Significance
Cellular homeostasis requires the sensing of and adaptation to dynamically changing levels of oxygen and reactive oxygen species (ROS). Here, we show that the Cys-branch of the Arg/N-degron pathway represents a cellular sensor for acute and chronic hypoxia, as well as oxidative stress. When the environment oscillates between normoxia and acute hypoxia, the enzymatic oxidation of the N-terminal cysteine (Nt-Cys) residues adjusts the levels of signaling molecules via the ubiquitin-proteasome system. However, if hypoxia becomes chronic, the Nt-Cys is chemically oxidized by consequent ROS to generate an N-degron that redirects the proteolytic flux of cognate substrates to the autophagy-lysosome system through reprograming of the ubiquitin code. These results provide critical insight into a first-line sensor and defense against oxidative stress.
Keywords: N-degron pathway, Cys/N-degron pathway, Arg/N-degron pathway, oxygen sensor, oxidative stress sensor
Abstract
Cellular homeostasis requires the sensing of and adaptation to intracellular oxygen (O2) and reactive oxygen species (ROS). The Arg/N-degron pathway targets proteins that bear destabilizing N-terminal residues for degradation by the proteasome or via autophagy. Under normoxic conditions, the N-terminal Cys (Nt-Cys) residues of specific substrates can be oxidized by dioxygenases such as plant cysteine oxidases and cysteamine (2-aminoethanethiol) dioxygenases and arginylated by ATE1 R-transferases to generate Arg-CysO2(H) (R-CO2). Proteins bearing the R-CO2 N-degron are targeted via Lys48 (K48)–linked ubiquitylation by UBR1/UBR2 N-recognins for proteasomal degradation. During acute hypoxia, such proteins are partially stabilized, owing to decreased Nt-Cys oxidation. Here, we show that if hypoxia is prolonged, the Nt-Cys of regulatory proteins can be chemically oxidized by ROS to generate Arg-CysO3(H) (R-CO3), a lysosomal N-degron. The resulting R-CO3 is bound by KCMF1, a N-recognin that induces K63-linked ubiquitylation, followed by K27-linked ubiquitylation by the noncanonical N-recognin UBR4. Autophagic targeting of Cys/N-degron substrates is mediated by the autophagic N-recognin p62/SQTSM-1/Sequestosome-1 through recognition of K27/K63-linked ubiquitin (Ub) chains. This Cys/N-degron–dependent reprogramming in the proteolytic flux is important for cellular homeostasis under both chronic hypoxia and oxidative stress. A small-compound ligand of p62 is cytoprotective under oxidative stress through its ability to accelerate proteolytic flux of K27/K63-ubiquitylated Cys/N-degron substrates. Our results suggest that the Nt-Cys of conditional Cys/N-degron substrates acts as an acceptor of O2 to maintain both O2 and ROS homeostasis and modulates half-lives of substrates through either the proteasome or lysosome by reprogramming of their Ub codes.
Cellular homeostasis requires the sensing of and adaptation to dynamically changing environmental factors such as hypoxia, oxidative stress, extreme temperatures, and nutrient starvation. Known sensing mechanisms involve GCN2 and AMPK, which respectively sense amino acid and glucose starvation (1, 2), as well as hypoxia-inducible factor 1α (HIF-1α), which senses oxygen (O2) levels (3). As an O2 sensor, the transcription factor HIF-1α is normally hydroxylated by prolyl-hydroxylase and degraded through ubiquitylation by the von Hippel-Lindau E3 ligase. Under hypoxia, HIF-1α is not oxidized and is thus metabolically stabilized, leading to the transcriptional induction of hypoxia-responsive proteins (4–6). However, as this chronic sensor of hypoxia typically requires at least 2 to 4 h (7) to adjust stress response pathways, it remains an outstanding question how cells sense and react to acute hypoxia.
Dysregulation of O2 homeostasis can cause the excessive accumulation of reactive oxygen species (ROS), leading to oxidative stress (8). In response to the increases in ROS levels, cells attempt to maintain homeostasis by activating stress-specific signaling cascades, such as the Keap1-Nrf2, NF-κB, AKT, and MAPK pathways (9). While the functions and mechanisms of antioxidative stress pathways are now fairly well understood, little is known about how cells initially sense the accumulation of ROS. Moreover, despite the fact that O2 and ROS are chemically and physiologically related to each other, it has remained unclear how sensing systems for O2 and ROS are in crosstalk with each other, especially when ROS begin to accumulate as a consequence of chronic O2 deficiency (i.e., hypoxia).
The Arg/N-degron pathway (previously called the “Arg/N-end rule pathway”) can target a protein that bears a destabilizing N-terminal (Nt) residue for degradation by the proteasome or via autophagy (10–15). The set of N-degrons encompasses Nt-arginine (Nt-Arg). While this N-degron can be present constitutively, it can also be generated conditionally, through arginylation of Nt-aspartate (Asp) or Nt-glutamate (Glu) by arginyl-tRNA protein transferase 1 (ATE1 R-transferase). The resulting Nt-Arg is bound by the UBR box of the N-recognins UBR1 and UBR2 that promote ubiquitylation and proteasomal degradation of native substrates (16). In addition to Nt-Asp and Nt-Glu, Nt-Cys can be Nt-arginylated following its chemical or enzymatic oxidation (17–20). The Nt-Cys–carrying substrates of arginylation include a set of GTPase-activating proteins (RGS4, RGS5, and RGS16) and interleukin-32 (IL-32) that carry the Nt-Met-Cys motif as a pro-N-degron (17, 18, 21, 22). The RGS proteins are normally degraded through the ubiquitin (Ub)-proteasome system (UPS), during which their Cys2 residue is N-terminally exposed as a pro-N-degron, following Nt-Met excision. This Nt-Cys residue is subsequently oxidized into CysO2 (Cys-sulfinic acid) by mammalian cysteamine (2-aminoethanethiol) dioxygenases (ADOs) or plant cysteine oxidases (PCOs) and arginylated by ATE1, leading to substrate ubiquitylation and proteasomal degradation (19, 22). However, under hypoxia, the oxidation of the Nt-Cys residue is inhibited, resulting in the metabolic stabilization and adjustment of downstream signaling pathways (18, 19). Through O2-dependent degradation, the Nt-Cys can function as an O2 acceptor in a cellular sensing system for acute hypoxia (19, 22–24). However, the role of the Nt-Cys residue in chronic hypoxia and oxidative stress remains unknown.
Here, we show that the Nt-Cys2 of the N-degron pathway is an acceptor of O2 in cellular sensing systems for acute and chronic hypoxia as well as oxidative stress. When O2 levels are altered, the half-lives of Cys/N-degron substrates are acutely modulated through oxidation of the Nt-Cys into CysO2(H), a proteasomal N-degron. If hypoxia is prolonged, the Nt-Cys residue is chemically oxidized by ROS, generating CysO3(H), an autophagic N-degron. The chemical oxidation of the Nt-Cys also functions as a sensor of oxidative stress. Substrates bearing the CysO3(H) degron are K27/K63-ubiquitylated by the E3 ligases/N-recognins KCMF1 and UBR4 for p62-dependent macroautophagic proteolysis. Thus, the Cys/N-degron pathway functions as a sensor of O2 and ROS under various physiological conditions.
