Abstract
Background and Aims
Bowel urgency and abdominal pain are impactful, yet under-appreciated ulcerative colitis symptoms and not commonly assessed in clinical trials. We evaluated how these symptoms may improve with upadacitinib treatment and correlate with clinical and health-related quality of life [HRQOL] outcomes in the phase 2b U-ACHIEVE study.
Methods
Patients aged 18–75 years, with moderately to severely active ulcerative colitis, were randomised to receive placebo or upadacitinib (7.5, 15, 30, or 45 mg once daily [QD]). Bowel urgency and abdominal pain were evaluated at baseline and Weeks 2, 4, 6, and 8. Week 8 correlations were evaluated between bowel urgency/abdominal pain with clinical [Mayo subscores and high-sensitivity C-reactive protein and faecal calprotectin measurements] and HRQOL outcomes [Inflammatory Bowel Disease Questionnaire and 36-Item Short Form Health Survey scores].
Results
A greater proportion of patients [n = 250] reported no bowel urgency and less abdominal pain with upadacitinib treatment compared with placebo, with improvements observed as early as 2 weeks. At Week 8, patients receiving the 45-mg QD dose had the greatest improvements versus placebo, with 46% reporting no bowel urgency [vs 9%; p ≤ 0.001] and 38% reporting no abdominal pain [vs 13%; p = 0.015]. At Week 8, moderate correlations were found between bowel urgency or abdominal pain and most clinical and HRQOL outcomes.
Conclusions
Induction treatment with upadacitinib demonstrated significant reductions in bowel urgency and abdominal pain compared with placebo. These symptoms also correlate to clinical and HRQOL outcomes, supporting their use to monitor disease severity and other treatment outcomes.
Keywords: Abdominal pain, bowel urgency, upadacitinib
1. Introduction
Ulcerative colitis [UC] is a chronic, relapsing inflammatory disease of the large intestine characterised by inflammation and ulceration of the colonic mucosa.1 The reported prevalence of UC is highest in Europe [505 per 100 000 in Norway] and North America [286 per 100 000 in the USA].2 The goals of medical treatment in UC are to control inflammation, reduce symptoms, and maintain remission, as well as to improve health-related quality of life [HRQOL].3,4 Despite the availability of various therapies, many patients are not able to achieve or maintain clinical remission, indicating a significant medical need.5,6
Upadacitinib, an oral selective Janus kinase [JAK]-1 inhibitor, is currently being assessed in patients with UC and Crohn’s disease.7,8 In the phase 2b study of the U-ACHIEVE programme for UC [NCT02819635], clinical remission was achieved after 8 weeks of treatment with upadacitinib once daily [QD].7 Adverse events with upadacitinib were consistent with those observed from other clinical trials with JAK inhibitors for moderate to severe inflammatory bowel disease and rheumatoid arthritis.7,9–18
One of the challenges in the development of new treatments for UC is the identification of measures reflecting symptoms which are important to patients and can serve as relevant tools for determining disease severity and assessment of treatment success.19 Current treatment guidelines use tools such as the Mayo score to define UC severity, which includes evaluations of stool frequency, rectal bleeding, endoscopic findings, and Physician Global Assessment.4,20
However, these tools do not incorporate bowel urgency and abdominal pain symptoms in their assessments.20 Both bowel urgency and abdominal pain are reported by more than 50% of patients with UC and are meaningful symptoms considered by patients in their treatment decisions.21–23 Bowel urgency is particularly distressing to patients, as it can lead to incontinence and inability to distinguish liquid and gas from solid stool.24 Tools commonly used in observational studies, such as the Simple Clinical Colitis Activity Index [SCCAI] and patient-SCCAI, include bowel urgency but not abdominal pain.25,26
In this study we evaluated bowel urgency and abdominal pain in patients with moderately to severely active UC, who received upadacitinib or placebo from the U-ACHIEVE study. We assessed improvements in bowel urgency and abdominal pain with upadacitinib treatment versus placebo and performed similar analyses for rectal bleeding, stool frequency, and HRQOL. Correlation of bowel urgency and abdominal pain improvements with clinical outcomes, HRQOL, and biomarker levels were also determined.
2. Materials and Methods
The methods for the U-ACHIEVE study have been described in detail elsewhere7 and are summarised briefly below.
2.1. Study design and participants
This was a post hoc analysis of the phase 2b, double-blind, placebo-controlled, randomised, dose-ranging, 8-week induction therapy portion of the U-ACHIEVE study. Patients were randomised to receive placebo or upadacitinib at doses of 7.5, 15, 30, or 45 mg QD.
Included in the study were patients aged 18–75 years, with moderately to severely active UC defined as an adapted Mayo score [Mayo score without Physician’s Global Assessment] of 5 to 9 points and Mayo endoscopic subscore of 2 to 3 [confirmed by central reader]. Patients also had to have UC for ≥ 90 days before baseline, confirmed by colonoscopy, and to have demonstrated an inadequate response to, loss of response to, or intolerance to corticosteroids, immunosuppressants, and/or biologic therapies. Patients were excluded if they had: a diagnosis of Crohn’s disease or indeterminate colitis, UC limited to the rectum, clinical signs of fulminant colitis, toxic megacolon, or a history of colectomy. Patients were permitted to concomitantly receive oral corticosteroids, inhaled or topical dermatological corticosteroids, aminosalicylates, topical non-steroidal anti-inflammatory drugs, or methotrexate, but could not receive biologics, cyclosporine, intravenous corticosteroids, tacrolimus, azathioprine, 6-mercaptopurine, or topical rectal therapies.
