Abstract
Introduction
Excess visceral fat increases the risk of type 2 diabetes and cardiovascular disease and is influenced by sex hormones. Our aim was to investigate changes in visceral fat and the ratio of visceral fat to total body fat (VAT/TBF) and their associations with changes in lipids and insulin resistance after 1 year of hormone therapy in trans persons.
Methods
In 179 trans women and 162 trans men, changes in total body and visceral fat estimated with dual-energy X-ray absorptiometry before and after 1 year of hormone therapy were related to lipids and insulin resistance [homeostatic model assessment of insulin resistance (HOMA-IR)] with linear regression analysis.
Results
In trans women, total body fat increased by 4.0 kg (95% CI 3.4, 4.7), while the amount of visceral fat did not change (−2 grams; 95% CI −15, 11), albeit with a large range from −318 to 281, resulting in a decrease in the VAT/TBF ratio of 17% (95% CI 15, 19). In trans men, total body fat decreased with 2.8 kg (95% CI 2.2, 3.5), while the amount of visceral fat did not change (3 g; 95% CI −10, 16; range −372, 311), increasing the VAT/TBF ratio by 14% (95% CI 10, 17). In both groups, VAT/TBF was not associated with changes in blood lipids or HOMA-IR.
Conclusions
Hormone therapy in trans women and trans men resulted in changes in VAT/TBF, mainly due to changes in total body fat and were unrelated to changes in cardiometabolic risk factors, which suggests that any unfavorable cardiometabolic effects of hormone therapy are not mediated by changes in visceral fat or VAT/TBF.
Keywords: visceral fat, transgender, hormone therapy, insulin resistance, lipids
Abdominal obesity—in particular, an excess of visceral fat—is strongly related to cardiovascular risk factors such as insulin resistance, dyslipidemia (1-3), and the development of type 2 diabetes and cardiovascular disease (4,5). The hypothesized mechanisms for a specific harmful role of excess visceral fat are its high secretion rate of proinflammatory cytokines and the high rate of lipolysis, resulting in an excess of free fatty acids drained by the portal vein to the liver (1).
Sex hormones play a major role in the regulation of both adipose tissue distribution and function (6). Whereas women have more total body fat and subcutaneous adipose tissue than men, men have more visceral fat than women (7). In a population-based study, higher testosterone concentrations were associated with increased visceral fat in women and decreased visceral fat in men (8). Likewise, in hypogonadal men, low testosterone concentrations are associated with visceral obesity (9). On the contrary, women with polycystic ovary syndrome (PCOS) have higher testosterone concentrations and visceral fat compared to women without PCOS (10). In addition, during menopause, estradiol levels decrease, while the amount of visceral fat increases (11). Altogether, this suggests a role for sex steroid hormones in the deposition of visceral fat (7).
Trans women (birth-assigned males, female gender identity) receive antiandrogen and estradiol treatment to induce feminization, and trans men (birth-assigned females, male gender identity) may be treated with testosterone to induce masculinization. In a previous study among trans women and trans men, we observed major changes in total body fat during the first year of hormone therapy (12). Based on the changes in visceral fat observed in hypogonadal men and women with PCOS, we hypothesize increases in visceral fat in trans women with suppressed testosterone levels and in trans men with increased testosterone levels.
In the 1990s, Elbers et al (13) pioneered by measuring visceral fat with magnetic resonance imaging (MRI) in small groups of lean trans women (n = 20) and trans men (n = 17). After 1 year of hormone therapy, they showed an increase in visceral fat of 18% in trans women and 13% in trans men (13-16). However, studies investigating changes in visceral fat in larger populations with a wider body mass index (BMI) range and contemporary treatment protocols have not been performed yet.
Our study group previously showed favorable changes in blood lipids in trans women and unfavorable lipid changes in trans men after 1 year of hormone therapy (17). Another recent study reported that masculinizing hormone therapy decreased insulin resistance, while feminizing hormone therapy induced insulin resistance (18). However, the underlying mechanisms responsible for the metabolic effects observed during hormone therapy are unknown and may be associated with changes in visceral fat.
The aim of the present study is therefore twofold. First, we aimed to investigate changes in the amount of visceral fat and the ratio of visceral fat to total body fat (VAT/TBF), as a more accurate measure of body fat distribution in transgender individuals after the first year of hormone therapy. Second, we aimed to examine whether changes in visceral fat and VAT/TBF were related to changes in blood lipids and insulin resistance.
Methods
Study Design and Study Population
This study is embedded in the ENIGI project (European Network for the Investigation of Gender Incongruence), a multicenter prospective observational study (19, 20). From 2010, transgender persons were eligible to participate in the study when they were 18 years or older and had gender dysphoria according to definition in the fourth edition, text revision, or fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (21, 22). Persons were not eligible when they started in a different treatment protocol than described in the following discussion or in case of previous gender-affirming hormone use or genital surgery, insufficient knowledge of the Dutch language, or psychological vulnerability. Participants visited the outpatient clinic every 3 months during the first year of hormone therapy for clinical data collection (19).
Different type of dual-energy X-ray absorptiometry (DXA) scanners were used in participating gender clinics (Amsterdam and Ghent: Hologic Discovery A; Oslo: Lunar; Florence: Hologic Delphi). Because the use of different types of DXA scanners results in noncomparable body composition data, only participants from Amsterdam and Ghent were selected for the present study. Further, persons were included if they started hormone therapy between February 2010 and March 2015 and if they completed the first year of hormone therapy. Also, the baseline DXA had to be performed within 3 months before the start of hormone therapy or 1 month after the start of hormone therapy. The follow-up DXA had to be performed between 10 months and 14 months after the start of hormone therapy. The participant inclusion flow chart has been previously published (23).
The Ethics Committee of Ghent University Hospital, Belgium, approved the overall study protocol. The other participating centers also obtained approval of their local ethical committees. Informed consent was obtained according to the institutional guidelines.
