Abstract
Numerous clinical observations and exploitation of cellular and animal models indicate that glucosylceramide (GlcCer) and galactosylceramide (GalCer) are involved in many physiological and pathological phenomena. In many cases, the biological importance of these monohexosylcermides has been shown indirectly as the result of studies on enzymes involved in their synthesis and degradation. Under physiological conditions, GalCer plays a key role in the maintenance of proper structure and stability of myelin and differentiation of oligodendrocytes. On the other hand, GlcCer is necessary for the proper functions of epidermis. Such an important lysosomal storage disease as Gaucher disease (GD) and a neurodegenerative disorder as Parkinson’s disease are characterized by mutations in the GBA1 gene, decreased activity of lysosomal GBA1 glucosylceramidase and accumulation of GlcCer. In contrast, another lysosomal disease, Krabbe disease, is associated with mutations in the GALC gene, resulting in deficiency or decreased activity of lysosomal galactosylceramidase and accumulation of GalCer and galactosylsphingosine. Little is known about the role of both monohexosylceramides in tumor progression; however, numerous studies indicate that GlcCer and GalCer play important roles in the development of multidrug-resistance by cancer cells. It was shown that GlcCer is able to provoke immune reaction and acts as a self-antigen in GD. On the other hand, GalCer was recognized as an important cellular receptor for HIV-1. Altogether, these two molecules are excellent examples of how slight differences in chemical composition and molecular conformation contribute to profound differences in their physicochemical properties and biological functions.
Keywords: biological functions, galactosylceramide, glucosylceramide, glycosphingolipids, neurological diseases
Chemical structure, metabolism and occurrence of glucosylceramide and galactosylceramide
Glucosylceramide (GlcCer) and galactosylceramide (GalCer) are essential molecules found in three out of six kingdoms of life: animal, plant and fungus. GlcCer is a founder molecule for synthesis of hundreds of glycosphingolipids (GSLs) (Hirabayashi 2012), which are subdivided in mammals according to the sugar sequence and configuration into GSLs of ganglio-, globo-, isoglobo-, lacto- and neolacto-series (Sandhoff and Sandhoff 2018). However, GalCer may be converted only to sulphated GalCer (3-sulfo-GalCer, SM4) or sialylated GalCer (3-Neu5Ac-GalCer, GM4) or just a few galactosphingolipids (Table I) (D'Angelo et al. 2013; Schnaar and Kinoshita 2017).
Table I.
Names and structures of glycosphingolipids referred in the manuscript
| Full name | Abbreviation | Structure |
|---|---|---|
| Glucosylceramide | GlcCer |
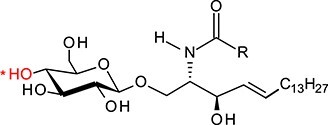
|
| Galactosylceramide | GalCer |
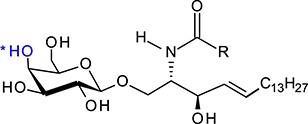
|
| Lactosylceramide | LacCer |
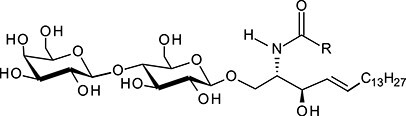
|
| 3-sulfo-GalCer | SM4 |
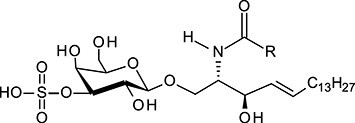
|
| 3-Neu5Ac-GalCer | GM4 |
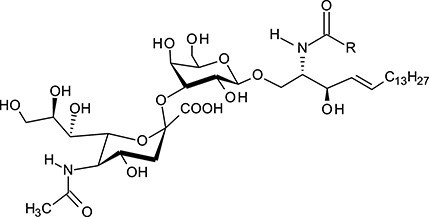
|
| Monosialodihexosylceramide | GM3 |
|
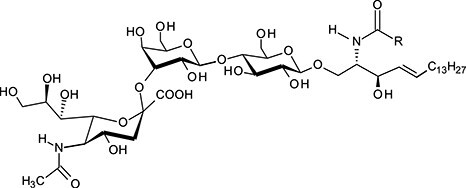
| Monosialotetrahexosylceramide | GM1 |
|
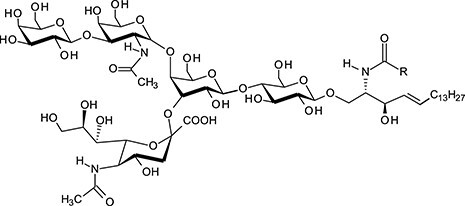
| Globotriaosylceramide | Gb3Cer |
|
*The difference in the position of the hydroxyl group at the C-4 atom of Gal and Glc is pointed out by asterisk and shown, respectively, in blue and red.
GlcCer and GalCer consist, respectively, of d-glucose (Glc) and d-galactose (Gal) residue linked by a β1-1′-glycosidic bond to ceramide (Cer) composed of d-erythro-sphingosine and long-chain fatty acid (Table I). Therefore, these two compounds represent very similar structures since d-galactose is an epimer of d-glucose and these two sugars differ only in the configuration at C-4. Fatty acids attached to sphingosine may vary significantly in length (C14–C26), with stearic acid (C18) being the most abundant. The galactosylceramides are enriched in very-long-chain α-hydroxy fatty acids (C18–C26), whereas glucosylceramides consist of ceramides with primarily nonhydroxylated shorter chain fatty acids (usually C16 or C24) (Schweppe et al. 2010). Various fatty acid residues have different biological roles, e.g., affecting the localization and trafficking of GSLs. The presence of monohexosylceramides with specific fatty acid residues is cell- and/or tissue-specific, which is determined by the expression of specific ceramide synthase/synthases (Levy and Futerman 2010).
Synthesis of GlcCer and GalCer starts with the generation of Cer in endoplasmic reticulum (ER) (Hannun and Obeid 2018; Ogretmen 2018). Transfer of Glc from UDP-Glc on ceramide is catalyzed by UDP-glucose: N-acetylsphingosine d-glucosyltransferase (EC2:4.1.80), also known under the names: glucosylceramide synthase (GCS, GlcCer synthase), ceramide glucosyltransferase (CGT) or UDP-glucose: ceramide glucosyltransferase (UGCG, GLCT-1) (Basu et al. 1968; Paul et al. 1996; Ishibashi et al. 2013) (Figure 1). The enzyme is encoded by the UGCG gene. The synthesis of GlcCer takes place on the cytosolic side of the Golgi apparatus (Coste et al. 1986; Futerman and Pagano 1991; Jeckel et al. 1992), as glucosylceramide synthase (GCS) (this name and acronym was chosen since it is the most frequently used), along with O-GlcNAc transferase (Ong et al. 2018), is the only glycosyltransferase, whose active site is located on the cytoplasmic side of the Golgi (Ichikawa et al. 1996a). GCS is localized on the pre-Golgi, and cis-, medial- and trans-Golgi membranes (Futerman and Pagano 1991; Jeckel et al. 1992) as well as perinuclear ER (Kohyama-Koganeya et al. 2004). It is generally accepted that GlcCer synthesized in the cis-Golgi is transported to the trans-Golgi and translocated to the luminal side, where synthesis of complex GSLs takes place (Sandhoff and Sandhoff 2018) (Figure 2). In addition to vesicular transport, the non-vesicular transport of GlcCer from the early Golgi to distal Golgi compartments is mediated by the glycolipid-binding protein FAPP2 (4-phosphate adaptor protein-2) (D'Angelo et al. 2007). However, according to Halter et al. (2007), GlcCer is synthesized in the trans-Golgi network (TGN). Subsequently, most GlcCer is transported back to the cytoplasmic leaflet of ER with the help of the FAPP2. There, GlcCer is translocated to the luminal side and transported again to the trans-Golgi apparatus, where complex GSLs are synthesized. Remaining GlcCer is transported by FAPP2 from the cytosolic surface of the TGN to the cytosolic surface of the plasma membrane or endosome. In the plasma membrane, GlcCer is translocated to the cell surface or remains on the cytoplasmic side (Halter et al. 2007; Quinn 2011).
Fig. 1.
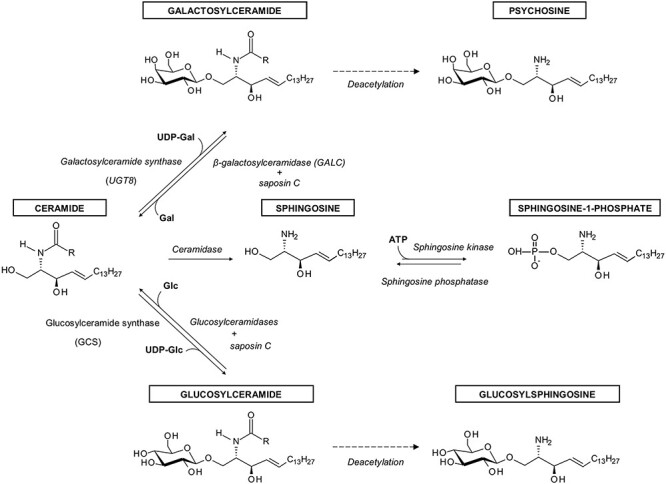
The synthesis and degradation of GlcCer and GalCer and their metabolites referred to in the review.
Fig. 2.
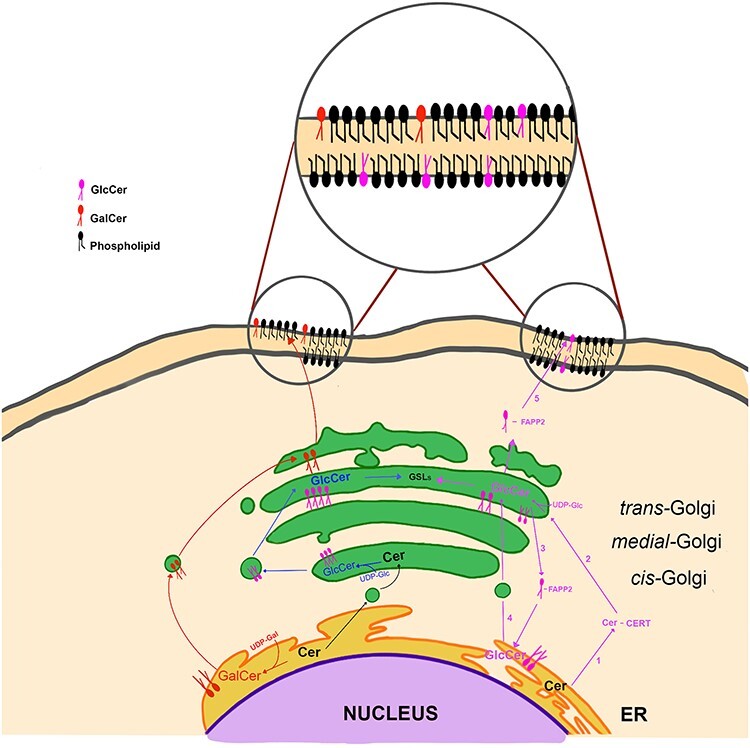
Intracellular localization and transport of GlcCer and GalCer. In the “classical” model (shown in blue), GlcCer, synthesized on the cytosolic side of the cis-Golgi, is transported to the trans-Golgi and translocated to the luminal side, where synthesis of complex GSLs takes place. In the alternative model (shown in violet), ceramide is transported from the ER to the cytosol with the help of CERT (1), then GlcCer is synthesized on the cytosolic side of the trans-Golgi (2), from there most GlcCer is transported back to the cytoplasmic leaflet of ER with the help of the FAPP2 (3). There, GlcCer is translocated to the luminal side and transported again to the trans-Golgi apparatus, where complex GSLs are synthesized (4). Remaining GlcCer is transported by FAPP2 from the cytosolic surface of the trans-Golgi to the cytosolic surface of the plasma membrane (5). GalCer is synthesized on the luminal side of the ER (shown in red). From there, it is transported to the trans-Golgi compartment, where complex galactosphingolipids and sulfatides are synthesized. FAPP2—4-phosphate adaptor protein-2, CERT—ceramide transport protein.
Similar to the synthesis of GlcCer, GalCer is generated by the transfer of Gal from UDP-galactose to the same hydroxyl group at the C-1 position of the ceramide backbone by galactosylceramide synthase (EC2.4.2.62) (Morell and Radin 1969; Sprong et al. 1998), described also as ceramide galactosyltransferase or UDP-galactose:ceramide galactosyltransferase, and known under the acronyms CGT and UGT8 (Figure 1). This enzyme is encoded by the UGT8 gene (Kapitonov and Yu 1997). However, the amino acid sequence of UGT8 shows no significant homology to GCS, which indicates different evolutionary origins of these enzymes (Ichikawa et al. 1996b). Also, the addition of Gal to ceramide moiety takes place in a different cellular compartment. GalCer is synthesized on the luminal side of the ER, as UGT8 is a type I membrane glycoprotein with its active site directed into the lumen of this organelle (Sprong et al. 1998; Sprong et al. 2003) (Figure 2). From there, GalCer is transported to the trans-Golgi compartment, where larger galactosphingolipids and sulfatides are synthesized.
Degradation of GlcCer to glucose and ceramide is carried out by several glucosylceramidases: GBA1, GBA2 and GBA3 (Astudillo et al. 2016) (Figure 1). GBA1 (EC3.2.1.45), also known as acid β-glucosidase, β-glucoceramidase, glucocerebrosidase, d-glucosyl-N-acylsphingosine glucohydrolase and GlcCerase, is a lysosomal hydrolase. GBA2 (EC3.2.1.45), i.e., bile acid β-glucosidase, is a ubiquitous nonlysosomal enzyme located on the cytosolic surface of the ER and/or Golgi apparatus. GBA3 (EC3.2.1.21), known under the names Klotho-related or KLrP, is another cytosolic glucosyl-ceramidase found in the liver, spleen, kidney and in some other tissues, but whose function is presently unclear. In the case of GalCer degradation, the only enzyme known so far is lysosomal galactosylceramidase (EC3.2.1.46), known also as β-galactocerebrosidase, and under the acronym GALC (Beier and Gorogh 2005). In both cases, ceramides that are released from GlcCer and GalCer are hydrolysed by ceramidases to sphingoid bases and fatty acids (Ogretmen 2018).
In animal cells, the main site for degradation of GlcCer and GalCer is lysosome. Monohexosyl-ceramides, after endocytosis of plasma membrane fragments, are transported to lysosome by endocytic vesicular membrane flow through early and late intraendosomal vesicles (Figure 3). Reaching the lysosomal compartment, the late endosome fuses with primary lysosome, allowing the vesicles to be transferred into this compartment. GSLs are then subsequently degraded on the surface of intralysosomal vesicles (Sandhoff and Kolter 1996; Schulze et al. 2009; Kolter and Sandhoff 2010).
Fig. 3.
