Abstract
Purpose
This study aimed to characterize the tear film immunologic profile in keratoconus (KC) patients compared with healthy individuals (control group) and to investigate the correlation between the tear film immunologic profile and atopy, disease severity, and disease status over time.
Methods
The study involved 30 KC patients and 18 healthy individuals. Tear collection was obtained using microcapillary tubes. Tear film levels of fractalkine, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17A, IL-21, IL-23, interferon-inducible T-cell alpha chemoattractant (ITAC), macrophage inflammatory protein-1 alpha (MIP-1α), MIP-1β, MIP-3α, and tumor necrosis factor (TNF)-α were detected. Keratometric measurements and topographic patterns were used to diagnose and define disease progression. Tear immunologic profiles were compared, emphasizing the presence or absence of ocular allergy. Correlations between the cytokine profile, disease severity, and disease status were also analyzed longitudinally in the KC patients.
Results
Lacrimal cytokine concentrations were higher in the KC patients than they were in the controls in 14 of 21 cytokines analyzed. IL-6 was the most relevant cytokine found in KC patients, especially when associated with ocular allergy. There was no correlation between KC progression and the level of inflammatory cytokines when analyzed longitudinally. KC severity correlated with IL-6 concentration, where the more severe KC presented a higher IL-6 concentration in tears.
Conclusions
Inflammatory activity seems to be involved in the pathogenesis of KC. Out of 21 cytokines, 14 were more concentrated in the tears of KC patients than healthy subjects. IL-6 was significantly higher in KC patients’ tears and was related to disease severity. Disease progression did not correlate with cytokine levels when analyzed longitudinally.
Introduction
Keratoconus (KC) is a noninflammatory ectatic and asymmetric corneal disorder [1]. Epidemiologic data have reported worldwide variability in its prevalence, which might be related to ethnicity, geographic differences, and different diagnostic criteria used in epidemiologic investigations [2]. KC is characterized by corneal thinning, increased apical curvature of the cornea, and loss of spherical shape, resulting in irregular astigmatism, which impairs visual acuity and quality of life. Worse yet, the disease mainly affects young working-age people and can cause severe low vision [3,4]. A better understanding of the etiology and risk factors associated with its progression can reduce visual impairment and labor limitations.
KC has a multifactorial etiology and is not yet fully understood. Proinflammatory biochemical environments [5], altered structural morphology [6], and multiple genetic factors [7] likely all play a role. An interlinked association seems to exist among these factors, but the identification of the precipitating ones that ultimately result in clinical KC is still unclear. Ocular allergy has played an essential role in the etiology of KC in the past few years, and several studies have found a higher prevalence of allergic diseases in KC patients than in healthy subjects [8-15].
Currently, there is a growing interest in tear film evaluation to understand how proteins, lipids, and other molecules are affected in the context of systemic and eye diseases. The presence and concentration of cytokines and other inflammatory tear molecules have been investigated and associated with inflammatory activity in different conditions affecting the ocular surface, especially in dry eye [16-18], ocular allergy [19-21], and graft versus host disease [22-26]. Similar methodological studies, involving KC patients reported increased IL-6; epidermal growth factor; matrix metalloproteinase (MMP)-1, 3, 7, 9, and 13; and metalloproteinase-1 tissue inhibitor (TIMP-1). Other studies have reported lower concentrations of interferon (IFN)-γ, interleukin (IL)-4, IL-5, IL-6, IL-8 (C-X-C motif chemokine ligand 8 [CXCL8]), IL-12, IL-13, C-C motif chemokine ligand 5 [CCL5], and vascular endothelial growth factor [27-31]. Elevated tear levels of MMPs, IL-1, IL-6, and tumor necrosis factor-α (TNF-α); increased expression of proteolytic and lysosomal enzymes; and decreased concentrations of protease inhibitors may be related to corneal tissue damage in KC patients [32]. This study aimed to characterize and compare the tear immunologic profile of KC patients with a control group and to investigate the correlation between the tear film immunologic profile and ocular allergy, disease severity, and disease status (progression or stability) over time.
Methods
This prospective study was performed at Sorocaba Ophthalmological Hospital (HOS) and the Department of Ophthalmology and Visual Sciences, Federal University of São Paulo (UNIFESP), in collaboration with the Department of Immunology, University of São Paulo (USP). The UNIFESP Ethics Committee approved this study (672.479/2014), and our study adheres to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants. Over 4 years, 48 individuals (30 with KC and 18 controls) were included. Inclusion criteria were KC patients with regular follow-up at the Cornea Clinic at HOS. Exclusion criteria included hydrops, previous history of herpetic keratitis, collagen diseases, autoimmune diseases, other systemic diseases (e.g., diabetes mellitus), topical or systemic use of hormonal or nonhormonal anti-inflammatory drugs, use of rigid or soft contact lenses within 1 month before tear collection, and pregnancy and lactation in women.
Tear collection was performed without touching the ocular surface and previous instillation of any eye drops. Samples were collected by capillarity using 10 µl microcapillary tubes (Microcaps, Drummond Scientific Co., Broomall, PA), transferred to 1.5 ml Eppendorf tubes (Eppendorf, Fremont, CA), and stored at −80 °C until immunoassay analysis. Processing and analysis of tear samples were performed upon completion of the sample collection.
