Abstract
Objectives: One of the serious problems after lung transplantation is chronic lung allograft dysfunction (CLAD). Most CLAD patients pathologically characterized by obliterative bronchiolitis (OB). Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)-Ig is a combination protein of the Fc fragment of human IgG1 linked to the extracellular domain of CTLA4. The aim of the study was to examine the effect of CTLA4-Ig therapy on OB using a mouse intrapulmonary tracheal transplantation (IPTT) model.
Methods: IPTT was performed between BALB/c (donor) and C57BL/6 (recipient) mice. Abatacept, which is a commercially available form of CTLA4-Ig, was intraperitoneally injected in recipient mice immediately after surgery, on days 7, 14, and 21. The mice in the control group received human IgG.
Results: We performed semi-quantitative analysis of graft luminal obliteration at post-transplant day 28. We calculated the obliteration ratio of the lumen of the transplanted trachea in each case. The obliteration ratio was significantly lower in the CTLA4-Ig group than that in the control group (91.2 ± 2.1% vs. 47.8 ± 7.9%, p = 0.0008). Immunofluorescent staining revealed significantly decreased lymphoid neogenesis in the lung.
Conclusions: CTLA4-Ig therapy attenuated tracheal obliteration with fibrous tissue in the mouse IPTT model. The attenuation of fibrous obliteration was correlated with the inhibition of lymphoid neogenesis.
Keywords: lung transplantation, chronic lung allograft dysfunction, obliterative bronchiolitis, cytotoxic T-lymphocyte-associated antigen 4
Introduction
Lung transplantation is an effective therapeutic option for patients with end-stage lung diseases. One of the serious problems in the chronic phase after lung transplantation is chronic rejection, which has been described as chronic lung allograft dysfunction (CLAD). Surprisingly, the morbidity rate of CLAD is approximately 50% at 5 years after lung transplantation and CLAD is the leading cause of death after 1 year since transplantation.1) CLAD can be divided into two major phenotypes: restrictive allograft syndrome (RAS) and bronchiolitis obliterans syndrome (BOS).2) Most of the CLAD patients are classified as BOS, which pathologically shows obliterative bronchiolitis (OB), a collagen fibrous tissue remodeling in small airways.
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)-Ig is a combination protein of the Fc fragment of human IgG1 linked to the extracellular domain of CTLA4. CTLA4-Ig attaches to CD80/86 on antigen-presenting cells and blocks their interaction with CD28 on activated T cells. Therefore, CTLA4-Ig selectively prevents T-cell activation through the co-stimulation blockage. It has been reported that the parameters of acute Inflammation in rat lung allograft rejection were significantly reduced in animals treated with CTLA4-Ig.3) Belatacept, which is a commercially available form of CTLA4-Ig, was approved by the US Food and Drug Administration and the European Medicines Agency in 2011. After kidney transplantation, the overall survival, graft survival rate, and the mean estimated glomerular filtration rate (eGFR) were significantly higher in the patients with belatacept regimens than in those with cyclosporine-based regimens.4) In a small number of case series of lung transplantation, belatacept also has been reported to be used in recipients as a conversion treatment from calcineurin inhibitor-based immunosuppressive regimens.5)
Lymphoid neogenesis, de novo formation of B-cell follicles and T cell areas, which are similar to secondary lymphoid organs and also known as tertiary lymphoid organ (TLO) formation, is observed in chronic inflammation related to autoimmune diseases and chronic infections.6,7) Sato et al. demonstrated lymphoid neogenesis in the lung affected by BOS after human lung transplantation.8,9) They have also reported that intrapulmonary lymphoid neogenesis occurs in a rat intrapulmonary tracheal transplantation (IPTT) model, which is an established model of BOS that pathologically shows OB.9) Moreover, Matsuda et al. have reported that inhibition of spleen tyrosine kinase (Syk), an immunoregulatory kinase that plays a critical role in B-cell and humoral maturation, prevents lymphoid neogenesis, and attenuates tracheal obliteration in a mouse IPTT model.10) These previous studies suggest that lymphoid neogenesis may be a therapeutic target of BOS.
Although Yamada et al. demonstrated that the development of OB can be inhibited by CTLA4-Ig in a murine heterotopic airway model,11) it remains to be clarified whether CTLA4-Ig has any effect on OB in the pulmonary milieu. We presume that the mouse IPTT model is a more clinically relevant model than a heterotopic airway model in which the trachea is transplanted heterotopically into a subcutaneous pocket, in the aspect that OB occurs accompanied with lymphoid neogenesis in the lung. The objective of the present study was to examine whether CTLA4-Ig prevents OB in the pulmonary milieu via the suppression of lymphoid neogenesis in a mouse IPTT model.
Materials and Methods
Mouse IPTT
BALB/c and C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). All mice were used at 7–8 weeks of age. Mouse IPTT between major histocompatibility complex (MHC) incompatible recipients and donors was performed according to the previous report by Wagnetz and colleagues.12) Briefly, a peripheral venous catheter (18G Angiocath; Becton Dickinson, Franklin Lakes, NJ, USA) was loaded with a trachea retrieved from a BALB/c mouse (donor) and the trachea was injected into the left lung parenchyma of a C57BL/6 mouse (recipient). The injection site was sealed with a ligation clip (LIGACLIP, Ethicon, Bridgewater, NJ, USA). The animals were housed under pathogen-free conditions in the Animal Research Facility, Tohoku University School of Medicine. All experimental protocols for mice were reviewed and approved by the Tohoku University School of Medicine Institutional Animal Care and Use Committee.
