Significance
Traditionally, fish sex determination was considered to be governed by genetic or environmental factors. However, many teleost species defy this dichotomy. We combined genomic and transcriptomic approaches to characterize the temperature-dependent polygenic sex determination of European sea bass. We observed that the estimated genetic sex tendency (eGST) provides an accurate estimation of the phenotypic sex. Our data support the hypothesis that sexually dimorphic growth is the consequence rather than the cause of sex determination. We also showed that temperature-induced masculinization involves the up-regulation of sox3 and sox9a for individuals in the middle of the eGST distribution. We depict a sex determination system influenced by both continuous genetic and environmental variation that results in variable proportions of males and females.
Keywords: sex determination, genomics, temperature, fish, epigenetic
Abstract
In most animals, sex determination occurs at conception, when sex chromosomes are segregated following Mendelian laws. However, in multiple reptiles and fishes, this genetic sex can be overridden by external factors after fertilization or birth. In some species, the genetic sex may also be governed by multiple genes, further limiting our understanding of sex determination in such species. We used the European sea bass (Dicentrarchus labrax) as a model and combined genomic (using a single nucleotide polymorphism chip) and transcriptomic (RNA-Sequencing) approaches to thoroughly depict this polygenic sex determination system and its interaction with temperature. We estimated genetic sex tendency (eGST), defined as the estimated genetic liability to become a given sex under a liability threshold model for sex determination, which accurately predicts the future phenotypic sex. We found evidence that energetic pathways, concerning the regulation of lipids and glucose, are involved in sex determination and could explain why females tend to exhibit higher energy levels and improved growth compared to males. Besides, early exposure to high-temperature up-regulated sox3, followed by sox9a in individuals with intermediate eGST, but not in individuals showing highly female-biased eGST, providing the most parsimonious explanation for temperature-induced masculinization. This gonadal state was maintained likely by DNA methylation and the up-regulation of several genes involved in histone modifications, including jmjd1c. Overall, we describe a sex determination system resulting from continuous genetic and environmental influences in an animal. Our results provide significant progress in our understanding of the mechanisms underlying temperature-induced masculinization in fish.
Sex determination is a central biological process with consequences relevant for natural population dynamics and livestock production. A plethora of systems, from purely genetic sex determination (GSD) to environmental sex determination (ESD), have been described in the animal kingdom (1). Sex determination involves the interaction of promale and profemale genetic pathways in birds and mammals (1), but interestingly, those pathways are often impacted by various environmental factors in reptiles and fish (2, 3). Undoubtedly, fishes represent the taxon exhibiting the widest diversity of sex determination systems (4), in which biotic (e.g., density) (5) or abiotic factors (e.g., pH and temperature) (6, 7) can interact with, or even override, the genetic background of sex.
These external factors affecting the sex of individuals are then transduced at the physiological level in different manners, depending on the species. Yet, two main routes have been identified in fishes, one involving the stress-axis pathway (8) and another one involving epigenetic mechanisms (differential methylation or histone modification) (9). Interestingly, the latter seems more conserved in reptiles (10, 11) when compared to the former (12). Temperature, the most studied environmental factor affecting fish sex, has been shown to either increase cortisol production (the main stress hormone) with cascading effects on sex (13) or to change methylation profiles in the promoters of key genes mostly involved in sex differentiation (9).
Temperature-dependent sex determination (TSD) has been detected in various fish species including the Atlantic silverside (Menidia menidia) (3), the Nile tilapia (Oreochromis niloticus) (14), the olive flounder (Paralichthys olivaceus) (15), the African spiny catfish (Clarias gariepinus) (16), the pejerrey (Odontesthes hatcheri), or the cobaltcap silverside (Hypoatherina tsurugaen) (17). In these species, natural temperatures within the thermal range of what fish usually encounter in the wild can impact sexual fate. Moreover, even in species with a supposedly strong GSD, extreme water temperatures outside the natural thermal range can sometimes override their sex determination pathway (18–20). In most of the above-mentioned species, sex reversal (usually from female to male) induced by temperature fluctuations is relatively easy to detect since the genetic sex can be identified either at the gene (21–25) or at the chromosome level (26, 27), which enables the investigation of the underlying physiological mechanisms of sex reversal on an individual basis. Identifying cases of sex reversal becomes much more complicated when species exhibit a polygenic sex determination system (28). In such instances, each individual presents a specific combination of promale and profemale genes involved in sex determination resulting in a genetic sex tendency (GST), defined as the genetic liability to become a male or a female under a liability threshold model (29) for sex determination. This GST is by definition continuous, as opposed to the dichotomic pattern found in species with a master sex–determining gene, in which the presence or the absence of such gene governs sex at conception.
