Abstract
Wound healing is a complex process of communication between growth factors, reactive species of oxygen, cells, signalling pathways, and cytokines in the extracellular matrix, in which growth factors are the key regulators. In humans, the main regulators of the cellular responses in wound healing are five growth factors, namely EGF, bFGF, VEGF, and TGF‐β1. On the other hand, antioxidants such as astaxanthin, beta‐carotene, epigallocatechin gallate, delphinidin, and curcumin have been demonstrated to stimulate cell proliferation, migration and angiogenesis, and control inflammation, to suggest a practical approach to design new strategies to treat non‐healing cutaneous conditions. Based on the individual effects of growth factors and antioxidants, it may be envisioned that the use of both types of bioactives in wound healing formulations may have an additive or synergistic effect on the healing potential. This review addresses the effect of growth factors and antioxidants on wound healing‐related processes. Furthermore, a prospective on their potential additive or synergistic effect on wound healing formulations, based on their individual effects, is presented. This may serve as a guide for the development of a new generation of wound healing formulations.
Keywords: antioxidants, growth factors, wound healing, signalling pathway
1. INTRODUCTION
The skin has multiple layers that protect against harmful external factors such as pathogens, radiation, heat, and wound. 1 A wound compromises the structure and function of the skin, and the type of wound is divided into acute (eg, trauma, burns, and surgery) and chronic (eg, diabetic and pressure ulcers). 2 , 3 The damage of a wound increases by external factors and patient condition producing reactive oxygen species (ROS), which affect the elements involved in skin repair such as in chronic wounds. 2 , 4
The wound healing process involves a diversity of elements, such as growth factors, cells, signalling pathways, and cytokines, which all work in synergy. 5 Growth factors and their signalling pathways coordinate cell responses in the wound healing process. 6 , 7 The mechanism of these elements helps in developing accurate treatments for impaired healing wounds, wherein growth factors are used in wound healing treatments because they coordinate cell responses in the wound healing phases.
Proliferation, migration, angiogenesis, and inflammation are cellular responses activated by growth factors. Uncontrolled rates of ROS alter the modulation of these cellular responses, resulting in excessive scarring or impaired wounds. 6 , 7 In this regard, antioxidants have shown regulation of cellular responses activated by growth factors, which aid in the repair of different types of wounds. 4 , 8 , 9 The potential effect of the combination of growth factors and antioxidants could lead the search to find diverse alternatives in cutaneous repair with high quality and reduced time. 8 This review aims to present the role of growth factors and antioxidants on skin wound healing‐related processes. Furthermore, based on their individual effects, a prospective analysis on their potential combinatorial effect on wound‐healing formulations is presented. This information may serve as a guide to envision further studies focused on the confirmation of those effects, allowing the rational design and development of novel skin wound‐healing formulations.
2. SKIN WOUND HEALING PHASES: ROLE OF GROWTH FACTORS AND ROS
The wound healing occurs in four overlapped and sequential phases, namely (a) haemostasis, (b) inflammation, (c) proliferation, and (d) remodelling. 10 They are synchronised by particular endogenous polypeptides, called growth factors, secreted by six specific cells recruited at the wound site: platelets, macrophages, keratinocytes, fibroblasts, mast cells, and neutrophils. 11 , 12 These growth factors activate the paracrine and autocrine cell communication by binding to their specific receptors, and also they are essential for the cellular function, namely proliferation, migration, angiogenesis, and inflammation. 11 , 13 Growth factors that have been demonstrated to have a major role in the wound healing process are platelet‐derived growth factor (PDGF), transforming growth factor‐beta 1 (TGF‐β1), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF). 10 Such growth factors are key elements in wound healing because they are responsible for the cellular communication and regulation of cellular responses that trigger the proliferation, migration, and differentiation of damaged cells and events when the balance of the inflammatory response, neovascularisation, and modulation of extracellular matrix (ECM) occurs. 12 The secondary key element in wound healing is ROS. 14 ROS produced at controlled levels stimulate haemostasis, pathogen defence, tissue repair, and lymphocyte recruitment in the wound healing process. 14 The deficiency of these growth factors and the excess of ROS levels is related to non‐healing conditions. 12 Nonetheless, growth factor administration is a promising strategy for wound healing management or treatment. Figure 1 shows the effect of the key growth factors and antioxidants involved in each wound healing phase.
FIGURE 1.
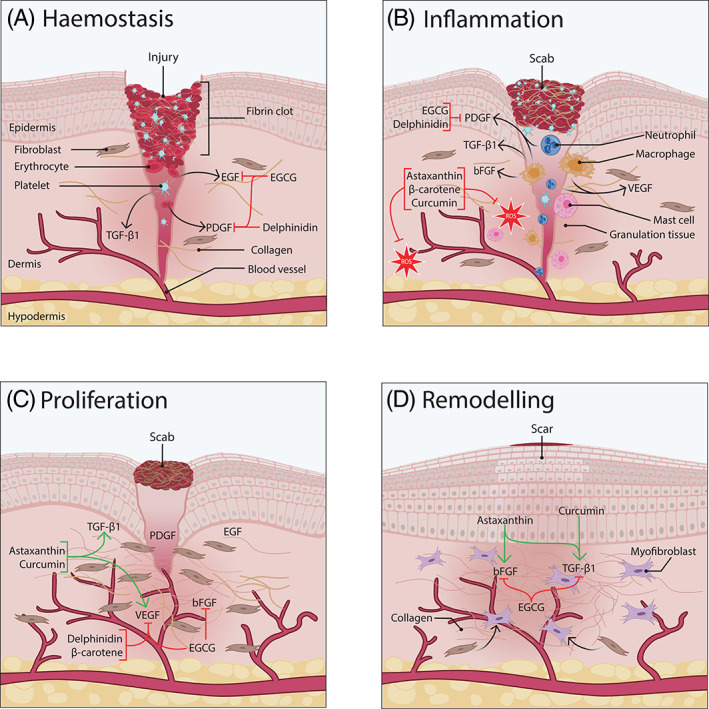
Effect of growth factors and antioxidants on each stage of the skin wound‐healing process. The four phases involved in the wound healing process are presented. In addition, selected growth factors and antioxidants are added to the phase wherein they have an enhancing effect. (a) Haemostasis phase: platelets produce platelet‐derived growth factor (PDGF), epidermal growth factor (EGF), and transforming growth factor‐β1 (TGF‐β1). They act as chemoattractant for inflammatory immune cells. Epigallocatechin gallate (EGCG) binds to PDGF and EGF receptors inhibiting the signalling cascade of them for the inflammatory phase. EGCG also inhibits the expression of EGF receptor (EGFR), which delays the beginning of the epithelialisation. Delphinidin inhibits PDGF signalling after binding to PDGFR, which delays wound repair. (b) Inflammation phase: inflammatory immune cells such as neutrophils, mast cells, and macrophages secrete cytokines, reactive oxygen species (ROS), and growth factors. Microvascularity increases to deliver nutrients, immune cells, and oxygen at the wound site. Monocytes matured to macrophages which produce PDGF, TGF‐β1, basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF) that act as chemoattractant for keratinocyte and fibroblasts. Through this phase, EGCG and delphinidin still inhibit PDGFR. Astaxanthin, β‐carotene, and curcumin aid wound healing when balance ROS. (c) Proliferation phase: bFGF, VEGF, PDGF, and TGF‐β activate cellular responses that promote angiogenesis, keratinocyte migration and proliferation, fibroblasts migration and differentiation, and collagen synthesis. Astaxanthin stimulates TGF‐β1 and VEGF signalling pathways and expression that enhance differentiation of fibroblast, migration of keratinocytes, granulation tissue formation and angiogenesis. Curcumin stimulates TGF‐β1 signalling pathways that enhance the expression of VEGF. Delphinidin and β‐carotene inhibit VEGF pathways and receptor reducing angiogenesis. EGCG inhibits VEGFR and bFGF expression, which reduce angiogenesis and collagen synthesis, and delays wound closure. (d) Remodelling phase: scar maturation and remodelling of extracellular matrix (ECM) occur when collagen type I (COLI) replaces collagen type III (COLIII), and keratinocytes and fibroblasts organise, apoptosis, and differentiate via bFGF and TGF‐β1 signalling pathways and by bFGF and astaxanthin stimulate the expression of bFGF and TGF‐β1 modulating collagen production and scar maturation. Curcumin enhance TGF‐β and collagen synthesis, which promotes remodelling of ECM. EGCG inhibits TGF‐β1 signalling, which reduces fibroblast proliferation, fibroblast differentiation to myofibroblast, and collagen synthesis that modulate scarring. Additionally, EGCG still inhibits the expression of bFGF that aids in scarring control
2.1. Haemostasis phase
After an injury occurs, the first stage is vasoconstriction, also known as haemostasis. 12 During this phase, platelets make contact with fibronectin and collagen forming a fibrin clot that stops the bleeding and blocks the entry of pathogens. 15 The generation of early ROS from platelets reduces the blood flow allowing to promote the vasoconstriction. 14 Along with the monocytes, platelets secrete PDGF, EGF, and TGF‐β1, which act as chemoattractants of inflammatory cells and promote the adaptive immune response of the inflammatory phase. 12 , 15 , 16 Table 1 describes each role and different pathways regulated by growth factors during haemostasis.