Results
The Nt-Cys Residue Plays a Key Role in Sensing Acute and Chronic Hypoxia as well as Oxidative Stress through Its Oxidation.
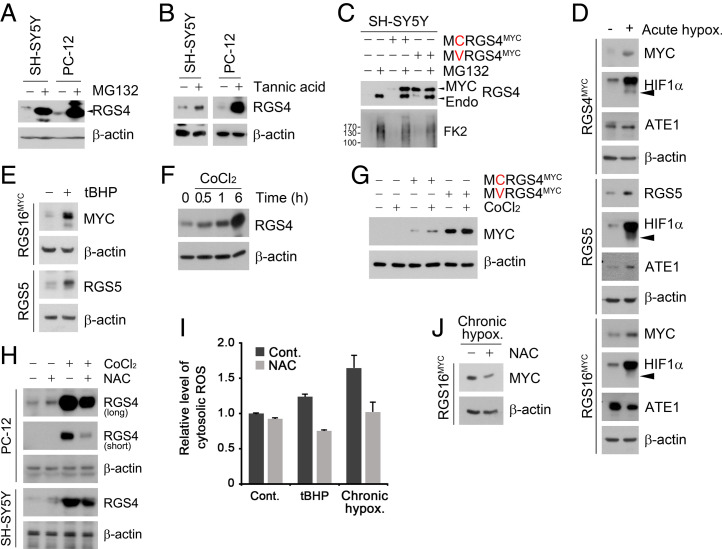
To understand the role of the Nt-Cys residue in sensing O2 and ROS and how the two sensing systems are intertwined with each other, we first characterized the metabolic fate and their Nt modifications of Nt-Cys proteins under acute versus chronic hypoxia. Degradation assays showed that RGS4, RGS5, RGS16, and IL-32 were normally degraded via the UPS (Fig. 1A and SI Appendix, Fig. S1A). This degradation required Nt-arginylation of their Cys2 residues, as evidenced by their stabilization under chemical inhibition of ATE1 by tannic acid (Fig. 1B) or Cys2 mutation to Val (Fig. 1C). Consistent with our previous finding that the Nt-Cys is oxidized to facilitate proteasomal degradation of N-degron substrates (22), the Nt-Cys proteins were drastically stabilized under hypoxia (Fig. 1D). These results reiterate the role of Nt-Cys in proteolysis via the N-degron pathway and sensing acute hypoxia in mammalian cells.
Fig. 1.
Proteasomal substrate Cys/N-degron proteins are degraded by autophagy under O2-mediated stress conditions. (A and B) WB of endogeneous RGS4 in SH-SY5Y and PC-12 cells treated with (A) MG132 (10 μΜ, 6 h) or (B) tannic acid (35 μΜ, 24 h). (C) Same as A but with SH-SY5Y cells expressing WT or C2V RGS4MYC. (D) WB of SH-SY5Y cells expressing RGS4MYC, RGS5, or RGS16FLAG and exposed to acute hypoxia (1% O2, 6 h). (E) WB of 293T cells expressing RGS5 or RGS16MYC and treated with 6 h tBHP (250 μΜ, 6 h). (F) WB of SH-SY5Y cells treated with CoCl2 (250 μΜ) for indicated time. (G) Same as C but with treatment of CoCl2 (250 μΜ, 6 h). (H) SH-SY5Y cells were treated with CoCl2 and NAC (0.5 mM) for 6 h and immunoblotted. (I) Relative cytosolic ROS level measured upon exposure to tBHP (250 μΜ, 6 h) under chronic hypoxia (1% O2, 48 h) with combination treatment of NAC (0.5 mM) on 293T cells. (J) WB of 293T cells expressing RGS16MYC and exposed to chronic hypoxia (1% O2, 48 h).
We also determined whether the Nt-Cys acts as a sensor of ROS under oxidative stress. Importantly, normally short-lived Cys/N-degron substrates turned to long-lived when cells under normoxia were exposed to ROS-inducing chemicals such as tert-butyl hydroperoxide (tBHP) and cobalt chloride (CoCl2) (Fig. 1E and SI Appendix, Fig. S1B). This ROS-induced stabilization occurred as early as 0.5 h and continued up to 6 h posttreatment with ROS inducers (Fig. 1F), independent of transcriptional alteration (SI Appendix, Fig. S1 C and D). These Nt-Cys proteins, metabolically stabilized by tBHP or CoCl2, returned back to be short-lived when ROS was scavenged using N-acetyl cysteine (NAC) (Fig. 1 G and H and SI Appendix, Fig. S1E). In contrast, C2V-RGS4 mutant was unconditionally long-lived under all conditions tested and insensitive to NAC (SI Appendix, Fig. S1F). These results suggest that normally short-lived Nt-Cys proteins are metabolically stabilized under oxidative stress through chemical oxidation of their Nt-Cys by ROS.
To determine the role of the Nt-Cys residue as a sensor of chronic hypoxia, we also tested the hypothesis that chronic hypoxia generates ROS, which in turn chemically oxidizes Nt-Cys bearing proteins. Indeed, DCHF-DA assays showed that prolonged hypoxia led to the excessive production of ROS, which was counteracted by NAC (Fig. 1I). Consistently, RGS16 was long-lived under chronic hypoxia and became short-lived following NAC treatment (Fig. 1J). These results collectively suggest that the Nt-Cys residue represents a cellular sensing system for acute and chronic hypoxia as well as oxidative stress in an HIF-1α–independent manner.
Proteolytic Flux in the Cys/N-degron Pathway Is Shifted from the Proteasome to the Lysosome under Chronic Hypoxia and Oxidative Stress.
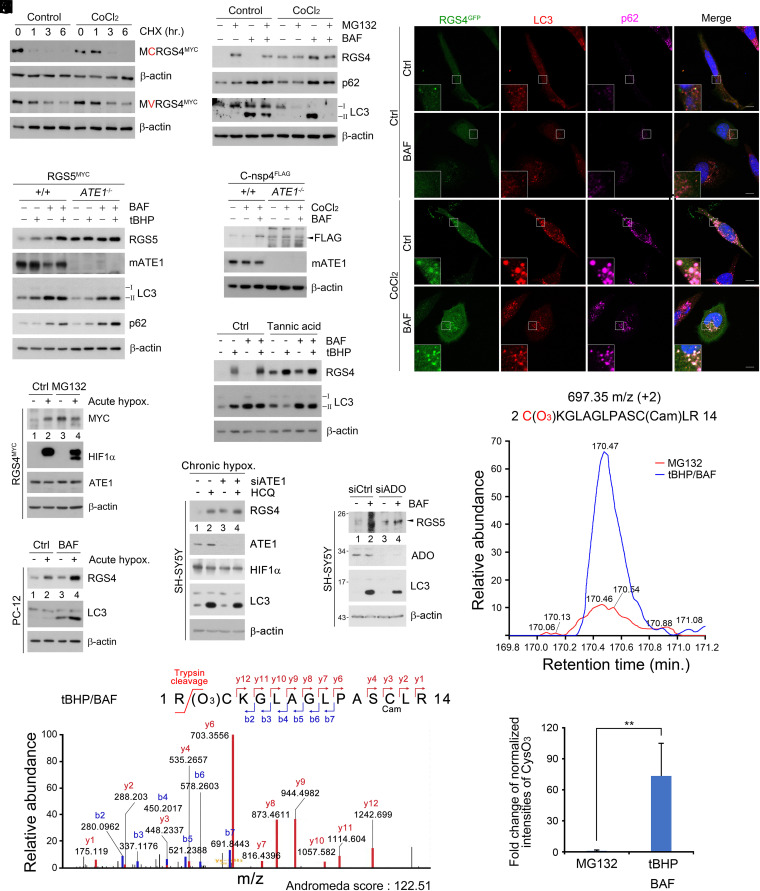
To investigate the role of the Nt-Cys residue as an oxidative stress-specific N-degron, we characterized the metabolic fates of Nt-Cys–carrying proteins. Cycloheximide-chase assays showed that Cys/N-degron substrates were stabilized in an Nt-Cys–dependent manner when cells were treated with CoCl2 (Fig. 2A and SI Appendix, Fig. S2A). When proteolytic flux was analyzed using the proteasomal inhibitor MG132 or the lysosomal inhibitor bafilomycin A1, they were normally degraded via the UPS but redirected to autophagy in response to oxidative stress induced by CoCl2 or tBHP (Fig. 2B and SI Appendix, Fig. S2 B–F). Such a proteolytic rerouting occurred as early as 0.5 h postexposure to ROS (SI Appendix, Fig. S2G). In colocalization analyses, Nt-Cys proteins under oxidative stress were targeted to p62+LC3+ autophagic membranes through the activity of their Nt-Cys (Fig. 2C and SI Appendix, Fig. S2 H–K). Their degradation via macroautophagy was abolished by chemical inhibition or genetic ablation of ATE1 (Fig. 2 D and F). Thus, the Nt-Cys residue exposed to oxidative stress redirects proteolytic flux from the UPS to macroautophagy through its oxidation and arginylation.
