Abstract
The immune checkpoint molecule, B7-H3, which belongs to the B7 family, has been shown to be overexpressed in various cancers. Its role in tumors is not well defined, and many studies suggest that it is associated with poor clinical outcomes. The effect of B7-H3 on laryngeal cancer has not been reported. This study investigated the expression of B7-H3 in laryngeal squamous cell carcinoma (LSCC), and its relationship with clinicopathological factors and prognosis of LSCC patients. The gene expression quantification data and clinical data of LSCC retrieved from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) database were analyzed to determine the diagnostic and prognostic roles of B7-H3. Quantitative real-time polymerase chain reaction (qRT-PCR) was then performed to determine the gene expression level of B7-H3 between LSCC tissues and paired normal adjacent tissues. In addition, TCGA RNA-seq data was analyzed to evaluate the expression level of B7 family genes. Next, the protein expression of B7-H3 and CD8 in LSCC was determined using immunohistochemistry and immunofluorescence. qRT-PCR results showed that the expression level of B7-H3 mRNA was significantly higher in LSCC tissues than in adjacent normal tissues. Similar results were obtained from the TCGA analysis. The expression of B7-H3 was significantly associated with T stage, lymph node metastasis, and pathological tumor node metastasis (TNM) stage, and it was also an independent factor influencing the overall survival time (OS) of patients with LSCC. In addition, B7-H3 was negatively correlated with CD8+T cells. These results show that B7-H3 is upregulated in LSCC. Therefore, B7-H3 may serve as a biomarker of poor prognosis and a promising therapeutic target in LSCC.
Keywords: B7-H3, biomarker, LSCC - laryngeal squamous cell carcinoma, diagnosis, prognosis
Introduction
Laryngeal cancer is ranked as the second most prevalent head and neck tumor after lip and oral cavity cancers worldwide (1). A previous study reported that the incidence and number of deaths due to laryngeal cancer increased steadily from 1997 to 2017 worldwide (2). The main causes of deaths due to this cancer are tumor recurrence and distant metastasis. To date, there is no effective treatment method for advanced laryngeal cancer. This calls for in-depth research to identify new diagnostic biomarkers and therapeutic targets in laryngeal cancer.
As a member of the newly discovered immunomodulatory protein B7 family, B7-H3 has been found to play an important role in a variety of tumors and immune diseases. One study reported that B7-H3 can regulate activation of T cells and promote secretion of inflammatory factors (3). Recent studies have also confirmed that B7-H3 has an immunosuppressive function in several cancers (4, 5), including non-small cell lung cancer (6), breast cancer (7), and other tumors. In these cancers, it affects the prognosis of patients. Moreover, evidence suggests that overexpression of B7-H3 is closely associated with tumor cell apoptosis, cell metabolism, tumor angiogenesis, tumor drug resistance, and other aspects (8–11). However, the role of B7-H3 in laryngeal cancer has not been elucidated.
Therefore, this study investigated the expression level of B7-H3 in laryngeal squamous cell carcinoma (LSCC) tissues, and explored its association with clinicopathological features, cancer prognosis, and tumor-infiltrating CD8+ T cells. In addition, clinical results were combined with data retrieved from The Cancer Genome Atlas (TCGA) to determine the functions of B7-H3 on LSCC. To the best of our knowledge, this is the first study that has elaborated the role of B7-H3 in laryngeal cancer.
Materials and Methods
Data Retrieval and Determination of Differential Expression of B7 Family mRNAs
Clinical characteristics and gene expression data of LSCC patients of the HTSeq-FPKM type were acquired from TCGA (http://tcga-data.nci.nih.gov/tcga/) and GEO (https://www.ncbi.nlm.nih.gov/geo/) on 4th May 2021. The mRNA expression data of LSCC patients was also downloaded from the TCGA database and used to determine differentially expressed genes (DEGs) of B7 family in LSCC tissues. All samples were analyzed using the bioconductor package of edgeR. Results were visualized using gplots packages in R software (version 3.0.1.2). The DEGs were identified using |log2-fold change| ≥ 1.0 as the cut-off. Next, the survival R package was used to generate Kaplan-Meier survival curves of the DEGs, with P< 0.05 being the cut-off for statistical significance.
