Abstract
Purpose: Pulmonary arterial hypertension (PAH) is a formidable disease with no effective treatment at present. With the goal of developing potential therapies, we attempted to determine whether ethyl pyruvate (EP) could alleviate PAH and its mechanism.
Methods: Pulmonary smooth muscle cells were cultured in conventional low-oxygen environments, and cellular proliferation was monitored after treatment with either EP or phosphate-balanced solution (PBS). Expression of high mobility group protein B1 (HMGB1) and receptor for advanced glycation end-products (RAGE) protein were detected by western blot. After hyperkinetic PAH rat models were treated with EP, hemodynamic data were collected. Right ventricular hypertrophy and pulmonary vascular remodeling were evaluated. Expression of HMGB1 and RAGE protein was also detected.
Results: In vitro, proliferative activity increased in low-oxygen environments, but was inhibited by EP treatment. Furthermore, Western blotting showed the decreased expression of HMGB1 and RAGE protein after EP treatment. In vivo, pulmonary artery pressures were attenuated with EP. Right ventricular hypertrophy and pulmonary vascular remodeling were also reversed. Additionally, the expression levels of HMGB1 and RAGE were reduced in lung tissues.
Conclusions: EP can alleviate PAH by suppressing the proliferation of pulmonary artery smooth muscle cells via inhibition of HMGB1/RAGE expression.
Keywords: pulmonary hypertension, ethyl pyruvate, high mobility group protein B1, receptor for advanced glycation end-products
Introduction
Pulmonary arterial hypertension (PAH) is a refractory cardiovascular disease with atypical presentation, and hence, a high rate of misdiagnosis. If the opportunity for early diagnosis and treatment is lost, most patients succumb to cardiac failure within 2–3 years. The mechanism of PAH has not been fully elucidated and requires exploration from a fresh perspective to devise effective treatment strategies. Our team’s previous studies confirmed that hepatocyte growth factor (HGF) gene transfection could be effectively used to treat PAH in rabbits,1,2) and that endothelial nitric oxide synthase (eNOS) gene therapy for PAH was also effective.3) Alternatively, autologous mesenchymal stem-cell transplantation has been shown to reduce PAH established by shunting,4) and hHIF-1 gene transfection in endothelial progenitor cells may reduce hyperkinetic PAH and reverse pulmonary vascular remodeling.5,6)
A few studies have identified inflammation as a possible mechanism involved in the development of PAH.7,8) Several perivascular inflammatory cells including macrophages, dendritic cells, T and B lymphocytes, and mast cells have been observed in pathological sections of patients with PAH. Furthermore, circulating levels of certain cytokines and chemokines have been associated with clinical outcomes, and anti-inflammatory treatment has been an effective treatment option.
Ethyl pyruvate (EP) is a pyruvic acid derivative that is safe, stable, and easily absorbed by cells. It is widely used in the food industry as an additive. EP is converted to pyruvate by removal of acetic groups upon spontaneous or enzymatic hydrolysis, and then enters the tricarboxylic acid cycle to meet the body’s energy requirements.9) Thus, its by-products exert a minimal influence on the body. The structure of EP is more stable than pyruvate, and possesses similar antioxidant effects. Several studies have shown that EP is an effective anti- inflammatory factor that can regulate cytokine expression, cellular proliferation, and apoptosis.10) At present, due to our in-depth understanding of EP and its protective effects against a wide range of conditions (atherosclerosis, ischemia-reperfusion injury of the liver and myocardium, nervous system disorders, endotoxins, hemorrhagic shock, acute pancreatitis, sepsis, organic dust-induced airway inflammation, and acute lung damage following trauma and hemorrhagic shock),11) the compound is now widely used in clinical practice. In the treatment of pulmonary hypertension, we previously reported that EP can prevent and reverse pulmonary vascular remodeling; however, the precise molecular mechanism requires further clarification.12,13)
High mobility group protein B1 (HMGB1) exists as a nucleoprotein in almost all eukaryotic cells. It was originally discovered in the calf thymus, and it was so named because of its rapid migration via polyacrylamide gel electrophoresis. Many studies have shown that the HMGB1 and the receptor for advanced glycation end-product (RAGE) axis are involved in vascular endothelial apoptosis, inflammatory heart disease, kidney injury, and other diseases.14–16) As an extracellular signaling molecule, HMGB1 promotes release of pro-inflammatory factors, the maturation of dendritic cells, and the chemotaxis and proliferation of smooth muscle cells. These inflammatory factors and cells participate in the pathogenesis of PAH.17) Therefore, HMGB1 may be a potential therapeutic target for PAH. Notably, EP can suppress HMGB1 synthesis.18)
Therefore, we devised in-vitro and in-vivo experiments in an attempt to determine whether EP reduces pulmonary artery smooth muscle cell proliferation by inhibiting the HMGB1/RAGE axis, thereby improving PAH. This can be a valuable outcome in PAH secondary to hypoxia or increased blood flow, as seen in congenital heart disease accompanied by PAH, for example. This study has the potential to provide a theoretical basis for PAH progression and generate new ideas for the diagnosis and treatment of patients with PAH.
