Abstract
Aims
To assess left atrial (LA) function in sinus rhythm in veteran athletes with a history of paroxysmal atrial fibrillation (AF) exposed to prolonged endurance exercise compared with veteran athletes without AF and controls with and without paroxysmal AF from a non-athletic population.
Methods and results
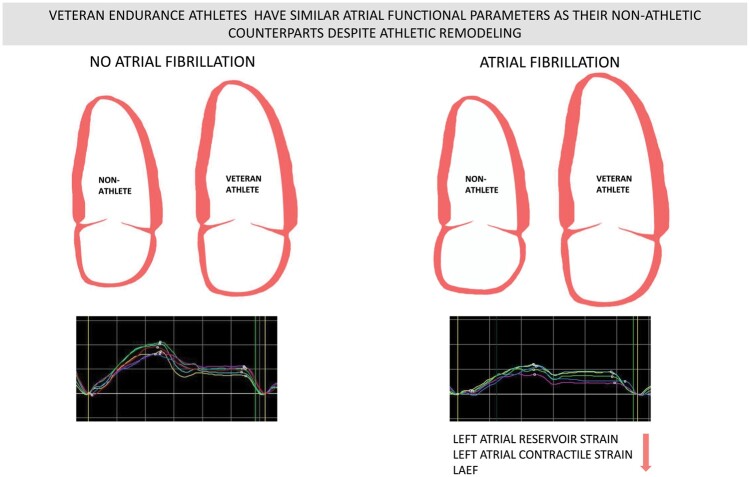
Three hundred and two male participants from four groups, veteran recreational skiers with paroxysmal AF (n = 62), veteran skiers without AF (n = 89), and controls from a non-athletic population with (n = 62) and without paroxysmal AF (n = 89) underwent an echocardiographic examination in sinus rhythm to evaluate LA anatomy and function. The skiers (mean age 70.86.7 years) reported an average exposure to regular endurance exercise for 40–50 years. LA maximum and minimum volumes were larger in skiers (P < 0.001). LA volumes differed within the athletic and non-athletic groups with larger volumes in the AF groups ( P < 0.001). We observed a considerable overlap in LA volumes among non-athletes with AF and athletes without AF. LA reservoir strain (33.6% 4.8% vs. 28.3% 6.7% P < 0.001) and contractile strain (18.3% 4.0% vs. 15.0% 5.2% P < 0.001) were lower in both AF groups regardless of athletic status. LA reservoir strain was superior to volumetric measurements at identifying participants with AF (area under the curve 0.740 0.041).
Conclusion
Male veteran athletes had significantly larger LA volumes than non-athletes. In contrast, LA strain values were similar in athletes and non-athletes with paroxysmal AF, and significantly lower than in subjects without AF.
Keywords: atrial fibrillation, strain, athlete, atrium, diastolic function, exercise
Graphical Abstract
Introduction
Atrial fibrillation (AF) is the most common heart rhythm disorder, associated with an increased risk of stroke, heart failure, dementia, and death.1,2 Moderate intensity exercise lowers the probability of AF, whereas prolonged endurance exercise seems to increase this risk.3–5 Long-distance endurance races have become increasingly popular, and changes in exercise habits could cause an increased incidence of exercise-induced AF.6
Although not fully understood, the pathophysiology of AF in otherwise healthy athletes is likely to include exercise-related adverse cardiac adaptations, such as dilation of the atria, changes in autonomic activation, inflammation, and myocardial fibrosis.3,7 Cardiac output increases with increasing exercise intensity.8 Simultaneously, the relative duration of the diastole decreases, which in turn requires a high flow across the atrioventricular valves, made possible by increased atrial pressure and greater left ventricular (LV) lusitropy.9 While younger athletes adapt to the increased demands during exercise by symmetrical enlargement of all four chambers with preserved and even enhanced diastolic properties (‘athlete’s heart’), diastolic function attenuate with increasing age, increasing LV filling pressures, and the load on the atria during exercise.10,11 Improved understanding of the interplay between exercise-related cardiac remodelling and physiological remodelling promoted by aging is warranted. It may guide clinicians facing veteran athletes to distinguish physiological from pathological atrial remodelling and enable us to provide evidence-based exercise recommendations.
Over the last few years, novel echocardiographic techniques using speckle tracking to assess atrial function have emerged, and atrial function is established to be strongly associated with AF in the general population.12,13 In athletes, however, data are limited and studies in younger elite athletes are not necessarily relevant to elderly recreational athletes.14,15 This study is the first to investigate the atrial function and AF in a large group of elderly recreational athletes.
The main aim of this study was to investigate structural and functional characteristics of the atria in relation to AF in a cohort of male veteran endurance athletes exposed to endurance exercise over decades. The secondary aim was to assess if left atrial (LA) strain by speckle tracking echocardiography is better than volumetric measurements to identify the athletes diagnosed with paroxysmal AF.
