Abstract
Resveratrol is a well‐known antioxidant that harbours many health beneficial properties. Multiple studies associated the antioxidant, anti‐inflammatory, and cell protective effects of resveratrol. These diverse effects of resveratrol are also potentially involved in cutaneous wound healing, scarring, and (photo‐)aging of the skin. Hence, this review highlighted the most relevant studies involving resveratrol in wound healing, scarring, and photo‐aging of the skin. A systematic review was performed and the database PubMed was searched for suitable publications. Only original articles in English that investigated the effects of resveratrol in wound healing, scarring, and (photo‐)aging of the skin were analysed. The literature search yielded a total of 826 studies, but only 41 studies met the inclusion criteria. The included studies showed promising results that resveratrol might be a feasible treatment approach to support wound healing, counteract excessive scarring, and even prevent photo‐aging of the skin. Resveratrol represents an interesting and promising novel therapy regime but to confirm resveratrol‐associated effects, more evidence based in vitro and in vivo studies are needed.
Keywords: chronic wound, resveratrol, scarring, skin aging, wound healing
1. INTRODUCTION
Resveratrol (3,5,4′‐trihydroxy‐trans‐stilbene) belongs to the stilbenoid group of polyphenols. It consists of two phenol rings linked by an ethylene bridge, which gives rise to two geometric isomers: the active trans‐isomer and an inactive cis‐isomer. 1 Resveratrol has been found in more than 70 different plant species and important dietary sources are grapes (skin and seeds), red wine, peanuts, and soy. 2 The so‐called “French Paradox” that describes the potential health benefits associated with regular and moderate wine consumption was first correlated to wine polyphenols, especially resveratrol, by Renaud and De Logeril in 1992. 3 Since then, resveratrol has received increasing scientific attention regarding its diverse biological activities, including antioxidant, anti‐inflammatory, antiobesity, and anticancer properties.
Among these diverse biological effects of resveratrol, the antioxidant properties are most prominent. Resveratrol has been shown to protect cells against hydrogen peroxide‐induced oxidative stress, as well as UV‐irradiation‐mediated cell death. These results were partially attributed to direct effects of resveratrol as a radical scavenger but indirect effects were also observed, where resveratrol was able to modulate cellular antioxidant pathways. 4 , 5 , 6 , 7
Resveratrol has also been known to interfere with inflammatory responses and to reveal anti‐inflammatory properties, as it targets nuclear factor‐kB (NFkB), expression of pro‐inflammatory cytokines (e.g. interleukin 6, IL6), and also genes involved in eicosanoid production. 1 , 8 Resveratrol also targets toll‐like receptor (TLR) signalling, such as TLR2, TLR3, and TLR4 signalling. 9 , 10 , 11 , 12 , 13 In skin, resveratrol reduces imiquimod‐induced, TLR7, TLR8, TLR9‐mediated, psoriatic inflammation in animal models, most likely via interfering with NF‐kB activation. 14 , 15 This anti‐inflammatory activity of resveratrol can be associated with health benefits for several autoimmune and chronic inflammatory conditions. 8
Numerous in vitro and in vivo studies confirmed the inhibitory effects of resveratrol on carcinogenesis in all stages, such as initiation, promotion, and progression. 16 , 17 Resveratrol serves as a chemopreventive agent 18 , 19 and displays chemotherapeutic properties that are attributed to its antioxidant, anti‐inflammatory, proapoptotic, and antiproliferative features. 17 , 20 Resveratrol is supposed to target intracellular signalling pathway components including regulators of cell survival and apoptosis, pro‐inflammatory mediators, and key components of tumour angiogenesis and metastasis by interfering with a distinct set of transcription factors, upstream kinases, and their regulators. 21 , 22 , 23
In vivo studies demonstrated that resveratrol additionally exerted beneficial effects on metabolic syndrome‐related alterations, for example, glucose and lipid homeostasis improvement and a reduction in fat mass, blood pressure, low‐grade inflammation, and oxidative stress. 24 , 25 , 26 , 27 , 28 Further studies verified that resveratrol mimics caloric restriction via the activation of sirtuin 1 (SIRT 1) 26 , 29 resulting in improved exercise performance and insulin sensitivity, as well as showing body fat‐lowering effects by inhibiting adipogenesis, and increasing lipid mobilisation in adipose tissue. 30 , 31
These diverse effects of resveratrol are also potentially involved in cutaneous wound healing, scarring, and (photo‐) aging of the skin, which is reflected by an increasing number of publications in this field over the past decade. This review will therefore summarise the most relevant studies involving resveratrol in wound healing, scarring, and photo‐aging of the skin.
2. METHODS
The online database PubMed was used to review the medical literature covering resveratrol application in wounds, skin aging, and scars. The day the database was accessed was on 28 November 2020, and the last 10 years have been analysed. The following MeSH terms have been used [“Resveratrol”[Mesh] AND (“Wounds and Injuries”[Mesh] OR “Wound Healing”[Mesh] OR “Re‐Epithelialization”[Mesh] OR “Skin Aging”[Mesh] OR “Aging”[Mesh] OR “Cicatrix”[Mesh])]. To minimise the risk of missing relevant data, the search term [resveratrol AND (“Wound*” OR “Anti‐Aging” OR “Cicatrix” OR “Scar”)] has been used additionally.
All search results were listed in an excel sheet (Microsoft Excel 2016 MSO [16.44] 32‐bit) and duplicates have been removed. Titles, abstracts, and if available, full‐text articles have been analysed, which concern the exact study purpose. Non‐English articles, reviews, commentaries, and studies that aimed at wrong topics were excluded. The remaining studies were included and analysed for their specific content. In order to minimise the bias of wrongful exclusion, each study was evaluated by at least two reviewers (AH and MS), and exclusion decisions were based on the consensus of the reviewing authors. If a discrepancy between the reviewers occurred, the literature was evaluated by a third reviewer (EH).
The initial literature search yielded a total of 942 studies with 539 results in PubMed with MeSH‐terms and 403 results in PubMed without MeSH terms. After duplicate exclusion (116 duplicates), 784 studies of the remaining 826 studies were excluded according to the prior mentioned exclusion criteria.
Thirty studies were excluded due to non‐English, 217 studies because they were reviews or commentaries, and 538 studies because of wrong topic. In total, 41 studies about the effects of resveratrol have been included. A flowchart, summarising the systematic algorithm, as well as the quantitative results can be found in Figure 1. A summary of in vitro, in vivo, and clinical studies investigating the effects of resveratrol in the context of wound healing, scarring and photo‐aging, are presented in Tables 1, 2, 3. The included studies can be divided into these major effects: wound healing, antiscarring, and antiaging properties of resveratrol, which will be presented in the following.
FIGURE 1.
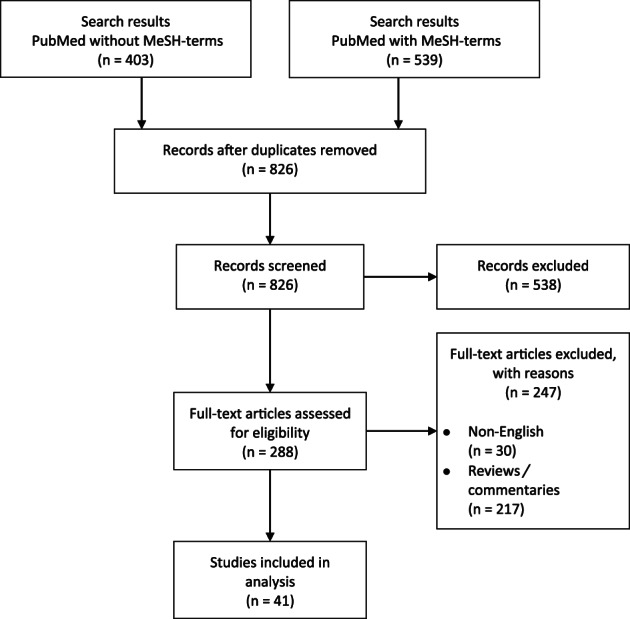
Review process depicted in a flowchart
TABLE 1.