Fig. 2.
CysO3 degron is a marker of cellular oxidative stress. (A) CHX chase assay in HEK293T cells expressing WT or C2V RGS4-MYC and treated with CoCl2 (250 μΜ). (B) WB of endogeneous RGS4 with combination of MG132, BAF, and CoCl2 treatment for 6 h in SH-SY5Y cells. (C) Colocalization assay of the GFP, p62, and LC3 puncta in RGS4-GFP–expressing HeLa cells treated with 6 h BAF (200 nM, 6 h), CoCl2, or both. (Scale bar, 10 µm.) (D and E) WB of WT and ATE1−/−MEF cells expressing (D) RGS5MYC or (E) C-nsp4FLAG treated with combination of BAF, CoCl2, or tBHP (same as B). (F) WB of endogeneous RGS4 in SH-SY5Y treated with combination of BAF (200 nM, 6 h), CoCl2 (250 μΜ, 6 h), and tannic acid treatment (35 μΜ, 24 h). (G) WB of 293T cells expressing RGS4MYC and exposed to acute hypoxia (1% O2, 6 h) and MG132 (10 μΜ, 6 h). (H) WB of PC-12 cells exposed to acute hypoxia (1% O2, 6 h) and BAF (200 nM) for 6 h. (I) WB of SH-SY5Y cells with siATE1 and exposure to chronic hypoxia(1% O2, 24 h) and HCQ (25 nM) for 24 h. (J) WB of SH-SY5Y cells under ADO1 interference (20 nM, 48 h) treated with BAF (200 nM, 6 h). (K) LC/MS spectrum of CysO3 containing peptide. The sequence contains the Nt-Arg which is expected to be cleaved off during trypsin digestion. (L and M) LC/MS assay of HEK293T cells expressing RGS4MYC and treated with tBHP (250 μΜ, 6 h) and BAF (200 nM, 6 h), shown in extracting ion current graph (L) and bar graph (M) reflecting relative fold change of the normalized intensity of CysO3 containing peptide purified from MG132 (10 μΜ, 6 h) and tBHP and BAF cotreated samples.
Next, we determined the role of the Nt-Cys residue in autophagic proteolysis under chronic hypoxia. Proteolytic flux assays showed that Cys/N-degron substrates were normally subject to acute degradation via the UPS with cooperation of ADO1 but drastically stabilized when hypoxia was prolonged to 6 h (Fig. 2G and SI Appendix, Fig. S2 L and M). The apparent stabilization was attributed to rerouting from UPS-mediated acute degradation to lysosome-mediated chronic degradation (Fig. 2H). The autophagic degradation was abrogated by genetic depletion (Fig. 2I) or chemical inhibition (SI Appendix, Fig. S2N) of ATE1. Thus, proteolytic flux of Cys/N-degron substrates is redirected from the UPS to autophagy if hypoxia is prolonged.
The Nt-Cys under Oxidative Stress Is Oxidized and Arginylated to Generate the N-degron Arg-CysO3.
Previous studies showed that when cells sense acute changes in O2 levels, the Nt-Cys residue is enzymatically oxidized to the CysO2 N-degron by plant PCO1 or mammalian ADO1 (19, 22, 24). We therefore tested whether ADO1 mediates oxidation of the Nt-Cys under chronic hypoxia. Importantly, ADO1 knockdown cells under chronic hypoxia retained significant autophagic flux of Cys/N-degron substrates (Fig. 2J), suggesting that Cys/N-degron substrates may be at least in part chemically oxidized by ROS under chronic hypoxia or oxidative stress. We therefore characterized the modifications of Nt-Cys under oxidative stress and chronic hypoxia by employing mass spectrometry of RGS4. Liquid chromatography–mass spectrometry (LC/MS) analysis showed that, while an expected modifications of the Nt-Cys product of RGS4 protein following oxidation under normoxia is CysO2 (19, 22), Nt-Cys residue of RGS4 in cells exposed to tBHP was mostly modified into CysO3 (Cys sulfonic acid) (Fig. 2K). Critically, the levels of Nt-CysO3–carrying RGS4 peptides destined to oxidative stress-associated autophagy were 73-fold higher than those destined to the UPS under normoxia (Fig. 2 L and M). Thus, under chronic hypoxia or oxidative stress, the Nt-Cys residue is chemically oxidized and arginylated into the autophagic N-degron Arg-CysO3.
Autophagic Targeting of Cys/N-degron Substrates Is Mediated via K63- and K27-Linked Ubiquitylation.
