excessive alcohol consumption and HBV infection in both Model 1 and Model 2 (see Tables 2 and 3).
Table 2.
Associations of metabolic dysfunction–associated fatty liver disease and nonalcoholic fatty liver disease with incident chronic kidney disease
| Population | No.of participants | No. of cases | Incidence rate (95% CI) | Model 1b RR (95% CI) | P | Model 2c RR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| Non-FL | 3311 | 273 | 8.2 (7.3-9.2) | Reference | Reference | ||
| MAFLD | 2837 | 366 | 12.9 (11.7-14.1) | 1.64 (1.39-1.94) | < .001 | 1.64 (1.39-1.94) | < .001 |
| With metabolic dysfunctiona only | 2429 | 328 | 13.5 (12.1-14.9) | 1.71 (1.44-2.03) | < .001 | 1.71 (1.44-2.04) | < .001 |
| With excessive alcohol consumption | 285 | 23 | 8.1 (4.9-11.2) | 1.11 (0.70-1.75) | .666 | 1.09 (0.69-1.73) | .714 |
| With HBV infection | 136 | 15 | 11.0 (5.8-16.3) | 1.34 (0.77-2.33) | .300 | 1.35 (0.78-2.35) | .288 |
| Non-FL | 3311 | 273 | 8.2 (7.3-9.2) | Reference | Reference | ||
| NAFLD | 2452 | 328 | 13.4 (12.0-14.7) | 1.69 (1.43-2.01) | < .001 | 1.70 (1.43-2.01) | < .001 |
Abbreviations: HBV, hepatitis B virus; MAFLD, metabolic dysfunction–associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; non-FL, non–fatty liver; RR, risk ratio.
a Metabolic dysfunction was defined as the presence of at least 1 of 3 criteria: overweight/obesity, diabetes, or metabolic dysregulation.
b Model 1 was adjusted for sex and age.
c Model 2 was adjusted for sex, age, educational background, smoking status, and leisure-time exercise at baseline.
Table 3.
Associations of metabolic dysfunction–associated fatty liver disease and nonalcoholic fatty liver disease with incident cardiovascular disease
| Population | No. of participants | No. of cases | Incidence rateb (95% CI) | Model 1c HR (95% CI) | P | Model 2d HR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| Non-FL | 3417 | 134 | 8.7 (7.4-10.3) | Reference | Reference | ||
| MAFLD | 2950 | 162 | 12.3 (10.6-14.4) | 1.43 (1.14-1.80) | .002 | 1.44 (1.15-1.81) | .002 |
| With metabolic dysfunctiona only | 2522 | 142 | 12.6 (10.7-14.9) | 1.48 (1.16-1.87) | .001 | 1.48 (1.17-1.89) | .001 |
| With excessive alcohol consumption | 298 | 12 | 9.0 (5.1-15.8) | 0.99 (0.54-1.80) | .961 | 1.01 (0.55-1.85) | .975 |
| With HBV infection | 141 | 8 | 12.8 (6.4-25.7) | 1.47 (0.72-3.01) | .287 | 1.48 (0.73-3.03) | .279 |
| Non-FL | 3417 | 134 | 8.7 (7.4-10.3) | Reference | Reference | ||
| NAFLD | 2545 | 143 | 12.6 (10.7-14.9) | 1.47 (1.16-1.87) | .001 | 1.48 (1.17-1.88) | .001 |
Abbreviations: HBV, hepatitis B virus; HR, hazard ratio; MAFLD, metabolic dysfunction–associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; non-FL, non–fatty liver.
a Metabolic dysfunction was defined as the presence of at least 1 of the 3 criteria: overweight/obesity, diabetes, or metabolic dysregulation.
b Incidence rate was calculated as the number of incident cases divided by per 1000 person-years of follow-up.
c Model 1 was adjusted for sex and age.
d Model 2 was adjusted for sex, age, educational background, smoking status, and leisure-time exercise at baseline.
Discussion
In this community-based retrospective cohort of 6873 middle-aged and elderly Chinese individuals, the prevalence and incidence rate of MAFLD were 46.7% and 27.0%. Compared to NAFLD, the prevalence and incidence rate increased by 6.4% and 4.3%, respectively. Both MAFLD and NAFLD increased incident risks of diabetes, CKD, and CVD, but these risks were practically equivalent between the two. Furthermore, the MAFLD definition identified an additional sizable portion of patients with metabolically fatty liver concomitant with excessive alcohol consumption and HBV infection, who had higher prevalent and incident risks of diabetes compared with those of non-FL.
