Abstract
Objective
To examine the association between the inflammatory potential of diet and the degree of cognitive function in an older adult Korean population.
Methods
A total of 239 participants (88 men and 151 women) ages ≥65 y were selected from various health centers in Korea. To assess inflammatory potential of diet, energy-adjusted Dietary Inflammatory Index (E-DII) scores were computed based on a single 24-h recall. Cognitive function was assessed using the Korean Mini-Mental State Examination. Multiple linear and logistic regression models were fit to estimate the association between E-DII scores and the degree of cognitive function.
Results
E-DII scores were significantly inversely associated with Korean Mini-Mental State Examination score in both unadjusted and adjusted models, after controlling for gender, age, BMI, sleep hours, supplement use, education level, self-reported health conditions, history of dementia, and physical activity (β = −1.33, 95 % CI = −1.95, −0.71, P value <0.0001; β = −0.58, 95 % CI = −1.11, −0.06, P value = 0.03, respectively). Participants in the highest E-DII tertile had increased risk for mild or moderate cognitive impairment compared with those in the lowest E-DII tertile (adjusted odds ratio 6.32, 95 % CI 1.18 - 33.78; P for trend = 0.0031).
Conclusions
Higher E-DII scores are associated with increased risk of cognitive impairment, suggesting that consuming a pro-inflammatory diet is associated with increased risk for cognitive impairment in the older Korean adults.
Keywords: Cognitive function, K-MMSE, Energy-adjusted dietary inflammatory index (E-DII), Older Koreans, Inflammation
Introduction
Substantial increases in the number of older adults have been recorded across most populations globally. The number of persons >60 y of age in the world is expected to reach around 2.1 billion by 2050 [1]. Simultaneously, a dramatic increase in the number of older adult population in Korea has been reported, with the number of those ≥65 y of age projected to increase from 14.3% in 2018 to 22.9% by 2028 [2].
Cognitive impairment is one of the major health problems of older adults. Cognitive impairment in older adults has been associated with adverse health consequences, including congestive heart failure [3], poor diabetes control [4], functional decline in activity of daily living [5], depressive symptoms [6] and reduced survival rates [7]. The direct medical cost per person is significantly higher for the older adults with cognitive impairment compared with those without cognitive impairment [8]. Given the anticipated increase in the number of the older adults with cognitive impairment, associated adverse health consequences, and medical costs, it is imperative that a practical means to reduce cognitive impairment among older adults (e.g., through diet) be identified.
Currently, inflammation, as indicated by elevated levels of C-reactive protein (CRP), interleukin (IL)-6 and tumor necrosis factor (TNF)-α has been found to be associated with cognitive impairment [9–11]. Because inflammation has been closely linked to diet [12], the dietary inflammatory index (DII) was developed recently by researchers at University of South Carolina to measure the inflammatory potential of individuals’ diets [13]. The DII has been found to be associated with cognitive decline in France and the United States [14, 15], where diets with proinflammatory properties, including energy-dense foods, foods with high saturated fat and cholesterol content, and meat products indicated by higher DII score, have been found to be associated with lower cognitive functioning in French and the US populations [14, 15]. The Korean diet is carbohydrate based but it is distinct in that it predominantly contains rice as a staple [16] (with distinct constituents compared with other refined or whole grains). This is one feature that distinguishes the Korean diet from the US and French diets. Specifically, high consumption of refined carbohydrates, including white rice, has been associated with increased risks for cognitive impairments [17–19]. As such, the Korean dietary pattern is different from Western dietary patterns in terms of dietary composition, and therefore it is important to assess the association between the inflammatory potential of the Korean diet and cognitive function. Limited data exist regarding the role of dietary inflammation on cognitive function in an older Korean population.
The objective of this study is to investigate the association between the inflammatory potential of diet and cognitive function in a cross-sectional study of Korean adults aged ≥65 y old. We hypothesized that a proinflammatory diet is associated with lower cognitive function in older Korean adults.
Material and methods
Study Participants
A convenience sample of 316 older adults aged ≥65 y residing in the district of Seoul, Gyeonggi province, and Incheon in Korea were recruited through one-on-one interviews, using structured questionnaires, from March 2012 to July 2012. During the initial planning of the study, research assistants contacted officers at the welfare center in Korea, and after a discussion regarding our ability to obtain data on cognitive functions and sociodemographic factors, we estimated that we could reach out to at least 300 older adults. Among 316 participants, 239 (88 men and 151 women) participants with good communication skills assessed by research assistants through one-on-one interviews completed a 24-h recall (24 HR). The refusals (n=77) were those with cognitive challenges as a result of poor communication skills. The survey was conducted in the University Hospital clinics, the Elderly Welfare Centers, and the Health Welfare Center in the district of Seoul, Gyeonggi province, and Incheon in Korea. The study was reviewed and approved by the Institutional Review Board at Hannam University (2012-01K).
