Abstract
This healthy volunteer study aimed to explore phenoxymethylpenicillin (penicillin-V) pharmacokinetics (PK) to support the planning of large dosing studies in adults. Volunteers were dosed with penicillin-V at steady state. Total and unbound penicillin-V serum concentrations were determined, and a base population PK model was fitted to the data.
Keywords: penicillin-V, pharmacokinetics, oral dosing, population PK modeling, healthy volunteers
Phenoxymethylpenicillin (penicillin-V) is a narrow-spectrum, beta-lactam antimicrobial that is widely used for the treatment and prevention of infection, particularly in community settings. It has activity against encapsulated bacteria including streptococci, gonococci, and meningococci. Penicillin-V is relatively stable to gastric acid and can therefore be administered orally [1].
Penicillin-V is commonly prescribed for treatment of pharyngitis where infection with Streptococcus pyogenes is confirmed or suspected [1]. While recommendations in the United Kingdom advise 500-mg 6-hourly dosing, there is a paucity of pharmacokinetic (PK) data in adults to support this [1, 2]. Furthermore, recent evidence for higher doses and shorter durations suggests that current dosing strategies remain to be optimized [3].
Penicillin-V is also commonly used for the prevention of infection in patients with asplenia [4] and sickle cell disease [5, 6] and for suppression of infection in recurrent cellulitis [1]. A dose of 250mg every 12 hours is recommended for adults in these scenarios [1]. However, there is a paucity of PK and clinical trial data in adults to support these dosing recommendations. In sickle cell disease, this is especially important as data supporting penicillin-V prophylaxis in adults have been extrapolated entirely from studies in pediatric populations [7–9].
With the rise in antimicrobial resistance and its threat to human health, there is a need to optimize the use of narrow-spectrum, front-line antimicrobials to prolong their efficacy, reduce collateral impact, and safeguard broader-spectrum agents [10]. This has been reflected by the World Health Organization’s Access, Watch, and Reserve (AwARe) index, which classifies penicillin-V within its Access group [11]. Being designated as an Access antibiotic emphasizes that penicillin-V is a core antibiotic that must be consistently available globally at an appropriate quality, dose, duration, formulation, and price [11]. Despite this designation, very few data are available to support dosing recommendations for penicillin-V in adult populations.
We report the evaluation of penicillin-V PK in 10 healthy volunteers. This study aimed to provide up-to-date base population PK estimates for penicillin-V in adults. This will provide data to support the planning and evaluation of dosing studies in larger patient populations, exploring the optimization of penicillin-V dosing for both prevention and treatment of infection.
METHODS
Setting
This single-center, population PK study in healthy volunteers took place at the Wellcome/NIHR Imperial Clinical Research Facility (CRF), Imperial College London, between April and August 2018. Ethical approval was granted by the London-Harrow Research Ethics Committee (Ref:18/LO/0054). Prior written consent was provided by all study participants before entry into the study.
Study Population
Healthy volunteers were recruited via a healthy volunteer database of >2000 subscribers held within Imperial CRF. Volunteers were eligible for inclusion in the study if they were age ≥18 years, had no clinical or biochemical evidence of active infection, and had no history of penicillin allergy.
Study Protocol
Before the study visit, participants took 5 doses of penicillin-V at home (500mg, 6-hourly) on an empty stomach (1 hour before or 2 hours after food), recording doses on a self-reported diary card. The sixth dose was directly observed during the study visit (time [T] = 0 minutes) on an empty stomach. Participants were asked to fast from midnight before their study visit for the observed dose. On the study day, participants underwent blood sampling via a cannula inserted into the antecubital fossa. A maximum of 15 bloods samples were obtained at T = –30, 0, 10, 20, 30, 45, 60, 75, 90, 105, 120, 150, 180, 210, and 240 minutes from the commencement of the final (sixth) dose.
Samples were allowed to clot for 10 minutes and then placed on ice. They were centrifuged within 30 minutes of being taken at 2700rpm for 10 minutes, separated, and plasma-stored at –80°C until analysis.
Penicillin-V Assay
Analysis was performed using high-performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) at Bristol Antimicrobial Reference Laboratory. Both total and free (unbound) drug concentrations were determined. Free penicillin-V concentration was determined for individuals by performing ultrafiltration (Centrifree ultrafiltration device, 30-kDa molecular weight cutoff, Sigma-Aldrich, Germany) before analysis. The limit of quantification for the LC-MS/MS assay was 0.10mg/L. The interday and intrarun performance of the assay met standards set out by the Food and Drug Administration. Assay error calculated from calibration runs using inter-run standard deviations was described by a second-order polynomial.