The European sea bass (Dicentrarchus labrax) is a gonochoristic species that possesses a GST whereby the genetic architecture (likely involving many genes) interacts with temperature during a labile period where sex can be altered before the sexual fate of the gonad is definitively fixed (30–32). The labile period encompasses the larval and the juvenile stages (SI Appendix, Fig. S1). Thus, as it occurs in many other fish species, exposure to relatively high temperature (HT) (>17 °C) during the larval stage promotes male differentiation (30–32). However, long-term exposure to relatively low temperature (LT) (<17 °C) before gonadal sex differentiation is complete (i.e., during the juvenile stage) can also trigger masculinization (31, 33). In the European sea bass, future females are already bigger when compared to future males (34), and it has been shown that sex-related differences in growth are established well before the appearance of the first currently known molecular markers of sex (34). However, whether being female induces enhanced growth rate or, conversely, if high early growth rate promotes feminization needs to be further studied.
The fact that this species possesses a polygenic sex determination system, where temperature also influences sex determination, has complicated the studies aiming at deciphering the underpinning mechanisms. Indeed, environmental effects have commonly been detected at the group level (35–37) or after the labile period (36), and genetic effects are deduced from the propensity of specific parents to produce a biased sex ratio (32, 38). While these earlier studies have improved our knowledge of the potential mechanisms involved, they did not allow the identification of the earliest molecular signs of environmental effects, when the gonad is not yet differentiated, even at the molecular level.
Here, we took advantage of the recently developed 57K single nucleotide polymorphism (SNP) chip in the European sea bass (39) to determine the estimated genetic sex tendency (eGST) of individuals, exposed to either high or low temperatures. A prediction equation for eGST can be obtained by combining multilocus SNP genotypes and sex phenotypes in a training population and then this equation can be used to estimate the eGST of fish for which only the SNP genotype is available, in a genomic evaluation framework (40). We predicted that the sex of individuals at both extremes of the eGST distributions would not be impacted by temperature, while those exhibiting intermediate values would be sensitive to temperature. To test this hypothesis, individually genotyped fish were sampled at four key time points during the labile period (SI Appendix, Fig. S1). We then used RNA-Sequencing (RNA-Seq) approaches, both at the whole-body and at the gonadal level, during gonadal sex differentiation. Based on the transcriptomic analysis of individually genotyped fish, we combined gene expression data with sex prediction through eGST data, enabling the investigation of the pathways involved in sex determination of the European sea bass at an early stage. Furthermore, our experimental design allowed us to test the overlooked hypothesis that masculinization by elevated temperature may result from sex-specific mortality of females rather than from induced female-to-male sex reversal, which could not be tested previously, as mortality in larval temperature treatments occurs before any phenotypic or molecular difference between sexes is visible.
Results
Sex Ratio Analysis of Control and HT-Exposed 1-y-Old Fish Validates the eGST Model.
In individuals reared at 21 °C (HT, n = 493) from 8 to 390 d post hatching (dph), the sex ratio was highly biased toward males (75 ± 8.5% males). On the contrary, those fish that were kept at 16 °C (LT, n = 537) from 2 to 59 dph before being switched to 21 °C had a more balanced sex ratio (46.5 ± 1.4% males), confirming a strong effect of early exposure to HT on the sex of European sea bass (z-value = −6.3, P value < 0.001). When combining genomic relationships with the phenotypic sex in a single trait threshold model, where low and high temperatures were considered a fixed effect, sex was found to be highly heritable, with a heritability estimate of h2 = 0.56 ± 0.06. In the multitrait analysis, where sex in each temperature treatment was considered a specific trait, both sex at LT (sex_LT) and at HT (sex_HT) were also found highly heritable: h2sex_LT = 0.65 ± 0.06 and h2sex_HT = 0.51 ± 0.08, with a strong genetic correlation between both temperature-specific sex traits, rG = 0.91 ± 0.09. From a leave-one-out cross-validation approach, where we predicted the sex of an individual based on a genomic prediction equation established without providing information on its phenotype, the genomic prediction successfully classified animals in 74.5% of the cases for the fish reared at LT and 72.5% for the fish at HT, based on the area under the curve values of the receiver operator characteristic curves (SI Appendix, Fig. S2). Importantly, we did not detect any skew in the distribution of eGST over time (randomly sampled at the four time points, SI Appendix, Fig. S1) when comparing one temperature to the other (SI Appendix, Fig. S3), emphasizing that the skew in sex ratio observed at high temperatures is due to sex reversal rather than to genotype-specific mortality. Based on the observed sex ratio of 1-y-old fish, we predicted that about 25% of the whole population had sex reversed at HT and that this would likely concern those individuals with an eGST lower than 0.5 (i.e., “weak” genetic females exhibiting male sex differentiation at HT, reference SI Appendix, Fig. S4).