Treatment with CTLA4-Ig
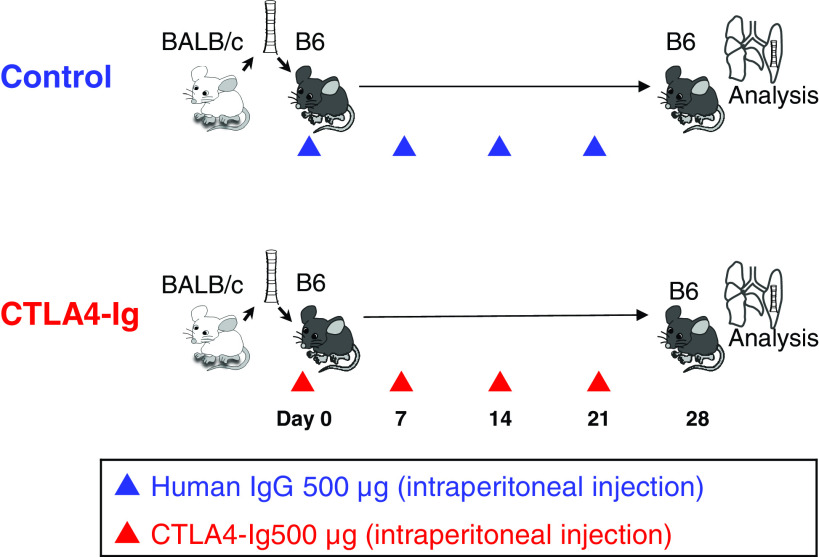
Abatacept, which is a commercially available form of CTLA4-Ig, was purchased from Bristol Myers Squibb JAPAN (Tokyo, Japan). For treatment with CTLA4-Ig, C57BL/6 recipients were intraperitoneally injected with 500 μg of abatacept diluted in 200 μL phosphate-buffered saline (PBS) immediately after surgery on days 7, 14, and 21. The dosage of the drug was determined by our pilot study, with reference to previous studies using mouse models.13,14) The mice in the control group received human IgG (isotype control) in the same manner (Fig. 1). We sacrificed the recipient mice at post-transplant day 28.
Fig. 1. Study design. C57BL/6 recipients were intraperitoneally injected with 500 μg of abatacept, which is a commercially available form of CTLA4-Ig, diluted in 200 μl PBS immediately after surgery, on days 7, 14, and 21. The recipients in the control group received human IgG (isotype control) in the same manner. On post-transplant day 28, the mice in both control and CTLA4-Ig groups were sacrificed and lungs were extracted. CTLA4: cytotoxic T-lymphocyte- associated antigen 4; PBS: phosphate-buffered saline.
Semi-quantification of luminal area and lymphoid aggregate
Formalin-fixed paraffin-embedded tissues of 5-µm section were used for hematoxylin and eosin or Masson’s trichrome staining. For semi-quantitative analysis of graft luminal obliteration and lymphoid aggregate (TLO formation) size, Image J software (version 1.51; NIH, Bethesda, MD, USA) was used, as described previously.15) The semi-quantitative analysis was done in a blinded manner.
Immunofluorescent staining for CD3/B220
Formalin-fixed paraffin-embedded tissues of 5-µm section were used for immunofluorescent staining. The following primary antibodies were used for the analysis: rabbit-anti mouse CD3 antibody from Abcam (Cambridge, UK), rat- anti-mouse B220 antibody from BD pharmngen (CA, USA). The following were used for the secondary antibodies: alexa Flour 647 donkey anti-rat from abcam, Alexa flour goat anti-rabbit from Abcam. The following primary antibodies were used as an isotype: rabbit IgG polyclonal isotype for CD3 (Abcam), purified rat IgG2a kappa Isotype control for B220 (Thermo Fisher Scientific K.K., Tokyo, Japan). DAPI was purchased from Vector laboratory (Burlingame, CA, USA). Images were acquired by a fluorescence microscope (BZ 9000, Keyence, Osaka, Japan).
Quantitative reverse transcription polymerase chain reaction for analysis of cytokines and chemokines
Quantitative real-time reverse transcription polymerase chain reaction (qPCR) was performed to analyze the intragraft mRNA expressions of cytokines and chemokines at day 28 after transplantation. Total RNA was extracted from the lung and purified with the Sepasol®-RNA I Super G (Nacalai Tesque Inc., Kyoto, Japan). cDNA was synthesized with the Super Script III (Thermo Fisher Scientific K.K., Tokyo, Japan). qPCR for the following genes was performed with SYBR qPCR Mix (TOYOBO, Osaka, Japan) and Gene Amp PCR system 7000 (Thermo Fisher Scientific K.K., Tokyo, Japan). The mRNA levels of mouse collagen type 1 alpha chain (COL1a1), matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-14 (MMP-14), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), C-C motif chemokine ligand 19 (CCL19), C-C motif chemokine ligand 21 (CCL21), C-X-C motif chemokine ligand 12 (CXCL12), C-X-C motif chemokine ligand 13 (CXCL13), and β-actin were measured using the primers listed in Table 1. Threshold cycle (Ct) values were determined by a real-time PCR system (ABI PRISM 7000 Sequence Detection System; Applied Biosystems, Tokyo, Japan). Change in expression was calculated using the 2-ΔΔCt method normalized to beta-actin (β-actin) expression and expressed as fold-change compared with the control group. All assays were performed in triplicate.
Table 1. Primers for quantitative real-time reverse transcription polymerase chain reaction.
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| COL1a1 | CGACCTCAAGATGTGCCACT | GCAGTAGACCTTGATGGCGT |
| MMP2 | ACGATGATGACCGGAAGTGG | GTGTAGATCGGGGCCATCAG |
| MMP14 | TTCGTGTTGCCTGATGACGA | TTCCCGTCACAGATGTTGGG |
| TNFα | ATGGCCTCCCTCTCATCAGT | TTTGCTACGACGTGGGCTAC |
| IFN-γ | AGACAATCAGGCCATCAGCA | CTCATTGAATGCTTGGCGCT |
| CCL19 | AGACTGCTGCCTGTCTGTGA | GCCTTTGTTCTTGGCAGAAG |
| CCL21 | AGACTCAGGAGCCCAAAGCA | GTTGAAGCAGGGCAAGGGT |
| CXCL12 | AGACAATCAGGCCATCAGCA | CTCATTGAATGCTTGGCGCT |
| CXCL13 | ATTCAAGTTACGCCCCCTGG | TTGGCACGAGGATTCACACA |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
CCL19: C-C motif chemokine ligand 19; CCL21: C-C motif chemokine ligand 21; COL1a1: collagen type 1 alpha 1 chain; CXCL12: C-X-C motif chemokine ligand 12; CXCL13: C-X-C motif chemokine ligand 13; IFN-γ: interferon-gamma; MMP-2: matrix metalloproteinase-2; MMP-14: matrix metalloproteinase-14; TNF-α: tumor necrosis factor-alpha
Quantification of total plasma IgG
Total plasma IgG was analyzed using the EesyTiter IgG Assay kits (Thermo Scientific, Rockford, IL, USA). The optical densities were measured using a microplate reader (SpectraMax multiplatereader. Molecular Devices, Tokyo, Japan) at 450 nm. The assay was performed in triplicate.
