Abstract
Glomus tumors originate from a neuroarterial structure called the glomus body, and grow mostly in soft tissue. It is rare for glomus tumors to develop in the respiratory system. The patient of the present case had an abnormal shadow in the right lung on chest X-ray, and computed tomography (CT) findings displayed a lung tumor in the right S6. Bronchoscopy was performed for the diagnosis of the lung tumor, and a polypoid bronchial tumor was unexpectedly found to occupy the right B3. The bronchial tumor was diagnosed as a glomus tumor, and the lung tumor was diagnosed as an adenocarcinoma. The bronchial glomus tumor was cauterized by argon plasma coagulation (APC). Three weeks after the cauterization by APC, the right lower lobectomy was performed for the treatment of the lung adenocarcinoma. The patient has remained disease free for 2 years.
Keywords: bronchial tumor, glomus tumor, argon plasma coagulation
Introduction
Glomus tumors are rare neoplasms, originating from the glomus body, which surrounds neuroarterial structures,1) and accounts for less than 2% of soft tissue tumors.2) They are usually found in the deep dermis of the extremities, particularly in the hands, upper limbs, and lower limbs.3) Bronchopulmonary glomus tumors are extremely rare.4–6) Most glomus tumors are benign, and bronchopulmonary glomus tumors are treated by resection or cauterization. We herein experienced a glomus tumor in a segmental bronchus, and it was treated.
Case Report
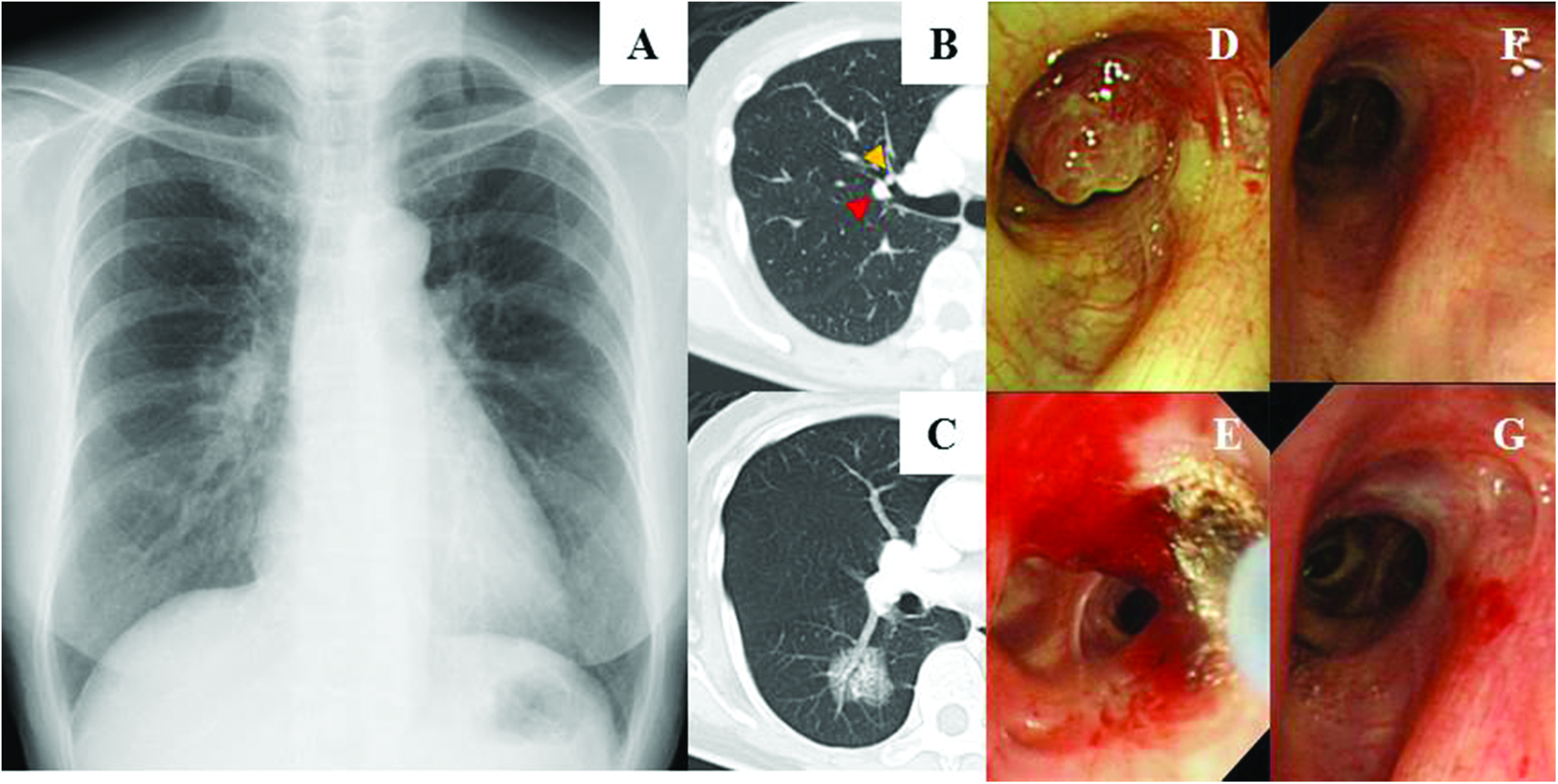
