Abstract
Background and Aims
Risankizumab, an interleukin-23 antibody, demonstrated efficacy and acceptable safety in a phase 2 study of patients with moderate-to-severe refractory Crohn’s disease. This open-label extension investigated the long-term safety, pharmacokinetics, immunogenicity and efficacy of risankizumab in responders to risankizumab in the parent phase 2 study.
Methods
Enrolled patients had achieved clinical response [decrease in Crohn’s Disease Activity Index from baseline ≥100] without clinical remission [Crohn’s Disease Activity Index <150] at Week 26, or clinical response and/or remission at Week 52 in the parent phase 2 study and received open-label subcutaneous risankizumab 180 mg every 8 weeks.
Results
Sixty-five patients were enrolled, including four who had lost response in the parent study and were first reinduced with risankizumab 600 mg every 4 weeks [three infusions]. Patients received risankizumab for a median of 33 months [total: 167.0 patient-years]. The rate of serious adverse events was 24.6 events/100 patient-years; the majority were gastrointestinal in nature. Rates of serious infections, opportunistic infections and fungal infections were 4.2, 1.8, and 6.6 events/100 patient-years, respectively. No deaths, malignancies, adjudicated major adverse cardiovascular events, latent/active tuberculosis or herpes zoster were reported. Treatment-emergent anti-drug antibodies developed in eight patients [12.3%]; none were neutralizing. Efficacy outcomes were maintained during the study, including the proportions of patients [observed analysis] with clinical remission [>71%] and endoscopic remission [>42%].
Conclusions
Long-term maintenance treatment with subcutaneous risankizumab 180 mg every 8 weeks was well tolerated by patients with Crohn’s disease, with no new safety signals.
Clinical trial registration number: NCT02513459
Keywords: Crohn’s disease, long-term safety, open-label extension
1. Introduction
Crohn’s disease [CD] is an inflammatory bowel disease characterized by chronic relapsing inflammation in the gastrointestinal tract. Signs and symptoms of CD include abdominal pain, chronic diarrhoea, fever, weight loss and fistula drainage, which represent a considerable systemic burden for patients.1,2 The reported worldwide incidence [0.0–29.3 per 100 000 person-years] and prevalence [0.9–322.0 per 100 000 persons] of CD vary greatly across geographical regions, with a prevalence of >0.3% in North America, Oceania and many countries in Europe.3 While the incidence in newly industrialized countries in Africa, Asia and South America is typically lower than in westernized countries, the incidence of CD is also rising in these countries.3,4
Current treatments for CD include corticosteroids, thiopurines, methotrexate, tumour necrosis factor [TNF] antagonists, the integrin antagonist vedolizumab and the interleukin [IL]-12/23 inhibitor ustekinumab.2,5 The European Crohn’s and Colitis Organisation and the American College of Gastroenterology recommend using corticosteroids, TNF antagonists [adalimumab, infliximab and certolizumab pegol], vedolizumab and ustekinumab to induce remission in patients with moderate-to-severe CD; and recommend thiopurines, methotrexate, TNF antagonists, vedolizumab and ustekinumab for the maintenance of remission.2,5 However, approximately one-third of patients do not respond to TNF antagonists or lose their response to TNF antagonists over time.1
Risankizumab is a fully human immunoglobulin G monoclonal antibody that binds with high affinity to the p19 subunit of IL-23, which has been approved for the treatment of moderate-to-severe plaque psoriasis.6,7 Results from the phase 2 M15-993 study showed that risankizumab was effective and well tolerated through 52 weeks in patients with moderate-to-severe CD, the majority of whom had failed prior therapy with one or more TNF antagonists.8,9 In the blinded induction period, a greater proportion of patients achieved clinical and endoscopic remission after 12 weeks of treatment with risankizumab 600 mg intravenous [IV] every 4 weeks [Q4W] compared with placebo. Risankizumab 600 mg IV treatment in the open-label reinduction period increased remission rates at Week 26, and remission was effectively maintained with risankizumab 180 mg subcutaneous [SC] through Week 52. Over 52 weeks, treatment-emergent anti-drug antibodies [ADAs] were observed in 8% of patients who received at least one dose of risankizumab; none were neutralizing.9 Pooled phase 1 and 2 population pharmacokinetics data from patients with psoriasis or CD demonstrated that body weight had a modest effect on risankizumab exposure, while baseline serum albumin levels were statistically correlated with risankizumab clearance but had no clinically meaningful impact on serum concentrations of risankizumab.10
Patients who responded to risankizumab in the phase 2 study were eligible to enrol in the open-label extension [OLE] M15-989 study reported here. The objective of this OLE was to investigate the long-term safety, pharmacokinetics, immunogenicity, efficacy and health-related quality of life [HRQoL] of risankizumab, in patients with moderate-to-severe active CD who achieved a clinical response or remission on previous treatment with risankizumab.
