The number of symptomatic and severe COVID-19 patients and deaths caused by COVID-19 were reduced by the large-scale vaccination campaign in 2021 [1].
Since the worldwide campaign started, vaccine-related occasional findings were described in patients performing PET/CT for other purposes. Increased tracer uptake at the injection site in the deltoid muscle and concomitant ipsilateral hypermetabolic axillary lymph nodes are the most frequently reported findings at [18F]FDG PET/CT (pooled prevalence of 30% (95% CI 20–41) and 37% (95% CI 27–47), respectively [2]). Lymph nodes can be normal or enlarged. Other ipsilateral supraclavicular and cervical lymph nodes have been more rarely observed [3]. The incidence of hypermetabolic axillary lymph nodes is higher in the first few weeks after the vaccination and decreases with time, although they may be visible even after 10 weeks [2]. After the third vaccination dose, the reported duration of hypermetabolic nodes is shorter (up to 5 days) [4]. These findings are detected more frequently in immunocompetent and young patients compared to immunocompromised and elderly [2]. Although deltoid uptake and hypermetabolic lymph nodes post-COVID-19 vaccination are typically reported with [18F]FDG, similar findings have also been described with other tracers and in patients recently vaccinated for influenza. These findings are related to the transient inflammation from immune system activation [5], and in the majority of cases, it can be easily correlated with the history of recent vaccination (Fig. 1). However, patients evaluated for certain diseases, such as lymphoma, melanoma, and breast and head and neck cancer, can be confusing and hard to interpret. Medical history should be taken into account during vaccination, and accordingly, the vaccination injection should be performed in the arm contralateral to the disease or even elsewhere (e.g. thigh). This shrewdness may reduce false-positive or inconclusive examinations.
Fig. 1.
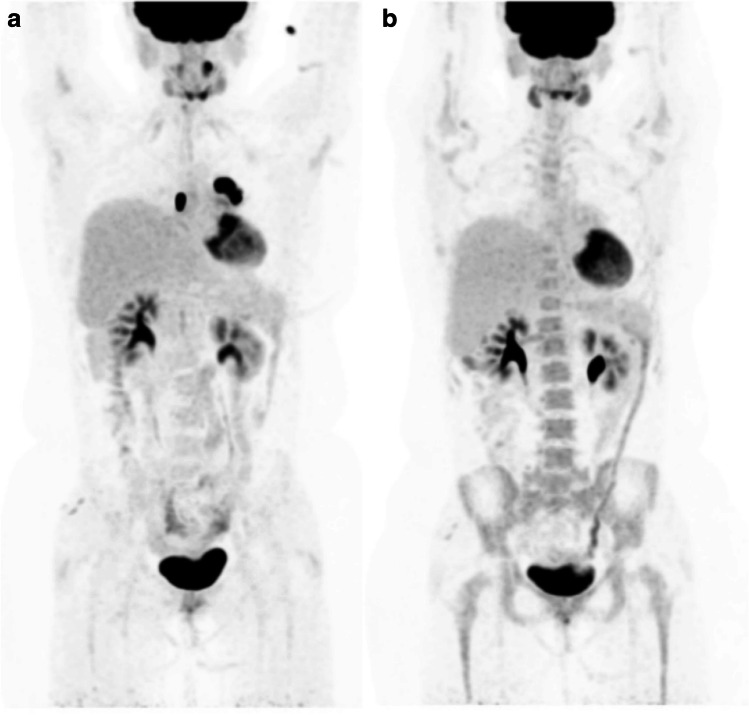
Restaging [18F]FDG PET/CT after neoadjuvant chemotherapy in a patient who has right breast cancer. Six days before the examination, she received the vaccine for influenza in the right deltoid muscle and the third dose of COVID-19 vaccination on the left side. Images show hypermetabolic lymph nodes in the right and left axilla related to influenza and COVID-19 vaccinations, respectively
In the last months, rare cases of autoimmune diseases that occurred or worsened after the Covid-19 vaccination were also claimed. Recently, von Tresckow et al. [6] reported the case of a patient imaged by PET/CT for an autoimmune disease possibly related to vaccination. After mRNA vaccination (Spikevax, Moderna, TX), the patient complained of cephalgia, cervicalgia, ostealgia, and pain in multiple large joints and muscles associated with increased inflammatory indices. The clinical picture was suspected for large vessel vasculitis. Images showed increased [18F]FDG uptake on vertebral and femoral arteries (associated with vessel wall thickening) and on thoracolumbar inter-spinous and pelvic bursae, findings highly suspicious for rheumatic polymyalgia [6].
