Abstract
Background
Parents and family carers of children with complex needs experience a high level of pressure to meet children's needs while maintaining family functioning and, as a consequence, often experience reduced well‐being and elevated psychological distress. Peer support interventions are intended to improve parent and carer well‐being by enhancing the social support available to them. Support may be delivered via peer mentoring or through support groups (peer or facilitator led).
Peer support interventions are widely available, but the potential benefits and risks of such interventions are not well established.
Objectives
To assess the effects of peer support interventions (compared to usual care or alternate interventions) on psychological and psychosocial outcomes, including adverse outcomes, for parents and other family carers of children with complex needs in any setting.
Search methods
We searched the following resources.
• Cochrane Central Register of Controlled Trials (CENTRAL; latest issue: April 2014), in the Cochrane Library.
• MEDLINE (OvidSP) (1966 to 19 March 2014).
• Embase (OvidSP) (1974 to 18 March 2014).
• Journals@OVID (22 April 2014).
• PsycINFO (OvidSP) (1887 to 19 March 2014).
• BiblioMap (EPPI‐Centre, Health Promotion Research database) (22 April 2014).
• ProQuest Dissertations and Theses (26 May 2014).
• metaRegister of Controlled Trials (13 May 2014).
We conducted a search update of the following databases.
• MEDLINE (OvidSP) (2013 to 20 February 2018) (search overlapped to 2013).
• PsycINFO (ProQuest) (2013 to 20 February 2018).
• Embase (Elsevier) (2013 to 21 February 2018).
We handsearched the reference lists of included studies and four key journals (European Child and Adolescent Psychiatry: 31 March 2015; Journal of Autism and Developmental Disorders: 30 March 2015; Diabetes Educator: 7 April 2015; Journal of Intellectual Disability Research: 13 April 2015). We contacted key investigators and consulted key advocacy groups for advice on identifying unpublished data.
We ran updated searches on 14 August 2019 and on 25 May 2021. Studies identified in these searches as eligible for full‐text review are listed as "Studies awaiting classification" and will be assessed in a future update.
Selection criteria
Randomised and cluster randomised controlled trials (RCTs and cluster RCTs) and quasi‐RCTs were eligible for inclusion. Controlled before‐and‐after and interrupted time series studies were eligible for inclusion if they met criteria set by the Cochrane Effective Practice and Organisation of Care Review Group. The comparator could be usual care or an alternative intervention. The population eligible for inclusion consisted of parents and other family carers of children with any complex needs. We applied no restriction on setting.
Data collection and analysis
Inclusion decisions were made independently by two authors, with differences resolved by a third author. Extraction to data extraction templates was conducted independently by two authors and cross‐checked. Risk of bias assessments were made independently by two authors and were reported according to Cochrane guidelines. All measures of treatment effect were continuous and were analysed in Review Manager version 5.3. GRADE assessments were undertaken independently by two review authors, with differences resolved by discussion.
Main results
We included 22 studies (21 RCTs, 1 quasi‐RCT) of 2404 participants. Sixteen studies compared peer support to usual care; three studies compared peer support to an alternative intervention and to usual care but only data from the usual care arm contributed to results; and three studies compared peer support to an alternative intervention only.
We judged risk of bias as moderate to high across all studies, particularly for selection, performance, and detection bias.
Included studies contributed data to seven effect estimates compared to usual care: psychological distress (standardised mean difference (SMD) ‐0.10, 95% confidence interval (CI) ‐0.32 to 0.11; 8 studies, 864 participants), confidence and self‐efficacy (SMD 0.04, 95% CI ‐0.14 to 0.21; 8 studies, 542 participants), perception of coping (SMD ‐0.08, 95% CI ‐0.38 to 0.21; 3 studies, 293 participants), quality of life and life satisfaction (SMD 0.03, 95% CI ‐0.32 to 0.38; 2 studies, 143 participants), family functioning (SMD 0.15, 95% CI ‐0.09 to 0.38; 4 studies, 272 participants), perceived social support (SMD 0.31, 95% CI ‐0.15 to 0.77; 4 studies, 191 participants), and confidence and skill in navigating medical services (SMD 0.05, 95% CI ‐0.17 to 0.28; 4 studies, 304 participants). In comparisons to alternative interventions, one pooled effect estimate was possible: psychological distress (SMD 0.2, 95% CI ‐0.38 to 0.79; 2 studies, 95 participants). No studies reported on adverse outcomes.
All narratively synthesised data for psychological distress (compared to usual care ‐ 2 studies), family functioning (compared to usual care ‐ 1 study; compared to an alternative intervention ‐ 1 study), perceived social support (compared to usual care ‐ 2 studies), and self‐efficacy (compared to alternative interventions ‐ 1 study) were equivocal. Comparisons with usual care showed no difference between intervention and control groups (perceived social support), some effect over time for both groups but more effect for intervention (distress), or mixed effects for intervention (family function). Comparisons with alternative interventions showed no difference between the intervention of interest and the alternative. This may indicate similar effects to the intervention of interest or lack of effect of both, and we are uncertain which option is likely.
We found no clear evidence of effects of peer support interventions on any parent outcome, for any comparator; however, the certainty of evidence for each outcome was low to very low, and true effects may differ substantially from those reported here.
We found no evidence of adverse events such as mood contagion, negative group interactions, or worsened psychological health.
Qualitative data suggest that parents and carers value peer support interventions and appreciate emotional support.
Authors' conclusions
Parents and carers of children with complex needs perceive peer support interventions as valuable, but this review found no evidence of either benefit or harm. Currently, there is uncertainty about the effects of peer support interventions for parents and carers of children with complex needs. However, given the overall low to very low certainty of available evidence, our estimates showing no effects of interventions may very well change with further research of higher quality.
Plain language summary
Peer support interventions for parents and carers of children with complex needs
Review question
This review assessed whether peer support interventions improve outcomes for parents and others caring for children with a wide range of complex needs (such as chronic or severe acute illness, disability, or delayed development).
Background
Parents and other family carers who care for children with complex needs may experience increased distress and reduced well‐being. Peer support interventions are intended to assist people caring for children to find social support from others who understand their situation. Peer support can be provided in groups, which sometimes are led by a facilitator, or can occur when people are matched with individual parents who have experience caring for a child with a similar condition.
Study characteristics
We included research up to 21 February 2018. Randomised controlled trials (RCTs), cluster RCTs, quasi‐RCTs, controlled before‐and‐after studies, and interrupted time series studies were all eligible for inclusion. Studies were included if they measured distress, confidence, feelings of coping, quality of life, how families functioned, feelings of support, or confidence in dealing with services among parents or any other family carers. Children being cared for could have any condition (for example, chronic or severe acute illness, disability, any kind of delayed or atypical development).
Results
We found 22 studies of 2404 participants who were caring for children with a wide range of conditions. All studies were RCTs or quasi‐RCTs and compared peer support to usual care (comparison 1) or to another intervention (comparison 2). Peer support was delivered in hospitals and in the community. Although we found studies that evaluated effects of peer support on all outcomes in comparison 1, and several outcomes in comparison 2, we did not see any benefit from peer support compared to usual care or compared to another intervention. We found no studies that reported on adverse effects (such as stress from hearing others' stories or conflicts with group members). Feedback from parents and carers suggests that they value emotional support, validation of their experiences, and access to knowledge that they find in peer support groups. More information is needed about training and supervision of peer support leaders, and about whether many participants withdraw from groups (and if so, why).
The overall quality of evidence for each outcome was low to very low, and because of this, our certainty about these findings is low. This means that further research is likely to change these findings while making clearer the possible benefits or harms of peer support interventions.
Conclusion
At the moment, we are uncertain about whether peer support helps or harms parents and carers of children with complex needs.
Summary of findings
Background
Many studies have found that parents and other family carers of children with complex needs, such as disability, developmental delay or learning difficulties, or other chronic or complex conditions such as autism spectrum disorder, experience exceptional pressure to meet the emotional and physical needs of the affected child or children, while at the same time maintaining family functioning (Cheshire 2010; Lee 2007; McGuire 2004; Resch 2010; Strunk 2010). (In this review, we use 'carers' to refer to family carers only, not paid professional carers).
Parents (and carers in a parenting role) of children with complex needs often show poor results on markers of psychosocial well‐being such as quality of life and life satisfaction, and they show elevated levels of psychological distress such as depression, anxiety, or stress (Cheshire 2010; McGuire 2004; Resch 2010).
The daily caregiving activities and responsibilities of parents of children with complex needs can be more time‐consuming than parenting a typically developing child, and can be physically and emotionally demanding (McGuire 2004; Resch 2010). These demands on the parent’s/carer’s time and energy can reduce the resources available for other meaningful and health‐protective activities such as employment, social activities, exercise, and hobbies. Family and social relationships can be disrupted and parents (or carers) left feeling overwhelmed, isolated, and lacking support (McGuire 2004; Resch 2010; Strunk 2010).
Description of the condition
Families of children with complex needs report experiencing more stress than families of children who do not have complex needs, regardless of the child's particular condition (Tak 2002; Van Riper 1992).
Demands of caregiving
Parents caring for children with complex needs experience anxiety about their child's diagnosis and prognosis, and may experience short‐term emotional distress, loneliness, uncertainty, and symptoms of depression (Barlow 2006). Physical caregiving activities, supporting provision of therapy, and advocating for the child can prove extremely time‐consuming (McGuire 2004). Parents may have difficulty gaining access to the services and resources they need (Banach 2010).
Behavioural problems may cause stress for families regardless of the underlying condition. For example, in families where children have developmental delay, behavioural problems resulting from the delay were reported to be a greater contributor to increased parenting stress than the developmental delay itself (Baker 2002). Parents may lack confidence in dealing with behavioural issues and may experience difficulty finding or accessing support services (Twoy 2007).
Changes to family life
Reviews have found that chronic diseases in children interfere with daily family life, increasing parents' burden of care (Barlow 2006). Balancing the healthcare needs of the affected child against other family needs, with reduced time for other necessary or enjoyable activities, is a source of family stress (Banach 2010). In addition to stresses directly related to the child's condition, families of children with complex needs must adapt to new roles, adjust their lives to cope with the needs of the child, and accommodate increased strain on family resources. As well as managing the needs of the affected child and of any other children in the family, parents must cope with their own chronic stress and periodic family crises (Bourke‐Taylor 2010; Dellve 2006).
Social stigma and isolation
Families that include a child with a chronic illness are at increased risk of isolation from formal and informal social support mechanisms (Tak 2002). Challenging behaviours and those due to emotional or cognitive conditions, which are seen by others as 'odd', may make social outings difficult ‐ a problem exacerbated by lack of understanding of the underlying condition among members of the community (Twoy 2007). This means that parents may choose isolation over the frustrations of taking their child out in public (Tak 2002). Physical frailty of the child, which may limits the child's ability to travel beyond the home, may similarly restrict parents' ability to maintain social networks.
Parents often feel the need to assist family and friends in handling their feelings about their child's condition, and to educate them and others such as workmates and acquaintances about the condition (Dellve 2006). Parents may feel stigmatised, either through the direct actions and comments of others, or indirectly through their own attributions and anxieties about what others might be thinking. As a result, they may restrict social activities or may socialise only with other families whose children have a similar diagnosis. In some cases, families may be excluded by others from social gatherings (Gray 2002).
Positive outcomes for families
Although families of children with complex needs face a range of challenges, in recent decades the benefits and rewards of raising a child with challenging behaviours or complex needs have been increasingly recognised. Families report feeling an enhanced sense of meaning or purpose and personal growth, and their positive perceptions of their role may be as high or higher than that of families of typically developing children (Blacher 2007; Hastings 2002). Social‐cultural constraints (such as service inefficiencies, perceived stigma, financial hardship, and low levels of support) have been found to contribute more to the negative impact of raising a child with complex needs than demands strictly associated with raising the child (Green 2007; Mas 2016; McConnell 2014).
Description of the intervention
The intervention of interest is peer support in the form of networks or groups for parents and carers of children with complex needs. Peer support may encompass peer‐led or facilitator‐led interventions, where the focus is on fostering peer‐to‐peer interactions and increasing social support. Peer support can be provided one‐to‐one or in a group, and may be given face‐to‐face or may be technology‐assisted (e.g. conducted by telephone or internet).
The aims of peer support interventions vary. However, for the purposes of this systematic review, we were interested in interventions intended to enhance the social support (perceived and/or actual) available to participants and to improve the well‐being of parents and carers across a range of psychological and psychosocial indicators.
How the intervention might work
Peer support interventions are assumed to work by increasing the amount of social support available to parents and carers of children with complex needs and providing that support in a form that is most useful and acceptable to families. It has been suggested that families of children with complex needs who display similar levels of function to families of children without such needs do so because they had current or prior affiliation with a support group (together with time and resources to adjust to the diagnosis) (Van Riper 1992). The perceived availability of support may play as great a role in determining stress levels in affected families as the actual support provided (Duarte 2005).
Peer support interventions are intended to supplement parents' existing social networks and to reduce feelings of isolation and stigma by introducing individuals who otherwise might not meet to others who can appreciate and understand their experiences (Shilling 2013). Participants' circumstances should be similar but need not be identical (Dale 2008). For example, parents who are in the early stages of adjusting to a diagnosis may benefit from the expertise of parents who have been coping longer with a diagnosis; parents who have been living with their child's condition for some time benefit from feeling that their experiences have meaning for others and from taking on an expert role.
Given the complexity of the emotional experience of raising a child with complex needs, peer support may work not by decreasing the negative impact or difficulties that parents experience, but rather by enhancing or encouraging the development of a new sense of meaning and purpose, opportunities for growth, and positive appraisals of their child and their caring role (Green 2007; Hastings 2002; Mas 2016; McConnell 2014). Although social support is the primary goal, peer support interventions may additionally increase instrumental (tangible) support for parents by increasing access to local social and health services, and may improve parents' knowledge about and confidence in managing their child's illness and other family issues.
Benefits of social support
Social support, defined as a combination of emotional concern, instrumental (tangible) aid, information, and appraisal, may mediate the stress experienced by families of children with serious physical, emotional, or behavioural challenges by contributing to coping resources (Coppola 2013; Dunkel‐Schetter 1987; Lazarus 1987). Social support may be of benefit for those who provide it as well as for those who receive it (Ignaki 2017). Such support has been found to reduce stress, for example, among parents of children with severe learning difficulties (Quine 1991). Social integration and support protect against the potentially harmful effects of stressful family circumstances and have beneficial effects on well‐being, whether or not a person is currently under stress (Armstrong 2005).
Emotional support and hope
Reports from practitioners working with parents of children with complex needs reveal that these parents want emotional support (e.g. someone to listen and understand), want to know of others in a similar situation who are doing well, and want to hear stories from others that give them hope for the future and make them feel less alone (Kirk 2015; Santelli 1996).
Reduction of isolation and stigma
Stigma, whether experienced or feared, can lead parents to avoid contact with others. Combined with the time‐consuming care tasks undertaken by these parents, this may mean increased risk of isolation for the families of children with complex needs. Social support can be an effective buffer against isolation (Kerr 2000).
Incidental learning
As well as buffering against stress, social support can have a direct effect on parenting stress by increasing exposure to incidental learning opportunities and competence‐promoting social interactions. Parents can benefit from the experience and knowledge of their peers without taking part in overt training and information sessions. A general survey of interactions between socio‐economic status, positive and negative parenting behaviours, and child difficulties recommended interventions to strengthen parents' social relationships with the goals of reducing stress and creating opportunities for parents to learn from and affirm one another (McConnell 2011).
Advocacy and self‐efficacy
Social support has been linked with enhanced advocacy skills and confidence in parents of children with complex needs (Banach 2010).
Instrumental support
Instrumental (tangible) support that parents and carers value includes information about specific disabilities and caring for children with complex needs, as well as ways to find and gain access to services and community resources (Santelli 1996).
Reciprocity
Social support provided by peers is suggested to provide reciprocal benefits: those receiving support gain the advantages described above, and people providing support report enhanced quality of life and validation of their previous experiences (Santelli 1997; Schwartz 1999). It has been suggested that social support must be reciprocal (or the possibility of reciprocity must at least exist) to be maximally effective (Hogan 2002). A group of peers of similar status provide a plausible arena for this egalitarian give‐and‐take of mutual support, in contradistinction to the power imbalance that may exist between service provider and service recipient.
Why it is important to do this review
A large number of self‐help and peer support groups and programmes target parents and carers of children with complex needs (Canary 2008; Davies 2005; Hastings 2004; Law 2001). These groups aim to provide “social support, practical information, and a sense of shared purpose or advocacy” (King 2000, p. 226). It is widely believed that these groups and programmes improve parental well‐being through social support mechanisms and peer support provided through sharing of experiences, information, and understanding, and provision of adaptive and credible models of coping (Davies 2005; King 2000; Lee 2007; McGuire 2004). However, despite anecdotal reports that these benefits are derived from participation in such groups and programmes (Ainbinder 1998; Davies 2005; Hartman 1992; Law 2001), little research has been undertaken to investigate the outcomes of participation in these groups and programmes for the parent/carer and the family in general.
Social support networks are not always uniformly positive in effect (Ortega 2002). Peer support groups have the potential to damage self‐esteem by reinforcing parents' self‐image as a member of a stigmatised group, and social comparison can lead to negative affect (Hogan 2002); so it is important to find out if, when, and how peer support interventions help, what barriers might exist to people's access to peer support, and if negative effects are known.
Some reviews in this general area already exist. We identified seven Cochrane Reviews relevant to this topic, but all were concerned with peer support for adult participants who were directly experiencing a condition or were supporting another adult with a condition, rather than supporting a child with a condition; some assessed interventions for which peer support was one component. Doull 2005 and Doull 2004 are protocols only. They have been withdrawn and will not be proceeding. Dale 2008 assessed the effectiveness of peer support telephone calls in improving physical, psychological, and behavioural health outcomes among adults; Lavender 2013 investigated telephone support for women during pregnancy and six weeks postpartum; and Chamberlain 2017 investigated psychosocial interventions for smoking cessation among pregnant women. Treanor 2019 assessed psychosocial interventions designed to improve quality of life and other outcomes for caregivers of people living with cancer; some of those interventions included a peer support component. González‐Fraile 2021 assessed the effectiveness of psychoeducational interventions (including remotely delivered support interventions promoting interactions with peers) in preventing or reducing caregiver burden among family members of people with dementia.
Some interventions examined were similar to those considered in this review, although we are considering a potentially broader range of groups and settings. However, there is only limited overlap with our population ‐ adults, but adults considered in their role as carers of children ‐ and with our conditions and outcomes of interest, which are sequelae to caring for children with complex needs rather than conditions directly affecting adults.
We also identified two recent non‐Cochrane reviews that are relevant to this review. Niela‐Vilén 2014 conducted a review of internet‐based peer support for parents. This is a highly relevant type of intervention, but the population (any parents, not only parents of children with complex needs) is less relevant.
Shilling 2013 conducted an integrative review of parent‐to‐parent (mentoring) support interventions. Again, this is a relevant intervention type, and unlike the other reviews we identified was of interventions for parents of children with chronic disabling conditions. However, the current review considers a broader range of intervention types including face‐to‐face and online parent support groups.
Objectives
Primary
To assess the effects of peer support interventions (compared to usual care or alternative interventions) on a range of psychological and psychosocial outcomes, including adverse outcomes, for parents and other family carers of children with complex needs in any setting
Secondary
Given that caring and financial demands on these parents and carers are high, to collect and report data related to barriers to participation, as evaluated in any qualitative research on intervention effectiveness
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and cluster RCTs, controlled before‐and‐after (CBA) studies, and interrupted times series (ITS) studies were eligible for inclusion in this review. Quasi‐RCTs (trials in which random allocation was attempted, but a method of allocation that was not strictly random, such as alternation, day of the week, or date of birth, was used) were also accepted.
To be included in this review, CBA and ITS studies had to meet the Cochrane Effective Practice and Organisation of Care Review Group (EPOC) criteria (Ryan 2011). These specify, for CBA designs, at least two intervention sites and two control sites, comparable timing of measurements for control and intervention groups, and comparable key characteristics for control and intervention groups; and for ITS designs, a clearly specified intervention time point and at least three data collection points before and after that intervention.
We included studies with a broad range of control groups, including no‐treatment, wait list, and usual care controls. When a study compared peer support with an alternative intervention (with or without a usual care control), the study was included but could not be included in meta‐analyses with studies in which peer support was compared with a no‐treatment control.
We incorporated evidence from quantitative studies that also had a qualitative component (Noyes 2011b). We did not conduct a separate search for qualitative studies and therefore could neither include qualitative‐only studies nor conduct an exhaustive review of mixed‐methods studies.
Types of participants
Participants were parents and other family carers of children with complex needs (as reported in the included study) for whom complex needs include chronic or severe acute illness, disability, or delayed/atypical development or an enduring condition in the physical, psychological, developmental, or intellectual domain. 'Parents' could be encompassed in studies where participants were biological, adoptive, or foster parents; mothers only; fathers only; or both parents. 'Family carers' could include any adults acting in a parenting role, including grandparents. Studies in which participants were professional (paid) carers were excluded from this review.
'Children with complex needs' was defined in the broadest possible terms to include children with any acute or chronic medical or psychological condition with a relatively long‐lasting course or sequelae.
Children were defined as individuals aged 18 years or younger.
Types of interventions
The target intervention was provision of peer support through networks or groups. Peer support was defined in this review as the existence of a community of common interest where people gather (in person or virtually by telephone or computer) to share experiences, ask questions, provide emotional support, and gain self‐help (Eysenbach 2004; Iscoe 1985). This is consonant with definitions used in published Cochrane Reviews (e.g. Dale 2008; Kew 2016, with the additional stipulation that specific knowledge possessed by the peer group is concrete, pragmatic, and derived from personal experience rather than through formal training; and that the group consists of individuals who are perceived to be equal (Dale 2008). Peer support interventions can range from purely emergent, informal, and member‐driven approaches to those that are mandated, professionally driven, and formal (Doull 2005).
Peer support encompasses a continuum of interventions of various degrees of formality, all of which emphasise the role of personal experience in the provision of peer support. Interventions that utilise a formal or professional facilitator were included, provided the facilitator's role was to manage group interpersonal processes rather than solely to provide counselling or psychoeducation.
We included one‐to‐one mentor (also known as peer‐to‐peer) and group parent/carer support interventions and both face‐to‐face interventions and those that were technology‐assisted (i.e. conducted over the telephone or internet). This range of intervention types were classified into two categories: (1) support groups for parents and/or carers, with or without a facilitator, that were conducted online or face‐to‐face; and (2) mentor arrangements, in which a 'novice' parent or carer was matched with a more experienced parent or carer.
We included the following comparisons in this review.
Any peer support intervention delivered to parents or carers of children with complex needs versus control (no‐treatment, wait list, or usual care).
Any peer support intervention versus another psychosocial intervention.
We excluded studies if we judged that effects of the peer intervention could not be separated from those of other intervention components, or if peer support was a 'side effect' of participation in some other intervention. Thus, we excluded studies in which the primary focus was something other than developing and supporting peer networks (e.g. where professionals deliver an educative component or formal therapy) and in which improved peer networks were an incidental outcome.
If the peer support intervention was used as an active control for a trial evaluating a more intensive intervention (e.g. a non‐directive peer support group versus a psychoeducation or therapy group), we included the study; however, such studies could not be included in meta‐analyses unless there was also a no‐treatment control condition.
We included studies in which the client or the focus of the intervention is the child only if direct outcomes for parents and carers were measured. For example, interventions for which the child is the primary client (such as play groups and early intervention programmes) and in which parent peer support is an incidental assumed outcome were excluded from the review, unless this support and other parental outcomes were directly measured.
Types of outcome measures
Primary outcomes
The primary outcome of interest was the psychosocial well‐being of parents and carers, as measured by a range of psychological, psychosocial, and skills acquisition outcomes for participants.
As social support (the target of the intervention) and psychosocial well‐being (the primary intervention outcome) are somewhat nebulous concepts, we operationalised the primary outcome using constructs developed by the Cochrane Consumers and Communication Review Group (CHCP 2012).
We used the following parent outcome categories.
-
Psychological health outcomes.
Psychological distress.
Confidence and self‐efficacy.
Perception of coping.
-
Psychosocial outcomes.
Quality of life and life satisfaction.
Family functioning.
Perceived social support.
-
Skills acquisition outcomes.
Confidence and skill in navigating medical services.
When a study used both sub‐scales and full scales related to the same outcome (e.g. a full psychiatric distress scale and anxiety and depression sub‐scales from other measures), we followed advice from Cochrane Australia and used the full, and most general, scale in analyses. In one case in which a study used both depression and anxiety sub‐scales but no more general scale, we chose the sub‐scale that led to a more even distribution of depression versus anxiety sub‐scales across all studies.
-
Adverse outcomes.
Mood contagion.
Increased feelings of stigma from identifying with the group.
Negative group interactions.
Any decrease in psychological health on the measures listed above.
As the measures for adverse outcomes fit the same broad categories as those for beneficial outcomes, we adopted the strategy outlined in the Cochrane Handbook for Systematic Reviews of Interventions of assessing beneficial and adverse effects together by the same method, with common eligibility criteria for included studies (Higgins 2011a).
The Cochrane Handbook for Systematic Reviews of Interventions suggests grouping outcomes into short‐term, medium‐term, and long‐term, and taking no more than one of each from each study (Higgins 2011a). We have preferred longer‐ over shorter‐term outcomes when conducting meta‐analyses.
Secondary outcomes
Satisfaction with the intervention (when data were available)
Incidental learning/improved knowledge (when data were available)
Process factors
Process factors that may influence outcomes include the following.
Facilitators of and barriers to uptake of peer support interventions.
Participant and provider satisfaction or dissatisfaction with peer support interventions.
Search methods for identification of studies
Electronic searches
We searched the following databases and resources.
Cochrane Central Register of Controlled Trials (CENTRAL; latest issue: April 2014), in the Cochrane Library.
MEDLINE (OvidSP) (1966 to 19 March 2014).
Embase (OvidSP) (1974 to 18 March 2014).
Journals@OVID (22 April 2014).
PsycINFO (OvidSP) (1887 to 19 March 2014).
BiblioMap (EPPI‐Centre, Health Promotion Research database) (22 April 2014).
ProQuest Dissertations and Theses (26 May 2014).
metaRegister of Controlled Trials (13 May 2014).
We conducted a search update of the following databases.
MEDLINE (OvidSP) (2013 to 20 February 2018) (search overlapped to 2013 to ensure subsequent additions to 2013‐2014 were captured).
PsycINFO (ProQuest) (2013 to 20 February 2018).
Embase (Elsevier) (2013 to 21 February 2018).
Our search strategy was developed with the assistance of John Kis‐Rigo, Information Specialist at the Cochrane Consumers and Communication Review Group, and is presented in Appendices 1 through 6.
A further search update was conducted on 14 August 2019, and again on 25 May 2021, by Anne Parkhill of the Cochrane Consumers and Communication Review Group, using the existing search strategy.
The Cochrane Library (2015‐).
Embase Classic + Embase (2018‐).
MEDLINE (2018‐).
PsycINFO (2018‐).
We placed no restrictions on publication date, publication status, or language. We sought unpublished studies, and we translated abstracts of potentially relevant studies to determine suitability for inclusion.
Searching other resources
ClinicalTrials.gov (13 May 2014).
World Health Organization Clinical Trials Registry (13 May 2014).
SCOPUS (13 May 2014).
Evidence in Health and Social Care (15 May 2014).
New York Academy of Medicine (8 May 2014).
OpenGrey (15 May 2014).
We conducted handsearches of the reference lists of included studies and relevant journals to identify other potentially eligible studies.
European Child and Adolescent Psychiatry (31 March 2015).
Journal of Autism and Developmental Disorders (30 March 2015).
Diabetes Educator (7 April 2015).
Journal of Intellectual Disability Research (13 April 2015).
We consulted advocacy and support groups via our existing professional connections with disability support and early childhood agencies to request information on any studies they were aware of: Down Syndrome Victoria, the Cerebral Palsy League, Tresillian Family Care Centres, and Women's and Children's Health Network. We contacted key investigators identified through other searches for advice on identifying other unpublished data and studies.
Data collection and analysis
Selection of studies
Bibliographic details of all search results were consolidated and duplicate records removed using EndNote. These were exported to Excel and were rated for potential inclusion independently by two review authors (GS/AP and GS/VL) based on title and abstract review. Differences were resolved by a third review author (VL or AP). Decisions about inclusion and exclusion were recorded in Excel.
We retrieved full texts for all studies assessed as possibly relevant on the basis of title and abstract review. The same two review authors (GS/AP) assessed studies for inclusion using Criteria for considering studies for this review, with the third review author (VL) also assessing studies on which the first two review authors disagreed. If decisions still were not clear, differences were resolved by discussion amongst all three review authors. Any studies examined in full text but excluded are listed in the Characteristics of excluded studies table along with reasons for exclusion. When several papers were related to the same trial, the trial ‐ not the papers related to it ‐ was counted. The unit of reporting is the trial ‐ not the number of papers.
Data extraction and management
For included studies, two review authors (GS/AP) independently extracted data, using the data extraction template provided by the Cochrane Consumers and Communication Review Group for quantitative data related to intervention effectiveness (see Appendix 1) (Ryan 2011). Any qualitative data associated with included studies were recorded on a template adapted from the qualitative data extraction template used by Noyes and Popay (2007, cited in Noyes 2011a), with modifications to enable extraction of data related to the process outcomes described above (Types of outcome measures) (see Appendix 2).
When details were not included in the published study or were unclear, we attempted to contact study authors for further information (see Appendix 3 for details of contacts attempted).
Data extracted by one review author were cross‐checked and confirmed by another; any discrepancies were resolved by discussion. All data were pasted from the checked data extraction sheets directly into RevMan software (Review Manager 2014).