2. Methods
2.1. Study design and patients
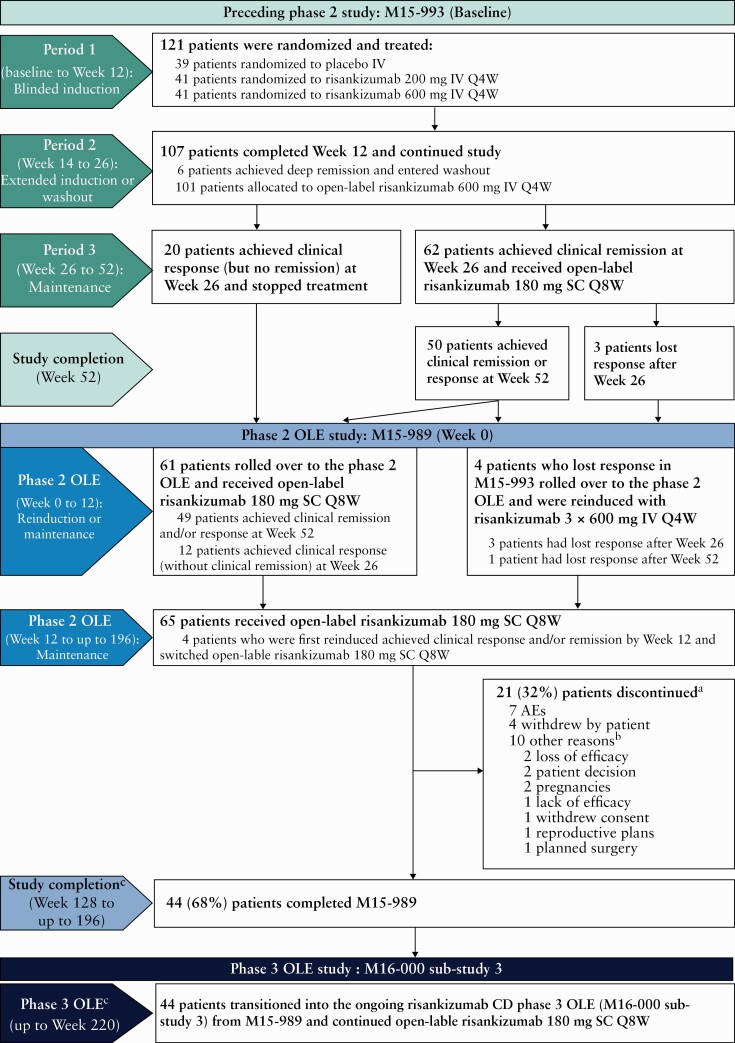
Patients who successfully completed the phase 2 M15-993 study [NCT02031276] were eligible to enrol in this single-group OLE [M15-989; NCT02513459; US Clinical Trials Registry]. Patients were included from 38 referral sites across Europe, Asia and North America. All patients received maintenance therapy with open-label risankizumab 180 mg SC every 8 weeks [Q8W] up to 196 weeks [Figure 1]. Successful completion of M15-993 was defined as achievement of clinical response [decrease in Crohn’s Disease Activity Index [CDAI] from baseline with ≥100 points] without clinical remission [CDAI <150] at Week 26 or achievement of clinical response and/or remission at Week 52.9 Patients who had lost clinical remission at the end of the trial visit for the M15-993 study, or at screening for the M15-989 OLE, received open-label reinduction of risankizumab with three infusions of 600 mg IV Q4W, after which eligibility was reassessed. If clinical response or remission was achieved, patients were switched to maintenance treatment of risankizumab 180 mg SC Q8W from visit 5 for the remainder of the OLE. Patients who completed the M15-989 study could elect to enrol in the M16-000 sub-study 3, an ongoing phase 3 OLE [NCT03105102], in which they continue to receive open-label risankizumab 180 mg SC Q8W and are eligible to receive risankizumab rescue therapy. The M15-989 study was terminated by the sponsor to provide the option for patients to roll over for continuous open-label treatment within the ongoing phase 3 OLE.
Figure 1.
Study design and patient disposition. aPrimary reasons for discontinuation. bOther reasons are as reported by the investigator [as free text] and have not been grouped to avoid inaccuracy in reporting. cPatients rolled over to the ongoing phase 3 OLE [M16-000 sub-study 3] between Weeks 128 and 184 of M15-989. AE, adverse event; CD, Crohn’s disease; IV, intravenous; OLE, open-label extension; Q4/8W, every 4/8 weeks; SC, subcutaneous.
Inclusion and exclusion criteria in the parent M15-993 study have been previously described.8 In brief, patients were adults [aged 18–75 years] with moderate-to-severe CD, defined as CDAI 220–450 and Crohn’s Disease Endoscopic Index of Severity [CDEIS] ≥7 [or ≥4 for patients with isolated ileitis] with mucosal ulcers in the ileum and/or colon. Patients could have been biologic-naïve or previously treated with one or more TNF antagonists or vedolizumab, while patients who had received ustekinumab were ineligible for enrolment.
During the OLE, patients were permitted to continue oral corticosteroids [prednisone equivalent dose of ≤20 mg/day], oral 5-aminosalicylates and immunosuppressive agents [azathioprine, 6-mercaptopurine or methotrexate] if they had been on a stable dose for ≥4 weeks prior to visit 2. Patients who entered the OLE on concomitant treatments were required to continue on the stable dose throughout the OLE. Initiation of systemic antihistamines and IV steroids was permitted in the case of infusion reactions during or after risankizumab infusion in accordance with the severity of the reaction and the local standard of care.
The M15-989 OLE was conducted in accordance with the International Conference on Harmonization guidelines, applicable regulations and the Declaration of Helsinki. Study-related documents were approved by institutional ethics committees and review boards. All patients provided written, informed consent. An independent data monitoring committee was utilized to ensure that the welfare of patients participating in this study was maintained.