This study showed that MAFLD and NAFLD were highly prevalent. After a 4.6-year follow-up, among middle-aged and elderly Chinese participants, nearly one-quarter developed MAFLD or NAFLD. Given the fact that exclusion of other concomitant liver diseases was not a prerequisite for the diagnosis of MAFLD, as expected, there was a higher prevalence of MAFLD than that of NAFLD in our study. Similarly, 3 studies (7, 20, 21) in Asia reported a higher prevalence of MAFLD than that of NAFLD, whereas the Third National Health and Nutrition Examination Survey (6) observed an opposite finding, which might be due to (i) a lack of a viral hepatitis test; or (ii) lower proportions of metabolic abnormalities. Our study for the first time reports the incidence rate of MAFLD diagnosed by ultrasound and showed a slightly higher incidence rate of MAFLD than that of NAFLD. Another cohort study from Hong Kong, however, showed that the incidence rate of MAFLD was 25% lower than that of NAFLD (9). These differences in the prevalence and incidence rates of MAFLD and NAFLD can be affected by the proportions of metabolic abnormalities and other coexisting conditions among their study populations.
Recently, an updated meta-analysis of 33 studies found about a 2.2-fold increased risk of incident diabetes associated with NAFLD over a median 5-year follow-up period (22). Consistent with these previous findings, our results showed that NAFLD was associated with a 2.01-fold increased risk of incident diabetes over a 4.6-year follow-up period. We also found a similar association between MAFLD and incident diabetes with an RR of 2.08. To date, there is a lack of data on the association between MAFLD (diagnosed by ultrasound) and incident diabetes.
Compared with the NAFLD definition, excessive alcohol consumption was no longer excluded for diagnosing MAFLD, making it possible to assess the interaction between alcohol consumption and metabolic risk factors. In this study, 4.7% of participants were diagnosed with MAFLD with excessive alcohol consumption. Previous cohort studies showed that in the general population, excessive alcohol consumption was associated with a 1.4- to 1.8-fold greater risk of incident diabetes (23-25). Our study observed that MAFLD with excessive alcohol consumption (> 140 g/week for men; > 70 g/week for women) was associated with an approximately 2.5-fold greater risk of incident diabetes. Similarly, a cohort study of 9948 Japanese men demonstrated that individuals with fatty liver concomitant with excessive alcohol consumption (> 280 g/week) had 3.45 times the risk of diabetes compared with those without fatty liver and consuming less than 40 g alcohol per week over a median 6-year follow-up period (25). In addition, our study found that MAFLD patients with excessive alcohol consumption, compared to those with metabolic dysfunction only, have a slightly higher risk of diabetes. This finding indicates the possible synergistic effect of excessive alcohol consumption, fatty liver, and metabolic dysfunction on the development of diabetes. Therefore, patients with MAFLD should be advised to avoid excessive alcohol consumption to prevent diabetes, which is a major health issue affecting nearly half a billion people worldwide and causing many health-threatening complications (26).
Apart from metabolic risk factors and excessive alcohol consumption, HBV infection was included in the MAFLD definition. In our study, 2.3% of participants had MAFLD with HBV infection. HBV infection can cause liver injury and further lead to dysregulation in glucose homeostasis and even diabetes. Our results show that MAFLD with HBV infection was associated with an around 2-fold higher risk of incident diabetes. Previous prospective studies demonstrated that NAFLD and HBV infection could collectively exacerbate liver injury and increase the risk of liver fibrosis and hepatocellular carcinoma (3, 27). Additionally, diabetes was associated with significantly increased hepatocellular carcinoma risk in individuals with HBV infection (28, 29). Given that there are still an estimated 77 to 97 million people with HBV infection in China (30), it was more practical to use the definition of MAFLD to identify more patients with fatty liver and HBV infection for managing disease progression. Further clinical trials should be designed to evaluate the clinical benefits of specific interventions on MAFLD patient subgroups with different etiologies.
Two comprehensive meta-analyses found increased risks of CKD and CVD associated with NAFLD (31, 32). Our study indicated that MAFLD and NAFLD were both associated with increased risks of CKD and CVD, but no associations were observed between MAFLD and CKD or CVD in the subgroups with excessive alcohol consumption and HBV infection. Recently, one retrospective cohort study of more than 8 million South Koreans showed that MAFLD (identified by fatty liver index) concomitant with another etiology was associated with a significantly higher risk of CVD after a median follow-up period of 10.1 years (20). No positive association between MAFLD with excessive alcohol consumption and HBV infection and CVD was found in our study, which could be attributed to a smaller sample size and shorter follow-up duration.