Statistical analysis
Data are expressed as means ± standard error of the mean. Group comparisons of the mRNA expression levels and serum IgG were performed using Welch’s t-test for normally distributed data or Mann–Whitney U test for non-normally distributed data. Linear regression was used to determine correlations. Differences were considered significant at the p <0.05 level. Statistical analyses were performed using Prism 5 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Fibrous obliteration and lymphoid neogenesis after MHC mismatched IPTT
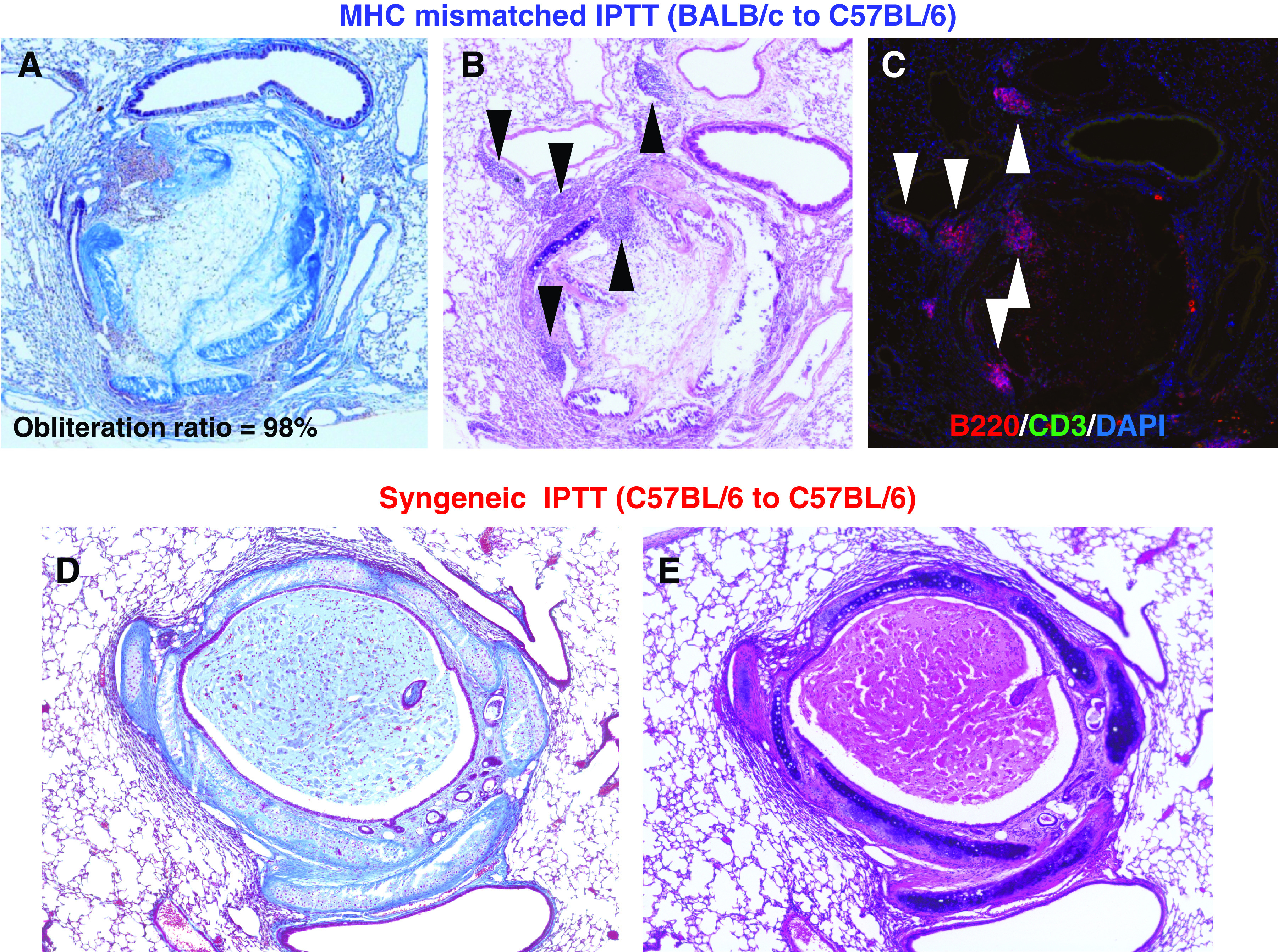
Figure 2 (A and B) shows the pathological findings from a case of MHC mismatched IPTT (BALB/c to C57BL/6). On day 28 after IPTT, the transplanted trachea shows typical luminal obliteration with collagen and fibrous tissue (Fig. 2A). The pathological findings resemble human OB as previously reported.9) Destruction of the tracheal structure (tracheal ring) is also apparent. Lymphoid aggregates in the recipient lung are seen adjacent to the transplanted trachea, which indicates the typical development of lymphoid neogenesis. Immunofluorescent staining demonstrated areas of CD3 positive T cells and B220 positive B cells in the lymphoid aggregates, which are similar to findings of previous reports (Fig. 2C).8,10,12) The number of T cells seems to a little smaller than that in previous reports. In contrast, in a syngeneic IPTT (C57BL/6 to C57BL/6) case on day 28, there is no fibrous obliteration in the tracheal lumen and the structure of the transplanted trachea is well preserved. There is no lymphoid aggregate in the recipient lung (Fig. 2D and 2E).
Fig. 2. Fibrous obliteration and lymphoid neogenesis after MHC mismatched IPTT on day 28 after IPTT. (A and B) Pathological findings from a case of MHC mismatched IPTT, BALB/c to C57BL/6. Masson’s trichrome and hematoxylin–eosin staining, magnification 100. The transplanted trachea shows typical luminal obliteration with collagen and fibrous tissue. The destruction of the tracheal structure (tracheal ring) is also observed. Lymphoid aggregates in the recipient lung are seen adjacent to the transplanted trachea. (C) Immunofluorescent staining from a case of MHC mismatched IPTT. Immunofluorescent staining, magnification 100. Areas of CD3 positive T cells and B220 positive B cells in the lymphoid aggregates are observed. (D and E) Pathological findings from a case of syngeneic IPTT (C57BL/6 to C57BL/6). Masson’s trichrome and hematoxylin–eosin staining, magnification 100. There is no fibrous obliteration in the tracheal lumen, and the structure of the transplanted trachea is well preserved. There is no lymphoid aggregate in the recipient lung. IPTT: intrapulmonary tracheal transplantation; MHC: major histocompatibility complex.
