Abstract
The COVID-19 pandemic has necessitated novel approaches and collaborative efforts across multiple disciplines. It is known that various aspects of our physiology and response to pathogens are under tight clock control. However, the assimilation of circadian biology into our clinical and research practices is still evolving. Using a focused review of the literature and original analyses of the UK Biobank, we discuss how circadian biology may inform our diagnostic and therapeutic strategies in this pandemic.
Keywords: vaccines, translational medicine, chronotherapy, COVID-19, circadian, immunity
The coronavirus disease 2019 (COVID-19) epidemic, which as caused by severe acute respiratory coronavirus-2 (SARS-CoV2), has prompted a scramble for safe and effective treatments. Our focus remains on vaccine development and repurposing approved drugs. However, reviewing our current understanding of chronobiology as applicable to other viral pathogens may help us realize opportunities to harness the power of circadian biology for SARS-CoV-2.
Circadian rhythms are evolutionarily conserved pathways that serve as a means for living organisms to adapt to the environment. It is an anticipatory system, preparing us to deal with challenges to our resilience, including infections. Molecular clockworks are highly conserved, tightly regulated, and exhibit a high degree of redundancy (Takahashi, 2017). Directed by a master clock in the suprachiasmatic nucleus, peripheral tissue clocks are among the most prominent biological networks that integrate cross-tissue functions, such as are intrinsic to cardiovascular homeostasis, metabolism, and the immune response (Yang et al., 2013).
The clock may be relevant to the pathophysiology and treatment of COVID-19 in the following ways:
-
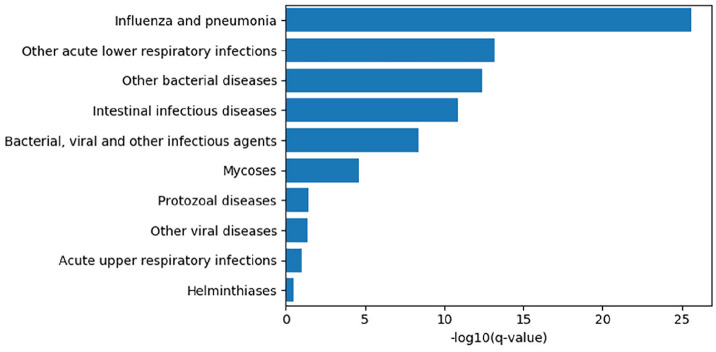
The time of infection relative to the circadian day: The molecular clock determines the response to infection via a wide range of pathogens in many species because of circadian control of the immune system (Curtis et al., 2014; Man et al., 2016; Zhuang et al., 2019; Stone et al., 2012; Ehlers et al., 2018). For example, we have demonstrated that mice infected with influenza virus at dusk have a 3-fold higher mortality than those infected at dawn. This did not result from attenuation of the viral burden but rather from modulation of the immune response to infection. This circadian variation in lethality was abolished when the clock was broken in immune cells and pulmonary epithelial cells by deletion of the nonredundant core clock gene, Bmal1 (Sengupta et al., 2019). Consistent with this observation, we discovered an interesting association between circadian rhythms and the need for hospitalization for influenza. Approximately 100,000 participants in the UK Biobank have activity measurement for 1 week using a wrist-worn accelerometer device (Doherty et al., 2017). Activity was classified using a machine-learning algorithm (Willetts et al., 2018) and summarized to participant-level measures of sleep, activity, and rhythmic patterns. We found that having a robust circadian amplitude in activity levels was associated with less likelihood of having been treated for influenza (false-discovery rate–corrected q value <2 × 10−26) and other lower respiratory infections, as well as other viral and bacterial infections (q value <10−9; Fig. 1). Might the time of infection contribute to the diversity of clinical response to SARS-CoV-2? The entry of SAR-CoV2 virus depends on the cellular receptor angiotensin converting enzyme 2 (ACE2; Hoffmann et al., 2020), a common target for antihypertensive therapy. ACE2 itself is a negative regulator of the renin-angiotensin-aldosterone system (RAAS), which is known to oscillate under circadian control (Ohashi et al. (Ohashi et al., 2017; Nonaka et al., 2001). There are no published reports of a circadian pattern of expression of ACE2 protein or mRNA. However, given its close connection to the oscillatory RAAS system, it is still plausible that ACE2 expression is affected by circadian rhythms, thus directly influencing cellular access by SARS-CoV-2.
