Abstract
Context
Confirming a diagnosis of Cushing disease (CD) remains challenging, yet is critically important before recommending transsphenoidal surgery for adenoma resection.
Objective
To describe predictive performance of preoperative biochemical and imaging data relative to post-operative remission and clinical characteristics in patients with presumed CD.
Design, Setting, Patients, Interventions
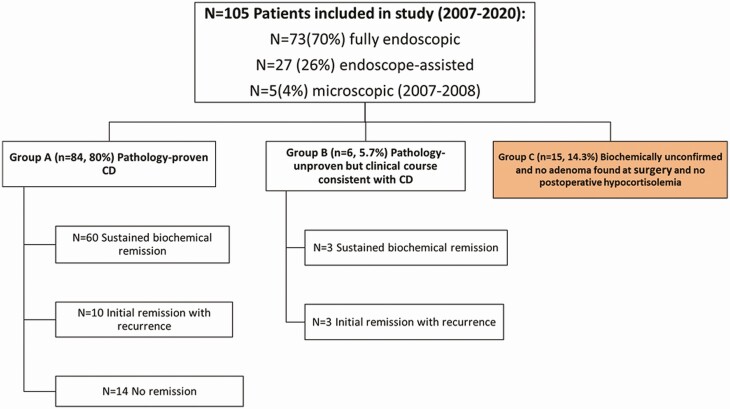
Patients (n = 105; 86% female) who underwent surgery from 2007 through 2020 were classified into 3 groups: group A (n = 84) pathology-proven ACTH adenoma; group B (n = 6) pathology-unproven but with postoperative hypocortisolemia consistent with CD; and group C (n = 15) pathology-unproven, without postoperative hypocortisolemia. Group A + B were combined as confirmed CD and group C as unconfirmed CD.
Main outcomes
Group A + B was compared with group C regarding predictive performance of preoperative 24-hour urinary free cortisol (UFC), late night salivary cortisol (LNSC), 1-mg dexamethasone suppression test (DST), plasma ACTH, and pituitary magnetic resonance imaging (MRI).
Results
All groups had a similar clinical phenotype. Compared with group C, group A + B had higher mean UFC (P < 0.001), LNSC (P = 0.003), DST (P = 0.06), and ACTH (P = 0.03) and larger MRI-defined lesions (P < 0.001). The highest accuracy thresholds were: UFC 72 µg/24 hours; LNSC 0.122 µg/dL, DST 2.70 µg/dL, and ACTH 39.1 pg/mL. Early (3-month) biochemical remission was achieved in 76/105 (72%) patients: 76/90(84%) and 0/15(0%) of group A + B vs group C, respectively, P < 0.0001. In group A + B, nonremission was strongly associated with adenoma cavernous sinus invasion.
Conclusions
Use of strict biochemical thresholds may help avoid offering transsphenoidal surgery to presumed CD patients with equivocal data and improve surgical remission rates. Patients with Cushingoid phenotype but equivocal biochemical data warrant additional rigorous testing.
Keywords: Cushing’s disease, pituitary adenoma, transsphenoidal surgery, ACTH, salivary cortisol, 24-hour urinary free cortisol
Cushing disease (CD) is characterized by hypercortisolemia caused by a pituitary corticotroph adenoma with ACTH secretion (1) and is the most common cause of endogenous Cushing syndrome (CS). Untreated patients can develop serious complications including cardiovascular events, coagulopathy, unusual infections, neuropsychological impairment, and death. Long-term sequelae often persist despite biochemical control (2, 3). Once diagnosed, first-line treatment of CD is transsphenoidal surgery and selective adenectomy. Current surgical remission rates at experienced pituitary centers range from 65% to 85% depending on remission criteria (4-10).
As per published guidelines, ACTH-dependent CS diagnosis requires abnormal 24-hour urinary free cortisol (UFC), late-night salivary cortisol (LNSC), or 1-mg dexamethasone suppression test (DST) results along with high or nonsuppressed plasma ACTH levels (11, 12). However, a clear diagnosis is not always achieved before recommending transsphenoidal surgery as many patients who present with a Cushingoid phenotype that may include progressive obesity, stria, hypertension, diabetes, weakness, and/or mood disorders have discordant biochemical data and imaging. As a result, the diagnosis may remain unknown if surgery does not result in biochemical remission and/or resection of an ACTH-staining adenoma (13-15).
Potential causes of discordant results or confounding factors include variable endogenous cortisol secretion (cyclical CS), conditions that cause mild nonneoplastic hypercortisolism (pregnancy, depression, alcohol dependence), inappropriate sample collection and processing, immunoassay limitations, neurosurgeon expertise, and pathology sample adequacy (14-17).
At our pituitary center, we have recognized a subset of patients with a Cushingoid phenotype yet discordant biochemical data and imaging, in whom no adenoma was found at surgery, remission was not achieved, and CD was never proven. The goal of this analysis was to develop preoperative biochemical thresholds that allow the endocrine and neurosurgical teams to confidently offer (or not offer) transsphenoidal surgery. We describe the sensitivity, specificity, positive and negative predictive values, and accuracy of tests used in CS diagnosis. To our knowledge, such an analysis and perspective on diagnostic tests has not been reported in a large group of patients. We hope our findings will lead to a more personalized and precise diagnosis and help prevent unnecessary surgical interventions.
Methodology
Study Design
This retrospective study aims to describe the sensitivity, specificity, and accuracy of standard tests in diagnosing CS including UFC, LNSC, 1-mg DST, as well as the utility of tests used to diagnose CD (ACTH and pituitary magnetic resonance imaging [MRI]) in a population of patients with presumed CD. The study was approved by the Saint John’s Cancer Institute institutional review board, protocol JWCI-19-1101; informed consent was waived.
Eligibility Criteria
We reviewed the medical records of all patients with a clinical diagnosis of CD who underwent transsphenoidal surgery by 1 of 2 neurosurgeons (D.K. and G.B.) between July 2007 and June 2020 at the Pacific Neuroscience Institute, Saint John’s Health Center in Santa Monica, California (18, 19). Of 109 patients, 4 were excluded, including 2 who underwent total or near total hypophysectomy and 2 who did not have a pathology-proven ACTH adenoma, did not achieve early remission, and then underwent bilateral adrenalectomy, or had minimal follow-up. Presumptive diagnosis of CD was based on an elevated mean preoperative UFC and LNSC, serum cortisol concentrations ≥ 1.8 µg/dL after DST, and plasma ACTH levels ≥ 20 pg/mL (20).
Surgical Technique and Postoperative Testing
Transsphenoidal surgery for pituitary adenoma has transitioned over the past 2 decades from a microscopic to endoscopic technique at most pituitary centers (6, 9, 10, 21-24). In this cohort, 5 procedures used a microscopic approach (2007-2008), 27 used an endoscope-assisted technique (2007-2011), and 73 (71%) (2010-2020) were performed with a fully endoscopic approach, with a goal of selective and complete adenomectomy using the pseudocapsule technique (25) with attempted removal or cauterization of tumor-infiltrated dura. Briefly, in its present state, endoscopic pituitary surgery uses a binostril technique with a neurosurgeon and otolaryngologist with 0° 4-mm endoscope and 30° and 45° endoscopes available (Karl Storz-America, El Segundo, CA). In patients with visible tumor on preoperative 3T sellar MRI (without and with gadolinium), selective adenomectomy is often initiated with an incision to reach the adenoma pseudocapsule. When the preoperative MRI does not indicate the adenoma location, the gland is carefully explored through multiple vertical incisions. If no tumor is found, partial hemihypophysectomy is performed on the most suspicious side; no patient in this analysis underwent total hypophysectomy.
