Abstract
The ubiquitin-proteasome pathway regulates gene expression through protein degradation. Here we show that the F-box protein βTrCP, the receptor component of the SCF E3 ubiquitin ligase responsible for IκBα and β-catenin degradation, is colocalized in the nucleus with ATF4, a member of the ATF-CREB bZIP family of transcription factors, and controls its stability. Association between the two proteins depends on ATF4 phosphorylation and on ATF4 serine residue 219 present in the context of DSGXXXS, which is similar but not identical to the motif found in other substrates of βTrCP. ATF4 ubiquitination in HeLa cells is enhanced in the presence of βTrCP. The F-box-deleted βTrCP protein behaves as a negative transdominant mutant that inhibits ATF4 ubiquitination and degradation and, subsequently, enhances its activity in cyclic AMP-mediated transcription. ATF4 represents a novel substrate for the SCFβTrCP complex, which is the first mammalian E3 ubiquitin ligase identified so far for the control of the degradation of a bZIP transcription factor.
Proteasome-mediated protein degradation requires the covalent attachment of polyubiquitin to the substrate proteins (11, 25, 38). The cascade of ubiquitin transfer reactions involves the ubiquitin-activating enzyme E1, an E2 ubiquitin-conjugating enzyme that operates with specificity factor E3. The selectivity of the reaction is due to the E3 ubiquitin ligase, which interacts with both E2 and the substrate.
SCF (Skp1/Cullin/F-box protein) complexes were initially shown to function as E3 ubiquitin ligases for a variety of phosphorylated proteins involved in the yeast cell cycle (1, 15, 35, 52, 60). The core components of these complexes include Skp1, Cul-1 (Cdc53), and the newly identified protein Rbx1 (Roc1 or Hrt1), which is thought to stabilize the interaction between Cul-1 and the E2 enzyme Cdc34 (12, 14, 30, 61). The SCF complexes also contain a variable receptor subunit, an F-box-containing protein, that provides substrate specificity. The F-box motif serves to anchor the receptor subunit to the SCF complex by its interaction with Skp1 (12, 14, 52, 60). For ubiquitination, substrate proteins are recruited to the SCF E3 ubiquitin ligase complexes through interaction with the substrate binding domain (WD-40 or leucine-rich repeats) of the F-box receptor subunits. SCF complexes are the largest and most versatile class of E3 ubiquitin ligases. To date, only two human SCF complexes, SCFSkp2 and SCFβTrCP, have been analyzed in detail and have had some of their substrates characterized (6, 7, 23, 36, 39, 41–44, 49, 62, 63, 67, 68). We made the first identification of human βTrCP (beta-transducin repeat-containing protein) as the F-box receptor component of the E3 ubiquitin ligase SCFβTrCP responsible for the degradation of CD4 induced by the human immunodeficiency virus type 1 (HIV-1) protein Vpu (43). Subsequently, we and others showed that SCFβTrCP is also responsible for phosphorylation-dependent ubiquitination and then for the degradation of IκBα and β-catenin (23, 36, 39, 42, 62, 67, 68). Vpu, IκBα, and β-catenin share a common motif, DSGXXS. Phosphorylation of its serine residues is required for interaction with βTrCP. IκBα is phosphorylated on the DS32GXXS36 motif by IKKα and IKKβ protein kinases activated through various signaling events (31). It is commonly thought that IκBα is subsequently ubiquitinated and degraded in the cytoplasm, resulting in NF-κB nuclear translocation and transcription stimulation of target genes (31). Similarly, it is also thought that β-catenin ubiquitination and subsequent degradation take place in the cytoplasm, preventing nuclear translocation of the protein which is required for TCF/LEF transcriptional activation of target genes (53).
ATF4 is a member of the ATF/CREB proteins that include CREB (cAMP responsive element binding protein), CREM (CRE modulator), ATF1, ATF2, and ATF3 (for reviews, see references 4, 22, 46, 57 and 69). These proteins bind to DNA via their basic region and dimerize via their leucine zipper domain to form a large variety of homodimers and/or heterodimers that allow the cell to coordinate signals from different pathways (4, 22, 46, 57, 69). Thus far, two other names have been used to refer to human ATF4 (21): CREB2 (32) and TAXREB67 (65). In addition, mouse cDNAs with 85% homology to human ATF4 have been referred to as mATF4 (47), C/ATF (66), or mTR67 (8). In the rest of this report, we will refer to it as ATF4. The E2 ubiquitin-conjugating enzymes involved in the degradation of some members of the ATF/CREB family have recently been identified; they include Cdc34 and Rad6B for hICERγ and ATF5 (ATFx), respectively (51), and hUBC9 for ATF2 (16). However, the E3 ubiquitin ligases required to make mammalian ATF/CREB transcription factor substrates for the proteasome remain unknown. While this paper was in preparation, Meimoun et al. identified the E3 ubiquitin ligase SCFCdc4 complex as responsible for degradation of the bZIP transcription factor Gcn4 in yeast (45).
There has been considerable interest in the role of ATF/CREB transcription factors and regulation of their activity by phosphorylation. In eukaryotes, cyclic (cAMP)-mediated transcription regulates multiple physiological processes, including gametogenesis, circadian rhythm, and neuroendocrine functions (13). Stimulation of this pathway is mediated via phosphorylation by protein kinase A (PKA) of a single serine in the structurally similar transcription factors CREB, CREM, or ATF1 (13). However, ATF4 lacks potential PKA phosphorylation sites and has been demonstrated to be a negative regulator of CRE-dependent transcription (2, 32). In contrast, ATF4 was proposed to be a positive regulator of transcription (40), increasing the expression of genes, such as somatostatin, serotonin, or interleukin-2 (2, 5, 66), and that of the human T-cell leukemia virus type 1 by interacting with the viral transactivator Tax (19, 54). How the versatile effects of ATF4 are regulated in these different pathways has not been investigated yet.
We found that ATF4 and βTrCP are associated in vivo and colocalized in the nucleus. Our results suggest that SCFβTrCP tightly modulates the stability of the transcription factor ATF4 and therefore also modulates its transcriptional activity following activation of the cAMP pathway.
MATERIALS AND METHODS
Two-hybrid assays.
Yeast two-hybrid screening was performed as described previously (3). Saccharomyces cerevisiae HF7 cells were transformed with the bait plasmid containing seven WD-40 repeats of human βTrCP (residues 251 to 569) fused to the Gal4 DNA binding domain in the pGBT10 plasmid and with a human Jurkat cDNA library fused to the GAL4 transactivation domain in the pGAD13-18 plasmid. Transformants were screened on plates lacking tryptophan, leucine, and histidine and were then assayed for β-galactosidase activity. For interaction assays, βTrCP, Slimb (27), or Fbw2 (9) and ATF4 or Skp1 were fused, respectively, to Escherichia coli LexA or the Gal4 binding domain and the Gal4 activation domain. The yeast reporter strain L40 or HF7 expressing the indicated hybrid protein pairs was analyzed for β-galactosidase expression or for histidine auxotrophy.
Plasmid construction and mutagenesis.