Treatment Protocol
Hormone therapy in trans women included in this study consisted of cyproterone acetate 50 mg/day in combination with oral estradiol valerate 4 mg/day or a transdermal estradiol patch 100 mcg/24 h twice a week. The estradiol patch was preferred if persons were above 40 years old or if they had a history of cardiovascular disease or thromboembolic events. Trans men were treated with 1 of the following testosterone formulations: testosterone gel 50 mg/day, testosterone undecanoate 1000 mg intramuscular once per 12 weeks, or testosterone esters 250 mg intramuscular once per 2 weeks. Participants were eligible for genital surgery after 12 months of hormone therapy. Thus, no participants in the current study had received genital surgery during the observation period.
Clinical Data Collection
Body height was measured to the nearest centimeter using a Harpenden stadiometer. Body weight was measured to the nearest 0.1 kg. BMI was calculated as body weight in kilograms divided by height in meters squared (kg/m2).
Total Body Fat, Lean Body Mass, and Visceral Adipose Tissue Estimation by DXA
Total body fat, lean body mass, and visceral fat were estimated using DXA (24-26) before and after approximately 12 months of hormone therapy. All DXA scans were analyzed with Hologic software version 13.5.3 according to the sex assigned at birth using the user’s instruction manual.
Whole body DXA is a 2-dimensional method to examine body composition. The DXA scanner produces 2 beams of high and low energies that are attenuated in the body (27). Adipose tissue has a different X-ray attenuation than lean body mass because adipose tissue has a higher hydrogen content. In every pixel, the attenuation is measured, and every high and low energy attenuation pair is related to a unique combination of fat mass and fat-free mass. The amount of total body fat or total lean body mass was calculated as the sum of body fat and lean body mass in every pixel and is expressed in both grams and percentages.
For the estimation of visceral fat, we restricted to the adipose tissue within the abdominal cavity. When body fat is measured as previously described, the body fat in this 2-dimensional image is a sum of the visceral fat inside the abdominal cavity and the subcutaneous fat on the anterior and posterior side of the body. Therefore, additional software was used to estimate solely visceral fat (24, 25). A 5-cm-wide region was placed across the abdomen just above the iliac crest at a level that approximately coincides with the fourth lumbar vertebrae. In this region, the abdominal cavity is indicated by a lighter grey color because the musculature in the abdominal wall appears lighter than the (darker) subcutaneous fat tissue outside the abdominal cavity. To estimate the amount of visceral fat, the amount of anterior and posterior subcutaneous fat is estimated by measuring the subcutaneous fat on the sides of the body. Then, a total amount of subcutaneous fat is estimated, and this is subtracted from the total amount of abdominal fat, which gives the amount of visceral fat in grams (24, 25). VAT/TBF was calculated and expressed in percentage.
Laboratory Measurements
Fasting venous blood samples were obtained at the start of hormone therapy and after 12 months of treatment. In Ghent, total cholesterol, glucose, insulin, high-density lipoprotein (HDL)-cholesterol, and triglycerides were measured using Roche Cobas chemistry analyzers (c701 module or c501 module; Modular, Roche Diagnostics, Mannheim, Germany). The interassay coefficients of variation (CV) were total cholesterol 1.3%, glucose 0.9%, insulin 2.3%, HDL-cholesterol 1.8%, and triglycerides 2.5%. In Amsterdam, total cholesterol, glucose, HDL-cholesterol, and triglycerides were measured using Roche Cobas chemistry analyzers (c701 module or c502 module; Modular, Roche Diagnostics, Mannheim, Germany). The interassay CV were as follows: total cholesterol 1.4%, glucose 1.1%, HDL-cholesterol 0.9%, and triglycerides 1.8%. Insulin was measured using an immunometric assay (Luminescence Advia Centaur, Siemens Medical Solutions Diagnostics, USA) with a CV of 7%. The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = (fasting glucose/fasting insulin in mU/L)/22.5 (28). low-density lipoprotein (LDL)-cholesterol was calculated using the Friedewald formula (29).
In Amsterdam, estradiol was measured using a competitive immunoassay (Delfia; PerkinElmer, Turku, Finland) with an interassay CV range of 10% to 13% and a lower limit of quantitation (LOQ) of 20 pmol/L until July 2014. After July 2014, estradiol was measured using liquid chromatography tandem mass spectrometry (VUmc, Amsterdam, the Netherlands) with an interassay CV of 7%and an LOQ of 20 pmol/L. For conversion of the Delfia values, the following formula was used: liquid chromatography-tandem mass spectrometry = 1.60 * Delfia − 29. Testosterone was measured using a radioimmunoassay (RIA; Coat-A-Count; Siemens, Los Angeles, CA, USA) with an interassay CV range of 7% to 20% and an LOQ of 1 nmol/L until January 2013. Thereafter, testosterone was measured using a competitive immunoassay (Architect; Abbott, Abbott Park, IL, USA) with an interassay CV range of 6% to 16% and an LOQ of 0.1 nmol/L. The RIA values were converted to the competitive immunoassay values. The following formula was used: Architect = 1.34 * RIA − 1.65. In Ghent, estradiol was measured using an E170 Modular (Gen II; Roche Diagnostics, Mannheim, Germany) until March 19, 2015. Thereafter, estradiol was measured using a E170 Modular (Gen III; Roche Diagnostics), with an interassay CV of 3.2% and an LOQ of 25 pmol/L. For conversion of estradiol values measured before March 19, 2015, the following formula was used: Gen III = 6.687940 + 0.834495 * Gen II. E170 Modular was also used to measure testosterone and had an interassay CV of 2.6% and an LOQ of 0.4 nmol/L.
Statistical Analyses
Baseline characteristics of trans women and trans men were expressed as median (interquartile range) or as percentage. First, linear mixed model regression analyses with observations clustered within center and within participants were performed to examine changes in visceral fat, total body fat, VAT/TBF, lean body mass, blood lipids, and HOMA-IR during the first year of hormone therapy. For all analyses with HOMA-IR, participants with diabetes mellitus were excluded. Both absolute and relative changes in measures of body fat distribution and body composition were calculated.