Summary of in vitro studies investigating the effects of resveratrol on wound healing, scarring, and photo‐aging
| Study topic | Author, year | Cohort | Test substance | Setting | Effects |
|---|---|---|---|---|---|
| Wound healing | Brakenhielm et al (2001) 32 | Bovine capillary endothelial cells, chick chorioallantoic membrane | 1–100 μg RES discs |
Effect of RES on fibroblast growth factor 2 (FGF‐2)‐induced activation of mitogen‐activated protein kinases in bovine capillary endothelial cells; effect of RES in porcine aortic endothelial cell lines; antiangiogenic activity of RES in chick chorioallantoic membrane (1–100 μg per disc.) Parameters evaluated: FGF‐2 and vascular endothelial growth factor (VEGF) receptor‐mediated endothelial cell growth and chemotaxis |
RES inhibits FGF‐2 and VEGF receptor‐mediated endothelial cell growth and chemotaxis. Dose‐dependent inhibitory effect on angiogenesis of RES. |
| Khanna et al (2001) 33 | Human keratinocytes (line HaCaT) | GSPE (grape seed proanthocyanidin extract) containing 5000 ppm trans‐RES |
Keratinocytes treated with GSPE; washed with GSPE‐free medium before, then challenged with H2O2 (hydrogen peroxide) or TNF‐α Parameters evaluated: VEGF |
Treatment with GSPE upregulated H2O2 and TNF‐α induced VEGF expression and release | |
| Chan et al (2002) 34 | Bacteria and dermatophytes | 3.12, 6.25, 12.5, 25, 20, 100 μg/mL RES | Susceptibility testing of bacteria (Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa) and dermatophytes (Trichophyton mentagrophytes, Trichophyton tonsurans, Trichophyton rubrum, Epidermophyton floccosum, Microsporum gypseum) in control medium, dimethyl sulphoxide solvent, and RES. | RES inhibited the growth of bacteria and dermatophytes in a dose‐dependent manner. Higher RES concentration resulted in better growth inhibition of bacteria and dermatophytes. | |
| Pastore et al. (2013) 35 | Human epidermal keratinocytes (HEK) | 50 μM RES |
HEK from 4 healthy donors: verbascoside (control), RES 50 μM Parameters evaluated: IL‐8 expression, extracellular‐signal regulated kinase (ERK), p65, c‐Fos, epidermal growth factor receptor (EGFR), NHEK proliferation |
IL‐8 overexpression, ERK phosphorylation was transiently inhibited, enhanced p65 and EGFR phosphorylation, c‐Fos upregulation, NHEK proliferation inhibited | |
| Eroğlu et al (2014) 36 | Normal skin‐derived fibroblasts (NSFBs); | RES loaded into microparticles consisting of dipalmitoylphosphatidylcholine and hyaluronic acid | Cell culture of NSFBs: RES‐loaded microparticles Parameters evaluated: cellular proliferation (CP), total glutathione (GSH), oxidised glutathione (GSSG), glutathione peroxidase (GPx), malondialdehyde (MDA), superoxide dismutase (SOD) | RES‐loaded microparticles increased CP, decreased oxidation in cells by GSH/GSSHG ratio reduction. No effect of RES in GPx, MDA, and SOD. | |
|
Abbas et al (2019) 37 |
Human fibroblasts | Flavonoids (chrysin, naringenin, RES) with β‐sitosterol |
Scratch‐wound migration assay: Parameters evaluated: closure rate, cytotoxicity, fibroblasts migration |
β‐sitosterol combined with RES and β‐sitosterol combined with naringenin achieved the best closure rates. No toxic effect on fibroblasts was detected. | |
| Comotto et al (2019) 38 | Human keratinocytes | Alginate dressings with natural antioxidants (curcumin; RES) |
Determination of the optimal concentration of the active compounds Parameters evaluated: release rate, cellular growth, bacterial growth |
Release rate: burst release followed by a gradual release; 300 μg/mL of RES did not induce any toxicity; curcumin and RES showed an improved cell viability; curcumin was superior regarding anti‐microbial properties | |
| Meng et al (2019) 39 | Human adipose stem cells | Bacterial cellulose conjugated with RES, collagen conjugated with RES |
In vitro biocompatibility: adipose stem cells were cultured in keratinocyte serum‐free medium with foetal bovine serum. Examination of the samples by immunocytochemical staining. |
RES‐conjugated bacterial cellulose created a biocompatible environment for stem cell attachment and cell growth. | |
| Huang et al (2019) 40 | Human umbilical vein endothelial cells (HUVECs) | 10 μM RES |
HUVECs in high glucose medium (HGM), HGM + 10 μM RES, normal glucose medium (NGM), NGM + 10 μM RES Parameters evaluated: CP, cell migration, apoptosis |
RES treatment increased hyperglycemia‐impaired endothelial CP. HGM induced cell migration and apoptosis alleviated by RES |
|
| Kaleci et al (2020) 41 | 3 T3 Swiss Albinofibroblasts | 1, 5, and 10 μM RES in combination with 500 μM H2O2 |
Fibroblasts, 4 groups: control group, 500 μM H2O2, RES, 500 μM, H2O2 + RES group Parameters evaluated: CP, OS (oxidative stress) level, collagen‐I‐expression, cell migration |
RES application showed better CP rates compared to the other groups. Best CP results with 1 μM RES. RES decreased H2O2‐induced high OS. No impact of RES in collagen‐I‐expression or cell migration compared to control group. |
|
| Scarring | Zeng et al (2013) 42 | Hypertrophic scar‐derived fibroblasts (HSFBs) | RES 25, 75, 150, 300 and 400 μM |
Cell culture of HSFBs and NSFBs from two young female donors: control, RES 25 μM, RES 75 μM, RES 150 μM, RES 300 μM, and RES 400 μM over the time (24 h, 48 h and 72 h) Parameters evaluated: cell proliferation (CP), cell cycle progression, apoptosis, hydroxyproline, collagen |
RES suppressed cell growth, arrested cell cycle progression, triggered apoptosis in a dose‐ and time‐dependent manner (increased effect with longer duration and higher concentration). RES downregulated mRNA expression of type I and III procollagen in fibroblasts, resulting in significant decreases in hydroxyproline and collagen |
| Zhai et al (2015) 43 | Human pathologic scar‐derived fibroblasts (PSFBs) | RES 10, 50, and 100 μmol/L |
Cell culture with fibroblast from 20 patients. 4 groups: control, RES 10, RES 50, 100 μmol Parameters evaluated: morphological changes in target cells, CP, TGF‐β1, Smad‐2,3,4,7 |
Apoptotic morphological alterations and reduced CP in RES‐treated pathological scar fibroblasts. Inhibitory effect in CP enhanced with increasing RES concentration. TGF‐β1, Smad‐2,3,4 negatively and Smad‐7 positively correlated with RES concentration. | |
| Bai et al (2016) 44 | HSFBs and NSFBs | RES 2.5, 5, 10, 20, and 40 mM |
Cell culture of HSFBs and NSFBs from 9 patients. SIRT1 upregulation by RES. Parameters evaluated: SIRT1, collagen 1, collagen 3, α‐smooth muscle Actin (α‐SMA), TGF‐β1 |
SIRT1 intensity lower in HSFBs compared to NSFBs. RES down‐regulated mRNA levels of collagen 1, collagen 3, α‐SMA due to upregulation of SIRT1 in a dose‐dependent manner. RES inhibited TGF‐β1‐induced mRNA/protein level increase of collagen 1, collagen 3, and α‐SMA. | |
| Tang et al (2017) 45 | PSFBs and NSFBs | RES 10, 50, and 100 μmol/L |
Cell culture of PSFBs and NSFBs from patients: control, RES 10 μmol/L, RES 50 μmol/L, 100 μmol/L Parameters evaluated: mammalian target of rapamycin (mTOR), ribosomal protein S6 kinase (70S6K) |
Strengthened mTOR and 70S6K expression in PSFBs compared to NSFBs. Decreased expression of mTOR and 70S6K in dose‐dependent manner. |
|
| Pang et al (2020) 46 | HSFBs and NSFBs | RES 0, 1, 10, 100 μmol/L |
Cell culture of HSFBs and NSFBs: control, RES 1 μmol/L, RES 10 μmol/L, 100 μmol/L over the time (24, 48, 72 h) Parameters evaluated: cell viability (CV), microRNA‐4654, Rheb, 1A/1B‐light chain (LC3), Beclin 1 |
RES decreased CV in dose‐dependent manner (increased effect with higher concentration). RES upregulated microRNA‐4654 expression level in dose‐dependent manner, thus downregulated Rheb expression level and upregulated autophagy markers LC3 and Beclin 1 |
|
| Photo‐aging | Subedi et al (2017) 47 | NSFBs | RES and RESl‐enriched rice (RR) |
Cell culture with NSFBs from a healthy young male donor UV‐B irradiation and treatment with normal rice, RR and RES afterwards Parameters evaluated: ROS (reactive oxygen species), MMP‐1 (matrix metalloproteinase 1), collagen I |
RR demonstrated the most effective reduction of ROS production compared to normal rice or RES alone. RR induced a downregulation of MMP‐1 (matrix metalloproteinase 1), inhibition of inflammatory cascades, and upregulation of collagen type I. |
| Zhou et al (2018) 48 | Human keratinocytes (HaCaT cell line) | RES (<99%): 2.5, 5, 7.5, and 10 mm |
Cell culture with keratinocytes s, UV‐B irradiation for 5 min at 10 cm below the lamp (irradiation intensity: 0.1 mW/cm2) Parameters evaluated: CV, apoptotic rate |
Dose‐dependent increase of CV of RES pretreated cells and decrease of apoptotic rate. Increase of HSP27 expression resulting in antiapoptotic effects through inhibiting NF‐kB and caspase‐3 activation. |
Abbreviations: α‐SMA, α‐smooth muscle actin; CP, cellular proliferation; CV, cell viability; EGFR, epidermal growth factor receptor; ERK, extracellular‐signal regulated kinase; FGF‐2, fibroblast growth factor 2; GPx, glutathione peroxidase; GSH, total glutathione; GSPE, grape seed proanthocyanidin extract; GSSG, oxidised glutathione; H2O2, hydrogen peroxide; HGM, high glucose medium; HSFBs, hypertrophic scar‐derived fibroblasts; HUVECs, human umbilical vein endothelial cells; LC3, 1A/1B‐light chain; MDA, malondialdehyde; MMP‐1, matrix metalloproteinase 1; NGM, normal glucose medium; NSFBs, normal skin‐derived fibroblasts; OS, oxidative stress; PSFBs, pathologic scar‐derived fibroblasts; RES: resveratrol; ROS, reactive oxygen species (ROS); RR, resveratrol‐enriched rice; SOD, superoxide dismutase; TNF‐α, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor.