Effect of CTLA4-Ig treatment on fibrous obliteration in allografts
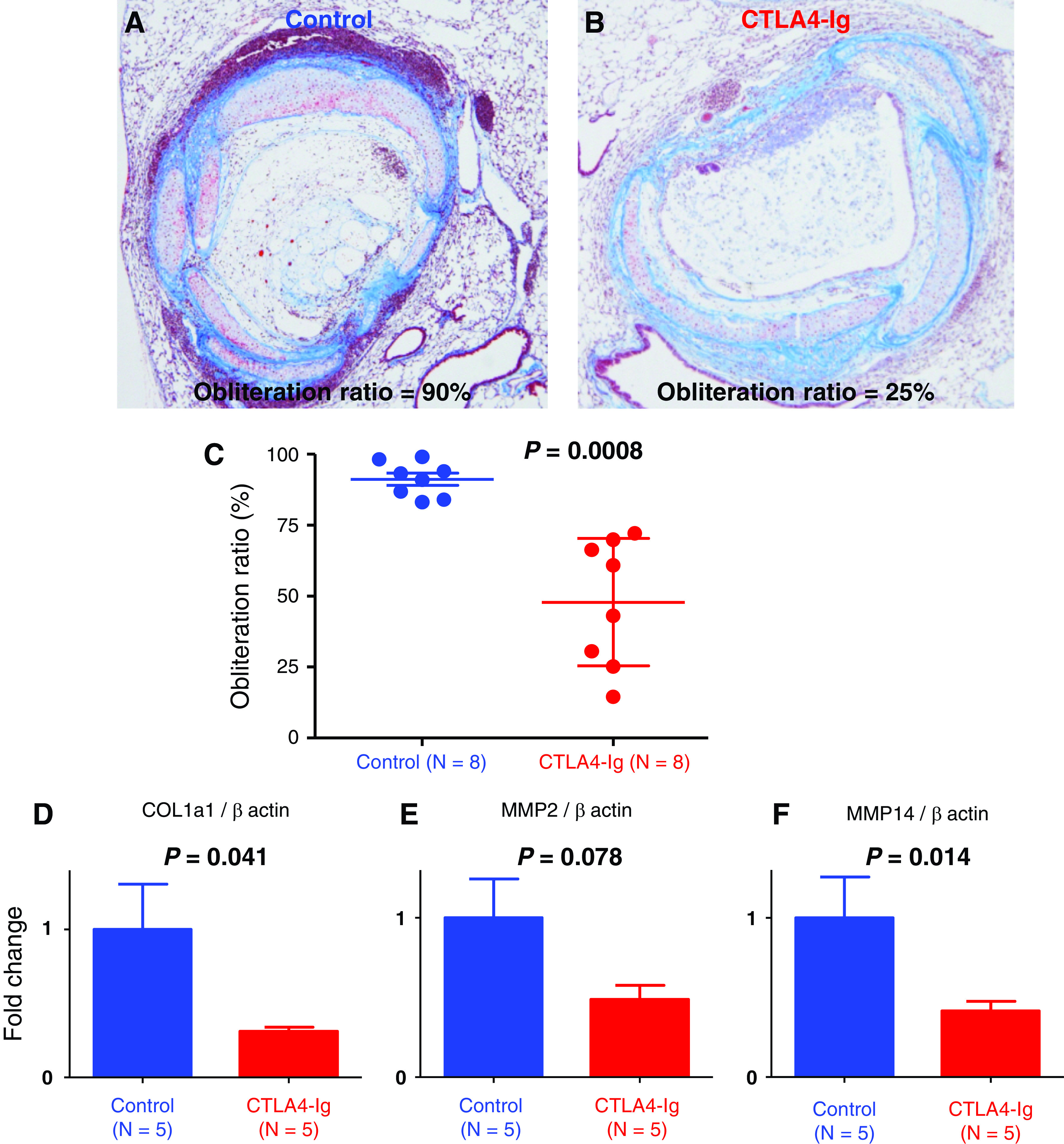
We performed semi-quantitative analysis of graft luminal obliteration in an MHC mismatched IPTT model on post-transplant day 28 to investigate the effect of CTLA4-Ig treatment. We calculated the obliteration ratio of the lumen of the transplanted trachea in the control group (mice treated with human IgG) and the CTLA4-Ig group (mice treated with CTLA4-Ig). Figure 3 (A and B) shows the representative histology of a case from each group. Figure 3A shows the severe fibrous obliteration of the transplanted trachea in a case from the control group and the obliteration ratio is 90%. On the other hand, the obliteration is mild in a case from the CTLA4-Ig group and the obliteration ratio is 25% (Fig. 3B). The obliteration ratio was significantly lower in the CTLA4-Ig group than that in the control group (91.2 ± 2.1% vs. 47.8 ± 7.9%, p = 0.0008; control group, n = 8; CTLA4-Ig group, n = 8; Fig. 3C).
Fig. 3. Effect of CTLA4-Ig treatment on fibrous obliteration in allografts. (A and B) Representative histology of a case from the control group (mice treated with human IgG) and from the CTLA4-Ig group (mice treated with CTLA4-Ig) on post-transplant day 28. Masson’s trichrome staining, magnification 100. A case from the control group shows severe fibrous obliteration of the transplanted trachea and the obliteration ration is 90%. A case from the CTLA4-Ig group 3B shows mild obliteration and the obliteration ration is 25%. (C) Obliteration ratio of the transplanted trachea on post-transplant day 28. The obliteration ratio was significantly lower in the CTLA4-Ig group than that in the control group (91.2 ± 2.1% vs. 47.8 ± 7.9%, p <0.001; control group, n = 8; CTLA4-Ig group, n = 8). (D) Analysis of mRNA expression of COL1a1 gene in lung grafts on post-transplant day 28 (control group, n = 5; CTLA4-Ig group, n = 5). The expression of COL1a1 in the CTLA4-Ig group was significantly decreased compared with that in the control group (p = 0.041). (E and F) Analysis of mRNA expression of MMP-2 and MMP-14 gene in lung grafts on post-transplant day 28 (control group, n = 5; CTLA4-Ig group, n = 5). The expression of MMP-2 tended to decrease in the CTLA4-Ig group (p = 0.078). The expression of MMP-14 in the CTLA4-Ig group was significantly decreased compared with that in the control group (p = 0.014). COL1a1: collagen type I alpha 1; CTLA4: cytotoxic T-lymphocyte- associated antigen 4; MMP-2: matrix metalloproteinase-2.
