Dear Editor,
The coronavirus disease 2019 (COVID-19) pandemic, originating from Wuhan City in China, has been a serious global concern (1). One of the cornerstones of ending the COVID-19 pandemic is vaccination. However, several vaccination-related neurological complications have been reported (2). There was an unexpectedly high incidence of acute transverse myelitis (ATM) as a neurological complication following COVID-19 infection (approximately 1.2–3.2%) (3,4), and 11 cases of ATM after inoculation of several types of COVID-19 vaccines had been reported by October 7, 2021 ([5], [6], [7]). Herein, we describe a case of ATM with a fatal prognosis following vaccination with an mRNA-based COVID-19 vaccine (BNT162b2, Pfizer) and review the clinical features of ATM in COVID-19 vaccine recipients.
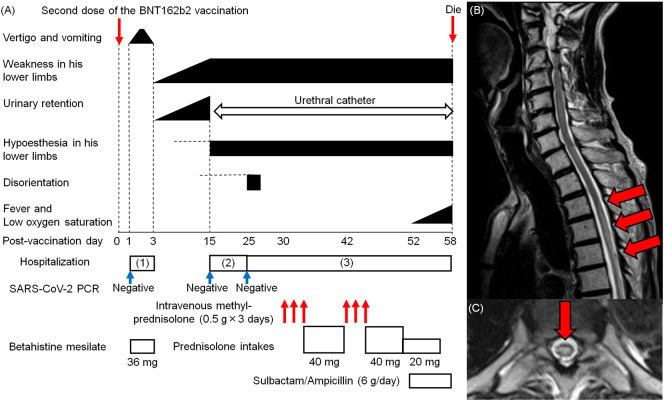
An 85-year-old man presented with vertigo and vomiting one day after receiving the second dose of the BNT162b2 vaccine (Fig. 1A). At admission, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) reverse transcription-polymerase chain reaction (RT-PCR) test using nasopharyngeal swabs was negative. He did not present with fever or other symptoms suggestive of infection. Neurological examination revealed rotatory nystagmus on the right side in the absence of weakness and sensory disturbance in the lower limbs. Blood tests showed no hematologic abnormalities. Thoracic CT findings were unremarkable. The vertigo and vomiting improved gradually, and the patient was discharged from the hospital on the 3rd day after vaccination. Subsequently, he presented with progressive gait disturbance and urinary retention, and he was admitted to the hospital because of gait disturbance on the 15th post-vaccination day. He had no fever or other symptoms suggestive of infection. The SARS-CoV-2 RT-PCR test was negative. Neurological examination showed proximal-dominant weakness and distal-dominant hypoesthesia in the lower extremities bilaterally. Hematological examinations and thoracic CT revealed no abnormalities. Brain CT and MRI were unremarkable, however, on the 16th day after vaccination, thoracic MRI revealed a longitudinal hyperintense lesion from the Th3–5 vertebral levels on T2-weighted imaging (Fig. 1B and C). On the 25th day after vaccination, the patient was referred to our hospital. Routine physical examination and RT-PCR for COVID-19 were normal. He was bedridden and neurological examination revealed that he was disoriented, with right-dominant paraplegia. He also had hypoesthesia in both lower limbs and hyporeflexia in both his upper and lower limbs, without an extensor plantar response. His consciousness immediately improved the following day. Blood tests revealed elevated levels of HbA1c (6.9%) and C-reactive protein (CRP) (3.26 mg/dL). Serologic tests confirmed the absence of anti-aquaporin (AQP)-4 antibodies. Cerebrospinal fluid (CSF) analysis demonstrated predominantly monomorphonuclear pleocytosis (11 cells/μL), with elevated protein levels (120 mg/dL) and the absence of oligoclonal bands. The IgG index (0.67) and myelin basic protein (MBP) level (58.1 pg/mL) were normal. Bacterial cultures and CSF cytology results were unremarkable. The patient was diagnosed with ATM, and intravenous methylprednisolone (0.5 g) for three days, followed by oral prednisolone (40 mg/day), was administered from the 30th day after vaccination. However, the patient's neurological symptoms did not improve. A second course of intravenous methylprednisolone (0.5 g) for three days from the 42nd day after vaccination still did not resolve his neurological symptoms. On the 52nd day after vaccination, he presented with fever and oxygen desaturation. Blood tests revealed pancytopenia with an elevated CRP level (15.72 mg/dL). Chest radiography revealed bilateral pneumonia. Treatment with intravenous sulbactam/ampicillin (6 g/day) did not improve the patient's condition, and on the 58th day after vaccination, he died. His family declined a postmortem examination.
Fig. 1.