TABLE 2.
Summary of in vivo studies investigating the effects of resveratrol wound healing, scarring, and photo‐aging
| Study type | Author, year | Cohort | Test substance | Setting | Effects | Adverse events |
|---|---|---|---|---|---|---|
| Wound healing |
Brâkenhielm et al (2001) 32 |
Mice (neovascularisation group) Murine T241 fibrosarcoma (fibrosarcoma model) C57Bl6/J mice (wound model) |
5.7 mg/mL RES |
Neovasculariaation group: corneas of mice implanted with VEGF and FGF‐2. Oral application of RES. Drinking solutions for mice with RES (equivalent of 3 glasses of red vine) compared to control group (drinking water). Fibrosarcoma group: oral administration of RES (1 mg/kg per day) for mice with fibrosarcoma Full‐thickness skin wound group: oral administration of RES (1 mg/kg per day) for C57Bl6/J mice Parameters evaluated: VEGF, FGF‐2 vessel density, fibrosarcoma growth, wound healing |
Inhibition of VEGF and FGF‐2 in RES group and vessel density reduction compared to control group. RES treatment inhibited fibrosarcoma growth and delayed wound healing. |
No minor or major adverse events |
| Khanna et al (2002) 49 |
9 BalbC mice |
GSPE containing 5000 ppm trans‐RES |
Two full‐thickness excisional wound in each mice. One of the two wounds treated with 25 mL of 100 mg/mL GSPE for 5 d. Second wound served as control. Parameters evaluated: wound healing, histology, glutathione/glutathione disulphide ratio |
RES treatment increased wound healing and glutathione/glutathione disulphide ratio RES application presented more well‐defined hyperproliferative epithelial region, higher cell density, enhanced deposition of connective tissue, improved histological architecture. |
No minor or major adverse events |
|
| Lin et al (2016) 50 | 30 male Balb/C mice | RES ointment (1, 100, 500 ng/mL) |
7 groups, full‐thickness excision of burn skin wound: vaseline (control), aloe emodin (1, 100, and 500 ng/mL), RES (1, 100, and 500 ng/mL). Parameters evaluated: wound healing, histology, IL‐1β, MCP‐1, VEGF |
Decreased healing time in RES‐treated group compared to control group. Histology: increased cellular infiltration IL‐1β, MCP‐1, and VEGF increased in RES group compared to control group |
No minor or major adverse events |
|
| Poornima et al (2017) 51 | 9 female albino Wistar rats | RES and ferulic acid |
3 groups of 3 rats, full‐thickness excision wounds: controls, chitosan polycaprolactone nanofibres, RES‐ferulic‐acid‐loaded nanofibres. Parameters evaluated: wound closure rate, wound vicinity, tensile strength, histology |
Accelerated healing time in RES‐treated group compared to control (15 d vs 20 d). Smaller wound vicinity in the RES‐treated group. histology showed a higher collagen synthesis and more collagen deposition with tight packing in the RES‐treated group |
No minor or major adverse events |
|
| Gokce et al(2017) 52 | 42 male Wistar albino rats | RES‐loaded hyaluronic acid and dipalmitoylphosphatidylcholine (DPPC) microparticles in dermal matrix |
7 groups of 6 rats, full‐thickness wound. Two control groups: non‐diabetic control, diabetic control (streptozotocin induced). Five intervention groups (diabetic wounds, streptozotocin induced): control, RES solution, RES‐loaded microparticles, dermal matrix, RES‐loaded microparticle impregnated dermal matrix (DM‐MP‐RES) Parameters evaluated: histology, wound healing, glutahione (GSH), oxidised glutathione (GSSG), glutahione peroxidase (GPx), malondialdehyde (MDA), superoxide dismutase (SOD) |
Highest amount of collagen fibres and efficient re‐epithelisation in DM‐MP‐RES group. DM‐MP‐RES application enhanced wound healing process in diabetic conditions, decreased SOD compared to diabetic wound control group and decreased GPx compared to diabetic control group. |
No minor or major adverse events | |
| Zhao et al (2017) 53 |
female C57BL/6 mice 24 Sprague–Dawley rats (12 wk old) 24 Sprague–Dawley rats (18 mo old) female New Zealand White rabbits |
RES 50 μM |
Full‐thickness wound 4 groups: control (ethanol), 2 μM metformin, 200 nM rapamycin, 50 μM RES. Agents locally applied to wound beds (100 μL per time in mice / rabbits and 225 μL per time in rats). Chronic application: one time every day and intermittent application: three times every other day in week 1 followed by a treatment‐free week. Treatment for 2 wk. Parameters evaluated: wound healing, vascularisation |
RES treatment improved wound healing in young rodents compared to control group. RES group in young rodents presented smaller wound size, thicker epidermis, more collagen deposition, higher number of hair follicles compared to control group. RES treatment improved vascularisation/number of capillary vessels in young and old rodents compared to control group. |
No minor or major adverse events |
|
| Berce et al (2017) 54 | 20 male Crl:CD1(ICR) mice | Chitosan‐sodium hyaluronate‐RES polymer sponge (RES: 20 mg) |
2 groups of 10 mice, full‐thickness wounds: control, chitosan‐sodium hyaluronate‐RES polymer sponge for 14 d. Parameters evaluated: wound healing, histology |
chitosan‐sodium hyaluronate‐RES polymer stimulated wound healing, showed bacteriostatic properties and inhibited inflammation. |
No minor or major adverse events |
|
|
Lakshmanan et al (2019) 55 |
C57BL/6 mice | Electrospun scaffold loaded with RES |
Full‐thickness ischaemic wound, 4 groups: no treatment, collagen patch, blank scaffolds without RES, scaffolds with RES. Parameters evaluated: wound healing, histology, thioredoxin‐1, hemeoxygenase‐1, vascular endothelial growth factor (VEGF), Bcl‐2 protein |
Treatment with RES‐loaded scaffold improved wound healing compared to control group. Activation of thioredoxin‐1, hemeoxygenase‐1, VEGF, and Bcl‐2 protein in RES‐loaded scaffold group. |
No minor or major adverse events |
|
| Christovam et al (2019) 56 | 32 male Wistar rats | 2% RES |
4 groups of 8 rats, full‐thickness wound after 18 d of caloric restriction (CR) / ad libitum diet: control, 2% RES, 30% CR + control solution, 30% CR + 2% RES. Parameters evaluated: collagen I + III, thiobarbituric acid‐reactive substances (TBARS), total sulfhydryl content (TSC), VEGF, SIRT1 |
Increased expression of collagen I and collagen III in RES groups compared to control. RES treatment decreased TBARS concentration and inhibited an increase of TSC. VEGF protein expression in 30% CR + 2% RES group was higher compared to control / 2% RES group. SIRT1 was higher in 2% RES group compared to control. |
No minor or major adverse events |
|
| Meng et al. (2019) 39 | 18 male Sprague–Dawley rats | Bacterial cellulose‐conjugated scaffold with 50 μM RES, collagen‐conjugated scaffold with 50 μM RES |
5 groups, excisional wounds: control, bacterial cellulose (BC), BC + RES, collagen, collagen + RES. Parameters evaluated: wound area, histology |
BC + RES conjugated scaffold induced re‐epithelisation and preserved normal collagen pattern. |
No minor or major adverse events |
|
| Li et al (2019) 57 | 12 Male db/db mice | 5 mmol/L trans‐RES + 5 mmol/L hesperetin |
2 groups of 6 mice, full‐thickness excision: control, 5 mmol/L tRES +5 mmol/L hesperetin to induce glyoxalase 1 expression. Parameters evaluated: wound healing |
tRES‐ hesperetin treatment induced faster wound closure with more capillary formation compared to control. |
No minor or major adverse events |
|
| Ávila‐Salas et al. (2019) 58 | 25 Sprague‐Dawley rats | Hydrogels loaded with bioactive compounds (RES, allantoin, dexpanthenol, caffeic acid) |
Full‐thickness excisional wounds: Madecassol (control), film dressing without bioactive compounds, hydrogels loaded with bioactive compounds (RES, allantoin, dexpanthenol, caffeic acid). Parameters: wound closure rate, wound size, histology |
All four bioactive compounds showed a better and faster re‐epithelialisation and a better organisation of the granulation tissue compared to controls. All formulations were associated with a faster wound closure rate than controls. |
No minor or major adverse events |
|
| Huang et al (2019) 40 | 6 db/db mice | RES 50 mg/kg/d |
Two full‐thickness excision wounds in each db/db mice: control wound, 10 μM RES. RES application 50 mg/kg/d for 4 wk Parameters evaluated: Ki67, apoptosis, c‐Caspase‐3, wound healing |
Ki67 increased due to RES treatment. Endothelial cells apoptosis alleviated by RES treatment. RES decreased c‐Caspase‐3 intensity. RES treatment accelerated wound healing. |
No minor or major adverse events |
|
| Orlowski et al (2020) 59 | C57BL6 female mice | Bimetallic Au@AgNPs modified with different polyphenols (with 200 μM RES) |
Wound model in vivo: 5 mice per group, splint mouse model, Au@AgNPs with different polyphenols. Parameters: Wound healing, transforming growth factor β (TGF‐β), VEGF, matrix metallopeptidase 9 (MMP‐9), tumour necrosis factor α (TNF‐α) Local lymph node assay: 3 mice per group, splint mouse model: negative control, positive control and a 25% w/v of polyphenol‐modified Au@AgNPs in vehicle or corresponding tannin solution were applied Parameters: lymphocyte proliferation |
