Abstract
Background
The burden of insulin resistance (IR) among young American adults has not been previously assessed. We evaluated (1) the prevalence and trends of IR and cardiometabolic risk factors and (2) the association between measures of adiposity and IR among adults 18 to 44 years of age without diabetes and preexisting cardiovascular disease.
Methods
Cross-sectional survey data from six consecutive National Health and Nutrition Examination Survey (2007-2008 to 2017-2018) cycles were analyzed. IR was defined by the homeostatic model assessment for IR (HOMA-IR) of ≥2.5. The temporal trends of IR, cardiometabolic risk factors, and the relationship between IR and measures of adiposity were assessed using multivariable-adjusted regression models.
Results
Among 6247 young adults 18 to 44 years of age, the prevalence of IR was 44.8% (95% CI: 42.0%-47.6%) in 2007-2010 and 40.3% (95% CI: 36.4%-44.2%) in 2015-2018 (P for trend = 0.07). There was a modest association of HOMA-IR with higher body mass index (BMI), waist circumference, total lean fat mass, and total and localized fat mass (all Ps < 0.001). Participants with IR had a higher prevalence of hypertension [31.3% (95% CI: 29.2%-33.5%) vs 14.7% (95% CI: 13.2%-16.2%)], hypercholesterolemia [16.0% (95% CI: 12.4%-19.5%) vs 7.0% (95% CI: 5.8%-8.5%)], obesity [56.6% (95% CI: 53.9%-59.3%) vs 14.7% (95% CI: 13.0%-16.5%)], and poor physical activity levels [18.3% (95% CI: 16.4%-20.2%) vs 11.7% (95%CI: 10.3–13.1%)] compared to participants without IR (all Ps < 0.05).
Conclusions
Four-in-10 young American adults have IR, which occurs in a cluster with cardiometabolic risk factors. Nearly half of young adults with IR are nonobese. Screening efforts for IR irrespective of BMI may be required.
Keywords: cardiovascular disease, cardiovascular risk, diabetes, hypercholesterolemia, hypertension, insulin resistance, young adults
Insulin resistance (IR) is associated with metabolic disturbances that contribute to the development of atherosclerotic cardiovascular diseases (ASCVD). IR is associated with obesity, hypertension, reduced high-density lipoprotein cholesterol (HDL-C) levels, and elevated triglyceride levels, which are also components of metabolic syndrome (1-3).
It is well-established that individuals with IR have an increased risk of adverse cardiovascular events and all-cause mortality (2-7). The presence of IR in early adulthood is associated with an early and significant predisposition to ASCVD events later in life (2,3,8-11). However, the contemporary trends of IR in young adults have not been evaluated in a nationwide population. Additionally, the important racial and sex-related differences in IR remain unclear in this age group (12-18). Adiposity has been associated with worsening IR and diabetes (19-22). However, the relationship of IR with various measures of generalized and localized adiposity among young American adults is also not known. Given the clinical implications of IR in the development of ASCVD, characterization of the prevalence of IR among young American adults is crucial. Since IR may not occur in isolation, it is important to assess the prevalence and trends of cardiometabolic risk factors alongside IR among young American adults. Furthermore, the distribution of social determinants of health, which are known to influence the incidence and prevalence of ASCVD, in a nationally representative population of young American adults with IR is not known (23-37).
We examined young adult participants without diabetes, 18 to 44 years of age, in the National Health and Nutrition Examination Survey (NHANES) 2007-2018 to evaluate (1) the association of measures of adiposity with IR, (2) the prevalence and temporal trends in IR (overall and stratified by sex and race), and (3) the prevalence and trends of cardiometabolic risk factors stratified by IR status.
Methods
Data Sources
The latest NHANES data from 2007-2018 was used for this study. The NHANES is a consecutive (nonoverlapping), cross-sectional, nationally representative, multistage probability survey of the noninstitutionalized American population. This population-based survey, which involves a telephone interview and in-person examination at mobile health clinics, is conducted once every 2 years by the National Center for Health Statistics and Centers for Disease Control and Prevention (37-40). The NHANES participants answer a series of questionnaires on their demographic details, health conditions such as diabetes and hypertension, use of medications, and dietary intake. The participants who consent for medical examination undergo a rigorous clinical assessment that includes anthropometric measures, complete physical examination, vital sign measurement, and blood collection for laboratory measurements (37-40).
Study Population
The data from 6 consecutive NHANES cycles (ie, 2007-2008 to 2017-2018) were used in this study. Individuals in the 18- to 44-year-old age group who participated in both the interview and the examination were included in this study. We excluded pregnant or breastfeeding females, those with a history of heart failure, coronary artery disease, angina, myocardial infarction, or chronic kidney disease, as well as those with missing fasting insulin and glucose measurements. Individuals with diabetes (defined as taking antidiabetic medications, having a fasting blood glucose ≥126 mg/dL, or a hemoglobin A1c ≥ 6.5%) were excluded.
Study Measures
Self-reported age, sex (male and female), and race (non-Hispanic white, non-Hispanic black, Mexican American, and others) were obtained from a written questionnaire. Self-reported smoking status (current, former, and never smoker), educational status (less than or equal to high school, some college, and a college degree or higher), health insurance status (yes or no), family income, and the total number of healthcare visits in the past year (0, 1-2, or >2 visits) were used in our study (37,41). Based on the family income and poverty guidelines, the family income poverty ratio was categorized as ≥3.5, 1.3 to 3.49, and <1.3 (37,41).
IR was defined based on the homeostatic model for IR (HOMA-IR), which estimates IR using fasting plasma glucose and serum insulin levels [fasting glucose (mg/dL) × fasting insulin (uIU/mL)/405]. Clinically, HOMA-IR is the most commonly used surrogate measure of IR (42-47). There is a strong correlation between IR estimation using HOMA-IR and the gold standard euglycemic-hyperinsulinemic clamp method (48). IR was defined as a HOMA-IR ≥2.5, and noninsulin resistant as a HOMA-IR <2.5 (42-47). In sensitivity analysis, we used the third tertile of HOMA-IR distribution of the study population to define IR.
The cardiometabolic risk factors evaluated in our study included hypertension [as per 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines], hypercholesterolemia (total cholesterol ≥ 240 mg/dL or taking lipid-lowering medication), obesity [body mass index (BMI) ≥ 30 kg/m2], smoking status (current, former, or never smoker), physical activity status (ideal, intermediate, or poor), and poor diet. Standardized NHANES laboratory methods were used for the assessment of the laboratory parameters. Plasma glucose was measured using a hexokinase-based enzymatic assay. Insulin was measured using the immunoenzymometric assay (Merocodia Insulin ELISA) in 2007-2008, chemiluminescent immunoassay (Roche Elecsy 2010) in 2009-2012 cycles, and immunoenzymometric assay (Tosoh Bioscience AIA-900) from 2013 onward.