2.2. Outcomes
The primary objective was to assess long-term safety in patients with CD receiving maintenance risankizumab 180 mg SC Q8W. Adverse events [AEs], vital signs and laboratory assessments were collected throughout the study and up to 20 weeks after the last dose of risankizumab for patients who prematurely discontinued the study. AEs were coded using the Medical Dictionary for Regulatory Activities version 21.1, and severity was graded based on the Rheumatology Common Toxicity Criteria version 4.0. Major cardiac, cerebrovascular and thrombotic events were adjudicated by an independent cardiovascular adjudication committee.
Treatment-emergent AEs [TEAEs] were defined as events occurring or worsening either on or after the first dose of risankizumab in the M15-989 study; within 20 weeks after the last dose of risankizumab for patients who prematurely terminated the study drug in the M15-989 study; and before the first dose of risankizumab in the phase 3 OLE. Duration of risankizumab exposure was defined as the date of last risankizumab dose minus the date of first risankizumab dose in the M15-989 OLE plus 8 weeks. AEs are presented as the number of patients [%] experiencing any event, the absolute number of events and events per 100 patient-years [E/100 PY].
Secondary objectives included the assessment of pharmacokinetics, immunogenicity, efficacy and HRQoL. Plasma samples were collected at scheduled visits, and were analysed at a later date using an enzyme-linked immunosorbent assay to measure risankizumab plasma concentration and an electrochemiluminescence assay with a three-tiered approach for the detection of ADAs.8 Due to sparse sampling, only trough plasma concentrations at the time relative to the first SC dose were summarized. Key efficacy outcomes included clinical remission [CDAI <150] and endoscopic remission [CDEIS ≤4, or ≤2 for patients with isolated ileitis]; HRQoL outcomes included Inflammatory Bowel Disease Questionnaire [IBDQ] response [increase from baseline in IBDQ total score ≥16] and remission [IBDQ total score ≥170]; and biomarker outcomes included median change from baseline in serum albumin, high-sensitivity C-reactive protein [hs-CRP] and faecal calprotectin. CDAI scores and hs-CRP levels were assessed at every visit; IBDQ scores and serum albumin concentrations were assessed every 24 weeks; faecal calprotectin was assessed at baseline, Week 24 and every 32 weeks thereafter; and ileocolonoscopies were performed yearly. Central scoring of endoscopy results was performed by independent, blinded reviewers for all endoscopic assessments.
2.3. Statistical analysis
Safety data were reported for patients who received at least one dose of risankizumab [safety analysis set]. The pharmacokinetics and immunogenicity analysis set included all patients who had pharmacokinetics and ADA data. Efficacy and HRQoL data were reported for the intent-to-treat population with non-responder imputation [NRI] of missing data up to Week 128. This was because from that time point onwards patients could roll over to the phase 3 OLE, resulting in a decrease in the number of patients continuing in the current phase 2 OLE study towards its completion and prior to the roll over into the phase 3 OLE for further risankizumab treatment and follow-up. Therefore, only observed cases were reported beyond Week 128. Note that the final time point reported may vary for different efficacy outcomes according to the frequency of assessments described in the previous section, and that patients completed M15-989 at different time points to roll over into the phase 3 OLE. No sample size calculation or statistical comparisons were conducted; all analyses were summarized descriptively. Week 0 denotes the first dose of study drug in the M15-989 OLE. However, for baseline characteristics and changes from baseline, baseline was defined as being prior to the first dose of study drug in the parent M15-993 study.8
3. Results
3.1. Patient disposition
Of the 121 patients randomized in the M15-993 study,9 65 rolled over to this OLE [all risankizumab group] from September 2015 onwards. This included patients who achieved clinical response [without clinical remission] at Week 26 [n = 12], patients who achieved clinical remission and/or response at Week 52 [n = 49] and four patients who had lost response at the end of the M15-993 study and were reinduced with risankizumab 600 mg IV Q4W at the beginning of the OLE [Figure 1]. Forty-four patients [68%] completed the M15-989 study and transitioned into the phase 3 OLE, while 21 [32%] patients prematurely discontinued the current study. Seven patients discontinued the study due to AEs and seven patients withdrew consent; all reasons for discontinuation are detailed in Figure 1. The M15-989 study concluded in June 2019; it was terminated by the sponsor to provide the option for patients to roll over to continuous open-label treatment within the ongoing phase 3 OLE.
Baseline characteristics and disease activity [i.e. at entry into the M15-993 study] of the OLE population are presented in Table 1. Median CD duration was 10.0 years [range: 2.0–38.0], and 92.3% of patients had been previously treated with at least one TNF antagonist.
Table 1.