Exposure Variable
Dietary Assessment and Dietary Inflammatory Index (DII)™
A trained interviewer assessed dietary intake using a single 24-hour dietary recall interview (24 HR). Nutrient intakes were computed using the Computer-Aided Nutritional Analysis Program for Professionals 4.0 (Can-Pro 4.0), developed by the Korean Nutrition Society. Using data from the 24 HR to assess the primary exposure of interest, the DII was computed based on the following macro and micronutrients (termed food parameters throughout): energy, carbohydrates; fat; protein; vitamins A, B1, B2, B3, B6, B12, C, and E; saturated, monounsaturated, and polyunsaturated fatty acids; fiber; cholesterol; iron; zinc and folic acid. Research from nearly 2000 peer-reviewed publications formed the basis of the DII. Inflammatory effect scores were informed by these peer-reviewed publications for each of the DII food parameters based on their effect on inflammatory cytokines [13]. Energy-adjusted DII (E-DII) scores were calculated per 1000 kcal/d consumed to control for the effect of total energy intake differences among participants. Once calculated from the 24 HR, the E-DII scores were standardized to a regionally representative world database consisting of dietary consumption from 11 populations around the world and also standardized to 1000 kcal/d. The world database provided a standard mean and standard deviation for all DII food parameters. For each food parameter, a z score was created by subtracting the individual’s estimated intake from the standard mean. This was then divided by the world standard deviation and then converted to a proportional distribution, with values ranging from 0 to 1. These values were then centered on 0 by doubling the proportion and subtracting 1 (resulting in an E-DII score range with a lower bound of −1 and an upper bound of +1. This value was then multiplied by the inflammatory effect score for each food parameter. These values were then summed across all food parameters to create the overall E-DII score. More positive scores indicate more proinflammatory diets and more negative values are more antiinflammatory [13].
Outcome Variable
Cognitive Function Assessment
Cognitive function was examined by the Korean-adjusted version of Mini-Mental State Examination (K-MMSE), the most widely used screening tool for quantitative assessment of the cognitive status of the older adult population, developed by Kwon & Park [20], although adapted from Folstein et al. [21]. The K-MMSE includes 19 items, and the scores range from 0 to 30. The study participants were classified into four groups by score: normal (25-30); boundary zone (20-24); mild cognitive impairment (15-19); and moderate cognitive impairment (10-14).
Statistical Analyses
Descriptive statistics were generated for K-MMSE, body mass index (BMI), sociodemographic, and lifestyle variables including age, sex, daily supplement use, family type, education level, self-reported health conditions, history of dementia, smoking status, sleep durations, and physical activity across the distributions of E-DII and degrees of cognitive functions. To judge the significance among variables, analysis of variance was performed for continuous variables, and χ2 testing was performed for categorical variables. Unadjusted and covariate-adjusted linear regression models were tested to estimate β coefficients and 95% confidence intervals (CI) for the association among the continuous variables, E-DII, and cognitive function (K-MMSE score). The E-DII scores were divided into tertiles (three groups) by scoring from 1 to 3 according to the order of E-DII scores. Thus, tertile 1 of E-DII, the lowest scoring group of E-DII, is the most antiinflammatory group, whereas tertile 3 of E-DII, the highest scoring group of E-DII, is the most proinflammatory group. Unadjusted and adjusted multiple logistic regression models were used to calculate odds ratios and 95% CI for the association among tertiles of E-DII and mild and moderate cognitive impairment (K-MMSE score 10-19). Covariates included in adjusted models were variables that significantly differed in relation to the degree of cognitive function. To assess the association between E-DII score and cognitive impairment using logistic regression models, subgroup analyses were performed by stratifying the older adults into two groups; those who slept for <7 versus ≥7 h/d (median sleep hours in the present study).
Receiver operating curve (ROC) and the area under the ROC curve were used to determine whether E-DII score was a predictor of cognitive function. All data analyses were performed using the SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA). Significance was set at a two-sided P value of <0.05.
Results
The distributions of study participants’ cognitive function and sociodemographic and lifestyle variables are shown in Table 1. K-MMSE and BMI significantly increased across E-DII score-based tertiles (all P values <0.05). Hours of sleep per day appeared to decrease with increasing E-DII score (P value=0.06). Categories of daily supplement usage, education level, and physical activity levels significantly differed by the tertiles of E-DII (all P values <0.05). Older adults who were in E-DII tertile 1 (n=79) were more likely to report daily supplement use and had higher education level and physical activity level compared to those in E-DII tertile 3 (n=80).
Table 1.
Distribution of study participants across tertiles of energy-adjusted dietary inflammatory index (E-DII), Korean Study of Diet and Aging, 2012.