The ATF4 cDNA found in the screen corresponds to the protein accession number P18848. ATF4ΔBZ was obtained by PCR with specific primers and was subcloned in the pGAD13-18 vector (3). ATF4 point mutations (D218N, S219N, G220A, S224N) were constructed by PCR mutagenesis. pGADSkp1 plasmid and pcDNA3 expression vectors for βTrCP-Myc and βTrCPΔF-Myc have already been described (23, 36). βTrCPΔW1, βTrCPΔW2-7, and βTrCPΔN were obtained by PCR with appropriate primers and subcloned in the pcDNA3.1A/Myc-His vector (Invitrogen). Hemagglutinin (HA)-ATF4, HA-βTrCP, and HA-βTrCPΔF were obtained following direct subcloning of ATF4, βTrCP, and βTrCPΔF in the pAS1B vector (59). The Myc-His sequence in the pcDNA3.1/Myc-His vector was replaced by the green fluorescent protein (GFP) coding sequence, resulting in pcDNA3-βTrCP-GFP. The pAS2 expression vector for Fbw2 was kindly provided by J. Hsu and P. Jackson.
Coimmunoprecipitation experiments.
Samples containing 8 × 106 HeLa cells were transiently cotransfected by electroporation with 10 μg of either pAS1B, pAS1B-ATF4, or pAS1B-ATF4S219N and with 15 μg of the pcDNA3.1A/Myc-His plasmid containing wild-type or mutant βTrCP sequences. Cells were either not pretreated or were pretreated with 20 μM Z-LLL-H (Nα-benzyloxycarbonyl-l-leucyl-l-leucyl-l-leucinal; MG132; Sigma) for 4 h and then cells were harvested 48 h after transfection and lysed in 1% NP-40 lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1 mM EDTA). For immunoprecipitations, cell lysates were incubated with 5 μg of rat monoclonal anti-HA antibody per ml (clone 3F10; Boehringer) or 5 μg of mouse monoclonal anti-Myc antibody per ml (clone 9E10; Santa Cruz) for 90 min and then incubated with protein G-agarose beads (Boehringer) for 1 h. To study the association of ATF4 and βTrCP endogenous proteins, we performed a coimmunoprecipitation in untransfected 293T cells lysed using the NP-40 lysis buffer. The lysate was precleared with rabbit nonimmune antibodies and protein A-agarose (Sigma) for 90 min. Supernatant was incubated with anti-βTrCP antibody (43) or anti-ATF4 antibody [raised in rabbits by immunization with the fragment 207-351 of human ATF4 expressed in BL21(DE3)LysS bacteria and purified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)] or nonimmune rabbit polyclonal antibodies for 90 min and then were incubated with protein A-agarose (Sigma) for 30 min. The beads were washed with lysis buffer. Immune complexes were eluted with Laemmli sample buffer, separated by SDS–12% PAGE, and revealed by chemiluminescence using rat anti-HA (clone 3F10; Boehringer) or mouse anti-Myc (clone 9E10; Santa Cruz) or goat polyclonal anti-βTrCP (C-18; Santa Cruz) antibodies. To demonstrate the specificity of the anti-ATF4 antibodies, we used both immunoprecipitation and Western blot experiments with cells transfected by HA-ATF4 and compared the results with those obtained by Western blotting with untransfected 293T cells. When needed, quantitation of the chemiluminescent signal was performed using NIH Image software. Where indicated, treatment of immunoprecipitates with 32 U of alkaline bovine phosphatase corresponding to a final concentration of 180 μg/ml (P6774; Sigma) was administered to immune complexes for 45 min at 30°C in 50 mM Tris (pH 7.5)–1 mM MgCl2.
Ubiquitination assay.
In vivo ubiquitination was assayed as described by Treier et al. (64). Six-His tag–ubiquitin (10 μg) and HA-ATF4 (8 μg) expression vectors were transiently cotransfected by electroporation in HeLa cells. Twenty-four hours later, cells were lysed with 6 M guanidinium-HCl (pH 8), and His-tagged proteins were purified by nickel resins Ni-nitrilotriacetic acid agarose (Qiagen) as described by Treier et al. (64), eluted with Laemmli sample buffer, and separated by SDS–10% PAGE. Ubiquitin-conjugated HA-ATF4 proteins were revealed by chemiluminescence using anti-HA antibody (clone 3F10; Boehringer).
Immunostaining of cells.
For ATF4 and βTrCP localization, HeLa cells were transiently transfected by electroporation with 15 μg of pAS1B-ATF4 and 15 μg of pcDNA3-βTrCP-GFP and seeded onto glass plates at a density of 50,000 cells/plate. At 48 h, cells were washed with phosphate-buffered saline (PBS) and fixed for 20 min in 4% paraformaldehyde, quenched for 10 min with 0.1 M glycine in PBS, and permeabilized for 30 min at room temperature with 0.05% saponin in PBS–0.2% bovine serum albumin. Cells were incubated with anti-HA antibody (1/10 dilution), washed in PBS, and incubated with goat anti-rat Texas-RedR secondary antibodies (1/125 dilution; Jackson Immunoresearch). Confocal microscopy was carried out under fluorescent light.
35S metabolic labeling and pulse-chase experiments.
At 24 h after electroporation with various plasmids (pAS1B for ATF4 proteins and pcDNA3 for βTrCP proteins), 106 HeLa cells were incubated for 30 min in Met and Cys-free Dulbecco modified Eagle medium (DMEM), and then 125 μCi of [35S]methionine-cysteine (NENlife) per ml was added to the same medium for 1 h. Similarly, to study the stability of ATF4 endogenous protein, metabolic labeling was performed with 293T cells that were untransfected or were transfected with pcDNA3-βTrCP and were not pretreated or were pretreated with Z-LLL-H (Sigma). Cells were washed in PBS and harvested (time zero) or incubated for 0.5, 1, 2, 3, 3.5, or 4 h in complete DMEM, washed again in PBS, and lysed as described previously. Cell lysates were immunoprecipitated with mouse monoclonal anti-HA (12CA5; Boehringer) or rabbit polyclonal anti-ATF4 antibodies and incubated with protein G-agarose or protein A-agarose (Sigma) beads. Beads were washed with lysis buffer supplemented with NaCl (300 mM final concentration), and immune complexes were eluted with Laemmli sample buffer, separated on SDS–12% PAGE gels, fixed in acetic acid (10%)–methanol (30%), dried, and exposed to Kodak X-Omat film. Quantitation was performed with an ImageQuant phosphorimager (Molecular Dynamics).
Luciferase assays.
293T cells were plated in 6-well flat-bottom plates on the day prior to transfection at a density of 5 × 10−4 cells/35-mm-diameter well in DMEM. Transfections were performed using the calcium phosphate coprecipitation method with the Mammalian Transfection Kit (Stratagene). We used the luciferase reporter pSS-CRE-LUC containing a sequence of rat somatostatin gene from −71 to 53 (37) (including the CRE site) placed 5′ to the gene for luciferase. Cells were cotransfected with 0.2 μg of pSS-CRE-LUC, 3 ng of pRL-TK-renilla (PRL-TK from Promega), and various amounts of plasmids expressing HA-ATF4, HA-TrCP, or HA-TrCPΔF as indicated. Six hours prior to harvesting, half of the transfected dishes were incubated in a mixture of 10−5 M forskolin (Sigma) and 0.5 mM 3-isobutyl-1-methylxanthine (IBMX; Sigma). At 24 h posttransfection, cells were lysed and luciferase and renilla activities were measured with luciferase assay reagent (Dual-Luciferase Reporter Assay System; Promega) by using a Lumat LB9507 luminometer (EG&G Instruments).
RESULTS
βTrCP interacts with ATF4.