2.2. Bowel urgency and abdominal pain symptoms
Bowel urgency and abdominal pain symptoms were collected by the patient in an electronic diary [e-diary] using a handheld device and recorded daily using a 24-h recall period. Data from e-diary entries over the most recent consecutive 3-day period within 10 days before each study visit were calculated for study symptoms.
Bowel urgency was reported by the patient [yes or no] from their daily diary, with the total number of days experiencing bowel urgency measured over 3 consecutive days before each study visit, within a range of 0–3. Patients were divided into two groups, those with any bowel urgency [1, 2, or 3 days] and those with no bowel urgency [0 days]. Abdominal pain was scored as 0 [no pain], 1 [mild pain], 2 [moderate pain], and 3 [severe pain], with average abdominal pain calculated over 3 consecutive days before each study visit, with a range of 0–3.
Bowel urgency and abdominal pain were collected from the patient daily diary and evaluated at baseline and at Weeks 2, 4, 6, and 8. UC symptoms evaluated across trial arms were the percentage of patients reporting the absence of bowel urgency and an average abdominal pain of 0 over the 3 examined days and the mean change from baseline to Weeks 2, 4, and 8 in abdominal pain.
2.3. Additional UC symptoms
Rectal bleeding was assessed using the Mayo rectal bleeding subscore27 with 0 meaning no blood seen; 1, streaks of blood with stool less than half the time; 2, obvious blood with stool most of the time; and 3, blood alone passed. Stool frequency was determined using the Mayo stool frequency subscore,27 with 0 meaning normal number of stools for respective patient; 1 meaning 1–2 stools more than normal; 2 meaning 3–4 stools more than normal; and 3 meaning ≥ 5 stools more than normal. The average values over the most recent consecutive 3-day period within 10 days before each study visit were calculated for rectal bleeding and stool frequency symptoms.
Rectal bleeding and stool frequency symptoms evaluated across trial arms were the percentage of patients reporting a Mayo rectal bleeding subscore of 0 and Mayo stool frequency subscore of ≤ 1 over the 3 examined days and the mean change from baseline to Weeks 2, 4, 6, and 8 in Mayo rectal bleeding subscore and Mayo stool frequency subscore.
2.4. Clinical outcomes
The full Mayo score was used to assess UC severity with all the subscores: stool frequency subscore, rectal bleeding subscore, endoscopy subscore, and Physician’s Global Assessment subscore. Clinical remission was determined per adapted Mayo score [defined as stool frequency subscore ≤ 1, rectal bleeding subscore of 0, and endoscopic subscore ≤ 1] at Week 8. Clinical response was defined as a decrease from baseline of ≥ 2 points in the adapted Mayo score and ≥ 30% from baseline PLUS a decrease in rectal bleeding subscore ≥ 1 or an absolute rectal bleeding subscore ≤ 1.
Biomarkers assessed were high-sensitivity C-reactive protein [hs-CRP] and faecal calprotectin [CRP] at baseline and Weeks 2, 4 [hs-CRP only], and 8.
2.5. HRQOL measures
HRQOL measures assessed were Inflammatory Bowel Disease Questionnaire [IBDQ] and 36-Item Short Form Health Survey [SF-36]. IBDQ is a disease-specific instrument composed of 32 items graded on a 7-point Likert scale.28,29 For IBDQ, total score ranges from 32 to 224, with higher scores indicating better HRQOL. SF-36 is composed of a Physical Component Summary [PCS] and Mental Component Summary [MCS].30,31 PCS and MCS scores range from 0 to 50, with higher scores indicating better HRQOL.
HRQOL measures were assessed at baseline and at Weeks 2, 4, and 8. HRQOL measure outcomes evaluated across trial arms were the percentage of patients with IBDQ remission [defined as IBDQ score ≥ 170 points] and with IBDQ response [defined as an increase of ≥ 16 points from baseline] at Weeks 2, 4, and 8.
2.6. Statistical analyses
Analyses were performed in the intention-to-treat population, which consists of all patients who were randomised and received one dose of study drug. Comparisons between bowel urgency and abdominal pain with clinical outcomes and HRQOL measures were determined using the Mann‐Whitney test at Week 8. Correlations between bowel urgency and abdominal pain with clinical outcomes, HRQOL measures, and biomarker levels were evaluated with Spearman’s correlation coefficients at Week 8. Estimates of correlation coefficient between 0 and 0.3 [−0.3] indicate weak correlation, >0.3 to 0.7 [<−0.3 to − 0.7] indicate moderate correlation, >0.7 to 0.9 [<−0.7 to − 0.9] indicate strong correlation, and >0.9 to 1.0 [<−0.9 to − 1.0] indicate very strong correlation.32
Comparisons between upadacitinib dose groups and placebo for percentage of patients with bowel urgency and abdominal pain symptoms, other UC symptoms, IBDQ response, and IBDQ remission were based on Cochran‐Mantel‐Haenszel tests, adjusting for previous biologic use, baseline corticosteroid use, and adapted Mayo score [≤7, >7]. Missing data were reported using non-responder imputation. For abdominal pain, Mayo rectal bleeding subscore, and Mayo stool frequency subscore, mean changes from baseline to Weeks 2, 4, and 8 were assessed using analysis of covariance. Missing data were reported using last observation carried forward. All data analyses were performed using SAS version 9.4 [SAS Institute, Cary, NC, USA].