| E-DII |
|||||||
|---|---|---|---|---|---|---|---|
| Tertile 1 (n=79) | Tertile 2 (n=80) | Tertile 3 (n=80) | |||||
|
| |||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | P * | |
| K-MMSE (range: 0-30) | 27.4 | (3.3) | 26.8 | (4.3) | 24.0 | (5.7) | <0.0001 |
| Age (y) | 73.0 | (6.1) | 73.7 | (5.9) | 75.4 | (6.2) | 0.04 |
| BMI (kg/m2) | 22.6 | (3.2) | 20.8 | (3.5) | 22.1 | (4.1) | 0.006 |
| Sleep durations (h/d) | 7.0 | (1.4) | 6.9 | (1.6) | 6.4 | (2.2) | 0.06 |
| n | (%) | n | (%) | n | (%) | P † | |
| Sex | |||||||
| Men | 33 | (41.8) | 29 | (36.3) | 26 | (32.5) | 0.48 |
| Women | 46 | (58.2) | 51 | (63.8) | 54 | (67.5) | |
| Supplements | |||||||
| Yes | 41 | (51.9) | 40 | (50.0) | 27 | (33.8) | 0.04 |
| No | 38 | (48.1) | 40 | (50.0) | 53 | (66.3) | |
| Family type | |||||||
| With spouse | 33 | (41.8) | 39 | (48.8) | 36 | (45.0) | 0.07 |
| With children | 31 | (39.2) | 25 | (31.3) | 26 | (32.5) | |
| Spouse and children | 5 | (6.3) | 8 | (10.0) | 3 | (3.8) | |
| Alone | 9 | (11.4) | 8 | (10.0) | 8 | (10.0) | |
| Other | 1 | (1.3) | 0 | (0.0) | 7 | (8.8) | |
| Education | |||||||
| Less than elementary | 13 | (16.5) | 12 | (15.0) | 26 | (32.5) | 0.04 |
| Elementary | 27 | (34.2) | 37 | (46.3) | 29 | (36.3) | |
| Middle school | 17 | (21.5) | 15 | (18.8) | 16 | (20.0) | |
| High school | 18 | (22.8) | 14 | (17.5) | 9 | (11.3) | |
| College or higher | 4 | (5.1) | 2 | (2.5) | 0 | (0.0) | |
| Self-reported health conditions | |||||||
| Very good | 3 | (3.8) | 6 | (7.5) | 3 | (3.8) | 0.16 |
| Good | 17 | (21.5) | 18 | (22.5) | 13 | (16.3) | |
| Fair | 46 | (58.2) | 40 | (50.0) | 38 | (47.5) | |
| Poor | 11 | (13.9) | 13 | (16.3) | 16 | (20.0) | |
| Very Poor | 2 | (2.5) | 3 | (3.8) | 10 | (12.5) | |
| History of dementia | |||||||
| Yes | 3 | (3.8) | 5 | (6.3) | 4 | (5.0) | 0.7782 |
| No | 76 | (96.2) | 75 | (93.8) | 76 | (95.0) | |
| Smoking status | |||||||
| Yes | 13 | (16.5) | 10 | (12.5) | 12 | (15.0) | 0.78 |
| No | 66 | (83.5) | 70 | (87.5) | 68 | (85.0) | |
| Physical activity | |||||||
| None | 20 | (25.3) | 13 | (16.3) | 35 | (43.8) | 0.001 |
| 1–2/wk | 27 | (34.2) | 35 | (43.8) | 24 | (30.0) | |
| 3–4/wk | 18 | (22.8) | 12 | (15.0) | 5 | (6.3) | |
| Every day | 14 | (17.7) | 20 | (25.0) | 16 | (20.0) | |
BMI, body mass index; K-MMSE, Korean-adjusted Mini-Mental State Examination
Analysis of variance test was performed for continuous variables.
A ꭕ2 test was performed for categorical variables.
The distribution of study participants’ cognitive function by E-DII and sociodemographic and lifestyle variables are presented in Table 2. E-DII and age significantly decreased as cognitive functions increased from the moderate cognitive impairment group to the normal cognitive function group (all P values <0.05). BMI and hours of sleep significantly increased from the moderate cognitive impairment group to the normal cognitive function group (all P values <0.05). Categories of sex, daily supplement use, education level, self-reported health conditions, history of dementia, and physical activity significantly differed by E-DII tertile (all P values <0.05). The majority of men (86%) were classified as having normal cognitive function. However, comparatively more women were found to have higher rates of normal cognitive function than men (53.1% versus 46.9%). The older adults who were categorized as having normal cognitive function reported more daily supplement use compared with those who had moderate cognitive impairment (51.9% versus 0%). Individuals with higher educational attainment, better self-reported health conditions, no history of dementia, and adequate physical activity were more likely to be in the normal cognitive functioning group than in the moderate cognitive impairment group (all P values <0.05).
Table 2.