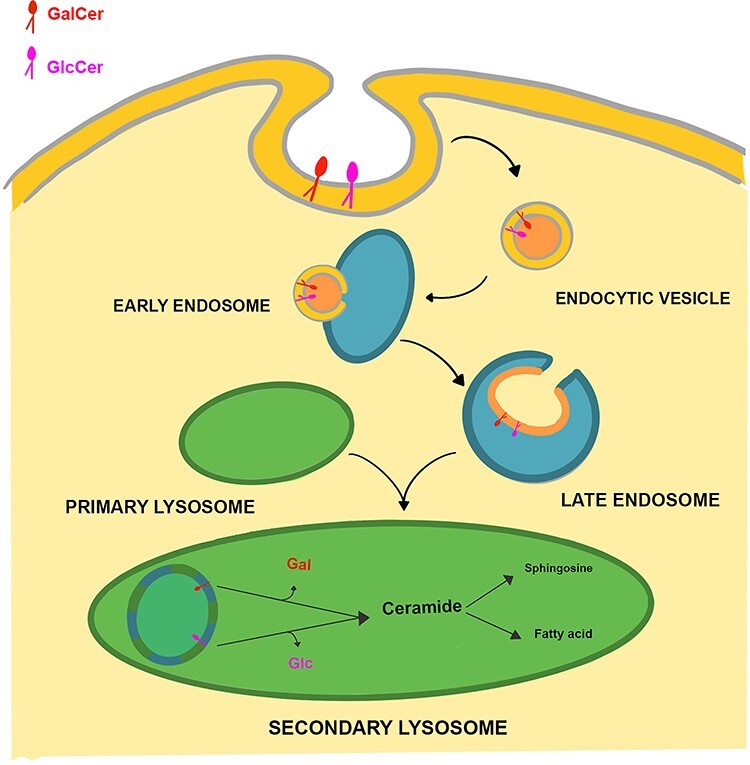
Lysosomal degradation of GlcCer and GalCer. GlcCer and GalCer degradation is initiated by the invagination of the plasma membrane. During endocytosis, GSLs are assimilated as vesicles into early and late endosome. These vesicles reach the lysosome compartment after the fusion of late endosome with primary lysosome. GlcCer and GalCer are then degraded to ceramide by lysosomal enzymes, which are further hydrolyzed by ceramidase to spingoid base and fatty acid.
Besides the enzymes, essential components of the intra-lysosomal degradation of GSLs with less than four sugar residues are sphingolipid activator proteins (SAPs): saposins (Saps) A, B, C and D and GM2-activator protein (GM2-AP) (Schulze et al. 2009). The four saposins arise by proteolytic processing of a single precursor protein, prosaposin, which is synthesized in the ER, transported to the Golgi apparatus for glycosylation and finally transported to the lysosomes. Two mechanisms were proposed to explain the role of saposins in the activation of GSL degradation. (1) Saponins facilitate the interaction between the GSLs and exohydrolases by binding, extracting and presenting the membrane localized lipids to water-soluble enzymes (Sandhoff and Kolter 1996); (2) SAPs bind directly to enzymes, not to GSLs, generating a more active enzymatic complex to hydrolyze GSLs (Fabbro and Grabowski 1991). It was found that deficiency of saposin C led to accumulation of GlcCer within the cells and resulted in a GlcCer storage disease resembling a neurologic form of Gaucher disease (GD; see GlcCer and lysosomal GBA1 in GD section), showing the crucial role of this cofactor in the metabolism of this monohexosylceramide (Kang et al. 2018). Also, the saposin A and probably saposin C are necessary components to activate the degradation of GalCer by galactosylceramidase in vivo (Harzer et al. 1997). Mice lacking, due to mutation, mature saposin A, accumulate GalCer and develop a late-onset form of chronic globoid cell leukodystrophy (Matsuda et al. 2001).
Despite structural similarities, the cellular and tissue localization of GlcCer and GalCer are different. GlcCer is present in essentially all cell types (Makita and Yamakawa 1962; Svennerholm and Svennerholm 1963; Makita 1964; Ishibashi et al. 2013), serving as a precursor for the synthesis of hundreds of different GSLs (Sandhoff and Sandhoff 2018). For example, the presence of GlcCer was analyzed in the mucosa of the gastrointestinal tract (Costantino-Ceccarini and Morell 1973; Breimer et al. 2012), liver (Nilsson and Svennerholm 1982) and adrenal medulla (Ariga et al. 1980). Data are also available on the expression of the UGCG gene in normal human tissues. The highest levels of GCS mRNA were found in the kidney, vulva, urinary bladder, stomach, pancreas and colon. Intermediate levels were seen in the prostate, lung, skin, cervix, rectum and thyroid gland, while the lowest levels of GCS mRNA were present in the breast, uterus, ovary and testis (Liu et al. 2011).
In contrast to GlcCer, GalCer is present only in a limited number of mammalian tissues/cells. GalCer is the most abundant single component of the myelin sheath (20–25% of the total lipid content) produced by oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (Norton and Autilio 1966). This monohexosylceramide is also present in larger amounts in the mucosa of the human gastrointestinal tract (Natomi et al. 1993; Breimer et al. 2012), adrenal medulla (Ariga et al. 1980), liver (Nilsson and Svennerholm 1982), testis (Vos et al. 1994) and milk (Bouhours and Bouhours 1979).
Interestingly, in nerves of protostome animals, only GlcCer and GlcCer-containing GSLs were found. This is in contrast to deuterostomes, whose myelin is rich in GalCer and 3-sulfo-GalCer (Okamura et al. 1985). These changes in GSL composition correlate with the evolution of the nervous system from loosely structured membrane-enwrapped axons to multilamellar highly structured myelin.
The analytical methods for the identification of GalCer and GlcCer
Because of the high structural similarity, identification and differentiation of GlcCer from GalCer is difficult using classical analytical lipid methods and requires either additional steps within the protocol or a simultaneous combination of several methods. Due to their amphipatic structure, GalCer and GlcCer can be separated by thin-layer chromatography (TLC) or high-performance-TLC (HP-TLC). For this purpose, borate-coated TLC plates are commonly used and separation is based on the differences in ability to form glucose-borate or galactose-borate complexes due to a different arrangement of the hydroxyl group at carbon atom 4 in these hexoses (Kean 1966). Ogawa et al. (1988) demonstrated a protocol for boron-free TLC separation using developing system (2-propanol/15 M ammonia solution/methyl acetate/water 75/10/5/15) which allows separation of GalCer from GlcCer in 1D chromatography. More recently, high-performance liquid chromatography of perbenzoylated monohexosylceramide derivatives was adopted for GlcCer/GalCer separation (McCluer et al. 1981). GlcCer and GalCer on HP-TLC plates can also be detected by specific antibodies (Vielhaber et al. 2001; Suchanski et al. 2018). A useful tool in the studies of GlcCer/GalCer trafficking, distribution and metabolism is radioactive or fluorescent analogues of these monohexosylceramides. Such experiments were usually carried out with short-chain fluorescent analogues of a biological active derivative of GalCer and GlcCer tagged with a fluorescent C-6 nitrobenzoxadiazole (C6-NBD) (Kok et al. 1995; Khiste et al. 2017).
Complete structural analysis of GSLs requires a combination of techniques to determine the composition, sequence, linkage positions, anomeric configurations of the sugars, the fatty acid and sphingoid base of the ceramide moiety. GSLs are often structurally characterized by mass spectrometry (MS) and/or nuclear magnetic resonance NMR spectroscopy. Traditional MS approaches are based on the fragmentation of organic molecules, followed by the differentiation of the resulting fragments according to their mass/charge ratio. Electrically neutral GalCer and GalCer are lipids which can be ionized (typically by electrospray) into two ion modes (negative and positive). In the negative-ion mode, cerebroside forms chlorine adducts ([M + Cl]−) (Han and Cheng 2005). In the positive ion mode, they are ionized as proton or alkaline adducts ([M + X]+, X = H, Li, Na, K), depending on the availability and affinity of the small cations (Han and Cheng 2005). GalCer and GlcCer are indistinguishable by MS analysis; therefore, their differentiation requires the use of gas-chromatography (GC) or liquid-chromatography (LC). In order to separate GalCer from GlcCer LC is used preferentially and their analysis is performed using tandem mass spectrometry (Shaner et al. 2009; Gegg et al. 2015; Hamler et al. 2017). Such analysis can often be performed without the need for the release of glycans. However, if necessary, the glycans can be released by enzymatic treatment or chemical methods. For monohexosylceramides, endoglycoceramidase I (EGCase I) can be used, which is the enzyme that catalyzes hydrolysis of the β-glycosidic linkage between oligosaccharides and ceramides in various GSLs (Albrecht et al. 2016). Chemical methods include acid hydrolysis or ozonolysis (Wang et al. 2019). For monohexosylceramides and other GSLs, quantitative release of monosaccharides is achieved by treatment with 4–6 M hydrochloric acid for 3–6 h at 100°C (Townsend 1993). The structure of monosacharide after release can be analyzed by different chromatographic and electrophoretic methods, e.g., GC-MS and its alternative, high-pH anion exchange chromatography with pulsed amperometric detection, which has a strong anion-exchange property and does not require monosaccharide derivatization (Schnaar and Kinoshita 2017). These methods allow for simple, sensitive and specific determination of monosaccharide composition and most importantly enable the discrimination of compounds with the same chemical composition such as glucose and galactose (Corradini et al. 2013). Other popular techniques used to specify monosaccharide composition in GSLs are HPLC and high-performance capillary electrophoresis (Liu and Chan 1991) or capillary electrophoresis with laser-induced fluorescence (Rossdam et al. 2019).
Biological functions of GlcCer and GalCer
Molecular level
GSLs play a role in membrane organization regulating the fluidity of the lipid bilayer and are involved in including or excluding proteins from membrane microdomains. They also act as bioactive lipid messengers on two levels, directly affecting membranous protein functions and regulating the expression level of specific genes. For example, several receptors can be directly regulated by GSLs present in cell membranes (Zhang et al. 2019), modulating specific signaling cascades by GSL-enriched lipid rafts (Modrak et al. 2006), also called GSL-enriched microdomains or “glycosynapses” (Hakomori 2002).
So far, it has been shown that on the molecular level, GlcCer and GalCer are involved in membrane organization. They are localized in the external lipid leaflet of cell membranes and are found predominantly within tightly packed lateral domains of lipids, called membrane rafts (Thompson and Tillack 1985; Brown and Rose 1992; Morrow et al. 1992; Westerlund and Slotte 2009; Varela et al. 2016). As very-long fatty acid chains (C22–C24 and longer) often present in such GSLs are able to penetrate far into the inner leaflet, it was proposed that such interactions allow for better interleaflet coupling and can affect association with cytosolic proteins and intracellular signaling (Skotland and Sandvig 2019). Using atomistic molecular dynamics simulations, Hall et al. (2010) demonstrated that GalCer significantly increased the thickness of raft membranes, while the average area per lipid and lipid conformational order were unchanged. They also showed that interdigitation of GalCer slows down the lateral diffusion of raft lipids and affects membrane viscosity between the two membrane leaflets, augmenting the friction between the monolayers. In addition, interdigitation of GalCer alters the lateral pressure profile, which may lead to a change in membrane protein activation (Niemela et al. 2007; Ollila et al. 2007). GalCer has the ability to form multiple hydrogen bonds with surrounding molecules; however, it preferentially interacts with cholesterol molecules shielding them from direct contact with water (Hall et al. 2010). Similar to GalCer, GlcCer is also involved in the formation of highly ordered gel domains and increases the order of the membranous fluid phase (Varela et al. (2013). The presence of GlcCer promotes morphological alterations in lipid vesicles, which leads to the formation of flexible tubule-like structures extending from the lipid surface. However, despite tiny differences in the structures of their headgroups—stereochemical orientation of one hydroxyl group in the sugar residues—palmitoyl GalCer and palmitoyl GlcCer differ significantly in their domain forming behavior (Maunula et al. 2007). GalCer formed ordered domains which dissociated with increasing temperature, and GlcCer at the same concentration formed domains with a more cooperative dissociation behavior, but lower thermostability.
Cellular level
On the cellular level, GSLs have important functions in such cellular processes as adhesion and recognition, growth, differentiation and development, angiogenesis, inflammation and multidrug resistance in cancer cells (Hannun and Obeid 2018). Several lines of evidence suggest that GlcCer affects the proliferation potential of various cell types. Using a mouse model, it was shown that intraperitoneal injection of emulsified GlcCer and inhibition of glucosylceramidase resulted in enlargement of the liver (Datta and Radin 1988). Furthermore, the inhibition of GCS activity in renal epithelial cells decreased their proliferation rate (Shayman et al. 1991). GlcCer also affected the proliferation of Schwann cells (Yao and Yoshino 1994) and stimulated mitogenesis of murine epidermis (Marsh et al. 1995; Marchell et al. 1998) (see GlcCer and lysosomal GBA1 in Parkinson’s disease section). On the other hand, RNA interference experiments showed that the loss of GCS expression and therefore inhibited synthesis of GlcCer resulted in enhanced apoptosis of cells in the Drosophila melanogaster embryo (Kohyama-Koganeya et al. 2004). These proproliferative and antiapoptotic effects are exerted not directly by GCS and GlcCer themselves, but by affecting the intracellular pool of ceramides, as increased synthesis of GlcCer decreases the level of antiproliferative ceramide and decreased synthesis of GlcCer increases the level of proapoptotic ceramide (Kohyama-Koganeya et al. 2004; Ishibashi et al. 2013). Treatment of human keratinocytes with exogenous sphingomyelinase, which is known as a potent stress inducer, first caused increased production of ceramide and therefore decreased proliferation of cells and then increased synthesis of GlcCer, which was associated with restoration of cell proliferation (Uchida et al. 2002). In the case of NIH 3T3 cells, it was shown that inhibition of GCS activity by N-[2-hydoxy-1-(4-morpholinymethyl)-2-phenylethyl]-decanamide (PDMP) resulted in a decreased GlcCer level and an increase in ceramide levels, which was associated with arrest of the cell cycle at G1/S and G2/M transition and decrease in the activities of two cyclin-dependent kinases, p34cdc2 kinase and cdk2 kinase (Rani et al. 1995). Incubation of human neuroepithelial CHP-100 cells with C6-ceramide induced their apoptosis. This effect was significantly increased when cells were simultaneously treated with the same GCS inhibitor (Spinedi et al. 1998). It is now broadly accepted that ceramide is a key molecule involved in specific signaling pathways related to apoptotic and proliferative cellular responses to many stressors, including chemotherapeutics (Hannun and Obeid 2018; Ogretmen 2018) (see Monohexosylceramides and cancer section). However, the exact molecular mechanisms of how ceramides affect proliferation and apoptosis are unknown. It should be mentioned that the role of GCS and therefore GlcCer in the accumulation of the intercellular pool of ceramide was not confirmed by others, e.g., in Jurkat cells during apoptosis induced by CD95 (Tepper et al. 2000).
In the case of GalCer, it was found that expression of UGT8 and accumulation of GalCer in breast cancer cells increased their resistance to apoptosis induced by doxorubicin in vitro (Owczarek et al. 2013; Suchanski et al. 2018) (see Monohexosylceramides and cancer section).