Methods
Study population
To cover the whole range of physical activity in the population, The Birkebeiner (2012) study comprised two independent cohorts: Men who had participated in The Birkebeiner cross-country ski race (veteran recreational skiers), and men who had participated in a population-based health survey, (The Oslo Health study). Altogether 3545 men aged 53 years and older participated.16 Aiming to investigate atrial function related to exercise-induced AF, we invited 520 out of these to attend a cross-sectional study in 2019:134 skiers with a history of AF, 123 skiers without AF, and age and gender-matched control groups with (n = 81) and without (n = 147) a history of AF from the general population. To increase the size of the non-athletic control group with AF, every male patient at the proper age visiting the AF outpatient clinic at Diakonhjemmet Hospital between May and September 2020, were consecutively invited to participate until reaching the same number of participants as in the athletic AF-group.
The annual Birkebeiner ski race, with a course length of 54 km covering over a 1000 m uphill, has an average finishing time for the top 25% 70-year-old men of 4 h. It is regarded as one of the most challenging cross-country ski races globally and requires regular endurance exercise for preparation.
All participants answered a questionnaire and were physically examined. Regular endurance exercise was defined in the questionnaire as an exercise for ≥30 min ≥3 times a week with the purpose of increasing physical endurance capacity and reported on an 8-level ordinal scale. CHA2DS2-VASc score was estimated based on data from the questionnaire (age, sex, history of heart failure, hypertension, stroke, coronary heart disease, and diabetes mellitus).
We performed a resting electrocardiogram (ECG) and a 24-h Holter monitoring to assess undetected AF. A comprehensive echocardiographic examination in sinus rhythm was performed, assessing all phases of atrial function. The history of AF was verified by reviewing medical records, identifying paroxysmal AF according to guidelines.17 As it was difficult to differentiate paroxysmal from persistent AF in 13 participants (5 athletes and 8 non-athletes), these were included as having paroxysmal AF.
The study was approved by the Regional Committee for Medical and Health Research Ethics (ref.nr: 2016/565) and complies with the Declaration of Helsinki. All participants gave informed written consent.
Transthoracic echocardiography
Resting echocardiograms were recorded by the main investigator (ES) in the left lateral decubitus position using the same digital ultrasound scanner in all participants (Vivid E9, GE Vingmed Ultrasound, Horten, Norway). We obtained 2D-greyscale, colour Doppler, and tissue Doppler images in two parasternal and three standard apical views according to recommendations.18 Three to five consecutive loops were obtained and saved in cine loop format, including 200 ms before and after all beats.
Registrations were analysed offline (Echo PAC version 203, GE Vingmed Ultrasound, Horten, Norway) by a physician (ES) blinded for the patients’ other data.
Parameters of LV size were obtained, calculated, and indexed to body surface area according to recommendations.18
LA 2D acquisitions were obtained from atria-focused apical views, and LA volumes were calculated by the summation of discs in apical four- and two-chamber views.18 Maximum LA volume (LAmax) was measured tracing the LA inner border at end-systole on the frame before mitral valve opening, and minimum LA volume (LAmin) was measured at end diastole on the frame before mitral valve closing. Pre-atrial contraction volume (LAPa) was measured on the frame before atrial contraction. Atrial functional parameters were computed using the following formulas: LA ejection fraction (LAEF): (LAmax − LAmin/LAmax) × 100; passive emptying fraction (p-LAEF): (LAmax − LAPa/LAmax) × 100; LA active emptying fraction (a-LAEF): (LAPa − LAmin/LAPa) × 100. Atrial volumes were indexed to body surface areas, and an average from three consecutive loops was computed.
Mitral inflow parameters E, A, and E deceleration time were assessed from an apical four-chamber view by pulsed-wave Doppler, and E/A ratio was computed from these values. Pulsed-wave tissue Doppler images were obtained, and values of early diastolic (e′) velocities were assessed. E/e′ was calculated from an average of septal and lateral e′ velocities.19
Atrial stiffness was computed by dividing E/e′ by peak LA reservoir strain.20 The ratio of LAmax to LV end-diastolic volume (LA/LV) was computed to evaluate potentially unbalanced remodelling.14
Strain analysis
LV global longitudinal strain (GLS) was computed according to recommendations from apical four-chamber, two-chamber, and apical long-axis views, using the 18-segment model.21 End-diastole was defined by the frame prior to mitral valve closure. GLS was defined as the peak negative strain during systole.