During treatment, persons who were overweight (BMI 25-30 kg/m2) or obese (BMI > 30 kg/m2) were advised to maintain a healthy lifestyle to lose body weight. In the latter group, this was even more emphasized because of requirements regarding body weight when applying for genital surgery (BMI between 18 and 30 kg/m2 for vaginoplasty or phalloplasty). Since these lifestyle advices might have affected changes in measured of body fat distribution, BMI at start and the interaction between BMI at start and time were added to the linear mixed model to examine the influence of BMI at start on the change in VAT/TBF. BMI at start was defined by the following categories: BMI < 25 kg/m2, BMI 25-30 kg/m2, and BMI > 30 kg/m2.
Next, linear regression analyses were performed to examine the associations between changes in VAT/TBF and changes in cardiometabolic risk factors. These analyses were adjusted for change in lean body mass. Additional regression analyses were performed to study associations between visceral fat, total body fat, and lean body mass with changes in cardiometabolic risk factors. As a sensitivity analysis, we evaluated the cross-sectional associations between visceral fat and VAT/TBF with cardiometabolic risk factors at baseline, adjusted for either lean body mass and total body mass (visceral fat) or lean body mass (VAT/TBF).
Results
In this study, 179 trans women and 162 trans men who started hormone therapy between February 2010 and March 2015 in Amsterdam (n = 266) and Ghent (n = 75) and who completed the first year of treatment were included. Baseline characteristics and hormone levels before and during treatment are shown in Table 1. Estradiol levels increased in transgender women, while testosterone sharply decreased. In transgender men, testosterone levels increased. Estradiol levels slightly increased due to the aromatization of testosterone to estradiol. For trans women at baseline, the mean amount of visceral fat was 353 g, and the mean amount of total body fat was 19.1 kg. For trans men, the mean amount of visceral fat was 277 g, and the mean amount of total body fat was 26.0 kg.
Table 1.
General characteristics
| Trans women | Trans men | |
|---|---|---|
| (n = 179) | (n = 162) | |
| At baseline | ||
| Age (years) | 29 (23, 43) | 24 (21, 33) |
| Body mass index (kg/m2) | 23.0 (20.5, 26.6) | 24.6 (21.7, 29.1) |
| Smokers (%) | 25 | 28 |
| Alcohol consumption (unit/day) | 0 (0, 2) | 0 (0, 2) |
| White (%) | 98 | 93 |
| Median hormone concentrations at start | ||
| Estradiol level (pmol/L) | 97 (80, 116) | 144 (76, 311) |
| Testosterone level (nmol/L) | 18.9 (14.0, 22.0) | 1.3 (1.0, 1.6) |
| Median hormone concentrations during treatment | ||
| Estradiol level (pmol/L) | 204 (145, 327) | 173 (121, 254) |
| Testosterone level (nmol/L) | 0.7 (0.5, 0.9) | 27.5 (19.0, 39.0) |
Data are presented as median (25th, 75th percentile).
Changes in Visceral Fat, Other Body Composition Measures, and Cardiometabolic Risk Factors
The mean change in visceral fat in trans women was −2 g (95% CI −15, 11) with reflects −1% (95% CI −5, 3), with a large range from −318 g to 281 g. In trans men, the mean change in visceral fat was 3 g (95% CI −10, 16), which reflects 1% (95% CI −4, 5), ranging from −372 to 311 g. VAT/TBF decreased by 17% (95% CI −19, −15) in trans women and increased by 14% (95% CI 11, 18) in trans men. Absolute changes in measures of body fat distribution and body composition are reported in Table 2, and the relative changes of these measures are shown in Figure 1. Table 2 additionally presents the absolute changes in cardiometabolic risk factors. In trans women, total cholesterol (− 12%, 95% CI −15, −11), HDL-cholesterol (−11%, 95% CI −15, −9), LDL-cholesterol (−12%, 95% CI −16, −9), and triglycerides (−18%, 95% CI −28, −9) decreased. HOMA-IR increased by 47% (95% CI 32, 64). In trans men, total cholesterol did not change (0%, 95% CI −3, 3), but HDL-cholesterol (−15%, 95% CI −19, −11) decreased, and LDL-cholesterol (7%, 95% CI 3, 10) and triglycerides (19%, 95% CI 10, 27) increased. HOMA-IR decreased with 25% (95% CI −39, −13). The changes in VAT/TBF were similar among different BMI categories (Fig. 2).
Table 2.
Absolute changes in measures of body fat distribution, body composition, and cardiometabolic risk factors during 1 year of hormone therapy in trans women and trans men
| Start of treatment | Mean change (95% CI) | |
|---|---|---|
| Trans women (n = 179) | ||
| Body composition | ||
| Visceral fat (g) | 353 (322, 384) | −2 (−15, 11) |
| VAT/TBF (%) | 1.84 (1.75, 1.92) | −0.31 (−0.35, −0.27) |
| Total body fat (kg) | 19.1 (17.9, 20.2) | 4.0 (3.4, 4.7) |
| Lean body mass (kg) | 57.2 (56.0, 58.4) | −1.7 (−2.1, −1.3) |
| Cardiometabolic parameters | ||
| Total cholesterol (mmol/L) | 4.6 (4.5, 4.8) | −0.6 (−0.7, −0.5) |
| HDL-cholesterol (mmol/L) | 1.4 (1.3, 1.4) | −0.2 (−0.2, −0.1) |
| LDL-cholesterol (mmol/L) | 2.7 (2.6, 2.9) | −0.3 (−0.4, −0.3) |
| Triglycerides (mmol/L) | 1.1 (1.0, 1.2) | −0.2 (−0.3, −0.1) |
| HOMA-IRa | 2.0 (1.8, 2.3) | +0.9 (0.6, 1.3) |
| Trans men (n = 162) | ||
| Body composition | ||
| Visceral fat (g) | 277 (249, 306) | 3 (−10, 16) |
| VAT/TBF (%) | 1.02 (0.95, 1.09) | 0.14 (0.11, 0.18) |
| Total body fat (kg) | 26.0 (24.4, 27.6) | −2.8 (−3.5, −2.2) |
| Total lean body mass (kg) | 46.9 (45.7, 48.2) | +4.7 (4.2, 5.1) |
| Cardiometabolic parameters | ||
| Total cholesterol (mmol/L) | 4.6 (4.4, 4.7) | 0.0 (−0.1, 0.1) |
| HDL-cholesterol (mmol/L) | 1.5 (1.5, 1.6) | −0.2 (−0.3, −0.2) |
| LDL-cholesterol (mmol/L) | 2.6 (2.5, 2.7) | 0.2 (0.1, 0.3) |
| Triglycerides (mmol/L) | 0.9 (0.8, 1.0) | 0.2 (0.1, 0.3) |
| HOMA-IRa | 2.6 (2.3, 2.9) | −0.7 (−1.0, −0.3) |
Data are shown as mean (95% CI).