Patient characteristics at baselinea and Week 0b [intent-to-treat analysis set]
| Patient characteristics at baselinea | All risankizumab [N = 65] | ||
|---|---|---|---|
| Age, years, median [range] | 34.0 [19–67] | ||
| Female, n [%] | 36 [55.4] | ||
| Race, white, n [%] | 55 [84.6] | ||
| Weight, kg, median [range] | 66.0 [41.0–123.9] | ||
| Disease duration, years, median [range] | 10.0 [2.0–38.0] | ||
| CD location, n [%] | |||
| Ileocolonic | 37 [56.9] | ||
| Colonic only | 20 [30.8] | ||
| Ileum only | 8 [12.3] | ||
| Prior TNF antagonist use, n [%] | 60 [92.3] | ||
| Prior use of one TNF antagonist | 20 [30.8] | ||
| Prior use of two TNF antagonists | 32 [49.2] | ||
| Prior use of three TNF antagonists | 8 [12.3] | ||
| Concomitant treatment,cn [%] | |||
| Corticosteroid only | 13 [20.0] | ||
| IMM only | 21 [32.3] | ||
| Corticosteroid and IMM | 9 [13.8] | ||
| Disease activity | Baseline,a median [range] | Baseline,a mean [SD] | Mean [SD] change from baseline at Week 0b |
| CDAI | 298.0 [109.0–477.6] | 304.8 [78.0] | −198.5 [101.8] |
| CDEIS | 11.9 [5.2–25.0] | 13.3 [5.6] | −8.1 [6.0] |
| IBDQ | 114.0 [52.0–182.0] | 114.3 [34.4] | 62.5 [38.8] |
| hs-CRP, mg/L | 10.2 [0.2–109.0] | 20.3 [23.2] | −14.6 [22.8] |
| Faecal calprotectin, mg/kg | 1364.0 [40.0–25 252.0] | 2436.5 [3707.0] | −1983.9 [3402.1] |
CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CDEIS, Crohn’s Disease Endoscopic Index of Severity; hs-CRP, high-sensitivity C-reactive protein; IBDQ, Inflammatory Bowel Disease Questionnaire; IMM, immunomodulator; OLE, open-label extension; SD, standard deviation; TNF, tumour necrosis factor.
aBaseline is defined as prior to first dose of study drug in the parent M15-993 study.
bWeek 0 is defined as first dose of study drug in the M15-989 OLE.
cPatients were permitted to continue oral corticosteroids [prednisone equivalent dose of ≤20 mg/day] and immunomodulators [azathioprine, 6-mercaptopurine or methotrexate] if they had been on a stable dose for at least 4 weeks prior to visit 2. Patients who entered the OLE on concomitant treatments were required to continue on a stable dose of this treatment throughout the M15-989 OLE.
3.2. Safety
In the all risankizumab group [n = 65], the treatment duration of risankizumab ranged from 114 to 1317 days, with a median of 1014 days [33 months] and a total of 167.0 PY. Forty patients [62%] received risankizumab for >924 days. All patients who required IV reinduction [n = 4] received the full reinduction with three infusions of 600 mg IV risankizumab Q4W [median duration: 84 days] and achieved clinical response by Week 8; they then continued risankizumab 180 mg SC Q8W from Week 12 onwards.
Most patients experienced at least one TEAE [Table 2]. The most common TEAEs were nasopharyngitis, gastroenteritis and fatigue [Supplementary Table 1]. Serious AEs [SAEs] were reported in 23 patients [35.4%; 24.6 E/100 PY]. SAEs observed in more than one patient were worsening CD, intestinal stenosis, viral gastroenteritis, peritonitis and post-procedural complications [n = 2 each]. Six patients [9.2%; 4.2 E/100 PY] discontinued the study drug due to the following TEAEs: worsening CD [n = 5], including one patient with colon injury [intraluminal laceration of the colon with no perforation during an endoscopy], and ileal stenosis [n = 1]. No deaths were reported.
Table 2.
Overview of TEAEs [safety analysis set]
| All risankizumab [N = 65; PY = 167.0] | ||
|---|---|---|
| Number of patients [%] | E/100 PY | |
| Any AE | 60 [92.3] | 408.4 |
| AE possibly related to study druga | 26 [40.0] | 49.7 |
| Serious AE | 23 [35.4] | 24.6 |
| Severe AEb | 14 [21.5] | 15.0 |
| AE leading to discontinuation of study drug | 6 [9.2] | 4.2 |
| All deaths | 0 | 0 |
| Infection | 48 [73.8] | 112.0 |
| Serious infection | 6 [9.2] | 4.2 |
| Opportunistic infection | 3 [4.6]c | 1.8 |
| Fungal infection | 7 [10.8]c | 6.6 |
| Tuberculosis | 0 | 0 |
| Herpes zoster | 0 | 0 |
| Malignancy excluding NMSC | 0 | 0 |
| Hypersensitivity | 16 [24.6] | 10.2 |
| Anaphylactic reaction | 1d [1.5] | 0.6 |
| Hepatic event | 6 [9.2] | 10.2 |
| Adjudicated MACE | 0 | 0 |
AE, adverse event; E/100 PY, events per 100 patient-years; MACE, major adverse cardiovascular event; NCI, National Cancer Institute; NMSC, non-melanoma skin cancer; PY, patient-years; TEAE, treatment-emergent adverse event.
aAs assessed by the investigator.
bAEs with NCI toxicity ≥grade 3 or unknown severity.
cOf all fungal infection cases, fungal oesophagitis [n = 2] and oral fungal infection [n = 1] were also coded as opportunistic infections.
dSerious anaphylactic reaction to intravenous iron carboxymaltose administration.