Assessment of risk of bias in included studies
We assessed and reported the risk of bias of included studies using the guidelines listed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), in keeping with advice provided by the Cochrane Consumers and Communication Review Group (Ryan 2011). These guidelines assess the following domains: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data (attrition), selective outcome reporting; and other sources of bias (e.g. pre‐existing significant differences in characteristics likely to affect parent outcomes; aspects of treatment as usual that may have been a confound; issues with agency recruitment). We judged each item as being at high, low, or unclear risk of bias, as set out in the data extraction template adapted by the Cochrane Consumers and Communication Review Group (Ryan 2011, adapted from Higgins 2011a).
RCTs were deemed to be at highest risk of bias if they were scored as being at high risk of bias in the sequence generation, allocation concealment, or incomplete outcome data domain. RCTs were assessed as at unclear risk of bias if they were rated as unclear in at least one of these three domains. Low risk of bias studies were defined as those receiving a low risk of bias rating in all three of the sequence generation, allocation concealment, and incomplete outcome data domains of the tool.
Quasi‐RCTs were rated as being at high risk of bias on the random sequence generation item of the 'Risk of bias' tool.
No CBA or ITS studies were identified as eligible for inclusion.
Two review authors (GS/AP) independently assessed the risk of bias of included studies, with any disagreements resolved by discussion and consensus. When necessary, we attempted to contact study authors for clarification of methods (see Appendix 3).
All studies meeting inclusion criteria were included in our data synthesis, regardless of the outcome of the 'Risk of bias' assessment. If future updates of this review include non‐randomised studies (such as CBA or ITS designs), we will assess risk of bias with regard to selection bias, performance bias, detection bias, attrition bias, and reporting bias, as suggested in Section 13.5.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Assessment of qualitative data
The qualitative data extraction template given as an example in Noyes 2011a was adapted for use in this review, with quality assessed for the domains of credibility, transferability, dependability, and confirmability.
Measures of treatment effect
All outcomes from studies included in meta‐analyses were continuous. We used final values scores in preference to change‐from‐baseline scores.
There was variability in the types of measures used to assess outcomes. For example, psychological distress was measured on the Psychiatric Symptom Index (total and sub‐scales) the Center for Epidemiologic Studies Depression Scale, the Parenting Stress Index, and the Profile of Mood States (short form). We calculated the standardised mean difference (SMD) and the 95% confidence interval (CI) for each outcome using mean, standard deviation, and numbers of people assessed in control and intervention groups via the inverse variance method in Review Manager 5 software (Section 9.4.3.2; Higgins 2011a; Review Manager 2014).
When standard deviations were not available, we calculated them from reported confidence intervals using the RevMan calculator.
Some outcomes were assessed on scales with differing directions (e.g. on the Caregiver Strain Questionnaire, a reduction in score indicates an improved outcome; on the Parent Coping Efficacy Scale, an increased score represents a better outcome). In these instances, the method outlined in Section 9.2.3.2 of Higgins 2011a was used to ensure that all scales had the same direction.
Qualitative data
When qualitative outcomes were reported in the studies included in this review, we performed a qualitative evidence synthesis to supplement our main quantitative data synthesis.
We extracted qualitative data on process factors affecting implementation, such as facilitators and barriers to participation in peer support interventions.
Unit of analysis issues
We considered the level at which randomisation occurred (e.g. individual, cluster, cross‐over) in included studies. We identified no cluster‐randomised trials for inclusion; therefore we did not need to perform corrections for inappropriate units of analysis.
Dealing with missing data
When data were missing, we attempted to contact study authors as described above. When missing data could not be obtained, we imputed these, following consultation with staff at Cochrane Australia, with appropriate adjustments to the standard error to account for added uncertainty in the results. Meta‐analyses did not include a significant quantity of imputed data: for Ireys 1996 we imputed standard deviations (SDs) from two other studies using the same scale; and for Preyde 2003, Flores 2009, and Swallow 2014, we calculated SDs using confidence intervals (CIs). Generally, when study data were insufficient for inclusion, the data were so incomplete as to make it impossible to include the study in the meta‐analysis. When a study was omitted from an outcome meta‐analysis due to lack of data, we have noted this in the narrative synthesis for that outcome.
Assessment of heterogeneity
When we considered studies similar enough, based on consideration of populations and intervention settings, to allow pooling of data using meta‐analysis, we assessed the degree of heterogeneity by visually inspecting forest plots and by examining the Chi² test for heterogeneity. We quantified heterogeneity by using the I² statistic. We considered an I² value of 50% or more to represent substantial levels of heterogeneity, but we also interpreted this value in light of the size and direction of effects and the strength of evidence for heterogeneity, based on the P value from the Chi² test (Higgins 2011a).
Assessment of reporting biases
We intended to assess the existence of reporting bias by testing for asymmetry of the funnel plot of intervention effect estimates against the standard error of intervention effect estimates. However, this was not appropriate, as there were no outcomes for which at least 10 studies were included in the meta‐analysis (Higgins 2011a; Sterne 2011).
Data synthesis
Although we noted some heterogeneity in participants, settings, and interventions, this was expected, and we considered that they were sufficiently similar to allow for meta‐analyses when data were available. For the one outcome (psychological distress) for which there were enough included studies to allow meta‐analysis by intervention type, we checked this, but it did not lead to different assessments of effectiveness by intervention type. Outcomes did differ across studies, and we present meta‐analyses and narrative syntheses separately by broad outcomes. As expected, our included studies were clinically heterogeneous, and we used a random‐effects model to calculate SMDs. We had intended to convert any outcome SMDs back to differences on a single, well‐understood scale, but given the lack of any intervention effects, this proved unnecessary.
We included RCT (including quasi‐RCT) studies in our meta‐analyses. No CBA or ITS studies were eligible for inclusion. As we noted substantial risk of bias for nearly all outcomes, we did not stratify meta‐analyses into low versus high or unclear risk of bias.
Results for studies included in the review but not suitable for meta‐analysis were presented in the narrative synthesis for the appropriate outcome. We used the summary of risk of bias of an outcome across studies to judge the robustness of this evidence (Cash‐Gibson 2012). We used tables of results to form a narrative assessment of the evidence, clustering studies by intervention type and setting. For each study, we reported the same elements of information in the same order (Section 11.7.2; Higgins 2011a).
We conducted statistical analyses by using the latest version of RevMan software (Review Manager 2014).
Synthesis of qualitative data
A limited quantity of qualitative data were available for review; we followed advice from the Cochrane Consumers and Communication Review Group ‐ Ryan 2016 ‐ and the Economic and Social Research Council (ESRC) Methods Programme ‐ Popay 2006 ‐ in synthesising these data.
Subgroup analysis and investigation of heterogeneity
As discussed in the Background, subgroups of the population may differ in their capacity to benefit from the intervention, and intervention mode and setting may influence outcomes for different subgroups. We had intended to investigate the effects of variables such as existing social connectedness of participants, as well as delivery mode, setting, duration, and size of effects of interventions on outcomes. However, data were insufficient for subgroup analyses to be appropriate. Heterogeneity of data was taken into account when the certainty of evidence for each outcome was judged.
Sensitivity analysis
It was possible to use only SMDs for continuous outcomes due to the wide range of outcome measures used, and we found no dichotomous outcome measures for which it was necessary to make any decisions regarding types of ratios to be used. Therefore it was not appropriate to conduct sensitivity analyses for these variables. When we needed to impute data, we checked the effects of differing assumptions on our analyses and found that none were discernible.
Summary of findings and assessment of the certainty of the evidence
'Summary of findings' tables were based on the methods described in Schünemann 2011. We assessed the quality of evidence using the GRADE system ‐ Schünemann 2011 ‐ and GRADEpro software (www.guidelinedevelopment.org). Two review authors independently assessed the certainty of evidence for each outcome; when GRADE scores differed for an outcome, we discussed how we had applied the relevant criterion and come to a consensus score.
Consumer participation
The review authors have strong links with early childhood and disability advocacy agencies in Australia. We sent our contacts in these agencies drafts of the protocol and review and sought their comments, especially on recommendations regarding consumer‐important outcomes reported in the 'Summary of findings'. We used our connections with local consumer agencies to seek input from consumers overseas. Both the protocol and the review received input from a consumer as part of standard Cochrane Consumers and Communication Review Group editorial processes.
Results
Description of studies
Results of the search
We identified 95,732 records from electronic database searches and 5084 records through other sources (see flow chart of study selection in Figure 1). After removing duplicates, we screened 55,961 records by title and abstract. We excluded 55,773 records at this stage. We obtained 182 records in full text, excluding 160 records. Reasons for exclusion of key excluded studies are detailed in the Characteristics of excluded studies table. A summary of reasons (chiefly methodological) for excluding other studies is provided in Table 3. Six studies are awaiting classification. These were identified through recent updates to the searches, or they are papers for which we have not been able to obtain full text copies (details are in Studies awaiting classification). We approached authors of 9 subsequently excluded and 10 subsequently included studies for further information (see Appendix 3). One author of an included study could not be contacted. Authors of 13 studies provided further details, and authors of 6 studies did not respond.
1.
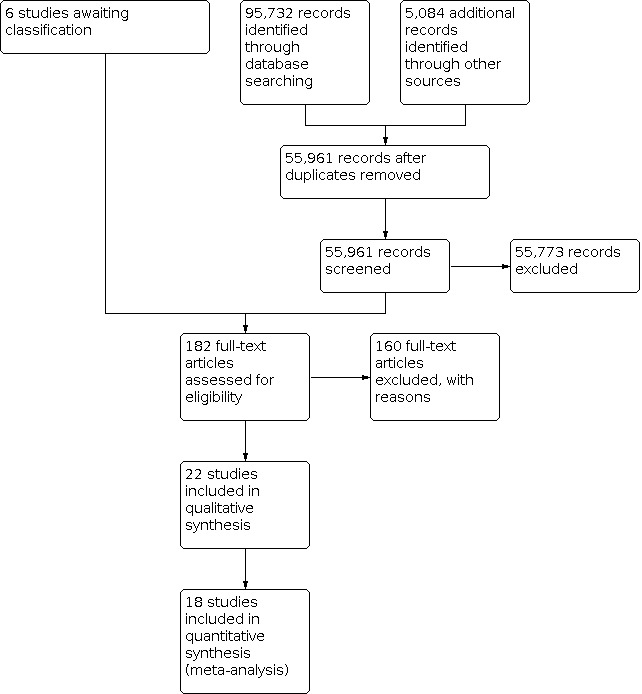
Study flow diagram.
1. Summary of reasons for excluding studies.
| Reason for exclusion | N |
| Intervention was not focused on parents/caregivers | 2 |
| Participants were carers of adults or the elderly | 6 |
| Participants were carers of a child without complex needs | 2 |
| Intervention was not peer support | 30 |
| Effects of the intervention were not able to be separated from other intervention components | 6 |
| Intervention outcomes not focused on parent/caregiver | 2 |
| Intervention outcomes not focused on psychological, psychosocial, or skills acquisition domains | 2 |
| Study design did not meet criteria for RCT, quasi‐RCT, CBA, or ITS | 50 |
| Study design was not an evaluation (e.g. descriptive, case study) | 26 |
| Qualitative data only | 3 |
| Review | 5 |
| Duplicates only identified at full text stage | 10 |
| TOTAL (does not include 16 studies described separately) | 144 |
Included studies
Twenty‐two studies including 2404 participants met selection criteria for inclusion in this review. Of these, 16 compared peer support interventions with a usual care control (Aiello 2015; Boogerd 2017; Boylan 2013; Flores 2009; Ireys 1996; Ireys 2001; Kutash 2011; Kutash 2013; McCallion 2004; Preyde 2003; Ruffolo 2005; Silver 1997; Singer 1999; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010; Swallow 2014). Three other studies compared peer support with an alternative intervention, as well as a usual care control (Roberts 2011; Scharer 2009; Wysocki 2008). In meta‐analyses of these studies, only peer support and usual care control findings were included. Three studies compared peer support with an alternative intervention only (Ferrin 2014; Rhodes 2008; Singer 1994). These studies could not be included in meta‐analyses but are part of our narrative synthesis. Additionally, Aiello 2015 did not include data in a format suitable for inclusion in a meta‐analysis.
Detailed information from all included studies is provided in the Characteristics of included studies tables. Key information is summarised below, and interventions evaluated by included studies are summarised in Table 4.
2. Characteristics of interventions.
| ID | Intervention type | Intervention details | Design | Population |
| Aiello 2015 | Parent groups | Asynchronous online tools used to share text messages, photos, and videos, and participate in discussion forums. Moderated by Speech Language Pathologists and Psychologist who proposed topics and answered questions but did not interfere in direct interactions between facilitators | RCT | Mothers of children with severe and profound bilateral sensorineural hearing loss |
| Boogerd 2017 | Parent groups | Secure web‐based portal, one for each participating clinic. Peer support facilitated through chat‐application and forum. Site also provides for one‐to‐one communication with health professionals and downloadable information. Forum is moderated by nurse practitioners. Free access for the duration of the evaluation |
RCT | Parents of children with type 1 diabetes. |
| Boylan 2013 | Parent groups | Ninety minute sessions held weekly for eight weeks. Small and large group discussions of relevant issues. Aim was to provide social support and improve communication and problem‐solving skills. | RCT | Parents and carers of young people with deliberate self‐harm/suicidal behaviour. |
| Ferrin 2014 | Parent groups | Groups of eight‐ten families met for 12 weekly 90 minute sessions. Families could share thoughts and experiences in a safe, non‐directive environment. Therapist present but precluded from providing feedback, psycho‐education, information or advice. Control also included 12 weekly, 90 minutes sessions. Psycho‐education was provided for the first 9 sessions. The last 3 sessions included behavioural strategies for managing ADHD symptoms and reducing defiant behaviour. There was also some opportunity for group discussion and support. |
RCT Comparator is psycho‐education |
Parents of children and adolescents (5‐18) with attention deficit hyperactivity disorder. |
| Flores 2009 | Parent mentors | Home visit from mentor within three days of child’s emergency department visit or hospitalisation. Then monthly meetings with assigned families at community centre. (Thus, there was a strong support group element as well). Numbers attending meetings varied. | RCT | African‐American and Latino parents of children (2‐18) with asthma. |
| Ireys 1996 | Parent mentors | Each parent mentor had child (now aged 18‐24) with JRA and was matched with five families of children with JRA. Over 15 months, mentors had fortnightly telephone contact and six‐weekly face‐to‐face meetings with mothers. They also hosted occasional group events. Thirty hours’ training for mentors. | RCT | Mothers of children (2‐11) with juvenile rheumatoid arthritis. |
| Ireys 2001 | Parent mentors | Parents of child with chronic illness linked with veteran “Network Mother” who’s child with chronic illness was then in young adulthood. Over 15 months, mentors had fortnightly telephone contact and seven face‐to‐face meetings with mothers. They also hosted occasional group events. Thirty hours’ training for mentors. | RCT | Mothers of children (7‐11) with chronic illnesses. |
| Kutash 2011 | Parent mentor | School‐based peer‐to‐peer. Veteran parents telephoned participants once per week during school year for support. Veteran parents had 30 hours’ training in communication skills, active listening, reframing, empowerment, boundary issues, ED topics, confidentiality. Weekly group supervision with clinically trained staff member. | RCT | Parents of middle school youth in special education programs for emotional disturbance. |
| Kutash 2013 | Parent mentor | School‐based peer‐to‐peer. Veteran parents telephoned participants once per week during school year for support. Veteran parents had 30 hours’ training in communication skills, active listening, reframing, empowerment, boundary issues, ED topics, confidentiality. Weekly group supervision with clinically trained staff member. | RCT | Parents of middle school youth in special education programs for emotional disturbance. |
| McCallion 2004 | Parent groups | Facilitated support groups; 8‐10 grandparent caregivers attended six fortnightly sessions of 90 minutes. Respite care and transport assistance provided. In addition to educational topics, sessions covered self‐care such as stress reduction, relaxation, nutrition, and own health needs. Led by community agency workers, trained and supervised by first author. Also included active case management. | RCT | Grandparents with primary care of at least one grandchild (mean age 11, 5 aged >21) with an intellectual or other developmental disability, learning problem or attention deficit and hyperactivity disorder. |
| Preyde 2003 | Parent mentors | Parents invited to connect with parent buddy (experienced with the NICU) within one week of birth. Support delivered primarily via telephone. Very few details provided. | Quasi‐RCT | Mothers of very pre‐term infants in a neonatal intensive care unit. |
| Rhodes 2008 | Parent group | Ten weekly group sessions of 90 minutes. Facilitated by a counsellor, who took an active role only to answer questions the group could not answer themselves and to encourage/regulate participation. | CBA | Parents of children with an intellectual disability. |
| Roberts 2011 | Parent groups | Manualised, weekly meetings with other parents to discuss topics based on individual interests and needs. Duration uncertain, but child playgroups ran for two hours, for 40 weeks, and it is stated that parent groups were ‘concurrent’. Delivered by transdisciplinary team of teachers, speech pathologists, occupational therapists, psychologists—but not clear if this was for child, parent, or both groups. | RCT | Parents of preschool children with a diagnosis of autism spectrum disorder who were also eligible for a centre‐based manualized playgroup intervention. |
| Ruffolo 2005 | Parent groups | Highly‐structured problem‐solving groups. Two‐hour meetings held twice monthly, for a minimum of six months. Five to nine parents per group plus leader to facilitate (each group had a parent and a professional leader). Onsite childcare, transport support, and refreshments provided. Parent leaders were parents of children who had previously been engaged in the intensive case management program. Professionals were mental health professionals. Parent and professional leaders were trained together over one day in group processes. |
RCT | Parents of children with a serious emotional disorder. |
| Scharer 2009 | Parent groups | Support group conducted via facilitated online chat room, available weekly. Duration unknown. Facilitated by child and adult psychiatric nurses. Participants could access other parts of the intervention website outside chat room times. | RCT | Mothers of children (5‐12) in child psychiatric units with a serious mental illness. |
| Silver 1997 | Parent mentors | Parent‐to‐Parent Network. Participants attended six face‐to‐face meetings (home or hospital) and received bi‐weekly phone calls over 12 months. Occasional group activities offered. Lay ‘intervenors’ were women who had raised children with ongoing health conditions. Forty hours’ training for mentors. | RCT | Mothers of children (5‐8) with ongoing health conditions (not necessarily chronic: lasting 3 months or requiring hospitalisation for >=30 days in a year). |
| Singer 1994 | Parent groups | Information and emotional support groups held weekly for nine weeks. Duration unclear. Facilitated, with discussion topics chosen by parents. No skills training or homework provided. | RCT Comparator is stress management group |
Parents of children and youths (2‐20) with acquired brain injury. |
| Singer 1999 | Parent mentor | Parents referred to nearest Parent‐to‐Parent program to call and find out details of their matched supporting parent. Supporting parents instructed to make a minimum of four phone calls to participant over two months. Supporting parents received eight to ten hours of training in communication skills, local services, and advocacy and support. | RCT | Parents, carers, or grandparents of a child with a disability. |
| Sullivan‐Bolyai 2004 | Parent mentor | Initial home visit from parent mentor, then negotiated number of home visits and phone calls. On average, three home visits and 13 phone call or emails over six months; however, the ranges were quite wide for each contact type. Mentors were mothers judged by the research team to be successful at managing their child’s diabetes. Matched on child age group (1‐5 or 6‐10 years). |
RCT | Mothers of children with Type I diabetes. |
| Sullivan‐Bolyai 2010 | Parent mentor | Initial home visit or phone call, then further contacts by negotiation, with a minimum of one face‐to‐face visit over 12 months. Mentors as in 2004 study. | RCT | Mothers of children with Type I diabetes. |
| Swallow 2014 | Peer support | Social networking group as part of an online app providing (1) clinical care‐giving support and (2) psychosocial support for caregiving. Very few details about the social networking component provided. App available on secure server via computer, mobile phone, smartphone, tablet. | RCT | Parents of children with chronic kidney disease (stages 3‐5). |
| Wysocki 2008 | Parent groups | Facilitated education and support groups; 12 sessions of 45 minutes with three to five families over six months. | RCT | Parents of children with diabetes. |
Design
Most included studies (21 of 22) were randomised controlled trials (RCTs) (Aiello 2015; Boogerd 2017; Boylan 2013; Ferrin 2014; Flores 2009; Ireys 1996; Ireys 2001; Kutash 2011; Kutash 2013; McCallion 2004; Rhodes 2008; Roberts 2011; Ruffolo 2005; Scharer 2009; Silver 1997; Singer 1994; Singer 1999; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010; Swallow 2014; Wysocki 2008).
One study was a quasi‐RCT with allocation to treatment condition by alternation (Preyde 2003). We did not identify any controlled before‐and‐after or interrupted time series studies that met inclusion criteria.
Sample size
Studies ranged in size from 15 participants in Singer 1994 to 343 participants in Silver 1997. The median number of participants was 71.
We were able to include in at least one meta‐analysis 1927 participants from 14 studies (Boogerd 2017; Flores 2009; Ireys 1996; Ireys 2001; Kutash 2011; Kutash 2013; McCallion 2004; Preyde 2003; Roberts 2011; Ruffolo 2005; Scharer 2009; Silver 1997; Singer 1999; Swallow 2014); the largest meta‐analysis (8 studies) included 864 participants (psychological distress).
Settings
Studies were based in the United States (14) (Flores 2009; Ireys 1996; Ireys 2001; Kutash 2011; Kutash 2013; McCallion 2004; Ruffolo 2005; Scharer 2009; Silver 1997; Singer 1994; Singer 1999; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010; Wysocki 2008); Australia (2) (Rhodes 2008; Roberts 2011); and Brazil (Aiello 2015), Canada (Preyde 2003), Ireland (Boylan 2013), Spain (Ferrin 2014), The Netherlands (Boogerd 2017), and the UK (Swallow 2014).
Peer support interventions were conducted in the following settings.
Community (14) (Ferrin 2014; Flores 2009; Ireys 1996; Ireys 2001; Kutash 2011; Kutash 2013; McCallion 2004; Roberts 2011; Ruffolo 2005; Silver 1997; Singer 1999; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010; Wysocki 2008).
Online (4) (Aiello 2015; Boogerd 2017; Scharer 2009; Swallow 2014).
Hospital (3) (Boylan 2013; Preyde 2003; Singer 1994).
Outpatient therapy (1) (Rhodes 2008).
Participants
Participants were parents and carers of children with a wide range of chronic physical, developmental, and psychiatric conditions.
Parents of children with diabetes (4 studies) (Boogerd 2017; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010; Wysocki 2008).
Parents of children with emotional disturbance (3 studies) (Kutash 2011; Kutash 2013; Ruffolo 2005).
Parents of children with chronic illness (2 studies) (Ireys 2001; Silver 1997).
Parents of children with a disability (1 study) (Singer 1999).
Parents of children with acquired brain injury (1 study) (Singer 1994).
Parents of children with anorexia nervosa (1 study) (Rhodes 2008).
Parents of children with attention deficit hyperactivity disorder (1 study) (Ferrin 2014).
Parents of children with autism spectrum disorder (1 study) (Roberts 2011).
Parents of children with chronic kidney disease (1 study) (Swallow 2014).
Parents of children with juvenile rheumatoid arthritis (1 study) (Ireys 1996).
Parents of young people with self‐harm or suicidal behaviour (1 study) (Boylan 2013).
Parents of children with serious mental illness (1 study) (Scharer 2009).
Minority parents of children with asthma (1 study) (Flores 2009).
Mothers of very preterm infants (1 study) (Preyde 2003).
Mothers of children with severe and profound sensorineural hearing loss (1 study) (Aiello 2015).
Grandparents with primary care of children with intellectual disability, other developmental disability, or learning problems, or with attention deficit and hyperactivity disorders (1 study) (McCallion 2004).
Interventions
Peer support interventions identified in this review fell into two broad categories.
Support groups for parents and/or carers, with or without a facilitator, which were conducted online or face‐to‐face (11 studies) (Aiello 2015; Boogerd 2017; Boylan 2013; Ferrin 2014; McCallion 2004; Roberts 2011; Ruffolo 2005; Scharer 2009; Singer 1994; Swallow 2014; Wysocki 2008).
Mentor arrangements, whereby a 'novice' parent or carer was matched with a more experienced parent or carer, sometimes referred to as peer‐to‐peer support (11 studies) (Flores 2009; Ireys 1996; Ireys 2001; Kutash 2011; Kutash 2013; Preyde 2003; Rhodes 2008; Silver 1997; Singer 1999; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010).
Parent/carer support groups (11 studies)
The typical purpose of support groups was to encourage parents and carers to share information, concerns, and achievements and to form a mutually supportive network (Roberts 2011). Support group interventions had a wide range of stated aims, such as to:
provide social support and information (Aiello 2015; Boogerd 2017; Boylan 2013);
reduce parental stress, depression, and anxiety (Aiello 2015; Boogerd 2017; Boylan 2013; McCallion 2004; Roberts 2011; Singer 1994);
improve communication, problem‐solving skills, and coping skills (Boylan 2013; Ruffolo 2005); or
increase parent knowledge (Aiello 2015 Boogerd 2017), self‐efficacy, sense of empowerment and caregiving mastery, and perception of competence in managing the child's condition (McCallion 2004; Roberts 2011; Swallow 2014).
Not all trial authors cited a theoretical basis for their support group intervention. Those that were cited included social support as a way of reducing role strain and life disruptions (e.g. McCallion 2004). References in these cases refer to studies citing the models, not to the original models.
Group structures ranged from formal peer support programmes, as in Boylan 2013 and Ruffolo 2005, to self‐help groups with content determined collaboratively by participants and facilitator, as in McCallion 2004, to completely non‐directive groups, as in Ferrin 2014. The format for groups identified in this review was either face‐to‐face (as in Boylan 2013, Ferrin 2014, McCallion 2004, Roberts 2011, Ruffolo 2005, Singer 1994, and Wysocki 2008) or online (as in Aiello 2015, Boogerd 2017, Scharer 2009, and Swallow 2014).
Whether online or face‐to‐face, support groups might include large and small group discussions of relevant information (e.g. Boylan 2013; McCallion 2004; Singer 1994; Wysocki 2008); problem‐solving activity‐based discussions (Ruffolo 2005); and encouragement of emotional expression (Singer 1994). Other components of support groups included homework, skill‐building exercises, free time for socialising (Ruffolo 2005; Wysocki 2008), testimonials from peers, and advice on managing stress (Swallow 2014). To increase parents' access to groups, some interventions also provided in‐home or onsite respite care and transport assistance (McCallion 2004).
Support groups were generally (although not always) led by a facilitator with input from participants (Boylan 2013; Ferrin 2014; McCallion 2004), for example, choosing topics for discussion (McCallion 2004; Singer 1994; Wysocki 2008). If facilitators were involved, these groups were usually non‐directive, with facilitators in some interventions explicitly prohibited from offering feedback, psychoeducation, or advice (Boogerd 2017; Ferrin 2014). When facilitators were more directive, they tended to intervene only to manage group processes, so as to ensure smooth running and full participation of members (Scharer 2009). This aspect of facilitation seemed particularly important in online settings, where peer discussion took place in an online chat room with the facilitator present to monitor.
Some groups included both a professional facilitator and a parent leader (Ruffolo 2005). Only one intervention appeared to have no facilitator or group leaders: an online intervention in which participants had open access to the psychosocial support site (Swallow 2014).
Support group interventions may have been developed following parent focus groups (Boogerd 2017; Boylan 2013), or during a pilot phase (Boogerd 2017). Grandparent recommendations were used to identify material to be covered in grandparent support group sessions (McCallion 2004), and consumers were involved in the development of one online system (Swallow 2014). In many cases, it was not clear whether there was consumer involvement in the design or conduct of the groups.
In most cases, the support group was the main intervention under investigation; in some cases, peer support was an alternate intervention control for more intensive interventions such as a structured psychoeducation programme (Ferrin 2014), an individual home‐based intervention (Roberts 2011), a stress management group (Singer 1994), or a family therapy group (Wysocki 2008). In some instances, peer support was a component of a broader online application (Aiello 2015; Boogerd 2017; Swallow 2014).
Parent/carer mentors (11 studies)
The common purpose of parent mentor (or peer‐to‐peer) interventions was to enhance the mental health of participants (Ireys 2001), while improving parent quality of life (Flores 2009). These interventions included named programmes such as Parent‐to‐Parent (Singer 1999; Silver 1997), Family‐to‐Family Network (Ireys 2001), Parent Connect (Kutash 2011; Kutash 2013), HOMEWARD (Sullivan‐Bolyai 2004), Social Support to Empower Parents (Sullivan‐Bolyai 2010), and unnamed mentoring arrangements (Flores 2009; Ireys 1996; Preyde 2003). The stated aims of interventions were to provide informational, affirmational, and emotional support (Ireys 1996; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010) with the goals of:
reducing parent anxiety, depression, and stress (Ireys 1996; Ireys 2001);
improving carer quality of life (Flores 2009);
reducing caregiver strain (Kutash 2011; Kutash 2013);
improving parents' confidence in managing the child's condition (Rhodes 2008; Sullivan‐Bolyai 2004); and
successfully adapting to the challenges of raising a child with a chronic condition (Singer 1999; Sullivan‐Bolyai 2004).
Theoretical bases cited for parent mentor interventions included consideration of the impact of social support on parent behaviour and attitudes (Ireys 2001), social support as an extension of coping (Singer 1999), as a determinant of parental mental health (Silver 1997), and as an element of planned behaviour (Kutash 2013).
Parent mentor interventions typically linked participants with a veteran parent who had experience raising a child with a comparable chronic condition (Ireys 2001; Preyde 2003; Rhodes 2008). Peer support might be provided via home visits (Ireys 2001), meetings in community venues (Ireys 2001), or therapeutic settings (Rhodes 2008). Interventions often included telephone contact in addition to face‐to‐face contact (Flores 2009; Ireys 1996; Ireys 2001; Preyde 2003; Silver 1997; Sullivan‐Bolyai 2010), or instead of face‐to‐face contact (Kutash 2011; Singer 1999).