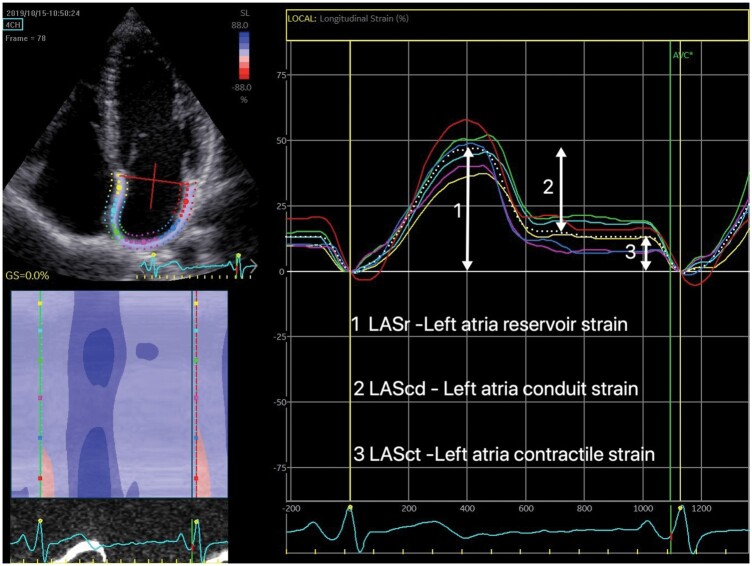
LA strain was obtained from apical four-chamber 2D views, optimized to avoid LA foreshortening, with a narrow image sector at frame rates > 60 fps. The LA border was traced from one side of the mitral annulus to the other along the endocardium. A region of interest was automatically defined, then adjusted manually to fit the thickness of the LA wall as recommended.22 The software automatically divided the LA into six segments, scoring their tracking quality with the possibility for manual correction. All strain values were computed as the average of the accepted segments and averaged over three heart cycles. Zero strain reference was set at LV end-diastole. LA strain values are stated as LASr - LA reservoir strain, LAScd - LA conduit strain, and LASct - LA contractile strain (Figure 1).22
Figure 1.
Left atria strain curves from apical four-chamber view. The six different segments were measured individually, and the average of accepted segments computed.
Statistical analysis
Continuous variables are reported as mean standard deviation (SD) for normally distributed data. Other continuous variables are reported as median (25th–75th percentile). Categorical values are reported using frequencies and percentages. Two-way between groups analysis of variance was conducted to explore the main effects of athletic status, AF status, and the interaction between AF status and athletic status. To assess independent associations between the atrial strain parameters LASr and LASct and AF, we performed a logistic regression for each parameter. Each regression was adjusted for weight, height, CHA2DS2VASc score, GLS, LV mass index, LAVImax, and E/e′. We performed a hierarchical logistic regression to evaluate the incremental value of LASr in identifying athletes with AF, LASct was excluded from this model due to collinearity.
Receiver operating curves (ROC) for atrial volumes and functional parameters were created, and areas under curves were calculated for these parameters’ ability to identify athletes suffering from AF. A sensitivity analysis excluding ablated patients was performed. Reproducibility analyses were conducted offline on anonymized recordings in 15 randomly selected participants using a two-way mixed model with absolute agreement between measures. Intra-observer analysis was done by the main investigator (ES) with a 2-week interval between measurements. Inter-rater analysis was conducted by rater two (MS) separated in time and place from rater one. Analysis was performed on the same set of recordings from each participant. The raters could randomly select different pictures and loops for analysis and re-analysis. Tests with P-values <0.05 were considered statistically significant. All analyses were performed using SPSS Statistics version 26 (IBM, Armonk, NY, USA).
Results
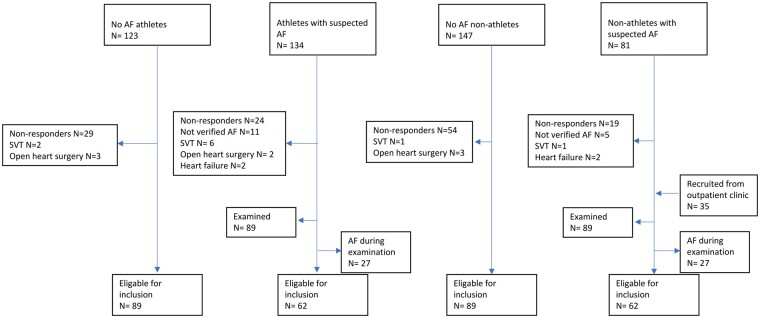
Three hundred and ninety-four out of the 520 invited individuals (76%) participated in the study. After exclusion of participants with AF during the examination, supraventricular tachycardia other than AF, heart failure (EF < 35%), open-heart surgery, and clinically significant valvular disease, we included 302 participants; 62 athletes with paroxysmal AF, 89 athletes without AF, 89 non-athletes without AF, and 62 non-athletes with paroxysmal AF (Figure 2). We did not identify any cases of AF during resting ECG or Holter monitoring in study participants without priorly known AF.