2.7. Ethical requirements
This study was conducted as per the International Conference on Harmonization guidelines, applicable regulations, and the Declaration of Helsinki, and was approved by respective institutional review committees.7 All patients provided informed consent before study participation.
3. Results
3.1. Study population
Of the 250 patients randomised between treatment groups, baseline UC symptoms were generally similar between treatment cohorts, although there was some variability in the percentage of patients with a given number of bowel urgency days [Table 1]. Bowel urgency was reported by 83% of patients over 3 days at baseline [71% for 3 days, 8% for 2 days, 4% for 1 day] and 7% experienced no bowel urgency. Abdominal pain was experienced at some level by 82% of patients at baseline [7% severe, 34% moderate, 41% mild] and 8% had no abdominal pain. The percentage of patients with missing data for bowel urgency and abdominal pain was 10% and ranged from 4% for the 7.5-mg QD cohort to 16% for the 45-mg QD cohort.
Table 1.
Patient demographics and baseline characteristics.
| Placebo | Upadacitinib 7.5 mg QD | Upadacitinib 15 mg QD | Upadacitinib 30 mg QD | Upadacitinib 45 mg QD | |
|---|---|---|---|---|---|
| Variable | [n = 46] | [n = 47] | [n = 49] | [n = 52] | [n = 56] |
| Age [years], median [range] | 40.0 [21–67] | 41.0 [18–75] | 47.0 [22–71] | 42.0 [20–72] | 37.0 [19–74] |
| Female, n [%] | 17 [37.0] | 24 [51.1] | 19 [38.8] | 21 [40.4] | 19 [33.9] |
| White, % | 37 [80.4] | 36 [76.6] | 37 [75.5] | 37 [71.2] | 38 [67.9] |
| Disease duration [years], mean ± SD | 7.5 ± 6.7 | 9.0 ± 7.9 | 9.3 ± 9.8 | 7.3 ± 5.7 | 7.9 ± 6.8 |
| Prior biologic use, % | 35 [76.1] | 36 [76.6] | 38 [77.6] | 42 [80.8] | 43 [76.8] |
| Adapted Mayo score, mean ± SDa | 7.0 ± 1.1 | 7.0 ± 1.2 | 7.0 ± 1.1 | 7.0 ± 1.2 | 6.7 ± 1.2 |
| Number of bowel urgency days, n [%] | |||||
| 0 day | 2 [4.3] | 5 [10.6] | 4 [8.2] | 4 [7.7] | 3 [5.4] |
| 1 day | 3 [6.5] | 2 [4.3] | 2 [4.1] | 1 [1.9] | 3 [5.4] |
| 2 days | 3 [6.5] | 3 [6.4] | 5 [10.2] | 1 [1.9] | 8 [14.3] |
| 3 days | 33 [71.7] | 35 [74.5] | 34 [69.4] | 42 [80.8] | 33 [58.9] |
| Missing | 5 [10.9] | 2 [4.3] | 4 [8.2] | 4 [7.7] | 9 [16.1] |
| Abdominal pain score, n [%]b | |||||
| 0 to 1 | 24 [52.2] | 23 [48.9] | 24 [49.0] | 26 [50.0] | 26 [46.4] |
| >1 to 2 | 14 [30.4] | 17 [36.2] | 17 [34.7] | 19 [36.5] | 18 [32.1] |
| >2 to 3 | 3 [6.5] | 5 [10.6] | 4 [8.2] | 3 [5.8] | 3 [5.4] |
| Missing | 5 [10.9] | 2 [4.3] | 4 [8.2] | 4 [7.7] | 9 [16.1] |
| Rectal bleeding subscore, mean ± SDa | 1.7 ± 1.0 | 1.6 ± 1.0 | 1.5 ± 0.9 | 1.5 ± 1.0 | 1.4 ± 0.9 |
| Stool frequency score, mean ± SDa | 2.6 ± 0.7 | 2.6 ± 0.6 | 2.7 ± 0.6 | 2.6 ± 0.7 | 2.6 ± 0.6 |
| IBDQ, mean ± SDa | 129.0 ± 36.0 | 120.6 ± 36.9 | 121.3 ± 34.7 | 123.0 ± 31.0 | 128.4 ± 29.3 |
| SF-36 PCS, mean ± SDa | 43.9 ± 8.0 | 40.0 ± 9.4 | 42.7 ± 9.0 | 41.5 ± 7.3 | 43.0 ± 7.0 |
| SF-36 MCS, mean ± SDa | 41.6 ± 10.4 | 41.9 ± 11.1 | 40.1 ± 12.5 | 40.8 ± 11.9 | 42.1 ± 10.4 |
| HS-CRP, n [%] | |||||
| ≤0.5 mg/L | 21 [45.7] | 24 [51.1] | 11 [22.4] | 18 [34.6] | 24 [42.9] |
| >0.5 mg/L | 25 [54.3] | 23 [48.9] | 38 [77.6] | 34 [65.4] | 32 [57.1] |
| Faecal calprotectin [mg/kg], mean ± SD | 3299 ± 4901 | 2713 ± 3644 | 4153 ± 5009 | 3031 ± 3575 | 2954 ± 3503 |
HS-CRP, high-sensitivity C-reactive protein; IBDQ, Inflammatory Bowel Disease Questionnaire; MCS, Mental Component Summary; PCS, Physical Component Summary; QD, once a day; SD, standard deviation; SF-36, 36-Item Short Form Health Survey.