As previously published, blood draws for serum cortisol and ACTH were performed on postoperative day 1 and 2 (sometimes extending to day 3 postsurgery) at 6- to 8-hour intervals and those with cortisol below 5 µg/dL during the first 3 days were established as early remission (9). Cortisol supplementation was given in the perioperative period when biochemical evidence of hypocortisolemia was documented and/or clinical evidence of adrenal insufficiency was present (eg, nausea, anorexia, fatigue, headache, arthralgias).
Assessment of corticotroph function and overall pituitary function (including thyrotroph, gonadotroph, and somatotroph function) were generally performed at 3 and 6 months after surgery (24). Sustained remission was defined as the need for glucocorticoid replacement for at least 6 months postsurgery and with clinical and biochemical evidence of eucortisolemia thereafter until the last follow-up contact. All patients were weaned off glucocorticoids as clinically tolerated until biochemical evidence of a recovered hypothalamic-pituitary-adrenal axis function was achieved. For patients able to be weaned off glucocorticoids, subsequent assessments of corticotroph function included 24-hour UFC collections, and more recently with LNSC levels at 6-month intervals, as well as ACTH stimulation testing in most patients (often at the discretion of the referring endocrinologist). Sustained remission was defined as ongoing need for glucocorticoid therapy or normal 24-hour UFC and/or midnight salivary cortisol tests at the last follow-up.
Data Collection
Clinical predictors of interest were obtained from medical records. All available preoperative biochemical testing (UFC, LNSC, DST, ACTH), pituitary MRI findings, tumor pathology (including ACTH staining, reticulin disruption), and additional treatments (radiation, medical therapy, and bilateral adrenalectomy) were analyzed. Data excluded from analysis because of high rates of missing data comprised 3/13 clinical covariates (low libido, osteoporosis, recurrent infections), additional differential diagnosis studies (8-mg DST, corticotropin releasing hormone stimulation test, and inferior petrosal vein sampling). Because many patients early in the series did not have pathology assessment of Crooke hyaline change, this data point was not assessed.
For each patient, surgical variables included transsphenoidal surgical technique, extent of pseudocapsular dissection technique, use of multiple gland incisions, percentage/amount of gland resection, presence or absence of dural or cavernous sinus invasion, 3-month and long-term remission rates, postoperative nadir serum cortisol and plasma ACTH levels, surgical complications, length of hospital stay, and need for additional treatments. The were no missing surgical data. Early biochemical remission criteria was defined as nadir serum cortisol < 5 µg/dL within 48 hours of surgery and need for hydrocortisone supplementation for at least 3 months postsurgery (9). Notably, at the time of serum cortisol measurements, no females in this study were receiving estrogen supplementation, which could increase serum cortisol measurements by increasing cortisol binding protein concentrations.
Hormonal Assays
Immunoassays (IAs) and liquid chromatography using tandem mass spectrometry (LC-MS/MS) were used over the study period following laboratory reference ranges to measure serum and urinary cortisol. For blood obtained at our center, plasma ACTH was measured by IAs (ARUP Laboratories; reference range 7-63 pg/mL). For urine and saliva obtained at our center, UFC by LC-MS/MS (reference range: 4-50 µg/24 hours) and LNSC by LCMS/MS (reference range: < 0.09 µg/dL) were performed by Quest Diagnostics. For hormonal assay results obtained at other outside laboratories for IAs, the laboratory-specific reference ranges were followed.
Study Groups
The study cohort of 105 patients was categorized into 3 groups (Fig. 1) based upon pathology and postoperative clinical course: group A (n = 84) pathology-proven ACTH adenoma (adenomatous tissue with ACTH-positive immunostaining); group B (n = 6) pathology-unproven adenoma removed at surgery or ≤ 50% hypophysectomy performed and clinical course consistent with CD (preoperative ACTH levels ≥ 20 pg/mL, postoperative nadir serum cortisol < 5 µg/dL, and requiring hydrocortisone before discharge); and group C (n = 15) pathology-unproven, no adenoma found at surgery, and no postoperative hypocortisolemia achieved. Given that all group B patients had unequivocal preoperative biochemical testing consistent with CD, early postoperative hypocortisolemia requiring hydrocortisone before hospital discharge and 5 of 6 had clear adenomatous tissue identified at surgery (but not seen on pathology), they are grouped with group A for the main analysis, as has been done in prior CD reports (26-29). Analyses were performed comparing groups A vs B (Table 1) and groups A + B vs group C (Table 2).
Figure 1.
Patients included in the study were classified into 3 groups: group A (pathology-proven CD), group B (pathology-unproven but clinical course consistent with CD), and group C (biochemically unconfirmed and no adenoma found at surgery and no postoperative hypocortisolemia). CD, Cushing disease.
Table 1.
Comparison of group A (pathology-proven) vs group B (pathology-unproven)
| Characteristic | Pathology-proven CD (n = 84) Group A |
Pathology-unproven CD (n = 6) Group B |
P value |
|---|---|---|---|
| Mean age, y (±SD) | 43 (15) | 49 (17) | 0.33 |
| Female sex, n (%) | 72 (86) | 5 (83) | 1.00 |
| Mean preoperative BMI (±SD) | 33 (6.9) | 28(5.5) | 0.07 |
| Prior surgery, n (±SD) | 27 (32) | 2 (33) | 1.00 |
| Signs and symptoms, n (%) | |||
| Weight gain (n = 90) | 81 (96) | 5 (83) | 0.25 |
| Fatigue (n = 39) | 31 (89) | 3 (75) | 0.44 |
| Hypertension (n = 89) | 56 (68) | 4 (67) | 1.00 |
| Diabetes (n = 88) | 26 (32) | 3 (50) | 0.39 |
| Irregular menstrual cycles (n = 4) | 4 (100) | 0 (0) | NA |
| Hirsutism (n = 23) | 20 (91) | 1 (100) | 1.000 |
| Violaceous striae (n = 21) | 12 (67) | 3 (100) | 0.52 |
| Easy bruising (n = 28) | 22 (82) | 1 (100) | 1.00 |
| Depression (n = 25) | 13 (57) | 1 (50) | 1.00 |
| Anxiety or psychosis (n = 22) | 13 (65) | 2 (100) | 1.00 |
| Laboratory and imaging | |||
| 24-h UFC LC-MS/MS (±SD) (n: 4-50 mcg/24 h) (n = 70) |
238.6 ± 33.0 | 114.8 ± 32.5 | 0.34 |
| LNSC LC/MS (±SD) (n: <0.09 mcg/dL) (n = 29) |
0.47 ± 0.09 | 0.10 ± 0.01 | 0.06 |
| Serum cortisol after 1 mg DST (±SD) (n: < 1.8 mcg/dL) (n = 41) |
17.9 ± 3.2 | 17.1 ± 5.0 | 0.57 |
| Plasma ACTH (±SD) (n = 87) |
62.2 ± 5.4 | 54.0 ± 15.6 | 0.65 |
| Pituitary lesion present on MRI, n (%) | 71 (85) | 5 (83) | 1.00 |
| Mean pituitary lesion size (±SD), mm | 9.5 (8.0) | 3.6 (1.9) | 0.11 |
Missing data were not included in the analysis. P values were obtained via nonparametric methods.
