Abstract
Clinical application of skin substitute is typically a two‐stage procedure with application of skin substitute matrix to the wound followed by engraftment of a split‐thickness skin graft (STSG). This two‐stage procedure requires multiple interventions, increasing the time until the wound is epithelialised. In this study, the feasibility of a one‐stage procedure by combining bioengineered collagen‐chondroitin‐6‐sulfate (DS1) or decellularised fetal bovine skin substitute (DS2) with autologous skin cell suspension (ASCS) in a porcine full‐thickness wound healing model was evaluated. Twelve full‐thickness excisional wounds on the backs of pigs received one of six different treatments: empty; ASCS; DS1 with or without ASCS; DS2 with or without ASCS. The ASCS was prepared using a point‐of‐care device and was seeded onto the bottom side of DS1, DS2, and empty wounds at 80 000 cells/cm2. Wound measurements and photographs were taken on days 0, 9, 14, 21, 28, 35, and 42 post‐wounding. Histological analysis was performed on samples obtained on days 9, 14, 28, and 42. Wounds in the empty group or with ASCS alone showed increased wound contraction, fibrosis, and myofibroblast density compared with other treatment groups. The addition of ASCS to DS1 or DS2 resulted in a marked increase in re‐epithelialisation of wounds at 14 days, from 15 ± 11% to 71 ± 20% (DS1 vs DS1 + ASCS) or 28 ± 14% to 77 ± 26 (DS2 vs DS2 + ASCS) despite different mechanisms of tissue regeneration employed by the DS used. These results suggest that this approach may be a viable one‐stage treatment in clinical practice.
Keywords: autologous skin cell suspension, porcine, skin reconstruction, skin substitutes, wound healing
1. INTRODUCTION
Severe burn injuries are traumatic and physically debilitating leading to significant morbidity and mortality. Excision of nonviable tissue followed by autologous split‐thickness skin grafting (STSG) remains the gold standard treatment. Clinical results have demonstrated the efficacy of skin grafting in hastening the wound healing process for rapid closure. 1 The use of an autologous STSG alone is associated with wound contracture and is limited by donor site availability in large total body surface area (TBSA) burns. Widely expanded mesh grafts are then used in large TBSA burns to conserve skin, resulting in worse functional and aesthetic outcomes. The donor sites are often harvested multiple times to cover large wounds. This process is associated with significant pain, donor site scarring, chronic wounds, and increased risk of donor site infection. 2 Recently, the availability of an autologous skin cell suspension (ASCS) has been approved by the FDA and is commercially available for use to minimise grafting requirements and morbidity associated with donor site harvesting for both small and large burns (up to 50% TBSA). 3 ASCS is indicated for direct application to acute partial‐thickness thermal burn wounds or application combined with meshed autografting for acute full‐thickness thermal burn wounds.
Reconstruction of the damaged or missing dermis is essential to restore functional skin with less contracture and improve aesthetic outcomes. Skin substitutes (DS) have been developed to address some of these shortcomings in skin reconstruction procedures. They are typically composed of extracellular matrix (ECM) that provide wound coverage, prevent fluid loss while allowing cellular infiltration, and undergo progressive remodelling to restore functional tissue. ECM‐based DS differ in the source species, tissue origin, manufacturing processing, and sterilisation process, and these attributes affect DS in vivo biocompatibility and result in wound healing. 4 ECM‐based DS provides a natural three‐dimensional structure, including the diverse composition of functional ECM proteins and growth factors for repairing damaged or missing dermal components. 1 , 4 There are many ECM‐based DS options available commercially, such as allografts, xenografts, and bioengineered alternatives. 1 , 5
The development of DS has fundamentally changed the management of cutaneous wounds resulting from burn, trauma, or surgery. In a two‐stage procedure, an avascular DS is first placed in the wound bed to incorporate and vascularise before being covered with STSG to obtain definitive closure. This technique has gained wide acceptance to provide durable, definitive coverage of wounds large and small, including those with exposed bone or tendon. 1 , 6 Although the two‐stage procedure is successful, significant drawbacks remain, including the need for two or more operations, increased time to wound closure, STSG donor site morbidity, and limited donor site availability for large TBSA injuries. 2
Consequently, the application of ASCS to a DS in a one‐stage procedure is hypothesised to reduce the number of operative procedures, maximise the limited skin graft expansion ratio, and lower healthcare expenditures. Previously, Wood et al demonstrated the feasibility of combining bioengineered collagen‐chondroitin‐6‐sulfate (C6S) DS with non‐cultured ASCS obtained using an autologous cell harvesting device (ACHD) and then implanting in porcine full‐thickness wounds as a one‐stage procedure. 7 This pilot study was conducted with two animals over 21 days and utilised only bioengineered collagen‐C6S DS. Other studies have attempted to develop a simplified one‐stage procedure. Still, most of the approaches used are difficult to translate into clinical application due to lengthy and complicated processes involving centrifugation of cells into DS or culturing cells before implantation. 8 , 9 , 10 , 11 In this study, the feasibility of a one‐stage procedure was further evaluated by directly combining bioengineered collagen‐C6S DS or decellularised fetal bovine DS with ASCS obtained using ACHD. Additionally, bioengineered collagen‐C6S DS and decellularised fetal bovine DS were compared for dermal and epidermal reconstruction procedures in a porcine full‐thickness wound healing model.
2. MATERIALS AND METHODS
2.1. Porcine model of full‐thickness wounds
This study was conducted according to a research proposal approved by the Wake Forest Institutional Animal Care and Use Committee (IACUC). Eleven female Yorkshire domestic swine, weighing approximately 43 to 52 kg each, were assigned to the study and kept under standard conditions in the animal care unit in Wake Forest Innovations of the Wake Forest University School of Medicine. On day 0, each animal was administered glycopyrrolate (0.01 mg/kg IM), buprenorphine (0.03 mg/kg IM), and Telazol (4 mg/kg IM). Sevoflurane delivered by facemask was used to supplement the injectable anaesthetic. According to a standardised template, the corners and numbers of 12 wound sites were tattooed on each animal, as shown in Figure 1. Wounds were 4 cm × 4 cm in size and spaced at least 4 cm from the spine and approximately 3 cm apart. The surgical areas were scrubbed with chlorhexidine diacetate, followed by 70% isopropyl alcohol, and allowed to dry. According to the tattooed template, the skin was excised down to fat with a #10 surgical scalpel. Haemostasis was achieved with pressure and minimal electrocautery.