a n values different from those listed in header: placebo: 45 [IBDQ, SF-36]; upadacitinib 7.5 mg: 44 [IBDQ, SF-36]; upadacitinib 15 mg: 48 [IBDQ, SF-36]; upadacitinib 30 mg: 49 [IBDQ] and 48 [SF-36]; upadacitinib 45 mg: 55 [IBDQ, adapted Mayo, rectal bleeding, stool frequency] and 54 [SF-36].
bAbdominal pain was scored as 0 [no pain], 1 [mild pain], 2 [moderate pain], and 3 [severe pain].
3.2. Change in bowel urgency and abdominal pain with upadacitinib treatment
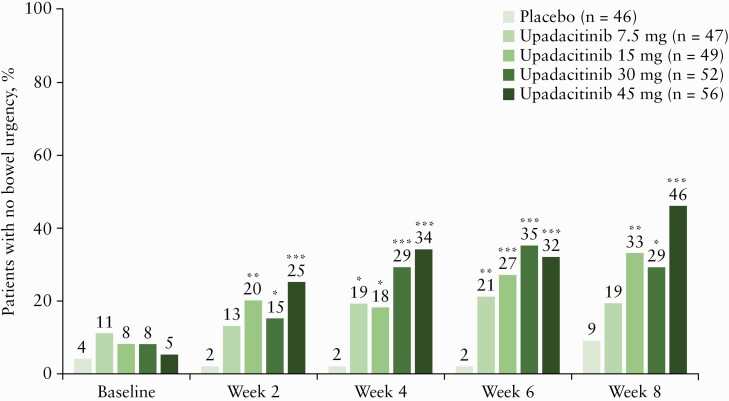
A greater proportion of patients who received upadacitinib reported no bowel urgency during the study compared with those who received placebo, with improvements observed by 2 weeks [Figure 1]. Improvements in bowel urgency with upadacitinib were dose dependent. Patients receiving upadacitinib 45 mg QD had the greatest improvements, with 46.4% reporting no bowel urgency at Week 8 compared with 8.7% for placebo, a 37.7% difference (95% confidence interval [CI], 18.1, 54.0; p ≤ 0.001 vs placebo].
Figure 1.
Percentage of patients reporting no bowel urgency over time with upadacitinib. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared with placebo. NRI, ITT population. ITT, intent-to-treat; NRI, non-responder imputation.
Similarly, patients receiving upadacitinib reported lower abdominal pain scores during the study compared with those receiving placebo, with differences observed by Week 2 [Figure 2]. With upadacitinib 45 mg QD, a 24.5% difference [95% CI, 4.0, 37.9; p = 0.015] in the percentage of patients reporting an abdominal pain score of 0 was observed compared with placebo [37.5% vs 13.0%] at Week 8 [Figure 2A]. Mean abdominal pain score was reduced with upadacitinib treatment by 2 weeks and continued over the course of the study, with reductions significant for most doses and time points versus placebo [p ≤ 0.01, Figure 2B].
Figure 2.
Improvements in abdominal pain with upadacitinib. A. Percentage of patients reporting an abdominal pain score of 0 from Weeks 2 to 8. B. Reduction in mean abdominal pain from Weeks 2 to 8. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared with placebo. Panel A is analysed NRI. Panel B is analysed LOCF ITT population. ITT, intent-to-treat; LOCF, last observation carried forward; NRI, non-responder imputation.
3.3. Change in other UC symptoms and IBDQ with upadacitinib treatment
Significantly greater percentages of patients reported a Mayo rectal bleeding subscore of 0 in the upadacitinib 30- and 45-mg QD groups compared with placebo, from Week 2 to Week 8 [p ≤ 0.05, Supplementary Figure S1A, available as Supplementary data at ECCO-JCC online]. Reductions in mean Mayo rectal bleeding subscore with upadacitinib were also noted throughout the study, with changes occurring as early as Week 2 [Supplementary Figure S1B].
Stool frequency was also substantially reduced with upadacitinib treatment, with a significantly greater percentage of patients at Week 8 reporting a Mayo stool frequency score of ≤ 1 for upadacitinib 30 mg and 45 mg QD compared with placebo [p ≤ 0.01, Supplementary Figure S2A, available as Supplementary data at ECCO-JCC online]. Mean Mayo stool frequency subscore reductions were observed with upadacitinib as early as Week 2, which continued during the study [Supplementary Figure S2B].