Abbreviations: 24-h UFC, 24-hour urinary free cortisol; BMI, body mass index; CD, Cushing disease; DST, dexamethasone stimulation test; LC-MS/MS, liquid chromatography tandem mass spectometry; LNSC, late night salivary cortisol; MRI, magnetic resonance imaging; NA, not applicable; UFC, urinary free cortisol.
Table 2.
Demographic characteristics of 105 patients comparing groups A + B (confirmed CD) vs group C (unconfirmed CD)
| Characteristic | Confirmed CD (n = 90) Group A + B |
Unconfirmed CD (n = 15) Group C |
P value |
|---|---|---|---|
| Age (y), mean (±SD) | 44 (15) | 41 (17) | 0.57 |
| Female, n (%) | 77 (86) | 13 (87) | 1.00 |
| Preoperative BMI, mean (±SD) | 33 (6.9) | 32 (5.8) | 0.61 |
| Prior surgery (%) | 29 (32) | 1 (7) | 0.06 |
| Signs and symptoms (%) | |||
| Weight gain (n = 105) | 86 (96) | 14 (93) | 0.54 |
| Fatigue (n = 50) | 34 (87) | 11 (100) | 0.57 |
| Hypertension (n = 104) | 60 (67) | 7 (47) | 0.15 |
| Diabetes (n = 103) | 29 (33) | 4 (27) | 0.77 |
| Irregular menstrual cycles (n = 30) | 21 (27) | 5 (39) | 0.41 |
| Hirsutism (n = 29) | 16 (21) | 7 (54) | 0.01 |
| Violaceous striae (n = 31) | 15 (71) | 7 (70) | 1.00 |
| Easy bruising (n = 35) | 23 (82) | 6 (85) | 1.00 |
| Depression (n = 32) | 14 (56) | 5 (71) | 0.67 |
| Anxiety or psychosis (n = 29) | 15 (68) | 6 (86) | 0.63 |
| Laboratory and imaging | |||
| 24-h UFC LC-MS/MS (n: 4-50 µg/24 h) (n = 82) |
230.5 ± 262.3 (n = 70) |
52.3 ± 19.3 (n = 12) |
<0.001 |
| LNSC LC-MS/MS (±SD) (n: <0.09 µg/dL) (n = 41) |
0.44 ± 0.46 (n = 29) |
0.19 ± 0.33 (n = 12) |
0.003 |
| Serum cortisol after 1-mg DST (±SD) (n: <1.8 µg/dL) (n = 48) |
18.0 ± 18.6 (n = 41) |
8.1 ± 11.1 (n = 7) |
0.06 |
| Plasma ACTH (±SD) (n: 6-50 pg/mL) (n = 102) | 61.7 ± 47.9 (n = 87) |
45.8 ± 41.6 (n = 15) |
0.03 |
| Pituitary lesion present on MRI, n (%) | 76 (84) | 10 (67) | 0.14 |
| Mean pituitary lesion size on MRI (±SD), mm | 9.1 (7.9) | 4.0 (1.8) | <0.001 |
Missing data were not included in the analysis. P values were obtained via nonparametric methods.
Abbreviations: 24-h UFC, 24-hour urinary free cortisol; BMI, body mass index; CD, Cushing disease; DST, dexamethasone stimulation test; LC-MS/MS, liquid chromatography tandem mass spectometry; LNSC, late night salivary cortisol; MRI, magnetic resonance imaging; UFC, urinary free cortisol.
Statistical Methods and Analysis of Diagnostic Test Performance
Continuous variables are expressed as mean ± SD or median (ranges) for variables with a skewed distribution. Categorical variables are presented as the number of patients and corresponding percentages. Categorical variables (ie, biological sex, symptoms) were compared using the χ 2 test or Fisher exact test when appropriate. The Wilcoxon rank-sum test was used for between group comparisons of continuous variables with a skewed distribution. Logistic regression analysis was used to test associations between the CD diagnosis and the hormonal test of interest.
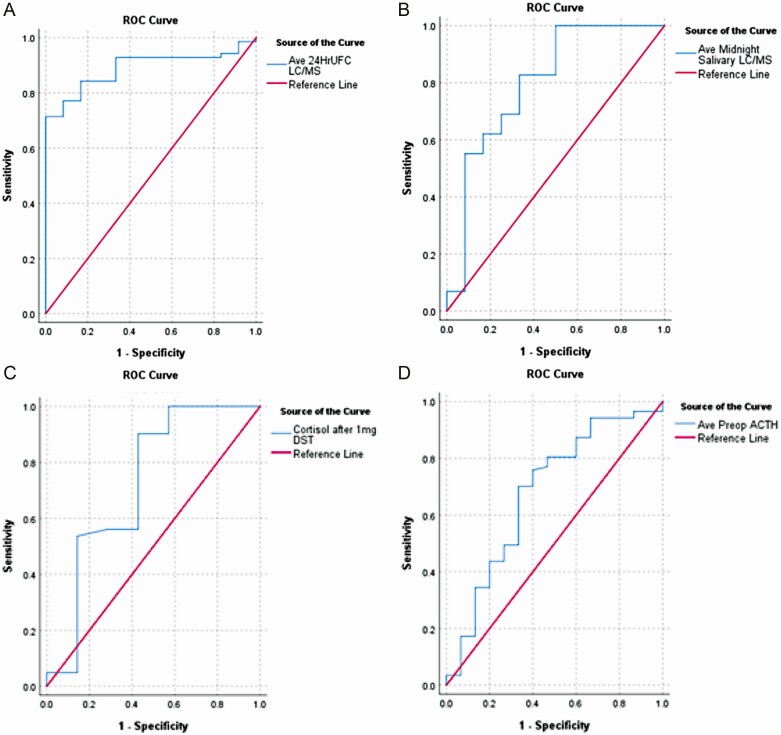
To assess the accuracy of discrimination for CS/CD, a receiver operating characteristic (ROC) analysis was performed for UFC and LNSC by LC-MS/MS, serum cortisol after DST, ACTH levels, and pituitary MRI findings using logistic regression probabilities predicting a diagnosis of CD. An abnormal value was defined as any value above the stated reference range for a given test. Sensitivity and specificity were examined along with area under the ROC curve (AUC) and their corresponding 95% CIs. AUCs were compared for statistical difference using the Venkatraman method (30). The threshold of highest diagnostic accuracy for each test was defined to be the point minimizing the distance from the point (0,1) (31, 32), which theoretically maximizes sensitivity and specificity. Positive and negative predictive values corresponding to each threshold value were calculated subsequently. ROC curves are presented (Fig. 3) to show the overall performance of each diagnostic test.
Figure 3.
ROC analysis. (A) 24-hour UFC LC-MS/MS, AUC 0.89. (B) Midnight salivary cortisol LC-MS/MS, AUC 0.80. (C) Serum cortisol after 1-mg DST, AUC 0.72. (D) Plasma ACTH pg/mL, AUC: 0.68. AUC, area under the curve; DST, dexamethasone suppression test; LC-MS/MS, liquid chromatography tandem mass spectometry; ROC, receiver operating characteristic.