Lipids and lipoproteins were assessed using the previously described standardized protocol (40). The total cholesterol and triglyceride levels were estimated using the enzymatic reaction method. HDL-C was measured using direct immunoassay. Among those with triglyceride ≤400 mg/dL, the Friedewald equation was used to calculate low-density lipoprotein cholesterol [low-density lipoprotein cholesterol = total cholesterol − (HDL-C + triglycerides/5)]. Hypercholesterolemia was defined as a total cholesterol level ≥240 mg/dL or those taking lipid-lowering medications.
The systolic and diastolic blood pressure was estimated based on the average of 3 readings taken after 5 min of rest. Hypertension was defined by the 2017 ACC/AHA guidelines (37,38). Hypertension was defined by a systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥80 mmHg, or current antihypertensive medication use (a yes response to “Because of high blood pressure/hypertension, have you ever been told to take prescribed medicine?”).
Physical activity levels were stratified into ideal (defined as ≥150 min/week of moderate exercise, ≥75 min/week vigorous exercise, or ≥150 min/week of moderate + vigorous exercise), intermediate (defined as 1-149 min/week of moderate exercise, 1-74 min/week of vigorous exercise, or 1-149 min/week moderate + vigorous exercise), or poor (no physical activity). BMI was calculated from height and weight measured during the study examination. A BMI ≥30 kg/m2 was defined as obese in our study.
We estimated the AHA’s Healthy Diet Score using the Food Patterns Equivalents Database created by the US Department of Agriculture (38, 39). The diet score was based on the results from two 24-h recall dietary interviews. Daily fruit and vegetable consumption was added to each 24-h recall and then averaged. We estimated the daily fish consumption based on the frequency of fish consumption in the last 30 days, with each fish serving assumed to be at least 3.5 ounces. This was done for the conversion of participant-reported fish intake into dietary score component. The mean of 2 dietary recall interviews was used to estimate the daily intake of fiber-rich whole grain. The daily sugar intake was estimated using the Food Patterns Equivalents Database data on added sugars. The dietary score involved assigning 1 point to each of the following: consumption of whole-grains ≥3 oz/day, fish ≥2 times/week, fruit and vegetable ≥4.5 cups/day, sodium <1500 mg/day, and/or added sugar <37.5 g/day for men and <25 g/day for women. Cumulating the score from each dietary component yielded the total healthy diet score. As per the AHA healthy diet score (0-5 points), the score of <2 indicates a poor diet. We used the AHA’s Life Simple 7 definition for the estimation of hypercholesterolemia, healthy diet, and physical activity (38, 39).
Among participants who underwent a whole-body dual-energy X-ray absorptiometry (DEXA) scan (i.e., NHANES 2011-2018 cycles), the relationship between the different adiposity measures and HOMA-IR levels were assessed. The measure of adiposity utilized in our analyses included BMI, waist circumference, total lean mass, total fat mass, leg fat mass, arm fat mass, and trunk fat mass (49-52).
Statistical Analysis
All statistical analyses were performed using the PROC SURVEY procedures in SAS version 9.4 (Cary, NC, USA) to account for the multistage sampling survey design. The prevalence of IR and cardiometabolic risk factors was performed by merging the NHANES cycles into 3 groups: 2007-2010, 2011-2014, and 2015-2018. The full examination weights for 4 years were used to generate the weighted population estimates, as recommended by NHANES. The age-standardized prevalence estimates for IR and cardiometabolic risk factors were computed by using 2005, 2010, and 2015 US Census population proportion estimates for the age groups of 18 to 24, 25 to 34, and 35 to 44 years, for the 2007-2010, 2011-2014, and 2015-2018 study periods, respectively. We stratified the data by sex and race to estimate the prevalence and temporal trends in IR across these subgroups. The prevalence and trends of cardiometabolic risk factors were assessed after stratifying by IR status (yes or no). The homogeneity of prevalence rates across survey cycles was tested using multivariable-adjusted logistic regression with Bonferroni correction for multiple comparisons. Due to the plausible impact of demographic and socioeconomic factors, the trends were adjusted for sex, race, education level, family income poverty ratio, health insurance status, and the number of healthcare visits in the previous year. As recommended by the National Center for Health Statistics, the linear trends for the prevalence estimates were examined (37,38,40). We also estimated IR predictors among young American adults using logistic regression, including the following variables in the model: age, sex, race, education, family income poverty ratio, health insurance status, number of health visits, hypertension, hypercholesterolemia, poor diet, poor physical activity, obesity, and smoking status (37). Among those who underwent full body DEXA scan in the NHANES 2011-2018 cycles, the linear association of the measures of adiposity (BMI, waist circumference, total fat mass, total lean mass, leg fat mass, arm fat mass, and truncal fat mass) with HOMA-IR on a continuous scale was assessed after adjusting for the aforementioned variables.
In sensitivity analyses, we stratified the study population based on HOMA-IR tertiles, and those in the third tertile were defined as having IR. The trends of IR based on this definition were computed. We also calculated the prevalence and trends of cardiometabolic risk factors using this definition.
Results
The derivation of a young, relatively healthy, nondiseased study population is described in Supplementary Figure 1 (53). Among 6247 young adults 18 to 44 years old, 42.1% had IR. The baseline characteristics of the study population stratified by the study period are described in Supplementary Tables 1 and 2 (54).
Relationship Between Insulin Resistance and Measures of Adiposity
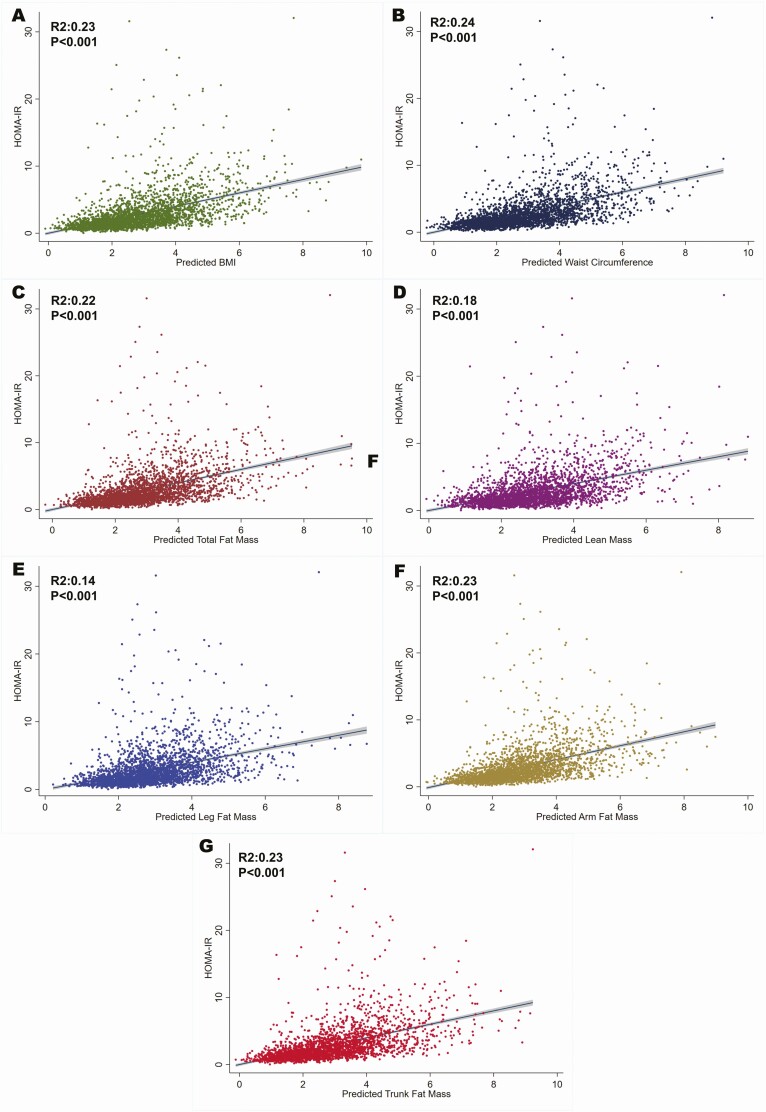
Among those who underwent full body DEXA scan in the NHANES 2011-2018 cycles (n = 3205), we noted that there was a modest linear association between BMI (R2:0.23), waist circumference (R2:0.24), total lean mass (R2:0.18), total fat mass (R2:0.22), leg fat mass (R2:0.14), arm fat mass (R2:0.23), and trunk fat mass (R2:0.23; all Ps < 0.001) (Fig. 1).