At Week 8, IBDQ remission was reported by a significantly greater percentage of patients treated with upadacitinib 15 mg, 30 mg, and 45 mg QD than with placebo [p ≤ 0.05, Supplementary Figure S3A, available as Supplementary data at ECCO-JCC online]. IBDQ response at Week 2 through Week 8 was also reported by a significantly greater percentage of patients treated with upadacitinib 15 mg, 30 mg, and 45 mg QD than with placebo [p ≤ 0.05, Supplementary Figure S3B].
3.4. Correlation of bowel urgency and abdominal pain with clinical outcomes, HRQOL measures, and biomarker levels
Patients who achieved clinical remission as per adapted Mayo score at Week 8 had significantly fewer bowel urgency days than those who did not achieve remission [p ≤ 0.01, Figure 3A]. At baseline, significantly lower IBDQ and SF-36 PCS scores were obtained for patients with any bowel urgency compared with those with no bowel urgency [p ≤ 0.05, Figure 3B]. When outcomes were correlated with bowel urgency at Week 8, a moderate correlation was found for most clinical outcomes, HRQOL measures, abdominal pain, and biomarker levels, except for the SF-36 MCS [Table 2].
Figure 3.
Association of bowel urgency with clinical outcomes and HRQOL measures. A. Bowel urgency severity in patients who attained clinical remission per adapted Mayo score at Week 8. B. Burden associated with bowel urgency based on HRQOL measures at baseline. Adapted Mayo score defined as stool frequency subscore ≤ 1, RBS of 0, and endoscopic subscore ≤ 1. *p ≤ 0.05, **p ≤ 0.01, using Mann–Whitney U test for patients between groups [panel A] or with any bowel urgency vs no bowel urgency [panel B]. Observed data. ITT population. HRQOL, health-related quality of life; IBDQ, Inflammatory Bowel Disease Questionnaire; ITT, intent-to-treat; MCS, Mental Component Summary; PCS, Physical Component Summary; RBS, rectal bleeding subscore; SF-36, 36-Item Short Form Health Survey.
Table 2.
Correlation of bowel urgency days and abdominal pain at Week 8 with clinical outcomes, HRQOL measures, and biomarker levels.
| Bowel urgency days | Abdominal pain | |||
|---|---|---|---|---|
| Measures | n | Spearman correlation [95% CI] | n | Spearman correlation [95% CI] |
| Full Mayo score | 206 | 0.59 [0.49, 0.68] | 206 | 0.41 [0.28, 0.53] |
| Mayo rectal bleeding subscore | 210 | 0.40 [0.27, 0.51] | 210 | 0.43 [0.31, 0.54] |
| Mayo stool frequency subscore | 210 | 0.55 [0.45, 0.65] | 210 | 0.35 [0.21, 0.47] |
| Mayo physician global assessment subscore | 210 | 0.50 [0.38, 0.60] | 210 | 0.42 [0.29, 0.53] |
| Mayo endoscopic subscore | 222 | 0.42 [0.30, 0.54] | 222 | 0.20 [0.05, 0.33] |
| Abdominal pain | 189 | 0.54 [0.43, 0.63] | – | – |
| Bowel urgency | – | – | 189 | 0.54 [0.43, 0.63] |
| IBDQ | 214 | −0.50 [−0.61, −0.38] | 214 | −0.55 [−0.64, −0.20] |
| SF-36 PCS | 214 | −0.41 [−0.53, −0.28] | 214 | −0.52 [−0.62, −0.41] |
| SF-36 MCS | 214 | −0.27 [−0.41, −0.13] | 214 | −0.31 [−0.44, −0.16] |
| HS-CRP | 231 | 0.34 [0.21, 0.46] | 231 | 0.32 [0.18, 0.44] |
| Faecal calprotectin | 211 | 0.41 [0.28, 0.53] | 211 | 0.22 [0.08, 0.36] |
Estimates between 0 and 0.3 [−0.3] indicate weak correlation [bold text] and > 0.3 to 0.7 [<−0.3 to − 0.7] indicate moderate correlation [plain text].32 All correlations in the table were statistically significant.
CI, confidence interval; HRQOL, health-related quality of life; HS-CRP, high-sensitivity C-reactive protein; IBDQ, Inflammatory Bowel Disease Questionnaire; MCS, Mental Component Summary; PCS, Physical Component Summary; SF-36, 36-Item Short Form Health Survey.
For abdominal pain, patients who achieved clinical remission per adapted Mayo score at Week 8 had lower mean scores versus those who did not achieve remission [p ≤ 0.01, Figure 4A]. A trend of increasing abdominal pain severity with greater HRQOL impairment at baseline was also demonstrated [Figure 4B]. At baseline, patients with severe abdominal pain had significantly lower IBDQ, SF-36 PCS, and SF-36 MCS scores compared with those with no abdominal pain [p ≤ 0.001, Figure 4B]. At Week 8, moderate correlations were observed between abdominal pain and most clinical outcomes, HRQOL measures, bowel urgency, and biomarker levels, except for Mayo endoscopic score and faecal calprotectin [Table 2].
Figure 4.