Figure 2.
Recruitment of the study sample. AF, atrial fibrillation; SVT, supraventricular tachycardia.
Baseline characteristics
All participants were men with a mean age of 70.8 6.7 years. Baseline characteristics are presented in Table 1. Athletes with and without AF reported a similar lifelong exposure to endurance sports, with an average of 40–50 years of regular endurance exercise and 16 completed Birkebeiner races. The prevalence of cardiovascular disease and risk factors in athletes was very low. AF burden was low in both athletes and non-athletes, with three out of four participants reporting less than one monthly episode of AF.
Table 1.
Baseline characteristics
| Athletes |
Non-athletes |
|||
|---|---|---|---|---|
| AF | No AF | AF | No AF | |
| Age (years) | ||||
| Weight (kg) | ||||
| Height (m) | ||||
| Body mass index (kg/m2) | ||||
| DBP (mmHg) | ||||
| SBP (mmHg) | ||||
| Heart rate (beats/min) | ||||
| CHA2DS2-VASc score | ||||
| Higher education, n (%) | ||||
| Anti-hypertensive treatment, n (%) | ||||
| Diabetes, n (%) | ||||
| Current smoking, n (%) | ||||
| Previous ablation, n (%) | 22 (35.5) | 14 (22.6) | ||
| Beta-blocker, n (%) | 12 19.4) | 24 (38.7) | ||
| Calsium-channel blocker, n (%) | 1 (1.6) | 4 (6.5) | ||
| Amiodarone, n (%) | 2 (3.2) | 3 (4.8) | ||
| Flecainid, n (%) | 3 (4.8) | 7 (11.3) | ||
| Digoxin, n (%) | 0 (0.0) | 0 (0.0) | ||
| Use of oral anticoagulants CHA2DS2-VASc score 2 (%) | 29/37 (84%) | |||
| Use of oral anticoagulants CHA2DS2-VASc-score 2 (%) | 13/25 (52%) | |||
| Number of completed Birkebeiner ski races | ||||
| Still participating in regular endurance exercise (%) | 46 (75.8%) | 68 (75.8%) | 11 (17.7%) | 8 (9.3%) |
Values are expressed as meanSD, n (%), or median (interquartile range).
DBP, diastolic blood pressure; SBP, systolic blood pressure.
CHA2DS2-VASc score estimated based on age, sex, CHF history, hypertension, stroke, coronary heart disease and diabetes mellitus.
Blood pressure was similar in all groups. Individuals with AF were taller, independent of athletic status. Athletes had a lower mean body mass index. While a higher proportion of athletes had undergone AF ablation, antiarrhythmic drug use was more prevalent among non-athletes. P-values for the main effects of athletic status and AF status as well as the interaction effect of these parameters regarding baseline characteristics are reported in Supplementary material online, Table S9.
Atrial parameters
LA parameters are summarized in Table 2. There was no significant interaction between AF status and athletic status regarding atrial parameters. Athletes had larger atrial volumes than non-athletes (P < 0.001), but there were no significant differences in atrial function as assessed by strain nor LAEF between athletes and non-athletes. The AF-groups had larger atrial volumes, higher atrial stiffness, and lower atrial function as assessed both by atrial strain and dynamic volumetric parameters than the non-AF groups regardless of athletic status. LASr remained highly significantly associated with AF (P < 0.001) in all participants combined and in the athletic group alone after adjusting for height, weight, CHA2DS2VASc score, E/e′, LV mass index, LAVImax, and GLS. The sensitivity analysis excluding ablated participants demonstrated consistent results. Results are reported as Supplementary data online.
Table 2.
Left atrium echocardiographic values
P-value interaction athletic status and AF status
| Athletes |
Non-athletes |
||||||
|---|---|---|---|---|---|---|---|
| AF | No AF | AF | No AF | P-value athletes vs. non-athletes | P-value AF vs. No AF | P-value interaction athletic status and AF status | |
| LASr (%) | 0.410 | <0.001 | 0.673 | ||||
| LAScd (%) | 0.788 | <0.001 | 0.955 | ||||
| LASct (%) | 0.215 | <0.001 | 0.591 | ||||
| LAEF (%) | 0.204 | <0.001 | 0.546 | ||||
| LAVImax (mL/m2) | <0.001 | <0.001 | 0.867 | ||||
| LAVImin (mL/m2) | <0.001 | <0.001 | 0.997 | ||||
| LA passive emptying fraction (%) | 0.247 | 0.020 | 0.090 | ||||
| LA active emptying fraction (%) | 0.013 | <0.001 | 0.970 | ||||
| LA stiffness | 0.052 | <0.001 | 0.948 | ||||
Values are expressed as meanSD. Two-way between groups analysis of variance was conducted. P-values for the main effects of athletic status and AF status as well as the interaction are presented in the table.