Infections were reported in 48 patients [73.8%; 112.0 E/100 PY]. The most common infections were nasopharyngitis, lower respiratory tract infection, oral herpes and urinary tract infection. Serious infections were reported in six patients [9.2%; 4.2 E/100 PY]; these included pelvic peritonitis secondary to anal dilatation and viral gastroenteritis [reported in the same patient], peritonitis following ileocaecal resection surgery, Campylobacter infection, subcutaneous abscess, viral gastroenteritis and perianal abscess. None of the serious infections led to discontinuation of risankizumab. Of the six patients with serious infections, two were receiving concomitant immunomodulators [n = 1] or corticosteroids [n = 1] throughout the OLE. Opportunistic infections were reported in three patients [4.6%; 1.8 E/100 PY] and included fungal oesophagitis and oral fungal infection; all were resolved during the study period. Fungal infections were reported in seven patients [10.8%; 6.6 E/100 PY]. Oral fungal infection and vulvovaginal mycotic infection were most frequently reported [n = 2 each]. The remaining fungal infections were single events of Blastocystis infection, fungal infection of the hands and feet, fungal oesophagitis, fungal skin infection, tinea capitis and vulvovaginal candidiasis. None of these were serious [all grade 1 or 2], and none led to treatment discontinuation. No events of tuberculosis or herpes zoster infections were reported.
Hypersensitivity reactions were reported in 16 patients [24.6%; 10.2 E/100 PY]. The most frequently reported hypersensitivity reactions were rash [n = 5] and eczema [n = 3]. Five events were considered to be related to the study drug according to the investigator, and no events led to discontinuation of risankizumab treatment. All hypersensitivity reactions were assessed as grade 1 or 2, except for one SAE, which was an anaphylactic reaction due to iron carboxymaltose [Ferinject] infusion.
Hepatic events were reported in six patients [9.2%; 10.2 E/100 PY]; 15 events involved increased liver enzymes. A single event of hepatic steatosis was observed in a patient with a body mass index >30 kg/m2. All hepatic events were assessed as grade 1, none were serious and none led to discontinuation of risankizumab. Potentially clinically important increases in liver enzymes were rare [Supplementary Table 2], and increases in alanine aminotransferase and aspartate aminotransferase returned to normal levels during the OLE. No patients met the criteria for Hy’s Law.
No malignancies or adjudicated major adverse cardiovascular events were reported during this OLE study.
In general, changes in laboratory values were not considered clinically meaningful [Supplementary Table 2]. There were few individual cases of ≥grade 3 laboratory values and most of them were transient. Three patients had ≥grade 3 decreases in lymphocytes, of whom two had ≥grade 3 decreases only once during the study, and all decreases resolved without discontinuation. Six patients had ≥grade 3 increases in creatine kinase, five of whom experienced increases only once during the study, and three of whom experienced increases during the follow-up period. All increases resolved without discontinuation. Most of these events were assessed by the investigator as temporary and not clinically meaningful. Five patients experienced ≥grade 3 increases in triglycerides, one of whom had increased triglycerides prior to this study. No persistent trend was observed. All increases in triglycerides resolved, and no patients discontinued risankizumab due to increases in triglycerides.
Three pregnancies were reported during this OLE: two pregnancies occurred during maternal treatment with risankizumab 180 mg SC Q8W, and the third was the pregnancy of a patient’s partner. Maternal use of risankizumab was discontinued after pregnancy was confirmed. One of the patients with maternal use of risankizumab pre-conception and during the first trimester [having received risankizumab treatment for 575 days in total] surgically terminated her pregnancy because of fetal defects [fetal cystic hygroma and hydrops fetalis] 78 days after risankizumab was discontinued; this was considered by the investigator as having a reasonable possibility of being related to risankizumab. This patient subsequently had a second pregnancy outside the OLE reporting period [after discontinuation of risankizumab for >20 weeks and therefore not included as one of the three pregnancies that occurred during the OLE], which resulted in a miscarriage. The other two pregnancies concluded with live births without any reported complications or abnormalities.
3.3. Pharmacokinetics and immunogenicity
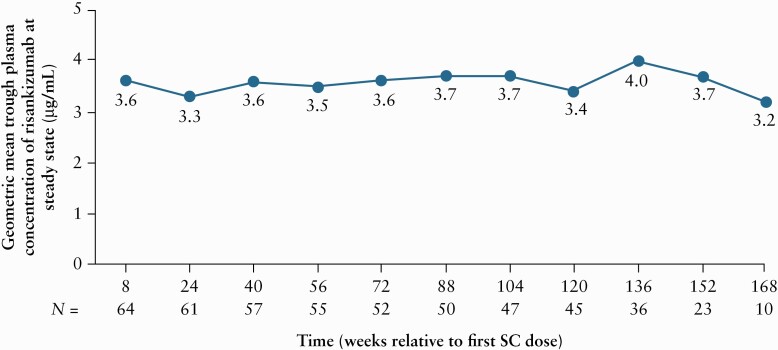
Steady-state plasma exposures of risankizumab were reached by Week 24 and were maintained throughout the study, with geometric mean trough concentrations ranging from 3.2 to 4.0 μg/mL [Figure 2].
Figure 2.
Risankizumab trough plasma concentration over time in all patients. Analysis set included all patients with available pharmacokinetics and ADA data. Patients rolled over to the ongoing phase 3 OLE [M16-000 sub-study 3] between Weeks 128 and 184, and as a result had different last visit time points. ADA, anti-drug antibody; OLE, open-label extension; SC, subcutaneous; SD, standard deviation.