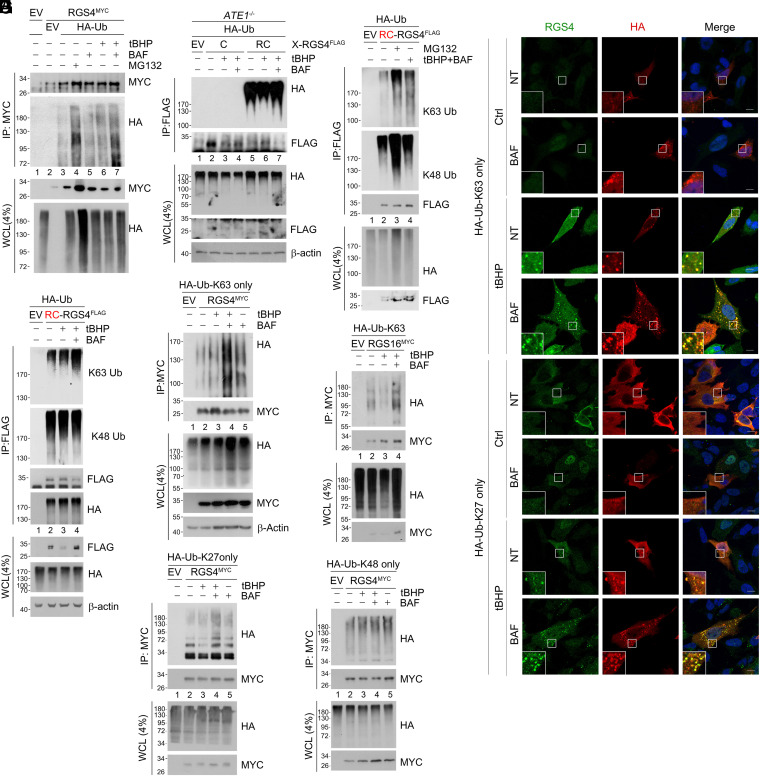
Previous studies showed that RGS4 and RGS5 under normoxia are assembled with K48-linked Ub chains by the N-recognin UBR1 and UBR2 (25). To elucidate the mechanisms by which the Cys/N-degron pathway modulates bidirectional proteolysis via the Arg-CysO2 or Arg-CysO3 degron, we characterized the Ub codes of Nt-Cys proteins under oxidative stress and chronic hypoxia. Denaturation immunoprecipitation (IP) assays under normoxia confirmed that polyubiquitylation of RGS4 led to acute degradation by the proteasome (Fig. 3 A, lanes 3 vs. 4). Notably, RGS4 was also polyubiquitylated in the presence of excessive ROS, which led to degradation by the lysosome but not the proteasome when proteolytic flux was analyzed using MG132 and bafilomycin A1 (lanes 6 vs. 7). To determine whether the Cys2 residue is essential for RGS4 ubiquitylation, X-nsP4 (X = Cys or Arg) was expressed in ATE1−/− mouse embryonic fibroblasts (MEFs) using Ub fusion technique (SI Appendix, Fig. S3A). Whereas Arg-RGS4 was ubiquitylated in both +/+ and ATE1−/− cells, Cys-RGS4 was conjugated with poly-Ub chains only in +/+ cells but not ATE1−/− cells (Fig. 3B). These results suggest that Nt-arginylation is essential for ubiquitylation of Nt-Cys–carrying proteins for degradation via both the UPS and autophagy.
Fig. 3.
K63, K27-linked ubiquitin chain reprimation induces autophagic degradation of the Cys substrates. (A) Denaturation IP of RGS4MYC and HA-Ub expressing HEK293T cells treated with a combination of BAF (200 nM), tBHP (250 μΜ), and MG132 (10 μΜ) for 6 h. (B and C) Same as A but with HA-Ub, and (B) C and RC-RGS4FLAG expressing ATE1−/− MEF cells or (C) RC-RGS4FLAG and treated with MG132 or BAF with tBHP. (D) Same as C but with tBHP and BAF only treatment. (E) Immunocytochemistry in SH-SY5Y cells expressing K63 only and K27 only HA-Ub treated with BAF, tBHP, or both (same as A). (F–I) Denaturation IP of HEK293T cells expressing RGS4MYC (F, H, I) or RGS16MYC (G) with a combination of K63 only (F and G), K27 only (H), or K48 only (I) HA-Ub mutant treated with tBHP, BAF, or both (same as A).
Our results suggest that the Nt-Cys residue is enzymatically oxidized to Arg-CysO2 under normoxia and chemically oxidized to Arg-CysO3 under chronic hypoxia and oxidative stress. We therefore characterized the differences in Ub chain linkages conjugated to substrates bearing the Arg-CysO2 or Arg-CysO3 degron with respect to their proteolysis via the UPS and autophagy, respectively. As expected, K48-ubiquitylated RGS4 was selectively enriched upon inhibition of the proteasome (Fig. 3 C, lanes 3 vs. 2, K48-Ub) but not the lysosome under oxidative stress (lanes 4 vs. 2). In contrast to K48-Ub chains, K63-Ub chains exhibited significant proteolytic flux upon lysosomal inhibition in cells treated with tBHP (lanes 4 vs. 2, K63-Ub). Such a proteolytic flux of K63-chains via autophagy was diminished in ATE1-deficient cells (SI Appendix, Fig. S3 B, lanes 3 vs. 4, K63-Ub). Under these oxidative stress conditions, autophagic flux was observed with K63-Ub chains (Fig. 3 D, lanes 4 vs. 3) but not K48-Ub chains (lanes 4 vs. 3). These results suggest that Cys/N-degron substrates are tagged with K63-linked Ub chains.
To identify additional Ub modifications on RGS4 that facilitate autophagic flux, we used single-lysine (Lys)-only Ub mutants in which all Lys residues except one are substituted with Arg. When visualized using immunostaining analyses, RCox-RGS4 formed cytosolic puncta that colocalized solely with K63-only or K27-only mutant Ub bodies (Fig. 3E). In denaturation IP assays under oxidative stress, autophagic flux was observed with K63-only (Fig. 3 F and G) and K27-only (Fig. 3H) Ub mutants conjugated to Nt-Cys substrates but not with K48-only mutants (Fig. 3I). These results suggest that RGS4 conjugated with K63/K27-linked Ub chains are selectively degraded by autophagy. We therefore asked whether K63/K27-ubiquitylation occurs on other Nt-arginylation substrates that enter autophagic flux independent of Nt-Cys. RD-CDC6FLAG, a model Nt-Arg substrate known to be destined to autophagic degradation under proteotoxic condition (26), was not K63-ubiquitylated in either oxidative or proteotoxic stress (SI Appendix, Fig. S3 C and D). These results show that the trioxidized Nt-Cys residue functions as an autophagic N-degron that targets substrates to the lysosome via arginylation-dependent Ub repriming with K63/K27 chains under oxidative stress.
KCMF1 Is an Oxidative Stress-Specific N-recognin that K63-Ubiquitylates Nt-Cys Substrates.