FIGURE 1.
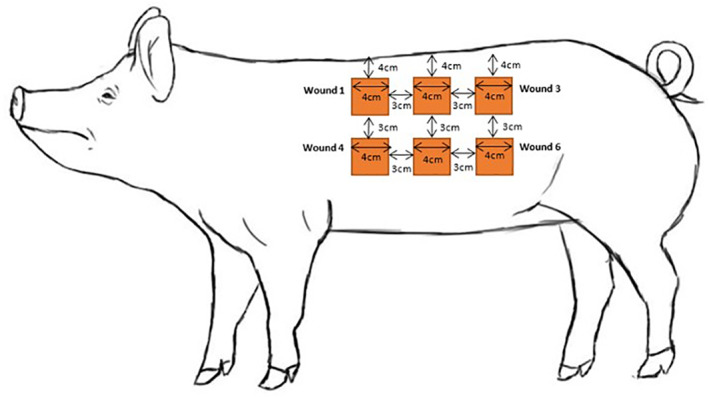
Wound positioning diagram
2.2. ASCS preparation
A 12 cm2 skin sample (3 cm × 4 cm) was harvested using a dermatome (Padgett Model S, Integra LifeSciences, Princeton, NJ) with a 2‐in. cover plate set at ten‐thousandths of an inch over the gluteal muscles. The skin samples were then processed using ACHD (RECELL Autologous Cell Harvesting Device, AVITA Medical) following the manufacturer's instructions. Briefly, the biopsy was incubated in a heated enzyme solution for ~20 minutes. The biopsy was then rinsed to remove residual enzymes and gently scraped until complete skin disintegration. The resulting cell suspension was combined with buffer and filtered with a cell strainer to achieve the final cell suspension of ~3 to 4 mL. The cell suspension was kept on ice for no more than 2 hours until implantation per animal. Cell counts were performed using a microscope, haemocytometer, and manual cell counter clicker. The average total cell number harvested per skin biopsy was ~1.2 × 107 cells. The final cell suspension volume was adjusted to achieve 3 × 106 cells/mL and kept on ice until implantation.
2.3. Experimental groups and implantation
Animals were assigned to two cohorts as described in Figure 2A. Six different treatment groups were evaluated (Figure 2B): empty, ASCS alone, bioengineered collagen‐C6S DS (DS1, Integra Meshed Dermal Regeneration Template, Integra LifeSciences, Corp, Princeton, NJ), and decellularised fetal bovine DS (DS2; PriMatrix Meshed, Integra LifeSciences, Corp, Princeton, NJ), DS1 + ASCS, and DS2 + ASCS. For the ASCS group, ASCS was applied dropwise on the wound bed (~8 × 104 cells per cm2 in each wound). For DS + ASCS groups, the same volume of ASCS was applied dropwise to the underside of DS1 or DS2 before implantation. DS was secured at the wound edges with simple interrupted 3‐0 nylon sutures (Ethicon). The dressings for all treatment groups consisted of a tie‐over bolster with non‐adherent Telfa Clear (Covidien, Medtronic, Minneapolis, MN) closest to the wound bed and covered with gauze sandwiched by two layers of Optifoam (MedLine, Northfield, IL) (Figure 2C). For wounds receiving ASCS, the Telfa was immediately secured to the wound site with care being taken to ensure the suspension did not leave the wound area. The surgical wounds were covered with absorbent pads and secured with tape.
FIGURE 2.

Study design (A) where biopsies were taken on day 9 (cohort 1) and day 28 (cohort 2). The five pigs were sacrificed on day 14, and 6 pigs were sacrificed on day 42. Six different treatment groups were applied to the pig (B). Bolster dressings scheme (C) was applied on each wound after treatment application
2.4. Digital photographs and wound measurements
Digital photographs were obtained at days 0, 9, 14, 21, 28, 35, and 42. Pictures of each wound were taken with a greyscale for calibration, and a centimetre‐scale placed parallel to the wound site. Images included the tattooed wound number and a photograph label with the following information, at a minimum: study number, animal number, study day, and date. The wound perimeter was traced and evaluated via digital planimetry using Image‐Pro Plus 7 (Media Cybernetics, Rockville, MD). 12 Wound margins were defined by the edge of epithelial growth or extension around the perimeter of each wound as assessed by colour and texture of the tissue.
2.5. Histological analysis
Wound biopsies and wound bed excision were performed as outlined in Figure 2A. Each biopsy was fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned via microtome. Haematoxylin and eosin (H&E), alpha smooth muscle actin (αSMA), Herovici, and CD31 immunostaining were used in the analysis. Histological assessment and scoring of granulation tissue, degree of epithelisation, collagen deposition, inflammation, and vascularisation were performed by an independent board‐certified veterinary pathologist blinded to the study design and treatment groups. 13 , 14
2.5.1. Inflammation analysis
Samples were stained with H&E and demarcated zonally by superficial (epidermal and superficial dermis), middle/deep (dermal bed) for evaluation, and proximal to the test skin substitute wherever applicable. Inflammation scoring was based on counts and inflammatory cell counts, including neutrophils, eosinophils, lymphocytes, plasma cells, macrophages, and multinucleated giant cells in each zone. Scoring ranged from 0 to 4: 0—no inflammation, 1—minimal inflammation, 2—mild inflammation, 3—moderate inflammation, and 4—marked inflammation.