A genome-wide association study identified five genomic regions explaining more than 2% of the total genetic variance of the GST (SI Appendix, Fig. S5), which we considered as putative quantitative trait loci (QTL). The region with the highest association with sex was in LG7, between positions 6.3 and 6.8 Mb (QTL_LG7) with a 8% variance explained. Two other regions in LG19 in the region 6.6 to 7.3 Mb (QTL_LG19a) and in the region 17.0 to 17.9 Mb (QTL_LG19b) explained 3.2 and 2.7% of the genetic variance, respectively. In LG13, the region 24.0 to 24.6 Mb (QTL_LG13) explained 2.3% of the genetic variance. A last region in LG1A (25.7 to 26.3 Mb, QTL_LG1A) explained 2.1% of the genetic variance for GST.
Sox Genes and Genes Linked to Histone Modification Processes Are Affected by HT in Fish with Intermediate eGST Values at the “Flexion” Stage.
Whole-body RNA-Seq was performed at the “flexion” stage (25 to 40 dph), coinciding with the early stages of the labile period for sex determination (SI Appendix, Fig. S1). At this stage, only one germ cell per future gonad is observable in transversal fish sections (41). A total of 10 individuals (5 per treatment) were selected for their intermediate, though positive, eGST. The DESeq2 analysis highlighted 341 differentially expressed genes (P value < 0.05) between HT and LT individuals. The gene ontologies (GOs) response to steroid hormone and cellular response to steroid hormone stimulus were among the biological processes up-regulated at HT (SI Appendix, Fig. S6). Specifically, we found that two sox (Sry-related HMG box) genes classically involved in sex determination and differentiation, sox3 and sox9b, were up-regulated at HT (Fig. 1 A and B). Seven genes were linked to histone modification processes, two of them up-regulated at HT (ncoa6 and lpin1) and five up-regulated at LT: ube2a, zbtb7b, setd5, suz12, and auts2.
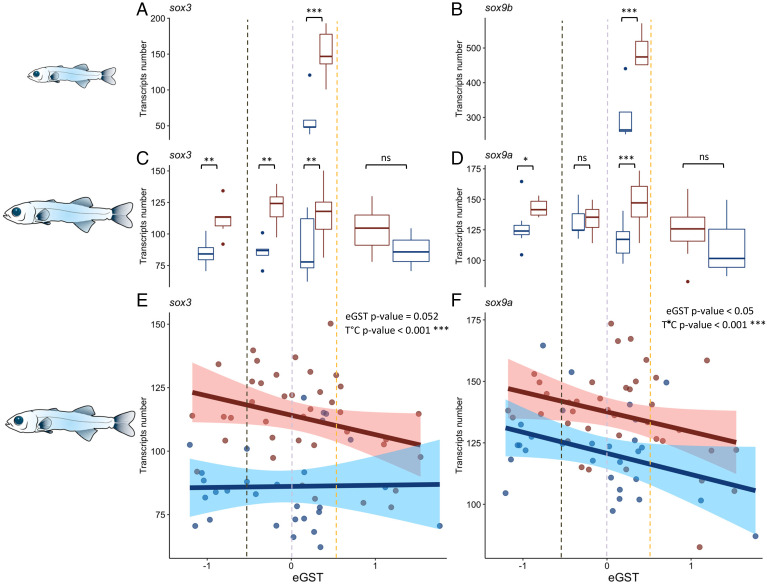
Fig. 1.
Both (A) sox3 and (B) sox9b were differentially expressed following the DeSeq2 analysis between neutral individuals (0 < eGST < 0.5) at the “flexion” stage kept at HT (in red) and LT (in blue). The number of RNA-Seq transcripts of (C) sox3 and (D) sox9a differed according to the temperature between groups of eGST < −0.5, −0.5 < eGST < 0, 0 < eGST < 0.5, and eGST > 0.5 in fish sampled at the “all fins” stage following the DeSeq2 analysis. The RNA-Seq analysis at the all fins stage revealed an overall negative and significant correlation between (E) sox3 and eGST and (F) sox9a and eGST, as well as a temperature effect. Abbreviations: *** = P value < 0.001; ** = P value < 0.01, * = P value < 0.05; ns = not significant.
Sox Genes and Genes Linked to Energy Regulation Correlate with eGST at the “All Fins” Stage.
Whole-body RNA-Seq was performed at the “all fins” stage (53 to 78 dph period) on 68 individuals (29 LT and 39 HT). This period coincides with rapid primordial germ cell proliferation (41). Among the 17,303 genes that respected our inclusion criteria (>30 reads per gene), we detected 584 genes for which expression was correlated with the eGST (eGST P value < 0.05; eGST × T (°C) > 0.5). In total, 12 and 2 genes, respectively (SI Appendix, Table S1), were part of the GOs sex differentiation and sex determination, with sox9a and sox3 among them (P value = 0.052) (Fig. 1 E and F). For both these genes, we also detected a strong temperature effect on transcript number (P < 0.001), with a higher number of transcripts at HT compared to LT (Fig. 1 C and D).