A Bio-Plex assay (Merck Millipore, St. Louis, MO) was used to detect fractalkine, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-17A, IL-21, IL-23, , interferon-inducible T-cell alpha chemoattractant (ITAC), macrophage inflammatory protein-1 alpha (MIP-1α), MIP-1β, MIP-3α, and TNF-α, following the manufacturer’s manual. Cytokine readings were obtained using a Luminex 200 (Luminex Corporation, Austin, TX), and quantitative data were obtained using Milliplex Analyst software (Merck Millipore). The Th1 to Th2 ratios, represented by the IFN-γ to IL-10 ratios, were obtained as respective representatives of T-helper cells. These ratios were used to compare KC and control groups and analyzed in the KC group over time.
Keratometric measurement defined disease severity and determined four stages of classification: mild (<45 diopters [D] in both meridians), moderate (between 45 D and 52 D in both meridians), advanced (between 52 D and 62 D in both meridians), and severe (>62 D in both meridians) [33]. KC progression was defined as an increase of at least 0.75 D in the apical keratometry (K) value of the corneal topography within 6–12 months. Regarding the presence of ocular allergy, the allergic component in patients with KC and controls was evaluated using a questionnaire based on the International Study of Asthma and Allergies in Childhood (ISAAC) model, which defined and characterized patients with ocular allergy [34].
KC patients were followed up after 12–18 months, and tear samples were analyzed at two time points (baseline: when patients were included in the study and at 12–18 months), allowing longitudinal analysis of cytokine concentrations associated with disease status (progression or stability). In the longitudinal arm of the study, to investigate the relationship between KC severity and cytokine concentration, KC patients were subdivided into the two following groups: KC with disease progression (n = 10) and KC without disease progression (n = 20). Tear immunologic profiles were compared between the groups, emphasizing the presence or absence of ocular allergy. Correlations between cytokine profile, disease severity, and disease status were analyzed longitudinally in the KC group.
The R version 3.3.2 statistical program was used for descriptive and inferential statistical analysis. Pearson’s chi-square test, the Mann–Whitney U test, or the Student t test was used for independent samples. The Kruskal–Wallis test or analysis of variance (ANOVA) with a fixed factor and Wilcoxon or Student t test was used for dependent samples. We also used ANOVA with two fixed factors (control or KC group; ocular allergy or not), as well as the Bonferroni comparison test, to compare IL-6 levels [11,13-15]. The alpha significance level equal to 5% was used in all conclusions obtained through inferential analyses.
Results
The study involved tear analyses of 48 individuals—18 (37.5%) in the control group and 30 (62.5%) in the KC group. The control group was mostly composed of female individuals (61.1%) with no ocular allergy (88.9%) and with an average age of 16.2 years (range, 10–21 years). The KC group was mostly composed of male individuals (60.0%) with ocular allergy (73.3%) and a mean age of 14.3 years (range, 11–17 years). The most frequent degrees of severity in the KC group were advanced (43.3%), moderate (30.0%), severe (23.3%), and mild (3.3%; Table 1). The control and KC groups presented the same profile regarding gender (p = 0.156), but the KC group had a younger population (p = 0.003) and a higher prevalence of ocular allergy (p<0.001).
Table 1. Distribution of control and keratoconus (KC) groups according to gender, age, presence of atopy, and disease severity.
| Variety | Control | KCd |