Patients and Clinical Samples
A total of 122 laryngeal cancer patients who underwent surgery between December 2012 and November 2015 were included in this study. The included patients met the following inclusion criteria: underwent surgery as the first treatment, preoperative pathological diagnosis was laryngeal squamous cell carcinoma, and did not receive preoperative radiotherapy, chemotherapy or other treatments. Exclusion criteria were: patients with other tumors or recurrent tumors and patients with other severe diseases or autoimmune disease. All patients were followed up for at least five years. During surgery, paired fresh laryngeal cancer specimens and normal adjacent tissues were collected from 12 patients. Patient clinicopathological variables were collected by reviewing medical records. Data extracted included: age, sex, primary tumor site, tumor differentiation, TNM stage, smoking index, and alcohol consumption. The TNM stage was defined according to the American Joint Committee on Cancer (AJCC). Overall survival (OS) was defined as the period from the date of surgery to the time of death or last follow-up, which was 1st November 2020. This study was approved by the institutional ethical review board of Sun Yat-Sen Memorial Hospital and signed informed consent form was obtained from all patients before the study.
RNA Extraction and Real-Time Quantitative PCR
Total RNA was extracted from homogenized frozen tissues using TRIzol reagent (Guangzhou IGE Bio.) and reverse transcribed to cDNA using a RevertAid First Strand cDNA Synthesis Kit (Guangzhou IGE Bio.) according to the manufacturer’s instructions. Next, quantitative real-time polymerase chain reaction (qRT-PCR) was performed on a Roche LightCycler 480 Real-Time PCR system using a SYBR Premix Ex Taq (Takara) according to the manufacturer’s instructions. The reaction protocol was as follows:1 min at 95°C, 40 cycles of 10 seconds at 95°C and 12 min at 60°C, and finally a 65°C to 95°C ramp up to determine the melting curve. Relative mRNA level of B7-H3 was normalized to that of GAPDH mRNA. The relative mRNA expression was calculated using the comparative 2-△△Ct method.
Immunohistochemistry
A total of 152 wax samples, including 122 LSCC samples and 30 adjacent normal tissues, were obtained from the pathology department of Sun Yat-sen Memorial Hospital. All samples were deparaffinized and rehydrated through a series of alcohol baths: 100% xylene (2×20 min), 100% ethanol (2×5 min), 95% ethanol, 80% ethanol, 75% ethanol and H2O (1×2 min each). Next, the samples were incubated in 10 mM sodium citrate buffer (pH 6.0) for antigen retrieval, followed by incubation with goat anti-human B7-H3 antibody (Cell Signaling Technology, 1:50) overnight at 4°C. Finally, 24 samples were further incubated with CD8 antibody (Cell Signaling Technology, 1:100) overnight at 4°C.
Evaluation of Immunohistological Staining
The staining intensity of B7-H3 was evaluated by two experienced pathologists using an independent double-blind method. Ten high-power fields (magnification×200) were selected to examine the positively stained area. The intensity of stained area was scored as follows: negative (0), weak (1), moderate (2), and strong (3). In addition, the percentage of positively stained cells was scored as: 0% to 5% (0), 6% to 25% (1), 26% to 50% (2), and >50% (3). To calculate the weighted scores for each section, the percentage of stained cells was multiplied with the staining intensity scores. Subsequently, a score of 4 was used as the cut-off to distinguish low B7-H3 expression low (<4) from high expression (≥4). The number of CD8+ T-cells in LSCC tissues was counted in three randomly selected tumor areas at a magnification of ×200. The average count was finally.
Double Immunofluorescence Staining
Paraffin‐embedded (FFPE) slides of LSCC tissues were dewaxed, rehydrated, and incubated in the retrieval buffer solution for antigen retrieval as described above. Next, the samples were incubated with a mixture of two primary antibodies [B7-H3 (goat antibody) and CD8 (rabbit antibody)] overnight at 4°C, followed by a mixture of FITC- and Cy3-conjugated secondary antibodies. The cell nuclei were counterstained blue with a DAPI solution. Images were acquired from at least three randomly high‐power fields using the Olympus BX51 microscope at the appropriate excitation wavelength for the fluorophore.
Statistical Analysis
All statistical analyses were performed using SPSS version 25.0 (IBM, Armonk New York). Data are presented as the mean ± S.E.M. Wilcoxon rank sum test was used to compare mRNA expression level between LSCC and noncancerous tissues. Categorical variables were analyzed using Fisher’s exact or Pearson’s chi-squared test. Moreover, the overall survival (OS) curve of patients was estimated using the Kaplan-Meier method. Univariate and multivariate analysis for hazard estimation was done using Cox regression. A P < 0.05 was considered statistically significant, and GraphPad Prism software version 8.0 was used to generate the figures.
Results
Identification of Differentially Expressed Genes of B7 Family in LSCC Tissues
To identify the association of B7 family genes with prognosis of LSCC, the RNA-Seq data of 32 LSCC samples and 7 normal tissues were obtained from the TCGA database. The mRNA expression profiles of seven B7 family genes were also extracted from the database and are shown in Figure 1A . Among them, B7-1, B7-DC, and B7-H3 were up-regulated in LSCC tissues (absolute logFC≥2.0, adjusted p<0.05). To validate results from TCGA, we used the GEO data for LSCC patients. The results also indicated significant upregulation of B7-1, B7-DC, and B7-H3 in LSCC samples compared to normal levels ( Figure 1B ). Kaplan-Meier analysis showed that none of them was significantly correlated with OS (P>0.05, Figure 1C ). However, patients with high B7-H3 expression had shorter OS time than patients with low B7-H3 expression.
