Abstract
Amniotic tissues have been long utilised to treat chronic wounds; however, there are few studies evaluating how the wound microenvironment responds to these therapies. The goal of this study was to evaluate the changes in wounds treated with a hypothermically stored amniotic membrane (HSAM). In this prospective single‐arm study, 15 female patients with venous leg ulcers were treated with HSAM from male donors and standard of care for 12 weeks. Over the course of the study, wound exudate was collected and evaluated using proteomic microarrays. Biopsies were collected during the course of treatment to detect the presence of HSAM tissue. By 4 weeks, 60% of subjects achieved 50% or greater reduction in wound size, and by 12 weeks, 53% of subjects achieved 100% re‐epithelialization. HSAM DNA was detected in 20% of biopsies as determined by the detection TSPY4, indicating HSAM was no longer present within the wound bed approximately 7 days from the last treatment for the majority of wounds. Proteomic analysis of wound exudate found that wounds on a healing trajectory had significantly higher levels of MMP‐10, MMP‐7, and TIMP‐4 and significantly lower levels of CX3CL1, FLT‐3 L, IL‐1ra, IL‐1a, IL‐9, IL‐2, IL‐3, MCP‐1, and TNF‐b compared with other wounds.
Keywords: amnion allograft, hypothermically stored amniotic membrane (HSAM), placental membrane, venous leg ulcer, wound fluid
1. INTRODUCTION
Venous leg ulcers (VLUs) are a common cause of lower extremity ulceration, with estimates of prevalence ranging from 0.25 to 0.33 per 1000 individuals. 1 Non‐healing VLUs are a significant detriment to an individual's quality of life and pose a significant challenge due to their difficulty to heal and frequent rate of recurrence. Studies have reported up to 30% of VLUs fail to heal within 12 months with standard compression therapy, 2 and ulcer recurrence has been reported at 28% at 12 months. 3 Due in part to the high recurrence rate and associated morbidities, there is a high economic burden on healthcare systems for managing VLUs. A recent report estimated that within the United Kingdom the annual cost of treating VLUs was as high as £941.13 million. 4 Within the United States, the total Medicare spending for VLUs has been estimated to range from $720 million to up to $1.5 billion annually. Consequently, there is a strong need to develop reliable treatment solutions for VLUs.
While the development of leg ulcers is associated with venous hypertension, the precise mechanism responsible for the development and chronicity of VLUs is not clear. 5 VLUs are characterised by high levels of pro‐inflammatory cytokines including interleukin (IL)‐1 alpha, IL‐1β, and tissue necrosis factor alpha (TNFα), upregulated protease activity including matrix metalloproteases (MMPs), and serine proteases. 6 In addition to these high levels of cytokines and proteases, aberrant signalling pathways have also been identified within VLUs including Wnt/β‐catenin, TGFβ, and EGF pathways. 7 , 8 , 9 , 10 Additionally, VLUs often have a hyperproliferative wound edge and decreased angiogenesis. 7 , 11
Due to the challenges associated with treating VLUs, advanced wound care therapies are often utilised for these wounds, including bi‐layered skin substitutes, 12 collagen dressings, 13 and placental based allografts. Placental membranes including both amnion only and amnion/chorion grafts have a long history of clinical use for chronic and acute wounds dating back as early as 1913, 14 , 15 including utilising the amniotic membrane specifically for the treatment of VLUs. 16 , 17 More recently, additional clinical studies have supported the utilisation of placental‐derived membranes for the treatment of VLUs. 18 , 19 , 20 , 21 Several in vitro studies have characterised the proteomic make up of these grafts and have identified numerous growth factors and cytokines known to be important to the wound microenvironment. 22 , 23 , 24 Additionally, preclinical studies have demonstrated that amnion and amnion/chorion membranes can promote cellular proliferation and migration, stimulate angiogenesis, modulate inflammation, and inhibit protease activity in in vitro models. 22 , 24 , 25 , 26 , 27 , 28 While placental derived grafts have been well characterised using in vitro and in vivo models, there is a dearth of knowledge regarding how chronic wounds, including VLUs, may respond to the application of amniotic membranes.
Recently, an aseptically processed, hypothermically stored amniotic membrane (HSAM) has been developed for use as a wound covering. HSAM is designed to maintain the native ECM, cells and cytokines characteristic of fresh amniotic membranes. In vitro studies have demonstrated that HSAM contains growth factors and cytokines and released factors from these grafts promotes proliferation and migration of cells found within the wound microenvironment. 29 In vivo, the application of HSAM on full thickness acute wounds in a rodent model resulted in an improved quality of regenerated tissue. 30 Clinically, a randomised controlled trial of HSAM versus standard of care for diabetic foot ulcers demonstrated that treatment with HSAM significantly increased the frequency of wound closure compared with standard of care alone. 31 The goal of this study is to evaluate the response of VLUs to HSAM and additionally how the wound microenvironment of responds to HSAM.
2. METHODS
2.1. Statement of informed consent
The study was conducted in accordance with the clinical protocols and in accordance with principles consistent with Good Clinical Practice, 21 CFR 312, ICH E6, HIPAA regulations in 45 CFR Part 164. Western Institutional Review Board (WIRB; WA, USA) acted as the central institutional review board for the study. Subjects read and understood an institutional review board‐ (IRB) approved informed consent prior to undergoing study activities.