In order to find new substrates of βTrCP, we used the two-hybrid system. The C-terminal substrate-recruiting domain of βTrCP, which contains the seven WD-40 repeats (residues 251 to 569), was chosen as a bait (Fig. 1A) and was fused to the Gal4 DNA binding domain (43). A cDNA library from Jurkat cells, constructed in fusion with the Gal4 activation domain, was screened as previously described (3). Four clones interacting specifically with the WD-40 repeats of βTrCP and which corresponded to human ATF4 cDNA, with different 5′ ends within the N-terminal region, were isolated. Interaction between βTrCP and full-length ATF4 is shown in Fig. 1B, lane 1. No other proteins from the ATF/CREB family were isolated from our screen. The structure of βTrCP and ATF4 proteins is schematized in Fig. 1A. Deletion experiments allowed us to map the interacting domains between the two proteins, respectively, on the seven WD-40 repeats at the C terminus of βTrCP and between residues 87 to 279 of ATF4 (data not shown). The deletion of the basic region and the leucine zipper domain of ATF4 (ATF4ΔBZ), both located at the C terminus of the protein, did not affect the interaction with βTrCP (Fig. 1B, lane 2). The interaction of ATF4 with human βTrCP was specific since it was not found with other members of the WD-40 and F-box proteins, such as Fbw2 (the human homolog of mouse MD6; 9, 48) (Fig. 1C, lane 3) or the Drosophila melanogaster homolog of βTrCP, Slimb (Fig. 1C, lane 1) (27), although these proteins both interact with Skp1 (Fig. 1C, lanes 2 and 4). The human isoform of βTrCP, βTrCP2, interacts with ATF4 (data not shown).
FIG. 1.
Interaction of βTrCP with ATF4 is impaired by mutation on the D218 and S219 ATF4 residues. (A) Scheme of protein structures of βTrCP and ATF4. The diagram of βTrCP shows the F box responsible for proteasome targeting through Skp1 binding and the seven WD-40 repeats involved in binding substrates. For ATF4, the basic region (b) and the leucine zipper domain (Z) are indicated in addition to the DSGXXXS motif. (B) The yeast reporter strain L40 expressing the indicated hybrid protein pairs was analyzed for β-galactosidase expression. βTrCP was fused to the E. coli LexA binding domain in the pLex plasmid. ATF4 sequences were fused to the Gal4 activation domain in pGAD13-18. (C) The yeast reporter strains L40 or HF7 expressing the indicated hybrid protein pairs were analyzed for histidine auxotrophy.
Serine 219 of ATF4 is required for interaction with βTrCP.
The phosphorylation motif DSGXXS required for interaction of βTrCP with its substrates HIV-1 Vpu (43), IκBα, and β-catenin (23, 36, 39, 42, 62, 67, 68) was not found in the ATF4 sequence. However, a closely related motif of the type DSGXXXS occurred at positions 218 to 224 (Fig. 1A). Single amino acid substitutions in this motif, D218N or S219N, were sufficient to impair ATF4 interaction with βTrCP, whereas mutation of ATF4 residues G220 and S224 did not affect such interaction (Fig. 1B, lanes 3 to 6).
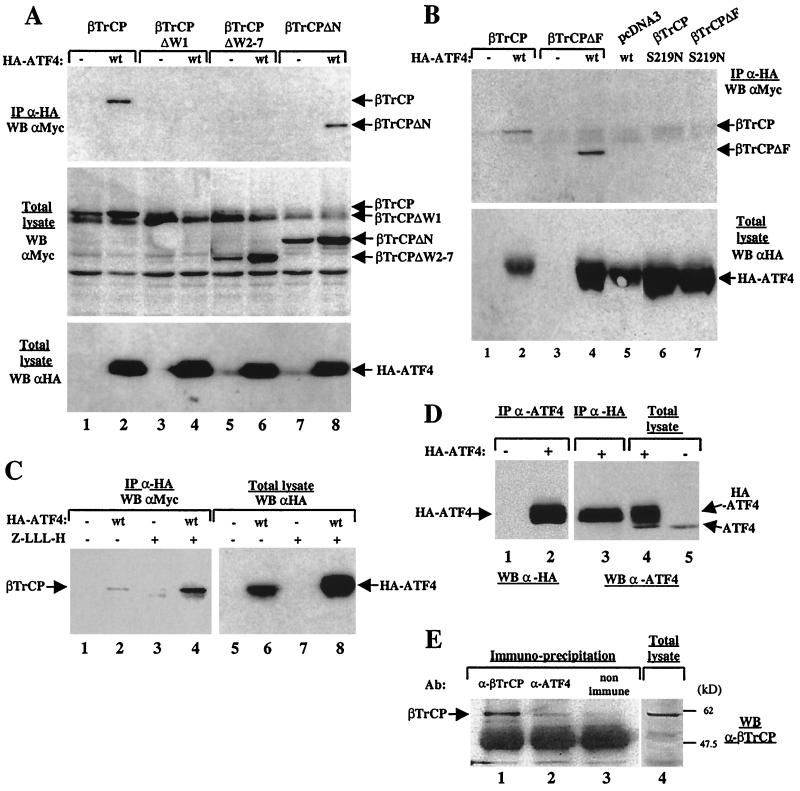
ATF4 and βTrCP were tagged with HA and Myc epitopes, respectively, and were transiently expressed in HeLa cells to determine whether the ATF4-βTrCP interaction can take place in vivo. HA-ATF4 was then immunoprecipitated using anti-HA antibody. Wild-type βTrCP was coprecipitated with HA-ATF4 (Fig. 2A, top, lane 2). Deletion of the first WD-40 repeat or the last six WD-40 repeats in βTrCP (βTrCPΔW1 and βTrCPΔW2-7) abolished the association with ATF4, confirming that the WD-40 repeats are the binding site for ATF4 (Fig. 2A, top, lanes 4 and 6). Expression of HA-ATF4 was checked and was shown to be identical irrespective of the βTrCP mutant proteins expressed (Fig. 2A, bottom). In addition, deletion in βTrCP of the F-box motif (βTrCPΔF) or of the N-terminal region upstream of the F-box motif (βTrCPΔN) did not affect association with ATF4 (Fig. 2B, top, lane 4, and Fig. 2A, top, lane 8, respectively). In ATF4, mutation of the serine residue at position 219 again abolished the interaction with βTrCP or βTrCPΔF (Fig. 2B, top; compare lanes 6 and 7 with lanes 2 and 4, respectively), although the level of the ATF4 S219N mutant was higher than that of wild-type ATF4 (Fig. 2B, bottom; compare lane 6 to lane 2). In the presence of the proteasome inhibitor Z-LLL-H, the amount of accumulated ATF4 was fivefold higher (Fig. 2C, right; compare lane 8 to lane 6), and subsequently, the ATF4-βTrCP interaction was stabilized (Fig. 2C, left; compare lane 4 to lane 2).
FIG. 2.
