Abstract
Introduction
To assess the impact of the COVID-19 pandemic impact on hemodialysis (HD) centers, The Dialysis Outcomes and Practice Patterns Study and ISN collaborated on a web-survey of centers.
Methods
A combined approach of random sampling and open invitation was used between March 2020 and March 2021. Responses were obtained from 412 centers in 78 countries and all 10 ISN regions.
Results
In 8 regions, rates of SARS-CoV-2 infection were <20% in most centers, but in North East Asia (NE Asia) and Newly Independent States and Russia (NIS & Russia), rates were ≥20% and ≥30%, respectively. Mortality was ≥10% in most centers in 8 regions, although lower in North America and Caribbean (N America & Caribbean) and NE Asia. Diagnostic testing was not available in 33%, 37%, and 61% of centers in Latin America, Africa, and East and Central Europe, respectively. Surgical masks were widely available, but severe shortages of particulate-air filter masks were reported in Latin America (18%) and Africa (30%). Rates of infection in staff ranged from 0% in 90% of centers in NE Asia to ≥50% in 63% of centers in the Middle East and 68% of centers in NIS & Russia. In most centers, <10% of staff died, but in Africa and South Asia (S Asia), 2% and 6% of centers reported ≥50% mortality, respectively.
Conclusion
There has been wide global variation in SARS-CoV-2 infection rates among HD patients and staff, personal protective equipment (PPE) availability, and testing, and the ways in which services have been redesigned in response to the pandemic.
Keywords: advocacy, chronic dialysis, nephrology
HD centers are especially vulnerable during the COVID-19 pandemic because patients regularly come into close contact with each other (in transit to and from the center, while waiting to start dialysis, and while being dialyzed) and staff.1 Dialysis patients are at particularly high risk of developing COVID-19 and subsequently dying.2 The incidence of SARS-CoV-2 infection has been reported to vary from 1.0% to 19.9% of the dialysis population, with case fatality reported to range from 0% to 30.5%.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15
Several organizations have developed guidelines on how to mitigate the spread of SARS-CoV-2 in dialysis centers.16, 17, 18, 19, 20 Globally, HD centers have had to rapidly adopt new infection prevention and control and coronavirus testing policies and adapt established dialysis practices to safeguard patients and staff while still supporting continuation of dialysis services, depending on local resources.21 The ISN has advocated for equitable access to kidney care and has highlighted challenges that some regions are facing.16
We leveraged the ISN’s network of national nephrology societies and registries to understand the impact of the pandemic on HD centers across the world. We adopted a novel strategy to lessen survey response bias, cultivated over the past 25 years by the Dialysis Outcomes and Practice Pattern Study (DOPPS),22 to randomly sample centers to provide an understanding of the impact of the COVID-19 pandemic on HD centers and services from frontline providers. The experience of peritoneal dialysis centers and comparisons between low- and middle-income countries and high-income countries are explored in companion manuscripts.
Methods
Survey Development and Validation
An initial survey to assess the impact of COVID-19 on dialysis services was developed by DOPPS investigators and administered in May 2020 to June 2020 to sites participating in DOPPS phase 7 (HD centers in Gulf Cooperation Council, USA, China, and 7 European countries) and PDOPPS (peritoneal dialysis centers in Thailand and South Korea). In May 2020, DOPPS and ISN agreed to collaborate in revising and extending the survey to countries not participating in DOPPS. The survey was reviewed by clinicians working in low- and middle-income countries, and questions relevant to these settings were added (additional questions) or adapted (additional response options). Pilot testing was then performed by purposively sampled nephrology colleagues working in a range of settings, with an average survey completion time of 30 minutes for the main questions. After professional translation into French, Russian, and Spanish, the survey was deployed in SurveyMonkey (www.surveymonkey.com). The format was specifically designed to be user-friendly and completed on a desktop computer, tablet, or smartphone. Completion was voluntary, and no compensation was provided.
Sampling of Countries and Invitation of Countries and Individual Dialysis Centers
The adapted survey was subsequently disseminated through 2 additional stages, detailed in the Supplementary Methods (Supplementary Appendix S2). In stage 1, individual centers were selected from a list of centers in that country, provided by ISN member-societies. For countries with >40 centers nationwide, 20 centers stratified by region and dialysis patient population, were randomly selected. In stage 2, an open invitation of the survey was advertised via social media. This second stage was added after we noted that responses from many important countries and centers within countries were not received despite extensive efforts to engage with country and region societies and email reminders.
Ethics Approval
The ethical and independent review services approved this study (IRB000007807). The confidentiality statement is included in Supplementary Appendix S1.
Data Analysis
For the primary analysis, responses obtained through the representative (stage 1) and the open invitation samples (stage 2) were combined and presented stratified by ISN region (Africa, Eastern and Central Europe [E&C Europe], Western Europe [W Europe], Middle East, NIS & Russia, NE Asia, S Asia, South East Asia and Oceania [SE Asia & Oceania], N America & Caribbean, and Latin America). A timeline of the different survey stages is summarized in Figure 1. Quantitative differences between the responses received by the 2 approaches were explored in secondary analyses.
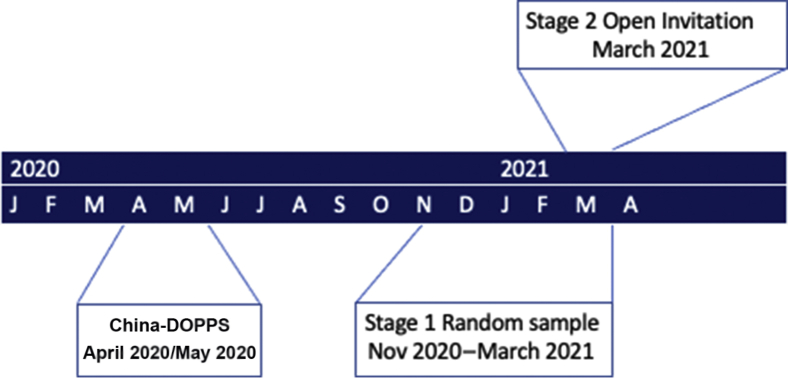
Figure 1.
Timeline of survey deployment. China was surveyed in April 2020/May 2020, randomly selected centers were surveyed in November 2020 to March 2021, and the survey was advertised via social media in March 2021. DOPPS, Dialysis Outcomes and Practice Patterns Study.
Descriptive statistics (counts and proportions, median [interquartile range]) were calculated, as appropriate. Complete case analyses were performed; nonmissing counts of responses are detailed in the first row of tables and adjacent to the respective region in figures. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NJ). The CONSORT checklist is provided in Supplementary Appendix S4.
Results
Country Outreach and Responses Received
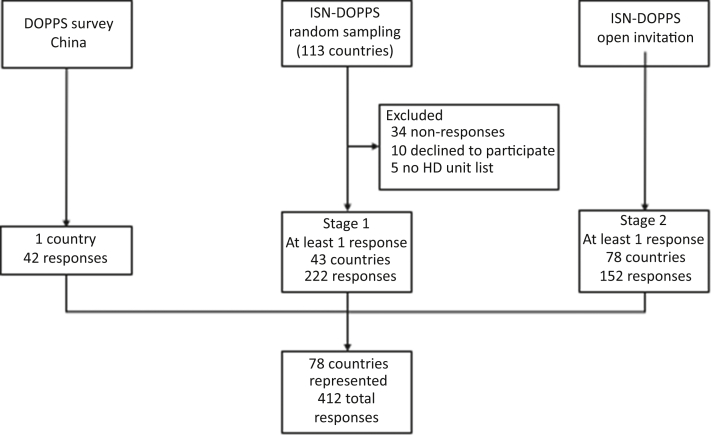
Lists of dialysis centers were received for sampling from 47 countries (Figure 2), with relatively good representation of several ISN regions: E&C Europe (65% of countries of this region), S Asia (56%), Latin America (48%), and W Europe (50%). Combining these responses with those from the open invitation and the China-DOPPS survey yielded 412 responses: representative sample 222, open invitation 152, and China-DOPPS 42. The highest number of responses was from Africa (n = 76, 18 countries).