LA, left atrial; LAEF, left atrial ejection fraction; LAScd, Left atrial conduit strain; LASct, Left atrial contractile strain; LASr, Left atrial reservoir strain; LAVImax, maximum left atrial volume index; LAVImin, minimum left atrial volume index.
Evaluation of LA parameters in identifying AF in athletes
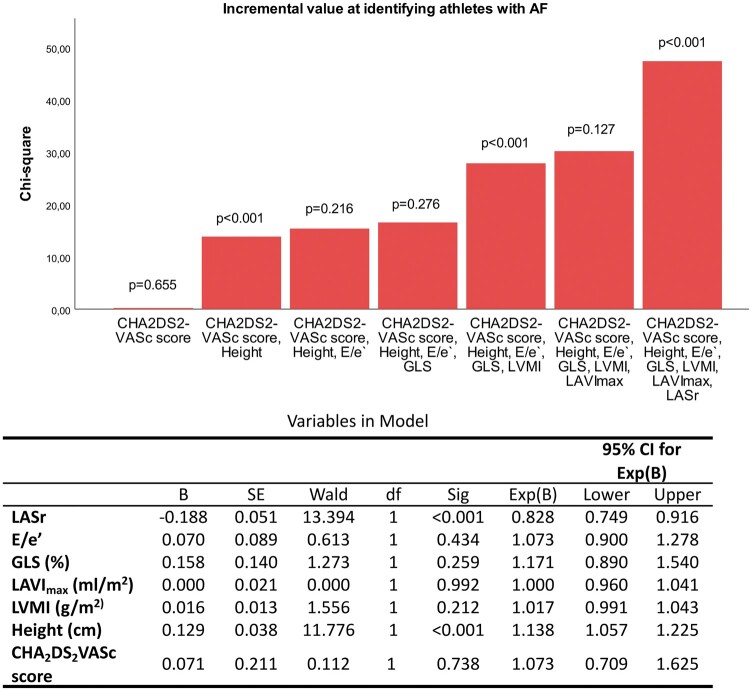
ROC curve analysis recognizes atrial strain parameters of the reservoir and contractile phase as best at identifying athletes suffering from AF. LASr demonstrated the highest area under the curve = 0.740 0.041, slightly above dynamic volumetric measurements followed by absolute volumes (Table 3). Figure 3 illustrates the independent and incremental value of LASr at identifying athletes with AF on top of clinical characteristics and standard echocardiographic parameters in a hierarchical logistic regression model. Height and LV mass index provided significant incremental value, though only height was significant independent of other covariates.
Table 3.
Performance of atrial volumes and functional parameters at identifying athletes with AF
| Variable | AUC | P-value |
|---|---|---|
| LASr | 0.000 | |
| LAScd | 0.012 | |
| LASct | 0.000 | |
| LAEF | 0.000 | |
| LA conduit fraction | 0.928 | |
| LA pump fraction | 0.000 | |
| LAVImax | 0.008 | |
| LAVIPa | 0.034 | |
| LAVImin | 0.000 |
AUC, area under the curve; LAEF, left atrial ejection fraction; LAScd, left atrial conduit strain; LASct, left atrial contractile strain; LASr, left atrial reservoir strain; LAVImax, maximum left atrial volume indexed; LAVImin, minimum left atrial volume indexed; LAVIPa, left atrial preatrial contraction volume indexed.
Figure 3.
Independent and incremental value of LASr at identifying athletes with AF on top of clinical characteristics (CHA2DS2-VASc score, height, and weight) and standard echocardiographic parameters (E/e′, mitral inflow velocity and mitral annular early diastolic velocity; GLS, global longitudinal strain; LAVImax, maximal left atrial volume indexed; LVMI, left ventricular mass index) in a hierarchical logistic regression model.
Ventricular parameters
LV values are summarized in Table 4. There was no significant interaction between AF status and athletic status regarding ventricular parameters except for LV mass index. Volumes were larger in athletes compared with non-athletes, whereas E/e′ was lower in athletes. LV mass index was higher in athletes than non-athletes and higher in athletes with AF than in athletes without AF. GLS was lower in athletes but unaffected by AF status.
Table 4.