Some parent mentor interventions also had a support group component, although this was generally informal and incidental to the mentoring relationship. For example, some parent mentors also convened group meetings with all their assigned families in community venues to encourage social networking and support (Flores 2009). In a school‐based programme (Kutash 2011; Kutash 2013), teachers received extra training and parents received written information (this was provided in control groups as well). One intervention was an adjunct to a well‐established family‐based treatment in which participants had access to a parent mentor and to the mentor's therapist throughout their own family therapy (Rhodes 2008). In at least one case, parent mentors had mandated topics to cover with participants (Silver 1997).
Parent mentors may have received extensive paid or volunteer training in interpersonal skills (as in Ireys 2001 Preyde 2003 and Sullivan‐Bolyai 2010) and in providing affirmational support that recognised the participant's existing competencies (Ireys 1996).
Some but not extensive consumer input was reported for parent mentor/peer‐to‐peer interventions. Parents had input into intervention design in one study (Singer 1999), and participants determined the amount of contact they received from mentors in two other interventions (Silver 1997; Sullivan‐Bolyai 2004).
Both intervention types varied widely on salient details such as number and length of group and mentoring sessions, structure of sessions (if any), and training and qualifications of facilitators and mentors. In most studies, interventions tended to be poorly specified; it would not be possible to implement most interventions faithfully from the information published. This was the case whether the peer support intervention was the focus of the study or was used as an alternative treatment control. Generally, measures for ensuring programme fidelity were not reported, although several trial authors reported that fidelity was checked (e.g. Flores 2009; Kutash 2011; Kutash 2013; Roberts 2011).
Because there was such wide variation within intervention categories, we have followed our original protocol and have not conducted separate syntheses for each intervention type, although we considered doing so. With future studies and better‐specified interventions, conducting separate meta‐analyses and narrative syntheses for each intervention type would be an approach worth considering.
Outcomes
Many studies collected data on multiple child and family outcomes, as is shown in the Characteristics of included studies table. In most cases, the peer support intervention was compared with a treatment as usual control only. In other cases, there was a usual care control and another comparator; in a small number of cases, there was no usual care control and peer support was compared only with another intervention; data from these studies could not be included in meta‐analyses. These are noted in the Characteristics of included studies table.
Outcomes included in meta‐analyses and the specific scales used to measure those outcomes are listed below. Nearly all measures were self‐reported; however, this is to be expected given the field of research and the nature of the outcomes. Many of the scales used, particularly for psychological distress and for confidence and self‐efficacy, are well established with reasonable psychometric profiles. Although we considered the measures used when assessing risk of bias (especially performance bias), use of self‐report measures such as those listed here should be considered standard for this research.
-
Psychological distress.
Global psychological distress: Psychiatric Symptom Index (Ireys 1996; Silver 1997); Profile of Mood States (Scharer 2009).
Stress: Parenting Stress Index (Aiello 2015; Boogerd 2017; Ferrin 2014; Roberts 2011); Parent Stress Scale (Boylan 2013).
Anxiety: Psychiatric Symptom Index (anxiety sub‐scale) (Ireys 2001); State Anxiety Inventory (Preyde 2003).
Depression: Center for Epidemiological Studies Depression Scale (McCallion 2004).
-
Confidence and self‐efficacy.
Self‐efficacy: Parent Asthma Management Self‐Efficacy Scale (Flores 2009); Parent Efficacy Scale (Rhodes 2008); Parental Confidence Questionnaire (Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010).
Locus of control: Parenting Locus of Control (Ruffolo 2005).
Feelings of mastery: Caregiving Mastery Scale (McCallion 2004); Family Management Measure (condition management sub‐scale) (Swallow 2014); confidence in managing the child's condition (Roberts 2011).
Positive attitude: Kansas Inventory of Parental Perceptions (Singer 1999).
-
Perception of coping.
Caregiver strain: Caregiver Strain Questionnaire (Kutash 2011; Kutash 2013).
Perception of coping: Parent Coping Efficacy Scale (Singer 1999).
-
Quality of life.
Pediatric Asthma Caregiver's QoL Scale (Flores 2009); Beach QoL Scale (Roberts 2011).
-
Family functioning.
Family Empowerment Scale, Family sub‐scale (Kutash 2011; McCallion 2004).
Family Empowerment Scale, Services sub‐scale (McCallion 2004).
Impact on Family Scale (Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010).
Video‐recorded family discussion coded using the Interaction Behavior Coded (Wysocki 2008).
PedsQL™ Family Impact Module (Ferrin 2014).
-
Perceived social support.
Ireys Social Support Inventory (Ireys 1996; Sullivan‐Bolyai 2010); Multidimensional Scale of Perceived Social Support (Boylan 2013; Preyde 2003); Arizona Social Support Scale (emotional support sub‐scale) (Ruffolo 2005); MOS Social Support Scale (Scharer 2009).
-
Confidence and skill in navigating medical systems.
Service efficacy: Vanderbilt Mental Health Service Efficacy Questionnaire (Kutash 2011; Kutash 2013).
Empowerment: Family Empowerment Scale (Services sub‐scale) (McCallion 2004; Swallow 2014).
Outcomes for which no data were available
No studies specifically reported adverse events or outcomes. Given that all other outcomes were measured on continuous scales, it is always possible that participants might have scored worse on such scales, relative to control participants, following an intervention. We considered results carefully with this possibility in mind.
Most data came from published studies, with the exception of Scharer 2009, for which data were provided by trial authors. Several trial authors provided additional data or clarification; this is noted in the Characteristics of included studies table.
Consumer involvement
Consumer involvement was not widely reported by study authors. Some interventions were developed following parent focus groups or a pilot phase (Boogerd 2017; Boylan 2013), or on the basis of carer recommendations (McCallion 2004; Singer 1999; Swallow 2014). Two studies reported that participants could determine their own preferred level of contact with mentors (Silver 1997; Sullivan‐Bolyai 2004), and participants were responsible for determining the materials discussed in several support group interventions (Ferrin 2014; McCallion 2004).
Excluded studies
We excluded 160 studies at the full‐text review stage. Of these, we excluded 151 for methodological reasons, as specified in Table 3. Reasons for excluding the 16 potentially most relevant studies are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
We present risk of bias information in Characteristics of included studies, Figure 2 (summary of all studies by risk of bias (ROB) items), and Figure 3 (individual studies by ROB items).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We judged risk of bias to be moderate to high across studies as a whole. This was particularly so for selection bias (random sequence generation and allocation concealment), performance bias, and detection bias, with around 50% to 90% of studies rated as being at unclear or high risk of bias in these domains. Performance bias was particularly high, reflecting the difficulty of blinding participants and personnel to the provision of peer support delivered in comparison to usual care. A high proportion of studies provided insufficient information about their design and conduct, and so we judged them as being unclear in these domains.
Risk of bias was generally lower for other domains of the tool, with a majority (sometime a bare majority) of studies rated as being at low risk of attrition bias, reporting bias, and other biases.
Allocation
Random sequence generation
Twelve studies described using a truly random sequence generation method and were rated as being at low risk of bias (Boogerd 2017; Ferrin 2014; Flores 2009; Ireys 1996; Ireys 2001; Kutash 2013; Rhodes 2008; Roberts 2011; Ruffolo 2005; Singer 1999; Sullivan‐Bolyai 2010; Swallow 2014).
Nine studies stated that they used a random sequence but did not describe their method of sequence generation. They were rated as being at unclear risk of bias for this item (Aiello 2015; Boylan 2013; Kutash 2011; McCallion 2004; Scharer 2009; Silver 1997; Singer 1994; Sullivan‐Bolyai 2004; Wysocki 2008).
One study reported using random assignment but actually used quasi‐random methods. This study was rated as being at high risk of bias (Preyde 2003).
Allocation concealment
Allocation concealment was less well reported than was random sequence generation. Only three studies described adequate allocation concealment and were rated at low risk of bias for this item (Ireys 2001; Rhodes 2008; Swallow 2014).
By contrast, risk of bias for allocation concealment was unclear in 16 studies due to insufficient information (Aiello 2015; Boylan 2013; Ferrin 2014; Flores 2009; Ireys 1996; Kutash 2011; Kutash 2013; McCallion 2004; Ruffolo 2005; Scharer 2009; Silver 1997; Singer 1994; Singer 1999; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010; Wysocki 2008).
In three studies, allocation did not appear to have been concealed; these studies were rated at high risk of bias (Boogerd 2017; Preyde 2003; Roberts 2011).
Blinding
Due to the nature of interventions in this field, blinding of participants and of staff delivering interventions can be difficult. When a peer support intervention was compared with usual care, it seems unlikely that participants could have been truly blind to their allocation to intervention, but this depends on the nature of the usual care provided and what participants were led to expect on recruitment to the study.
If in doubt, we have coded this domain as 'unclear risk' rather than 'high risk' because it is possible ‐ depending on how the research was described to prospective participants ‐ that participants were not aware whether they had been allocated to intervention or control. We rated as 'high risk' if it seemed clear, in our judgement, that participants could not have been blind to allocation due to the nature of the intervention. Research personnel could have been blinded to allocation during data collection, particularly if they did not also deliver the intervention, and in some cases, baseline measures at least were taken prior to allocation to intervention or control.
Outcome measures were a mixture of self‐report and structured interview, which represents a potential form of bias. However, the self‐report surveys generally used established, validated measures, and these may not represent substantially higher risk of bias than usual in this domain than for properly blinded participants.
We rated risk of bias as low if participants were blinded, even if personnel were not, especially if checks were in place to ensure fidelity of programme delivery and outcome measurement.
Performance bias
Only one study was rated as being at low risk of performance bias (Ferrin 2014). Risk of performance bias was unclear in eight studies (Boylan 2013; Flores 2009; Kutash 2011; Kutash 2013; McCallion 2004; Roberts 2011; Scharer 2009; Singer 1994).
The remaining 13 studies were at high risk of performance bias (Aiello 2015; Boogerd 2017; Ireys 1996; Ireys 2001; Preyde 2003; Rhodes 2008; Ruffolo 2005; Silver 1997; Singer 1999; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010; Swallow 2014; Wysocki 2008).
Detection bias
Five studies were at low risk of detection bias (Flores 2009; Roberts 2011; Silver 1997; Swallow 2014; Wysocki 2008), and risk was unclear for 12 studies (Boylan 2013; Ferrin 2014; Ireys 1996; Kutash 2011; Kutash 2013; McCallion 2004; Rhodes 2008; Ruffolo 2005; Scharer 2009; Singer 1994; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010).
Five studies were assessed as being at high risk of detection bias (Aiello 2015; Boogerd 2017; Ireys 2001; Preyde 2003; Singer 1999).
Incomplete outcome data
Attrition bias appeared to be at generally lower risk than bias in previous domains. Strategies used by trial authors to address potential attrition bias included full reporting of missing data; checking whether attrition differed between intervention and control; and using intention‐to‐treat analyses and checking against per‐protocol analyses. When there appeared to be high risk of attrition bias, this was due to issues such as discarding final outcome measures due to attrition and using earlier measures (considered here rather than in selective reporting below), reporting unequal attrition rates without reporting potential reasons, or reporting attrition rates that differed by participant characteristics.
Fourteen studies were assessed as being at low risk of bias (Aiello 2015; Boogerd 2017; Ferrin 2014; Flores 2009; Ireys 1996; Ireys 2001; Kutash 2013; McCallion 2004; Preyde 2003; Silver 1997; Singer 1999; Sullivan‐Bolyai 2004; Swallow 2014; Wysocki 2008).
Risk of bias was unclear in four studies (Boylan 2013; Roberts 2011; Ruffolo 2005; Singer 1994), and risk was high in four studies (Kutash 2011; Rhodes 2008; Scharer 2009; Sullivan‐Bolyai 2010).
Selective reporting
Seventeen studies were assessed as being at low risk of reporting bias (Aiello 2015; Boogerd 2017; Ferrin 2014; Flores 2009; Kutash 2011; Kutash 2013; McCallion 2004; Preyde 2003; Roberts 2011; Ruffolo 2005; Scharer 2009; Silver 1997; Singer 1994; Sullivan‐Bolyai 2004; Sullivan‐Bolyai 2010; Swallow 2014; Wysocki 2008).
Risk of reporting bias was rated as unclear in two studies (Boylan 2013; Singer 1999), and risk was high in three studies (Ireys 1996; Ireys 2001; Rhodes 2008).
Other potential sources of bias
Six studies were assessed as being at high risk of bias from other potential sources of bias (Ireys 1996; McCallion 2004; Rhodes 2008; Roberts 2011; Ruffolo 2005; Singer 1994).
Differences between intervention and control participants in functional status of the child, severity of child diagnosis, and rates of relevant child comorbidity (Ireys 1996; Roberts 2011; Ruffolo 2005; Singer 1994).
Differences between intervention and control participant demographics that could plausibly affect outcomes (family composition and rates of obsessive‐compulsive disorder and depression) (Rhodes 2008).
Potential baseline differences in major outcomes, not corrected for in analysis (Roberts 2011).
Agencies delivering interventions and responsible for recruitment of participants, when it was not clear whether participants could self‐select the intervention (McCallion 2004).
Unknown dose of treatment as usual when this was something that might act as a confounder, such as intensive case management (Ruffolo 2005).
Bias from other potential sources was unclear in three studies (Boylan 2013; Kutash 2011; Scharer 2009).
Effects of interventions
Summary of findings 1. Peer support compared to usual care for parents and carers of children with complex needs.
| Peer support compared to usual care for parents and carers of children with complex needs | |||||
| Patient or population: parents and carers of children with complex needs Setting: Community, hospital, online Intervention: Peer support Comparison: usual care | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Risk with Peer support | |||||
| Psychological distress Measures: ‐ Parenting Stress Index (PSI‐SF) ‐ Psychiatric Symptom Index (PSI) ‐ PSI anxiety sub‐scale ‐ State anxiety inventory ‐ Center for Epidemological Studies Depression Scale (CES‐D) ‐ Profile of Mood State Inventory (POMS) Higher score = greater symptom severity, for all scales. Timing of follow‐up measure varied from 3 to 18 months; timing of 2 studies was unknown. |
SMD 0.1 SD lower (0.32 lower to 0.11 higher) | ‐ | 864 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Included one quasi‐RCT, per protocol Two RCTs not included in meta‐analysis showed mixed results: in one study, no difference in mean distress scores for intervention and control group participants; in the other, participants in both groups showed reduced distress at follow‐up but this reduction was significantly greater for intervention participants. GRADE scores contributing to very low certainty: Risk of bias ‐2 Inconsistency ‐1 Other components not serious or not detected |
| Confidence and self‐efficacy Measures: ‐ Parent Asthma Management Self‐efficacy Scale (PAMSES) (higher score = greater self‐efficacy) ‐ Caregiving Mastery Scale (CMS) (higher score = higher mastery) ‐ Parent Perception Questionnaire (higher score = higher confidence) ‐ Parenting Locus of Control (higher score = greater externality; reversed) ‐ Kansas Inventory of Parental Perceptions (source of strength sub‐scale; higher score = greater perceived benefit) ‐ Parental Confidence Questionnaire (higher score = greater confidence) ‐ Family Management Measure (FMM) condition sub‐scale (higher score = better able to manage condition) Timing of follow‐up measure varied from 2 to 18 months. |
SMD 0.04 higher (0.14 lower to 0.21 higher) | ‐ | 542 (8 RCTs) | ⊕⊕⊝⊝ LOW 3 | GRADE scores contributing to low certainty: Risk of bias ‐2 Other components not serious or not detected |
| Perception of coping Measures: ‐ Caregiver Strain Questionnaire (CGSQ) (higher score = more strain) ‐ Parent Coping Efficacy Scale (higher score = better coping) Timing of follow‐up measure varied from 2 months (1 study) to 9 months (2 studies) |
SMD 0.08 SD lower
(0.38 lower to 0.21 higher) |
‐ | 293 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 5 | GRADE scores contributing to very low certainty: Risk of bias ‐2 Imprecision ‐1 Other components not serious or not detected |
| Quality of life Measures: ‐ Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ) ‐ Beach Centre Family Quality of Life Scale (higher scores = better quality of life) Timing of follow‐up measure was 12 months for 1 study and unknown in the other. |
SMD 0.03 higher
(0.32 lower to 0.38 higher) |
‐ | 143 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 45 | GRADE scores contributing to very low certainty: Risk of bias ‐2 Imprecision ‐1 Other components not serious or not detected |
| Family functioning Measures: ‐ Family Empowerment Scale (FES) (higher score = less impact) ‐ Impact on Family Questionnaire (higher score = more impact) Timing of follow‐up measure varied from 3 to 9 months |
SMD 0.15 higher (0.09 lower to 0.38 higher) | ‐ | 272 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW4 5 | One study not included in the meta‐analysis showed some improvement for some measures of problematic family communication for peer support compared to usual care control, for mothers but not for fathers, at later times points (18 months) but not earlier (6‐12 months). GRADE scores contributing to very low certainty: Risk of bias ‐2 Imprecision ‐1 Other components not serious or not detected |
| Perceived social support Measures: ‐ Irey's Social Support Inventory ‐ Multidimensional Scale of Perceived Social Support ‐ Arizona Social Support Scale ‐ MOS Social Support scale (higher scores = more support) Timing of follow‐up measure varied from 4 to 18 months. Timing was unknown in one study. |
SMD 0.31 higher (0.15 lower to 0.77 higher) | ‐ | 191 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW2 5 6 | Included one quasi‐RCT, per protocol. Two studies could not be included in the meta‐analysis. One reported no significant change in perceived social support over time or between groups; the other did not report usable data. GRADE scores contributing to very low certainty: Risk of bias ‐2 Inconsistency ‐1 Imprecision ‐1 Other components not serious or not detected |
| Confidence and skill at navigating medical services Measures: ‐ Vanderbilt Mental Health Services Efficacy Questionnaire (VMHSEQ) ‐ Family Empowerment Scale (service system sub‐scale) (higher score = more confidence and skill) Timing of follow‐up measure varied from 3 to 9 months. |
SMD 0.05 higher
(0.17 lower to 0.28 higher) |
‐ | 304 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 5 7 | GRADE scores contributing to low certainty: Risk of bias ‐2 Imprecision ‐1 Other components not serious or not detected |
| Adverse events | None reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Of 8 studies, 1 was quasi‐RCT with attendant risk of confounding. High proportion of high/unknown RoB in random sequence generation, allocation concealment, and blinding
2 Minimal overlap of CIs; tests of heterogeneity suggest departure from null hypothesis. Effects were in different directions (positive and negative)
3 Majority of studies had high or unclear ROB in allocation concealment and blinding of participants and personnel
4 Majority of ROB criteria are high or unknown risk, including nearly all sequence generation, allocation concealment, and blinding criteria
5 Total sample was underpowered to detect small effect size according to GRADE guideline of 200 participants each in intervention and control
6 Of 4 studies, 1 was quasi‐RCT. Majority of ROB criteria are high or unknown risk, including nearly all sequence generation, allocation concealment, and blinding criteria
7 High proportion of ROB criteria are unknown risk
Summary of findings 2. Peer support compared to alternative interventions for parents and carers of children with complex needs.
| Peer support compared with alternative interventions for parents and carers of children with complex needs | |||||
|
Patient or population: parents and carers of children with complex needs Settings: Community, hospital, outpatient Intervention: peer support Comparison: alternative intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Risk with peer support | |||||
| Psychological distress Measures: ‐ Parenting Stress Index (PSI‐SF) ‐ State‐Trait Anxiety Inventory (STAI) |
SMD 0.2 SD higher (0.38 lower to 0.79 higher) | ‐ | 95 (2 RCTs) | ⊕⊝⊝⊝ very low1 2 | GRADE scores contributing to very low certainty: Risk of bias ‐1 Imprecision ‐2 Other components not serious or not detected |
| Confidence and self‐efficacy | A single study of 20 participants found that peer support plus family‐based treatment had no effect on parent confidence relative to family‐based treatment alone. | ||||
| Quality of life | No studies reported findings for this outcome | ||||
| Perception of coping | No studies reported findings for this outcome | ||||
| Family functioning | A single study of 81 found no difference in quality of life between peer support group participants and participants of a psycho‐educational group | ||||
| Perceived social support | No studies reported findings for this outcome | ||||
| Confidence and skill at navigating medical services | No studies reported findings for this outcome | ||||
| Adverse events | None reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 High proportion of high/unknown risk of bias across the two RCTs
2 Very small sample sizes, especially Singer 1994.
&&In preparing this section of the review, we have relied upon guidance from the Cochrane Consumers and Communication Group (Ryan 2016) in addition to the Cochrane Handbook (Higgins 2011a).
See: Table 1 for seven outcomes in comparison 1 (peer support interventions versus usual care).
Comparison 1. Peer support interventions versus usual care
Primary outcomes
1.1 Psychological distress
Ten studies were identified which assessed psychological distress following peer support interventions compared with usual care. Eight studies assessing psychological distress in 864 participants were able to be included in a meta‐analysis (Boogerd 2017 Ireys 1996 Ireys 2001 Preyde 2003 Silver 1997 McCallion 2004 Roberts 2011 Scharer 2009). The pooled interventions arms showed no evidence of effect on psychological distress (SMD ‐0.10 (95% CI ‐0.32 to 0.11), see Analysis 1.1).
1.1. Analysis.

Comparison 1: Peer support vs usual care, Outcome 1: Psychological distress
Two RCTs could not be included in the meta‐analysis due to missing data. In Aiello 2015 (22 participants), no significant difference in participant distress scores between intervention and control participants was observed at either baseline or follow‐up. In Boylan 2013 (147 participants), both the intervention and the usual care control participants reported significantly improved well‐being (reduced distress) at follow‐up, but with significantly greater improvement for intervention participants. More detail on these results is provided in Table 5.
3. Studies measuring outcomes but not included in meta‐analysis.
| Outcome | Study name | Measure | Mean (SD) | Significance |
| 1.1 Psychological distress | Aiello 2015 | Parenting Stress Index‐Short Form | Means and SDs not provided (requested from authors) | No change in either group: p=0.64 control pre‐post, p=0.88 experimental pre‐post |
| Boylan 2013 | General Health Questionnaire | Means and SDs not provided | ANOVA showed significant improvement in parental well‐being across both groups: F(2,126)=24.11, p=0.00, g2=0.277. The interaction effect of intervention X time was significant, F (2,126)=4.75, p=0.01, g2 = 0.07, as was the main effect of intervention, F (1, 63)=8.66, p=0.005, g2=0.121, which indicated a significantly greater improvement in well‐being for the intervention group | |
| 1.5 Family functioning | Wysocki 2008 | Negative communication (mothers and fathers) | 6‐month Mothers (91) ES: 4.1 (2.8) SC: 3.5 (2.2) BFST: 2.5 (2.2) Fathers (93) ES: 2.3 (1.7) SC: 1.9 (1.5) BFST: 2.1 (1.5) (lower scores are better) |
Educational support (ES) vs Standard Care (SC) vs Behavioural Family Systems therapy (BFST). Peer support control (ES) performed worse than BFST at 6 and 12‐month follow ups, but as well as BFST at 18‐month follow‐up. This should be interpreted to mean that there was no effect of peer support in reducing negative communication. |
| 1.6 Perceived social support | Boylan 2013 | Multimodal scale of perceived social support | Means and SDs not provided | 'There was no change in perceived social support or family communication over time for either group' |
| Sullivan‐Bolyai 2010 | Irey's social support inventory | Not applicable | Authors used a dichotomous adaptation (not the continuous Irey's social support scale used elsewhere) and we could not find information about how to score. Also, this describes contact with mothers in the intervention condition, which was the main aim of that intervention. Not appropriate to compare this with number of contacts in the control condition. |
The certainty of the evidence relating to this outcome (see Table 1, for this and subsequent outcomes in this comparison) was very low, meaning we can have very little confidence in the effect estimates. The eight studies contributing to the pooled estimate of effect included interventions in a range of settings, delivery of interventions varied widely, there was a wide range of follow‐up times, and there is some degree of heterogeneity in estimates (I2 51%). Additionally, most studies had high or unclear risk of bias for allocation concealment and blinding (participants and personnel, and outcome assessors).
Based on this evidence, we are uncertain about the effect of peer support interventions on parents' psychological distress. The true effect is likely to differ substantially from the estimate of effect reported here.
1.2 Confidence and self‐efficacy
We identified eight studies which compared confidence and self‐efficacy outcomes for peer support interventions compared with usual care in 542 participants (Flores 2009 McCallion 2004 Roberts 2011 Ruffolo 2005 Singer 1999 Sullivan‐Bolyai 2004 Sullivan‐Bolyai 2010 Swallow 2014). The pooled intervention arms showed no evidence of effect on parents' confidence and self‐efficacy (SMD 0.04 (95% CI ‐0.14 to 0.21), see Analysis 1.2). Note that one scale measuring this outcome (used in the two Sullivan‐Bolyai studies) was scored negatively, so a decrease represents an improvement on that scale.
1.2. Analysis.

Comparison 1: Peer support vs usual care, Outcome 2: Confidence and self‐efficacy
The certainty of the evidence relating to this outcome (see Table 1) was low, meaning we can have only limited confidence in the effect estimates. The majority of the studies had a high or unclear risk of bias for allocation concealment and blinding of participants and personnel.
Based on this evidence, peer support may have little or no effect on parent confidence and self‐efficacy. The true effect may differ substantially from that reported here.
1.3 Perception of coping
We identified three studies assessing perception of coping in 293 participants, and these were able to be included in a meta‐analysis (Kutash 2011 Kutash 2013 Singer 1999). The pooled intervention arms showed no evidence of effect on parents' perception of coping (SMD ‐0.08 (95% CI ‐0.38 to 0.21), see Analysis 1.3). Note that two of the three scales used to assess this outcome are scored in such a way that a lower score represents improved perception (better) of coping.
1.3. Analysis.

Comparison 1: Peer support vs usual care, Outcome 3: Perception of coping
The certainty of the evidence relating to this outcome (see Summary of findings table 1) was very low, meaning we can have very little confidence in the effect estimate. Delivery of interventions varied widely, there was a wide range of follow‐up times, and the majority of risk of bias items are at high or unknown risk including all allocation concealment, and blinding criteria. The total sample was underpowered according to GRADE guidelines.
Based on this evidence, we are uncertain about the effect of peer support interventions on parents' perception of coping. The true effect is likely to differ substantially from the estimate of effect reported here.
1.4 Quality of life
We identified two studies which assessed quality of life in 143 participants, following peer support intervention or usual care (Flores 2009 Roberts 2011). Both were eligible for inclusion in a meta‐analysis. The pooled effect estimate showed no evidence of effect of the intervention on parents' quality of life (SMD 0.03 (95% CI ‐0.32 to 0.38), Analysis 1.4).
1.4. Analysis.

Comparison 1: Peer support vs usual care, Outcome 4: Quality of life
The certainty of the evidence relating to this outcome (see Table 1) was very low, meaning we can have very little confidence in the effect estimate. Delivery of interventions varied widely, there was a wide range of follow‐up times, and the total sample was underpowered to detect small effect size according to GRADE guidelines (Schünemann 2013). Additionally, several key risk of bias criteria were rated as at high or unknown risk, including allocation concealment in both studies.
Based on this evidence, we are uncertain about the effect of peer support interventions on quality of life in parents and carers. The true effect is likely to differ substantially from the estimate of effect reported here.
1.5 Family functioning
We identified five studies which compared family functioning following peer support intervention or usual care. Four studies assessing family functioning in 272 participants were able to be included in a meta‐analysis (Kutash 2011 McCallion 2004 Sullivan‐Bolyai 2004 Sullivan‐Bolyai 2010). The pooled effect estimate showed no evidence of effect of the intervention on family functioning (SMD 0.15 (95% CI‐0.09 to 0.38), see Analysis 1.5).
1.5. Analysis.

Comparison 1: Peer support vs usual care, Outcome 5: Family functioning
One study could not be included in the meta‐analysis as there was no suitable data reported and the authors could not be contacted (Wysocki 2008, 104 participants). This study was very difficult to interpret as analysis of variance tables focused on the authors' preferred intervention (Behavioural Family Systems Therapy) while we were interested in their Educational Support (peer support) condition, an active control using peer support; however both interventions appeared to perform better than a usual care control on some measures of family communication and problem‐solving skills, for mothers but not for fathers, and at the later (18 months) but not earlier (6 to 12 month) time point.
The certainty of the evidence was very low. Only a small proportion of risk of bias criteria were rated as low risk, and almost all studies were at unclear risk due to sequence generation and allocation concealment. There were also differences in intervention delivery and follow‐up times and the total sample was underpowered by GRADE guidelines.
Based on this evidence, we are uncertain about the effect of peer support interventions on family functioning and the true effect is likely to differ substantially from the estimate of effect reported here.
1.6 Perceived social support
We identified six studies assessing perceived social support. Four studies assessing perceived social support in 191 participants following peer support intervention or usual care were able to be included in a meta‐analysis (Ireys 1996 Preyde 2003 Ruffolo 2005 Scharer 2009). The pooled effect estimate showed no evidence of an effect of the intervention on parents' perceived social support (SMD 0.31 (95% CI ‐0.15 to 0.77), see Analysis 1.6).
1.6. Analysis.

Comparison 1: Peer support vs usual care, Outcome 6: Perceived social support
Two studies (Boylan 2013 Sullivan‐Bolyai 2010) could not be included in the meta‐analysis. The authors reported no significant change in perceived social support over time for either intervention or usual care group in either study.
The certainty of the evidence was very low. Of the four studies, one was a quasi‐RCT and the majority of risk of bias criteria were rated as high or unknown risk, including all allocation concealment and blinding criteria. Intervention delivery and timing of follow‐up varied widely. Tests of heterogeneity suggest some heterogeneity of individual results (I2 60%) and the total sample was underpowered by GRADE guidelines.
Based on this evidence, we are uncertain about the effect of peer support interventions on parents and carers' perceived social support. The true effect is likely to differ substantially from the estimate of effect reported here.
1.7 Confidence and skill in navigating medical services
W identified four studies assessing confidence and skill at navigating medical services in 304 participants, following peer support intervention or usual care (Kutash 2011 Kutash 2013 McCallion 2004 Swallow 2014). All were able to be included in a meta‐analysis. The pooled effect estimate showed no evidence of effect of the intervention on parents' confidence and skill at navigating the medical service system (SMD 0.05 (95% CI ‐0.17 to 0.28), see Analysis 1.7).