Monohexosylcermides under physiological conditions
GalCer in myelin function and oligodendrocyte differentiation
As it was mentioned earlier, GalCer and its sulphated and sialylated analogues, 3-sulfo-GalCer (SM4) and 3-Neu5Ac-GalCer (GM4), respectively, are essential components of unique plasma membranes elaborated by oligodendrocytes (OLs) in the CNS and Schwann cells in the peripheral nervous system in the form of myelin sheaths (Norton and Cammer 1984; Jackman et al. 2009; Schnaar and Kinoshita 2017). Using knock-out mice, it was shown that these galactolipids play a role in (1) the formation of normal myelin in CNS, (2) development of normal axo-glial interactions at nodes of Ranvier and (3) promote axo-glial adhesion during myelinogenesis (Marcus and Popko 2002; Jackman et al. 2009). However, there are indications that GalCer and 3-sulfo-GalCer are not essential for the formation of myelin, but are necessary for the proper structure and stability of myelin, e.g., mice without GalCer wrap their axons in myelin sheathes that are enriched in GlcCer but non-functional due to altered structures at nodes of Ranvier (Boggs 2014). The molecular mechanisms that underlie these cellular phenomena are not fully understood. However, it has been proposed that the proper structure of multilayered myelin sheaths is dependent on the carbohydrate–carbohydrate interactions between GalCer and 3-sulfo-GalCer molecules forming domains localized on the apposed surfaces of these myelin sheaths (Coetzee et al. 1998; Boggs 2014). Furthermore, such GalCer/3-sulfo-GalCer-enriched microdomains form glycosynapses (Hakomori 2002), which are involved in signal transduction and loss of the cytoskeleton (Boggs 2014).
On the cellular level, GalCer and 3-sulfo-GalCer are also involved in terminal maturation of OLs. OL differentiation can be divided into several stages distinguished by the expression of specific cell surface markers and changes in morphology (Butts et al. 2008). In brief, the bipolar early oligodendrocyte progenitor cells (stage 1) differentiate into pro-OLs (stage 2), then, immature OLs (stage 3) and finally mature OLs (stage 4). Stage 3 immature OLs are characterized by the synthesis of GalCer and expression of 2′,3′-cyclic nucleotide 3′-phosphohydrolase and O4 marker. Interestingly, when GalCer and 3-sulfo-GalCer are synthesized and transported to plasma membrane, OL progenitors stop to proliferate and begin terminal differentiation (Bansal et al. 1999). It was shown that binding of Ranscht monoclonal antibody (R-mAb) to GalCer and 3-sulfo-GalCer present on the surface of OL progenitors inhibited their terminal differentiation, suggesting that both galactolipids are involved in the regulation of OL differentiation (Bansal and Pfeiffer 1989). However, in subsequent studies, using specific antibodies directed against 3-sulfo-GalCer or GalCer, it was found that rather 3-sulfo-GalCer, not GalCer, acted as the main inhibitory molecule in the regulation of oligodendrocyte terminal differentiation (Bansal et al. 1999). This was further confirmed using knock-out mice lacking cerebroside sulfotransferase (Hirahara et al. 2004). Using transgenic mice unable to express UGT8, Bansal et al. (1999) showed that the lack of GalCer and 3-sulfo-GalCer resulted in a 2- to 3-fold increase in the number of terminally differentiated oligodendrocytes. The authors proposed that both galactosphingolipids act as ligands for endogenous receptors generating inhibitory signals to block differentiation. However, other studies revealed that GalCer affects the differentiation of OLs indirectly as the constituent of lipid rafts present on ER membranes (Hayashi and Su 2004). Such GalCer/cholesterol microdomains are enriched in Sigma-1 receptors (Sig-1Rs) that are involved in lipid distribution in ER membranes and their transport to plasma membrane in neuronal cells (Hayashi and Su 2003a; Hayashi and Su 2003b). Moreover, it was found that in OL progenitors and myelin of developing rat brains, the amounts of lipid-raft-localized Sig-1Rs and GalCer increase as cells differentiate, and such structures remain present in myelin sheets of mature OLs (Hayashi and Su 2004). Importantly, the differentiation ability of OLs was positively affected by expression of Sig-1Rs. Altogether, these data suggest that GalCer rafts enriched with Sig-1Rs are important for OL differentiation. However, the question about the precise molecular mechanism by which GalCer affects OL differentiation remains open.
GlcCer and epidermal functions
GlcCer plays an important role in the biology of keratinocytes as well as the formation and maintenance of an epidermal permeability barrier. The outermost epidermal layer, called stratum corneum, consists of dead keratinocytes embedded with extracellular lipids such as cholesterol, ceramides and fatty acids (Elias et al. 1983; Long et al. 1985), which protect against excessive transepidermal water loss and eventual pathogen entry. These lipid components form an array of lamellar membranes derived from lamellar bodies after fusion of these organelles with the apical plasma membrane of the uppermost granular cells and subsequent release of their content into the intercellular spaces by exocytosis (Bouwstra et al. 2003; Madison 2003). The ceramides, essential components of lamellar membranes, are the result of GlcCer hydrolysis, which is stored in lamellar bodies (Holleran et al. 1993) (Figure 4). GlcCer represents only about 4% of all epidermal lipids but is one of the main components of lamellar membranes (Madison et al. 1986; Doering et al. 1999). Enzymatic hydrolysis of GlcCer takes place after the release of the contents of lamellar bodies into the intercellular domains during epidermal terminal differentiation (Gray and Yardley 1975; Elias 1981). Part of the GlcCer present in lamellar bodies is converted to acylglucosylceramide with ester-linked linoleic acid on the ω-oxygen function (Abraham et al. 1985; Hansen and Jensen 1985; Schoephoerster et al. 1985).
Fig. 4.
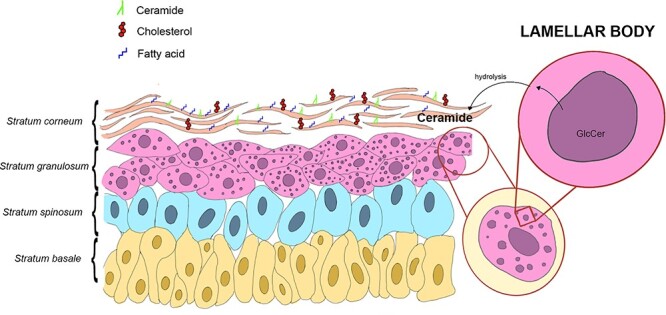
GlcCer and epidermal functions. Lamellar bodies after fusion with the apical plasma membrane of the uppermost granular cells release GlcCer into the intercellular spaces by exocytosis. There, GlcCer is hydrolyzed and the released ceramide becomes a part of stratum corneum consisting of dead keratinocytes embedded with extracellular lipids such as cholesterol, ceramides and fatty acids.
Jennemann et al. (2007) developed a mouse model with an epidermis-specific GCS knock-out, showing that the lack of GlcCer leads to a disrupted arrangement of the epidermal lamellar bodies, which results in excessive desquamation, uncontrolled transepidermal water loss and animal death on the fourth day after birth. Based on these results, the authors propose that the presence of GlcCer is crucial for the proper formation of lamellar bodies and subsequently ceramide-rich lamellar membranes. A recent study using a mouse model also showed that an oral intake of rice-derived GlcCer would prevent transepidermal water loss by accelerating GlcCer metabolism, which increases the amounts of ceramides in epidermis (Shimoda et al. 2012).
GlcCer and GalCer under pathological conditions
GlcCer and lysosomal GBA1 in GD
GD remains the most common lysosomal storage disorder (LSD). It is mainly caused by mutations in GBA1 gene coding for lysosomal GBA1. Generally, the mutations greatly decrease the activity of the enzyme; however, such enzymatic deficiency is not complete, as the total absence of GBA1 is lethal for humans and mice. In the visceral GD variant (type I), mutations lead to GlcCer accumulation in lysosomes of leukocytes, primarily macrophages and antigen presenting cells (APCs) in the spleen, liver, lungs and bone marrow. The GBA1-defective macrophages accumulate GlcCer as the result of ingestion of exogenous lipids from senescent or/and apoptotic erythrocytes, leukocytes and platelets (Kattlove et al. 1969). The exact mechanisms by which GlcCer accumulation leads to GD are not known. However, there are several hypotheses about the role of excessive amounts of GlcCer in the pathogenesis of this disease. Using cellular models of GD constructed by treatment of human fibroblasts or murine RAW macrophages with inhibitor of GBA1—conduritol B epoxide (CBE), it was found that an increase in the level of GlcCer induced altered lactosylceramide (Table I) trafficking from the plasma membrane to late endosomes and lysosomes, instead of to the Golgi apparatus (Sillence et al. 2002). These data suggest that accumulation of GlcCer disrupts proper lipid transport and sorting. According to the “jamming of the endosomal system” hypothesis, accumulation of GlcCer in late endosomal membranes prevents subsequent recruitment of cholesterol and other sphingolipids, which in turn leads to a jam in the membrane transport system (Simons and Gruenberg 2000). However, the exact molecular mechanisms of this phenomenon are not known. Similarly, Hein et al. (2007), using a macrophage model of GD, showed that intracellular GlcCer accumulation caused its (as well as other GSLs) increased localization not only in lysosomes but also in other cellular compartments. In plasma membrane, GlcCer and other GSLs accumulated primarily in lipid rafts. According to the authors, aberrant localization of GSLs negatively affects different biochemical pathways, leading to macrophage activation and enhanced pro- and antiinflammatory cytokine production. This agrees with clinical observations on the prevalence of inflammatory processes in tissues of Gaucher patients. Recently, Pandey et al. (2017), based on a mouse model of GD, proposed that the mechanism connecting the accumulation of GlcCer in visceral macrophages with chronic inflammation developed in target organs. According to them, the continuous release of GlcCer from macrophages causes differentiation of B lymphocytes into plasma cells producing anti-GlcCer autoantibodies forming GlcCer-anti-GlcCer IgG immune complexes, which activate the classical pathway of complement or lead to systemic generation of C5a by C5 cleavage. Activation of C5a receptor 1 (C5aR1) by C5a on dendritic cells (DCs) up-regulates expression of co-stimulatory molecules—CD80, CD86 and CD40, which in turn activate T cells producing proinflammatory cytokines: IFNγ, IL-17, TNF, IL-1β and IL-6. Interestingly, generated C5a binds and activates C5aR1 present also on macrophages, causing increased expression of GCS, which in combination with the GBA1 deficiency, promotes the accumulation of even higher amounts of GlcCer within macrophages. Such a mechanism further propels the autoimmune response against GlcCer, as more GlcCer is released, and therefore, more anti-GlcCer antibodies are produced and more GlcCer-anti-GlcCer IgG complexes are formed. This further activates a complement pathway and promotes inflammation and tissue damage observed in GD. It should be emphasized that this model is supported by clinical data. Elevated levels of GlcCer and other GSLs in plasma membrane of Gaucher-type macrophages also affect its biophysical properties (Batta et al. 2018). It was found that such plasma membranes are characterized by decreased fluidity, leading to the enlargement and fusion of raft-like domains, which in turn highly restrict the lateral mobility of non-raft lipids and proteins. These alterations significantly affected the biological properties of Gaucher-type macrophages, as evidenced by the inhibition of clathrin-dependent endocytosis and reduction in IFNγ induced STAT1 phosphorylation.
In a chemically induced model of neurological GD variant (types II and III), accumulation of GlcCer in neurons, as the result of GBA1 inhibition by CBE, makes them more sensitive to calcium-induced neurotoxicity due to increased calcium released via ryanodine receptors (RyaRs), whose activity is modulated by GlcCer (Korkotian et al. 1999; Lloyd-Evans et al. 2003). Since RyaRs are calcium channels localized in ER, the depletion of ER calcium stores makes cells susceptible to ER stress and induction of apoptosis (Kacher and Futerman 2006). Similar results were obtained with microsomes prepared from human brains of patients with type II GD (Lloyd-Evans et al. 2003). Using models of GD, it was also shown that increased amounts of GlcCer in neurons and brain tissue affected phospholipid metabolism, as GlcCer directly increased the activity of CTP:phosphocholine cytidylyltransferase. It was proposed that elevated synthesis of phosphatydylcholine affects the growth rate of neuronal cells, which is observed in GD (Bodennec et al. 2002). Similar changes in phosphatidylcholine metabolism were also observed in a chemically induced macrophage model of GD (Trajkovic-Bodennec et al. 2004). In types II and III of GD involving the CNS, accumulation of GlcCer in neuronal cells leads to the production of glucosylsphingosine (Grabowski 2012) (Figure 1). This neurotoxin induces apoptosis of neuronal cells, which in turn activates astroglial and microglial cells. As a result, the loss of neurons and subsequent neuroinflammatory effects are observed.
GlcCer and lysosomal GBA1 in Parkinson’s disease
Mutations in the GBA1 gene are one of the most common genetic risk factors for Parkinson’s disease (PD) suggesting a link between PD and GD (Sidransky et al. 2009; Belarbi et al. 2020). The involvement of lysosomal GBA1 in PD pathophysiology was further supported by the discovery that this enzyme was present in Lewy bodies from brains of PD patients carrying GBA1 mutations (Goker-Alpan et al. 2010). Lewy bodies, which contribute to PD, are protein aggregates composed mainly of α-synuclein (SNCA), a presynaptic protein regulating synaptic vesicle cycling (Spillantini et al. 1997; Taguchi et al. 2017). It was proposed that mutated GBA1 binds directly or indirectly to SNCA, which enhances aggregation of the latter or soluble SNCA oligomers trap misfolded mutant GBA1, and such complexes are converted to insoluble fibrils in Lewy bodies (Westbroek et al. 2011). Based on another model, it was suggested that misfolded mutated GBA1 does not localize in lysosomes but undergoes ubiquitination by parkin (E3 ubiquitin ligase) and subsequently ER-associated degradation. Occupation of parkin with mutated GBA1 blocks its interaction with natural substrates, which causes their accumulation to be detrimental to neuronal survival and induces apoptotic cell death (Ron et al. 2010; Westbroek et al. 2011). However, it was also shown that inhibition of GBA1 activity in neuroblastoma cells and mice subjected to treatment with CBE leads to elevated levels of SNCA and its accumulation within nigral cell bodies and astroglia (Manning-Bog et al. 2009). Recently, it was found that GlcCer and its metabolites (glucosylsphingosine, sphingosine and sphingosine-1-phosphate), which accumulate as the result of GBA1 gene mutation in PD, are able to accelerate aggregation and induce pathologic SNCA species in neurons and other human cells (Taguchi et al. 2017).