Methods
Experiments were carried out at Shandong University, Jinan, China with prior approval from the Institutional Review Board.
In-vitro experiment
Fifth passage pulmonary smooth muscle cells (Provided by Jiekai Bio, Jinan, China) were cultured in Medium 200 (Thermo Fisher Scientific, Waltham, MA, USA). Four groups were randomly divided, as follows: in the conventional oxygen group, cells were cultured in a conventional oxygen environment (21% O2); in the low-oxygen group, cells were cultured in a low-oxygen environment (2% O2); in the low-oxygen + EP group, cells were cultured with EP (12 μml/L, this concentration selected as per the pretest) in a low-oxygen environment (2% O2); and in the low-oxygen + phosphate-balanced solution (PBS) group, cells were cultured with PBS in a low-oxygen environment (2% O2).
In-vivo experiment
Male Wistar rats (n = 50, weighting 121.3 ± 4.6 g) were provided by the Laboratory Animal Center of Shandong University. All rats had received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Research (ILAR), published by the National Academies Press. They were placed in an environment with constant ambient temperature and humidity. The animals were also allowed to acclimatize for 1 week and had free access to water.
The PAH model was created by anastomosis of the common carotid and jugular veins.6) After measuring pulmonary pressure, we selected 30 successful modeling rats (systolic pulmonary arterial pressure [SPAP] = 34.53 ± 2.66 mmHg). They were randomly divided into three groups, as follows: a model group consisting of rats with PAH; a therapy group consisting of rats with PAH treated using EP via intraperitoneal injections for 28 days (50 mg/kg, a dose selected as per the pretest); and a control group consisting of rats with PAH treated with PBS via intraperitoneal injection for 28 days (According to our pretest and previous study, vascular remodeling of Male Wistar rats could be reversed significantly within 4 weeks.).
Detection of pulmonary smooth muscle cell proliferation
The 3H-TdR incorporation assay was performed using a BrdU Cell Proliferation ELISA Kit (Abcam, USA, ab126556). Cells were seeded on 96-well plates, and a Brd U-labeled solution (1:300 dilution) was added separately. After 60 min, peroxidase-labeled anti-Brd U antibody (1:2000 dilution) was added at room temperature (25°C) for 90 min. The cells were washed, and the substrate solution was incubated at room temperature (25°C) for 5 min.
Detection of HMGB1 and RAGE protein expression by Western blot
Total proteins were extracted from the cells via treatment with lysis buffer and benzylsulfonyl fluoride (PMSF) (both from Beyotime, Shanghai, China), and then the protein supernatants were centrifuged at 10000 rpm for 10 min (at 4°C) to remove tissue fragments from sediments. The precipitate was then quantified using a bicinchoninic acid protein assay. Next, protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membrane was blocked with 5% fat-free milk for 2 hours, and incubated with either anti-HMGB1 antibody (ab77302, 1:1000 dilution, Abcam, Cambridge, MA, USA) or anti-RAGE antibody (ab54741, 1:200 dilution, Abcam) at 4°C, overnight. The blots were incubated with horseradish peroxidase-conjugated secondary antibodies (ab205718, 1:2000 dilution, Abcam) at room temperature (25°C) for 1 hour with continuous shaking. After washing, proteins within Western blots were detected using an enhanced chemiluminescence kit (Millipore, MA, USA), and then the blots were exposed to an X-ray film. Bands were quantified using Fluorchem (9900) (Alpha Innotech, Sam Leandro, CA, USA) equipment, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference.
Measurement of hemodynamic parameters
Four weeks after EP treatment, intravenous injection of pentobarbital sodium (30 mg/kg) was administered to anesthetize rats. Catheterization (as described in our preliminary research6,12)) was performed to collect hemodynamic data. The parameters were recorded three times per animal, and the average pressure was noted.
Disposal of heart and lung tissues
After collecting the hemodynamic data, animals were sacrificed by cervical dislocation. The heart and lung were rapidly dissected, and heart tissue was cut and separated into the right ventricle (RV) and left ventricle plus septum (LV + S). The extent of hypertrophy of the RV was evaluated using the right heart hypertrophy index, which is the ratio of the weight of the RV to LV + S (RV/LV + S). The left lung was fixed in phosphate-buffered 4% paraformaldehyde. The residual lung tissues were subsequently flash-frozen in liquid nitrogen and stored at −80°C for Western blotting.