Composite test performance for UFC + ACTH for CS/CD discrimination was then analyzed with the newly identified diagnostic thresholds and with the thresholds recommended by the Endocrine Society clinical practice guidelines separately (20). A similar ROC analysis was performed using combined UFC and ACTH measurements. The analysis following guidelines recommendations incorporated the pool of measurements by LC-MS/MS (UFC normal: 4-50 µg/24 hours) or other methodologies (laboratory-specific reference ranges were followed). Composite testing was not performed using LNSC or DST given small sample size for these variables.
SAS software version 9.4 (Cary, NC), SPSS Version 26 (IBM Corporation, Armonk NY), and R 4.0.3 (R Core Team, 2020) including the OptimalCutpoints (v1.1.4; Lopez-Raton and Rodriguez-Alvarez, 2019) and pROC (33) packages were used for statistical analysis. Two-sided P values less than 0.05 were considered significant.
Results
A total of 105 patients (mean age, 43.2 ± 14.8 years; 86% female) underwent endonasal surgery between January 2007 and June 2020. Table 1 compares group A patients (pathology-proven) vs group B patients, (pathology-unproven). No significant differences were found between groups A and B in their clinical symptoms/signs (except body mass index) or preoperative biochemical testing, or in remission rates by surgical technique. As a result, groups A and B were combined as confirmed CD.
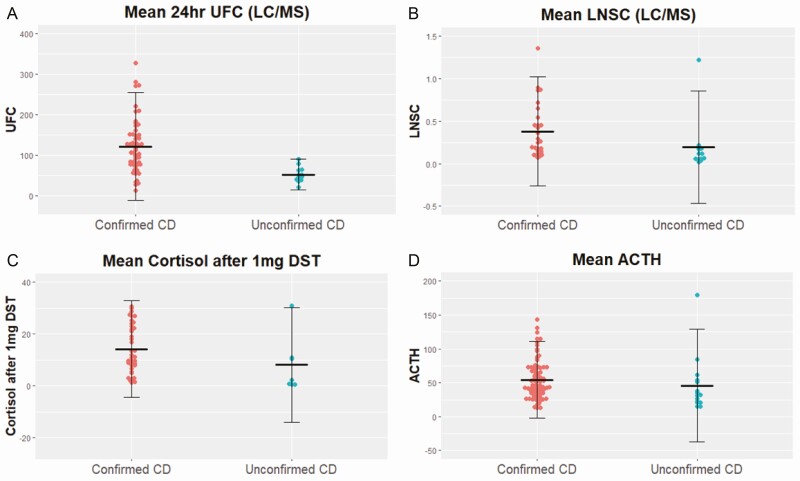
Table 2 and Fig. 2 compare groups A + B (confirmed CD; n = 90) vs group C (unconfirmed CD, n = 15). No significant differences were found for age, sex, preoperative body mass index, or CD symptoms. By biochemical testing and imaging, the confirmed CD group had higher mean UFC LC-MS/MS (230.5 ± 262.3 vs 52.3 ± 19.3 µg/24 hours; P ≤ 0.001), LNSC LC-MS/MS (0.44 ± 0.46 vs 0.19 ± 0.33 µg/dL; P = 0.003), serum cortisol after 1-mg DST (18.0 ± 18.6 vs 8.1 ± 11.1µg/dL; P = 0.06), plasma ACTH (61.7 ± 47.9 vs 45.8 ± 41.6 pg/mL; P = 0.03), and larger pituitary lesion size on MRI (9 vs 4 mm; P ≤ 0.001).
Figure 2.
Comparing groups A + B (confirmed CD, n = 90) vs group C (unconfirmed CD, n = 15) for (A) mean 24-hour urinary free cortisol (with crossbars denoting standard deviation), (B) mean late night salivary cortisol, (C) mean serum cortisol after 1-mg DST, and (D) mean plasma ACTH. CD, Cushing disease; DST, dexamethasone suppression test.
Table 3 and Fig. 3 summarize biochemical test performance and ROC analysis. As shown in Table 3, for individual tests, the highest accuracy thresholds were: UFC (by LC-MS/MS) 72 µg/24 hours; LNSC (by LC-MS/MS) 0.122 µg/dL, serum cortisol after DST (IA) 2.70 µg/dL, and ACTH 39.1 pg/mL. In addition, it shows the proportion of patients above these thresholds in the confirmed (A + B) vs unconfirmed (C) groups. Table 4 shows that composite testing of UFC + ACTH using the highest accuracy thresholds had superior performance over standard of care thresholds for AUC, specificity, and positive and negative predictive values, but similar sensitivity.
Table 3.
Diagnostic test performance of individual tests to distinguish confirmed CD from unconfirmed CD and performance of tests in confirmed vs unconfirmed groups
| Diagnostic test, technique, and normal range | Highest accuracy threshold | AUC | Sensitivity % | Specificity % | PPV (95% CI) | NPV (95% CI) | Confirmed (group A + B) above threshold | Unconfirmed (group C) above threshold | P value |
|---|---|---|---|---|---|---|---|---|---|
| UFC LC-MS/MS (n: 4-50 µg/24h) n = 82 |
72.0 | 0.89 (0.82-0.96) | 84 | 83 | 97 (86-98) | 48 (32-90) | 59/70 (84%) | 2/12 (17%) | <0.001 |
| LNSC LC-MS/MS (n: <0.09 µg/dL) n = 41 |
0.122 | 0.80 (0.63-0.97) | 83 | 67 | 86 (62-95) | 62 (37-88) | 24/29 (83%) | 4/12 (33%) | 0.004 |
| Cortisol after DST IA (n: <1.8 µg/dL) n = 48 |
2.70 | 0.72 (0.45-0.99) | 90 | 57 | 93 (68-99) | 50 (26-87) | 36/41 (88%) | 3/7 (43%) | 0.02 |
| ACTH IA (n: 6-50 pg/mL) n = 102 |
39.1 | 0.68 (0.51-0.84) | 70 | 67 | 92 (79-95) | 28 (19-59) | 60/87 (69%) | 5/15 (33%) | 0.02 |
| Adenoma size (mm) on pituitary MRI n = 87 | 5.0 | 0.81 (0.64-0.91) | 75 | 70 | 95 (81-97) | 27 (17,69) |
Abbreviations: AUC, area under the curve; DST, dexamethasone stimulation test; IA, immunoassay; LC-MS/MS, liquid chromatography tandem mass spectometry; LNSC, late night salivary cortisol; MRI, magnetic resonance imaging; NPV, negative predictive value; PPV, positive predictive value; UFC, urinary free cortisol.
Table 4.
Composite diagnostic test performance of UFC + ACTH using normal upper limit vs highest accuracy threshold
| Diagnostic test, technique, and normal reference range | Highest accuracy threshold | AUCa | Sensitivity, % | Specificity, % | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| Composite test following highest accuracy thresholds | ||||||
| UFC (LC-MS/MS) + ACTH, n = 82 | 72.0, 39.1 | 0.93 (0.87,0.98) | 84 | 100 | 100 (93-100) | 52 (36-100) |
| Composite tests following standard-of-care thresholds | ||||||
| Pool UFC + ACTH n = 97 |
UFC ≥ ULN + ACTH > 20 ng/mL | 0.67 (0.84,0.50) | 86 | 47 | 89 (73-95) | 39 (25-67) |
Abbreviations: AUC, area under the curve; LC-MS/MS, liquid chromatography tandem mass spectometry; NPV, negative predictive value; PPV, positive predictive value; UFC, urinary free cortisol; ULN, upper limit of normal.
a Comparing AUC using highest accuracy thresholds versus standard of care thresholds, P = 0.015.