2.5.2. Epithelialisation
Excised sections were evaluated for re‐epithelialisation via histomorphometry analysis. H&E‐stained tissue strips excised along the midline of each wound enabled visualisation of the entire bed length. The zone of the histologically identifiable epithelium was recorded as a percentage of the initial wound bed length corresponding to the orientation of the histological sample.
2.5.3. Epithelial hyperplasia
H&E stained samples were evaluated for epithelial hyperplasia throughout the wound bed by identifying the degree of epithelial cell and structure presence within the skin layer. Scores ranged from 0 to 4: 0—absent, 1—minimal, 2—mild, 3—moderate, and 4—marked.
2.5.4. Collagen maturation
Tissue proximal to DS1 or DS2 was stained with Herovici and evaluated for collagen maturation. Herovici staining enables differentiation between immature and mature collagen via colorimetric changes. Immature collagen and reticulum are stained blue, and mature collagen is stained red. The intensity of red staining was characterised on a scale from 0 to 4: 0—red staining absent, 1—minimal red staining, 2—mild red staining, 3—moderate red staining, and 4—marked red staining.
2.5.5. Vascular density
The vascular density was quantified using morphometric analysis of calibrated images of all wound sections using Image‐Pro Plus 7. CD31 stain (an endothelial marker) was used to visually identify the blood vessels within each treatment group. Before analysis, digital images of CD31 stained slides were marked by an independent pathologist to delineate the region of interest (ROI). The blood vessels were then counted per ROI (mm2). Briefly, digital image contrast was enhanced using image editing software (photoshop) to enable identification of a region of interest (ROI, mm2) corresponding to the areas of dense CD31 signal. Automated area measurements were then made of the ROI's, which a blinded reviewer verified. The vascular density was then calculated by dividing the total number of CD31‐positive vessels by the area of ROI (vessels/mm2).
2.5.6. Myofibroblasts
Myofibroblasts were identified with αSMA stain. The presence and zonal location of myofibroblasts were scored according to the scoring outlined in Table 1.
TABLE 1.
Myofibroblast density scoring rubric
| Score | Description |
|---|---|
| 0 | No myofibroblast activity |
| 1 | Minimal myofibroblasts, mainly located in deepest aspects of the wound bed |
| 2 | Mild myofibroblasts located within some levels of wound bed but lacking in some areas or in areas of dermal substitute |
| 3 | Moderate myofibroblasts located within deep, middle, and superficial wound bed with few areas lacking myofibroblasts |
| 4 | Marked myofibroblasts located throughout the wound bed |
2.6. Statistical analysis
Statistical analyses were conducted using Graphpad Prism 8.4.3 (San Diego, CA). The ordinal data sets, including 0 to 4 histological grading scales, were analysed using a non‐parametric test, Kruskal‐Wallis, to determine statistical significance, followed by the Dunn test for post hoc multiple comparisons. The data sets, including continuous variables, including length‐scale data, were tested for normality (Shapiro‐Wilk test) followed by Levene's equal variance test. Statistical significance was determined using one‐way analysis of variance followed by post hoc multiple comparisons using Tukey's test. The P < .05 was considered statistically significant.
3. RESULTS
The gross wound photographs for groups with skin substitutes qualitatively demonstrated less wound contracture when compared to empty and ASCS (Figure 3). At day 0, 4 cm × 4 cm full‐thickness wounds measured on average 20 cm2 following the relaxation of the tissue typical for dorsal porcine full‐thickness defects. The planimetry wound area measurements at each time point were then normalised to day 0 and shown as normalised wound area in Figure 4A. On day 9, the average wound area of the empty and ASCS treatment groups was significantly smaller than all DS groups (P < .001) (Figure 4B). By day 14 (Figure 4C), DS1 and DS1 + ASCS had significantly larger wound area compared to other groups (P < .05). From 21 days through the duration of the study, the wound area significantly decreased for all treatment groups and had no significant differences between treatments.
FIGURE 3.

Gross wounds pictures over the study duration for each treatment at days 0 (before treatment application), 9, 14, 28, and 42
FIGURE 4.
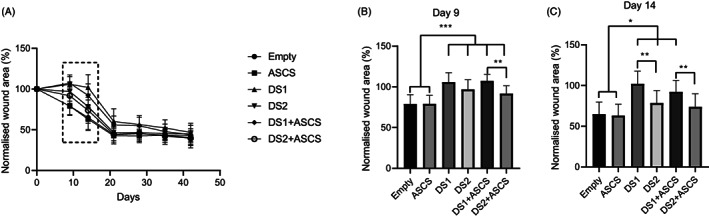
Percentage of normalised wound area to day 0 (n = 24), 9 (n = 24), 14 (n = 24), 21 (n = 14), 28 (n = 14), 35 (n = 14) and 42 (n = 14) for all treatments (A). Results shown as mean ± SD. Statistical analysis performed on day 9 (B) where **P < .01 and ***P < .001 and day 14 (C) where *P < .05, **P < .01 for normalised wound area
3.1. Histological observations
3.1.1. Epithelialisation of wound bed
The percentage of re‐epithelialisation was quantified histomorphometrically and plotted for day 14 (Figure 5A), and representative H&E‐stained histology micrographs for days 9, 14, 28, and 42 are demonstrated in Figure 5B. Epithelialisation increased over time in all treatments. On day 14, empty, ASCS, and DS2 had comparable mean epithelialisation, but DS1 had the lowest mean epithelialisation. Notably, the wounds treated with DS1 + ASCS (71 ± 20%) and DS2 + ASCS (77 ± 26%) demonstrated significant increase in epithelialisation (P < .01) compared with DS1 (15 ± 11%) or DS2 (28 ± 14%) at day 14. By 42 days (Table S1), epithelialisation was nearly complete and comparable between the empty, ASCS, DS2, and DS2 + ASCS treatments, with DS2 + ASCS having the greatest average percent epithelialisation at 42 days (99%). In comparison, DS1 and DS1 + ASCS treatments had slightly decreased epithelialisation percentages (81% and 88%, respectively) at 42 days.
