Abstract
Background
The first case of a novel coronavirus (COVID-19) infection was detected in Wuhan, fever and respiratory symptoms have been frequently reported in patients infected with this virus.
Aim
It was aimed to compare the symptoms of patients with COVID-19 positivity and patients without COVID-19 positivity hospitalized with suspicion of COVID-19.
Methods
Patients presenting to the Sakarya University Training and Research Hospital with suspicion of COVID-19 were included in the study. Samples were obtained from the patients and PCR tests were performed; the patients were grouped as COVID-19 positive and COVID-19 negative; these two groups were questioned for 15 symptoms and the results were compared.
Results
A total of 297 patients with suspicion of COVID-19 were included in the study. COVID-19 was positive in 143 patients and negative in 154 patients. The most common symptoms in the COVID-19 positive group were: cough (56.6%), weakness (56.6%), taste disorder (35.7%), myalgia (34.3%), and fever (33.6%); and in the COVID-19 negative group: cough (63%), weakness (45.5%), dyspnea (29.9%), headache (27.3%) and fever (24.7%). When these two groups were compared, taste disorder, smell disorder and diarrhea were significantly higher in the COVID-19 positive group (p = <0,00001, p = 0,00001 and p = 0,02).
Conclusion
Our study showed that taste and smell disorders and diarrhea were important markers in COVID-19 infection.
Keywords: COVID-19, symptom, taste disorder, smell disorder, diarrhea
Introduction
Coronavirus is an enveloped, positive-sense single-stranded RNA virus belonging to the Ortho Coronaviridae subfamily.1 These viruses are common in animals but are also known to affect humans. They cause symptoms such as fever, cough, and shortness of breath during their infection.2
A mysterious pneumonia epidemic, characterized by fever, dry cough and fatigue started from a market in Wuhan (Hubei, China) in late December 2019. The disease spread to thousands of people and many cities in China in January and was also seen in other countries.3 The pathogen of the epidemic has been identified as a new betacoronavirus, and the World Health Organization (WHO) has declared the name of this new coronavirus as SARS-CoV-2.4
The mean incubation period of SARS-CoV-2 is 5.2 days.5 It begins with symptoms such as fever, dry cough, fatigue, and may affect respiratory, gastrointestinal, musculoskeletal and nervous system. Symptoms may vary depending on the organ system involved. Confirmatory laboratory diagnosis is usually the RT-PCR test to detect viral RNA; chest CT (computed tomography) usually shows multiple ground-glass opacities and bilateral pneumonia.3
Non-specific symptoms such as fever, cough and weakness are common in viral diseases that involve the respiratory system.
Symptoms of COVID-19 differ from country to country. In this study, we aimed to investigate the symptoms of patients presenting to our hospital with suspicion of COVID-19, and to compare the symptoms of patients with COVID-19 positivity with RT-PCR test and those without COVID-19 positivity.
Material-method
According to the guideline for COVID-19 of the General Directorate of Public Health division of the Ministry of Health, 297 patients who adhered to the possible/definitive case definition and who should be followed up by hospitalization were admitted to the COVID inpatient clinic of Sakarya University Training and Research Hospital.
According to the guideline for COVID-19 of the General Directorate of Public Health division of the Ministry of Health:
Possible Case was defined as follows: Presence of fever or at least one of the signs and symptoms of acute respiratory disease (cough and respiratory distress), and having a clinical picture which cannot be explained for any other reason, and a history of him/herself or his/her relative having been abroad within 14 days before the onset of symptoms; or the presence of fever or at least one of the signs and symptoms of acute respiratory disease (cough and respiratory distress) and close contact with a confirmed COVID-19 case within 14 days prior to the onset of symptoms, or presence of fever; or at least one of the signs and symptoms of acute respiratory disease (cough and respiratory distress), and the need for hospitalization and the inability to explain the clinical picture for any other reason; or; the presence of cough or shortness of breath with a sudden onset of fever and no runny nose.