Association of abdominal pain with clinical outcomes and HRQOL measures. A. Abdominal pain severity in patients who attained clinical remission per adapted Mayo score at Week 8. B. Burden associated with abdominal pain based on HRQOL measures at baseline. *p ≤ 0.01, **p ≤ 0.001 using Mann–Whitney U test for patients between groups [panel A] or with abdominal pain vs no pain [panel B]. ITT population. Observed data. HRQOL, health-related quality of life; IBDQ, Inflammatory Bowel Disease Questionnaire; ITT, intent-to-treat; MCS, Mental Component Summary; PCS, Physical Component Summary; SF-36, 36-Item Short Form Health Survey.
4. DISCUSSION
In this report, we demonstrated that 8 weeks of induction therapy with upadacitinib resulted in statistically significant improvements in patient symptoms, including bowel urgency and abdominal pain, in patients with moderately to severely active UC. Upadacitinib treatment also resulted in significant and clinically meaningful positive changes in IBDQ. Both symptom and IBDQ improvements with upadacitinib occurred as early as Week 2. These results extend the positive findings that were recently reported for the primary and key secondary endpoints from the phase 2b study of the U-ACHIEVE programme.7 At Week 8, clinical remission [defined based on the adapted Mayo score as a stool frequency subscore ≤ 1, rectal bleeding subscore = 0, and endoscopic subscore ≤ 1] was achieved by 8.5%, 14.3%, 13.5%, and 19.6% of patients receiving upadacitinib 7.5 mg, 15 mg, 30 mg, or 45 mg, respectively, with none in the placebo group [p = 0.052, p = 0.013, p = 0.011, and p = 0.002, compared with placebo, respectively]. Endoscopic improvement [defined as endoscopic subscore ≤ 1] at Week 8 was also obtained by a greater percentage of patients receiving upadacitinib [14.9%, 30.6%, 26.9%, and 35.7% for the 7.5 mg, 15 mg, 30 mg, and 45 mg doses, respectively] compared with placebo [2.2%] [p = 0.033, p < 0.001, p < 0.001, p < 0.001, respectively].
There is a considerable need for novel treatments and measurement tools for UC, particularly as current therapeutic options, including mesalamine, glucocorticoids, immunosuppressants, and biologics, are ineffective in a substantial percentage of patients.33,34 For those patients who have a response, it can be inadequate or may not be maintained.33,34 Additionally, these treatments can be associated with adverse effects that can limit their use.33,34
Bowel urgency and abdominal pain are highly prevalent in patients with UC and have a serious impact on their mental and physical quality of life.21–23,35,36 In a study of 501 patients with UC, bowel urgency and abdominal pain were reported in 63% and 58%, respectively, of patients with active disease.23 This resulted in a significant impact on the daily life of approximately 40% of these patients.23 Bowel urgency and abdominal pain have also been defined as meaningful attributes from a patient perspective for treatment.21
However, to our knowledge, the use of bowel urgency and abdominal pain to measure disease severity or treatment effectiveness has never been evaluated in comparison with other more commonly used UC measurement tools such as the Mayo score. In this phase 2b study of patients with moderately to severely active UC treated with upadacitinib or placebo for 8 weeks, we found a moderate correlation of improvements in bowel urgency and abdominal pain with positive outcomes for most clinical outcomes, IBDQ measures, and biomarker level changes. Furthermore, we observed statistically significant improvements with upadacitinib versus placebo in bowel urgency and abdominal pain at Week 8 with improvements seen as early as Week 2. Similar results were also seen for rectal bleeding, stool frequency, and disease-specific and general HRQOL. Therefore, bowel urgency and abdominal pain are relevant, related to clinical outcomes, and responsive to upadacitinib treatment.
Previously, the validated Monitor Inflammatory bowel disease At Home [MIAH] questionnaire, which included questions on bowel urgency and abdominal pain, was used with the combination of a calprotectin home test and shown to be highly accurate in predicting endoscopic inflammation.37 In the current study, Mayo endoscopic subscore was moderately correlated with bowel urgency but weakly with abdominal pain. The findings in this current study provide further support for the use of bowel urgency and abdominal pain evaluations in patient-reported outcome measurements, in addition to the commonly used stool frequency and rectal bleeding assessments.
This study reported the important, but under-appreciated, patient-reported outcome measurements of bowel urgency and abdominal pain from a patient perspective in the context of a phase 2b clinical trial. We observed that upadacitinib can significantly relieve symptoms that are highly prevalent and burdensome to these patients compared with placebo by 2 weeks of treatment, with the caveat that this study was not pre-specified to statistically power the evaluation of changes in bowel urgency and abdominal pain. One limitation of this study was that this was a post hoc analysis which was not designed to directly evaluate the association of improvements in these individual symptoms with HRQOL. Although this analysis was based on data collected in a phase 2b study with a limited sample size, the reported positive findings provide an incentive to confirm this association in a larger phase 3 trial.
Additional avenues of research that could not be addressed in this current study are the long-term outcomes for patients based on bowel urgency and abdominal pain response and the characterisation of patients with differential improvements in bowel urgency and abdominal pain relative to other measures, including the Mayo score, rectal bleeding, and stool frequency. These will be addressed in future studies, including the larger phase 3 portion of the U-ACHIEVE study and its long-term follow-up.