βTrCP-ATF4 association in HeLa and 293T cells. (A) HeLa cells were transfected with HA-ATF4 expression vector (lanes 2, 4, 6, and 8) or the corresponding empty vector (lanes 1, 3, 5, and 7) and plasmids expressing βTrCP protein tagged with the Myc epitope, wild-type βTrCP (lanes 1 and 2), or βTrCP deleted either from its first WD-40 repeat (βTrCPΔW1, lanes 3 and 4) or from WD-40 repeats 2 to 7 (βTrCPΔW2-7, lanes 5 and 6) or from its N-terminal region from residues 1 to 144 (βTrCPΔN, lanes 7 and 8). Proteins from total cytoplasmic lysates were immunoprecipitated with anti-HA antibodies (IP α-HA) and probed with anti-Myc antibodies (WB αMyc) (top) or directly probed with anti-Myc antibodies (middle) or anti-HA antibodies (bottom). (B) HeLa cells were transfected with wild-type (wt) ATF4 (lanes 2, 4 and 5), empty vector (lanes 1 and 3), or the S219N ATF4 mutant (lanes 6 and 7) and with βTrCP (lanes 1, 2, and 6), βTrCP deleted from its F-box motif (βTrCPΔF, lanes 3, 4, and 7), or the corresponding empty vector (lane 5). Anti-HA immunoprecipitates (IP α-HA) were analyzed by immunoblotting with anti-Myc antibodies (WB αMyc) (top) or lysates were directly probed with anti-HA antibodies (bottom). (C) At 48 h after transfection of vectors expressing βTrCP (lanes 1 to 8) and ATF4 (lanes 2, 4, 6, and 8) or empty vector (lanes 1, 3, 5, and 7), cells were exposed to Z-LLL-H for 4 h (lanes 3, 4, 7, and 8) or were left untreated (lanes 1, 2, 5, and 6). Anti-HA immunoprecipitates were analyzed with anti-Myc antibodies (lanes 1 to 4) or lysates were directly probed with HA antibodies (lanes 5 to 8). (D) Anti-ATF4 (lanes 1 and 2) or anti-HA (lane 3) antibodies were used to immunoprecipitate lysates from untransfected 293T cells (lane 1) or transfected cells expressing HA-ATF4 (lanes 2 and 3). The immunoprecipitates were probed with anti-HA (lanes 1 and 2) or with anti-ATF4 (lane 3) antibodies. Anti-ATF4 antibodies were used in Western blotting with total lysates from HA-ATF4 transfected cells (lane 4) or from untransfected cells (lane 5). (E) Proteins from 293T cell total lysate (lane 4) were immunoprecipitated with rabbit anti-βTrCP (lane 1), anti-ATF4 (lane 2), or nonimmune (lane 3) antibodies and were probed with goat anti-βTrCP antibodies. Western blotting from a total lysate of untransfected cells with goat anti-βTrCP antibodies is shown in lane 4.
Endogenous βTrCP coimmunoprecipitates with endogenous ATF4.
In order to check that both endogenous ATF4 and βTrCP proteins were also associated, we performed immunoprecipitations of untransfected cell lysates with anti-ATF4 antibodies, and the immunoprecipitate was probed by Western blotting with anti-βTrCP antibodies. The anti-βTrCP antibodies have been previously characterized by us (43) or by the supplier (Santa Cruz). In order to assess the specificity of the anti-ATF4 antibodies, we checked, as shown in Fig. 2D, that these antibodies can recognize specifically HA-ATF4, in both Western blot and immunoprecipitation experiments (Fig. 2D, lanes 2, 3, and 4). In transfected cells expressing HA-ATF4, two bands were detected by Western blotting using anti-ATF4 antibodies (Fig. 2D, lane 4). The upper band corresponds to the exogenous HA-ATF4 since it comigrates with HA-ATF4 precipitated by anti-HA antibodies (Fig. 2D, lane 3). The lower band, with an approximate molecular mass of 35 kDa, the only one detected in the untransfected cells, corresponds to the endogenous ATF4 (Fig. 2D, lanes 4 and 5). As shown in Fig. 2D, lane 2, anti-ATF4 antibodies efficiently immunoprecipitated HA-ATF4. Thus, these antibodies could be used for coimmunoprecipitation experiments of endogenous ATF4 and endogenous βTrCP proteins.
As shown in Fig. 2E (lane 2), a protein recognized by anti-βTrCP antibodies and migrating as a band with a molecular mass of 60 kDa was present in the anti-ATF4 immunoprecipitate. This band was clearly identified as βTrCP since it was also found in the immunoprecipitate with anti-βTrCP antibodies (Fig. 2E, lane 1) but not in the immunoprecipitate with non-immune antibodies (Fig. 2E, lane 3). Thus, this experiment demonstrates that the endogenous proteins interact in untransfected cells.
ATF4-βTrCP interaction depends on ATF4 phosphorylation.
In vivo interaction between ATF4 and βTrCP was confirmed by coimmunoprecipitation of HA-ATF4 with Myc-tagged βTrCP or Myc-tagged βTrCPΔF by using anti-Myc antibody (Fig. 3, lanes 2 and 4). To assess whether ATF4 that coprecipitated with βTrCP proteins was a phosphorylated form of ATF4, treatment of the immunoprecipitates with alkaline phosphatase was performed prior to denaturation and loading on SDS-PAGE gels. As shown in Fig. 3 (compare lane 3 to lane 2 and lane 5 to lane 4), such phosphatase treatment gave rise to a faster migrating band of HA-ATF4. This result is consistent with the fact that ATF4 which was associated with βTrCP in the anti-Myc immunoprecipitate was phosphorylated. Interestingly, when HA-ATF4 was immunoprecipitated with anti-HA antibody, two bands of HA-ATF4 were detected in the immunoprecipitate (Fig. 3, lane 6). From these results, one can conclude that only the phosphorylated forms of ATF4 can associate with βTrCP.
FIG. 3.
ATF4-βTrCP interaction depends on ATF4 phosphorylation. After transfection of vectors expressing HA-ATF4 (lanes 1 to 6) and βTrCP-Myc (lanes 2 and 3) or βTrCPΔF-Myc (lanes 4 and 5) or the empty vector pcDNA3 (lanes 1 and 6), anti-Myc or anti-HA immunoprecipitates (IP α-Myc and IP αHA, respectively) were left untreated (lanes 1, 2, 4, and 6) or were exposed to phosphatase treatment (lanes 3 and 5). Immunoprecipitates were probed with anti-HA antibodies (WB αHA).
ATF4 ubiquitination is enhanced in the presence of wild-type βTrCP.
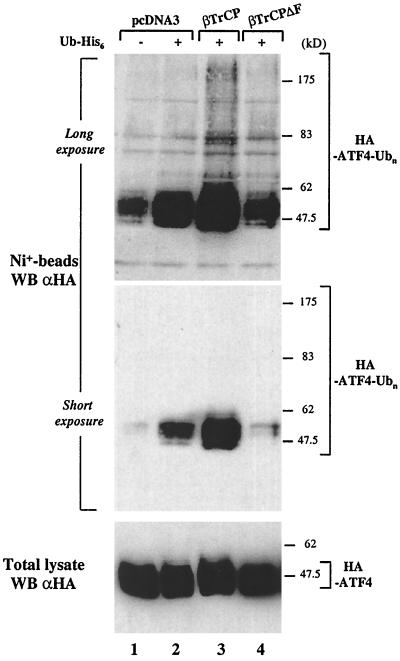
To investigate the role of βTrCP as an E3 ubiquitin ligase for ATF4, we decided to establish an in vivo ubiquitination assay of ATF4. HA-ATF4 was transiently coexpressed with a six-His tag-ubiquitin in HeLa cells. Ubiquitin-conjugated proteins were purified using nickel beads, and ubiquitin-conjugated ATF4 proteins were revealed by immunoblotting with HA antibodies. In the absence of the proteasome inhibitor Z-LLL-H, no ubiquitin-conjugated ATF4 was detected on the nickel beads (data not shown). In the presence of Z-LLL-H, ubiquitin-conjugated ATF4 was retained on the beads (Fig. 4, lane 2). In the absence of six-His tag-ubiquitin, no HA-ATF4 was detected on the beads after a short exposure (Fig. 4, lane 1, middle) although some background signal could be seen after a long exposure (Fig. 4, lane 1, top). To further confirm the role of βTrCP in ATF4 ubiquitination, we investigated the effect of the expression of βTrCP and of the βTrCPΔF mutant on ATF4 ubiquitination. In the presence of βTrCP, ATF4 ubiquitination was enhanced (Fig. 4, lane 3, short exposure) and higher-molecular-weight ubiquitinated forms could be detected (Fig. 4, lane 3, long exposure). In contrast, the expression of βTrCPΔF, previously identified as a negative transdominant able to inhibit degradation of IκBα, β-catenin, or CD4 (23, 36, 43), decreased ATF4 ubiquitination (Fig. 4, lane 4, short exposure). βTrCPΔF behaves as a negative transdominant because it binds substrates, but the deletion of the F-box motif impairs its association to Skp1, which is required for its recruitment to the SCF-E3 ubiquitin ligase complex, and the targeting of substrates to the proteasome (23, 36, 43). As a control, we checked that the total amount of ATF4 in the cells was roughly similar (Fig. 4, bottom). Thus, these results further support the notion that SCFβTrCP is the E3 ubiquitin ligase required for ATF4 ubiquitination. However, most of the ubiquitinated ATF4 that was recovered from nickel beads had a low molecular mass and corresponded in majority to monoubiquitinated ATF4 or to protein-conjugated ATF4 with a very small number of ubiquitin residues. In order to observe higher-molecular-weight forms of ATF4 representing polyubiquitinated protein, the longer exposure was needed. This result raises the question of whether ATF4 is preferentially targeted for monoubiquitination by SCFβTrCP.