Effect of CTLA4-Ig treatment on mRNA expression of COL1a1 and fibrous tissue mediators
We examined the mRNA expression of COL1a1 for the assessment of fibrosis, and the mRNA expression of fibrous tissue mediators that are implicated in the fibrosis process of OB by qPCR (control group, n = 5; CTLA4-Ig group, n = 5). The expression of COL1a1 in the CTLA4-Ig group was significantly decreased compared with that in the control group (p = 0.041, Fig. 3D). The expression of MMP-2 tended to decrease in the CTLA4-Ig group (p = 0.078, Fig. 3E). The expression of MMP-14 in the CTLA4-Ig group was significantly decreased compared with that in the control group (p = 0.014, Fig. 3F).
Effect of CTLA4-Ig treatment on lymphoid neogenesis
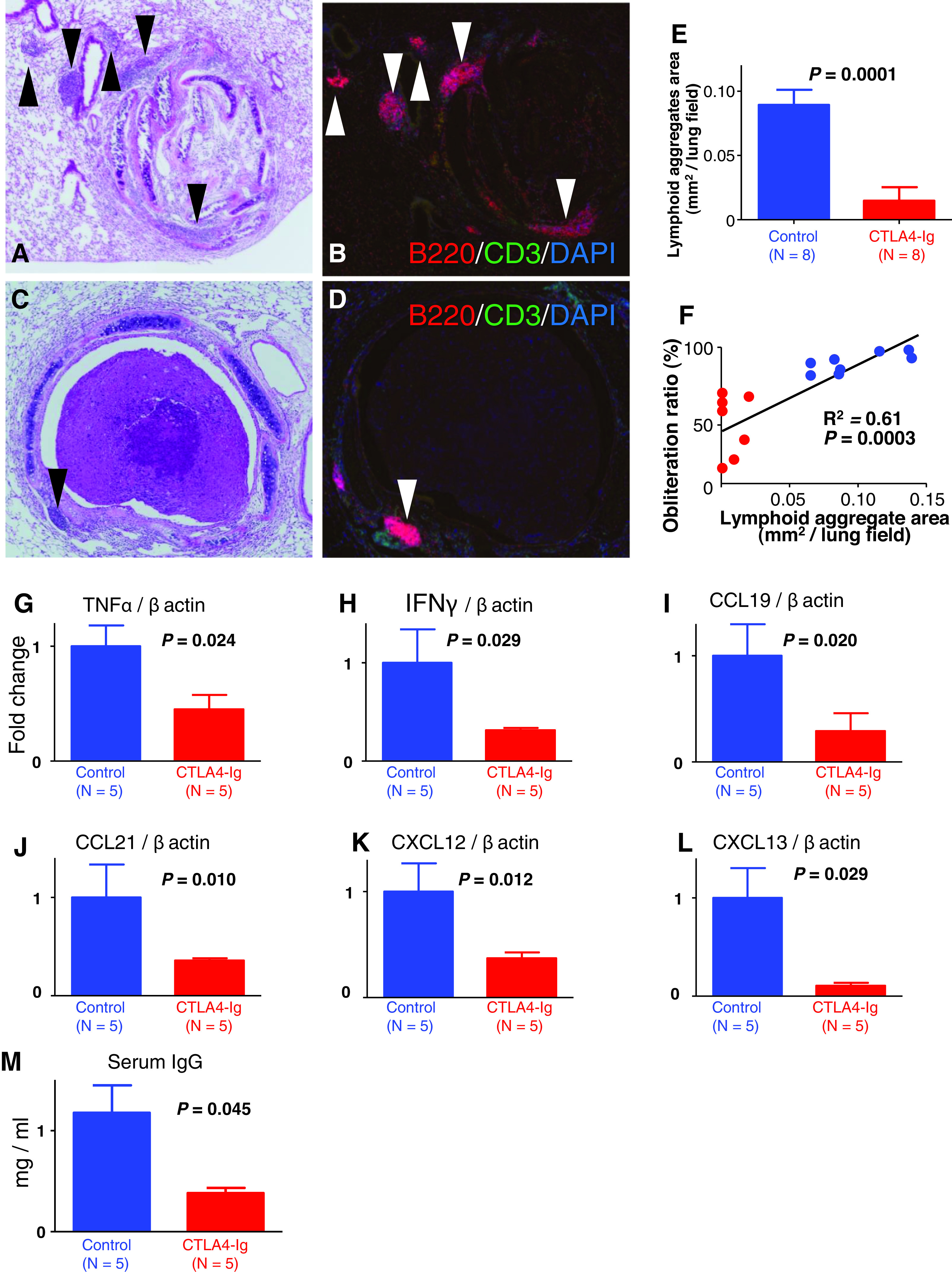
We performed semi-quantitative analysis of the lymphoid aggregate to investigate the effect of CTLA4-Ig treatment on the lymphoid neogenesis in the MHC mismatched IPTT model (control group, n = 8; CTLA4-Ig group, n = 8). We measured the total area of the lymphoid aggregates in a section in each case in the control group and the CTLA4-Ig group on post-transplant day 28. Figure 4 (A and B) shows the lymphoid aggregates of a typical case in the control group. Compared to this case, Fig. 4C and 4D from a case in the CTLA4-Ig group show much fewer lymphoid aggregates. Total area of the lymphoid aggregates in the CTLA4-Ig group was significantly decreased compared to the control group (0.096 ± 0.010 mm2 vs. 0.0066 ± 0.0028 mm2, p = 0.0001, Fig. 4E). Then, we investigated the correlation between the total lymphoid aggregates area and the obliteration ratio in each case from both groups. There was a significant positive correlation between them (R² = 0.61, p = 0.0003, Fig. 4F).