Another interesting report has been recently published by Boursier et al. [7]. They presented digital-PET/CT images from two patients who experienced myocarditis a few days after the second shot of the mRNA vaccine (Spikevax and COMIRNATY, respectively). Images acquired up to 3 days from peak troponin showed increased myocardial uptake related to myocardial inflammatory cells in the infiltrate overexpressing somatostatin receptors [7].
Other reports on autoimmune disease possibly related to COVID-19 vaccination are summarized in Table 1. We also observed some anecdotal cases of autoimmune diseases after the COVID-19 vaccination (Figs. 2 and 3).
Table 1.
Reports on autoimmune disease possibly related to COVID-19 vaccination
| Reference | Sex, age | Tracer | Vaccination | Days between vaccination and examination | Main findings at PET/CT | Injection site uptake | Hypermetabolic lymph nodes |
|---|---|---|---|---|---|---|---|
| [8] | F, 35 yr | [18F]FDG | 1st administration of mRNA-1273 | 10 | Thymic hyperplasia | nr | Yes |
| [9] | M, 67 yr | [18F]FDG | 2nd administration of mRNA-1273 | 4 | Radiation recall pneumonitis | nr | nr |
| [10] | F, 46 yr | [18F]FDG | nr | 7 | Splenic and bone marrow uptake | nr | Yes |
| [11] | F, 65 yr | [18F]FDG | 1st administration of mRNA-1273 | 5 | Splenic uptake | Yes | Yes |
| [7] | M, 18 yr | [68Ga]Ga-DOTATOC | 2nd administration of mRNA-1273 | 2 | Myocarditis | nr | nr |
| M, 21 yr | 2nd administration of BNT162b2 | 3 | nr | nr | |||
| [6] | F, 78 yr | [18F]FDG | 1st administration of mRNA-1273 | nr | Rheumatic polymyalgia | nr | nr |
F, female; M, male; nr, not reported; yr, years
Fig. 2.
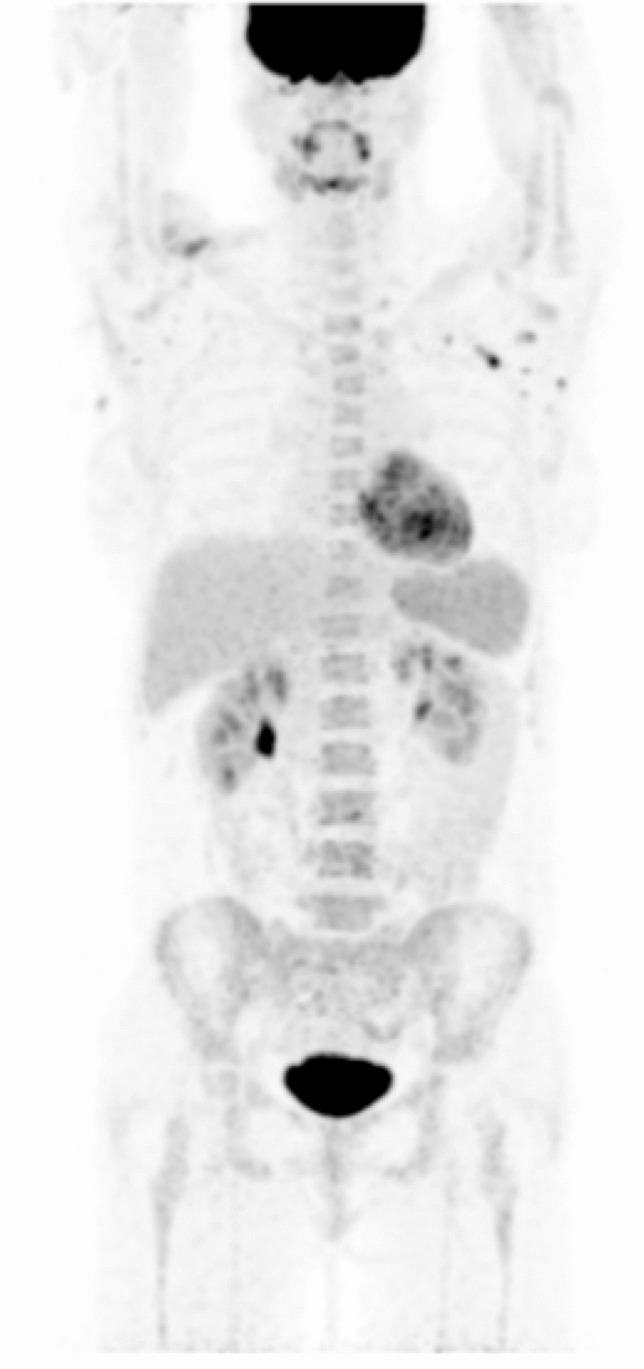