2.2. Patient screening and eligibility
The inclusion and exclusion criteria for this study are found in Table 1. Female patients over the age of 18 who were capable of giving consent with a VLU were assessed by a clinical investigator. Initial patient assessment was conducted at a screening visit which occurred 7 days prior to the baseline and initial treatment visit. During the initial screening visit, informed consent was completed, the subject's medical history was collected, and patient eligibility was determined. To be eligible for this study, VLUs needed to be between 2.0 and 25.0 cm2 and extend through the full thickness of the skin but not down to muscle, tendon or bone. Additionally, during the 7 ± 3 day run in period the target ulcer was required to be treated with adequate compression therapy. Lastly, VLUs had to have a clean granulation base with minimal adherent slough at the randomization visit. Ulcers which were infected, treated with other grafts or non‐standard or care therapies, or deemed by the investigator to be caused by a medical condition other than venous insufficiency were excluded from this study (Table 1). Additionally, ulcers which healed more than 20% from the time of the screening visit to the baseline visit were excluded from this study.
TABLE 1.
Study inclusion and exclusion criteria
|
2.3. Study treatments
Following screening, subjects who were eligible for inclusion into the study underwent an assessment for adverse events (AE), a review of concomitant medications, and a pain assessment. VLUs were treated using standard wound care approaches; study‐specific activities included sharp debridement of VLUs, wound imaging and measurements, application of the HSAM graft and the reapplication of standard multilayer compression bandaging. Weekly wound care techniques and reapplication of HSAM was completed for up to 12 weeks or until the ulcer achieved closure drainage free and 100% re‐epithelialized as determined by the investigator.
For the HSAM utilised in this study, all manufacturing, recovery, and processing of donated tissues were performed in accordance with all federal, state and local regulations, including the US FDA regulations 21 CFR 1270 and 1271. Serological testing for bacterial, viral, and infectious diseases as performed on blood specimens from each donor and exceeded the requirements of FDA and the American Association of Tissue Banks. Viral testing included antibodies to HIV‐1, HIV‐2, HTLV‐1, HTLV‐2, Hepatitis B Core, Hepatitis C, and CMV total. Tests for HIV‐1 nucleic acid, Hepatitis B Surface Antigen, and other adventitious viruses and pathogens were also conducted.
At treatment visits 2 to 5 and the end of study visit (13 weeks after the initial treatment visit) for wounds not achieving complete closure, wound exudate was collected from the ulcer using previously established methods. 32 Prior to debridement of the study ulcer, the ulcer was cleaned with sterile saline to remove loose debris and necrotic tissue. After the achievement of haemostasis, the wound area was moistened with saline, with care taken not to flood the area with excessive saline. Sterile foam tipped applicators (Puritan Medical Products, Guilford, Maine) were then used to collect the wound fluid by pressing the head of the applicator flat against the base of the wound and gently rotating it back and forth several times while applying pressure until the foam applicator head was coloured tan/yellow by wound fluid. When collecting the wound fluid care was taken not to swab areas containing blood, necrotic tissue or fibrinous tissue. At each collection time point, up to six swabs were taken. Swabs were then stored at −80°C until analysis.
At treatment visits 2, 3, and 5, biopsies were collected from the wound bed in order to evaluate the wound bed for the presence of remaining HSAM. Biopsies were collected following wound exudate collection and before debridement. First wounds were gently cleaned with saline in order to remove loose or necrotic tissue and the ulcer was numbed using 1% lidocaine as a local anaesthetic. A 4‐mm biopsy punch was then used to collect tissue 3 mm away from the wound margin, and the tissue was then transferred into a cryovial with 2 mL of RNAlater (Qiagen, Manchester, UK) and stored at −80°C until analysis.
2.4. Study outcomes
For this study, clinical endpoints included the proportion of VLUs that achieved complete wound closure at 12 weeks, the proportion of VLUs that achieved 50% or greater reduction in size by 4 and 8 weeks, time to initial wound closure, and the number of applications of HSAM. Scientific endpoints included measuring the changes in cytokines, growth factors, and MMPs found in the wound fluid, comparing levels of cytokines, growth factors, and MMPs in wound exudate between healed and non‐healed wounds, and the presence of HSAM in wound biopsies collected 1 week after application. Safety endpoints included the number and types of AE, serious adverse events (SAE), and the number of patients with worsening of VLUs (as defined by a 50% increase in wound size).
2.5. Proteomic evaluation of wound exudate
Cytokine, growth factors, and MMPs were measured from collected wound fluid samples using the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel ‐ Premixed 41 Plex ‐ Immunology Multiplex Assay, MILLIPLEX MAP Human MMP Magnetic Bead Panel 2, MILLIPLEX MAP Human TIMP Magnetic Bead Panel 2, and the MILLIPLEX MAP TGFß Magnetic Bead 3 Plex Kit, Arrays (EMD Millipore, Burlington, Massachusetts). The extraction of wound fluid from the foam applicators was performed as previously described. 33 Briefly, applicator tips were submerged and mixed in 110 μL of sample diluent before centrifuging both the applicator and diluent in a 0.45 μm pore centrifuge tube filter at 930g for 2 minutes. All arrays were then conducted as per the manufacturer's instructions. The resulting protein levels were then reported as pg/mL of wound fluid samples.
Samples which were detected below the array's reported level of detection were set to 0 and samples above the reported maximum levels of detection were set to the maximum value for analysis. Proteomic data were then evaluated by comparing protein levels in patients on a healing trajectory (termed “healers”) compared with those not on a healing trajectory (termed “non‐healers”); for the purposes of this analysis, healers were defined as wounds which achieved greater than 85% closure by treatment visit 12 or were healed during the course of the study. Ratios of MMPs to TIMPs were also compared between healing and non‐healing wounds as well as the ratio of IL‐1b to IL‐1ra. Additionally, the correlation between protein levels in wound fluid and the percent area reduction (PAR) of wound size was evaluated for study ulcers with PARs between −50% and 50%.