FIG. 4.
ATF4 ubiquitination is enhanced in the presence of wild-type βTrCP. HeLa cells were transfected with HA-ATF4 expression vector (lanes 1 to 4), with plasmids expressing βTrCP (lane 3) or βTrCPΔF (lane 4) proteins or the corresponding empty vector (lanes 1 and 2), and with a six-His tag-ubiquitin expression vector (lanes 2 to 4). In vivo ubiquitin-conjugated proteins were purified, and ubiquitin-conjugated HA-ATF4 proteins were revealed by immunoblotting with HA antibody after a long exposure (top) or a short exposure (middle) of the film. The lower panel shows a control immunoblotting analysis of whole-cell extracts of HA-ATF4 expression.
βTrCP colocalizes with ATF4 in the nucleus.
The βTrCP-ATF4 interaction was further investigated in HeLa cells by confirming the codistribution of the proteins by confocal microscopy. In transient-transfection assays, we found by indirect immunofluorescence that βTrCP tagged either with GFP at the C terminus or HA epitope at the N terminus is mainly in the nucleus (Fig. 5A and B). In these assays, the nucleoli were unlabeled. After cotransfection with vectors expressing βTrCP-GFP and HA-ATF4, which is a nuclear protein (10), βTrCP-GFP and HA-ATF4 were found colocalized in the nucleus (Fig. 5C). All cells examined which had been successfully cotransfected with vectors expressing βTrCP-GFP and HA-ATF4 showed such colocalization of the two proteins in the nucleus.
FIG. 5.
βTrCp and ATF4 are codistributed in the nucleus. HeLa cells were transfected with a plasmid expressing βTrCP fused to the GFP protein at its C terminus (βTrCP-GFP) (A), with a plasmid expressing βTrCP fused to the HA epitope at its N terminus (HA-βTrCP) (B), or with both βTrCP-GFP and HA-ATF4 expression vectors (C). (B and C) Cells were stained with a Texas red-conjugated anti-HA antibody. Cells were analyzed by confocal microscopy.
ATF4 is an unstable protein, and βTrCP modulates its stability.
By 35S metabolic labeling and pulse-chase experiments, we studied the stability of the endogenous ATF4 protein in the presence or absence of the proteasome inhibitor Z-LLL-H in untransfected cells and in transfected cells overexpressing βTrCP. As shown in Fig. 6, the addition of the proteasome inhibitor Z-LLL-H stabilized ATF4 considerably, since most of the protein was still detectable after 3.5 h of chase (Fig. 6A and B). Overexpression of βTrCP resulted in a significant enhancement of the instability of the ATF4 protein, with its half-life decreasing from 30 to 15 min (Fig. 6B). These results demonstrate that the endogenous protein is an unstable protein with a half-life of about 30 min which is stabilized in the presence of proteasome inhibitors. In addition, we found that the protein is further destabilized by overexpression of βTrCP (Fig. 6A and B).
FIG. 6.
ATF4 is an unstable protein and βTrCP modulates its stability. (A) Untransfected 293T cells were not treated or were treated with the proteasome inhibitor MG132 (Z-LLL-H) as indicated, cells transfected with expression vector for βTrCP were [35S]Met-Cys metabolically labeled and chased for 0, 0.5, or 3.5 h, and lysates were immunoprecipitated with anti-ATF4 antibodies and analyzed following SDS–12%PAGE and autoradiography. (B) Scanning of the gel shown in panel A was performed using a phosphorimager. The ratios of the amounts of ATF4 left undegraded at each time point relative to that of the starting material were plotted as a function of time. (C) The ATF4 S219N mutant which has lost its ability to interact with βTrCP is stabilized in HeLa cells. Vectors expressing wild-type HA-ATF4 or HA-ATF4 S219N were transfected, cells were [35S]Met-Cys metabolically labeled and chased for 0, 1, 2, 3, and 4 h, and lysates were immunoprecipitated using anti-HA antibody and analyzed following SDS–12% PAGE and autoradiography. Immunoprecipitation with a lysate from labeled nontransfected cells (NT) was also performed. (D) Scanning of the gel shown in panel C. (E) Expression of the βTrCPΔF mutant inhibits ATF4 degradation. ATF4 was transfected in the presence of βTrCP, βTrCPΔF, or the corresponding empty vector pcDNA3, labeled, and immunoprecipitated as described for panel A. (F) Scanning of the gel shown in panel E.
Because of the critical importance of ATF4 residue serine 219 for interaction with βTrCP, we hypothesized that if such interaction plays a role in the control of the stability of this transcription factor, the ATF4 S219N mutant should be stabilized by comparison with wild-type ATF4 when expressed in cells. The ATF4 wild type and mutant S219N, both tagged with the HA epitope at the N terminus, were transfected in HeLa cells. At 24 h after transfection, 35S metabolic labeling and pulse-chase experiments from 0 to 4 h were carried out prior to immunoprecipitation using anti-HA antibody and SDS-PAGE analysis (Fig. 6C). HA-ATF4 and HA-ATF4 S219N half-lives were determined after quantification of the amounts of protein immunoprecipitated by anti-HA antibodies at each time point by using a phosphorimager. The half-life of HA-ATF4 was found to be of about 1 h (Fig. 6D). Of note is that the protein ran as a multiple set of bands, probably because phosphorylation events had taken place. The mutation S219N stabilized the protein, giving rise to a half-life of about 2.5 h (Fig. 6C and D). These results are in favor of a role for the βTrCP-ATF4 interaction in the stability of ATF4. Interestingly, the half-life of the overexpressed exogenous HA-ATF4 protein is significantly longer than that of the ATF4 endogenous protein (1 h instead of 30 min). This observation may indicate that the activity of the SCFβTrCP machinery to target ATF4 for degradation by the proteasome could be relatively limited in a context of overexpression of exogenous ATF4.