In total, 16 genes were involved in the GO lipid biosynthetic process and 19 in the GO regulation of growth (SI Appendix, Table S1). The gene encoding the growth hormone (gh) was one of these genes and was positively and significantly correlated with the eGST. Three other genes (prkca, gfi1b, and eya2) that are in close vicinity of the three previously detected QTL (QTL_LG7, QTL_LG19b, and QTL_LG1A) exhibited a significant correlation with the eGST, though their expression was independent of their SNP genotype of the QTLs (AA, AB, or BB).
The “response to glucose” was among the biological processes presenting a positive correlation with the eGST and thus more expressed in females (SI Appendix, Fig. S7). A total of 11 genes involved in the GO histone modification were also significantly correlated to the eGST (SI Appendix, Table S1). Interestingly, the genes from the GO “histone H3-K27 methylation” and “histone H3-K4 methylation” were negatively correlated with the eGST (thus more expressed in males; SI Appendix, Fig. S7).
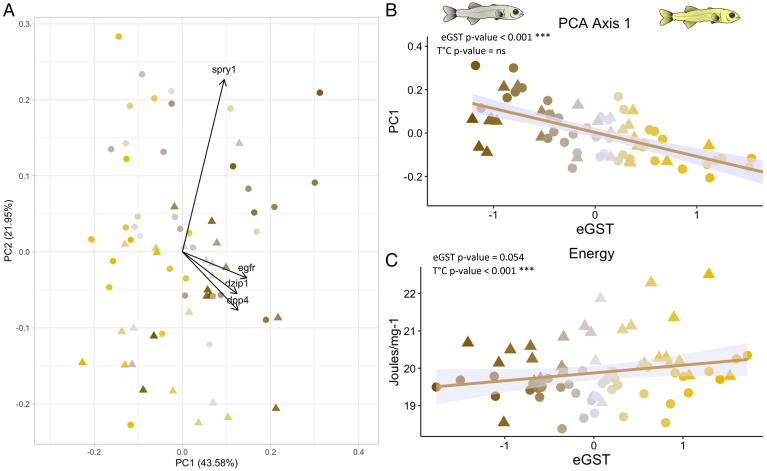
Using a more stringent significance threshold (P value < 0.001), four genes (spry1, egfr, dpp4, and dzip1) were correlated to the eGST. Only the gene encoding the Daz interacting protein 1 (dzip1), involved in spermatogenesis, showed a clear dimorphic expression higher for individuals with negative eGST. The three other genes have a role in growth rate (Epidermal growth factor receptor, Egfr; Sprouty rtk signaling antagonist 1, Spry1) and glucose (The dipeptidyl-peptidase IV, Dpp4) regulation (based on their GO). The first axis of a Principal Component Analysis (PCA), representing these four genes (Fig. 2A), was highly correlated to the eGST (Fig. 2B). With a quadratic model, only seven genes (SI Appendix, Table S1) showed both an overall linear relationship with eGST (P < 0.01) and significant interaction with temperature, revealed by a temperature-specific quadratic component (P value < 0.05). Three of these genes were involved in epigenetic processes: sgsm2, entpd2, and map3k3.
Fig. 2.
(A) PCA of four genes (dzip1, dpp4, egfr, and spry1) having a highly significant (P value < 0.001) and linear correlation with the sex tendency (eGST) and detected from the RNA-Seq analysis of whole individuals at the “all fins” stage. (B) The first component axis strongly correlated to the eGST. (C) The energy content (joules ⋅ mg−1 of tissue) of fish sampled at the “all fins” stage correlated positively with eGST so that genetic females displayed slightly higher energy content than males. Fish kept at low temperatures also displayed higher energy content than those kept at HT. Individuals are represented with a color gradient, from maroon to yellow, representing their eGST. Circles represent fish kept at HT (HT = 21 °C; n = 39) and triangles those kept at LT (LT = 16 °C; n = 29). ns = not significant.
The Juvenile Gonadal Transcriptome Faithfully Reflects the Underlying eGST Independently of Temperature Influences.