Total | P value | ||
|---|---|---|---|---|---|---|
| Gender |
Male |
7 |
18 |
|
25 |
0.156a |
|
|
Female |
11 |
12 |
|
23 |
|
|
|
Total |
18 |
30 |
|
48 |
|
| Age (years) |
Mean ± SDc |
16.2±3.2 |
14.3±1.9 |
|
15.0±2.6 |
0.003b |
| Atopy |
Yes |
2 |
22 |
|
24 |
<0.001a |
|
|
No |
16 |
8 |
|
24 |
|
|
|
Total |
18 |
30 |
|
48 |
|
| Severity (KC) |
Mild |
- |
1 |
3.3% |
|
|
|
|
Moderate |
- |
9 |
30% |
|
|
|
|
Advanced |
- |
13 |
43.3% |
|
|
|
|
Severe |
- |
7 |
23.3% |
|
|
| Total | 30 | 100% | ||||
aPearson’s chi-square; bMann–Whitney; cStandard deviation. dKeratoconus
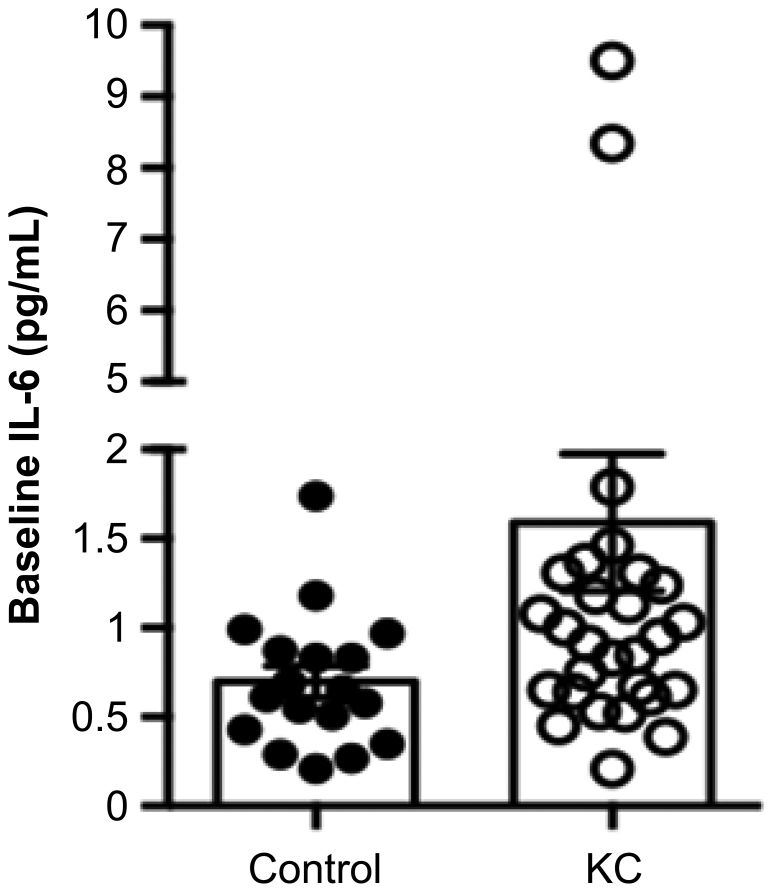
Twenty-one cytokines and the INF-γ to IL-10 ratio (representing the Th1/Th2 ratio) were measured at the first ophthalmic evaluation (baseline). The KC group presented higher concentrations in 14 of 21 cytokines than controls did. Both groups showed comparative concentrations of IL-2 (Table 2). IL-6 was significantly higher in the KC group (p = 0.012; Figure 1), and IL-5 also tended to differ in both groups (p = 0.06). The effect of ocular allergy on the IL-6 increase in the KC group (p = 0.003) is presented in Figure 2. The effect of ocular allergy on IL-6 levels was also analyzed using two fixed factors (KC and ocular allergy). In the control group, the presence or absence of ocular allergy did not interfere with the IL-6 level (p = 0.325), whereas in the KC group, the presence of ocular allergy demonstrated a tendency toward an increased IL-6 level (p = 0.08), albeit without statistical significance. The comparison among controls and KC patients with and without disease progression was an important object of investigation (Table 3). The three distinct groups had the same profile for gender but not age (p = 0.007) or ocular allergy (p<0.001). The KC without progression group had the same frequency of ocular allergy as the KC with progression group (p = 0.682). KC patients with and without progression had more cases of ocular allergy than controls did (p<0.001).
Table 2. Baseline cytokines concentration in control and KC group.
| Variety |
Control |
|
KCd |
|
Total |
|
P value |
|---|---|---|---|---|---|---|---|
|
|
(n=18) |
|
(n=30) |
|
(n=4) |
|
|
| Mean | SDa | Mean | SDa | mean | SDa | ||
| Cytokines (pg/ml) |
|
|
|
|
|
|
|
| itac |
89.76 |
50.51 |
108.36 |
129.88 |
101.38 |
106.84 |
0.544b |
| gm-csf |
0.76 |
0.33 |
1.13 |
0.94 |
0.99 |
0.79 |
0.241b |
| Fractalkine |
55.26 |
25.77 |
79.37 |
65.27 |
70.33 |
54.85 |
0.418b |
| ifn-Ɣ |
3.02 |
1.84 |
4.15 |
4.23 |
3.73 |
3.54 |
0.733b |
| il-10 |
1.43 |
1.23 |
2.08 |
2.3 |
1.84 |
1.97 |
0.462b |
| mip-3α |
24.11 |
12.2 |
22.75 |
14.92 |
23.26 |
13.85 |
0.746c |
| il-12p |
1.2 |
0.61 |
1.74 |
1.7 |
1.54 |
1.41 |
0.655b |
| il-13 |
4.04 |
1.9 |
5.91 |
4.93 |
5.21 |
4.14 |
0.343b |
| il-17 |
1.44 |
0.67 |
2 |
1.86 |
1.79 |
1.54 |
0.757b |
| il-1b |
0.69 |
0.3 |
0.84 |
0.68 |
0.79 |
0.57 |
0.949b |
| il-2 |
0.03 |
0.05 |
0.03 |
0.05 |
0.03 |
0.05 |
0.627b |
| il-21 |
0.65 |
0.71 |
0.96 |
0.97 |
0.85 |
0.88 |
0.213b |
| il-4 |
33.47 |
22.07 |
51.8 |
47.21 |
44.93 |
40.4 |
0.163b |
| il-23 |
41.82 |
29.36 |
59.29 |
71.35 |
52.73 |
59.38 |
0.873b |
| il-5 |
0.37 |
0.26 |
0.67 |
0.6 |
0.56 |
0.52 |
0.064b |
| il-6 |
0.7 |
0.38 |
1.59 |
2.11 |
1.26 |
1.73 |
0.012b |
| il-7 |
22.79 |
11.52 |
28.9 |
18.5 |
26.61 |
16.37 |
0.365b |
| il-8 |
19.02 |
20.7 |
17.17 |
18.88 |
17.86 |
19.38 |
0.701b |
| mip-1α |
6.35 |
4.89 |
8.61 |
11.28 |
7.76 |
9.4 |
0.890b |
| mip-1b |
6.64 |
2.87 |
7.73 |
8.27 |
7.32 |
6.74 |
0.233b |
| tnf-α |
0.12 |
0.07 |
0.16 |
0.15 |
0.15 |
0.12 |
0.798b |
| Ifn-Ɣ/il-10 | 2.73 | 1.02 | 2.45 | 1.01 | 2.56 | 1.01 | 0.375c |
aStandard deviation; bMann–Whitney; cStudent t test for independent samples; dKeratoconus
Figure 1.