RES‐Au@AgNPs improved wound healing and induced TGF‐β expression during the inflammatory phase as well as the expression of VEGF in the remodelling phase of wound healing. RES‐Au@AgNPs inhibited MMP‐9 and TNF‐α expression. RES‐Au@AgNPs induced down‐regulation of lymphocyte proliferation. |
No minor or major adverse events |
|
| Shevelev et al (2020) 60 | 200 male Wistar rats | RES |
4 groups of 50 rats, full‐thickness excision including (1) no infection, (2) S aureus, (3) P aeruginosa, and (4) C albicans. Each group divided into five treatment subgroups: placebo, positive control, RES, dihydroquercetin, dihydromyricetin. 14 d wound evaluation. Parameters evaluated: wound healing, histology |
Decreased healing time in RES‐treated group compared to placebo group in a pathogen type‐dependent manner. In P aeruginosa RES‐treatment subgroup, no wound square reduction compared to placebo group over 14 d. Increased healing rate in RES treatment group on days 10–14. Decreased infiltration of mast cells and increased infiltration of lymphocytes and macrophages in histological wound area. |
No minor or major adverse events |
|
| Zheng et al (2020) 61 | 18 male Sprague‐Dawley rats | Host‐guest gelatin hydrogel (HGM) loaded with RES and histatin‐1 |
Four full‐thickness burn wounds in each rat. Treatment with 4 hydrogels / dressings in each rat: control, HGM, HGM + RES, HGM + Histatin‐1, HGM + RES + histatin‐1 for 2 wk. Parameters evaluated: wound healing, interleukin‐6 (IL‐6), interleukin‐1β (IL‐1β), TNF‐α, transforming growth factor β1 (TGF‐β1) |
HGM + RES + histatin‐1 hydrogel treatment decreased wound area compared to other groups. HGM + RES + histatin‐1 hydrogel treatment inhibited IL‐6, IL‐1β, and TNF‐α and increased TGF‐β1. |
No minor or major adverse events |
|
| Bilgic et al (2020) 62 | 18 adult female Sprague‐Dawley rats | RES (0.5 mg/kg) |
3 groups of 6 rats, full‐thickness wounds: control, RES intraperitoneal (systemic), subcutaneous RES (local) daily for 14 d. Parameters evaluated: wound healing, histology |
RES treatment (local and systemic) improved wound healing, collagen deposition, and tensile strength compared to control group. |
No minor or major adverse events |
|
| Scarring | Bai et al (2016) 44 | 24 Balb/C mice | RES (4.4 mM, 0.5 mL/100 g bodyweight) |
Cutaneous excision wound models, intradermal injection of RES or SIRT1 shRNA (SIRT1 knock out). After 4 wk wound tissue evaluation. Parameters evaluated: histology, SIRT1, α‐smooth muscle Actin (α‐SMA) |
More neatly arranged and thinner collagen fibres compared to SIRT1 shRNA group. Increased SIRT1‐expression and decreased α‐SMA expression. |
No minor or major adverse events |
| Zhao et al (2020) 63 | 24 adult male Sprague‐Dawley rats | RES 8 and 32 μg/mL loaded into peptide hydrogel to form wound dressing |
4 groups of 6 rats, full‐thickness skin wound: no treatment, 200 μL peptide hydrogel, 200 μL peptide hydrogel + 8 μg/mL RES and 200 μL peptide hydrogel + RES 32 μg/mL. Treatment for 2 wk. Parameters evaluated: Histology |
Treatment with RES‐loaded peptide hydrogel showed more regular and thinner collagen deposition compared to both treatments without RES. | No minor or major adverse events | |
| Photo‐aging | Abbas et al (2018) 64 | 40 adult male Wistar rats (6–8 wk) | Compritol ATO‐based RES colloidal carriers (CCCs) |
4 groups of 10 rats, 10 consecutive days: daily UVB irradiation/UVB irradiation + CCC6/UVB irradiation + resveratrol suspension /no irradiation. Parameters evaluated: catalase, GSH, superoxide dismutase, IL‐6, IL‐8, NF‐kB, matrix metalloproteinase 1 (MMP‐1), GM‐CSF |
RES showed a protective effect when applied prior UVB irradiation compared to the positive control group. A superior dermal photoprotection was detected in the binary mixture of surfactants (P0407/P188). |
No minor or major adverse events |
| Kim et al (2019) 65 | 56 mice 25–30 g of bodyweight | RES (2/10/50 mg/kg) dissolved in a vehicle containing 10% ethanol and 10% Tween‐80 in drinking water (oral administration) |
3 RES groups (out of 7): Oral administration of RES 3×/wk for 6 wk. UVB irradiation from week 2 to week 6. Parameters evaluated: nuclear factor erythroid 2‐related factor 2 (Nrf2) level, MMP‐1; MMP‐9 Histopathological assessment, wrinkle formation |
Groups orally administered with RES tended to attenuate UVB‐caused wrinkle formation to the extent to the control group. RES administration enhanced Nrf2 level in liver and skin, suppressed UVB‐induced MMP‐1 and MMP‐9 expression. |
No minor or major adverse events |
Abbreviations: α‐SMA, α‐smooth muscle actin; BC, bacterial cellulose; CR, caloric restriction; CCCs, colloidal carriers; DPPC, dipalmitoylphosphatidylcholine; GSH, total glutathione; GSSG, oxidised glutathione; GPx, glutahione peroxidase; HGM, host‐guest gelatin hydrogel; IL‐6, interleukin‐6; IL‐8, interleukin‐8; IL‐1β, interleukin‐1β; MDA, malondialdehyde; MMP‐1, matrix metalloproteinase 1; MMP‐9, matrix metalloproteinase 9; MCP‐1, monocyte chemoattractant protein‐1; Nrf2, nuclear factor erythroid 2‐related factor 2; RES, resveratrol; DM‐MP‐RES, RES‐loaded microparticle‐impregnated dermal matrix; SOD, superoxide dismutase; TSC, total sulfhydryl content; TGF‐β1, transforming growth factor β1; TNF‐α, tumour necrosis factor α; VEGF, vascular endothelial growth factor,
TABLE 3.
Summary of clinical studies investigating the effects of resveratrol on wound healing and photo‐aging
| Study topic | Author, year | Cohort | Test substance | Setting | Effects | Dropout | Follow‐up | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Wound healing | Bashmakov et al (2014) 66 |
15 male and 9 female participants; average age of 56.4 y (±9.1) with diabetic foot ulcers type 2 diabetes |
50 mg trans‐RES |
Double blind, parallel‐group, randomised, controlled clinical trial. 2 groups: 14 patients with 50 mg trans‐RES, 10 patients with placebo; application twice a day over a 60‐d time period. Parameters evaluated: diabetic ulcer size, plasma fibrinogen level, C‐reactive protein level |
RES treatment induced a reduction of foot ulcer size and reduced plasma fibrinogen level compared to control group. | No dropout | every 2 wk |
No minor or major adverse events |
| Çetinkalp et al (2020) 67 | 48 male and female participants; age 18–80, with diabetic foot syndrome and type 1 or type 2 diabetes | RES and hyaluronic acid (Dermalix; 3D dermal matrix impregnated with RES and HA) |
Open, prospective, comparative parallel‐armed, randomised medical device clinical study of Dermalix. Parameters evaluated: wound closure, cytokine tissue concentration (TNF‐α, complement component 3) |
Dermalix treatment significantly enhanced wound closure compared to standard wound treatment. Dermalix significantly decreased TNF‐α tissue concentration and reduced/oxidised glutathione levels. | 3 drop outs | 2 mo follow‐up | No adverse events related to Dermalix | |
| Photo‐aging | Moyano‐Mendez et al (2013) 68 | 8 women aged between 45 and 70 y | RES, RES + ßCD (β‐cyclodextrin) binary system |
In vitro: cell culture of human keratinocytes (HaCaT cell line). Investigation of cytotoxicity and reactive oxygen species (ROS) inhibition. Clinical: daily application of RES, RES + βCD for 30 consecutive days. Parameters evaluated: skin hydration, luminosity, and elasticity |
Application of RES reduced ROS generation by 32.26% (0.34%). The binary system showed a ROS reduction of 61.82 (0.22%). For the hemiface treated with the binary system, the skin parameters measured increased to a higher extent compared to RES alone. |
No dropout | No follow‐up | No minor or major adverse events |
| Fariss et al (2014) 69 |
Dermatomed human skin (3 donors) 55 healthy female subjects aged between 40 y and 60 y |
Topical antioxidant (RES, Baicalin, vitamin E) |
In vitro: application of 2 mg/cm2 for 24 h at 32°C. Clinical: daily application to face and neck areas at bedtime for 12 wk. Parameters evaluated: firmness, elasticity, laxity, radiance, skin tone evenness, roughness, hyperpigmentation, tactile density, overall appearance, gene expression changes (via biopsy analysis) |
Induction of the nuclear factor erythroid 2‐related factor 2 (Nrf2) pathway, decrease of ROS prevalence. Increase in collagen type‐II alpha‐1 gene. Improvement in fine lines, wrinkles, elasticity, laxity, skin tone, hyperpigmentation, radiance and tactile roughness was noted after week 4. |
No dropout |
No follow up |
No minor or major adverse events |
|
| Igielska‐Kalwat et al (2019) 70 | 20 healthy volunteers | RES Cream |
6 consecutive weeks: daily application of RES cream or placebo cream twice a day. Parameters evaluated: skin hydration measured every 2 wk |
Hydration level in the epidermis increased during a regular use of the formulation containing RES from week 0 to week 6. Due to an increased hydration level in the stratum corneum, transepidermal water loss (TEWL) can be inhibited. | No Dropout |
No Follow Up |
No minor or major adverse events |
Abbreviations: βCD, β‐cyclodextrin; Nrf2, nuclear factor erythroid 2‐related factor 2; RES, resveratrol; ROS, reactive oxygen species; TEWL, transepidermal water loss; TNF‐α, tumour necrosis factor alpha.