1.7. Analysis.

Comparison 1: Peer support vs usual care, Outcome 7: Confidence and skill at navigating medical services
The quality of the evidence was very low. Of the four studies, a high proportion of risk of bias criteria were rated as at unknown risk, including the majority of studies for allocation concealment and blinding. The total sample was underpowered by GRADE guidelines.
Based on this evidence, we are uncertain about the effect of peer support parents' confidence and skill at navigating medical services. The true effect may differ substantially from that reported here.
1.8 Adverse events
No authors reported discrete adverse events. A relative worsening of outcomes following intervention (for example an increase in psychological distress) would also have counted as an adverse event in this review; however, given that there was no evidence of effect in either direction for any of the assessed outcomes, peer support interventions do not appear to worsen any outcomes for participants as far as we currently are aware. However, the very low certainty of evidence overall indicates that we are currently uncertain about possible adverse events and should continue to monitor for these, rather than indicating that none are possible with the use of peer support interventions.
Comparison 2. Peer support interventions versus alternative intervention
A small number of studies compared peer support with an alternative intervention only ‐ that is, there was no usual care control (several studies in comparison 1 had all three arms, but we reported only the peer support and usual care arms in this review). In these studies, the authors' assumption was presumably that peer support would be less effective than the comparator intervention, as peer support was being used as a control.
Primary outcomes
2.1 Psychological distress
Two studies assessed psychological distress in 95 participants (Ferrin 2014 (comparator was psycho‐education), Singer 1994 (comparator was stress management group)). The pooled estimates of effect did not differ between peer support and alternative interventions (SMD 0.2 (95% CI ‐0.38 to 0.79), see Analysis 2.1). However, the certainty of evidence was rated as very low, as the pooled estimate was based on a very small sample size, and there was a high proportion of risk of bias criteria rated as being at high or unknown risk of bias, particularly in Singer 1994.
2.1. Analysis.

Comparison 2: Peer support vs alternative intervention, Outcome 1: Psychological distress
Based on this evidence, we are uncertain about the effect of peer support interventions on parents' psychological distress, compared with alternative interventions. The true effect is likely to differ substantially from the estimate of effect reported here.
2.2 Confidence and self‐efficacy
One study (Rhodes 2008, 20 participants) assessed parent confidence and indicated that peer support plus family‐based treatment had no significant effect on parent confidence, relative to family‐based treatment alone. As this is a single study, we are very uncertain about the effect and expect that the true effect may differ substantially from that reported here.
2.3 Family functioning
Ferrin 2014 assessed impact on family life (PedsQL family function module) in 81 participants. The authors found no significant difference in quality of life between peer support group participants and a psycho‐educational group. As this is a single study, we are very uncertain about the effect and expect that the true effect may differ substantially from that reported here.
Secondary outcomes
Satisfaction with the intervention (Comparison 1, peer support interventions versus usual care)
Only three studies reported on parent satisfaction with the intervention, but data is not comparative and should therefore be viewed only as indicative. Boogerd 2017 found moderate acceptability of and demand for the intervention in parents, and high acceptability and demand in health care professionals (however, this was not a direct measure of satisfaction, but is inferred from reported rates of uptake and continued use). Kutash 2011 reported that parents were very satisfied with the intervention (98% (40 of 41) of participants responding to that question). Similarly Preyde 2003 reported that of 24 participants (out of 32) who responded to a question about helpfulness, 21 (87.5%) indicated that the intervention was helpful or very helpful.
Incidental learning/improved knowledge
No included studies in either comparison addressed this outcome.
Qualitative findings: process factors
Some authors reported on process factors that influenced the implementation of interventions and participants' experience of those interventions:
Contextual factors influencing the development of peer support: facilitators and barriers to uptake of peer support interventions,
User and provider experiences of peer support interventions.
We have assessed these factors through qualitative data identified in our search for studies evaluating the effectiveness of intervention. Our intention was to provide insight into how or why interventions work (if they in fact do so), we make no claim to be providing a completely comprehensive or systematic account of qualitative findings. The qualitative component of this review was intended to provide suggestions as to why particular interventions may or may not be effective for particular participants or particular outcomes. We have used guidance from Popay 2006 in writing this section of the review, which is best considered as a preliminary implementation synthesis given the lack of significant pooled effects and scarcity of qualitative evidence from included studies.
Five studies included a qualitative component, either providing additional context in a single publication, or as an additional publication arising from an included study (Rearick 2011 (additional data from Sullivan‐Bolyai 2010); Ruffolo 2005 Scharer 2009 Singer 1999 Sullivan‐Bolyai 2004). The qualitative data collection and analysis methods used included:
Open‐ended satisfaction interview (Sullivan‐Bolyai 2004), analysis method not reported
Interview as part of a concurrent nested mixed‐methods study. Content analysis of interview transcripts conducted independently of quantitative analysis (Rearick 2011)
Random selection of telephone support transcripts, thematic analysis, sampling ended when no new themes or differences in content emerged (Scharer 2009).
Semi‐structured interview, constant‐comparative procedures to identify emerging themes (Singer 1999)
Qualitative methods not described (Ruffolo 2005)
Factors shaping access to and acceptability of peer support
Authors reported a variety of factors shaping‐‐whether positively or negatively‐‐access to and availability of peer support. As there is very little cross‐over in the factors cited, they are listed below by author, as well as being tabulated below.
Rearick 2011 (method extremely well described)
High level of comfort with mentors and ease of discussion
Readily available support from other sources decreased need for parent mentor support
Availability of mentor, ability to contact through a range of methods
Parent mentor initiating contact
Parent mentors whose child had the same diagnosis as participant's child
Structured format
Collaboration between parent and professional leaders
Onsite childcare, transportation support
Group (online) available in the evenings
Personality of nurse (chat room facilitator) determined acceptability of format
Perceived similarity of parent mentor
Practical and logistical obstacles to contact
Consistency of parent mentor follow‐up/initiation of contact
Perceived availability of mentor
Perceived interest of mentor
Calling mentor less intimidating than calling medical team
| Factors shaping access to and acceptability of support (risk of bias for these factors is presented in Table 3) | Specific factor | Type of peer support | Participant | Child diagnosis |
| Interpersonal and group dynamics (Rearick 2011, Ruffolo 2005, Singer 1999, Sullivan‐Bolyai 2004) | Comfort with mentors and ease of discussion | Peer‐to‐peer | Parent | Type 1 Diabetes |
| Parent mentor initiates contact | Peer‐to‐peer | Parent | Type 1 Diabetes | |
| Similarity of diagnosis between mentor's and participant's child | Peer‐to‐peer | Parent | Type 1 Diabetes | |
| Perceived similarity of parent mentor | Peer‐to‐peer | Parent | Disability | |
| Perceived interest of mentor | Peer‐to‐peer | Parent | Type 1 Diabetes | |
| Structured format | Support group | Parent | Emotional disturbances | |
| Collaboration between parent and professional leaders | Support group | Parent | Emotional disturbances | |
| Perceived availability of mentor/facilitator (Rearick 2011, Singer 1999, Sullivan‐Bolyai 2004) | Mentor is readily available | Peer‐to‐peer | Parent | Type 1 Diabetes |
| Perceived availability of mentor | Peer‐to‐peer | Parent | Type 1 Diabetes | |
| Perceived approachability of mentor | Peer‐to‐peer | Parent | Type 1 Diabetes | |
| Consistency of mentor in initiation/follow‐up | Support group | Parent | Acquired brain injury | |
| Pragmatic issues (Ruffolo 2005, Scharer 2009, Singer 1999) | Onsite childcare | Support group | Parent | Emotional disturbances |
| Transportation support | Support group | Parent | Emotional disturbances | |
| Availability after business hours | Support group | Parent | Serious mental illness | |
| Pratical and logistical obstacles | Support group | Parent | Acquired brain injury |
Factors shaping effectiveness of peer support
Given the lack of significant pooled effects, this should perhaps more properly be phrased as 'factors participants consider important for peer support to work for them'. These were identified by study participants as contributing to or detracting from the support they received.
Rearick 2011. Contributed: perceived availability of mentor, ability of mentor to provide practical tips about condition management, 'common ground' between mentor and parent (enables mentor to provide validation of emotion, build parent's confidence, assist with adaptation to child diagnosis and the 'new normal'.
Ruffolo 2005. Nothing reported relevant to facilitators of or barriers to effectiveness.
Scharer 2009. Contributed: professional (nurse) facilitator, providing empathy and emotional support, information, clarification, encouragement, and affirmation.
Singer 1999. Contributed: social comparisons between parent and mentor, exchange of practical information. Detracted: differences in circumstances, especially relating to child condition and behaviour.
Sullivan‐Bolyai 2004. Contributed: parent belief that they could call mentor for advice and support; mentor validated parent experience; mentor encouraged parent to call medical team when necessary.
We assessed the quality of the qualitative data, where possible, against the criteria outlined in Noyes 2011a. These assessments are summarised in Table 6 Credibility/internal validity was generally at high or unknown risk of bias, with only one study (Singer 1999) clearly reporting steps taken to reduce response bias. On the other hand, we rated transferability/external validity as being at low risk of bias overall, with good contextual and demographic data provided by most authors. Dependability/reliability was addressed by most authors, with some form of triangulation, inter‐rater agreement check, peer debriefing or audit trail (or a combination of these) used by most authors. Confirmability/objectivity was moderate. Three authors provided context and background for the research and research team or attempted to do so, but in the remaining four this was unclear.
4. Risk of bias assessment, qualitative findings.
| Interpersonal and group dynamics | Credibility | Transferability | Dependability | Confirmability |
| Rearick 2011 | Unclear: potential response bias | Detailed contextual and demographic data | Audit trails, inter‐rater agreement, and triangulation methods used | Confirmability not extensive, but attempted |
| Ruffolo 2005 | Not reported | Good contextual and demographic data | No reliability or reflexivity procedures evident | Unclear |
| Sullivan‐Bolyai 2004 | Unclear | Reasonalble context, but not all demographic details reported or methods specified | Multiple methods of data collection used | Researcher context and background provided |
| High risk of bias | Moderate risk of bias | Low risk of bias | Moderate risk of bias | |
| Perceived availability of mentor/facilitator | Credibility | Transferability | Dependability | Confirmability |
| Rearick 2011 | Unclear: potential response bias | Detailed contextual and demographic data | Audit trails, inter‐rater agreement, and triangulation methods used | Confirmability not extensive, but attempted |
| Singer 1999 | Clear and appropriate data collection/analysis methods, including interviewers blind to participant outcomes. Negative cases used | Good contextual and demographic data | Triangulation undertaken; inter‐rater agreement measured; attempt to minimise researcher bias | Researcher context and background provided |
| Sullivan‐Bolyai 2001 | Unclear | Reasonable context, but not all demographic details reported or methods specified | Multiple methods of data collection used | Researcher context and background provided |
| Moderate risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | |
| Factors related to pragmatic issues | Credibility | Transferability | Dependability | Confirmability |
| Ruffolo 2005 | Not reported | Good contextual and demographic data | No reliability or reflexivity procedures evident | Unclear |
| Singer 1999 | Clear and appropriate data collection/analysis methods, including interviewers blind to participant outcomes. Negative cases used | Good contextual and demographic data | Triangulation undertaken; inter‐rater agreement measured; attempt to minimise researcher bias | Researcher context and background provided |
| Moderate risk of bias | Low risk of bias | Moderate risk of bias | Moderate risk of bias |
Discussion
Summary of main results
Twenty‐two studies of 2,404 participants met criteria for inclusion in this review. Studies varied in design (twenty‐one RCTs and one quasi‐RCT), setting (community, hospital, online, and outpatient), participants (mothers only, parents, and grandparents), and child diagnosis (a wide range of disabilities, chronic conditions, and mental illnesses).
Interventions fell into two broad categories: parent/carer support groups and parent mentoring arrangements. Both intervention types varied widely in their reported characteristics such as number and length of sessions, structure of sessions, and the training and qualifications of personnel. Interventions of both types also varied widely in the degree of detail in which these characteristics were described, but the majority were fairly poorly described. Measures for ensuring fidelity of implementation were generally not described.
Risk of bias was moderate to high across the studies as a whole, and was used as a basis for downgrading certainty of the evidence. Most risk of bias concerns related to selection, performance, and detection bias. With regard to performance bias, our rating of moderate to high bias on this domain results primarily from the difficulty of blinding participants and personnel to participation intervention versus control. Of lesser concern was the high proportion of self‐report measures, as this can be considered typical of the field and measures with acceptable psychometric profiles tended to be used. However, there was also some inconsistency in measures used and effect estimates were sometimes in different (opposite) directions. A high proportion of studies provided insufficient information across a range of bias domains and were rated as being at unclear risk of bias. Risk of bias was generally lower for attrition and reporting biases.
Eight outcomes were assessed in this review. Meta‐analyses were conducted for all outcomes other than adverse events, for which no data were reported, but not all studies measuring a given outcome could be included in the relevant meta‐analysis. Effects of peer support interventions were assessed on outcomes compared to usual care control or to an alternative intervention.
Relative to usual care control:
Psychological distress (8 studies in meta‐analysis, 2 could not be included). We are uncertain about the effect of peer support on distress.
Confidence and self‐efficacy (6 studies in meta‐analysis, 2 could not be included). We are uncertain about the effect of peer support on confidence and self‐efficacy.
Perception of coping (3 studies in meta‐analysis). We are uncertain about the effect of peer support on perception of coping.
Quality of life (2 studies included in meta‐analysis). We are uncertain about the effect of peer support on quality of life.
Family functioning (4 studies in meta‐analysis, 1 could not be included). We are uncertain about the effect of peer support on family functioning.
Perceived social support (4 studies in meta‐analysis, 2 could not be included). We are uncertain about the effect of peer support on perceived social support.
Confidence and skill at navigating medical services (4 studies in meta‐analysis). We are uncertain about the effect of peer support on confidence and skill at navigating medical services.
Adverse events: could not be assessed as no studies reported on this outcome.
Relative to alternative intervention (psychoeducation or stress management groups):
Psychological distress (2 studies in meta‐analysis). We are uncertain about the effect of peer support on distress.
Confidence and self efficacy (1 study). We are uncertain about the effect of peer support on confidence and self‐efficacy.
Family functioning (1 study). We are uncertain about the effect of peer support on quality of life.
In addition to pooled estimates of effect lacking significance, individual studies varied widely in whether they found significant or non‐significant effects, and whether those effects supported (or tended to support) intervention or control. The variance of included studies (see forest plots) means that there was very little unequivocal evidence supporting the intervention. In fact, only three results favoured peer support, two of which came from Preyde 2003 (psychological distress and perceived social support). This was the only included quasi‐RCT, a design at a much higher risk of bias ‐ and hence less able to increase the certainty of our conclusions ‐ than the RCT studies. In addition, the small sample size of some (although not all) studies meant that they lacked power and were therefore imprecise; that is, would have been unable to detect a statistically (more importantly, a clinically) significant effect even if one existed. Many individual studies reported non‐significant findings where the direction of effect favoured intervention; however some reported non‐significant findings where the direction of effect favoured control. The narratively synthesised data was similarly equivocal in terms of the effects of interventions across comparisons and outcomes.
Some qualitative data were available, mainly relating to what participants valued about peer support interventions.
Factors shaping access to support included interpersonal considerations and group dynamics (such as the perceived similarity and interest of the mentor and collaboration between parent and professional leaders), the perceived availability and approachability of the mentor/facilitator, and pragmatic issues such as the availability of onsite childcare and transportation support.
Potential barriers to accessing peer support included: difficulty of initiating sensitive conversations, the perceived availability of mentor, insufficient resources from facilitator, and differences in other participants' circumstances and experiences.
Factors that participants considered important for peer support to work for them included: the perceived availability and similarity of the mentor, the ability of the mentor to provide practical tips about condition management, 'common ground' between mentor and parent, and socially comparable parents and mentors. Parents valued the exchange of practical information, feeling able to call mentor for advice and support, and having the mentor validate their experience.
The results of this review must be interpreted in the light of the overall very low certainty of evidence, as discussed in more detail below. On the other hand, they should also be interpreted in light of the fact that peer support may perform as well as alternative, generally more intensive, forms of intervention such as psycho‐education and stress management (although the evidence was uncertain and based on a very small number of studies). These alternative interventions may often necessarily involve a health professional for their delivery, unlike peer support interventions (even if a facilitator is involved, such a facilitator need not be a health professional, although they should have expertise in supporting groups). This has implications for the ease and relative cost of implementation of peer support groups. Peer support may potentially be beneficial, but the extent and quality of currently available evidence is insufficient to demonstrate benefit.
Overall completeness and applicability of evidence
All studies identified were conducted in high‐income countries. The majority were conducted in the US (14 studies), and the remainder in Australia (2 studies), and Brazil, Canada, Ireland, South Korea, Spain, The Netherlands, and the UK (1 each). The majority of studies took place in community settings (14 studies), with the remainder spread across online, hospital, and outpatient settings (4, 3, and 1 study respectively). Most studies (18 of 22) have been conducted since 2000, with nine of those conducted between 2010‐2017. Apart from the lack of data from middle‐ and low‐income countries, the studies identified cover a range of relevant participants, interventions, and outcomes, for parents and carers of children with a very broad range of chronic conditions.
For the most part, the gender identity, relationship status, CALD status, LGBTIQ status, and educational level of participants was not reported. 720 participants were noted as being the mother of the child with the condition; all other participants were recorded as being the parent or carer or grandparent of the child. 220 participants were noted as being either African‐American or Latino (Flores 2009). It is therefore difficult to comment on the completeness and applicability of the evidence in relation to such factors.
In most studies, the theoretical model underpinning the intervention was not reported, although many authors provided a general rationale for the benefits of social support. The range of scales for each outcome was quite broad. The outcomes of psychological distress and confidence/self‐efficacy were thosewith the greatest number of measurement scales, and arguably, the most used and best established scales. On the other hand, these outcomes are the most general and least condition‐specific compared to, for example, perception of coping (caregiver strain) and confidence at navigating medical systems. It remains an open question whether an outcome with well‐established but very general measures, such as distress, is more or less appropriate than a condition‐specific but less established outcome measure. The more global the outcome, the more difficult it may be for a relatively low‐intensity intervention to affect it. On the other hand, such outcomes may be more important to consumers and practitioners. For this reason, we chose a mix of outcomes for this review. However, researchers and practitioners should consider whether the scales for measuring their outcomes of interest are relevant to them when considering the applicability of this review.
It will be noted that we pooled several measures of distress for outcomes 1.1 and 2.1. The studies for these comparisons used a mix of anxiety scales, depression scales, indicators of mood change, and general psychiatric symptoms. We were not interested in any specific parent diagnosis because the link between peer support for a child's condition and a formal diagnosis of (for example) depression seems fairly tenuous. It is unlikely that a parent would seek a diagnosis for themselves in this context, and our intervention of interest is not an intervention for any particular diagnosis. Similar to the reasoning behind using the Kessler K6 scale (Kessler 2010), we decided to view elevated symptoms of common mental health concerns as indicating elevated distress without being concerned about whether the participant met formal diagnostic criteria for a condition. Psychiatric distress (due to family stressors) seems like a more appropriate target for peer support interventions, rather than an entrenched and possibly pre‐existing condition.
We contacted several authors for clarification and further details. While many responded, and some provided further data, six studies could not be included in meta‐analyses because no suitable data were available. Findings from all these studies were addressed narratively. No studies reported adverse events.
There was considerable variation in how well particular interventions were described, but in most cases the detail about content and delivery would be insufficient to replicate the intervention. A certain amount of variability is unavoidable in any case, given that both peer support groups and parent‐to‐parent mentoring interventions generally rely on unstructured free discussion. The range of peer support interventions available, the variability of interventions included in this review, and the incomplete details reported all potentially limit the applicability of this review. Practitioners and researchers should think carefully about the content of their intervention when assessing the applicability of this review to their work.
Generally, authors did not provide information about the training and on‐going supervision of those identified as peer support leaders. This is an area in which potentially open to harmful outcomes might arise, if leaders are not appropriately supported, but we cannot comment one way or another. Similarly, it would be useful to track participant withdrawal from peer support programs and investigate reasons for withdrawal, as this could shed further light on the acceptability of peer support interventions, as well as any potentially negative effects encountered or arising from such interventions.
Quality of the evidence
We assessed the degree of certainty of the evidence for each outcome using the GRADE system and presented findings in summary of findings tables for each comparison (Table 1; Table 2). Two authors independently assessed the evidence for each outcome; where GRADE scores differed, we discussed how we had each applied the relevant criterion and came to a consensus score. Where individual studies could not be included in meta‐analyses, we commented briefly on them in the narrative results.
The degree of certainty relating to all outcomes was very low or low. We downgraded on all outcomes for high or unknown risk of bias in random sequence generation, allocation concealment, and blinding. Two outcomes (psychological distress and perceived social support) included a quasi‐RCT.
Evidence for all outcomes was affected by the wide variation in intervention delivery and follow‐up times. The total samples for quality of life, family functioning, perceived social support, and confidence and skill at navigating medical services were underpowered to detect small effect sizes, according to GRADE guidelines. This problem also affected the psychological distress outcome in the peer support versus alternative intervention comparison.
We assessed the quality of the evidence for the limited qualitative data which was available relating to factors affecting access to and acceptability of support as moderate to low overall (Table 6).
We have very little confidence in the effect estimates (which all suggest no effect of peer support interventions) for all outcomes in this review. The true effects may differ substantially from those reported here.
Potential biases in the review process
We minimised bias in this review by adhering to our published protocol (Sartore 2013). Changes to this protocol are detailed below; they include expanding inclusion criteria to account for studies where peer support was an active control, and combining depression and anxiety outcomes into a general psychological distress outcome. These changes were made in consultation with Cochrane Australia. We also did not conduct our planned subgroup analyses, stratification by design, and sensitivity analyses, as these were no longer appropriate given the lack of main effects. In any case subgroup analyses could not be conducted because of the small number of studies contributing data to each outcome, within each comparison.
These changes aside, we adhered to a comprehensive search strategy, and two reviewers independently assessed eligibility criteria, extracted data, assessed risk of bias, and used GRADE criteria to evaluate our degree of confidence in the evidence. A third reviewer was available to resolve differences.
As discussed in previous sections, the range of scales for each outcome was quite broad, and we had to use our best judgement in grouping them to derive standardized mean difference estimates. While psychometric and other information was available for most scales and we attempted the most sensible groupings given the scales we had, it is possible that other reviewers would have made different decisions as to which scales should be allocated to which outcomes.
We attempted to contact authors for additional outcome data, to resolve uncertainties, and to request any unpublished studies. Despite these activities, and our extensive searches for relevant literature, it is possible that we may have missed studies relevant for inclusion in the review.
Agreements and disagreements with other studies or reviews
We identified five completed Cochrane reviews relevant to this area. All concerned peer support for adult participants who were directly experiencing a condition or supporting another adult, rather than children living with a condition.
Dale 2008 assessed the effectiveness of peer support telephone calls to improve physical, psychological, and behavioural health outcomes in adults. While there was some evidence of effect for some outcomes (such as improving screening rates, increasing healthy behaviours, and reducing symptoms of depression) the authors concluded, as we did, that few of the studies were of high quality and that methodological limitations decrease confidence in and generalizability of their findings.
Lavender 2013 investigated telephone support for women during pregnancy and six weeks postpartum. Peer support was provided only in a small minority of trials (four trials out of twenty nine meeting inclusion criteria). The review did not find clear evidence of effectiveness for telephone support, and could not draw any conclusions regarding the desirability of peer support.
In a recently updated review (Chamberlain 2017) of psychosocial interventions for smoking cessation in pregnant women, the authors found that high‐quality evidence suggests that effectiveness is unclear for interventions provided by peers (compared to clear evidence of effects for counselling and incentive interventions, and borderline evidence for health education interventions). However, this review identified only a single study of peer social support.
A recent review of psychosocial interventions for informal caregivers of people living with cancer (Treanor 2019) found some support for psychosocial interventions compared to usual care for improving caregiver quality of life, including 18 interventions with a component of support. However, these components formed part of a hierarchical framework (comprising information, support, coping skills training, psychotherapy, and spiritual or existential therapy) and could not be distinguished from other intervention components of the hierarchy in the analysis. Peer‐led support interventions were excluded from the review.
A very recent review of remotely delivered information, training, and support for informal caregivers of people with dementia (González‐Fraile 2021) found that interventions including support or training or both, with or without information, may slightly reduce caregiver burden and improve caregiver depressive symptoms compared with provision of information alone, but not compared with usual treatment, waiting list, or attentional control. Both peer support and professional support interventions were eligible for inclusion in the review. Such interventions may not add significantly to usual care in settings where social and health resources are well developed and available; the authors could not comment on their efficacy in settings when usual care services cannot be accessed or are less developed.
We identified two recent non‐Cochrane reviews that are relevant to this review. Niela‐Vilén 2014 conducted a systematic review (four databases, independent screening and quality assessment but no GRADE or equivalent reporting) of internet‐based peer support for parents. Note that this review covered any parents, and not only parents of children with complex needs. Thirty‐eight publications met inclusion criteria. The authors concluded that internet‐based peer support provided informational, emotional, and affirmational support to parent; however similarly to our review they found only inconclusive evidence that this translated into improved mental well‐being. Shilling 2013 identified 17 studies (qualitative, quantitative, and mixed‐methods) of parent‐to‐parent (mentoring) support interventions. This integrative review searched a wide range of databases, journals, and grey literature and used independent screening and assessment against formal quality criteria. Again, consistent with our review the authors found that parents perceived benefit from peer support programs, but that evidence of positive effects on psychological health was inconsistent and was not able to be aggregated across studies.
Authors' conclusions
Implications for practice.
Parents and carers of children with complex needs perceive peer support programs as valuable, but there is currently no evidence of effect and the existing evidence is of low or very low certainty. At present, we are uncertain of the potential benefits and harms of peer support interventions in this population of parents and carers of children with complex needs. Although we are unsure of the effects of these interventions from the evidence evaluated in trials to date, qualitative data from these same trials suggest that people often valued these interventions, particularly when peer support is provided by peers perceived as available, approachable, and with similar experiences to themselves. Participants valued exchanges of practical information about condition management and having their experiences validated.
Implications for research.
Ongoing randomised controlled trials could help to clarify whether peer support interventions improve outcomes for parents and carers of children with complex needs.
Of most use would be RCTs with larger samples than is generally seen here, which use and describe rigorous randomisation and allocation concealment strategies. For the kinds of outcomes assessed in this review, self‐report measures are likely to remain the most appropriate and easily‐administered; however care should be taken to select well‐validated measures with broad applicability. Researchers might also consider whether researcher‐rating scales are feasible.
In order to allow for replication and to facilitate comparisons across different studies, researchers should specify both intervention and control in as much detail as possible. This is particularly important where the control is usual care, given that participants may be participating in other programs or otherwise receiving condition‐related support with their child as part of standard care.
While qualitative data would not have changed our estimates of effect, given the nature of the interventions it is possible that mixed‐method research incorporating rigorous qualitative data would shed valuable light on participants' perceptions of peer support.
Given the low certainty of evidence to date, and the low degree of certainty in the evidence, our estimates showing no effect of intervention may change in the light of future research, especially future research of higher quality. Ideally, sufficient high‐quality research will be conducted in coming years to determine the effect of peer support programs generally, and to permit subgroup analyses for intervention type (group vs mentoring) and other important variables such as program setting, duration, and key features of participants that may importantly influence effectiveness.
History
Protocol first published: Issue 6, 2013
Acknowledgements
We thank Dr Megan Prictor, Dr Rebecca Ryan and Anne Parkhill for their extensive assistance in the preparation of this review, and Mr John Kis‐Rigo for his help in drafting our MEDLINE search strategy. Dr Prictor, Dr Ryan, Ms Parkhill and Mr Kis‐Rigo are from the Cochrane Consumers and Communication Review Group, based at La Trobe University's Centre for Health Communication and Participation. We thank Dr Michelle Macvean from the Parenting Research Centre for her comments on the full draft of this review.
We also thank the staff at Cochrane Australia for advice and assistance, especially Miranda Cumpston, Kelly Allen, and Dr Jo McKenzie.
Appendices
Appendix 1. Quantitative data extraction template
| Version 1.5.0, UPDATED MAY 2011 (uploaded to PRC d.m.yy) FOR EACH NEW STUDY, SAVE FILE TO SHAREPOINT ‘MyTime/Review data’ USING STUDY ID AS FILENAME |
| Peer support interventions for parents and carers of children with complex needs Study yyyy Extracted by Date extracted Notes and queries to be raised with co‐authors for discussion and/or things to follow up. |
|
Method Details of study Aim: From trial report. What was the problem that this intervention was designed to address? Design: Qualitative component? Initiate a file using the qualitative DET. Study ID will be same, use _qual on filename. Method of recruitment of participants: Inclusion/exclusion criteria for study participants: Informed consent obtained? (Yes/no/unclear) Ethics approval obtained? (Yes/no/unclear) Funding: (source, amount/not stated) Statistical methods and their appropriateness: Consumer involvement? (intervention design/delivery/evaluation; study design/interpretation) |
|
Participants Description: (patients/consumers, carers, parents of patients/consumers, health professionals, community members) Location: (city/state/country; urban/rural if indicated) Setting: (community, home, primary health centre, acute care hospital, extended care facility) Number: (eligible/excluded/refused/randomised to intervention/randomised to control/excluded post‐randomisation/withdrawn/lost to follow‐up/died/included in analysis/included for each outcome) Age: (range, mean(SD)) Gender Ethnicity Principal health problem or diagnosis (of relevant child/children) Other child health problem/s Stage of problem/illness Treatment received/receiving (relevant child/children) Other social or demographic details |
GO TO ‘RISK OF BIAS’ BELOW
Assessment of Risk of Bias for RCTs, quasi‐RCTs and CBAs (used to complete the ‘Risk of Bias’ tables in RevMan 5.)