TABLE 1.
Function and signalling pathway of selected growth factors during wound healing
| Growth factor | Function | Signalling pathway | References |
|---|---|---|---|
| PDGF | Stimulate cell proliferation and migration | Ras/Erk1/2/MAPK | 81 |
| Up‐regulation of IGF‐1 and VEGF expression | PLCγ | ||
| PI3K/AKT | |||
| Stimulate angiogenesis | PI3K/AKT/eNOS | 82 | |
| Stimulate migration and cytoskeletal remodelling | Rho GTPase | 83 | |
| PI3K/AKT | |||
| Regulate proinflammatory cytokines | PI3K/AKT/NF‐κβ | 74 | |
| VEGF | Activate proliferation of endothelial cells in angiogenesis | PLCγ/ PKC/ Ras/Raf/ MEK/ ERK | 84 |
| Stimulate cell migration of keratinocyte and endothelial cells | FAK | 84, 85 | |
| p38/MAPK | |||
| Stimulate permeability and vasodilation | PI3K/AKT/eNOS | 85 | |
| EGF | Activate migration and proliferation of keratinocyte | Erk1/2/MAPK | 81, 86 |
| Activate production of type I collagen | PI3K/AKT | 86 | |
| Induce migration and formation of vascular tubes in endothelial cells (angiogenesis) | PI3K | 81 | |
| MAPK | |||
| Induce production of MMP and cell proliferation in fibroblasts | PI3K | 81 | |
| Rac | |||
| ERK | |||
| Inhibition of infiltration of inflammatory cells | RANTES | 86 | |
| MCP‐1 | |||
| Suppress secretion of IL‐1α, IL‐8, and TNF‐α in inflammatory cells | NF‐κβ | 87 | |
| bFGF | Stimulate fibroblast and endothelial cells proliferation, migration, and differentiation | Ras/Raf/MEK/MAPK | 88 |
| PI3K/AKT | |||
| Activate production of ROS to induce fibroblast migration and collagen production | PI3K/AKT/Rac1/JNK/NOX | 89 | |
| FAK/Paxillin | |||
| Stimulate and collagenase production | PLCγ/ IP3‐Ca2+/ DAG/PKC | 10, 90 | |
| Activate inflammatory response | NF‐κβ/JNK | 91 | |
| Regulate scar formation activating TGF‐β signalling. | Wnt/β‐catenin | 19, 91 | |
| Activate angiogenesis producing ROS | Wnt/β‐catenin | ||
| TGF‐β1 | Fibroblast proliferation, migration, and differentiation | Wnt/β‐catenin | 78 |
| Regulate differentiation of fibroblast to myofibroblast | Smad/Erk | 78 | |
| Enhance collagen deposit | TGF‐β/Smad | 78, 92 | |
| β‐catenin | |||
| Stimulate collagen synthesis in fibroblast | JNK/ET‐1/c‐Jun | 93 |
Note: For each of the five main growth factors involved in wound healing their functions (related to one or several healing stages) and signalling pathway are presented.
Abbreviations: AKT, protein kinase B; bFGF, fibroblast growth factor; DAG, diacylglycerol; EGF, epithelial growth factor; eNOS, endothelial nitric oxide synthase; ET‐1, endothelin‐1; JNK, c‐Jun N‐terminal kinase; FAK, focal adhesion kinase; IP3, inositol trisphosphate; MCP‐1, monocyte chemoattractant protein‐1; NF‐κβ, nuclear factor kappa beta; NOX, NADPH oxidase; PI3K, phosphatidylinositol 3‐kinase; PDGF, platelet‐derived growth factor; Rac1, Ras‐related C3 botulinum toxin substrate 1; RANTES, regulated on activation, normal T cell expressed and secreted; Smad, small mothers against decapentaplegic; TGF‐β, transforming growth factor; VEGF, vascular endothelial growth factor; Wnt, wingless‐related integration site.
2.2. Inflammatory phase
The inflammatory phase begins with the activation of the adaptive immune response, and the migration of inflammatory cells, such as macrophages, T cells, monocytes, mast cells, and neutrophils, to control pathogens, regulate ROS, and degrade foreign material. 16 , 17 They balance inflammatory responses secreting the growth factors and cytokines, also producing ROS, that regulate this process. 16 , 18
The inflammatory balance is mediated by pro‐inflammatory and anti‐inflammatory agents. 16 The pro‐inflammatory agents promote ROS production in the inflammatory microenvironment. Neutrophils act as pro‐inflammatory agents because they can generate ROS that function as pathogen inhibitors, 16 , 18 and secrete chemoattractants, such as VEGF, and cytokines especially IL‐6, TNF‐α, and IL‐1. 12 Macrophages, maturated from monocytes, are the key agents in the inflammatory phase because they release pro‐inflammatory cytokines, such as IL‐1 and TNF‐α, along with growth factors, such as bFGF, PDGF, and VEGF, that promote proliferation of fibroblasts, keratinocytes, and epithelial cells through MAPK and PI3K‐AKT pathways; also PI3K‐Akt‐eNOS, NF‐kB, and FAK‐ERK‐MCP1 pathways of VEGF and PDGF produce ROS. 16 , 17 , 19 The later function of these growth factors is the attraction of more inflammatory cells to further stimulate its secretion. 16 , 18
As new cells form the new tissue by the activation of growth factor signalling, macrophages and T cells secrete anti‐inflammatory cytokines and growth factors, such as IL‐10 and TGF‐β1, to suppress the pro‐inflammatory response and balance the inflammatory microenvironment at the site. 16 Chronic and excessive scarring wounds have uncontrolled inflammatory agents and ROS excess that induces a prolonged inflammation phase. 18 On the contrary, when a proper inflammatory balance is achieved in acute wounds, the wound healing process proceeds into the following stage. Table 1 presents the role of different growth factors during the inflammatory phase.
2.3. Proliferative phase
This phase consists of four processes that occur simultaneously and depend on each other, being the angiogenesis, granulation tissue formation, re‐epithelialisation, and wound contraction. 15 , 18 All these phenomena are modulated by VEGF, PDGF, bFGF, and TGF‐β1 (Figure 1), and diverse signalling pathways are involved.
