Abstract
Background
Medical and interventional therapies for older adults with acute myocardial infarction (AMI) reduce mortality and improve outcomes in selected patients, but there are also risks associated with treatments. Shared decision making (SDM) may be useful in the management of such patients, but to date patients’ and cardiologists’ perspectives on SDM in the setting of AMI remain poorly understood. Accordingly, we performed a qualitative study eliciting patients’ and cardiologists’ perceptions of SDM in this scenario.
Methods
We conducted 20 in-depth, semi-structured interviews with older patients (age ≥70) post-AMI, and 20 interviews with cardiologists. The interviews were transcribed, and analyzed using ATLAS.ti. Two investigators independently coded transcripts using the constant comparative method, and an integrative, team-based process was utilized to identify themes.
Results
Six major themes emerged: (1) patients felt their only choice was to undergo an invasive procedure; (2) patients placed a high level of trust and gratitude towards physicians; (3) patients wanted to be more informed about the procedures they underwent; (4) for cardiologists, patients’ age was not a major contraindication to intervention, while cognitive impairment and functional limitation were; (5) while cardiologists intuitively understood the concept of SDM, interpretations varied; and (6) cardiologists considered SDM to be useful in the setting of non-ST elevated myocardial infarction (NSTEMI), but not ST-elevated myocardial infarction (STEMI).
Conclusions
Patients viewed intervention as “the only choice,” whereas cardiologists saw a need for balancing risks and benefits in treating older adults post-NSTEMI. This discrepancy implies there is room to improve communication of risks and benefits to older patients. A decision aid informed by the needs of older adults could help to better convey patient-specific risk and increase choice awareness.
Keywords: Shared-decision making, Acute myocardial infarction, geriatrics, qualitative
Among older adults with acute myocardial infarction (AMI), there has been an increasing use of invasive coronary angiography and coronary revascularization in the U.S.,1 Canada2 and Europe3 over recent decades. For example in the U.S, the use invasive coronary angiography for non-ST-elevation acute coronary syndromes increased from 49.7% in 2004 to 66.0% in 2014 among patients aged 71–80, and from 27.2% to 37.9% among patients >80.1 While these procedures have multiple benefits including the potential for reduced mortality and recurrent AMI,4,5 they also confer risk. This risk increases with advancing age, as physiologic changes coupled with comorbidities increase the risk of treatment-related complications including bleeding, acute kidney injury, vascular complications, and stroke.6–10 However, individual risk in older adults is quite variable. Some older adults have exceptional outcomes while in select cases the impact of these adverse events outweigh the potential benefits of intervention. Moreover, because the damage in an ST-elevation myocardial infarction (STEMI) is extensive (involving the full thickness of the myocardium) urgent revascularization is essential to reperfuse and salvage as much myocardium as possible. The standard of care is to revascularize within 90 minutes of presentation in these cases. It is less urgent in the setting of a non-ST-elevation myocardial infarction (NSTEMI) which denotes more limited sub-endothelial ischemia, and therefore lends more time to weigh the risks/benefits of such an invasive procedure in this higher risk population.
Shared decision making (SDM)11—which can be defined as the process by which patients and clinicians make health care decisions together, taking into account the best clinical evidence as well as patients’ values and goals of care—has emerged as a central component of a more patient-centered health system.12 SDM has significant potential to improve management of older patients with AMI by aligning the treatment received with individuals’ values and preferences.11,13 However, to date patients’ and cardiologists’ familiarity with and willingness to incorporate SDM in the management of older adults post-AMI has not been thoroughly investigated.
Accordingly, we performed a qualitative study to better understand patients’ and cardiologists’ perspectives on SDM in older adults with AMI, with distinct objectives for each group. In patients, our objective was to explore decisional needs related to invasive coronary angiography and revascularization, and their interest in shared decision making. In cardiologists, our objective was to elucidate their familiarity with SDM, their perception of its usefulness in the treatment of older adults, and specific barriers to its adoption in practice. Our overall goal was to identify themes through these interviews that would serve as foundational data for efforts to improve implementation of SDM in practice among older adults with AMI.