To further confirm the role of βTrCP in the control of ATF4 degradation, we investigated the effect on HA-ATF4 stability of the expression of the βTrCP wild type and of the βTrCPΔF mutant (Fig. 6E). In the presence of βTrCPΔF, we observed that wild-type ATF4 was stabilized, with its half-life increasing from 60 to 120 min (Fig. 6F). By contrast, expression of wild-type βTrCP shortened the half-life of the protein from 60 to 45 min (Fig. 6F). We checked that expression of βTrCP or βTrCPΔF did not affect the half-life of the ATF4 S219N mutant (data not shown). From these results, we conclude that ATF4 behaves like the other substrates of βTrCP (Vpu/CD4, IκBα, and β-catenin) whose degradations were impaired by the negative transdominant mutant of βTrCP (βTrCPΔF). Thus, ATF4 can be considered the fourth substrate of the SCFβTrCP identified so far.
Transcriptional activity of ATF4 is inhibited by βTrCP.
To check whether modulation of ATF4 stability by βTrCP had any functional consequences, we examined the effect of βTrCP or βTrCPΔF expression on the transcriptional activity of ATF4. ATF4 was identified as a transcriptional factor capable of inhibiting or activating transcription mediated through CRE (2, 5, 19, 32, 40, 54, 66). The activity of a luciferase reporter gene under the control of the somatostatin promoter, which contains the classical CRE binding site for the ATF/CREB transcription factors, was analyzed in 293T human embryo kidney cells after transfection with ATF4 and βTrCP or βTrCPΔF expression vectors and treatment with forskolin as a cAMP pathway stimulator. Consistent with the results obtained by Karpinski et al. (32), overexpression of ATF4 inhibited the CRE-dependent transcriptional stimulation induced by forskolin treatment (Fig. 7A). Expression of βTrCP impaired the repressor activity of ATF4, whereas expression of βTrCPΔF stimulated its activity (Fig. 7B). These results are consistent with the stimulation of ATF4 degradation provoked by βTrCP overexpression (Fig. 6E) and with the stabilization of ATF4 resulting from inhibition of ATF4 degradation induced by the expression of the βTrCPΔF-negative transdominant mutant (Fig. 6E).
FIG. 7.
Repression transcriptional activity of ATF4 on the somatostatin promoter stimulated by forskolin is controlled by βTrCP. (A) 293T cells were transiently cotransfected with a reporter plasmid expressing luciferase under the control of the somatostatin CRE sequence and different amounts of ATF4 expression vectors. Cells were left untreated or were stimulated in the presence of forskolin and IBMX. Experiments were repeated three times with duplicate samples, and results of a representative experiment are shown. The stimulation factor is reported to the transcription level observed in the cells without transfected ATF4. (B) Increasing amounts of βTrCP or βTrCPΔF expression vectors were transfected in the presence of the CRE luciferase reporter plasmid and 0.2 μg of ATF4 vector.
DISCUSSION
The ubiquitin-proteasome degradation pathway is important at the level of transcription regulation to assure the controlled and timely termination of signaling by irreversible destruction of the activated transcription factors. Because the E3 ubiquitin ligases are the factors responsible for the specificity of the ubiquitin-dependent proteolysis process, it is essential to identify which E3 targets which transcription factors. Here we have identified SCFβTrCP as the first mammmalian E3 ubiquitin ligase responsible for degradation of ATF4, a transcription factor belonging to the important bZIP family. ATF4 binds to βTrCP, the component receptor of the E3 complex by phosphorylation-dependent interaction, and is colocalized in the nucleus with this protein. We have found that βTrCP controls the stability of ATF4 and subsequently its transcriptional activity. However, the results of our in vivo ubiquitination experiments raise the question of whether ATF4 is preferentially targeted for monoubiquitination by SCFβTrCP.
It was recently reported by Kaiser et al. (29) that the ubiquitination of the transcription factor Met4 mediated by the F-box protein Met30 in yeast would not lead to its degradation as previously shown by Rouillon et al. (56) but nevertheless results in the inhibition of Met4 transcriptional activity. Since, among the yeast F-box proteins, Met30 is the closest homolog to βTrCP, one can wonder whether the same phenomenon of regulation of transcription by ubiquitination without proteolysis described for Met30 could also be observed with βTrCP. Although this cannot be ruled out from our results, we do not favor this hypothesis because in contrast with Met4, which was found to be a relatively stable protein (29), we observed that endogenous ATF4 is an unstable protein with a half-life of 30 min, further destabilized by overexpression of βTrCP, which shortens the half-life of the protein by a factor of two.
ATF4 has been described as both a negative regulator of CRE-dependent transcription (2, 32) and a positive regulator of transcription (5, 40, 66). One possibility for the difference is that forskolin, which activates the PKA pathway, was used in one experiment in which repression was observed (32) but was not used in experiments in which activation was observed. Another possibility is that different amounts of ATF4 were used in the experiments. As suggested previously (40), at high concentrations, ATF4 may repress transcription due to squelching. Alternatively, the difference in activity may be due to dimerization of ATF4 with other bZip proteins (20, 21, 57, 66). In our experiments, we showed that the inhibitory effect of ATF4 on CRE-dependent transcription of the somatostatin promoter is modulated by SCFβTrCP-mediated ATF4 degradation. It remains to be demonstrated whether the other ATF4 transcriptional activities are also regulated by βTrCP.
Our observation that βTrCP is a nuclear protein differs from the previously reported localization of βTrCP by Winston et. al. (67) as being largely cytoplasmic. However, our result is in agreement with the nuclear localization found by Hatakeyama et al. (reference 24 and personal communication). Such descrepancy with the results reported by Winston et al. (67) remains to be further evaluated. One possibility is that the cytoplasmic localization of βTrCP observed by Winston et al. could correspond to that of the βTrCP2 homolog of βTrCP (also called KIAA0696 [18, 26]). Indeed, we also found that βTrCP2, in addition to the nucleus, can be seen in the cytoplasm (unpublished results). The nuclear localization of βTrCP and its involvement in controlling the degradation of ATF4 illustrates the role that E3 ubiquitin ligases of the SCF type could play in the nucleus in mammalian cells. The question then arises whether the degradation of SCFβTrCP substrates takes place in the nucleus rather than in the cytoplasm, as it is currently thought for IκBα and β-catenin, two substrates of βTrCP (31, 53). This hypothesis is consistent with recent results which reveal that tumor necrosis factor alpha can induce the degradation of nuclear IκBα (28, 55). Interestingly, other components of the ubiquitination machinery have also been seen in the nucleus. These include the E2 ubiquitin-conjugating enzyme UBC9, which has been implicated in the proteolytic control of ATF2 (16), Cdc34, the E2 enzyme typically recruited by SCF complexes (41), Skp1 (17), Cullin1 (41), and the F-box protein Skp2 (41). Because several similar forms of βTrCP have been identified (26, 43), one can speculate that these isoforms could be differentially localized in cells, directing degradation of their substrates in different cellular compartments. For instance, we cannot at this point rule out the hypothesis that ATF4 is transported to the cytoplasm for its ubiquitination and degradation.
Interaction of ATF4 with βTrCP relies on motif DSGXXXS, similar but not identical to that found in the other substrates of βTrCP (HIV-1 Vpu, IκBα, and β-catenin), which is DSGXXXS (23, 36, 39, 42, 43, 62, 67, 68). It is interesting to note that the first serine residue of this motif seems to play an essential role in this interaction while the second serine residue, which is important in the case of Vpu and β-catenin (unpublished results), is not required for the interaction of ATF4 with βTrCP. The recent discovery of the role of βTrCP in the processing of NF-κB p105 supports the idea that the DSGXXS motif is not the only determinant of interaction with βTrCP (50). The βTrCP recognition motif in p105 is very different from the DSGXXS motif although it contains three phosphorylation sites important for interaction. However, p105 is a special case in the way it undergoes limited processing rather than complete destruction by the proteasome. Further work will be needed to determine which sequence requirements in addition to the DSGXXS motif are playing a role in interaction with βTrCP.