Angiogenesis, the formation of vascularity, provides oxygen and growth factors to induce the formation of granulation tissue. 18 Angiogenesis is stimulated by bFGF, VEGF, and TGF‐β signalling pathways (Table 1). VEGF is the mainly responsible for endothelial proliferation and migration, and blood vessel maturation promoted via MAPK and PI3K‐Akt‐eNOS, and the later signalling pathway produces ROS. 20 , 21 At the same time, the low generation of ROS stimulates the proliferation and migration of fibroblast enhancing collagen production to prepare granulation tissue formation and wound closure. 20 Granulation tissue formation and type III collagen are promoted principally by bFGF and TGF‐β and provide the structure for fibroblast and keratinocyte migration and vascular formation. 10 , 18
Re‐epithelialisation, known by the proliferation and migration of keratinocytes, promotes the closure of wounds mainly stimulated by signalling pathways in Table 1, such as MAPK, FAK‐paxillin, PI3K‐Akt‐mTOR pathways of VEGF, EGF, bFGF, TGF‐β, and ROS. 18 , 19 , 22 Dysfunction of angiogenesis is present in diabetic foot ulcers and burns, 16 and this highlights the relevance of this event in non‐healing conditions. 11
2.4. Remodelling phase
The remodelling or maturation phase is where the scar is formed, the fibroblast matures to myofibroblasts and collagen structure is remodelled. 18 The TGF‐β1 and bFGF stay at last to enhance ECM maturing or known as replacement and degradation of type III collagen by type I collagen by the action of collagenases, metalloproteinases, and fibroblasts (MMP). 2 , 4 In this process, ROS has an active role in enhancing bFGF expression, modulating the production of collagen, and remodelling the ECM. 14 , 20 The principal activated signalling pathways in this phase are MAPK, Smad, and β‐catenin pathways (Table 1 ). The complications associated with this phase are the overexpression of MMP and collagenases that constantly destruct ECM structure in chronic wounds, and the underexpression of the later enzymes and elevated synthesis of type III collagen in excessive scarring wounds such as hypertrophic wounds, burns, and infected wounds. 4
Signalling pathways are the mediators of the cellular responses in which redox signalling is also a critical point in all the wound healing phases. 20 Therefore, ROS at low or controlled concentration function as pathogen controller and help to activate proliferation, migration, inflammation, and angiogenesis cell responses. Nonetheless, ROS in excess or without control induce a chronic inflammatory response at the inflammation phase occurring in an impaired wound. 14 , 20 In this regard, antioxidants play a key role in the efficiency and speed of the wound healing process.
3. ANTIOXIDANTS IN WOUND HEALING
ROS, and the respective pro‐inflammatory cell signalling, have a key role in wound healing. 23 , 24 When enzymatic endogenous antioxidants in cell are not capable to overcome the high rate of oxidative stress, the administration of exogenous antioxidants allows the balance of ROS and inhibition of inflammatory signalling pathways 20 enhancing wound healing. 25 , 26
Cutaneous antioxidants are mainly classified as non‐enzymatic and enzymatic. 27 The enzymatic antioxidants are endogenous molecules found in oxidative cell mechanism, with catalase, glutathione peroxidase, and superoxide dismutase being some of the examples. 27 The non‐enzymatic kinds are both endogenous and exogenous molecules, mainly obtained from plants and found in a wide variety, classified as carotenoids and polyphenols. 26 , 27
Carotenoids and polyphenols with anti‐inflammatory, antioxidant, and antibacterial properties are used in cancer and wound healing therapies. 4 , 28 , 29 The mechanisms of oxidative stress control and NF‐κB inflammatory signalling in the wound healing phases are leading to the discovery of therapies for non‐healing and aberrant scarring wounds. 4 , 20 Scientific literature concerning exogenous supplementation of antioxidants for wound healing enhancement focuses on carotenoids and polyphenols. 1 , 4 This makes sense as these two bioactive families are among the most characterised in terms of antioxidant activity, given their availability in natural generally recognised as safe (GRAS) sources. 29 , 30 , 31 , 32 Both carotenoids and polyphenols have been reported to play a key role during the inflammation, proliferation, migration, and angiogenesis stages in wound healing. Figure 1 presents the effect of selected antioxidants in wound healing. It is important to remark that, as part of such a role, antioxidants may have a direct effect on the expression and activity of different growth factors. This opens the opportunity of harnessing such interactions to develop wound healing formulations with enhanced effectiveness. In this section, the reported effects of selected, well‐characterised antioxidants in wound healing are presented.
3.1. Carotenoids
Carotenoids are present in vegetables, fruits, marine animals, and microalgae, characterised by its red and orange‐coloured pigment. 33 Carotenoids have a lipophilic structure and are classified in (a) carotenes (non‐polar), and (b) xanthophylls (amphipathic, given their terminal hydroxyl groups). 33 They are strong scavengers of ROS resulting in well controllers of oxidative stress in wound healing. 34 Table 2 shows the reported effect of β‐carotene and astaxanthin, two well‐characterised carotenoids, over the wound healing process.
TABLE 2.
Target signalling pathway and response of selected antioxidants applied in wound healing
| Antioxidant | Response | Target signalling pathway | References |
|---|---|---|---|
| Astaxanthin | Regulate collagen through inhibition MMP‐1 and production of collagen | TIMP1 | 33 |
| Inhibit inflammatory signalling | NF‐κβ/eNOS | 33 | |
| Promote angiogenesis | Wnt/β‐catenin | 94 | |
| Promote cell migration in keratinocytes | Rac1/Cdc42/RhoA | 39 | |
| β‐carotene | Inhibit angiogenesis | VEGF/NF‐κβ | 62 |
| CREB | |||
| Inhibit metalloproteinases: MMP‐1, MMP‐10, MMP‐2, and MMP‐9 | ROS‐induced MAPK | 33, 95 | |
| Inhibit inflammatory signalling | NF‐κβ/PI3K/eNOS | 62 | |
| Curcumin | Act as anti‐inflammatory, antioxidant, anti‐infectious, and antiangiogenesis | IKKα/β/NF‐κβ | 4, 96 |
| NF‐κβ/VEGF | |||
| Inhibit angiogenesis and granulation tissue formation in fibroblasts | VEGFR2/Erk‐1/2 | 66 | |
| AKT | |||
| Inhibit synthesis of collagen, proliferation, and differentiation of fibroblasts | TGF‐β/Smad2 | 97 | |
| Inhibit proliferation of fibroblast and synthesis of collagen in hypertrophic scarring | Wnt/ β‐catenin | 97 | |
| Delphinidin | Inhibit inflammatory responses and ROS | NF‐κβ | 47 |
| Act as anti‐angiogenesis effector | VEGFR | 98 | |
| Anti‐proliferative | VEGFR/Erk‐1/2 | 45, 98 | |
| PLCγ | |||
| EGCG | Regulate inflammatory response and ROS | NF‐κβ | 99 |
| Inhibit proliferation of fibroblast and synthesis of collagen in hypertrophic scarring | Wnt/β‐catenin | 97 | |
| Act as antitumour an antiangiogenesis effector | VEGFR | 68 | |
| EGFR | |||
| PI3K/AKT | |||
| Erk1/2/p‐p38/MAPK |
Abbreviations: AKT, protein kinase B; Cdc42, cell division cycle 42; CREB, cAMP‐response element‐binding protein; EGCG, epigallocatechin gallate; EGFR, EGF receptor; eNOS, endothelial nitric oxide synthase; Erk‐1/2, extracellular signal‐regulated kinase‐1/2; JNK, c‐Jun N‐terminal kinase; NF‐κβ, nuclear factor kappa beta; MAPK, mitogen‐activated protein kinase; PI3K, phosphatidylinositol 3‐kinase; p‐p38, phosphorylated p38; PLCγ, phospholipase C gamma; Rac1, Ras‐related C3 botulinum toxin substrate 1; RhoA, Ras homologue family member A; ROS, reactive oxygen species; Smad, small mothers against decapentaplegic; TGF‐β, transforming growth factor; TIMP1, tissue inhibitor of metalloproteinases‐1; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; Wnt, wingless‐related integration site.