[18F]FDG PET/CT in a patient with a known history of psoriatic arthritis and erythema nodosum, still under treatment with corticosteroids. He complained of the onset of indolent lateral-cervical lymphadenopathies and bilateral salivary glands swelling after COVID-19 vaccination. PET/CT shows a pattern suspicious for sarcoidosis, with symmetric lymphadenopathies with high [18F]FDG uptake in the mediastinum, lateral-cervical and pulmonary hilum bilaterally, and in sub-diaphragmatic stations. Mediastinal lymphadenopathy biopsy confirmed the diagnosis of non-necrotizing granulomatous sarcoidosis
Fig. 3.
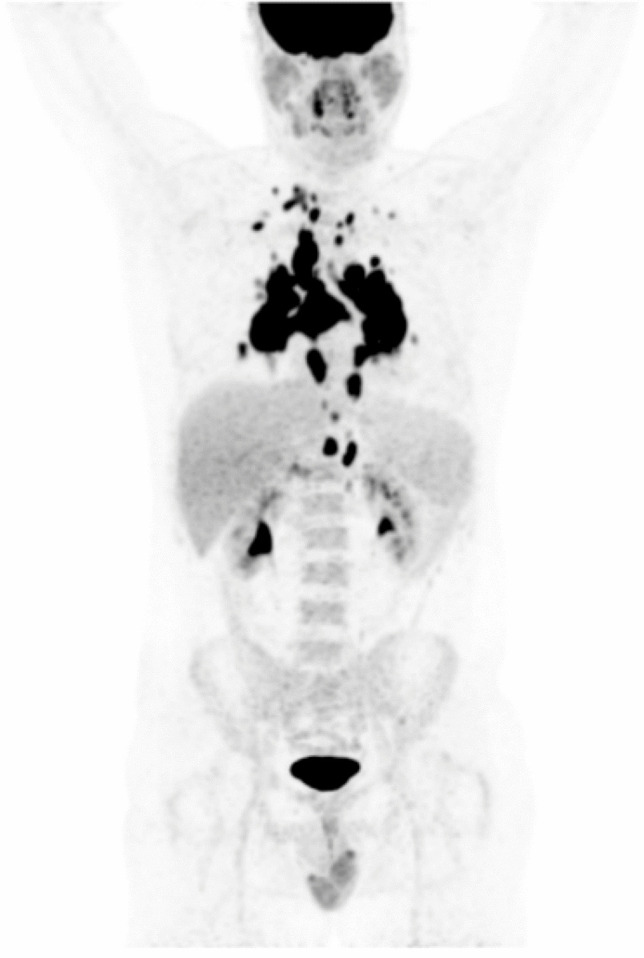
[18F]FDG PET/CT images in a patient who experienced a herpes zoster–associated erythema on the left thigh with associated ipsilateral inguinal lymphadenopathies that occurred after the second dose of COVID-19 vaccination. PET/CT images show [18F]FDG-avid left external inguinal lymphadenopathies associated with known herpes zoster–related erythema. COVID-19 vaccine-related swelled lymph nodes with [18F]FDG uptake are also visible in the left supraclavicular and ipsilateral axillary region
However, we must be cautious not to confound coincidental events with cause-effect relationships. Since the development of vaccines, there have been several complaints about the association with various autoimmune diseases, which were then disproved, such as between the hepatitis B vaccine and multiple sclerosis. Only in a few cases, the relationship has been scientifically proven (i.e. swine influenza vaccine and Guillain-Barré syndrome), with much lower risk than the disease itself and its related complications [12]. Also, regarding anti-COVID-19 vaccines, there is no solid scientific evidence that they may directly trigger autoimmune disorders. The above examples are predominantly rare isolated case reports. These phenomena might be explained by a genetic predisposition to develop such diseases, which probably would be precipitated equally by different factors and mechanisms. In addition, the risk of developing a severe form of COVID-19 is much higher in patients affected by autoimmune diseases than in the healthy population. Accordingly, the benefits of the vaccine are far greater than the risk of worsening the autoimmune disease. Therefore, clinical guidelines strongly recommend its administration also in patients suffering from autoimmune diseases [13].
Moreover, COVID-19 vaccination may also trigger positive effects. Indeed, the hyper-activation of innate immunity supporting the strong COVID-19-induced immune-stimulation also has potential effects on the tumour microenvironment. Cases of spontaneous regression post-COVID-19 infection have been described in lymphoma, renal carcinoma, and colorectal carcinoma patients [14–18], and the hyper-activation of innate immunity with anticancer effects generated by acute infection may be elicited similarly also by anti-COVID-19 vaccination. A case of disease spontaneous regression after BNT162b2 vaccination (COMIRNATY, BioNTech/Pfizer) has been recently described in lymphoma [19].
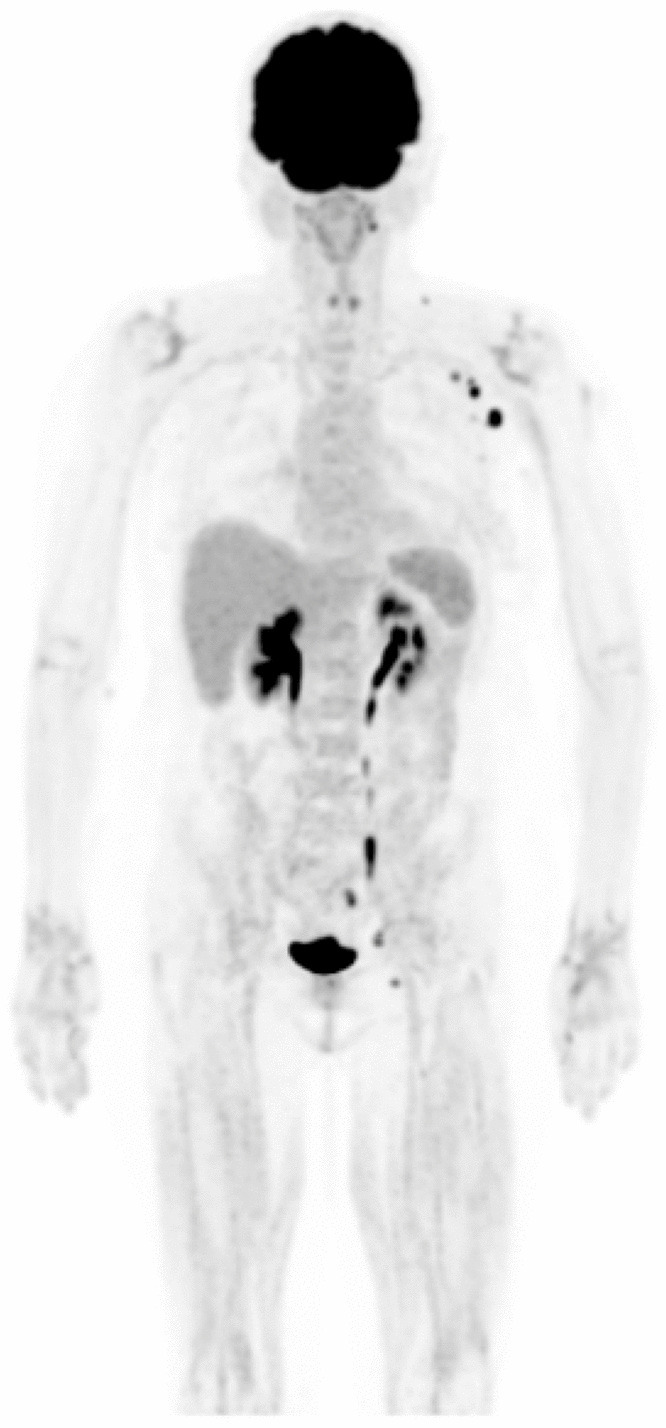
We recently observed a similar case in a multi-refractory diffuse large B-cell lymphoma (DLBCL) patient who experienced a spontaneous metabolic complete response after COVID-19 vaccination (Fig. 4).