IL-6 levels in KC and control groups at baseline (p = 0.012).
Figure 2.
IL-6 levels in KC and control groups according to ocular allergy at baseline (p = 0.003).
Table 3. Distribution of control and KC with and without progression groups according to gender, age, and presence of atopy.
| Variety |
Control |
KCd |
p value |
|
|---|---|---|---|---|
| Without progression | With progression | |||
| Gender |
|
|
|
|
| Male |
7 |
12 |
6 |
0.424a |
| Female |
11 |
8 |
4 |
|
| Total |
18 |
20 |
10 |
|
| Age (years) |
|
|
|
|
| Mean ± SDc |
16.2±3.2 |
14.6±2.1 |
13.9±1.4 |
0.007b |
| Atopy |
|
|
|
|
| Yes |
2 |
14 |
8 |
<0.001a |
| No |
16 |
6 |
2 |
|
| Total | 18 | 20 | 10 | |
aPearson’s chi-square; bKruskal–Wallis; cStandard deviation; dKeratoconus
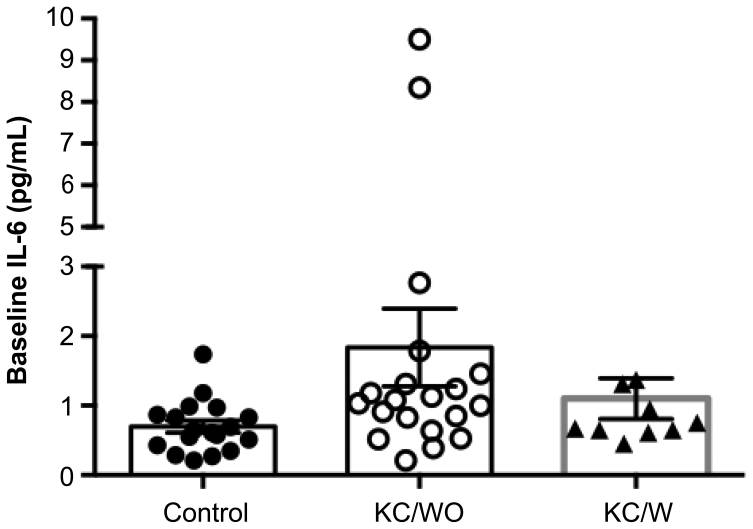
A comparison of 21 cytokines was also performed among the three groups at baseline. Only IL-6 showed a significant difference (p = 0.032; Figure 3).
Figure 3.
IL-6 levels between control and KC groups with and without progression at baseline (p = 0.032).
The correlation between disease severity and cytokine concentrations in the KC group indicated that disease severity correlated only with IL-6. Here, the higher the severity was, the higher the IL-6 tear concentration became (s = 0.391; p = 0.033; Figure 4).
Figure 4.
IL-6 levels according to KC severity (p = 0.033).
The Th1 to Th2 ratio, represented by the IFN-γ to IL-10 ratio, was used to compare the inflammatory profile between the KC and control groups at baseline, but it did not demonstrate any statistical difference between them (p = 0.375; Figure 5). This analysis was also performed in the KC subgroups over time and based on disease status (with and without progression), but again, there were no statistical differences between the KC subgroups (Table 2 and Table 4; Figure 6). Longitudinal IL-5 and IL-6 measurements in the KC subgroups indicated no statistically significant change between baseline and 12–18 months, which means that progression was not related to tear film cytokine levels (Figure 6).
Figure 5.
IFN-γ to IL-10 ratio in KC and control groups at baseline (p = 0.375).
Table 4. Th1 to Th2 (IFN-γ/IL-10) ratio in KC patients according to disease status over time (with and without progression).
| Cytokines | Baseline (mean ± SDa) | 12–18 months (mean ± SDa) | P value |
|---|---|---|---|
| Ifn-Ɣ/il10 |
2.47±1.08 (n=20) |
2.23±0.76 (n=20) |
0.370b |
| (KCc without progression) |
|
|
|
| Ifn-Ɣ/il10 |
2.42±0.93 (n=10) |
2.42±1.24 (n=10) |
0.959b |
| (KCc with progression) |
aStandard deviation; bWilcoxon; cKeratoconus
Figure 6.