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; VAT/TBF, visceral adipose tissue/total body fat ratio.
a Eight trans women and 6 trans men were excluded from the specific analysis for having diabetes mellitus.
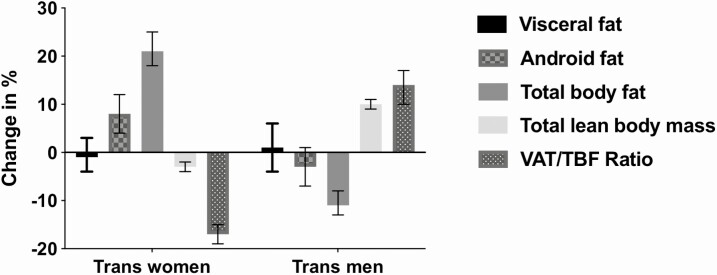
Figure 1.
Relative changes in measures of body fat distribution and body composition during 1 year of hormone therapy in 179 trans women and 162 trans men. Trans women: visceral adipose tissue: −1% (95% CI −4, 3); total body fat 21% (95% CI 18, 25); VAT/TBF: −17% (95% CI −19, −15); and lean body mass: −3% (95% CI −4, −2). Trans men: visceral adipose tissue: 1% (95% CI −4, 6); total body fat: −11% (95% CI −13, −8); VAT/TBF: 14% (95% CI 10, 17); and lean body mass 10% (95% CI 9, 11). Abbreviation: VAT/TBF, visceral adipose tissue/total body fat ratio.
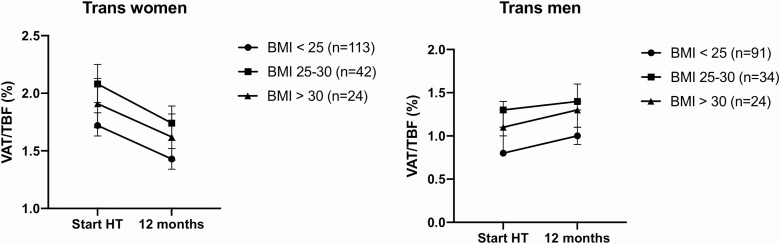
Figure 2.
Change in VAT/TBF per BMI category in trans women and trans men during 1 year of hormone therapy. Trans women: relative change in VAT/TBF: BMI < 25 kg/m2: −17% (95% CI −21, −14); BMI 25-30 kg/m2: −16% (95% CI −22, −1); and BMI > 30 kg/m2: −15% (95% CI −23, −9). Trans men: relative change in VAT/TBF: BMI < 25 kg/m2: 15% (95% CI −10, −18); BMI 25-30 kg/m2: 6% (95% CI 11, 16); and BMI > 30 kg/m2: 15% (95% CI 9, 19). Abbreviation: BMI, body mass index; VAT/TBF, visceral adipose tissue/total body fat ratio.
Relation Between Changes in Measures of Body Fat Distribution Lean Body Mass With Changes in Cardiometabolic Risk Factors
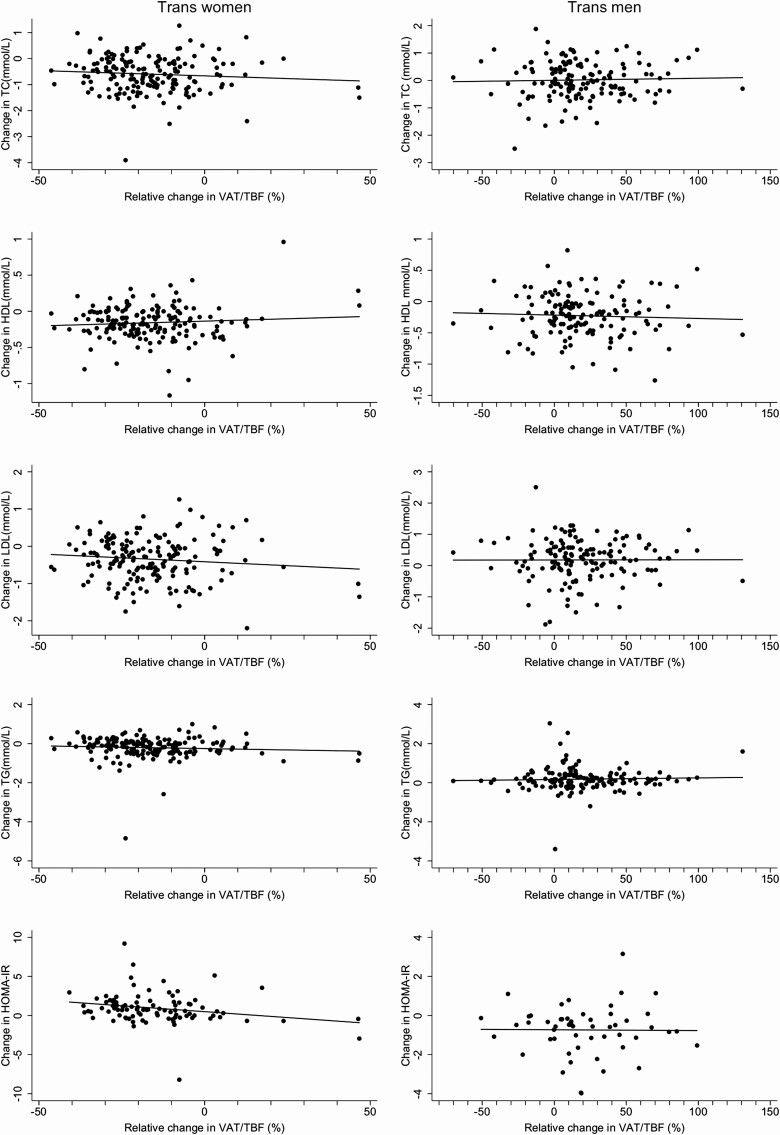
No statistically significant or clinically relevant associations were observed between changes in VAT/TBF and changes in blood lipids or HOMA-IR after 1 year of hormone therapy. Figure 3 shows the associations between the relative change in the ratio of VAT/TBF and the change in blood lipids and HOMA-IR in the total group of trans women and trans men. Table 3 contains an overview of the associations between 1 SD change in visceral fat, VAT/TBF, total body fat, and lean body mass with changes in cardiometabolic risk factors. In trans women, changes in visceral fat and total body fat were associated with changes in total and LDL-cholesterol. An increase in total body fat was additionally associated with an increase in HOMA-IR. In trans men, changes in measures of body fat distribution and body composition were not associated with changes in cardiometabolic risk factors.