Clinical course and MRI findings of our patient. (A) An 85-year-old man presented with vertigo and vomiting one day after receiving the second dose of the Pfizer-BioNTech coronavirus disease 2019 vaccine (BNT162b2). On the 3rd day after vaccination, the patient's symptoms improved. However, he presented with progressive paraplegia, hypoesthesia in the lower limbs bilaterally, and urinary retention, which prompted referral to our hospital. He was diagnosed with acute transverse myelitis. A three-day course of intravenous methylprednisolone (0.5 g) was initiated. However, the patient's neurological symptoms did not improve. Subsequently, he developed bilateral pneumonia. Treatment with sulbactam/ampicillin (6 g/day) did not resolve his condition. On the 58th day after vaccination, he died. (B, C) Thoracic MRI demonstrates a longitudinal hyperintense lesion at the Th3–5 vertebral levels (red arrow) (B: sagittal image, C: axial image at the Th4 vertebral level).
Our patient presented with progressive paraplegia, hypoesthesia in both lower limbs, and urinary retention following a two-day history of vertigo and vomiting after receiving the second dose of the BNT162b2 vaccine. Based on the Brighton case definition for myelitis, our patient was diagnosed with myelitis with level 2 diagnostic certainty (8). Treatment with continuous steroid therapy did not improve his neurological symptoms. Subsequently, he died of a poor general condition.
An increasing number of people are having the opportunity to get access to COVID-19 vaccines, and several neurological manifestations have been reported in COVID-19 vaccine recipients (2). Among the cases of severe neurological complications following COVID-19 vaccination, 11 of ATM had been reported by October 7, 2021 ([5], [6], [7]). We have shown the clinical features of ATM after COVID-19 vaccination (Table 1 ). The remarkable difference between the previously reported cases and ours was the prognosis after treatment. Both our patient and the previously reported patients were treated with immunological therapies (Table 1). The previously reported patients had recovery of neurological symptoms after treatment; however, our patient did not respond to treatment, and he died 58 days after receiving the second dose of the BNT162b2 vaccine (Table 1). The BNT162b2 vaccine is a lipid-nanoparticle-formulated mRNA vaccine against SARS-CoV-2 (9). Once administered, the mRNA is translated into SARS-CoV-2 spike proteins, which are expressed on the surface of host cells. The transient expression of spike antigens induces the production of antibodies against SARS-CoV-2 (9). Although the precise pathophysiologic mechanisms of the neurological complications following BNT162b2 vaccination remain uncertain, an abnormal immune-mediated inflammation has been implicated (10). This study highlights that ATM after COVID-19 vaccination may be refractory to conventional steroid therapy, and that ATM in COVID-19 vaccine recipients could have a poor prognosis.
Table 1.
Summary of the clinical features of acute transverse myelitis in coronavirus disease 2019 vaccine recipients.
| Patient (y.o./sex) | Past history | Vaccine types | Onset | Neurological symptoms | MRI findings | Treatments | Prognosis |
|---|---|---|---|---|---|---|---|
| 36/M5) | (−) | AZD1222 | 8 days after first vaccination | abnormal sensations in bilateral lower limbs, impaired vibration up to sternum | C6–7 (mild enhancement) | IV mPSL (1 g × 5 days), oral mPSL (16 mg/day, 12 hourly) | recovery |
| 45/M5) | dermatitis | AZD1222 | 11 days after first vaccination | acute flaccid tetraparesis, thoracic back pain, urinary retention | C3-Th2 (no enhancement) | IV mPSL (1 g × 5 days), oral PSL (100 mg/day) | recovery |
| 44/F5) | (−) | AZD1222 | 4 days after first vaccination | bilateral plantar feet paresthesia, hypoesthesia in her lower back | C6–7, Th2–3 (partial enhancement) | IV mPSL (1 g × 5 days), oral mPSL (1 mg/kg/day) | recovery |
| 40/F5) | RRMS | AZD1222 | 2 weeks after first vaccination | binocular blindness, back pain, lower-limb weakness and numbness | C4–5, C7-Th1, Th7–10 (no information about enhancement) | IV mPSL (2 g × 2 days), plasma exchange | recovery |
| 58/M5) | diabetes mellitus, pulmonary sarcoidosis | AZD1222 | 7 days after first vaccination | hyperesthesia below Th7, urinary retention, lower-limb numbness | Th2–10 (partial enhancement) | IV mPSL (1 g × 5 days), oral PSL (60 mg/day), plasma exchange | recovery |
| 41/M5) | diabetes mellitus | AZD1222 | 2 weeks after first vaccination | paresthesia below Th4, lower-limb weakness and clumsiness, loss of joint position and vibration | Th1–6 (no information about enhancement) | IV mPSL (1 g × 5 days), oral PSL (1 mg/kg/day) | recovery |