Figure 2.
Flowchart of country outreach and participation. Of the 113 invited countries for which ISN held contact information, 64 were interested. Of these, only 47 provided lists or directly invited units themselves (n = 13). Only 43 of 47 countries responded to the survey. DOPPS, Dialysis Outcomes and Practice Patterns Study.
The characteristics of responding centers are presented by ISN region in Table 1. Most centers (79%) were located in an urban area, except in N America & Caribbean, where only 29% were in an urban area, with 32% in a suburban area and 39% in a rural area. Pediatric services were represented in 30% of responding centers (28% combined with adult services and 2% pediatric only), with this rising to 66% in Africa (63% combined with adult services and 3% pediatric only). Characteristics of the survey respondents are presented in Supplementary Table S1.
Table 1.
Characteristics of kidney units that responded, by ISN region
| Characteristics | All | ISN region |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Africaa | E&C Europeb | W Europec | Middle Eastd | NIS & Russiae | S Asiaf | Oceania & SE Asiag | NE Asiah | N America & Caribbeani | Latin Americaj | ||
| n facilities | 412 | 76 | 45 | 59 | 11 | 20 | 25 | 54 | 60 | 28 | 34 |
| n countries | 78 | 18 | 13 | 12 | 4 | 4 | 5 | 5 | 2 | 3 | 12 |
| World bank classification, %k | |||||||||||
| Low income | 8 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lower-middle income | 26 | 39 | 8 | 0 | 17 | 50 | 100 | 20 | 0 | 0 | 25 |
| Upper-middle income | 27 | 22 | 33 | 0 | 33 | 50 | 0 | 40 | 50 | 0 | 50 |
| High income | 40 | 6 | 59 | 100 | 50 | 0 | 0 | 40 | 50 | 100 | 25 |
| Healthcare sector, % | |||||||||||
| Public healthcare | 46 | 49 | 58 | 62 | 91 | 65 | 52 | 41 | 3 | 54 | 41 |
| Private healthcare | 27 | 27 | 16 | 16 | 0 | 25 | 20 | 41 | 31 | 32 | 47 |
| Academic/university hospital | 27 | 24 | 27 | 22 | 9 | 10 | 28 | 19 | 66 | 14 | 12 |
| Location, % | |||||||||||
| Rural area | 10 | 8 | 4 | 20 | 0 | 0 | 0 | 15 | 0 | 39 | 0 |
| Urban area | 79 | 72 | 87 | 68 | 91 | 100 | 88 | 81 | 92 | 29 | 97 |
| Suburban area | 12 | 20 | 9 | 12 | 9 | 0 | 12 | 4 | 8 | 32 | 3 |
| Services offered, % | |||||||||||
| Adults only | 70 | 34 | 82 | 98 | 73 | 100 | 64 | 56 | 63 | 100 | 71 |
| Children only | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 3 |
| Both | 28 | 63 | 16 | 2 | 27 | 0 | 36 | 39 | 37 | 0 | 27 |
| Modalities available, % | |||||||||||
| HD only | 49 | 79 | 53 | 22 | 36 | 50 | 56 | 48 | 18 | 64 | 47 |
| PD only | 1 | 0 | 4 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 6 |
| HD and PD | 50 | 21 | 42 | 78 | 55 | 50 | 44 | 52 | 82 | 36 | 47 |
DOPPS, Dialysis Outcomes and Practice Patterns Study; E&C Europe, Eastern and Central Europe; HD, hemodialysis; N America & Caribbean, North America and Caribbean; NE Asia, North East Asia; NIS & Russia, Newly Independent States and Russia; PD, peritoneal dialysis; REF, reference; S Asia, South Asia; SE Asia & Oceania, South East Asia and Oceania; W Europe, Western Europe.
Algeria, Cameroon, Democratic Republic of Congo, Republic of the Congo, Cote d’Ivoire, Ghana, Guinea, Kenya, Malawi, Mali, Mauritania, Mauritius, Namibia, Nigeria, Somalia, South Africa, Sudan, Tunisia.
Albania, Croatia, Cyprus, Czech Republic, Estonia, Lithuania, Moldova, Montenegro, North Macedonia, Romania, Serbia, Slovenia, Turkey.
Austria, Belgium, France, Germany, Greece, Israel, Italy, Norway, Portugal, Sweden, Switzerland, UK.
Iran, Kuwait, Oman, Syria.
Belarus, Georgia, Russia, Ukraine.
Bangladesh, India, Nepal, Pakistan, Sri Lanka.
Australia, Indonesia, Malaysia, New Zealand, Philippines.
South Korea, China (results for China included a prior survey wave of China between May and June 2020 as part of the DOPPS Study. Although other countries were also surveyed, only China is displayed here because there were no responses from China in the ISN-DOPPS survey wave, and the number of positive cases, availability of PPE and testing are thought to be consistent across the pandemic [REF]).
Canada, The Bahamas, USA.
Argentina, Bolivia, Brazil, Chile, Colombia, El Salvador, Guatemala, Mexico, Paraguay, Peru, Uruguay, Venezuela.
Income classification is based on the World Bank (worldbank.org). China-DOPPS was not asked whether pediatric/adult services were offered.
Burden and Fatality Among In-Center HD Patients
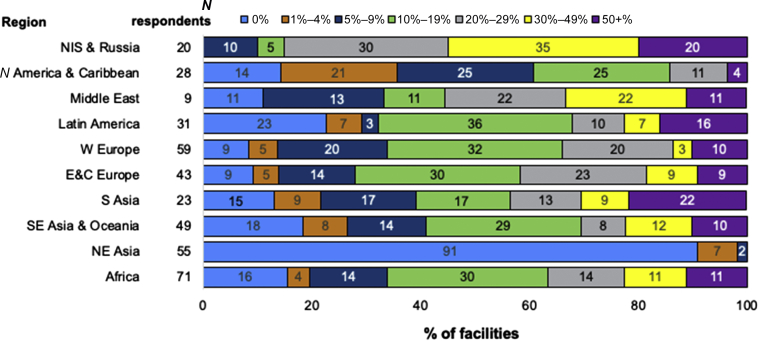
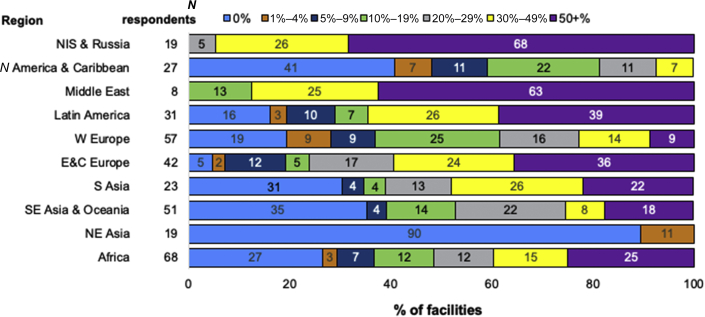
In 8 of the 10 ISN regions, rates of confirmed or suspected SARS-CoV-2 infection were reported to be <20% in the majority of centers; this included NE Asia, where the vast majority of centers reported no infections. The regions with higher rates were the Middle East, where the majority reported rates ≥20%, and NIS & Russia, where the majority reported rates ≥30% (Figure 3). In terms of severity of SARS-CoV-2 infection, the most commonly reported rate of hospitalizations was ≥50% in all regions (Figure 4).