Surgical Highlights, Clinical Remission Rates, and Complications
Surgical and postoperative outcomes are outlined in Table 5. Early (3-month) biochemical remission for the entire cohort was 76/105 (72%). For groups A, B, and C, remission rates were 70/84 (83%), 6/6 (100%), and 0/15(0%), respectively, and for confirmed (A + B) vs unconfirmed (C) groups were 76/90(84%) and 0/15(0%), respectively; P < 0.0001. Additional notable differences in confirmed vs unconfirmed groups include higher rates of pseudocapsular dissection, lower rates of partial hypophysectomy, and lower rates of multiple gland incisions for confirmed CD. Of first-time surgery patients in the confirmed group, pseudocapsular dissection was performed in 34/62 (55%) patients and in 0/15 patients in the unconfirmed group (P < 0.001). Cavernous sinus invasion also was documented in 30% of the confirmed group and none of the unconfirmed. Of 90 confirmed cases, 3-month remission rates were 60/63 (95%) without cavernous sinus invasion and 16/27 (59%) with cavernous sinus invasion; P < 0.001. Of 4 patients without cavernous sinus invasion who did not achieve early remission, 2 had macroadenomas with apoplexy and 1 had a no adenoma seen on MRI. Overall, 5 of 105 patients (all in confirmed CD group) (4.7%) sustained major postoperative complications including: (1) cerebrospinal fluid leak and basilar artery injury, (2) cerebrospinal fluid leak, (3) permanent diabetes insipidus, (4) pulmonary embolism, and (5) aortic thrombosis with pulmonary embolism. Five patients had minor complications, including 4 with delayed hyponatremia and 1 with sinusitis; 1 case of hyponatremia occurred in the unconfirmed group.
Table 5.
Surgical and postoperative characteristics
| Characteristic | Confirmed CD (n = 90) Group A + B |
Unconfirmed CD (n = 15) Group C |
P value |
|---|---|---|---|
| Intraoperative technique | |||
| Pseudocapsular technique, n (%) | |||
| None | 52 (58) | 15 (100) | 0.007 |
| Partial | 18 (20) | 0 | |
| Complete | 20 (22) | 0 | |
| Multiple gland incision, n (%) | 10 (11) | 15 (100) | <0.001 |
| Hemihypophysectomy, n (%) | |||
| None | 56 (62) | 1 (7) | <0.001 |
| Minimal | 17 (19) | 1 (7) | |
| Partial | 16 (18) | 12 (80) | |
| Near total | 1 (1) | 1 (7) | |
| Dural invasion, n (%) | 18 (20) | 0 | 0.07 |
| Cavernous sinus invasion, n (%) | 27 (30) | 0 | 0.01 |
| Endoscopy | |||
| Microscopic | 5 (6) | 0 | 0.51 |
| Endoscope-assisted | 24 (27) | 3 (20) | |
| Fully endoscopic | 61 (68) | 12 (80) | |
| Major surgical complication, n (%): | 5 (5.6) | 0 | 1.00 |
| Postoperative 3-month remission | 76 (84) | 0 (0) | <0.0001 |
| Long-term surgical remission | 63 (70) | NA | NA |
| Long-term remission without CS invasion | 49/63 (77) | NA | NA |
| Long-term remission with CS invasion | 14/27 (52) | NA | NA |
| Postoperative predictors | |||
| Nadir serum cortisol median (range); NR | 1.49 (0.5-26.2); 0 | 4.70 (1.91-20.1);0 | <0.001 |
| Nadir postoperative plasma ACTH; median (range); NR | 9 (1-73); 5 | 11 (5-19); 0 | 0.16 |
| Length of hospital stay, days, median (range); NR | 2 (1-27); 0 | 2 (2-5); 0 | 0.78 |
| Survival n (%); NR | 86 (96); 0 | 15 (100); 0 | 1.00 |
| Additional treatments | |||
| Repeat operation | 20 (22) | 2 (13) | 0.43 |
| Medical treatment | 5 (6) | 3 (20) | 0.09 |
| Stereotactic radiation | 4 (4) | 0 | 1.00 |
| Bilateral adrenalectomy | 2 (2) | 5 (33) | <0.001 |
| Long-term remission after all therapies | 70 (78) | NA | NA |
Postoperative ACTH values were calculated before discharge. Gradient postoperative serum cortisol (maximal serum cortisol – nadir serum cortisol).
Abbreviations: CD, Cushing disease; CS, Cushing syndrome; NA, not applicable; NR, none reported/missing data.
Subgroup Analysis and Characteristics of Patients With Unconfirmed CD
Table 6 provides individual clinical data for the 15 unconfirmed CD patients, showing that all had evidence of mild or moderate hypercortisolemia in a least 1 assay, but none had evidence of persistent hypercortisolemia in more than 2 tests. Preoperative MRI showed an adenoma or cystic lesion in 11 (73%), ranging from 2 to 6 mm. Five of 15 patients had localization testing, mostly with inferior petrosal sinus sampling. However, persistent hypercortisolemia preceding inferior petrosal sinus sampling (IPSS) was not documented. Only 1 of 12 (8.3%) patients (#12) had Crooke hyaline changes in pathology (Crooke staining was unavailable in 3 patients). Pathology confirmed normal pituitary gland (n = 10), Rathke cleft cyst (n = 1), other pituitary cysts (n = 2), fibrosis (n = 1), and a microprolactinoma (n = 1). None of these 15 patients achieved sustained hypocortisolemia in the early postoperative period. Of these 15 patients, 1 ultimately underwent hypophysectomy and 3 underwent bilateral adrenalectomies. All 3 patients who underwent adrenalectomy were first treated medically (2 with mifepristone and 1 with ketoconazole followed by mifepristone). Medical therapy resulted in significant regression of the cushingoid phenotype in all 3 patients, thereby providing the rationale to proceed with bilateral adrenalectomy, after which symptoms remain controlled.
Table 6.