Left ventricular echocardiographic parameters
| Athletes |
Non-athletes |
||||||
|---|---|---|---|---|---|---|---|
| AF | No AF | AF | No AF | P-value athletes vs. non-athletes | P-value AF vs. No AF | P-value interaction athletic status and AF status | |
| GLS (%) | 0.009 | 0.606 | 0.169 | ||||
| Ejection fraction (%) | <0.001 | 0.016 | 0.070 | ||||
| Peak mitral E wave vel (cm/s) | <0.001 | 0.842 | 0.750 | ||||
| Peak mitral A wave vel (cm/s) | <0.001 | <0.001 | 0.525 | ||||
| E/A | 0.427 | <0.001 | 0.600 | ||||
| E/e′ | 0.003 | 0.417 | 0.156 | ||||
| Average e′ (cm/s) | 0.789 | 0.509 | 0.177 | ||||
| EDecT (ms) | 0.006 | 0.410 | 0.670 | ||||
| LVEDV indexed (mL/m2) | <0.001 | 0.028 | 0.157 | ||||
| LVESV indexed (mL/m2) | <0.001 | 0.008 | 0.068 | ||||
| LV mass index (g/m2) | <0.001 | 0.075 | 0.004 | ||||
| Indexed LA/LV ratio | 0.368 | 0.079 | 0.271 | ||||
| Stroke volume indexed (mL/beat/m2) | 0.005 | 0.967 | 0.189 | ||||
Values are expressed as meanSD. Two-way between groups analysis of variance was conducted. P-values for the main effects of athletic status and AF status as well as interaction are presented in the table.
AF, atrial fibrillation; EDecT, E deceleration time; EDV, end-diastolic volume; ESV, end-systolic volume; GLS, Global longitudinal strain; LA, Left atrium; LV, left ventricle.
Reproducibility
For LAVImax intra-observer intraclass correlation coefficient (ICC) was 0.985 (0.957–0.995) and inter-observer ICC was 0.984 (0.954–0.995). For LAVImin the corresponding numbers were 0.980 (0.922–0.994) and 0.959 (0.881–0.986). For LASr intra-observer ICC was 0.846 (0.543–0.948) and inter-observer ICC 0.893 (0.676–0.964). LAScd intra-observer ICC was 0.872 (0.614–0.956), inter-observer ICC 0.854 (0.557–0.951), and for LASct the intra-observer ICC was 0.869 (0.614–0.956) and the inter-observer ICC 0.820 (0.452–0.940). Other ICC results are reported as Supplementary data online.
Discussion
In this large cohort of male veteran recreational athletes with strong exposure to endurance exercise through several decades, we found enlarged left atria compared with non-athletes. Both athletes and non-athletes with paroxysmal AF had significantly reduced LA strain values compared with subjects without AF.
This is the first study exploring the relationship between atrial size, function, and AF in an elderly population of recreational athletes. The enlarged left atria in athletes compared with non-athletes suggests atrial remodelling due to prolonged exposure to exercise. This complies with the findings of Letnes et al.23 that demonstrated how LAVImax increases with improved cardiorespiratory fitness, regardless of diastolic function, and how this effect augments with increasing age.
LAVImax and LAVImin are associated with AF in non-athletic populations, and we found a strong association between these parameters and AF status in athletes as well as non-athletes.12,24 However, as LAVImax and LAVImin are also strongly associated with athletic status, cardiorespiratory fitness needs to be taken into consideration when evaluating left atrial volumes.
As opposed to absolute volumes, we found that functional parameters, both assessed by dynamic volumetric measurements and atrial strain, were less influenced by athletic status. This complies with studies of LA function in healthy athletes, showing minute differences between athletes and non-athletic controls with borderline significant lower reservoir strain and no difference in contractile strain, despite a highly significant difference in LAVImax.25
Despite a higher indexed stroke volume, GLS was lower in athletes, reflecting that a larger ventricle demands less deformation to produce the same stroke volume. LV mass index was higher in athletes than non-athletes as expected and higher in athletes with AF than without AF, suggesting LV mass index as a potential co-marker of AF in athletes as seen in non-athletic populations.26
Diastolic properties decline with increasing age.27 While exercise seems to promote LV relaxation properties and slow the age-related progression of diastolic dysfunction, our study supports the notion that long-term exercise does not prevent a gradual decline in resting diastolic function in athletes.14,28,29 We identified significantly lower E/e′ in athletes, but the numerical difference was slight with values close to their age-expected value.30 In addition, we observed a high contractile strain relative to conduit and reservoir strain regardless of athletic status, concordant with findings in non-athletic populations, where age-induced atrial remodelling reduces early diastolic emptying and results in a compensatory increase in active LA function.31,32 These findings imply that athletes cannot mitigate the impact of aging on diastolic properties and atrial compliance. To provide further insight as to whether these changes result in a lower atrial contractile reserve, studies examining atrial function in elderly athletes during exercise are warranted.