Adapted from Cochrane Handbook Table 8.5.a: The Cochrane Collaboration’s tool for assessing risk of bias
| Domain | Review authors’ judgement | Support for judgement |
| Random sequence generation* |
High risk Unclear Low risk |
Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. Quasi‐RCTs and Controlled Before and After (CBA) studies must be rated as ‘High Risk’ for random sequence generation as the methods were not, by definition, truly random. |
| Allocation concealment |
High risk Unclear Low risk |
Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. CBA Studies should be rated ‘High Risk. Quasi‐RCTs are likely to be rated ‘High Risk but there may be some exceptions. |
|
Blinding of participants and personnel Assessments should be made for each main outcome (or class of outcomes). |
High risk Unclear Low risk |
Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. |
|
Blinding of outcome assessment Assessments should be made for each main outcome (or class of outcomes). |
High risk Unclear Low risk |
Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. If the outcome is objective (eg. length of hospital stay) the rating should be ‘Low risk. |
|
Incomplete outcome data Assessments should be made for each main outcome (or class of outcomes). |
High risk Unclear Low risk |
Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. |
| Selective reporting |
High risk Unclear Low risk |
State how the possibility of selective outcome reporting was examined by the review authors, and what was found. |
|
Other sources of bias See the Cochrane Handbook 8.15.1 for further examples of potential threats to validity, as well as 16.3.2 for issues relating to cluster trials and 16.4.3 for cross‐over trials. |
Note: all answers should follow the format: High risk Unclear Low risk |
State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. |
GO TO ‘INTERVENTION’ BELOW
|
Intervention Details (for each arm if multiple interventions) Theoretical basis (with key references): Aim: Content (format, media, source, setting): Details of control/usual/routine care Details of co‐interventions in all groups (Co‐interventions may be separate to the intervention of interest for this review; or may be other similar elements in a suite of interventions with a common purpose. Record all relevant information) Delivery of intervention (for each arm if multiple) Stages, timing, frequency, duration Providers Who delivers? How many? What training (in the relevant intervention)? Fidelity Delivered as intended? How assessed? |
|
Outcomes Principal (as identified by study authors): Secondary: Method of assessing outcomes (phone survey/questionnaire/physical measure; record for each): Validity and reliability of outcome measures: Methods of follow‐up for non‐respondents: Timing of assessment (frequency, length of follow‐up; for each outcome) Adverse events (complaints, levels of dissatisfaction, adverse incidents) |
GO TO ‘NOTES’ BELOW
|
Notes For publication in “Characteristics of Included Studies” table Possible entries: Contact with author? (Yes (information obtained)/no) (Must be attempted if clarification required. Use open‐ended questions) Power calculation? Record if study was translated from a language other than English Record if study was a duplicate publication. |
GO TO ‘RESULTS’ BELOW
Results
These data will be used in the “Comparisons and Data” section in RevMan (not the table “Characteristics of Included Studies”) and as the basis for the “Results” section of review text.
All data are numbers (of patients/units), not percentages.
Dichotomous outcomes
| Outcome | Timing of outcome assessment (days/months) | Intervention group* | Control group | Notes | ||
| Observed (n) | Total (N) | Observed (n) | Total (N) | |||
*Note: add additional columns if there is more than one intervention group, e.g. Intervention Group A, Intervention Group B…
Continuous outcomes
| Outcome | Timing of outcome assessment (days/months) | Intervention group | Control group | Notes | ||||
| *Mean / Mean change | Standard deviation | N | *Mean / Mean change | Standard deviation | N | |||
*delete as appropriate
Appendix 2. Qualitative data extraction template
| Version 1, 8.5.2013 FOR EACH NEW STUDY, SAVE FILE TO SHAREPOINT ‘MyTime/Review data’ USING STUDY ID_qual AS FILENAME |
| Peer support interventions for parents and carers of children with complex needs Study ID (Surname Year): Person completing form:. Date form completed: Notes (e.g. references to follow up, source of information (quantitative study details)) |
|
Study focus and methods: Aim: Research question(s): Method: Sample: Context: Approaches to data analysis and interpretation:. |
|
Qualitative findings: list factors, barriers, facilitators, etc. reported by authors Factors shaping access to and acceptability of peer support Factors shaping effectiveness of peer support |
| Other notes:. |
| Go to Quality Assessment (next page) |
|
Quality assessment (see protocol for full QA method) Credibility (cf internal validity) (cf external validity) Dependability (cf reliability) Confirmability (cf objectivity) |
Appendix 3. Record of author contacts
| Study ID | Included/excluded | Date of contact | Response |
| Aiello 2015 | Included | 10/05/19 | No response |
| Boogerd 2014 | Excluded | 10/12/14; 10/3/15 | Full publication later identified in updated search |
| Boylan et al 2013 | Included | 5/4/16 | No response |
| Brach et al 1998 | Excluded | 10/3/15; 27/10/15 | No formal evaluation completed |
| Carreon 2010 | Excluded | 10/12/14; 20/1/15 | Provided paper |
| Centeno‐Collado 2010 | Excluded | 29/7/14 | Provided details which allowed identification with Ferrin |
| Ireys et al 1996 and 2001 | Included | 9/5/16 | No response |
| Iwata 2012 | Excluded | 11/3/14 | Provided poster |
| Kelly 2014 | Excluded | 10/3/15; 27/10/15 | No response |
| Koren & Kupriyanova, 2013 | Excluded | 29/7/14; 30/9/14 | No response |
| Kutash et al 2013 | Included | 8/4/16 | Clarification provided |
| Lewis 1972 | Included | 5/4/14 | Contact not possible |
| Rhodes et al 2008 | Included | 9/5/16 | No further data available |
| Scharer et al 2009 | Included | 5/4/16 | Raw data provided |
| Shekarabi Ahari 2012 | Excluded | 4/8/14; 30/9/14 | No response |
| Singer et al 1994 and 1999 | Included | 9/5/16 | No further data available |
| Sliver et al 1997 | Included | 8/4/16 | Extra data provided |
| Sullivan‐Bolyai 2011 | Included | 9/5/16 | No further data available |
| Swallow 2012 | Excluded | 12/8/14; 30/3/15 | Manuscript under review; copy to be provided if accepted |
| Wysocki et al 2008 | Included | 29/7/14; 30/9/14 | No further data available |
Appendix 4. CENTRAL search strategy
1. child* or infan* or toddler* or newborn or neonat* or baby or babies or preschool* or pre‐school* or boy or boys or girl or girls or schoolchild* or school‐age or adolescen* or pediatric* or paediatric* or youth* or juvenile* or teen* or "minors"
2. [mh pediatrics]
3. {or #1‐#2}
4. [mh parents]
5. (parent or parents or parental or parenting or mother* or maternal or father* or paternal or carer* or caregiver* or care‐giver* or guardian* or stepparent* or foster‐care or foster‐home* or childrearing or child‐rearing):ti,ab,kw
6. [mh "parent child relations"]
7. {or #4‐#6}
8. #3 and #7
9. chronic*:ti,ab,kw
10. ((persistent or long*‐term or ongoing or degenerative) near/3 (disease* or disab* or ill* or condition or impairment)):ti,ab,kw
11. long*‐term‐care:ti,ab,kw
12. [mh "cardiovascular diseases"]
13. ((coronary or artery) next (disease* or disorder*)):ti,ab,kw
14. (angina‐pectoris or hypertension or high‐blood‐pressure):ti,ab,kw
15. ((heart or *cardia* or cardio*) next (disease* or disorder* or failure)):ti,ab,kw
16. ((myocardial or brain or cerebral) next (ischemia or infarction)):ti,ab,kw
17. (cerebrovascular or stroke or epilep* or seizure*):ti,ab,kw
18. [mh "lung diseases obstructive"]
19. (obstructive‐lung‐disease* or obstructive‐pulmonary‐disease* or obstructive‐airway*‐disease* or copd or asthma* or bronchitis):ti,ab,kw
20. [mh emphysema]
21. [mh "pulmonary emphysema"]
22. emphysema:ti,ab,kw
23. (cystic‐fibrosis or respiratory‐distress):ti,ab,kw
24. [mh "nervous system diseases"]
25. ((neurologic* or brain) next (disease* or damage* or injur*)):ti,ab,kw
26. (neurodegenerative or Huntington* or Parkinson* or amyotrophic‐lateral‐sclerosis or multiple‐sclerosis or motor‐neuron*‐disease):ti,ab,kw
27. (down* near/2 syndrome):ti,ab,kw
28. (palsy or paralys* or quadriplegi* or tetraplegi* or paraplegi* or locked‐in‐syndrome):ti,ab,kw
29. ((communication or learning or consciousness or language or speech or voice or vision or visual or hearing) next disorder*):ti,ab,kw
30. (hearing‐loss or hearing‐aid* or deaf* or blind* or stutter*):ti,ab,kw
31. [mh "gastrointestinal diseases"]
32. ((intestinal or bowel or colon*) next (disease* or disorder*)):ti,ab,kw
33. (((inflammatory or irritable) next (colon or bowel)) or colitis or crohn* or gastroenter*):ti,ab,kw
34. [mh "nutrition disorders"]
35. (underweight or malnutrition or malnourished or overweight or obes*):ti,ab,kw
36. ((renal or kidney) next (failure or insufficienc* or disease*)):ti,ab,kw
37. (diabetes or diabetic*):ti,ab,kw
38. [mh arthritis]
39. [mh "rheumatic diseases"]
40. (*arthritis or rheumati* or fibromyalgia):ti,ab,kw
41. (((back or neck) next (pain or ache)) or backache):ti,ab,kw
42. [mh "thyroid diseases"]
43. thyroid‐disease*:ti,ab,kw
44. [mh hypersensitivity]
45. (hypersensitivit* or allerg* or intolerance or anaphyla*):ti,ab,kw
46. [mh neoplasms]
47. (cancer* or oncolog* or neoplasm* or carcinom* or tumo*r* or malignan* or leuk*emia):ti,ab,kw
48. [mh "hiv infections"]
49. (hiv‐infect* or hiv‐disease or human‐immunodeficiency‐virus‐infection):ti,ab,kw
50. [mh "mental disorders"]
51. [mh "behavioral symptoms"]
52. ((mental* or psychiatr* or psychological* or behavioral*) next (ill* or disorder* or disease* or distress or disab* or dysfunction* or problem* or health* or patient* or treatment*)):ti,ab,kw
53. ((personality or mood or dysthymic or cognit* or anxiety or stress or eating or adjustment or reactive or somatoform or conversion or behavior* or percept* or thought or psycho* or impulse‐control or development* or attention‐deficit or hyperactivity or conduct or motor‐skills or movement or tic or substance‐related) next disorder*):ti,ab,kw
54. (psychosis or psychoses or psychotic* or paranoi* or schizo* or neurosis or neuroses or neurotic* or delusion* or depression or depressive or bipolar or mania or manic or obsessi* or compulsi* or panic or phobic or phobia or anorexia or bulimia or neurastheni* or dissociative or autis* or Asperger* or Tourette or dyslex* or affective or borderline or narcissis* or suicid* or self‐injur* or self‐harm or adhd):ti,ab,kw
55. (((substance or drug or alcohol) next abuse) or "substance use" or "illegal drug use" or addict* or alcoholism or (problem* near/1 drinking)):ti,ab,kw
56. [mh "congenital hereditary and neonatal diseases and abnormalities"]
57. (congenital* or abnormalit*):ti,ab,kw
58. [mh "chromosome aberrations"]
59. ((genetic or hereditary or chromosome) next (disease* or disorder*)):ti,ab,kw
60. [mh "disease susceptibility"]
61. (susceptib* or predispos*):kw
62. [mh "infant low birth weight"]
63. [mh "infant premature"]
64. (preterm or birth‐weight or (premature near/1 (infant* or birth))):ti,ab,kw
65. ((development* or growth) near/1 (delay* or disorder*)):ti,ab,kw
66. ((newborn or neonatal or infant or child* or adolescent or juvenile) next (disease* or disorder*)):ti,ab,kw
67. [mh "disabled persons"]
68. (disabled or disabilit* or handicap* or impaired or impairment* or dysfunction*):ti,ab,kw
69. ((behavio* or emotion*) near/1 (problem* or disorder*)):ti,ab,kw
70. (sensory‐dysfunction* or sensory‐system‐disorder*):ti,ab,kw
71. [mh "special education"]
72. (special near/1 education):ti,ab,kw
73. ((complex or special) near/3 need*):ti,ab,kw
74. {or #9‐#73}
75. #8 and #74
76. ((lay or user*) near/2 (led or run)):ti,ab,kw
77. (lay near/2 (expert* or person* or worker* or advisor* or consultant* or leader* or educator* or tutor*)):ti,ab,kw
78. (layperson* or expert‐patient* or non‐professional* or nonprofessional* or non‐medical or nonmedical):ti,ab,kw
79. (peer or peers):ti,ab,kw
80. (((self‐help or support*) next (group* or network*)) or ((parent* or carer* or caregiv* or care‐giv*) near/2 (group* or network*)) or (mutual next (aid or support)) or ((social or community) near/2 network*)):ti,ab,kw
81. (parent‐to‐parent):ti,ab,kw
82. social‐support:kw
83. (mentor* or befriend* or buddy or buddies):ti,ab,kw
84. volunteer*:kw
85. ((trained or aide*) near/1 volunteer*):ti,ab,kw
86. ((voluntary or volunteer) next (work* or care* or service* or involvement or health* or help* or counsel* or staff or personnel or provider* or group* or organi*ation* or agenc* or sector or program*)):ti,ab,kw
87. ((online or on‐line or internet or web or electronic or virtual) next communit*):ti,ab,kw
88. {or #76‐#87}
89. #75 and #88
Appendix 5. Journals @ Ovid search strategy
1. (child* or infan* or toddler* or newborn or neonat* or baby or babies or preschool* or pre‐school* or boy? or girl? or schoolchild* or school‐age or adolescen* or pediatric* or paediatric* or youth* or juvenile* or teen* or minors).ti,ab.
2. (parent? or parental or parenting or mother* or maternal or father* or paternal).ti,ab.
3. (carer* or caregiver* or care giver* or guardian* or stepparent* or foster care or foster home* or childrearing or child rearing).ti,ab.
4. 1 and (2 or 3)
5. chronic*.ti,ab.
6. ((persistent or long* term or ongoing or degenerative) adj3 (disease* or ill* or condition* or insufficienc* or disorder*)).ti,ab.
7. long* term care.ti,ab.
8. (cardi* disease* or heart disease* or heart failure or myocardial ischemia or coronary disease* or coronary artery disease* or myocardial infarction or hypertension or high blood pressure).ti,ab.
9. sickle cell.ti,ab.
10. (obstructive lung disease* or obstructive pulmonary disease* or copd or asthma or bronchitis).ti,ab.
11. emphysema.ti,ab.
12. (cystic fibrosis or respiratory distress).ti,ab.
13. (brain adj (disease* or damage* or injur*)).ti,ab.
14. (cerebrovascular or brain ischemia or cerebral infarction or carotid artery disease* or stroke or epilep* or seizure*).ti,ab.
15. (neurodegenerative or Huntington* or Parkinson* or amyotrophic lateral sclerosis or multiple sclerosis or motor neuron disease).ti,ab.
16. (paralys* or quadriplegi* or tetraplegi* or paraplegi* or locked‐in syndrome).ti,ab.
17. ((communication or learning or consciousness or perceptual or language or speech or voice or vision or hearing or psychomotor) adj disorder*).ti,ab.
18. (hearing loss or hearing aid* or deaf* or blind* or stutter*).ti,ab.
19. down* syndrome.ti,ab.
20. cerebral palsy.ti,ab.
21. (gatroenter* or intestinal or bowel or colonic).ti,ab.
22. ((renal or kidney) adj (failure* or insufficienc*)).ti,ab.
23. (diabetes or diabetic*).ti,ab.
24. (underweight or malnutrition or malnourished or overweight or obes*).ti,ab.
25. (arthritis or osteoarthritis or rheumati* or fibromyalgia).ti,ab.
26. ((back or neck) adj pain).ti,ab.
27. thyroid.ti,ab.
28. (hypersensitivit* or allerg* or intolerance or anaphyla*).ti,ab.
29. (cancer* or oncolog* or neoplasm* or carcinom* or tumo?r* or malignan* or leuk?emia).ti,ab.
30. (hiv infect* or hiv disease*).ti,ab.
31. ((mental* or psychiatr* or psychological*) adj (ill* or disorder* or disease* or distress* or disab* or dysfunction* or problem* or health* or patient* or treatment)).ti,ab.
32. ((personality or mood or dysthymic or cognit* or anxiety or stress or eating or adjustment or reactive or somatoform or conversion or behavior or percept* or psycho* or impulse control or development* or attention deficit or hyperactivity or conduct or motor skills or movement or tic or substance related) adj disorder*).ti,ab.
33. (psychos#s or psychotic* or paranoi* or schizo* or neuros#s or neurotic* or delusion* or depression or depressive or bipolar or mania or manic or obsessi* or compulsi* or panic or phobic or phobia or anorexia or bulimia or neurastheni* or dissociative or autis* or Asperger* or Tourette or dyslex* or affective or borderline or narcissis* or suicid* or self injur* or self harm or adhd).ti,ab.
34. (((substance or drug or alcohol) adj abuse) or "substance use" or "illegal drug use" or addict* or alcoholism or (problem* adj1 drinking)).ti,ab.
35. (congenital* or abnormalit*).ti,ab.
36. ((genetic or hereditary or chromosome) adj (disease* or disorder*)).ti,ab.
37. (preterm or low birth weight).ti,ab.
38. growth disorder*.ti,ab.
39. (development* adj1 delay*).ti,ab.
40. ((newborn or neonatal or infant or child* or adolescent or juvenile) adj (disease* or disorder*)).ti,ab.
41. (disabled or disabilit* or handicap* or impaired or impairment* or dysfunction*).ti,ab.
42. ((behavio* or emotion*) adj1 (problem* or disorder*)).ti,ab.
43. ((complex or special) adj3 need*).ti,ab.
44. or/5‐43
45. 4 and 44
46. ((lay or user*) adj2 (led or run)).ti,ab.
47. (lay adj2 (expert* or person* or worker* or advisor* or consultant* or leader* or educator* or tutor*)).ti,ab.
48. (layperson* or expert patient* or non professional* or nonprofessional* or non medical or nonmedical).ti,ab.
49. peer?.ti,ab.
50. (((self help or support*) adj (group* or network*)) or ((parent* or carer* or caregiv* or care giv*) adj2 (group* or network*)) or (mutual adj (aid or support)) or ((social or community) adj2 network*)).ti,ab.
51. parent to parent.ti,ab.
52. (mentor* or befriend* or buddy or buddies).ti,ab.
53. ((trained or aide*) adj1 volunteer*).ti,ab.
54. ((voluntary or volunteer) adj (work* or care* or service* or involvement or health* or help* or counsel* or staff or personnel or provider* or group* or organi#ation* or agenc* or sector)).ti,ab.
55. ((online or on‐line or internet or web or electronic or virtual) adj communit*).ti,ab.
56. or/46‐55
57. 45 and 56
58. random*.ti,ab.
59. (experiment* or intervention*).ti,ab.
60. trial*.ti,ab.
61. placebo*.ti,ab.
62. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab.
63. (pre test or pretest or post test or posttest).ti,ab.
64. (preintervention or postintervention).ti,ab.
65. (cross over or crossover or factorial* or latin square).ti,ab.
66. (assign* or allocat* or volunteer*).ti,ab.
67. (control* or compar* or prospectiv*).ti,ab.
68. (impact* or effect? or chang* or evaluat*).ti,ab.
69. or/58‐68
70. 57 and 69
Appendix 6. BiblioMap search strategy
1 Freetext: "child*" or "infan*" or "toddler*" or "newborn" or "neonat*" or "baby" or "babies" or "preschool*" or "pre‐school*" or "boy" or "boys" or "girl" or "girls" or "schoolchild*" or "school‐age" or "adolescen*" or "pediatric*" or "paediatric*" or "youth*" or "juvenile*" or "teen*" or "minors"
2 Freetext: "parent" or "parents" or "parental" or "parenting" or "mother*" or "maternal" or "father*" or "paternal" or "carer*" or "caregiver*" or "care giver*" or "guardian*" or "stepparent*" or "foster care" or "foster home*" or "childrearing" or "child rearing"
3 Person(s) providing the intervention: community OR community worker OR counsellor OR health promotion practitioner OR lay therapist OR parent OR peer
4 What type of study does this report describe?: intervention OR outcome evaluation OR process evaluation OR RCT OR trial
5 1 AND 2 AND 3 AND 4
Appendix 7. MEDLINE search strategy
1. exp child/
2. exp infant/
3. adolescent/
4. minors/
5. pediatrics/
6. (child* or infant* or newborn* or baby or babies or neonat* or perinatal or adolescen* or youth* or juvenile or teen* or pediatric*).tw,hw.
7. or/1‐6
8. exp parents/
9. exp parent child relations/
10. parenting/
11. child rearing/
12. foster home care/
13. (parent? or parental or parenting or mother* or maternal or father* or paternal).tw.
14. caregivers/
15. (carer* or caregiver* or care giver* or guardian* or foster care or foster home*).tw.
16. or/8‐15
17. 7 and 16
18. chronic*.mp.
19. ((persistent or long* term or ongoing or degenerative) adj3 (disease* or ill* or condition* or insufficienc* or disorder*)).tw.
20. long term care/
21. long* term care.tw.
22. exp cardiovascular diseases/
23. (heart disease* or heart failure or myocardial ischemia or coronary disease* or coronary artery disease* or myocardial infarction or hypertension or high blood pressure).tw.
24. sickle cell.mp.
25. exp lung diseases obstructive/
26. (obstructive lung disease* or obstructive pulmonary disease* or copd or asthma or bronchitis).tw.
27. exp emphysema/
28. exp pulmonary emphysema/
29. emphysema.tw.
30. (cystic fibrosis or respiratory distress).mp.
31. exp nervous system diseases/
32. (brain adj (disease* or damage* or injur*)).tw.
33. (cerebrovascular or brain ischemia or cerebral infarction or carotid artery disease* or stroke or epilep* or seizure*).tw.
34. (neurodegenerative or Huntington* or Parkinson* or amyotrophic lateral sclerosis or multiple sclerosis or motor neuron disease).tw.
35. (paralys* or quadriplegi* or tetraplegi* or paraplegi* or locked‐in syndrome).tw.
36. ((communication or learning or consciousness or perceptual or speech or voice or vision or hearing or psychomotor) adj disorder*).tw.
37. (hearing loss or hearing aid* or deaf* or blind* or stutter*).tw.
38. down* syndrome.tw.
39. cerebral palsy.tw.
40. exp gastrointestinal diseases/
41. (gastroenter* or intestinal or bowel or colonic).tw.
42. renal insufficiency/
43. ((renal or kidney) adj (failure* or insufficienc*)).tw.
44. diabetes mellitus/
45. (diabetes or diabetic*).tw.
46. exp nutrition disorders/
47. (underweight or malnutrition or malnourished or overweight or obes*).tw.
48. exp arthritis/
49. exp rheumatic diseases/
50. (arthritis or osteoarthritis or rheumati* or fibromyalgia).tw.
51. ((back or neck) adj pain).tw.
52. exp thyroid diseases/
53. thyroid.tw.
54. exp hypersensitivity/
55. (hypersensitivit* or allerg* or intolerance or anaphyla*).mp.
56. exp neoplasms/
57. (cancer* or oncolog* or neoplasm* or carcinom* or tumo?r* or malignan* or leuk?emia).tw.
58. exp hiv infections/
59. (hiv infect* or hiv disease*).tw.
60. exp mental disorders/
61. exp behavioral symptoms/
62. ((mental* or psychiatr* or psychological*) adj (ill* or disorder* or disease* or distress* or disab* or problem* or health* or patient* or treatment)).tw.
63. ((personality or mood or dysthymic or cognit* or anxiety or stress or eating or adjustment or reactive or somatoform or conversion or behavior or perception or psycho* or impulse control or development* or attention deficit or hyperactivity or conduct or motor skills or movement or tic or substance related) adj disorder*).tw.
64. (psychos#s or psychotic* or paranoi* or schizo* or neuros#s or neurotic* or delusion* or depression or depressive or bipolar or mania or manic or obsessi* or compulsi* or panic or phobic or phobia or anorexia or bulimia or neurastheni* or dissociative or autis* or Asperger* or Tourette or dyslex* or affective or borderline or narcissis* or suicid* or self injur* or self harm or adhd).tw.
65. (((substance or drug or alcohol) adj abuse) or "substance use" or "illegal drug use" or addict* or alcoholism or (problem* adj1 drinking)).tw.
66. exp "Congenital, Hereditary, and Neonatal Diseases and Abnormalities"/
67. (congenital* or abnormalit*).mp.
68. exp chromosome aberrations/
69. ((genetic or hereditary or chromosome) adj (disease* or disorder*)).mp.
70. exp disease susceptibility/
71. exp infant low birth weight/
72. infant premature/
73. (preterm or low birth weight).tw.
74. growth disorder*.mp.
75. exp disabled persons/
76. (disabled or disabilit* or handicapped or impaired or impairment* or dysfunction*).tw.
77. developmental delay*.tw.
78. ((behavio* or emotion*) adj1 (problem* or disorder*)).mp.
79. exp education special/
80. ((complex or special) adj3 need*).tw.
81. or/18‐80
82. 17 and 81
83. ((lay or user*) adj2 (led or run)).tw.
84. (lay adj2 (expert* or person* or worker* or advisor* or consultant* or leader* or educator* or tutor*)).tw.
85. (layperson* or expert patient* or non professional* or nonprofessional* or non medical or nonmedical).tw.
86. peer group/
87. peer?.tw.
88. self help groups/
89. (((self help or support*) adj (group* or network*)) or ((parent* or carer* or caregiv* or care giv*) adj2 (group* or network*)) or (mutual adj (aid or support)) or ((social or community) adj2 network*)).tw.
90. parent to parent.tw.
91. community networks/
92. mentors/
93. (mentor* or befriend* or buddy or buddies).tw.
94. voluntary workers/
95. ((trained or aide*) adj1 volunteer*).tw.
96. ((voluntary or volunteer) adj (work* or care* or service* or involvement or health* or help* or counsel* or staff or personnel or provider* or group* or organi#ation* or agenc* or sector)).tw.
97. ((online or on‐line or internet or web or electronic or virtual) adj communit*).tw.
98. or/83‐97
99. 82 and 98
100. randomized controlled trial.pt. 101. controlled clinical trial.pt. 102. clinical trial.pt. 103. evaluation studies.pt. 104. comparative study.pt. 105. random*.tw. 106. placebo*.tw. 107. trial.tw. 108. research design/ 109. follow up studies/ 110. prospective studies/ 111. cross over studies/ 112. (experiment* or intervention*).tw. 113. (pre test or pretest or post test or posttest).tw. 114. (preintervention or postintervention).tw. 115. time series.tw. 116. (cross over or crossover or factorial* or latin square).tw. 117. (assign* or allocat* or volunteer*).tw. 118. (control* or compar* or prospectiv*).tw. 119. (impact* or effect? or chang* or evaluat*).tw. 120. or/100‐119 121. exp animals/ not humans.sh. 122. 120 not 121 123. 99 and 122
Appendix 8. Embase search strategy
1. exp child/
2. exp newborn/
3. exp adolescent/
4. exp pediatrics/
5. (child* or infan* or toddler* or newborn or neonat* or baby or babies or preschool* or pre‐school* or boy? or girl? or schoolchild* or school‐age or adolescen* or pediatric* or paediatric* or youth* or juvenile* or teen* or minors).mp.
6. or/1‐5
7. exp parental behavior/
8. (parent? or parental or parenting or mother* or maternal or father* or paternal).mp.
9. (carer* or caregiver* or care giver* or guardian* or stepparent* or foster care or foster home* or childrearing or child rearing).mp.
10. or/7‐9
11. 6 and 10
12. chronic*.mp.
13. ((persistent or long* term or ongoing or degenerative) adj3 (disease* or disorder* or disab* or ill* or condition*)).ti,ab,kw.
14. long term care/
15. long* term care.ti,ab,kw.
16. exp degenerative disease/
17. (neurodegenerative or Huntington* or Parkinson* or amyotrophic lateral sclerosis or motor neuron* disease).ti,ab,kw.
18. exp neurologic disease/
19. (brain adj (damag* or injur*)).ti,ab,kw.
20. multiple sclerosis.ti,ab,kw.
21. exp paralysis/
22. (palsy or paralys* or quadriplegi* or tetraplegi* or paraplegi* or locked‐in syndrome).ti,ab,kw.
23. exp arthritis/
24. exp rheumatic disease/
25. (arthritis or osteoarthritis or rheumati*).ti,ab,kw.
26. exp obstructive airway disease/
27. (obstructive lung disease* or obstructive pulmonary disease* or copd or asthma* or bronchitis).ti,ab,kw.
28. exp emphysema/
29. emphysema.ti,ab,kw.
30. exp diabetes mellitus/
31. (diabetes or diabetic).ti,ab,kw.
32. exp hypertension/
33. (hypertension or high blood pressure).ti,ab,kw.
34. exp cerebrovascular disease/
35. (cerebrovascular disease* or cerebrovascular disorder* or brain ischemia or cerebral infarction or carotid artery disease* or stroke).ti,ab,kw.
36. exp epilepsy/
37. epilep*.ti,ab,kw.
38. exp ischemic heart disease/
39. (myocardial ischemia or angina pectoris or coronary disease* or coronary artery disease* or myocardial infarction).ti,ab,kw.
40. exp heart failure/
41. ((heart or cardiac) adj (failure or disease* or disorder*)).ti,ab,kw.
42. kidney disease/
43. ((renal or kidney) adj (failure* or insufficien* or disease*)).ti,ab,kw.
44. exp colon disease/
45. (colon* disease* or colon* disorder* or colitis or crohn*).ti,ab,kw.