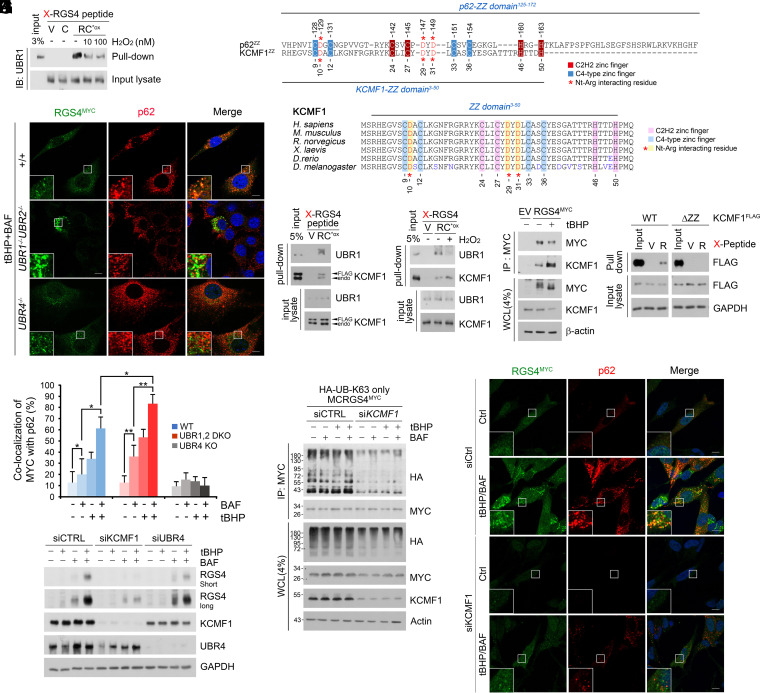
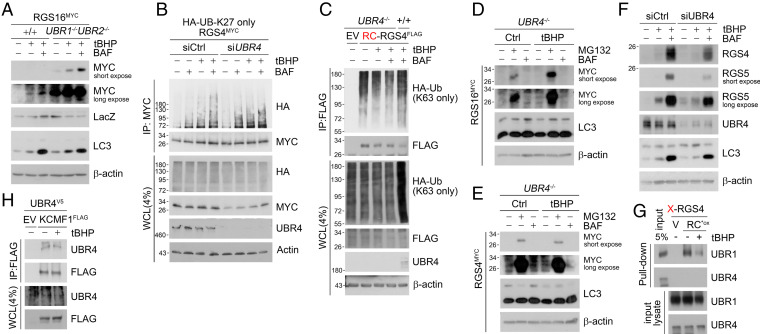
To identify the E3s that mediate K63-linked and/or K27-linked Ub chains on Nt-Cys bearing proteins, we measured the binding of UBR1 to RGS4. Co-IP analyses showed that the binding affinity significantly weakened under oxidative stress as compared with normal conditions (Fig. 4A). Moreover, the genetic ablation of both UBR1 and UBR2 did not block but rather accelerated autophagic targeting of Nt-Cys substrates (Fig. 4 B and C and SI Appendix, Fig. S4 A, B, and D). As these results suggest that UBR1 and UBR2 preferentially recognize Arg-CysO2 over Arg-CysO3, we screened for oxidative stress-specific E3s that recognize Arg-CysO3 and promote K27 or K63-ubiquitylation. Co-IP analyses coupled with mass spectrometry identified KCMF1 as an E3 responsible for K63-ubiquitylation of Nt-Cys substrates. Mapping analyses showed that KCMF1 bound Arg-CysO3 through the ZZ domain (Fig. 4D). The ZZ domain of KCMF1 shared sequence similarity with the UBR box of UBR1 and the ZZ domain of the autophagic N-recognin p62, including those required for binding Nt-Arg (Fig. 4E). We therefore determined how the binding affinity of KCMF1 and UBR1 to the RCox degron is modulated under oxidative stress using in vitro peptide pulldown and co-IP assays. In basal conditions, similar amounts of KCMF1 and UBR1 were coprecipitated with RGS4 (Fig. 4F). However, under oxidative stress, the RCox exhibited higher affinity to KCMF1 and weaker affinity to UBR1 as compared with basal conditions (Fig. 4 G and H). Pulldown assays confirmed that the ZZ domain of KCMF1 is essential for the recognition of the Nt-Arg-CysO3 degron (Fig. 4I). These results suggest that KCMF1 is an oxidative stress-specific N-recognin that K63-ubiquitylates Nt-Cys substrates.
Fig. 4.
KCMF1 is oxidative stress-specific E3 ligase responsible for K63-linked polyubiquitin chain ligation. (A) In vitro X-RGS4 (X = V, C, or RCO3) peptide pulldown assay with H2O2 added to lysate with indicated concentrations. (B) ICC of WT, UBR1/UBR2−/−, and UBR4−/− MEFs expressing RGS4MYC and treated with treated with tBHP (250 μΜ, 6 h) and BAF (200 nM, 6 h). (Scale bar, 10 μm.) (C) Quantification of B (n = 50 cells). (D and E) Sequence alignment of the ZZ domain of p62 and (D) KCMF1 and (E) evolutionarily conserved ZZ domain of KCMF1. (F and G) In vitro peptide pulldown assay of X-RGS4 (X = V, or RCO3) 12-mer peptide using (F) KCMF1FLAG transfected or (G) endogenous HEK293T cell lysates added with H2O2 (100 mM). (H) Co-IP of HEK293T cells expressing RGS4MYC treated with tBHP (250 μΜ, 6 h). (I) X-RGS4 and X-nsp4 (X = V, RCO3, or R) peptides were mixed with HEK293T cell lysates transfected with WTFLAG and ZZ domain deficient mutant KCMF1FLAG to pullout recombinant KCMF1 proteins. (J) Denaturation IP of HEK293T cells expressing RGS4MYC and HA-Ub K63 only mutant with KCMF1 siRNA. (K) WB of SH-SY5Y cells with KCMF1 or UBR4 interference and treated with tBHP (250 μΜ, 6 h), BAF (200 nM, 6 h), or both. (L) ICC of KCMF1-interfered SH-SY5Y cells treated with tBHP and BAF (same as K). (Scale bar, 10 μm.)
Next, we characterized the role of KCMF1 in targeting Nt-Cys proteins to autophagy. Denaturation IP assays confirmed that the genetic interference of KCMF1 (Fig. 4J) abolished the conjugation of K63-linked Ub chains on Nt-Cys substrates and their ROS-induced autophagic turnover (Fig. 4K). Colocalization assays revealed that interference of KCMF1 abolished both the formation and subsequent colocalization of recombinant RGS4+p62+ punctate structures (Fig. 4L). In contrast, no such impairment in K63-ubiquitylation was observed in UBR1−/−UBR2−/− cells (SI Appendix, Fig. S5 A and B). These results suggest that KCMF1 is essential for targeting Cys/N-degron substrates to autophagy.
UBR4 Assembles K27-Linked Ub Chains on K63-Ubiquitylated Nt-Cys Substrates.
To identify the E3 responsible for the assembly of K27-linked Ub chains on K63-ubiquitylated Nt-Cys bearing proteins, we screened UBR box proteins. Autophagic flux assays showed that the loss of both UBR1 and UBR2 accelerated the autophagic degradation of Nt-Cys proteins under oxidative stress (Fig. 5A). UBR5-knockdown cells also retained the capacity to target K63-ubiquitylated RGS4 to autophagy (SI Appendix, Fig. S5C). By contrast, UBR4 deficiency did not affect K63-ubiquitylation of RGS4 (Fig. 5 C, lanes 4 vs. 3) but abolished its K27-ubiquitylation (Fig. 5B). Consistently, UBR4−/− MEFs under oxidative stress failed to target RGS4 to p62+ autophagic membranes, which otherwise would form RGS4+p62+ cytosolic puncta (Fig. 4 B and C and SI Appendix, Fig. S4 C and E). As a consequence, lysosomal degradation of Nt-Cys substrates was disrupted in UBR4−/− MEFs (Fig. 5 D and E and SI Appendix, Fig. S5D) as well as UBR4 knockdown cells (Fig. 5F). Thus, UBR4 is essential for the assembly of K27-linked Ub chains on K63-ubiquitylated Nt-Cys proteins.
Fig. 5.