Clinical characteristics and biochemical data of 15 patients with unconfirmed CD
| # (Year) | Age, sex | Signs and symptoms | UFC LC-MS/MS | LNSC LC-MS/MS | Cortisol after 1-mg DST | ACTH | MRI | Localizing studies | Pathology |
|---|---|---|---|---|---|---|---|---|---|
| 1 (2008) |
51 F | WG (+40 lb/2 y), HTN, facial plethora, dorsocervical/ supraclavicular fat | 1/7: 135.0 |
0/2 | 1.04 | 1/5: 75 |
Neg | Postop IPSS with oCRH: Peak ACTH petrosal to peripheral ratio: 8.5 at 10 min; max basal ACTH: 69 pg/mL; max ACTH value: 357 pg/mL |
Normal pituitary |
| 2 (2009) | 21 M | WG (+60 lb/3 y), fatigue, abdominal striae, depression | 1/3: 66.8 |
0/2 | 4.3 0.5 |
1/3: 46 |
6 mm | Postoperative IPSS with oCRH: Peak ACTH petrosal to peripheral ratio: 17.5 at 5 min; Max basal ACTH: 14 pg/mL; Max ACTH value: 211 pg/mL |
RCC |
| 3 (2010) | 34 F | WG (+80 lb/4 y), HTN, DM, abdominal striae | 5/10: 82.2a 108.8vs 109.0a 143.1a 114.0a |
1/6: 0.240 |
5.0 17.0 20.0 21.0 12.0 |
0/1 | 3 mm | None | Prolactinoma |
| 4 (2011) | 29 F | WG (+80 lb/5 y), irregular menses | 2/2: 103.0a 78.9a |
Not done | 31.0 | 1/1: 132 |
3 mm | Postoperative IPSS with oCRH: peak ACTH petrosal to peripheral ratio: 17.3 at 10 min; max basal ACTH: 22 pg/mL; max ACTH value: 104 pg/mL |
Normal pituitary |
| 5 (2012) | 20 F | WG (+40 lb/3 y), irregular menses, fatigue, hirsutism, abdominal striae, anxiety | 3/8: 91.0 88.0 68.0 |
1/7: 0.120 |
Not done | 1/4: 45 |
Neg | None | Normal pituitary |
| 6 (2013) | 62 F | WG (+120 lb/6 y, BMI 43.8 kg/m2), HTN, proximal myopathy, osteoporosis, abdominal striae | 2/4: 67.1a 124.3a |
0/1 | Not done | 4/7: 48 49 49 77 |
Neg | IPSS with oCRH: peak ACTH petrosal/peripheral ratio: 24 at 8 min; max basal ACTH: 355 pg/mL Max ACTH value 1120 pg/mL |
Normal pituitary |
| 7 (2013) | 52 M | HTN, decreased libido, muscle wasting, fatigue, easy bruising, depression | 4/5: 131.4 102.3 71.5 56.7 |
0.170 | Not done | 1/2: 37 |
2 mm | None | Normal pituitary |
| 8 (2014) | 45 F | WG (+40 lb/4 y, BMI 28.2 kg/m2), irregular menses, fatigue, depression | 5/21: 62.7 64.4 68.7 154.6 68.2 |
5/6: 0.18 0.16 0.10 0.09 0.51 |
0.5 | 0/3 | Neg | IPSS with DDAVP: peak ACTH petrosal/peripheral ratio: 21 at 2 min Max basal ACTH: 29 pg/mL Max ACTH value 211 pg/mL |
Normal pituitary |
| 9 (2015) |
39 F | WG (+50 lb/several months, BMI 43.62 kg/m2), HTN, fatigue, hirsutism, easy bruising, depression | 4/4: 51.0 116.0 130.0 64.0 |
4/6: 0.148 5.984 0.262 0.852 |
21.0 25.4 12.0 11.0 8.0 |
2/3 | 4 mm | None | Fibrotic tissue |
| 10 (2016) | 20 F | WG (+60 lb/1.5 y, BMI 36.75 kg/m2), irregular menses, fatigue, hirsutism, abdominal striae, easy bruising, depression | 0/4 | 1/18: 0.090 |
0.6 | 2/4 45 252.7 |
Neg | DDAVP stimulation test: negative for CD | Normal pituitary |
| 11 (2017) | 59 F | WG (+100 lb/8 y, BMI 36.64 kg/m2), HTN, DM, irregular menses, myopathy, fatigue, osteoporosis, easy bruising, depression, anxiety | 1/6: 127.0 |
0/7 | Not done | 3/10: 72 199 140 |
4 mm | None | Pituitary cyst |
| 12 (2017) | 69 F | Fatigue, easy bruising, anxiety | 4/4: 67.3a 114.6a 123.2a 105.3a |
Not done | Not done | 2/3: 40 33 |
6 mm; pituitary cyst | None | Pituitary cyst |
| 13 (2017) |
43 F | WG (60 lb/2 y, BMI 34.7), DM, fatigue, abdominal striae, anxiety | 3/4: 54.2 50.2 70.0 |
1/1: 0.120 |
Not done | 1/5: 43 |
2 mm | None | Normal pituitary |
| 14 (2019) | 33 F | WG (10 y, BMI 42.61 kg/m2), HTN, DM, irregular menses, fatigue, hirsutism, anxiety | 3/4: 71.0 93.0 57.0 |
1/1: 0.119 |
10.35 | 2/3: 33 37 |
4.5 mm | None | Normal pituitary |
| 15 (2019) |
28 F | WG (30 lb/2 y, BMI 30.2 kg/m2), irregular menses, abdominal striae, easy bruising | 1/3: 64.8 |
2/7: 0.110 0.130 |
Not done | 1/3: 85.9 |
2 mm | None | Normal pituitary |
For biochemical data, where multiple results were available, the number of abnormal values out of the total is shown (n/T), followed by the abnormal results. Reference range: UFC by LC-MS/MS was 4-50 mcg/24 h; LNSC by LC-MS/MS: <0.09 µg/dL; DST (1 mg): normal <1.8 mcg/dL. ACTH: all values ≥20 pg/mL are noted.
Abbreviations: BMI, body mass index; CD, Cushing disease; DM, diabetes mellitus; F, female; HTN, hypertension; IPSS, inferior petrosal sinus sampling; LC-MS/MS, liquid chromatography tandem mass spectometry; LNSC, late night salivary cortisol; MRI, magnetic resonance imaging; M, male; Neg, negative; oCRH, ovine corticotropin releasing hormone; RCC, Rathke cleft cyst; UFC, 24-h urinary free cortisol; WG, weight gain.
a Unknown assay methodology. The fraction of elevated values is shown for each patient for UFC, LNSC, and ACTH.
Discussion
Overview
This study describes diagnostic test reliability and clinical outcomes in a relatively large cohort of patients presenting with a Cushingoid phenotype, all of whom ultimately underwent transsphenoidal surgery for presumed CD. Early biochemical remission rates were 76/90 (84%) for the confirmed CD group and 0/15 for the unconfirmed CD group. Despite the inherent limitations of retrospective studies and the relatively high missing data rates for some covariates, this analysis reveals variability in the performance of individual diagnostic tests and their combinations for different patient subgroups. The unconfirmed CD group had similar clinical signs and symptoms as the confirmed CD group as well as some degree of hypercortisolemia and nonsuppressed ACTH levels, leading experienced endocrinologists and neurosurgeons to pursue surgical exploration. However, our retrospectively analysis shows clear biochemical differences in UFC, LNSC, DST, and ACTH between the confirmed and unconfirmed groups, raising the question—did group C have CD or even CS? Despite the relatively small proportion (14%) of such “suspicious” cases, this unconfirmed CD group presents real diagnostic and treatment challenges. These results highlight 3 important points: (1) clinical and biochemical characteristics of confirmed and unconfirmed CD overlap when considering current guideline thresholds; (2) diagnostic accuracy improves when using optimized thresholds of highest accuracy for diagnosis of CS/CD; and (3) there is a need for new biomarkers and diagnostic strategies to facilitate the correct diagnosis, reduce the interval from diagnosis to surgery, and avoid unnecessary surgical interventions.