Figure 3.
Proportion of confirmed or suspected (combined) SARS-CoV-2 infections among HD patients, by ISN region. Reported as the percentage of center dialysis population. The number of responses per region are shown next to the respective region (N respondents). The number inside the horizontal bar is the % of units in each category. E&C Europe, Eastern and Central Europe; HD, hemodialysis; N America & Caribbean, North America and Caribbean; NE Asia, North East Asia; NIS & Russia, Newly Independent States and Russia; S Asia, South Asia; SE Asia & Oceania, South East Asia and Oceania; W Europe, Western Europe.
Figure 4.
Proportion of HD patients hospitalized with SARS-CoV-2 infection, by ISN region. Reported as the % of center dialysis population. The number of responses per region are shown next to the respective region (N respondents). The number inside the horizontal bar is the % of units in each category. E&C Europe, Eastern and Central Europe; HD, hemodialysis; N America & Caribbean, North America and Caribbean; NE Asia, North East Asia; NIS & Russia, Newly Independent States and Russia; S Asia, South Asia; SE Asia & Oceania, South East Asia and Oceania; W Europe, Western Europe.
Patient mortality was reported to be ≥10% in >60% of centers in 8 of the 10 ISN regions, the exceptions being North America and NE Asia, where mortality was reported to be lower. In Latin America and Africa, 25% and 26% of HD centers reported mortality rates over 50%, respectively (Figure 5).
Figure 5.
Proportion of HD patients who died of SARS-CoV-2 infection, by ISN region. Reported as the % of center dialysis population. The number of responses per region are shown next to the respective region (N respondents). The number inside the horizontal bar is the % of units that selected each category. E&C Europe, Eastern and Central Europe; HD, hemodialysis; N America & Caribbean, North America and Caribbean; NE Asia, North East Asia; NIS & Russia, Newly Independent States and Russia; S Asia, South Asia; SE Asia & Oceania, South East Asia and Oceania; W Europe, Western Europe.
SARS-CoV-2 Diagnostic and Antibody Testing of Symptomatic Dialysis Patients
Reporting on the situation in the early months of the pandemic (April 2020 and May 2020), diagnostic testing (e.g., polymerase chain reaction) was only reported to be widely available in SE Asia & Oceania (69% of responding centers) (Table 2). Later, at the time of the survey (November 2020–March 2021), testing had improved but was still not available in a significant percentage of centers in some regions: Latin America (33%), Africa (37%), and E&C Europe (61%). Access to antibody testing was even more limited (Supplementary Table S2).
Table 2.
Proportion of SARS-CoV-2 diagnostic and antibody testing availability, by ISN region
| Testing availability | All | ISN region |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | E&C Europe | W Europe | Middle East | NIS & Russia | S Asia | Oceania & SE Asia | NE Asia | N America & Caribbean | Latin America | ||
| n facilities | 384 | 74 | 41 | 57 | 10 | 20 | 23 | 51 | 52 | 26 | 30 |
| Availability of PCR testing | |||||||||||
| In April 2020/May 2020, % | |||||||||||
| Not available | 13 | 24 | 7 | 5 | 10 | 5 | 9 | 16 | 4 | 16 | 27 |
| Limited | 26 | 28 | 32 | 13 | 30 | 16 | 44 | 42 | 2 | 20 | 53 |
| Moderate | 33 | 30 | 27 | 57 | 40 | 47 | 39 | 26 | 25 | 40 | 13 |
| Widespread | 27 | 18 | 34 | 25 | 20 | 32 | 9 | 16 | 69 | 24 | 7 |
| Now, % | |||||||||||
| Not available | 5 | 11 | 2 | 0 | 11 | 0 | 4 | 4 | 0 | 12 | 7 |
| Limited | 8 | 21 | 5 | 0 | 11 | 0 | 0 | 4 | 0 | 4 | 20 |
| Moderate | 21 | 30 | 32 | 4 | 0 | 11 | 17 | 26 | 11 | 8 | 40 |
| Widespread | 66 | 37 | 61 | 96 | 78 | 90 | 78 | 66 | 89 | 77 | 33 |
| Anticipated in 3-6 months, % | |||||||||||
| Not available | 7 | 12 | 5 | 2 | 0 | 0 | 5 | 4 | 0 | 16 | 13 |
| Limited | 9 | 20 | 5 | 0 | 20 | 0 | 0 | 6 | 0 | 8 | 20 |
| Moderate | 19 | 29 | 27 | 6 | 10 | 11 | 18 | 20 | 11 | 4 | 37 |
| Widespread | 66 | 39 | 63 | 92 | 70 | 89 | 77 | 69 | 89 | 72 | 30 |
| Availability of antibody testing | |||||||||||
| In April 2020/May 2020, % | |||||||||||
| Not available | 58 | 77 | 56 | 48 | 44 | 47 | 83 | 40 | 45 | 71 | 60 |
| Limited | 33 | 18 | 37 | 45 | 44 | 32 | 17 | 50 | 28 | 25 | 37 |
| Universal testing | 10 | 5 | 7 | 7 | 11 | 21 | 0 | 10 | 28 | 4 | 3 |
| Now, % | |||||||||||
| Not available | 31 | 54 | 34 | 9 | 40 | 16 | 35 | 20 | 61 | 23 | 27 |
| Limited | 42 | 37 | 54 | 41 | 40 | 53 | 44 | 32 | 11 | 50 | 57 |
| Universal testing | 27 | 10 | 12 | 50 | 20 | 32 | 22 | 48 | 28 | 2 | 17 |
| Anticipated in 3-6 months, % | |||||||||||
| Not available | 26 | 46 | 27 | 6 | 11 | 15 | 33 | 17 | 44 | 17 | 33 |
| Limited | 41 | 34 | 59 | 42 | 56 | 50 | 33 | 30 | 22 | 50 | 43 |
| Universal testing | 33 | 20 | 15 | 52 | 33 | 35 | 33 | 53 | 33 | 33 | 23 |
DOPPS, Dialysis Outcomes and Practice Patterns Study; E&C Europe, Eastern and Central Europe; N America & Caribbean, North America and Caribbean; NE Asia, North East Asia; NIS & Russia, Newly Independent States and Russia; PCR, polymerase chain reaction; S Asia, South Asia; SE Asia & Oceania, South East Asia and Oceania; W Europe, Western Europe.
Limited availability was defined as the test being challenging to obtain even for symptomatic persons; moderate availability, tests were readily obtained for symptomatic persons but broadly restricted otherwise; and widespread, readily obtained for persons with mild symptoms and contacts. “Now” for China-DOPPS was May 2020/June 2020 and November 2020 to March 2021 for the remaining responses. The number of responses is shown in the first row, that is, some respondents did not fully answer this question.
Infection Prevention and Control
Surgical masks were mostly worn for direct contact with all patients, with particulate-air filter respirators being used sparingly (Table 3). Surgical masks were not available at all in 1% of centers, and there was a severe shortage in 5% and a moderate shortage in 22% (Table 4). Access to particulate-air filter masks was even more limited, with a severe shortage reported in 30% of African and 18% of Latin American centers. In all regions, many centers reported extending the shelf-life of masks beyond that recommended by the manufacturer: Africa 38%, E&C Europe 34%, Latin America 41%, Middle East 50%, NIS & Russia 10%, N America & Caribbean 31%, NE Asia 11%, SE Asia & Oceania 40%, S Asia 54%, W Europe 22%.
Table 3.