2.6. Biopsy collection and detection for male DNA
In order to determine whether HSAM remained present in the wound bed 1 week after application, biopsies collected from the wound bed were evaluated for the presence of Y chromosome specific genes as HSAM grafts were sourced from male donors and all enrolled subjects were female. Specifically, SRY and TSPY4 genes, which have been utilised by others, 34 , 35 , 36 were evaluated in this study. Biopsies post‐collection were stored at −80°C in RNAlater. For analysis, biopsies were thawed and minced using a sterile scalpel and placed in a microcentrifuge tube with 600 μL of Buffer RLT Plus (Qiagen) with 10 μL β‐mercaptoethanol (Gibco, Waltham, Massachusetts) and homogenised for approximately 60 seconds (OmniGLI homogenizer, Omni International, Kennesaw, Georgia). After homogenization of biopsies, DNA was extracted from the samples using All prep DNA/RNA Mini Kits (Qiagen). DNA concentration was then measured using a Take3 Trio plate in a Synergy H1 plate reader (BioTek, Winooski, Vermont), and samples with an OD 260/280 between 1.8 and 2.0 were used. Taqman probes (ThermoFisher, Waltham, Massachusetts) for SRY (Hs00976796_s1) and TSPY4 (Hs005049137_g1) were used with GAPDH (Hs03929097_g1) as the housekeeping gene along with TaqMan™ Fast Advanced Master Mix.
In order to determine the overall percentage of male DNA in a sample, standards from 0.01% to 100% male to female DNA were evaluated using male and female genomic DNA standards (Promega, Madison, Wisconsin). For all standards and samples, a 20 μL reaction volume was used with 100 ng DNA per reaction. PCR reactions were performed using a QuantStudio 3 (Applied Biosystems, ThermoFisher) with a UNG incubation step at 50°C for 2 minutes, a polymerase step at 95°C for 20 seconds, and 40 PCR cycles at 95°C for 1 second and 60°C for 20 seconds. The resulting Ct values were then analysed by calculating the δCt (CtSRY − CtGAPDH, or CtTSPY4 − CtGAPDH) value, standards were then plotted against the standards with known % male DNA and the resulting standard curve was used to calculate the % male DNA for each sample.
2.7. Statistical methods
All continuous variables were reported as mean with standard deviation, categorical variables were reported in terms of frequencies and percentages. For comparing wound fluid proteomic data in healing and non‐healing patients, a Mann–Whitney test was used to compare each protein where P < .05 was considered significant. When evaluating correlations between protein levels and percent area reduction of wounds, Pearson correlation coefficients were calculated for each protein where P < .05 was considered significant.
3. RESULTS
A total of 19 subjects were screened for inclusion in the study: there were 4 screen failures, and 15 subjects were enrolled in this study. Enrolment and treatment for this study was conducted from March 2017 through April 2018. A summary of the subject demographic information can be found in Table 2. The average initial wound size was 8.6 ± 7.9 cm2 and had been present for an average of 50.3 weeks ±103.3 (minimum ulcer duration was 8 weeks and the maximum duration was 8 years). On average the total number of HSAM Applications was 8 ± 3 over the course of the study. By 4 weeks 60% (9/15) of subjects had achieved 50% closure, by 8 weeks 73% (11/15) of subjects had achieved 50% or greater wound closure, and by 12 weeks 73% (11/15) of subjects achieved 50% or greater wound closure. Complete wound closure was achieved for 53% (8/15) subjects at 12 weeks. The percent area reduction over the course of the study for each subject is plotted in Figure 1.
TABLE 2.
Patient demographics and ulcer history. Values reported as either the mean (standard deviation) or percent of subject population
| Total population N = 15 | Healing trajectory N = 11 | Non‐healed N = 4 | |
|---|---|---|---|
| Age (years) | 62.6 (12.7) | 62.6 (10.4) | 62.5 (19.8) |
| History of tobacco use | 6.7% (1/15) | 0.0% (0/11) | 25.0% (1/4) |
| Diabetes | 40.0% (6/15) | 36.4% (4/11) | 50.0% (2/4) |
| History of recurrent ulcers | 26.7% (4/15) | 27.3% (3/11) | 25.0% (1/4) |
| BMI | 41.9 (14.01) | 41.3 (10.2) | 43.5 (22.5) |
| ABI | 0.99 (0.14) | 1.00 (0.15) | 0.97 (0.11) |
| Study ulcer age (weeks) | 50.3 (103.3) | 51.8 (121.04) | 46.25 (30.5) |
| Study ulcer initial size (cm2) | 8.1 (7.9) | 7.1 (5.9) | 12.6 (11.9) |
Notes: The “Healed” category includes subjects with wounds which achieved greater than 85% closure by treatment visit 12 or were healed during the course of the study. The “Non‐Healed” category includes subjects with wounds which did not achieved greater than 85% closure by treatment visit 12.
Abbreviations: ABI, ankle‐brachial index; BMI, body mass index.
FIGURE 1.