Distribution of study participants by the degree of cognitive function, Korean Study of Diet and Aging, 2012
| Cognitive Function (K-MMSE score; n) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate Cognitive Impairment (10-14; n=6) | Mild Cognitive Impairment (15-19; n=26) | Boundary Zone Cognition (20-24; n=45) | Normal Cognition (25-30; n=162) | Total | |||||||
|
| |||||||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | P * | |
| Energy-adjusted Dietary Inflammatory Index (E-DII) | −0.4 | (0.8) | −0.6 | (0.8) | −0.9 | (1.0) | −1.3 | (0.9) | −1.1 | (1.0) | 0.0005 |
| Age (y) | 77.3 | (4.4) | 80.0 | (4.5) | 75.6 | (5.7) | 72.5 | (5.8) | 74.0 | (6.1) | <0.0001 |
| BMI (kg/m2) | 19.8 | (3.0) | 19.5 | (4.9) | 22.2 | (4.2) | 22.2 | (3.2) | 21.8 | (3.7) | 0.003 |
| Sleep durations (h/d) | 4.0 | (3.2) | 6.1 | (2.4) | 7.1 | (2.2) | 6.8 | (1.3) | 6.7 | (1.8) | 0.0001 |
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | P † | |
| Sex | |||||||||||
| Men | 0 | (0.0) | 6 | (23.1) | 6 | (13.3) | 76 | (46.9) | 88 | (36.8) | <0.0001 |
| Women | 6 | (100.0) | 20 | (76.9) | 39 | (86.7) | 86 | (53.1) | 151 | (63.2) | |
| Daily supplement use | |||||||||||
| Yes | 0 | (0.0) | 4 | (15.4) | 20 | (44.4) | 84 | (51.9) | 108 | (45.2) | 0.0006 |
| No | 6 | (100.0) | 22 | (84.6) | 25 | (55.6) | 78 | (48.2) | 131 | (54.8) | |
| Family type | |||||||||||
| With spouse | 2 | (33.3) | 10 | (38.5) | 23 | (51.1) | 73 | (45.1) | 108 | (45.2) | 0.88 |
| With children | 3 | (50.0) | 9 | (34.6) | 14 | (31.1) | 56 | (34.6) | 82 | (34.3) | |
| Spouse and children | 0 | (0.0) | 2 | (7.7) | 1 | (2.2) | 13 | (8.0) | 16 | (6.7) | |
| Alone | 1 | (16.7) | 4 | (15.4) | 4 | (8.9) | 16 | (9.9) | 25 | (10.5) | |
| Other | 0 | (0.0) | 1 | (3.9) | 3 | (6.7) | 4 | (2.5) | 8 | (3.4) | |
| Education | |||||||||||
| Less than elementary | 4 | (66.7) | 13 | (50.0) | 16 | (35.6) | 18 | (11.1) | 51 | (21.3) | <0.0001 |
| Elementary | 2 | (33.3) | 10 | (38.5) | 20 | (44.4) | 61 | (37.7) | 93 | (38.9) | |
| Middle school | 0 | (0.0) | 1 | (3.9) | 6 | (13.3) | 41 | (25.3) | 48 | (20.1) | |
| High school | 0 | (0.0) | 1 | (3.9) | 3 | (6.7) | 37 | (22.8) | 41 | (17.2) | |
| College or higher | 0 | (0.0) | 1 | (3.9) | 0 | (0.0) | 5 | (3.1) | 6 | (2.5) | |
| Self-reported health conditions | |||||||||||
| Very good | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 12 | (7.4) | 12 | (5.0) | <0.0001 |
| >Good | 2 | (33.3) | 6 | (23.1) | 4 | (8.9) | 36 | (22.2) | 48 | (20.1) | |
| Fair | 2 | (33.3) | 8 | (30.8) | 24 | (53.3) | 90 | (55.6) | 124 | (51.9) | |
| Poor | 0 | (0.0) | 10 | (38.5) | 10 | (22.2) | 20 | (12.4) | 40 | (16.7) | |
| Very Poor | 2 | (33.3) | 2 | (7.7) | 7 | (15.6) | 4 | (2.5) | 15 | (6.3) | |
| History of dementia | |||||||||||
| Yes | 2 | (33.3) | 0 | (0.0) | 6 | (13.3) | 4 | (2.5) | 12 | (5.0) | 0.0002 |
| No | 4 | (66.7) | 26 | (100.0) | 39 | (86.7) | 158 | (97.5) | 227 | (95.0) | |
| Smoking status | |||||||||||
| Yes | 0 | (0.0) | 2 | (7.7) | 5 | (11.1) | 28 | (17.3) | 35 | (14.6) | 0.34 |
| No | 6 | (100.0) | 24 | (92.3) | 40 | (88.9) | 134 | (82.7) | 204 | (85.4) | |
| Physical activity | |||||||||||
| None | 4 | (66.7) | 14 | (53.9) | 19 | (42.2) | 31 | (19.1) | 68 | (28.5) | <0.0001 |
| 1-2/wk | 0 | (0.0) | 4 | (15.4) | 16 | (35.6) | 66 | (40.7) | 86 | (36.0) | |
| 3-4/wk | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 35 | (21.6) | 35 | (14.6) | |
| Every day | 2 | (33.3) | 8 | (30.8) | 10 | (22.2) | 30 | (18.5) | 50 | (20.9) | |
K-MMSE, Korean-adjusted Mini-mental State Examination.
Analysis of variance test was performed for continuous variables.
χ2 test was performed for categorical variables.
β Coefficients for the association between E-DII score and cognitive functions are presented in Table 3. Increases in E-DII scores were significantly and negatively associated with cognitive functions, in both crude analyses and after adjusting for sex, age, BMI, sleep hours, supplement use, education level, self-reported health conditions, history of dementia, and physical activity (β=−1.33, 95% CI=−1.95, −0.71, P <0.0001; β=−0.58, 95% CI = −1.11, −0.06, P =0.03, respectively). When stratified by sex, there was no significant association between E-DII and cognitive function in men. In women, a statistically significant inverse association was found between E-DII and K-MMSE after adjusting for covariates (β=−0.96, 95% CI=−1.69, −0.22, P =0.01).
Table 3.
β Coefficient estimates for association of energy-adjusted Dietary Inflammatory Index (E-DII) and cognitive functions in total and stratified by sex, Korean Study of Diet and Aging, 2012
| β | (95% CI) | P | |
|---|---|---|---|
| Total (n=239) | |||
| Unadjusted | −1.33 | (−1.95, −0.71) | <0.0001 |
| Multivariable* | −0.58 | (−1.11, −0.06) | 0.03 |
| Men (n=88) | |||
| Unadjusted | −0.63 | (−1.33, 0.07) | 0.08 |
| Multivariable † | −0.21 | (−0.88, 0.47) | 0.54 |
| Women (n=151) | |||
| Unadjusted | −1.77 | (−2.64, −0.90) | <0.0001 |
| Multivariable2† | −0.96 | (−1.69, −0.22) | 0.01 |
BMI, body mass index; CI, confidence interval.