Definite Case was defined as follows: Patients with SARS-CoV-2 positivity confirmed with molecular methods, among the cases that match the possible case definitions.6
First an oropharyngeal swab was obtained, then the nasal sample was obtained using the same swab; they were placed in the transport medium and sent to the laboratory. The samples were used for the reverse transcription polymerase chain reaction (RT-PCR) test. Chest tomographies of the patients were carried out.
A total of 297 patients admitted to the Sakarya University Training and Research Hospital with possible/definitive COVID-19 case were grouped according to their PCR test results as COVID-19 positive and COVID-19 negative. They were questioned for fever, cough, sputum production, dyspnea, sore throat, myalgia, lumbago, weakness, rhinorrhea, headache, taste disorder, smell disorder, diarrhea, conjunctivitis and skin rash, and the symptoms in both groups were compared.
Epi Info 7 was used in the statistical analysis. A p value of less than 0.05 was considered significant.
Results
Of the 297 patients who had been hospitalized in the Sakarya University Training and Research Hospital with possible/definitive COVID-19 diagnosis, 164 were male and 133 were female. The mean age of the patients was 57.8 years. COVID-19 positivity was detected in 143 patients with RT-PCR, and at least two RT-PCR results were negative in 154 patients.
The mean age and gender distribution of the patients according to COVID-19 positivity have been presented in Table 1.
Table 1.
Gender and age according to COVID-19 positivity.
| COVID-19 | Age (mean) | Male | Female |
|---|---|---|---|
| Positive | 55,63 | 77 | 66 |
| Negative | 59,83 | 87 | 67 |
The patients were questioned for 15 major COVID-19 symptoms. Comparison of the patients' symptoms has been displayed in Table 2.
Table 2.
Symptoms of the patients.
| Symptom | COVID-19 positive (n=143) | % | COVID-19 negative (n=154) | % | P | |
|---|---|---|---|---|---|---|
| 1 | Fever | 48 | 33,6 | 38 | 24,7 | 0,09 |
| 2 | Cough | 81 | 56,6 | 97 | 63 | 0,26 |
| 3 | Sputum production | 32 | 22,4 | 35 | 22,7 | 0,91 |
| 4 | Dyspnea | 31 | 21,7 | 46 | 29,9 | 0,107 |
| 5 | Myalgia | 49 | 34,3 | 32 | 20,8 | 0,08 |
| 6 | Lumbago | 24 | 16,8 | 19 | 12,3 | NS |
| 7 | Diarrhea | 31 | 21,7 | 18 | 11,7 | 0,02 |
| 8 | Rhinorrhea | 7 | 4,9 | 6 | 3,9 | NS |
| 9 | Sore throat | 14 | 9,8 | 17 | 11 | NS |
| 10 | Taste Disorder | 51 | 35,7 | 21 | 13,6 | <0,00001 |
| 11 | Smell Disorder | 33 | 23,1 | 17 | 11 | 0,00001 |
| 12 | Weakness | 81 | 56,6 | 70 | 45,5 | 0,053955 |
| 13 | Headache | 43 | 30,1 | 42 | 273 | NS |
| 14 | Conjunctivitis | 0 | 0 | 0 | 0 | NS |
| 15 | Skin rash | 0 | 0 | 0 | 0 | NS |
NS: non-significant.
The most common symptom in COVID-19 positivity was cough (57%) and weakness (57%) and the most common symptoms in COVID-19 negative patients were again cough (63%) and weakness (46%). Taste disorder, smell disorder and diarrhea were significantly higher in COVID-19 positive patients. Of the patients with taste disorder, 26(%39) were female, 25(%32) were male. Of the patients with smell disorder, 18(%27) were female, 15(%19) were female. Most common accompanying symptoms to taste disorder were cough (%69), weakness (%69), smell disorder (%63), myalgia (%49) and headache (%41). Most common accompanying symptoms to smell disorder were taste disorder (%97), weakness (%67), cough (%64), myalgia (%55) and headache (%45). All of the patients with taste and smell disorders were mild (%31, %57) to moderate (%69, %43) patients with clinical course.