Methods
Study Design
We conducted in-depth, semi-structured one-on-one interviews with older adult patients (n=20) and cardiologists (n=20) with experience making treatment decisions post-myocardial infarction. We initially chose our sample size based on prior qualitative studies in cardiovascular disease which reached thematic saturation after enrolling this number of participants.14,15,16 We then ensured in our own sample through an iterative process (coding transcripts after each interview), that thematic saturation was achieved with 20 individuals in both groups. We chose to explore this subject qualitatively because of the complexity involved in making decisions, and our interest in achieving a more comprehensive understanding of the factors involved. This investigation was performed at Tisch Hospital, an urban teriary academic medical center that is part of the New York University Langone Health (NYULH) network. The study was approved by the NYULH Institutional Review Board (IRB), and verbal informed consent was obtained from each study participant prior to the start of the interview.
Recruitment
Patients
We interviewed twenty older adults (age ≥70) hospitalized with AMI. Eligible participants were identified by research coordinators (EVG, JS) through daily electronic medical record screening of admission diagnoses on all inpatient medical units at NYULH Tisch Hospital. Patients were only approached with permission from their inpatient cardiologist. Participants were provided informed consent in hospital, and then interviewed within one month post-discharge via telephone.
Cardiologists
Twenty cardiologist members of the NYULH Faculty Group Practice (FGP) with experience treating older adults hospitalized for AMI at NYULH within the past year were interviewed. Cardiologists were initially invited to participate via a group email to members of the NYULH Leon H. Charney Division of Cardiology. The first twenty respondents were then selected as study participants. No demographic data was collected from cardiologist participants due to their status as a vulnerable population (potentially identifiable as institution employees) according to NYULH IRB policy at the time of the study.
Interviewers (EVG, JS) were research assistants trained in qualitative interview methods by a member of our study team (VVD) with extensive prior experience in qualitative research methodologies.
Interview Format
Each interview followed a semi-structured format that consisted of a series of open-ended questions followed by more direct probes, with the purpose of focusing the interview while allowing the participant to speak freely and distinguish essential aspects of their narratives (Box 1, Box 2). Separate interview guides were developed for the patient and cardiologist sessions. Interviews lasted 15–30 minutes depending on length of responses. In interviews with patients, concepts included (1) decisional needs related to invasive coronary angiography and revascularization; (2) desire to participate in decision making process during AMI, and (3) perceived benefits and risks of invasive coronary angiography and revascularization. In interviews with cardiologists, concepts included general experiences treating older adults with AMI, as well as their familiarity with SDM concepts, whether they envisioned a role for SDM in the care of older adults with AMI, and what specific tools may facilitate the implementation of SDM. Our question probes focused on a hypothetical patient age ≥80, which was informed by preliminary discussions with our cardiologists whereby they considered these patients to be of an advanced age that may warrant different considerations in treatment. Notably, this differed from our cutoff for patient interviews (age ≥70), which was determined based on the increasing prevalence of aging-related functional impairments after this age, as well as by concerns over finding an adequate sample of patients at a more extreme age cutoff (e.g. age ≥80) who would meet criteria for enrollment.
Box 1. Interview guide for patients.
| Interview Guide (Patients) | ||
|---|---|---|
| Topic/Rationale | Question | Follow Up/Probe |
| For all patients | 1. Tell me about your heart attack. | What symptoms were you having before you came in to the hospital? What did you think was wrong when you first began having these symptoms? |
| 2. Tell me about any procedures you remember having in the hospital. | Did you have a coronary artery bypass graft (CABG) procedure? Did you have heart catheterization procedure or percutaneous coronary intervention (PCI)? What did you know about this procedure before you came in to the hospital? |
|
| If participant underwent procedure | 3. Tell me about any benefits, or upsides, to this procedure that your doctor may have talked with you about. | Can you talk to me about how well you understood these benefits at the time? What questions do you remember asking about these benefits? |
| 4. Tell me about any potential downsides, or complications, of this procedure that your doctor may have talked with you about before having it. | Can you tell me about your understanding of these downsides at the time? Can you tell me about any questions you may have asked about these potential complications? |
|
| 5. Describe your level of comfort with your procedure before having it. | What other information do you wish you had been given before your procedure, or did you feel pretty well informed? What other kinds of questions did you ask your doctor prior to your procedure? How could your doctor or your care team have made you feel more comfortable with the procedure? |
|
| 6. Describe how comfortable you would have been with saying “no” to the procedure that your doctor recommended. | What was it that allowed you to feel that you could say “no” to the procedure? Why, do you think, you felt as if you couldn’t turn down the procedure? |
|
| 7. How long were you in the hospital after your procedure? | How did you feel about your length of stay in the hospital? Why did you think that your stay should have been longer/shorter? |
|
| 8. How important is it for you to fully understand your procedure? | How were you feeling about the procedure before having it? How well did you understand what was going to happen? |
|
| If participant did not undergo procedure | 9. Tell me about any heart procedures your doctor may have discussed with you. | Can you tell me about the procedures they discussed with you (if any)? Did you say “no” to having the procedure? Can you tell me your reasons for not wanting to have the procedure? |
| 10. How has your recovery been after getting discharged from the hospital? | Can you describe any issues you may have had related to your procedure after you left the hospital? How concerned are you that you will have another heart attack? | |
| 11. How important is it for you to fully understand your heart disease? | Tell me more about your heart disease. What would help you to further understand it? |
|
| Closure | Is there anything else you would like to tell me about your hospitalization for a heart attack? | ———— |
Box 2. Interview guide for cardiologists.
