Abstract
Lucilia sericata bottle fly worms can be used to heal infected, chronic, or necrotic wounds, including those associated with ulceration and diabetic foot. The study aimed to evaluate changes in the microflora in patients treated with L sericata larvae due to leg ulcers and diabetic foot. One hundred twenty‐nine patients diagnosed with lower limb ulceration and diabetic foot were enrolled in the study, of which 80 of them met the eligibility criteria for maggot debridement therapy (MDT). On the contrary, 49 unqualified patients were offered ozone therapy (22 with leg ulcers; 27 with diabetic foot). In each of these patients, a microbiological swab was performed before and after the start of therapy. The group of 80 patients was further divided into four equal groups in terms of the treated area (lower leg vs foot) and the number of larvae/cm2 (5 vs 10). Twenty‐three particular species of bacteria in the infected wound were studied microbiologically in terms of presence/absence within the wound environment before and after treatment of patients with diabetic foot and lower limb ulceration. It was noted that there was a more intensive bacterial accumulation in the feet of patients compared to legs; furthermore, this applies to almost all analysed species. Diabetes status is also a clinical factor that generates a lower chance of bacterial appearance in the wound environment. Densification of MDT larvae per wound area unit also reduced the chance of the presence of Corynebacterium species, Enterobacteriaceae, Pseudomonas aeruginosa, Staphylococcus aureus MSSA, and Streptococcus coagulase negativa; however, it increased the likelihood of occurrence for Proteus mirabilis and the Proteus species. A microbiological analysis in this non‐reference study shows the efficacy of larval therapy for leg and foot ulcers. Rearrangement of the microflora within the wound has been reported as a result of the therapy.
Keywords: diabetic foot, leg ulcers, Lucilia sericata larvae, microbiology
1. INTRODUCTION
Diabetes mellitus (DM) is a global healthcare issue and according to the International Diabetes Federation, 1 in 11 adults (20–79 years) have diabetes (463 million people). 1 DM and its concurrent complications impact a significant proportion of the world population and create a large financial burden on worldwide healthcare systems. 2 One of the most common complications of both type 1 and type 2 diabetes is ulceration of the lower leg or feet. 3 It is suggested that more frequent occurrences of ulcerations may lead to death in patients with diabetes with coexisting cardiovascular and kidney disorders. 4 , 5 Brownrigg et al indicate that the risk of death in persons with diabetes having ulcerations of the lower leg or feet was approximately 1.89 times (95% CI: 1.60–2.23) higher than in the group without these changes. 6 In the treatment of ulcerations in the course of diabetes, the following, among others, are utilised: growth factors; somatic stem cells; antidiabetic drugs; herbal preparations; biological agents, such as the acid peptide matrix. 7
Therefore, a traditional wound healing approach using the application of sterile laboratory‐reared Lucilia sericata larvae, which has recently been approved for the treatment of chronic wounds, is a cost‐effective and successful treatment for diabetic foot ulcers and many other medical conditions. 8
The maggots of the green bottle fly L sericata can be used to treat infected, chronic, or necrotic wounds, particularly those that cannot be cured using conventional approaches. Besides the efficient removal of necrotic tissue and the acceleration of wound healing, the benefits of maggot debridement therapy (MDT) include wound disinfection. 9 , 10 A variety of growth factors and antimicrobial peptides delivered by the larvae in maggot excretions to the wound environment have been already reported in the literature with the aim of enhancing wound healing. 11 , 12
The study aim was to evaluate changes in the microflora in patients treated with L sericata larvae due to leg ulcers and diabetic foot.
2. MATERIALS AND METHODS
One‐hundred twenty‐nine patients diagnosed with lower limb ulceration and diabetic foot were enrolled in the study (Bioethics Committee of the Opole Doctor's Chamber agreement No. 156/08) in the “ChirMedicus” doctor's surgery in Kędzierzyn‐Koźle, Poland, in the years 2010 to 2012. From all of these patients, who took part in the study, informed and voluntary consent was obtained for them to participate in this study.
With regard to inclusion criteria, for the conducted 5 and 10 larvae/cm2 MDT (depending on the patients' expected pain level during the larval therapy), there were qualified patients who had fulfilled a few clinical conditions. Firstly, subjects with chronic wounds covered with necrotic tissue as well as with purulent exudates in the vascular calf and feet area of diabetic aetiology were qualified. Moreover, a condition that allowed for patients to undergo MDT was compensated diabetes, which was confirmed by the use of a test of current blood glucose concentration, efficient circulation, and respiration, together with a correct result in the examination of the coagulation system and ultrasound examination of the venous and arterial vessels of the lower limb at the current time, performed with the use of Doppler ultrasonography techniques (colour and spectral). Criteria for excluding patients were patients with wounds lacking necrosis and exudates, acute and traumatic wounds, bedsores, cancer ulcers, and burn wounds. Subjects treated with anticoagulants and those with symptoms of chronic circulatory failure in the form of oedema of the lower limbs and dyspnea at rest were also excluded from MDT. Additionally, patients with symptoms of uncompensated or decompensated diabetes confirmed using testing of current blood glucose in serum, and those without Doppler ultrasound examination the veins and arteries of the lower limbs (both colour and spectral) also did not undergo larval therapy. Therefore, patients who did not qualify for larval therapy or did not consent to its use were referred to the group treated with ozone therapy.