GalCer and lysosomal galactocerebrosidase in Krabbe disease
Krabbe disease also known as globoid cell leukodystrophy or galactosylceramide lipidosis is an autosomal recessive LSD involving the white matter of the peripheral and CNSs. This disease, characterized by the loss of myelin, is caused by mutations in the GALC gene, resulting in deficiency and/or decreased activity of lysosomal GALC (Wenger et al. 2000), which leads to accumulation of GalCer and galactosylsphingosine (psychosine) (Figure 1) in macrophages and neural cells, especially in oligodendrocytes and Schwann cells (Won et al. 2016). According to the “psychosine hypothesis,” galactosylsphingosine, not GalCer, is a highly cytotoxic compound directly linked to demyelination of the central and peripheral nervous systems. When there is a deficiency of GALC, large amounts of psychosine are produced by deacylation of accumulated GalCer by N-deacylase (Svennerholm et al. 1980; Kanazawa et al. 2000) and galactosylation of sphingosine by UGT8 (Cleland and Kennedy 1960). GalCer does not accumulate in the absence of GALC and therefore does not appear to be directly linked to demyelination processes, since in the absence of this enzyme, GalCer is degraded by GM1 (Table I) β-galactosidase (Won et al. 2016). Psychosine, because of its detergent-like properties, destabilizes cellular membranes, which leads to cell lysis, induces oxidative stress and mitochondrial damage, resulting in cell apoptosis, and on the cellular level causes inflammation, as well as vascular, neuronal and axonal dysfunction (Won et al. 2016).
GalCer in juvenile neuronal ceroid lipofuscinosis
Juvenile neuronal ceroid lipofuscinosis (JNCL) represents one of the genetic diseases known under the collective name of neuronal ceroid lipofuscinoses or Batten disease. It is one of the most common childhood neurodegenerative disorders. JNCL is caused by mutations in the CLN3 gene and is characterized by abnormal accumulation of lipopigments in lysosomes of neurons (Cotman and Staropoli 2012). The loss of function by the CLN3 gene is accompanied by massive neuronal death in the cerebrum and cerebellum. This confirms the proposal that the CLN3 protein (battenin) acts as an anti-apoptotic molecule, and the absence of which highly increases the level of the intracellular pool of pro-apoptotic ceramide (Puranam et al. 1999; El-Sitt et al. 2019).
CLN3 is a transmembrane protein characterized by the presence of a GalCer binding domain (Persaud-Sawin et al. 2004). Normal CLN3, localized in the Golgi apparatus and plasma membranes, takes part in anterograde transport of GalCer from the trans-Golgi to lipid rafts of plasma membrane involving early recycling endosomes (Persaud-Sawin et al. 2004; Cotman and Staropoli 2012). This proposal is supported by observations that mutations in the CLN3 gene found in JNCL patients affect the proper structure of a GalCer binding domain, which prevents mutant CLN3 protein from normal movement between these compartments and therefore prevent delivery of GalCer to plasma membrane, which is associated with accumulation of GalCer in ER and Golgi (Rusyn et al. 2008). The absence of CLN3 protein and GalCer affects the proper composition, structure and function of lipid rafts in the Golgi and plasma membranes, which in turn leads to deregulation of ceramide levels with an end effect of increased apoptosis (Persaud-Sawin et al. 2004).
Monohexosylceramides and cancer
The presence of GlcCer and GalCer and expression of UGCG and UGT8 genes in cancer tissues
There is a lack of detailed studies comparing the presence of GlcCer in cancer cells and human tumors with corresponding normal cells and tissues. However, more information is available on the expression of the UGCG gene in human cancers. GCS mRNA levels were significantly higher in tumors of the rectum, small intestine, cervix and breast than in corresponding normal tissues (Liu et al. 2011). GCS mRNA expression was significantly up-regulated in metastases of breast cancer in comparison to primary tumors, benign fibroadenoma and normal mammary tissue. Also, significantly higher expression of GCS mRNA was found in Stage III tumors than Stage I and II tumors and in node-positive tumors than in node-negative tumors (Lucci et al. 1998; Liu et al. 2011). Using immunohistochemistry, it was further shown that GCS protein levels in breast cancer tissue specimens and lymph node metastases were significantly higher than in normal tissues. Furthermore, GCS expression correlated positively with ER-positive and HER2-positive metastatic breast cancer, suggesting that GCS can be a potential marker of tumor aggressiveness. However, these data have not been fully confirmed by others. Ruckhaberle et al. (2009), using transcriptome profiling, showed that the expression level of the UGCG gene correlated positively with positive estrogen receptor (ER) status but was inversely associated with lower histological grading, low Ki67 levels and HER2-negativity. Using the same approach, it was shown that UGCG is one of several genes whose elevated expression was found in metastatic tumors of clear cell renal cell cancer in comparison to primary tumors (Jones et al. 2005).
There is little information available on GalCer expression in human tumors. In studies on molecular markers in human astrocytomas and oligodendrogliomas, it was found that high amounts of GalCer were present more frequently in oligodendrogliomas than in astrocytomas (Sung et al. 1996; Popko et al. 2002). Enhanced synthesis of GalCer was found in MDR human colon cancer HT29col cells derived from HT-29 G+ compared with parental HT-29 cells (Kok et al. 2000). However, more is known about expression of the UGT8 gene in cancer cells and tissues. Transcriptome profiling of prostate cancer cell lines showed that metastatic cells express much higher levels of UGT8 mRNA than non-metastatic cells (Oudes et al. 2005). It was also found that elevated expression of the UGT8 gene in breast cancer was significantly associated with ER-negativity, and therefore with a more malignant phenotype (Yang et al. 2006; Ruckhaberle et al. 2008). Furthermore, Landemaine et al. (2008) using DNA microarray analysis have shown that UGT8 is one of six genes whose elevated expression correlated with a significantly increased risk of lung metastases in breast cancer patients. These results were verified by PCR and immunohistochemical staining using antibodies against UGT8 protein. Expression of UGT8 is significantly higher in breast cancer metastases to the lung than in corresponding primary tumors and in primary tumors of UGT8 node-positive patients than in UGT8 node-negative patients (Dziegiel et al. 2010). Also, the amounts of this enzyme in cancerous tissue correlated with higher malignancy grades. These data suggest that UGT8 could be a significant index of breast tumor malignancy and a potential marker for the prognostic evaluation of lung metastases. This proposal is supported by studies of Cao et al. (2018). In agreement with these findings, it was also shown that UGT8 and GalCer are only present in malignant “mesenchymal-like” cell lines forming metastases in nude mice (Dziegiel et al. 2010).
The role of monohexosylceramides in tumor progression
Very little is known about the role of GlcCer and GalCer in tumor progression. Inhibition of GCS activity with specific imino sugar or suppression of UGCG gene expression using an antisense mRNA approach resulted in overall inhibition of ganglioside synthesis and importantly suppressed murine melanoma growth in vivo (Deng et al. 2002; Weiss et al. 2003), suggesting that GlcCer may be involved in proliferation of melanoma cells. However, based on these data, it is impossible to discriminate between the direct involvement of GlcCer in this process and its role as a precursor molecule for more complex gangliosides.
Studies on the role of GalCer in breast cancer progression suggest that this monohexosylceramide acts as an antiapoptotic molecule and its presence facilitates tumor cells to survive in the hostile microenvironment of tumors (Owczarek et al. 2013; Suchanski et al. 2018). In agreement with this hypothesis, the increased expression of UGT8 resulting in accumulation of GalCer was observed in Madin-Darby canine kidney cells in response to hyperosmotic and heat stresses (Niimura and Nagai 2008; Niimura et al. 2010). Ceramide is one of the key proapoptotic molecules (Bieberich 2004; Patwardhan et al. 2016; Ogretmen 2018), and it was originally suggested that the anti-apoptotic effects of GalCer are associated with decreased levels of ceramide as a result of increased synthesis of GalCer (Owczarek et al. 2013). However, further study revealed that cells with high or low levels of GalCer contain essentially the same amounts of ceramide, suggesting that in breast cancer cells, the key antiapoptotic molecule is GalCer itself (Suchanski et al. 2018).
The role of GlcCer and GalCer in multidrug-resistance of cancer cells
There is a lot of information available on the involvement of GlcCer in multidrug-resistance (MDR) of cancer cells. Studies on MDR breast, ovarian, colon and cervix epitheloid cancer cell lines as well as leukemic cell lines revealed that such cells are characterized by increased synthesis and accumulation of GlcCer (Lavie et al. 1996; Lavie et al. 1997; Nicholson et al. 1999; Kok et al. 2000; Morjani et al. 2001) and increased expression/activity of GCS (Liu et al. 2001). Furthermore, clinical studies demonstrated elevated levels of GlcCer in tumor specimens from breast cancer and melanoma patients who failed chemotherapy, but this marker was absent in patients who showed a clinical response (Lucci et al. 1998). In a gain-of-function cellular model, overexpression of the UGCG gene resulted in accumulation of GlcCer in MDR breast cancer MCF-7-AdrR cells, which made them even more resistant to doxorubicin, daunorubicin and actinomycin D (Liu et al. 2001). On the other hand, in loss-of-function cellular models, transfection of the same MCF-7-AdrR cells with antisense cDNA restored their sensitivity to anthracyclines, Vinca alkaloids, taxans and other anticancer drugs (Liu et al. 2000; Liu et al. 2001). In in vivo studies, such MCF-7-AdR cells with suppressed expression of the UGCG gene were characaterized by the siginificant loss of tumorigenicity and enhanced response to chemotherapy (Sun et al. 2010).
To explain increased resistance of GlcCer-overexpressing breast cancer cells to various anti-cancer drugs and TNF-α-induced apoptosis, it was proposed that increased glycosylation of ceramides by GCS, leading to accumulation of GlcCer, effectively decreases the intracellular pool of ceramides (Liu, Han, Giuliano, Cabot 1999; Liu, Han, Giuliano, Ichikawa, et al. 1999). Similar results were obtained in chemoresistant leukemia patients and MDR HL-60 cells, as the intracellular ceramide levels were clearly lower than in chemosensitive patients and drug-sensitive HL-60 cells, which was correlated with increased activity of GCS and sphingomyelin synthase (Itoh et al. 2003). On the other hand, inhibition of ceramide glycosylation by transfecting MDR cancer cells with GCS antisense cDNA (Liu et al. 2000) or antisense oligodeoxyribonuclotides (Liu et al. 2004) or using specific small molecule inhibitors of GCS or anticancer drugs increased the levels of ceramides, making cells more sensitive to ceramide- and drug-induced apoptosis (Lavie et al. 1997; Cabot et al. 1998; Spinedi et al. 1998; Maurer et al. 1999; Lucci, Giuliano, et al. 1999; Lucci, Han, et al. 1999; Olshefski and Ladisch 2001; Senchenkov et al. 2001). Interestingly, it was found that increased expression of the UGCG gene in cancer cells of breast, ovary, cervical and colon origin in response to doxorubicin treatment is dependent on the generation of ceramide (Liu et al. 2008). Therefore, it seems that ceramide is not only the substrate for GCS but also plays a role as its regulatory molecule, increasing expression of the UGCG gene (Abe et al. 1996; Komori et al. 2000). How the ceramide increases the expression of the UGCG gene is unknown; however, it was shown that signaling cascades leading to increased expression of the UGCG gene involve transcription factor Sp1 (Uchida et al. 2004; Liu et al. 2008). According to Liu et al. (2008), such transcriptional up-regulation of the UGCG gene by ceramides creates a vicious circle, since it further decreases the ceramide level and deepens the MDR phenotype of cancer cells.
However, it was also shown that overexpression of the UGCG gene in Jurkat cells did not affect the intracellular pool of ceramide (Tepper et al. 2000). Furthemore, in breast cancer MCF-7-AdrR cells, the inhibition of UGCG gene expression or inhibition of GCS activity by 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP) in MDR human cervical carcinoma KB-V0.01 cells not only had no effect on the level of intracellular ceramide but also highly decreased the expression of P-glycoprotein (P-gp, MDR1) (Gouaze et al. 2005; Sun et al. 2010), which is one of the major efflux pumps, removing chemotherapy drugs from cancer cells and inducing MDR phenotype (Bradley et al. 1988; Sikic et al. 1997). On the other hand, ceramide and GlcCer upregulated the expression of the MDR1 gene (Gouaze-Andersson et al. 2007), although the exact role of GlcCer in the regulation of MDR1 gene expression was not evaluated. Subsequent studies confirmed that silencing of the UGCG gene down-regulated P-gp expression and sensitized cultured cancer cells and experimental tumors to chemotherapy (Liu, Gupta, et al. 2010). It was proposed that GCS upregulates expression of the MDR1 gene indirectly by increasing the synthesis of globo-series GSLs, which, as components of plasma membrane, activate the cSrc and β-catenin signaling pathways, which in turn directly leads to increased expression of the MDR1 gene. On the other hand, there are some indications that P-glycoprotein is involved in the translocation of GlcCer from the cytosolic to the luminal side of the Golgi apparatus, which increases the possibilities for synthesis of LacCer and subsequently Gb3Cer (Table I). This resulted in the negative feedback control and therefore increased the activity of GCS, which enhances synthesis of GlcCer, in this way decreasing the intracellular ceramide pool (Lala et al. 2000; Shabbits and Mayer 2002; De Rosa et al. 2004). Based on these results and others (van den Heuvel-Eibrink et al. 2001), it was proposed that expression of P-gp protein increases resistance of acute myeloid leukemia cells to ceramide-induced apoptosis by increasing the activity of GCS, which in turn decreases the level of ceramide (Turzanski et al. 2005). However, the pharmacological inhibition of GCS in T-cell leukemia cell line CCRF-CEM had no effect on expression and function of P-gp (Olshefski and Ladisch 2001).
In breast cancer MCF-7-AdR cells with inhibited expression of the UGCG gene, not only decreased expression of P-gp but also increased activity of caspase-3 were observed, which suggested a link between the accumulation of GlcCer, MDR phenotype and increased resistance to apoptosis. An association between the accumulation of GlcCer and decreased sensitivity of cancer cells to drug-induced apoptosis has been shown by studies on drug-resistant and drug-sensitive human chronic myelogenous leukaemia K562 cells. It was revealed that inhibition of UGCG gene expression by siRNA or inhibition of GCS activity by PPMP in drug-resistant K562 correlated with down-regulation of key anti-apoptotic Bcl-2 protein and increased sensitivity to doxorubicin (Liu, Xie, et al. 2010).
In summary, it is now widely accepted that accumulation of GlcCer in cancer cells caused by overexpression of GCS attenuates the accumulation of ceramide and contributes to drug resistance in multidrug-resistant cancer cells (Ryland et al. 2011). However, despite the large amount of literature supporting the role of GCS and GlcCer in cancer cell MDR, there are some indications that their effect may be cell type and/or drug-specific (Segui et al. 2006). For example, in mouse melanoma cells, the presence of GCS did not alter their sensitivity to anticancer drugs (Veldman et al. 2003). It was also shown that inhibition of the UGCG gene in drug-sensitive U937 and HL-60 cells actually protects them from daunorubicin-induced apoptosis (Grazide et al. 2004). Interestingly, such treatment did not increase intracellular ceramide concentrations but instead increased GalCer levels. Furthermore, in cells enriched in exogenous GalCer, daunorubicin-induced apoptosis was significantly inhibited. Similarly, Krabbe cells with high levels of GalCer were more resistant to daunorubicin- and cytosine arabinoside-induced apoptosis than Gaucher cells with lower levels of GalCer. Therefore, these data pointed to the possible role of GalCer in MDR of cancer cells. Confirming these observations, it was found that GalCer increases the resistance of breast cancer cells to apoptosis induced by doxorubicin, suggesting that this monohexosylceramide may be involved in MDR of breast cancer cells (Owczarek et al. 2013), which supports previous findings that UGT8 expression is associated with poorer prognosis in breast tumors (Dziegiel et al. 2010). This hypothesis was supported by other studies. It was shown that GalCer expression is elevated in multidrug resistant colon cancer and ovarian cancer cells (Kok et al. 2000; Veldman et al. 2002). Also, Krabbe cells with high levels of GalCer were more resistant to daunorubicin- and cytosine arabinoside-induced apoptosis than Gaucher cells with lower levels of this monohexosylceramide (Grazide et al. 2004). Treatment of U937 and HL60 cells with inhibitors of GCS such as PDMP or PPMP protects them from-daunorubicin-induced apoptosis, which was accompanied by increased levels of GalCer (Grazide et al. 2004). In addition, cells enriched in exogenously added GalCer were significantly more resistant to apoptosis induced by this chemotherapeutic.