At Week 0 [prior to first risankizumab dose in M15-989], pre-existing ADAs were detected in 2/65 patients [3%] who received at least one dose of risankizumab in the study, possibly due to prior exposure to risankizumab in the parent phase 2 study M15-993. Treatment-emergent ADAs developed in 8/65 patients [12%] during this OLE, none of whom developed neutralizing antibodies. Four patients with treatment-emergent ADAs discontinued from the study for the following reasons: AE, pregnancy, withdrawal of consent and lack of efficacy, respectively. ADA titres ranged from 1 to 64 with no apparent impact on risankizumab plasma exposure in all but one patient. Further pharmacokinetic and immunogenicity data will be collected and their correlations with safety and efficacy outcomes will be analysed in phase 3 studies.
3.4. Efficacy, HRQoL and biomarkers
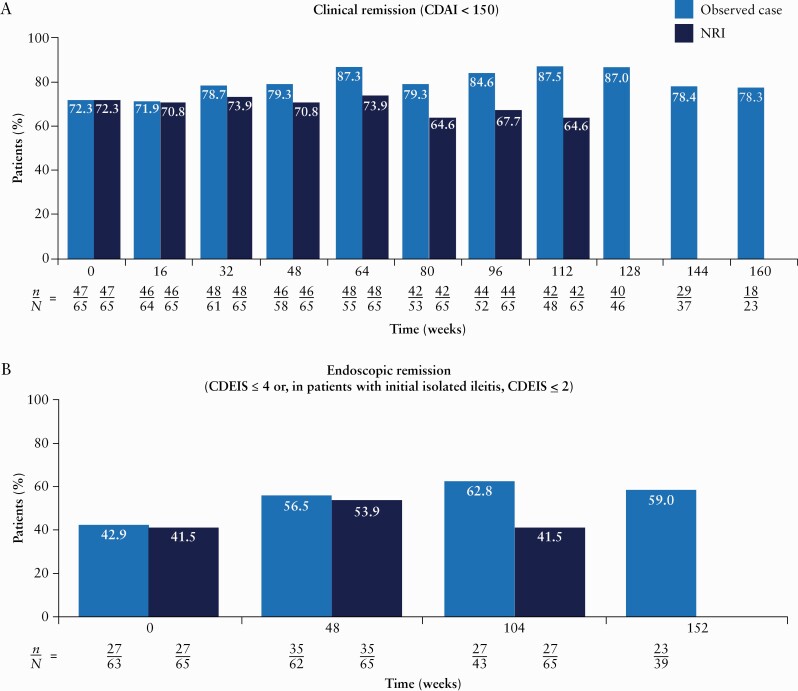
The proportions of patients in clinical remission and endoscopic remission were maintained with long-term risankizumab treatment throughout the duration of the study. Central reviewer scoring was used for all endoscopic efficacy assessments. Clinical remission was achieved in >71% of patients in the observed analysis and >64% of patients in the NRI analysis at any given visit through Week 112 of OLE therapy [Figure 3A]. Endoscopic remission was achieved in >42% of patients in the observed analysis and >41% of patients in the NRI analysis at any given visit through Week 104 [Figure 3B].
Figure 3.
Proportions of patients achieving clinical remission [A], CDEIS remission [B], IBDQ response [C] and IBDQ remission [D] over time [intent-to-treat analysis set]. From Week 128 onwards, patients could roll over to the ongoing phase 3 OLE [M16-000 sub-study 3]. This resulted in a decrease in the number of patients continuing towards completion in the current phase 2 OLE study and prior to rollover into the phase 3 OLE for further risankizumab treatment and follow-up. Therefore, NRI for missing data was not presented after Week 128. Only observed cases were presented beyond Week 128. n denotes the number of responders and N denotes the number of patients at risk. CDAI, Crohn’s Disease Activity Index; CDEIS, Crohn’s Disease Endoscopic Index of Severity; IBDQ, Inflammatory Bowel Disease Questionnaire; NRI, non-responder imputation; OLE, open-label extension.
A high proportion of patients reported HRQoL improvements from baseline [prior to the first dose of risankizumab in the M15-993 study], assessed by IBDQ response [>88% of patients in the observed analysis and >70% of patients in the NRI analysis; Figure 3C] and IBDQ remission [>58% of patients in the observed analysis and >52% of patients in the NRI analysis; Figure 3D] at any given visit through Week 120. These improvements were observed across all four domains of the IBDQ [bowel symptoms, systemic symptoms, social function and emotional function].
Median reductions from baseline [prior to the first dose of risankizumab in the M15-993 study] in hs-CRP and faecal calprotectin observed at Week 0 of this OLE were generally maintained over the course of the OLE through Week 152 [Supplementary Figure 1A and B]. Increased serum albumin levels at Week 0 were also generally maintained through Week 152 [Supplementary Figure 1C].
4. Discussion
In this OLE, risankizumab 180 mg SC Q8W maintenance treatment was well tolerated by patients with CD with over 3 years and 167 PY of treatment, and no new safety signals were observed. As secondary outcomes, the pharmacokinetics of risankizumab were consistent with previous observations,9,10 and efficacy was maintained during the study. Patients included in this study had treatment-refractory CD at M15-993 baseline and completed both the M15-993 and M15-989 studies before rolling over to the phase 3 OLE, suggesting that the safety and efficacy of risankizumab were compelling enough to warrant continued risankizumab.