Fig. 4. Effect of CTLA4-Ig treatment on lymphoid neogenesis, mRNA expression of cytokines and chemokines in the lung and plasma IgG. (A and B) Representative histology of a case from the CTLA4-Ig group (mice treated with CTLA4-Ig) on post-transplant day 28. Hematoxylin–eosin staining and immunofluorescent staining for B220/CD3/DAPI, magnification 100. Black and white arrow heads indicate lymphoid aggregates. (C and D) Representative histology of a case from the control group (mice treated with human IgG) on post-transplant day 28. Hematoxylin–eosin staining and immunofluorescent staining for B220/CD3/DAPI, magnification 100. Each arrow head indicates (black and white) lymphoid aggregates on post-transplant day 28. (E) Total area of the lymphoid aggregates in each group on post-transplant day 28 (control group, n = 8; CTLA4-Ig group, n = 8). The total area of the lymphoid aggregates in the CTLA4-Ig group was significantly decreased compared to the control group (0.096 ± 0.010 mm2 vs. 0.0066 ± 0.0028 mm2, p = 0.001). (F) Correlation between the total lymphoid aggregates area and the obliteration on post-transplant day 28 (control group, red dots, n = 8; CTLA4-Ig group, blue dots, n = 8). There was a significant positive correlation between them (R² = 0.61, p = 0.001). (G and H) Analysis of mRNA expressions of TNF-α and in the lung on post-transplant day 28 (control group, n = 5; CTLA4-Ig group, n = 5). The expression of TNF-α was significantly lower in the CTLA4-Ig group than that in the control group (p = 0.024). The expression of IFN-γ in the CTLA4-Ig group (p = 0.29). (I–L) Analysis of mRNA expressions of CCL19, CCL21, CXCL 12 and CXCL 13 in the lung on post-transplant day 28 (control group, n = 5; CTLA4-Ig group, n = 5). The expression of CCL19 in the CTLA4-Ig group was significantly lower than that in the control group (p = 0.020). The expression of CCL21 showed a trend toward a decrease with the CTLA4-Ig treatment (p = 0.10). The expression of CXCL12 in the CTLA4-Ig group was significantly lower than that in the control group (p = 0.012). The expression of CXCL13 in the CTLA4-Ig group was significantly lower than in the control group (p = 0.042). (M) Analysis of plasma IgG level on post-transplant day 28 (control group, n = 5; CTLA4-Ig group, n = 5). Plasma IgG was significantly lower in the CTLA4-Ig group than that in the control group (p = 0.042). CCL19: C-C motif chemokine ligand 19; CTLA4: cytotoxic T-lymphocyte-associated antigen 4; CXCL 12: C-X-C motif chemokine ligand 12; IFN-γ: interferon-gamma; TNF-α: tumor necrosis factor-alpha.
Effect of CTLA4-Ig treatment on mRNA expression of cytokines
We analyzed mRNA expressions of cytokines in the lung by qPCR. As for proinflammatory cytokines, we measured the expressions of TNF-α and IFN-γ (control group, n = 5; CTLA4-Ig group, n = 5). The expression of TNF-α was significantly lower in the CTLA4-Ig group than that in the control group (p = 0.024, Fig. 4G). The expression of IFN-γ in the CTLA4-Ig group (p = 0.29, Fig. 4H).
Effect of CTLA4-Ig treatment on mRNA expression of chemokines and plasma IgG
We measured the mRNA expression of chemokines in the lung by qPCR (control group, n = 5; CTLA4-Ig group, n = 5). The expression of CCL19 in the CTLA4- Ig group was significantly lower than that in the control group (p = 0.020; Fig. 4I). The expression of CCL21 showed a trend toward a decrease with the CTLA4-Ig treatment (p = 0.10; Fig. 4J). The expression of CXCL12 in the CTLA4-Ig group was significantly lower than that in the control group (p = 0.012; Fig. 4K). The expression of CXCL13 in the CTLA4-Ig group was significantly lower than in the control group (p = 0.042; Fig. 4L). We next analyzed serum IgG on day 28 after transplantation. Plasma IgG was significantly lower in the CTLA4-Ig group than that in the control group (1.17 ± 0.27 mg/mL vs. 0.35 ± 0.059 mg/mL, p = 0.042; Fig. 4M, control group, n = 5; CTLA4-Ig group, n = 5).
Discussion
We have shown that CTLA4-Ig therapy attenuated tracheal obliteration with fibrous tissue in a mouse IPTT model. We further demonstrated that the inhibition of the lymphoid neogenesis was correlated with the attenuation of fibrous obliteration by CTLA4-Ig therapy.
The mouse subcutaneous tracheal transplant model was developed as a heterotopic tracheal transplantation model of OB.16) Due to the simple surgical techniques and the reproducibility of results, this model has been used in many publications for studying the pathogenesis of OB.12,17) One of the advantages of the model is the reproducibility of the airway obliteration in the pulmonary milieu.17) The pathological change at each time point was reported by the previous study.18) We adopted the mouse IPTT model in the present study because it shows inflammatory cell infiltrations in the surrounding lung tissue and obliterative fibrosis of the transplanted trachea, which is similar to fibrous obliterations in bronchioles observed in human BOS.12) More importantly, intrapulmonary lymphoid neogenesis, which we consider to be a therapeutic target of BOS, occurs in this model. Indeed, we demonstrated that there are lymphoid aggregates, which indicate the typical development of lymphoid neogenesis, in the recipient lung of cases of MHC mismatched IPTT (Fig. 2A and 2B). A minor-alloantigen-mismatched mouse orthotopic single lung transplant model (C57BL/10 donor to C57BL/6 recipient) has proven to show OB. Whereas this model might to be more clinically relevant from a surgical point of view; however; the percentage of mice with prominent fibrotic obliteration was only 44%, which is much lower than the IPTT model, and the fibrosis variability may depend on technical or surgical factors in the mouse lung transplant model.19) We are currently aiming to establish a new mouse orthotopic single lung transplant model and planning a future experiment with CTLA4-Ig.