GlcCer as a self-antigen
Lipids, including GSLs, are able to induce cell-mediated immunity, when they are presented to the subset of T lymphocytes called Natural Killer T (NKT) cells, characterized by the expression of markers typical for T lymphocytes as well as NK cells. NKT cells are further divided into type I or invariant natural killer (iNKT) cells and type II or non-iNKT cells. iNKT cells recognize and bind lipid antigens and are characterized by a highly restricted repertoire of TCRs (Popovic et al. 2017). Lipid antigens are recognized and bound by iNKT TCRs only when they are presented by the CD1 molecules expressed by APCs (Salio et al. 2014; Kaczmarek et al. 2017). In humans, CD1 isoforms are divided into group I, represented by CD1a, CD1b, CD1c and CD1e and group II, represented by CD1d. Muroids express only CD1d (Barral and Brenner 2007), while ruminants express all CD1 proteins except CD1c (Van Rhijn et al. 2006; Thi et al. 2013). In chicken, two CD1 genes identified so far are not similar to any mammalian genes (Salomonsen et al. 2005). As one CD1 molecule interacts with different GSLs, it is suggested that binding involved ceramide, not carbohydrate moiety. This hypothesis was directly confirmed by crystallographic data (Zajonc et al. 2003; Koch et al. 2005). Each CD1 binds a defined set of GSLs, has tissue-specific expression and presents antigens T cells with a specific repertoire of TCRs (Salio et al. 2014). It was initially thought that iNKT cells are primarily activated by exogenous glycolipid antigens with a primary α-linked monohexose such as α-galactosylceramide from Agelas mauritanus or bacterial glycolipids, e.g., from Borrelia burgdorferi and Sphingomonas (Kinjo and Kronenberg 2005; Kinjo et al. 2006), and therefore play an important role in microbial immunity (Cohen et al. 2009). However, it should be remembered that primary α-glycosidic linkages are not present in most microbes or in mammalian glycolipids, and importantly, iNKT cells were activated in situations where no foreign glycolipid antigens were present, such as auto-inflammation, virus infection or activation by Toll-like receptors (TLRs). Therefore, based on the data obtained from an experimental model consisting of iNKT cells activated by APCs exposed to lipopolysaccharides (LPS) or other TLR agonists, it was proposed that activation of iNKT cells requires two signals (Brigl et al. 2003). The first signal includes binding the CD1d-lipid complex by TCR. The second signal is delivered by cytokines (mainly IL-12) secreted by APCs. Subsequent studies revealed that this lipid antigen, which specifically activates iNKT cells, is most probably GlcCer, acting as a self-antigen (Brennan et al. 2011). This proposal was further supported by the following findings: (1) GlcCer accumulated in APCs in response to LPS or bacterial infection and (2) inhibition of GlcCer (but not gangliosides or LacCer) synthesis in human bone marrow-derived DCs reduced autoreactivity and iNKT cell activation after LPS addition. Recently, based on results from a mouse model, it was also proposed that GlcCer may induce an immune response acting as a self-antigen in GD (see GlcCer and lysosomal GBA1 in GD section).
Regarding GalCer, there is still a lack of evidence that β-GalCer is able to provoke an immune reaction. So far, only the α-GalCer has been shown to be an activator of NKT cells and a mediator of immune response in some cancer or virus infections (Ko et al. 2005). However, it is important to remember that α-GalCer is not produced by mammals.
GalCer as a receptor for viruses and bacteria
Even though the major HIV-1 cell receptor present on the surface of T lymphocytes is a CD4 molecule (Bour et al. 1996), CD4-negative brain-, muscle- and intestinal-derived cells and fibroblastoid cells are sensitive to virus infection (Adachi et al. 1987; Chiodi et al. 1987; Dewhurst et al. 1987; Clapham et al. 1989; Tateno et al. 1989; Fantini et al. 1991). These findings indicated that there are alternative ways for the virus to enter the cells, possibly involving another receptor (Harouse et al. 1989). Therefore, using a variety of antibodies directed against surface antigens, it was found that anti-GalCer antibodies specifically inhibited infection of CD4-negative U373-MG and SK-N-MC cells (Harouse et al. 1991). The sensitivity to HIV-1 infection was also associated with GalCer expression in human colon cancer HT-29 and Caco-2 cells (Fantini et al. 1993). Furthermore, it was shown that HIV-1 gp120 binds to mono- and polyhydroxylated forms of GalCer but not to GalCer molecules with nonhydroxylated fatty acids (Bhat et al. 1991; Fantini et al. 1993). In HT-29 cells, binding of gp120 to GalCer localized in lipid rafts caused the release of intracellular calcium and depolymerization of microtubules (Fantini et al. 2000). These results may explain why HIV-1 can affect the absorptive and secretory functions of intestinal cells (Fantini et al. 1992; Asmuth et al. 1994). It was shown that binding of HIV-1 to GalCer is mediated by the V3 loop of HIV-1surface envelope glycoprotein gp120 (Cook et al. 1994; Yahi et al. 1995), which represents common GalCer-binding domain/structural motif present in Alzheimer β-amyloid peptide and human PrP protein (Mahfoud et al. 2002), and earlier described CLN3 protein (see GalCer in juvenile neuronal ceroid lipofuscinosis section).
GalCer is one of several GSLs-3-sulfo-GalCer, GM3 and LacCer (Table I)—receptors for Helicobacter pylori (Saitoh et al. 1991), a bacterium responsible for gastritis and duodenal ulcers.
Concluding remarks
Numerous studies unequivocally demonstrated that GlcCer and GalCer contribute to many physiological and pathological phenomena. However, in many cases, more information is available on abnormal expression and/or activities of enzymes responsible for their synthesis and/or degradation than on the involvement of these monohexosylcermides themselves. A lot of information about their biological activities is purely descriptive, meaning that in the future, the major challenge will be to carry out mechanistic studies, where the functions will be examined carefully on molecular and biological levels simultaneously. Since it is generally accepted that GSLs directly affect the functions of membrane proteins and/or regulate the expression of specific genes, the identification of specific signaling pathways and partner molecule/molecules interacting directly with GlcCer and GalCer or their metabolites as well as target genes will be required. It can be done, only by multidisciplinary research, using not only modern genetic, cell biology and biochemistry methods but also biophysical techniques. Considering the complexity of sphingolipid metabolism, such studies must be supported by the analysis of whole sphingolipidome. The elucidation of the mechanisms by which monohexosylceramides exert their biological functions is necessary in order to design effective therapeutic strategies and develop new agents with GalCer and GlcCer and their enzymes as molecular targets to treat important human diseases described in this review. For example, there are numerous reports describing GlcCer as a target for anticancer therapy to decrease the multidrug resistance of cancer cells; however, because of the lack of detailed knowledge on the action of GlcCer on the molecular level, its usefulness is questionable.
Funding
Grant support for this work was provided by the National Science Center (Poland): grant no. 2019/35/B/NZ5/01392. The publication is financed/co-financed under the Leading Research Groups support project from the subsidy increased for the period 2020–2025 in the amount of 2% of the subsidy referred to Art. 387 (3) of the Law of 20 July 2018 on Higher Education and Science, obtained in 2019.
Contributor Information
Safoura Reza, Department of Biochemistry and Molecular Biology, Wroclaw University of Environmental and Life Sciences, C.K. Norwida 31, Wroclaw 50-375, Poland.
Maciej Ugorski, Department of Biochemistry and Molecular Biology, Wroclaw University of Environmental and Life Sciences, C.K. Norwida 31, Wroclaw 50-375, Poland.
Jarosław Suchański, Department of Biochemistry and Molecular Biology, Wroclaw University of Environmental and Life Sciences, C.K. Norwida 31, Wroclaw 50-375, Poland.
Conflict of interest statement
None declared.
References
- Abe A, Radin NS, Shayman JA. 1996. Induction of glucosylceramide synthase by synthase inhibitors and ceramide. Biochim Biophys Acta. 1299(3):333–341. [DOI] [PubMed] [Google Scholar]
- Abraham W, Wertz PW, Downing DT. 1985. Linoleate-rich acylglucosylceramides of pig epidermis: Structure determination by proton magnetic resonance. J Lipid Res. 26(6):761–766. [PubMed] [Google Scholar]
- Adachi A, Koenig S, Gendelman HE, Daugherty D, Gattoni-Celli S, Fauci AS, Martin MA. 1987. Productive, persistent infection of human colorectal cell lines with human immunodeficiency virus. J Virol. 61(1):209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht S, Vainauskas S, Stockmann H, McManus C, Taron CH, Rudd PM. 2016. Comprehensive profiling of glycosphingolipid glycans using a novel broad specificity endoglycoceramidase in a high-throughput workflow. Anal Chem. 88(9):4795–4802. [DOI] [PubMed] [Google Scholar]
- Ariga T, Ando S, Takahashi A, Miyatake T. 1980. Gangliosides and neutral glycolipids of human adrenal medulla. Biochim Biophys Acta. 618(3):480–485. [PubMed] [Google Scholar]
- Asmuth DM, Hammer SM, Wanke CA. 1994. Physiological effects of HIV infection on human intestinal epithelial cells: An in vitro model for HIV enteropathy. AIDS. 8(2):205–211. [DOI] [PubMed] [Google Scholar]
- Astudillo L, Therville N, Colacios C, Segui B, Andrieu-Abadie N, Levade T. 2016. Glucosylceramidases and malignancies in mammals. Biochimie. 125:267–280. [DOI] [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE. 1989. Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proc Natl Acad Sci USA. 86(16):6181–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Winkler S, Bheddah S. 1999. Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J Neurosci. 19(18):7913–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral DC, Brenner MB. 2007. CD1 antigen presentation: How it works. Nat Rev Immunol. 7(12):929–941. [DOI] [PubMed] [Google Scholar]
- Basu S, Kaufman B, Roseman S. 1968. Enzymatic synthesis of ceramide-glucose and ceramide-lactose by glycosyltransferases from embryonic chicken brain. J Biol Chem. 243(21):5802–5804. [PubMed] [Google Scholar]
- Batta G, Soltesz L, Kovacs T, Bozo T, Meszar Z, Kellermayer M, Szollosi J, Nagy P. 2018. Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease. Sci Rep. 8(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier UH, Gorogh T. 2005. Implications of galactocerebrosidase and galactosylcerebroside metabolism in cancer cells. Int J Cancer. 115(1):6–10. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Cuvelier E, Bonte MA, Desplanque M, Gressier B, Devos D, Chartier-Harlin MC. 2020. Glycosphingolipids and neuroinflammation in Parkinson's disease. Mol Neurodegener. 15(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S, Spitalnik SL, Gonzalez-Scarano F, Silberberg DH. 1991. Galactosyl ceramide or a derivative is an essential component of the neural receptor for human immunodeficiency virus type 1 envelope glycoprotein gp120. Proc Natl Acad Sci USA. 88(16):7131–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich E. 2004. Integration of glycosphingolipid metabolism and cell-fate decisions in cancer and stem cells: Review and hypothesis. Glycoconj J. 21(6):315–327. [DOI] [PubMed] [Google Scholar]
- Bodennec J, Pelled D, Riebeling C, Trajkovic S, Futerman AH. 2002. Phosphatidylcholine synthesis is elevated in neuronal models of Gaucher disease due to direct activation of CTP:phosphocholine cytidylyltransferase by glucosylceramide. FASEB J. 16(13):1814–1816. [DOI] [PubMed] [Google Scholar]
- Boggs JM. 2014. Role of galactosylceramide and sulfatide in oligodendrocytes and CNS myelin: Formation of a glycosynapse. Adv Neurobiol. 9:263–291. [DOI] [PubMed] [Google Scholar]
- Bouhours JF, Bouhours D. 1979. Galactosylceramide is the major cerebroside of human milk fat globule membrane. Biochem Biophys Res Commun. 88(4):1217–1222. [DOI] [PubMed] [Google Scholar]
- Bour S, Schubert U, Peden K, Strebel K. 1996. The envelope glycoprotein of human immunodeficiency virus type 2 enhances viral particle release: A Vpu-like factor? J Virol. 70(2):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M. 2003. Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res. 42(1):1–36. [DOI] [PubMed] [Google Scholar]
- Bradley G, Juranka PF, Ling V. 1988. Mechanism of multidrug resistance. Biochim Biophys Acta. 948(1):87–128. [DOI] [PubMed] [Google Scholar]
- Breimer ME, Hansson GC, Karlsson KA, Larson G, Leffler H. 2012. Glycosphingolipid composition of epithelial cells isolated along the villus axis of small intestine of a single human individual. Glycobiology. 22(12):1721–1730. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. 2011. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 12(12):1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. 2003. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 4(12):1230–1237. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68(3):533–544. [DOI] [PubMed] [Google Scholar]
- Butts BD, Houde C, Mehmet H. 2008. Maturation-dependent sensitivity of oligodendrocyte lineage cells to apoptosis: Implications for normal development and disease. Cell Death Differ. 15(7):1178–1186. [DOI] [PubMed] [Google Scholar]
- Cabot MC, Han TY, Giuliano AE. 1998. The multidrug resistance modulator SDZ PSC 833 is a potent activator of cellular ceramide formation. FEBS Lett. 431(2):185–188. [DOI] [PubMed] [Google Scholar]
- Cao Q, Chen X, Wu X, Liao R, Huang P, Tan Y, Wang L, Ren G, Huang J, Dong C. 2018. Inhibition of UGT8 suppresses basal-like breast cancer progression by attenuating sulfatide-alphaVbeta5 axis. J Exp Med. 215(6):1679–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi F, Fuerstenberg S, Gidlund M, Asjo B, Fenyo EM. 1987. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 61(4):1244–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham PR, Weber JN, Whitby D, McIntosh K, Dalgleish AG, Maddon PJ, Deen KC, Sweet RW, Weiss RA. 1989. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 337:368–370. [DOI] [PubMed] [Google Scholar]