46. ((inflammatory or irritable) adj (colon or bowel)).ti,ab,kw.
47. exp obesity/
48. (obesity or obese).ti,ab,kw.
49. exp human immunodeficiency virus infection/
50. (hiv infect* or hiv disease*).ti,ab,kw.
51. exp osteoporosis/
52. osteoporosis.ti,ab,kw.
53. fibromyalgia/
54. fibromyalgia*.ti,ab,kw.
55. endometriosis.mp.
56. exp thyroid disease/
57. (thyroid adj (disease* or disorder*)).ti,ab,kw.
58. exp neoplasm/
59. (cancer* or oncolog* or neoplasm* or carcinom* or tumo?r* or malignan*).ti,ab,kw.
60. exp mental disease/
61. ((mental* or psychiatr* or psychological*) adj (ill* or disorder* or disease* or distress* or disab* or dysfunction* or problem* or health* or patient* or treatment)).ti,ab,kw.
62. ((personality or mood or dysthymic or cognit* or anxiety or stress or eating or adjustment or reactive or somatoform or conversion or behavior or communication or language or learning or percept* or thought or psycho* or impulse control or development* or attention deficit or hyperactivity or conduct or motor skills or movement or tic or substance related) adj disorder*).ti,ab,kw.
63. (psychos#s or psychotic* or paranoi* or schizo* or neuros#s or neurotic* or delusion* or depression or depressive or bipolar or mania or manic or obsessi* or compulsi* or panic or phobic or phobia or anorexia or bulimia or neurastheni* or dissociative or autis* or Asperger* or Tourette or dyslex* or affective or borderline or narcissis* or suicid* or self injur* or self harm or adhd).ti,ab,kw.
64. (((substance or drug or alcohol) adj abuse) or "substance use" or "illegal drug use" or addict* or alcoholism or (problem* adj1 drinking)).ti,ab,kw.
65. exp "genetic and familial disorders"/
66. exp infant disease/
67. (congenital* or abnormalit*).mp.
68. ((genetic or hereditary or chromosome) adj (disease* or disorder*)).mp.
69. exp disease predisposition/
70. (predispos* or susceptib*).ti,ab,kw.
71. (preterm or low birth weight or premature infant*).ti,ab,kw.
72. exp disability/
73. disabled person/
74. (disabled or disabilit* or handicap* or impaired or impairment* or dysfunction*).mp.
75. exp childhood disease/
76. exp adolescent disease/
77. exp developmental disorder/
78. exp growth disorder/
79. ((development* or growth) adj1 (delay* or disorder*)).ti,ab,kw.
80. ((newborn or neonatal or infant or child* or adolescent or juvenile) adj (disease* or disorder*)).ti,ab,kw.
81. ((behavio* or emotion*) adj1 (problem* or disorder*)).ti,ab,kw.
82. exp sensory dysfunction/
83. (deaf* or blind* or ((vision or visual or hearing) adj disorder*) or hearing loss or hearing aid*).ti,ab,kw.
84. exp speech disorder/
85. (((speech or voice) adj disorder*) or stutter*).ti,ab,kw.
86. exp special education/
87. ((complex or special) adj3 need*).ti,ab,kw.
88. or/12‐87
89. 11 and 88
90. ((lay or user*) adj2 (led or run)).ti,ab,kw.
91. (lay adj2 (expert* or person* or worker* or advisor* or consultant* or leader* or educator* or tutor*)).ti,ab,kw.
92. (layperson* or expert patient* or non professional* or nonprofessional* or non medical or nonmedical).ti,ab,kw.
93. peer group/
94. peer counseling/
95. peer?.ti,ab,kw.
96. (((self help or support*) adj (group* or network*)) or ((parent* or carer* or caregiv* or care giv*) adj2 (group* or network* or support)) or (mutual adj (aid or support)) or ((social or community) adj network*)).mp.
97. parent to parent.ti,ab,kw.
98. (mentor* or befriend* or buddy or buddies).ti,ab,kw.
99. voluntary worker/
100. voluntary program/
101. volunteer/
102. ((trained or aide*) adj1 volunteer*).ti,ab,kw.
103. ((voluntary or volunteer) adj (work* or care* or service* or involvement or health* or help* or counsel* or staff or personnel or provider* or group* or organi#ation* or agenc* or sector)).ti,ab,kw.
104. ((online or on‐line or internet or web or electronic or virtual) adj communit*).ti,ab,kw.
105. or/90‐104
106. 89 and 105
107. randomized controlled trial/
108. controlled clinical trial/
109. single blind procedure/ or double blind procedure/
110. crossover procedure/
111. random*.tw.
112. trial.tw.
113. placebo*.tw.
114. ((singl* or doubl*) adj (blind* or mask*)).tw.
115. (experiment* or intervention*).tw.
116. (pre test or pretest or post test or posttest).tw.
117. (preintervention or postintervention).tw.
118. (cross over or crossover or factorial* or latin square).tw.
119. (assign* or allocat* or volunteer*).tw.
120. (control* or compar* or prospectiv*).tw.
121. (impact* or effect? or chang* or evaluat*).tw.
122. time series.tw.
123. or/107‐122
124. 106 and 123
Appendix 9. PsycINFO search strategy
1. ("100" or "120" or "140" or "160" or "180" or "200").ag.
2. (child* or infan* or toddler* or newborn or neonat* or baby or babies or preschool* or pre‐school* or boy? or girl? or schoolchild* or school‐age or adolescen* or pediatric* or paediatric* or youth* or juvenile* or teen* or minors).ti,ab,hw,id.
3. or/1‐2
4. exp parents/
5. exp parenting/
6. (parent? or parental or parenting or mother* or maternal or father* or paternal).ti,ab,hw,id.
7. (carer* or caregiver* or care giver* or guardian* or stepparent* or foster care or foster home* or childrearing or child rearing).ti,ab,hw,id.
8. or/4‐7
9. 3 and 8
10. chronic*.ti,ab,hw,id.
11. ((persistent or long* term or ongoing or degenerative) adj3 (disease* or disorder* or disab* or ill* or condition*)).ti,ab,hw,id.
12. long term care/
13. long* term care.ti,ab,id.
14. exp nervous system disorders/
15. (neurodegenerative or Huntington* or Parkinson* or amyotrophic lateral sclerosis or motor neuron* disease).ti,ab,hw,id.
16. (paralys* or palsy or quadriplegi* or tetraplegi* or paraplegi* or locked‐in syndrome).ti,ab,hw,id.
17. multiple sclerosis.ti,ab,hw,id.
18. (arthritis or osteoarthritis or rheumati*).ti,ab,hw,id.
19. (obstructive lung disease* or obstructive pulmonary disease* or copd or asthma* or bronchitis or bronchial).ti,ab,hw,id.
20. emphysema.ti,ab,hw,id.
21. (diabetes or diabetic).ti,ab,hw,id.
22. ((cardiovascular or cerebrovascular or coronary or artery) adj (disease* or disorder*)).ti,ab,hw,id.
23. ((myocardial or brain or cerebral) adj (ischemia or infarction)).ti,ab,hw,id.
24. (stroke or epilep* or seizure*).ti,ab,hw,id.
25. ((heart or cardiac) adj (disease* or disorder* or failure)).ti,ab,hw,id.
26. (hypertension or high blood pressure).ti,ab,hw,id.
27. (dementia or alzheimer*).ti,ab,hw,id.
28. ((renal or kidney) adj (failure* or insufficienc* or disease*)).ti,ab,hw,id.
29. (colon* disease* or colon* disorder* or colitis or irritable bowel syndrome).ti,ab,hw,id.
30. (obesity or obese).ti,ab,hw,id.
31. exp hiv/
32. (hiv infect* or hiv disease*).ti,ab,id.
33. osteoporosis.ti,ab,hw,id.
34. fibromyalgia*.ti,ab,hw,id.
35. exp neoplasms/
36. (cancer* or oncolog* or neoplasm* or carcinom* or tumo?r* or malignan*).ti,ab,hw,id.
37. exp mental disorders/
38. exp behavior disorders/
39. ((mental* or psychiatr* or psychological*) adj (ill* or disorder* or disease* or distress* or disab* or dysfunction* or problem* or health* or patient* or treatment)).ti,ab,hw,id.
40. ((personality or mood or dysthymic or cognit* or anxiety or stress or eating or adjustment or reactive or somatoform or conversion or behavior or communication or language or learning or percept* or thought or psycho* or impulse control or development* or attention deficit or hyperactivity or conduct or motor skills or movement or tic or substance related) adj disorder*).ti,ab,hw,id.
41. (psychos#s or psychotic* or paranoi* or schizo* or neuros#s or neurotic* or delusion* or depression or depressive or bipolar or mania or manic or obsessi* or compulsi* or panic or phobic or phobia or anorexia or bulimia or neurastheni* or dissociative or autis* or Asperger* or Tourette or dyslex* or affective or borderline or narcissis* or suicid* or self injur* or self harm or adhd).ti,ab,hw,id.
42. (((substance or drug or alcohol) adj abuse) or "substance use" or "illegal drug use" or addict* or alcoholism or (problem* adj1 drinking)).ti,ab,hw,id.
43. exp disabilities/
44. (disabled or disabilit* or handicap* or impaired or impairment* or dysfunction*).ti,ab,hw,id.
45. exp genetic disorders/
46. exp congenital disorders/
47. (congenital* or abnormalit*).ti,ab,hw,id.
48. ((genetic or hereditary or chromosome) adj (disease* or disorder*)).ti,ab,hw,id.
49. "susceptibility (disorders)"/
50. predisposition/
51. exp neonatal disorders/
52. premature birth/
53. birth weight/
54. (preterm or low birth weight or premature infant*).ti,ab,hw,id.
55. growth disorder*.ti,ab,hw,id.
56. exp intellectual development disorder/
57. down* syndrome.ti,ab,hw,id.
58. exp delayed development/
59. (development* adj1 delay*).ti,ab,hw,id.
60. ((newborn or neonatal or infant or child* or adolescent or juvenile) adj (disease* or disorder*)).ti,ab,hw,id.
61. ((behavio* or emotion*) adj1 (problem* or disorder*)).mp.
62. exp communication disorders/
63. exp sensory system disorders/
64. (deaf* or blind* or hearing disorder* or vis* disorder* or hearing loss or hearing aid*).ti,ab,hw,id.
65. special education/
66. ((complex or special) adj3 need*).ti,ab,hw,id.
67. or/10‐66
68. 9 and 67
69. ((lay or user*) adj2 (led or run)).ti,ab,hw,id.
70. (lay adj2 (expert* or person* or worker* or advisor* or consultant* or leader* or educator* or tutor*)).ti,ab,hw,id.
71. (layperson* or expert patient* or non professional* or nonprofessional* or non medical or nonmedical).ti,ab,id.
72. peer?.ti,ab,hw,id.
73. (((self help or support*) adj (group* or network*)) or ((parent* or carer* or caregiv* or care giv*) adj2 (group* or network*)) or (mutual adj (aid or support)) or ((social or community) adj2 network*)).ti,ab,hw,id.
74. parent to parent.ti,ab,id.
75. social support/
76. mentor/
77. (mentor* or befriend* or buddy or buddies).ti,ab,id.
78. volunteers/
79. ((trained or aide*) adj1 volunteer*).tw.
80. ((voluntary or volunteer) adj (work* or care* or service* or involvement or health* or help* or counsel* or staff or personnel or provider* or group* or organi#ation* or agenc* or sector)).ti,ab,hw,id.
81. ((online or on‐line or internet or web or electronic or virtual) adj communit*).ti,ab,hw,id.
82. or/69‐81
83. 68 and 82
84. random*.ti,ab,hw,id.
85. (experiment* or intervention*).ti,ab,hw,id.
86. trial*.ti,ab,hw,id.
87. placebo*.ti,ab,hw,id.
88. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
89. treatment effectiveness evaluation/
90. mental health program evaluation/
91. (pre test or pretest or post test or posttest).ti,ab,hw,id.
92. (preintervention or postintervention).ti,ab,hw,id.
93. (cross over or crossover or factorial* or latin square).ti,ab,hw,id.
94. (assign* or allocat* or volunteer*).ti,ab,hw,id.
95. (control* or compar* or prospectiv*).ti,ab,hw,id.
96. (impact* or effect? or chang* or evaluat*).ti,ab,hw,id.
97. time series.ti,ab,hw,id.
98. exp experimental design/
99. ("0430" or "0450" or "0451" or "1800" or "2000").md.
100. or/84‐99
101. 83 and 100
Data and analyses
Comparison 1. Peer support vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Psychological distress | 8 | 864 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.11] |
| 1.2 Confidence and self‐efficacy | 8 | 542 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.14, 0.21] |
| 1.3 Perception of coping | 3 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.38, 0.21] |
| 1.4 Quality of life | 2 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.32, 0.38] |
| 1.5 Family functioning | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.09, 0.38] |
| 1.6 Perceived social support | 4 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.15, 0.77] |
| 1.7 Confidence and skill at navigating medical services | 4 | 304 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.17, 0.28] |
Comparison 2. Peer support vs alternative intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Psychological distress | 2 | 95 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.38, 0.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aiello 2015.
| Study characteristics | ||
| Methods | Randomized controlled trial; usual care control. | |
| Participants | 22 mothers of children with severe and profound bilateral sensorineural hearing loss (11 in experimental group, 11 in control). The mean age of the children in experimental group was 18.2 months (SD = 6.2), and in control was 22 months (SD = 7.5) | |
| Interventions |
Online facilitated peer support Parents have free access to asynchronous communication tools to share text messages, photos, videos, and participate in discussion forums. The tools are moderated by speech pathologists and a psychologist, who propose topics and answer questions and comments but do not interfere in direct interactions between participants. Majority of participants accessed the network less than once per week (n=6, 54%), and majority contributed to matters already under discussion (n=7, 44%), rather than generate their own posts. Usual care not described |
|
| Outcomes |
Primary
Secondary
Measured at baseline and at 3 months following baseline. |
|
| Notes | Contacted authors to request complete outcomes data as published data is not suitable for meta‐analysis. No response to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization asserted but no method described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were, necessarily, aware of their allocation. No mention is made of blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some measures (e.g. rating content of posts) were unsuitable for blind assessment. Others (via online collection form) may have been assessed blindly but processes not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Main outcome of interest reported |
| Selective reporting (reporting bias) | Low risk | This is a small study and given that the main measure (parenting stress) is reported as not being as expected, selective reporting seems unlikely to be a major issue. |
| Other bias | Low risk | None apparent |
Boogerd 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial; wait list control | |
| Participants | 189 parents of children aged under 13 years with type 1 diabetes initially agreed to participate. Forty‐seven parents dropped out before filling out the final questionnaire, leaving N=105 in total (54 in intervention, 51 in control). | |
| Interventions |
Online parent support group with facilitator Online peer support facilitated through a chat application, a forum, and a blog. Parents and health professionals communicate in real time via the chat app. Parents and professionals can read and post messages on the forum. Nurse practitioners moderate the forum daily and answer questions via the app. OTHER COMPONENTS: 1. Information sharing relating to treatment goals between parent and relevant professionals (private, 1‐to‐1) 2. Downloadable documents and web links Care as usual: This included multidisciplinary care provided by a team of pediatric diabetologists, diabetes nurse practitioners, dietitians, and psychologists. Pediatric diabetologists and nurse practitioners were seen 4x per year. Dieticians and psychologists were available on request, or by referral from the treating team. The care team was contactable during business hours by all parents, and an emergency line was set up for after hours use. Participants in the experimental group received both the online parent support group and care as usual. The control group received only care as usual. |
|
| Outcomes |
Primary
Secondary
Measured at baseline and 6 months following baseline. Protocol also mentions a 12 month follow‐up but this was not reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation conducted by independent researcher. Random sequence of red/green cards used to allocate (colour cannot be discerned prior to allocation) |
| Allocation concealment (selection bias) | High risk | Authors state “when participants have sent back their filled out baseline questionnaire, they are informed about the allocation”. Control is wait‐list so allocation would in any case be problematic |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors state “this study is not blinded”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report (measure of interest, parenting stress). Site use metrics and child health outcomes derived from computer and medical files. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition is well reported across the life of the study. Some reasons suggested, and attempts made to assess extent of and reasons for incompleteness (likely because this is a feasibility study and they are trying to determine what attrition to expect in a full study). |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to have been reported, regardless of whether they showed a significant difference between intervention and control. |
| Other bias | Low risk | None apparent |
Boylan 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial; wait‐list control | |
| Participants | 147 parents and full‐time carers of young people (16 years and under) with deliberate self‐harm (DSH) or suicidal behaviour. 65 completed all 3 assessment blocks. | |
| Interventions |
Parent support groups with facilitator Face‐to‐face and powerpoint sessions with small and large group discussions and handouts. Covers support, information on DSH, parent‐child communication, parenting adolescents, managing an episode of DSH, services, and parent self‐care. Eight sessions of 90 minutes' duration. |
|
| Outcomes |
Primary Parent mental health well‐being
Secondary Social support, stress, satisfaction with parenting (no data provided for these measures).
Outcomes measured at baseline and 3 months following baseline. |
|
| Notes | Contacted authors for more information; no reply as at August 2016. Abstract as written has between/within subjects ANOVA for participants completing all 3 assessment blocks (n=65) on GHQ only. This total N was not split into intervention and control, so it was not possible to derive a standardised mean difference using the F‐statistic (Lipsey and Wilson, 2001) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data provided for GHQ measure only; however this publication is an abstract only so other outcome data may have been available |
| Selective reporting (reporting bias) | Unclear risk | Data provided for GHQ measure only; however this publication is an abstract only so other outcome data may have been available |
| Other bias | Unclear risk | Insufficient information to judge |
Ferrin 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial, psycho‐education vs parent support group. | |
| Participants | 81 Parents of children and adolescents with ADHD intervention (for review purposes, parent support group) n=37, control (psycho‐education therapy) n=44. Following attrition, n=36 intervention and n=40 control at follow up. Children were aged from 5‐18 years. | |
| Interventions | For the purposes of this review, the intervention is the study support group control. Parent support group with facilitator. Weekly sessions where families could share thoughts and experiences in safe, non‐directive environment. Therapist present but precluded from providing feedback, psycho‐education, information or advice. Control was psycho‐education on ADHD and some behavioural strategies; there was also opportunity provided for group discussion and support. |
|
| Outcomes |
Primary Child ADHD Secondary Parenting stress, impact on family; other child measures.
Outcomes were measured at baseline, at the completion of the 12‐week program, and at 12‐month follow‐up (latter used in meta‐analysis). |
|
| Notes | The intervention of interest in this review (peer support) is the control group in this trial report. It is expected the control would do better in this case. Therefore, this study contributes to comparison 2 in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was asserted but not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. Personnel running study could not be, but fidelity checks were used to ensure lack of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors and clinicians rating outcomes were described as blinded, but method was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data available via supplement. Conflicting Ns published, but the peer‐reviewed version has more information. Assuming the discrepancy is an error that was not caught because supplement not peer reviewed. |
| Selective reporting (reporting bias) | Low risk | All measures available in supplement |
| Other bias | Low risk | No other sources of bias apparent |
Flores 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial, usual care control | |
| Participants | 220 parents (112 intervention and 108 control) of children with asthma. Ages of children included in the study ranged from 2‐12 years. | |
| Interventions |
Parent mentors Mentors for parents of minority children with asthma, acting as peer counsellors and parent resources, collaborating with the clinical team. Parent mentors held an initial meeting with their assigned families in their home, then monthly community meetings, to provide social networking and peer support. Second home visit 6 months after first. Monthly phone calls for first year after initial hospital visit; mentor was also available to families by phone 24/7. Parent mentors were experienced African‐American or Latino parents of children with asthma. matched with participants by race/ethnicity, primary language, and zip code. Control was pediatric asthma care as usual. Care as usual was not described. |
|
| Outcomes | Review‐relevant outcomes: Primary Caregiver's quality of life; parent asthma management self‐efficacy; satisfaction with care
Outcomes measured at baseline and 12 months. |
|
| Notes | This study also measured cost effectiveness via costs of intervention, costs of care (differences between intervention and control), incremental cost‐effectiveness ratio, and person‐level indirect costs. SDs derived from confidence intervals. Authors conducted post‐hoc analysis with participants split into high and low participation groups, according to their rates of attending meetings and completing scheduled phone calls. We used high participation vs control data for our meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used SAS for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes measured by telephone interview conducted by RA blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported in both groups. IT and per‐protocol analyses done depending on measure; effect was apparent in both, and per‐protocol analyses were clearly indicated |
| Selective reporting (reporting bias) | Low risk | All measures mentioned in hypotheses were reported |
| Other bias | Low risk | No other sources of bias apparent |
Ireys 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial, usual care control. | |
| Participants | 42 mothers of children with Juvenile Rheumatoid Arthritis (23 intervention, 19 control). Children were aged between 2 and 11. | |
| Interventions |
Parent mentors Each mentor supports 5 families. Individual support (informational, affirmational, and emotional) via fortnightly telephone contact and six‐weekly individual meetings. Occasional group events,such as picnics or lunches. Support provided over 15 months. Parent mentors were mothers of children aged 18‐24 who had had JRA since childhood. Mentors supervised by psychologist and social worker. Details of control not provided; implied that usual care was given. |
|
| Outcomes |
Primary Mental health, social support
Outcomes measured at baseline, 7.5 months following baseline (not reported) and 15 months following baseline. |
|
| Notes | Perceived availability of support used two dichotomous/count measures in addition to Ireys' social support inventory (we were not able to obtain further information about this latter scale). SDs for means were not available and were imputed. We attempted to contact the author but could not. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Names drawn from hat |
| Allocation concealment (selection bias) | Unclear risk | Concealment method not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unable to be blinded due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if those collecting data were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors reported overall attrition, which was quite low and relatively equal across groups. Although the reasons for attrition were not given, as the numbers were low, it is unlikely to have introduced bias. No mention was made of any data being excluded from analysis. No mention of re‐inclusions were made. |
| Selective reporting (reporting bias) | High risk | Standard deviations were not given for any scale at any time point. In addition, T2 was not reported on or included in analysis. |
| Other bias | High risk | There was a significant difference between experimental and control group by functional status of child. Although this was reported to be controlled for in analysis, the scale used to measure functional status was not described in method section and the data for this was not given. The full statistics for the analysis used were also not given. The statistical analysis used is also not clear. T‐test is mentioned before one analysis, however, in a later analysis ‘covariates’ are mentioned, which alludes to analysis of variance. |
Ireys 2001.
| Study characteristics | ||
| Methods | Randomised controlled trial; control was usual care plus phone access to experienced parent if wanted. | |
| Participants | 161 parents of children aged 7‐11 with a chronic illness (86 intervention, 75 control). Following attrition, experimental = 73, control = 66 at T2. | |
| Interventions |
Parent mentor Parent mentors providing informational, affirmational, and emotional support. Parents of child with chronic illness linked with a veteran "Network mother (NM)" who had a child (now older) who had a chronic illness in childhood. NM made 7 visits of 60‐90 minutes to assigned family, plus biweekly telephone contact of at least 5 minutes. Parents participated in 3 social events to meet other parents in program. Control: parents had phone access to another experienced parent, but with no training and who did not initiate contact. 3% of control mothers called the experienced parent. |
|
| Outcomes |
Primary maternal anxiety, maternal depression, stressful life events
Secondary physical health of mother, dose of intervention Outcomes were measured at baseline and 12 months following baseline. Some outcomes were also measured at 4, 8, and 16 months following but these were not reported. |
|
| Notes | NB: no data available for PEDRI‐LS or BDI. We attempted to contact the author but could not. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants' names drawn from hat. |
| Allocation concealment (selection bias) | Low risk | Participants not allocated to groups until after baseline interview. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could not be blinded due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Interviewers were reported to be extensively trained in collecting data and were kept blind to group assignment. Post‐test questionnaires pertaining to group assignment were asked only after all other parts of the interview were completed to ensure interviewers could be kept blind during this time. However, the study authors were in charge of reviewing all interviews once completed for any missing information or clarification. The study authors were not blind to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion numbers reported in appropriate detail. Reasons for attrition not given (lost to follow up only). Attrition numbers between experimental and control group were small and comparable. Stated that an intention to treat analysis was conducted in the method section, however, this was not reported on or described in the results section. Numbers were small, so unlikely to affect outcome. Individuals lost to follow up were reported as being more likely to be on welfare. No statistical data for this analysis was reported. |
| Selective reporting (reporting bias) | High risk | No data relating to gender, age, health status, depression scores or life events scale reported, included Ms and SDs. The statistical analysis conducted for these were described as not showing any effects, but data not given. PSI Anxiety sub‐scales scores were reported on and analyses shown (these were the only analyses to show significance). However, data used was baseline and 12 months after baseline. The intervention duration was 15 months. No data collected at 4, 6, 8 and 16 months was reported. It is also unclear what data was collected at this time. |
| Other bias | Low risk | No other sources of bias evident |
Kutash 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial, usual care control | |
| Participants | 115 parents and children (60 in intervention, 55 in comparison) attending self‐contained special education classrooms for students with emotional disturbances (ED) (n=46 intervention and n=47 comparison at follow up). Children were aged between 12 ‐ 17 years. | |
| Interventions |
Peer‐to‐peer support from veteran parents (Parent Connectors (PCs)). PCs contact participants by telephone weekly during the school year, to provide emotional, informational, and instrumental support. PCs were mothers of youths with ED who had a history of relative success in negotiating school and mental health systems. Control was usual care; parents were indirectly exposed to teacher training on parent involvement which was offered in both intervention and comparison conditions. |
|
| Outcomes |
Primary Parent function (mental health service efficacy, strain, family empowerment, need for support, hopefulness); perceived benefit of engagement with services; influence of social norms; perceived services efficacy Secondary Parent engagement with school and MH systems; student engagement with MH services; student school function.
Outcomes were measured at baseline and 9 months following baseline. |
|
| Notes | Same intervention as Kustash 2013; participant numbers are similar but not identical and comparison seems to be different so we have classed this as a separate study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Parents in PC group were told a PC would be contacting them to engage in weekly phone conversations. Comparison group were told that research staff would work with child’s teachers on strategies to build positive relationships with parents. Possible but not certain that intervention group knew they were in intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Baseline and follow‐up interviews were conducted by telephone. Very possible that interviewers could have been blinded to intervention status but not made clear. Data also from school MH providers and school records; again, presumably this could have been blinded but not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data in all outcome measures, both from attrition and from incomplete answers in 2nd questionnaire. |
| Selective reporting (reporting bias) | Low risk | Appears to be complete reporting across outcome measures, including non significant findings for several outcome measures. |
| Other bias | Unclear risk | As teachers from both conditions completed training on engaging parents, this is a possible confound. Although the control participants also had teachers who received the training (which should have controlled for any extra benefit over and above the parent support) participation in the training was voluntary and numbers are not reported. It may be that any effect of training was not controlled for in practice. |
Kutash 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial, information mailing control | |
| Participants | 128 parents and children attending self‐contained special education classrooms for students with emotional disturbances (ED) (66 intervention, 62 control). 112 completed the follow up (56 intervention, 56 control). Children were aged between 12‐16 years. | |
| Interventions |
Peer‐to‐peer support from veteran parents (Parent Connectors (PCs)). PCs contact participants by telephone weekly during the school year, to provide emotional, informational, and instrumental support. PCs were mothers of youths with ED who had a history of relative success in negotiating school and mental health systems. Control was informational mailings (three over the year, to intervention and control parents) on topics related to special education, mental illness, and parenting. |
|
| Outcomes |
Primary Expected benefit mental health; expected benefit education; social norms mental health; social norms education; perceived influence over education and mental health systems Secondary Parent engagement; student engagement; student performance, parent satisfaction.
Parent strain was provided as additional data from the authors, measured pre‐ and post‐intervention for both conditions. Outcomes were measured at baseline, and 9 months following baseline. |
|
| Notes | This is the same intervention as Kutash 2011 but is a separate study. Each continuous outcome was modelled as a function of the outcome prior to the intervention, level of caregiver strain, level of child emotional impairment, and treatment group. Caregiver strain was not an outcome of interest for the authors, but it is the most appropriate outcome for the purpose of this review. We contacted the authors for pre‐post means, SDs, and Ns (rather than the effects estimates provided in the publication). These were provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Students paired according to school attended and time entered study (to ensure balanced group sizes within schools); assigned to intervention or control using random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Parents would have been aware that they were receiving the PC intervention; both groups received three informational mailings but it is possible that participants knew that they were receiving the intervention versus control. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Baseline and follow‐up interviews were conducted by telephone. Very possible that interviewers could have been blinded to intervention status but not made clear. Data also from school mh providers and school records; again, presumably this could have been blinded but not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data seems to be complete for proximal outcomes; for distal outcomes the only information is ‘n varied between 41 and 60 depending on measures'. Attrition rates by group were provided by authors on request. |
| Selective reporting (reporting bias) | Low risk | Appears to be complete reporting across outcome measures, including non significant findings for several outcome measures. |
| Other bias | Low risk | No other sources of bias apparent |
McCallion 2004.
| Study characteristics | ||
| Methods | Partial crossover with wait‐list control | |
| Participants | 97 grandparents with primary care of at least one grandchild with a developmental delay or disability (49 intervention, 48 control at baseline; 49 intervention, 46 control after 3 months). These included intellectual or other developmental disabilities, learning problems or attention deficit and hyperactivity disorders. Children were an average age of 11. Five children in the study were 21 years old. | |
| Interventions |
Support groups with trained leaders (from local community agencies) and education (topics chosen by members). 8‐10 grandparent caregivers attended 6 fortnightly group meetings of 90 minutes duration. In addition to educational topics, sessions covered self‐care such as stress reduction, relaxation, nutrition, and own health needs. Participants also received active case management. Control was active case management by a single trained agency staffer. |
|
| Outcomes |
Primary Depression; sense of empowerment; caregiving mastery.