IL-5, IL-6, and IFN-γ to IL-10 ratio levels in the KC group according to disease status (with or without progression) over time.
Discussion
This study investigated cytokines in the tear film of KC patients and healthy individuals to determine whether the altered inflammatory response is a factor contributing to its etiology. Tear analyses of 48 individuals were included as follows: 18 (37.5%) in the control group and 30 (62.5%) in the KC group. The control and KC groups presented the same gender profile (p = 0.156), but the KC group constituted a younger population (p = 0.003) and a higher prevalence of ocular allergy (p<0.001). KC was more prevalent in male than in female patients. In a systematic review and meta-analysis, Hashemi et al. reported that the prevalence of KC in the general population is estimated at 1.38 per 1,000 population (20.6 per 1,000 men and 18.33 per 1,000 women) [35]. In a nationwide study in South Korea, Hwang et al. found a similar prevalence in men and women [36]. In contrast, in another study, Hashemi et al. found a higher prevalence in women [37].
Different definitions of atopy in the ophthalmic literature may foster controversial results about its role or correlation with KC. There are several ways to classify allergies, and the need for standardization is vital for data collection. A questionnaire (face-to-face interview) based on ISAAC was used to evaluate and define an ocular allergic component in all patients [34]. Comparison between the control group and the KC group subdivided into two subgroups (with and without progression) revealed a difference in the prevalence of ocular allergy (p<0.001). The KC without progression group had the same frequency of ocular allergy as the KC with progression group (p = 0.682), which means that ocular allergy did not interfere in disease progression in our study. The KC with and without progression groups presented more cases of ocular allergy than controls did (p<0.001). In accordance with our findings, Millodot et al. [38] and Harrison et al. [39] also reported an association between atopy allergy and KC and considered it a risk factor. Therefore, dissociating ocular allergy from KC was complicated. In contrast, atopic disease, such as ocular allergy, is the most important cause of eye rubbing, which has been considered a significant risk factor for KC and has been intrinsically considered in its causal etiology pathway.
Disease severity was defined by keratometric measurements obtained by axial topographic maps (Orbscan®, Bausch & Lomb, Rochester, NY; Table 1). KC progression was defined as an increase of at least 0.75 D in the apical K value of the corneal topography within 6–12 months. We used similar criteria to define KC progression to those other researchers have used [40,41]. Great progress has been made in corneal imaging during the last decade, mostly focused on improving the sensitivity toward early diagnosis of KC and identifying disease progression. There is no doubt that steepening of the posterior corneal surface, changes in corneal thinning, and epithelial thickness mapping would add helpful information to identify disease progression. By the time this study was conducted, the apical K value of the corneal topography was considered the gold standard method to define disease progression [42-45]. Since there is no quantitative consensus on analyzing other parameters (posterior cornea and corneal thickness), anterior cornea K is still the most used parameter to define progression in recent literature. However, for some corneal specialists, reproducible and consistent quantitative data that define KC progression are lacking [46].
The etiopathogenesis of KC has not yet been fully elucidated. The role of cytokines, proteases, and oxidative stress has been a central theme because these mediators seem to degrade corneal tissue and stimulate ectasia progression [47,48]. At baseline measurement, KC patients presented higher tear cytokine levels in 14 of the 21 cytokines tested than controls did, which may indicate the presence of proinflammatory activity in the tear film of KC patients, as reported in several studies [27,47,49-51]. In our study, despite the global trend toward a higher concentration of proinflammatory molecules, the only significant difference observed was in IL-6.
A significant increase in IL-6 in KC patients’ tears has already been reported, and this finding has been reproduced in several studies, regardless of the methodology applied [49,50]. In addition, using an immune bead–based multiplex kit, Ionescu et al. reported significant expressions of IL-1β, IL-4, IL-6, IL-10, IFN-γ, and TNF-α in the tears of eyes of KC patients compared with control subjects [51]. Jun et al. analyzed lacrimal proteins and found elevated levels of IL-6 and reduction of IL-4, CCL5, IFN-γ, and TNF-α in KC compared with control tears [30]. Sorkhabe et al. found a significant increase in the levels of IL-1b, IL-6, and IFN-γ and a decrease in IL-10 in KC patients compared with the control group [52]. However, some controversies have been observed regarding the IL-6 level in tear film. Andrade et al. reported that tears, corneal cells, and tissues of KC patients had higher levels of MMP-9 and no significant differences in the tear concentrations of IL-6, IL-8, MMP-2, galectin 1 (Gal-1), and Gal-3 compared with controls [53]. These differences might be explained by factors related to the studied population (age, genetic, and geographic factors) and by the methodology of analysis employed. Interestingly, the IL-6 level correlated with disease severity (p = 0.033) in our study: The higher the severity was, the higher the IL-6 tear concentration became. Published data on KC severity and cytokine concentration in tear film is limited. Jun et al. analyzed cytokine levels, stratifying the KC group into mild to moderate and severe groups. No significant differences were evident when mild to moderate disease was compared with controls [30].