- Cleland WW, Kennedy EP. 1960. The enzymatic synthesis of psychosine. J Biol Chem. 235(1):45–51. [PubMed] [Google Scholar]
- Coetzee T, Dupree JL, Popko B. 1998. Demyelination and altered expression of myelin-associated glycoprotein isoforms in the central nervous system of galactolipid-deficient mice. J Neurosci Res. 54(5):613–622. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Garg S, Brenner MB. 2009. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 102:1–94. [DOI] [PubMed] [Google Scholar]
- Cook DG, Fantini J, Spitalnik SL, Gonzalez-Scarano F. 1994. Binding of human immunodeficiency virus type I (HIV-1) gp120 to galactosylceramide (GalCer): Relationship to the V3 loop. Virology. 201(2):206–214. [DOI] [PubMed] [Google Scholar]
- Corradini C, Lantano C, Cavazza A. 2013. Innovative analytical tools to characterize prebiotic carbohydrates of functional food interest. Anal Bioanal Chem. 405(13):4591–4605. [DOI] [PubMed] [Google Scholar]
- Costantino-Ceccarini E, Morell P. 1973. Synthesis of galactosylceramide and glucosylceramide by mouse kidney preparations. J Biol Chem. 248(23):8240–8246. [PubMed] [Google Scholar]
- Coste H, Martel MB, Got R. 1986. Topology of glucosylceramide synthesis in Golgi membranes from porcine submaxillary glands. Biochim Biophys Acta. 858(1):6–12. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Staropoli JF. 2012. The juvenile Batten disease protein, CLN3, and its role in regulating anterograde and retrograde post-Golgi trafficking. Clin Lipidol. 7(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo G, Capasso S, Sticco L, Russo D. 2013. Glycosphingolipids: Synthesis and functions. FEBS J. 280(24):6338–6353. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, et al. 2007. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 449(7158):62–67. [DOI] [PubMed] [Google Scholar]
- Datta SC, Radin NS. 1988. Normalization of liver glucosylceramide levels in the "Gaucher" mouse by phosphatidylserine injection. Biochem Biophys Res Commun. 152(1):155–160. [DOI] [PubMed] [Google Scholar]
- De Rosa MF, Sillence D, Ackerley C, Lingwood C. 2004. Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis. J Biol Chem. 279(9):7867–7876. [DOI] [PubMed] [Google Scholar]
- Deng W, Li R, Guerrera M, Liu Y, Ladisch S. 2002. Transfection of glucosylceramide synthase antisense inhibits mouse melanoma formation. Glycobiology. 12(3):145–152. [DOI] [PubMed] [Google Scholar]
- Dewhurst S, Stevenson M, Volsky DJ. 1987. Expression of the T4 molecule (AIDS virus receptor) by human brain-derived cells. FEBS Lett. 213(1):133–137. [DOI] [PubMed] [Google Scholar]
- Doering T, Holleran WM, Potratz A, Vielhaber G, Elias PM, Suzuki K, Sandhoff K. 1999. Sphingolipid activator proteins are required for epidermal permeability barrier formation. J Biol Chem. 274(16):11038–11045. [DOI] [PubMed] [Google Scholar]
- Dziegiel P, Owczarek T, Plazuk E, Gomulkiewicz A, Majchrzak M, Podhorska-Okolow M, Driouch K, Lidereau R, Ugorski M. 2010. Ceramide galactosyltransferase (UGT8) is a molecular marker of breast cancer malignancy and lung metastases. Br J Cancer. 103(4):524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sitt S, Soueid J, Maalouf K, Makhoul N, Al Ali J, Makoukji J, Asser B, Daou D, Harati H, Boustany RM. 2019. Exogenous galactosylceramide as potential treatment for CLN3 disease. Ann Neurol. 86(5):729–742. [DOI] [PubMed] [Google Scholar]
- Elias PM. 1981. Lipids and the epidermal permeability barrier. Arch Dermatol Res. 270(1):95–117. [DOI] [PubMed] [Google Scholar]
- Elias PM, Chung JC, Orozco-Topete R, Nemanic MK. 1983. Membrane glycoconjugate visualization and biosynthesis in normal and retinoid-treated epidermis. J Invest Dermatol. 81(1 Suppl):81s–85s. [DOI] [PubMed] [Google Scholar]
- Fabbro D, Grabowski GA. 1991. Human acid beta-glucosidase. Use of inhibitory and activating monoclonal antibodies to investigate the enzyme's catalytic mechanism and saposin A and C binding sites. J Biol Chem. 266(23):15021–15027. [PubMed] [Google Scholar]
- Fantini J, Baghdiguian S, Yahi N, Chermann JC. 1991. Selected human immunodeficiency virus replicates preferentially through the basolateral surface of differentiated human colon epithelial cells. Virology. 185(2):904–907. [DOI] [PubMed] [Google Scholar]
- Fantini J, Cook DG, Nathanson N, Spitalnik SL, Gonzalez-Scarano F. 1993. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc Natl Acad Sci USA. 90(7):2700–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J, Maresca M, Hammache D, Yahi N, Delezay O. 2000. Glycosphingolipid (GSL) microdomains as attachment platforms for host pathogens and their toxins on intestinal epithelial cells: Activation of signal transduction pathways and perturbations of intestinal absorption and secretion. Glycoconj J. 17(3/4):173–179. [DOI] [PubMed] [Google Scholar]
- Fantini J, Yahi N, Baghdiguian S, Chermann JC. 1992. Human colon epithelial cells productively infected with human immunodeficiency virus show impaired differentiation and altered secretion. J Virol. 66(1):580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Pagano RE. 1991. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 280(Pt 2):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Sweet L, Wang BH, Shihabuddin LS, Sardi SP, Schapira AHV. 2015. No evidence for substrate accumulation in Parkinson brains with GBA mutations. Mov Disord. 30(8):1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. 2010. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 120(5):641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaze-Andersson V, Yu JY, Kreitenberg AJ, Bielawska A, Giuliano AE, Cabot MC. 2007. Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim Biophys Acta. 1771(12):1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. 2005. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 65(9):3861–3867. [DOI] [PubMed] [Google Scholar]
- Grabowski GA. 2012. Gaucher disease and other storage disorders. Hematology Am Soc Hematol Educ Program. 2012(1):13–18. [DOI] [PubMed] [Google Scholar]
- Gray GM, Yardley HJ. 1975. Different populations of pig epidermal cells: Isolation and lipid composition. J Lipid Res. 16(6):441–447. [PubMed] [Google Scholar]
- Grazide S, Terrisse AD, Lerouge S, Laurent G, Jaffrezou JP. 2004. Cytoprotective effect of glucosylceramide synthase inhibition against daunorubicin-induced apoptosis in human leukemic cell lines. J Biol Chem. 279(18):18256–18261. [DOI] [PubMed] [Google Scholar]
- Hakomori SI. 2002. The glycosynapse. Proc Natl Acad Sci USA. 99(1):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Rog T, Karttunen M, Vattulainen I. 2010. Role of glycolipids in lipid rafts: A view through atomistic molecular dynamics simulations with galactosylceramide. J Phys Chem B. 114(23):7797–7807. [DOI] [PubMed] [Google Scholar]
- Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Maziere AM, Vieira OV, Mattjus P, Klumperman J, van Meer G, Sprong H. 2007. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol. 179(1):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamler R, Brignol N, Clark SW, Morrison S, Dungan LB, Chang HH, Khanna R, Frascella M, Valenzano KJ, Benjamin ER, et al. 2017. Glucosylceramide and glucosylsphingosine quantitation by liquid chromatography-tandem mass spectrometry to enable in vivo preclinical studies of neuronopathic Gaucher disease. Anal Chem. 89(16):8288–8295. [DOI] [PubMed] [Google Scholar]
- Han XL, Cheng H. 2005. Characterization and direct quantitation of cerebroside molecular species from lipid extracts by shotgun lipidomics. J Lipid Res. 46(1):163–175. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. 2018. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 19(3):175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HS, Jensen B. 1985. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and alpha-linolenate. Biochim Biophys Acta. 834(3):357–363. [DOI] [PubMed] [Google Scholar]
- Harouse JM, Bhat S, Spitalnik SL, Laughlin M, Stefano K, Silberberg DH, Gonzalez-Scarano F. 1991. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 253(5017):320–323. [DOI] [PubMed] [Google Scholar]
- Harouse JM, Kunsch C, Hartle HT, Laughlin MA, Hoxie JA, Wigdahl B, Gonzalez-Scarano F. 1989. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 63(6):2527–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzer K, Paton BC, Christomanou H, Chatelut M, Levade T, Hiraiwa M, O'Brien JS. 1997. Saposins (sap) A and C activate the degradation of galactosylceramide in living cells. FEBS Lett. 417(3):270–274. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. 2003a. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 306(2):726–733. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. 2003b. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: Roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther. 306(2):718–725. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. 2004. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci USA. 101(41):14949–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein LK, Meikle PJ, Hopwood JJ, Fuller M. 2007. Secondary sphingolipid accumulation in a macrophage model of Gaucher disease. Mol Genet Metab. 92(4):336–345. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y. 2012. A world of sphingolipids and glycolipids in the brain-novel functions of simple lipids modified with glucose. P Jpn Acad B-Phys. 88(4):129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara Y, Bansal R, Honke K, Ikenaka K, Wada Y. 2004. Sulfatide is a negative regulator of oligodendrocyte differentiation: Development in sulfatide-null mice. Glia. 45(3):269–277. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Takagi Y, Menon GK, Legler G, Feingold KR, Elias PM. 1993. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J Clin Invest. 91(4):1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Sakiyama H, Suzuki G, Hidari KI, Hirabayashi Y. 1996a. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc Natl Acad Sci USA. 93:12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Sakiyama H, Suzuki G, Hidari KI, Hirabayashi Y. 1996b. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc Natl Acad Sci USA. 93(10):4638–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y, Kohyama-Koganeya A, Hirabayashi Y. 2013. New insights on glucosylated lipids: Metabolism and functions. BBA-Mol Cell Biol L. 1831(9):1475–1485. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kitano T, Watanabe M, Kondo T, Yabu T, Taguchi Y, Iwai K, Tashima M, Uchiyama T, Okazaki T. 2003. Possible role of ceramide as an indicator of chemoresistance: Decrease of the ceramide content via activation of glucosylceramide synthase and sphingomyelin synthase in chemoresistant leukemia. Clin Cancer Res. 9(1):415–423. [PubMed] [Google Scholar]
- Jackman N, Ishii A, Bansal R. 2009. Oligodendrocyte development and myelin biogenesis: Parsing out the roles of glycosphingolipids. Physiology (Bethesda). 24:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel D, Karrenbauer A, Burger KN, van Meer G, Wieland F. 1992. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J Cell Biol. 117(2):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennemann R, Sandhoff R, Langbein L, Kaden S, Rothermel U, Gallala H, Sandhoff K, Wiegandt H, Grone HJ. 2007. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J Biol Chem. 282(5):3083–3094. [DOI] [PubMed] [Google Scholar]
- Jones J, Otu H, Spentzos D, Kolia S, Inan M, Beecken WD, Fellbaum C, Gu X, Joseph M, Pantuck AJ, et al. 2005. Gene signatures of progression and metastasis in renal cell cancer. Clin Cancer Res. 11(16):5730–5739. [DOI] [PubMed] [Google Scholar]
- Kacher Y, Futerman AH. 2006. Genetic diseases of sphingolipid metabolism: Pathological mechanisms and therapeutic options. FEBS Lett. 580(23):5510–5517. [DOI] [PubMed] [Google Scholar]
- Kaczmarek R, Pasciak M, Szymczak-Kulus K, Czerwinski M. 2017. CD1: A singed cat of the three antigen presentation systems. Arch Immunol Ther Ex. 65(3):201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T, Nakamura S, Momoi M, Yamaji T, Takematsu H, Yano H, Sabe H, Yamamoto A, Kawasaki T, Kozutsumi Y. 2000. Inhibition of cytokinesis by a lipid metabolite, psychosine. J Cell Biol. 149(4):943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Zhan X, Ye J, Han L, Qiu W, Gu X, Zhang H. 2018. A rare form of Gaucher disease resulting from saposin C deficiency. Blood Cells Mol Dis. 68:60–65. [DOI] [PubMed] [Google Scholar]
- Kapitonov D, Yu RK. 1997. Cloning, characterization, and expression of human ceramide galactosyltransferase cDNA. Biochem Bioph Res Co. 232(2):449–453. [DOI] [PubMed] [Google Scholar]
- Kattlove HE, Williams JC, Gaynor E, Spivack M, Bradley RM, Brady RO. 1969. Gaucher cells in chronic myelocytic leukemia: An acquired abnormality. Blood. 33(2):379–390. [PubMed] [Google Scholar]
- Kean EL. 1966. Separation of Gluco- and Galactocerebrosides by Means of Borate Thin-Layer Chromatography. J Lipid Res. 7(3):449–452. [PubMed] [Google Scholar]
- Khiste SK, Hosain SB, Dong YX, Uddin MB, Roy KR, Hill RA, Liu ZJ, Liu YY. 2017. Incorporation of fluorescence ceramide-based HPLC assay for rapidly and efficiently assessing glucosylceramide synthase in vivo. Sci Rep. 7:2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo Y, Kronenberg M. 2005. V alpha14 i NKT cells are innate lymphocytes that participate in the immune response to diverse microbes. J Clin Immunol. 25(6):522–533. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. 2006. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 7(9):978–986. [DOI] [PubMed] [Google Scholar]
- Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. 2005. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 175(5):3309–3317. [DOI] [PubMed] [Google Scholar]
- Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, et al. 2005. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol. 6(8):819–826. [DOI] [PubMed] [Google Scholar]
- Kohyama-Koganeya A, Sasamura T, Oshima E, Suzuki E, Nishihara S, Ueda R, Hirabayashi Y. 2004. Drosophila glucosylceramide synthase: A negative regulator of cell death mediated by proapoptotic factors. J Biol Chem. 279(34):35995–36002. [DOI] [PubMed] [Google Scholar]