3.1.1. β‐carotene
β‐carotene, found in several vegetables and fruits, works as a preventive element for photo‐aging and carcinogenesis, through the inhibition of the signalling pathways NF‐Kβ in haemostasis and inflammatory phase, as well as MAPK pathway in the proliferative phase. It has a long chain of conjugated double bonds with two β‐ionic rings 35 contributing to prevent photodamage, inhibit proliferation and migration in carcinogenesis of epithelial cells, and inhibition of metalloproteinases (MMP) degradation in collagen deposit in the proliferative and the remodelling phase of wound healing. 33 , 36
3.1.2. Astaxanthin
Astaxanthin has shown similar properties as beta‐carotene has. The astaxanthin has a hydroxy group at a β‐ionone ring, on each end of the polyene chain. 37 Its role in the inhibition of photo‐aging has been reported, decreasing the production of the MMP‐1 enzyme and the inflammatory signalling pathway and promoting the migration of the keratinocyte in the proliferative phase of wound healing. 38 , 39 , 40 Owing to its characteristics, it is a promising molecule in accelerating the wound healing process through migration and collagen production. 41
3.2. Polyphenols
Polyphenols are abundant and found in a wide diversity of natural sources such as cereals, vegetables, tea leaves, fruits, yeast, and crustacea. 33 , 41 They are much more diverse, from a molecular structure point of view, than carotenoids and are classified in (a) flavonoids and (b) non‐flavonoids. They are commonly used in cosmeceuticals and wound healing applications because of their strong scavenging activity. 34 , 42 Table 2 shows the reported effect of delphinidin, epigallocatechin gallate (EGCG), and curcumin, three well‐characterised polyphenols, over the wound healing process.
3.2.1. Delphinidin
The delphinidin is a flavonoid‐anthocyanidin compound found in plant foods such as blackcurrants hibiscus, and bilberries. 43 , 44 , 45 It is a benzopyrylium with three hydroxy substituents 46 acting as a good antioxidant for the inflammatory phase in wound healing. 47 Blackcurrants extract rich in delphinidin modulates type I collagen expression, reduces NF‐kB inflammatory signalling, and modulates oxidative stress on skin fibroblast cells, which can aid in the remodelling phase. 45
3.2.2. Epigallocatechin gallate
The last flavonoid compound is the flavan‐catechin EGCG, having three hydroxyphenyl and trydoxybenzoate moieties, 48 which confers properties such as down‐regulating inflammatory pathways that are used in the production of cosmetics and dermatology. 49 Their antimutagenic and antiproliferative actions are the main applications in chemoprotective drugs 50 for cancer patients, so is the inhibition of the MAPK signalling pathway of VEGF and EGF. Additionally, curcumin is the most trending antioxidant in wound healing in diabetic patients.
3.2.3. Curcumin
Curcumin is a non‐flavonoid hydroxycinnamic acid with anti‐infectious, antiapoptotic, and anti‐inflammatory actions, for which it is used in the wound healing treatment for diabetic patients. 1 , 4 It is a hydrophobic compound containing B‐diketone moiety and two o‐methoxy phenolic groups that confer strong antioxidant activity to reduce ROS during the inflammatory phase. 51 Curcumin is the most studied antioxidant in wound healing, and it has been demonstrated to stimulate the expression of TGF‐β1, which promotes VEGF expression via the TGF‐β pathway that will be discussed in the following section. 52
4. POTENTIAL INTERACTION OF GROWTH FACTORS AND EXOGENOUS ANTIOXIDANTS DURING WOUND HEALING
As previously stated, there are some scientific reports in literature describing the interaction effect of growth factors and antioxidants during wound healing. 52 , 53 Although the information available in the literature is still limited, the study of potential interaction of growth factors and antioxidants is of the essence for the design of wound healing formulations. Based on the reported individual effects of both growth factors and exogenous antioxidants (Tables 1 and 2 and Figure 1), it is possible to propose combinations that may have a potential additive or synergistic effect over the wound healing process. Table 3 shows the reported and potential growth factor—antioxidant interactions, based on the analysis of the information presented in this review. These prospected growth factor‐antioxidant interactions may serve as a starting point to envision further experimental work focused on the study and characterisation of such interactions, looking forward the rational design of wound healing formulations. Although these potential growth factor‐antioxidant interactions (Table 3) have not yet been studied based on reported scientific evidence regarding their individual effect on wound healing, they would be expected to exert the prospected result at least to some extent.
TABLE 3.
Potential synergetic effect of growth factor with antioxidants for a wound‐healing formulation
| Antioxidant | PDGF | EGF | VEGF | TGF‐β1 | bFGF | Reference |
|---|---|---|---|---|---|---|
| Astaxanthin | ↓ Inflammation | ND | ↑ Angiogenesis | ND | ↑ FB Migration | 24, 29, 39, 41, 54, 72, 100 |
| ■ ● | ↑ KC migration | ↑KC Migration | ||||
| ■ ● | ● | |||||
| β‐carotene | ↓ Inflammation | ND | ND | ND | ND | 59, 62, 73 |
| ● | ||||||
| Curcumin | ↓ Inflammation | ↑KC migration | ↑ Angiogenesis | ↑ FB migration | ND | 4, 52, 53, 64, 66, 67, 101, 102, 103 |
| ■ ● | ↑ KC proliferation | ↑KC migration | ↑ FB proliferation | |||
| ■ ● | ↑ KC proliferation | ● | ||||
| ■ | ||||||
| Delphinidin | ↓ Inflammation | ND | ND | ND | ND | 58, 98, 104 |
| ● | ||||||
| EGCG | ↓ Inflammation | ND | ND | ND | ND | 55, 63, 68, 78, 79, 101 |
| ■ ● |
Note: The potential additive or synergistic effect of the combination of growth factors and exogenous antioxidants over the regulation of different wound healing‐related pathways is presented. Consequently, different combinations are proposed based on the type of injury (acute full‐thickness wound, chronic wound, or burn) to be treated. Based on reported individual effect of antioxidants, these are the prospective effect with the combined application of growth factor and antioxidant. ↓, decrease cellular response; ↑, enhance cellular response; ND, no data reported. Type of wound: ●, acute full‐thickness wound (surgery, trauma, etc.); ■, chronic wound (diabetic foot ulcer, vascular ulcer, etc.).
Abbreviations: bFGF, fibroblast growth factor; EGCG, epigallocatechin gallate; EGF, epidermal growth factor; FB, fibroblast; KC, keratinocyte; PDGF, platelet‐derived growth factor; TGF‐β, transforming growth factor; VEGF, vascular endothelial growth factor.