Adjusted for sex (men/women), age (continuous), BMI (continuous), sleep hours (continuous), supplemental use (yes/no), education level (uneducated, elementary, middle school, high school, college or higher), self-reported health conditions (very good, good, fair, poor, very poor), history of dementia (yes/no), and physical activity (none, 1-2/wk, 3-4/wk, every day).
Adjusted for age (continuous), BMI (continuous), sleep hours (continuous), supplemental use (yes/no), education level (less than elementary, elementary, middle school, high school, college or higher), self-reported health conditions (very good, good, fair, poor, very poor), history of dementia (yes/no), and physical activity (none, 1-2/wk, 3-4/wk, every day)
Unadjusted and adjusted odds ratios for mild or moderate cognitive impairment by E-DII tertile and as continuous values of E-DII score are presented in Table 4. In the unadjusted model, the older adults in the highest tertile of E-DII score (most proinflammatory diet) had increased odds of being in the moderate or mild cognitive impairment group compared with those older adults in the lowest tertile of DII score (most antiinflammatory diet) (OR=11.67, 95% CI=3.27-41.68). After controlling for sex, age, BMI, sleep hours, daily supplement use, education level, self-reported health conditions, history of dementia, and physical activity, the inverse association between E-DII and moderate or mild cognitive impairment still remained (adjusted OR [AOR]=6.32, 95% CI=1.18-33.78; P for trend=0.003). When E-DII was fit as a continuous variable, each one-point increase in E-DII score was associated with 2.54 times increased odds for mild or moderate cognitive impairment (AOR=2.54, 95% CI=1.14-5.70).
Table 4.
Unadjusted and adjusted odds ratios for mild or moderate cognitive impairment by the tertiles of E-DII, Korean Study of Diet and Aging, 2012
| Mild or Moderate Cognitive Impairment |
|||
|---|---|---|---|
| OR* (95% CI) | Adjusted OR† (95% CI) | ||
| E-DII Tertiles | |||
| Tertile 1 (most antiinflammatory) | 1.00 (Reference) | 1.00 (Reference) | |
| Tertile 2 | 2.99 (0.76-11.80) | 2.57 (0.40-16.63) | |
| Tertile 3 (most proinflammatory) | 11.67 (3.27-41.68) | 6.32 (1.18-33.78) | |
| P for trend‡ | <0.0001 | 0.003 | |
| E-DII Continuous | 2.66 (1.23-5.76) | 2.54 (1.14-5.70) | |
BMI, body mass index; CI, confidence interval; E-DII, energy-adjusted Dietary Inflammatory Index; OR, odds ratio.
Unadjusted model
Adjusted for sex (men/women), age (continuous), BMI (continuous), sleep hours (continuous), supplemental use (yes/no), education level (less than elementary, elementary, middle school, high school, college or higher), self-reported health conditions (very good, good, fair, poor, very poor), history of dementia (yes/no), and physical activity (none, 1-2/wk, 3-4/wk, every day)
Tests of linear trend between moderate or mild cognitive impairment and DII score were calculated by assigning the median value of each tertile to each participant in the tertile, and this value was inputted into the model as ordinal values.
The older adult population who slept less than 7 h/night and consumed a proinflammatory diet (highest tertile of E-DII) had increased odds for mild or moderate cognitive impairment compared with those who consumed an antiinflammatory diet (lowest tertile of E-DII) in the covariate-adjusted model (AOR=14.82, 95% CI=1.13-194.73; P for trend=0.009). On division of E-DII into tertiles, there was no significant association between the tertiles and cognitive impairment for the older adults who slept ≥7 h/night. However, a significant linear trend was found between E-DII and cognitive impairment in this group (P for trend=0.01) (Table 5). The prediction of mild or moderate cognitive impairment using the ROC curve of DII is presented in Figure 1. Area under ROC curve was 0.72, which is high.
Table 5.
Unadjusted and adjusted odds ratios of mild or moderate cognitive impairment by tertiles of E-DII in subgroups of hours of sleep, Korean Study of Diet and Aging, 2012.
| E-DII Tertiles |
||||||
|---|---|---|---|---|---|---|
| n | Tertile 1 | Tertile 2 | Tertile 3 | P for trend* | P interaction† | |
| Subgroup analyses for mild or moderate cognitive impairment | ||||||
| Unadjusted model | ||||||
| Sleep durations (h/d) | 0.03 | |||||
| <7 | 108 | 1.00 (Reference) | 11.79 (1.36-102.12) | 20.22 (2.43-168.20) | <0.0001 | |
| ≥7 | 131 | 1.00 (Reference) | 1.54 (0.24-9.78) | 3.85 (0.69-21.49) | 0.005 | |
| Adjusted model‡ | ||||||
| Sleep durations (h/d) | 0.11 | |||||
| <7 | 108 | 1.00 (Reference) | 13.27 (0.99-178.08) | 14.82 (1.13-194.73) | 0.009 | |
| ≥7 | 131 | 1.00 (Reference) | 1.11 (0.13-9.27) | 4.91 (0.69-35.17) | 0.01 | |
BMI, body mass index; E-DII, energy-adjusted Dietary Inflammatory Index.
Tests of linear trend between moderate or mild cognitive impairment and DII score were calculated by assigning the median value of each tertile to each participant in the tertile, and this value was inputted into the model as ordinal values.
Potential effect modification of the association between the DII and moderate or mild cognitive impairment by hours of sleep was investigated by stratifying the models by levels of potential effect modifiers. Significant effect modification was considered at a P value of 0.15 for the DII covariate interaction term.
Adjusted for sex (men/women), age (continuous), BMI (continuous), supplemental use (yes/no), and history of dementia (yes/no)
Figure 1.
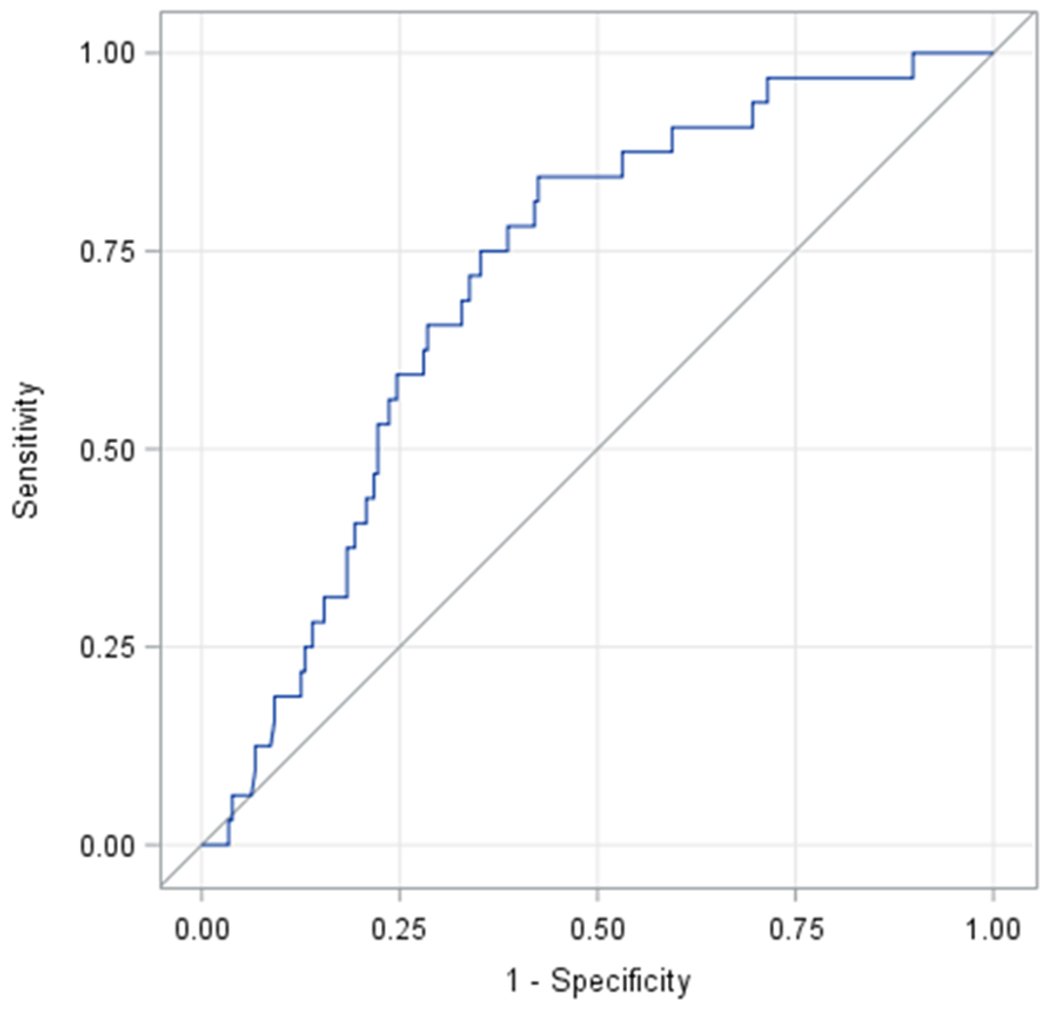
Receiver operating characteristic (ROC) curve of energy-adjusted Dietary Inflammatory Index (E-DII) in the prediction of moderate or mild cognitive impairment (area under ROC curve, 0.72 [95% confidence interval 0.63-0.80]).
Discussion
In the present study, older adults Koreans who consumed a proinflammatory diet had increased risks for mild or moderate cognitive impairment, compared with those who consumed anti-inflammatory diet, regardless of sociodemographic and lifestyle variables. Possible underlying mechanisms that could explain the inverse association between E-DII scores and the risk of cognitive impairment involve the role of proinflammatory diet on systemic inflammation [22]. The traditional Korean diet is high in carbohydrates, mainly composed of rice [23]. Consuming foods that have a high proinflammatory potential, such as refined carbohydrates, elevates inflammation levels, which in turn is associated with increased risks for cognitive impairment in older adults [17–19]. Consistent with this finding, higher DII scores were associated with cognitive decline over 10 y among women ages 65 to 79 y in the Women’s Health Initiative Memory Study, a randomized, double-blind, placebo-controlled trial [14]. In the SU.VI.MAX (supplementation with antioxidant vitamins and minerals) cohort, proinflammatory diet was significantly and inversely associated with cognitive functioning [15]. As reported in earlier studies, elevated inflammatory biomarkers such as CRP and IL-6 have been cross-sectionally associated with cognitive impairment at baseline, in a longitudinal cohort study of healthy older adults ages 70 to 79 [17]. Consistent with this finding, in a cross-sectional study of an older adults ages 60 to 75 with type 2 diabetes [18], inflammatory biomarkers, including IL-6 and TNF-α were statistically inversely associated with cognitive test scores - specifically, matrix reasoning, trail making test, and faces and family pictures subtest - after age and sex were adjusted. Another prospective population-derived cohort study reported that in community-dwelling participants ages 70 to 90 y [19], high levels of IL-12 were significantly associated with lower scores on attention on attention/processing speed domain, a crucial component for normal cognitive functioning. Nonetheless, conflicting results on inflammation and cognitive function also have been reported [24, 25]. In a cross-sectional study in a community-dwelling older adults population [24], serum cytokines, including IL-1B, sIL-4R, IL-6, IL-8, IL-10, IL-12, and TNF- α were not significantly associated with MMSE scores. In the Longitudinal Aging Study Amsterdam, CRP and IL-6 of the older adults ages 62 to 85 y [25] were not significantly longitudinally associated with cognitive decline assessed by MMSE. Conflicting results on inflammation and cognitive decline may be the single measurement of inflammation or the assessment tool to quantify cognitive functions varied in each study. A causal inference between inflammation and cognitive function can be drawn from a randomized, double-blind, placebo-controlled trial [14], and cohort studies [15, 19], whereas casual inference from cross-sectional studies requires additional, corroborative evidence [17, 18].
Previous research on the role of dietary patterns or factors on cognitive function has been inconclusive. Adherence to the Mediterranean diet has been found to be associated with lower risk of cognitive impairment in a cohort, multiethnic community study in New York [26]. The older adults in the highest tertile of the Mediterranean Diet Score had a lower risk for incidence of mild cognitive impairment (hazard ratio=0.85, 95% CI=0.72-1.00) compared with those in the lowest tertile of Mediterranean diet score. A Mediterranean diet, characterized by high consumption of antiinflammatory foods such as fruits and vegetables, omega-3 and omega-6 fatty acids, polyunsaturated fatty acids, and antioxidants has been closely linked to a lower DII score in a prospective study [27]. The common antiinflammatory foods and nutrients such as high polyunsaturated fatty acids and antioxidants that can be found in both Mediterranean and antiinflammatory diets (lower DII) may be closely link to higher K-MMSE score.
When we stratified by sex in this study, the significant inverse association between E-DII score and cognitive function was found only in women, not in men. In women, each unit increase in E-DII score was significantly negatively associated with a 0.96-point decrease in K-MMSE after adjusting for age, BMI, sleep hours, supplement use, education level, self-reported health conditions, history of dementia, and physical activity (P =0.01). This is not because of the sample size difference in men and women (88 versus 151, respectively) because the regression coefficient in men was only about one-third of that in women. Moreover, no man was defined as having moderate cognitive impairment. This may have resulted from inaccurate responses of the men to the K-MMSE questionnaire.
In a subgroup analysis to assess the association between E-DII score tertiles and mild or moderate cognitive impairment with regard to sleep hours per day (<7 versus ≥7), higher E-DII scores were negatively associated with cognitive functions in both groups (P for trend <0.05). Specifically, the older adults who slept fewer than 7 h/d and consumed proinflammatory diet had a 15-fold increased risk of cognitive impairment. This highlights the importance of securing enough sleep and eating a healthy diet to ensure healthy cognitive function; as found in a cohort study of Chinese older adults, less sleep has been reported to be a risk factor for cognitive impairment [28]. Also, a healthy older population who reported poor sleep quality, as assessed by the Pittsburgh Sleep Quality Index, had decreased functional symptoms such as decreased concentration [29].
There are several limitations that pertain to this study. First, because of its cross-sectional design, a cause-effect relationship on dietary inflammation and cognitive function cannot be drawn from this study. Second, a single 24 HR was used to assess dietary intake in the present study, so this may not necessarily represent the usual dietary intake of the older adults. Also, a single 24 HR may not capture the intraindividual variability of dietary intake. Third, because cognitive function was assessed at one single time point in the present study, any cognitive decline or improvement over the period among the older adults could not be captured. Research has found that only 30% of the older adults maintained cognitive function over 8 y [30]. Last, stratified analyses produced small sample sizes in this study of older adults with moderate cognitive impairment (n=6).
Despite its limitations, the study has a number of strengths. To the best of our knowledge, this is the first study to assess inflammatory potential from the diet in relation to degrees of cognitive function in older Korean adults. Additionally, a number of important confounding variables, including BMI, sleep duration, daily supplement use, history of dementia, smoking status, and physical activity, were assessed and controlled in the present study. Finally, the ROC curve analysis allowed us to evaluate the predictability and accuracy [31] of the DII on cognitive impairment. We evaluated our data with the understanding that K-MMSE is a screening tool rather than a diagnostic tool for assessing cognitive impairment.