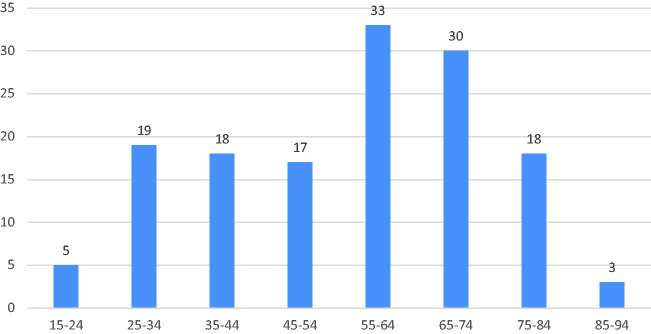
When we evaluated 143 COVID-19 positive patients, most patients were between the ages of 55-74 years (Figure 1) and the male patient rate was 53.8%.
Figure 1.
Age distribution of COVID-positive patients.
We evaluated the 5 most common symptoms in our COVID-19 positive patients by age (over 65 and below); when we evaluated the rate of fever by age, we found that fever was more common in patients under 65 years of age.
When we evaluated the cough symptom by age in our COVID-19 positive patients, we found that it was higher in patients over 65 years of age.
When we evaluated the fatigue complaint by age in our COVID-19 positive patients, we found that patients over 65 years of age had more fatigue complaints.
In our COVID-19 positive patients, we found that patients under 65 years of age had more complaints of myalgia when we evaluated it according to age.
We found that the taste disorder complaint was higher in patients under 65 years of age in our COVID-19 positive patients when we evaluated it according to age.
In COVID-19 patients, fever, myalgia and taste disorder were observed more frequently under 65 years of age, and the weakness and cough complaints were observed more in patients who were above 65 years of age.
Conclusion
There is no specific clinical symptom that allows COVID-19 to be reliably differentiated from other viral respiratory infections. The severity of symptomatic infection ranges from mild to critical, but most infections are not severe. However, some patients with mild symptoms at the beginning may progress to more severe symptoms after a week.7
SARS-CoV-2 is the pathogen responsible for the emergence of acute respiratory disease in Wuhan, China's Hubei Province, in December 2019. While most infected individuals have mild symptoms, pneumonia, multiple organ failure, and death may be seen in some cases.8
The most frequently reported symptoms are fever, cough, myalgia, fatigue, dyspnea, and less often, headache, diarrhea and runny nose.2 The most common symptoms in the COVID-19 positive group were: cough (56.6%), weakness (56.6%), taste disorder (35.7%), myalgia (34.3%) and fever (33.6%); and in COVID-19 negative group: cough (63%), weakness (45.5%), dyspnea (29.9%), headache (27.3%) and fever (24.7%), in our study. The most common symptoms in both groups were weakness and cough, whereas smell disorder, taste disorder and diarrhea were significantly higher in Covid-19 positive patients.