| Interview Guide (Cardiologists) | ||
|---|---|---|
| Topic/Rationale | Question | Follow Up/Probe |
| Clinical care | 1. How do you approach management of acute myocardial infarction (AMI) in patients aged 80 y or older? | ———— |
| 2. What influences your management decisions? | How do you assess things like frailty, cognitive impairment, and family support in a patient? | |
| 3. What is your practice with cardiac catheterization in patients over the age of 80 y? | Can you explain your answer? | |
| 4. In older adults, what complications do you worry about the most? | ———— | |
| 5. How do you explain the risks and benefits of cardiac catheterization to patients? | What do you believe is the greatest risk/benefit? | |
| Shared decision making | 6. What do you know about shared decision making (SDM)? | SDM is a collaborative process that allows patients and their providers to make healthcare decisions together. It takes into account the best clinical evidence available, as well as the patient’s values and preferences. |
| 7. Do you or have you ever used SDM with your AMI patients? How have you used SDM in your practice? How did you carry this out? |
Tell me about a time when you used SDM for an older adult with an AMI? Tell me about a time that you would have liked to use SDM but did not or felt you were not able to? |
|
| 8. Do you think SDM is useful in AMI? How useful do you believe SDM to be? What are the benefits and what are the downsides? |
Can you explain why/why not? | |
| 9. What would help you explain the risks and benefits of (1) catheterization and (2) medications to patients after AMI? | As part of our research, we’re looking to help physicians make decisions with older adults and AMI. What would be helpful to you? | |
| 10. Tell me about your most memorable experience treating an older adult with AMI. | ———— | |
| Closure | Is there anything else you would like to say in relation to SDM and clinical care of your patients post-AMI? | ———— |
All interviews were audiotaped and subsequently transcribed professionally. Any identifiable respondent information in these transcripts was removed prior to analysis in order to preserve anonymity.
Data Analysis
De-identified transcripts were entered into ATLAS.ti qualitative software17 and coded by trained members of the study team (JAD, EVG, JS). Both inductive and deductive approaches were employed in developing the codes.18 Inter-coder reliability was ensured with regular meetings between coders (JAD, EVG, JS) to discuss coded data, reconcile differences, and reach consensus on code meanings. Using the constant comparative method,19 essential ideas from the interviews were coded and compared over subsequent interviews. Recurrent themes were extracted until thematic saturation was reached. Our thematic analysis was then corroborated by a larger multidisciplinary team composed of representatives from Cardiology (JAD), Geriatrics (DM, SDK, SIC), and Nursing (VVD).20 Methodological rigor throughout the analysis was assured by following the consolidated criteria for reporting qualitative research guidelines (COREQ). 21
Results
Themes: Patients
We enrolled 29 patients in the hospital in order to obtain 20 interviews (9 patients could not be contacted after discharge). The mean age of participants was 79 years (standard deviation 6 years), 40% were female, and 15% were nonwhite. Three quarters of participants had a diagnosis of NSTEMI on admission; the remainder had STEMI. Only one person did not undergo invasive coronary angiography. Other demographics are summarized in Table. Major themes that emerged were: (1) patients felt the only choice was to have the procedure (invasive coronary angiography); (2) patients placed a high level of trust and gratitude towards physicians; and (3) patients wanted to be more informed about the procedures they underwent.