It is noteworthy that during both MDT and ozone therapy, patients were not treated with antibiotics. Of the total set of patients—129, 80 underwent MDT, while, on the other hand, the remaining 49 underwent ozone therapy. The clinical characteristics of the patients are presented in Table 1.
TABLE 1.
Clinical characteristics of patients treated with either larvae or ozone
| Group | Larvae therapy | Ozone therapy | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Location | Foot | Leg | Foot | Leg | ||
| Number | 20 | 20 | 20 | 20 | 22 | 27 |
| Density of larvae per 1 cm2 | 5 | 10 | 5 | 10 | 0 | 0 |
| DM status (%) | 100 | 100 | 30 | 35 | 100 | 26 |
| Age | 63 ± 11.9 | 60 ± 10.4 | 69.5 ± 8.8 | 66.8 ± 17.8 | 60.9 ± 11.2 | 71.1 ± 7.4 |
| Days of MDT | 13.5 ± 6.98 | 8.0 ± 3.0 | 2.3 ± 0.7 | 3.8 ± 1.6 | 0 | 0 |
| Months of ulceration | 3.6 ± 1.8 | 5.2 ± 2.9 | 3.0 ± 2.4 | 3.0 ± 2.4 | 4.7 ± 1.8 | 5.8 ± 2.8 |
| Larvae application | 4.6 ± 2.7 | 2.7 ± 1.1 | 2.3 ± 0.7 | 1.3 ± 0.5 | 0 | 0 |
| Wound area before treatment (cm2) | 16.0 ± 13.9 | 24.9 ± 29.2 | 220.7 ± 123.9 | 39.1 ± 46.5 | 12.8 ± 13.7 | 133.6 ± 130.7 |
| Wound area after treatment (cm2) | 10.2 ± 13.5 | 8.6 ± 11.0 | 66.6 ± 71.9 | 2.4 ± 4.4 | 9.4 ± 11.3 | 105.5 ± 111.6 |
Abbreviations: DM, diabetes mellitus; MDT, maggot debridement therapy.
The clinical factors listed in Table 1, that is, location of wounds, the density of larvae, DM status, days of MDT, gender fractions, patient ages, months of ulceration, number of larvae applications, and wound areas, were set as predictors generating a statistical chance of occurrence or disappearance of bacterial species before and after treatment. In turn, in Figure 1, selected photos of ulcers are presented.
FIGURE 1.
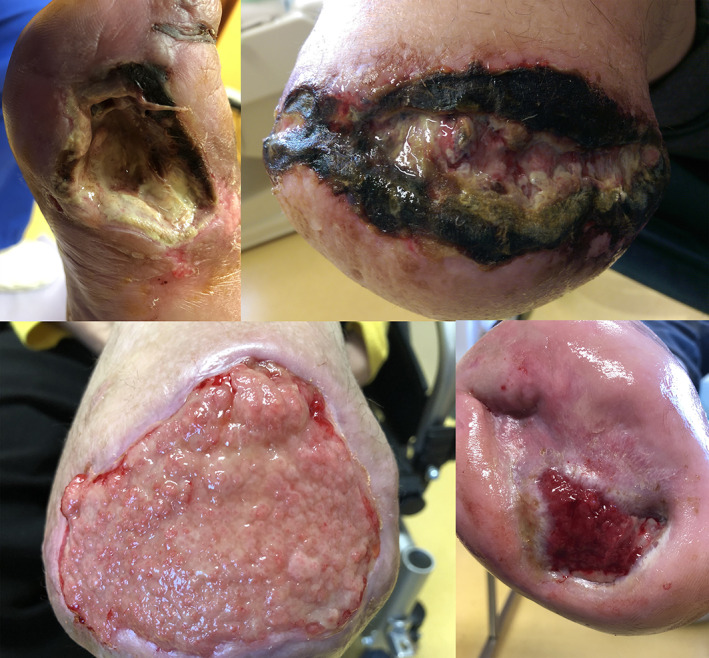
Presenting ulcerations in patients qualified for larvae therapy
In the next stage of the study, the microbiological analysis of the wound environment was performed. Before applying the first larvae‐containing dressing, the wound surface was rinsed with a 0.9% NaCl solution. After that, using a sterile stick ended with a viscose ball, microbial material was collected from the wound and sealed in a transport container with a Stuart medium. Within 48 hours, this container was sent to the Unit of Microbiological Diagnostics at the Kędzierzyn‐Koźle County Hospital for qualitative assessment of bacterial flora. Subsequent microbiological assessments of the wound were performed identically after completion of larval therapy (in this study, a clinical event of healing was established if there was a decrease of at least >50% in the wound area after the conclusion of observation).