Analyzing Biochemical Diagnostic Thresholds
In assessing the biochemical data, UFC demonstrated the highest sensitivity and specificity (84% and 83%, respectively), similar to the recent report by Ceccato et al (34), followed by cortisol by IA after DST (90% and 57%, respectively) and LNSC LC/MS (83% and 67%, respectively). Diagnostic accuracy of DST was not reduced by changes in cortisol binding globulin concentrations because females were not exposed to estrogen compounds during testing.
This analysis also showed that composite testing with UFC and ACTH using optimal cutoff thresholds improves diagnostic accuracy for ACTH-dependent CS. In addition, the cutoffs obtained from the individual tests performed better than cutoff values based on standard-of-care thresholds, as noted by a statistically higher AUC and a higher specificity for a CD diagnosis. The limited LNSC and DST data precluded evaluation of other test combinations in this study, but such an analysis should be pursued in the future.
Comparing Confirmed vs Unconfirmed Cohorts
In the 105 patients, there were no significant phenotypic differences between the confirmed and unconfirmed CD cohorts; however, there were differences in preoperative biochemical data and in surgical details and outcomes. Specifically, UFC, LNSC, ACTH values, and MRI-detected adenoma/lesion size were all significantly greater in the confirmed vs unconfirmed cohorts. Only the 1-mg DST cortisol level comparison between confirmed and unconfirmed patients was not significant (serum cortisol 18 ± 18.6 vs 8.1 ± 11.1 µg/dL, respectively), possibly due in part to the small number of patients in each cohort who had a DST and/or differences in cortisol metabolism.
From a surgical perspective, important differences also were noted between the 2 cohorts. Importantly, in the confirmed cases, tumor identification followed by pseudocapsular adenoma dissection was more common, with less need for multiple gland incisions and partial hypophysectomy. Most importantly, there were no cases of CD remission (sustained hypocortisolemia) in the unconfirmed cohort vs 84% early remission in the confirmed cohort. Within the confirmed cohort, early remission was 95% vs 59% in patients without and with cavernous sinus invasion, respectively. However, when the entire cohort is assessed, including the 15 unconfirmed (group C) cases, the overall early remission rate falls to 72%.
Regarding possible confounders of including the 6 pathology-unproven patients (group B) in the larger confirmed cohort with group A, this has been an accepted methodology in multiple prior CD series (26-29). It is well appreciated that a subset of patients who often have small, presumed adenomas or no adenoma seen on 3T thin-cut sellar MRIs may go into early remission yet not have pathologically proven ACTH-staining adenoma. These 6 patients all had unequivocal preoperative biochemical data consistent with CD, none had more than a hemihypophysectomy, 5 of 6 had clear adenomatous tissue removed at surgery, and all had immediate postoperative hypocortisolemia consistent with early remission. Thus, including them in the confirmed cohort seems appropriate and justified.
Understanding the Unconfirmed CD Cohort
In the unconfirmed CD group, all 15 patients had a clinical phenotype suggestive of CD with equivocal/discordant biochemical data without pathology-proven ACTH adenoma nor postoperative hypocortisolemia (Table 6). These findings are similar to previous published data from several surgical case series (13-16) in which it was speculated that significant minorities of patients who did not achieve remission may not have had CD or CS. It has been hypothesized that such cases may have a supraglandular or other nonpituitary source of their episodic and relatively mild cortisol excess. Some cases might represent pseudo-Cushing secondary to obesity, depression, undiagnosed obstructive sleep apnea, or other conditions associated with mild hypercortisolemia or disruptions of the hypothalamic-pituitary-adrenal axis stress response (35, 36). The exact diagnosis for these patients remains unknown and it is likely that this challenging population encompasses several diagnoses. In retrospect, in some patients, we clearly should have performed a more thorough assessment before recommending surgery. For example, none underwent dexamethasone-corticotropin releasing hormone suppression test, which may help distinguish pseudo-Cushing from CD. Furthermore, some patients did not undergo localization studies such as IPSS. In addition, when IPSS was performed in 2 of 15 patients, there was no documentation of hypercortisolemia at the time of IPSS, which may have led to an erroneous assumed diagnosis of CD.
However, we have been reluctant to pursue IPSS testing in patients with equivocal biochemical data such as this cohort of 15 patients, given that such testing almost always demonstrates a central-to-peripheral ACTH gradient and further “encourages” a surgical exploration. We have learned that some of these patients have had transsphenoidal surgery elsewhere after we advocated against surgical exploration. Our data provide additional validation of a “watch and wait” approach, with further diagnostic testing to firmly establish the diagnosis of CS in patients with mild abnormalities in screening tests before evaluation of the differential diagnosis of ACTH-dependent CS. The use of ketoconazole or mifepristone (as we did in 3 patients in group C) as a therapeutic trial warrants further consideration in the diagnostic approach for these patients, using quantifiable endpoints (eg, weight, blood pressure, glucose) to assess if cortisol reduction would improve symptoms.
If given the opportunity to reevaluate the 15 unproven CD patients based on our current analysis, we would perform the following additional tests: 1-mg DST in those that did not have it (including a dexamethasone level, with repeat testing at a higher dexamethasone dose in those with suboptimal levels), and a dexamethasone-corticotropin releasing hormone stimulation test to exclude patients with pseudo-CS (11). We also would perform repeat endocrinological testing for those with equivocal UFC and salivary cortisol values. It is also noteworthy that 4 of these 15 patients ultimately received additional pituitary and/or adrenal directed therapies for hypercortisolemia, after which their Cushingoid phenotype regressed. Indeed, this challenging patient group highlights the need for diagnostic strategy optimization and more reliable biomarkers that confirm or rule out CD preceding a recommendation for surgery. Validation of our findings in a prospective and ideally multisite registry may help corroborate the identified thresholds of highest accuracy for CD diagnosis. Incorporation of new diagnostic strategies with higher performance and less variability may further optimize care and avoid unnecessary surgery.
Conclusion
An accurate diagnosis of CS and CD is essential to optimize chances for surgical remission and to minimize the chances of exposing patients who ultimately likely do not have CD to unnecessary surgical interventions. The identified diagnostic thresholds for UFC, LNSC, DST, and ACTH in this study may assist clinicians in accurately confirming or ruling out CD. UFC had the highest accuracy in detection of CD over LNSC and DST and combining UFC with ACTH improved diagnostic accuracy. These findings can hopefully enhance diagnostic strategies that facilitate the correct identification of CD before recommending transsphenoidal surgery.
Acknowledgments
Funding: This study was funded in part by the intramural program of National Institutes of Health, 1 ZIA DK075122-04
Glossary
Abbreviations
- AUC
area under the curve
- CD
Cushing disease
- CS
Cushing syndrome
- DST
dexamethasone stimulation test
- IA
immunoassay
- IPSS
inferior petrosal sinus sampling
- LC-MS/MS
liquid chromatography tandem mass spectometry
- LNSC
late night salivary cortisol
- ROC
receiver operating characteristic
- UFC
24-h urinary free cortisol.
Additional Information
Disclosures: D.F.K. receives royalties from Mizuho, Inc., and G.B. is a consultant for Vascular Technologies and Cerevasc, Inc. No other authors have conflicts of interests to disclose.