Figure 1.
Association of measures of adiposity with homeostatic model for insulin resistance (HOMA-IR) among National Health and Nutrition Examination Survey participants 18-44 years of age. The figure depicts the linear association of HOMA-IR values and the measures of adiposity, which includes body mass index (A), waist circumference (B), total fat mass (C), total lean mass (D), leg fat mass (E), arm fat mass (F) and trunk fat mass (G).
Prevalence and Temporal Trends in Insulin Resistance
The prevalence of IR was 44.8% (95% CI: 42.0%-47.6%) in 2007-2010, which numerically decreased to 40.3% (95% CI: 36.4%-44.2%) in 2015-2018 (P for trend = 0.07) (Table 1). The prevalence of IR declined among males from 50.2% (95% CI: 45.8%-54.7%) in 2007-2010 to 41.0% (95% CI: 35.8%-46.2%) in 2015-2018 (P for trend = 0.02). The prevalence of IR remained stable among females during the study period (P for trend = 0.60) and was 39.5% (95% CI: 34.5%-44.5%) in 2015-2018. While the temporal trends for IR prevalence remained stable across the study years and across all racial groups (all Ps for trend > 0.05), Mexican Americans had the highest prevalence of IR. The estimated prevalence of IR (in millions) among young adults across study periods is depicted in Supplementary Figure 2 (53).
Table 1.
Prevalence and temporal trends for insulin resistance among young adults without diabetes from National Health and Nutrition Examination Survey, 2007-2018
| NHANES study period (years) | Prevalence (95% CI) | Trend P-valuea | ||
|---|---|---|---|---|
| 2007-2010 (n = 2219) | 2011-2014 (n = 2151) | 2015-2018 (n = 1877) | ||
| Overall | 44.8 (42.0-47.6) | 41.3 (37.9-44.7) | 40.3 (36.4-44.2) | 0.07 |
| Stratified by sex | ||||
| Males | 50.2 (45.8-54.7) | 43.8 (39.3-48.3) | 41.0 (35.8-46.2) | 0.02 |
| Females | 39.9 (35.8-43.9) | 38.6 (34.6-42.5) | 39.5 (34.5-44.5) | 0.60 |
| Stratified by race | ||||
| Non-Hispanic whites | 40.5 (36.8-44.3) | 37.9 (33.3-42.6) | 35.7 (30.4-41.0) | 0.22 |
| Non-Hispanic blacks | 49.3 (41.9-56.7) | 46.5 (42.2-50.7) | 37.9 (32.9-43.1) | 0.08 |
| Mexican Americans | 60.1 (56.8-63.4) | 55.5 (49.7-61.3) | 53.8 (49.3-58.3) | 0.25 |
| Others | 46.2 (40.4-52.1) | 40.2 (33.9-46.5) | 45.7 (41.1-50.4) | 0.20 |
Data are presented as percentages (95% CIs). Bonferroni adjustment was performed for multiple comparisons. When sex and race stratified temporal trends were assessed, sex and race, respectively, were excluded in the models.
aAdjusted for sex, race, education status, family income to poverty ratio, health insurance status, and number of health visits.
Predictors of Insulin Resistance Among Young Adults
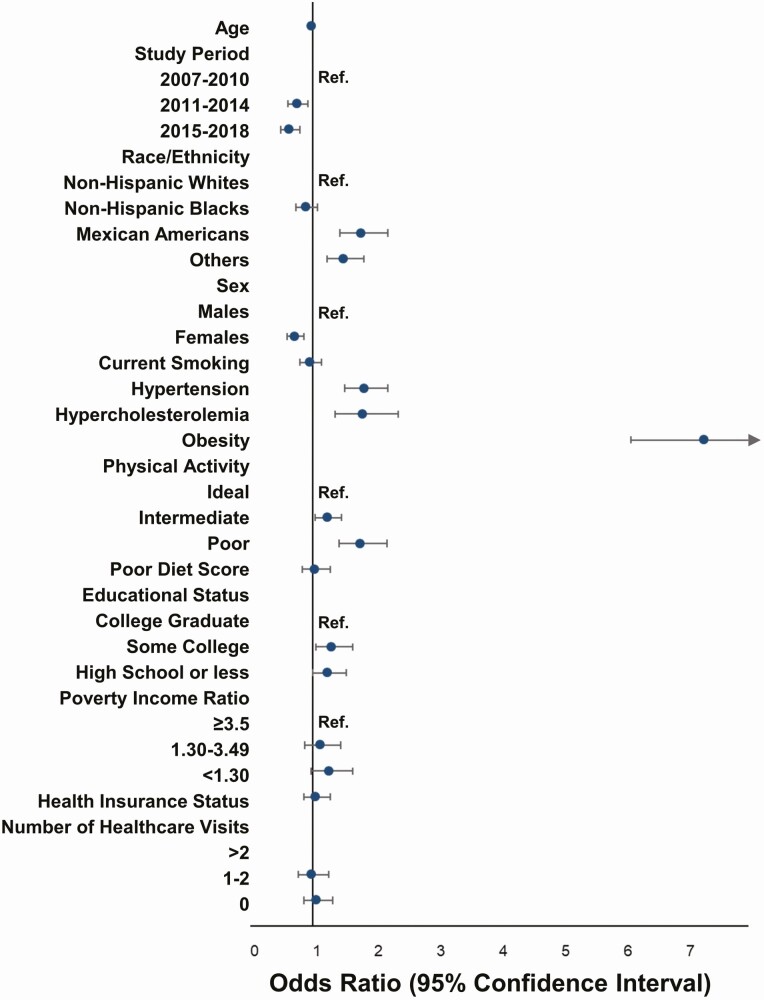
NHANES participants from 2011-2014 [odds ratio (OR): 0.75 (95% CI: 0.61-0.93)], and 2015-2018 [OR: 0.63 (95% CI: 0.50-0.80)] had lower odds of having IR compared with those from 2007-2010. Mexican Americans [OR: 1.78 (95% CI: 1.44-2.21)] and participants of other races [OR: 1.50 (95% CI: 1.23-1.82)] had higher odds of having IR. Females had lower odds of having IR [OR: 0.71 (95% CI: 0.59-0.86)]. Individuals with hypertension [OR: 1.83 (95% CI: 1.52-2.21)], hypercholesterolemia [OR: 1.80 (95% CI: 1.36-2.38)], obesity [OR: 7.29 (95% CI: 6.11-8.70)], intermediate physical activity levels [OR: 1.23 (95% CI: 1.04-1.46)], poor physical activity levels [OR: 1.77 (95% CI: 1.43-2.19)], and who were not college graduates [some college, OR: 1.23 (95% CI:1.00-1.54); high school or less, OR: 1.77 (95% CI: 1.43-2.19)] had greater odds of having IR (Fig. 2).