To model the probability of presence/absence of certain bacteria before and after the treatment, logistic regression was used, which adopts a logistic function to model a binary (“0/1”, “yes/no”, etc.) response based on a linear combination of one or more independent variables and express the statistical effects via odds ratios (ORs).
Since repeated measurement of dichotomous data (presence/absence of bacteria) and explanatory predictors (wound areas before and after treatment) appeared in the statistical material, an extension of the logistic regression with random effects that models correlation among observations within a cluster 13 was adopted in the statistical analysis.
The classical statistics fit the logistic regression utilising an iterative procedure like maximum likelihood. Due to some missingness of data in the second microbiological measurements and also high dimension and analytically intractable regression, which was consisting of eight (listed earlier) explanatory variables, the estimation in classical statistics resulted in non‐convergence modelling, alternatively, the Bayesian methodology was employed in this study, which enables statistical inference on posterior distributions. Additionally, in Bayesian statistics, the Markov chain Monte Carlo (MCMC) algorithm approach was used to provide an estimate of complicated distribution. The performance of the MCMC chains based on an initial “burn‐in” 1000 samples and following 10 000 “production run” cycles of the Gibbs sampler were determined with the Geweke statistic using the WinBUGS software. 14
3. RESULTS
twenty‐three different species of bacteria in the wound infection were studied microbiologically in terms of presence/absence within the wound environment before and after treatment of patients with diabetic and lower limb ulceration. Ultimately, therefore, each patient was to be tested twice microbiologically (before and after therapy).
In the group of patients with diabetic foot undergoing MDT, the most numerous bacteria before treatment in group 1 were as follows: Streptococcus B haemolyticus (24%); Streptococcus coagulase negativa (19%); Enterobacteriaceae, and Enterococcus faecalis (14% each), whereas in group 2: Staphylococcus aureus (37%); Enterococcus faecalis, and Pseudomonas aeruginosa (11% each). After the therapy, it was noted that, in group 1, the wound was colonised primarily by the Proteus species (46%) and Enterobacteriaceae (23%), whereas in group 2 it was the Proteus species (29%) and Proteus mirabilis (21%). In turn, among patients with diabetic foot qualified for ozone therapy (group) before therapy began, the dominating bacteria were as follows: Staphylococcus aureus (23%), Streptococcus B haemolyticus (23%), and Pseudomonas aeruginosa (11%), whereas after the completion of treatment: the Proteus species (32%) and Staphylococcus aureus (32%).
However, in the case of lower limb ulceration before larval therapy, in the microbiological culture, the greatest amount of bacteria was determined for, in group 3: Pseudomonas aeruginosa (20%) and Staphylococcus aureus (14%); in group 4, this was Staphylococcus aureus (32%), Streptococcus B haemolyticus (16%), and Streptococcus coagulase negativa (10%). After the completion of therapy, the following can be observed for group 3: Pseudomonas aeruginosa and Staphylococcus aureus (17% each); Serratia marcescens, Enterobacteriaceae, and Klebsiella oxytoca (11% each), whereas in group 4: Proteus species (53%) and Proteus mirabilis (29%).
On the other hand, in group 6, represented by patients with lower limb ulceration, treated with ozone therapy, the greatest percentage was noted for Staphylococcus aureus (20%) and Pseudomonas aeruginosa (18%); and after the treatment this was Staphylococcus aureus (30%), Enterococcus faecalis (22%), and Streptococcus coagulase negativa (15%). The results are presented in Figure 2.
FIGURE 2.
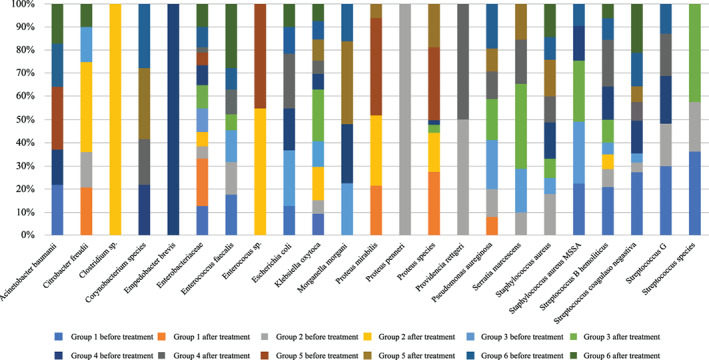
Bacterial microflora in the wound environment before and after treatment of patients with diabetic foot and lower limbs' ulceration
The estimated significant (P < .05, one‐sided) ORs with 95% credible intervals (CIs) in the multivariable regression approach for the microbiological response compared to clinical predictors are reported in Table 2.