Figure 1.

The expression levels of B7 family members in LSCC samples and normal tissues. The data was obtained from TCGA database (A) and GEO database (B). ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001. Overall survival of LSCC patients grouped based on B7-1, B7-DC, and B7-H3 expression level by Kaplan-Meier analysis (C). p < 0.05 considered as significant.
B7-H3 Is Overexpressed in LSCC Tissues
Next, the mRNA expression level of B7-H3 in LSCC and adjacent normal tissues (n=12) was determined. Results showed that expression of B7-H3 mRNA was significantly higher in LSCC tissues than in adjacent normal tissues (P< 0.01, Figure 2 ).
Figure 2.

Overexpression of B7-H3 in LSCC tissues compared with noncancerous tissues. B7-H3 mRNA was tested by qRT-PCR, relative to the internal endogenous control GAPDH. B7-H3 show significant increase in expression in LSCC compared to noncancerous tissues. **p < 0.01 (n = 12).
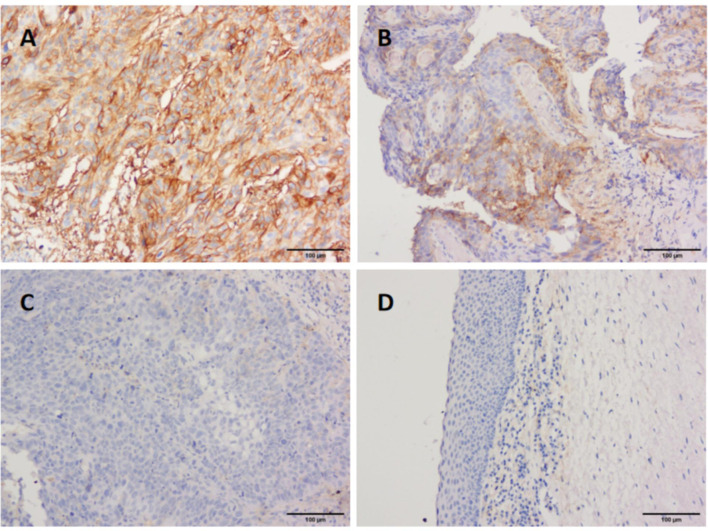
In addition, IHC was performed on 122 wax LSCC samples and 30 wax adjacent normal tissues to further evaluate the role of B7-H3. B7-H3 positive staining was mainly found in membranes of cancer cells and partially in the cytoplasm. However, central keratinized area of cancer nests was negatively stained. The positive expression of B7-H3 was also found in tumor-infiltrating immune cells. Results indicated that the expression of B7-H3 was significantly higher in LSCC tissues than in the adjacent normal tissues ( Figure 3 ), suggesting that B7-H3 is overexpressed in LSCC.
Figure 3.
Immunohistological staining of LSCC tissues for B7-H3 expression. Representative tissues of, strong staining (A), moderate (B), weak (C), and adjacent normal tissues (D) for B7-H3 expression in LSCC samples. (magnification ×200).
Correlation of B7-H3 Expression With Clinicopathological Features of LSCC Patients
Among the 122 LSCC cases, 75 (61.5%) stained strongly positive for B7-H3. Table 1 , shows that the expression of B7-H3 in LSCC correlated with T stage, lymph node metastasis, and TNM stage (P<0.05), but not with age, sex, tumor location, pathological differentiation, alcohol consumption, smoking, or cancer recurrence.
Table 1.
Clinicopathological factors associated with B7-H3 expression levels.