Fig. 4.
[18F]FDG PET/CT images before (A) and after (B) COVID-19 vaccination in a multi-refractory diffuse large B-cell lymphoma (DLBCL) patient. She was treated with immunotherapy, and PET/CT images obtained after 5 cycles showed a disease progression (A). The treatment was stopped immediately, and she underwent a biopsy which confirmed DLBCL. About 1 month after, the patient underwent a new [18F]FDG PET/CT for restaging (B). Unexpectedly, previously reported lesions were no longer visible on either PET or CT images. Between the biopsy and the last PET/CT, the patient received the COVID-19 vaccination.
After all, vaccine-induced immune system stimulation for anti-tumour purposes is currently used in daily clinical practice. Bacillus Calmette-Guérin (BCG) is an attenuated vaccine from Mycobacterium bovis developed as an anti-tuberculosis vaccine. BCG is the standard of therapy as intravesical adjuvant treatment for non-invasive high- and medium-risk muscle bladder cancer. BCG induces a local immune response resulting in an anti-tumour effect called trained immunity [20, 21].
We can conclude that the COVID-19 vaccine and the acute COVID-19 disease cause a strong activation of the immune system that may result in several phenomena that can be observed at PET/CT images. These “incidental” findings range from classic local inflammation signs to more unusual events that may occur in predisposed individuals, such as the triggering of autoimmune diseases or tumour regression. The cause-effect relationship of these events, being rare, cannot be demonstrated yet.
Acknowledgements
We thank the physicians who cured and managed patients included in this editorial.
Author contribution
MS and AC conceptualized the editorial; MS, FG, and MB drafted the paper; FG, MB, and MS provided original images and cases; AC critically commented the paper; all the authors critically revised the paper and approved the submitted version of the editorial.