Sato et al.8) reported lymphoid neogenesis in the lung affected by CLAD after human lung transplantation as a de novo lymphoid tissue. Wagnetz et al.12) demonstrated that intrapulmonary lymphoid neogenesis contributed to allograft airway rejection in the mouse IPTT model and mentioned that lymphoid neogenesis is a potential novel mechanism of CLAD. Actually, in the present study, we demonstrated that lymphoid aggregates developed in the lung around the transplanted trachea in the mouse MHC mismatched IPTT model and that they comprised a number of B220 positive B lymphocytes. Matsuda et al.10) demonstrated that inhibition of B lymphocyte maturation by spleen tyrosine kinase resulted in a significant decrease in intrapulmonary lymphoid aggregates and significant attenuation of lumenal obstruction in a mouse IPTT model. They also showed that loss of spleen tyrosine kinase activity impaired B-cell recruitment to the lung.10)
Matsumura et al.3) demonstrated that CTLA4-Ig modified parameters of inflammation in rat lung acute rejection. In the present study, the expression of TNF-α, which is a proinflammatory cytokine, was significantly decreased in the lung by CTLA4-Ig. CTLA4-Ig was previously reported to abrogate the development of OB in a murine11) and a rat20 subcutaneous tracheal transplant model; however, it had remained unclear whether CTLA4-Ig has any effect on the fibrous obliteration of intrapulmonary transplanted trachea and lymphoid neogenesis.
We showed that weekly administration of CTLA4-Ig significantly ameliorated the tracheal obliteration on post-transplant day 28 in the mouse IPTT model and suppressed the expression of COL1a1 in the lung. Whereas MMP-2, which plays an important role in the degradation of the extracellular matrix in pulmonary diseases, was not upregulated, MMP-14 was significantly increased in the current study. MMP-14 is an activator of MMP-2 and has been reported to be associated with fibrosis in OB in a mouse subcutaneous tracheal transplant model.21) Abatacept, which was used in the current study, is a commercially available form of CTLA4-Ig and has been already used clinically for rheumatoid arthritis patients; therefore, we expect that CTLA4-Ig could be a therapeutic option for BOS after human lung transplantation in the future.We demonstrated that CTLA4-Ig significantly decreased lymphoid neogenesis and that a significant positive correlation between the total area of lymphoid aggregates and the obliteration ratio of the transplanted trachea. We speculated that the underlying mechanism of the amelioration of graft luminal obliteration might be the inhibition of lymphoid neogenesis by CTLA4-Ig.In previous studies, both CCL19 and CCL21 were found to be upregulated at sites of chronic inflammation6) and induce invasive infiltrates containing predominantly T cells.22) Sanjiv et al.22) demonstrated that CCL19- and CCL21-mediated induction of lymphotoxin α1β2 on naive T cells may participate in promoting the development of endogenous and disease-associated lymphoid tissues. Both chemokines have been reported to play an important role in the induction and maintenance of TLOs in the lung.23) In the current study, CTLA4-Ig treatment significantly downregulated the expressions of CCL19 and CCL21 in the lung and may have inhibited the development of lymphoid neogenesis.
Expressions of CXCL12 and CXCL13 were required in the development of TLOs in the lung,24) and were especially related to the recruitment of B cells to the lymphoid aggregates in the lung.25) In the present study, we found that CTLA4-Ig treatment significantly decreased the expressions of both CXCL12 and CXCL13.
Recently, antibody-mediated rejection (AMR) by donor-specific antibodies (DSAs) has attracted attention as a possible contributor to both acute rejection and CLAD after lung transplantation.26) In our study, CTLA4-Ig treatment significantly decreased the plasma IgG level. Post-transplant donor-specific B-cell development is T-cell dependent and leads to long-lasting, anti-donor antibody-producing plasma cells,27) therefore; CTLA4-Ig may have indirectly inhibited the plasma IgG level in the present study.
We acknowledge that there are limitations in the present study. First, we initiated CTLA4-Ig treatment immediately after tracheal transplantation and it remains to be clarified whether the treatment has only preventive effects or is effective for already established OB as a therapeutic option. We are planning to investigate the effect of CTLA4-Ig started on post-transplant day 21 or 28, which would be important to determine the regimen of CTLA4-Ig for future clinical use, especially to decide the timing of the administration.
Second, whereas CTLA4-Ig has been reported to directly prevent T-cell activation on by blocking the costimulation, and we demonstrated the improvement of OB by CTLA4-Ig via the inhibition of the lymphoid neogenesis and that related cytokines and chemokines were significantly downregulated, it has not been revealed yet how CTLA4-Ig reduces the recruitment of B cells to lymphoid aggregates. Leibler et al.28) reported that CTLA4-Ig modifies the pattern of expression of costimulatory molecules on the surface of B cells by occupying both CD80 and CD86 ligands, decreasing the free expression of CD86 and increasing the expression of PDL1. On the other hand, we found the decreased expressions of CXCL12 and CXCL13, which are both responsible for recruitment of B cells to TLOs. We intend to elucidate the underlying mechanism in the downregulation of these chemokines by CTLA4-Ig in future experiments.
Third, the effect of CTLA4-Ig at each time point after transplantation remained unknown. Specifically, CTLA4-Ig does not necessarily decrease the influx of lymphocytes in acute phase after transplantation. Matsumura et al.3) reported that the degree of lymphocytic infiltration was greater in the lung allograft treated with CTLA4-Ig in a rat lung transplantation model at day 7. In the IPTT model used in the present study, T cells and macrophages are infiltrating into the lumen of allografts on day 718) whether CTLA4-Ig ameliorates the cell infiltration. More importantly, it should be clarified whether the lymphoid neogenesis observed on day 28 is donor-specific and we are currently planning a new experiment.
Lastly, there is a major difference between clinical lung transplantation and the IPTT model that lymphoid aggregates are formed in the transplanted lung in clinical setting or in the native (recipient) lung in the animal model. However, functional assessment of the lymphoid neogenesis is unable in clinical lung transplantation8) and the IPPT model is currently the best model. Maybe a new mouse orthotopic single lung transplant model will be established and overcome the limitation in the future.
Conclusion
CTLA4-Ig therapy attenuated tracheal obliteration with fibrous tissue in a mouse IPTT model. The attenuation of fibrous obliteration correlated to the inhibition of lymphoid neogenesis.