| Clinicopathological Features | n | B7-H3 expression | P | |
|---|---|---|---|---|
| low | high | |||
| (≤3) | (>3) | |||
| gender | ||||
| male | 118 | 45 | 73 | 0.632 |
| female | 4 | 2 | 2 | |
| age | ||||
| <60 | 54 | 22 | 32 | 0.654 |
| ≥60 | 68 | 25 | 43 | |
| T stage | ||||
| T1+T2 | 86 | 38 | 48 | 0.047 |
| T3+T4 | 36 | 9 | 27 | |
| lymph node metastasis | ||||
| N0 | 105 | 45 | 60 | 0.015 |
| N+ | 17 | 2 | 15 | |
| TNM stage | ||||
| I+II | 81 | 39 | 42 | 0.002 |
| III+IV | 41 | 8 | 33 | |
| pathological differentiation | ||||
| high | 57 | 25 | 32 | 0.257 |
| moderate,low | 65 | 22 | 43 | |
| alcohol consumption | ||||
| positive | 33 | 14 | 19 | 0.59 |
| negative | 89 | 33 | 56 | |
| smoking index | ||||
| <400 | 38 | 15 | 23 | 0.885 |
| ≥400 | 84 | 32 | 52 | |
| recurrence | ||||
| positive | 17 | 6 | 11 | 0.768 |
| negative | 105 | 41 | 64 | |
Overexpression of B7-H3 Confers Poor Prognosis to LSCC Patients
The median follow-up was 75.0 months in the total cohort. A Kaplan–Meier curve showed that overexpression of B7-H3 was significantly associated with poor prognosis at 60 months (P = 0.003, Figure 4 ). Next, univariate and multivariate Cox regression analyses were performed to determine whether B7-H3 is an independent prognostic factor for OS. Univariate analyses showed that T stage (P=0.039), lymph node metastasis (P=0.002), alcohol consumption (P=0.023), cancer recurrence (P=0.021), and B7-H3 expression (P=0.045) had a significant association with OS. On the other hand, multivariate analysis indicated that lymph node metastasis, alcohol consumption, cancer recurrence, and B7-H3 expression (P=0.038) were independent factors influencing the OS ( Table 2 ). Collectively, these results suggest that overexpression of B7-H3 is significantly associated with poor prognosis.
Figure 4.
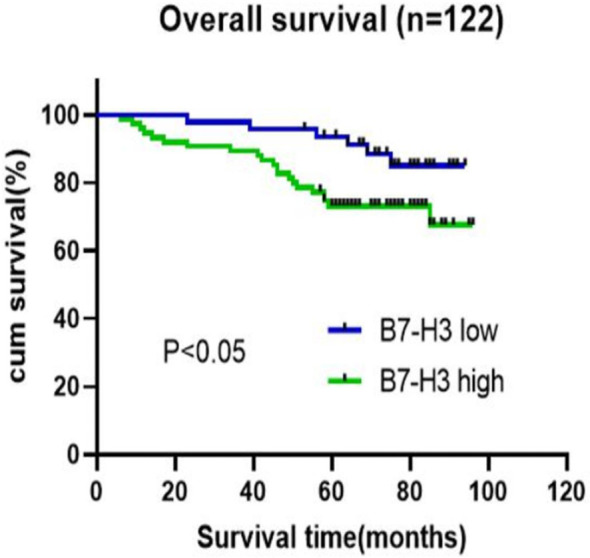
Survival analysis of correlation of overexpression of B7-H3 and overall survival in LSCC patients. The overall survival rate was calculated with Kaplan-Meier method and compared with log-rank test (n=122).
Table 2.
Univariate and Multivariate Cox proportional hazards analysis for overall survival in 122 LSCC patients.
| Variable | Group | Univariate Cox regression | Multivariate Cox regression | ||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| age | >60 (vs.≤60) | 0.789 (0.348-1.789) | 0.259 | ||
| gender | Male (vs. female) | 0.696 (0.094-5.172) | 0.776 | ||
| smoking index | ≥400 (vs.<400) | 1.346 (0.53-3.413) | 0.556 | ||
| pathological differentiation | well (vs.moderate,low) | 1.735 (0.735-4.094) | 0.303 | ||
| T stage | T3+T4 (vs. T1+T2) | 2.182 (0.956-4.978) | 0.039 | 1.774 (0.696-4.521) | 0.23 |
| lymph node metastasis | N+ (vs. N0) | 3.809 (1.564-9.277) | 0.002 | 5.708 (1.974-16.506) | 0.001 |
| alcohol consumption | Positive (vs. negative) | 2.747 (1.204-6.267) | 0.023 | 4.571 (1.829-11.425) | 0.001 |
| recurrence | Positive (vs. negative) | 2.758 (1.134-6.71) | 0.021 | 5.54 (2.036-15.079) | 0.001 |
| B7-H3 | low (vs. strong) | 3.423 (1.164-10.067) | 0.045 | 3.08 (1.066-8.899) | 0.038 |
HR, hazard ratio; CI, confidence interval.