Table.
Demographics of Patient Respondents
| Characteristic | No. (%) |
|---|---|
| Age, y, mean (SD) | 79 (6) |
| Female | 8 (40) |
| Nonwhite | 3 (15) |
| AMI type | |
| STEMI | 5 (25) |
| NSTEMI | 15 (75) |
| Comorbidities | |
| Hypertension | 17 (85) |
| Hyperlipidemia | 13 (65) |
| Diabetes | 7 (35) |
| Chronic lung disease | 1 (5) |
| Other | 15 (75) |
AMI, acute myocardial infarction; NSTEMI, non-ST elevated myocardial infarction; STEMI, ST-elevated myocardial infarction.
Theme 1: Patients felt their only choice was to undergo invasive coronary angiography
Most patients interviewed were surprised by the question of choice in their care, and did not see any alternative to the care they were offered:
“I had no choice”
“I didn’t know it was an option”
Moreover, some patients pointed to their own acute vulnerability as the basis for their hastened decision making:
“I think every option should be given to you, but like I said like it was desperate”
Many patients were surprised by the question asking if they would have been comfortable saying “no” to the procedure, sometimes naming death as the alternative:
“Of course I could have said “no” but that would have been foolish on my part”
“there was no ‘no’ to this procedure”
“I would not have come to the hospital if I wanted to commit suicide”
However patients often claimed ownership of their decision.
“I would say 90% towards me, it was my decision”
“It was my own decision and judgement”
Theme 2: Patients placed a high level of trust in their cardiologists
Most patients expressed a desire to follow their cardiologist’s recommendation, and trusted them greatly, often valuing their perspective more than their own.
“I believe her. She’s my doctor. If I don’t believe them, who am I going to talk to? They’re professionals. They went to school, I didn’t”
“I trusted that they knew what they were doing and what needed to be done”
“I really have total faith in my cardiologist, and that means everything to me”
Theme 3: Patients wanted to be more informed about the procedures they underwent
Many patients claimed they had no idea what was going on and would have liked to have more information especially after the procedure (if not possible before).
“I had no knowledge and I still don’t have much knowledge about what the complications could have been”
“I think after the procedure the nurse or some knowledgeable person should have walked me through what was done, how, when, why, and where. I really wasn’t informed”
Some patients also noted that they needed help from their family to get all the information from the doctors:
“Sometimes, doctors will give you information and just like the tip of the iceberg. I like to have my daughter along when we’re talking to a doctor because she has some very pointed questions that she puts to them. I get a lot of information through my daughter’s questioning”
Moreover, many patients did not recall the potential complications of the procedure being explained to them:
“No, they didn’t mention any complications, no”
Themes: Cardiologists
Three themes emerged for cardiologists, which are summarized below.
Theme 1: Age was not a major contraindication to intervention for cardiologists, while cognitive impairment and functional limitation were
None of the cardiologists interviewed said they would forego intervention in a patient strictly due to age. Cardiologists recognized that age was not always a reliable way to triage patients, explicitly distinguishing between biological and chronological age.
“I haven’t been using age as a cutoff”
“Numeric age doesn’t necessarily deter me. It’s really what kind of 80”
Moreover, most cardiologists claimed to approach management in the same way they approach younger patients, explaining that it was always an individualized decision based on a discussion of the balance between risks and benefits.
“I think that fundamentally, I approach the management of acute myocardial infarction similarly to younger patients under the age of 80”
“I would say it a balance of, as with any patient, risks and benefits”
Still, some cardiologists expressed heightened concern for patients over the age of 80 undergoing cardiac catheterization on the basis of their different physiology.
“I mean as a general philosophy, I tend to be less aggressive… I have a respect for both the procedure and the procedural complications due to the vessel tortuosity and calcification that happens to older people”
However—whether explicitly or implicitly noted, many cardiologists claimed that advanced cognitive impairment or poor functional status would be the major contraindications to recommending invasive coronary angiography.
“Unless there’s something really extraordinary on their presentation, like extremely advanced dementia or they can’t provide and fend for themselves, then…I’d say we treat them the same”
“For example, a patient with dementia is going to have a different approach than someone without dementia”
Notably, some cardiologists saw cognitive impairment as a more important contraindication to intervention than functional limitation.