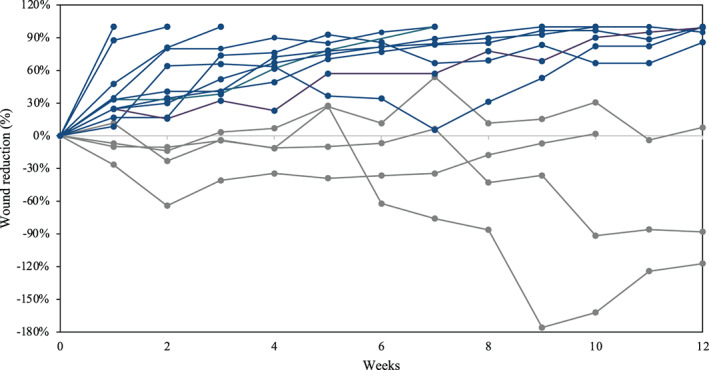
Percent area reduction of venous leg ulcers (VLUs) treated with hypothermically stored amniotic membrane (HSAM). Blue lines represent VLUs which were identified as on a trajectory of healing and grey lines represent VLUs identified as non‐healing
Over the course of the study a total of 18 AE including 1 SAE were reported, and 2 of the subjects' study ulcers increased more than 50% by the end of the study. Of the 18 AE reported, 13 were deemed to be not related to the study treatment, and 5 were infections to either the study ulcer or study limb. Of the five infections reported in this study, three occurred on subjects who had a stagnant or worsening ulcer (as shown in Figure 1) during the 12‐week duration of this study. The SAE was an amputation to the 1st metatarsal.
A total of 73 wound exudate samples were collected for proteomic analysis with 47 samples collected from 11 subject with ulcers that healed and 24 samples from 4 subjects with ulcers that did not heal. Heat maps show the relative change in growth factor and cytokine levels from the initial wound fluid collected prior to the first application of HSAM (Figure 2). Red colour indicates an increase from baseline and green indicates a downregulation of the target. Interestingly, we found the greatest changes in protein levels, relative to baseline values, was 1 week after the first application of HSAM (Figure 2A). When comparing protein levels within the wound exudate between healers and non‐healers across all time points, 12 targets were identified that were significantly different between these two populations (Table 3). These targets included TIMP‐4 (P = .0477), MMP‐7 (P = .0205), and MMP‐10 (P = .0305) which were higher in wounds on a healing trajectory compared with non‐healing ulcers, and CX3CL1 (P = .477), FLT‐3 L (P = .474), IL‐1ra (P = .0029), IL‐1a (P = .0131), IL‐9 (P = .0001), IL‐2 (P = .0075), IL‐3 (P = .0153), MCP‐1 (P = .032), and TNF‐b (P = .0005) were higher in ulcers which were non‐healing ulcers compared with those on a healing trajectory. Additionally, we compared protein levels within wound exudate to the percent reduction of wounds at 1, 2, 3, 4, and 12 weeks, and we found levels of several protein targets that correlated with the change in wound area (Figure 3). TIMP2, MMP‐10, and EGF had a significant positive correlation between increased protein levels and reduction in wound size. IL‐1ra, IL‐7, and IL‐8 had a significant negative correlation between protein levels and change in wound size.
FIGURE 2.
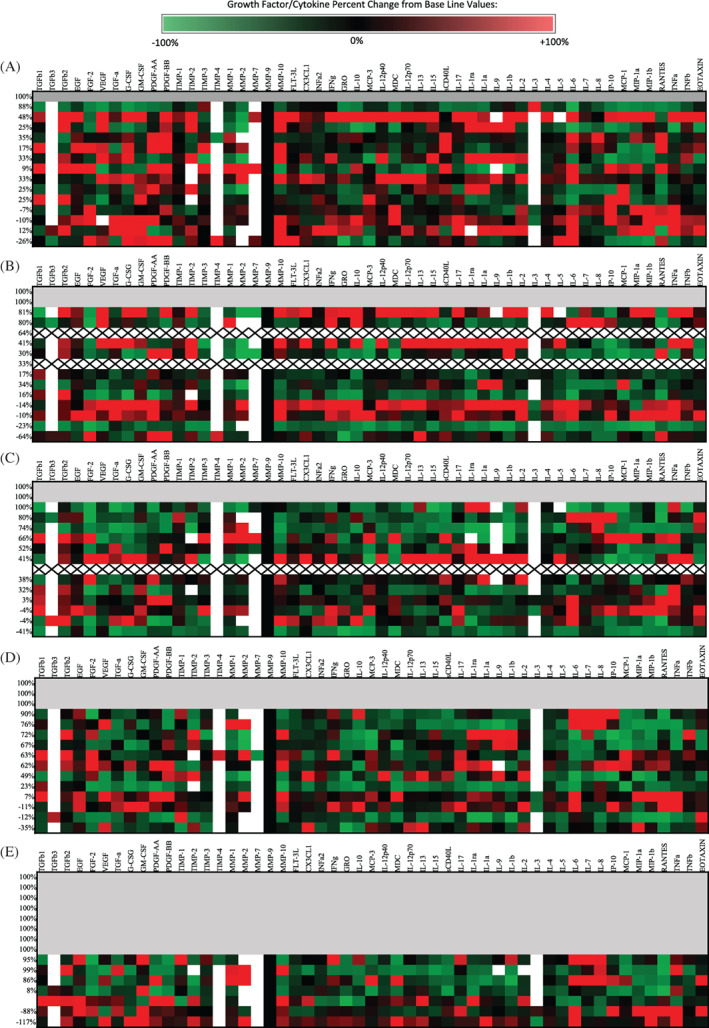
Heat maps illustrating the changes in cytokine concentrations within wound exudate of venous leg ulcers (VLUs) relative to baseline values at (A) 1 week, (B) 2 weeks, (C) 3 weeks, and (D) 4 weeks. The respective wound closure for each subject at each time point is included along the left of each table. Grey shading indicates healed wounds, “X” indicates missing data due to missed visit, and white represent values which were outside the range of detection
TABLE 3.