Relationship Between B7-H3 Expression and Infiltration of CD8+ T Cells
Furthermore, we analyzed a TCGA cohort with head and neck squamous cell carcinoma (HNSCC) individuals using six different algorithms and found that the expression of B7-H3 was inversely correlated with the infiltration level of CD8+ T cells ( Figure 5 ). To determine the potential relationship between B7-H3 expression and tumor infiltrating CD8+ T cells in LSCC, immunohistochemical staining was performed in 24 cases for CD8. Results showed that the number of CD8+ T cells per 3HPF of high B7-H3 expression cases ranged from 50 to 238 (mean ± SD: 130 ± 58.2), and the number of low B7-H3 expression cases ranged from 111 to 381 (mean ± SD: 239 ± 87.0), indicating significant differences ( Figure 6 ). Given that previous IHC results showed a positive expression of B7-H3 in tumor-infiltrating immune cells, immunofluorescence was further performed to examine B7-H3 expression in CD8+ T cells. The results revealed a trend toward higher stroma CD8+ T-cell infiltration in the tumors relative to lower B7-H3 expression ( Figure 7 ). However, B7-H3 was not detected in CD8+ T cells. Collectively, these results suggest that B7-H3 is negatively correlated with CD8+ T-cell infiltrate density.
Figure 5.
Correlation of B7-H3 expression with infiltration of CD8+ T cells. The data was obtained from TCGA HNSCC datasets and analyzed by 6 different algorithms from TIMER 2.0.
Figure 6.

The number of tumor-infiltrating CD8+ T-cell was inversely correlated with B7-H3 expression in 24 patients with LSCC (**p < 0.01). This correlation was tested by Wilcoxon rank sum test.
Figure 7.
Representative immunofluorescence images of B7-H3 and tumor-infiltrating CD8+ T cells in LSCC tissues. Original magnification ×100; scale bar, 100 μm. No apparent colocalization of the B7-H3 (red) and CD8 (green) signal was found.
Discussion
Previous studies have reported that B7 family ligands are overexpressed in many types of malignancies and B7-H3 is highly expressed in HNSCC (12–14). This study examined the expression of a set of B7 family genes in LSCC tissues using TCGA and GEO cohorts. It was found that three DEGs including B7-H3 were upregulated in LSCC tissues. According to our PCR analysis, B7-H3 mRNA was also overexpressed in LSCC tissues. Furthermore, results of IHC test revealed that high B7-H3 expression level was associated with advanced T stage, lymph node metastasis, and advanced TNM stage. Moreover, high B7-H3 expression level was correlated with poor prognosis of LSCC. To the best of our knowledge, this study provides the first evidence that overexpression of B7-H3 can be used to predict the prognosis of patients with LSCC.
As a novel member of B7 family ligands, B7-H3 has been identified as an immune checkpoint in many types of cancers. It has been detected in several cancers, including melanoma, oral squamous carcinoma, colorectal cancer, and numerous cancers (10, 15–22). Overexpression of B7-H3 is associated with poor prognosis in most cancers. Previous studies demonstrated that B7-H3 was highly expressed in HNSCC tissues and thus it may be a prognostic marker (5, 14, 16). However, the role of B7-H3 in LSCC has not been elucidated. In this study, we investigated the expression of B7-H3 in LSCC tissues, and found that B7-H3 was extensively expressed on membranes of cancer cells and slightly expressed in the cytoplasm. In addition, B7-H3 was highly expressed at the invasive front of tumor. Recent studies have reported that high expression of B7-H3 is associated with adverse clinicopathologic features and poor survival. In the present study, B7-H3 was found to be associated with advanced T stage, lymph node metastasis, and advanced TNM stage (III-IV), indicating that B7-H3 may regulate LSCC progression. However, B7-H3 was not correlated with recurrence in LSCC, unlike the results obtained for glioblastoma (23) and some other cancers (24, 25). Therefore, we will continue to follow up these patients to observe the possibility of recurrence. Next, the ability of B7-H3 to predict the prognosis of patients was tested. Results showed that B7-H3 was significantly associated with poor prognosis, suggesting that it might be an independent predictor of OS. Overall, this study reveals that B7-H3 may be used as a marker for predicting the prognosis of LSCC. While, as the adjacent tumor sample cohort size was limited, larger cohorts are required to validate these observations in the future.
As an immune checkpoint, B7-H3 acts as a costimulatory and coinhibitory molecule that influence T cell proliferation (26). Chapoval et al. (3) reported that B7-H3 promoted proliferation of both CD4+ and CD8+ T-cells, and enhanced function of the cytotoxic T-cell (CTL). However, some studies have shown that B7-H3 has a negative effect on proliferation of T-cells (27, 28). Similarly, B7-H3 was inversely associated with the infiltration of CD8+ T cells, and B7-H3 blockade decreased the number of cancer stem cells in HNSCC (29). In this study, IHC staining results revealed that immune cells in the stromal tissue stained positive for B7-H3. To further determine the relationship between B7-H3 and CD8+ T cell infiltration, immuno-fluorescence staining experiment was done and tissues were examined under a confocal microscope. It was found that CD8+ infiltration was mainly occurred in stromal areas and positive staining for B7-H3 was mostly seen in tumor tissues. In addition, low B7-H3 expression was associated with high CD8+ infiltration. However, B7-H3 was not expressed on CD8+ T cells. Similar results were obtained from IHC staining tests. CD8+ T lymphocytes have been shown to be correlated with good prognosis in many cancer types and may be an independent favorable prognostic factor in HNSCC (30). Based on results of this study and previous studies (4, 19, 25), we infer that B7-H3 might inhibit proliferation of CD8+ T cells and impair antitumor immunity in LSCC. However, the specific mechanism of B7-H3 regulating CD8+ T cells infiltration needs further study.