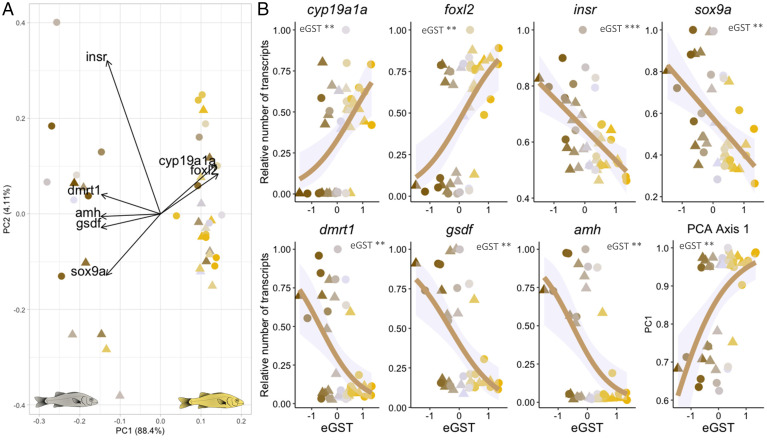
RNA-Seq was performed on total RNA extracted from the gonads of 42 individuals (21 HT and 21 LT) sampled at the juvenile stage (117 to 124 dph), before the first signs of morphological sex differentiation (SI Appendix, Fig. S1). Among the 15,724 genes that respected our inclusion criteria, 1,297 showed a significant (P value < 0.01) linear correlation, either positive or negative, between their expression level and the eGST, independently of the initial temperature treatment (HT versus LT). Among those genes, 19 and 6 genes (SI Appendix, Table S3) were within the GOs of sex differentiation and sex determination, respectively, including cyp19a1a (gonadal aromatase), foxl2 (forkhead box l2), dmrt1 (doublesex and mab-3 related transcription factor 1), gsdf (gonadal soma derived factor), amh (anti-Müllerian hormone), sox9a (sry-related HMG box 9a), and insr (insulin receptor). Those genes, well described to be involved in sexual development, allowed to distinguish two groups on the first axis of the PCA: the differentiating males as opposed to the differentiating females (Fig. 3A). As expected, the correlation was positive for genes involved in ovarian development and negative for those involved in testis development (Fig. 3B and SI Appendix). This was confirmed by genes involved in the GO steroids metabolic process, namely, hsd17b1, cyp26a, and 3β-hsd (SI Appendix, Fig. S8).
Fig. 3.
(A) PCA of seven genes involved in sex determination and differentiation. Data are from the RNA-Seq analysis of the gonads of fish at the juvenile stage (n = 42). The PC1 separated the sex horizontally and explained 88.4% of the variance. The PC2 separated the variables vertically and explained 4.1% of the variance. The contribution of the variables (genes) are represented by the arrows. (B) Significant (P < 0.01) linear correlation between the eGST and both insr and sox9a (relative number of transcripts on the y-axis). For five genes, cyp19a1a, foxl2, dmrt1, amh, gsdf, and the PC1 axis, a dichotomic distribution was observed and modeled with a “quasibinomial” function. Circles represent fish kept at HT (HT = 21 °C; n = 21) and triangles those kept at LT (LT = 16 °C; n = 21). Individuals are represented with a color gradient, from maroon to yellow, representing their lower or higher eGST. Abbreviations: *** = P value < 0.001; ** = P value < 0.01.
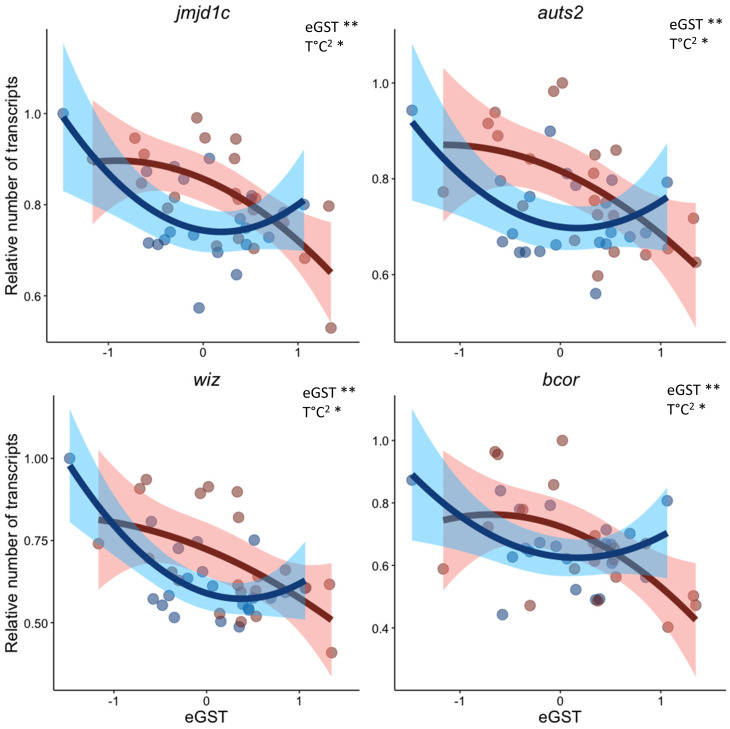
Overall, this allowed ascertaining the high relevance of the GST estimated with the Gibbs model (eGST), especially for individuals at both extremes of the distribution, independently of the temperature. Our results were further validated at the group level (eGST > 0 = genetic females versus eGST < 0 = genetic males) with DESeq2 on the GO of sex determination (SI Appendix, Fig. S9). A total of 52 genes involved in the GO histone modification were also significantly correlated to the eGST (SI Appendix, Table S2), which was confirmed with the “without a priori approach” showing that genes involved in histone methylation and acetylation were also up- or down-regulated in differentiating gonads (SI Appendix, Fig. S10). Interestingly, other epigenetic processes such as those involved in the microRNA (miRNA) production, were negatively correlated with the eGST and thus positively with maleness (SI Appendix, Fig. S10). With the quadratic model used for detecting changes linked to the temperature in the middle of the eGST distribution, only seven genes (thop1, paxip1, sik3, jmjd1c, bcor, wiz, and auts2) showed both an overall linear correlation with eGST (P < 0.01) and a significant interaction with temperature for the quadratic term (P value < 0.05). Four of these genes are involved in epigenetic processes: jmjd1c, bcor, wiz, and auts2. The expression of these four genes increased in individuals with an eGST in the middle of the distribution and that were reared at HT, which are the ones with a weak GSD that are expected to be more influenced by the environment (Fig. 4).