“There are plenty of patients that may be functionally limited but have an excellent quality of life, and that needs to be taken into account. There are patients who may be functionally independent, but so cognitively impaired that it may change the calculus in the other direction”
Theme 2: Interpretations of SDM varied considerably among cardiologists
Generally, cardiologists understood that SDM was a tool to involve the patient in the decision, recognizing a broader shift away from paternalism and towards patient centered care.
“It’s the idea that patients’ family and physicians would come together to share their thoughts and come together to a mutually beneficial decision where the physician sort of guides the conversation but offers enough information to the family and the patient so that they can really actively participate in the decision rather than the old model of the doctor being “this is what you’re going to do”
In fact, many cardiologists reported using SDM in their practices.
“I would think I’m doing some version of it every time I’m talking to a patient”
However, there were slight variations in the way cardiologists interpreted SDM. Some saw it as shared because it involved family members.
“Typically it’s not just the patient. It’s the patient and family member. That’s where it’s shared”
Others saw it as a stepwise discussion between the patient and the physician, with an emphasis on the physician’s role as educator.
“I think the sharing is really my attempt to educate the patient, and then hearing the patient’s response. If the patient has a reservation about the proposed process, then I would try to probe the ingredients of the reservation and try to answer in whatever way would clarify the decision making for the patient”
Others understood SDM as the professional collaboration between care providers prior to discussing the options with the patient.
“We make the decision as a team whether or not the patient should go for a cath. I don’t frequently give patients--if I’m sending a patient, if I make the decision that this is appropriate, then we go through the risks and benefits”
Theme 3: SDM was considered useful among patients hospitalized for NSTEMI, but not STEMI
Because of the urgency involved in the treatment of STEMI (including the high risk of adverse sequelae with failure to intervene promptly), all cardiologists agreed that immediate intervention was the recommended choice in every patient barring an extreme contraindication. Consequently, cardiologists felt that SDM was much more feasible in NSTEMI, where there is greater clinical equipoise and considerably more time for in-depth conversations.
“If it’s a…[STEMI], I’m going to be more likely to take them to invasive angiography and explain to the patient that it really needs to be done. If it’s a non-ST segment elevation MI…depending on the extent of the damage or systems or EKG changes, I would say they always have the option of medical therapy first”
Logically, lack of time was cited as the most important barrier to SDM in the STEMI population, with the implication that it is not feasible in acute situations.
“I guess the shared decision making process is shortened in a STEMI, and so it may be more of a definitive recommendation…if you’re including NSTEMI’s, you have a lot more time usually with those patients, so you can have a full discussion”
When it was feasible however, many cardiologists stressed the importance of SDM.
“Shared decision making is really important in the older population, and we employ it, particularly in the non-acute setting when it’s feasible. It usually is”
“It’s not only useful, it’s integral in the discussions”
Discussion
To our knowledge this is the first first study of SDM in the setting of AMI that incorporates both patient and cardiologist perspectives. Previous studies have acknowledged the importance of SDM for a range of conditions in older adults, often exploring the question through the patient perspective only.22–24 The lack of investigation in the AMI setting may be due to the fact that older adults hospitalized for AMI are more acutely ill than other groups considering cardiovascular interventions where SDM tools have already been established, such as in the setting of outpatients facing implantable cardioverter-defibrillator 14,25 or left ventricular assist device implantation.15 Time constraints in AMI are greater, and the dynamics of inpatient hospitalization are different with patients typically having less control and simultaneously facing a wider range of time-sensitive decisions. Nonetheless, we found that both patients and cardiologists were generally receptive to SDM concepts, at least in the setting of NSTEMI.
From the patient perspective, we identified several key themes. Patients in our sample reported feeling that their only option was to undergo cardiac catheterization (with many claiming “I had no choice”), and they also placed a high level of trust in the cardiologists caring for them. This passivity in decision making may be due to patients’ acute vulnerabilities in the hospital as inpatients—particularly in the setting of AMI. Of the patients interviewed, many noted feeling desperate and weak at the time of discussion. This is in line with the literature indicating patients are more likely to cede their decisionmaking when hospitalized.16
However, it was also clear in most interviews that patients wanted to be more informed about the invasive procedure they underwent. Some struggled to understand the reasoning behind needing the procedure, and the options available to them. In our view, this last theme supports the creation of a decision support tools that would provide information about the risks and benefits of cardiac catheterization, in clear language understandable to the patient.