Data Availability
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Sharma ST; AACE Adrenal Scientific Committee . An individualized approach to the evaluation of cushing syndrome. Endocr Pract. 2017;23(6):726-737. [DOI] [PubMed] [Google Scholar]
- 2. Wengander S, Trimpou P, Papakokkinou E, Ragnarsson O. The incidence of endogenous Cushing’s syndrome in the modern era. Clin Endocrinol. 2019;91(2):263-270. [DOI] [PubMed] [Google Scholar]
- 3. Kreitschmann-Andermahr I, Psaras T, Tsiogka M, et al. From first symptoms to final diagnosis of Cushing’s disease: experiences of 176 patients. Eur J Endocrinol. 2015;172(3):285-289. [DOI] [PubMed] [Google Scholar]
- 4. Kelly DF. Transsphenoidal surgery for Cushing’s disease: a review of success rates, remission predictors, management of failed surgery, and Nelson’s Syndrome. Neurosurg Focus. 2007;23(3):E5. [DOI] [PubMed] [Google Scholar]
- 5. Dallapiazza RF, Oldfield EH, Jane JA Jr. Surgical management of Cushing’s disease. Pituitary. 2015;18(2):211-216. [DOI] [PubMed] [Google Scholar]
- 6. Starke RM, Reames DL, Chen CJ, Laws ER, Jane JA Jr. Endoscopic transsphenoidal surgery for cushing disease: techniques, outcomes, and predictors of remission. Neurosurgery. 2013;72(2):240-7; discussion 247. [DOI] [PubMed] [Google Scholar]
- 7. Chandler WF, Barkan AL, Hollon T, et al. Outcome of transsphenoidal surgery for cushing disease: a single-center experience over 32 years. Neurosurgery. 2016;78(2):216-223. [DOI] [PubMed] [Google Scholar]
- 8. Patil CG, Veeravagu A, Prevedello DM, Katznelson L, Vance ML, Laws ER Jr. Outcomes after repeat transsphenoidal surgery for recurrent Cushing’s disease. Neurosurgery. 2008;63(2):266-70; discussion 270. [DOI] [PubMed] [Google Scholar]
- 9. Esposito F, Dusick JR, Cohan P, et al. Early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab. 2006;91(1):7-13. [DOI] [PubMed] [Google Scholar]
- 10. Broersen LHA, Biermasz NR, van Furth WR, et al. Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary. 2018;21(5):524-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society . Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stolyarov Y, Mirocha J, Mamelak AN, Ben-Shlomo A. Consensus-driven in-hospital cortisol assessment after ACTH-secreting pituitary adenoma resection. Pituitary. 2018;21(1):41-49. [DOI] [PubMed] [Google Scholar]
- 14. Meinardi JR, Wolffenbuttel BH, Dullaart RP. Cyclic Cushing’s syndrome: a clinical challenge. Eur J Endocrinol. 2007;157(3):245-254. [DOI] [PubMed] [Google Scholar]
- 15. Alexandraki KI, Kaltsas GA, Isidori AM, et al. The prevalence and characteristic features of cyclicity and variability in Cushing’s disease. Eur J Endocrinol. 2009;160(6):1011-1018. [DOI] [PubMed] [Google Scholar]
- 16. Oldfield EH, Vance ML, Louis RG, Pledger CL, Jane JA Jr, Lopes MB. Crooke’s changes in Cushing’s syndrome depends on degree of hypercortisolism and individual susceptibility. J Clin Endocrinol Metab. 2015;100(8):3165-3171. [DOI] [PubMed] [Google Scholar]
- 17. Alwani RA, Schmit Jongbloed LW, de Jong FH, van der Lely AJ, de Herder WW, Feelders RA. Differentiating between Cushing’s disease and pseudo-Cushing’s syndrome: comparison of four tests. Eur J Endocrinol. 2014;170(4):477-486. [DOI] [PubMed] [Google Scholar]
- 18. Casanueva FF, Barkan AL, Buchfelder M, et al. ; Pituitary Society, Expert Group on Pituitary Tumors . Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): a Pituitary Society statement. Pituitary. 2017;20(5):489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McLaughlin N, Laws ER, Oyesiku NM, Katznelson L, Kelly DF. Pituitary centers of excellence. Neurosurgery. 2012;71(5):916-24; discussion 924. [DOI] [PubMed] [Google Scholar]
- 20. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conger A, Zhao F, Wang X, et al. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg. 2018;130(3):861-875. [DOI] [PubMed] [Google Scholar]
- 22. Barkhoudarian G, Cutler AR, Yost S, Lobo B, Eisenberg A, Kelly DF. Impact of selective pituitary gland incision or resection on hormonal function after adenoma or cyst resection. Pituitary. 2015;18(6):868-875. [DOI] [PubMed] [Google Scholar]
- 23. Bora SK, Suri A, Khadgawat R, et al. Management of Cushing’s disease: changing trend from microscopic to endoscopic surgery. World Neurosurg. 2020;134:e46-e54. [DOI] [PubMed] [Google Scholar]
- 24. Thakur JD, Corlin A, Mallari RJ, et al. Complication avoidance protocols in endoscopic pituitary adenoma surgery: a retrospective cohort study in 514 patients. Pituitary. 2021:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oldfield EH, Vortmeyer AO. Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg. 2006;104(1):7-19. [DOI] [PubMed] [Google Scholar]
- 26. Pouratian N, Prevedello DM, Jagannathan J, Lopes MB, Vance ML, Laws ER Jr. Outcomes and management of patients with Cushing’s disease without pathological confirmation of tumor resection after transsphenoidal surgery. J Clin Endocrinol Metab. 2007;92(9):3383-3388. [DOI] [PubMed] [Google Scholar]
- 27. Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325(13):897-905. [DOI] [PubMed] [Google Scholar]
- 28. Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH. Outcome of surgical treatment of 200 children with Cushing’s disease. J Clin Endocrinol Metab. 2013;98(3):892-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedman RB, Oldfield EH, Nieman LK, et al. Repeat transsphenoidal surgery for Cushing’s disease. J Neurosurg. 1989;71(4):520-527. [DOI] [PubMed] [Google Scholar]
- 30. Venkatraman E. A permutation test to compare receiver operating characteristic curves. Biometrics. 2000;56(4):1134-1138. [DOI] [PubMed] [Google Scholar]
- 31. Metz C, Starr S, Lusted L, Rossmann K.. Progress in evaluation of human observer visual detection performance using the ROC curve approach. Int J Nucl Med Biol. 1976;3(3–4):178-179. [Google Scholar]
- 32. Vermont J, Bosson JL, François P, Robert C, Rueff A, Demongeot J. Strategies for graphical threshold determination. Comput Methods Programs Biomed. 1991;35(2):141-150. [DOI] [PubMed] [Google Scholar]
- 33. Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ceccato F, Barbot M, Zilio M, et al. Screening tests for cushing’s syndrome: urinary free cortisol role measured by LC-MS/MS. J Clin Endocrinol Metab. 2015;100(10):3856-3861. [DOI] [PubMed] [Google Scholar]
- 35. Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol. 2010;22(1):165-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alwani RA, Schmit Jongbloed LW, de Jong FH, van der Lely AJ, de Herder WW, Feelders RA. Differentiating between Cushing’s disease and pseudo-Cushing’s syndrome: comparison of four tests. Eur J Endocrinol. 2014;170(4):477-486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.