Proportion of use of different types of PPE worn by HD staff, by ISN region
| Types of PPE | All | ISN region |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | E&C Europe | W Europe | Middle East | NIS & Russia | S Asia | Oceania & SE Asia | NE Asia | N America & Caribbean | Latin America | ||
| n facilities | 381 | 70 | 41 | 55 | 10 | 20 | 23 | 51 | 56 | 26 | 29 |
| Particulate-air filter respirators (e.g., N95 masks), % | |||||||||||
| For direct contact—with all patients | 36 | 32 | 44 | 48 | 11 | 42 | 44 | 35 | 26 | 8 | 52 |
| For direct contact—only for patients with suspected/confirmed cases | 51 | 52 | 51 | 40 | 67 | 53 | 48 | 47 | 67 | 58 | 33 |
| Not available in this dialysis unit | 10 | 13 | 5 | 10 | 11 | 5 | 9 | 8 | 6 | 15 | 15 |
| Available but not used | 4 | 3 | 0 | 2 | 11 | 0 | 0 | 10 | 2 | 19 | 0 |
| Surgical mask, % | |||||||||||
| For direct contact—with all patients | 89 | 90 | 89 | 79 | 80 | 100 | 96 | 90 | 90 | 96 | 83 |
| For direct contact—only for patients with suspected/confirmed cases | 6 | 3 | 5 | 11 | 20 | 0 | 0 | 4 | 8 | 4 | 7 |
| Not available in this dialysis unit | 1 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 7 |
| Available but not used | 4 | 6 | 3 | 11 | 0 | 0 | 4 | 6 | 0 | 0 | 3 |
| Gloves, % | |||||||||||
| For direct contact—with all patients | 84 | 93 | 90 | 67 | 60 | 90 | 91 | 92 | 80 | 81 | 83 |
| For direct contact—only for patients with suspected/confirmed cases | 15 | 7 | 10 | 27 | 40 | 10 | 9 | 6 | 20 | 19 | 17 |
| Not available in this dialysis unit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Available but not used | 1 | 0 | 0 | 6 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Eye protection, % | |||||||||||
| For direct contact—with all patients | 63 | 45 | 71 | 59 | 44 | 70 | 43 | 84 | 51 | 92 | 79 |
| For direct contact—only for patients with suspected/confirmed cases | 30 | 32 | 27 | 40 | 56 | 30 | 38 | 14 | 47 | 8 | 14 |
| Not available in this dialysis unit | 4 | 16 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 7 |
| Available but not used | 3 | 7 | 2 | 2 | 0 | 0 | 14 | 2 | 2 | 0 | 0 |
| Isolation gown, % | |||||||||||
| For direct contact—with all patients | 47 | 25 | 49 | 28 | 63 | 70 | 30 | 75 | 32 | 77 | 79 |
| For direct contact—only for patients with suspected/confirmed cases | 46 | 56 | 49 | 67 | 38 | 30 | 61 | 22 | 64 | 19 | 21 |
| Not available in this dialysis unit | 4 | 13 | 2 | 4 | 0 | 0 | 4 | 0 | 2 | 0 | 0 |
| Available but not used | 3 | 6 | 0 | 2 | 0 | 0 | 4 | 4 | 2 | 4 | 0 |
| Plastic apron,% | |||||||||||
| For direct contact—with all patients | 45 | 38 | 50 | 40 | 20 | 33 | 71 | 71 | 43 | 26 | 26 |
| For direct contact—only for patients with suspected/confirmed cases | 31 | 35 | 33 | 45 | 50 | 33 | 10 | 14 | 57 | 9 | 44 |
| Not available in this dialysis unit | 16 | 22 | 10 | 0 | 10 | 27 | 19 | 6 | 0 | 52 | 30 |
| Available but not used | 8 | 6 | 8 | 15 | 20 | 7 | 0 | 8 | 0 | 13 | 0 |
DOPPS, Dialysis Outcomes and Practice Patterns Study; E&C Europe, Eastern and Central Europe; HD, hemodialysis; N America & Caribbean, North America and Caribbean; NE Asia, North East Asia; NIS & Russia, Newly Independent States and Russia; PPE, personal protective equipment; S Asia, South Asia; SE Asia & Oceania, South East Asia and Oceania; W Europe, Western Europe.
Answers reference the entire pandemic period as of the survey completion date: November 2020 to March 2021 for all countries except China-DOPPS (May 2020/June 2020).
Table 4.
Proportion of perceived PPE shortages, by ISN region
| Perceived shortages | All | ISN region |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | E&C Europe | W Europe | Middle East | NIS & Russia | S Asia | Oceania & SE Asia | NE Asia | N America & Caribbean | Latin America | ||
| n facilities | 342 | 69 | 41 | 55 | 10 | 20 | 23 | 51 | 18 | 26 | 29 |
| Particulate-air filter respirators (e.g., N95 masks), % | |||||||||||
| No shortage | 39 | 15 | 55 | 35 | 50 | 58 | 30 | 41 | 100 | 32 | 41 |
| Moderate shortage | 38 | 44 | 30 | 38 | 30 | 37 | 52 | 39 | 0 | 56 | 35 |
| Severe shortage | 14 | 30 | 8 | 18 | 0 | 0 | 9 | 12 | 0 | 4 | 14 |
| Not available (before or during pandemic) | 9 | 12 | 8 | 9 | 20 | 5 | 9 | 8 | 0 | 8 | 10 |
| Surgical mask, % | |||||||||||
| No shortage | 72 | 54 | 76 | 67 | 80 | 90 | 74 | 80 | 89 | 81 | 76 |
| Moderate shortage | 22 | 39 | 17 | 26 | 20 | 11 | 22 | 14 | 11 | 15 | 17 |
| Severe shortage | 5 | 4 | 7 | 7 | 0 | 0 | 4 | 6 | 0 | 4 | 3 |
| Not available (before or during pandemic) | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Gloves, % | |||||||||||
| No shortage | 80 | 73 | 85 | 78 | 80 | 90 | 74 | 86 | 94 | 77 | 72 |
| Moderate shortage | 17 | 23 | 12 | 18 | 20 | 11 | 22 | 8 | 6 | 19 | 24 |
| Severe shortage | 3 | 3 | 2 | 4 | 0 | 0 | 4 | 6 | 0 | 4 | 3 |
| Not available (before or during pandemic) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eye protection, % | |||||||||||
| No shortage | 63 | 35 | 66 | 71 | 60 | 65 | 35 | 78 | 94 | 89 | 66 |
| Moderate shortage | 25 | 35 | 24 | 24 | 40 | 35 | 44 | 14 | 6 | 12 | 17 |
| Severe shortage | 7 | 9 | 7 | 6 | 0 | 0 | 17 | 6 | 0 | 0 | 10 |
| Not available (before or during pandemic) | 6 | 21 | 2 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 7 |
| Isolation gown, % | |||||||||||
| No shortage | 58 | 25 | 66 | 57 | 60 | 80 | 39 | 73 | 100 | 69 | 66 |
| Moderate shortage | 31 | 45 | 20 | 30 | 40 | 20 | 52 | 26 | 0 | 31 | 28 |
| Severe shortage | 7 | 12 | 12 | 11 | 0 | 0 | 9 | 2 | 0 | 0 | 7 |
| Not available (before or during pandemic) | 4 | 19 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Plastic apron, % | |||||||||||
| No shortage | 57 | 42 | 62 | 58 | 60 | 67 | 48 | 76 | 88 | 33 | 50 |
| Moderate shortage | 22 | 20 | 23 | 31 | 20 | 22 | 35 | 16 | 12 | 13 | 25 |
| Severe shortage | 5 | 12 | 5 | 6 | 10 | 0 | 4 | 2 | 0 | 4 | 4 |
| Not available (before or during pandemic) | 16 | 26 | 10 | 6 | 10 | 11 | 13 | 6 | 0 | 50 | 21 |
DOPPS, Dialysis Outcomes and Practice Patterns Study; E&C Europe, Eastern and Central Europe; N America & Caribbean, North America and Caribbean; NE Asia, North East Asia; NIS & Russia, Newly Independent States and Russia; PPE, personal protective equipment; S Asia, South Asia; SE Asia & Oceania, South East Asia and Oceania; W Europe, Western Europe.
Shortage related to any time during the pandemic. No definition of moderate and severe was provided. Answers reference the entire pandemic period as of the survey completion date: November 2020 to March 2021. This question was not included in the initial DOPPS survey and is therefore unavailable for China-DOPPS. “Moderate” and “severe” were not predefined in the survey.
Adoption of the recommended 2 m (6 ft) distance between HD machines was variable between regions: Africa 21%, E&C Europe 27%, Latin America 21%, Middle East 30%, NIS & Russia 15%, N America & Caribbean 65%, NE Asia 6% (this was not asked of China-DOPPS HD centers), SE Asia & Oceania 16%, S Asia 5%, W Europe 40%. A few centers across all regions were not able to achieve at least 1 m distance between machines (12% overall), a particular difficulty in 18% of African and S Asian, 20% of NIS & Russian, and 33% of NE Asian centers.
Isolation of patients who were suspected of having or tested positive for SARS-CoV-2 on separate dialysis shifts was implemented in 65% of N American & Caribbean centers, despite specific Centers for Disease Control and Prevention guidelines,17 and was implemented more widely elsewhere: 70% to 79% (Latin America, NIS & Russia, NE Asia, W Europe) and 80% to 89% (E&C Europe, Middle East, SE Asia & Oceania, S Asia).
Access to HD and Missed Visits
Transportation to/from the dialysis center was reported to be challenging in many or most centers across all regions: Africa (65%), E&C Europe (73%), Latin America (79%), Middle East (70%), NIS & Russia (45%), N America & Caribbean (77%), NE Asia (55%), SE Asia (70%), S Asia (82%), and W Europe (74%).
Compared with before the pandemic, N America & Caribbean (4%) and W Europe (4%) reported very few missed HD sessions, whereas Africa (52%), E&C Europe (12%), Latin America (68%), Middle East (50%), NIS & Russia (25%), NE Asia (14%), SE Asia & Oceania (48%), and S Asia (76%) reported more sessions missed during the pandemic.
Changes to Center Practice
Globally, few centers reported having to decrease HD session length either because of logistic (limited slots, staff, or supplies; 6%), infection control (to limit exposure; 5%) or for both reasons (8%). Likewise, most respondents reported having made no changes to the number of HD sessions per week.
Although home dialysis was reported to be widely available in N America & Caribbean and W Europe, it was rarely available in many surveyed centers: Africa (62%), E&C Europe (46%), Latin America (46%), NIS & Russia (60%), N Asia (19%), SE Asia & Oceania (46%), and S Asia (76%). Centers were divided as to whether they were more likely to recommend initiating home dialysis for new patients requiring kidney replacement therapy during the pandemic. Despite transplantation being available in all centers surveyed in Middle East, N America & Caribbean, and NE Asia, only 20%, 15%, and 18% of respective respondents said that they were more likely to recommend a kidney transplant to dialysis patients because of the COVID-19 pandemic. Transplantation was reported to be rarely available in Africa (40%), Latin America (29%), and SE Asia & Oceania (28%).
Impact on Staff
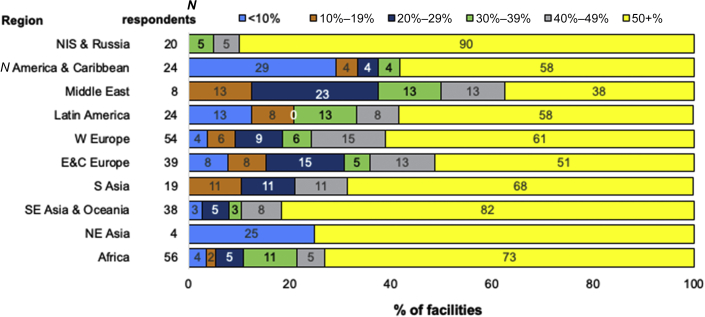
The most commonly reported rate of confirmed or suspected SARS-CoV-2 infection among HD staff was ≥50% in NIS & Russia, Middle East, Latin America, and EC Europe, whereas in N America & Caribbean, W Europe, S Asia, and Africa, it was 0% (Figure 6). Hospitalization of staff was most commonly reported as <10% in all regions, except in SE Asia & Oceania and NE Asia, where ≥50% of infected staff were hospitalized (Supplementary Figure S1). Considering mortality, ≥50% of staff with suspected or confirmed SARS-CoV-2 were reported to have died in 6% of HD centers in SE Asia & Oceania and 2% of HD centers in Africa, whereas in other regions, no centers reported mortality rates ≥10%.
Figure 6.
Proportion of confirmed or suspected (combined) SARS-CoV-2 infection among HD staff, by ISN region. Reported as the % of center dialysis population. The number of responses per region are shown next to the respective region (N respondents). The number inside the bar is the % of units in each category. E&C Europe, Eastern and Central Europe; HD, hemodialysis; N America & Caribbean, North America and Caribbean; NE Asia, North East Asia; NIS & Russia, Newly Independent States and Russia; S Asia, South Asia; SE Asia & Oceania, South East Asia and Oceania; W Europe, Western Europe.
Globally, 29% of respondents reported redeployment of dialysis center staff to other clinical areas during the pandemic: centers located in N America & Caribbean (12%), SE Asia & Oceania (18%), S Asia (23%), and E&C Europe (27%) redeployed dialysis staff relatively less commonly, whereas centers in Africa (32%), NIS & Russia (35%), W Europe (39%), Latin America (41%), and Middle East (50%) were often required to reorganize their staff. Furthermore, a severe shortage of personnel was reported in S Asia (18%), whereas other regions reported a severe shortage in 5% to 10% of centers.
Overall, mental health and wellbeing services were only made available to staff in 48% of HD centers, with this varying considerably by region: S Asia (25%), NIS & Russia (30%), E&C Europe (38%), Africa (40%), Middle East (40%), SE Asia & Oceania (41%), W Europe (47%), Latin America (63%), NE Asia (66%), and N America & Caribbean (84%). Where these services were available, there was no cost to staff for their use, except in 6% of African centers, 10% of N American & Caribbean centers, and 4% of W European centers. With the exception for Latin America and S Asia, most regions reported that staff were not always aware of these services.
Differences Between Responses From Representative Sample and Open Invitation
There were 209 surveys returned as part stage 1 (random sample) and 203 as part of stage 2 (open invitation). A comparison of center characteristics between the 2 approaches is displayed in Supplementary Table S3. The surveyed centers were very similar overall, for example in terms of facility type, availability of testing, and use of and shortages of PPE (Supplementary Tables S2, S4, and S5).
Discussion
We report the results of an international, comprehensive, and first of its kind web-survey on the impact of the COVID-19 pandemic on in-center HD centers—a collaboration between the DOPPS and the ISN. Using a combination of sampling approaches, and guaranteeing anonymized reporting, responses were received from a wide range of countries demonstrating a wide global variation in the rates of SARS-CoV-2 infection among staff and patients, the availability of PPE and testing, and the ways in which HD services were redesigned as a response to the pandemic.23 We provide evidence of deficiencies in resource allocation, infrastructure, and compromised HD care that we hope can be used to leverage governments and policymakers to advocate improvements in HD services in future COVID-19 waves, pandemics, and crises.
In most regions, centers reported a high number of SARS-CoV-2 infections and associated deaths, with 2 notable exceptions—in NE Asia (representing S Korea and China only) most centers reported having no cases and no fatalities among their HD patients, consistent with data published elsewhere.15 In NIS & Russia, in contrast, most centers reported suspected or confirmed SARS-CoV-2 infection rates over 30%. The incidence of SARS-CoV-2 infection in high-income countries (USA, UK, Italy, France, Spain, Canada, and China) has previously been estimated to be between 1% and 30%.6, 7, 8,10,13, 14, 15 Centers in India, Iran, South Africa, and Turkey have reported SARS-CoV-2 infection rates between 7% and 47% among their dialysis patients, with fatality rates of 2% to 38%.3, 4, 5,11,12,23 Whereas infection rates in low- and middle-income countries were generally higher than in high-income countries, differences may reflect the community infection burden at that time rather than within center transmission,6,8,24,25 although outbreaks within centers have been reported globally.6,7 Center layout, including available side rooms for isolation, and patient factors have also been associated with the risk of infection.24 Diagnostic testing was not widely available in most centers surveyed, especially in Africa (37% widely available) and Latin America (33%), and this would have made screening and isolation policies difficult to implement to reduce spread. Furthermore, because testing was not always available, the burden was likely to be underestimated, especially early in the pandemic when targeted testing was performed.26
The ISN and other national kidney organizations have issued guidelines on how to safely continue in-center HD services through the COVID-19 pandemic.16, 17, 18, 19,27,28 These have mostly been consistent and practical.29 Paramount to being able to follow recommendations is the availability of adequate resources and, in particular, PPE. A significant number of centers reported PPE shortages not only because of an increased demand but also because of national and international disruptions in supply chains.30
These shortages in respiratory protection were particularly frequently reported in Africa, where resources are often limited, regardless of pandemic status.31 Respiratory protection is critical to reduce viral transmission during the COVID-19 pandemic, because SARS-CoV-2 is particularly spread by respiratory droplets and aerosols.32 Asymptomatic patient and staff masking has been shown to decrease the risk of hospitalization of infected dialysis patients.24 Despite shortages and some evidence that reuse of masks after decontamination was acceptable,33 staff did not report having to use masks that were beyond the manufacturer’s suggested expiration date or to reuse masks after decontamination.
Although still being tested in the TWOPLUS-HD trial, twice weekly dialysis may not be associated with risk in some patients if delivered with concomitant potassium-lowering medication. In a pandemic situation, the risk benefit balance is likely to shift toward fewer dialysis sessions to minimize time at risk of infection in a dialysis center. Reflecting this, many of the COVID-19 guidelines recommend considering decreasing the number of HD sessions per week or hours per session. We found that centers reduced dialysis sessions or time infrequently, necessitated by either limited dialysis slots, staff, or consumables, or because of the risk of transmission that could occur between patients and staff.34 This was despite recommendations16, 17, 18 and twice weekly dialysis potentially being safe.34,35
E&C Europe, Africa, Middle East, Latin America, and S Asia reported relatively more patients missing HD sessions because of the pandemic. Missed visits may have been a challenge for centers because of cessation of shared transportation services to/from HD, which was problematic in all regions, disruptions to HD shifts, or fear of being exposed to SARS-CoV-2.8,11 Shared transportation has been found to be an independent risk factor for infection,36 but for many patients, shared and public transport is the only available means of accessing dialysis.
As part of social distancing recommendations, few centers reported increasing the distance between machines to >2 m (25% across all regions), whereas most reported achieving 1 to 2 m (62%). This may have been because adopting a 2 m policy would have resulted in a significant reduction in the number of people who could be treated in the same HD center and the consequences of that being weighed against the risks of SARS-CoV-2 infection. Furthermore, it has been suggested in general population studies that even a distance 2 m may not be enough to prevent SARS-CoV-2 transmission.37 However, in the HD population, there is some evidence from the UK that every 1-m increase in spacing between machines decreases the transmission of SARS-CoV-2 and admission to hospital by 32%.24
For patients with confirmed SAR-CoV-2 infection, dedicated shifts were used to isolate these patients from others in 65% (N America & Caribbean) to 95% (E&C Europe, and S Asia) of centers. This may have been due to space/time/staff limitations in the N America & Caribbean region or due to a greater ability to find alternative solutions, such as isolation rooms or zones within the same dialysis center.7,24,36
The nephrology workforce under nonpandemic conditions is insufficient in many regions and is essential to maintain HD services.38 Some regions reported more than half of their staff being infected with SARS-CoV-2. This is much higher than hospital staff seroprevalence (6.4% of Belgian healthcare workers) or dialysis staff seroprevalence (16.5% and 6% of dialysis staff in New York and Wuhan, China, respectively) in other studies.39, 40, 41 Our findings point to the imperative of assuring that dialysis centers worldwide are resourced to sufficiently implement recommended infection prevention and control measures not only to protect patients but also to protect staff. Many respondents reported staff being redeployed to other clinical areas. This combination of hits to HD staffing levels will have made the ongoing delivery of HD services very challenging, in addition to the enormous physical and psychological pressures experienced by staff.42 Staff in many locations will also have had to deal with the additional burden of providing consults and acute dialysis services to people with acute kidney injury associated with SARS-CoV-2 infection and potentially having had to ration treatment.43 The COVID-19 pandemic has put unprecedented pressures on healthcare services and professionals, not least those providing care to people with kidney disease,38,44 yet psychological support services were only available in about half of surveyed centers.
Strengths
By bringing together the survey design expertise of DOPPS and the global clinical network of ISN, this survey has captured the impact of the COVID-19 pandemic on a broad range of centers from almost 50 countries, with care to lessen survey response bias. These 50 countries represent 2.3/7.8 billion (29%) people of the world in 2020 (ourworldindata.org), although we were unable to obtain responses from populous countries such as India and Indonesia (stage 1 of survey invitation). The targeted invitation ensured that a range of frontline HD providers was surveyed, not only overly optimistic or pessimistic respondents keen to forward a particular agenda. Responses were anonymous and cannot be traced back to specific people or centers. Deficiencies in resources were anticipated to be most acute and politically sensitive in low-income countries, and it was recognized that the smaller number of HD centers in these countries could make identification of respondents easier. For these reasons, respondents in low-income countries were assured that their data would only be reported at a country level. Importantly, there were no differential missing data patterns for particular survey questions, and missing responses were overall low.
Limitations
There were a few challenges in the distribution of the survey. The number of countries that responded to invitations to share HD center lists and the number of centers that eventually responded were limited. Overwhelmed clinical services, survey fatigue, and, in some cases, death of colleagues from SARS-CoV-2 infection resulted in nonresponse. Consequently, initial plans to survey a random, representative sample were adapted, and the invitation was extended to anyone who wished to complete the survey via mailing lists and social media advertising. Facility type, testing, and PPE use and shortages were not dissimilar between representative and open invitation responses, overall, however. Aggregating the results was therefore appropriate. Although the time window for respondents to complete the survey for the random sample (stage 1) was longer than for the open invitation via social media (stage 2), the number of responses received were approximately equal. It is not possible to rule out a response bias, but this could have been in either direction because some may have been more motivated to complete the survey via social media because they were in the midst of a pandemic peak or, on the contrary, have more time to complete the survey now that infection numbers and pressures have abated. Last, countries responded at different points in their local peak and wave, and the number of SARS-CoV-2 infections may have been underestimated because of limitations in diagnostic testing, although diagnostic testing questions were not limited to only polymerase chain reaction technology.
Future Research
We have identified that some regions may have been particularly affected. We therefore have developed a companion manuscript that explores differences between high-income countries and low- and middle-income countries. The experience of peritoneal dialysis centers will also be explored separately. Data are available, on request, for non–low-income countries with >5 responses, to leverage change in practice and policy locally. The nephrology community has highlighted that there is variable access to vaccines and divergent vaccine policies across the world and have vehemently advocated for the prioritization of at-risk dialysis patients and staff.45 A survey on the availability and implementation of vaccine rollout programs is therefore planned.
Conclusion
This collaborative, wide-reaching survey using a novel sampling approach has found that the COVID-19 pandemic has had a significant impact on dialysis services and staffing worldwide. Guidance needs to be consistent, adaptable to availability of resources and infrastructure, and evidence based. Policymakers and governments need to ensure that adequate resources are provided to implement such policies. Equitable provision of resources to healthcare facilities serving high risk-patients attending multiple times a week for life-sustaining treatment—in this case, for HD care—is essential if patients, staff, and services in future COVID-19 waves and future pandemics are to be protected.
Disclosure
RPF has received research grants from Fresenius Medical Care, consulting fees from Astra Zeneca, Bayer, Novo Nordisk, Boeringer-Lilly, and Rethrophin. RPF has also conducted voluntary work for the ISN and KDIGO. VJ has received grants from Baxter Healthcare, GlaxoSmithKline, NephroPlus, and Biocon, received honoraria from AstraZeneca and Baxter healthcare, participated in Zydus and GlaxoSmithKline data safety monitoring boards, and is the past president of the ISN. AL is a member of ISN ExCom, OSEA Regional Board, and Chair of the ISN Disaster Preparedness Working Group. BR has received consultancy fees or travel reimbursement since 2019 from AstraZeneca, GlaxoSmithKline, and Kyowa Kirin Co. All the other authors declared no competing interests.
Acknowledgments
The authors would like to thank national Society and Registry country and ISN Regional Board leads who provided lists of dialysis centers that we were then able to contact. Supplementary Appendix S3 lists these contributors and surveyed respondents who wished to be acknowledged (NB we are unable to link respondents with individual responses). We are grateful to Charu Malik and Paul Laffin at ISN for their support, and Silvia Salaro at ISN who assisted with contacting country leads and deploying the survey. We are grateful to staff at Arbor Research Collaborative for Health for supporting survey development and deployment. This research has previously been presented at Kidney Week 2021 (American Society of Nephrology). Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. Funding is provided to Arbor Research Collaborative for Health and not to investigators. For details see https://www.dopps.org/AboutUs/Support.aspx.
Author Contributions
BR, RPF, MG, and RP (DOPPS) developed the study idea and initial questionnaire. FC, RA, ET, GD, AL, VL, DS, CP, RE, and VJ adapted the survey. RA and FC disseminated the survey to ISN-members (with the support of ISN). BB performed the analysis. RA wrote a first draft of the manuscript, with edits by FC, RPF, and BR. All authors reviewed the manuscript and approved its final draft.
Footnotes
Appendix S1. Final ISN-DOPPS deployed survey including confidentiality statement.
Appendix S2. Supplementary methods and results.
Table S1. Characteristics of survey respondents, by ISN region.
Table S2. Unit characteristics, by random sample (stage 1) versus open invitation (stage 2), overall and by ISN Region.
Table S3. Availability of diagnostic and antibody testing, compared between random sample (stage 1) versus open invitation (stage 2), overall and by ISN region.
Table S4. Comparison between random sample (stage 1) and open invitation (stage 2) in terms of PPE use, overall and by ISN region.
Table S5. PPE shortages, by random sample (stage 1) versus open invitation (stage 2), overall and by ISN region.
Figure S1. Proportion of confirmed or suspected SARS-CoV-2 infected staff who required hospitalization, by ISN region.
Appendix S3. List of contributors.
Appendix S4. STROBE checklist.
Supplementary Material
Appendix S1. Final ISN-DOPPS deployed survey including confidentiality statement.
Appendix S2.Supplementary Methods and Results.
Table S1. Characteristics of survey respondents, by ISN region.
Table S2. Unit characteristics, by random sample (stage 1) versus open invitation (stage 2), overall and by ISN Region.
Table S3. Availability of diagnostic and antibody testing, compared between random sample (stage 1) versus open invitation (stage 2), overall and by ISN region.
Table S4. Comparison between random sample (stage 1) and open invitation (stage 2) in terms of PPE use, overall and by ISN region.
Table S5. PPE shortages, by random sample (stage 1) versus open invitation (stage 2), overall and by ISN region.
Figure S1. Proportion of confirmed or suspected SARS-CoV-2 infected staff who required hospitalization, by ISN region.
Appendix S3. List of contributors.
Appendix S4. STROBE checklist.
References
- 1.Naicker S., Yang C.W., Hwang S.J., Liu B.C., Chen J.H., Jha V. The Novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilbrands L.B., Duivenvoorden R., Vart P., et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration [published correction appears in Nephrol Dial Transplant. 2021;36:1962] Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones E.S.W., Davidson B.J., Barday Z., et al. COVID-19 and the kidney: a South African state healthcare experience. Clin Nephrol. 2021;95:171–181. doi: 10.5414/CN110390. [DOI] [PubMed] [Google Scholar]
- 4.Ossareh S., Bagheri M., Abbasi M., Abolfathi S., Bohlooli A. Role of screening for COVID-19 in hemodialysis wards, results of a single center study. Iran J Kidney Dis. 2020;14:389–398. [PubMed] [Google Scholar]
- 5.Tayebi Khosroshahi H., Mardomi A., Niknafs B., et al. Current status of COVID-19 among hemodialysis patients in the East Azerbaijan Province of Iran. Hemodial Int. 2021;25:214–219. doi: 10.1111/hdi.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taji L., Thomas D., Oliver M.J., et al. Covid-19 in patients undergoing long-term dialysis in Ontario. CMAJ. 2021;193:E278–E284. doi: 10.1503/cmaj.202601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yau K., Muller M.P., Lin M., et al. COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis. 2020;76:690–695.e1. doi: 10.1053/j.ajkd.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett R.W., Blakey S., Nitsch D., et al. Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol. 2020;31:1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibernon M., Bueno I., Rodríguez-Farré N., et al. The impact of COVID-19 in hemodialysis patients: experience in a hospital dialysis unit. Hemodial Int. 2021;25:205–213. doi: 10.1111/hdi.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y., Diao B., Lv X., et al. COVID-19 in Hemodialysis (HD) Patients: Report From One HD Center in Wuhan, China. MedRxiv. https://www.medrxiv.org/content/10.1101/2020.02.24.20027201v3.full.pdf Publshed June 17, 2020. Accessed August 16, 2021.
- 11.Deshpande R., Dash S., Bahadur M.M., et al. Study of covid-19 pandemic in representative dialysis population across Mumbai, India: an observational multicentric analysis. J Assoc Physicians India. 2020;68:13–17. [PubMed] [Google Scholar]
- 12.Trivedi M., Shingada A., Shah M., Khanna U., Karnik N.D., Ramachandran R. Impact of COVID-19 on maintenance haemodialysis patients: the Indian scenario. Nephrology (Carlton) 2020;25:929–932. doi: 10.1111/nep.13760. [DOI] [PubMed] [Google Scholar]
- 13.Keller N., Chantrel F., Krummel T., et al. Impact of first-wave COronavirus disease 2019 infection in patients on haemoDIALysis in Alsace: the observational COVIDIAL study. Nephrol Dial Transplant. 2020;35:1338–1411. doi: 10.1093/ndt/gfaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dian S., Simeone M., Rossi B., et al. Going to war with COVID-19: strategies for SARS-CoV-2 management in the Padua Nephrology and Dialysis Unit’s hemodialysis facility. Clin Nephrol. 2021;95:151–156. doi: 10.5414/CN110330. [DOI] [PubMed] [Google Scholar]
- 15.Robinson B.M., Guedes M., Alghonaim M., et al. Worldwide early impact of COVID-19 on dialysis patients and staff and lessons learned: a DOPPS roundtable discussion. Kidney Med. 2021;3:619–634. doi: 10.1016/j.xkme.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ISN The International Society of Nephrology COVID-19 Recommendations. ISN, https://www.theisn.org/initiatives/covid-19/recommendations/ Accessed May 12, 2021.
- 17.Center for Disease Control and Prevention Interim additional guidance for infection prevention and control recommendations for patients with suspected or confirmed COVID-19 in outpatient hemodialysis facilities. Center for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis.html Updated December 17, 2020. Accessed May 12, 2021.
- 18.Basile C., Combe C., Pizzarelli F., et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant. 2020;35:737–741. doi: 10.1093/ndt/gfaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NICE, National Institute for Health and Care Excellence (UK). COVID-19 rapid guideline: dialysis service delivery. NICE, National Institute for Health and Care Excellence (UK) https://www.nice.org.uk/guidance/ng160%0Ahttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medp&NEWS=N&AN=33497146 Accessed August 16, 2021. [PubMed]
- 20.Wadee S., Zaki M., Elsayed H.M., et al. Guidelines for the prevention, detection and management of the renal complications of COVID-19 in Africa. Afr J Nephrol. 2020;23:109–126. [Google Scholar]
- 21.Mitchell K.R., Bomm A., Shea B.S., Shemin D., Bayliss G. Inpatient dialysis planning during the COVID-19 pandemic: Aasingle-center experience and review of the literature. Int J Nephrol Renovasc Dis. 2020;13:253–259. doi: 10.2147/IJNRD.S275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson B.M., Bieber B., Pisoni R.L., Port F.K. Dialysis outcomes and practice patterns study (DOPPS): its strengths, limitations, and role in informing practices and policies. Clin J Am Soc Nephrol. 2012;7:1897–1905. doi: 10.2215/CJN.04940512. [DOI] [PubMed] [Google Scholar]
- 23.Turgutalp K., Ozturk S., Arici M., et al. Determinants of mortality in a large group of hemodialysis patients hospitalized for COVID-19. BMC Nephrol. 2021;22:29. doi: 10.1186/s12882-021-02233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caplin B., Ashby D., McCafferty K., et al. Risk of COVID-19 disease, Dialysis Unit Attributes, and Infection Control Strategy among London in-center hemodialysis patients. Clin J Am Soc Nephrol. 2021;16:1237–1246. doi: 10.2215/CJN.03180321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thwin O., Grobe N., Tapia Silva L.M., et al. SARS-CoV-2 seropositivity rates in patients and clinical staff in New York City dialysis facilities: association with the general population. Kidney Med. 2021;3:678–679. doi: 10.1016/j.xkme.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke C., Prendecki M., Dhutia A., et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31:1969–1975. doi: 10.1681/ASN.2020060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akbarialiabad H., Kavousi S., Ghahramani A., Bastani B., Ghahramani N. COVID-19 and maintenance hemodialysis: a systematic scoping review of practice guidelines. BMC Nephrol. 2020;21:470. doi: 10.1186/s12882-020-02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suri R.S., Antonsen J.E., Banks C.A., et al. Management of outpatient hemodialysis during the COVID-19 pandemic: recommendations from the Canadian Society of Nephrology COVID-19 Rapid Response Team. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120938564. 2054358120938564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kliger A.S., Cozzolino M., Jha V., Harbert G., Ikizler T.A. Managing the COVID-19 pandemic: international comparisons in dialysis patients. Kidney Int. 2020;98:12–16. doi: 10.1016/j.kint.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golan M.S., Jernegan L.H., Linkov I. Trends and applications of resilience analytics in supply chain modeling: systematic literature review in the context of the COVID-19 pandemic. Environ Syst Decis. 2020;40:222–243. doi: 10.1007/s10669-020-09777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massinga Loembé M., Tshangela A., Salyer S., Varma J.K., Ouma A.E.O., Nkengasong J.N. COVID-19 in Africa: the spread and response. Nat Med. 2020;26:999–1003. doi: 10.1038/s41591-020-0961-x. [DOI] [PubMed] [Google Scholar]
- 32.Taylor D., Lindsay A.C., Halcox J.P. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2010;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Côrtes M.F., Espinoza E.P.S., Noguera S.L.V., et al. Decontamination and re-use of surgical masks and respirators during the COVID-19 pandemic. Int J Infect Dis. 2021;104:320–328. doi: 10.1016/j.ijid.2020.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murea M., Moossavi S., Fletcher A.J., et al. Renal replacement treatment initiation with twice-weekly versus thrice-weekly haemodialysis in patients with incident dialysis-dependent kidney disease: rationale and design of the TWOPLUS pilot clinical trial. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodge M.D.S., Abeygunaratne T., Alderson H., et al. Safely reducing haemodialysis frequency during the COVID-19 pandemic. BMC Nephrol. 2020;21:532. doi: 10.1186/s12882-020-02172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rincón A., Moreso F., López-Herradón A., et al. The keys to control a COVID-19 outbreak in a haemodialysis unit. Clin Kidney J. 2020;13:542–549. doi: 10.1093/ckj/sfaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones N.R., Qureshi Z.U., Temple R.J., Larwood J.P.J., Greenhalgh T., Bourouiba L. Two metres or one: what is the evidence for physical distancing in covid-19? BMJ. 2020;370:m3223. doi: 10.1136/bmj.m3223. [DOI] [PubMed] [Google Scholar]
- 38.Osman M.A., Alrukhaimi M., Ashuntantang G.E., et al. Global nephrology workforce: gaps and opportunities toward a sustainable kidney care system. Kidney Int Suppl (2011) 2018;8:52–63. doi: 10.1016/j.kisu.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steensels D., Oris E., Coninx L., et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Self W.H., Tenforde M.W., Stubblefield W.B., et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 Academic Medical Centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J., Yang Y., Gong M., et al. Aggressive quarantine measures reduce the high morbidity of COVID-19 in patients on maintenance hemodialysis and medical staff of hemodialysis facilities in Wuhan, China. Kidney Dis (Basel) 2020;6:271–283. doi: 10.1159/000508579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sever M.S., Ortiz A., Maggiore U., Bac-García E., Vanholder R. Mass disasters and burnout in nephrology personnel: From earthquakes and hurricanes to COVID-19 pandemic. Clin J Am Soc Nephrol. 2021;16:829–837. doi: 10.2215/CJN.08400520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta S., Coca S.G., Chan L., et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2021;32:161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moisoglou I., Yfantis A., Tsiouma E., Galanis P. The work environment of haemodialysis nurses and its mediating role in burnout. J Ren Care. 2021;47:133–140. doi: 10.1111/jorc.12353. [DOI] [PubMed] [Google Scholar]
- 45.Francis A., Baigent C., Ikizler T.A., Cockwell P., Jha V. The urgent need to vaccinate dialysis patients against severe acute respiratory syndrome coronavirus 2: a call to action. Kidney Int. 2021;99:791–793. doi: 10.1016/j.kint.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.