- Kok JW, Babia T, Klappe K, Hoekstra D. 1995. Fluorescent, short-chain C-6-Nbd-Sphingomyelin, but not C-6-Nbd-Glucosylceramide, is subject to extensive degradation in the plasma-membrane—Implications for signal-transduction related to cell-differentiation. Biochem J. 309(3):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok JW, Veldman RJ, Klappe K, Koning H, Filipeanu CM, Muller M. 2000. Differential expression of sphingolipids in MRP1 overexpressing HT29 cells. Int J Cancer. 87(2):172–178. [DOI] [PubMed] [Google Scholar]
- Kolter T, Sandhoff K. 2010. Lysosomal degradation of membrane lipids. FEBS Lett. 584(9):1700–1712. [DOI] [PubMed] [Google Scholar]
- Komori H, Ichikawa S, Hirabayashi Y, Ito M. 2000. Regulation of UDP-glucose:ceramide glucosyltransferase-1 by ceramide. FEBS Lett. 475(3):247–250. [DOI] [PubMed] [Google Scholar]
- Korkotian E, Schwarz A, Pelled D, Schwarzmann G, Segal M, Futerman AH. 1999. Elevation of intracellular glucosylceramide levels results in an increase in endoplasmic reticulum density and in functional calcium stores in cultured neurons. J Biol Chem. 274(31):21673–21678. [DOI] [PubMed] [Google Scholar]
- Lala P, Ito S, Lingwood CA. 2000. Retroviral transfection of Madin-Darby canine kidney cells with human MDR1 results in a major increase in globotriaosylceramide and 10(5)- to 10(6)-fold increased cell sensitivity to verocytotoxin. Role of p-glycoprotein in glycolipid synthesis. J Biol Chem. 275(9):6246–6251. [DOI] [PubMed] [Google Scholar]
- Landemaine T, Jackson A, Bellahcene A, Rucci N, Sin S, Abad BM, Sierra A, Boudinet A, Guinebretiere JM, Ricevuto E, et al. 2008. A six-gene signature predicting breast cancer lung metastasis. Cancer Res. 68(15):6092–6099. [DOI] [PubMed] [Google Scholar]
- Lavie Y, Cao H, Bursten SL, Giuliano AE, Cabot MC. 1996. Accumulation of glucosylceramides in multidrug-resistant cancer cells. J Biol Chem. 271(32):19530–19536. [DOI] [PubMed] [Google Scholar]
- Lavie Y, Cao H, Volner A, Lucci A, Han TY, Geffen V, Giuliano AE, Cabot MC. 1997. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem. 272(3):1682–1687. [DOI] [PubMed] [Google Scholar]
- Levy M, Futerman AH. 2010. Mammalian ceramide synthases. IUBMB Life. 62(5):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xie KM, Yang GQ, Bai XM, Shi YP, Mu HJ, Qiao WZ, Zhang B, Xie P. 2010. GCS induces multidrug resistance by regulating apoptosis-related genes in K562/AO2 cell line. Cancer Chemother Pharmacol. 66(3):433–439. [DOI] [PubMed] [Google Scholar]
- Liu YB, Chan KFJ. 1991. High-performance capillary electrophoresis of gangliosides. Electrophoresis. 12(6):402–408. [DOI] [PubMed] [Google Scholar]
- Liu YY, Gupta V, Patwardhan GA, Bhinge K, Zhao Y, Bao J, Mehendale H, Cabot MC, Li YT, Jazwinski SM. 2010. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol Cancer. 9(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Han TY, Giuliano AE, Cabot MC. 1999. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J Biol Chem. 274(2):1140–1146. [DOI] [PubMed] [Google Scholar]
- Liu YY, Han TY, Giuliano AE, Cabot MC. 2001. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 15(3):719–730. [DOI] [PubMed] [Google Scholar]
- Liu YY, Han TY, Giuliano AE, Hansen N, Cabot MC. 2000. Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance. J Biol Chem. 275(10):7138–7143. [DOI] [PubMed] [Google Scholar]
- Liu YY, Han TY, Giuliano AE, Ichikawa S, Hirabayashi Y, Cabot MC. 1999. Glycosylation of ceramide potentiates cellular resistance to tumor necrosis factor-alpha-induced apoptosis. Exp Cell Res. 252(2):464–470. [DOI] [PubMed] [Google Scholar]
- Liu YY, Han TY, Yu JY, Bitterman A, Le A, Giuliano AE, Cabot MC. 2004. Oligonucleotides blocking glucosylceramide synthase expression selectively reverse drug resistance in cancer cells. J Lipid Res. 45(5):933–940. [DOI] [PubMed] [Google Scholar]
- Liu YY, Patwardhan GA, Xie P, Gu X, Giuliano AE, Cabot MC. 2011. Glucosylceramide synthase, a factor in modulating drug resistance, is overexpressed in metastatic breast carcinoma. Int J Oncol. 39(2):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, Holleran WM, Giuliano AE, Jazwinski SM, Gouaze-Andersson V, et al. 2008. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 22(7):2541–2551. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Pelled D, Riebeling C, Futerman AH. 2003. Lyso-glycosphingolipids mobilize calcium from brain microsomes via multiple mechanisms. Biochem J. 375(3):561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SA, Wertz PW, Strauss JS, Downing DT. 1985. Human stratum corneum polar lipids and desquamation. Arch Dermatol Res. 277(4):284–287. [DOI] [PubMed] [Google Scholar]
- Lucci A, Cho WI, Han TY, Giuliano AE, Morton DL, Cabot MC. 1998. Glucosylceramide: A marker for multiple-drug resistant cancers. Anticancer Res. 18(1B):475–480. [PubMed] [Google Scholar]
- Lucci A, Giuliano AE, Han TY, Dinur T, Liu YY, Senchenkov A, Cabot MC. 1999. Ceramide toxicity and metabolism differ in wild-type and multidrug-resistant cancer cells. Int J Oncol. 15(3):535–540. [DOI] [PubMed] [Google Scholar]
- Lucci A, Han TY, Liu YY, Giuliano AE, Cabot MC. 1999. Multidrug resistance modulators and doxorubicin synergize to elevate ceramide levels and elicit apoptosis in drug-resistant cancer cells. Cancer. 86(2):300–311. [DOI] [PubMed] [Google Scholar]
- Madison KC. 2003. Barrier function of the skin: "la raison d'etre" of the epidermis. J Invest Dermatol. 121(2):231–241. [DOI] [PubMed] [Google Scholar]
- Madison KC, Wertz PW, Strauss JS, Downing DT. 1986. Lipid composition of cultured murine keratinocytes. J Invest Dermatol. 87(2):253–259. [DOI] [PubMed] [Google Scholar]
- Mahfoud R, Garmy N, Maresca M, Yahi N, Puigserver A, Fantini J. 2002. Identification of a common sphingolipid-binding domain in Alzheimer, prion, and HIV-1 proteins. J Biol Chem. 277(13):11292–11296. [DOI] [PubMed] [Google Scholar]
- Makita A. 1964. Biochemistry of organ glycolipids. II. Isolation of human kidney glycolipids. J Biochem. 55(3):269–276. [DOI] [PubMed] [Google Scholar]
- Makita A, Yamakawa T. 1962. Biochemistry of organ glycolipid. I. Ceramide-oligohexosides of human, equine and bovine spleens. J Biochem. 51(2):124–133. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, Schule B, Langston JW. 2009. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: A biological link between Gaucher disease and parkinsonism. Neurotoxicology. 30(6):1127–1132. [DOI] [PubMed] [Google Scholar]
- Marchell NL, Uchida Y, Brown BE, Elias PM, Holleran WM. 1998. Glucosylceramides stimulate mitogenesis in aged murine epidermis. J Invest Dermatol. 110(4):383–387. [DOI] [PubMed] [Google Scholar]
- Marcus J, Popko B. 2002. Galactolipids are molecular determinants of myelin development and axo-glial organization. Biochim Biophys Acta. 1573(3):406–413. [DOI] [PubMed] [Google Scholar]
- Marsh NL, Elias PM, Holleran WM. 1995. Glucosylceramides stimulate murine epidermal hyperproliferation. J Clin Invest. 95(6):2903–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J, Vanier MT, Saito Y, Tohyama J, Suzuki K, Suzuki K. 2001. A mutation in the saposin A domain of the sphingolipid activator protein (prosaposin) gene results in a late-onset, chronic form of globoid cell leukodystrophy in the mouse. Hum Mol Genet. 10(11):1191–1199. [DOI] [PubMed] [Google Scholar]
- Maunula S, Bjorkqvist YJ, Slotte JP, Ramstedt B. 2007. Differences in the domain forming properties of N-palmitoylated neutral glycosphingolipids in bilayer membranes. Biochim Biophys Acta. 1768(2):336–345. [DOI] [PubMed] [Google Scholar]
- Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. 1999. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 91(13):1138–1146. [DOI] [PubMed] [Google Scholar]
- McCluer RH, Williams MA, Gross SK, Meisler MH. 1981. Testosterone effects on the induction and urinary-excretion of mouse kidney glycosphingolipids associated with lysosomes. J Biol Chem. 256:3112–3120. [PubMed] [Google Scholar]
- Modrak DE, Gold DV, Goldenberg DM. 2006. Sphingolipid targets in cancer therapy. Mol Cancer Ther. 5(2):200–208. [DOI] [PubMed] [Google Scholar]
- Morell P, Radin NS. 1969. Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry. 8(2):506–512. [DOI] [PubMed] [Google Scholar]
- Morjani H, Aouali N, Belhoussine R, Veldman RJ, Levade T, Manfait M. 2001. Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets. Int J Cancer. 94(2):157–165. [DOI] [PubMed] [Google Scholar]
- Morrow MR, Whitehead JP, Lu D. 1992. Chain-length dependence of lipid bilayer properties near the liquid crystal to gel phase transition. Biophys J. 63(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natomi H, Saitoh T, Sugano K, Iwamori M, Fukayama M, Nagai Y. 1993. Systematic analysis of glycosphingolipids in the human gastrointestinal tract: Enrichment of sulfatides with hydroxylated longer-chain fatty acids in the gastric and duodenal mucosa. Lipids. 28(8):737–742. [DOI] [PubMed] [Google Scholar]
- Nicholson KM, Quinn DM, Kellett GL, Warr JR. 1999. Preferential killing of multidrug-resistant KB cells by inhibitors of glucosylceramide synthase. Br J Cancer. 81(3):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela PS, Ollila S, Hyvonen MT, Karttunen M, Vattulainen I. 2007. Assessing the nature of lipid raft membranes. PLoS Comput Biol. 3(2):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura Y, Moue T, Takahashi N, Nagai KI. 2010. Modification of sphingoglycolipids and sulfolipids in kidney cell lines under heat stress: Activation of monohexosylceramide synthesis as a ceramide scavenger. Glycobiology. 20(6):710–717. [DOI] [PubMed] [Google Scholar]
- Niimura Y, Nagai K. 2008. Metabolic responses of sulfatide and related glycolipids in Madin-Darby canine kidney (MDCK) cells under osmotic stresses. Comp Biochem Physiol B Biochem Mol Biol. 149(1):161–167. [DOI] [PubMed] [Google Scholar]
- Nilsson O, Svennerholm L. 1982. Characterization and quantitative determination of gangliosides and neutral glycosphingolipids in human liver. J Lipid Res. 23(2):327–334. [PubMed] [Google Scholar]
- Norton WT, Autilio LA. 1966. The lipid composition of purified bovine brain myelin. J Neurochem. 13(4):213–222. [DOI] [PubMed] [Google Scholar]
- Norton WT, Cammer W. 1984. Isolation and characterization of myelin. In: Morell P, editor. Myelin. 2nd ed. New York: Plenum. p. 147–180. [Google Scholar]
- Ogawa K, Fujiwara Y, Sugamata K, Abe T. 1988. Thin-layer chromatography of neutral glycosphingolipids—An improved simple method for the resolution of GlcCer, GalCer, LacCer and Ga2Cer. J Chromatogr-Biomed. 426:188–193. [DOI] [PubMed] [Google Scholar]
- Ogretmen B. 2018. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 18(1):33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Stoskopf M, Hendricks F, Kishimoto Y. 1985. Phylogenetic dichotomy of nerve glycosphingolipids. Proc Natl Acad Sci USA. 82(20):6779–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollila S, Hyvonen MT, Vattulainen I. 2007. Polyunsaturation in lipid membranes: Dynamic properties and lateral pressure profiles. J Phys Chem B. 111(12):3139–3150. [DOI] [PubMed] [Google Scholar]
- Olshefski RS, Ladisch S. 2001. Glucosylceramide synthase inhibition enhances vincristine-induced cytotoxicity. Int J Cancer. 93(1):131–138. [DOI] [PubMed] [Google Scholar]
- Ong QX, Han WP, Yang XY. 2018. O-GlcNAc as an integrator of signaling pathways. Front Endocrinol. 9:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudes AJ, Roach JC, Walashek LS, Eichner LJ, True LD, Vessella RL, Liu AY. 2005. Application of affymetrix array and massively parallel signature sequencing for identification of genes involved in prostate cancer progression. BMC Cancer. 5(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owczarek TB, Suchanski J, Pula B, Kmiecik AM, Chadalski M, Jethon A, Dziegiel P, Ugorski M. 2013. Galactosylceramide affects tumorigenic and metastatic properties of breast cancer cells as an anti-apoptotic molecule. PLoS One. 8(12):e84191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey MK, Burrow TA, Rani R, Martin LJ, Witte D, Setchell KD, McKay MA, Magnusen AF, Zhang W, Liou B, et al. 2017. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature. 543(7643):108–112. [DOI] [PubMed] [Google Scholar]
- Patwardhan GA, Beverly LJ, Siskind LJ. 2016. Sphingolipids and mitochondrial apoptosis. J Bioenerg Biomembr. 48(2):153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P, Kamisaka Y, Marks DL, Pagano RE. 1996. Purification and characterization of UDP-glucose:ceramide glucosyltransferase from rat liver Golgi membranes. J Biol Chem. 271(4):2287–2293. [DOI] [PubMed] [Google Scholar]
- Persaud-Sawin DA, McNamara JO 2nd, Rylova S, Vandongen A, Boustany RM. 2004. A galactosylceramide binding domain is involved in trafficking of CLN3 from Golgi to rafts via recycling endosomes. Pediatr Res. 56(3):449–463. [DOI] [PubMed] [Google Scholar]
- Popko B, Pearl DK, Walker DM, Comas TC, Baerwald KD, Burger PC, Scheithauer BW, Yates AJ. 2002. Molecular markers that identify human astrocytomas and oligodendrogliomas. J Neuropathol Exp Neurol. 61(4):329–338. [DOI] [PubMed] [Google Scholar]
- Popovic ZV, Rabionet M, Jennemann R, Krunic D, Sandhoff R, Grone HJ, Porubsky S. 2017. Glucosylceramide synthase is involved in development of invariant natural killer T cells. Front Immunol. 8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranam KL, Guo WX, Qian WH, Nikbakht K, Boustany RM. 1999. CLN3 defines a novel antiapoptotic pathway operative in neurodegeneration and mediated by ceramide. Mol Genet Metab. 66(4):294–308. [DOI] [PubMed] [Google Scholar]
- Quinn PJ. 2011. The structure of complexes between phosphatidylethanolamine and glucosylceramide: A matrix for membrane rafts. Biochim Biophys Acta. 1808(12):2894–2904. [DOI] [PubMed] [Google Scholar]
- Rani CS, Abe A, Chang Y, Rosenzweig N, Saltiel AR, Radin NS, Shayman JA. 1995. Cell cycle arrest induced by an inhibitor of glucosylceramide synthase. Correlation with cyclin-dependent kinases. J Biol Chem. 270(6):2859–2867. [DOI] [PubMed] [Google Scholar]
- Ron I, Rapaport D, Horowitz M. 2010. Interaction between parkin and mutant glucocerebrosidase variants: A possible link between Parkinson disease and Gaucher disease. Hum Mol Genet. 19(19):3771–3781. [DOI] [PubMed] [Google Scholar]