Outcomes were measured at baseline and 3 months following baseline. |
|
| Notes | FES was given as three separate sub‐scales: Family, Services, and Community. Looking at the items (Family Empowerment Scale, Koren, DeChillo, & Friesen 1992) it makes most sense to include the Family and Services sub‐scales under Family Functioning. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment asserted but not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition, and data analysed as intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | No indication of possible selective reporting; program developers’ stated outcomes of interest were all reported in this study. |
| Other bias | High risk | Possible bias from recruitment. Agencies running the intervention were responsible for recruiting; not reported how many eligible participants declined to participate. All participants (eventually) received the intervention; without knowing whether or not this was disclosed during recruitment, and what proportion of potential participants declined, can’t know how many people self‐selected in to the study. Recruiters may have consciously or unconsciously selected for inclusion. |
Preyde 2003.
| Study characteristics | ||
| Methods | Quasi RCT with usual care control | |
| Participants | 60 mothers with single or twin preterm births; 32 recruited to intervention, 28 recruited to control (24 intervention and 25 control completed trial). Children were aged between 25 to 29 weeks. | |
| Interventions |
Parent‐to‐parent support Participants invited to connect with parent buddy. Buddies were mothers who appeared to have adjusted successfully to their previous experience of very preterm births (as determined by social work clinical assessment) and who had attended 5 hours of communication skills and self‐awareness training. Number and duration of buddy contacts varied according to participant preference; assessed as an outcome rather than a set amount being mandated as part of intervention. Intervention participants also attended parent group support meetings; details not reported. Control was medical care and social work services as usual. |
|
| Outcomes |
Primary Parental stress, anxiety, depression. Secondary Perceived social support, proneness to anxiety in response to stressful events, number and duration of buddy contacts and group sessions, satisfaction (scale not described, possibly Likert).
Outcomes measured at baseline, 4 weeks following baseline, and 16 weeks following baseline. We used the 16‐week outcomes for meta‐analysis. |
|
| Notes | Consulted with Cochrane method advisor on classification as quasi‐RCT design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation to intervention or control depended on referral to one of two NICUs. Both NICUs took referrals, on alternate days, from the same geographic region. Allocation took place prior to recruitment to study and was not within control of researchers. Cochrane lists admission date‐based sequence rules as at high risk of bias. |
| Allocation concealment (selection bias) | High risk | No allocation concealment possible. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured by survey instruments completed by participants who were not blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participation and attrition rates were reported; differences in attrition were reported and did not differ significantly between groups. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported. |
| Other bias | Low risk | No other sources of bias apparent. |
Rhodes 2008.
| Study characteristics | ||
| Methods | Randomised controlled trial, Alternative treatment | |
| Participants | 20 parents of children aged 12‐16 years with diagnosis of anorexia nervosa (10 intervention, 10 control) | |
| Interventions |
Parent‐to‐parent support The "Maudsley approach" is a family‐based approach to treating anorexia nervosa which gives parents responsibility for re‐feeding their child in the home. The intervention added to this by linking parents with 'consultant' veteran parents. Parents met with their therapist, the consultant parent, and the therapist of the consultant parent. In most sessions interaction was strictly controlled by therapists, with questions to consultant parent directed through their therapist. Howevever, 10 minutes were set aside in each session for parents to discuss privately with consultant parent, without either therapist being present. Up to 20 sessions, completing earlier if child weight successfully restored earlier. Control was Maudsley model of family‐based treatment alone. |
|
| Outcomes |
Primary Parent self‐efficacy; child distress; child weight outcomes
Outcomes were measured at 2 weeks prior to intervention, and weekly during the intervention. Outcomes for week 6 were reported. |
|
| Notes | Only means and Ns available for parent self‐efficacy. No effect of intervention compared with control (difference in log likelihood = 0.93, df = 1, Cohen's omega = 0.07, observed power = 0.16). Authors approached for more data, none available. Could not include this study in meta‐analysis. Qualitative data published separately, in Rhodes et al. 2009. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated using SPSS. |
| Allocation concealment (selection bias) | Low risk | Therapists given a sealed envelope containing group allocations at week 1 of treatment; unaware until invited to consultation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Allocation concealment would have helped to reduce the risk of bias. However, as therapists were involved in the parent consultation process, both parents and therapist would have been aware of what group participants were in following the interview. It is unclear if participants knew they were in a different group to controls, as the control in this study was an alternate treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Those conducting interviews knew which group participants were in. It is unclear if those entering quantitative data and scoring outcomes were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All attrition was reported with most reasons given. However, reasons for dropping out of the treatment were not given. Appeared to manage missing data appropriately. However, To reduce the impact of attrition, data included past week 6 was not included in the analysis. This leads to a high risk of bias. There may be something in the treatment at week 6 that participants find adverse in some way; treatment continues up to 20 sessions so this is potentially a large proportion of the intervention which is missed. Also did not give a break down of the attrition by intervention vs control. |
| Selective reporting (reporting bias) | High risk | Results of statistical analysis given. However, M, SDs and other raw data not included for all outcomes. This was only included for %IBW. |
| Other bias | High risk | There appeared to be differences between the intervention and control in regards to the family make up and also rate of other disorders (OCD, Depression). Statistical analysis were not run, so it is unclear if these were significant differences. This would have been an under‐powered analysis even so, so it is unclear if these differences would have an impact on outcomes. There were more single parents in the intervention group. As the treatment relies solely on the parent, there may have been added difficulty for intervention parents to carry out these tasks without the support of a partner. It is difficult to state then whether adding parent consultation to a group of mostly single parents would have added extra treatment benefit, or perhaps ‘levelled the playing field’ between intervention and control. |
Roberts 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial, alternative treatment and wait‐list controls. | |
| Participants | 84 parents of pre‐school aged children with an Autism Spectrum Disorder referred for treatment (29 intervention, 27 home‐based control, 28 wait‐list control; 95 enrolled but 10 withdrew before start of intervention, and 1 was withdrawn by staff due to the small group environment proving too stressful for the child). | |
| Interventions |
Facilitated support groups Held at the same centre as associated child playgroups. The parent component was manualized, with set topics chosen and discussed by members. Parents encouraged to share information, concerns, and achievements and to form a support network. 40 weekly sessions of one hour, running over a year. 4‐6 children per playgroup, corresponding groups for parents. Two comparison conditions: alternative treatment of home visits with child and parent (2hrs each fortnight over 40 weeks, max 20 sessions), focusing on helping parent and child work effectively together; and a wait‐list control. |
|
| Outcomes |
Primary Parent stress, perception of competence in managing child, and quality of life; various child outcomes.
Secondary Autism diagnosis, child mental development, demographics, other service use. Timing of baseline and follow‐up outcomes measurement was unclear. |
|
| Notes | For the purposes of meta‐analysis, only the intervention and wait‐list control outcomes are used; comparisons with alternative treatment controls are not made. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by statistician using computer‐generated random number tables |
| Allocation concealment (selection bias) | High risk | Allocation was reported as not concealed from child or family. ~30 participants chose to withdraw from the study as they did not prefer the group they were allocated to, and participated in preferred group instead. No analysis was completed on these individuals. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Staff running the home‐based and centre‐based programs were not aware of which children and families were enrolled in research. Families and children were aware of their allocation, however, most measures were completed via diagnostic/assessment interview. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, data entry team, and baseline and outcome assessors reported as blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A large group of participants opted out of the study as they did not get randomised to their chosen intervention. Their data was not included in analysis, but this represents incomplete data from original recruitment. No intention to treat analysis was conducted with other participants who chose to withdraw from the study. |
| Selective reporting (reporting bias) | Low risk | All measures and demographics reported. |
| Other bias | High risk | Differences on main outcome measures were not tested for at baseline. There may have been a significant difference between groups on baseline PSI scores. This was alluded to but not statistically examined. Important because although there was a random sequence generated, this was not maintained over allocation with participants withdrawing, potentially non‐uniformly, depending on whether or not they liked the condition they were allocated to. Additionally, there were more participants in the control group with severe ASD and severe language difficulties. Participants in the wait‐list control group accessed a greater number of additional services. Authors also made the decision to not include children who they did not believe were ready for group participation from the study as a whole. Although the rationale for this is clear, the criteria for determining this was not transparent. Required sample size (30 in each group) was not met. |
Ruffolo 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial, treatment as usual control | |
| Participants | 94 parents of children with serious emotional disturbance (58 intervention and 36 control; following attrition, 26 intervention and 17 control at follow up). Children were aged between 8‐11 years and had a diagnosis of major depression, dysthymic disorder or bipolar disorder. | |
| Interventions |
Facilitated support group Facilitated support group with structured problem‐solving format, meeting twice‐monthly for 2 hours, for a minimum of 6 months. 5‐9 parents per group. Co‐facilitated by mental health professionals and parent leaders (veteran parents). Control was usual care, which included a case manager, crisis intervention workers being available 24/7 and home visits. |
|
| Outcomes |
Primary Parent social support network use; parent problem solving and coping skills; youth behavioural problems.
Outcomes were measured at baseline and 9 and 18 months following baseline. 18‐month outcomes were used in the meta‐analysis. |
|
| Notes | Qualitative data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin procedure used to generate random sequence |
| Allocation concealment (selection bias) | Unclear risk | Concealment method not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unable to be blind to condition, due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blind to intervention condition |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition rate was reported; not broken down by intervention vs control. High rates of missing data at each time point. |
| Selective reporting (reporting bias) | Low risk | All hypothesised measures were reported on. |
| Other bias | High risk | Children in intervention group had higher externalising behaviour scores; parents in intervention had higher locus of control scores. Dyads in the intervention condition were therefore more severe in presentation, but possibly more amenable to the intervention. Dose of intensive case management (usual care) was not reported for intervention condition. Authors do note that the effectiveness of ICM may have made differences due to intervention difficult to detect. |
Scharer 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial; alternative treatment and usual care controls. | |
| Participants | 129 mothers or maternal caregivers of children aged 5‐12 years with serious mental illness (primary DSM‐IV diagnosis). | |
| Interventions |
Facilitated support groups Web social support: weekly 1‐hour chat room (offered as part of a wider website) facilitated by a psychiatric nurse. Intention was for mothers to provide peer support to each other; only minimal details available. Usual care control. (Another comparison condition was one‐to‐one telephone support, provided by psychiatric nurses fortnightly for 15‐20 mins. This condition is outside the scope of the review and outcomes are not reported here). |
|
| Outcomes |
Primary Social support; psychiatric distress.
Outcomes were measured at baseline and follow‐up (timing unknown). |
|
| Notes | The only available publication was an interim qualitative paper published while the RCT was in progress. Although it seems to have been intended that a full quantitative paper be published at the end of the trial, this does not appear to have occurred.The research was supported by a grant from the (US) National Institute for Nursing Research. We could not locate a protocol for this study. Contact with the first author was not possible. A co‐author supplied SAS data file which was converted to STATA. No information regarding timing of outcome measures or other details of the experiment was available. Means and SDs for two key measures were computed using scale scoring instructions. Information on timing of follow up measures was not available; the putative latest measure (variables named with a leading c) was used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Asserted but not described. Using unclear rather than high risk for this study because only publication available is the linked qualitative paper—a full quantitative paper was intended but never published (and authors could not provide an unpublished draft or protocol, only data). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details not provided in this qualitative paper. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not provided in this qualitative paper. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No discussion/details provided in this qualitative paper, but raw data shows high attrition overall (127/74) and attrition unequal across groups (particularly high in telephone social support group, 45/17) from baseline to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Raw data file provided, all initial measures have post and follow‐up data |
| Other bias | Unclear risk | Difficult to assess, as full RCT was not published and protocol (presumably specified to obtain National Institute for Nursing Research grant) could not be found. |
Silver 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial; usual care control | |
| Participants | 365 mothers (343 following attrition) of children aged 5‐8 with a variety of ongoing health conditions (183 intervention, 182 control; following attrition, 174 intervention and 169 control). | |
| Interventions |
Parent‐to‐parent support Group meetings and individual support phone calls, using veteran parents ("lay intervenors"). Aim was for parents to attend 6 face‐to‐face meetings and receive biweekly phone calls for over 12 months. Dose was tracked and varied widely amongst participants, with fewer contacts received, but at a longer duration than was anticipated. Control condition was standard care through relevant paediatric inpatient units, primary care, and subspecialty clinics. |
|
| Outcomes |
Primary Psychiatric distress, stressful life events
Secondary Child health
Outcomes measured at baseline and 6, 12, and 18 months following. 18 month outcomes data was used in the meta‐analysis. |
|
| Notes | Authors contacted, and provided 18 month data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation completed 'using a preestablished randomization procedure by a member of the research team'‐‐details not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given that support program was described, participants would have been aware of their allocation. Personnel would have been aware due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers were blind to intervention status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for attrition and attempts at follow‐up were reported clearly. ITT analyses were used |
| Selective reporting (reporting bias) | Low risk | Missing time points were provided by authors on request |
| Other bias | Low risk | Difference in baseline PSI scores was found and controlled for in subsequent analyses. |
Singer 1994.
| Study characteristics | ||
| Methods | Randomised controlled trial; alternate treatment control. | |
| Participants | 15 parents of children with acquired brain injury (8 in intervention, 7 in control). Child age range from 5‐20yrs. | |
| Interventions | Parent support group. Sessions were run weekly, of 2hrs duration and held over 9 weeks. Topics were determined by parents. Aim was to provide information and a parents could provide mutual support. Unclear if groups had facilitator; possibly yes as author states that no skills training was provided. The control for the purposes of this review was a stress management group (the focus of the reported study). Also run weekly for 9 weeks, and of 2hrs duration. Classes were run by a PhD level psychologist. |
|
| Outcomes |
Primary Depression, anxiety
Outcomes were measured at baseline and follow‐up (timing unknown). |
|
| Notes | Approached author for further details on intervention (as it was the comparison condition in the study and therefore not as well specified as the "focus" intervention). Timing of outcome measures is not described. No further information is available. No usual care control condition, comparison was with alternate intervention only. Data from this study could not be included in meta‐analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Prior to consenting to the study, participants were told they would be in a support group and were not informed of the difference between the two interventions. Participants are therefore likely to have been blind to difference between groups. No blinding of personnel as far as can be determined. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐report by participants blind to allocation. Not reported who scored questionnaires and if they were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported. |
| Selective reporting (reporting bias) | Low risk | Both outcome measures reported in method were also reported in results |
| Other bias | High risk | Due to small sample size and more marital dyads in the control group, there is a high risk that participant characteristics (such as all acquired brain injuries in the intervention group being result of trauma) biased the results. |
Singer 1999.
| Study characteristics | ||
| Methods | Randomised controlled trial; wait list control. | |
| Participants | 174 parents/carers of children with a disability participated. 128 in final analysis (56 experimental/72 control). | |
| Interventions | Parent‐to‐parent support. Participants were matched (following interview) to supporting parents who received training in communication skills, local services, and advocacy and support. Participants made initial call to coordinating centre to be matched; thereafter supporting parents were instructed to make a minimum of 4 phone calls to participants over 2 months. Wait‐list control. |
|
| Outcomes |
Primary Cognitive adaptation; empowerment; coping efficacy
Secondary Abstract mentions satisfaction measure but the qualitative analysis (of responses from a small subset of participants) was about possible mechanisms by which the intervention might provide support. See notes. Outcomes were measured at baseline and at 8 weeks following baseline. |
|
| Notes | Means and SDs for the empowerment outcome were sought from the author but were not available. The reported ANCOVA "suggests that initial contacts in Parent to Parent do not change parents' perceptions of empowerment". Qualitative data available 24 parents drawn from the pool of subjects who participated in Parent to Parent (12 from intervention, 12 from wait list) completed a standardised telephone interview. Transcribed interviews were coded according to identified themes. These themes pertained to how P2P makes a difference. Degree of satisfaction with intervention was not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss used |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of intervention and control. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Post‐test questionnaire asked about amount of contact with support parent, revealing group allocation. Self‐report nature of data collection means this should not have affected outcome assessments by research personnel, but may be bias from participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusions well reported |
| Selective reporting (reporting bias) | Unclear risk | Statistical analyses reported for all outcomes, but not means and SDs for all outcomes |
| Other bias | Low risk | No other sources of bias found |
Sullivan‐Bolyai 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial, usual care control. | |
| Participants | 42 mothers of children aged 1‐10yrs newly diagnosed with Type 1 diabetes (22 intervention and 20 control; 22 intervention and 19 control at follow‐up). | |
| Interventions | Parent mentors matched to participants' child age made home visits and supportive phone calls. Average of 3 home visits per parent (range 1‐8) and 13 phone calls or emails (range 5‐38); average total of 7.6 hours of contact over 6 months. Parent mentors‐‐mothers who were successfully managing their children's diabetes‐‐were selected and trained, and debriefed after every home visit or phone contact. Control was usual care (including daily medical call from diabetes nurse immediately post‐discharge, then 3‐4 calls/week). |
|
| Outcomes |
Primary Parent concern and confidence, impact on family, community resource use
Outcomes were measured at baseline and at 1 and 6 months following baseline. 6‐month follow‐up data was used in the meta‐analysis. |
|
| Notes | Qualitative data available. Family function outcomes could be included in meta‐analysis, but measures of confidence and self‐esteem could not be converted to a form suitable for inclusion in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation asserted but not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could not be blinded due to the nature of the intervention, likely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported |
| Selective reporting (reporting bias) | Low risk | Some consultation numbers not given, not relevant to outcomes of interest |
| Other bias | Low risk | No other sources of bias evident |
Sullivan‐Bolyai 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial, usual care control. | |
| Participants | 60 mothers of children aged 1‐12yrs newly diagnosed with Type 1 Diabetes (32 intervention, 28 control; 30 intervention and 21 control at follow‐up). | |
| Interventions | Trained parent mentors delivering initial face‐to‐face or home visits, followed by further contacts as agreed over following 12 months. Participants had on average 5 contacts (range, 1‐25) with an average duration of 63mins (range 5‐195). Including both face‐to‐face and phone contacts (phone utilised most often). Mentors were matched to participants where possible and received weekly supervision by the study author. Control participants had access to a parent contact (not trained; very few control participants made contact (2 in total). |
|
| Outcomes |
Primary: Parent concerns and worries about diabetes, parent confidence in caring for child, perceived impact of illness on family, perceived amount of care/helpfulness of father involvement, use of social support
|
|
| Notes | Authors contacted for further details on Irey's social support inventory. No further details available. Qualitative data available. Family function outcomes could be included in meta‐analysis, but measures of confidence and self‐esteem could not be converted to a form suitable for inclusion in meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by 'statistical permutation'. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition well described; participants who were less educated, divorced or separated, and working full time were significantly more likely to not complete data collection |
| Selective reporting (reporting bias) | Low risk | All outcomes described in method reported |
| Other bias | Low risk | No other sources of bias apparent |
Swallow 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 55 parents of children aged 4‐16 yrs with chronic kidney disease, stage 3‐5 (26 intervention, 29 control; 19 intervention at completion, 22 control). | |
| Interventions | Online parent information and support program; support and social networking with peers and professionals. Named OPIS (Online Parent Information and support) App. Focussed on clinical care‐giving support (e.g., information, video tutorials, quizzes and puzzles) and psychosocial support (e.g., social networking, testimonials, advice). Had access to the password controlled app for 20 weeks. Also received usual care. Control was usual care: Discussions with members of the multidisciplinary team, home visits from specialist nurse for children at CKD stage 5. |
|
| Outcomes |
Primary: Family condition management, family empowerment, father involvement
Outcomes were measured at baseline and at 20 weeks following baseline. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An author not involved in data collection generated randomised allocation sequence using computer program |
| Allocation concealment (selection bias) | Low risk | Sequence concealed from parents and researchers using sequentially numbered opaque paper envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was deemed not feasible. Blinding of personnel not stated but possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Baseline measures collected blind. Outcome data collected by team member blind to condition. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants excluded from analysis; missing data broken down by measure, comparable across control and intervention |
| Selective reporting (reporting bias) | Low risk | Reporting appears to be complete and transparent |
| Other bias | Low risk | No other sources of bias apparent |
Wysocki 2008.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | 104 parents/caregivers of children aged 11‐16yrs with type 1 Diabetes (36 educational support, 32 standard care control, 36 behavioural family systems therapy (BFST); complete data for 31 educational support, 26 standard care and 28 BFST). | |
| Interventions | Facilitated support groups plus educational lectures. Included 12 sessions of 3‐5 families over six months covering diabetes care topics. Face to face sessions; 45 minute lecture from facilitator and 45 minutes of family interaction on the topic, led by the facilitator. Also received standard care. Standard care was routine diabetes care. Included quarterly clinic visits with endocrinologist, and diabetes education including self‐monitoring, meal planning and exercise planning. Referral to psychologist/psychiatrist as needed. Behavioural Family Systems Therapy modified for diabetes patients and their families (BFST‐D): 12 individual family therapy sessions over six months, delivered by trained and licensed therapists. Comprised problem‐solving training, communication training, cognitive restructuring, and functional‐structural family therapy. Also received standard care. |
|
| Outcomes |
Primary: Family communication (negative and positive) and problem solving (discussions scored using interaction behaviour code).
Secondary: Disease control scores. Outcomes were measured at baseline, at completion of intervention (6 months), and at 12 and 18 months following baseline. |
|
| Notes | BFST‐D group is not used for outcomes comparisons in this review. Insufficient data from to enable inclusion in meta‐analysis‐‐only summary statistics provided. Author contacted, no further data available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process asserted but not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but from study description generally there appears to have been no attempt made to do this |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to family identity and group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was well reported and uniform across conditions. Baseline scores of families lost to follow‐up did not differ significantly from those of families completing all measures |
| Selective reporting (reporting bias) | Low risk | All outcomes subject to hypothesis appear to have been reported |
| Other bias | Low risk | No other sources of bias apparent |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chesney 1989 | No randomisation: non‐participants were selected by liaison staff to complete questionnaires. Analysis was regression at a single time point, so not a CBA study |
| Clifford 2013 | No randomisation: participants were allocated to intervention or control on the basis of participant preference. Did not meet criteria for CBA study. |
| Flores 2008 | This was an abstract only, could not locate more information. Potentially the same study as that reported in Flores 2009, but not sure. Outcomes are for children rather than parents/carers |
| Ji 2014 | Design stated as quasi‐experimental with cluster randomisation but did not meet criteria |
| Kang 2012 | Allocation to experimental condition was by participant preference; did not meet Cochrane definition of quasi‐RCT |
| Kreuger 1998 | Unclear if this was CBA; given that intervention was located in a single agency, we determined that this did not meet criteria for multiple sites. |
| Lewis 1972 | No randomisation: participants were allocated on the basis of availability. Did not meet criteria for CBA study. |
| Madden 2010 | This study was intended to run as a waitlist RCT, but in practice ran as CBA which did not meet criteria for inclusion. |
| Osman 2017 | Neither intervention nor control group (wait‐list) were peer support interventions |
| Picard 2014 | Participants allocated to intervention or control on the basis of preference. Does not meet criteria for inclusion as CBA |
| Samadi 2012 | Design stated as pre‐post crossover. Allocation to groups on basis of parent preference so not a crossover RCT. Does not meet criteria for inclusion as CBA. |
| Schultz 1993 | No randomisation. |
| Shu 2005 | Design stated as quasi‐experimental. No randomisation; allocation on basis of parent preference. Does not meet criteria for inclusion as CBA. |
| Swallow 2012 | Protocol only. May relate to Swallow 2014, but proposed numbers of participants are quite different and protocol is for a larger study. Some measures in common. |
| Vadasy 1985 | Compared participants in pilot program with new enrolments to full program at baseline. Does not meet criteria for inclusion as CBA. |
| Verduyn 2003 | Child difficulties are secondary to mother's diagnosis of depression. Not clear that child behaviour issues were part of chronic condition as no independent diagnosis was made and maternal depression may have affected reporting of issues. |
Characteristics of studies awaiting classification [ordered by study ID]
Carty 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Chien 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Jamison 2017.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Koren 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to contact authors for further information (abstract only) |
Pugh 1981.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Shekarabi Ahari 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to access journal |
Differences between protocol and review
INCLUSION: We originally specified that peer support intervention versus no‐treatment control studies would be included, but interventions where peer support was an incidental component of a more intensive intervention would be excluded. Our search identified a third option, where peer support was used as an active control for a more intensive intervention (for example, a non‐directive support group versus a psycho‐education or therapy group). We included such studies in the review as a whole, although they could not be included in meta‐analyses unless the study also used a no‐treatment control group.
OUTCOMES: Following discussion with Cochrane Australia, it was decided to subsume protocol outcomes 1 and 2 (anxiety and depression) in a single broader outcome, psychological distress. Several studies used a general psychiatric symptom scale (the PSI), the sub‐scales for depression and/or anxiety from that general scale, or some combination of the three. From the point of view both of consumers and of agencies deciding what support to offer consumers, it is more important to know if overall psychological distress is reduced than to know the precise symptomatology. This is particularly so because none of the scales or sub‐scales were used to make formal diagnoses, nor would such diagnoses be appropriate in this context. Additionally, general distress outcomes fit better with the broad outcome categories listed above.This replaces 'level of anxiety and depression' in the protocol. Similarly, the outcomes 'confidence and skill at navigating medical, community, and service support networks' and 'knowledge of local resources' were combined, and the single outcome 'confidence and skill at navigating medical services' was reported.
Also on the advice of Cochrane Australia, we changed our method of selecting from multiple scales measuring a single outcome. We preferred full, general, scales to specific scales and sub‐scales; where there was no general scale and several specific scales and/or sub‐scales we selected one at random.
SUBGROUP ANALYSES: A number of subgroup analyses were planned to determine if particular subgroups of parents receive more or less benefit than others, or if particular settings and modes of delivery are more effective than others. As no overall effect of peer support programs was apparent, these subgroup analyses were not conducted. They should be included in any update of this review, should new studies be located and overall effectiveness demonstrated for any outcome. Any new RCT of peer support programs would benefit from considering these questions.
How socially connected are parents before commencing the intervention?
How is the peer support delivered: in pairs, in a group, peer‐led, facilitator‐led?
Should peer support be delivered face‐to‐face, or assisted by technology?
Is there an optimum group size (apart from the support pairs considered above)?
Is there an optimum duration, either of individual sessions or of the intervention?
Is there an optimum timing for peer support interventions?
Is it better for peer groups or pairs to be homogeneous (that is, age‐ or condition‐specific)?
STRATIFICATION BY DESIGN: we had intended to stratify studies for meta‐analysis according to whether they were RCT or quasi‐RCT. As none of our meta‐analyses showed any effect of intervention, this was not considered necessary.
SENSITIVITY ANALYSES: we had intended to investigate the effect of variables such as existing social connectedness of participants, and delivery mode, setting, duration, and size of interventions on outcomes. We also intended to check the effects of choice of SMD across all scales versus individual mean differences and other assumptions about outcome measures on pooled estimates. In the event, there was insufficient data for such analyses to be appropriate. It was only possible to use SMDs for continuous outcomes due to the wide range of outcome measures used.
Contributions of authors
GS conceived the review question; VL provided advice on formulating the review question
GS and AP ran searches, screened retrieved studies, determined inclusions/exclusions, extracted data from included studies
VL checked studies where there was screening disagreement
GS and VL resolved outstanding screening disagreements
GS and AP completed risk of bias assessments
GS, AP, and VL checked extracted data and final inclusion decisions
GS conducted analyses and prepared the first draft of full review
GS and AP conducted and cross‐checked GRADE assessments
AP assisted with first draft of full review
VL commented on first draft of full review.
GS and AP conducted top‐up searches, screening, and extraction where updates were needed
AP and VL checked and commented on the full review, abstract, and plain language summary
GS and AP incorporated feedback and prepared the final draft.
Sources of support
Internal sources
-
Parenting Research Centre, Australia
Staff time
External sources
No sources of support provided
Declarations of interest
The first author was previously the manager of a federally‐funded peer support program for parents and carers of children with complex needs in Australia. All three authors worked at The Parenting Research Centre, which administers the program on behalf of the Australian government, for a substantial portion of the time taken to complete this review. An evaluation of the effectiveness and acceptability of this program was conducted; no publications or data from this evaluation to date met inclusion criteria for this review.