UBR4 is responsible for K27-linked polyubiquitin chain ligation. (A) WB of RGS16MYC transfected in WT and UBR1−/−UBR2−/− MEF cells with tBHP (250 μΜ, 6 h), BAF (200 nM, 6 h) treatment. (B) Denaturation IP of HEK293T cells transfected with HA-Ub-K27 only mutant and RGS4MYC and treated with tBHP (250 μΜ, 6 h), BAF (200 nM, 6 h), or both under UBR4 interference (48 h). (C) Denaturation IP in WT and UBR4−/− MEF cells expressing RCRGS4FLAG and HA-Ub-K63 only mutant and treated with tBHP, BAF, or both (same as A). (D and E) WB of RGS4MYC and RGS16MYC overexpressed in UBR4−/− MEF cells with tBHP (250 μM, 6 h), MG132 (10 μM, 6 h), and BAF (200 nM, 6 h) treatment. (F) SH-SY5Y cells with UBR4 interference (48 h) and tBHP (250 μΜ, 6 h), BAF (200 nM, 6 h) treatment (G) In vitro peptide pulldown assay of X-RGS4 (X = V, or RCO3) 12-mer peptide using endogenous HEK293T cell lysates added with tBHP (250 μΜ). (H) Co-IP of HEK293T cells expressing UBR4V5 and KCMF1FLAG treated with tBHP (250 μΜ, 6 h).
Next, we determined whether UBR4 acts as an N-recognin that directly binds the Arg-CysO3 N-degron of Nt-Cys. In pulldown analyses, UBR4 did not bind Nt-CysO3 carrying RGS4 peptides (Fig. 5G). Instead, UBR4 bound KCMF1 to form a complex (Fig. 5H), suggesting that KCMF1 serves as a scaffold on which UBR4 K27-ubiquitylates Nt-Cys substrates without a direct interaction.
p62 Recognizes K63/K27-Linked Ub Chains to Target Cys/N-degron Substrates to Macroautophagy.
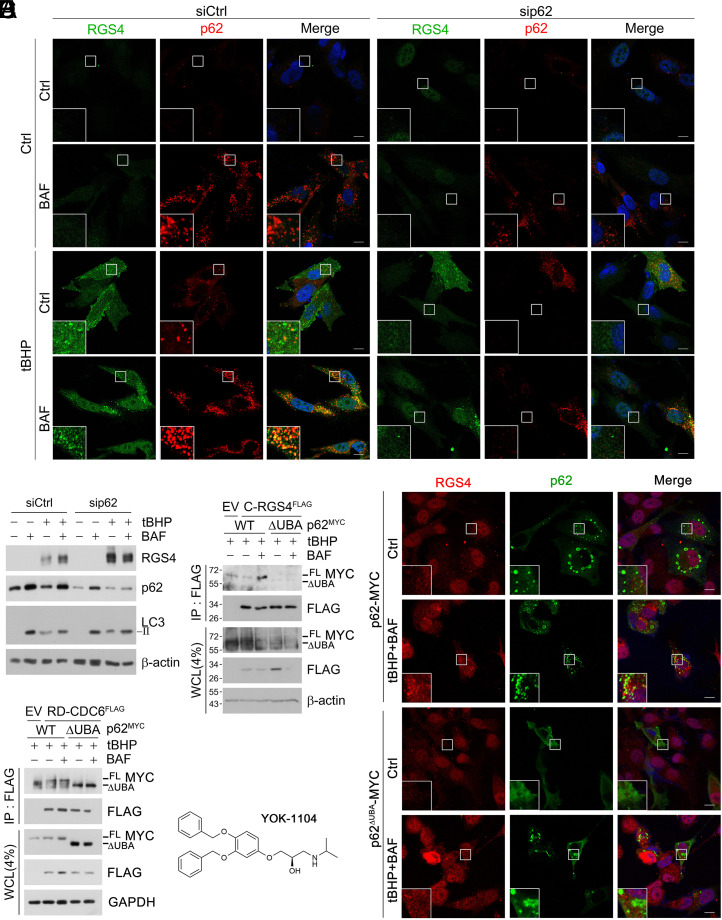
We investigated how ubiquitylated Cys/N-degron substrates are delivered to autophagy. Loss-of-function analyses of candidate proteins revealed that the autophagy receptor p62 is critical for autophagic targeting (Fig. 6A) and degradation (Fig. 6B) of RGS4. Mapping analyses using p62 fragments showed that UBA domain-lacking p62 mutant (ΔUBA) could not bind Nt-Cys-RGS4 (Fig. 6C and SI Appendix, Fig. S6A). Given our earlier finding that p62 is an autophagic N-recognin that binds the Nt-Arg (12, 13), these results suggest that both the Arg-CysO3 and K63/K27-linked Ub chains may synergistically facilitate interaction between p62 and RGS4. This is in direct contrast to non-Cys Arg/N-degron substrates such as RD-CDC6FLAG (Fig. 6D) that bind p62 solely via their ZZ domains (26). We therefore determined whether p62 is essential for targeting Nt-Cys proteins to autophagic membranes. In immunostaining analyses, RGS4 failed to form autophagic puncta in p62 knockdown SH-SY5Y cells as well as p62−/− MEFs expressing p62ΔUBA-MYC (Fig. 6 B and E). These results suggest that p62 delivers K63/K27-ubiquitylated RGS4 to autophagic membranes via UBA domain.
Fig. 6.
p62 targets Cys/N-degron substrates to autophagy via UBA domain. (A) ICC analysis of SH-SY5Y cells under p62 interference and treated with BAF (200 nM, 6 h), tBHP (250 μΜ, 6 h) or both. (Scale bar, 10 μm.) (B) WB with same conditions as A. (C) Co-IP assay of HEK293T cells expressing C-RGS4-FLAG with full-length or ΔUBA p62MYC and treated with BAF, Tbhp, or both (same as A). (D) Same as C but with RD-CDC6FLAG. (E) Same as A but expressing p62 WT and ΔUBA. (F) Chemical structure of the Nt-Arg mimic ZZ-ligand YOK-1104.
We have previous developed a chemical mimicry (YOK-1104; Fig. 6F) of the Arg N-degron that binds and activates p62 as an autophagic receptor (13). To test whether the Arg-CysO3 activates p62 in targeting Nt-Cys proteins to autophagy, cells were treated with YOK-1104. Indeed, YOK-1104 facilitated the cotargeting of RGS4 and p62 to autophagy under oxidative stress, leading to the formation of RGS4+p62+ puncta and their lysosomal degradation (SI Appendix, Fig. S6B). In contrast, YOK-1104 did not alter KCMF1 mediated K63-ubiquitylation status of RGS4 (SI Appendix, Fig. S7A). These results suggest that the Arg-CysO3 binds p62 and facilitates codegradation of p62 in complex with oxidized and ubiquitylated RGS4 via autophagy.
The Cys/N-degron Pathway Is Required for Cellular Homeostasis in Response to Oxidative Stress and Chronic Hypoxia.