Fig. 4.
Quadratic correlation between the eGST and genes involved in histone modification, detected from the RNA-Seq analysis of the gonads of fish at the juvenile stage. The four genes exhibit a significant (**= P value < 0.01) linear correlation with the eGST, plus a significant (* = P value < 0.05) interaction with the temperature for the quadratic term (T °C2). Red and blue points represent respectively fish kept at HT (HT = 21 °C; n = 21) or LT (LT = 16 °C; n = 21).
DNA Methylation Levels of 1-y-Old Fish Gonads.
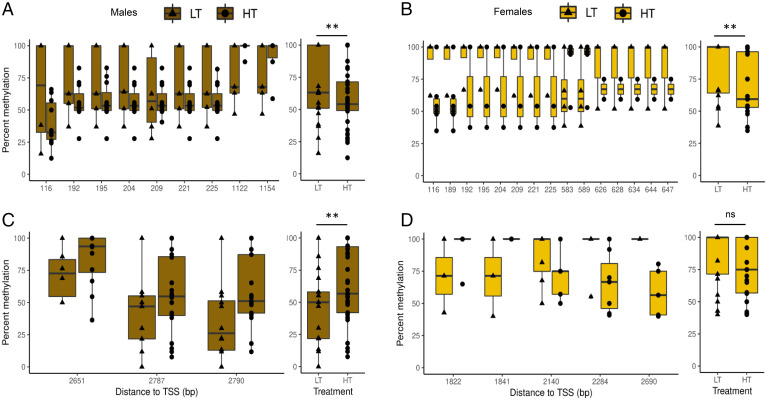
Reduced representation bisulfite sequencing (RRBS) was conducted at the 1-y-old fish stage using gonadal tissue from 65 males and 42 females. The statistical analysis of methylation data showed several differentially methylated cytosines (DMCs) between fish reared at LT and HT in sox3 and sox9a genes (Fig. 5). For sox3, there was a decrease of methylation levels at HT in both males (P = 0.01844) and females (P = 0.0001636). In males, this gene showed seven hypomethylated DMCs in the first exon, close (<200 base pair) from the transcription start site (TSS; Fig. 5A). In the females, the same positions were hypomethylated in the first exon, with a total of up to 15 DMCs detected, among which two of them, found around 600 bp from the TSS, were hypermethylated (Fig. 5B). The methylation levels of sox9a showed an increase at HT in males (P value = 0.01069) but no significant difference in females (P value = 0.1866) between LT and HT (Fig. 5C). In males, there were three hypermethylated DMCs toward the end of the gene body (Fig. 5C). However, three out of the five DMCs identified in this region were hypomethylated in females at HT (Fig. 5D).
Fig. 5.
Box plots of DNA methylation levels of sox3 (A and B) and sox9a (C and D) in 1-y-old fish testes (maroon) and ovaries (yellow), respectively. Individual DMCs identified within the gene region (Left), and average methylation levels of the gene body ± 2,000 bp (Right). The black line within the box indicates the median of the distribution, and the lower and upper hinges display the distribution of values between the first and third quartiles. The upper whisker extends to the maximum value (1.5 * interquartile range [IQR]), and the lower whisker extends to the minimum value (1.5 * IQR). Individual DMCs are defined as CpGs with methylation differences >15% and q-value <0.01, while significant differences between average data were assessed with the t test. Abbreviations: ** = P value < 0.01; ns = not significant. Circles represent fish kept at HT (HT = 21 °C; n = 65) and triangles those kept at LT (LT = 16 °C; n = 42).
Gonadal Histology.