TABLE 2.
The estimated significant (P < .05, one‐sided) ORs of the microbiological response vs clinical predictors (multivariable regression)
| Bacteria (microbiological response) | Risk factor (predictor) | OR | 95% CI | P (one‐sided) |
|---|---|---|---|---|
| Acinetobacter baumannii | Leg vs foot | 0.11 | (0.02, 0.50) | .0017 |
| Citrobacter freudii | Leg vs foot | 0.10 | (0.02, 0.43) | <.0001 |
| Clostridium sp. | Leg vs foot | 0.12 | (0.03, 0.43) | <.0001 |
| DM | 0.23 | (0.05, 0.99) | .0247 | |
| Corynebacterium species | No. of larvae per cm2 | 0.62 | (0.33, 0.98) | .0196 |
| No. of applications | 3.81 | (1.01, 15.3) | .0227 | |
| DM | 0.25 | (0.06, 1.03) | .0261 | |
| Time of MDT (day) | 0.60 | (0.35, 1.00) | .0319 | |
| Empedobacter brevis | Leg vs foot | 0.10 | (0.02, 0.39) | <.0001 |
| Age of patients | 1.15 | (1.01, 1.39) | .0285 | |
| Enterobacteriaceae | No. of larvae | 0.80 | (0.63, 0.96) | .0047 |
| Age of patients | 0.95 | (0.89, 1.00) | .0425 | |
| Enterococcus faecalis | Wound area (cm2) | 1.020 | (1.010, 1.034) | <.0001 |
| Leg vs foot | 0.13 | (0.03, 0.46) | .0005 | |
| Enterococcus sp. | Leg vs foot | 0.12 | (0.03, 0.41) | .0001 |
| Escherichia coli | Leg vs foot | 0.19 | (0.04, 0.81) | .0068 |
| Klebsiella oxytoca | Leg vs foot | 0.09 | (0.02, 0.36) | <.0001 |
| Morganella morgani | Leg vs foot | 0.21 | (0.07, 0.69) | .0064 |
| Proteus mirabilis | Leg vs foot | 0.20 | (0.06, 0.60) | .0014 |
| DM | 0.22 | (0.08, 0.70) | .0063 | |
| No. of larvae per cm2 | 1.22 | (1.03, 1.46) | .0125 | |
| Wound area (cm2) | 0.980 | (0.955, 0.999) | .0226 | |
| Proteus penneri | Leg vs foot | 0.09 | (0.02, 0.40) | <.0001 |
| Proteus species | Wound area (cm2) | 0.965 | (0.942, 0.985) | <.0001 |
| No. of applications | 2.66 | (1.33, 5.61) | .0027 | |
| Time of MDT (day) | 0.71 | (0.54, 0.93) | .0042 | |
| Time of ulceration (month) | 0.81 | (0.65, 0.97) | .0095 | |
| DM | 0.36 | (0.13, 0.90) | .0146 | |
| No. of larvae per cm2 | 1.14 | (1.01, 1.29) | .0193 | |
| Providencia rettgeri | Leg vs foot | 0.13 | (0.03, 0.54) | .0010 |
| Pseudomonas aeruginosa | No. of larvae per cm2 | 0.25 | (0.08, 0.58) | <.0001 |
| Wound area (cm2) | 1.035 | (1.011, 1.069) | .0006 | |
| Leg vs foot | 0.21 | (0.05, 0.99) | .0255 | |
| Age of patients | 1.27 | (1.01, 1.74) | .0370 | |
| Serratia marcescens | Leg vs foot | 0.08 | (0.02, 0.35) | <.0001 |
| Staphylococcus aureus | Wound area (cm2) | 1.005 | (1.001, 1.011) | .0471 |
| Staphylococcus aureus MSSA | Time of ulceration (month) | 1.41 | (1.08, 1.87) | .0070 |
| Leg vs foot | 0.22 | (0.05, 0.83) | .0111 | |
| No. of larvae per cm2 | 0.72 | (0.49, 0.98) | .0149 | |
| Age of patients | 0.92 | (0.84, 0.99) | .0302 | |
| Streptococcus B haemolyticus | Leg vs foot | 0.19 | (0.06, 0.58) | .0010 |
| Time of ulceration (month) | 0.64 | (0.37, 0.92) | .0034 | |
| Age of patients | 1.12 | (1.03, 1.27) | .0071 | |
| No. of larvae per cm2 | 0.76 | (0.51, 0.99) | .0216 | |
| Streptococcus G | Leg vs foot | 0.23 | (0.06, 0.78) | .0031 |
| Time of ulceration (month) | 0.60 | (0.33, 0.95) | .0121 | |
| DM | 0.23 | (0.05, 0.89) | .0135 | |
| Streptococcus species | Leg vs foot | 0.11 | (0.02, 0.43) | .0003 |
Abbreviations: DM, diabetes mellitus; MDT, maggot debridement therapy.