Morphological analysis
The left lung was fixed in phosphate-buffered 4% paraformaldehyde, dehydrated, embedded in paraffin, and cut into 5 μm slices. Hematoxylin–eosin staining was applied to the slices and they were observed under an optical microscope. The external and internal diameters of the pulmonary arteries per lung section were measured by a blinded observer. We then calculated and recorded the medial wall thickness (WT) and medial wall area (WA) using the following formula: % WT = [(external diameter−internal diameter)/external diameter] × 100 and % WA = [(total area−internal area)/total area] × 100.
Detection of HMGB1 and RAGE protein expression in lung tissues by Western blot
Total proteins were extracted from the lung tissues and the methods used were the same as previously described in the cells experiment.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). One-way analysis of variance with a post hoc Tukey's test was used for statistical analysis. SPSS version 25.0 (IBM, Armonk, NY, USA) was used for all analyses and P <0.05 was regarded as statistically significant.
Results
Cell experiment
The incorporation of 3H-TdR in the low-oxygen and low-oxygen + PBS groups was significantly higher than that of the conventional oxygen group. The low- oxygen + EP group had lower 3H-TdR incorporation than the low-oxygen group, and the level was not significantly different from the conventional oxygen group (Fig. 1A).
Fig. 1. Pulmonary smooth muscle cell proliferation and the expression of HMGB1 and RAGE. (A) A 3H-TdR incorporation test is shown. Expression levels of (B, D) HMGB1 and (C, E) RAGE are shown versus those of the conventional oxygen group (*P <0.05; compared with the low-oxygen