Biomarkers identified with significant differences between healing and non‐healing VLUs, values represented as mean with the lower and upper 95% confidence interval values
| Biomarker | Healing trajectory (N = 47) | Non‐healed (N = 24) | P value |
|---|---|---|---|
| CX3CL1 (Fractalkine) | 134.9 (113.2, 156.5) | 181.4 (135.7, 227.0) | .0477 |
| FLT‐3 L | 18.70 (17.21, 20.18) | 24.29 (19.75, 28.83) | .474 |
| IL‐1a | 2355 (1782, 2928) | 4610 (3194, 6027) | .0131 |
| IL‐1ra | 5250 (4388, 6113) | 7328 (6232, 8423) | .0029 |
| IL‐2 | 2.4 (1.7, 3.1) | 4.6 (2.2, 7.1) | .0075 |
| IL‐3 | 0.3 (0.09, 0.50) | 0.58 (0.27, 0.89) | .0153 |
| IL‐9 | 0.93 (0.66, 1.2) | 1.9 (1.4, 2.3) | .0001 |
| MCP‐1 | 1556 (984.5, 2128) | 3409 (1901, 4917) | .0320 |
| MMP‐7 | 2921 (−74.05, 5917) | 326 (−50.3, 702.3) | .0205 |
| MMP‐10 | 6910 (5234, 8585) | 4158 (2274, 6042) | .0305 |
| TIMP‐4 | 41.2 (19.8, 63.0) | 2.7 (−0.6, 6.1) | .0251 |
| TNFb | 2.2 (1.7, 2.7) | 3.1 (2.6, 3.6) | .0005 |
FIGURE 3.
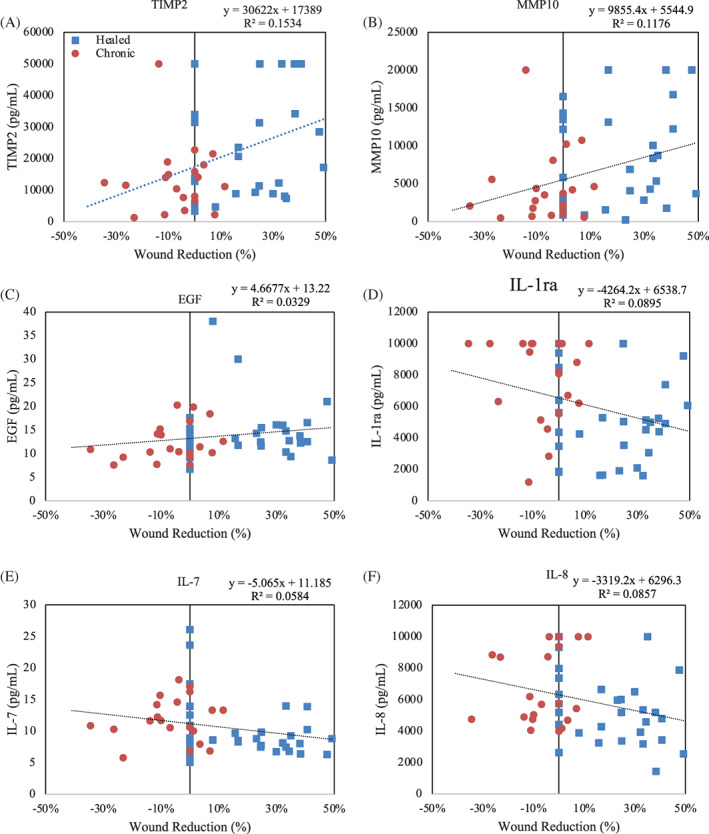
Biomarker correlation to percent wound reduction for: (A) TIMP‐2, (B) MMP10, (C) EGF, (D) IL‐1ra, (E) IL‐7, and (F) IL‐8. Red data points are from non‐healing venous leg ulcers (VLUs) and blue data points are form healing VLUs
To get a better idea of the status of the wound microenvironment, we evaluated ratios of targets and compared between groups. When evaluating ratios of MMPs to TIMPs, only the MMP‐10/TIMP‐1 ratio was found to be significantly different (P = .0134) between wounds on a healing trajectory (mean = 3.81, lower and upper 95% confidence interval = 2.86, 4.76) and non‐healed wounds (mean = 2.11, lower and upper 95% confidence interval = 1.13, 3.09). Additionally, the ratio of IL‐1b/IL‐1ra and MMP‐10/TIMP‐1 had a significant positive correlation to the change in wound size whereas the MMP‐2/TIMP‐3 and MMP‐2/TIMP‐2 ratios had a significant negative correlation with the change in wound size (Figure 4).
FIGURE 4.
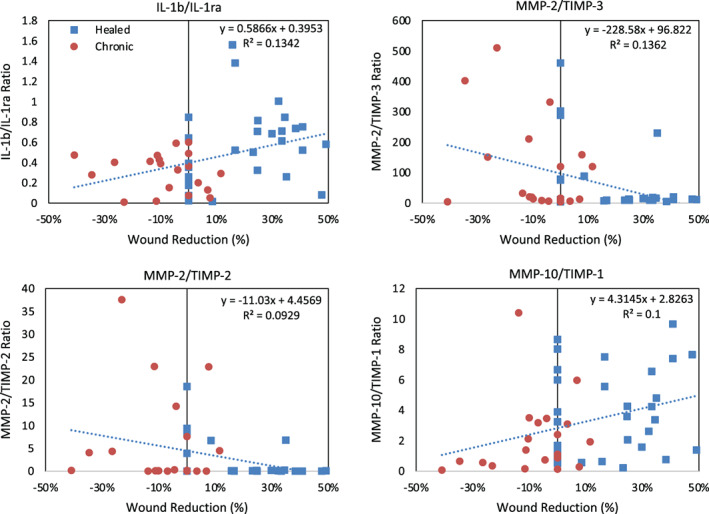
Biomarker ratios correlation to percent wound reduction for: (A) IL‐1b/IL‐1ra, (B) MMP‐2/TIMP‐3, (C) MMP‐2/TIMP‐2, and (D) MMP‐10/TIMP‐1. Red data points are from non‐healing venous leg ulcers (VLUs) and blue data points are form healing VLUs
A total of 33 biopsies were collected from study ulcers over the course of this study from 13 patients. Of these samples, 10 samples met the required 260/280 threshold for further analysis. While expression of SRY was undetected in all samples, we found TSPY4 in two of the samples, when compared with standards these samples contained approximately 0.79% and 0.17% male DNA (Table 4).