From the results presented in Table 2, at first glance, there is a tendency towards more intensive bacterial accumulation in the feet of patients compared to the legs, as it can be observed that there is a radical disparity between these locations. Furthermore, this applies to almost all analysed species. The status of diabetes is another clinical factor that generates a lower chance of bacterial appearance in the wound environment. Densification of MDT larvae per wound area unit also reduced the chance of Corynebacterium species, Enterobacteriaceae, Pseudomonas aeruginosa, Staphylococcus aureus MSSA, Streptococcus coagulase negativa presence; however, it increases the likelihood of Proteus mirabilis and Proteus species existence within the wound environment. With the shorter duration of the ulcer, a reduction in the Proteus species, Streptococcus coagulase negativa, and Streptococcus G could be expected as well as an increase in the likelihood of Staphylococcus aureus MSSA ulcer colonisation. Although the increased number of applications elongates the duration of larval therapy, the larger the number of applications enhances the microbial response in the Corynebacterium and Proteus species, whereas MDT duration is a factor that reduces their presence.
4. DISCUSSION
The treatment of difficult‐to‐heal wounds is a significant clinical problem, especially in the case of the coexistence of diseases and factors impeding its surgical development. One of the methods used to clean cavities from necrotic tissues is larvae therapy. There exists evidence of deliberate use of larvae for cleaning wounds by many ancient cultures such as the Aboriginal Ngemba tribe, residents of Burma (Myanmar), or the Mayans. Therefore, as these larvae consume only necrotic tissues and there also exist methods of sterilising their eggs, in practice, most often fly larvae of the blowfly family are used of the L sericata species. They are bred and propagated according to a strictly defined protocol, under aseptic conditions on special sterile substrates, and the eggs are sterilised three times with 0.25% chloramine. 8 , 10 , 15 MDT is a recommended form of cleaning wounds in the situation where the surgical cleaning of necrotic tissue cannot be used. The cleansing of wounds with the use of L sericata larvae is particularly recommended in situations where necrosis has penetrated deep into the tissue, which cannot be removed mechanically using a traditional technique. The therapeutic effect resultant from L sericata is an implication of necrotic tissue being removed by the larvae, crawling on the wound surface, which results in the stimulation of granulation and angiogenesis as well as the secretion of enzymes by them, which have an effect similar to trypsin and chymotrypsin, deoxyribonucleases, and metalloproteinases. Consequently, an increase to a more alkaline pH is noted, which translates to a decrease or halting of an increase in bacteria. 15 , 16
In this study, we focused on assessing the changes in the bacterial microflora of wounds appearing in ulcerations in the lower limbs as well as diabetic foot under the influence of MDT.
Aldhyfan et al conducted a retrospective analysis of the microbiome of infected wounds in patients who underwent surgical cleansing or amputation of limbs. They determined the occurrence of anaerobic microflora in 89.6% of patients, with a predominance of growth of Escherichia coli (22% of cases) in patients with diabetic foot. The bacterial microflora isolated before therapy is characterised by diversity. However, the advantage of pathogenic microflora, which does not occur under physiological conditions, should be noted. 17 Both Gram‐positive and Gram‐negative bacteria can be distinguished, as well as aerobic and anaerobic bacteria and relative anaerobes. Such a complicated microbiological picture is characteristic of chronic conditions. 18 , 19 We observed a more varied microflora composition as well as heightened colonisation by it in ulcer cases located on feet, compared to changes in the lower legs. This could be connected with better vascularisation of this area, while simultaneously having a greater influx of oxygen and nutrients and therefore better conditions for the existence of microorganisms. 20
Moreover, we noted that in patients with diabetes, the number of isolated microorganisms was lower than in the group without comorbid diabetes. Our observations coincide with the results obtained by Kim et al, who assessed the diversity of ulcer bacterial microflora in mice with type 2 diabetes, compared with a group without this illness. They determined that a significant factor determining a lower microbiological diversity in the group with diabetes is a phenomenon of wound‐induced and aggravated oxidative stress in the course of diabetes. 21 Furthermore, these authors indicated that colonisation by Pseudomonas aeruginosa is characteristic for chronic ulcers, whereas the presence of Escherichia coli and Enterococcus faecalis are the characteristics for fresh changes. 21 It is also possible that the cause of a lower microbiological diversity in ulcerations in patients with comorbid diabetes is the fact that the disease also negatively impacts the blood supply to tissues, which delivers oxygen and nutrients 22 , 23 to these tissues, which seems to result in a failure to meet the proper conditions for the growth in certain types of bacteria. 