Acknowledgment
The authors would like to express their gratitude to Brent Bell for assistance in editing this manuscript.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No.18K08777).
Disclosure Statement
All authors have no conflict of interest.
References
- 1). Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report—2018. Focus theme: Multiorgan Transplantation. J Heart Lung Transplant 2018; 37: 1169– 83. [DOI] [PubMed] [Google Scholar]
- 2). Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment—A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019; 38: 493– 503. [DOI] [PubMed] [Google Scholar]
- 3). Matsumura Y, Zuo XJ, Prehn J, et al. Soluble CTLA4Ig modifies parameters of acute inflammation in rat lung allograft rejection without altering lymphocytic infiltration or transcription of key cytokines. Transplantation 1995; 59: 551– 8. [PubMed] [Google Scholar]
- 4). Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 2016; 374: 333– 43. [DOI] [PubMed] [Google Scholar]
- 5). Iasella CJ, Winstead RJ, Moore CA, et al. Memory T cells in transplantation: Old challenges define new directions. Transplantation 2018; 104: 2024– 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 2006; 6: 205– 17. [DOI] [PubMed] [Google Scholar]
- 7). Cupedo T, Jansen W, Kraal G, et al. Induction of secondary and tertiary lymphoid structures in the skin. Immunity 2004; 21: 655– 67. [DOI] [PubMed] [Google Scholar]
- 8). Sato M, Hirayama S, Hwang DM, et al. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J Immunol 2009; 182: 7307– 16. [DOI] [PubMed] [Google Scholar]
- 9). Sato M, Hirayama S, Matsuda Y, et al. Stromal activation and formation of lymphoid-like stroma in chronic lung allograft dysfunction. Transplantation 2011; 91: 1398– 405. [DOI] [PubMed] [Google Scholar]
- 10). Matsuda Y, Wang X, Oishi H, et al. Spleen tyrosine kinase modulates fibrous airway obliteration and associated lymphoid neogenesis after transplantation. Am J Transplant 2016; 16: 342– 52. [DOI] [PubMed] [Google Scholar]
- 11). Yamada A, Konishi K, Cruz GLE, et al. Blocking the CD28-B7 T-cell costimulatory pathway abrogates the development of obliterative bronchiolitis in a murine heterotopic airway model. Transplantation 2000; 69: 743– 9. [DOI] [PubMed] [Google Scholar]
- 12). Wagnetz D, Sato M, Hirayama S, et al. Rejection of tracheal allograft by intrapulmonary lymphoid neogenesis in the absence of secondary lymphoid organs. Transplantation 2012; 93: 1212– 20. [DOI] [PubMed] [Google Scholar]
- 13). Kim I, Wu G, Chai NN, et al. Immunological characterization of de novo and recall alloantibody suppression by CTLA4Ig in a mouse model of allosensitization. Transpl Immunol 2016; 38: 84– 92. [DOI] [PubMed] [Google Scholar]
- 14). Boleto G, Guignabert C, Pezet S, et al. T-cell costimulation blockade is effective in experimental digestive and lung tissue fibrosis. Arthritis Res Ther 2018; 20: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Sato M, Hwang DM, Guan Z, et al. Regression of allograft airway fibrosis: the role of MMP-dependent tissue remodeling in obliterative bronchiolitis after lung transplantation. Am J Pathol 2011; 179: 1287– 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Hertz MI, Jessurun J, King MB, et al. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol 1993; 142: 1945– 51. [PMC free article] [PubMed] [Google Scholar]
- 17). Sato M, Keshavjee S, Liu M. Translational research: animal models of obliterative bronchiolitis after lung transplantation. Am J Transplant 2009; 9: 1981– 7. [DOI] [PubMed] [Google Scholar]
- 18). Sato M, Liu M, Anraku M, et al. Allograft airway fibrosis in the pulmonary milieu: a disorder of tissue remodeling. Am J Transplant 2008; 8: 517– 28. [DOI] [PubMed] [Google Scholar]
- 19). Martinu T, Oishi H, Juvet SC, et al. Spectrum of chronic lung allograft pathology in a mouse minor- mismatched orthotopic lung transplant model. Am J Transplant 2019; 19: 247– 58. [DOI] [PubMed] [Google Scholar]
- 20). Tikkanen JM, Lemström KB, Koskinen PK. Blockade of CD28/B7-2 costimulation inhibits experimental obliterative bronchiolitis in rat tracheal allografts: suppression of helper T cell type1-dominated immune response. Am J Respir Crit Care Med 2002; 165: 724– 9. [DOI] [PubMed] [Google Scholar]
- 21). Inaki N, Tsunezuka Y, Kawakami K, et al. Increased matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase activity and expression in heterotopically transplanted murine tracheas. J Heart Lung Transplant 2004; 23: 218– 27. [DOI] [PubMed] [Google Scholar]
- 22). Luther SA, Bidgol A, Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 2002; 169: 424– 33. [DOI] [PubMed] [Google Scholar]
- 23). Fleige H, Bosnjak B, Permanyer M, et al. Manifold roles of CCR7 and its ligands in the induction and maintenance of bronchus-associated lymphoid tissue. Cell Rep 2018; 23: 783– 95. [DOI] [PubMed] [Google Scholar]
- 24). Frija-Masson J, Martin C, Regard L, et al. Bacteria- driven peribronchial lymphoid neogenesis in bronchiectasis and cystic fibrosis.Eur Respir J 2017; 49: 1601873. [DOI] [PubMed] [Google Scholar]
- 25). Fleige H, Ravens S, Moschovakis GL, et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med 2014; 211: 643– 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Tikkanen JM, Singer LG, Kim SJ, et al. De novo DQ donor-specific antibodies are associated with chronic lung allograft dysfunction after lung transplantation. Am J Respir Crit Care Med 2016; 194: 596– 606. [DOI] [PubMed] [Google Scholar]
- 27). McManigle W, Pavlisko EN, Martinu T. Acute cellular and antibody-mediated allograft rejection. Semin Respir Crit Care Med 2013; 34: 320– 35. [DOI] [PubMed] [Google Scholar]
- 28). Leibler C, Thiolat A, Hénique C, et al. Control of humoral response in renal transplantation by belatacept depends on a direct effect on B cells and impaired T follicular helper-B cell crosstalk. J Am Soc Nephrol 2018; 29: 1049– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]