The sampling at the juvenile stage (117 to 124 dph; n = 10 fish per temperature) confirmed that gonads were still not morphologically differentiated. Nevertheless, some oocytes were sparsely observable, but it was impossible to conclude with confidence on the actual phenotypic sex of individuals based on histological analyses alone. Furthermore, the number of oocytes was not correlated to the eGST (data not shown).
Relationship between Energy Content, Body Size, and eGST.
The energy content ( joules ⋅ mg−1) was not significantly correlated to the eGST at any stage, though it almost reached significance (P = 0.054) at the “all fins” stage, with genetic females tending to have higher values than males regardless of their size (Fig. 2C). Individuals from LT presented significantly (P value < 0.001) higher energy content than those from HT at the “all fins” stages (Fig. 2C), likely because LT fish were older. The size of fish was not correlated to the eGST (length: t-value = 1.56, P value = 0.13; wet weight: t-value = 1.3, P value = 0.2), while there was an effect of temperature (length: t-value = 4.6, P value < 0.001; wet weight: t-value = 4.75, P value < 0.001).
Discussion
Our analysis combining genomic and transcriptomic data allowed us to shed light on the mechanisms involved in temperature-induced masculinization of the European sea bass. The results confirm the high heritability of the GST. Furthermore, the high genetic correlation between sex_LT and sex_HT suggested low genotype-by-temperature interaction. In other words, the ranking of animals based on their eGST remains very similar at least across the two tested thermal environments. Hence, the effect of larval rearing temperature on sex was mostly additive. This appears to contradict previous results where such interaction occurred (31, 38). However, these previous results were obtained using between-family variation, while we used a genomic relationship matrix for the present study, which is expected to accurately estimate the true genetic parameters (42). Note that the low genotype-by-environment interaction for GST between the two temperature treatments accounts for what happens globally. But it does not impede local G × E interaction occurring at the genes level or the existence of G × E interactions in other populations and/or under other environmental circumstances. The prediction equation for GST, which allowed us to estimate the eGST of genotyped individuals, was established on phenotypically sexed individuals at 1 y of age and predicted their sex with a 72.5 to 74.5% success. The relevance of eGST was further confirmed by transcriptomic analysis of gonads at 117 and 124 dph, that is, when the first signs of molecular differentiation can be identified (43). Indeed, we detected a strong correlation between key genes involved in sex differentiation in the gonad and the eGST at this juvenile stage. This good match between phenotypic and genetic sex allowed us to determine the eGST of 1- to 3-mo-old individuals, when the temperature is known to act on the sex of European sea bass (30). Five putative QTLs, explaining a low but significant part of the variance of GST, were identified in four different chromosomes (LG7, LG19, LG13, and LG1). Faggion et al. (44) already identified QTL_LG7 in Northern Atlantic and Mediterranean populations of European sea bass and the two LG19 QTLs in Mediterranean populations only (origin of the present population). The minor QTLs found in LG13 and LG1A were, however, specific of this study. None of the QTLs previously found in LG6, LG11, and LG18-21 (45) were detected in the present study. Overall, our results are consistent with those of previous studies, pinpointing a GST strongly driven by polygenic variation (∼90% of the variance) with a low contribution of minor QTLs (∼10%).
These QTLs however participate to the accuracy of the model, which appeared to be strong (100%) for individuals at both extremes of the eGST distribution. However, some mismatches were detected in the middle of the distribution, with some individuals with low negative eGST value that likely were phenotypic females (15% at HT and 29% at LT) based on the dichotomic expression of cyp19a1a, foxl2, dmrt1, gsdf, and amh and some individuals with positive eGST values that were likely phenotypic males (25% at HT and 14% at LT). The proportion of fish that are supposedly genetic females, but that exhibit a male phenotype (25%), could well be explained by precocious and relatively long-term exposure to HT (31, 46, 47). It also corresponds well to the supposed percentage of masculinized genetic females observed at the end of the experiment: 25%, a figure within the range of masculinization typically observed in European sea bass exposed to HT (48). The mismatch occurring at LT (14%) could also be due to the masculinization of fish kept too long at this temperature, since there is what could be regarded as a second period of sensitivity to adverse environmental conditions, including prolonged exposure to LT, in European sea bass (31, 37, 46). However, temperature itself might not explain the pattern detected for individuals that are supposedly genetic males. This could come from the fact that some errors occurred in the estimation of eGST (as detected in 1-y-old fish, with the leave-one-out approach), which is expected with a polygenic trait that typically has incomplete penetrance, when heritability is lower than 1, which is the case here (49, 50). It could also be that phenological events linked to gonad development are involved. In the European sea bass, the sex is considered “fixed” once animals reach a size of 8 to 10 cm according to some studies (41) but at a size of 4 to 6 cm according to others (34). It is thus possible that individuals in the middle of the distribution can still develop a phenotypic sex not predicted by their eGST, according to the polygenic nature of sex determination in this species, and that their observed transcript values of sex-related genes remain transitory at this stage/age (7.2 cm and 4.5 g). In this sense, the histological analysis did not permit to unambiguously identify males and females at the early juvenile stage, but some intratesticular oocytes were detected in some individuals, showing that they probably had undergone sex reversal (51). In European eels (Anguilla anguilla), individuals presenting intratesticular oocytes showed higher levels of cyp19a1a than males (52). It is thus possible that the presence of both tissues in the same gonad drives the mismatch observed between transcript values of sex-related genes and eGST for intermediate individuals. A last plausible explanation involves the sampling design at this stage for transcriptomic analysis. To ensure having sufficient tissue quantity (>100 ng total RNA), we sampled the biggest fish. Since early growth rate is known to impact sex, it is possible that some of the genetic males (eGST < 0) developed as phenotypic females at this stage.