Increasingly, patient-reported outcomes in UC are including bowel urgency and abdominal pain questions, such as for the paediatric daily UC signs and symptoms scale [DUCS].38 Consequently, as part of the UC patient-reported outcomes, bowel urgency and abdominal pain results would need to be incorporated for use in clinical trials. These findings are relevant, as the association between symptoms of bowel urgency and abdominal pain and quality of life in patients with UC tends to be underestimated by health care professionals.39,40
In conclusion, we found that improvements with upadacitinib in bowel urgency and abdominal pain correlate with positive changes in various clinical outcomes, HRQOL measures, and biomarker levels, including those that are commonly used to measure disease severity in clinical trials. These results support the use of bowel urgency and abdominal pain measurements to monitor disease severity and treatment outcomes. As patients treated with upadacitinib had superior IBDQ remission and response rates compared with those given placebo, these results indicate a potential HRQOL benefit with symptom relief for patients with UC. These results support the further evaluation of upadacitinib for patients with moderate to severe UC in phase 3 trials.
Supplementary Material
Acknowledgements
Medical writing assistance was provided by Alan Saltzman, PhD, CMPP, of Fishawack Facilitate Ltd, part of Fishawack Health, and was funded by AbbVie Inc., North Chicago, IL.
Funding
This work was supported by AbbVie Inc. AbbVie sponsored the study; contributed to the design; and participated in collection, analysis, and interpretation of data and in writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship.
Conflict of Interest
SG received lecture fee[s] from AbbVie, Janssen, Takeda, Pfizer, Celltrion, Eli Lilly, Shield, Ferring, Falk Pharma; is steering/advisory committee member for Pfizer, Janssen, AbbVie, Bristol Myers Squibb, Celgene, Boehringer Ingelheim, Celltrion, Gilead, Galapagos, Eli Lilly, Takeda; and received research support from GSK, AbbVie, and Vertex. YSG, WZ, RC, and WX are AbbVie employees and may own AbbVie stock/stock options. EL received financial support for research from Takeda, Pfizer, and Janssen; received lecture fee[s] from AbbVie, Celgene, Falk, Ferring, MSD, Takeda, Janssen, and Pfizer; received consultancy fees from AbbVie; received educational grants from AbbVie, Takeda, and Janssen; and served on advisory boards for AbbVie, Ferring, MSD, Takeda, Celgene, Janssen, Gilead-Galapagos, Arena, Pfizer, and Eli Lilly. EVL Jr received financial support for research from AbbVie, Takeda, Janssen, UCB, Amgen, Pfizer, Genentech, Gilead, Celgene, Robarts Clinical Trials, Receptos, Bristol Myers Squibb, Theravance; and received consultancy fees from AbbVie, Allergan, Boehringer Ingelheim, Takeda, Janssen, UCB, Amgen, Pfizer, Eli Lilly, Celgene, Celltrion Healthcare, Bristol Myers Squibb, Gilead, Genentech, Iterative Scopes, Ono Pharma, Calibr, Arena, and Sun Pharma. JP received financial support for research from AbbVie and Pfizer; received lecture fee[s] from AbbVie, Ferring, Janssen, and Takeda; and received consultancy fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Genentech, Janssen, Origo, Pandion, Pfizer, Robarts, Roche, Second Genome, Takeda, Theravance, and Wassermann. SD received speaker fees from Ferring, Merck Sharp & Dohme, and UCB Pharma; received consulting fees from Abbott Laboratories, AbbVie, AstraZeneca, Schering Plough, Takeda, and Millennium; and served as board member of Merck Sharp & Dohme.
Author Contributions
SG contributed to the concept, draft structure, data interpretation, writing of manuscript, final revision and approved the final version. YSG contributed to the concept, draft structure, data interpretation, writing of manuscript, and final revision, and approved the final version. WZ contributed to data acquisition, data interpretation, statistical analyses, and revising the manuscript, and approved the final version. RC contributed to data interpretation and revising the manuscript, and approved the final version. WX contributed to data acquisition, data interpretation, statistical analyses, and revising the manuscript, and approved the final version. EL contributed to data interpretation and writing of the manuscript and approved the final version. EVL contributed to data acquisition, data interpretation, and revising the manuscript, and approved the final version. JP contributed to data interpretation and revising the manuscript, and approved the final version. SD contributed to data interpretation and revising the manuscript, and approved the final version. Conference presentations: 14th Congress of European Crohn’s and Colitis Organisation, Copenhagen, March 6–9, 2019; 26th United European Gastroenterology Week [UEGW], Vienna, October 20–24, 2018.