New
References
References to studies included in this review
Aiello 2015 {published data only (unpublished sought but not used)}
- Aiello CP, Ferrari DV. Teleaudiology: efficacy assessment of an online social network as a support tool for parents of children candidates for cochlear implant. CoDAS 2015;27(5):411-8. [DOI] [PubMed] [Google Scholar]
Boogerd 2017 {published data only}
- Boogerd E, Maas-Van Schaaijk NM, Sas TC, Clement-de Boers A, Smallenbroek M, Nuboer R, Noordam C, Verhaak CM. Sugarsquare, a web-based patient portal for parents of a child with Type 1 diabetes: A multicenter randomized controlled feasibility trial. Journal of Medical Internet Research 2017;19(8):e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogerd EA, Noordam C, Verhaak CM. The Sugarsquare study: protocol of a multicenter randomized controlled trial concerning a web-based patient portal for parents of a child with type 1 diabetes. BMC Pediatrics 2014;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boylan 2013 {published data only (unpublished sought but not used)}
- Boylan C, Morgan S, Carthy A, Crowley S, Lyons J, Fitzpatrick C, Rickard E, Guerin S. A randomised controlled trial of a programme for parents and full-time carers of young people with self-harm and suicidal behaviour. European Child and Adolescent Psychiatry 2013;22((Suppl 2)):S87-S313. [Google Scholar]
Ferrin 2014 {published data only}
- Ferrin M, Moreno-Granados JM, Salcedo-Marin MD, Ruiz-Veguilla M, Perez-Ayala V, Taylor E. Evaluation of a psychoeducation programme for parents of children and adolescents with ADHD: immediate and long-term effects using a blind randomized controlled trial. European Child and Adolescent Psychiatry 2013;23(8):637-47. [DOI: DOI 10.1007/s00787-013-0494-7] [DOI] [PubMed] [Google Scholar]
Flores 2009 {published data only}
- Flores G, Bridon C, Torres S, Perez R, Walter T, Brotanek J, Hua L, Tomany-Korman S. Improving asthma outcomes in minority children: A randomized, controlled trial of parent mentors. Pediatrics 2009;124:1522-0230. [DOI] [PubMed] [Google Scholar]
Ireys 1996 {published data only (unpublished sought but not used)}
- Ireys HT, Sills EM, Kolodner KB. A social support intervention for parents of children with Juvenile Rheumatoid Arthritis: Results of a randomized trial. Journal of Pediatric Psychology 1996;21(5):633-641. [DOI] [PubMed] [Google Scholar]
Ireys 2001 {published data only (unpublished sought but not used)}
- Ireys HT, Chernoff R, DeVet KA, Kim Y. Maternal outcomes of a randomized controlled trial of a community-based support program for families of children with chronic illness. Archives of Pediatric & Adolescent Medicine 2001;155:771-777. [DOI] [PubMed] [Google Scholar]
Kutash 2011 {published data only}
- Kutash K, Duchnowski AJ, Green AL, Ferron JM. Supporting parents who have youth with emotional disturbances through a Parent-to-Parent support program: A proof of concept study using random assignment. Administration and Policy in Mental Health 2011;38:412-427. [DOI] [PubMed] [Google Scholar]
Kutash 2013 {published and unpublished data}
- Kutash K, Duchnowski AJ, Green AL, Ferron J. Effectiveness of the Parent Connectors Program: Results from a randomized controlled trial. School Mental Health 2013;5:192-208. [Google Scholar]
McCallion 2004 {published data only}
- McCallion P, Janicki MP, Kolomer SR. Controlled evaluation of support groups for grandparent caregivers of children with developmental disabilities and delays. American Journal on Mental Retardation 2004;109(5):352-361. [DOI] [PubMed] [Google Scholar]
Preyde 2003 {published data only}
- Preyde M, Ardal F. Effectiveness of a parent "buddy" program for mothers of very preterm infants in a neonatal intensive care unit. Canadian Medical Association Journal April 15, 2003;168(8):969-973. [PMC free article] [PubMed] [Google Scholar]
Rhodes 2008 {published data only (unpublished sought but not used)}
- Rhodes P, Baillee A, Brown J, Madden S. Can parent-to-parent consultation improve the effectiveness of the Maudsley model of family-based treatment for anorexia nervosa? A randomized control trial. Journal of Family Therapy 2008;30:96-108. [Google Scholar]
Roberts 2011 {published data only}
- Roberts J, Williams K, Carter M, Evans D, Parmenter T, Silove N, Clark T, Warren A. A randomised controlled trial of two early intervention programs for young children with autism: Centre-based with parent program and home-based. Research in Autism Spectrum Disorders 2011;5:1553-1566. [Google Scholar]
Ruffolo 2005 {published data only}
- Ruffolo MC, Kuhn MT, Evans ME. Support, Empowerment, and Education: A study of multiple family group psychoeducation. Journal of emotional and behavioral disorders 2005;13(4):200-212. [Google Scholar]
Scharer 2009 {published and unpublished data}
- Scharer K, Colon E, Moneyham L, Hussey J, Tavakoli A, Shugart M. A comparison of two types of social support for mothers of mentally ill children. Journal of Child and Adolescent Psychiatric Nursing 2009;22(2):86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silver 1997 {published and unpublished data}
- Silver EJ, Ireys HT, Bauman LJ, Stein REK. Psychological outcomes of a support intervention in mothers of children with ongoing health conditions: the Parent-to-Parent Network. Journal of Community Psychology 1997;25(3):249-264. [Google Scholar]
Singer 1994 {published data only (unpublished sought but not used)}
- Singer GHS, Glang A, Nixon C, Cooley E, Kerus KA, Williams D, Powers LE. A comparison of two psychosocial interventions for parents of children with acquired brain injury: An exploratory study. Journal of Head Trauma Rehabilitation 1994;9(4):38-49. [Google Scholar]
Singer 1999 {published data only (unpublished sought but not used)}
- Singer GHS, Marquis J, Powers LK, Blanchard L, Divenere N, Santelli B, Ainbinder JG, Sharp M. A multi-site evaluation of Parent to Parent programs for parents of children with disabilities. Journal of Early Intervention 1999;22(3):217-229. [Google Scholar]
Sullivan‐Bolyai 2004 {published data only}
- Sullivan-Bolyai S, Grey M, Deatrick J, Gruppuso P, Giraitis P, Tamborlane W. Helping other mothers effectively work at raising young children with Type 1 Diabetes. The Diabetes Educator 2004;30(3):476-484. [DOI] [PubMed] [Google Scholar]
Sullivan‐Bolyai 2010 {published data only (unpublished sought but not used)}
- Rearick EM. Parents of children newly diagnosed with Type 1 Diabetes.. The Diabetes Educator 2011;37(4):508-518. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Bova C, Lee M, Gruppuso PA. Mentoring fathers of children newly diagnosed with T1DM. American Journal of Maternal and Child Nursing 2011;36(4):224-231. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Bova C, Leung K, Trudeau A, Lee M, Gruppuso. Social Support to Empower Parents (STEP): An intervention for parents of young children newly diagnosed with Type 1 Diabetes. The Diabetes Educator 2010;36:88-97. [DOI] [PubMed] [Google Scholar]
Swallow 2014 {published data only (unpublished sought but not used)}
- Swallow VM, Knafl K, Santacroce S, Campbell M, Hall AG, Smith T, Carolan I. An interactive health communication application for supporting parents managing childhood long-term conditions: Outcomes for a randomized controlled feasibility trial. JMIR Research Protocols 2014;3(4):e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wysocki 2008 {published data only (unpublished sought but not used)}
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Sobel Lochrie A, Taylor A, Sadler M, White NH. Randomized, controlled trial of behavioral family systems therapy for diabetes: Maintenance and generalization of effects on Parent-Adolescent Communication. Behavior Therapy 2008;39:33-46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chesney 1989 {unpublished data only}
- Chesney BCK. Empowering parents of children with cancer: The role of self-help groups. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Sociology) in The University of Michigan 1989.
Clifford 2013 {published data only}
- Clifford T, Minnes P. Logging on: evaluating an online support group for parents of children with Autism Spectrum Disorders. Journal of Austism and Developmental Disorders 2013;43:1662-1675. [DOI] [PubMed] [Google Scholar]
Flores 2008 {published data only}
- Flores G, Snowden-Bridon C, Torres S, Walter T, Brotanck S, Tomany-Korman S. A randomised controlled trial (RCT) of the effectiveness of Parent Mentors in improving asthma outcomes in minority children.. In: Journal of Allergy and Clinical Immunology. Vol. 121, issue 2, Supplement 1. February 2008:S794.
Ji 2014 {published data only}
- Ji B, Sun M, Yi R, Tang S. Multidisciplinary parent education for caregivers of children with Austism Spectrum Disorders. Archives of Psychiatric Nursing 2014;28:319-326. [DOI] [PubMed] [Google Scholar]
Kang 2012 {published data only}
- Kang HS, Kim WO, Jeong Y, Kim SY, Yoo KY. Effect of a self-help program for mothers of hemophilic children in Koria. Haemophilia 2012;18:892-897. [DOI] [PubMed] [Google Scholar]
Kreuger 1998 {unpublished data only}
- Kreuger BD. Social support intervention for mothers of typical and special needs infants and toddlers: An outcome evaluation. A dissertation submitted to the Department of Curriculum, Instruction, and Educational Psychology, Loyola University Chicago, in candidacy for the degree of Doctor of Philosophy 1998.
Lewis 1972 {published data only}
- Lewis J. Effects of group procedure with parents of MR children.. Mental retardation 1972;10(6):14-15. [PubMed] [Google Scholar]
Madden 2010 {unpublished data only}
- Madden LM. Parent support groups: Impact of parental adjustment and perceptions of children's behaviour difficulties. A thesis submitted to McGill University in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in School/Applied Child Psychology 2010.
Osman 2017 {published data only}
- Osman F, Flacking R, Schön U-K, Klingberg-Alvin M. A support program for Somali-born parents on child behaviour problems. Pediatrics 2017;139(3):e20162764. [DOI] [PubMed] [Google Scholar]
Picard 2014 {published data only}
- Picard I, Morin D, De Mondehare L. Psychoeducational program for parents of adolescents with intellectual disabilities. Journal of Policy and Practice in Intellectual Disabilities 2014;11(4):279-292. [Google Scholar]
Samadi 2012 {published data only}
- Samadi SA, McConkey R, Kelly G. Enhancing parental well-being and coping through a family centred short course for Iranian parents of children with an autism spectrum disorder. Autism 2013;17(1):27-43. [DOI] [PubMed] [Google Scholar]
Schultz 1993 {published data only}
- Schultz CL, Schultz NC, Bruce EJ, Smyrnios KX, Carey LB, Carey CL. Psychoeducational support for parents of children with intellectual disability: An outcome study. International Journal of Disability, Development and Education 1993;40(3):205-216. [Google Scholar]
Shu 2005 {published data only}
- Shu B-C, Lung F-W. The effect of support group on the mental health and quality of life for mothers with autistic children. Journal of Intellectual Disability Research 2005;49(1):47-53. [DOI] [PubMed] [Google Scholar]
Swallow 2012 {published data only}
- Swallow V, Knafl K, Santacroce S, Hall A, Smith T, Campbell M, Webb NJA. The Online Parent Information adn Support project, meeting parents' information and support needs for home-based management of childhood chronic kidney disease: research protocol. Journal of Advanced Nursing 2012;68(9):2095-2102. [DOI] [PubMed] [Google Scholar]
Vadasy 1985 {published data only}
- Vadasy PF, Fewell RR, Meyer DJ, Greenberg MT. Supporting fathers of handicapped young children: Preliminary findings of program effects. Analysis and Intervention in Developmental Disabilities 1985;5:151-163. [Google Scholar]
Verduyn 2003 {published data only}
- Verduyn C, Barrowclough C, Roberts J, Tarrier N, Harrington R. Maternal depression and child behaviour problems: Randomised placebo-controlled trial of a cognitive-behavioural group intervention. British Journal of Psychiatry 2003;183:342-348. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Carty 2018 {published data only}
- Carty CL, Soghier LM, Kritikos KI, Tuchman LK, Jiggetts M, Glass P, Streisand R, Fratantoni KR. The Giving Parents Support Study: A randomized clinical trial of a parent navigator intervention to improve outcomes after neonatal intensive care unit discharge. Contemporary Clinical Trials 2018;70:117-134. [DOI] [PubMed] [Google Scholar]
Chien 2018 {published data only}
- Chien WT, Bressington D, , Chan SWC. A randomized controlled trial on mutual support group intervention for families of people with recent-onset psychosis: A four-year follow-up. Frontiers in psychiatry 2018;9:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamison 2017 {published data only}
- Jamison JM, Fourie E, Siper PM, Trelles MP, George-Jones J, Buxbaum Grice A, Krata J, Holl E, Shaoul J, Hernandez B, et al. Examining the efficacy of a family peer advocate model for Black and Hispanic caregivers of children with autism spectrum disorder. Jounal of autism and developmental disorders 2017;Article in press:10.1007/s10803-017-3045-0. [DOI] [PubMed] [Google Scholar]
Koren 2013 {published data only}
- Koren E, Kupriyanova T. Family burden-focused psychosocial therapy with parents and social functioning of children and adolescents with schizophrenic spectrum disorders. European Psychiatry 2013;28(Supplement 1):1. [Google Scholar]
Pugh 1981 {published data only}
- Pugh G, Hattersley J, Tennant L, Wilcock P. Parents as partners: intervention schemes and group work with parents of handicapped children. National Children's Bureau, London (UK).
Shekarabi Ahari 2012 {published data only}
- Shekarabi-Ahari G, Younesi J, Borjali A, Damavandi SA. The effectiveness of group hope therapy on hope and depression of mothers with children suffering from cancer in Tehran. Iranian Journal of Cancer Prevention 2012;5(4):183. [PMC free article] [PubMed] [Google Scholar]
Additional references
Ainbinder 1998
- Ainbinder JG, Blanchard LW, Singer GHS, Sullivan ME, Powers LK, Marquis JG, et al. A qualitative study of Parent to Parent support for parents of children with special needs. Journal of Pediatric Psychology 1998;23(2):99-109. [DOI] [PubMed] [Google Scholar]
Armstrong 2005
- Armstrong MI, Birnie-Lefcovitch S, Ungar MT. Pathways between social support, family well being, quality of parenting, and child resilience: what we know. Journal of Child and Family Studies 2005;14(2):269-81. [Google Scholar]
Baker 2002
- Baker B, Blacher J, Crnic K, Edelbrock C. Behavior problems and parenting stress in families of three-year-old children with and without developmental delays. American Journal on Mental Retardation 2002;107(6):433-44. [DOI] [PubMed] [Google Scholar]
Banach 2010
- Banach M, Iudice J, Conway L, Couse LJ. Family support and empowerment: Post autism diagnosis support group for parents. Social Work with Groups: A Journal of Community and Clinical Practice 2010;33(1):69-83. [Google Scholar]
Barlow 2006
- Barlow J, Ellard D. The psychosocial well-being of children with chronic disease, their parents and siblings: an overview of the research evidence base. Child: Care Health & Development 2006;32(1):19-31. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation.. Clinical Psychology Review 1988;8(1):77-100. [Google Scholar]
Behr 1992
- Behr SK, Murphy DL, Summers JA. User’s Manual: Kansas Inventory of Parental Perceptions.. The University of Kansas Beach Center on Disability 1992.
Blacher 2007
- Blacher J, Baker BL. Positive impact of intellectual disability on families. American Journal on Mental Retardation 2007;112(5):330-348. [DOI] [PubMed] [Google Scholar]
Blanchard 1996
- Blanchard L, Powers L, Ginsberg C, Marquis J, Singer GHS. The Coping Efficacy Inventory: Measuring parents perceptions of coping with a child with a disability and family problems.. Unpublished manuscript. University of North Carolina Medical School, Chapel Hill, N.C. 1996.
Bourgeois 2010
- Bourgeois A, LeUnes A, Meyers M. Full-scale and short-form of the profile of mood states: a factor analytic comparison. Journal of Sport Behavior 2010;33(4):355–376. [Google Scholar]
Bourke‐Taylor 2010
- Bourke-Taylor H, Howie L, Law M. Impact of caring for a school-aged child with a disability: understanding of mothers' perspectives. Australian Occupational Therapy Journal 2010;57:127-69. [DOI] [PubMed] [Google Scholar]
Campagnolo 2002
- Campagnolo DI, Filart RA, Millis SR, Lann DE. Appropriateness Of The Ilfeld Psychiatric Symptom Index as a screening tool for depressive symptomatology in persons with spinal cord injury. The Journal of Spinal Cord Medicine 2002;25(2):129-132. [DOI] [PubMed] [Google Scholar]
Canary 2008
- Canary HE. Creating supportive connections: a decade of research on support for families of children with disabilities. Health Communication 2008;23(5):413-26. [DOI] [PubMed] [Google Scholar]
Cash‐Gibson 2012
- Cash-Gibson L, Felix LM, Minorikawa N, Pappas Y, Gunn, LH, Majeed A, et al. Automated telephone communication systems for preventive healthcare and management of long-term conditions. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD009921. [DOI: 10.1002/14651858.CD009921] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O'Mara-Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD001055. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
CHCP 2012
- Cochrane Consumers and Communication Review Group. Outcomes of Interest to the Cochrane Consumers & Communication Review Group. http://www.latrobe.edu.au/chcp/assets/downloads/Outcomes.pdf January 2012 (accessed 30 May 2012).
Cheshire 2010
- Cheshire A, Barlow JH, Powell LA. The psychosocial well-being of parents of children with cerebral palsy: a comparison study. Disability and Rehabilitation 2010;32:1673-7. [DOI] [PubMed] [Google Scholar]
Coppola 2013
- Coppola G, Cassibba R, Bosco A, Papagna S. In search of social support in the NICU: features, benefits and antecedents of parents' tendency to share with others the premature birth of their baby. The Journal of Maternal-Fetal & Neonatal Medicine 2013;26(17):1737-1741. [DOI] [PubMed] [Google Scholar]
Dale 2008
- Dale J, Caramlau IO, Lindenmeyer A, Williams SM. Peer support telephone calls for improving health. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD006903. [DOI: 10.1002/14651858.CD006903.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2005
- Davies S, Hall D. "Contact A Family": professionals and parents in partnership. Achives of Disease in Childhood 2005;90:1053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dellve 2006
- Dellve L, Samuelsson L, Tallborn A, Fasth A, Lillemor R-M. Stress and well-being among parents of children with rare diseases: a prospective intervention study. Journal of Advanced Nursing 2006;53(4):392-402. [DOI] [PubMed] [Google Scholar]
Doull 2004
- Doull M, O'Connor AM, Wells GA, Tugwell P, Welch V. Peer-based interventions for reducing morbidity and mortality in HIV-infected women. Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No: CD004774. [DOI: 10.1002/14651858.CD004774] [DOI] [Google Scholar]
Doull 2005
- Doull M, O'Connor AM, Welch V, Tugwell P, Wells GA. Peer support strategies for improving the health and well-being of individuals with chronic diseases. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD005352. [DOI: 10.1002/14651858.CD005352] [DOI] [Google Scholar]
Duarte 2005
- Duarte CS, Bordin IA, Yazigi L, Mooney J. Factors associated with stress in mothers of children with autism. Autism 2005;9(4):416-27. [DOI] [PubMed] [Google Scholar]
Dunkel‐Schetter 1987
- Dunkel-Schetter C, Folkman S, Lazarus RS. Correlates of social support receipt. Journal of Personality and Social Psychology 1987;53(1):71-80. [DOI] [PubMed] [Google Scholar]
Eysenbach 2004
- Eysenbach G, Powell J, Englesakis M, Rizo C, Stern A. Health related virtual communities and electronic support groups: systematic review of the effects of online peer to peer interactions. BMJ 2004;328:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
González‐Fraile 2021
- González-Fraile E, Ballesteros J, Rueda J-R, Santos-Zorrozúa B, Solà I, McCleery J. Remotely delivered information, training and support for informal caregivers of people with dementia. Cochrane Database of Systematic Reviews 04 January 2021, Issue 1. Art. No: CD006440. [DOI: 10.1002/14651858.CD006440.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2002
- Gray DE. 'Everybody is just embarrassed': felt and enacted stigma among parents of children with high functioning autism. Sociology of Health & Illness 2002;24(6):734-49. [Google Scholar]
Green 2007
- Green SE. "We’re tired, not sad": Benefits and burdens of mothering a child with a disability. Social Science & Medicine 2007;64(1):150-163. [DOI] [PubMed] [Google Scholar]
Hartman 1992
- Hartman A, Radin M, McConnell B. Parent-to-parent support: a critical component of health care services for families. Issues in Comprehensive Pediatric Nursing 1992;15(1):55-67. [DOI] [PubMed] [Google Scholar]
Hastings 2002
- Hastings SE, Taunt HM. Positive perceptions in families of children with developmental disabilities. American Journal on Mental Retardation 2002;107(2):116-127. [DOI] [PubMed] [Google Scholar]
Hastings 2004
- Hastings R, Beck A. Practitioner review: stress intervention for parents of children with intellectual disabilities. Journal of Child Psychology and Psychiatry 2004;45(8):1338-49. [DOI] [PubMed] [Google Scholar]
Hays 1995
- Hays RD, Sherbourne CD, Mazel RM. User's manual for the medical outcomes study (MOS) core measures of health-related quality of life. Santa Monica, California: RAND, 1995. [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8 Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hoffman 2006
- Hoffman L, Marquis J, Poston D, Summers JA, Turnbull A. Assessing family outcomes: psychometric evaluation of the beach center family quality of life scale. Journal of Marriage and Family 2006;68(4):1069-1083. [Google Scholar]
Hogan 2002
- Hogan BE, Lindena W, Najarian B. Social support interventions: do they work? Clinical Psychology Review 2002;22:381-440. [DOI] [PubMed] [Google Scholar]
Ignaki 2017
- Ignaki TK, Orehek E. On the benefits of giving social support: when, why, and how support providers gain by caring for others. Current Directions in Psychological Science 2017;26(2):109-113. [Google Scholar]
Iscoe 1985
- Iscoe L, Bordelon K. Pilot parents: peer support for parents of handicapped children. Children's Health Care 1985;14(2):103-9. [Google Scholar]
Kerr 2000
- Kerr SM, McIntosh J. Coping when a child has a disability: exploring the impact of parent-to-parent support. Child: Care, Health and Development 2000;26(4):309-21. [DOI] [PubMed] [Google Scholar]
Kessler 2010
- Kessler RC, Green JG, Gruber MJ, Sampson NA, Bromet E, Cuitan M, et al. Screening for serious mental illness in the general population with the K6 screening scale: results from the WHO World Mental Health (WMH) survey initiative.. International Journal of Methods in Psychiatric Research 2010;19(Supplement 1):4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kew 2016
- Kew KM, Carr R, Crossingham I. Lay-led and peers support interventions for adolescents with asthma. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2000
- King G, Stewart D, King S, Law M. Organizational characteristics and issues affecting the longevity of self-help groups for parents of children with special needs. Qualitative Health Research 2000;10(2):225-41. [DOI] [PubMed] [Google Scholar]
Kirk 2015
- Kirk S, Fallon D, Fraser C, Robinson G, Vassallo G. Supporting parents following childhood traumatic brain injury: a qualitative study to examine information and emotional support needs across key care transitions. Child: Care, Health and Development. 2015;41(2):303-313. [DOI] [PubMed] [Google Scholar]
Knight 1983
- Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. British Journal of Clinical Psychology 1983;22:245–249. [DOI] [PubMed] [Google Scholar]
Koeske 1992
- Koeske GF, Koeske RD. Parenting locus of control: measurement, construct validation, and a proposed conceptual model. Social Work Research & Abstracts 1992;28(3):37-46. [Google Scholar]
Lavender 2013
- Lavender T, Richens Y, Milan SJ, Smyth RMD, Dowswell T. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD009338. [DOI: 10.1002/14651858.CD009338.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2001
- Law M, King S, Stewart D, King G. The perceived effects of parent-led support groups for parents of children with disabilities. Physical & Occupational Therapy in Pediatrics 2001;21(2/3):29-48. [PubMed] [Google Scholar]
Lazarus 1987
- Lazarus RS, Folkman S. Transactional theory and research on emotions and coping. European Journal of Personality 1987;1:141-69. [Google Scholar]
Lee 2007
- Lee S, Yoo JS, Yoo IY. Parenting stress in mothers of children with congenital heart disease. Asian Nursing Research 2007;2(3):116-24. [DOI] [PubMed] [Google Scholar]
Lovibond 1995
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour research and therapy 1995;33(3):335-343. [DOI] [PubMed] [Google Scholar]
Mas 2016
- Mas JM. The adaptation process of families with children with intellectual disabilities in Catalonia. Infants and Young Children 2016;29(4):335-351. [Google Scholar]
McConnell 2011
- McConnell D, Breitkreuz R, Savage A. From financial hardship to child difficulties: main and moderating effects of perceived social support. Child: Care, Health and Development 2011;37(5):679-91. [DOI] [PubMed] [Google Scholar]
McConnell 2014
- McConnell D, Savage A, Breitkreuz R. Resilience in families raising children with disabilities and behavior problems. Research in Developmental Disabilities 2014;35(4):833-848. [DOI] [PubMed] [Google Scholar]
McGuire 2004
- McGuire BK. Mothers of children with disabilities: occupational concerns and solutions. OTJR-Occupational Participation and Health 2004;24(2):54-63. [Google Scholar]
Montazeri 2003
- Montazeri A, Harirchi AM, Shariati M, Garmaroudi G, Ebadi M, Fateh A. The 12-item General Health Questionnaire (GHQ-12): translation and validation study of the Iranian version.. Health and Quality of Life Outcomes 2003;1:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niela‐Vilén 2014
- Niela-Vilén H, Axelin A, Salanterä S, Melender H-L. Internet-based peer support for parents: a systematic integrative review. International Journal of Nursing Studies 2014;51(11):1527-1537. [DOI] [PubMed] [Google Scholar]
Noyes 2011a
- Noyes J, Lewin S. Chapter 5: Extracting qualitative evidence. In: Noyes J, Booth A, Hannes K, Harden A, Harris J, Lewin S, et al, editors(s). Supplementary Guidance for Inclusion of Qualitative Research in Cochrane Systematic Reviews of Interventions. Cochrane Collaboration Qualitative Methods Group, 2011. [Google Scholar]
Noyes 2011b
- Noyes J, Popay J, Pearson A, Hannes K, Booth A. Chapter 20: Qualitative research and Cochrane reviews. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Ortega 2002
- Ortega D. How much support is too much? Parenting efficacy and social support. Children and Youth Service Review 2002;24(11):853-76. [Google Scholar]
Popay 2006
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews: a product from the ESRC Methods Programme. Provided by authors via http://www.lancaster.ac.uk/shm/research/nssr/research/dissemination/publications.php 2006.
Quine 1991
- Quine L, Pahl J. Stress and coping in mothers caring for a child with severe learning difficulties: a test of Lazarus' transactional model of coping. Journal of Community & Applied Social Psychology 1991;1:57-70. [Google Scholar]
Rearick 2011
- Rearick EM, Sullivan-Bolyai S, Bova C, Knafl K. Parents of children newly diagnosed with type 1 diabetes: experiences with social support and family management. The Diabetes Educator 2011;37(4):508-518. [DOI] [PubMed] [Google Scholar]
Reitman 2002
- Reitman D, Currier RO, Stickle TR. A critical evaluation of the Parenting Stress Index-Short Form (PSI-SF) in a Head Start population. Journal of Clinical Child and Adolescent Psychology 2002;31(3):384-392. [DOI] [PubMed] [Google Scholar]
Resch 2010
- Resch JA, Mireles G, Benz MR, Grenwelge C, Peterson R, Zhang DL. Giving parents a voice: a qualitative study of the challenges experienced by parents of children with disabilities. Rehabilitation Psychology 2010;55(2):139-50. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 2005
- Rhodes P, Brown J, Baillee A. The Parents Versus Anorexia Scale: A brief measure of parental efficacy in the family-based treatment of anorexia. European Eating Disorders Review 2005;13:399-405. [Google Scholar]
Ryan 2011
- Ryan R, Hill S, Prictor M, McKenzie J. Study quality guide. Cochrane Consumers and Communication Review Group May 2011;http://www.latrobe.edu.au/chcp/cochrane/resources.html; accessed 30 April 2012.
Ryan 2016
- Ryan R, Synnot A, Hill S. Describing results. Cochrane Consumers and Communication Group, available at: http://cccrg.cochrane.org/author-resources June 2016.
Santelli 1996
- Santelli B, Turnbull A, Sergeant J, Lerner EP, Marquis JG. Parent to parent programs: parent preferences for supports. Infants and Young Children 1996;9(1):53-62. [Google Scholar]
Santelli 1997
- Santelli B, Turnbull A, Marquis J, Lerner E. Parent-to-parent programs: a resource for parents and professionals. Journal of Early Intervention 1997;21(1):73-83. [Google Scholar]
Sartore 2013
- Sartore G, Lagioia V, Mildon R. Peer support interventions for parents and carers of children with complex needs. Cochrane Database of Systematic Reviews 2013, Issue CD010618. Art. No: CD010618. [DOI: 10.1002/14651858.CD010618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of Findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach.. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html Updated October 2013.
Schwartz 1999
- Schwartz CE, Sendor M. Helping others helps oneself: response shift effects in peer support. Social Science and Medicine 1999;48:1563-75. [DOI] [PubMed] [Google Scholar]
Shilling 2013
- Shilling V, Morris C, Thompson-Coon J, Ukoumunne O, Rogers M, Logan S. Peer support for parents of children with chronic disabling conditions: a systematic review of quantitative and qualitative studies. Developmental Medicine and Child Neurology 2013;55(7):602-609. [DOI] [PubMed] [Google Scholar]
Stein 1980
- Stein RK, Riessman CK. The development of an Impact-on-family scale: preliminary findings.. Medical Care 1980;28(4):465-472.. [DOI] [PubMed] [Google Scholar]
Sterling 1996
- Sterling YM, Jones LC, Johnson DH, Bowen MR. Parents' resources and home management of the care of chronically ill infants. Journal of the Society of Pediatric Nurses 1996;1(3):103-109. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;342:d4002. [DOI: 10.1136/bmj.d4002] [DOI] [PubMed] [Google Scholar]
Strunk 2010
- Strunk JA. Respite care for families of special needs children: a systematic review. Journal of Developmental and Physical Disabilities 2010;22(6):615-30. [Google Scholar]
Tak 2002
- Tak Y, McCubbin M. Family stress, perceived social support and coping following the diagnosis of a child's congenital heart disease. Journal of Advanced Nursing 2002;39(2):190-8. [DOI] [PubMed] [Google Scholar]
Treanor 2019
- Treanor CJ, Santin O, Prue G, Coleman H, Cardwell CR, O'Halloran P, et al. Psychosocial interventions for informal caregivers of people living with cancer. Cochrane Database of Systematic Reviews 17 June 2019, Issue 6. Art. No: CD009912. [DOI: 10.1002/14651858.CD009912.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Twoy 2007
- Twoy R, Connolly P, Novak J. Coping strategies used by parents of children with autism. Journal of the American Academy of Nurse Practitioners 2007;19:251-60. [DOI] [PubMed] [Google Scholar]
Van Riper 1992
- Van Riper M, Ryff C, Pridham K. Parental and family well-being in families of children with Down Syndrome: a comparative study. Research in Nursing and Health 1992;15:227-35. [DOI] [PubMed] [Google Scholar]
Varni 2004
- Varni JW, Sandra AS, Tasha MB, Paige ED, Pamela D. The PedsQL™ family impact module: Preliminary reliability and validity. Health and Quality of Life Outcomes 2;1:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimet 1998
- Zimet GD, Dahlem NW, Zimet SG, Gordon KF. The multidimensional scale of perceived social support. Journal of Personality Assessment 1998;52:30-41. [Google Scholar]