4.1. Inhibition of pathogen growth and ROS production in the haemostasis phase
Pathogen blockage is a critical step in the haemostasis. Pathogen presence strongly activates immune response and produces ROS that will prolong the inflammatory phase, alter the proliferative and the remodelling phase. Astaxanthin, EGCG, curcumin, and delphinidin inhibit bacteria in skin disorders and injuries when they are administrated exogenously. 1 , 54 , 55 , 56 Their antibacterial and scavenging activity aids in the haemostasis phase reducing the proliferation of present pathogens and controlling the ROS produced by them. They exhibit promotion of vasoconstriction process and wound healing to the exception of EGCG that delays wound healing. 55 , 57 Delphinidin inhibits the action of platelet‐derived growth factor receptor (PDGFR) and its ligand (PDGF) in endothelial cells and smooth muscle cells, delaying the angiogenesis activation. 58 Platelets are essential in fibrin clotting, wherein both platelet activation and aggregation are compromised by delphinidin in the haemostasis phase. 43
4.2. Reduction of pro‐inflammatory agents in the inflammatory phase
The excess of ROS increases tissue damage and delays the wound healing process. The five antioxidants inhibit transcription of pro‐inflammatory agents (eg, TNF‐α, IL‐1, IL‐6) via nuclear factor κβ (NF‐κβ) and exhibit well control of ROS on dysregulated inflammation of acute or chronic wounds. 24 , 59 Astaxanthin, EGCG, and curcumin inhibit NF‐κβ in the PDGF pathway in inflammatory cells improving chronic wound healing. 60 , 61 They are a promising treatment though at a specific concentration to enhance a cellular response. 33 Either β‐carotene or delphinidin suppresses inflammatory response that delays the proliferative and remodelling phases. They could be used in wounds with prolonged inflammatory response and also impaired scarring. 44 , 62
4.3. Enhanced proliferation, migration, and angiogenesis in the proliferative phase
Antioxidants have a direct effect on the inhibition or stimulation of angiogenesis pathways. As inhibitors, astaxanthin blocks pathological angiogenesis pathway JAK/STAT3, 41 involved in tumorigenesis, while delphinidin and EGCG have a strong inhibition of VEGFR2 and VEGF blocking angiogenesis response. 63 Also related to the suppression of angiogenesis, β‐carotene and delphinidin exhibit receptor blockage delaying the wound closure rate. Furthermore, curcumin has been reported to increase the expression of VEGF and TGF‐β1, promoting angiogenesis and collagen synthesis in chronic (eg, diabetic foot) and acute wounds. 64 Astaxanthin‐richalgal extract stimulates VEGF expression enhancing vascularity and wound closure in fibroblasts. 65
Curcumin and astaxanthin enhance the migration of keratinocyte and fibroblast cells through MAPK and FAK signalling pathways, thus improving wound closure in chronic and acute wounds. 41 , 66 , 67 β‐carotene, delphinidin, and EGCG down‐regulate migration, proliferation, and angiogenesis responses in the involved cells, which aid in wounds with hypertrophic scarring.
On proliferation, curcumin can induce the expression of the TGF‐β1, promoting the proliferation of fibroblast, inhibiting the collagen expression by Smad2/3 pathway in the TGF‐β1 signalling cascade, and increasing the expression of VEGF (Table 2). These mechanisms enhance the wound healing process in deep acute and chronic wounds.
4.4. Increase of collagen deposit and inhibition of collagen degradation in the remodelling phase
Polyphenols modulate collagen production in the skin. EGCG and curcumin exhibit an increase in the synthesis of collagen improving wound healing in acute and chronic wounds. 68 , 69 Delphinidin in plant extracts also enhances collagen deposit, whereas it suppresses cellular responses in the proliferative phase in hypertrophic scarring wounds. 45 Astaxanthin, EGCG, and β‐carotene regulate the remodelling of collagen through the inhibition of metalloproteinases: MMP‐1, MMP‐2, MMP‐9, and MMP‐10. Astaxanthin improves the expression of bFGF and TGF‐β1 enhancing the vascularity and wound closure in fibroblasts. 41 , 70
5. PROSPECTIVE WOUND HEALING FORMULATION DESIGN BASED ON POTENTIAL GROWTH FACTOR—ANTIOXIDANT INTERACTIONS
According to the available results of different treatments in animal models, antioxidants combined with growth factors have better wound healing rate than separate treatments of each one. 66 Antioxidants applied in wound healing treatments would have promising effects either individually or in combination with growth factors, enhancing the action of the growth factors, increasing wound closure rate, and improving scarring quality. The excess of ROS and uncontrolled inflammatory process drive to impairment of wound healing in different types of wounds, divided into (a) acute wounds and (b) chronic wounds. Based on the information presented in Table 3, potential additive or synergistic growth factor—antioxidant combination for the treatment of these two types of wounds are proposed. As previously stated, these potential interaction effects still require to be studied and confirmed (Table 3). Nevertheless, based on reported scientific evidence regarding the individual effect of growth factors and antioxidants on wound healing, they would be expected to exert the prospected result at least in some extent.
5.1. Acute wounds
Acute wounds (eg, surgeries, burns, trauma) heal in the expected time under unaltered physiological conditions. 2 Deep injuries required a stimulation of angiogenesis and collagen deposition but minimal scarring. According to Table 3, curcumin with PDGF or EGF could significantly improve wound closure rate fibroblasts and keratinocytes. Li et al. in 2016 showed that the administration of EGF‐curcumin nanoparticles into a polymeric bandage in male Sprague‐Dawley rats accelerated significantly the wound closure and reduced levels of inflammatory cytokines. 52 Curcumin with VEGF or TGF‐β1 stimulates their expression expecting an increase in the synergistic effect. Curcumin with EFG proved that the combination of both increases significantly the angiogenesis, collagen remodelling, and wound closure than the treatment with EGF. 52 PDGF acts in every wound healing phase, though PDGF in inflammatory pathways can be inhibited by the mentioned antioxidants and consequently stimulate wound closure. 44 , 59 , 67 , 69 , 71 The suitable combination that could have a synergistic effect in deep incision is astaxanthin with PDGF, VEGF, TGF‐β1, and bFGF, in order to reduce wound closure time and improve scarring of full‐thickness or deep wounds. 41 , 65 , 70 , 72 Systemic administration of β‐carotene is expected to decrease inflammatory cytokines and ROS, improving wound healing in acute wounds. 73 PDGF produces inflammatory cytokines through NF‐κβ pathway, the use of antioxidants with PDGF could have a synergistic effect in reducing inflammation that could improve the closure of injury. 74 Nonetheless, topical concentrations between 25 and 200 μM reduce proliferation and control the scarring process in hypertrophic injuries improving the quality of wound closure . 75 , 76 Delphinidin is expected to reduce inflammatory response and stimulate wound healing rate. 47 , 58 Moreover, its inhibitory action on VEGF and PDGF could regulate angiogenesis, collagen synthesis, and ROS in hypertrophic wounds. 44 , 58 , 77
In second‐degree burns, astaxanthin modulates collagen remodelling, reduces oxidative stress, and accelerates wound closure rate in adult male rats. 54 Astaxanthin also significantly increases expression of bFGF. 41 Therefore, bFGF combined with astaxanthin into a delivery system (eg, hydrogel, nanofibers, nanogel) could prevent pathological angiogenesis and aberrant scarring, modulate pro‐inflammatory response, promote proper wound microenvironment conditions, and stimulate collagen synthesis and modulation. Hence, they will increase the therapeutic effect of bFGF. 3 , 41 , 54 On the other hand, EGCG combined with PDGF can reduce inflammation, the expression of bFGF, VEGF, TGF‐β1, controlling the synthesis of collagen, inflammation, and fibroblast migration. 55 , 69 , 78 , 79 , 80
5.2. Chronic wounds
Adverse physiological conditions of the patient (eg, diabetes, immune diseases, malnutrition) complicate the healing process prolonging the inflammatory phase in chronic wounds (eg, diabetic, vascular, or pressure ulcers). 14 , 20 In Table 3, it is reported that curcumin and astaxanthin can improve the quality of scarring because both control collagen disposition in patients with diabetes. The combination of astaxanthin with PDGF, VEGF, or TGF‐β1 would enhance angiogenesis in chronic ulcers with deficiency of angiogenesis and inhibition of inflammatory transcription agents. Curcumin is also an efficient antioxidant in the treatment of chronic wounds as it regulates EGF and VEGF expression. 52 , 53 , 66 EGCG in green tea extract shows wound healing effect, from 100 to 1000 ppm, it reduces the wound closure. In addition, it is a good modulator of ECM components, accelerating wound closure at 10 ppm EGCG in chronic or hypertrophic scarring wounds in diabetic patients. 71
Prevention of infection in second‐degree burns, full‐thickness or chronic wounds could be aided by the antimicrobial activity of the antioxidants. Growth factors and antioxidants into a hydrogel should improve wound management, including pathogen blockage, stop bleeding, and absorb swelling. 2 , 11
6. CONCLUSION
Wound healing formulation design is an ever‐growing field. In this regard, some authors have reported the combined use of growth factors and exogenous antioxidants to enhance the wound healing process. However, the information available in the literature is still limited. In this review, based on the reported individual effects of both, growth factors and exogenous antioxidants, it was possible to propose prospected combinations that may have a potential additive or synergistic effect over the wound healing process.