As we reopen institutions and design schedules and workspaces, we could test whether time of day influences the susceptibility to or severity of COVID-19 infection. Historically, shift workers have been known to have increased susceptibility to many diseases (Smith and Eastman, 2012) and demonstrate differences in their immunological profile modified by their chronotype and the time from last night shift (Loef et al., 2019). However, in the context of the pandemic, the central question is whether the risk of infection among shift workers is influenced by the time of their shift. This is applicable to all shift workers, including health care workers, but it has particular relevance to the clustered outbreaks in workplaces where social distancing has been a challenge, such as the meat-processing industry (Waltenberg et al., 2020). In many ways, the COVID-19 pandemic presents us with a natural experiment. Thus, an intriguing possibility would be to leverage the many well-designed contact-tracing initiatives to address the time-of-day–specific susceptibility of various populations.
Circadian variation in the immune system across the day: Control of the immune system by the molecular clock includes regulation of inflammatory cytokines (Gibbs et al., 2012; Early et al., 2018; Keller et al., 2009), neutrophil maturation (Adrover et al., 2019), myeloid and lymphoid cell trafficking (Druzd et al., 2017), and migration as well as development and differentiation of immune cells, such as Th17 cells (Yu et al., 2013). Similarly, various aspects of human physiology have been shown to oscillate across the circadian day (Skarke et al., 2017). Thus, although there is evidence that the various components of the immune system oscillate in a circadian manner, this is not usually factored into sample collection for immunophenotyping (Mathew et al., 2020). Sample collection is typically driven by the availability of patient and staff and/or the relation to drugs already administered, and it is agonistic to timing. Given the magnitude of oscillations in immune cell number and function in model systems and humans, time stamping such samples to account for this variable can limit the source of biological noise, refine data interpretation, and reduce the required sample size for studies of COVID-19.
-
Circadian biology and time of vaccination: There is evidence from animal studies that both the innate immune system (Silver et al., 2012) and the adaptive system (Suzuki et al., 2016), such as the CD8 cell-intrinsic clock (Nobis et al., 2019), influence the immune response to vaccination in model systems. In a cluster randomized design study in humans, morning vaccination with the inactivated influenza vaccine resulted in higher antibody responses than afternoon vaccination in adults older than 65 years (Long et al., 2016). However, this may be confounded by factors such as vaccination history (Cobey and Hensley, 2017). In the case of a novel pathogen such as SARS-CoV-2, there is the opportunity to perform a controlled comparison of morning versus evening vaccination to determine if time conditions the immune response. Another related factor that has been shown to affect vaccine response, at least for influenza immunization, is sleep deprivation (Benedict et al., 2012; Brown et al., 1989; Spiegel et al., 2002). Further, we speculate that chronotype, which refers to the endogenous propensity of an individual to sleep during particular periods of the day, contributes to the heterogeneity of response to vaccinations. While there are no studies that clearly implicate chronotype in determining vaccine response, the effect of the time of day on vaccination response would likely be modified by the endogenous chronotype. Thus, although the approach to leveraging the principles of chronobiology in improving the efficacy of the vaccine response against SARS-CoV2 is multilayered, simply time stamping vaccination across the day would allow us to rapidly scale up to define the impact of time in observational studies. An additional consideration is that circadian rhythms tend to deconsolidate in the elderly, so the likelihood of a time-dependent response to vaccination may be conditioned by the age of the patient.
Further, these differences in the immune system based on time of day should be considered when working on animal models of COVID-19. That there are differences between the mouse and man, in terms of immune architecture and response, is well documented (Mestas and Hughes, 2004; Shay et al., 2013). However, specifically relevant here is the fact that mice are nocturnal and humans are not. Promising results from mice containing a humanized ACE2 (Hassan et al., 2020) and other models need to be interpreted in the light of the nocturnal habitus of mice. Appreciation of the differences in their circadian rhythms may improve the reproducibility of data and directly affect the translation of findings from animal to human studies.
Using circadian rhythms to optimize the effect of antivirals and anti-inflammatory agents: Just like the immune system, many drug targets, transporters, and metabolizing enzymes are subject to circadian variation under the control of the clock (Zhang et al., 2014). The response to many drugs, particularly those with shorter half-lives, is greatly influenced by time of dosing (Sulli et al., 2018). While targeting drugs to a particular time of day to enhance efficacy and reduce adverse effects is common practice for some medications, it is still not practiced for all drugs with shorter half-lives (Erkekoglu and Baydar, 2012). Similarly, chronotherapy may be relevant to antivirals such as remdesivir and anti-inflammatory drugs such as aspirin (Chen et al., 2018; Bonten et al., 2015; Buurma et al., 2019), which are being used to treat COVID-19, but might also be relevant to antithrombotic medications used to treat COVID-19 complications. Finally, clock genes (Kojetin and Burris, 2014) and melatonin (Farias et al., 2019; Prado et al., 2018) have been shown to have anti-inflammatory effects in other models, and the use of melatonin to reentrain the clock in cases of lifestyle-related misalignment may also be considered to reduce the risk of infection.
Figure 1.