FIGURE 5.

Percent epithelialisation was quantified histomorphometrically and plotted for day 14 (A) as mean ± SD (n = 10, **P < .01). Representative histology micrographs from the centre of each wound (B) demonstrating re‐epithelialisation for each treatment at days 9, 14, 28, and 42. Scale bars = 100 μm
3.1.2. Epithelial/squamous hyperplasia
Epithelial/squamous hyperplasia was histologically scored and summarised in Table 2. Higher epithelial/squamous hyperplasia occurred in the DS1 + ASCS treatment at 9 (mean score = 3.2 ± 1.8) and 14 (mean score = 3.9 ± 0.3) days and was statistically significant when compared with DS2 + ASCS (day 14 = 0.9 ± 0.6; P < .01) at day 14. The epithelial hyperplasia occurred as large islands and nests of epithelial cells at days 9 and 14 that extended from the superficial dermis into the mid‐dermis and tracked adjacent to DS1 (Figure 6C). A similar, though much less severe, finding occurred in the DS2 + ASCS group at 9 and 14 days, with a full resolution by 28 days. Thus, epithelial hyperplasia within the wound bed was associated with the presence of DS1/DS2 + ASCS treatment. Empty, ASCS, DS1, and DS2 had low levels of epithelial hyperplasia occurring on the edges of the wound bed only at 9 days, which completely resolved by 42 days.
TABLE 2.
Histological observations
| Treatments | 9 days (n = 5) | 14 days (n = 10) | 28 days (n = 6) | 42 days (n = 12) |
|---|---|---|---|---|
| Epithelial hyperplasia | ||||
| Empty | N/A | N/A | N/A | N/A |
| ASCS | N/A | N/A | N/A | N/A |
| DS1 | N/A | 0.1 ± 0.3 | N/A | N/A |
| DS1 + ASCS | 3.2 ± 1.8 | 3.9 ± 0.32 a | 0.7 ± 0.8 | N/A |
| DS2 | N/A | N/A | N/A | N/A |
| DS2 + ASCS | 1.2 ± 1.3 | 0.9 ± 0.60 | N/A | N/A |
| Inflammation | ||||
| Empty | 1.8 ± 0.4 | 2.2 ± 0.4 | 1.3 ± 0.5 | 1.3 ± 0.5 |
| ASCS | 1.6 ± 0.5 | 2.1 ± 0.3 | 1.0 ± 0.0 | 1.1 ± 0.3 |
| DS1 | 2.2 ± 0.4 | 2.9 ± 0.3 b | 1.7 ± 0.5 | 1.4 ± 0.5 |
| DS1 + ASCS | 1.4 ± 0.5 | 2.3 ± 0.7 | 2.2 ± 0.8 | 1.3 ± 0.8 |
| DS2 | 2.8 ± 0.4 | 2.5 ± 0.5 | 1.7 ± 0.5 | 1.2 ± 0.4 |
| DS2 + ASCS | 2.4 ± 0.5 | 1.7 ± 0.7 | 1.3 ± 0.6 | 1.0 ± 0.0 |
| Multinucleated giant cells | ||||
| Empty | 0.6 ± 0.5 | 0.8 ± 0.4 | 0.5 ± 0.5 | 0.8 ± 0.4 |
| ASCS | 0.2 ± 0.4 | 0.8 ± 0.4 | 0.5 ± 0.5 | 0.7 ± 0.5 |
| DS1 | 1.0 ± 0.7 | 1.9 ± 0.3 c | 2.0 ± 0.9 | 1.9 ± 0.7 e |
| DS1 + ASCS | 0.8 ± 0.4 | 1.8 ± 0.4 c | 2.3 ± 0.8 d | 1.4 ± 0.5 f |
| DS2 | 0.4 ± 0.5 | 0.9 ± 0.3 | 0.8 ± 0.8 | 1.0 ± 0.0 |
| DS2 + ASCS | 0.6 ± 0.5 | 0.9 ± 0.3 | 0.7 ± 0.5 | 0.8 ± 0.4 |
| Collagen maturation | ||||
| Empty | 2.0 ± 0.0 | 2.5 ± 0.7 | 3.5 ± 0.5 | 3.8 ± 0.4 |
| ASCS | 1.6 ± 0.5 | 2.6 ± 0.5 | 3.5 ± 0.5 | 3.8 ± 0.4 |
| DS1 | 1.0 ± 0 | 1.7 ± 0.7 | 2.8 ± 0.4 | 2.2 ± 0.4 h |
| DS1 + ASCS | 0.8 ± 0.4 | 1.2 ± 0.4 g | 2.2 ± 0.8 | 2.3 ± 0.5 |
| DS2 | 0.8 ± .4 | 1.7 ± 0.8 | 2.5 ± 0.6 | 2.6 ± 1.0 |
| DS2 + ASCS | 1.0 ± 0.7 | 2.0 ± 0.8 | 2.0 ± 0.0 | 2.8 ± 0.8 |
| Myofibroblasts | ||||
| Empty | 3.2 ± 0.4 | 3.9 ± 0.3 i | 3.8 ± 0.4 | 3.8 ± 0.5 |
| ASCS | 3.6 ± 0.5 | 3.8 ± 0.4 j | 4.0 ± 0.0 | 3.8 ± 0.4 |
| DS1 | 2.4 ± 0.9 | 2.4 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.5 |
| DS1 + ASCS | 2.4 ± 0.5 | 2.4 ± 0.5 | 3.3 ± 0.8 | 3.8 ± 0.4 |
| DS2 | 2.6 ± 0.5 | 2.9 ± 0.3 | 3.5 ± 0.5 | 3.5 ± 0.5 |
| DS2 + ASCS | 2.8 ± 0.4 | 3.0 ± 0.0 | 3.8 ± 0.4 | 3.5 ± 0.5 |
DS1 + ASCS is significantly different from all groups (P < .01) at day 14.
DS1 significantly different from ASCS (P < .05).
DS1 (P < .01) and DS1 + ASCS (P < .01) significantly different from other groups at 14 days.
DS1 + ASCS significantly different from empty and ASCS groups (P < .05) at 28 days.
DS1 (P < .01) is significantly different from empty, ASCS, DS2 and DS2 + ASCS at 42 days.
DS1 + ASCS is significantly different from ASCS (P < .05) at 42 days.
DS1 + ASCS significantly different from empty and ASCS (P < .01) at 14 days.
DS1 (P < .001) and DS1 + ASCS (P < .01) significantly different from empty and ASCS groups at 42 days.
Empty is significantly different from other groups except for ASCS (P < .05) at 14 days.
ASCS is significantly different from DS1 and DS1 + ASCS (P < .001) at 14 days.
FIGURE 6.

Epithelial/squamous hyperplasia was scored histologically at day 9 (A) and day 14 (B) as mean ± SD, (n = 10, **P < .01). Representative micrographs for days 9 and 14 (B). Scale bars = 100 μm; arrows = DS material in wound bed; arrowheads = giant multinucleated cells around DS1/2; K = island of epithelial cells
3.1.3. Inflammation
Inflammation due to treatment was scored and is summarised in Table 2. Overall inflammation within the wound beds tended to decrease over time for all treatments. DS1 had the most significant overall inflammation response at 14 days than ASCS groups (P < .05). The presence of multinucleated giant cells (Table 2) was primarily associated with DS1 and DS1 + ASCS compared with all groups at all time points that often directly surrounded the DS1 material (Figure 6C). The addition of ASCS to DS1 and DS2 tended to decrease the overall inflammatory response compared with DS1 or DS2 treatments.
3.1.4. Collagen maturation
The newly deposited collagen adjacent to DS was characterised using the Herovici stain, and histological scoring is summarised in Table 2. Representative histology micrographs for days 14 and 42 are shown in Figure 7. At 9 days (not shown), all treatments were mainly equivalent with immature collagen directly surrounding the test material. By 14 days, DS2 + ASCS had higher average scores than the DS1 + ASCS treatments but was not statistically significant. By 28 days, all these four treatments were comparable with mostly mature collagen surrounding the DS. The addition of ASCS to either DS1 or DS2 did not affect the maturation of collagen directly covering the test material at 14 or 42 days. Empty and ASCS groups had higher mature collagen scores than either DS and DS + ASCS groups at all time points consistently.
FIGURE 7.

Herovici staining of immature collagen (blue) and mature collagen (pink/purple) fibrils. Scale bar = 100 μm; Arrowhead = collagen staining; Arrow = DS material in the wound bed
3.1.5. Myofibroblasts within wound bed
Myofibroblasts within the wound bed were histologically scored and are summarised in Table 2. Representative histology micrographs for day 14 are shown in Figure S1. At early time points (9 and 14 days), the DS1 and DS1 + ASCS treatments had lower average myofibroblast scores than all other groups. The DS2 and DS2 + ASCS treatments tended to have higher scores and empty, and ASCS treatments had significantly higher average myofibroblast scores (P < .05) at day 14. By 42 days, the myofibroblast scores were comparable between treatments, with DS1 having the lowest average score but was not statistically significant.
3.1.6. Vascular density
The vascular density was evaluated by counting CD31 stained vessels as shown in representative histology micrographs for day 14 (Figure S2) and quantified morphometrically and is summarised in Table S2. The vascular density was relatively high at 9 days (~81‐116 vessels/mm2), decreased slightly around 28 days (78‐95 vessels/mm2), and then increased to a vascular density that was greater than that found at 9 days (112‐129 vessels/mm2). DS1 + ASCS had a relatively low vascular density at 9 days (81 vessels/mm2) that remained low through 28 days (83 vessels/mm2) and then increased to the highest average level at 42 days (129 vessels/mm2). At 14 days, the vascular density appeared to be slightly greater in the superficial wound bed (increased numbers of small blood vessels) in empty, ASCS, DS2, and DS2 + ASCS. Statistically significant differences were not noted between groups.
4. DISCUSSION
Soft‐tissue injuries resulting from burns and extreme traumatic events often require reconstruction of damaged or missing skin. Typically, reconstruction of large area full‐thickness wounds is achieved via a two‐stage surgical procedure where a skin graft follows DS implantation. 1 These procedures require complex wound management, often with an initial staging period following DS placement to allow for dermal maturation followed by rounds of STSG harvesting and placement. The ACHD is a promising clinical innovation that can enzymatically digest a thin STSG to obtain a cell suspension consisting of epidermal and dermal cells. These cells could then be resuspended and sprayed directly onto a wound bed with continuous dermis or combined with meshed STSG in a full‐thickness injury, with or without a DS. 3 , 15 The present pilot study results support the feasibility of a one‐stage application by directly combining ASCS with DS1 or DS2 for full‐thickness wound healing in a porcine model by demonstrating enhanced rates of epithelialisation when compared with DS alone while retaining reduction in wound contraction observed in both DS1 and DS2.
The one‐stage technique aims to provide the deep‐partial to full‐thickness wound patient with the benefit of high‐quality functional skin encompassing both dermis and epidermis while limiting contracture hypertrophic scarring. A recent clinical study has shown utility in combining ASCS with STSG to treat chronic wounds and found that ASCS + STSG treatment significantly decreased the average time to re‐epithelialisation compared with STSG alone. 16 This highlights the potential for one‐stage applications for patients with large‐area burns where burn wound pathology is entirely a process of an open wound. When the wound closes, the metabolic insult and catabolic response ease while allowing the patient to rehabilitate. Mortality with a burn injury is directly related to the size of the burn. However, donor sites for skin grafting are often limited while increasing the risk of infections due to delayed wound closure and other complications leading to death. The application of DS + ASCS described in this study could decrease the mortality from burn injuries due to faster closure using the ASCS, allowing epithelialisation with fewer donor site limitations.
The choice of DS for one‐stage application is an essential factor as its three‐dimensional structure and composition dictate the regeneration of dermal tissue while simultaneously supporting ASCS survival. DS facilitates wound healing via influencing attachment, migration, differentiation, and proliferation of fibroblasts, keratinocytes, and other cell types and eventually undergo progressive remodelling to regenerate the dermis. 5 The wound area measurements reported in this study were measured using digital planimetry and indicate wound contracture and peripheral re‐epithelialisation, which were significantly reduced at the intermediate time points of 9 and 14 days, in groups without DS1 or DS2. The evidence of contracture is further substantiated by the significant increase in myofibroblast populations in empty and ASCS only groups compared with DS1 or DS2. This finding at the intermediate time points supports this mechanism as the inhibition of contraction was observed in DS1 and DS2, which may be attributable to their biophysical properties and controlled degradation kinetics during early wound healing. 17 , 18 , 19 , 20 However, at 21 days and later time points, all groups had similar wound contraction, indicating that a one‐stage application with DS + ASCS may not achieve the same results as a two‐stage technique. From a clinical standpoint, contracture may compromise skin quality, especially around joint articulation, and may require a higher need for secondary burn/wound reconstruction procedures. However, this finding highlights the limitations of a porcine model where wounds heal primarily through contraction and does not fully capture the wound healing in humans, which occurs via epithelialisation. Additional preclinical studies would need to be performed to determine further impact of contracture as related to the timing of ASCS application.
In this study, ASCS was applied dropwise at a density of 8 × 104 cells per cm2 to the underside of DS for a one‐stage procedure within the range of previously reported preclinical studies. 7 , 8 , 10 , 11 , 19 Our finding of significantly higher re‐epithelialisation for DS + ASCS groups is consistent with previous preclinical studies of cultured or non‐cultured keratinocytes seeded in DS1. 7 , 8 , 10 , 11 , 19 Empty wounds, and those treated with DS or ASCS alone did not demonstrate enhanced re‐epithelialisation. Therefore, this highlights the role of DS1 and DS2 as favourable substrates to facilitate ASCS signalling, attachment, and migration, resulting in the regeneration of the epidermis in a full‐thickness wound. ASCS contains a mixed cutaneous cell population of keratinocytes, fibroblasts, and a smaller population of melanocytes. 15
We hypothesise that ASCS combined with DS promotes paracrine signalling. Cell–cell interactions between keratinocytes and fibroblasts induce the release of growth factors and cytokines, enhancing keratinocyte migration, proliferation, and activation present in DS and within wound margins. 21 , 22 This phenomenon has previously been demonstrated in an in vitro assay where co‐culturing dermal fibroblasts with keratinocytes in collagen gels significantly enhanced the activation of keratinocytes outgrowth compared with applying fibroblasts conditioned media, thus highlighting the importance of fibroblasts interaction with keratinocytes. 22 Furthermore, increased epithelial hyperplasia was observed in DS + ASCS groups, especially in the DS1 + ASCS group, which demonstrated progressive nests of keratinocytes. These nests of keratinocytes are known to migrate through the DS to fuse with the developing epidermis between 2 and 3 weeks, after which epithelial hyperplasia resolves, consistent with our findings. 9 , 10 , 23 Cell seeding density at the time of implantation has shown to be directly correlated to the levels of epithelial hyperplasia observed and impacts the epithelialisation rate, further supporting the hypothesis of the direct contribution of ASCS.
In this study, the DS1 group elicited a heightened inflammation response with many multinucleated giant cells relative to DS2. This trend may be attributable to the presence and crosslinking of collagen and C6S in DS1 and the remodelling mechanism of the bioengineered material, which is resorbed during dermal regeneration by phagocytosis and other means. 13 , 19 , 20 , 24 Addition of ASCS to DS1 lowered the inflammation and multinucleated giant cells, which could be attributed to the rapid epithelialisation of these wounds. Still, the mechanism of action remains to be investigated. The absence of multinucleated giant cells in DS2 is consistent with a transient inflammatory response and slow matrix degradation. 4 , 25 These inherent differences between DS1 and DS2 serve to establish a set of critical variables in the concept of combined ASCS and DS. Despite these observed differences in modes of dermal regeneration, both DS1 and DS2 provided adequate structure, signalling, and remodelling kinetics to support both ASCS seeding and simultaneous dermal and epidermal regeneration observed via ultimately comparable levels of early re‐epithelialisation and noted epithelial hyperplasia.
The principal clinical concern associated with single application dermal and epidermal regenerative interventions is the potential for lack of “take” of the epidermal component (ASCS or STSG) or cell necrosis due to lack of proximity to an actively perfused capillary bed. The diffusion limit of oxygen is limited to ~200 μm in biological tissue, directly correlated with the structural tissue's metabolic activity, including ECM deposition and remodelling. 26 , 27 , 28 , 29 This distance is significantly less than the thickness of the human dermis, which ranges according to anatomic location but can be up to multiple mm thick. 27 Likewise, commercially available DS is also more than 200 μm in thickness. After introducing the viable component of the therapy proximal to the wound bed, diffusional limitations noted earlier to influence cell viability and ECM production were overcome, as evidenced by the observed “epithelial hyperplasia” and early re‐epithelialisation. Despite differences in inflammatory and remodelling responses, both DS1 and DS2 demonstrated sufficiently favourable regenerative kinetics to support the migrating ASCS populations in the preclinical model.
Despite previous efforts to provide physicians with a one‐stage reconstruction solution, two‐stage dermal and epidermal reconstruction procedures remain the gold standard for deep partial‐ and full‐thickness large area wounds. Typical staging times following dermal substitute application vary but are generally between 2 and 4 weeks following dermal substitute application. 1 Staging not only impacts the time for patients to return to daily activities but increases the duration of stay, which has a significant financial impact, with an estimated $6795 mean cost per patient per day on a burn unit calculated for three U.S. burn centres in 2017. 30 Patients with burns requiring staging have larger third‐degree burns. This population is associated with a greater length of stay. 31 , 32 Staging procedures between dermal coverage and epidermal grafting require specialised accommodations, which have been identified as a primary driver of the duration of stay in burn care. 33 From a translational perspective, combining DS1 or DS2 with ASCS for a one‐stage application is a simple technique with the potential to maximise STSG expansion for deep wounds with large burn wound surface area. It does not present a drastic departure from current practices. Furthermore, the application of sterile, readily available, and non‐immunogenic DS further obviates the need for extensive skin graft harvesting for dermal reconstruction, resulting in a potential reduction in time to grafting and overall patient stay. However, the application of ACHD does require surgeon training and is limited for use in an aseptically controlled operating room for inpatient procedures.
Our findings establish a proof of concept in a porcine preclinical model for one‐stage application by combining ASCS with well‐characterised DS. Variables of interest for future study not optimised here include DS thickness, ASCS cell density, ASCS cell composition, direct comparison with STSG in one and two‐stage reconstruction procedures, and a randomisation scheme focused on isolating the impact of wound anatomic location. Another limitation of this study is that using a healthy, porcine full‐thickness excisional model presents significant metabolic and etiological differences from a thermal burn in an adult human and serves primarily as an initial investigation into the basic biological principles for the technique. Further long‐term investigation using different wound healing pathologies in porcine models is warranted. Evaluation of a translationally relevant model such as burn‐excisional debridement models capable of better simulating clinical scenarios may further evaluate the translational potential of this one‐stage application in practice. An extension of the one‐stage technique to full‐thickness complex chronic wounds may be an additional avenue for development.
CONFLICT OF INTEREST
Sita M. Damaraju, Benjamin R. Mintz, Ankur Gandhi and Sunil Saini are the employees of Integra LifeSciences, Corp, USA. J. Genevieve Park and Joseph A. Molnar are the employees of Wake Forest University School of Medicine. Joseph A. Molnar is a consultant to Integra LifeSciences, Corp, USA.
AUTHOR CONTRIBUTIONS
Sita M. Damaraju, Benjamin R. Mintz, Ankur Gandhi, Sunil Saini, Joseph A. Molnar contributed to study design details and execution. Sita M. Damaraju and Benjamin R. Mintz contributed equally to data analysis and writing the original draft. Sunil Saini, Ankur Gandhi, J. Genevieve Park, and Joseph A. Molnar reviewed and edited the draft.
Supporting information
Figure S1: Immunohistochemical staining of myofibroblasts. The myofibroblasts were stained by an anti‐SMA antibody. Scale bars = 100 μm; arrows = DS1/2 material; arrowheads = myofibroblasts infiltrating DS1/2.
Figure S2: Immunohistochemical staining of endothelial cells. The endothelial cells were stained by an anti‐CD31 antibody. Scale bars = 100 μm; Arrows = DS1/2 material; arrowheads = small blood vessels infiltrating DS1/2. The vessel density was calculated as the number of vessels per mm2. N = 10 for day 14.
Table S1 Percent epithelialisation on wound surface. **DS1/2 + ASCS were significantly different from all groups (P < .01)
Table S2: Vascular density (vessels/mm2).
ACKNOWLEDGEMENTS
Integra LifeSciences, Corp, USA, funded this work. The authors thank AVITA Medical (Valencia, CA, USA) for providing RECELL devices for use during the study. The authors thank Wake Forest Innovations team for coordinating and organising the execution of the preclinical study.
Damaraju SM, Mintz BR, Park JG, Gandhi A, Saini S, Molnar JA. Skin substitutes with noncultured autologous skin cell suspension heal porcine full‐thickness wounds in a one‐stage procedure. Int Wound J. 2022;19(1):188–201. 10.1111/iwj.13615
Funding information Integra LifeSciences
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wang Y, Beekman J, Hew J, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3‐17. [DOI] [PubMed] [Google Scholar]
- 2. Singh M, Nuutila K, Kruse C, Robson MC, Caterson E, Eriksson E. Challenging the conventional therapy: emerging skin graft techniques for wound healing. Plast Reconstr Surg. 2015;136(4):524e‐530e. [DOI] [PubMed] [Google Scholar]
- 3. Cooper‐Jones B, Visintini S. A noncultured autologous skin cell spray graft for the treatment of burns. CADTH Issues in Emerging Health Technologies. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2018. [PubMed] [Google Scholar]
- 4. Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications: a comparative histologic study. JBJS. 2006;88(12):2673‐2686. [DOI] [PubMed] [Google Scholar]
- 5. van der Veen VC, van der Wal MBA, van Leeuwen MCE, Ulrich MMW, Middelkoop E. Biological background of dermal substitutes. Burns. 2010;36(3):305‐321. [DOI] [PubMed] [Google Scholar]
- 6. Rudnicki PA, Purt B, True D, Siordia H, Lohmeier S, Chan RK. Single‐stage composite skin reconstruction using a dermal regeneration template. Plast Reconstr Surg: Glob Open. 2020;8(2):e2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wood FM, Stoner ML, Fowler BV, Fear MW. The use of a noncultured autologous cell suspension and Integra® dermal regeneration template to repair full‐thickness skin wounds in a porcine model: a one‐step process. Burns. 2007;33(6):693‐700. [DOI] [PubMed] [Google Scholar]
- 8. Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A. 1989;86(3):933‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones I, James SE, Rubin P, Martin R. Upward migration of cultured autologous keratinocytes in Integra™ artificial skin: a preliminary report. Wound Repair Regen. 2003;11(2):132‐138. [DOI] [PubMed] [Google Scholar]
- 10. Butler CE, Yannas IV, Compton CC, Correia CA, Orgill DP. Comparison of cultured and uncultured keratinocytes seeded into a collagen‐GAG matrix for skin replacements. Br J Plast Surg. 1999;52(2):127‐132. [DOI] [PubMed] [Google Scholar]
- 11. Butler CE, Orgill DP, Yannas IV, Compton CC. Effect of keratinocyte seeding of collagen‐glycosaminoglycan membranes on the regeneration of skin in a porcine model. Plast Reconstr Surg. 1998;101(6):1572‐1579. [DOI] [PubMed] [Google Scholar]
- 12. Jørgensen LB, Sørensen JA, Jemec GBE, Yderstræde KB. Methods to assess area and volume of wounds–a systematic review. Int Wound J. 2016;13(4):540‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Truong A‐TN, Kowal‐Vern A, Latenser BA, Wiley DE, Walter RJ. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J Burns Wounds. 2005;4(e4):72‐82. [PMC free article] [PubMed] [Google Scholar]
- 14. Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wood FM, Giles N, Stevenson A, Rea S, Fear M. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns. 2012;38(1):44‐51. [DOI] [PubMed] [Google Scholar]
- 16. Hu ZC, Chen D, Guo D, et al. Randomized clinical trial of autologous skin cell suspension combined with skin grafting for chronic wounds. Br J Surg. 2015;102(2):e117‐e123. [DOI] [PubMed] [Google Scholar]
- 17. MacEwan MR, MacEwan S, Kovacs TR, Batts J. What makes the optimal wound healing material? A review of current science and introduction of a synthetic nanofabricated wound care scaffold. Cureus. 2017;9(10):e1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Philandrianos C, Andrac‐Meyer L, Mordon S, et al. Comparison of five dermal substitutes in full‐thickness skin wound healing in a porcine model. Burns. 2012;38(6):820‐829. [DOI] [PubMed] [Google Scholar]
- 19. Compton CC, Butler CE, Yannas IV, Warland G, Orgill DP. Organized skin structure is regenerated in vivo from collagen‐GAG matrices seeded with autologous keratinocytes. J Invest Dermatol. 1998;110(6):908‐916. [DOI] [PubMed] [Google Scholar]
- 20. Yannas IV, Tzeranis D, So PT. Surface biology of collagen scaffold explains blocking of wound contraction and regeneration of skin and peripheral nerves. Biomed Mater. 2015;11(1):14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ter Horst B, Chouhan G, Moiemen NS, Grover LM. Advances in keratinocyte delivery in burn wound care. Adv Drug Deliv Rev. 2018;123:18‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tuan T‐L, Keller LC, Sun D, Nimni ME, Cheung D. Dermal fibroblasts activate keratinocyte outgrowth on collagen gels. J Cell Sci. 1994;107(8):2285‐2289. [DOI] [PubMed] [Google Scholar]
- 23. De Angelis B, Orlandi F, Fernandes Lopes Morais D'Autilio M, et al. Long‐term follow‐up comparison of two different bi‐layer dermal substitutes in tissue regeneration: clinical outcomes and histological findings. Int Wound J. 2018;15(5):695‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wosgrau ACC, Da Silva Jeremias T, Leonardi DF, Pereima MJ, Di Giunta G, Trentin AG. Comparative experimental study of wound healing in mice: Pelnac versus integra. PLoS One. 2015;10(3):e0120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rennert RC, Sorkin M, Garg RK, Januszyk M, Gurtner GC. Cellular response to a novel fetal acellular collagen matrix: implications for tissue regeneration. Int J Biomater. 2013;2013:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lewis MC, MacArthur BD, Malda J, Pettet G, Please CP. Heterogeneous proliferation within engineered cartilaginous tissue: the role of oxygen tension. Biotechnol Bioeng. 2005;91(5):607‐615. [DOI] [PubMed] [Google Scholar]
- 27. Olsen LO, Takiwaki H, Serup J. High‐frequency ultrasound characterization of normal skin. Skin thickness and echographic density of 22 anatomical sites. Skin Res Technol. 1995;1(2):74‐80. [DOI] [PubMed] [Google Scholar]
- 28. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249‐257. [DOI] [PubMed] [Google Scholar]
- 29. Rouwkema J, Koopman BFJM, Blitterswijk CAV, Dhert WJA, Malda J. Supply of nutrients to cells in engineered tissues. Biotechnol Genet Eng Rev. 2014;26(1):163‐178. [DOI] [PubMed] [Google Scholar]
- 30. Kowal S, Kruger E, Bilir P, et al. Cost‐effectiveness of the use of autologous cell harvesting device compared to standard of care for treatment of severe burns in the United States. Adv Ther. 2019;36(7):1715‐1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dolp R, Rehou S, McCann MR, Jeschke MG. Contributors to the length‐of‐stay trajectory in burn‐injured patients. Burns. 2018;44(8):2011‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rehou S, Dolp R, McCann MR, Jeschke MG. 295 burn patients that exceed the average length of stay. J Burn Care Res. 2018;39(suppl_1):S117. [Google Scholar]
- 33. Abdelrahman I, Elmasry M, Olofsson P, Steinvall I, Fredrikson M, Sjoberg F. Division of overall duration of stay into operative stay and postoperative stay improves the overall estimate as a measure of quality of outcome in burn care. PLOS ONE. 2017;12(3):e0174579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Immunohistochemical staining of myofibroblasts. The myofibroblasts were stained by an anti‐SMA antibody. Scale bars = 100 μm; arrows = DS1/2 material; arrowheads = myofibroblasts infiltrating DS1/2.
Figure S2: Immunohistochemical staining of endothelial cells. The endothelial cells were stained by an anti‐CD31 antibody. Scale bars = 100 μm; Arrows = DS1/2 material; arrowheads = small blood vessels infiltrating DS1/2. The vessel density was calculated as the number of vessels per mm2. N = 10 for day 14.
Table S1 Percent epithelialisation on wound surface. **DS1/2 + ASCS were significantly different from all groups (P < .01)
Table S2: Vascular density (vessels/mm2).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