Astaxanthin and curcumin have potential effects combined with PDGF, VEGF, bFGF, and TGF‐β1 for acute and chronic wounds as they promote the healing effect, inducing angiogenesis, migration, proliferation, and modulating the inflammatory response and ROS production. EGCG, delphinidin, or β‐carotene has a potential effect in the regulation of inflammatory agents and ROS, and the modulation of collagen production and remodelling for burns and hypertrophic scarring wounds.
Microenvironment factors (eg, enzymes, UV, pH) degrade growth factors and antioxidants diminishing their effect. Particulate vehicles (eg, nanoparticles and microparticles) improve bioactive bioavailability and stability, thus enhancing their effect. They can be embedded in wound dressings, including fibres, hydrogels, or microneedles. They are expected to block pathogens, provide an adequate wound microenvironment (eg, moisture, pH), and absorb exudate that should promote wound healing.
Further studies should be focused on characterising the proposed growth factor—antioxidant combinations to confirm their synergistic effect on the wound healing process. Moreover, studies focused on the optimisation of growth factor‐antioxidant combination ratios are relevant to have a better understanding about the rational selection of bioactive principles for wound healing applications.
CONFLICT OF INTEREST
The other authors declare no conflicts of interest regarding the publication of this article.
ACKNOWLEDGEMENTS
PVM, MLS, and JB structured and contributed in equal parts in the article. PVM drew the figure. MLS and JB are corresponding authors. MLS is a member of CONICET. Research was funded by Consejo Nacional de Ciencia y Tecnología (1048769).
Viaña‐Mendieta P, Sánchez ML, Benavides J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int Wound J. 2022;19:100–113. 10.1111/iwj.13602
Mirna Lorena Sánchez and Jorge Benavides contributed equally to this work.
Funding information Consejo Nacional de Ciencia y Tecnología, Grant/Award Number: 1048769
Contributor Information
Mirna Lorena Sánchez, Email: mirna.sanchez@unq.edu.ar.
Jorge Benavides, Email: jorben@tec.mx.
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs
REFERENCES
- 1. 'Izzah Ibrahim N, Wong SK, Mohamed IN, et al. Wound healing properties of selected natural products. Int J Environ Res Public Health. 2018;15(11):2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12:1‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zarei F, Soleimaninejad M. Role of growth factors and biomaterials in wound healing. Artif Cells Nanomed Biotechnol. 2018;46:906‐911. [DOI] [PubMed] [Google Scholar]
- 4. Shah A, Amini‐Nik S. The role of phytochemicals in the inflammatory phase of wound healing. Int J Mol Sci. 2017;18(5):1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aldag C, Nogueira Teixeira D, Leventhal PS. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: a review of the literature. Clin Cosmet Investig Dermatol. 2016;9:411‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585‐601. [DOI] [PubMed] [Google Scholar]
- 7. Yamakawa S, Hayashida K. Advances in surgical applications of growth factors for wound healing. Burn Trauma. 2019;7:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration. Burns. 2014;40:966‐973. [DOI] [PubMed] [Google Scholar]
- 9. Pastore S, Lulli D, Fidanza P, et al. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxidants Redox Signal. 2012;16:317‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrientos S, Brem H, Stojadinovic O, Tomic‐Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park JW, Hwang SR, Yoon IS. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules. 2017;22:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cañedo‐Dorantes L, Cañedo‐Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflam. 2019;2019:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fitridge R, Thomson M. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists. Adelaide: University Adelaide Press; 2011. [PubMed] [Google Scholar]
- 14. Dunnill C, Patton T, Brennan J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS‐modulating technologies for augmentation of the healing process. Int Wound J. 2017;14:89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terijam AS, Kavalukas SL, Shupp JW, Barbul A. Wound healing. In: Ågren M, ed. Wound Healing Biomaterials Volume 1: Therapies and Regeneration. 1st ed. Woodhead Publishing; 2016:3‐39. [Google Scholar]
- 16. Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care. 2018;7:209‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinno H, Prakash S. Complements and the wound healing Cascade: an updated review. Plast Surg Int. 2013;2013:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonham CA, Kuehlmann B, Gurtner GC. Impaired neovascularization in aging. Adv Wound Care. 2020;9:111‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuehlmann B, Bonham CA, Zucal I, Prantl L, Gurtner GC. Mechanotransduction in wound healing and fibrosis. J Clin Med. 2020;9:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanchez MC, Lancel S, Boulanger E, Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: a systematic review. Antioxidants (Basel). 2018;7(8):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilgus TA, Matthies AM, Radek KA, et al. Novel function for vascular endothelial growth factor receptor‐1 on epidermal keratinocytes. Am J Pathol. 2005;167:1257‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rochette L, Mazini L, Meloux A, et al. Anti‐aging effects of GDF11 on skin. Int J Mol Sci. 2020;21:2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park YR, Sultan MT, Park HJ, et al. NF‐κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018;67:183‐195. [DOI] [PubMed] [Google Scholar]
- 24. Park JH, Yeo IJ, Han JH, Suh JW, Lee HP, Hong JT. Anti‐inflammatory effect of astaxanthin in phthalic anhydride‐induced atopic dermatitis animal model. Exp Dermatol. 2018;27:378‐385. [DOI] [PubMed] [Google Scholar]
- 25. Gaspar A, Craciunescu O, Moldovan L, Ganea E. New composites collagen—polyphenols as potential dressing for wound care. Rom J Biochem. 2012;49(2):173‐181. [Google Scholar]
- 26. Budovsky A, Yarmolinsky L, Ben‐Shabat S. Effect of medicinal plants on wound healing. Wound Repair Regen. 2015;23:171‐183. [DOI] [PubMed] [Google Scholar]
- 27. Addor FAS. Antioxidants in dermatology. An Bras Dermatol. 2017;92:356‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scarpa ES, Ninfali P. Phytochemicals as innovative therapeutic tools against cancer stem cells. Int J Mol Sci. 2015;16:15727‐15742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in skin health, repair, and disease: a comprehensive review. Nutrients. 2018;10:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cione E, La Torre C, Cannataro R, Caroleo MC, Plastina P, Gallelli L. Quercetin, epigallocatechin gallate, curcumin, and resveratrol: from dietary sources to human MicroRNA modulation. Molecules. 2020;25(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. U.S. Department of Agriculture . β‐Carotene Handling/Processing. Technical Evaluation Report, 2011. https://www.ams.usda.gov/sites/default/files/media/ColorsDerivedfromAgProductsTR.pdf
- 32. U.S. Department of Agriculture . Colors Handling/Processing. Technical Evaluation Report, 2015. https://www.ams.usda.gov/sites/default/files/media/BetaCaroteneSyntheticTR.pdf
- 33. Balić M. Do we utilize our knowledge of the skin protective effects of carotenoids enough? Antioxidants. 2019;8:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51‐54. [DOI] [PubMed] [Google Scholar]