Several notable themes emerged among the cardioliogists in our sample. First, age-related impairments (such as dementia and functional status) were more important contraindications to intervention than age alone. This underscores a paradigm shift in cardiology over the past several decades where older adults are routinely intervened on as technologies become less invasive and more widely available. Second, SDM was deemed useful in the setting of NSTEMI but not STEMI, which was our expectation. With current pressures on emergent revascularization among patients with STEMI (even the oldest old), as well as strong evidence that failure to treat can result in complications including shock, arrhythmia, and death, implementation of SDM is challenging to implement. However, with NSTEMI there is generally adequate time for an informed discussion as angiography is rarely considered emergent. The third theme was that interpretations of SDM varied widely; some cardiologists thought SDM involved shared decisions made by patients and families, while others thought that it included conversations between members of the clinical team. Most intuitively understood that in contemporary practice decisions were made collaboratively by both patients and clinicians, but also reported that optimal tools for this were lacking.
Notably, cardiologists in our sample also described guidelines as a rather limited tool to help with SDM in the older adult NSTEMI population, which underscores a lack of clinical trial evidence in the “oldest old.”26 These findings differ from a prior study by Matlock et al. focused on SDM in implantable cardioverter defibrillator placement, which found that cardiologists’ tendency to strictly adhere to guidelines was inhibiting SDM implementation.14 In this same study, cardiologists generally took beneficent/paternalistic approach to the decision and few had a patient centered/shared approach. Our findings may differ due to changes in practice among cardiologists over time (with acknowldgement of greater uncertainty and endorsement in clinical practice guidelines of SDM principles27), the different nature of AMI therapies, or because of the additional complexity of decision making in the setting of advanced age and comorbidities.
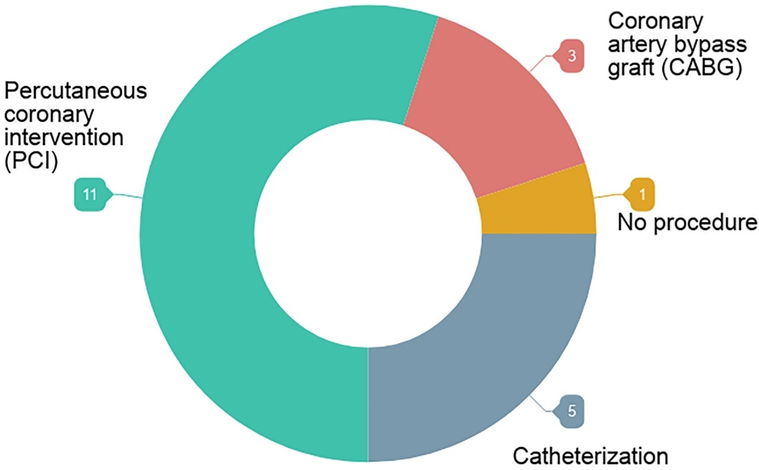
Since the majority of patients interviewed for our study had undergone catheterization (Figure), it can be argued that patients who’ve made the decision to undergo catheterization justify their choice further as “obvious” or “the only possible choice” in retrospective interviews. However, choice awareness has been established as a central feature in successful SDM,28 and many of our patients claim they had no alternatives to catheterization in treatment.
Figure.
Distribution of the procedures patients underwent.
The discrepancy between patient and cardiologist perspectives surrounding the need for risk/benefit analysis in decision making post-AMI (along with the physician’s amenability to tools which may facilitate SDM implementation) was one of our most salient findings. Physicians emphasized the importance of SDM and the lack of available tools, whereas patients noted a lack of adequate information. This discrepancy suggests that, from the patient perspective, conversations in practice were often inadequate to be fully informational. Solutions to this shortfall are complex, especially in light of time constraints in current inpatient settings. However, SDM tools may help to provide standardized information to patients for individualized risk prediction (e.g. embedding a calculator for risk of acute kidney injury) and can be designed to be left with patients to review by themselves or with family members, with subsequent facilitation by a member of the clinical team. We do not believe decisional aids would necessarily change the outcome of the decision in this population (intervention vs. medical management), however we do believe it could alleviate some of the stress and uncertainty involved in discussing evidence-based risks for physicians and it could make “choice awareness” more transparent for patients.