One crucial step which triggers the interaction of substrates with βTrCP is their phosphorylation by a distinct protein kinase, which thus confers specificity on the ubiquitination process. β-catenin, IκBα, and Vpu are phosphorylated on DSGXXS by GSK3/axin (53), IKK (31), and casein kinase II (58), respectively. To understand more of how ATF4 is regulated, it is thus crucial to identify the kinase that phosphorylates ATF4 on its DSGXXXS motif. It could be that ATF4 is phosphorylated by the nuclear protein kinase Zip-kinase/Dlk, recently identified as an ATF4 binding protein, favoring ATF4 interaction with βTrCP (33, 34).
ACKNOWLEDGMENTS
We thank F. Bantignies for the CRE-luciferase plasmid, M. Kroll for the βTrCPΔW1 and βTrCP ΔW2-7 expression vectors, J. Hsu and P. Jackson for the pAS2Fbw2 expression vector, I. Bouchaert for help in confocal microscopy analysis, Franck Letourneur for DNA sequencing, J. Bertherat, U. Hazan, Anne Gatignol and P. Chaffey for helpful discussions, and Owen Parkes for editing the manuscript.
C. Berlioz-Torrent is supported by FRM (Foundation pour la Recherche Medicale). This work was supported by ANRS (Agence Nationale pour la Recherche contre le SIDA), Sidaction, ARP, Association pour la Recherche sur le Cancer, and Ligue Nationale contre le Cancer.
REFERENCES
- 1.Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper J W, Elledge S J. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch D, Ghirardi M, Skehel P A, Karl K A, Herder S P, Chen M, Bailey C H, Kandel E R. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 3.Benichou S, Bomsel M, Bodeus M, Durand H, Doute M, Letourneur F, Camonis J, Benarous R. Physical interaction of the HIV-1 Nef protein with beta-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J Biol Chem. 1994;269:30073–30076. [PubMed] [Google Scholar]
- 4.Brindle P K, Montminy M R. The CREB family of transcription activators. Curr Opin Genet Dev. 1992;2:199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- 5.Butscher W G, Powers C, Olive M, Vinson C, Gardner K. Coordinate transactivation of the interleukin-2 CD28 response element by c-Rel and ATF-1/CREB2. J Biol Chem. 1998;273:552–560. doi: 10.1074/jbc.273.1.552. [DOI] [PubMed] [Google Scholar]
- 6.Carrano A C, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 7.Charrasse S, Carena I, Brondani V, Klempnauer K H, Ferrari S. Degradation of B-Myb by ubiquitin-mediated proteolysis: involvement of the Cdc34-SCF(p45Skp2) pathway. Oncogene. 2000;19:2986–2995. doi: 10.1038/sj.onc.1203618. [DOI] [PubMed] [Google Scholar]
- 8.Chevray P M, Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci USA. 1992;89:5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiaur D S, Murthy S, Cenciarelli C, Parks W, Loda M, Demetrick G, Pagano M. Five human genes encoding F-box proteins: chromosome mapping and analysis in human tumors. Cytogenet Cell Genet. 2000;88:255–258. doi: 10.1159/000015532. [DOI] [PubMed] [Google Scholar]
- 10.Cibelli G, Schoch S, Thiel G. Nuclear targeting of cAMP response element binding protein 2 (CREB2) Eur J Cell Biol. 1999;78:642–649. doi: 10.1016/S0171-9335(99)80049-1. [DOI] [PubMed] [Google Scholar]
- 11.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;15:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig K L, Tyers M. The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Prog Biophys Mol Biol. 1999;72:299–328. doi: 10.1016/s0079-6107(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 13.De Cesare D, Fimia G M, Sassone-Corsi P. Signaling routes to CREM and CREB: plasticity in transcriptional activation. Trends Biochem Sci. 1999;24:281–285. doi: 10.1016/s0968-0004(99)01414-0. [DOI] [PubMed] [Google Scholar]
- 14.Deshaies R J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–467. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- 15.Feldman R M, Correll C C, Kaplan K B, Deshaies R J. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell. 1997;91:221–230. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 16.Firestein R, Feuerstein N. Association of activating transcription factor 2 (ATF2) with the ubiquitin-conjugating enzyme hUBC9. Implication of the ubiquitin/proteasome pathway in regulation of ATF2 in T cells. J Biol Chem. 1998;273:5892–5902. doi: 10.1074/jbc.273.10.5892. [DOI] [PubMed] [Google Scholar]
- 17.Freed E, Lacey K R, Huie P, Lyapina S A, Deshaies R J, Stearns T, Jackson P K. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 1999;13:2242–2257. doi: 10.1101/gad.13.17.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs S Y, Chen A, Xiong Y, Pan Z Q, Ronai Z. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene. 1999;18:2039–2046. doi: 10.1038/sj.onc.1202760. [DOI] [PubMed] [Google Scholar]
- 19.Gachon F, Peleraux A, Thebault S, Dick J, Lemasson I, Devaux C, Mesnard J M. CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J Virol. 1998;72:8332–8337. doi: 10.1128/jvi.72.10.8332-8337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hai T W, Liu F, Coukos W J, Green M R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 22.Hai T, Wolfgang C D, Marsee D K, Allen A E, Sivaprasad U. ATF3 and stress responses. Gene Exp. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- 23.Hart M, Concordet J P, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 24.Hatakeyama S, Kitagawa M, Nakayama K, Shirane M, Matsumoto M, Hattori K, Higashi H, Nakano H, Okumura K, Onoe K, Good R A, Nakayama K I. Ubiquitin-dependent degradation of IkappaBalpha is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc Natl Acad Sci USA. 1999;96:3859–3863. doi: 10.1073/pnas.96.7.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa K, Nagase T, Suyama M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. X. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res. 1998;5:169–176. doi: 10.1093/dnares/5.3.169. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by F-box/WD400-repeat protein. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 28.Johnson C, Van Antwerp D, Hope T J. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IkappaBalpha. EMBO J. 1999;18:6682–6693. doi: 10.1093/emboj/18.23.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser P, Flick K, Wittenberg C, Reed S I. Regulation of transcription by ubiquitination without proteolysis: Cdc34/SCFMet30-mediated inactivation of the transcription factor Met4. Cell. 2000;102:303–314. doi: 10.1016/s0092-8674(00)00036-2. [DOI] [PubMed] [Google Scholar]
- 30.Kamura T, Conard M N, Yan Q, Conaway R C, Conaway J W. The Rbx1 subunit of SCF and VHL E3 ubiquitin ligase activates Rub1 modification of cullins Cdc53 and Cu12. Genes Dev. 1999;13:2928–2933. doi: 10.1101/gad.13.22.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 32.Karpinski B A, Morle G D, Huggenvik J, Uhler M D, Leiden J M. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc Natl Acad Sci USA. 1992;89:4820–4824. doi: 10.1073/pnas.89.11.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai T, Matsumoto M, Takeda K, Sanjo H, Akira S. ZIP kinase, a novel serine/threonine kinase which mediates apoptosis. Mol Cell Biol. 1998;18:1642–1651. doi: 10.1128/mcb.18.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kogel D, Plottner O, Landsberg G, Christian S, Scheidtmann K H. Cloning and characterization of Dlk, a novel serine/threonine kinase that is tightly associated with chromatin and phosphorylates core histones. Oncogene. 1998;17:2645–2654. doi: 10.1038/sj.onc.1202204. [DOI] [PubMed] [Google Scholar]