- 35. Bogacz‐Radomska L, Harasym J. β‐Carotene‐properties and production methods. Food Qual Saf. 2018;2:69‐74. [Google Scholar]
- 36. Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress‐implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014;34:907‐929. [DOI] [PubMed] [Google Scholar]
- 37. Brotosudarmo THP, Limantara L, Setiyono E. Heriyanto. Structures of Astaxanthin and their consequences for therapeutic application. Int J Food Sci. 2020;2020:2156582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meléndez‐Martínez AJ, Stinco CM, Mapelli‐Brahm P. Skin carotenoids in public health and nutricosmetics: the emerging roles and applications of the UV radiation‐absorbing colourless carotenoids phytoene and phytofluene. Nutrients. 2019;11(5):1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ritto D, Tanasawet S, Singkhorn S, et al. Astaxanthin induces migration in human skin keratinocytes via Rac1 activation and RhoA inhibition. Nutr Res Pract. 2017;11:275‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Imokawa G. Intracellular signaling mechanisms involved in the biological effects of the xanthophyll carotenoid Astaxanthin to prevent the photo‐aging of the skin in a reactive oxygen species depletion‐independent manner: the key role of mitogen and stress‐activated. Photochem Photobiol. 2019;95:480‐489. [DOI] [PubMed] [Google Scholar]
- 41. Meephansan J, Rungjang A, Yingmema W, Deenonpoe R, Ponnikorn S. Effect of astaxanthin on cutaneous wound healing. Clin Cosmet Investig Dermatol. 2017;10:259‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Evans JA, Johnson EJ. The role of phytonutrients in skin health. Nutrients. 2010;2:903‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Shi Z, Reheman A, et al. Plant food delphinidin‐3‐glucoside significantly inhibits platelet activation and thrombosis: novel protective roles against cardiovascular diseases. PLoS One. 2012;7(5):e37323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sogo T, Terahara N, Hisanaga A, et al. Anti‐inflammatory activity and molecular mechanism of delphinidin 3‐sambubioside, a hibiscus anthocyanin. Biofactors. 2015;41:58‐65. [DOI] [PubMed] [Google Scholar]
- 45. Nanashima N, Horie K, Maeda H, Tomisawa T, Kitajima M, Nakamura T. Blackcurrant anthocyanins increase the levels of collagen, elastin, and hyaluronic acid in human skin fibroblasts and ovariectomized rats. Nutrients. 2018;10(4):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Estévez L, Mosquera RA. Molecular structure and antioxidant properties of delphinidin. J Phys Chem A. 2008;112:10614‐10623. [DOI] [PubMed] [Google Scholar]
- 47. Palungwachira P, Tancharoen S, Phruksaniyom C, et al. Antioxidant and anti‐inflammatory properties of anthocyanins extracted from Oryza sativa L in primary dermal fibroblasts. Oxid Med Cell Longev. 2019;2019:2089817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dettlaff K, Stawny M, Ogrodowczyk M, et al. Formulation and characterization of EGCG forthe treatment of superficial bladder cancer. Int J Mol Med. 2017;40:329‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim E, Hwang K, Lee J, et al. Skin protective effect of epigallocatechin gallate. Int J Mol Sci. 2018;19(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vauzour D, Rodriguez‐Mateos A, Corona G, Oruna‐Concha MJ, Spencer JPE. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19:20091‐20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li X, Ye X, Qi J, et al. EGF and curcumin co‐encapsulated nanoparticle/hydrogel system as potent skin regeneration agent. Int J Nanomedicine. 2016;11:3993‐4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang J, Song M, Li W, Zhao F, Li Y. Curcumin inhibits proliferation and soluble collagen synthesis of NIH/3T3 cell line by modulation of miR‐29a and via ERK1/2 and β‐catenin pathways. Mol Immunol. 2019;116:191‐198. [DOI] [PubMed] [Google Scholar]
- 54. Fang Q, Guo S, Zhou H, Han R, Wu P, Han C. Astaxanthin protects against early burn‐wound progression in rats by attenuating oxidative stress‐induced inflammation and mitochondria‐related apoptosis. Sci Rep. 2017;7:41440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jeon J, Kim JH, Lee CK, Oh CH, Song HJ. The antimicrobial activity of (−)‐epigallocatehin‐3‐gallate and green tea extracts against Pseudomonas aeruginosa and Escherichia coli isolated from skin wounds. Ann Dermatol. 2014;26:564‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Silva S, Costa EM, Mendes M, Morais RM, Calhau C, Pintado MM. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin‐rich blueberry extract purified by solid phase extraction. J Appl Microbiol. 2016;121:693‐703. [DOI] [PubMed] [Google Scholar]
- 57. Minasyan H, Flachsbart F. Blood coagulation: a powerful bactericidal mechanism of human innate immunity. Int Rev Immunol. 2019;38:3‐17. [DOI] [PubMed] [Google Scholar]
- 58. Lamy S, Beaulieue É, Labbé D, et al. Delphinidin, a dietary anthocyanidin, inhibits platelet‐derived growth factor ligand/receptor (PDGF/PDGFR) signaling. Carcinogenesis. 2008;29:1033‐1041. [DOI] [PubMed] [Google Scholar]
- 59. Li R, Hong P, Zheng X. β‐Carotene attenuates lipopolysaccharide‐induced inflammation via inhibition of the NF‐κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. Anim Sci J. 2019;90:140‐148. [DOI] [PubMed] [Google Scholar]
- 60. Yamaguchi M, Tomihara K, Heshiki W, et al. Astaxanthin ameliorates cisplatin‐induced damage in normal human fibroblasts. Oral Sci Int. 2019;16:171‐177. [Google Scholar]
- 61. Said A, Naeem N, Siraj S, et al. Mechanisms underlying the wound healing and tissue regeneration properties of Chenopodium album . 3 Biotech. 2020;10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guruvayoorappan C, Kuttan G. β‐Carotene inhibits tumor‐specific angiogenesis by altering the cytokine profile and inhibits the nuclear translocation of transcription factors in B16F‐10 melanoma cells. Integr Cancer Ther. 2007;6:258‐270. [DOI] [PubMed] [Google Scholar]
- 63. Moyle CWA, Cerezo AB, Winterbone MS, et al. Potent inhibition of VEGFR‐2 activation by tight binding of green tea epigallocatechin gallate and apple procyanidins to VEGF: relevance to angiogenesis. Mol Nutr Food Res. 2015;59:401‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kamar SS, Abdel‐Kader DH, Rashed LA. Beneficial effect of Curcumin nanoparticles‐hydrogel on excisional skin wound healing in type‐I diabetic rat: histological and immunohistochemical studies. Ann Anat. 2019;222:94‐102. [DOI] [PubMed] [Google Scholar]
- 65. Chou HY, Lee C, Pan JL, et al. Enriched astaxanthin extract from Haematococcus pluvialis augments growth factor secretions to increase cell proliferation and induces MMP1 degradation to enhance collagen production in human dermal fibroblasts. Int J Mol Sci. 2016;17:955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bhattacharya D, Tiwari R, Bhatia T, et al. Accelerated and scarless wound repair by a multicomponent hydrogel through simultaneous activation of multiple pathways. Drug Deliv Transl Res. 2019;9:1143‐1158. [DOI] [PubMed] [Google Scholar]
- 67. Rezaii M, Oryan S, Javeri A. Curcumin nanoparticles incorporated collagen‐chitosan scaffold promotes cutaneous wound healing through regulation of TGF‐β1/Smad7 gene expression. Mater Sci Eng C. 2019;98:347‐357. [DOI] [PubMed] [Google Scholar]
- 68. Chen J, Li Y, Zhu Q, et al. Anti‐skin‐aging effect of epigallocatechin gallate by regulating epidermal growth factor receptor pathway on aging mouse model induced by D‐Galactose. Mech Ageing Dev. 2017;164:1‐7. [DOI] [PubMed] [Google Scholar]