In our study, diarrhea was observed in 21.7% of patients, at a higher rate than other studies. Diarrhea was observed in 3.2% of patients in Jun Chen et al.’s study.9 In another study, gastrointestinal symptoms were seen in 11.4% of patients in a total of 651 COVID-19 patients.10 In a study in Singapore, 17% of COVID-19 patients were reported to have diarrhea. The reason for a higher rate of diarrhea in our study may be due to mutations that the virus has gone through in its route from China to our country. The virus may have become more enterotropic within this period. It is assumed that SARS-CoV-2 binds to ACE-2 receptors, possibly by transmembrane serine protease 2. ACE-2 is considered an important regulator of intestinal inflammation and is seen as the mechanism that causes diarrhea in COVID-19 in many hypotheses.11
SARS-COV-2 virus RNA can be detected in the cerebrospinal fluid, virus has the neuro-invasive potential. The most common complaints in patients of nervous system involvement were taste and smell disorder.12 Although the current cohort studies do not emphasize this, it is not surprising that anosmia was reported as a distinctive symptom in patients diagnosed with COVID-19; other coronaviruses have also been associated with anosmia.13 Central nervous system involvement and epithelial damage may be cause of anosmia. In mouse models, SARS-COV revealed transneuronal penetration through the olfactory bulb and its infection resulted in the rapid, transneural spread of the virus to connected areas of the brain. For this reason, headache may be a symptom induced by of the central nervous system.14 Anosmia has been observed in COVID-19 positive patients, especially in those with no other clinical features. It is suggested that the virus causes olfactory nerve inflammation rather than damaging the structure of the receptors.13
In our study, taste disorder was observed in 35.7% of COVID-19 positive patients and smell disorder in 23.1%. In one study, smell and taste disorders were found in 68% and 71% of COVID-19 positive patients, and in 16% and 17% of COVID-19 negative patients, respectively (p < 0.001).15 A research group in Milan showed that 34% of 59 SARS-CoV-2 positive hospitalized patients had smell and taste disorders.16 In another study, they found that 59% of COVID-19 positive individuals had smell and taste loss, and in 18% of those with negative test results.17 Bagheri et al. detected persistent anosmia in 60.9 % of patients.18
In our study, we found that taste and smell disorder were significantly more common females and younger individuals. Lee et al. found that 15.3% patients had taste and smell disorder in the early stage of COVID-19, taste and smell disorder were more common among females and younger individuals.14 A European multicenter study reported 85,6% and 88% patients olfactory and gustatory dysfunction and found that females were significantly more affected by olfactory and gustatory dysfunction than males.19
In our study, taste and smell disorder were seemed in patients with mild-to moderate clinical. Similarly, Yan et al shown that anosmia seemed to be associated with a milder clinical course in patients with COVID-19.20
In the contrary of our study, in a systematic review, no significant of the prevalence taste and smell disorder by gender was noted.21
Bagheri et al. showed that fever, cough and dyspnea were less common in the patients with anosmia/hyposmia compliant (87,9% vs 37.38%, 67,7% vs 18,98% and 18,6% vs 14.38%).18 In our study, one of the accompanying common symptoms to taste and smell disorder was cough. Fever and dyspnea were less common in these patients.
In our study, the mean age was 55.6 years and 53.8% of COVID-19 positive patients were male. Recent studies have shown that COVID-19 mostly affects the middle-aged and elderly people.22 In a meta-analysis of 18 studies, the mean age of the patients was 51.97 and 55.9% were male. In the same review, fever was seen in 88.7%, cough in 57.6%, dyspnea in 45.6%, myalgia and weakness in 29.4%, sputum production in 28.5%, sore throat in 11%, headache in 8%, and diarrhea in 6.1% of the patients.23 In our study, the rates of symptoms in the COVID-19 positive group were as follows: cough, 56.6%; weakness, 56.6%; taste disorder, 35.7%; myalgia, 34.3%; fever, 33.6%; headache, 30.1%; smell disorder, 23.1%; sputum production, 22.4%; dyspnea and diarrhea, 21.7%; lumbago, 16.8%; sore throat, 9.8%; and rhinorrhea, 4.9%. Conjunctivitis and skin rash were not observed in any of our patients. In Nanshan et al.'s study, fever was detected in 83% of patients, cough in 82%, dyspnea in 31%, myalgia in 11%, headache in 8%, sore throat in 5%, rhinorrhea in 4% and diarrhea in 2%.24 In another study, fever was seen in 97%, cough in 93% and myalgia and fatigue in 33%, dyspnea in 30%,25 while in another study, fever was seen in 87%, dry cough in 60% and fatigue in 39%.26 In one study, the most common symptoms at the onset of the disease were fever (87.1%), cough (36.5%) and fatigue (15.7%). In one study, the main clinical findings of the patients were fever and cough, respectively. Shortness of breath was seen in 37.5%, muscle pain in 22.5% and headache in 16.25%; none of the patients had diarrhea.27 Contrary to the other studies, the rate of fever was low in our study.