Two smaller studies have previously evaluated LA strain and AF in athletes.14,15 In a group of younger elite athletes with AF, Trivedi et al.14 found no differences in atrial strain between athletes with and without AF, but athletes with AF had normal diastolic function as opposed to their non-athletic control group with AF. Among 27 athletes with AF and a mean age of 60 years, Hubert et al.15 found highly significant differences in all atrial strain parameters, with reservoir strain being the strongest identifier of AF. The age-induced cardiac remodelling in our elderly cohort may attenuate the favourable effect of exercise on diastolic properties in younger athletes, thus explaining much of the differences between the studies. The age-induced alterations in atrial strain parameters may also diminish the effect of athletic atrial remodelling and reduce the ability of atrial strain to identify AF compared to younger athletes. However, comparisons between different study populations are complicated, emphasizing the need for longitudinal studies in different athletic cohorts and caution when generalizing these results among different groups of athletes.
The majority of athletes without AF in our study surpass the upper limit of normality regarding LAVImax in guidelines despite having normal atrial function assessed by atrial strain.18 This illustrates the difficulties of using LAVImax to identify pathological atrial remodelling in elderly recreational endurance athletes.
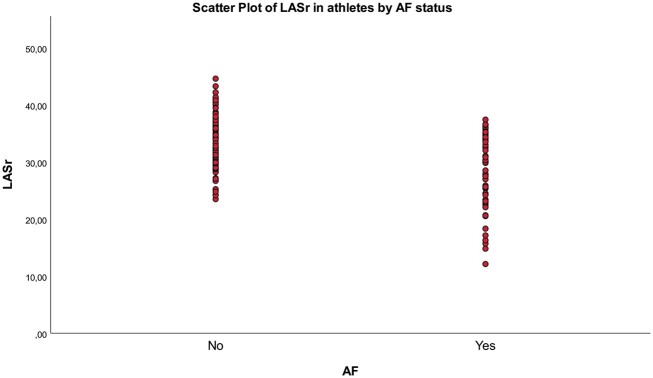
Even though atrial functional parameters and especially LASr prove a promising marker of atrial dysfunction in elderly endurance athletes with incremental value to clinical and conventional echocardiographic parameters in identifying athletes with AF, the values overlap between AF and non-AF athletes (Figure 4). Thus, it does not seem evident that LASr is suited to identify veteran athletes at risk of paroxysmal AF at an individual level. According to the NORRE study, the lower normal limit of LASr >60 years of age was 22.7%.33 We found 13 athletes having strain values below this point, all suffering from paroxysmal AF. This suggests that a LASr value below this limit is a reason for further investigating the presence of undiagnosed AF in elderly athletes.
Figure 4.
Scatter plot of left atrial reservoir strain (LASr) in athletes based on AF status.
Strengths and limitations
To our knowledge, this is the most extensive study to date exploring the relationship between endurance exercise, AF, and atrial function. The study comprises a unique cohort of elderly recreational athletes with an average of 40–50 years of performing endurance exercise, making the results relevant to a large group of recreational athletes.
The study may have seemed more appealing to individuals interested in endurance exercise, hence likely to attract the fittest members of our control cohort, diluting the effect of training in the athletic group. To increase the number of participants in the control group with AF, we supplemented the control group with patients from an outpatient clinic. Finally, this is a single-centre study, consisting of men only, due to the low number of female participants in the Birkebeiner study with exercise-induced AF. Considering the increasing number of female recreational endurance athletes, future studies should strive to include women.
Conclusions
Male veteran athletes had significantly larger LA volumes than non-athletes. In contrast, LA strain values were similar in athletes and non-athletes with paroxysmal AF, and significantly lower than in subjects without AF. Our findings suggest that LA functional parameters are more closely linked to AF than volumetric parameters in veteran athletes.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
This work was supported by Diakonhjemmet Hospital, Department of Medical Research Bærum Hospital, the Norwegian Institute of Public Health, the Raagholt foundation and the Dam foundation through a Ph.D. grant.