3. RESVERATROL IN SKIN WOUND HEALING
Wound healing is a complex, highly regulated process crucial for maintaining the skin's barrier function. 71 Numerous disease processes are associated with an impairment of pathways involved in wound healing, resulting in chronic, non‐healing wounds that expose the patient to significant discomfort. 71 Insufficient vascularisation, infections, and prolonged inflammatory responses cause poor and delayed wound healing. Resveratrol has become a subject of interest to improve wound healing by promoting anti‐inflammatory, antioxidant, and angiogenic effects. 1
Resveratrol has an impact on the regulation of inflammation and therefore, repair‐related processes in the skin. 50 According to Pastore et al, anti‐inflammatory and wound healing effects of resveratrol depend rather on their interaction with epidermal growth factor receptor (EGFR)‐controlled cytoplasmic and nuclear pathways than on their direct antioxidant and free radical scavenging activity. 35 An in vivo study in rats showed that resveratrol can limit the infiltration of mast cells toward the wounded area, while the infiltration of lymphocytes and macrophages is stimulated. 60 It is assumed that resveratrol is able to suppress an acute antigen‐dependent reaction (Th1‐type response) in favour of a Th2 response. The infiltration of neutrophils differs depending on the type of pathogen. 60
In vitro studies demonstrated protective effects on fibroblasts by decreasing oxidative stress levels. Due to its antioxidative properties, resveratrol can stabilise cell proliferation, and improve migration quality and ultrastructural preservation. 36 , 41 Also, in vivo results demonstrated significantly improved wound healing following resveratrol application based on antioxidant activity. 52
Angiogenesis also plays a central role in wound healing, and resveratrol is discussed to influence neovascularisation. 62 Among the known growth factors, vascular endothelial growth factor (VEGF) is believed to be one of the most prevalent, effective, and long‐term signal‐stimulating angiogenesis in wounds. 49 Several studies showed that resveratrol induces VEGF expression and subsequently regulates angiogenesis. 49 , 55 , 67 Christovam et al ascribed the proangiogenic properties of resveratrol to its activation of SIRT1. SIRT1 is associated with the induction of angiogenesis, fibroplasia, and collagen organisation. Therefore, resveratrol, as an activator of SIRT1, shows several beneficial effects for wound healing. 56
Khanna et al showed that the wound site is rich in oxidants, which promote VEGF expression and thereby support wound healing. According to their study, resveratrol treatment enhanced the oxidising environment at the wound site and was also associated with increased VEGF and tenascin expression in the wound edge. 49 Another in vitro study supports these findings and showed that resveratrol induced oxidant‐induced VEGF expression in keratinocytes. 33 Data from another in vivo study also found angiogenetic properties of resveratrol following chronic topical administration. Improved vascularisation of the wound bed was attributed to the stimulation of the AMPK pathway, a key mediator of wound healing. 53
However, Bråkenhielm et al proposed that resveratrol might block both VEGF‐ and FGF‐receptor‐mediated angiogenic responses, inhibiting capillary endothelial cell growth, and therefore the antiangiogenetic effect would cause a delay in wound healing. 32 To clarify the exact mechanism on how resveratrol treatment affects angiogenesis, more studies using suitable in vitro and in vivo models are needed.
Resveratrol is associated with antibacterial and antifungal properties and showed immune‐stimulating effects in the wound site. 34 , 60 Shevelev et al showed that topical resveratrol treatment exhibited a significant antimicrobial efficacy against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans, which are discussed as significant pathogens in the context of non‐healing wounds. 60 Resveratrols' antimicrobial effects seemed to be even superior than some commercial antimicrobial (Levomecol) and antifungal (Clotrimazole) ointments. 60 Further in vitro studies showed that resveratrol directly inhibited microbial growth in dose dependently and therefore resveratrol might be a promising agent in fighting skin infections. 34
3.1. Non‐healing wounds
Resveratrol also showed promising potential for the treatment of diabetic ulcers. 66 Resveratrol prevented hyperglycemia‐induced endothelial dysfunction and angiogenic impairment by activating endothelial SIRT1 and promoting forkhead box O1 (FOXO1) degradation, following a de‐repression of c‐Myc expression. 40 Furthermore, resveratrol is involved in improved angiogenesis induced by glyoxalase 1 (GLO1) overexpression in the context of diabetic non‐healing wounds. GLO1 might support wound healing through multiple pathways, in addition to supporting inositol‐requiring enzyme 1 α (IRE1α) action. 57
4. ANTISCARRING PROPERTIES OF RESVERATROL
Pathological scar formation is a type of tissue fibrosis caused by excessive deposition of extracellular matrix (ECM) components such as collagen and an unbalance in fibroblasts proliferation and apoptosis. 42 These pathophysiological processes are mediated by transforming growth factor beta 1 (TGF‐β1). Through its profibrotic properties, TGF‐β1 is one of the key players in pathological scar formation. 43 , 46 Currently, there are several treatment options available, but due to the lack of permanent improvement or absence of therapy‐related side effects, pathological scars remain a major health problem, but there is increasing evidence that resveratrol exhibits antifibrotic properties. 42 , 43
On a cellular level, Zeng et al were able to show that resveratrol treatment in human hypertrophic scar‐derived fibroblasts (HSFBs) suppressed cell proliferation, which lead to cell cycle arrest and induced apoptosis in a dose‐ and time‐dependent manner. 42 Treatment with resveratrol for 24 hours achieved an antiproliferative effect through an induction of apoptosis. The effect was enhanced with longer resveratrol treatment duration and higher resveratrol concentration. In addition, resveratrol downregulated Type I and III procollagen mRNA expression in HSFBs, which resulted in a decreased collagen deposition. 42 Similar results were reported by Zhai et al and by Pang et al regarding the apoptosis induction by resveratrol in HSFB. 43 , 46
A small number of studies investigated the genes and signalling pathways involved in the proliferation of pathological scar fibroblasts. In this context, Zhai et al showed that a resveratrol‐associated suppression of cell proliferation and induction of apoptosis in HSFBs is caused by altering TGF‐β1/Smad signalling. 43 Here, a resveratrol treatment suppressed TGF‐β1 and Smad‐2,3,4 protein expression in HSFBs and thereby induced antifibrotic effects. 43 Pang et al demonstrated that microRNA‐4654 expression, which is involved in cell proliferation, cell differentiation, apoptosis, and autophagy regulation, was significantly downregulated in HSFBs compared to normal skin‐derived fibroblasts (NSFBs). Resveratrol treatment of HSFBs induced autophagy via downregulation of Ras homologue enriched in brain (RHEB) expression, a GTP‐binding protein and target gene of microRNA‐4654, through the upregulation of microRNA‐4654. 46 Besides RHEB, the mammalian target of rapamycin (mTOR) signalling pathway also seems to have an influence on the specific proliferation mechanism in pathological scar formation. 45 An enhanced expression of mTOR and its corresponding downstream target protein ribosomal protein S6 kinase (70S6K) was found by Tang et al in HSFBs in 2017 while no explicit expression was found in NSFBs. Resveratrol treatment of HSFBs decreased the expression of mTOR and 70S6K mRNA. 45 Similar results regarding the relationship between mTOR signalling pathway and resveratrol treatment were confirmed by Tang et al in 2020. In addition, further key factors of the mTOR signalling pathway such as PI3K and AKT were identified to be elevated in HSFBs. The expression of AKT in HSFBs was reduced by a resveratrol intervention. 72 The molecular mechanisms underlying microRNA‐4654, TGF‐β1/Smad and mTOR signalling pathway and the influence of resveratrol on their regulation, may be a new approach for novel potential therapeutic strategies in pathological scar formation.