Figure 2.
Predictors of insulin resistance among young American adults 8 to 44 years: National Health and Nutrition Examination Survey 2007-2018. The figure depicts the odds ratio and CIs for the various predictors of insulin resistance derived from a multivariable logistic regression model.
Prevalence of Cardiometabolic Risk Factors From 2007-2018: Stratified by Insulin Resistance Status
The prevalence of obesity was 56.6% (95% CI: 53.9%-59.3%) among participants with IR, compared with 14.7% (95% CI: 13.0%-16.5%) in those without IR (P < 0.001) (Table 3). Individuals with IR had a higher prevalence of hypertension [31.3% (95% CI: 29.2%-33.5%) vs 14.7% (95% CI: 13.2%-16.2%)], hypercholesterolemia [16.0% (95% CI: 12.4%-19.5%) vs 7.0% (95% CI: 5.8%-8.5%)], and poor physical activity levels [18.3% (95% CI: 16.4%-20.2%) vs 11.7% (95% CI: 10.3%-13.1%); all Ps < 0.05]. The prevalence of smoking and poor diet was similar among those with and without IR (all Ps > 0.05). The combined prevalence of cardiovascular risk factors over the entire study period, stratified by IR status, is depicted in Table 2.
Table 3.
Prevalence of cardiovascular risk factors overall and stratified by insulin resistance: National Health and Nutrition Examination Survey, 2007-2018
| NHANES study period (years) | Prevalence (95% CIs) | Trend P-value | ||
|---|---|---|---|---|
| 2007–2010 (n = 2219) | 2011–2014 (n = 2151) | 2015–2018 (n = 1877) | ||
| Obesitya | ||||
| All | 27.8 (26.2-28.7) | 31.8 (29.1-34.5) | 35.3 (30.8-39.7) | 0.02 |
| +IR | 50.0 (46.0-54.0) | 58.4 (54.4-62.4) | 61.7 (55.9-67.6) | 0.004 |
| −IR | 12.2 (9.6-14.9) | 13.5 (11.5-15.4) | 18.5 (14.5-22.6) | 0.11 |
| Hypertensionb | ||||
| All | 23.9 (23.0-24.9) | 21.0 (18.7-23.2) | 22.8 (20.4-25.2) | 0.81 |
| +IR | 29.2 (25.8-32.5) | 29.1 (25.9-32.3) | 36.1 (31.6-40.7) | 0.33 |
| −IR | 11.3 (9.4-13.2) | 16.3 (13.8-18.7) | 16.0 (12.9-19.1) | 0.18 |
| Hypercholestrolemiac | ||||
| All | 9.7 (8.4-11.1) | 9.4 (7.5-11.3) | 8.0 (6.2-9.7) | 0.01 |
| +IR | 13.3 (10.8-15.9) | 12.4 (8.8-16.0) | 9.9 (7.5-12.3) | 0.01 |
| −IR | 6.8 (5.1-8.5) | 7.4 (5.3-9.5) | 6.7 (4.4-8.9) | 0.44 |
| Smoking | ||||
| Nonsmoker | ||||
| All | 59.1 (56.0-62.2) | 65.0 (62.0-67.9) | 66.0 (62.7-69.2) | 0.33 |
| +IR | 59.9 (56.7-63.1) | 64.9 (61.1-68.7) | 66.9 (62.8-71.1) | 0.64 |
| −IR | 58.4 (53.4-63.4) | 65.0 (61.0-69.0) | 65.3 (61.4-69.2) | 0.39 |
| Former smoker | ||||
| All | 10.6 (9.0-12.2) | 10.1 (8.5-11.7) | 14.5 (12.2-16.9) | 0.77 |
| +IR | 10.2 (8.1-12.4) | 10.3 (8.2-12.4) | 14.3 (11.8-16.8) | 0.67 |
| −IR | 10.9 (7.9-13.9) | 10.0 (8.0-11.9) | 14.7 (10.9-18.5) | 0.56 |
| Current smoker | ||||
| All | 23.2 (20.8-25.5) | 21.9 (18.6-25.2) | 19.5 (16.5-22.5) | 0.32 |
| +IR | 23.3 (20.1-26.4) | 21.5 (17.6-25.4) | 18.8 (14.9-22.7) | 0.44 |
| −IR | 23.2 (19.4-26.9) | 22.2 (17.8-26.6) | 19.9 (16.6-23.3) | 0.53 |
| Physical Activityd | ||||
| Ideal | ||||
| All | 57.4 (54.6-60.3) | 56.2 (53.0-59.3) | 59.8 (56.7-63.0) | 0.71 |
| +IR | 54.7 (51.3-58.0) | 52.9 (48.9-56.8) | 58.1 (53.8-62.5) | 0.62 |
| −IR | 59.7 (55.9-63.6) | 58.4(54.5-62.4) | 60.8 (55.8-65.9) | 0.74 |
| Intermediate | ||||
| All | 25.8 (23.0-28.6) | 27.6 (24.5-30.7) | 22.2 (19.1-25.3) | 0.39 |
| +IR | 24.6 (20.9-28.2) | 27.3 (23.8-30.8) | 20.5 (17.0-24.0) | 0.35 |
| −IR | 26.8 (22.7-30.9) | 27.8 (24.2-31.4) | 23.5 (19.4-27.7) | 0.55 |
| Poor | ||||
| All | 14.6 (12.9-16.3) | 13.3 (11.4-15.3) | 16.0 (13.0-18.2) | 0.43 |
| +IR | 19.0 (16.5-21.6) | 17.2 (14.3-20.0) | 18.8 (14.4-23.3) | 0.52 |
| −IR | 11.0 (8.8-13.2) | 10.7 (8.4-12.9) | 13.4 (10.7-16.1) | 0.66 |
| Poor diet scoree | ||||
| All | 77.4 (74.8-80.1) | 77.8 (75.3-80.3) | 79.3 (76.0-82.5) | 0.50 |
| +IR | 78.1 (75.7-80.6) | 79.6 (76.1-82.9) | 79.9 (75.9-83.9) | 0.33 |
| −IR | 76.8 (73.0-80.7) | 76.6 (73.2-79.9) | 78.9 (74.6-83.2) | 0.81 |
Abbreviation: IR, insulin resistance.