In addition to penicillin concentration, individual participant renal profile, liver function, and full blood count were determined at the study visit. Height, weight, age, sex, and ethnicity were also recorded for individuals during their study visit.
Pharmacokinetic Analysis
One-, 2-, and 3-compartment PK models with an absorptive time lag were evaluated. First-order and saturable absorption as well as first-order and Michaelis-Menten clearance (CL) from the central compartment were evaluated. PK data were weighted by the inverse of penicillin interassay variance at the given concentration.
The fit of models to data was evaluated using (i) the coefficients of determination (r2), the y-intercept, and slope of regression from observed vs predicted plots before and after the Bayesian plot; (ii) the log-likelihood value; (iii) the Akaike information criterion (AIC); and (iv) Normalised Prediction Distribution Error (NPDE) analysis.
Covariate Modeling
Given the small number of participants included in this study and therefore the potential for introduction of bias, covariate fitting was not performed.
RESULTS
Study Participants
Supplementary File 1 summarizes individual participant characteristics. The mean (SD) age of participants was 42 (14) years, with 7/10 (70%) being male. Participants’ mean (SD) height was 174 (11) cm, weight 74 (15) kg, and body mass index 24 (3) kg/m2. Mean (SD) creatinine clearance was 114 (29) mL/min. All participants reported taking 5 doses of penicillin-V before attending the study visit.
The mean (SD) total steady state drug AUC was 7.8 (2.5) mg∗h/L, Cmax 5.7 (2.3) mg/L, tmax 48 (18) minutes, and total serum t1/2 41 (13) minutes. The mean (SD) unbound penicillin-V serum percentage in the samples was 23% (4%). There was a linear association in protein binding across concentrations, with an r2 = 0.89. Free serum t1/2 was 55 (10) minutes.
Pharmacokinetic Analysis
In total, 130 samples making up 10 concentration-time profiles were analyzed. A 3-compartment model with an absorptive time lag was found to best describe penicillin-V PK. This included linear first-order absorption and elimination to and from the central compartment plus linear first-order transfer between bound and unbound drug states.
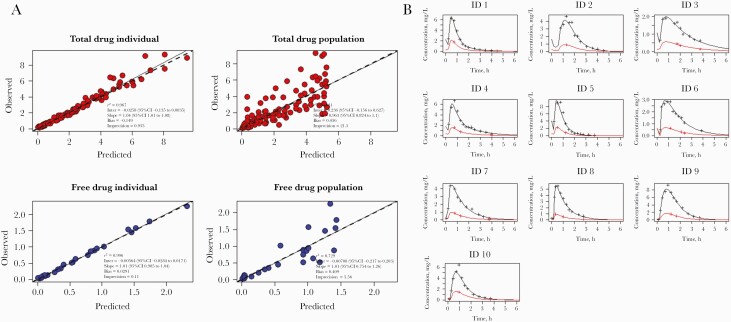
Figure 1 demonstrates goodness-of-fit plots for both individual and population observed vs predicted concentrations of total and free drug plus individual posterior estimated PK for each participant. The final model demonstrated good correlation for individual observed vs predicted concentrations. NPDE demonstrated good overall model performance, with a mean (SD) of 0.002 (0.10), variance of 1.19 (0.16), and global adjusted P value of .49 (Supplementary File 2). Table 1 summarizes the final base models’ population PK parameters. Supplementary File 3 provides additional model information.
Figure 1.
Phenoxymethylpenicillin pharmacokinetics and final model observed vs predicted plots. A, Individual and population observed vs posterior Bayesian predicted data plot for base pharmacokinetic model used. B, Individual data for 10 healthy volunteers showing posterior estimated pharmacokinetic profile for total (black) and free (red) serum penicillin-V concentrations.
Table 1.