group, #P <0.05). The following groups are shown: (a) conventional oxygen, (b) low oxygen, (c) low oxygen + EP, and (d) low oxygen + PBS. EP: ethyl pyruvate; HMGB1: high mobility group protein B1; PBS: phosphate-balanced solution; RAGE: receptor for advanced glycation end-product.
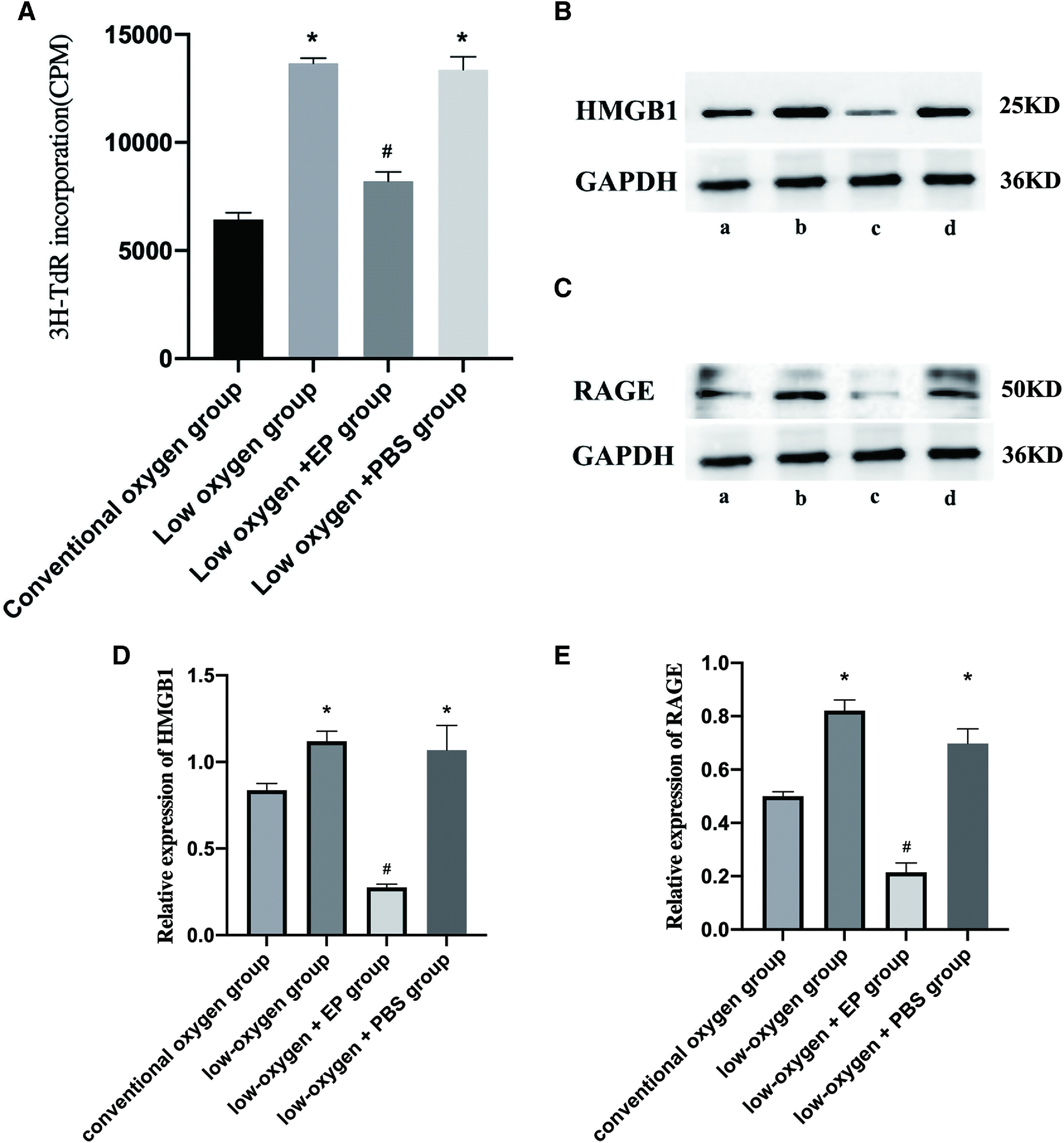
Western blot analysis showed that HMGB1 and RAGE increased significantly in the low-oxygen and low- oxygen + PBS groups compared with the conventional oxygen group. The low-oxygen + EP group, which was not significantly different from the conventional oxygen group, had lower HMGB1 and RAGE expression than the low-oxygen group (Fig. 1B–1E).
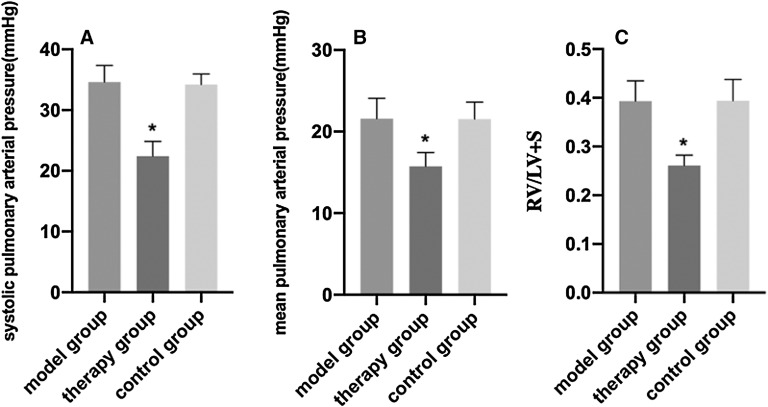
EP therapy attenuated PAH and RV hypertrophy
Hemodynamic analysis results and the RV hypertrophy index of the three groups of rats are shown in Fig. 2. Compared with the model group, SPAP, mean pulmonary arterial pressure (MPAP), and RV/LV + S were significantly decreased in the therapy group (P <0.05). No significant differences were noted between the model and control groups.
Fig. 2. Hemodynamic index and right ventricular hypertrophy index of the three groups. (A) SPAP, (B) MPAP, (C) right ventricular hypertrophy index (RV/LV + S) compared with the model group (*P <0.05) are shown. LV: left ventricle; MPAP: mean pulmonary arterial pressure; RV: right ventricle; SPAP: systolic pulmonary arterial pressure.
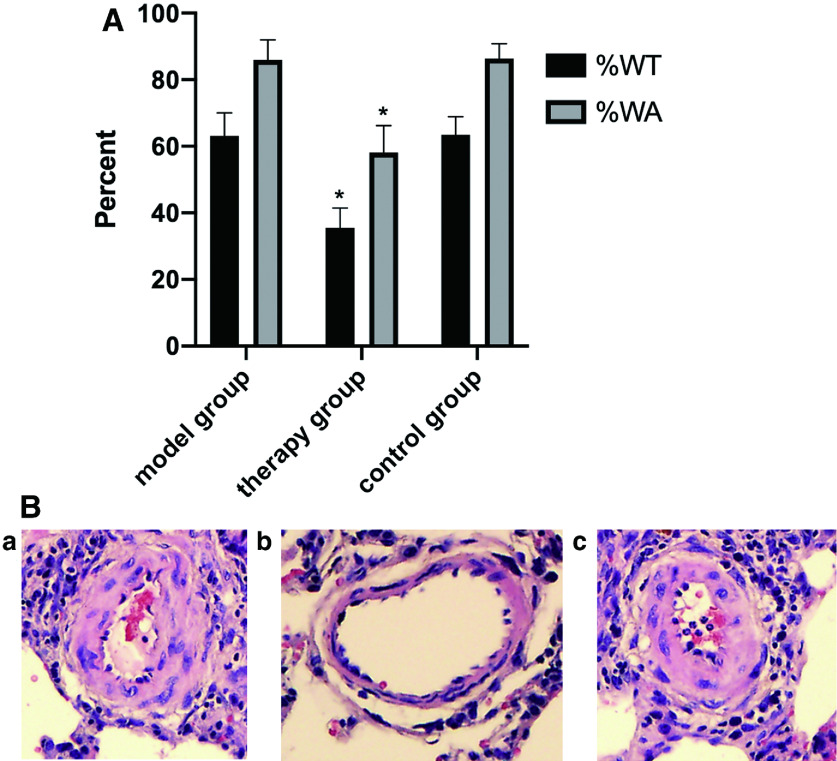
EP therapy reversed vascular remodeling
Compared with the model group, the therapy group had significantly decreased pulmonary artery WT and WA (P <0.05). There were no significant differences between the model and control groups (Fig. 3 and Table 1).
Fig. 3. The results of vascular remodeling. (A) WT and WA of the pulmonary arteries. WT and WA data compared with model group (*P <0.05) are shown. (B) Histopathological analysis of rat lung tissues. H&E findings at 100 × magnification of the (a) model group, (b) therapy group, and (c) control group are shown. H&E: hematoxylin–eosin; WA: wall area; WT: wall thickness.
Table 1. The effect of EP on the WT and WA of the pulmonary arteries.
| Groups | Small pulmonary artery | ||
|---|---|---|---|
| Diameter | %WT | %WA | |
| Model group | 35.97 ± 9.37 | 63.13 ± 6.94 | 85.98 ± 45.96 |
| Therapy group | 35.14 ± 8.13 | 35.51 ± 5.95* | 58.09 ± 8.16* |
| Control group | 35.81 ± 8.38 | 63.46 ± 5.43 | 86.39 ± 4.39 |
Compared with model group: * P <0.01
EP: ethyl pyruvate; WA: wall area; WT: wall thickness
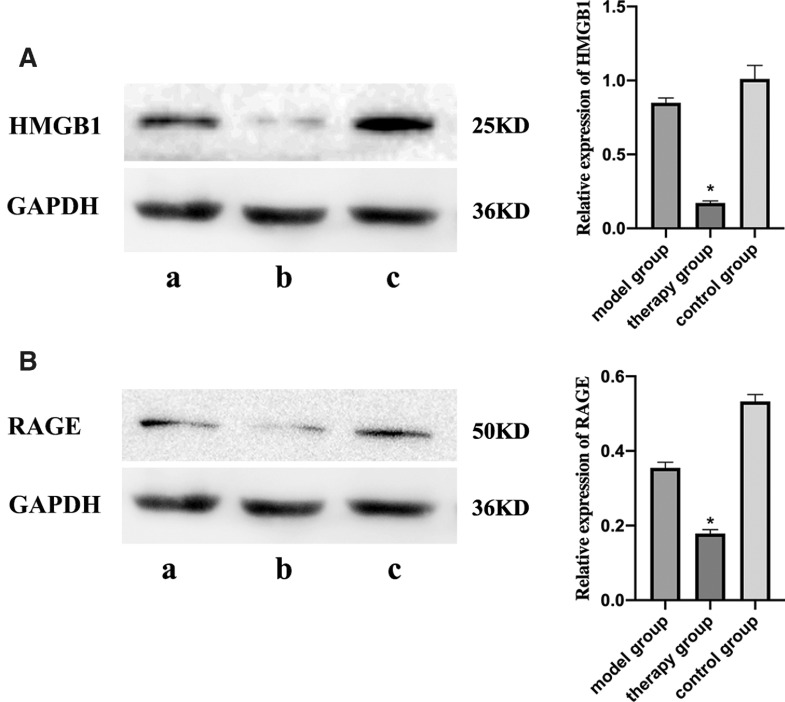
Mechanism underlying the alleviation of pulmonary hypertension by EP therapy
Western blot analysis showed that HMGB1 and RAGE levels decreased significantly in the therapy group compared with the model group, and there were no significant differences between the model and control groups (Fig. 4).
Fig. 4. Expression of HMGB1 and RAGE in lung tissues of individuals from each of the three groups assessed. (A) Expression of HMGB1 and (B) RAGE in the (a) model, (b) therapy, and (c) control group compared with the model group (*P <0.05). HMGB1: high mobility group protein B1; RAGE: receptor for advanced glycation end-product.
Discussion
In the present study, we established that the in-vitro proliferation of pulmonary artery smooth muscle cells can be inhibited by EP therapy, and that hyperkinetic PAH is established by anastomosis of the common carotid artery and jugular vein can be alleviated by in-vivo EP abdominal injection in rats. Furthermore, both effects were mediated by the HMGB1/RAGE axis.
It is well known that pulmonary artery smooth muscle cells are in a hypoxic state in PAH, and that their resultant proliferation contributes to pulmonary vascular remodeling, which further aggravates pulmonary hypertension.19) To confirm the mechanism by which EP attenuates PAH, we used in-vitro and in-vivo experiments. We previously reported that EP therapy could ameliorate monocrotaline-induced PAH in rats and that intratracheal instillation of EP nanoparticles prevented the development of shunt-flow-induced PAH in a rat model. However, PAH was restricted to monocrotaline-induced PAH, and EP to intratracheal EP therapy. Another limitation was that the detailed mechanism was not elucidated. Accordingly, in this study, we used pulmonary artery smooth muscle cells cultured in a low-oxygen environment to mimic the hypoxic state after PAH caused by a left-to-right shunt.
HMGB1 has been shown to be an inflammatory cytokine activated by extracellular lysophospholipid choline. Finally, it stimulates lysosomal exocytosis and is secreted extracellularly through an atypical vesicle-mediated pathway.20) Sadamura-Takenaka et al.21) found that HMGB1 expression increased in the mouse model of PAH, where it promoted the inflammatory response and thickened the pulmonary vascular wall. The work also revealed that anti-HMGB1 antibody could weaken the pulmonary inflammatory response and prevent thickening of the pulmonary vascular wall. In addition, it has been shown that anti-HMGB1 antibody reduces levels of endothelin-1 in alveolar lavage fluid and serum, indicating that HMGB1 may have a pro-inflammatory effect in the pathogenesis of PAH, and may control inflammation and pulmonary vascular reconstruction.22) HMGB1 binds to receptors such as RAGE, initiates signal transduction pathways, induces the translocation and activation of NF-κB, stimulates the production of inflammatory factors such as chemokines, triggers inflammation, and affects cell proliferation, differentiation, and migration.23)
RAGE is a specific receptor of HMGB1 and belongs to the immunoglobulin superfamily. The extracellular region of the receptor has a high affinity binding site for HMGB1, which can be expressed on the surface of various cells.24) It has been shown that under normal conditions, RAGE expression is low, but when expression of its ligand increases, RAGE expression is also upregulated to initiate a positive feedback cascade that produces multiple cytokines, and aggravates the inflammatory response.25) In addition, HMGB1 can bind to RAGE on the cell surface, activate downstream NF-κB and other signaling pathways, promote cell autophagy, and play an anti-apoptotic role.26) Therefore, the protein is capable of affecting PAH occurrence. Our study confirmed the relationship between PAH and the HMGB1/RAGE axis.
Many studies have previously shown that EP treatment improves various diseases (including organic dust-induced airway inflammation and lung cancer, etc.11,27)) by inhibiting HMGB1/RAGE, but our team discovered for the first time that EP is capable of ameliorating PAH using a mechanism related to the HMGB1/RAGE pathway. Pulmonary vascular endothelial cell injury and smooth muscle cell proliferation play an important role in the occurrence and development of PAH, and the latter is particularly important. Therefore, we selected pulmonary artery smooth muscle cells for our experiments and found that EP could inhibit the proliferation of pulmonary artery smooth muscle cells, and decrease levels of HMGB1/RAGE expression. The pathological characteristics of PAH are muscular pulmonary artery plexiform lesions, and the gradual occlusion of the vascular cavity.28) Therefore, in animal experiments, we measured the medial WT and medial WA of the muscular pulmonary artery which were significantly reduced by EP. In addition, expression of HMGB1/RAGE was significantly reduced. Our experiments confirmed the effect of EP in reducing PAH and its mechanism of action, which involved the inhibition of the HMGB1/RAGE axis to reduce the proliferation of pulmonary artery smooth muscle cells and reverse pulmonary artery muscular remodeling.
Hyperkinetic PAH mainly occurs in congenital heart disease with anomalous shunt, such as in individuals with ventricular septal and atrial septal defects. Prior to surgery, which is contraindicated if Eisenmenger syndrome occurs, drugs such as Sildenafil or Bosentan are needed to treat PAH. Nonetheless, outcomes remain unsatisfactory. Currently, there is no effective approach that is used to treat patients with hyperkinetic PAH. In this study, the PAH model was established by the anastomosis of the common carotid artery and jugular vein in an attempt to imitate hyperkinetic PAH caused by an anomalous shut, thereby allowing us to evaluate a new therapeutic approach for the treatment of hyperkinetic PAH.
In our previous study,12) we had found that EP had both preventive and therapeutic effect in PAH. Therefore, EP may suppress the whole process of inflammation during the pathogenesis and progress of PAH. In our present study, we only studied the therapeutic effect of EP. According to our pretest, the dose of EP in vitro and vivo was selected as 12 μmL/L and 50mg/kg, respectively. Additionally, our previous study had confirmed that vascular remodeling of Male Wistar rats could be reversed significantly within 2 weeks. And in that study, we used EP treatment for 14 and 28 days (two groups, respectively). In the present study, we select 4 weeks to obtain the more significant vascular remodeling.
EP is an antitumor agent. Unlike other compounds, EP is not toxic and has been determined to be safe.29) EP is widely used as a food additive and is found in caramel, brandy, rum, chocolate, and other foods. Several previous studies on animal models have confirmed its non- toxicity. EP treatment has also been shown to be of no risk to humans.30) Taken together, these prior findings and those of our study strongly support the use of EP as an adjunct therapy for PAH. In this study, we found that EP plays a key role in reducing PAH by regulating HMGB1/RAGE gene expression, but downstream regulation of gene expression and signaling processes have not been fully elucidated. Recently, we observed that autophagy and PI3K-Akt-mTOR signal transduction pathways are involved in mechanisms of EP treatment of PAH. The precise role of and PI3K-Akt-mTOR signal transduction pathways in PAH should be evaluated in future studies. Besides, based on our present animal experiment, we need to carry out clinical trials later. Then, EP amount can be selected according to weight. And administered intravenously may be convenient, the amount of dietary intake should be further studied in view of its safety.
Conclusion
In conclusion, there are no effective drugs for PAH treatment at present. Because of the poor prognosis associated with PAH, it is important to develop new treatments and elucidate underlying mechanisms. Our study provides evidence using cellular and animal models that EP can alleviate hyperkinetic PAH, and that associated changes are mediated by the suppression of pulmonary artery smooth muscle proliferation via inhibition of HMGB1/RAGE expression.
Ethical Approval
Approved by Animal Care and Use Committee of Shandong University.
Authors’ Contributions
Chuanzhen Liu contributed to the study design, in-vivo and in-vitro experiments, data analysis, and paper writing; Xiquan Zhang and Guangqing Cao contributed to the study design and paper review; Hourong sun, Mengmeng Tang, and Jianhua Li contributed to the data acquisition, data analysis, and experiments.
Funding
This work was supported by the Shandong Provincial Natural Science Foundation of China (no. ZR2019PH024). Medical Health Technology Development Plan of Shandong province (no. 2016WS0353).
Disclosure Statement
The authors declare that there is no conflict of interest.
References
- 1). Wang W, Liu K, Zhang F, et al. Recombinant human hepatocyte growth factor transfection alleviates hyperkinetic pulmonary artery hypertension in rabbit models. J Thorac Cardiovasc Surg 2013; 146: 198– 205. [DOI] [PubMed] [Google Scholar]
- 2). Liu R, Wu S, Cao G, et al. Transfection of human hepatocyte growth factor gene inhibits advancing pulmonary arterial hypertension induced by shunt flow in a rabbit model. Transplant Proc 2013; 45: 705– 12. [DOI] [PubMed] [Google Scholar]
- 3). Zhang F, Wu S, Lu X, et al. Gene transfer of endothelial nitric oxide synthase attenuates flow-induced pulmonary hypertension in rabbits. Ann Thorac Surg 2008; 85: 581– 5. [DOI] [PubMed] [Google Scholar]
- 4). Liu K, Liu R, Cao G, et al. Adipose-derived stromal cell autologous transplantation ameliorates pulmonary arterial hypertension induced by shunt flow in rat models. Stem Cells Dev 2011; 20: 1001– 10. [DOI] [PubMed] [Google Scholar]
- 5). Cao G, Liu C, Wan Z, et al. Combined hypoxia inducible factor-1α and homogeneous endothelial progenitor cell therapy attenuates shunt flow-induced pulmonary arterial hypertension in rabbits. J Thorac Cardiovasc Surg 2015; 150: 621– 32. [DOI] [PubMed] [Google Scholar]
- 6). Liu C, Yan Z, Fang C, et al. Establishment and comparison of two reliable hyperkinetic pulmonary hypertension models in rabbits. J Thorac Cardiovasc Surg 2014; 148: 2353– 9. [DOI] [PubMed] [Google Scholar]
- 7). Goldenberg NM, Steinberg BE. Inflammation drives pulmonary arterial hypertension. Anesthesiology 2019; 130: 820– 1. [DOI] [PubMed] [Google Scholar]