The top 10 infection phenotypes associated with relative circadian amplitude among participants of the UK Biobank, by −log10 of the Benjamini-Hochberg q-values. Cases were extracted by lifetime occurrence of ICD-10 codes and associations computed by a linear model controlling for age, sex, socioeconomic status, body mass index, smoking status, and self-reported overall health. Relative amplitude was computed as a ratio (M10-L5/M10+L5) where M10 and L5 are are the participant’s average activity levels of the ten hours of highest activity and five hours of the lowest activity, respectively ( Witting et al., 1990).
In conclusion, our molecular clocks may modulate viral cellular entry and the consequent immunological response to infection. Timing of administration may influence the response to administration of a vaccine or to the drugs used to attenuate the severity of COVID-19. The various intersections of the circadian clocks and immunobiology as well as the epidemiology of COVID-19 (Fig. 2) constitute biologically plausible hypotheses that are readily testable.
Figure 2.
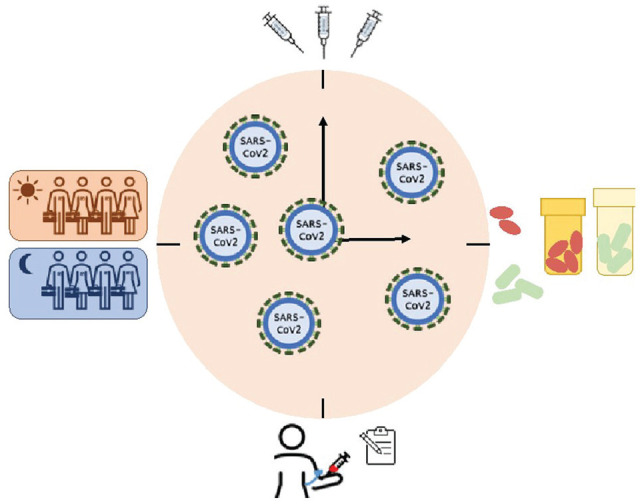
Summarized representation of the ways circadian rhythms interface and thus may be harnessed against COVID-19.
Acknowledgments
This work was supported by the NHLBI-K08HL132053 (to S.S.), NIH/NCRR RR023567 (to G.A.F.), a Merit Award from the American Heart Association (to G.A.F.), a grant from the Volkswagen Foundation (to G.A.F.), and 5T32MH106442-04 and NCATS-5UL1TR000003 (to T.G.B. and G.R.G). Dr. FitzGerald is the McNeil Professor of Translational Medicine and Therapeutics and a senior advisor to Calico Laboratories. This research has been conducted using the UK Biobank Resource under project ID 50398. We thank Kaitlyn Forrest for her help with the graphics.
Footnotes
ORCID iDs: Shaon Sengupta  https://orcid.org/0000-0001-5237-3835
https://orcid.org/0000-0001-5237-3835
Thomas G. Brooks  https://orcid.org/0000-0002-6980-0079
https://orcid.org/0000-0002-6980-0079
References
- Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, Huerga-Encabo H, Silvestre-Roig C, Rossaint J, Cossio I, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity 2019;50:390-402 e10. [DOI] [PubMed] [Google Scholar]
- Benedict C, Brytting M, Markstrom A, Broman JE, Schioth HB. Acute sleep deprivation has no lasting effects on the human antibody titer response following a novel influenza A H1N1 virus vaccination. BMC Immunol 2012;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonten TN, Snoep JD, Assendelft WJ, Zwaginga JJ, Eikenboom J, Huisman MV, Rosendaal FR, van der Bom JG. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: a randomized cross-over trial. Hypertension 2015;65:743-750. [DOI] [PubMed] [Google Scholar]
- Brown R, Pang G, Husband AJ, King MG. Suppression of immunity to influenza virus infection in the respiratory tract following sleep disturbance. Reg Immunol 1989;2:321-325. [PubMed] [Google Scholar]
- Buurma M, van Diemen JJK, Thijs A, Numans ME, Bonten TN. Circadian rhythm of cardiovascular disease: the potential of chronotherapy with aspirin. Front Cardiovasc Med 2019;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang G, Zhang J, Ren B, Tang S, Li X, FitzGerald GA. Time-dependent hypotensive effect of aspirin in mice. Arterioscler Thromb Vasc Biol 2018;38:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobey S, Hensley SE. Immune history and influenza virus susceptibility. Curr Opin Virol 2017;22:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity 2014;40:178-86. [DOI] [PubMed] [Google Scholar]
- Doherty A, Jackson D, Hammerla N, Plötz T, Olivier P, Granat MH, White T, van Hees VT, Trenell MI, Owen CG, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS One 2017;12:e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 2017;46:120-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, Palsson-McDermott EM, Angiari S, Ryan DG, Corcoran SE, et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci USA 2018;115:E8460-E846E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Xie W, Agapov E, Brown S, Steinberg D, Tidwell R, Sajol G, Schutz R, Weaver R, Yu H, et al. BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol 2018;11:97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkekoglu P, Baydar T. Chronopharmacodynamics of drugs in toxicological aspects: a short review for clinical pharmacists and pharmacy practitioners. J Res Pharm Pract 2012;1:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias T, Paixao RID, Cruz MM, Cavalcante da Cunha de Sa RD, de Jesus Simão J, Antraco VJ, Cardoso Alonso-Vale MI. Melatonin supplementation attenuates the pro-inflammatory adipokines expression in visceral fat from obese mice induced by a high-fat diet. Cells 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA 2012;109:582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AO, Case JB, Winkler ES, Thackray LB, Kafai NM, Bailey AL, McCune BT, Fox JM, Chen RE, Alsoussi WB, et al. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell 2020;182:744-753.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271-280 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk H-D, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA 2009;106:21407-21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov 2014;13:197-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef B, Nanlohy NM, Jacobi RHJ, van de Ven C, Mariman R, van der Beek AJ, Proper KI, van Baarle D. Immunological effects of shift work in healthcare workers. Sci Rep 2019;9:18220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC. Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 2016;34:2679-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K, Loudon A, Chawla A. Immunity around the clock. Science 2016;354:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Giles JR, Baxter AE, Greenplate AR, Wu JE, Alanio C, Oldridge DA, Kuri-Cervantes L, Pampena MB, D’Andrea K, et al. Deep immune profiling of COVID-19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. bioRxiv 2020. doi: 10.1101/2020.05.20.106401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731-2738. [DOI] [PubMed] [Google Scholar]
- Nobis CC, Dubeau Laramee G, Kervezee L, Maurice De, Sousa D, Labrecque N, Cermakian N. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc Natl Acad Sci USA 2019;116:20077-20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation 2001;104:1746-1748. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertens Res 2017;40:413-22. [DOI] [PubMed] [Google Scholar]
- Prado NJ, Ferder L, Manucha W, Diez ER. Anti-inflammatory effects of melatonin in obesity and hypertension. Curr Hypertens Rep 2018;20:45. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, Valenzuela A, Fisher DG, Grant GR, López CB, et al. Circadian control of lung inflammation in influenza infection. Nat Commun 2019;10:4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay T, Jojic V, Zuk O, Rothamel K, Puyraimond-Zemmour D, Feng T, Wakamatsu E, Benoist C, Koller D, Regev AImmGen Consortium. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci USA 2013;110:2946-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 2012;36:251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarke C, Lahens NF, Rhoades SD, Campbell A, Bittinger K, Bailey A, Hoffmann C, Olson RS, Chen L, Yang G, et al. A pilot characterization of the human chronobiome. Sci Rep 2017;7:17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Eastman CI. Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep 2012;4:111-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288:1471-1472. [DOI] [PubMed] [Google Scholar]
- Stone EF, Fulton BO, Ayres JS, Pham LN, Ziauddin J, Shirasu-Hiza MM. The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS Pathog 2012;8:e1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli G, Manoogian ENC, Taub PR, Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci 2018;39:812-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Hayano Y, Nakai A, Furuta F, Noda M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med 2016;213:2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 2017;18:164-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltenburg MA, Victoroff T, Rose CE, Butterfield M, Jervis RH, Fedak KM, Gabel JA, Feldpausch A, Dunne EM, Austin C, et al. Update: COVID-19 among workers in meat and poultry processing facilities—United States, April–May 2020. MMWR Morb Mortal Wkly Rep 2020;69:887-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts M, Hollowell S, Aslett L, Holmes C, Doherty A. Statistical machine learning of sleep and physical activity phenotypes from sensor data in 96,220 UK Biobank participants. Sci Rep 2018;8:7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF.Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry 1990;27:563-572. [DOI] [PubMed] [Google Scholar]
- Yang G, Paschos G, Curtis AM, Musiek ES, McLoughlin SC, FitzGerald GA. Knitting up the raveled sleave of care. Sci Transl Med 2013;5:212rv3. [DOI] [PubMed] [Google Scholar]
- Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, Rothman PB, Takahashi JS, Hooper LV. TH17 cell differentiation is regulated by the circadian clock. Science 2013;342:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 2014;111:16219-16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Magri A, Hill M, Lai AG, Kumar A, Rambhatla SB, Donald CL, Lopez-Clavijo AF, Rudge S, Pinnick K, et al. The circadian clock components BMAL1 and REV-ERBalpha regulate flavivirus replication. Nat Commun 2019;10:377. [DOI] [PMC free article] [PubMed] [Google Scholar]