TABLE 4.
Detection of male DNA from biopsies retrieved from VLUs
| Subject ID | Weeks following initial application | % male DNA (SRY) | % male DNA (TSPY4) |
|---|---|---|---|
| 001‐001 | 2 | ND | 0.79% |
| 001‐002 | 1 | ND | ND |
| 001‐002 | 2 | ND | ND |
| 001‐003 | 2 | ND | ND |
| 001‐007 | 2 | ND | 0.17% |
| 001‐013 | 2 | ND | ND |
| 001‐013 | 4 | ND | ND |
| 002‐001 | 2 | ND | ND |
| 002‐003 | 1 | ND | ND |
| 002‐003 | 2 | ND | ND |
4. DISCUSSION
The goal of this study was to investigate how VLUs respond to the application of HSAMs. In this study, we found that HSAM was well tolerated within the wound bed and over the 12‐week study period 53% (8/15) subjects achieved complete closure of VLUs treated with HSAM. Proteomic analysis of wound exudate identified 12 biomarkers which had significantly different concentrations between study wounds on a healing trajectory versus non‐healers. A more detailed analysis identified a number of correlations between biomarker concentration and percent area wound reduction. Lastly, DNA analysis of wound biopsies collected from the study ulcer generally failed to identify graft DNA within the study ulcer indicating that these grafts were predominately no longer present in the wound bed 7 days after application.
This study is novel as it is, to our knowledge, the first study to evaluate changes in the growth factor/cytokine levels within the VLU microenvironment over the course of treatment with placental membranes, and is also the first study to attempt to identify the dwell time of amniotic membranes applied to a wound bed. We found that HSAM, in addition to multilayer compression therapy, was effective in managing most chronic VLUs. While the single arm structure of this study prevents a direct comparison to standard of care, prior prospective studies have evaluated placental membranes for the treatment of VLUs. Serena et al. reported a percent wound reduction at 4 weeks of 48.1% for 53 VLUs treated with dehydrated human amnion/chorion (dHACM) and compression therapy alone compared with a 19% reduction in 31 VLUs with compression therapy alone. 19 Bianchi et al reported 12 week closure rates of 60% for dHACM and compression therapy for 52 VLUs, and 35% for standard of care alone for 57 VLUs. 18 In a 15‐patient study of VLUs that received frozen amniotic membrane, Mermet et al reported an average percent area reduction of 63.4% at 4 weeks and at 12 weeks 80% of subjects had a 50% or greater reduction in wound size including three subjects whose wounds achieved complete closure. 21 Varying parameters in these studies prevent direct comparison to these results, in particular, ulcer severity between the studies. VLUs in these studies had a mean size of 4.6 to 7.6 cm2 and a duration of 41.9 to 55.2 weeks, which is generally less severe than ulcers evaluated in our study which had an average size of 8.6 ± 7.9 cm2 and a duration or 50.3 ± 103.3 weeks. Despite these differences, in this small population we found comparable results with the use of HSAM with multilayer compression.
While this is the first study to investigate the proteomic changes within a VLU treated with a placental‐derived product, this is not the first study to investigate changes in wound fluid of healing and non‐healing chronic wounds. Stacey et al also reported a number of differentially expressed growth factors and cytokines in the wound fluid of healing and non‐healing VLUs including Eotaxin, GM‐CSF, ICAM‐1, IL‐6, IL‐16, MCP‐1, MIP‐1a, MMP‐13, PDGF‐BB, and TIMP‐4. 37 Similarly, Edsberg et al conducted a proteomic analysis on the wound fluid of pressure ulcers and identified 20 proteins which were also differentially expressed between chronic and healed wounds including GM‐CSF, I‐309, INF‐γ IL‐11, IL‐12p40, IL‐15, IL‐1a, IL‐1b, IL‐8, TIMP‐1, TIMP‐2 TNF RI, TNF RII, ICAM‐1, IL‐16, MIP‐1d, MMP‐10, MMP‐13, MMP‐3, and Eotaxin‐2. 38 In this study, we observed similar trends as seen in other studies with TIMP‐4, IL‐1a, and MMP‐10. 37 , 38 Prior studies have identified that protease levels within the wound bed of VLUs may be predictive of the outcome of the wound, specifically MMP‐1, 39 MMP‐2, 39 , 40 MMP‐7, 39 and MMP‐9 33 , 39 , 40 , 41 ; however, it should be noted that there is no current consensus that protease activity is an indicator of healing in VLUs. 42 In our study, we observed that MMP‐7 was elevated in VLUs on a healing trajectory relative to non‐healed, VLUs which is consistent with the results reported by Raffetto et al. 39 When looking at ratios of proteases to protease inhibitors, we did observe a negative correlation between MMP‐2/TIMP‐3 and wound reduction and MMP‐2/TIMP‐2 and wound reduction. Of particular interest, we found the largest changes in growth factor and cytokine levels appeared to occur following the first application of HSAM, potentially suggesting a shift from a chronic to a more acute state.