The patient was a 62-year-old woman with right chest pain. Chest X-ray displayed an abnormal shadow in the right lung (Fig. 1A). Computed tomography (CT) findings displayed a 3.3 cm ground-glass nodule (GGN) in the right S6 (Fig. 1C). Bronchoscopy was performed to diagnose the GGN, and we found a polypoid bronchial tumor with a glossy surface in the right B3 (Fig. 1D). It was also displayed on CT (yellow arrow in Fig. 1B). The bronchial tumor was diagnosed as a glomus tumor by transbronchial biopsy, and the GGN was diagnosed as a lung adenocarcinoma by transbronchial brushing.
Fig. 1. (A) Chest X-ray displaying an abnormal shadow on the right lung. (B) CT image displaying the polypoid tumor at the right B3 (yellow arrowhead) and the pulmonary artery passed along the bronchus (red arrowhead). (C) CT image displaying a 3.3 cm GGN in the right S6. (D) A bronchoscopic image displaying a glomus tumor in the right B3. (E) A bronchoscopic image of the glomus tumor in the right B3 immediately after the cauterization by APC (F) A bronchoscopic image of the glomus tumor 3 months after the cauterization by APC, displaying slight redness on the bronchial surface. (G) A bronchoscopic images of the bronchial glomus tumor 2 years after the cauterization by APC. APC: argon plasma coagulation; CT: computed tomography; GGN: ground-glass nodule.
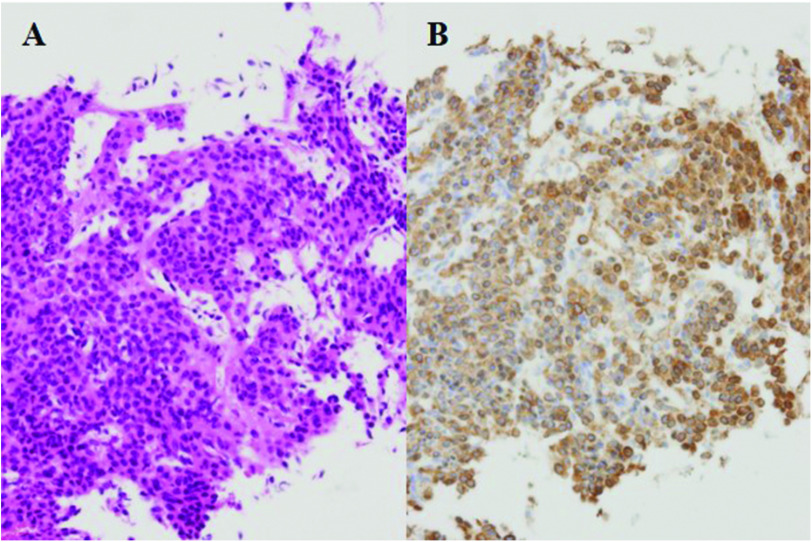
Microscopically, the tumor consisted specifically of proliferated epithelioid cells surrounding the capillary. The tumor cells had abundant eosinophilic cytoplasms and small round nuclei. No atypical or mitotic cells were observed (Fig. 2A). Immunohistochemical staining of the tumor cells demonstrated positive staining for α-smooth muscle actin (Fig. 2B), but the tumor was negative for cytokeratin, CD56, and chromogranin A. The tumor was hence diagnosed as a glomus tumor, which consisted of cells similar to smooth muscle cells.