- 69. Fatemi MJ, Nikoomaram B, Rahimi AAK, Talayi D, Taghavi S, Ghavami Y. Effect of green tea on the second degree burn wounds in rats. Indian J Plast Surg. 2014;47:370‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lephart ED. Equol's efficacy is greater than astaxanthin for antioxidants, extracellular matrix integrity & breakdown, growth factors and inflammatory biomarkers via human skin gene expression analysis. J Funct Foods. 2019;59:380‐393. [Google Scholar]
- 71. Kim H, Kawazoe T, Han DW, et al. Enhanced wound healing by an epigallocatechin gallate‐incorporated collagen sponge in diabetic mice. Wound Repair Regen. 2008;16:714‐720. [DOI] [PubMed] [Google Scholar]
- 72. Serra R, Grande R, Butrico L, et al. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J. 2016;13:88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Blass SC, Goost H, Tolba RH, et al. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clin Nutr. 2012;31:469‐475. [DOI] [PubMed] [Google Scholar]
- 74. van Steensel L, Paridaens D, Dingjan GM, et al. Platelet‐derived growth factor‐BB: a stimulus for cytokine production by orbital fibroblasts in graves' ophthalmopathy. Investig Ophthalmol Vis Sci. 2010;51:1002‐1007. [DOI] [PubMed] [Google Scholar]
- 75. Klass BR, Branford OA, Grobbelaar AO, Rolfe KJ. The effect of epigallocatechin‐3‐gallate, a constituent of green tea, on transforming growth factor‐β1‐stimulated wound contraction. Wound Repair Regen. 2010;18:80‐88. [DOI] [PubMed] [Google Scholar]
- 76. Syed F, Bagabir RA, Paus R, Bayat A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI‐1 independently inhibit growth and induce keloid shrinkage. Lab Invest. 2013;93:946‐960. [DOI] [PubMed] [Google Scholar]
- 77. Lamy S, Blanchette M, Michaud‐Levesque J, et al. Delphinidin, a dietary anthocyanidin, inhibits vascular endothelial growth factor receptor‐2 phosphorylation. Carcinogenesis. 2006;27:989‐996. [DOI] [PubMed] [Google Scholar]
- 78. Lingzhi Z, Meirong L, Xiaobing F. Biological approaches for hypertrophic scars. Int Wound J. 2020;17:405‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li M, Xu J, Shi T, Yu H, Bi J, Chen G. Epigallocatechin‐3‐gallate augments therapeutic effects of mesenchymal stem cells in skin wound healing. Clin Exp Pharmacol Physiol. 2016;43:1115‐1124. [DOI] [PubMed] [Google Scholar]
- 80. Pedro AC, Maciel GM, Rampazzo Ribeiro V, Haminiuk CWI. Fundamental and applied aspects of catechins from different sources: a review. Int J Food Sci Technol. 2020;55:429‐442. [Google Scholar]
- 81. Demidova‐Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing. Adv Skin Wound Care. 2012;25:349‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wu LW, Chen WL, Huang SM, Chan JYH. Platelet‐derived growth factor‐AA is a substantial factor in the ability of adipose‐derived stem cells and endothelial progenitor cells to enhance wound healing. FASEB J. 2019;33:2388‐2395. [DOI] [PubMed] [Google Scholar]
- 83. Yamada K, Hamashima T, Ishii Y, et al. Different PDGF receptor dimers drive distinct migration modes of the mouse skin fibroblast. Cell Physiol Biochem. 2018;51:1461‐1479. [DOI] [PubMed] [Google Scholar]
- 84. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3:647‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Melincovici C, Boșca AB, Șușman S, et al. Vascular endothelial growth factor (VEGF)—key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455‐467. [PubMed] [Google Scholar]
- 86. Chen J, Zeng F, Forrester SJ, Eguchi S, Zhang MZ, Harris RC. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol Rev. 2016;96:1025‐1069. [DOI] [PubMed] [Google Scholar]
- 87. Kim JM, Choo JE, Lee HJ, Kim KN, Chang SE. Epidermal growth factor attenuated the expression of inflammatory cytokines in human epidermal keratinocyte exposed to Propionibacterium acnes . Ann Dermatol. 2018;30:54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nunes QM, Li Y, Sun C, Kinnunen TK, Fernig DG. Fibroblast growth factors as tissue repair and regeneration therapeutics. PeerJ. 2016;4:e1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abdelhakim M, Lin X, Ogawa R. The Japanese experience with basic fibroblast growth factor in cutaneous wound management and scar prevention: a systematic review of clinical and biological aspects. Dermatol Ther (Heidelb). 2020;10:569‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. de Araújo R, Lôbo M, Trindade K, Silva DF, Pereira N. Fibroblast growth factors: a controlling mechanism of skin aging. Skin Pharmacol Physiol. 2019;32:275‐282. [DOI] [PubMed] [Google Scholar]
- 91. Wang X, Zhu Y, Sun C, et al. Feedback activation of basic fibroblast growth factor signaling via the Wnt/β‐catenin pathway in skin fibroblasts. Front Pharmacol. 2017;8. 10.3389/fphar.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun Q, Guo S, Wang CC, et al. Cross‐talk between TGF‐β/Smad pathway and Wnt/β‐catenin pathway in pathological scar formation. Int J Clin Exp Pathol. 2015;8:7631‐7639. [PMC free article] [PubMed] [Google Scholar]
- 93. Nikoloudaki G, Brooks S, Peidl AP, Tinney D, Hamilton DW. JNK signaling as a key modulator of soft connective tissue physiology, pathology, and healing. Int J Mol Sci. 2020;21:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xu Y, Zhang J, Jiang W, Zhang S. Astaxanthin induces angiogenesis through Wnt/β‐catenin signaling pathway. Phytomedicine. 2015;22:744‐751. [DOI] [PubMed] [Google Scholar]
- 95. Minami Y, Kawabata K, Kubo Y, et al. Peroxidized cholesterol‐induced matrix metalloproteinase‐9 activation and its suppression by dietary β‐carotene in photoaging of hairless mouse skin. J Nutr Biochem. 2009;20:389‐398. [DOI] [PubMed] [Google Scholar]
- 96. Chowdhury I, Banerjee S, Driss A, et al. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF‐κB signaling pathway. J Cell Physiol. 2019;234:6298‐6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Amini‐Nik S, Yousuf Y, Jeschke MG. Scar management in burn injuries using drug delivery and molecular signaling: current treatments and future directions. Adv Drug Deliv Rev. 2018;123:135‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Keravis T, Favot L, Abusnina AA, et al. Delphinidin inhibits tumor growth by acting on VEGF Signalling in endothelial cells. PLoS One. 2015;10:e0145291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sharifi‐Rad M, Pezzani R, Redaelli M, et al. Preclinical pharmacological activities of epigallocatechin‐3‐gallate in signaling pathways: an update on cancer. Molecules. 2020;25(3):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Suganya V, Anuradha V. In silico molecular docking of astaxanthin and sorafenib with different apoptotic proteins involved in hepatocellular carcinoma. Biocatal Agric Biotechnol. 2019;19:101076. [Google Scholar]
- 101. Bommu UD, Konidala KK, Pabbaraju N, Yeguvapalli S. QSAR modeling, pharmacophore‐based virtual screening, and ensemble docking insights into predicting potential epigallocatechin gallate (EGCG) analogs against epidermal growth factor receptor. J Recept Signal Transduct. 2019;39:18‐27. [DOI] [PubMed] [Google Scholar]
- 102. Wang TY, Chen JX. Effects of Curcumin on vessel formation insight into the pro‐and antiangiogenesis of Curcumin. Evid Based Complement Alternat Med. 2019;2019:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yen Y‐H, Pu C‐M, Liu C‐W, et al. Curcumin accelerates cutaneous wound healing via multiple biological actions: the involvement of TNF‐α, MMP‐9, α‐SMA, and collagen. Int Wound J. 2018;15:605‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Duluc L, Jacques C, Soleti R, Andriantsitohaina R, Simard G. Delphinidin inhibits VEGF induced‐mitochondrial biogenesis and Akt activation in endothelial cells. Int J Biochem Cell Biol. 2014;53:9‐14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs


