Abstract
Objective.
Folic acid supplementation during the periconceptual period has been shown to improve cognitive outcomes in children of women with epilepsy taking antiseizure medications (ASMs). The dose of folic acid necessary to provide positive cognitive outcomes is unclear. In many countries including the United States, food is fortified with folic acid, but no data exists on how food fortification may affect cognition in children with fetal-ASM exposure. This study evaluates the effect of dietary folate from natural folates plus folic acid fortification, separate from folic acid vitamin supplements, on age-6 year IQ in children with fetal-ASM exposure.
Methods.
Data from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study was retrospectively analyzed for this investigation. Assessment of nutrient intake was conducted using the Block Food Frequency Questionnaire-98. The primary outcome of the present study was to assess association of maternal pre-pregnancy nutrient levels to child age-6 IQ.
Results.
Folate from food alone without supplement was not associated with improvement of age-6 IQ in children with fetal ASM exposure (95% CI: −11.7 – 2.3, p = 0.187). Periconceptual folate supplement use was associated with a 10.1 point higher age-6 IQ (95% CI: 5.2 – 15.0, p <.001). Total combined folate from food plus supplement also showed that higher intake of folate was associated with higher age-6 IQ (Coefficient: 4.5, 95% CI: 2.0 – 6.9, p <.001). Six other nutrients from food and supplements were analyzed (Vitamin C, Vitamin D, Vitamin E, Omega 3, Gamma Tocopherol, and Vitamin B12) and had no significant association with age 6-IQ.
Significance.
Dietary content of folate, even in a country where food is fortified with folic acid, is not sufficient to provide improved cognitive outcomes for children of women taking ASMs during pregnancy. Folate supplementation is needed for significant improvement in cognitive outcomes, specifically age-6 IQ.
Keywords: antiepileptic drugs, epilepsy, pregnancy, folate, diet
INTRODUCTION
Folate is a general term used to describe the many different forms of vitamin B9 including folic acid. However, folate is also used specifically for the natural form of the vitamin occurring in some foods. Folic acid is a synthetic version of folate, which is more heat-stable than natural food folate; thus, folic acid is better suited for food fortification and vitamin supplements. Both food folate and folic acid have to be metabolized into active folates.
The effects of maternal diet on child development have been a question of concern for parents and caretakers for centuries, and folic acid has been in the spotlight since the 1960s. Hibbard studied folic acid levels in 1,484 patients from 1961–1963 in Liverpool, England and found that folic acid deficiency was common and was associated with anemia, miscarriages and likely fetal malformations (1). The article concludes, “Folic Acid deficiency in pregnancy commonly results from dietary intake which is inadequat.” This work was continued in 1980 and 1981 with two studies looking at the effect of vitamin supplementation in women who had previous pregnancies with neural tube defects (2, 3).These studies found that supplementation decreased the incidence of recurrent neural tube defects, and folic acid was suggested as the key agent, but the studies were not carried out in a randomized controlled fashion. The Medical Research Council Vitamin Study recruited 1,817 women who had one prior birth with neural tube defects between the years of 1983 – 1991. It was a randomized, double-blind placebo-controlled study that placed patients in four groups: no vitamin supplementation, folate supplementation only, folate and other vitamin supplementation, and other vitamins only without folate. The other vitamins studied included vitamin A 4000 U, D 400 U, Bl 1–5 mg, B2 1.5 mg, B6 10 mg, C 40 mg, and nicotinamide 15 mg. Children of women in the folate only or folate and other vitamins group had 72% less neural tube defects than in their prior pregnancies. Women in the other vitamins only group did not have a significantly lower risk of having children with neural tube defects compared to their prior pregnancies (4). The effect of vitamin supplementation specifically on cognition has been studied in the general population. The Norwegian Mother and Child Cohort Study recruited pregnant women between 1999 and December 2008 and measured the effect of folic acid supplementation on language delay through the use of survey questionnaires completed up to 3 years of age. This study found that maternal use of folic acid in early pregnancy decreased risk of severe language delay in children at 3 years of age (5). The Markers of Autism Risk in Babies: Learning Early Signs (MARBLES) study found that maternal prenatal vitamin intake during the first month of pregnancy may reduce recurrence of autism spectrum disorder (ASD) in siblings of children with ASD (6). Although uncertainty exists, many other studies have also shown a positive effect of periconceptional folate on cognition in the general population (7–13). This work laid the foundation for an understanding that folic acid supplementation is essential in decreasing the incidence of fetal malformations. It also suggested that folic acid may allow for better cognitive outcomes for children of women in the general population.
Children of women with epilepsy who take antiseizure medications (ASMs) are at increased risk for both lower cognitive performance and for congenital malformations (14–16). The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study was a prospective, observational, assessor-blinded study that was conducted between 1999–2004 and included 305 mothers and 311 children. Women with epilepsy who were taking one of four ASMs (carbamazepine, lamotrigine, phenytoin, or valproate) were enrolled in the study. This investigation examined the effects of each ASM on child cognition, finding that fetal valproate exposure has a dose-dependent detrimental effect on children’s cognitive outcomes. The investigation also found a positive association between maternal periconceptual folate use and higher IQ in children at ages 3 and 6 years (14,15). Subsequently, studies from the Norwegian Mother and Child Cohort study in women with epilepsy supported the NEAD observations by showing that periconceptional folate reduced risks of language delay and autistic traits in children with fetal ASM exposure (17,18). A later analysis of the NEAD data further detailed the relationship between periconceptual folate supplementation and cognitive development of children across many domains (19). Higher performance at 6 years of age was seen with periconceptual folate use in the Expressive Language Index, Nonverbal Index, and NEPSY (Developmental Neuropsychological Assessment) Executive Function.
Although the beneficial effects of folate supplementation have become evident, the role of dietary folic acid on cognition for children of women with epilepsy has not yet been examined. Many countries, including the United States, fortify their foods with folate and other vitamins, and the role this plays in the cognition of children exposed fetally to ASMs is not yet clear. In the present analyses, the NEAD database was used to evaluate the effect of dietary folic acid and other vitamins on cognition at 6 years in children of women with epilepsy using ASMs.
METHODS
DESIGN
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study was a prospective, observational and assessor-blinded study that enrolled 311 women and 305 children between the years of 1999 and 2004. Twenty-five epilepsy centers in the United States and UK enrolled pregnant women with epilepsy who were taking one of four ASMs: valproate, lamotrigine, carbamazepine, and phenytoin.
STANDARD PROTOCOL APPROVALS, REGISTRATIONS, AND PATIENT CONSENTS
The NEAD study is registered as NCT00021866 at clinicaltrials.gov. A Data Safety Monitoring Board appointed by the NIH monitored study conduct. The Institutional Review Board at each of the 25 sites approved the study. In accordance with the Declaration of Helsinki, written informed consent was obtained from participants.
PARTICIPANTS
Because maternal IQ is a significant predictor of child IQ (20, 21), women with an IQ less than 70 were excluded from the study. Exposure to known teratogens (other than ASMs), poor compliance with ASMs, drug abuse in the prior year and its sequelae, progressive cerebral disease, and positive HIV or syphilis serology were other exclusion criteria.
PROCEDURES
Assessment of potentially confounding variables was completed, e.g., maternal age, IQ, education, race/ethnicity, seizure and epilepsy types and frequency, medical history, socioeconomic status, alcohol/tobacco or other drug use, complications in present or prior pregnancies, unwanted pregnancy, enrollment and birth gestational age, breastfeeding and birth weight among others. Detailed description of maternal and child screening including confounding factors is available in the original NEAD publication (15).
Cognitive outcome of children was evaluated at the ages of 2, 3, 4.5, and 6 years by assessors who were blinded to treatment. IQ assessments were conducted at 2 years of age using the Bayley scales of infant development (BSID) (22), and at 3, 4.5, and 6 years of age was done with the differential ability scales (DAS) (23). Additional cognitive and behavioral evaluations were conducted as detailed in our prior publications (14, 15). In the present analyses, we focus on IQ outcomes as they relate to nutritional factors.
Assessment of nutrient intake was conducted using the Block Food Frequency Questionnaire 98 (i.e., Block FFQ) (24–27) at the USA sites. The USA and UK sites began as separate studies with very similar protocols, and later the two studies merged. Nutrition questionnaires were not given to the UK women, so the present analyses are limited to the USA cohort. The dietary content of seven nutrients (Folate, Vitamin C, Vitamin D, Vitamin E, Omega 3, Gamma Tocopherol, and Vitamin B12) from food and from supplements was extracted from the Block FFQ questionnaires. At enrollment, a separate NEAD questionnaire specific to folate supplementation was given to the women in the study and quantified folate in 5 categories (i.e., 0mg/d, >0 to 0.4 mg/d, >0.4 mg/d to 1.0 mg/d, >1.0 mg/d to <4.0 mg/d, and ≥4.0 mg/d). This questionnaire (i.e., NEAD Folate Suppl) had direct inquiries on amounts of folate supplement intake rather than estimated amounts as in the Block FFQ, and the NEAD Folate Suppl was used in our prior publications (14, 15). Thus, we employed the NEAD Folate Suppl as the primary data for folate supplements in the present analyses.
Food nutrient levels were estimated from the Block FFQ administered to women after enrollment. Women were asked to estimate their food and supplement intake in the month prior to pregnancy. Supplement intake for Folate, Vitamin C, Vitamin D, and Vitamin E were dichotomized into ‘any use’ versus ‘no use’. Estimated daily intake for Folate, Vitamin C, Vitamin D, Vitamin E and Vitamin B12 was analyzed both as amount of nutrients from food as reported in the Block FFQ and amount of nutrients from food plus supplements calculated by adding together the amount from food and supplements. Additional nutrients analyzed included amount of nutrients from food for Folate, Gamma Tocopherol and Omega 3, and daily folate equivalents from food, naturally occurring folate from food, and fortified folate from food.
Periconceptual folate supplement use and dose were collected separately from the Block FFQ using the NEAD study’s data collection forms (i.e., NEAD Folate Suppl), as described above. Periconceptual folate was analyzed both as a dichotomous variable of ‘any use’ versus ‘no use’ and by adding the reported dose amount to the amount of nutrients from food from folate estimated from the Block FFQ. Since dose values were collected in ranges on the NEAD Folate Suppl, they were changed for purposes of the present analyses to a single value prior to combining with the amounts from food from the Block FFQ as follows: 0mg = 0mg, >0 to ≤0.4mg = 0.4mg, >0.4 to ≤1 = 0.9mg, >1 to <4 = 2.0mg, and ≥4 = 5.0mg. The ‘Single Value’ was selected based on knowledge of most-common folate supplement doses.
STATISTICAL ANALYSIS
For the primary analysis, we examined the association between maternal pre-pregnancy nutrient levels and age 6 IQ in the multiple imputation analysis population (i.e. all children with mothers who completed a Block FFQ and at least one IQ assessment at age 2, 3, 4.5 or 6). Multiple imputation was used to impute missing age 6 IQ scores. Sensitivity analyses were conducted using only children with an age 6 IQ score (age 6 IQ completers) without imputations for missing data. Another sensitivity analysis was run removing children whose mothers were on valproate.
Continuous nutrients and model covariates (maternal IQ, ASM standardized dose, daily calories, and pre-pregnancy BMI) were summarized by ASM type for all subjects using n, mean, standard deviation, median, minimum, and maximum. There were no missing data for any covariates. The dose of ASM was standardized relative to the ranges observed within each group according to the formula 100×(observed dose – minimum dose)÷range of doses. The proportion of children with mothers taking nutrient supplements were summarized using counts and percentages within each ASM and overall.
Prior to modeling, diagnostic plots were used to assess distributional assumptions. A log transformation was applied to all continuous nutrients due to the right skewness of the data. Data were visualized with partial regression plots constructed by plotting Age 6 IQ (Completers Only) against the log transformed nutrients adjusted for maternal IQ. Scatter plots without adjusting for maternal IQ were also constructed. Best fit regression lines by ASM were fit for each of the plots.
For the multiple imputation analysis population, Markov chain Monte Carlo methods (28, 29) were used to impute missing outcomes at 6 years of age from available IQ at 2 years, 3 years, and 4.5 years of age and baseline variables related to outcome or likelihood of missing data. The imputation procedure generated 50 imputed datasets and fit regression models to each. The following variables were used in the imputation model: age 2-year, 3 year, and 4.5 year IQ (BSID and DAS), all Folate, Vitamin C, Vitamin D, Vitamin E, Omega 3, and Gamma Tocopherol variables, maternal IQ, ASM type, ASM standardized dose, daily calories, pre-pregnancy BMI, gestational age, unwanted pregnancy, maternal age, convulsions during pregnancy, employment status, maternal education, and socioeconomic status.
Estimates of means and Least-Square Means (LS Means) and their standard errors (SE) were computed for age 6 IQ by ASM and overall. LS Means were adjusted for ASM type, ASM standardized dose, daily calories, and pre-pregnancy BMI. Standard errors of age 6 IQ and LS Means incorporated imputation uncertainty.
Linear regression models with the log transformed nutrient as the exposure and age 6 IQ as the outcome were used to assess the association between the continuous nutrients and IQ. ANOVA models with dichotomous supplement use as the exposure were used to assess the association between supplement use and age 6 IQ. Unadjusted models and ANCOVA models adjusting for maternal IQ, ASM type, ASM standardized dose, daily calories, and pre-pregnancy BMI were fit. Nutrient regression parameter estimates were combined across imputations with standard errors that incorporated imputation uncertainty. LS Means for ASMs were computed adjusting for the model covariates for every nutrient model. P-Values for the LS Means by ASM nutrients were adjusted for multiple comparison using Bonferroni correction. Because valproate may have confounded the association between the nutrient levels and IQ, a sensitivity analysis was done removing valproate.
A post-hoc analysis was conducted analyzing the effect of Vitamin B12. A multiple imputation procedure and modeling approach was conducted similar to that for the primary analysis, with the addition of Vitamin B12 as a covariate. Unadjusted and adjusted models were run to assess the association between Vitamin B12 and age 6 IQ. An adjusted model including the interaction between total folate and total Vitamin B12 was also run to determine if there was an interactive effect on age 6 IQ.
Another post-hoc analysis was conducted to determine if there were different nutrient dose effects of ASM on age 6 IQ. For nutrients significantly associated with age 6 IQ, unadjusted and adjusted models including the nutrient, ASM type, and their interaction were fit. If the interaction term was significant, the slopes by ASM were computed and p-values were constructed comparing the equality of the slope to the reference slope (Valproate). P-values were adjusted for multiple comparisons using Bonferroni correction.
A sensitivity was run on all analyses using only the age 6 IQ completers population.
Statistical significance was set at a two-sided p-value with a type I error rate of 0.05. Analyses were done at the NEAD Data and Statistical Center using SAS 9.4.
RESULTS
In the NEAD study, there were 205 children born in the US (5 sets of twins). The Block FFQ regarding nutrition prior to conception was only disseminated to US mothers, and 180 children had mothers who completed the questionnaire (87.8% of US children, 4 sets of twins). There were 117 children who had age 6 IQ data and whose mothers had completed the Block FFQ (57.1% of US children, 3 sets of twins), and 159 children had at least one IQ assessment at age 2, 3, 4.5 or 6 and mothers who completed the questionnaire (77.6% of US children, 4 sets of twins). The multiple imputation analysis used 159 children and the completers sensitivity analysis used 117 children. The study population is summarized in Table 1.
Table 1:
Analysis Population Summary
| N | Percent | |
|---|---|---|
| Children (USA only)a | 205 | 100.0% |
| Mother with Nutrition Survey | 180 | 87.8% |
| Age 6 IQ and Nutrition Survey | 117 | 57.1% |
| Age 6 IQ (Multiple Imputation)b and Nutrition Survey | 159 | 77.6% |
5 sets of twins in USA
Age 6 IQ score imputed using multiple imputation method for children with at least one IQ score at age 2, 3, 4.5, or 6. All subsequent tables use the imputed Age 6 IQ Score
The distribution of the 159 children across the four ASMs was 24 valproate, 42 carbamazepine, 37 phenytoin, and 56 lamotrigine. Valproate had the lowest mean age 6 IQ (93.5±4.2 SE) and lamotrigine had the highest (112.8±2.0 SE). In the adjusted analysis, total folate from food (log scale) was not associated with age 6 IQ outcomes (95% CI: −11.7 – 2.3, p = 0.187). Periconceptual folate supplement use was associated with a 10.1 point higher age 6 IQ in the adjusted analysis (95% CI: 5.2 – 15.0, p <.001). The adjusted model with the combined folate supplement dose added to folate from food (log scale) also showed that higher intake of folate (including supplemental folate) was associated with higher age 6 IQ (Coefficient: 4.5, 95% CI: 2.0 – 6.9, p <.001). The information from the above analyses is depicted in Table 2. None of the other nutrients were significantly associated with age 6 IQ in the adjusted analyses (see Table 3). The results were very similar in the sensitivity analysis removing children with mothers on valproate.
Table 2:
Association between Age 6 IQ and Folate, N = 159
| Unadjusted Analysis | Adjusted Analysisa | |||
|---|---|---|---|---|
| Coefficient (95% CI) | P-Value | Coefficient (95% CI) | P-Value | |
| Log Daily Folate Equivalents from food, mcg | −5.4 (−9.9, −0.9) | 0.019 | −4.9 (−10.8, 0.9) | 0.100 |
| Log Naturally Occurring Folate from food, mcg | −4.0 (−9.0, 1.1) | 0.123 | −1.7 (−8.2, 4.8) | 0.607 |
| Log Fortified Folate from food, mcg | −4.5 (−8.4, −0.5) | 0.026 | −3.6 (−8.3, 1.1) | 0.136 |
| Log Total Folate from food, mcg | −5.9 (−10.9, −0.9) | 0.021 | −4.7 (−11.7, 2.3) | 0.187 |
| Log Folate from food and supplements, mcg | 5.4 (2.7, 8.1) | <.001 | 4.5 (2.0, 6.9) | <.001 |
| Folate supplements, (Yes/No) | 13.3 (8.3, 18.3) | <.001 | 10.1 (5.2, 15.0) | <.001 |
Adjusted for ASM, Maternal IQ, ASM Standardized Dose, Pre-pregnancy BMI, and Daily Calories.
See methods for explanation of use of log calculations for food nutrients. Note that folate refers to natural folate and/or folic acid from fortification or supplements.
Table 3:
Association between Age 6 IQ and Other Vitamins, N = 159
| Unadjusted Analysis | Adjusted Analysisa | |||
|---|---|---|---|---|
| Coefficient (95% CI) | P-Value | Coefficient (95% CI) | P-Value | |
| Log Vitamin D from food, IU | −4.3 (−7.5, −1.0) | 0.010 | −2.7 (−5.9, 0.6) | 0.109 |
| Log Vitamin D from food and supplements, IU | −1.7 (−5.0, 1.6) | 0.322 | −1.4 (−4.4, 1.6) | 0.369 |
| Log Vitamin E from food, aTE | −2.6 (−7.8, 2.6) | 0.328 | 0.9 (−5.7, 7.4) | 0.795 |
| Log Vitamin E from food and supplements, aTE | 0.9 (−2.1, 3.9) | 0.566 | 0.7 (−2.1, 3.5) | 0.618 |
| Log Vitamin C from food, mg | −3.3 (−7.3, 0.8) | 0.117 | −1.3 (−5.5, 2.8) | 0.531 |
| Log Vitamin C from food and supplements, mg | −1.1 (−4.9, 2.7) | 0.574 | 0.2 (−3.4, 3.7) | 0.930 |
| Log Vitamin B12 from food, mcg | −5.4 (−10.1, −0.7) | 0.026 | −3.4 (−8.7, 2.0) | 0.222 |
| Log Vitamin B12 from food and supplements, mcg | −1.6 (−5.9, 2.7) | 0.457 | −1.4 (−5.3, 2.5) | 0.478 |
| Log Omega 3 from food, g | −1.0 (−6.2, 4.3) | 0.717 | 1.2 (−4.8, 7.3) | 0.688 |
| Log Gamma Tocopherol from food, mg | −1.4 (−5.8, 2.9) | 0.521 | 0.3 (−5.0, 5.7) | 0.902 |
| Vitamin D supplements, IU (Yes/No) | 3.4 (−2.1, 8.9) | 0.221 | 0.1 (−4.9, 5.1) | 0.979 |
| Vitamin E supplements, aTE (Yes/No) | 1.5 (−4.1, 7.1) | 0.592 | −0.5 (−5.6, 4.6) | 0.856 |
| Vitamin C supplements, mg (Yes/No) | 1.2 (−4.4, 6.8) | 0.679 | −1.1 (−6.2, 4.0) | 0.676 |
| Vitamin B12 Supplement, mcg (Yes/No) | 3.4 (−2.0, 8.8) | 0.219 | 0.2 (−4.7, 5.0) | 0.951 |
Adjusted for ASM, Maternal IQ, ASM Standardized Dose, Pre-pregnancy BMI, and Daily Calories.
See methods for explanation of use of log calculations for food nutrients.
Vitamin B12 from food, supplements, and total was examined post-hoc and had no significant effect on age 6 IQ (Table 4). There was no interactive effect between total Vitamin B12 and total folate.
Table 4:
Association between Age 6 IQ and Vitamin B12, N = 159
| Unadjusted Analysis | Adjusted Analysisa | |||
|---|---|---|---|---|
| Coefficient (95% CI) | P-Value | Coefficient (95% CI) | P-Value | |
| Log Vitamin B12 from food, mcg | −5.4 (−10.1, −0.7) | 0.026 | −3.4 (−8.7, 2.0) | 0.222 |
| Log Vitamin B12 from food and supplements, mcg | −1.6 (−5.9, 2.7) | 0.457 | −1.4 (−5.3, 2.5) | 0.478 |
| Vitamin B12 Supplement, mcg (Yes/No) | 3.4 (−2.0, 8.8) | 0.219 | 0.2 (−4.7, 5.0) | 0.951 |
Adjusted for ASM, Maternal IQ, ASM Standardized Dose, Pre-pregnancy BMI, and Daily Calories
See methods for explanation of use of log calculations for food nutrients.
In the partial regression plots adjusted for maternal IQ and in the unadjusted scatter plots, the association between the nutrient levels and age 6 IQ showed a negative slope for valproate compared with the other ASMs (Figure 1). After adjusting for multiple comparisons, the LS Mean IQ scores for Valproate were significantly lower than Phenytoin and Lamotrigine in the adjusted analysis for every nutrient. The effect of the combined folate supplement dose added to folate from food (log scale) on age 6 IQ stratified by ASM is depicted in Table 5. Lamotrigine had a significantly different dose effect compared to valproate (adjusted p = 0.032). The effect of folate supplements alone on age 6 IQ was not found to be significant different across the ASMs.
Figure 1. Folate from Food and Supplements (log scale, mcg) by Age 6 IQ.
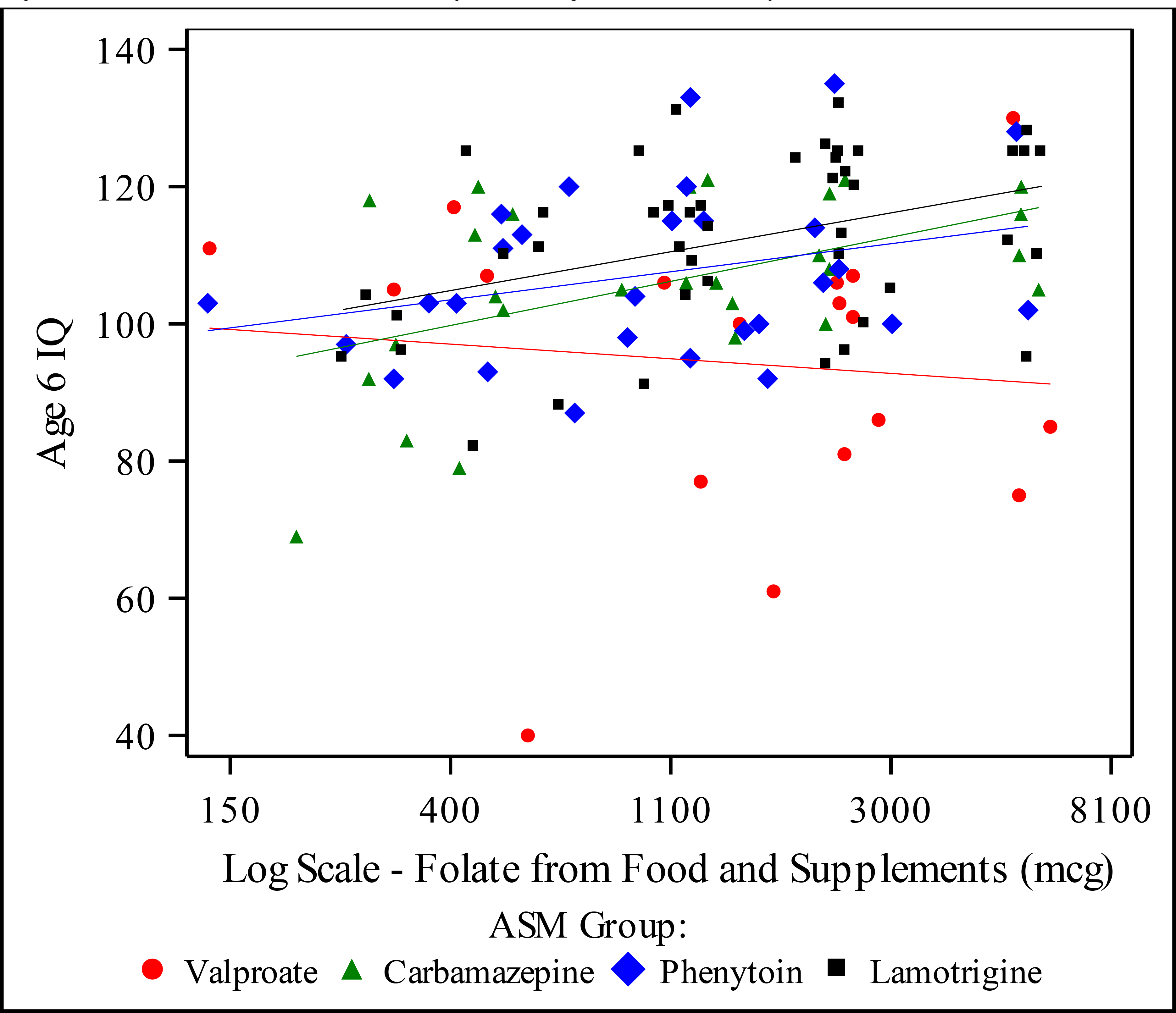
Figure depicts scatter plot with unadjusted regression lines by ASM. Includes 117 completers.
Table 5:
Adjusted Model - Slopes of Log Folate from Food and Supplements (mcg) by ASM
| ASM | Slope (95% CI) | P-Valuea | Adj. P-Valueb |
|---|---|---|---|
| Valproate | −1.9 (−7.2, 3.3) | Ref | Ref |
| Carbamazepine | 5.5 (0.9, 10.1) | 0.039 | 0.234 |
| Phenytoin | 4.9 (−0.1, 9.9) | 0.063 | 0.379 |
| Lamotrigine | 7.4 (3.3, 11.4) | 0.005 | 0.032 |
P-Value comparing equality of ASM slope to reference slope, Valproate
P-Value adjusted for multiple comparisons using Bonferonni correction.
All results were very similar in the sensitivity analysis using the age 6 IQ completers population. This analysis again showed that when folate supplements were added to folate from food, there was a significant effect on child IQ at age 6. The interactions between folate nutrient effect and each ASM were analyzed and depicted in Table 4. The effect of folate on IQ was analyzed for children taking each of four ASMs in the study, and this showed that folate has a significantly different dose-effect on lamotrigine compared to valproate.
DISCUSSION
In many countries including the United States, food is fortified with folic acid. The effect of dietary folate without supplements had not been previously examined in relation to cognitive effects on children of mothers with epilepsy taking ASMs. This study evaluated the effect of dietary folate (i.e., natural folate plus folic acid fortificatoin) without folic acid vitamin supplementation and found that it has no significant effect on age 6 IQ for this subset of children, even in the USA where food is fortified. In contrast, the addition of folic acid vitamin supplements has a significant positive effect on age 6 IQ for this at risk population. Six nutrients other than folate were also assessed and did not have effects on age 6 IQ ( Vitamin C, Vitamin D, Vitamin E, Vitamin B12, Omega 3, and Gamma Tocopherol).
The effects of maternal diet on child cognition is a topic of interest for the general population, and especially for women with epilepsy taking ASMs. Folate is a nutrient that has received a great deal of attention in this field, but the effects of dietary folate including folic acid food fortification on cognition in vulnerable populations, like children of women with epilepsy, have not been previously studied. Prior studies have shown that children of women with epilepsy who take ASMs are at increased risk for lower cognitive performance, behavioral problems, and congenital malformations (14,15). Several studies have also shown that folate taken as a supplement during the periconceptional period can improve cognitive and behavioral outcomes in children with fetal ASM exposures (14–18). The present study shows that dietary intake of periconceptual folate, even in a country with food fortified with folic acid like the United States, is not adequate to produce a positive effect on cognition in children with fetal ASM exposure. This study limited to our USA sample again shows that adding folic acid vitamin supplements during the periconceptual period improves cognitive outcomes in these children. In contrast, prior studies have not found that folic acid supplementations in women taking ASMs prevents major congenital malformations (30, 31). Nevertheless, our present findings provide further support for folic acid supplementation for women with epilepsy taking ASMs.
The optimal dose of folic acid supplementation necessary to allow a positive effect on IQ is not clear. Participants in the current study had a range of folic acid supplementation from 0mg to >4mg similar to our prior report with a larger cohort that showed significantly higher IQ for doses >0.4 mg/d compared to the no folate group (15). However, some studies in the general population have raised concerns that higher doses of folate supplementation might pose risks for behavioral problems (32). Since some ASMs interfere with folate metabolism, the folate dose for women with epilepsy may not be the same as the dose for the general population of women.
Strengths of this investigation include the prospective design of the original study, detailed monitoring and correction for potential confounding variables, and use of cognitive assessments with standardized measures and blinded assessors. Limitations of this study are retrospective analysis for the present findings on dietary folate, lack of randomization, no ASM-unexposed controls, loss to follow up, and a relatively small non-population-based sample. Adherence to folate dosing for participants cannot be confirmed because periconceptional folate use was assessed retrospectively at time of enrollment (mean 18 weeks), and no folate levels were obtained early in pregnancy. Although the large majority of literature on periconceptional folate has similar limitations (33), a prospective study assessing folate intake and levels for folate or homocysteine before and during early pregnancy would be preferable. Regardless, information on folate collection was completed in the first trimester before cognitive outcomes were known, making it less likely for misclassification due to errors in recollection about folate are associated with the outcome measures. Although our analyses controlled for baseline group differences, some residual confounding effects are possible due to inability to randomize pregnant women to antiseizure medications.
The findings of the current study underscore the importance of folic acid supplementation for women with epilepsy taking ASMs, supporting prior research in the field (14–21). The results of this investigation also point out that the folates (i.e., natural folate and folic acid) present in food, even in countries like the United States where food is fortified with folic acid, is not sufficient to produce positive cognitive effects in children of women with epilepsy taking antiseizure medications.
Highlights.
Fetal antiseizure medication (ASM) exposure impairs cognitive outcomes
Folate during periconceptual period improves outcomes in these children
Dietary folate even with fortified food is insufficient without supplementation
Folate supplementation is needed for significant improvement in cognition
Study funding:
This work was supported by the National Institutes of Health (NINDS, NICHD) [NS038455 to KJM] and NIH NINDS 2U01-NS038455 [KJM RM, PBP).
Author Disclosures: Dr. Sadat-Hossieny reports no disclosures. Ms. Roblino and Dr. May report no disclosures. Dr. Pennell has received research support from the National Institutes of Health and the Epilepsy Foundation, and honoraria and travel support from American Epilepsy Society, Epilepsy Foundation, National Institutes of Health, and academic institutions for CME lectures. Dr. Cohen receives royalties from The Psychological Corporation, Pearson as author of the Children’s Memory Scale. Dr. Loring has received support from the National Institutes of Health (2U01-NS038455), compensation for consulting services for NeuroPace and Supernus, and is an associate editor for Epilepsia, Critically Appraised Topics editor for The Clinical Neuropsychologist, and Editor-In-Chief for Neuropsychology Review. Mr. Block is the owner of Nutritionquest, which designed the questionnaire and generated the per-person nutrient and food group estimates used in the study. Mr. Swialto has no disclosures. Dr. Meador receives research support from the NIH NINDS and Eisai, serves on the editorial boards of Neurology, Epilepsy & Behavior, Epilepsy & Behavior Case Reports, and Genes & Diseases, has received travel support form Eisai, and has consulted for the Epilepsy Study Consortium that receives money from multiple pharmaceutical companies (in relation to his work for Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher Smith Laboratories, and UCB Pharma); the funds for consulting for the Epilepsy Study Consortium were paid to his university.
APPENDIX 1: NEAD Sites and Study Group.
NEAD Sites:
Arizona Health Sciences Center, Tucson, Arizona
Baylor Medical Center, Iving Texas
University of Texas-Southwestern, Dallas Texas
Western Reserve University, Cleveland, Ohio
Columbia University, New York City, NY
EMMES Corporation, Rockville, Maryland
Emory University, Atlanta, Georgia
Georgia Health Sciences University, Augusta, Georgia
Georgetown University, Washington, DC
Harvard - Brigham & Women’s, Boston, Massachusetts
Harvard - Massachusetts General, Boston, Massachusetts
Henry Ford Hospital, Detroit, Michigan
Medical College of Cornell University, New York City, New York
Minnesota Epilepsy Group, St. Paul, Minnesota
Ohio State University, Columbus, Ohio
Riddle Health Care, Media, Pennsylvania
Rush University Medical Center, Chicago, Illinois
St. Mary’s Hospital, Manchester, England
The Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, Missouri
University of Alabama, Birmingham, Alabama
University of Cincinnati, Cincinnati, Ohio
University of Kansas School of Medicine - Wichita, Wichita, Kansas
University of Liverpool, Liverpool, England
University of Manchester, Manchester, England
University of Miami, Miami, Florida
University of Southern California, Los Angeles, California
University of Utah, Salt Lake City, Utah
University of Washington, Seattle, Washington
Walton Centre for Neurology & Neurosurgery, Liverpool, England
Wake Forest University, Winston-Salem, North Carolina
NEAD Study Group:
Arizona Health Sciences Center, Tucson, Arizona: David Labiner MD Jennifer Moon PhD, Scott Sherman MD; Baylor Medical Center, Irving Texas: Deborah T. Combs Cantrell MD, University of Texas-Southwestern, Dallas Texas: Cheryl Silver PhD; Case Western Reserve University, Cleveland, Ohio: Monisha Goyal MD, Mike R. Schoenberg PhD; Columbia University, New York City, NY: Alison Pack MD, Christina Palmese, PhD, Joyce Echo, PhD; Emory University, Atlanta, Georgia: Kimford J. Meador MD, David Loring PhD, Page Pennell MD, Daniel Drane PhD, Eugene Moore BS, Megan Denham MAEd, Charles Epstein MD, Jennifer Gess PhD, Sandra Helmers MD, Thomas Henry MD; Georgia Health Sciences University, Augusta, Georgia: Gregory Lee PhD, Morris Cohen EdD; Georgetown University, Washington, DC: Gholam Motamedi MD, Erin Flax BS; Harvard- Brigham & Women’s, Boston, Massachusetts: Edward Bromfield MD, Katrina Boyer PhD, Barbara Dworetzky ScB, MD; Harvard – Massachusetts General, Boston, Massachusetts: Andrew Cole MD, Lucila Halperin BA, Sara Shavel-Jessop BA; Henry Ford Hospital, Detroit, Michigan: Gregory Barkley MD, Barbara Moir MS; Medical College of Cornell University, New York City, New York: Cynthia Harden MD, Tara Tamny-Young PhD; Minnesota Epilepsy Group, St. Paul, Minnesota: Patricia Penovich MD, Donna Minter EdD; Ohio State University, Columbus, Ohio: Layne Moore MD, Kathryn Murdock MA; Riddle Health Care, Media, Pennsylvania: Joyce Liporace MD, Kathryn Wilcox, BS; Rush University Medical Center, Chicago, Illinois: Andres Kanner MD, Michael N. Nelson PhD; The Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, Missouri: William Rosenfeld MD, Michelle Meyer MEd; St. Mary’s Hospital, Manchester, England: Jill Clayton-Smith MD, George Mawer MD, Usha Kini MD; University Alabama, Birmingham, Alabama: Roy Martin PhD; University of Cincinnati, Cincinnati, Ohio: Michael Privitera MD, Jennifer Bellman PsyD, David Ficker MD; University of Kansas School of Medicine - Wichita, Wichita, Kansas: Lyle Baade PhD, Kore Liow MD; University of Liverpool, Liverpool, England: Gus Baker PhD, Alison Gummery BSc, Rebekah Shallcross PhD; Rebecca Bromley PhD, University of Manchester, Jill Clayton-Smith MD, Maria Briggs RGN, George Mawer MD, Manchester, England; University of Miami, Miami, Florida: Eugene Ramsay MD, Patricia Arena PhD; University of Southern California, Los Angeles, California: Laura Kalayjian MD, Christianne Heck MD, Sonia Padilla PsyD; University of Washington, Seattle, Washington: John Miller MD, Gail Rosenbaum BA, Alan Wilensky MD; University of Utah, Salt Lake City, Utah: Tawnya Constantino MD, Julien Smith PhD; Walton Centre for Neurology & Neurosurgery, Liverpool, England: Naghme Adab, MD, Gisela Veling-Warnke MD; Wake Forest University, Winston-Salem, North Carolina: Maria Sam MD, Cormac O’Donovan MD, Cecile Naylor PhD, Shelli Nobles MS, Cesar Santos MD. Executive Committee: Dartmouth Medical School, Hanover, New Hampshire: Gregory L. Holmes MD; Stanford University, Stanford, California: Maurice Druzin MD, Martha Morrell MD, Lorene Nelson PhD; Texas A & M University Health Science Center, Houston, Texas: Richard Finnell PhD; University of Oregon, Portland, Oregon: Mark Yerby MD; University of Toronto, Toronto, Ontario: Khosrow Adeli PhD, Peter Wells PharmD. Data and Statistical Center: EMMES, Rockville, Maryland: Carrie Brown MS, Lisa Davis BA, Mark Friedman MS, Dominic Ippolito MS, Hayley Loblein PhD, Ryan May PhD, Chelsea Robalino MStat, Traci Scheer BA, Nilay Shah MD, Julia Skinner MS
REFERENCES
- 1.Hibbard BM. The role of folic acid in pregnancy with particular reference to anaemia, abruption and abortion. J Obstet Gynaecol Br Commonw 1964, 71: 529–42. [DOI] [PubMed] [Google Scholar]
- 2.Hibbard ED, Smithells RW. Folic acid metabolism and human embryopathy. Lancet 1965; i: 1254. [Google Scholar]
- 3.Smithells RW, Shephard S, Schorah CJ, et al. Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet 1980; i: 339–40. [DOI] [PubMed] [Google Scholar]
- 4.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991;338:131–7. [PubMed] [Google Scholar]
- 5.Roth C, Magnus P, Schjølberg S, et al. Folic Acid Supplements in Pregnancy and Severe Language Delay in Children. JAMA. 2011;306(14):1566–1573. doi: 10.1001/jama.2011.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt RJ, Iosif AM, et al. Association of Maternal Prenatal Vitamin Use With Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry. 2019. April 1;76(4):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Julvez J, Fortuny J, Mendez M, et al. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr Perinat Epidemiol 2009;23:199–206. [DOI] [PubMed] [Google Scholar]
- 8.Chatzi L, Papadopoulou E, Koutra K, et al. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: the mother-child cohort “Rhea” study in Crete, Greece. Public Health Nutr 2012;15:1728–1736. [DOI] [PubMed] [Google Scholar]
- 9.Villamor E, Rifas-Shiman SL, Gillman MW, Oken E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr Perinat Epidemiol 2012;26: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roza SJ, van Batenburg-Eddes T, Steegers EAP, et al. Maternal folic acid supplement use in early pregnancy and child behavioural problems: the Generation R Study. Br J Nutr 2010;103:445–452. [DOI] [PubMed] [Google Scholar]
- 11.Schlotz W, Jones A, Phillips DIW, et al. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J Child Psychol Psychiatry 2010;51:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehby GL, Murray JC. The effects of prenatal use of folic acid and other dietary supplements on early child development. Matern Child Health J 2008;12:180–187. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Sheng C, Xie RH, et al. New perspective on impact of folic acid supplementation during pregnancy on neurodevelopment. [DOI] [PMC free article] [PubMed]
- 14.Meador KJ, Baker GA, Browning N, et al. : Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009; 360: pp. 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meador KJ, Baker GA, Browning N et al. NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013. March;12(3):244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomson T, Battino D, Perucca E. Teratogenicity of antiepileptic drugs. Curr Opin Neurol. 2019. April;32(2):246–252. [DOI] [PubMed] [Google Scholar]
- 17.Husebye ESN, Gilhus NE, Riedel B, et al. Verbal abilities in children of mothers with epilepsy: association to maternal folate status. Neurology 2018;91:e811–e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjørk M, Riedel B, Spigset O, et al. Association of folic acid supplementation during pregnancy with the risk of autistic traits in children exposed to antiepileptic drugs in utero. JAMA Neurol 2018;75:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meador KJ, Pennell PB, May RC, et al. NEAD Investigator Group. Effects of periconceptional folate on cognition in children of women with epilepsy (NEAD study). Neurology. 2020;18;94(7):e729–e740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattler JM. Assessment of Children: Cognitive Foundations, 5th ed. San Diego: Jerome M. Sattler Publisher, Inc; 2008. [Google Scholar]
- 21.Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One 2015;10:e0131130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayley N: Bayley scales of infant development. San Antonio, TX: The Psychological Corporation, 1993. [Google Scholar]
- 23.Elliott CD: Differential abilities scales. San Antonio, TX: The Psychological Corporation, 1990. [Google Scholar]
- 24.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986; 124:453–469. [DOI] [PubMed] [Google Scholar]
- 25.Mares-Perlman JA, Klein BEK, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr 1993; 123:489–501. [DOI] [PubMed] [Google Scholar]
- 26.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990; 43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 27.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006. February;9(1):84–93. [DOI] [PubMed] [Google Scholar]
- 28.Li KH. Imputation using Markov chains. J Stat Comput Simul 1988; 30: 57–79. [Google Scholar]
- 29.Little RJA, Rubi n DB. Statistical analysis with missing data, 2nd edn. New York: John Wiley and Sons, 2002. [Google Scholar]
- 30.Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One 2015;10:e0131130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol 2018;17:530–538. [DOI] [PubMed] [Google Scholar]
- 32.Murray LK, Smith MJ, Jadavji NM. Maternal oversupplementation with folic acid and its impact on neurodevelopment of offspring. Nutr Rev 2018;76:708–721. [DOI] [PubMed] [Google Scholar]
- 33.De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015. December 14;(12):CD007950. [DOI] [PMC free article] [PubMed] [Google Scholar]