Population Pharmacokinetic Parameter Estimates for Penicillin-V in Healthy Volunteers
| Mean | SD | CV% | Var | Median | |
|---|---|---|---|---|---|
| V, L | 46.39 | 31.02 | 66.87 | 962.28 | 35.08 |
| CL, L/h | 97.88 | 26.60 | 27.17 | 707.44 | 95.79 |
| Ka, h-1 | 4.74 | 2.57 | 54.36 | 6.63 | 3.75 |
| Tlag1, h | 0.25 | 0.11 | 44.24 | 0.01 | 0.22 |
| Kub, h-1 | 60.33 | 19.82 | 32.85 | 392.69 | 60.07 |
| Kbu, h-1 | 19.01 | 5.21 | 27.39 | 27.10 | 19.95 |
| Kcp, h-1 | 12.66 | 9.70 | 76.60 | 94.07 | 13.05 |
| Kpc, h-1 | 16.84 | 11.30 | 67.09 | 127.67 | 16.21 |
| IC1, mg | 40.22 | 36.48 | 90.69 | 1330.59 | 28.64 |
| IC2, mg | 8.71 | 5.51 | 63.23 | 30.35 | 9.56 |
Abbreviations: CL, clearance; CV%, coefficient of variation; IC, initial condition for bound drug (IC1) and free drug (IC2); Ka, first-order rate constant for absorption; Kcp & Kpc, first-order inter-compartmental rate constants; Kub & Kbu, first-order rate constants for total and free serum drug; Tlag, Time lag from dosing to absorption into the central compartment; V, volume of distribution; var, variance.
DISCUSSION
This study provides a base PK model for oral penicillin-V developed using rich PK data from 10 healthy volunteers. It adds to historical clinical trial data, which are now >20 years old [8, 12, 13]. In this study, we observed that penicillin-V was 77% plasma protein bound and had a free serum t1/2 of 55 minutes, in keeping with published product characteristics [12]. This is something that has rarely been demonstrated beyond pediatric populations and will support the planning of large population dosing studies in adults.
Concern surrounding antimicrobial resistance means that there is an urgent need to prolong and maximize the effectiveness of current front-line, Access-classified antimicrobials [10]. An important and often neglected aspect of antimicrobial optimization is ensuring that an appropriate dose is delivered for each individual patient.
With reports of novel penicillin resistance mechanisms emerging in Streptococcus pyogenes, optimization of penicillin-V dosing is of critical importance [14]. Penicillin-V nonsusceptibility within Streptococcus pneumoniae has also been reported as an emerging threat that can be controlled through optimal antibiotic use [15]. Despite concerns over rising rates on penicillin-V nonsusceptibility, large prospective studies undertaken between 1999 and 2001 demonstrated that penicillin nonsusceptibility was not a risk factor for mortality in patients with pneumococcal pneumonia and concomitantly positive blood cultures [16]. The authors suggest that penicillin therapy may still therefore be an important agent even in the face of in vitro nonsusceptibility and highlight the importance of improving our understanding of penicillin PD in vivo for Streptococcus pneumoniae [16]. The development of a penicillin-V PK model is a first step in exploring PK-PD relationships for this agent.
Strengths and Limitations
This study had several limitations. Initial dosing before the study visit was unsupervised, meaning that variations in dosing beyond that recorded on participant diary cards cannot be excluded. The study was performed in a small number of healthy volunteers, meaning that generalizability may not be possible. Creatinine clearance was variable within our participants, but our limited study size means that covariate modeling was not appropriate.
CONCLUSIONS
Penicillin-V is an important front-line antimicrobial for the treatment and prevention of infection. Rich population PK data from this study will provide a basis to inform larger studies in adult patients, helping to guide optimal recommendations for the use of penicillin-V in clinical practice.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to acknowledge (i) the National Institute of Health Research Imperial Biomedical Research Centre and the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infection and Antimicrobial Resistance at Imperial College London in partnership with Public Health England and the NIHR Imperial Patient Safety Translational Research Centre; (ii) the Department of Health and Social Care–funded Centre for Antimicrobial Optimisation (CAMO), Imperial College London, which provides state-of-the-art research facilities and consolidates multidisciplinary academic excellence, clinical expertise, the Imperial’s NIHR/Wellcome–funded Clinical Research Facility (CRF), and partnerships with the NHS to support and deliver innovative research on antimicrobial optimization and precision prescribing; and (iii) the NIHR/Wellcome Trust Imperial Clinical Research Facility, Imperial College London, UK, where this study took place.