Our qualitative study has several limitations that should be considered when interpreting our findings. First, as with any qualitative study, the perspectives of the investigators may have influenced the results. For example both patients and cardiologists could have sensed that valuing SDM was important to the investigators as we were conducting a study about it, which may have overscored how much they actually consider it as a central part of care. In this report the investigators have interpreted results through the lens of patient-centered care, but we have attempted to reduce subjectivity by presenting the results to a multidisciplinary group. Second, our results may be biased by the characteristics of cardiologists who agreed to participate in our survey (and their views may have differed from non-participants). Third, nearly all patients interviewed underwent invasive coronary angiography (vs. conservative management with medications alone) which largely overshadows the decisional needs of patients who chose to decline the procedure. This finding likely reflects a combination of institutional practice (large academic medical center with rapid access to cardiac catheterization), national trends towards more invasive procedures in older adults, and selection bias (e.g. patients who did not undergo catheterization may have had comorbidities such as dementia which precluded study enrollment). Our results therefore may differ from other settings with limited access to invasive coronary angiography (e.g. where transfer for the procedure over a long distance is required, conservative management may be more common). Finally, our sample consisted of cardiologists practicing at a single academic institution, and findings may not extend to other centers where local practice patterns differ.
In conclusion, in the setting of evidence that SDM could improve outcomes including adherence, and satisfaction with care, there has been a movement in the healthcare system to implement SDM across a wide range of practice settings.29–, 32 Cardiologists in our study valued SDM in the setting of older adults hospitalized for NSTEMI, but they noted a lack of specific tools for implementation. On the other hand, patients often only perceived catheterization as a possible choice for treatment, and many would have liked to be more informed. A personalized risk calculator may serve as a tangible step towards translating choice awareness more clearly, and subsequently achieving higher-quality SDM among this population.
Acknowledgments
Funding: Dr. Dodson receives support from a Patient Oriented Career Development Award (K23 AG052463) from the NIH/National Institute of Aging.
Sponsor’s Role: The sponsor had no role in the design, analysis or preparation of this paper.
Footnotes
Conflict of Interest: All authors have no conflicts.
References
- 1.Rashid M, Fischman DL, Gulati M, et al. Temporal trends and inequalities in coronary angiography utilization in the management of non- ST-Elevation acute coronary syndromes in the U.S. Sci Rep 2019;9:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Page M, Doucet M, Eisenberg MJ, et al. Temporal trends in revascularization and outcomes after acute myocardial infarction among the very elderly. Can Med Assoc J. 2010;182(13):1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roffi M, Patrono C, Collet JP, Mueller C, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). 2015. Eur Heart J 37(3), 267–315. [DOI] [PubMed] [Google Scholar]
- 4.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13–20. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SR, Cannon CP, Fox KAA, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA. 2005;293(23):2908–2917. [DOI] [PubMed] [Google Scholar]
- 6.Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. [DOI] [PubMed] [Google Scholar]
- 7.Aronow HD, Steinhubl SR, Brennan DM, et al. Bleeding risk associated with 1 year of dual antiplatelet therapy after percutaneous coronary intervention: Insights from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2009;157(2):369–374. [DOI] [PubMed] [Google Scholar]
- 8.Mangano CM, Diamondstone LS, Ramsay JG, et al. Renal Dysfunction after Myocardial Revascularization: Risk Factors, Adverse Outcomes, and Hospital Resource Utilization. Ann Intern Med. 1998;128(3):194. [DOI] [PubMed] [Google Scholar]
- 9.Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural Bleeding and 1-Year Outcome After Percutaneous Coronary Interventions. J Am Coll Cardiol. 2008;51(7):690–697. [DOI] [PubMed] [Google Scholar]