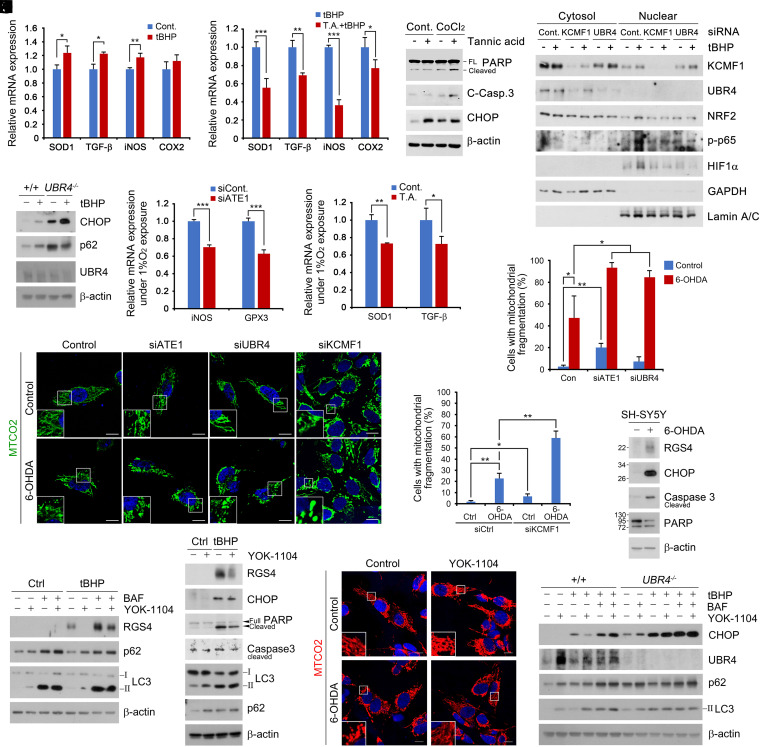
To determine the role of the Cys/N-degron pathway in cellular responses to oxidative stress, we monitored signaling pathways in cells challenged with tBHP. Chemical inhibition of Nt-arginylation using tannic acid significantly impaired the transcriptional activation of oxidative stress-responsive proteins, including SOD1, TGF-β, iNOS, and COX2 (Fig. 7 A and B). Consistently, tannic acid rendered cells hypersensitive to CoCl2-induced oxidative stress, leading to apoptosis as evidenced by the cleavage of caspase-3 and PARP (Fig. 7C). Next, we investigated whether the KCMF1-UBR4 circuit is also required for cellular responses to oxidative stress. Indeed, siRNA-mediated knockdown of either KCMF1 or UBR4 retarded the nuclear translocation of a set of transcription factors sensitive to oxidative stress including NRF2, p-p65, and HIF-1α (Fig. 7D). Such an impairment was associated with hypersensitivity to ROS-induced endoplasmic reticulum (ER) stress (Fig. 7E). Given the results with oxidative stress, we also performed analogous assays in cells under chronic hypoxia. A similar impairment in the transcription of iNOS, GPX3, SOD1, and TGF-β was observed when ATE1 was depleted using siRNA (Fig. 7F) or inactivated using tannic acid (Fig. 7G). These results suggest that the Cys/N-degron pathway is required for cellular responses to oxidative stress.
Fig. 7.
The Cys/N-degron pathway is required for cellular homeostasis in response to oxidative stress. (A and B) qRT assay of HEK293T cells treated with (A) tBHP (250 μΜ, 6 h) and (B) in combination with tannic acid (35 μΜ, 24 h). (C) WB of HEK293T treated with CoCl2 (250 μΜ) in combination of tannic acid (35 μΜ) for 24 h. (D) Nuclear fractionation assay of HEK293T with KCMF1 or UBR4 interference treated with tBHP (250 μΜ, 6 h). (E) WB of WT or UBR4−/− MEFs treated with tBHP (250 μΜ, 6 h). (F and G) Same as A but exposed to (F) chronic hypoxia (1% O2) 48 h (G) with combination of tannic acid (15 μΜ, 48 h). (H) ICC of SH-SY5Y cells with ATE1, UBR4, and KCMF1 interference or treated with CCG-50014 (1 nM, 6 h). (I and J) Quantification of H (n = 50 or 100 cells, respectively). (K) WB of SH-SY5Y cells treated with 6-OHDA (50 μM, 6 h). (L) tBHP (250 μM, 6 h), YOK-1104 (10 μM, 6 h), and BAF (200 nM, 6 h) treatment. (M) WB of SH-SY5Y cells treated with a combination of tBHP and YOK-1104 (same as L). (N) ICC analysis with same conditions as M but with 6-OHDA (50 μM, 6 h). (Scale bar, 10 μm.) (O) WB of WT or UBR4−/− MEFs treated with a combination of tBHP, BAF, and YOK-1104 treatment (same as L).
To further characterize the protective role of the Cys/N-degron pathway in the pathogenesis driven by oxidative stress, SH-SY5Y cells were treated with 6-hydroxydopamine (6-OHDA), a known oxidative stressor that induces mitochondrial damage leading to death of dopaminergic neurons and followed by development of Parkinsonism (27). When monitored using immunocytochemistry analyses, ATE1 knockdown cells exhibited drastically increased mitochondrial fragmentation in response to 6-OHDA treatment, resulting in apoptotic cell death (Fig. 7 H, I, and K). A similar degree of mitochondrial fragmentation and apoptosis was observed in cells deficient in KCMF1 or UBR4 (Fig. 7 H–J). These results highlight the importance of the Cys/N-degron pathway in cellular homeostasis in response to oxidative stress.
To obtain the pharmaceutical means to protect cells from oxidative stress, cells challenged with 6-OHDA were treated with YOK-1104. The chemical activation of p62 ZZ domain stimulated p62-associated autophagic degradation of Cys/N-degron substrates and rescued cells from mitochondrial fragmentation and apoptosis (Fig. 7 L–N). The cytoprotective efficacy was abolished when either macroautophagy or UBR4 was inactivated (Fig. 7O). These results suggest that the ATE1-KCMF1-UBR4-p62 circuit could be a therapeutic target in the pathogenesis driven by oxidative stress.
Discussion
Cellular homeostasis requires the sensing of dynamically fluctuating levels of O2-containing molecules (e.g., O2− and H2O2). In this study, we show that the Cys/N-degron pathway functions as a sensor for both acute and chronic hypoxia as well as oxidative stress. In this bimodular sensing system, the Nt-Cys residues in the presence of O2 is enzymatically oxidized by ADO1 and arginylated into the proteasomal N-degron R-CO2, leading to acute proteolysis via K48-linked ubiquitylation. When cells encounter hypoxia, Cys/N-degron substrates cannot be readily oxidized and metabolically stabilized to adjust signaling pathways. However, if hypoxia is prolonged, the Nt-Cys residue is chemically oxidized by ROS to generate the autophagic N-degron R-CO3 which enables cells to eradicate otherwise nondegradable substrates. Through this dual mechanism, the Nt-Cys residue acts as a common acceptor for O2 and ROS in response to various O2 dysregulations, generating two structurally distinct N-degrons that induce proteolysis via the UPS or autophagy, depending on the nature of O2 stress and duration (SI Appendix, Fig. S8).