| 67/F5) | coronary artery disease, CKD, colon rupture | mRNA-1273 | 1 day after vaccination | weakness in four limbs | C1–3 (partial enhancement) | IV methylprednisolone sodium succinate (1 g × 3 days), plasma exchange | recovery |
| 76/F6) | hypertention | mRNA-1273 | 4 days after vaccination | unsteadiness and abnormal sensations in four limbs | C2–5 (partial enhancement) | IV mPSL (1 g × 5 days), oral PSL (60 mg/day) | recovery |
| 44/F5) | (−) | Ad26.COV2·S | 10 days after vaccination | nausia, urinary retention, back pain, lower-limb numbness and weakness | C2–3 (no information about enhancement) | mPSL, plasma exchange | recovery |
| 78/F5) | hypertension, diabetes mellitus, breast cancer | CoronaVAC | 3 weeks after second vaccination | tetraparesis, paresthesia in bilateral upper limbs, urinary retention | C1-Th3 (no information about enhancement) | IV mPSL (1 g × 4 days), plasma exchange | recovery |
| 38/M7) | (−) | BNT162b2 | 2 days after first vaccination | pain and weaknes in his lower limbs, headache | Th5–6 (no information about enhancement) | N.D. | N.D. |
| Our patient | |||||||
| 85/M | hypertension, diabetes mellitus, interstitial pneumonia | BNT162b2 | 1 day after second vaccination | paraplegia, hypoesthesia in bilateral lower limbs, urinary retention | Th3–5 (no enhancement) | IV mPSL (0.5 g × 3 days, 2 courses), oral PSL (40 mg/day) | die |
y.o., years old; M, male; F, female; RRMS, relapsing remitting multiple sclerosis; CKD, chronic kidney disease; IV, intravenous; mPSL, methylprednisolone; PSL, prednisolone; N.D., not described.
Author agreement
All authors read and approved the final version of the manuscript. They warrant that the article is the author's original work, has not received prior publication, and is not under consideration for publication elsewhere.
Disclosure statement
We confirmed that there is no financial/personal interest or belief that could affect objectivity.
Funding
Not applicable.
Availability of data and material
Anonymized data and material regarding this case report not included in the manuscript are available on request to the corresponding author by any qualified investigator.
Author's contributions
HN, KY, KK, MA, and YM performed the clinical and bedside studies. HN wrote the initial draft of the paper and all authors contributed to its preparation. The final version was read and approved by all authors.
Ethics approval
This work was approved by the ethic committee in Ishikawa prefectural central hospital.
Consent to participate
Informed concent was obtained from his family.
Consent for publication
Informed concent was obtained from his family.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
Not applicable.
References
- 1.Munz M., Wessendorf S., Koretsis G., Tewald F., Baegi R., Krämer S., Geissler M., Reinhard M. Acute transverse myelitis after COVID-19 pneumonia. J. Neurol. 2020;267:2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goss A.L., Samudralwar R.D., Das R.R., Nath A. ANA investigates: neurological complications of COVID-19 vaccine. Ann. Neurol. 2021;89:856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Román G.C., Gracia F., Torres A., Palacios A., Gracia K., Harris D. Acute Transverse Myelitis (ATM): clinical review of 43 patients with COVID-19- associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulte E.C., Hauer L., Kunz A.B., Sellner J. Systematic review of cases of acute myelitis in individuals with COVID-19. Eur. J. Neurol. 2021;28:3230–3244. doi: 10.1111/ene.14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsiao Y.T., Tsai M.J., Chen Y.H., Hsu C.F. Acute transverse myelitis after COVID-19 vaccination. Medicina. 2021;57:1010. doi: 10.3390/medicina57101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J.J., Tseng H.P., Lin C.L., Shiu J.S., Lee M.H., Liu C.H. Acute transverse myelitis following COVID-19 vaccination. Vaccines (Basel) 2021;9:1008. doi: 10.3390/vaccines9091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alshararni A. Acute transverse myelitis associated with COVID-19 vaccine: a case report. Int. J. Res. Pharma. Sci. 2021;12:2083–2087. doi: 10.26452/ijrps.v12i3.4818. [DOI] [Google Scholar]
- 8.Sejvar J.J., Kohl K.S., Bilynsky R., Blumberg D., Cvetkovich T., Galama J., Gidudu J., Katikaneni L., Khuri-Bulos N., Oleske J., Tapiainen T., Wiznitzer M., Brighton Collaboration Encephalitis Working Group Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5771–5792. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 9.Lamb T.N. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy D., Ghosh R., Dubey S., Dubey M.J., Benito-León J., Ray B.K. Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can. J. Neurol. Sci. 2021;48:9–24. doi: 10.1017/cjn.2020.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data and material regarding this case report not included in the manuscript are available on request to the corresponding author by any qualified investigator.