In this study, although B7-H3 was found to be highly expressed in LSCC and associated with poor prognosis, the underlying molecular mechanism was not elucidated. However, we hypothesized that B7-H3 may inhibit CD8+T cells infiltration, hereby promote tumor immune escape. Similarly, a recent study reported that inhibition of B7-H3×m4-1BB suppressed tumor growth and increased the number of terminally differentiated CD8+ T cells in tumor models (31). B7-H3 was also found to be an inhibitor of CD4+T-cells, Th cells, Treg cells, and NK cells (32, 33). However, the receptor of B7-H3 is currently unknown. Some researchers have proposed that the triggering receptor expressed on myeloid cell (TREM)-like transcript 2 (TLT-2) may be a putative counter receptor for B7-H3 (34, 35). Therefore, further studies are advocated to identify the receptor of B7-H3, and elucidate the signaling mechanism of B7-H3. Immunotherapy has emerged as a promising treatment for malignancies. PD-L1/PD-1 blockade therapy has been approved for advanced head and neck cancer treatment, but a large fraction of patients do not respond to this therapy. Other studies have investigated the use of B7-H3 as an inhibitor of antibodies. For instance, 8H9, 131I-monoclonal antibodies targeting B7-H3, was found to be an immunotherapeutic agent in neuroectodermal tumors, and has been tested in a phase I trial to treat patients with diffuse intrinsic pontine glioma (36, 37). Therefore, this study suggests that B7-H3 may be a promising target for LSCC immunotherapy.
Conclusions
Overall, this study demonstrates that B7-H3 is upregulated in LSCC, and the high expression level of B7-H3 was closely associated with clinicopathological features and poor prognosis of LSCC patients. Moreover, B7-H3 may inhibit CD8+T-cell infiltration. These results suggest that B7-H3 may be a critical biomarker for predicting LSCC prognosis and a new target for LSCC immunotherapy.
Data Availability Statement
The datasets presented in this study can be found in online repositories, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of Sun Yat-Sen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YL performed the experiments and contributed to the writing of the manuscript. QC was as the cooperation teacher of the experiments and took charge of supplementary bioinformatic analysis; XS performed the experiments. XC took charge of the result analysis; ZG played a guiding role in conceiving and designing the study,and provided the overall writing guidance. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China No. 81572648.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Aupérin A. Epidemiology of Head and Neck Cancers: An Update. Curr Opin Oncol (2020) 32(3):178–86. doi: 10.1097/CCO.0000000000000629 [DOI] [PubMed] [Google Scholar]
- 2. Deng Y, Wang M, Zhou L, Zheng Y, Li N, Tian T, et al. Global Burden of Larynx Cancer, 1990-2017: Estimates From the Global Burden of Disease 2017 Study. Aging (Albany NY) (2020) 12(3):2545–83. doi: 10.18632/aging.102762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: A Costimulatory Molecule for T Cell Activation and IFN-Gamma Production. Nat Immunol (2001) 2(3):269–74. doi: 10.1038/85339 [DOI] [PubMed] [Google Scholar]
- 4. Katayama A, Takahara M, Kishibe K, Nagato T, Kunibe I, Katada A, et al. Expression of B7-H3 in Hypopharyngeal Squamous Cell Carcinoma as a Predictive Indicator for Tumor Metastasis and Prognosis. Int J Oncol (2011) 38(5):1219–26. doi: 10.3892/ijo.2011.949 [DOI] [PubMed] [Google Scholar]
- 5. Hu J, Jiang C, Zheng M, Guo Y, Tang X, Ren J, et al. Overexpression of B7-H3 as an Opportunity for Targeted Therapy in Head and Neck Cancers. Am J Trans Res (2019) 11(8):5183–96. [PMC free article] [PubMed] [Google Scholar]