The majority of SAEs were gastrointestinal in nature and may reflect underlying CD. Rates of serious infections, opportunistic infections and fungal infections in the OLE [4.2, 1.8, and 6.6 E/100 PY, respectively] were similar to those reported in the parent phase 2 M15-993 study [4.6, 2.7 and 7.3 E/100 PY, respectively].9 Serious infections were predominantly driven by gastrointestinal events, and no cases of tuberculosis [including latent tuberculosis] or herpes zoster were reported. Hypersensitivity reactions were observed in about 25% of patients and occurred at a lower rate than that observed in the M15-993 study. There was one serious anaphylactic reaction to IV iron carboxymaltose administration that did not lead to drug discontinuation. No deaths, malignancies or adjudicated cardiovascular events were reported.
Laboratory values ≥grade 3 were infrequent and usually transient. The safety profile of risankizumab 180 mg in patients with CD was generally consistent with previous reports in psoriasis and psoriatic arthritis, despite the difference in risankizumab doses evaluated across indications [150 mg every 12 weeks for psoriasis and 150 mg every 4 or 12 weeks for psoriatic arthritis].11–13 The types of TEAEs reported with risankizumab in patients with CD were similar to those reported by studies of ustekinumab, an IL-12/23 inhibitor.14–16
Of the three pregnancies reported during the OLE, two resulted in healthy births, while the third was surgically terminated due to fetal abnormalities. It is important to note that this OLE was not designed to evaluate the effects of risankizumab on pregnancies, as women of childbearing potential had agreed to use and remain on contraception from the date of screening until 20 weeks after the last dose of risankizumab treatment according to the protocol.
Steady-state plasma exposure was achieved by Week 24 following administration of risankizumab 180 mg SC Q8W. The development of largely low-titre, non-neutralizing ADAs in eight patients was not expected to affect target binding sites on risankizumab and did not appear to have a clear impact on risankizumab plasma exposures. These results are consistent with previous studies of psoriasis, in which only high-titre ADAs [>128] for risankizumab had a meaningful impact on pharmacokinetics.17,18 Therefore, no marked impact on risankizumab exposure, and thus efficacy and safety, was expected given that the majority of the ADAs in this study were of low titre. However, these results should be interpreted with caution due to the small sample size of the study. Although four of the eight patients who developed treatment-emergent ADAs discontinued from the study, based on review of the time-course data for ADA titres, risankizumab exposure and reasons for discontinuation there was no apparent correlation between immunogenicity and discontinuation.
The main limitation of this OLE was the relatively small sample size [n = 65]. Another limitation was that this study was not powered for statistical comparisons. The open-label study design and lack of comparator or control treatment arms meant that the study was more likely to be subject to bias than a randomized controlled trial. In addition, interpretation of safety outcomes is more challenging without a control group. However, objective evaluations including endoscopy were performed prospectively. As all patients were required to remain on any concomitant treatments throughout the OLE, the achievement of steroid-free response or remission could not be assessed. While the small sample size of this study may limit the generalizability of the findings, safety and efficacy data will be confirmed by ongoing phase 3 studies with larger patient populations.
In this OLE, maintenance treatment with risankizumab 180 mg SC Q8W for up to 184 weeks was well tolerated by patients with moderate-to-severe refractory CD, and no new safety signals were observed. Results from ongoing phase 3 studies, including the phase 3 OLE, will further inform the efficacy and safety of risankizumab in patients with CD.
Supplementary Material
Acknowledgments
AbbVie and the authors thank all study investigators for their contributions and the patients who participated in these studies. Medical writing support was provided by Hilary Wong, PhD, of 2 the Nth [Cheshire, UK], funded by AbbVie.
Conference Presentation
Part of this work was presented in an oral presentation at the European Crohn’s and Colitis Organisation Congress, Vienna, Austria in 2020 [Ferrante M, et al. J Crohns Colitis 2020;14[Suppl.1]:S024–5].
Funding
This work was supported by Boehringer Ingelheim and AbbVie. Boehringer Ingelheim and AbbVie participated in the study design, research, analysis, data collection, interpretation of data, review and approval of the publication. AbbVie provided writing support for this publication. All authors had access to relevant data and participated in the drafting, review and approval of this publication. No honoraria or payments were made for authorship.