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morseth B, Løchen ML, Ariansen I, Myrstad M, Thelle DS. The ambiguity of physical activity, exercise and atrial fibrillation. Eur J Prev Cardiol 2018;25:624–36. [DOI] [PubMed] [Google Scholar]
- 4. Myrstad M, Løchen ML, Graff-Iversen S, Gulsvik AK, Thelle DS, Stigum H et al. Increased risk of atrial fibrillation among elderly Norwegian men with a history of long-term endurance sport practice. Scand J Med Sci Sports 2014;24:e238-44–e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA et al. Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the Henry Ford Exercise Testing (FIT) Project. Circulation 2015;131:1827–34. [DOI] [PubMed] [Google Scholar]
- 6. Scheer V. Participation trends of ultra endurance events. Sports Med Arthrosc Rev 2019;27:3–7. [DOI] [PubMed] [Google Scholar]
- 7. Elliott AD, Mahajan R, Linz D, Stokes M, Verdicchio CV, Middeldorp ME et al. Atrial remodeling and ectopic burden in recreational athletes: implications for risk of atrial fibrillation. Clin Cardiol 2018;41:843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. La Gerche A, Claessen G, Van de Bruaene A, Pattyn N, Van Cleemput J, Gewillig M et al. Cardiac MRI: a new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging 2013;6:329–38. [DOI] [PubMed] [Google Scholar]
- 9. Reeves JT, Groves BM, Cymerman A, Sutton JR, Wagner PD, Turkevich D et al. Operation Everest II: cardiac filling pressures during cycle exercise at sea level. Respir Physiol 1990;80:147–54. [DOI] [PubMed] [Google Scholar]
- 10. Prior DL, La Gerche A. The athlete's heart. Heart 2012;98:947–55. [DOI] [PubMed] [Google Scholar]
- 11. Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A et al. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol (1985) 2008;104:1121–8. [DOI] [PubMed] [Google Scholar]
- 12. Schaaf M, Andre P, Altman M, Maucort-Boulch D, Placide J, Chevalier P et al. Left atrial remodelling assessed by 2D and 3D echocardiography identifies paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging 2017;18:46–53. [DOI] [PubMed] [Google Scholar]
- 13. Rivner H, Mitrani RD, Goldberger JJ. Atrial myopathy underlying atrial fibrillation. Arrhythm Electrophysiol Rev 2020;9:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trivedi SJ, Claessen G, Stefani L, Flannery MD, Brown P, Janssens K et al. Differing mechanisms of atrial fibrillation in athletes and non-athletes: alterations in atrial structure and function. Eur Heart J Cardiovasc Imaging 2020;21:1374–83. [DOI] [PubMed] [Google Scholar]
- 15. Hubert A, Galand V, Donal E, Pavin D, Galli E, Martins RP et al. Atrial function is altered in lone paroxysmal atrial fibrillation in male endurance veteran athletes. Eur Heart J Cardiovasc Imaging 2018;19:145–53. [DOI] [PubMed] [Google Scholar]
- 16. Myrstad M, Nystad W, Graff-Iversen S, Thelle DS, Stigum H, Aarønæs M et al. Effect of years of endurance exercise on risk of atrial fibrillation and atrial flutter. Am J Cardiol 2014;114:1229–33. [DOI] [PubMed] [Google Scholar]
- 17. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 19. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 20. Machino-Ohtsuka T, Seo Y, Tada H, Ishizu T, Machino T, Yamasaki H et al. Left atrial stiffness relates to left ventricular diastolic dysfunction and recurrence after pulmonary vein isolation for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:999–1006. [DOI] [PubMed] [Google Scholar]
- 21. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 2015;28:183–93. [DOI] [PubMed] [Google Scholar]
- 22. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018;19:591–600. [DOI] [PubMed] [Google Scholar]
- 23. Letnes JM, Nes B, Vaardal-Lunde K, Slette MB, Mølmen-Hansen HE, Aspenes ST et al. Left atrial volume, cardiorespiratory fitness, and diastolic function in healthy individuals: the HUNT Study. Norway. J Am Heart Assoc 2020;9:e014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tiwari S, Schirmer H, Jacobsen BK, Hopstock LA, Nyrnes A, Heggelund G et al. Association between diastolic dysfunction and future atrial fibrillation in the Tromsø Study from 1994 to 2010. Heart 2015;101:1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Left atrial function in elite athletes: a meta-analysis of two-dimensional speckle tracking echocardiographic studies. Clin Cardiol 2019;42:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seko Y, Kato T, Haruna T, Izumi T, Miyamoto S, Nakane E et al. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci Rep 2018;8:6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: links to heart disease. Circulation 2003;107:346–54. [DOI] [PubMed] [Google Scholar]
- 28. Beaumont AJ, Grace FM, Richards JC, Campbell AK, Sculthorpe NF. Aerobic training protects cardiac function during advancing age: a meta-analysis of four decades of controlled studies. Sports Med 2019;49:199–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nottin S, Nguyen LD, Terbah M, Obert P. Long-term endurance training does not prevent the age-related decrease in left ventricular relaxation properties. Acta Physiol Scand 2004;181:209–15. [DOI] [PubMed] [Google Scholar]
- 30. Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D et al. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging 2015;16:1031–41. [DOI] [PubMed] [Google Scholar]
- 31. Boyd AC, Richards DA, Marwick T, Thomas L. Atrial strain rate is a sensitive measure of alterations in atrial phasic function in healthy ageing. Heart 2011;97:1513–9. [DOI] [PubMed] [Google Scholar]
- 32. Stefani LD, Trivedi SJ, Ferkh A, Altman M, Thomas L. Changes in left atrial phasic strain and mechanical dispersion: effects of age and gender. Echocardiography 2021;38:417–26. [DOI] [PubMed] [Google Scholar]
- 33. Sugimoto T, Robinet S, Dulgheru R, Bernard A, Ilardi F, Contu L et al. Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2018;19:630–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.