Strengths and Limitations
The Shanghai Nicheng Cohort Study was initially designed as a community-based prospective cohort to investigate the prevalence and incidence of cardiometabolic diseases. This study collected at baseline comprehensive and detailed clinical data, such as alcohol consumption, HBsAg, hepatitis C virus antibody, HOMA-IR, and hs-CRP, and evaluated multiple outcome events. In addition, our study, for the first time, reported the incidence rate of ultrasound-diagnosed MAFLD and assessed the effects of MAFLD and its subgroups with excessive alcohol consumption and HBV infection on incident diabetes, CKD, and CVD.
There are some limitations to this study. First, ultrasound, rather than liver biopsy, was used to diagnose hepatic steatosis. It had a limited sensitivity at 60% to 94% (33) and did not accurately detect steatosis when the liver fat infiltration was less than 20% (34, 35), and its diagnostic accuracy was suboptimal in participants with a BMI greater than 40.0 (36). However, ultrasound is the first-choice imaging modality to detect hepatic steatosis in clinical practice and large-scale epidemiological studies (37, 38). Second, the duration of follow-up is comparatively shorter and might limit the findings of significant associations between MAFLD and CKD or CVD. Third, other potential confounders such as dietary, genetic factors, and medications were not evaluated. Finally, because we conducted our study with a middle-aged to older Chinese population, whose metabolic dysfunction was particularly common, and excluded 19 individuals for lack of data for the diagnosis of MAFLD, there might be selection bias and limitation to the generalizability of our results.
In summary, MAFLD and NAFLD are highly prevalent among middle-aged and elderly Chinese individuals. The change from NAFLD to MAFLD did not affect the incident risks of diabetes, CKD, and CVD. However, the MAFLD definition captured an additional sizable portion of patients with metabolically fatty liver accompanied by excessive alcohol consumption or HBV infection. These patients would have higher prevalent and incident risks of diabetes compared with those of non-FL individuals. Thus, more attention should be given to those at high risk of metabolic disorders and stratification for management in clinical practice.
Acknowledgments
We are grateful to all the investigators for their contributions to this study. We thank Yang Su, who holds a master’s degree in English from Shanghai International Studies University for proofreading draft versions of the manuscript.
Financial Support: This work was supported by the Natural Science Foundation of Shanghai (grant No. 18ZR1429000), the Shanghai Science and Technology Commission Foundation (grant No. 20DZ2270300), the Shanghai Health Commission Award (grant No. GWV-10.2-YQ24), and the Strategic Priority CAS Project (grant No. XDB38020000).
Author Contributions: Yebei Liang, Hongli Chen, Yuexing Liu, Xuhong Hou, and Weiping Jia made substantial contributions to the conception and design of the study. Yebei Liang, Hongli Chen, Yuexing Liu, and Xuhong Hou analyzed the data. Yebei Liang, Hongli Chen, Yuexing Liu, Xuhong Hou, and Weiping Jia drafted the manuscript. All the authors assisted in the acquisition and interpretation of data, contributed to the critical revision of the manuscript for important intellectual content, and approved the final version.
Glossary
Abbreviations
- BMI
body mass index
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- ELISA
enzyme-linked immunosorbent assay
- FPG
fasting plasma glucose
- HbA1c
glycated hemoglobin A1c
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HOMA-IR
homeostasis model assessment of insulin resistance
- hs-CRP
high sensitivity C-reactive protein
- MAFLD
metabolic dysfunction–associated fatty liver disease
- NAFLD
nonalcoholic fatty liver disease
- non-FL
non–fatty liver
- OR
odds ratio
- RR
risk ratio
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Deidentified data underlying the results of our study will be shared with individuals or organizations on approval of their stated purpose and their agreement to provide evidence of consistency with that purpose before submitting manuscripts or reports. Interested investigators can contact Weiping Jia at wpjia@sjtu.edu.cn (Shanghai Diabetes Institute, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 200233, China) to obtain the data set. All applications related to this data set are subject to the laws and guidelines regarding these matters of the People’s Republic of China.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84. [DOI] [PubMed] [Google Scholar]
- 2. Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70(4):1119-1133. [DOI] [PubMed] [Google Scholar]
- 3. Choi HSJ, Brouwer WP, Zanjir WMR, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539-548. [DOI] [PubMed] [Google Scholar]
- 4. Chiang DJ, McCullough AJ. The impact of obesity and metabolic syndrome on alcoholic liver disease. Clin Liver Dis. 2014;18(1):157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202-209. [DOI] [PubMed] [Google Scholar]
- 6. Lin S, Huang J, Wang M, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082-2089. [DOI] [PubMed] [Google Scholar]
- 7. Yamamura S, Eslam M, Kawaguchi T, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40(12):3018-3030. [DOI] [PubMed] [Google Scholar]