References
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence. Ulcerative Colitis: Management. NICE guideline. 2019. https://www.nice.org.uk/guidance/ng130 Accessed July 1, 2019. [PubMed]
- 4. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 5. Bilsborough J, Targan SR, Snapper SB. Therapeutic targets in inflammatory bowel disease: current and future. Am J Gastroenterol Suppl 2016;3:27–37. [Google Scholar]
- 6. Gordon JP, McEwan PC, Maguire A, Sugrue DM, Puelles J. Characterizing unmet medical need and the potential role of new biologic treatment options in patients with ulcerative colitis and Crohn’s disease: a systematic review and clinician surveys. Eur J Gastroenterol Hepatol 2015;27:804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology 2020;158:2139–49.e14. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology 2020;158:2123–38.e8. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W; Study A3921043 Investigators . A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:1485–93.e2. [DOI] [PubMed] [Google Scholar]
- 11. Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and Safety of ABT-494, a Selective JAK-1 Inhibitor, in a Phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol 2016;68:2857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kremer JM, Emery P, Camp HS, et al. A Phase IIb Study of ABT-494, a Selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol 2016;68:2867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Westhovens R, Taylor PC, Alten R, et al. Filgotinib [GLPG0634/GS-6034], an oral JAK1 selective inhibitor, is effective in combination with methotrexate [MTX] in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study [DARWIN 1]. Ann Rheum Dis 2017;76:998–1008. [DOI] [PubMed] [Google Scholar]
- 15. Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib [GLPG0634/GS-6034], an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study [DARWIN 2]. Ann Rheum Dis 2017;76:1009–19. [DOI] [PubMed] [Google Scholar]
- 16. Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 17. Genovese MC, Kremer J, Zhong S, Friedman A. Long-term safety and efficacy of upadacitinib [ABT-494], an oral JAK-1 inhibitor in patients with rheumatoid arthritis in an open label extension study. Ann Rheum Dis 2018;77:979.
- 18. Burmester GR, Kremer J, van Den Bosch F, et al. A phase 3, randomized, placebo controlled, double-blind study of upadacitinib [ABT-494], a selective JAK-1 inhibitor, in patients with active rheumatoid arthritis with inadequate response to conventional synthetic DMARDs. Ann Rheum Dis 2018;77:68‐9.
- 19. Danese S, Allez M, van Bodegraven AA, et al. Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis 2019;37:266–83. [DOI] [PubMed] [Google Scholar]
- 20. Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S; AGA Institute Clinical Guidelines Committee . AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Louis E, Ramos-Goñi JM, Cuervo J, et al. A qualitative research for defining meaningful attributes for the treatment of inflammatory bowel disease from the patient perspective. Patient 2020;13:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coates MD, Lahoti M, Binion DG, Szigethy EM, Regueiro MD, Bielefeldt K. Abdominal pain in ulcerative colitis. Inflamm Bowel Dis 2013;19:2207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hibi T, Ishibashi T, Ikenoue Y, Yoshihara R, Nihei A, Kobayashi T. Ulcerative colitis: disease burden, impact on daily life, and reluctance to consult medical professionals: results from a japanese internet survey. Inflamm Intest Dis 2020;5:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joyce JC, Waljee AK, Khan T, et al. Identification of symptom domains in ulcerative colitis that occur frequently during flares and are responsive to changes in disease activity. Health Qual Life Outcomes 2008;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bennebroek Evertsz’ F, Nieuwkerk PT, Stokkers PC, et al. The patient simple clinical colitis activity index [P-SCCAI] can detect ulcerative colitis [UC] disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis 2013;7:890–900. [DOI] [PubMed] [Google Scholar]
- 26. Ghosh S, Sensky T, Casellas F, et al. A global, prospective, observational study measuring disease burden and suffering in patients with ulcerative colitis using the pictorial representation of illness and self-measure tool. J Crohns Colitis 2020;15:228‐ 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005;54:782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s relapse prevention trial study group. Gastroenterology 1994;106:287–96. [DOI] [PubMed] [Google Scholar]
- 30. Ware JE Jr. SF-36 health survey update. Spine [Phila Pa 1976] 2000;25:3130–9. [DOI] [PubMed] [Google Scholar]
- 31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey [SF-36]. I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 32. Hinkle DE, Jurs SG, Wiersma W.. Applied Statistics for the Behavioral Sciences. Boston, MA: Houghton Mifflin; 2003. [Google Scholar]
- 33. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 34. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology . Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501–23; quiz 524. [DOI] [PubMed] [Google Scholar]
- 35. Rapport F, Clement C, Seagrove AC, Alrubaiy L, Hutchings HA, Williams JG. Patient views about the impact of ulcerative colitis and its management with drug treatment and surgery: a nested qualitative study within the CONSTRUCT trial. BMC Gastroenterol 2019;19:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rubin DT, Siegel CA, Kane SV, et al. Impact of ulcerative colitis from patients’ and physicians’ perspectives: Results from the UC: NORMAL survey. Inflamm Bowel Dis 2009;15:581–8. [DOI] [PubMed] [Google Scholar]
- 37. de Jong MJ, Roosen D, Degens JHRJ, et al. Development and validation of a patient-reported score to screen for mucosal inflammation in inflammatory bowel disease. J Crohns Colitis 2019;13:555–63. [DOI] [PubMed] [Google Scholar]
- 38. Flood E, Silberg DG, Romero B, Beusterien K, Erder MH, Cuffari C. Development of the pediatric daily ulcerative colitis signs and symptoms scale [DUCS]: qualitative research findings. BMC Res Notes 2017;10:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panés J, Domènech E, Aguas Peris M, et al. Association between disease activity and quality of life in ulcerative colitis: Results from the CRONICA-UC study. J Gastroenterol Hepatol 2017;32:1818–24. [DOI] [PubMed] [Google Scholar]
- 40. Schreiber S, Panés J, Louis E, Holley D, Buch M, Paridaens K. Perception gaps between patients with ulcerative colitis and healthcare professionals: an online survey. BMC Gastroenterol 2012;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.