Interestingly, we found 2 out of 11 samples had low levels of detectable male DNA. The overall lack of significant levels of detectable male DNA from VLUs suggests that applied HSAM grafts may remain in the wound bed for 1 to 2 weeks following application. Only in two cases were any male DNA detected, but should also be noted that the detection of DNA from the HSAM does not suggest graft viability, only the presence of tissue within the sample. 43 Of note in our study, only expression of TSPY4 was detected; these results seem to be consistent with prior studies which have shown that the TSPY family was a more sensitive marker of male DNA than SRY. 34 These results are similar to studies investigating the retention of bilayered living cellular constructs (BLCC, Apligraf, Organogenesis, Canton, Massachusetts) within chronic wounds, where in a majority of subjects DNA from the graft was not detected 1 week after application. 44 , 45
Study limitations include a single arm structure, the inclusion of only female subjects, and the quantification of proteases using multiplex MAP arrays alone. Future multi‐centre, randomised studies will need to be conducted to fully elucidate the efficacy of treating VLUs with HSAM compared with the standard of care. The inclusion of female only subjects was necessary in this study in order to detect HSAM using markers for male DNA; however, gender differences may play a role in tissue response. Finally, the proteomic evaluation of wounds using the multiplex MAP arrays does not provide information on protease activity; this is especially relevant for the detection of proteases where these arrays would have detected both active and inactive versions, that is, MMPs. Despite these limitations, the results presented herein provide the first data suggesting how VLUs may respond to the applications of placental membranes. It is the authors' opinion that studies evaluating how patient's tissue respond to therapies are essential to the further advancement of chronic wound care.
CONFLICT OF INTEREST
J. P. McQuilling and K. C. Mowry are employees of Organogenesis. M. J. Carter and T. E. Serena have provided consulting services to Organogenesis. All other authors have no disclosures.
McQuilling JP, Carter MJ, Fulton JA, et al. A prospective clinical trial evaluating changes in the wound microenvironment in patients with chronic venous leg ulcers treated with a hypothermically stored amniotic membrane. Int Wound J. 2022;19:144–155. 10.1111/iwj.13606
Funding information Organogenesis
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cullum N, Buckley H, Dumville J, et al. Wounds research for patient benefit: a 5‐year programme of research. Program Grants Appl Res. 2016;4(13):1‐304. [PubMed] [Google Scholar]
- 2. Ashby RL, Gabe R, Ali S, et al. Clinical and cost‐effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial. Lancet (London, England). 2014;383(9920):871‐879. [DOI] [PubMed] [Google Scholar]
- 3. de Carvalho MR. Comparison of outcomes in patients with venous leg ulcers treated with compression therapy alone versus combination of surgery and compression therapy. J Wound Ostomy Cont Nurs. 2015;42(1):42‐46. [DOI] [PubMed] [Google Scholar]
- 4. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that different wound types impose on the UK's National Health Service. Int Wound J. 2017;14(2):322‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alavi A, Sibbald RG, Phillips TJ, et al. What's new: management of venous leg ulcers. J Am Acad Dermatol. 2016;74(4):627‐640. [DOI] [PubMed] [Google Scholar]
- 6. Liu YC, Margolis DJ, Rivkah IR. Does inflammation have a role in the pathogenesis of venous ulcers?: a critical review of the evidence. J Invest Dermatol. 2011;131(4):818‐827. [DOI] [PubMed] [Google Scholar]
- 7. Stojadinovic O, Pastar I, Vukelic S, et al. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med. 2008;12(6b):2675‐2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds. Am J Pathol. 2005;167(1):59‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stojadinovic O, Pastar I, Nusbaum AG, Vukelic S, Krzyzanowska A, Tomic‐Canic M. Deregulation of epidermal stem cell niche contributes to pathogenesis of nonhealing venous ulcers. Wound Repair Regen. 2014;22(2):220‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pastar I, Stojadinovic O, Krzyzanowska A, et al. Attenuation of the transforming growth factor β‐signaling pathway in chronic venous ulcers. Mol Med. 2010;16(3–4):92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drinkwater SL, Smith A, Sawyer BM, Burnand KG. Effect of venous ulcer exudates on angiogenesis in vitro. Br J Surg. 2002;89(6):709‐713. [DOI] [PubMed] [Google Scholar]
- 12. Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol. 1998;134(3):293‐300. [DOI] [PubMed] [Google Scholar]
- 13. Westby MJ, Norman G, Dumville JC, Stubbs N, Cullum N. Protease‐modulating matrix treatments for healing venous leg ulcers. Cochrane Database Syst Rev. 2016;12:CD011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sabella N. Use of fetal membranes in skin grafting. Med Rec. 1913;83:478‐480. [Google Scholar]
- 15. Stern M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituing skin grafts. JAMA. 1913;60(13):973. [Google Scholar]
- 16. Shun A, Ramsey‐Stewart G. Human amnion in the treatment of chronic ulceration of the legs. Med J Aust. 1983;2(6):279‐283. [DOI] [PubMed] [Google Scholar]
- 17. Bennett JP, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet (London, England). 1980;1(8179):1153‐1156. [DOI] [PubMed] [Google Scholar]