- 6. Altan M, Pelekanou V, Schalper KA, Toki M, Gaule P, Syrigos K, et al. B7-H3 Expression in NSCLC and Its Association With B7-H4, PD-L1 and Tumor-Infiltrating Lymphocytes. Clin Cancer Res an Off J Am Assoc Cancer Res (2017) 23(17):5202–9. doi: 10.1158/1078-0432.CCR-16-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun J, Guo Y-D, Li X-N, Zhang Y-Q, Gu L, Wu P-P, et al. B7-H3 Expression in Breast Cancer and Upregulation of VEGF Through Gene Silence. OncoTargets Ther (2014) 7:1979–86. doi: 10.2147/OTT.S63424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang T, Jiang B, Zou ST, Liu F, Hua D. Overexpression of B7-H3 Augments Anti-Apoptosis of Colorectal Cancer Cells by Jak2-Stat3. World J Gastroenterol (2015) 21(6):1804–13. doi: 10.3748/wjg.v21.i6.1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, et al. B7-H3 Promotes Aerobic Glycolysis and Chemoresistance in Colorectal Cancer Cells by Regulating HK2. Cell Death Dis (2019) 10(4):308. doi: 10.1038/s41419-019-1549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, et al. B7-H3 Promotes Colorectal Cancer Angiogenesis Through Activating the NF-κb Pathway to Induce VEGFA Expression. Cell Death Dis (2020) 11(1):55. doi: 10.1038/s41419-020-2252-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu H, Tekle C, Chen YW, Kristian A, Zhao Y, Zhou M, et al. B7-H3 Silencing Increases Paclitaxel Sensitivity by Abrogating Jak2/Stat3 Phosphorylation. Mol Cancer Ther (2011) 10(6):960–71. doi: 10.1158/1535-7163.MCT-11-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janakiram M, Shah U, Liu W, Zhao A, Schoenberg M, Zang X. The Third Group of the B7-CD28 Immune Checkpoint Family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev (2017) 276(1):26–39. doi: 10.1111/imr.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burugu S, Dancsok A, Nielsen T. Emerging Targets in Cancer Immunotherapy. Semin Cancer Biol (2018) 52:39–52. doi: 10.1016/j.semcancer.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 14. Mao L, Fan T-F, Wu L, Yu G-T, Deng W-W, Chen L, et al. Selective Blockade of B7-H3 Enhances Antitumour Immune Activity by Reducing Immature Myeloid Cells in Head and Neck Squamous Cell Carcinoma. J Cell Mol Med (2017) 21(9):2199–210. doi: 10.1111/jcmm.13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang J, Chong K, Nakamura Y, Nguyen L, Huang S, Kuo C, et al. B7-H3 Associated With Tumor Progression and Epigenetic Regulatory Activity in Cutaneous Melanoma. J Invest Dermatol (2013) 133(8):2050–8. doi: 10.1038/jid.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Z, Liu J, Que L, Tang X. The Immunoregulatory Protein B7-H3 Promotes Aerobic Glycolysis in Oral Squamous Carcinoma via PI3K/Akt/mTOR Pathway. J Cancer (2019) 10(23):5770–84. doi: 10.7150/jca.29838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, et al. Expression and Clinical Significance of PD-L1, B7-H3, B7-H4 and TILs in Human Small Cell Lung Cancer (SCLC). J immunotherapy Cancer (2019) 7(1):65. doi: 10.1186/s40425-019-0540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang B, Zhang T, Liu F, Sun Z, Shi H, Hua D, et al. The Co-Stimulatory Molecule B7-H3 Promotes the Epithelial-Mesenchymal Transition in Colorectal Cancer. Oncotarget (2016) 7(22):31755–71. doi: 10.18632/oncotarget.9035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai D, Li J, Liu D, Hong S, Qiao Q, Sun Q, et al. Tumor-Expressed B7-H3 Mediates the Inhibition of Antitumor T-Cell Functions in Ovarian Cancer Insensitive to PD-1 Blockade Therapy. Cell Mol Immunol (2019) 17(3):227–36. doi: 10.1038/s41423-019-0305-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu CL, Zang XX, Huang H, Zhang H, Wang C, Kong YL, et al. The Expression of B7-H3 and B7-H4 in Human Gallbladder Carcinoma and Their Clinical Implications. Eur Rev Med Pharmacol Sci (2016) 20(21):4466–73. [PubMed] [Google Scholar]
- 21. Lupu CM, Eisenbach C, Lupu AD, Kuefner MA, Hoyler B, Stremmel W, et al. Adenoviral B7-H3 Therapy Induces Tumor Specific Immune Responses and Reduces Secondary Metastasis in a Murine Model of Colon Cancer. Oncol Rep (2007) 18(3):745–8. [PubMed] [Google Scholar]