In mammals, the HIF-1–based O2 sensing system is known to regulate stress-responsive pathways under hypoxia (4). In this O2 sensing system, metabolically stabilized HIF-1 migrates into the nucleus to induce the transcription of hypoxia-responsive proteins. The resulting heterogeneous nuclear RNAs are processed into mRNAs, which in turn are transported back to the cytosol and used for translation on the ER. The nascent polypeptides discharged from the ribosome are folded, migrate to their designated sites to participate in cellular stress responses. However, this chronic sensor of hypoxia typically requires at least 2 to 4 h for the cell to respond to hypoxia, one outstanding question had been how cells sense and react to rapidly changing O2 levels. Our earlier work has identified the Cys/N-degron pathway as a sensor of O2 and NO, which modulates the half-life of RGS4 through Nt-Cys oxidation under basal condition (17, 18). In this mechanism, RGS4 is “cotranslationally” stabilized under hypoxia and migrates to the plasma membrane, exerting its functions in 2 to 3 min. There is now an increasing consensus that mammalian cells send O2 through the Cys/N-degron pathway and the HIF signaling. In this study, we asked how metabolically stabilized RGS4 can be degraded if hypoxia is prolonged, despite the inability of its Nt-Cys to be oxidized. Our finding that the chemical oxidation of Nt-Cys by ROS under prolonged hypoxia and oxidative stress provides a comprehensive understanding on how cells adopt to the transition of transient hypoxia to chronic hypoxia. Given that the Met-Cys motif represents about 2% of the entire human proteome, the Nt-Cys degron may provide a wide-ranging means for cells to maintain O2 homeostasis.
In mammals, proteins are mainly degraded by the UPS or autophagy. To date, little is known about selectivity in proteolytic flux into either of the two distinct degradative pathways. Our results show that in normoxia, the canonical N-recognins UBR1 and UBR2 preferentially bind the R-CO2, inducing K48-ubiquitylation and proteasomal degradation (11, 15). However, under chronic hypoxia, the R-CO3 is selectively recognized by KCMF1, a noncanonical E3 ligase/N-recognin (Fig. 4). KCMF1 binds R-CO3 through its ZZ domain, which shares sequence similarity with the UBR box of UBR1 and the ZZ domain of the autophagic N-recognin p62. Upon binding to the R-CO3, KCMF1 induces K63-ubiquitylation, followed by UBR4-mediated K27-ubiquitylation. Thus, reprograming of the Ub code from K48-ubiquititination by UBR1 and UBR2 to K63/K48-ubiquitylation by KCMF1 and UBR4 redirect Cys/N-degron substrates from the UPS to autophagy. One remaining question to be addressed concerns the topology of K27, K63, and K48-linked Ub chains assembled on Cys/N-degron substrates. Given that UBR4 is known to assemble branched Ub chains (28, 29), K27-linked Ub chains may conjugate to already assembled K63-Ub chains ligated by KCMF1 as a branched chain conformation. Such possibility of a branched K63/K27-Ub chain may explain our previous finding (13, 26) that p62 functions as an N-recognin only for Nt-Arg-Asp/Glu substrates but not for Nt-Cys substrates, which instead interact with the KCMF1-ZZ domain. It also remains to be determined if the ZZ domain, in not only p62 and KCMF1 but also many other types of proteins, represents a generally applicable stress-specific N-recognin motif for cellular responses to said stresses.
Materials and Methods
Cell culture, immunoblotting, plasmids, reagents, antibodies, RNA interference analysis, peptide pulldown assays, protein degradation assays, immunocytochemistry, digitonin-based fractionation assays, LC/MS analysis, Co/denaturation IP assays, and quantitative real-time PCR assays were performed using standard techniques, which are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT [MSIT]) (Grant NRF-2020R1A5A1019023 to Y.T.K.), the Basic Science Research Program through the NRF funded by the Ministry of Education (Grant NRF-2021R1A2B5B03002614 to Y.T.K.), and Seoul National University Hospital (to Y.T.K.). A.C. was supported by the Dr. Miriam and Sheldon Adelson Medical Research Foundation and the Bio and Medical Technology Development Program (Project No. 2012M3A9B6055305) through the Korean Ministry of Education, Science and Technology, Korea. A.C. is an Israel Cancer Research Fund USA Professor. This work was also supported by an NRF grant funded by the Korea government (MSIT) (Grant NRF-2021R1A2C3004965 to B.Y.K.) and by the Korea Research Institute of Bioscience and Biotechnology Research Initiative Program (Grant KGM5292113 to B.Y.K.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107993118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Ye J., et al. , GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 29, 2331–2336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia D., Shaw R. J., AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 66, 789–800 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greijer A. E., van der Wall E., The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 57, 1009–1014 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G. L., Jiang B. H., Rue E. A., Semenza G. L., Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U.S.A. 92, 5510–5514 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruick R. K., McKnight S. L., A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Epstein A. C., et al. , C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Holmquist-Mengelbier L., et al. , Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10, 413–423 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Chen R., et al. , Reactive oxygen species formation in the brain at different oxygen levels: The role of hypoxia inducible factors. Front. Cell Dev. Biol. 6, 132 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans J. L., Goldfine I. D., Maddux B. A., Grodsky G. M., Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 23, 599–622 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Bachmair A., Finley D., Varshavsky A., In vivo half-life of a protein is a function of its amino-terminal residue. Science 234, 179–186 (1986). [DOI] [PubMed] [Google Scholar]
- 11.Tasaki T., Sriram S. M., Park K. S., Kwon Y. T., The N-end rule pathway. Annu. Rev. Biochem. 81, 261–289 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha-Molstad H., et al. , Amino-terminal arginylation targets endoplasmic reticulum chaperone BiP for autophagy through p62 binding. Nat. Cell Biol. 17, 917–929 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha-Molstad H., et al. , p62/SQSTM1/Sequestosome-1 is an N-recognin of the N-end rule pathway which modulates autophagosome biogenesis. Nat. Commun. 8, 102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji C. H., Kwon Y. T., Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Mol. Cells 40, 441–449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varshavsky A., N-degron and C-degron pathways of protein degradation. Proc. Natl. Acad. Sci. U.S.A. 116, 358–366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tasaki T., et al. , A family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons. Mol. Cell. Biol. 25, 7120–7136 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu R. G., et al. , The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature 437, 981–986 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Lee M. J., et al. , RGS4 and RGS5 are in vivo substrates of the N-end rule pathway. Proc. Natl. Acad. Sci. U.S.A. 102, 15030–15035 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White M. D., et al. , Plant cysteine oxidases are dioxygenases that directly enable arginyl transferase-catalysed arginylation of N-end rule targets. Nat. Commun. 8, 14690 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dissmeyer N., Conditional protein function via N-degron pathway-mediated proteostasis in stress physiology. Annu. Rev. Plant Biol. 70, 83–117 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Kwon Y. T., et al. , An essential role of N-terminal arginylation in cardiovascular development. Science 297, 96–99 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Masson N., et al. , Conserved N-terminal cysteine dioxygenases transduce responses to hypoxia in animals and plants. Science 365, 65–69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs D. J., et al. , Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479, 415–418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weits D. A., et al. , Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 5, 3425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P. C., Sowa M. E., Gygi S. P., Harper J. W., Alternative ubiquitin activation/conjugation cascades interact with N-end rule ubiquitin ligases to control degradation of RGS proteins. Mol. Cell 43, 392–405 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo Y. D., et al. , N-terminal arginylation generates a bimodal degron that modulates autophagic proteolysis. Proc. Natl. Acad. Sci. U.S.A. 115, E2716–E2724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum D., et al. , Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 65, 135–172 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Yau R. G., et al. , Assembly and function of heterotypic ubiquitin chains in cell-cycle and protein quality control. Cell 171, 918–933.e20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtake F., Tsuchiya H., Saeki Y., Tanaka K., K63 ubiquitylation triggers proteasomal degradation by seeding branched ubiquitin chains. Proc. Natl. Acad. Sci. U.S.A. 115, E1401–E1408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.