In a study examining the clinical course of COVID-19 in elderly patients, cough and sputum production were more common in the younger group, fever was more common in the younger group, while weakness and fatigue were more common in the older group; however, in our study, fever was more common in the younger group and cough in the older group.28 The fever being more common in young people may be due to rapid reactivation of the immune system and faster release of IL-6 and prostaglandin in young people. However, the fact that cough is seen more in the elderly may be due to the fact that its course with pneumonia is more common in the elderly.
As a result, it is difficult to differentiate COVID-19 from viral respiratory infections; in our study, taste and smell disorder and diarrhea were determined as important clinical symptoms in COVID-19 positive cases. In the current pandemic, taste and smell disorders and diarrhea should be carefully evaluated, and it should be taken into consideration that the symptoms such as fever and cough change according to age, and even if there are no symptoms of fever and cough, special attention should be paid to COVID-19 to reduce contagiousness and maintain health for patients with taste and smell disorders and diarrhea.
Declaration of Conflicting Interests
The author (s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author (s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Aylin Çalıca Utku https://orcid.org/0000-0002-9302-5842
References
- 1.Perlman S.Another decade, another coronavirus. N Engl J Med 2020; 382: 760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 2020; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu YC, Chen CS, Chan YJ.The outbreak of COVID-19: an overview. J Chin Med Assoc 2020; 83: 217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Novel coronavirus – China, www.who.int/csr/don/12- january-2020-novel-coronavirus-china/en/ (accessed 1 February 2020).
- 5.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382: 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Study of scientific board, COVID-19 (SARS-CoV-2 infection) Guide Republic of Turkey, Ministry of Health April 14th 2020, Ankara, https://hsgm.saglik.gov.tr (accessed 27 July 2020).
- 7.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J 2020;133(11): 1261--1267. [DOI] [PMC free article] [PubMed]
- 9.Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020; 80: e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020; 69: 1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong J, Young BE, Ong S.COVID-19 in gastroenterology: a clinical perspective. Gut 2020; 69: 1144–1142. [DOI] [PubMed] [Google Scholar]
- 12.Finsterer J and Stollberger C. Causes of hypogeusia/hyposmia in SARS-COV2 infected patients. J Med Virol 2020; 1--2. doi:10.1002/jmv.25903. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 13.Villalba NL, Maouche Y, Ortiz MBA, et al. Anosmia and dysgeusia in the absence of other respiratory diseases: should COVID-19 infection be considered? Eur J Case Rep Intern Med 2020; 7: 001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Min P, Lee S, et al. Prevalance and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci 2020; 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol 2020; 10(7): 806--813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis 2020; 71(15): 889--890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menni C, Valdes A, Freydin MB, et al. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. MedRxiv2020: 1, 4.
- 18.Bagheri SHR, Asghari AA, Farhadi M, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islamic Republic Iran 2020; 34(1): 446--452. [DOI] [PMC free article] [PubMed]
- 19.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277: 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan CH, Faraji F, Prajapati DP, et al. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol 2020; 10: 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agyeman AA, Chin KL, Landersdorfer CB, et al. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 22.Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020; 133(11): 1261--1267. [DOI] [PMC free article] [PubMed]
- 23.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin X, Qiu S, Yuan Y, et al. Characteristics and treatment of patients infected with COVID-19 in Shishou, China. Lancet Respir Med. 2020:Pre-Print. Available at SSRN: https://ssrn.com/abstract=3541147. Accessed February 20, 2020.
- 26.Han R, Huang L, Jiang H, et al. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. AJR 2020; 215: 1–6. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis 2020; XX: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu K, Chen Y, Lin R, et al. Clinical features of COVID-19 in elderly patients: a comparison with young and Middle-aged patients. J Infect 2020; 80: e14–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]