Bold P-values indicate P-values < 0.05.
aObesity was defined as a body mass index of ≥30 kg/m2.
bHypertension was defined based on the 2017 American College of Cardiology/American Heart Association guidelines.
cHypercholesterolemia control was defined as total cholesterol ≥240 mg/dL.
dPoor physical activity level was defined based on the American Heart Association Life Simple 7 score metrics.
ePoor diet score is defined as a diet score <2 based on American Heart Association Life Simple 7 score metrics.
Table 2.
Prevalence of cardiovascular risk factors among National Health and Nutrition Examination Survey, 2007-2018, participants 18-44 years of age, stratified by insulin resistance
| Cumulative (n = 6247) | IR+ (n = 2756) | IR− (n = 3491) | P-value (IR+ vs IR−) | |
|---|---|---|---|---|
| Obesitya | 32.3 (30.4-34.3) | 56.6 (53.9-59.3) | 14.7 (13.0-16.5) | <0.001 |
| Hypertensionb | 21.7 (20.6-22.9) | 31.3 (29.2-33.5) | 14.7 (13.2-16.2) | <0.001 |
| Hypercholestrolemiac | 9.1 (8.1-10.1) | 16.0 (12.4-19.5) | 7.0 (5.8-8.2) | <0.001 |
| Smoking | ||||
| Nonsmoker | 63.5 (61.7-65.3) | 63.9 (61.8-66.1) | 63.1 (60.6-65.6) | 0.58 |
| Former | 11.7 (10.6-12.9) | 11.5 (10.2-12.9) | 11.9 (10.1-13.7) | 0.69 |
| Current | 21.5 (19.8-23.2) | 21.2 (19.1-23.3) | 21.2 (19.1-23.3) | 0.81 |
| Physical activityd | ||||
| Ideal | 57.8 (56.0-59.6) | 55.3 (53.0-57.5) | 59.7 (57.2-62.2) | 0.003 |
| Intermediate | 25.2 (23.4-26.9) | 24.1 (22.0-26.3) | 26.0 (23.8-28.3) | 0.27 |
| Poor | 14.5 (13.3-15.7) | 18.3 (16.4-20.2) | 11.7 (10.3-13.1) | <0.001 |
| Poor diet scoree | 78.1 (76.5-79.7) | 79.1 (77.2-81.0) | 77.4 (75.2-79.6) | 0.20 |
Data are presented as percentages (95% CIs).
Abbreviations: HOMA-IR, homeostasis model assessment of insulin resistance; IR, insulin resistance.
aObesity was defined as a body mass index of ≥30 kg/m.2
bHypertension was defined based on the 2017 American College of Cardiology/American Heart Association guidelines.
cHypercholesterolemia control was defined as total cholesterol ≥240 mg/dL.
dPoor physical activity level was defined based on the American Heart Association Life Simple 7 score metrics.
ePoor diet score is defined as a diet score <2 based on American Heart Association Life Simple 7 score metrics.
Trends in Cardiovascular Risk Factors: Stratified by Insulin Resistance Status
The prevalence of obesity increased from 50.0% (95% CI: 46.0%-54.0%) in 2007-2010 to 61.7% (95% CI: 55.9%-67.6%) in 2015-2018 among those with IR (P for trend = 0.004) (Table 3). The prevalence of obesity was 12.2% (95% CI: 9.6%-14.9%) in 2007-2010 and increased to 18.5% (95% CI: 14.5%-22.6%) in 2015-2018 in those without IR (P for trend = 0.11). The prevalence of hypertension did not significantly change through the study period for those with and without IR (all Ps for trend > 0.05). The prevalence of hypercholesterolemia declined from 13.3% (95% CI: 10.8%-15.9%) in 2007-2010 to 9.9% (95% CI: 7.5%-12.3%) in 2015-2018 (P for trend = 0.01).
There was no significant difference in the temporal trend of current smoking status among young adults with IR (P for trend = 0.44). Similarly, among young adults without IR, the prevalence of current smoking did not demonstrate any temporal trend for change (P for trend = 0.53). The prevalence of former and nonsmokers remained stable throughout the study period among those with and without IR (all Ps for trend > 0.05). Among those with IR, the prevalence of poor physical activity levels was 19.0% (95% CI: 16.5%-21.6%) in 2007-2010 and 18.8% (95% CI: 14.4%-23.3%) in 2015-2018 (P for trend = 0.52). Among those without IR, the prevalence of poor physical activity levels was 11.0% (95% CI: 8.8%-13.2%) in 2007-2010 and 13.4% (95% CI: 10.7%-16.1%) in 2015-2018 (P for trend = 0.66). There was a stable prevalence of ideal and intermediate physical activity among young adults with and without IR (all Ps for trend > 0.05). The relatively high prevalence of poor diet among those with IR remained stable at 78.1% (95% CI: 75.7%-80.6%) in 2007-2010 and 79.9% (95% CI: 75.9%-83.9%) in 2015-2018 (P for trend = 0.33). The prevalence of poor diet among those without IR did not significantly change over study period (P for trend = 0.81). The mean diet score across study periods stratified by IR status is depicted in Supplementary Table 3 (54). The trends of cardiovascular risk factors during the study period in the cumulative study population irrespective of IR status are depicted in Table 3. The estimated prevalence of cardiovascular risk factors (in millions) among young adults across study periods is depicted in Supplementary Figure 3 (53).
In sensitivity analyses, the HOMA-IR tertiles were identified as <1.65, 1.65 to 3.06, and >3.06. Those in the third tertile were defined as having IR in the sensitivity analyses. The trends of IR using this definition are described in Supplementary Table 4 (54). The prevalence and trends of cardiometabolic risk factors among those with and without IR are described in Supplementary Table 5 (54).
Discussion
In this study, we observed that ~40% of young adults without diabetes have IR. Young adults with IR are more likely to be male; Mexican American; obese; and have lower educational attainment, hypertension, hypercholesterolemia, and poor physical activity levels. IR was modestly associated with a larger waist circumference, higher BMI, higher total and localized fat mass, and lean mass. Importantly, nearly half of those with IR did not have obesity. The prevalence of IR declined among male participants during the study period but not among young female participants. There was a numerical increase in hypertension, a statistically significant decrease in hypercholesterolemia, and an increase in obesity in the last 12 years among young adults with IR. In summary, we observed that IR is highly prevalent among young American adults even without obesity, and there is a clustering of cardiovascular risk factors and measures of adiposity among young American adults with IR.