Conflict of Interest
M.F. has received research grants from Amgen, Biogen, Janssen, Pfizer and Takeda; consultancy fees from AbbVie, Boehringer Ingelheim, Celltrion, Janssen, Lilly, Medtronic, MSD, Pfizer, Sandoz, Takeda and Thermo Fisher; and speakers’ fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, Sandoz, Takeda and Truvion Healthcare. B.G.F. has received consultancy fees from Abbott/AbbVie, ActoGeniX, Akros, Albireo Pharma, Amgen, AstraZeneca, Avaxia Biologics Inc., Avir Pharma, Axcan, Baxter Healthcare Corp., Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, enGene, Ferring, gIcare Pharma, Gilead Sciences, Given Imaging, GSK, Ironwood Pharma, Janssen Biotech [Centocor], JnJ/Janssen, Kyowa Hakko Kirin Co., Ltd, Lexicon, Lilly, Lycera BioTech, Mesoblast Pharma, Millennium, MSD, Nektar, Nestlé, Novartis, Novo Nordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Roche/Genentech, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, VHsquared Ltd., Warner Chilcott, Wyeth, Zealand and Zyngenia; and grants and research support from Abbott/AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Janssen Biotech [Centocor], JnJ/Janssen, Millennium, Pfizer, Receptos, Roche/Genentech, Sanofi, Santarus, Tillotts and UCB Pharma; and is on the board of directors for Robarts Clinical Trials. J.P. has received consultancy fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Genentech, GoodGut, GSK, Janssen, Origo, Pandion, Pfizer, Progenity, Robarts, Roche, Takeda, Theravance and Wassermann; speakers’ fees from AbbVie, Janssen and Takeda; and research funding from AbbVie and Pfizer. F.B. has received research grants from AbbVie, Amgen, Chiesi, Ipsen and MSD; and speakers’ and consultancy fees from AbbVie, Arena, Falk, Ferring, Janssen, MSD, Mundipharma, Pfizer, Takeda and Vifor Pharma. E.L. has received research grants from Janssen, Pfizer and Takeda; educational grants from AbbVie, Janssen, MSD and Takeda; speakers’ fees from AbbVie, Falk, Ferring, Hospira, Janssen, MSD, Pfizer and Takeda; and consultancy fees from AbbVie; and participated in advisory boards with AbbVie, Celgene, Ferring, Hospira, Janssen, MSD, Pfizer and Takeda. O.D. has received speakers’ or consultancy fees from AbbVie, Ferring, Janssen, MSD, Mylan, Pfizer, Sandoz and Takeda. A.K. has received consultancy fees from Applied Molecular Transport, AstraZeneca, Boehringer Ingelheim, Galapagos, Genentech, Gilead Sciences, Glenmark, GSK, Hospira, Janssen, Kintai Therapeutics, Novartis, Pfizer, Roche and VHsquared Ltd. G.R.D. has received consultancy and/or speakers’ fees from AbbVie, ActoGeniX, AIM, Allergan, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene/Receptos, Celltrion, Cosmo Technologies, Elan Pharmaceuticals, enGene, Falk, Ferring, Galapagos, Genentech, Gilead Sciences, Giuliani SpA, Given Imaging, Gossamer Bio, GSK, Janssen, Lilly, MSD, Neovacs, Novo Nordisk, Otsuka, PDL BioPharma, Pfizer, Progenity, Prometheus Laboratories, Robarts Clinical Trials, Salix, Schering-Plough, Seres/Nestlé, SetPoint, Shire, Takeda, Tillotts, UCB Pharma, Versant and Vifor Pharma; research grants from AbbVie, Falk, Given Imaging, Janssen, MSD and PhotoPill; and speaking honoraria from AbbVie, Ferring, MSD, Norgine, Shire, Tillotts, Tramedico and UCB Pharma. W.R.D., Y.P., W.-J.L., D.G., X.L., K.W. and J.K. are AbbVie employees and may own AbbVie stock and/or options.
Author Contributions
M.F., B.G.F., J.P., F.B., E.L., O.D., A.K., W.R.D., Y.P., W.-J.L., D.G., X.L., K.W., J.K. and G.R.D. contributed substantially to the conception and design of the work; the acquisition, analysis and interpretation of data for the work; drafting the manuscript and revising it critically for important intellectual content; and reviewing and providing final approval of the version to be published, with the authors maintaining control over the final content.
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials they sponsor. This includes access to anonymized, individual and trial-level data [analysis data sets], as well as other information [e.g. protocols and clinical study reports], as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. The clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan, and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1. Gomollón F, Dignass A, Annese V, et al. ; ECCO. 3rd European Evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 2. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 3. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 4. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013;145:158–65.e2. [DOI] [PubMed] [Google Scholar]
- 5. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020;14:4–22. [DOI] [PubMed] [Google Scholar]
- 6. AbbVie. Skyrizi (risankizumab) [package insert]. European Medicines Agency website. July 13, 2020. https://www.ema.europa.eu/en/documents/product-information/skyrizi-epar-product-information_en.pdf. Accessed November 11, 2020.
- 7. AbbVie. Skyrizi (risankizumab) [package insert]. U.S. Food and Drug Administration website. July 13, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed November 11, 2020.
- 8. Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017;389:1699–709. [DOI] [PubMed] [Google Scholar]
- 9. Feagan BG, Panés J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol 2018;3:671–80. [DOI] [PubMed] [Google Scholar]
- 10. Suleiman AA, Khatri A, Minocha M, Othman AA. Population pharmacokinetics of the interleukin-23 inhibitor risankizumab in subjects with psoriasis and Crohn’s disease: analyses of phase I and II trials. Clin Pharmacokinet 2019;58:375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018;392:650–61. [DOI] [PubMed] [Google Scholar]
- 12. Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019;394:576–86. [DOI] [PubMed] [Google Scholar]
- 13. Mease PJ, Kellner H, Morita A, et al. OP0307 Efficacy and safety of risankizumab, a selective il-23p19 inhibitor, in patients with active psoriatic arthritis over 24 weeks: results from a phase 2 trial. Ann Rheum Dis 2018;77:200–1. [Google Scholar]
- 14. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther 2018;48:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghosh S, Gensler LS, Yang Z, et al. Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of phase II/III clinical development programs. Drug Saf 2019;42:751–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khatri A, Suleiman AA, Polepally AR, Othman AA. Exposure–response relationships for efficacy and safety of risankizumab in phase II and III trials in psoriasis patients. Clin Pharmacol Ther 2020;107:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pang Y, Khatri A, Suleiman AA, Othman AA. Clinical pharmacokinetics and pharmacodynamics of risankizumab in psoriasis patients. Clin Pharmacokinet 2020;59:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials they sponsor. This includes access to anonymized, individual and trial-level data [analysis data sets], as well as other information [e.g. protocols and clinical study reports], as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. The clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan, and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.