Figure 3.
Associations between the change in VAT/TBF and change in cardiometabolic risk factors during 1 year of hormone therapy in 179 trans women and 162 trans men. Eight trans women and 6 trans men were excluded from the analysis on HOMA-IR for having diabetes mellitus. Abbreviations: HDL, high-density lipoprotein-cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; VAT/TBF, visceral adipose tissue/total body fat ratio.
Table 3.
Change in cardiometabolic risk factors per SD increase in visceral fat, visceral adipose tissue/total body fat ratio, total body fat, and lean body mass
| Trans women (n = 179) | Trans men (n = 162) | |||||||
|---|---|---|---|---|---|---|---|---|
| Visceral fat | VAT/TBF | Total body fat | Lean body mass | Visceral fat | VAT/TBF | Total body fat | Lean body mass | |
| (SD 87 g) | (SD 0.25%) | (SD 4468 g) | (SD 2832 g) | (SD 83 g) | (SD 0.23%) | (SD 4333 g) | (SD 2721 g) | |
| Total cholesterol (mmol/L) | 0.27 (0.17, 0.36) | 0.02 (−0.08, 0.13) | 0.28 (0.18, 0.37) | 0.16 (0.06, 0.26) | 0.04 (−0.07, 0.15) | 0.01 (−0.10, 0.12) | 0.08 (−0.03, 0.18) | 0.02 (−0.09, 0.12) |
| Adjusted | 0.16 (0.04, 0.27) | 0.06 (−0.04, 0.16) | 0.17 (0.03, 0.30) | 0.03 (−0.09, 0.14) | −0.01 (−0.15, 0.13) | 0.02 (−0.10, 0.13) | 0.09 (−0.06, 0.23) | −0.01 (−0.12, 0.11) |
| HDL-cholesterol (mmol/L) | −0.03 (−0.07, 0.01) | 0.02 (−0.02, 0.05) | −0.03 (−0.07, 0.00) | −0.03 (−0.07, 0.00) | −0.01 (−0.07, 0.05) | 0.00 (−0.06, 0.06) | −0.02 (−0.07, 0.04) | −0.03 (−0.08, 0.03) |
| Adjusted | −0.02 (−0.07, 0.02) | 0.01 (−0.03, 0.05) | −0.01 (−0.06, 0.04) | −0.02 (−0.07, 0.02) | −0.00 (−0.07, 0.07) | −0.00 (−0.06, 0.05) | −0.01 (−0.09, 0.06) | −0.02 (−0.08, 0.04) |
| LDL-cholesterol (mmol/L) | 0.23 (0.15, 0.31) | −0.02 (−0.10, 0.01) | 0.24 (0.16, 0.32) | 0.16 (0.08, 0.24) | −0.02 (−0.13, 0.09) | −0.02 (−0.13, 0.09) | 0.05 (−0.05, 0.16) | −0.02 (−0.13, 0.08) |
| Adjusted | 0.13 (0.04, 0.23) | 0.02 (−0.07, 0.10) | 0.13 (0.02, 0.24) | 0.05 (−0.04, 0.14) | −0.09 (−0.23, 0.05) | −0.03 (−0.14, 0.09) | 0.12 (−0.02, 0.26) | −0.05 (−0.16, 0.06) |
| Triglycerides (mmol/L) | 0.16 (0.08, 0.25) | 0.05 (−0.04, 0.13) | 0.16 (0.08, 0.24) | 0.08 (−0.00, 0.16) | 0.07 (−0.03, 0.17) | −0.00 (−0.10, 0.10) | 0.06 (−0.03, 0.16) | 0.08 (−0.02, 0.17) |
| Adjusted | 0.11 (0.00, 0.21) | 0.07 (−0.02, 0.15) | 0.09 (−0.02, 0.21) | 0.00 (−0.10, 0.10) | 0.05 (−0.08, 0.17) | −0.00 (−0.10, 0.11) | 0.01 (−0.11, 0.14) | 0.07 (−0.04, 0.17) |
| HOMA-IRa | 0.13 (−0.22, 0.49) | −0.37(−0.68, −0.05) | 0.67 (0.26, 1.08) | 0.42 (0.07, 0.78) | 0.18 (−0.13, 0.50) | 0.02 (−0.30, 0.35) | 0.19 (−0.17, 0.56) | 0.32 (−0.06, 0.70) |
| Adjusted | −0.20 (−0.60, 0.20) | −0.29 (−0.62, 0.02) | 0.72 (0.12, 1.32) | 0.10 (−0.34, 0.55) | 0.11 (−0.27, 0.50) | 0.05 (−0.27, 0.38) | 0.07 (−0.38, 0.52) | 0.30 (−0.11, 0.70) |
Data are expressed as mean change in risk factor with 95% CI per SD of measure of body fat or lean body mass. Adjusted: analyses between change in visceral fat and risk factors were adjusted for changes in total body fat and lean body mass. Analyses between change in VAT/TBF and risk factors were adjusted for change in lean body mass. Analyses on total body fat were adjusted for visceral fat and lean body mass. Analyses on lean body mass were adjusted for visceral fat and total body fat.
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; VAT/TBF, visceral adipose tissue/total body fat ratio.
a Eight trans women and 6 trans men were excluded from the specific analysis for having diabetes mellitus.
As a sensitivity analysis, cross-sectional associations between visceral fat and VAT/TBF with total cholesterol, LDL-cholesterol, triglycerides, and HOMA-IR at baseline were observed [see Supplemental Table 1 (30)].
Discussion
In this study, we aimed to investigate the effects of 1 year of hormone therapy on the amount of visceral fat and total body fat and the relationship between changes in VAT/TBF and changes in cardiometabolic risk factors in 179 trans women and 162 trans men. Hormone therapy resulted in only small mean changes in visceral fat. There was, however, a large individual range with regard to change in visceral fat in both trans women and trans men. Mean changes in VAT/TBF in both trans women and trans men were mainly the result of changes in total body fat. In both trans women and trans men, changes in VAT/TBF were unrelated to changes in cardiometabolic risk factors observed during 1 year of hormone therapy.
In cis women (gender identity equal to sex assigned at birth), a large cross-sectional study (8) observed positive associations between both testosterone and estradiol levels and visceral fat. Trans men have high testosterone levels and, instead of cyclic estradiol peaks, stable estradiol levels due to aromatization of testosterone into estradiol. Therefore, we hypothesized an increase of visceral fat in trans men. However, in the Multi-Ethnic Study on Atherosclerosis, estradiol was more strongly associated with visceral fat than testosterone, which suggests a more important role for estradiol in the accumulation of visceral fat in cis women (8). Since the estradiol level remained on luteal level and did not largely change in trans men (due to aromatization of testosterone to estradiol), this might explain why on average visceral fat did not change. Two previous MRI studies reporting on the effects of hormone therapy on visceral fat showed an increase in visceral fat in small groups of trans women (17% and 18%, respectively) and trans men (6%-18%) after 1 year of hormone therapy (13, 16), but these studies were very small (n range 17-20), all with a mean BMI of 21 kg/m2.
In this study, visceral fat did not significantly change in trans women (−1%) nor in trans men (1%). Possibly, the reported changes in previous small studies may have been by chance, as we have observed a large interindividual variation in the change in visceral fat, ranging from −57% to 52%. In cis men, both low testosterone levels and high estradiol levels (8) are associated with an increase in visceral fat (1, 31, 32). Therefore, we hypothesized that in trans women, with suppressed testosterone levels and high estradiol levels, visceral fat would increase. The mean change in visceral fat, however, was small and the concomitant increase in total body fat led to a decrease in VAT/TBF. The large interindividual range of change in visceral fat suggests that other factors (eg, lifestyle factors)—possibly in combination with testosterone—play a more important role in the accumulation of visceral fat.
Although a longer duration of hormone therapy may have resulted in more pronounced mean changes in visceral fat mass, previous studies in trans men and trans women did observe changes after 1 year of sex hormone therapy (13, 16). In addition, lifestyle interventions have shown changes in visceral fat after 12 weeks of diet or exercise (33). Therefore, a duration of 1 year should suffice to be able to observe changes in visceral fat and cardiometabolic risk factors. Future studies should investigate changes in visceral fat, preferably with MRI, and in cardiometabolic risk after a longer duration of hormone therapy.
In a previous study, favorable changes in lipid profile were observed in trans women, and unfavorable changes, in trans men (17). Based on earlier studies (1, 7, 31, 34-37), we hypothesized that unfavorable changes in lipid profile and HOMA-IR in trans men would be related to increases in visceral fat during the first year of hormone therapy. Although the mean changes in visceral fat were small, we still evaluated the associations between visceral fat and cardiometabolic risk factors due to the large interindividual range observed in the change of visceral fat over time. VAT/TBF was specifically used as it is a better measure of relative body fat distribution and accounts for changes in subcutaneous fat, which is often related with changes in visceral fat (38) and may even represent a translocation of lipids from the visceral to subcutaneous areas. Our results suggest that in transgender hormone therapy, the directional changes in visceral fat and total body fat seem to dissociate, opposite to what is often observed in the general population. Although we confirmed previously reported cross-sectional associations of visceral fat and VAT/TBF with cardiometabolic risk factors in the general population, in the present study changes in visceral fat and VAT/TBF were not associated with the unfavorable changes in blood lipids in trans men. Possibly, the lack of a concomitant decrease in visceral fat with the decrease in total body fat observed may explain why an unfavorable lipid profile was observed in trans men—opposite to what is expected with the degree of total body fat loss observed in this group. Similarly, the observation that visceral fat, on average, did not change in trans women, despite a large increase in total body fat, may explain why favorable, instead of expected unfavorable effects, on blood lipids were found in this group. However, both visceral fat and total body fat were independently positively associated with total cholesterol and LDL-cholesterol in trans women. An alternate explanation, which may be more likely, is that the metabolic alterations observed in both trans men and trans women are additionally affected by direct hepatic effects of hormone therapy (39-41).
We observed large changes in insulin resistance, with an increase in trans women and a decrease in trans men after 1 year of hormone therapy, which is in line with a previous report evaluating changes in HOMA-IR after 1 year of hormone therapy (18). Despite strong associations between visceral adiposity and insulin resistance in the general population (36), changes in visceral fat and VAT/TBF were not related to the changes in HOMA-IR. In trans women, the increase in HOMA-IR was solely associated with an increase in total body fat but not with VAT/TBF. The increase in total body fat and decrease in lean body mass may explain the increase in HOMA-IR in trans women, although we did not find an association between lean body mass and HOMA-IR in this study. Another plausible explanation for the increase in HOMA-IR can be found in a recent study by Gava et al (42)., who compared metabolic effects of the antiandrogen agents cyproterone acetate and gonadotropin-releasing hormone analogues. The authors observed an increase in HOMA-IR only in trans women using cyproterone acetate and suggested that cyproterone acetate may have direct effects on insulin resistance (42). A potential mechanism by which cyproterone acetate may induce insulin resistance is through activation of the glucocorticoid receptor (43). In contrast, the decrease in insulin resistance as seen in trans men is possibly explained by the increase in lean body mass as a result of the testosterone treatment. In the present study however, changes in lean body mass were also not associated with HOMA-IR. Possibly, the change in HOMA-IR in masculinizing hormone therapy is influenced by direct insulin-sensitizing effects on skeletal muscle at a cellular level instead. Testosterone, for example, is known to increase expression of glucose transporter type 4 on skeletal muscle and may enhance insulin-mediated glucose uptake (44).
Estradiol and testosterone seem to play a key role in body fat distribution in both men and women (45). However, there seems to be a sexual dimorphism in the relationship between androgens and body fat. In cis men, androgens are negatively related to visceral fat, while this relation is reversed in cis women. Testosterone levels of men with androgen deficiency and women with androgen excess overlap and are in both sexes associated not only with increased visceral fat but also with other adverse metabolic consequences such as insulin resistance, increased prevalence of type 2 diabetes, cardiovascular disease, and even premature mortality. These observations have led to the concept of the “metabolic valley of death” as a metabolically adverse window of low androgen concentrations (46). In both sexes, low testosterone levels would exert prolipogenic effects on body fat, resulting in body fat expansion and an increase in insulin resistance. At higher levels, testosterone would have a net anabolic effect resulting in an increase in lean body mass and a decrease in insulin resistance. This would explain why men with androgen deficiency and women with androgen excess have metabolic disturbances, and this is not seen in men using hormone replacement treatment (47-49). The present study shows that in trans men with high testosterone levels, lean body mass increases and blood lipids only slightly increase. These observations might suggest that in both sexes higher testosterone levels, in contrast to lower testosterone levels, are not metabolically disadvantageous. If this theory is correct, this would explain why, in contrast to PCOS women with lower testosterone levels and metabolic disturbances, trans men with higher testosterone levels do not show clinically relevant metabolic disturbances. In our study, it was unknown whether PCOS was present among the trans men. However, we do not we do not expect that any presence of PCOS and associated hyperandrogenism at baseline may have affected the longitudinal changes in visceral fat mass and cardiometabolic risk factors as reported in this study.
A strength of our study is that we examined longitudinal changes in visceral fat and VAT/TBF and relations with changes in cardiometabolic risk factors in a large cohort of trans persons receiving hormone therapy. This study is limited by the absence of a control group; thus, we cannot rule out that the natural course of changes over time has affected our results. Nevertheless, the opposite direction of changes of lipids and insulin resistance in trans men and trans women support that the observed changes are not merely due to age-related changes but more likely a consequence of the initiated hormone therapy. Likewise, the directional changes in visceral fat and total body fat seemed to dissociate, opposite to what is often observed in the general population. Another limitation is that in our study DXA was used to estimate the whole volume of visceral fat, whereas previous studies in the general population used transverse magnetic resonance images to assess visceral fat. DXA estimates visceral fat by using other measures of body fat and sex-specific formulas, which are developed from previous performed studies (24). Although DXA has been shown a valid and precise method to estimate visceral fat in cross-sectional studies in men and women (25, 26), DXA has not been validated for measurement of change in visceral fat over time.
Although DXA has been shown a valid method to estimate visceral fat in cross-sectional studies (25, 26), DXA has not been validated for measurement of change in visceral fat over time. In a cross-sectional analysis of the baseline of our study, visceral fat and VAT/TBF were associated with total cholesterol, LDL-cholesterol, triglycerides, and HOMA-IR, which conform to previous literature (2, 3). These results support that DXA provides an accurate estimation of visceral fat. However, an intervention study comparing changes in visceral fat by DXA and MRI measurements is necessary to assess whether DXA displays a precise representation of the total change in visceral fat, in particular in transgender persons during hormone therapy. Finally, all trans women in this study used cyproterone acetate as an antiandrogen agent. As the specific effects of this antiandrogen on accumulation of visceral fat mass are unknown, it is possible that our results do not apply to populations in which other antiandrogen agents are prescribed (eg, spironolactone or gonadotropin-releasing hormone analogues).
In conclusion, hormone therapy in trans women and trans men resulted in large changes in VAT/TBF, mainly due to changes in total body fat, with only small mean changes in visceral fat, albeit with a large interindividual variation. Changes in visceral fat and VAT/TBF were not associated with the unfavorable changes in cardiometabolic risk factors observed during hormone therapy. These results may suggest that the unfavorable changes in blood lipids in trans men and insulin resistance in trans women are likely due to direct effects of hormone therapy on insulin, the liver, skeletal muscle, or adipose tissue, instead of an indirect effect mediated by changes in visceral fat or VAT/TBF. Future studies with direct assessment of whole volume visceral fat using MRI are needed to confirm our results.
Additional Information
Disclose Summary: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359-404. [DOI] [PubMed] [Google Scholar]
- 2. Fox CS, Massaro JM, Hoffmann U, et al. . Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39-48. [DOI] [PubMed] [Google Scholar]
- 3. Rendell M, Hulthén UL, Törnquist C, Groop L, Mattiasson I. Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women. J Clin Endocrinol Metab. 2001;86(2):744-749. [DOI] [PubMed] [Google Scholar]
- 4. Mahabadi AA, Massaro JM, Rosito GA, et al. . Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30(7):850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gast KB, den Heijer M, Smit JW, et al. ; NEO study group. Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: The NEO study. Atherosclerosis. 2015;241(2):547-554. [DOI] [PubMed] [Google Scholar]
- 6. Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58(4):463-467. [DOI] [PubMed] [Google Scholar]
- 8. Mongraw-Chaffin ML, Andersson CAM, Allison MA, et al. . Association between sex hormones and adiposity: qualitative differences in women and men in the Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2015;100(4):596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton EJ, Gianatti E, Strauss BJ, et al. . Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf). 2011;74(3):377-383. [DOI] [PubMed] [Google Scholar]
- 10. Yildirim B, Sabir N, Kaleli B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2003;79(6):1358-1364. [DOI] [PubMed] [Google Scholar]