Financial support. The authors would like to acknowledge research grants from the Imperial Biomedical Research Centre (BRC) and Mérieux Research Grants, which provided funding to support the undertaking of this study. T.R., A.H., and P.G. are also supported by funding from the National Institute for Health Research Invention for Innovation Grant (i4i), Enhanced, Personalized and Integrated Care for Infection Management at Point of Care (EPIC IMPOC), II-LA-0214–20008. A.H. is an NIHR Senior Research Investigator.
Disclaimer. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the UK Department of Health.
Role of funders in research. This work was produced independently. The funders had no role in this work.
Potential conflicts of interest. The authors have no conflicts of interest to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. T.M.R. and A.H. conceived and designed the study. T.M.R., R.W., L.S.P.M., and M.G. performed the study. A.G., A.L., M.B., and M.K. performed sample analysis. T.M.R. performed data analysis and pharmacokinetic modeling, supported by R.W., J.R., and W.H. T.M.R. drafted the manuscript, with all authors having a significant contribution to revisions and finalizations for submission.
Patient consent. Ethical approval was granted by the London-Harrow Research Ethics Committee (Ref:18/LO/0054). Prior written consent was provided by all study participants before entry into the study.
References
- 1. National Institute for Health & Care Excellence (NICE). Phenoxymethylpenicillin. Available at: https://bnf.nice.org.uk/drug/phenoxymethylpenicillin.html. Accessed 11 January 2021.
- 2. Roos K, Grahn E, Ekedahl C, Holm SE.. Pharmacokinetics of phenoxymethylpenicillin in tonsils. Scand J Infect Dis 1986; 18:125–30. [DOI] [PubMed] [Google Scholar]
- 3. Skoog Ståhlgren G, Tyrstrup M, Edlund C, et al. Penicillin V four times daily for five days versus three times daily for 10 days in patients with pharyngotonsillitis caused by group A streptococci: randomised controlled, open label, non-inferiority study. BMJ 2019; 367:l5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies JM, Lewis MP, Wimperis J, et al. ; British Committee for Standards in Haematology. Review of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen: prepared on behalf of the British Committee for Standards in Haematology by a working party of the Haemato-Oncology Task Force. Br J Haematol 2011; 155:308–17. [DOI] [PubMed] [Google Scholar]
- 5. Sickle Cell Disease in Childhood. Standards and guidelines for clinical care. 2010. Available at: https://www.gov.uk/government/publications/sickle-cell-disease-in-children-standards-for-clinical-care. Accessed 11 January 2021.
- 6. Sickle Cell Society. Standards for clinical care of adults with sickle cell disease in the UK. 2018. Available at: https://www.sicklecellsociety.org/wp-content/uploads/2018/05/Standards-for-the-Clinical-Care-of-Adults-with-Sickle-Cell-in-the-UK-2018.pdf. Accessed 11 January 2021.
- 7. Prevention and therapy of bacterial infections for children with asplenia or hyposplenia. Paediatr Child Health 1999; 4:417–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barza M, Weinstein L.. Pharmacokinetics of the penicillins in man. Clin Pharmacokinet 1976; 1:297–308. [DOI] [PubMed] [Google Scholar]
- 9. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA 2002; 287:3103–9. [DOI] [PubMed] [Google Scholar]
- 10. Perry MR, Mcclean D, Simonet C, et al. Focussing on resistance to front-line drugs is the most effective way to combat the antimicrobial resistance crisis. Available at: https://www.biorxiv.org/content/biorxiv/early/2018/12/17/498329.full.pdf. Accessed 11 January 2021.
- 11. NICE. Cellulitis and erysipelas: antimicrobial prescribing. 2019. Available at: https://www.nice.org.uk/guidance/GID-NG10131/documents/draft-guideline12. Accessed 11 January 2021.
- 12. Josefsson K, Bergan T.. Pharmacokinetics of phenoxymethylpenicillin in volunteers. Chemotherapy 1982; 28:241–6. [DOI] [PubMed] [Google Scholar]
- 13. Preston S, Drusano G.. Penicillins. 2017. Available at: http://www.antimicrobe.org/d24.asp. Accessed 11 January 2021.
- 14. Vannice K, Ricaldi J, Nanduri S, et al. Streptococcus pyogenes pbp2x mutation confers reduced susceptibility to β-lactam antibiotics. Clin Infect Dis 2020; 71:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liñares J, Ardanuy C, Pallares R, Fenoll A.. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect 2010; 16:402–10. [DOI] [PubMed] [Google Scholar]
- 16. Yu VL, Chiou CCC, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis 2003; 37:230–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.