- 10.Bach RG, Cannon CP, Weintraub WS, et al. The effect of routine, early invasive management on outcome for elderly patients with non-ST-segment elevation acute coronary syndromes. Ann Intern Med. 2004;141(3):186–195. [DOI] [PubMed] [Google Scholar]
- 11.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinetti ME, Naik AD, Dodson JA. Moving From Disease-Centered to Patient Goals–Directed Care for Patients With Multiple Chronic Conditions. JAMA Cardiol. 2016;1(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spatz ES, Spertus JA, Montori VM. Shared decision making: a path toward improved patient-centered outcomes. Circ Cardiovasc Qual Outcomes. 2012;5(6):e75–77. [DOI] [PubMed] [Google Scholar]
- 14.Matlock DD, Nowells CT, Masoudi FA, et al. Patient and Cardiologist Perceptions on Decision Making for Implantable Cardioverter-Defibrillators: A Qualitative Study. Pacing Clin Electrophysiol. 2011;34(12):1634–1644. [DOI] [PubMed] [Google Scholar]
- 15.Thompson JS, Matlock DD, McIlvennan CK, et al. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra SR, Haldar S, Pollack AH, et al. “Not Just a Receiver”: Understanding Patient Behavior in the Hospital Environment. Proc SIGCHI Conf Hum Factor Comput Syst. 2016;2016:3103–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ATLAS.ti 7 User Guide and Reference ATLAS.ti 7.1 USER MANUAL 2 ATLAS.ti 7 User Manual. http://atlasti.com/wp-content/uploads/2014/05/atlasti_v7_manual_201312.pdf?q=/uploads/media/atlasti_v7_manual_201312.pdf. Accessed March 20, 2018.
- 18.Fereday J, Muir-Cochrane E. Demonstrating Rigor Using Thematic Analysis: A Hybrid Approach of Inductive and Deductive Coding and Theme Development. Int J Qual Methods. 5(1), 80–92. [Google Scholar]
- 19.Glaser BG, Strauss AL. The discovery of grounded theory. Int J Qual Methods. 1967;5:1–10. [Google Scholar]
- 20.Curry LA, Nembhard IM, Bradley EH. Qualitative and Mixed Methods Provide Unique Contributions to Outcomes Research. Circulation. 2009;119(10):1442–1452. [DOI] [PubMed] [Google Scholar]
- 21.COREQ (COnsolidated criteria for REporting Qualitative research) Checklist. https://www.elsevier.com/__data/promis_misc/ISSM_COREQ_Checklist.pdf. Accessed February 13, 2018.
- 22.Isaacs CG, Kistler C, Hunold KM, et al. Shared Decision-Making in the Selection of Outpatient Analgesics for Older Individuals in the Emergency Department. J Am Geriatr Soc. 2013;61(5):793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feder SL, Schulman-Green D, Dodson JA, et al. Risk Stratification in Older Patients With Acute Myocardial Infarction. J Aging Health. 2016;28(3):387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess EP, Hollander JE, Schaffer JT, et al. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial. BMJ. 2016;355:i6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green AR, Jenkins A, Masoudi FA, et al. Decision-Making Experiences of Patients with Implantable Cardioverter Defibrillators. Pacing Clin Electrophysiol. 2016;39(10):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodson JA, Chaudhry SI, Krumholz HM. Time for a New Approach to Studying Older People with Ischemic Heart Disease. J Am Geriatr Soc. 2017;65(11):2349–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes. J Am Coll Cardiol. 2014;64(24):e139–e228. [DOI] [PubMed] [Google Scholar]
- 28.Wilson T, Miller J, Teare S, et al. Patient perspectives on engagement in decision-making in early management of non-ST elevation acute coronary syndrome: a qualitative study. BMC Med Inform Decis Mak. 2017;17(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wieringa TH, Rodriguez-Gutierrez R, Spencer-Bonilla G, et al. Decision aids that facilitate elements of shared decision making in chronic illnesses: a systematic review. Syst Rev. 2019;8(1):121. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feder SL, Schulman-Green D, Geda M, et al. Physicians’ perceptions of the Thrombolysis in Myocardial Infarction (TIMI) risk score in older adults with acute myocardial infarction. Heart Lung. 2015;44(5):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wennberg JE, O’Connor AM, Collins ED, et al. Extending the P4P agenda, part 1: how Medicare can improve patient decision making and reduce unnecessary care. Health Aff. 2007;26(6):1564–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spatz ES, Spertus JA. Shared decision making: a path toward improved patient-centered outcomes. Circ Cardiovasc Qual Outcomes. 2012;5(6):e75–7. [DOI] [PubMed] [Google Scholar]