- 11. Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the study of women’s health across the nation (SWAN) fat patterning study. Obesity (Silver Spring). 2010;18(3):604-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klaver M, de Blok CJM, Wiepjes CM, et al. . Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol. 2018;178(2):163-171. [DOI] [PubMed] [Google Scholar]
- 13. Elbers JMH, Asscheman H, Seidell JC, Gooren LJG. Effects of sex steroids hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276:317-325. [DOI] [PubMed] [Google Scholar]
- 14. Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82(7):2044-2047. [DOI] [PubMed] [Google Scholar]
- 15. Giltay EJ, Elbers JM, Gooren LJ, et al. . Visceral fat accumulation is an important determinant of PAI-1 levels in young, nonobese men and women: modulation by cross-sex hormone administration. Arterioscler Thromb Vasc Biol. 1998;18(11):1716-1722. [DOI] [PubMed] [Google Scholar]
- 16. Elbers JM, Giltay EJ, Teerlink T, et al. . Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf). 2003;58(5):562-571. [DOI] [PubMed] [Google Scholar]
- 17. van Velzen DM, Paldino A, Klaver M, et al. . Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. J Clin Endocrinol Metab. 2019;104(6):1937-1947. [DOI] [PubMed] [Google Scholar]
- 18. Shadid S, Abosi-Appeadu K, De Maertelaere AS, et al. . Effects of gender-affirming hormone therapy on insulin sensitivity and incretin responses in transgender people. Diabetes Care. 2020;43(2):411-417. [DOI] [PubMed] [Google Scholar]
- 19. Dekker MJHJ, Wierckx K, van Caenegem E, et al. . A European network for the investigation of gender incongruence: endocrine part. J Sex Med. 2016:13(6):994-999. [DOI] [PubMed] [Google Scholar]
- 20. Kreukels BP, Haraldsen IR, De Cuypere G, Richter-Appelt H, Gijs L, Cohen-Kettenis PT. A European network for the investigation of gender incongruence: the ENIGI initiative. Eur Psychiatry. 2012;27(6):445-450. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Association; 2013. [Google Scholar]
- 22.American Psychiatric Association. American Psychiatric Association Diagnostic and Statistical manual of Mental Disorders. 4th ed, text revision. American Psychiatric Association; 1994. [Google Scholar]
- 23. Klaver M, de Blok CJM, Wiepjes CM, et al. . Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol.2018;178(2):163-171. [DOI] [PubMed] [Google Scholar]
- 24. Kelly TL, Wilson KE, Ruth C, inventors; Hologic Inc, assignee. Patent application publication estimating visceral fat by dual-energy X-ray absorptiometry. US Patent No. 2010/0234719 A1. November 10, 2015.
- 25. Mickelsfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20(5):1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaul S, Rothney MP, Peters DM, et al. . Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring). 2012;20(6):1313-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996;12(1):45-51. [DOI] [PubMed] [Google Scholar]
- 28. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 29. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 30.van Velzen DM, de Mutsert R. Supplemental data visceral fat. Figshare. 2021. doi: 10.6084/m9.figshare.14994453.v1. [DOI] [Google Scholar]
- 31. Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39(9):897-901. [DOI] [PubMed] [Google Scholar]
- 32. Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 2000;24(4):485-491. [DOI] [PubMed] [Google Scholar]
- 33. Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17(8):664-690. [DOI] [PubMed] [Google Scholar]
- 34. Sasai H, Brychta RJ, Wood RP, et al. . Does visceral fat estimated by dual-energy X-ray absorptiometry independently predict cardiometabolic risks in adults? J Diabetes Sci Technol. 2015;9(4):917-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Larochellière E, Côté J, Gilbert G, et al. . Visceral/epicardial adiposity in nonobese and apparently healthy young adults: association with the cardiometabolic profile. Atherosclerosis. 2014;234(1):23-29. [DOI] [PubMed] [Google Scholar]
- 36. de Mutsert R, Gast K, Widya R, et al. . Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: the netherlands epidemiology of obesity study. Metab Syndr Relat Disord. 2018;16(1):54-63. [DOI] [PubMed] [Google Scholar]
- 37. Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS. Association of changes in abdominal fat quantity and quality with incident cardiovascular disease risk factors. J Am Coll Cardiol. 2016;68(14):1509-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55(10):2622-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kelly DM, Akhtar S, Sellers DJ, Muraleedharan V, Channer KS, Jones TH. Testosterone differentially regulates targets of lipid and glucose metabolism in liver, muscle and adipose tissues of the testicular feminised mouse. Endocrine. 2016;54(2):504-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Angelin B, Olivecrona H, Reihnér E, et al. . Hepatic cholesterol metabolism in estrogen-treated men. Gastroenterology. 1992;103(5):1657-1663. [DOI] [PubMed] [Google Scholar]
- 41. Holmäng A, Larsson BM, Brzezinska Z, Björntorp P. Effects of short-term testosterone exposure on insulin sensitivity of muscles in female rats. Am J Physiol. 1992;262(6 Pt 1):E851-E855. [DOI] [PubMed] [Google Scholar]
- 42. Gava G, Mancini I, Alvisi S, Seracchioli R, Meriggiola MC. A comparison of 5-year administration of cyproterone acetate or leuprolide acetate in combination with estradiol in transwomen. Eur J Endocrinol. 2020;183(6):561-569. [DOI] [PubMed] [Google Scholar]
- 43. Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(suppl 1):3-63. [DOI] [PubMed] [Google Scholar]
- 44. Antinozzi C, Marampon F, Corinaldesi C, et al. . Testosterone insulin-like effects: an in vitro study on the short-term metabolic effects of testosterone in human skeletal muscle cells. J Endocrinol Invest. 2017;40(10):1133-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21(3):415-430. [DOI] [PubMed] [Google Scholar]
- 46. Schiffer L, Kempegowda P, Arlt W, O’Reilly MW. Mechanisms in endocrinology: the sexually dimorphic role of androgens in human metabolic disease. Eur J Endocrinol. 2017;177(3):R125-R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5(4):197-216. [DOI] [PubMed] [Google Scholar]
- 48. Gibb FW, Strachan MW. Androgen deficiency and type 2 diabetes mellitus. Clin Biochem. 2014;47(10-11):940-949. [DOI] [PubMed] [Google Scholar]
- 49. Nandi A, Chen Z, Patel R, Poretsky L. Polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2014;43(1): 123-147. [DOI] [PubMed] [Google Scholar]
- 50. Wander PL, Boyko EJ, Leonetti DL, McNeely MJ, Kahn SE, Fujimoto WY. Change in visceral adiposity independently predicts a greater risk of developing type 2 diabetes over 10 years in Japanese Americans. Diabetes Care. 2013;36(2):289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.