- 22. Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, et al. Relationship Between Co-Stimulatory Molecule B7-H3 Expression and Gastric Carcinoma Histology and Prognosis. World J Gastroenterol (2006) 12(3):457–9. doi: 10.3748/wjg.v12.i3.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Digregorio M, Coppieters N, Lombard A, Lumapat P, Scholtes F, Rogister B. The Expression of B7-H3 Isoforms in Newly Diagnosed Glioblastoma and Recurrence and Their Functional Role. Acta neuropathologica Commun (2021) 9(1):59. doi: 10.1186/s40478-021-01167-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen J-T, Chen C-H, Ku K-L, Hsiao M, Chiang C-P, Hsu T-L, et al. Glycoprotein B7-H3 Overexpression and Aberrant Glycosylation in Oral Cancer and Immune Response. Proc Natl Acad Sci USA (2015) 112(42):13057–62. doi: 10.1073/pnas.1516991112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim N, Park M, Lee J. Associations of B7-H3 and B7-H4 Expression in Ductal Carcinoma In Situ of the Breast With Clinicopathologic Features and T-Cell Infiltration. Appl immunohistochemistry Mol morphology AIMM (2020) 28(10):767–75. doi: 10.1097/PAI.0000000000000817 [DOI] [PubMed] [Google Scholar]
- 26. Ni L, Dong C. New B7 Family Checkpoints in Human Cancers. Mol Cancer Ther (2017) 16(7):1203–11. doi: 10.1158/1535-7163.MCT-16-0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahnke K, Ring S, Johnson TS, Schallenberg S, Schönfeld K, Storn V, et al. Induction of Immunosuppressive Functions of Dendritic Cells In Vivo by CD4+CD25+ Regulatory T Cells: Role of B7-H3 Expression and Antigen Presentation. Eur J Immunol (2007) 37(8):2117–26. doi: 10.1002/eji.200636841 [DOI] [PubMed] [Google Scholar]
- 28. Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 Family Member B7-H3 Preferentially Down-Regulates T Helper Type 1-Mediated Immune Responses. Nat Immunol (2003) 4(9):899–906. doi: 10.1038/ni967 [DOI] [PubMed] [Google Scholar]
- 29. Wang C, Li Y, Jia L, Kim JK, Li J, Deng P, et al. CD276 Expression Enables Squamous Cell Carcinoma Stem Cells to Evade Immune Surveillance. Cell Stem Cell (2021) 28(9):1597–613.e7. doi: 10.1016/j.stem.2021.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. So Y, Byeon S, Ku B, Ko Y, Ahn M, Son Y, et al. An Increase of CD8 T Cell Infiltration Following Recurrence is a Good Prognosticator in HNSCC. Sci Rep (2020) 10(1):20059. doi: 10.1038/s41598-020-77036-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. You G, Lee Y, Kang Y, Park H, Park K, Kim H, et al. B7-H3×4-1BB Bispecific Antibody Augments Antitumor Immunity by Enhancing Terminally Differentiated CD8 Tumor-Infiltrating Lymphocytes. Sci Adv (2021) 7(3):eaax3160. doi: 10.1126/sciadv.aax3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kreymborg K, Haak S, Murali R, Wei J, Waitz R, Gasteiger G, et al. Ablation of B7-H3 But Not B7-H4 Results in Highly Increased Tumor Burden in a Murine Model of Spontaneous Prostate Cancer. Cancer Immunol Res (2015) 3(8):849–54. doi: 10.1158/2326-6066.CIR-15-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa A, et al. Identification of 4Ig-B7-H3 as a Neuroblastoma-Associated Molecule That Exerts a Protective Role From an NK Cell-Mediated Lysis. Proc Natl Acad Sci USA (2004) 101(34):12640–5. doi: 10.1073/pnas.0405025101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering Receptor Expressed on Myeloid Cell-Like Transcript 2 (TLT-2) Is a Counter-Receptor for B7-H3 and Enhances T Cell Responses. Proc Natl Acad Sci USA (2008) 105(30):10495–500. doi: 10.1073/pnas.0802423105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang SS, Tang J, Yu SY, Ma LI, Wang F, Xie SL, et al. Expression Levels of B7-H3 and TLT-2 in Human Oral Squamous Cell Carcinoma. Oncol Lett (2015) 10(2):1063–8. doi: 10.3892/ol.2015.3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental Intrathecal Radioimmunotherapy: Results for Treatment for Metastatic CNS Neuroblastoma. J Neuro-Oncol (2010) 97(3):409–18. doi: 10.1007/s11060-009-0038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Souweidane M, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, et al. Convection-Enhanced Delivery for Diffuse Intrinsic Pontine Glioma: A Single-Centre, Dose-Escalation, Phase 1 Trial. Lancet Oncol (2018) 19(8):1040–50. doi: 10.1016/S1470-2045(18)30322-X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories, further inquiries can be directed to the corresponding authors.