- 18. Bianchi C, Cazzell S, Vayser D, et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix®) allograft for the treatment of venous leg ulcers. Int Wound J. 2018;15(1):114‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Serena TE, Carter MJ, Le LT, et al. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22(6):668‐693. [DOI] [PubMed] [Google Scholar]
- 20. Regulski M, Jacobstein DA, Petranto RD, Migliori VJ, Nair G, Pfeiffer D. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage. 2013;59(12):38‐43. [PubMed] [Google Scholar]
- 21. Mermet I, Pottier N, Sainthillier JM, et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen. 2007;15(4):459‐464. [DOI] [PubMed] [Google Scholar]
- 22. Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353‐1362. [DOI] [PubMed] [Google Scholar]
- 23. McQuilling JP, Vines JB, Kimmerling KA, Mowry KC. Proteomic comparison of amnion and chorion and evaluation of the effects of processing on placental membranes. Wounds. 2017;29(6):E38‐E42. [PMC free article] [PubMed] [Google Scholar]
- 24. McQuilling JP, Burnette M, Kimmerling KA, Kammer M, Mowry KC. A mechanistic evaluation of the angiogenic properties of a dehydrated amnion chorion membrane in vitro and in vivo. Wound Repair Regen. 2019;27(6):609‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duan‐Arnold Y, Uveges TE, Gyurdieva A, Johnson A, Danilkovitch A. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv Wound Care. 2015;4(9):513‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koob TJ, Lim JJ, Massee M, et al. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell. 2014;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duan‐Arnold Y, Gyurdieva A, Johnson A, Uveges TE, Jacobstein DA, Danilkovitch A. Retention of endogenous viable cells enhances the anti‐inflammatory activity of cryopreserved amnion. Adv Wound Care. 2015;4(9):523‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McQuilling JP, Kammer M, Kimmerling KA, Mowry KC. Characterisation of dehydrated amnion chorion membranes and evaluation of fibroblast and keratinocyte responses in vitro. Int Wound J. 2019;16(3):827‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McQuilling JP, Vines JB, Mowry KC. In vitro assessment of a novel, hypothermically stored amniotic membrane for use in a chronic wound environment. Int Wound J. 2017;14(6):993‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mowry KC, Bonvallet PP, Bellis SL. Enhanced skin regeneration using a novel amniotic‐derived tissue graft. Wounds. 2017;29(9):277‐285. [PubMed] [Google Scholar]
- 31. Serena TE, Yaakov R, Moore S, et al. A randomized controlled clinical trial of a hypothermically stored amniotic membrane for use in diabetic foot ulcers. J Comp Eff Res. 2020;9(1):23‐34. [DOI] [PubMed] [Google Scholar]
- 32. Serena TE. Development of a novel technique to collect proteases from chronic wounds. Adv Wound Care. 2014;3(12):729‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Serena TE, Cullen BM, Bayliff SW, et al. Defining a new diagnostic assessment parameter for wound care: elevated protease activity, an indicator of nonhealing, for targeted protease‐modulating treatment. Wound Repair Regen. 2016;24(3):589‐595. [DOI] [PubMed] [Google Scholar]
- 34. Jacot TA, Zalenskaya I, Mauck C, Archer DF, Doncel GF. TSPY4 is a novel sperm‐specific biomarker of semen exposure in human cervicovaginal fluids; potential use in HIV prevention and contraception studies. Contraception. 2013;88(3):387‐395. [DOI] [PubMed] [Google Scholar]
- 35. Zimmermann BG, Holzgreve W, Avent N, Hahn S. Optimized real‐time quantitative PCR measurement of male fetal DNA in maternal plasma. Ann N Y Acad Sci. 2006;1075:347‐349. [DOI] [PubMed] [Google Scholar]
- 36. Kastelic V, Budowle B, Drobnic K. Validation of SRY marker for forensic casework analysis. J Forensic Sci. 2009;54(3):551‐555. [DOI] [PubMed] [Google Scholar]
- 37. Stacey MC, Phillips SA, Farrokhyar F, Swaine JM. Evaluation of wound fluid biomarkers to determine healing in adults with venous leg ulcers: a prospective study. Wound Repair Regen. 2019;27(5):509‐518. [DOI] [PubMed] [Google Scholar]
- 38. Edsberg LE, Wyffels JT, Brogan MS, Fries KM. Analysis of the proteomic profile of chronic pressure ulcers. Wound Repair Regen. 2012;20(3):378‐401. [DOI] [PubMed] [Google Scholar]
- 39. Raffetto JD, Mosti G, Santi M, Ligi D, Mannello F. Matrix metalloproteinase profiles in chronic venous ulcer wound fluid of inflammatory and granulating venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2015;3(1):119‐120. [DOI] [PubMed] [Google Scholar]
- 40. Mwaura B, Mahendran B, Hynes N, et al. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase‐2, tissue inhibitor of matrix metalloproteinase‐2 and PDGF‐AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31(3):306‐310. [DOI] [PubMed] [Google Scholar]
- 41. Serra R, Buffone G, Falcone D, et al. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair Regen. 2013;21(3):395‐401. [DOI] [PubMed] [Google Scholar]
- 42. Westby MJ, Dumville JC, Stubbs N, et al. Protease activity as a prognostic factor for wound healing in venous leg ulcers. Cochrane Database Syst Rev. 2018;9:CD012841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yasuda T, Weisel RD, Kiani C, Mickle DAG, Maganti M, Li R‐K. Quantitative analysis of survival of transplanted smooth muscle cells with real‐time polymerase chain reaction. J Thorac Cardiovasc Surg. 2005;129(4):904‐911. [DOI] [PubMed] [Google Scholar]
- 44. Stone RC, Stojadinovic O, Rosa AM, et al. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci Transl Med. 2017;9(371):eaaf8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu S, Kirsner RS, Falanga V, Phillips T, Eaglstein WH. Evaluation of ApligrafR persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen. 2006;14(4):427‐433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