- 8. Sun DQ, Jin Y, Wang TY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433. [DOI] [PubMed] [Google Scholar]
- 9. Wai-Sun Wong V, Lai-Hung Wong G, Woo J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol. Published online October 31, 2020. doi:10.1016/j.cgh.2020.10.046 [Google Scholar]
- 10. Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41(6):1290-1293. [DOI] [PubMed] [Google Scholar]
- 11. Semmler G, Wernly S, Bachmayer S, et al. Metabolic dysfunction-associated fatty liver disease (MAFLD)—rather a bystander than a driver of mortality. J Clin Endocrinol Metab. 2021;106(9):2670-2677. [DOI] [PubMed] [Google Scholar]
- 12. Chen P, Hou X, Hu G, et al. Abdominal subcutaneous adipose tissue: a favorable adipose depot for diabetes? Cardiovasc Diabetol. 2018;17(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo F, Moellering DR, Garvey WT. The progression of cardiometabolic disease: validation of a new cardiometabolic disease staging system applicable to obesity. Obesity (Silver Spring). 2014;22(1):110-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luepker RV, Evans A, McKeigue P, Reddy KS.. Cardiovascular Survey Methods. 3rd ed. World Health Organization, 2004. [Google Scholar]
- 15. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 16. Farrell GC, Chitturi S, Lau GK, Sollano JD; Asia-Pacific Working Party on NAFLD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22(6):775-777. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S15-S33. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Becker GJ, Wheeler DC, Zeeuw DD, et al. Kidney Disease: Improving Global Outcomes (KDIGO) blood pressure work group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2(5):337-414. [Google Scholar]
- 20. Lee H, Lee YH, Kim SU, Chang Kim H. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. Published online December 22, 2020. doi:10.1016/j.cgh.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 21. Huang SC, Su HJ, Kao JH, et al. Clinical and histologic features of patients with biopsy-proven metabolic dysfunction-associated fatty liver disease. Gut Liver. 2021;15(3):451-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70(5):962-969. [DOI] [PubMed] [Google Scholar]
- 23. Lee DY, Yoo MG, Kim HJ, et al. Association between alcohol consumption pattern and the incidence risk of type 2 diabetes in Korean men: a 12-years follow-up study. Sci Rep. 2017;7(1):7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kao WH, Puddey IB, Boland LL, Watson RL, Brancati FL. Alcohol consumption and the risk of type 2 diabetes mellitus: Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2001;154(8):748-757. [DOI] [PubMed] [Google Scholar]
- 25. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Effect of alcohol consumption and the presence of fatty liver on the risk for incident type 2 diabetes: a population-based longitudinal study. BMJ Open Diabetes Res Care. 2020;8:e001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. International Diabetes Federation. IDF Diabetes Atlas. 9th ed.International Diabetes Federation;2019. [Google Scholar]
- 27. Chan AW, Wong GL, Chan HY, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32(3):667-676. [DOI] [PubMed] [Google Scholar]
- 28. Tan Y, Zhang X, Zhang W, et al. The influence of metabolic syndrome on the risk of hepatocellular carcinoma in patients with chronic hepatitis B infection in mainland China. Cancer Epidemiol Biomarkers Prev. 2019;28(12):2038-2046. [DOI] [PubMed] [Google Scholar]
- 29. Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135(1):111-121. [DOI] [PubMed] [Google Scholar]
- 30. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383-403. [DOI] [PubMed] [Google Scholar]
- 31. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600. [DOI] [PubMed] [Google Scholar]
- 32. Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. Published online December 10, 2020. doi:10.1136/gutjnl-2020-323082 [DOI] [PubMed] [Google Scholar]
- 33. Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51(3):433-445. [DOI] [PubMed] [Google Scholar]
- 34. Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745-750. [DOI] [PubMed] [Google Scholar]
- 35. Fishbein M, Castro F, Cheruku S, et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39(7):619-625. [DOI] [PubMed] [Google Scholar]
- 36. Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8(12):1114-1122. [DOI] [PubMed] [Google Scholar]
- 37. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang Y, Ryu S, Kim Y, et al. Low levels of alcohol consumption, obesity, and development of fatty liver with and without evidence of advanced fibrosis. Hepatology. 2020;71(3):861-873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data underlying the results of our study will be shared with individuals or organizations on approval of their stated purpose and their agreement to provide evidence of consistency with that purpose before submitting manuscripts or reports. Interested investigators can contact Weiping Jia at wpjia@sjtu.edu.cn (Shanghai Diabetes Institute, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 200233, China) to obtain the data set. All applications related to this data set are subject to the laws and guidelines regarding these matters of the People’s Republic of China.