Conclusions
The findings from this study suggest that diet-associated inflammation is inversely associated with the degree of cognitive function in Korean older adults. High consumption of a variety of antiinflammatory foods, such as fruits, vegetables, omega-3 fatty acids, and low consumption of saturated fatty acids and energy-dense foods may ensure adequate cognitive function in Korean older adults. Increased levels of inflammation not only have been linked to higher risk of cognitive decline but also to dementia [32]. Because cognitive impairment has been found to be an important predictor of dementia [33], establishing an antiinflammatory dietary recommendation for Korean older adults may be a successful strategy to reduce the risk of cognitive impairment.
Funding:
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2016S1A3A2924243).
Dr. Simona Kwon was supported by grants number P60MD000538 and UL1TR001445 from the National Institutes of Health, United States. Drs. Nitin Shivappa and James R. Hébert were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI.
References
- [1].United Nations, Department of Economic and Social Affairs, Population Division (2015). World Population Ageing 2015. ST/ESA/SER.A/390.
- [2].Statistics Korea. Population projections and summary indicators (Korea), http://kosis.kr/eng/statisticsList/statisticsList_01List.jsp?vwcd=MT_ETITLE&parentId=A#SubCont; 2017. [accessed 18 May 2017].
- [3].Almeida OP, Flicker L. The mind of a failing heart: a systematic review of the association between congestive heart failure and cognitive functioning. Intern Med J. 2001;31:290–5. [DOI] [PubMed] [Google Scholar]
- [4].Munshi M, Grande L, Hayes M, Ayres D, Suhl E, Capelson R, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes care. 2006;29:1794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. J Am Geriatr Soc. 2002;50:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vinkers DJ, Gussekloo J, Stek ML, Westendorp RG, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ. 2004;329:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Georgakis MK, Papadopoulos FC, Protogerou AD, Pagonari I, Sarigianni F, Biniaris-Georgallis SI, et al. Comorbidity of Cognitive Impairment and Late-Life Depression Increase Mortality: Results From a Cohort of Community-Dwelling Elderly Individuals in Rural Greece. J Geriatr Psychiatry Neurol. 2016;29:195–204. [DOI] [PubMed] [Google Scholar]
- [8].Zhu CW, Sano M, Ferris SH, Whitehouse PJ, Patterson MB, Aisen PS. Health-Related Resource Use and Costs in Elderly Adults with and without Mild Cognitive Impairment. J Am Geriatr Soc. 2013;61:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roberts RO, Geda YE, Knopman DS, Boeve BF, Christianson TJ, Pankratz VS, et al. Association of C-reactive protein with mild cognitive impairment. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2009;5:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol. 2010;67:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation. J Am Coll Cardiol. 2006;48:677–85. [DOI] [PubMed] [Google Scholar]
- [13].Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hayden KM, Beavers DP, Steck SE, Hebert JR, Tabung FK, Shivappa N, et al. The association between an inflammatory diet and global cognitive function and incident dementia in older women: The Women’s Health Initiative Memory Study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kesse-Guyot E, Assmann KE, Andreeva VA, Touvier M, Neufcourt L, Shivappa N, et al. Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU.VI.MAX study. Eur J Nutr. 2017;56:1647–55. [DOI] [PubMed] [Google Scholar]
- [16].Park SH, Lee KS, Park HY. Dietary carbohydrate intake is associated with cardiovascular disease risk in Korean: analysis of the third Korea National Health and Nutrition Examination Survey (KNHANES III). Int J Cardiol. 2010;139:234–40. [DOI] [PubMed] [Google Scholar]
- [17].Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marioni RE, Strachan MW, Reynolds RM, Lowe GD, Mitchell RJ, Fowkes FGR, et al. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes. Diabetes. 2010;59:710–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Trollor JN, Smith E, Agars E, Kuan SA, Baune BT, Campbell L, et al. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age. 2012;34:1295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kwon YC, Park J-H. Korean version of Mini-Mental State Examination (MMSE-K). Part I: development of the test for the elderly. J Korean Neuropsychiatr Assoc. 1989;28:125–35. [Google Scholar]
- [21].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [22].Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy. 2015;45:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chung H- K, Yang HJ, Shin D, Chung KR. Aesthetics of Korean foods: the symbol of Korean culture. Journal of Ethnic Foods. 2016;3:178–88. [Google Scholar]
- [24].Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, et al. Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiol Aging. 2008;29:937–44. [DOI] [PubMed] [Google Scholar]
- [25].Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–7. [DOI] [PubMed] [Google Scholar]
- [26].Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Archives of neurology. 2009;66:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hodge A, Bassett J, Shivappa N, Hébert J, English D, Giles G, et al. Dietary inflammatory index, Mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Control. 2016;27:907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu L, Jiang CQ, Lam TH, Zhang WS, Cherny SS, Thomas GN, et al. Sleep duration and memory in the elderly Chinese: longitudinal analysis of the Guangzhou Biobank Cohort Study. Sleep. 2014;37:1737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, et al. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011;48:277–87. [DOI] [PubMed] [Google Scholar]
- [32].Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci. 2014;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–9. [DOI] [PubMed] [Google Scholar]