Availability of data and material
The manuscript represents valid work, and neither this manuscript nor the one with substantially similar content under the same authorship has been published or is being considered for publication elsewhere.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate and consent for publication
Not applicable for an editorial.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Infection and Inflammation
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. 101056/NEJMoa2101765. Massachusetts Med Soc. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treglia G, Cuzzocrea M, Giovanella L, Elzi L, Muoio B. Prevalence and significance of hypermetabolic lymph nodes detected by 2-[18F]FDG PET/CT after COVID-19 vaccination: a systematic review and a meta-analysis. Pharmaceuticals. 2021;14:762. doi: 10.3390/ph14080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minamimoto R, Kiyomatsu T, et al. Glob Health Med. 2021;3:129. doi: 10.35772/ghm.2021.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen D, Hazut Krauthammer S, Wolf I, Even-Sapir E. A sigh of relief: vaccine-associated hypermetabolic lymphadenopathy following the third COVID-19 vaccine dose is short in duration and uncommonly interferes with the interpretation of [18F]FDG PET-CT studies performed in oncologic patients. Eur J Nucl Med Mol Imaging. 2021 Springer Science and Business Media Deutschland GmbH 1–7. [DOI] [PMC free article] [PubMed]
- 5.Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38:894–898. doi: 10.1007/s00259-011-1729-9. [DOI] [PubMed] [Google Scholar]
- 6.Schierz J-H, Merkel C, Kittner T, Ali F. Vasculitis and bursitis on [18F]FDG-PET/CT following COVID-19 mRNA vaccine: post hoc ergo propter hoc? Eur J Nucl Med Mol Imaging. Springer Berlin Heidelberg 2021 2–3. [DOI] [PMC free article] [PubMed]
- 7.Boursier C, Chevalier E, Filippetti L, Laetitia I, Roch V, Olivier H, et al. 68Ga-DOTATOC digital-PET imaging of inflammatory cell infiltrates in myocarditis following COVID-19 vaccination. Eur J Nucl Med Mol Imaging. 2021;2021:1–2. doi: 10.1007/s00259-021-05609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Tresckow J, von Tresckow B, Reinhardt HC, Herrmann K, Berliner C. Thymic hyperplasia after mRNA based COVID-19 vaccination. Radiol Case Rep. 2021;16:3744–3745. doi: 10.1016/j.radcr.2021.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes NM, Hammer MM, Awad MM, Jacene HA. Radiation recall pneumonitis on FDG PET/CT triggered by COVID-19 vaccination. Clin Nucl Med. 2021;Publish Ah:2300. [DOI] [PMC free article] [PubMed]
- 10.Nawwar AA, Searle J, Lyburn ID. Features of systemic immune response from COVID-19 vaccination on 18F-FDG PET/CT. Clin Nucl Med. 2021;47(1):e89–e90. doi: 10.1097/RLU.0000000000003859. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg J, Thomas A, Iravani A. 18F-fluorodeoxyglucose PET/CT findings in a systemic inflammatory response syndrome after COVID-19 vaccine. Lancet. 2021;397:e9. doi: 10.1016/S0140-6736(21)00464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362:1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 13.ACR COVID-19 Vaccine Clinical Guidance Task Force. COVID-19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases.
- 14.Kahraman S, Akinci MB, Sendur MAN, Yalcin B. Can the host immune response against SARS-CoV2 also cause an anticancer effect? Med Oncol. Springer; 2021;38:90. [DOI] [PMC free article] [PubMed]
- 15.Sollini M, Gelardi F, Carlo-Stella C, Chiti A. Complete remission of follicular lymphoma after SARS-CoV-2 infection: from the “flare phenomenon” to the “abscopal effect.” Eur J Nucl Med Mol Imaging. Eur J Nucl Med Mol Imaging. 2021;48:2652–2654. doi: 10.1007/s00259-021-05275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192(3):415. doi: 10.1111/bjh.17116. [DOI] [PubMed] [Google Scholar]
- 17.Buchler T, Fiser L, Benesova J, Jirickova H, Votrubova J. Spontaneous regression of metastatic renal cell carcinoma after SARS-CoV-2 infection: a report of two cases. Curr Oncol. 2021;28:3403–3407. doi: 10.3390/curroncol28050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottaiano A, Scala S, D’Alterio C, Trotta A, Bello A, Rea G, et al. Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-Cov-2 infection. London: SAGE Publications Sage UK; 2021. p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambichler T, Boms S, Hessam S, Tischoff I, Tannapfel A, Lüttringhaus T, et al. Primary cutaneous anaplastic large-cell lymphoma with marked spontaneous regression of organ manifestation after SARS-CoV-2 vaccination. 2021 Br J Dermatol. John Wiley & Sons, Ltd [DOI] [PMC free article] [PubMed]
- 20.Cardillo F, Bonfim M, da Silva Vasconcelos Sousa P, Mengel J, LRR C-B, Pinho RT. Bacillus Calmette–Guérin immunotherapy for cancer. Vaccines. 2021;9:439. doi: 10.3390/vaccines9050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer 2011 101. BioMed Central. 2011;10:1–20. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript represents valid work, and neither this manuscript nor the one with substantially similar content under the same authorship has been published or is being considered for publication elsewhere.
Not applicable.