Regarding the effect of ocular allergy on IL-6 level, there was no interference of ocular allergy on IL-6 level in the control group (p = 0.325), but there is a tendency to increase on IL-6 level when ocular allergy is associated to KC (p = 0.08). Importantly, these analyses should be treated with caution because of the low frequency of ocular allergy in the control group and the sample size in the KC group. A larger study including individuals with ocular allergy and KC or excluding individuals with this association might help to clarify the interference of ocular allergy in tear cytokine levels in KC patients.
The Th1 to Th2 ratio, represented by the IFN-γ to IL-10 ratio, did not indicate a significant difference between KC and control groups or between KC subgroups. This ratio has been used to find any imbalance between proinflammatory and anti-inflammatory cytokines [54]. Because IL-6 is sometimes related to the Th2 pathway and can interfere in its direction via different mechanisms, we extrapolated to investigate whether there was any shift toward the Th2 profile in KC patients’ tears. Jun et al. already suggested that there may be a complex imbalance between proinflammatory and anti-inflammatory tear cytokines altering epithelial and stromal functions [30].
To the best of our knowledge, no other studies have evaluated the behavior of inflammatory molecules in KC patients longitudinally. This study allowed for the analysis of inflammatory cytokines over time in KC patients (baseline and 12–18 months). The KC group did not present significant changes in cytokine levels over time compared with baseline except for a sustainable elevated level of IL-6. This study has some limitations, such as sample size and the already known high variability in cytokine measurements using immune bead–based multiplex assays [55]. This study does not exclude the possibility of other inflammatory mediators involved in the pathophysiology of KC, and it does not identify the source or target of the cell or receptor activity of mediators measured in tears. Correlations reported from these results might be useful for further investigation of KC’s pathogenesis and progression associated with inflammatory activity.
Inflammatory activity seems to be involved in the pathogenesis of KC. Fourteen of 21 cytokines were more concentrated in the tears of KC patients than they were in healthy subjects. IL-6 was significantly higher in KC patients’ tears and was related to disease severity. Disease progression did not correlate with cytokine levels when analyzed longitudinally.
Acknowledgments
Conflicts of interest: The authors declare that they neither have any competing interests nor any commercial interests in the subject of the manuscript or in the entities discussed in this work. Funding: This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Grant numbers 2014/15720–1 and 2017/02564–7), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), financial code 001.
References
- 1.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Gokhale NS. Epidemiology of keratoconus. Indian J Ophthalmol. 2013;61:382–3. doi: 10.4103/0301-4738.116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krachmer JH. Eye rubbing can cause keratoconus. Cornea. 2004;23:539–40. doi: 10.1097/01.ico.0000137168.24883.3e. [DOI] [PubMed] [Google Scholar]
- 4.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 5.Engler C, Chakravarti S, Doyle J, Eberhart CG, Meng H, Stark WJ, Kelliher C, Jun AS. Transforming growth factor-beta signaling pathway activation in keratoconus. Am J Ophthalmol. 2011;151:752–9.e2. doi: 10.1016/j.ajo.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khawaja AP, Rojas Lopez KE, Hardcastle AJ, Hammond CJ, Liskova P, Davidson AE, Gore DM, Hafford Tear NJ, Pontikos N, Hayat S, Wareham N, Khaw KT, Tuft SJ, Foster PJ, Hysi PG. Genetic variants associated with corneal biomechanical properties and potentially conferring susceptibility to keratoconus in a genome-wide association study. JAMA Ophthalmol. 2019;137:1005–12. doi: 10.1001/jamaophthalmol.2019.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Rabinowitz YS, Rotter JI, Yang H. Genetic epidemiological study of keratoconus: evidence for major gene determination. Am J Med Genet. 2000;93:403–9. [PubMed] [Google Scholar]
- 8.Agrawal VB. Characteristics of keratoconus patients at a tertiary eye center in India. J Ophthalmic Vis Res. 2011;6:87–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834–6. doi: 10.1136/bjo.84.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cingu AK, Cinar Y, Turkcu FM, Sahin A, Ari S, Yuksel H, Sahin M, Caca I. Effects of vernal and allergic conjunctivitis on severity of keratoconus. Int J Ophthalmol. 2013;6:370–4. doi: 10.3980/j.issn.2222-3959.2013.03.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaya V, Karakaya M, Utine CA, Albayrak S, Oge OF, Yilmaz OF. Evaluation of the corneal topographic characteristics of keratoconus with orbscan II in patients with and without atopy. Cornea. 2007;26:945–8. doi: 10.1097/ICO.0b013e3180de1e04. [DOI] [PubMed] [Google Scholar]
- 12.Khor WB, Wei RH, Lim L, Chan CM, Tan DT. Keratoconus in Asians: demographics, clinical characteristics and visual function in a hospital-based population. Clin Experiment Ophthalmol. 2011;39:299–307. doi: 10.1111/j.1442-9071.2010.02458.x. [DOI] [PubMed] [Google Scholar]
- 13.Nemet AY, Vinker S, Bahar I, Kaiserman I. The association of keratoconus with immune disorders. Cornea. 2010;29:1261–4. doi: 10.1097/ICO.0b013e3181cb410b. [DOI] [PubMed] [Google Scholar]
- 14.Weed KH, MacEwen CJ, Giles T, Low J, McGhee CN. The Dundee University Scottish keratoconus study: demographics, corneal signs, associated diseases, and eye rubbing. Eye (Lond) 2008;22:534–41. doi: 10.1038/sj.eye.6702692. [DOI] [PubMed] [Google Scholar]
- 15.Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H, Gordon MO. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study. Invest Ophthalmol Vis Sci. 1998;39:2537–46. [PubMed] [Google Scholar]
- 16.Carracedo G, Blanco MS, Martin-Gil A, Zicheng W, Alvarez JC, Pintor J. Short-term effect of scleral lens on the dry eye biomarkers in keratoconus. Optom Vis Sci. 2016;93:150–7. doi: 10.1097/OPX.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, McDermott AM, Miller WL. Elevated nerve growth factor in dry eye associated with established contact lens wear. Eye Contact Lens. 2009;35:232–7. doi: 10.1097/ICL.0b013e3181b3e87f. [DOI] [PubMed] [Google Scholar]
- 18.McMonnies CW. Inflammation and keratoconus. Optom Vis Sci. 2015;92:e35–41. doi: 10.1097/OPX.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 19.Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323–36. doi: 10.1016/j.iac.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Pacharn P, Vichyanond P. Immunomodulators for conjunctivitis. Curr Opin Allergy Clin Immunol. 2013;13:550–7. doi: 10.1097/ACI.0b013e328364d86a. [DOI] [PubMed] [Google Scholar]
- 21.Shetty R, Sureka S, Kusumgar P, Sethu S, Sainani K. Allergen-specific exposure associated with high immunoglobulin E and eye rubbing predisposes to progression of keratoconus. Indian J Ophthalmol. 2017;65:399–402. doi: 10.4103/ijo.IJO_217_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Ghosh A, Goldman S, Slone V, van de Ven C, Suen Y, Murphy L, Sender L, Cairo M. Immunological reconstitution and correlation of circulating serum inflammatory mediators/cytokines with the incidence of acute graft-versus-host disease during the first 100 days following unrelated umbilical cord blood transplantation. Bone Marrow Transplant. 1999;24:535–44. doi: 10.1038/sj.bmt.1701921. [DOI] [PubMed] [Google Scholar]
- 23.Hill GR, Krenger W, Ferrara JL. The role of cytokines in acute graft-versus-host disease. Cytokines Cell Mol Ther. 1997;3:257–66. [PubMed] [Google Scholar]
- 24.Mohty M, Blaise D, Faucher C, Vey N, Bouabdallah R, Stoppa AM, Viret F, Gravis G, Olive D, Gaugler B. Inflammatory cytokines and acute graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2005;106:4407–11. doi: 10.1182/blood-2005-07-2919. [DOI] [PubMed] [Google Scholar]
- 25.Mohty M, Gaugler B. Inflammatory cytokines and dendritic cells in acute graft-versus-host disease after allogeneic stem cell transplantation. Cytokine Growth Factor Rev. 2008;19:53–63. doi: 10.1016/j.cytogfr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Toubai T, Tanaka J, Paczesny S, Shono Y, Reddy P, Imamura M. Role of cytokines in the pathophysiology of acute graft-versus-host disease (GVHD): are serum/plasma cytokines potential biomarkers for diagnosis of acute GVHD following allogeneic hematopoietic cell transplantation (Allo-HCT)? Curr Stem Cell Res Ther. 2012;7:229–39. doi: 10.2174/157488812799859856. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramanian SA, Mohan S, Pye DC, Willcox MD. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012;90:e303–9. doi: 10.1111/j.1755-3768.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 28.Carreño E, Enríquez-de-Salamanca A, Tesón M, García-Vázquez C, Stern ME, Whitcup SM, Calonge M. Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol. 2010;88:e250–8. doi: 10.1111/j.1755-3768.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- 29.Dionne K, Redfern RL, Nichols JJ, Nichols KK. Analysis of tear inflammatory mediators: A comparison between the microarray and Luminex methods. Mol Vis. 2016;22:177–88. [PMC free article] [PubMed] [Google Scholar]
- 30.Jun AS, Cope L, Speck C, Feng X, Lee S, Meng H, Hamad A, Chakravarti S. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One. 2011;6:e16437. doi: 10.1371/journal.pone.0016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shetty R, Ghosh A, Lim RR, Subramani M, Mihir K, Reshma AR, Ranganath A, Nagaraj S, Nuijts RM, Beuerman R, Shetty R, Das D, Chaurasia SS, Sinha-Roy A, Ghosh A. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci. 2015;56:738–50. doi: 10.1167/iovs.14-14831. [DOI] [PubMed] [Google Scholar]
- 32.Balasubramanian SA, Pye DC, Willcox MD. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin Exp Optom. 2013;96:214–8. doi: 10.1111/cxo.12038. [DOI] [PubMed] [Google Scholar]
- 33.Rathi VM, Mandathara PS, Dumpati S. Contact lens in keratoconus. Indian J Ophthalmol. 2013;61:410–5. doi: 10.4103/0301-4738.116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goulart DA, Tacla MA, Marback PM, Solé D, Paranhos A, Junior, Perez HB, de Freitas D, Sato EH. Artificial neural networks applied to study allergic conjunctivitis screening questionnaire. Arq Bras Oftalmol. 2006;69:707–13. doi: 10.1590/s0004-27492006000500017. [DOI] [PubMed] [Google Scholar]
- 35.Hashemi H, Heydarian S, Hooshmand E, Saatchi M, Yekta A, Aghamirsalim M, Valadkhan M, Mortazavi M, Hashemi A, Khabazkhoob M. The prevalence and risk factors for keratoconus: A systematic review and meta-analysis. Cornea. 2020;39:263–70. doi: 10.1097/ICO.0000000000002150. [DOI] [PubMed] [Google Scholar]
- 36.Hwang S, Lim DH, Chung TY. Prevalence and incidence of keratoconus in South Korea: A nationwide population-based study. Am J Ophthalmol. 2018;192:56–64. doi: 10.1016/j.ajo.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 37.Hashemi H, Beiranvand A, Khabazkhoob M, Asgari S, Emamian MH, Shariati M, Fotouhi A. Prevalence of keratoconus in a population-based study in Shahroud. Cornea. 2013;32:1441–5. doi: 10.1097/ICO.0b013e3182a0d014. [DOI] [PubMed] [Google Scholar]
- 38.Millodot M, Shneor E, Albou S, Atlani E, Gordon-Shaag A. Prevalence and associated factors of keratoconus in Jerusalem: a cross-sectional study. Ophthalmic Epidemiol. 2011;18:91–7. doi: 10.3109/09286586.2011.560747. [DOI] [PubMed] [Google Scholar]
- 39.Harrison RJ, Klouda PT, Easty DL, Manku M, Charles J, Stewart CM. Association between keratoconus and atopy. Br J Ophthalmol. 1989;73:816–22. doi: 10.1136/bjo.73.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric [corrected] corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28:753–8. doi: 10.3928/1081597X-20121011-01. [DOI] [PubMed] [Google Scholar]
- 41.Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41:41–6. doi: 10.1016/j.jcrs.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012;119:2425–33. doi: 10.1016/j.ophtha.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sykakis E, Karim R, Evans JR, Bunce C, Amissah-Arthur KN, Patwary S, McDonnell PJ, Hamada S. Corneal collagen cross-linking for treating keratoconus. Cochrane Database Syst Rev. 2015;(3):CD010621. doi: 10.1002/14651858.CD010621.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–93. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Recalde JI, Duran JA, Rodriguez-Agirretxe I, Soria J, Sanchez-Tena MA, Pereiro X, Suarez T, Acera A. Changes in tear biomarker levels in keratoconus after corneal collagen crosslinking. Mol Vis. 2019;25:12–21. [PMC free article] [PubMed] [Google Scholar]
- 46.Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R, Guell JL, Malecaze F, Nishida K, Sangwan VS. Group of panelists for the Global Delphi Panel of keratoconus and ectatic diseases. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34:359–69. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 47.Galvis V, Sherwin T, Tello A, Merayo J, Barrera R, Acera A. Keratoconus: an inflammatory disorder? Eye (Lond) 2015;29:843–59. doi: 10.1038/eye.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wisse RP, Kuiper JJ, Gans R, Imhof S, Radstake TR, Van der Lelij A. Cytokine expression in keratoconus and its corneal microenvironment: A systematic review. Ocul Surf. 2015;13:272–83. doi: 10.1016/j.jtos.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Lema I, Durán JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005;112:654–9. doi: 10.1016/j.ophtha.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 50.Lema I, Sobrino T, Durán JA, Brea D, Díez-Feijoo E. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol. 2009;93:820–4. doi: 10.1136/bjo.2008.144253. [DOI] [PubMed] [Google Scholar]
- 51.Ionescu IC, Corbu CG, Tanase C, Ionita G, Nicula C, Coviltir V, Potop V, Constantin M, Codrici E, Mihai S, Popescu ID, Enciu AM, Dascalescu D, Burcel M, Ciuluvica R, Voinea LM. Overexpression of tear inflammatory cytokines as additional finding in keratoconus patients and their first degree family members. Mediators Inflamm. 2018;2018:4285268. doi: 10.1155/2018/4285268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorkhabi R, Ghorbanihaghjo A, Taheri N, Ahoor MH. Tear film inflammatory mediators in patients with keratoconus. Int Ophthalmol. 2015;35:467–72. doi: 10.1007/s10792-014-9971-3. [DOI] [PubMed] [Google Scholar]
- 53.Andrade FEC, Covre JL, Ramos L, Hazarbassanov RM, Santos MSD, Campos M, Gomes JÁP, Gil CD. Evaluation of galectin-1 and galectin-3 as prospective biomarkers in keratoconus. Br J Ophthalmol. 2018;102:700–7. doi: 10.1136/bjophthalmol-2017-311495. [DOI] [PubMed] [Google Scholar]
- 54.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–6. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 55.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP. Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61:35–9. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]