24 Diabetes‐related hyperglycaemia significantly disturbs the balance between the concentration of pro‐ and anti‐angiogenic factors, which in turn leads to abnormal wound healing and tissue regeneration. During diabetes, disturbances in the integrity of the blood vessel walls are also noted. An increased concentration of glucose in diabetics is the cause of micro‐ and macrovascular complications, which may ultimately affect angiogenesis and wound healing. Otherwise, assessment of the involvement of bacterial microflora in the induction and afterward development of the inflammation accompanying the foot and lower limb ulceration can be more difficult as the culture was only taken from the surface of the wound and not from within. Therefore, it could be observed the isolation of the physiological flora, such as coagulase‐negative Staphylococcus, Corynebacterium spp., and Propionibacterium spp., alongside bacteria with relatively low pathogenicity, including Enterococcus spp., Stenotrophomonas maltophilia, and Pseudomonas sp., which makes the clinical interpretation of our results harder. 24 , 25 , 26 This is one of the factors limiting our study. 25 , 26 A significant factor that hinders the healing of ulcers is the fact that bacteria, which colonise chronic wounds, indicate the ability to secrete biofilm. This causes a delay in wound healing, which may be due to the insufficient supply of nutrients for the growth of bacteria, which causes their slow growth, which in turn causes resistance to antibiotics. 26 It is also worth considering the molecular mechanisms related to wound healing and keratinocytes involved in this process. These cells are capable of secreting factors whose secretion influences the healing time of wounds or ulcers. This group consists of transforming growth factor‐beta (TGFβ); vascular endothelial growth factor‐A (VEGF‐A); connective tissue growth factor (CTGF); and antioxidants. In patients with diabetics, an impairment in the secretion of these factors by keratinocytes was observed due to the conditions that prevail in the wound microenvironment (elevated levels of glucose, advanced glycation end‐products (AGEs), reactive oxygen species (ROS), and inflammatory cytokines). An increased concentration of glucose and AGE decreases the proliferation of keratinocytes and their migrations, which impairs the healing process. 27
In our previous study, we assessed the influence of MDT on the healing process of wounds, and we noted that the used therapy gave expected results in the form of a decreased ulceration area. 28 Tantawi et al noted that there was a significant decrease in the number of bacteria as a result of larval therapy, before beginning they isolated 20 types of bacteria in a group of 14 patients, 29 whereas in our population of patients, we managed to isolate 23 different bacteria, of which most were aerobic bacteria. The reason for these differences can be individual and population variability, 30 as Tantawi et al. assessed the effects of biosurgery in the Egyptian population. 29 Tantawi et al. conducted their research on a group of 14 recumbent patients with pressure ulcers, who underwent a 3‐week therapy with L sericata larvae. The ulcers were examined each week in terms of changes to their size, the size of the necrosis, and the microflora before the start and after the end of the given therapy cycle (4.86 × 108 CFU/mL of exudate vs 1.92 × 104 CFU/mL of exudate; P < .05). 29 These results indicate that larval therapy seems to be a fast, simple, effective, and economically reasonable method for treating pressure ulcers, which do not respond to conventional treatment and surgical intervention. 29
What is significant is that recent studies indicate that obtaining an adequate clinical response to larval therapy is only resultant if there is a rearrangement in the microflora of the ulcer wound environment, as L sericata do not indicate having the ability to secrete cytokines and growth factors. 31 Furthermore, the antibacterial activity of L sericata via the Gram‐positive and Gram‐negative bacteria is ambiguous. In other studies, observations can be found that larval therapy has the same effectiveness towards both Gram‐positive and Gram‐negative bacteria, 32 as well as reports of larger effectiveness towards Gram‐positive bacteria, as Gram‐negative bacteria are capable of endotoxin secretion which, in turn, neutralises the secretion of L sericata larvae. 33 The results obtained by us may indirectly indicate greater bactericidal activity of larvae as a result of Gram‐positive bacteria. Firstly, of the 23 isolated bacteria, 13 of them are Gram‐negative microorganisms; therefore, it is possible that in the group of patients in which a larger number of larvae per cm2 of ulceration was used, the size of the ulcer decreased more noticeably compared to the group of patients, in which five larvae per cm2 were used. Secondly, the statistical analysis indicated that together with an increase in the number of larvae per cm2, there is also a larger likelihood of Gram‐positive bacteria being absent: Corynebacterium species, Enterobacteriaceae, S aureus MSSA, and Streptococcus coagulase negativa, whereas only one Gram‐negative bacteria—Pseudomonas aeruginosa. Finally, with a simultaneous decrease in the likelihood of contamination of the ulceration by Gram‐positive microorganisms, an increase in the chance of the Gram‐negative bacteria Proteus sp. being absent was noted.