- Rossdam C, Konze SA, Oberbeck A, Rapp E, Gerardy-Schahn R, von Itzstein M, Buettner FFR. 2019. Approach for profiling of glycosphingolipid glycosylation by multiplexed capillary gel electrophoresis coupled to laser-induced fluorescence detection to identify cell-surface markers of human pluripotent stem cells and derived cardiomyocytes. Anal Chem. 91(10):6413–6418. [DOI] [PubMed] [Google Scholar]
- Ruckhaberle E, Karn T, Hanker L, Gatje R, Metzler D, Holtrich U, Kaufmann M, Rody A. 2009. Prognostic relevance of glucosylceramide synthase (GCS) expression in breast cancer. J Cancer Res Clin Oncol. 135(1):81–90. [DOI] [PubMed] [Google Scholar]
- Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grosch S, Geisslinger G, Holtrich U, Karn T, et al. 2008. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 112:41–52. [DOI] [PubMed] [Google Scholar]
- Rusyn E, Mousallem T, Persaud-Sawin DA, Miller S, Boustany RM. 2008. CLN3p impacts galactosylceramide transport, raft morphology, and lipid content. Pediatr Res. 63:625–631. [DOI] [PubMed] [Google Scholar]
- Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. 2011. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther. 11:138–149. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Natomi H, Zhao WL, Okuzumi K, Sugano K, Iwamori M, Nagai Y. 1991. Identification of glycolipid receptors for Helicobacter pylori by TLC-immunostaining. FEBS Lett. 282:385–387. [DOI] [PubMed] [Google Scholar]
- Salio M, Silk JD, Jones EY, Cerundolo V. 2014. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 32:323–366. [DOI] [PubMed] [Google Scholar]
- Salomonsen J, Sorensen MR, Marston DA, Rogers SL, Collen T, van Hateren A, Smith AL, Beal RK, Skjodt K, Kaufman J. 2005. Two CD1 genes map to the chicken MHC, indicating that CD1 genes are ancient and likely to have been present in the primordial MHC. Proc Natl Acad Sci USA, 102:8668-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K, Kolter T. 1996. Topology of glycosphingolipid degradation. Trends Cell Biol. 6:98–103. [DOI] [PubMed] [Google Scholar]
- Sandhoff R, Sandhoff K. 2018. Emerging concepts of ganglioside metabolism. FEBS Lett. 592:3835–3864. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Kinoshita T. 2017. Glycosphingolipids. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. [Internet]. 3rd edition. Chapter 11. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press, p. 2015-2017. doi: 10.1101/glycobiology.3e.011. [DOI]
- Schoephoerster RT, Wertz PW, Madison KC, Downing DT. 1985. A survey of polar and non-polar lipids of mouse organs. Comp Biochem Physiol B. 82:229–232. [DOI] [PubMed] [Google Scholar]
- Schulze H, Kolter T, Sandhoff K. 2009. Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation. Biochim Biophys Acta. 1793:674–683. [DOI] [PubMed] [Google Scholar]
- Schweppe CH, Hoffmann P, Nofer JR, Pohlentz G, Mormann M, Karch H, Friedrich AW, Muthing J. 2010. Neutral glycosphingolipids in human blood: A precise mass spectrometry analysis with special reference to lipoprotein-associated Shiga toxin receptors. J Lipid Res. 51:2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segui B, Andrieu-Abadie N, Jaffrezou JP, Benoist H, Levade T. 2006. Sphingolipids as modulators of cancer cell death: Potential therapeutic targets. Biochim Biophys Acta. 1758:2104–2120. [DOI] [PubMed] [Google Scholar]
- Senchenkov A, Han TY, Wang H, Frankel AE, Kottke TJ, Kaufmann SH, Cabot MC. 2001. Enhanced ceramide generation and induction of apoptosis in human leukemia cells exposed to DT(388)-granulocyte-macrophage colony-stimulating factor (GM-CSF), a truncated diphtheria toxin fused to human GM-CSF. Blood. 98:1927–1934. [DOI] [PubMed] [Google Scholar]
- Shabbits JA, Mayer LD. 2002. P-glycoprotein modulates ceramide-mediated sensitivity of human breast cancer cells to tubulin-binding anticancer drugs. Mol Cancer Ther. 1:205–213. [PubMed] [Google Scholar]
- Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, Merrill AH. 2009. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 50:1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayman JA, Deshmukh GD, Mahdiyoun S, Thomas TP, Wu D, Barcelon FS, Radin NS. 1991. Modulation of renal epithelial cell growth by glucosylceramide. Association with protein kinase C, sphingosine, and diacylglycerol. J Biol Chem. 266:22968–22974. [PubMed] [Google Scholar]
- Shimoda H, Terazawa S, Hitoe S, Tanaka J, Nakamura S, Matsuda H, Yoshikawa M. 2012. Changes in ceramides and glucosylceramides in mouse skin and human epidermal equivalents by rice-derived glucosylceramide. J Med Food. 15:1064–1072. [DOI] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, et al. 2009. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 361:1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikic BI, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G. 1997. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 40(Suppl):S13–S19. [DOI] [PubMed] [Google Scholar]
- Sillence DJ, Puri V, Marks DL, Butters TD, Dwek RA, Pagano RE, Platt FM. 2002. Glucosylceramide modulates membrane traffic along the endocytic pathway. J Lipid Res. 43:1837–1845. [DOI] [PubMed] [Google Scholar]
- Simons K, Gruenberg J. 2000. Jamming the endosomal system: Lipid rafts and lysosomal storage diseases. Trends Cell Biol. 10:459–462. [DOI] [PubMed] [Google Scholar]
- Skotland T, Sandvig K. 2019. The role of PS 18:0/18:1 in membrane function. Nat Commun. 10:2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. 1997. Alpha-synuclein in Lewy bodies. Nature. 388:839–840. [DOI] [PubMed] [Google Scholar]
- Spinedi A, Bartolomeo SD, Piacentini M. 1998. Apoptosis induced by N-hexanoylsphingosine in CHP-100 cells associates with accumulation of endogenous ceramide and is potentiated by inhibition of glucocerebroside synthesis. Cell Death Differ. 5:785–791. [DOI] [PubMed] [Google Scholar]
- Sprong H, Degroote S, Nilsson T, Kawakita M, Ishida N, van der Sluijs P, van Meer G. 2003. Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol Biol Cell. 14:3482–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong H, Kruithof B, Leijendekker R, Slot JW, van Meer G, van der Sluijs P. 1998. UDP-galactose:ceramide galactosyltransferase is a class I integral membrane protein of the endoplasmic reticulum. J Biol Chem. 273:25880–25888. [DOI] [PubMed] [Google Scholar]
- Suchanski J, Grzegrzolka J, Owczarek T, Pasikowski P, Piotrowska A, Kocbach B, Nowak A, Dziegiel P, Wojnar A, Ugorski M. 2018. Sulfatide decreases the resistance to stress-induced apoptosis and increases P-selectin-mediated adhesion: A two-edged sword in breast cancer progression. Breast Cancer Res. 20:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang T, Gao P, Meng B, Gao Y, Wang X, Zhang J, Wang H, Wu X, Zheng W, et al. 2010. Targeting glucosylceramide synthase downregulates expression of the multidrug resistance gene MDR1 and sensitizes breast carcinoma cells to anticancer drugs. Breast Cancer Res Treat. 121:591–599. [DOI] [PubMed] [Google Scholar]
- Sung CC, Collins R, Li J, Pearl DK, Coons SW, Scheithauer BW, Johnson PC, Yates AJ. 1996. Glycolipids and myelin proteins in human oligodendrogliomas. Glycoconj J. 13:433–443. [DOI] [PubMed] [Google Scholar]
- Svennerholm E, Svennerholm L. 1963. The separation of neutral blood-serum glycolipids by thin-layer chromatography. Biochim Biophys Acta. 70:432–441. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Vanier MT, Mansson JE. 1980. Krabbe disease: A galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 21:53–64. [PubMed] [Google Scholar]
- Taguchi YV, Liu J, Ruan J, Pacheco J, Zhang X, Abbasi J, Keutzer J, Mistry PK, Chandra SS. 2017. Glucosylsphingosine promotes alpha-synuclein pathology in mutant GBA-associated Parkinson's disease. J Neurosci. 37:9617–9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno M, Gonzalez-Scarano F, Levy JA. 1989. Human immunodeficiency virus can infect CD4-negative human fibroblastoid cells. Proc Natl Acad Sci USA. 86:4287–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper AD, Diks SH, van Blitterswijk WJ, Borst J. 2000. Glucosylceramide synthase does not attenuate the ceramide pool accumulating during apoptosis induced by CD95 or anti-cancer regimens. J Biol Chem. 275:34810–34817. [DOI] [PubMed] [Google Scholar]
- Thi KAN, Koets AP, Vordermeier M, Jervis PJ, Cox LR, Graham SP, Santema WJ, Moody DB, van Calenbergh S, Zajonc DM, et al. 2013. The bovine CD1D gene has an unusual gene structure and is expressed but cannot present -galactosylceramide with a C26 fatty acid. Int Immunol. 25:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TE, Tillack TW. 1985. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 14:361–386. [DOI] [PubMed] [Google Scholar]
- Townsend RR. 1993. Quantitative monosaccharide analysis of glycoproteins—High-performance liquid-chromatography. Acs Sym Ser. 529:86–101. [Google Scholar]
- Trajkovic-Bodennec S, Bodennec J, Futerman AH. 2004. Phosphatidylcholine metabolism is altered in a monocyte-derived macrophage model of Gaucher disease but not in lymphocytes. Blood Cells Mol Dis. 33:77–82. [DOI] [PubMed] [Google Scholar]
- Turzanski J, Grundy M, Shang S, Russell N, Pallis M. 2005. P-glycoprotein is implicated in the inhibition of ceramide-induced apoptosis in TF-1 acute myeloid leukemia cells by modulation of the glucosylceramide synthase pathway. Exp Hematol. 33:62–72. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Itoh M, Taguchi Y, Yamaoka S, Umehara H, Ichikawa S, Hirabayashi Y, Holleran WM, Okazaki T. 2004. Ceramide reduction and transcriptional up-regulation of glucosylceramide synthase through doxorubicin-activated Sp1 in drug-resistant HL-60/ADR cells. Cancer Res. 64:6271–6279. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Murata S, Schmuth M, Behne MJ, Lee JD, Ichikawa S, Elias PM, Hirabayashi Y, Holleran WM. 2002. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J Lipid Res. 43:1293–1302. [PubMed] [Google Scholar]
- van den Heuvel-Eibrink MM, Wiemer EA, de Boevere MJ, van der Holt B, Vossebeld PJ, Pieters R, Sonneveld P. 2001. MDR1 gene-related clonal selection and P-glycoprotein function and expression in relapsed or refractory acute myeloid leukemia. Blood. 97:3605–3611. [DOI] [PubMed] [Google Scholar]
- Van Rhijn I, Koets AP, Im AS, Piebes D, Reddington F, Besra GS, Porcelli SA, van Eden W, Rutten VPMG. 2006. The bovine CD1 family contains group 1 CD1 proteins, but no functional CD1d. J Immunol. 176:4888–4893. [DOI] [PubMed] [Google Scholar]
- Varela AR, Goncalves da Silva AM, Fedorov A, Futerman AH, Prieto M, Silva LC. 2013. Effect of glucosylceramide on the biophysical properties of fluid membranes. Biochim Biophys Acta. 1828:1122–1130. [DOI] [PubMed] [Google Scholar]
- Varela ARP, Couto AS, Fedorov A, Futerman AH, Prieto M, Silva LC. 2016. Glucosylceramide reorganizes cholesterol-containing domains in a fluid phospholipid membrane. Biophys J. 110:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman RJ, Klappe K, Hinrichs J, Hummel I, van der Schaaf G, Sietsma H, Kok JW. 2002. Altered sphingolipid metabolism in multidrug-resistant ovarian cancer cells is due to uncoupling of glycolipid biosynthesis in the Golgi apparatus. FASEB J. 16:1111–1113. [DOI] [PubMed] [Google Scholar]
- Veldman RJ, Mita A, Cuvillier O, Garcia V, Klappe K, Medin JA, Campbell JD, Carpentier S, Kok JW, Levade T. 2003. The absence of functional glucosylceramide synthase does not sensitize melanoma cells for anticancer drugs. FASEB J. 17:1144–1146. [DOI] [PubMed] [Google Scholar]
- Vielhaber G, Pfeiffer S, Brade L, Lindner B, Goldmann T, Vollmer E, Hintze U, Wittern KP, Wepf R. 2001. Localization of ceramide and glucosylceramide in human epidermis by immunogold electron microscopy. J Invest Dermatol. 117:1126–1136. [DOI] [PubMed] [Google Scholar]
- Vos JP, Lopes-Cardozo M, Gadella BM. 1994. Metabolic and functional aspects of sulfogalactolipids. Biochim Biophys Acta. 1211:125–149. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Wen YN, Yang MF, Huang LJ, Wang ZF, Fan JB. 2019. High-sensitivity quantification of glycosphingolipid glycans by ESI-MS utilizing ozonolysis-based release and isotopic Girard's reagent labeling. Anal Biochem. 582:113355. [DOI] [PubMed] [Google Scholar]
- Weiss M, Hettmer S, Smith P, Ladisch S. 2003. Inhibition of melanoma tumor growth by a novel inhibitor of glucosylceramide synthase. Cancer Res. 63:3654–3658. [PubMed] [Google Scholar]
- Wenger DA, Rafi MA, Luzi P, Datto J, Costantino-Ceccarini E. 2000. Krabbe disease: Genetic aspects and progress toward therapy. Mol Genet Metab. 70:1–9. [DOI] [PubMed] [Google Scholar]
- Westbroek W, Gustafson AM, Sidransky E. 2011. Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol Med. 17:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund B, Slotte JP. 2009. How the molecular features of glycosphingolipids affect domain formation in fluid membranes. Biochim Biophys Acta. 1788:194–201. [DOI] [PubMed] [Google Scholar]
- Won JS, Singh AK, Singh I. 2016. Biochemical, cell biological, pathological, and therapeutic aspects of Krabbe's disease. J Neurosci Res. 94(11):990–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N, Sabatier JM, Baghdiguian S, Gonzalez-Scarano F, Fantini J. 1995. Synthetic multimeric peptides derived from the principal neutralization domain (V3 loop) of human immunodeficiency virus type 1 (HIV-1) gp120 bind to galactosylceramide and block HIV-1 infection in a human CD4-negative mucosal epithelial cell line. J Virol. 69(1):320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Foekens JA, Yu J, Sieuwerts AM, Timmermans M, Klijn JG, Atkins D, Wang Y, Jiang Y. 2006. Laser microdissection and microarray analysis of breast tumors reveal ER-alpha related genes and pathways. Oncogene. 25(9):1413–1419. [DOI] [PubMed] [Google Scholar]
- Yao JK, Yoshino JE. 1994. Association of glucocerebroside homolog biosynthesis with Schwann cell proliferation. Neurochem Res. 19(1):31–35. [DOI] [PubMed] [Google Scholar]
- Zajonc DM, Elsliger MA, Teyton L, Wilson IA. 2003. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 A. Nat Immunol. 4(8):808–815. [DOI] [PubMed] [Google Scholar]
- Zhang T, de Waard AA, Wuhrer M, Spaapen RM. 2019. The role of glycosphingolipids in immune cell functions. Front Immunol. 10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]