In addition to the before mentioned potential molecular targets for prevention or therapy of pathological scar formations, the deacetylase SIRT 1 seems to play an important role as a drug target in fibrotic diseases. 44 Compared with NSFBs, a decreased expression of SIRT 1 in HSFBs was observed by Bai et al. As an agonist to SIRT 1, resveratrol downregulated mRNA levels of collagen I, collagen III, and α‐smooth muscle actin (α‐SMA) due to upregulation of SIRT1 in a dose‐dependent manner. Furthermore, resveratrol inhibited TGF‐β1‐associated trans‐differentiation of fibroblasts into myofibroblasts due to SIRT 1 upregulation, resulting in a decreased expression of fibrotic markers such as collagen I, collagen III, and α‐SMA. 44 The same authors confirmed the in vitro results in a mouse wound model with full‐thickness wounds. By using intradermal injection of a recombinant lentiviral vector for silencing SIRT 1 (SIRT1 shRNA), the authors blocked SIRT 1 expression in the mouse wound. Compared to the SIRT1 shRNA‐treated mice, resveratrol treatment led to a more organised and thinner collagen fibres. 44 The results revealed that SIRT1 upregulation with resveratrol has the potential to prevent in vivo pathological scar formation. A novel resveratrol‐loaded peptide‐hydrogel wound dressing showed similar results with regard to collagen deposition in an in vivo full‐thickness rat wound model. 63
Although resveratrol improved antifibrotic properties within different targets in a small number of in vitro and in vivo studies, these resveratrol‐associated effects still need to be confirmed in more animal models and, especially, in more clinical studies. Yet, no clinical studies could be found which investigated the antifibrotic effect of resveratrol.
5. PHOTOPROTECTIVE AND ANTI‐AGING PROPERTIES OF RESVERATROL
Numerous studies have repeatedly revealed the damaging effects of UV irradiation as a major cause of skin aging: Chronic UV exposure induces reactive oxygen species (ROS) production, activating three major skin aging cascades. These are the matrix metalloproteinase 1 (MMP‐1)‐mediated aging, inflammation‐induced aging (“inflammaging”) via interleukin 6 (IL6) and interleukin 8 (IL8), and/or apoptosis‐induced aging via, for example, nuclear factor‐kappa B (NF‐kB) or caspase 3. 47 , 48 Resveratrol has been widely used to prevent photo‐aging through anti‐inflammatory, antioxidant, and antitumour pathways. However, the specific mechanism underlying the protective effect on UV‐induced damage on a cellular level has not fully been elucidated yet. 47 , 48 Recently, Subedi et al were able to show a promising downregulation of the abovementioned pathways by resveratrol in in vitro irradiated cells. Resveratrol and resveratrol enriched rice (RR) downregulated UVB‐induced ROS production in normal human dermal fibroblast cells (NHDFs). As a result, MMP‐1 expression and transcription of inflammatory cytokines responsible for “inflammaging” were reduced while the expression of type I collagen was increased. 47
Similar results were reported by Zhou et al where a photoprotective effect of resveratrol was achieved via the upregulation of heat shock protein 27 (HSP27) expression. Further, resveratrol treatment showed antiapoptotic effects through inhibition of NF‐kB and caspase‐3 activation. Consequently, the viability of cultured human keratinocytes (HaCaT cell line) pretreated with different doses of resveratrol significantly increased up to 94%. 48
Results of prior in vitro studies were also transferable to following in vivo investigations. A recent in vivo study using mice revealed that oral or topical administration of resveratrol resulted in a suppression of UVB‐induced epidermal thickening. 65 On a cellular level, the expression of MMP‐1 and MMP‐9 was decreased through resveratrol treatment. 65 Activation of the intracellular Nrf2‐mediated antioxidant signalling pathway provides protection from UV‐induced cellular damage. 65
A further in vivo study in adult male Wistar rats by Abbas et al also indicated a photoprotective effect of topically applied resveratrol. To enhance the penetration through the epidermis, the authors used lipid nanoparticles as a drug carrier. The compritol ATO‐based resveratrol colloidal carrier CCC6 containing P407/P188 as bisurfactant attained the highest drug loading and release efficiency during a span of 24 hours. This binary mixture of surfactants even showed superior dermal photoprotection compared to the resveratrol suspension alone. 64
The potential of resveratrol containing binary systems was also investigated by Moyano‐Mendez et al, who showed a significant inhibition of ROS in vitro, similar to Subedi et al. 47 , 68 Resveratrol and a combination with β‐cyclodextrin (βCD) as a solubilising excipient were subsequently tested in eight women aged between 45 and 70 years, showing clear clinical signs of photo‐aging considered as loss of luminosity, hydration, and elasticity. After 30 days of daily application, a visible improvement of the skin condition with a remarkable decrease of aging signs in the hemi face treated with the βCD‐containing formula could be detected in all patients. 68
Yet, only a small number of further clinical studies investigated the effect of resveratrol in photo‐aged skin or its protection. A recent study by Farris et al reported significant improvement in several skin parameters after a daily usage of a night‐time topical antioxidant containing resveratrol, baicalin, and vitamin E for 12 weeks. Besides an induction of the Nrf2 pathway and a decrease of ROS, the authors were able to detect a significant increase in collagen type II alpha 1 gene expression. This finding was supported with a clinical improvement in elasticity and skin thickness as measured by ultrasound. 69
In 2019, Igielska‐Kalwat et al were not only able to verify tightening but also skin‐moisturising properties of resveratrol, in an observation of 20 volunteers during a period of 6 weeks. During the regular use, a 20% increase in skin hydration was detected after 2 weeks of treatment. The authors showed that resveratrol is able to replenish and modify the natural water‐lipid layer and intercellular cement in skin deeper layers, leading to an inhibition of transepidermal water loss (TEWL). 70
6. CLINICAL VALUE
Even though several studies elucidate the potential for therapeutic application of resveratrol, the fast metabolisation and excretion, is the main issue for its clinical use. Therefore, improving the bioavailability of resveratrol is the subject of extensive biotechnology research. Studies aim to develop innovative drug delivery systems for local and topical resveratrol application or improve its bioavailability during systemic intake. Regarding wound healing, a controlled and prolonged release directly at the target site would ensure the beneficial effects of resveratrol. 51 The combination of specific wound dressing properties and resveratrol even lead to synergistic beneficial effects. 39 , 54 , 59 , 67 , 73 Especially hydrogels with encapsulated resveratrol seem to provide a gradual release of resveratrol, show excellent biocompatibility, and appear promising as dressing materials to support wound healing. 38 , 58 , 63 A small number of studies examined the effects of resveratrol in combination with other agents for wound healing such as histatin, ferulic acid, or β‐sitosterol, and report promising results. However, beneficial effects cannot only be ascribed to resveratrol itself. 37 , 51 , 58 , 61
Nevertheless, the studies that were included in this review show promising results that resveratrol might be a feasible approach to support dermal wound healing, counteract excessive scarring, and even prevent photo‐aging of the skin.
7. CONCLUSION
Despite numerous beneficial effects and mechanisms of action of resveratrol that have been studied intensively, there is still room for improvement in the context of skin wound healing and photo‐aging. Our literature research yielded only 41 relevant original research articles that studied the effects, outcomes, or applicability of resveratrol in the treatment of acute or chronic wounds, hypertrophic scars, and skin aging. Interestingly, the experimental in vitro and in vivo studies that were discussed here highlighted that similar pathways are targeted by resveratrol treatment in wound healing, scarring, or the prevention of photo‐aging and are further summarised in Figure 2. Although there is little clinical evidence for the efficacy and applicability of resveratrol treatment, the studies summarised in Table 3 show promising results and underline the importance of more systematical clinical investigation. Nevertheless, resveratrol represents an interesting and promising novel therapy regime or could also be used as a supplementary treatment for classical treatment regimes. Therefore, more studies that investigate and improve the resveratrol skin pharmacokinetics and their clinical relevance are urgently needed.
FIGURE 2.