- 8). Kumar R and Graham B. How does inflammation contribute to pulmonary hypertension? Eur Respir J 2018; 51: 1702403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Taylor MD, Grand TJ, Cohen JE, et al. Ethyl pyruvate enhances ATP levels, reduces oxidative stress and preserves cardiac function in a rat model of off-pump coronary bypass. Heart Lung Circ 2005; 14: 25– 31. [DOI] [PubMed] [Google Scholar]
- 10). Dong N, Xu X, Xue C, et al. Ethyl pyruvate protects against Salmonella intestinal infection in mice through down-regulation of pro-inflammatory factors and inhibition of TLR4/MAPK pathway. Int Immunopharmacol 2019; 71: 155– 63. [DOI] [PubMed] [Google Scholar]
- 11). Fink MP. Ethyl pyruvate. Curr Opin Anaesthesiol 2008; 21: 160– 7. [DOI] [PubMed] [Google Scholar]
- 12). Liu C, Fang C, Cao G, et al. Ethyl pyruvate ameliorates monocrotaline-induced pulmonary arterial hypertension in rats. J Cardiovasc Pharmacol 2014; 64: 7– 15. [DOI] [PubMed] [Google Scholar]
- 13). Liu K, Zhang X, Cao G, et al. Intratracheal instillation of ethyl pyruvate nanoparticles prevents the development of shunt-flow-induced pulmonary arterial hypertension in a rat model. Int J Nanomedicine 2016; 11: 2587– 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Mi L, Zhang Y, Xu Y, et al. HMGB1/RAGE pro- inflammatory axis promotes vascular endothelial cell apoptosis in limb ischemia/reperfusion injury. Biomed Pharmacother 2019; 116: 109005. [DOI] [PubMed] [Google Scholar]
- 15). Bangert A, Andrassy M, Müller AM, et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc Natl Acad Sci U S A 2016; 113: E155– 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Zhang C, Dong H, Chen F, et al. The HMGB1-RAGE/ TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia. Exp Ther Med 2019; 17: 17– 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Itoh T, Nagaya N, Ishibashi-Ueda H, et al. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology 2006; 11: 158– 63. [DOI] [PubMed] [Google Scholar]
- 18). Shin JH, Kim ID, Kim SW, et al. Ethyl pyruvate inhibits HMGB1 phosphorylation and release by chelating calcium. Mol Med 2015; 20: 649– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Zurlo G, Piquereau J, Moulin M, et al. Sirtuin 1 regulates pulmonary artery smooth muscle cell proliferation: role in pulmonary arterial hypertension. J Hypertens 2018; 36: 1164– 77. [DOI] [PubMed] [Google Scholar]
- 20). Elfeky M, Yoneshiro T, Okamatsu-Ogura Y, et al. Adiponectin suppression of late inflammatory mediator, HMGB1-induced cytokine expression in RAW264 macrophage cells. J Biochem 2018; 163: 143– 53. [DOI] [PubMed] [Google Scholar]
- 21). Sadamura-Takenaka Y, Ito T, Noma S, et al. HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS One 2014; 9: e102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Yang PS, Kim DH, Lee YJ, et al. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir Res 2014; 15: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Hudson BI, Lippman ME. Targeting RAGE signaling in inflammatory disease. Annu Rev Med 2018; 69: 349– 64. [DOI] [PubMed] [Google Scholar]
- 24). Huebener P, Pradere JP, Hernandez C, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest 2019; 130: 1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Yeo JG, Leong J, Arkachaisri T, et al. Proteolytic inactivation of nuclear alarmin high-mobility group box 1 by complement protease C1s during apoptosis. Cell Death Discov 2016; 2: 16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Mou K, Liu W, Han D, et al. HMGB1/RAGE axis promotes autophagy and protects keratinocytes from ultraviolet radiation-induced cell death. J Dermatol Sci 2017; 85: 162– 9. [DOI] [PubMed] [Google Scholar]
- 27). Liu Q, Huo Y, Zheng H, et al. Ethyl pyruvate suppresses the growth, invasion and migration and induces the apoptosis of non‐small cell lung cancer cells via the HMGB1/RAGE axis and the NF‐κB/STAT3 pathway. Oncol Rep 2019; 42: 817– 25. [DOI] [PubMed] [Google Scholar]
- 28). Tuder RM, Stacher E, Robinson J, et al. Pathology of pulmonary hypertension. Clin Chest Med 2013; 34: 639– 50. [DOI] [PubMed] [Google Scholar]
- 29). Pathak M, Mishra R, Agarwala PK, et al. Binding of ethyl pyruvate to bovine serum albumin: calorimetric, spectroscopic and molecular docking studies. Thermochim Acta 2016; 633: 140– 48. [Google Scholar]
- 30). Bennett-Guerrero E Swaminathan M Grigore AM, et al. A Phase II multicenter double-blind placebo- controlled study of ethyl pyruvate in high-risk patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothoracic Vasc Anesth 2009; 23: 324– 29. [DOI] [PubMed] [Google Scholar]