It was determined that the Proteus mirabilis bacteria constitute the natural intestinal microflora of L sericata, thus being a natural defence against the action of pathogenic microorganisms towards the larvae. 34 , 35 Hence, it is possible that the source of these bacteria in the ulcers after therapy can be the same larvae, which during their life released these bacteria into the environment of the wound together with the secretion, which is a beneficial effect of larval therapy. Nonetheless, however, it can be observed that together with an elongation of the treatment period, the likelihood of a decrease in the colonisation of the wound by the Corynebacterium and Proteus species is increased, which can suggest a gradual decrease in the therapeutic effectiveness or could be a result of the larvae themselves dying; however, this does require further research.
In conclusion, microbiological analysis carried out as part of this study indicates the effectiveness of larval therapy in the case of lower limb and foot ulceration. The rearrangement of microflora in the wound area under larval therapy was noted. Of course, it is recommended that further studies, including those on a larger population of patients as well as studies with the use of molecular biological methods, which allows for a more precise assessment of therapy effectiveness.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We would like to thank Mr. Oskar Ogloszka for improving our work and checking our English. We would like to thank Mrs. Sonia Banaszak for helping in the figures preparation.
Szczepanowski Z, Grabarek BO, Boroń D, Tukiendorf A, Kulik‐Parobczy I, Miszczyk L. Microbiological effects in patients with leg ulcers and diabetic foot treated with Lucilia sericata larvae. Int Wound J. 2022;19:135–143. 10.1111/iwj.13605
DATA AVAILABILITY STATEMENT
All data was included in the paper.
REFERENCES
- 1. Yoon D, Sheen SS, Lee S, Choi YJ, Park RW, Lim HS. Statins and risk for new‐onset diabetes mellitus: a real‐world cohort study using a clinical research database. Medicine. 2016;95(46):e5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng Y, Ley SH, Hu FB. Global etiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88‐98. [DOI] [PubMed] [Google Scholar]
- 3. Sarinnapakorn V, Sunthorntepwaraku T, Deerochanawong C, Niramitmahapanya S, Napartivaumnuay N. Prevalence of diabetic foot ulcers and risk classifications in type 2 diabetes mellitus patients at Rajavithi hospital. J Med Assoc Thai. 2016;99(2):S99‐S105. [PubMed] [Google Scholar]
- 4. Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: from ulceration to death, a systematic review. Int Wound J. 2016;13:892‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saluja S, Anderson SG, Hambleton I, et al. Foot ulceration and its association with mortality in diabetes mellitus: a meta‐analysis. Diabet Med. 2020;37(2):211‐218. [DOI] [PubMed] [Google Scholar]
- 6. Brownrigg JR, Davey J, Holt PJ, et al. The association of ulceration of the foot with cardiovascular and all‐cause mortality in patients with diabetes: a meta‐analysis. Diabetologia. 2012;55:2906‐2912. [DOI] [PubMed] [Google Scholar]
- 7. Adeghate J, Nurulain S, Tekes K, Fehér E, Kalász H, Adeghate E. Novel biological therapies for the treatment of diabetic foot ulcers. Expert Opin Biol Ther. 2017;17(8):979‐987. [DOI] [PubMed] [Google Scholar]
- 8. Martin C, Verheggen F. Behavioural response of Lucilia sericata to a decaying body infested by necrophagous insects. Physiol Entomol. 2018;43:188‐195. [Google Scholar]
- 9. Linger RJ, Belikoff EJ, Yan Y, et al. Towards next generation maggot debridement therapy: transgenic Lucilia sericata larvae that produce and secrete a human growth factor. BMC Biotechnol. 2016;16(30):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bazaliński D, Karnas M, Wołkowicz M, Kózk M, Więch P. Zastosowanie larw lucilia sericata w oczyszczaniu ran przewlekłych‐opis trzech przypadków. Leczenie Ran. 2018;15(3):153‐159. [Google Scholar]
- 11. Pöppel AK, Kahl M, Baumann A, et al. A Jonah‐like chymotrypsin from the therapeutic maggot Lucilia sericata plays a role in wound debridement and coagulation. Insect Biochem Mol Biol. 2016;70:138‐147. [DOI] [PubMed] [Google Scholar]
- 12. Naik G, Harding K. Maggot debridement therapy: the current perspectives. Chronic Wound Care Manag Res. 2017;4:121‐128. [Google Scholar]