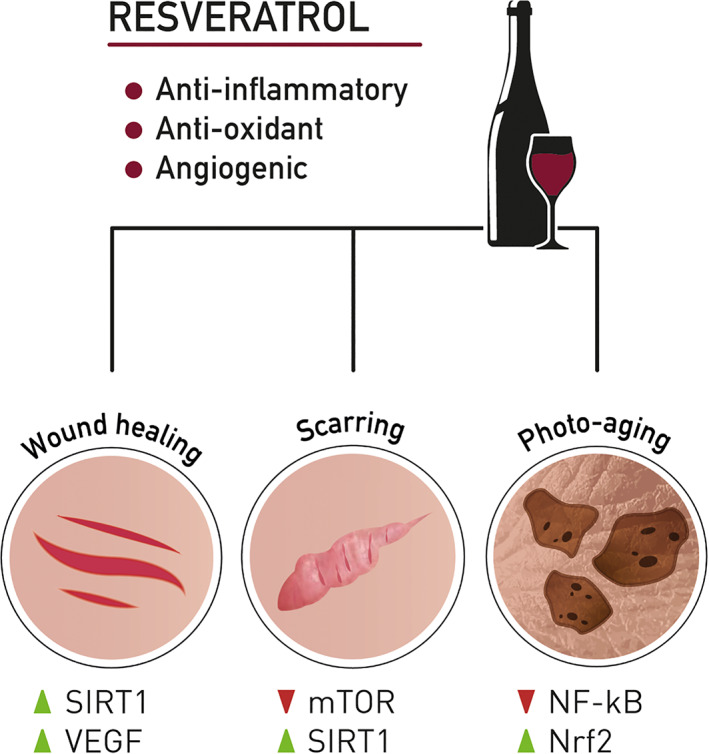
Resveratrol and its potential targets in wound healing, skin scarring, and photo‐aging
8. LIMITATIONS
In this review, some limitations occur that should be mentioned. Firstly, only studies that are published in the English language were investigated. Secondly, PubMed was the only database that was searched. These limitations leave the risk of studies being missed in this analysis. Five out of the 41 included studies investigated resveratrol within a clinical setting. These studies presented partially small cohorts, missing control groups that were judged as standard of care, and short or no follow‐up. The remaining 36 studies examined the effects of resveratrol in vivo and mostly in vitro studies. Hence, the results presented in this review offer limited insight in the context of clinical application.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualisation: AH, PK, EH, LPK; Methodology: AH, SPN; Investigation: AH, MS, EH, HL; Visualisation: HL; Formal Analysis: SPN, PK, LPK; Writing – Draft Preparation: AH, MS, HL, EH; Writing – Review & Editing: EH, PK, LPK; Supervision: PK, LPK. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
This research was funded by the Austrian Federal Ministry of Climate Action, Environment, Energy, Mobility, Innovation and Technology (BMK). We thank Attila Primus, MA, for conceptualisation and design of the graphics used in Figure 2 and Katharina Haslacher, BA, for proof reading.
Hecker A, Schellnegger M, Hofmann E, et al. The impact of resveratrol on skin wound healing, scarring, and aging. Int Wound J. 2022;19:9–28. 10.1111/iwj.13601
Funding information Austrian Federal Ministry of Climate Action, Environment, Energy, Mobility, Innovation and Technology (BMK).
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Salehi B, Mishra A, Nigam M, et al. Resveratrol: a double‐edged sword in health benefits. Biomedicine. 2018;6:91. 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burns J, Yokota T, Ashihara H, Lean MEJ, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337‐3340. 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 3. Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523‐1526. 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 4. Marques FZ, Markus MA, Morris BJ. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol. 2009;41:2125‐2128. 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 5. Malhotra A, Bath S, Elbarbry F. An organ system approach to explore the antioxidative, anti‐inflammatory, and cytoprotective actions of resveratrol. Oxid Med Cell Longev. 2015;2015:1‐15. 10.1155/2015/803971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konyalioglu S, Armagan G, Yalcin A, Atalayin C, Dagci T. Effects of resveratrol on hydrogen peroxide‐induced oxidative stress in embryonic neural stem cells. Neural Regen Res. 2013;8:485‐495. 10.3969/j.issn.1673-5374.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Means JC, Gerdes BC, Koulen P. Distinct mechanisms underlying resveratrol‐mediated protection from types of cellular stress in C6 glioma cells. Int J Mol Sci. 2017;18(7):1521. 10.3390/ijms18071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11(5):946. 10.3390/nu11050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zunino SJ, Hwang DH, Huang S, Storms DH. Resveratrol increases phagocytosis and lipopolysaccharide‐induced interleukin‐1β production, but decreases surface expression of toll‐like receptor 2 in THP‐1 monocytes. Cytokine. 2018;102:141‐144. 10.1016/j.cyto.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 10. Rahimifard M, Maqbool F, Moeini‐Nodeh S, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 2017;36:11‐19. 10.1016/j.arr.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 11. Saqib U, Faisal SM, Saluja R, Baig MS. Structural insights of resveratrol with its binding partners in the toll‐like receptor 4 pathway. J Cell Biochem. 2019;120:452‐460. 10.1002/jcb.27401. [DOI] [PubMed] [Google Scholar]
- 12. Jakus PB, Kalman N, Antus C, et al. TRAF6 is functional in inhibition of TLR4‐mediated NF‐κB activation by resveratrol. J Nutr Biochem. 2013;24:819‐823. 10.1016/j.jnutbio.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 13. Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88‐independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339‐3346. 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 14. Lai CY, Su YW, Lin KI, Hsu LC, Chuang TH. Natural modulators of endosomal toll‐like receptor‐mediated psoriatic skin inflammation. J Immunol res. 2017;2017:1‐15. 10.1155/2017/7807313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kjær TN, Thorsen K, Jessen N, Stenderup K, Pedersen SB. Resveratrol ameliorates imiquimod‐induced psoriasis‐like skin inflammation in mice. PLoS One. 2015;10:e0126599. 10.1371/journal.pone.0126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zykova TA, Zhu F, Zhai X, et al. Resveratrol directly targets COX‐2 to inhibit carcinogenesis. Mol Carcinog. 2008;47:797‐805. 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Varoni EM, Lo Faro AF, Sharifi‐Rad J, Iriti M. Anticancer molecular mechanisms of resveratrol. Frontiers in Nutrition. 2016;3: 8. 10.3389/fnut.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev res. 2009;2:409‐418. 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 19. Rimando AM, Suh N. Biological/chemopreventive activity of stilbenes and their effect on colon cancer. Planta Med. 2008;74:1635‐1643. 10.1055/s-0028-1088301. [DOI] [PubMed] [Google Scholar]
- 20. van Ginkel PR, Sareen D, Subramanian L, et al. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin Cancer Res. 2007;13:5162‐5169. 10.1158/1078-0432.CCR-07-0347. [DOI] [PubMed] [Google Scholar]
- 21. Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243‐261. 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 22. Baier A, Szyszka R. Compounds from natural sources as protein kinase inhibitors. Biomolecules. 2020;10:1‐30. 10.3390/biom10111546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1‐8. 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- 24. Chaplin A, Carpéné C, Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018;10(11):1651. 10.3390/nu10111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haghighatdoost F, Hariri M. Effect of resveratrol on lipid profile: an updated systematic review and meta‐analysis on randomized clinical trials. Pharmacol res. 2018;129:141‐150. 10.1016/j.phrs.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 26. Lagouge M, Argmann C, Gerhart‐Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC‐1α. Cell. 2006;127:1109‐1122. 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Ma W, Zhang P, He S, Huang D. Effect of resveratrol on blood pressure: a meta‐analysis of randomized controlled trials. Clin Nutr. 2015;34:27‐34. 10.1016/j.clnu.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 28. Zhu X, Wu C, Qiu S, Yuan X, Li L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta‐analysis. Nutr Metab. 2017;14:60. 10.1186/s12986-017-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high‐calorie diet. Nature. 2006;444:337‐342. 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Springer M, Moco S. Resveratrol and its human metabolites—effects on metabolic health and obesity. Nutrients. 2019;11(1):143. 10.3390/nu11010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Timmers S, Konings E, Bilet L, et al. Calorie restriction‐like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612‐622. 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brâkenhielm E, Cao R, Cao Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001;15:1798‐1800. 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]
- 33. Khanna S, Roy S, Bagchi D, Bagchi M, Sen CK. Upregulation of oxidant‐induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic Biol Med. 2001;31:38‐42. 10.1016/S0891-5849(01)00544-5. [DOI] [PubMed] [Google Scholar]
- 34. Chan MMY. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem Pharmacol. 2002;63:99‐104. 10.1016/S0006-2952(01)00886-3. [DOI] [PubMed] [Google Scholar]
- 35. Pastore S, Lulli D, Fidanza P, et al. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxidants Redox Signal. 2012;16:317‐328. 10.1089/ars.2011.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eroğlu I, Gökçe EH, Tsapis N, et al. Evaluation of characteristics and in vitro antioxidant properties of RSV loaded hyaluronic acid‐DPPC microparticles as a wound healing system. Colloids Surfaces B Biointerfaces. 2015;126:50‐57. 10.1016/j.colsurfb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 37. Abbas M, Al‐Rawi N, Abbas M, Al‐Khateeb I. Naringenin potentiated β‐sitosterol healing effect on the scratch wound assay. Res Pharm Sci. 2019;14:566‐573. 10.4103/1735-5362.272565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Comotto M, Saghazadeh S, Bagherifard S, et al. Breathable hydrogel dressings containing natural antioxidants for management of skin disorders. J Biomater Appl. 2019;33:1265‐1276. 10.1177/0885328218816526. [DOI] [PubMed] [Google Scholar]