- 35.Krek W. Proteolysis and the G1-S transition: the SCF connection. Curr Opin Genet Dev. 1998;8:36–42. doi: 10.1016/s0959-437x(98)80059-2. [DOI] [PubMed] [Google Scholar]
- 36.Kroll M, Margottin F, Kohl A, Renard P, Durand H, Concordet J P, Bachelerie F, Arenzana-Seisdedos F, Benarous R. Inducible degradation of IkappaBalpha by the proteasome requires interaction with the F-box protein h-betaTrCP. J Biol Chem. 1999;274:7941–7345. doi: 10.1074/jbc.274.12.7941. [DOI] [PubMed] [Google Scholar]
- 37.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CMP. Nature. 1996;308:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 38.Laney J D, Hochstrasser M. Substrate targeting in the ubiquitin system. Cell. 1999;94:427–430. doi: 10.1016/s0092-8674(00)80752-7. [DOI] [PubMed] [Google Scholar]
- 39.Latres E, Chiaur D S, Pagano M. The human F box protein beta-Trcp associates with the Cul1/Skpl complex and regulates the stability of beta-catenin. Oncogene. 1999;18:849–854. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 40.Liang G, Hai T. Characterization of human activating transcription factor 4, a transcriptional activator that intersects with multiple domains of cAMP-responsive element-binding protein (CREB)-binding protein. J Biol Chem. 1997;272:24088–24095. doi: 10.1074/jbc.272.38.24088. [DOI] [PubMed] [Google Scholar]
- 41.Lisztwan J, Marti A, Sutterluty H, Gstaiger M, Wirbelauer C, Krek W. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45(SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 1998;17:368–383. doi: 10.1093/emboj/17.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maniatis T. A ubiquitin ligase complex essential for the NF-kappaB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev. 1999;13:505–510. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- 43.Margottin F, Bour S P, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 44.Marti A, Wirbelauer C, Scheffner M, Krek W. Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlines the regulation of E2F-1 degradation. Nat Cell Biol. 1999;1:14–19. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- 45.Meimoun A, Holtzman T, Weissman Z, McBride H J, Stillman D J, Fink G R, Kornitzer D. Degradation of the transcription factor Gcn4 requires the kinase Pho85 and the SCFCDC4 ubiquitin-ligase complex. Mol Biol Chem. 2000;11:915–927. doi: 10.1091/mbc.11.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer T E, Habener J F. Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding protein. Endocr Rev. 1993;14:269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- 47.Mielnicki L M, Pruitt S C. Isolation and nucleotide sequence of a murine cDNA homologous to human activating transcription factor 4. Nucleic Acids Res. 1991;19:6332. doi: 10.1093/nar/19.22.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miura M, Hatakeyama S, Hattori K, Nakayama K. Structure and expression of the gene encoding mouse F-box protein, Fwd2. Genomics. 1999;62:50–58. doi: 10.1006/geno.1999.5965. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama K, Nagahama H, Minamishima Y A, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Nakayama K, Hatakeyama S. Targeted disruption of Skp2 results in accumulation of cyclic E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orian A, Gonen H, Bercovich B, Fajerman I, Eytan E, Israel A, Mercurio F, Iwai K, Schwartz A L, Ciechanover A. SCF(beta)(-TrCP) ubiquitin ligase-mediated processing of NF-kappaB p105 requires phosphorylation of its C-terminus by IkappaB kinase. EMBO J. 2000;19:2580–2591. doi: 10.1093/emboj/19.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pati D, Meistrich M L, Plon S E. Human Cdc34 and Rad6B ubiquitin-conjugating enzymes target repressors of cyclic AMP-induced transcription for proteolysis. Mol Cell Biol. 1999;19:5001–5013. doi: 10.1128/mcb.19.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patton E E, Willems A R, Tyers M. Combinatorial control in ubiquitin-dependent proteolysis: don't Skp the F-box hypothesis. Trends Genet. 1998;14:236–243. doi: 10.1016/s0168-9525(98)01473-5. [DOI] [PubMed] [Google Scholar]
- 53.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 54.Reddy T R, Tang H, Li X, Wong-Staal F. Functional interaction of the HTLV-1 transactivator Tax with activating transcription factor-4 (ATF4) Oncogene. 1997;14:2785–2792. doi: 10.1038/sj.onc.1201119. [DOI] [PubMed] [Google Scholar]
- 55.Renard P, Percherancier Y, Kroll M, Thomas D, Virelizier J L, Arenzana-Seisdedos F, Bachelerie F. Inducible NF-κB activation is permitted by simultaneous degradation of nuclear IκBα J. Biol Chem. 2000;275:15193–15199. doi: 10.1074/jbc.275.20.15193. [DOI] [PubMed] [Google Scholar]
- 56.Rouillon A, Barbey R, Patton E E, Tyers M, Thomas D. Feedback-regulated degradation of the transcriptional activator Met4 is triggered by the SCF(Met30) complex. EMBO J. 2000;19:282–294. doi: 10.1093/emboj/19.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sassone-Corsi P. Goals for signal transduction pathways: linking up with transcriptional regulation. EMBO J. 1994;13:4717–4728. doi: 10.1002/j.1460-2075.1994.tb06797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schubert U, Henklein P, Boldyreff B, Wingender E, Strebel K, Porstmann T. The human immunodeficiency virus type 1 encoded Vpu protein is phosphorylated by casein kinase-2 (CK-2) at positions Ser52 and Ser56 within a predicted alpha-helix-turn-alpha-helix-motif. J Mol Biol. 1994;236:16–25. doi: 10.1006/jmbi.1994.1114. [DOI] [PubMed] [Google Scholar]
- 59.Selig L, Pages J C, Tanchou V, Preveral S, Berlioz-Torrent C, Liu L X, Erdtmann L, Darlix J, Benarous R, Benichou S. Interaction with the p6 domain of the gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J Virol. 1999;73:592–600. doi: 10.1128/jvi.73.1.592-600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skowyra D, Craig K L, Tyers M, Elledge S J, Harper J W. F-box proteins are receptors that recruit phosphorylated substrates to the SCFubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 61.Skowyra D, Craig K L, Tyers M, Elledge S J, Harper J W. Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGrr1 and Rbx1. Science. 1999;284:662–665. doi: 10.1126/science.284.5414.662. [DOI] [PubMed] [Google Scholar]
- 62.Spencer E, Jiang J, Chen Z J. Signal-induced ubiquitination of IkappaBalpha by the F-box protein Slimb/beta-TrCP. Genes Dev. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 64.Treier M, Staszewski L M, Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 65.Tsujimoto A, Nyunoya H, Morita T, Sato T, Shimotohno K. Isolation of cDNAs for DNA-binding proteins which specifically bind to a tax-responsive enhancer element in the long terminal repeat of human T-cell leukemia virus type I. J Virol. 1991;65:1420–1426. doi: 10.1128/jvi.65.3.1420-1426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vallejo M, Ron D, Miller C P, Habener J F. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci USA. 1993;90:4679–4683. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winston J T, Strack P, Beer-Romero P, Chu C Y, Elledge S J, Harper J W. The SCFbeta-TRCP-ubiquitin ligase complex associated specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yaron A, Hatzubai A, Davis M, Lavon I, Amit S, Manning A M, Andersen J S, Mann M, Mercurio F, Ben-Neriah Y. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature. 1998;396:590–594. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- 69.Ziff E B. Transcription factors: a new family gathers at the cAMP response site. Trends Genet. 1990;6:69–72. doi: 10.1016/0168-9525(90)90081-g. [DOI] [PubMed] [Google Scholar]