Fig. 2. High-magnification images of hematoxylin and eosin staining (A) and α-smooth muscle actin staining (B) of the biopsy specimen.
The bronchial glomus tumor in the right B3 was cauterized by argon plasma coagulation (APC) with flexible bronchoscopy (Fig. 1E). Three weeks after the cauterization by APC, the right lower lobectomy with mediastinal lymph node dissection was performed for the treatment of the lung adenocarcinoma. Three months after cauterization of the bronchial glomus tumor, slight redness of the bronchial surface was observed (Fig. 1F). The patient has remained disease free for 2 years regarding the bronchial glomus tumor and the lung adenocarcinoma (Fig. 1G).
Discussion
Bronchopulmonary glomus tumors are rare, and they usually occur in the central airway. The patient of the present case had the bronchial glomus tumor in the segmental bronchus, which was treated by cauterization with flexible bronchoscopy.
Two devices for endobronchial cauterization are widely used, namely, the neodymium-doped yttrium aluminum garnet (Nd:YAG) laser and APC. The performance of Nd:YAG laser causes hemostasis and debulking of tissue. However, the absorption of the laser is affected by the color of the tissue surface, with dark-colored tissue having greater absorbance. Therefore, the laser depth is unpredictable, and there is a risk of tissue perforation. On the other hand, the depth cauterized by APC is constant at 2–3 mm of superficial coagulation. Therefore, the rate of complications of APC is lower than that of Nd:YAG lasers; however, it has a lower efficiency of tissue debulking. In the present case, the bronchial glomus tumor was tiny nodule and the pulmonary artery passed along the bronchus on CT (red arrow in Fig. 1B). The cauterization of APC was suitable for the treatment of the small tumor and to prevent the perforation into the pulmonary artery.
Conclusion
The appropriate treatment for bronchopulmonary glomus tumors should be selected according to their size and location, namely, resection or cauterization. Most glomus tumors are benign, and minimal treatment is required. In the present patient, APC was useful for treatment of the bronchial glomus tumor.
Acknowledgment
The authors thank the Department of International Medical Communications of Tokyo Medical University (Tokyo, Japan) for editing of the English manuscript.
Informed Consent
In this report, informed consent to participate was obtained from the patient.
Disclosure Statement
All of the authors did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors to perform this research.
References
- 1). Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am 1972; 54: 691– 703. [PubMed] [Google Scholar]
- 2). Shugart RR, Soule EH, Johnson EW. Glomus tumor. Surg Gynecol Obstet 1963; 117: 334– 40. [PubMed] [Google Scholar]
- 3). Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg 1992; 79: 345– 7. [DOI] [PubMed] [Google Scholar]
- 4). Choi IH, Song DH, Kim J, et al. Two cases of glomus tumor arising in large airway: well organized radiologic, macroscopic and microscopic findings. Tuberc Respir Dis 2014; 76: 34– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Oide T, Yasufuku K, Shibuya K, et al. Primary pulmonary glomus tumor of uncertain malignant potential: a case report with literature review focusing on current concepts of malignancy grade estimation. Respir Med Case Rep 2016; 19: 143– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Venegas O, Newton A, Vergara N, et al. Tracheal glomus tumor: a case report and review of literature. Rare Tumors 2017; 9: 26– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]