We found a modest continuous linear association between the surrogate measures of adiposity (BMI, waist circumference, total lean mass, total and localized fat mass) and HOMA-IR levels, validating the importance of adiposity in predicting IR. Higher levels of adiposity contribute to the development and worsening of IR (19-22). We found a temporal increase in the prevalence of obesity among young adults with IR while the prevalence of obesity among those without IR remained stable. Traditionally, BMI-defined obesity has been used as a surrogate for underlying IR, and it drives screening and primary prevention efforts. The current US Preventive Services Task Force guidelines only recommend diabetes screening for adults 40 to 70 years of age who are overweight or obese defined using the BMI cutoffs (55). The lack of obesity in nearly half of the young adults with IR, coupled with the clustering of cardiovascular risk factors, highlights the need to screen young adults for IR irrespective of BMI-defined obesity status. These findings also highlight the limitations of using BMI alone to identify individuals with metabolic syndrome (56). The other measures of adiposity such as waist circumference and visceral adiposity have been demonstrated to have a strong association with IR (57) and ASCVD risk (58). Total body visceral adipose tissue is considered to be the strongest predictor of metabolic syndrome and ASCVD risk (59). However, we noted only a modest association of adiposity measure with HOMA-IR, indicating that other biological factors may be contributing to the observed high prevalence of IR even among nonobese individuals. The contributors to IR other than body fat distribution, such as added sugars (60,61), neurohormonal dysregulation (62,63), and renin-angiotensin-aldosterone axis dysregulation (64), need further investigation to address this population of young adults with IR but without BMI-defined obesity.
The prevalence of IR from these NHANES data parallels the prevalence of the metabolic syndrome described in other studies (65,66). We noted that while females had a lower prevalence of IR, the prevalence of IR was declining only in young male adults and remained unchanged in young female adults. Prior studies have indicated that sex-related differences in visceral adiposity and hepatic lipid levels, alongside the lack of protective effect of estrogen, may contribute to the greater IR burden among males compared with females (67-69). A recent investigation indicates a greater adherence to physical activity guidelines among males (70). There may be other population-level factors such as differences in diet, healthcare-seeking behavior, access to healthcare, health education, socioeconomic status, and other social determinants of health contributing to the observed sex-related differences in the prevalence and trend of IR in young adults (70-73).
We demonstrated that prevalent hypertension was strongly associated with IR. IR increases sympathetic nerve activity and sodium retention (74), which leads to hyperinsulinemia. Hyperinsulinemia then independently increases blood pressure and also stimulates the sympathetic nervous system (62). High systolic blood pressure has a strong association with early coronary atherosclerosis among children and young adults (75). We have previously shown a high prevalence of hypertension and poor control among young adults, especially in individuals from racial minorities (37,38). The superimposed IR-associated cardiovascular disease burden among hypertensive young adults may increase cardiovascular events in the United States as the current young adult population ages. IR is strongly associated with a poor lipid profile (low HDL-C and elevated triglyceride levels) (5). In line with prior evidence, we observed a relatively higher prevalence of hypercholesterolemia among those with IR. Encouragingly, we noted a decline in hypercholesterolemia prevalence, especially among young adults with IR. As we have previously noted, this may be a result of the widespread implementation of the 2013 ACC/AHA lipid management guidelines (38,40).
There are several public health implications of our study. Our study highlights the role of aggressive screening and primordial prevention to mitigate the high prevalence of IR and associated cardiometabolic diseases among young American adults. The relatively high prevalence of IR and clustering of cardiometabolic risk factors among young adults with IR in the United States foreshadows an imminent epidemic of incident diabetes and ASCVD events nationally (37,38,76), which may burden the healthcare infrastructure (37,38,76). Our study findings encourage the intensification of IR screening across the spectrum of BMI, as well as early diagnosis and treatment of IR-associated cardiometabolic diseases among young adults. Screening for IR may also allow us to capture the associated cardiometabolic diseases, which already have very low awareness and treatment rates among young American adults (37,38). In our study, poor physical activity and poor diet were major contributors to the high prevalence of IR among young adults. Additionally, those with IR were less likely to be educated beyond high school, have health insurance, and seek outpatient medical care. These findings indicate the need to improve our health education efforts to inculcate the principles of healthy lifestyle measures as described in AHA’s Life Simple 7 measures starting from early childhood (37,38). Innovative approaches that involve education institutes to incentivize healthy diet, physical activity, and routine physical assessment may help integrate primordial cardiovascular disease prevention principles into everyday lives. Targeting the lifestyle interventions among high-risk young adults with IR and cardiometabolic risk factors to prevent further development of adverse cardiovascular events may have a higher yield of these interventions in the near future (77,78). A combination of population-level predictors of IR (eg, using sex, race, and socioeconomic status) along with a precision medicine approach (eg, using biomarkers) may help identify young adult populations with the highest yield of interventions (79,80). Newer therapies and combination pills may also be used to target the cluster of cardiometabolic risk factors accompanying IR and may have utility due to lower pill burden and higher adherence among young adults (81,82).
Mexican Americans and those from other minority racial groups had a relatively higher prevalence of IR. The accumulation of various socioeconomic factors may contribute to the clustering of cardiometabolic diseases among racial minorities. Increasing the community outreach and awareness about the prevention and control of cardiometabolic diseases among young adults from racial minorities may improve the cardiovascular health of disadvantaged communities. Targeting young adults, especially in geographical areas with high cardiometabolic disease prevalence, may yield significant benefits to reduce cardiovascular events and mortality in the United States (35).
Our study has important limitations. The NHANES is designed as a serial cross-sectional nationwide study. Hence, we cannot derive conclusions regarding incidence frequency, causality, and event frequency from these data (37-40,83). Alternative methods of ascertaining IR, such as intravenous glucose tolerance test or euglycemic clamp, may have yielded a more rigorous estimate of IR prevalence. However, these studies are challenging to implement, and HOMA-IR is a robust surrogate for IR and routinely used in large epidemiological investigations across diverse populations. The dichotomization of HOMA-IR to define IR may not be ideal. However, HOMA-IR ≥ 2.5 has been used as a validated measure of IR in prior studies (42-47). HOMA-IR is primarily an index of hepatic IR and may not adequately capture the peripheral IR, which may be higher in black individuals. Fasting insulin was measured using different assays in the different NHANES cycles, which may contribute to individual-level variance in measurement. However, a high degree of correlation in insulin measured across various analytical platforms, rigorous NHANES laboratory protocols, and lack of cross-reactivity with exogenous insulin administration (individuals without diabetes) may reduce the estimated variance at a population level (84). The study findings must be interpreted at a population level as there may be multiple factors contributing to individual-level variance in estimates. There may be potential misclassification of individuals due to the single assessment of blood pressure, other lab measures, and self-reported data measures. However, NHANES data are rigorously validated and routinely used for evaluating national-level healthcare trends. Thus, they provide an ideal nationally representative data source for providing a broad overview of the cardiometabolic risk profile in young American adults.
Conclusion
Approximately 40% of American adults 18 to 44 years of age without diabetes have IR. Nearly half of those with IR were not obese as per BMI. There is increasing clustering of cardiovascular risk factors among young adults with IR. Clinicians and policymakers need to aggressively pursue screening and primordial prevention measures to control IR and associated cardiovascular risk factors among young adults across the spectrum of BMI.
Acknowledgments
Funding: This work is supported by the National Institutes of Health Mentored Patient-Oriented Research Award (5K23 HL146887-03) to P.A.
Author Contributions: V.P., B.H., R.K., P.L., and P.A. contributed to the data acquisition, statistical analyses, and compilation of results. V.P., B.H., R.K., P.L.., GA., B.G., and P.A. contributed to the interpretation of the data. V.P. and B.H. wrote the initial draft of the manuscript with input from R.K., P.H., B.G., G.A., and P.A. All authors critically reviewed and revised the manuscript. V.P. and P.A. are guarantors of this work, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Additional Information
Disclosures: None of the authors had any conflicts of interest or financial disclosures to declare.
Data Availability
All data used for this analysis is publically available for download at https://www.cdc.gov/nchs/nhanes/index.htm.
References
- 1. Kekäläinen P, Sarlund H, Laakso M. Long-term association of cardiovascular risk factors with impaired insulin secretion and insulin resistance. Metabolism. 2000;49(10):1247-1254. [DOI] [PubMed] [Google Scholar]
- 2. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293-302. [DOI] [PubMed] [Google Scholar]
- 4. Hedblad B, Nilsson P, Engström G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabetic Med. 2002;19(6):470-475. [DOI] [PubMed] [Google Scholar]
- 5. Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49(21):2112-2119. [DOI] [PubMed] [Google Scholar]
- 6. Després JP, Lamarche B, Mauriège P, et al. . Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334(15):952-957. [DOI] [PubMed] [Google Scholar]
- 7. Ausk KJ, Boyko EJ, Ioannou GN. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care. 2010;33(6):1179-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tagi VM, Giannini C, Chiarelli F. Insulin resistance in children. Front Endocrinol (Lausanne). 2019;10:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kindler JM, Lobene AJ, Vogel KA, et al. . Adiposity, insulin resistance, and bone mass in children and adolescents. J Clin Endocrinol Metab. 2019;104(3):892-899. [DOI] [PubMed] [Google Scholar]
- 10. Sinaiko AR, Steinberger J, Moran A, et al. . Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111(15):1985-1991. [DOI] [PubMed] [Google Scholar]
- 11. Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444-2450. [DOI] [PubMed] [Google Scholar]
- 12. Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(suppl 1):60-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Gower BA, Goran MI. Dietary fat intake and insulin resistance in black and white children. Obes Res. 2005;13(9):1630-1637. [DOI] [PubMed] [Google Scholar]
- 14. Ingram KH, Lara-Castro C, Gower BA, et al. . Intramyocellular lipid and insulin resistance: differential relationships in European and African Americans. Obesity (Silver Spring). 2011;19(7):1469-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garr Barry V, Peterson CM, Gower BA. Membrane capacitance from a bioimpedance approach: associations with insulin resistance in relatively healthy adults. Obesity (Silver Spring). 2020;28(11):2184-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tay J, Goss AM, Garvey WT, et al. . Race affects the association of obesity measures with insulin sensitivity. Am J Clin Nutr. 2020;111(3):515-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167(7):642-648. [DOI] [PubMed] [Google Scholar]
- 19. Racette SB, Evans EM, Weiss EP, Hagberg JM, Holloszy JO. Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50-95 year olds. Diabetes Care. 2006;29(3):673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 2020;126(11):1549-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21(12):1443-1455. [DOI] [PubMed] [Google Scholar]
- 22. Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34(4):463-500. [DOI] [PubMed] [Google Scholar]
- 23. Clark ML, Utz SW. Social determinants of type 2 diabetes and health in the United States. World J Diabetes. 2014;5(3):296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 2014;129(suppl 2):19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hill-Briggs F, Adler NE, Berkowitz SA, et al. . Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silva-Tinoco R, Cuatecontzi-Xochitiotzi T, De la Torre-Saldaña V, et al. . Influence of social determinants, diabetes knowledge, health behaviors, and glycemic control in type 2 diabetes: an analysis from real-world evidence. BMC Endocr Disord. 2020;20(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim EJ, Abrahams S, Marrast L, Martinez J, Hanchate AD, Conigliaro J. Significance of multiple adverse social determinants of health on the diagnosis, control, and management of diabetes. J Gen Intern Med. 2021;36(7):2152-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walker RJ, Strom Williams J, Egede LE. Influence of race, ethnicity and social determinants of health on diabetes outcomes. Am J Med Sci. 2016;351(4):366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker RJ, Smalls BL, Egede LE. Social determinants of health in adults with type 2 diabetes–contribution of mutable and immutable factors. Diabetes Res Clin Pract. 2015;110(2):193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walker RJ, Smalls BL, Campbell JA, Strom Williams JL, Egede LE. Impact of social determinants of health on outcomes for type 2 diabetes: a systematic review. Endocrine. 2014;47(1):29-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schulz AJ, Zenk S, Odoms-Young A, et al. . Healthy eating and exercising to reduce diabetes: exploring the potential of social determinants of health frameworks within the context of community-based participatory diabetes prevention. Am J Public Health. 2005;95(4):645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ, Carnethon MR. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA. 2017;318(24):2457-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parcha V, Kalra R, Best AF, et al. . Geographic inequalities in cardiovascular mortality in the United States: 1999 to 2018. Mayo Clin Proc. 2021;96(5):1218-1228. [DOI] [PubMed] [Google Scholar]
- 34. Parcha V, Kalra R, Suri SS, et al. . Geographic variation in cardiovascular health among American adults. Mayo Clin Proc. 2021;96(7):1770-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parcha V, Malla G, Suri SS, et al. . Geographic variation in racial disparities in health and Coronavirus disease-2019 (COVID-19) mortality. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):703-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kalra R, Parcha V, Patel N, et al. . Increased awareness, inadequate treatment, and poor control of cardiovascular risk factors in American young adults: 2005-2016. Eur J Prev Cardiol. Published online March 2020. doi: 10.1177/2047487320905190 [DOI] [PubMed] [Google Scholar]
- 37. Parcha V, Patel N, Kalra R, Arora G, Arora P. Prevalence, awareness, treatment, and poor control of hypertension among young American adults: race-stratified analysis of the National Health and Nutrition Examination Survey. Mayo Clin Proc. 2020;95(7):1390-1403. [DOI] [PubMed] [Google Scholar]
- 38. Kalra R, Parcha V, Patel N, et al. . Increased awareness, inadequate treatment, and poor control of cardiovascular risk factors in American young adults: 2005-2016. Eur J Prev Cardiol. Published online March 2020: doi:10.1177/2047487320905190. [DOI] [PubMed] [Google Scholar]
- 39. Patel N, Kalra R, Bhargava A, Arora G, Arora P. Ideal cardiovascular health among American adults after the economic recession of 2008-2009: insights from NHANES. Am J Med. 2019;132(10):1182-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel N, Bhargava A, Kalra R, et al. . Trends in lipid, lipoproteins, and statin use among U.S. adults: impact of 2013 cholesterol guidelines. J Am Coll Cardiol. 2019;74(20):2525-2528. [DOI] [PubMed] [Google Scholar]
- 41. Wang DD, Leung CW, Li Y, et al. . Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vardeny O, Gupta DK, Claggett B, et al. . Insulin resistance and incident heart failure the ARIC study (Atherosclerosis Risk in Communities). JACC Heart Fail. 2013;1(6):531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 44. da Silva RC, Miranda WL, Chacra AR, Dib SA. Metabolic syndrome and insulin resistance in normal glucose tolerant Brazilian adolescents with family history of type 2 diabetes. Diabetes Care. 2005;28(3):716-718. [DOI] [PubMed] [Google Scholar]
- 45. Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res Care. 2017;5(1):e000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19(1):160-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cho SK, Huh JH, Yoo JS, Kim JW, Lee KJ. HOMA-estimated insulin resistance as an independent prognostic factor in patients with acute pancreatitis. Sci Rep. 2019;9(1):14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487-1495. [DOI] [PubMed] [Google Scholar]
- 49. Lee DH, Keum N, Hu FB, et al. . Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999-2006. Br J Nutr. 2017;118(10):858-866. [DOI] [PubMed] [Google Scholar]
- 50. Fan B, Shepherd JA, Levine MA, et al. . National Health and Nutrition Examination Survey whole-body dual-energy X-ray absorptiometry reference data for GE Lunar systems. J Clin Densitom. 2014;17(3):344-377. [DOI] [PubMed] [Google Scholar]
- 51. Hinton BJ, Fan B, Ng BK, Shepherd JA. Dual energy X-ray absorptiometry body composition reference values of limbs and trunk from NHANES 1999-2004 with additional visualization methods. PLoS One. 2017;12(3):e0174180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone. 2017;104:101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parcha V, Heindl BF, Kalra R, et al. Supplemental figures for: Insulin resistance and cardiometabolic risk profile among nondiabetic American young adults: insights from NHANES. 2021. doi: 10.6084/m9.figshare.14823627.v2 [DOI] [PMC free article] [PubMed]
- 54. Parcha V, Heindl BF, Kalra R, et al. Supplementary tables for: Insulin resistance and cardiometabolic risk profile among nondiabetic American young adults: insights from NHANES 2007-2018. Uploaded June 22, 2021. doi: 10.6084/m9.figshare.14825511.v3 [DOI] [PMC free article] [PubMed]
- 55. Siu AL; U S Preventive Services Task Force . Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. preventive services task force recommendation statement. Ann Intern Med. 2015;163(11):861-868. [DOI] [PubMed] [Google Scholar]
- 56. Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137(13):1391-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91(11):4459-4466. [DOI] [PubMed] [Google Scholar]
- 58. Cerhan JR, Moore SC, Jacobs EJ, et al. . A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clinic Proceedings. 2014;89(3):335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Neeland IJ, Ayers CR, Rohatgi AK, et al. . Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring). 2013;21(9):E439-E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang JW, Mark S, Henderson M, et al. . Adiposity and glucose intolerance exacerbate components of metabolic syndrome in children consuming sugar-sweetened beverages: QUALITY cohort study. Pediatr Obes. 2013;8(4):284-293. [DOI] [PubMed] [Google Scholar]
- 61. Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123(3):249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities—the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334(6):374-382. [DOI] [PubMed] [Google Scholar]
- 63. Parcha V, Patel N, Gutierrez OM, et al. . Chronobiology of natriuretic peptides and blood pressure in lean and obese individuals. J Am Coll Cardiol. 2021;77(18):2291-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150(11):776-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index; United States, 2003-2006. Natl Health Stat Report. 2009;5(13):1-7. [PubMed]
- 67. Aldhoon-Hainerová I, Zamrazilová H, Dušátková L, et al. . Glucose homeostasis and insulin resistance: prevalence, gender differences and predictors in adolescents. Diabetol Metab Syndr. 2014;6(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim HA, Lee SY, Kwon HS, et al. . Gender differences in the association of insulin resistance with metabolic risk factors among Korean adolescents: Korea National Health and Nutrition Examination Survey 2008-2010. Diabetes Res Clin Pract. 2013;99(1):54-62. [DOI] [PubMed] [Google Scholar]
- 69. Tester J, Sharma S, Jasik CB, Mietus-Snyder M, Tinajero-Deck L. Gender differences in prediabetes and insulin resistance among 1356 obese children in Northern California. Diabetes Metab Syndr. 2013;7(3):161-165. [DOI] [PubMed] [Google Scholar]
- 70. Du Y, Liu B, Sun Y, Snetselaar LG, Wallace RB, Bao W. Trends in adherence to the physical activity guidelines for americans for aerobic activity and time spent on sedentary behavior among US adults, 2007 to 2016. JAMA Netw Open. 2019;2(7):e197597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shan Z, Rehm CD, Rogers G, et al. . Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA. 2019;322(12):1178-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999-2012. JAMA. 2016;315(23):2542-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Peters SAE, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation. 2019;139(8):1025-1035. [DOI] [PubMed] [Google Scholar]
- 74. Masuo K, Rakugi H, Ogihara T, Esler MD, Lambert GW. Cardiovascular and renal complications of type 2 diabetes in obesity: role of sympathetic nerve activity and insulin resistance. Curr Diabetes Rev. 2010;6(2):58-67. [DOI] [PubMed] [Google Scholar]
- 75. Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338(23):1650-1656. [DOI] [PubMed] [Google Scholar]
- 76. Virani SS, Alonso A, Aparicio HJ, et al. ; American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S . Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021:143(8):e254-e743. [DOI] [PubMed] [Google Scholar]
- 77. Raitakari OT, Juonala M, Kähönen M, et al. . Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277-2283. [DOI] [PubMed] [Google Scholar]
- 78. Li S, Chen W, Srinivasan SR, et al. . Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290(17):2271-2276. [DOI] [PubMed] [Google Scholar]
- 79. Pandey A, Patel KV, Vongpatanasin W, et al. . Incorporation of biomarkers into risk assessment for allocation of antihypertensive medication according to the 2017 ACC/AHA high blood pressure guideline: a pooled cohort analysis. Circulation. 2019;140(25):2076-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Parcha V, Malla G, Kalra R, et al. . Coronary artery calcium score for personalization of antihypertensive therapy: a pooled cohort analysis. Hypertension. 2021:77(4):1106-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Muñoz D, Uzoije P, Reynolds C, et al. . Polypill for cardiovascular disease prevention in an underserved population. N Engl J Med. 2019;381(12):1114-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016;39(5):717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kalra R, Patel N, Arora P, Arora G. Cardiovascular health and disease among Asian-Americans (from the National Health and Nutrition Examination Survey). Am J Cardiol. 2019;124(2):270-277. [DOI] [PubMed] [Google Scholar]
- 84. Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem. 2007;53(5):922-932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for this analysis is publically available for download at https://www.cdc.gov/nchs/nhanes/index.htm.