- 39. Meng E, Chen CL, Liu CC, et al. Bioapplications of bacterial cellulose polymers conjugated with resveratrol for epithelial defect regeneration. Polymers (Basel). 2019;11(6):1048. 10.3390/polym11061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang X, Sun J, Chen G, et al. Resveratrol promotes diabetic wound healing via SIRT1‐FOXO1‐c‐Myc signaling pathway‐mediated angiogenesis. Front Pharmacol. 2019;10:421. 10.3389/fphar.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaleci B, Koyuturk M. Efficacy of resveratrol in the wound healing process by reducing oxidative stress and promoting fibroblast cell proliferation and migration. Dermatol Ther. 2020;33:e14357. 10.1111/dth.14357. [DOI] [PubMed] [Google Scholar]
- 42. Zeng G, Zhong F, Li J, Luo S, Zhang P. Resveratrol‐mediated reduction of collagen by inhibiting proliferationand producing apoptosis in human hypertrophic scar fibroblasts. Biosci Biotechnol Biochem. 2013;77:2389‐2396. 10.1271/bbb.130502. [DOI] [PubMed] [Google Scholar]
- 43. Zhai X, Ding J, Tang Z. Resveratrol inhibits proliferation and induces apoptosis of pathological scar fibroblasts through the mechanism involving TGF‐β1/Smads signaling pathway. Cell Biochem Biophys. 2015;71:1267‐1272. 10.1007/s12013-014-0317-6. [DOI] [PubMed] [Google Scholar]
- 44. Bai XZ, Liu JQ, Yang LL, et al. Identification of sirtuin 1 as a promising therapeutic target for hypertrophic scars. Br J Pharmacol. 2016;173:1589‐1601. 10.1111/bph.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang Z‐M, Zhai X‐X, Ding J‐C. Expression of mTOR/70S6K signaling pathway in pathological scar fibroblasts and the effects of resveratrol intervention. Mol Med Rep. 2017;15:2546‐2550. 10.3892/mmr.2017.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pang K, Li B, Tang Z, et al. Resveratrol inhibits hypertrophic scars formation by activating autophagy via the miR‐4654/Rheb axis. Mol Med Rep. 2020;22:3440‐3452. 10.3892/mmr.2020.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Subedi L, Lee TH, Wahedi HM, Baek S‐H, Kim SY. Resveratrol‐enriched Rice attenuates UVB‐ROS‐induced skin aging via downregulation of inflammatory cascades. Oxid Med Cell Longev. 2017;2017:1‐15. 10.1155/2017/8379539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou F, Huang X, Pan Y, et al. Resveratrol protects HaCaT cells from ultraviolet B‐induced photoaging via upregulation of HSP27 and modulation of mitochondrial caspase‐dependent apoptotic pathway. Biochem Biophys res Commun. 2018;499:662‐668. 10.1016/j.bbrc.2018.03.207. [DOI] [PubMed] [Google Scholar]
- 49. Khanna S, Venojarvi M, Roy S, et al. Dermal wound healing properties of redox‐active grape seed proanthocyanidins. Free Radic Biol Med. 2002;33:1089‐1096. 10.1016/S0891-5849(02)00999-1. [DOI] [PubMed] [Google Scholar]
- 50. Lin LX, Wang P, Wang YT, Huang Y, Jiang L, Wang XM. Aloe vera and Vitis vinifera improve wound healing in an in vivo rat burn wound model. Mol Med Rep. 2016;13:1070‐1076. 10.3892/mmr.2015.4681. [DOI] [PubMed] [Google Scholar]
- 51. Poornima B, Korrapati PS. Fabrication of chitosan‐polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr Polym. 2017;157:1741‐1749. 10.1016/j.carbpol.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 52. Gokce EH, Tuncay Tanrıverdi S, Eroglu I, et al. Wound healing effects of collagen‐laminin dermal matrix impregnated with resveratrol loaded hyaluronic acid‐DPPC microparticles in diabetic rats. Eur J Pharm Biopharm. 2017;119:17‐27. 10.1016/j.ejpb.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 53. Zhao P, Sui BD, Liu N, et al. Anti‐aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application. Aging Cell. 2017;16:1083‐1093. 10.1111/acel.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berce C, Muresan MS, Soritau O, et al. Cutaneous wound healing using polymeric surgical dressings based on chitosan, sodium hyaluronate and resveratrol. A preclinical experimental study. Colloids Surfaces B Biointerfaces. 2018;163:155‐166. 10.1016/j.colsurfb.2017.12.041. [DOI] [PubMed] [Google Scholar]
- 55. Lakshmanan R, Campbell J, Ukani G, et al. Evaluation of dermal tissue regeneration using resveratrol loaded fibrous matrix in a preclinical mouse model of full‐thickness ischemic wound. Int J Pharm. 2019;558:177‐186. 10.1016/j.ijpharm.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 56. Christovam AC, Theodoro V, Mendonça FAS, Esquisatto MAM, dos Santos GMT, do Amaral MEC. Activators of SIRT1 in wound repair: an animal model study. Arch Dermatol res. 2019;311:193‐201. 10.1007/s00403-019-01901-4. [DOI] [PubMed] [Google Scholar]
- 57. Li H, O'Meara M, Zhang X, et al. Ameliorating methylglyoxal‐induced progenitor cell dysfunction for tissue repair in diabetes. Diabetes. 2019;68:1287‐1302. 10.2337/db18-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ávila‐Salas F, Marican A, Pinochet S, et al. Film dressings based on hydrogels: simultaneous and sustained‐release of bioactive compounds with wound healing properties. Pharmaceutics. 2019;11(9):447. 10.3390/pharmaceutics11090447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Orlowski P, Zmigrodzka M, Tomaszewska E, et al. Polyphenol‐conjugated bimetallic au@agnps for improved wound healing. Int J Nanomedicine. 2020;15:4969‐4990. 10.2147/IJN.S252027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shevelev AB, la Porta N, Isakova EP, et al. In vivo antimicrobial and wound‐healing activity of resveratrol, dihydroquercetin, and dihydromyricetin against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans . Pathogens. 2020;9(4):296. 10.3390/pathogens9040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zheng Y, Yuan W, Liu H, Huang S, Bian L, Guo R. Injectable supramolecular gelatin hydrogel loading of resveratrol and histatin‐1 for burn wound therapy. Biomater Sci. 2020;8:4810‐4820. 10.1039/d0bm00391c. [DOI] [PubMed] [Google Scholar]
- 62. Bilgic T. Comparison of the effect of local and systemic injection of resveratrol on cutaneous wound healing in rats. Int J Low Extrem Wounds. 2021;20(1):55‐59. 10.1177/1534734620938168. [DOI] [PubMed] [Google Scholar]
- 63. Zhao C‐C, Zhu L, Wu Z, Yang R, Xu N, Liang L. Resveratrol‐loaded peptide‐hydrogels inhibit scar formation in wound healing through suppressing inflammation. Regen Biomater. 2020;7:99‐107. 10.1093/rb/rbz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Abbas H, Kamel R, El‐Sayed N. Dermal anti‐oxidant, anti‐inflammatory and anti‐aging effects of Compritol ATO‐based resveratrol colloidal carriers prepared using mixed surfactants. Int J Pharm. 2018;541:37‐47. 10.1016/j.ijpharm.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 65. Kim J, Oh J, Averilla JN, Kim HJ, Kim J, Kim J. Grape peel extract and resveratrol inhibit wrinkle formation in mice model through activation of Nrf2/HO‐1 signaling pathway. J Food Sci. 2019;84:1600‐1608. 10.1111/1750-3841.14643. [DOI] [PubMed] [Google Scholar]
- 66. Bashmakov YK, Assaad‐Khalil SH, Abou Seif M, et al. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014;2014:1‐8. 10.1155/2014/816307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Çetinkalp Ş, Gökçe EH, Şimşir IY, et al. Comparative evaluation of clinical efficacy and safety of collagen laminin–based dermal matrix combined with resveratrol microparticles (Dermalix) and standard wound Care for Diabetic Foot Ulcers. Int J Low Extrem Wounds. 2020;1534734620907773. 10.1177/1534734620907773. [DOI] [PubMed] [Google Scholar]
- 68. Moyano‐Mendez JR, Fabbrocini G, De Stefano D, et al. Enhanced antioxidant effect of trans ‐resveratrol: potential of binary systems with polyethylene glycol and cyclodextrin. Drug Dev Ind Pharm. 2014;40:1300‐1307. 10.3109/03639045.2013.817416. [DOI] [PubMed] [Google Scholar]
- 69. Farris P, Yatskayer M, Chen N, Krol Y, Oresajo C. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. J Drugs Dermatol. 2014;13:1467‐1472. [PubMed] [Google Scholar]
- 70. Igielska‐Kalwat J, Firlej M, Lewandowska A, Biedziak B. In vivo studies of resveratrol contained in cosmetic emulsions. Acta Biochim Pol. 2019. 66(3):371–374. 10.18388/abp.2019_2838. [DOI] [PubMed] [Google Scholar]
- 71. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34:599‐610. 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang Z, Ding J‐C, Zhai X‐X. Effects of resveratrol on the expression of molecules related to the mTOR signaling pathway in pathological scar fibroblasts. G Ital Dermatol Venereol. 2020;155(2):161–167. 10.23736/S0392-0488.17.05556-0. [DOI] [PubMed] [Google Scholar]
- 73. Amanat S, Taymouri S, Varshosaz J, Minaiyan M, Talebi A. Carboxymethyl cellulose‐based wafer enriched with resveratrol‐loaded nanoparticles for enhanced wound healing. Drug Deliv Transl res. 2020;10:1241‐1254. 10.1007/s13346-020-00711-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