- 13. Li B, Lingsma HF, Steyerberg EW, Lesaffre E. Logistic random effects regression models: a comparison of statistical packages for binary and ordinal outcomes. BMC Med Res Methodol. 2011;11(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spiegelhalter D, Thomas A, Best N, Lunn D. WinBUGS Version 1.4.3. Cambridge: Medical Research Council‐Biostatistics Unit; 2004. [Google Scholar]
- 15. Teh CH, Nazni WA, Nurulhusna AH, Norazah A, Lee HL. Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin‐based turbidometric assay. BMC Microbiol. 2017;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aldhfyan Y, Morgan A, Alsubaie M, Alzahrani A. Bacteria patterns in infected diabetic foot: is there a surgical implication? Egypt J Hosp Med. 2018;70(10):1842‐1846. [Google Scholar]
- 18. Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and management of lower‐extremity ulcers. New Engl J Med. 2017;377(16):1559‐1567. [DOI] [PubMed] [Google Scholar]
- 19. Sopata M, Jawień A, Mrozikiewicz‐Rakowska B, et al. Wytyczne postępowania miejscowego w ranach niezakażonych, zagrożonych infekcją oraz zakażonych–przegląd dostępnych substancji przeciwdrobnoustrojowych stosowanych w leczeniu ran. Zalecenia Polskiego Towarzystwa Leczenia Ran. Leczenie Ran. 2020;17:1‐21. [Google Scholar]
- 20. Zhang Z, Lv L. Effect of local insulin injection on wound vascularization in patients with diabetic foot ulcer. Exp Ther Med. 2016;11(2):397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim JH, Ruegger PR, Lebig EG, et al. High levels of oxidative stress create a microenvironment that significantly decreases the diversity of the microbiota in diabetic chronic wounds and promotes biofilm formation. Front Cell Infect Microbiol. 2020;10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peña‐Villalobos I, Casanova‐Maldonado I, Lois P, et al. Hyperbaric oxygen increases stem cell proliferation, angiogenesis and wound‐healing ability of WJ‐MSCs in diabetic mice. Front Physiol. 2019;9:995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18(7):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Bacterial contribution in chronicity of wounds. Microb Ecol. 2017;73(3):710‐721. [DOI] [PubMed] [Google Scholar]
- 25. Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infectious Disease. 2012;54(2012):e132‐e173. [DOI] [PubMed] [Google Scholar]
- 26. Jneid J, Lavigne JP, La Scola B, Cassir N. The diabetic foot microbiota: a review. Human Microbiome J. 2017;5:1‐6. [Google Scholar]
- 27. Wang Y, Graves DT. Keratinocyte function in normal and diabetic wounds and modulation by FOXO1. J Diabetes Res. 2020;2020:3714704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szczepanowski Z, Tukiendorf A, Krasowski G. Further data on wound healing rates after application of Lucilia sericata . Int J Low Extrem Wounds. 2021;20(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 29. Tantawi TI, Gohar YM, William SG, Kotb MM, Abou Zeid NA. Clinical and microbiological efficacy of medicinal maggots in the treatment of pressure ulcers in Egypt. J Biosci Appl Res. 2017;3(4):152‐177. [Google Scholar]
- 30. Tsiouris CG, Tsiouri MG. Human microflora, probiotics and wound healing. Wound Med. 2017;19:33‐38. [Google Scholar]
- 31. Sherman RA, Mumcuoglu KY, Grassberger M, Tantawi TI. Maggot therapy. In: Sherman OS MGRA, Gileva CM, Kim H, Mumcuoglu KY, eds. Biotherapy History, Principles and Practice: a Practical Guide to the Diagnosis and Treatment of Disease Using Living Organisms. Dordrecht, Netherlands: Springer; 2019:5‐29. [Google Scholar]
- 32. Malekian A, Djavid GE, Akbarzadeh K, et al. Efficacy of maggot therapy on Staphylococcus aureus and Pseudomonas aeruginosa in diabetic foot ulcers: a randomized controlled trial. J Wound Ostomy Cont Nurs. 2019;46(1):25‐29. [DOI] [PubMed] [Google Scholar]
- 33. Steenvoorde P, Jukema GN. The antimicrobial activity of maggots: in‐vivo results. J Tissue Viability. 2014;14(3):97‐101. [DOI] [PubMed] [Google Scholar]
- 34. Sherman RA. Mechanisms of maggot‐induced wound healing: what do we know, and where do we go from here? Evid Based Complement Alternat Med. 2014;2014:592419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maleki‐Ravasan N, Ahmadi N, Soroushzadeh Z, Raz AA, Zakeri S, Dinparast DN. New insights into culturable and unculturable bacteria across the life history of medicinal maggots Lucilia sericata (Meigen) (Diptera: Calliphoridae). Front Microbiol. 2020;11:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data was included in the paper.