Interestingly, the goodness of the linear fit between genetic and phenotypic sex was confirmed by the gene expression of sox9a and insr, even when considering individuals in the middle of the eGST distribution and at both temperatures. These two genes are within the GO of sex determination and play a key role in male sex differentiation (1, 53–55). None of them were pinpointed as essential for sex differentiation in previous studies on European sea bass (35, 43, 56). However, both genes are known to be overexpressed at high temperatures in TSD reptile species during sex determination (57–59). The fact that both genes present a very linear correlation (as opposed to the dichotomic expression of cyp19a1a, foxl2, dmrt1, gsdf, and amh) with the eGST suggests that they are involved early in the process of sex determination, while the other sex-related genes just transduce the future state of the gonad (male or female). This was confirmed at the “all fins” stage with sox9a exhibiting a negative correlation with the eGST, with higher expression at high temperatures for neutral fish. This gene was also shown to play a key role in the ovary-to-testis transition in the zebrafish (60), where it was strongly expressed in pre-Sertoli cells prior to oocyte apoptosis and degeneration. The DNA methylation levels of sox9a increased with high temperatures in testes of 1-y-old fish. This suggests that HT could affect sox9a gene expression already at the “flexion” stage and maintain its state through to adulthood in males. However, the higher expression of this gene found at HT for neutral eGST fish and the hypermethylation detected at HT in males would not match the standard association of hypermethylation with down-regulation of gene repression (61). This could be explained by the fact that the DMCs were identified toward the end of the gene body, while it is the methylation level of the first intron, and to a lesser extent, the first exon, which was shown to play an important role and inverse association with gene expression regardless of tissue and species (62).
Another gene that was also reported as a marker of germ cells and supporting cells that preferentially develop into testes in zebrafish juvenile ovary-to-testis transformation (60) was dzip1, which is concordant with our results where dzip1 shows a clear dimorphic expression at the “all fins” stage, predominantly at HT. Three other genes were strongly correlated with the eGST at this stage, namely, egfr, dpp4, and spry1. The specific function of these genes has not been described in fish, and much of our knowledge comes from murine models and humans. Sprouty1 (Spry1 gene product) is involved in fat and bone development (63). Spry1 gene knockout in mice adipocytes results in decreased bone mass and increased body fat (63), while adipose tissue-specific expression of the Spry1 gene in mice protects against fat accumulation and bone loss (64). EGFR is involved in protein kinase activity, and the inhibition of such protein was shown to improve glucose tolerance and favor insulin action (65). Interestingly, its expression was shown to be regulated by spry1 (66). DPP4 is also involved in glucose homeostasis, and targeted inactivation of this gene in mice yielded individuals with enhanced insulin secretion and improved glucose tolerance (67). All those studies advocate for genetic males having less capacity to produce fat and display appropriate glucose levels. Although this could be viewed as only a conjunction of facts, this hypothesis is enforced by the tendency of genetic females (individuals with high eGST values) to display higher energy content in their tissue (in joules ⋅ mg−1) when compared to males (individuals with low eGST values) at the “all fins” stage. At this stage (53 to 78 dph), no correlation was found between size and eGST, and the earliest sexual size dimorphism was found at 103 dph in another experiment (68). Overall, all these results support the hypothesis that early growth differences are the consequence rather than the cause of sex differentiation. In that scheme, the polygenic sex determination of the European sea bass provides information that is later transduced at the phenotypic level, as exemplified by the positive correlation between eGST and the gh gene at the “all fins” stage.
Regarding the role of temperature, at both the “flexion” and the “all fins” stages we detected enhanced expression of sox3 at an HT compared to an LT, while this was not detected for genetic females (eGST > 0.5) at the “all fins” stage following transcriptomic analysis. Sox3 is the evolutionary precursor of Sry (sex determining region Y) in mammals (69) and a major master-sex determination gene in three medaka fish species (70). Interestingly, ectopic expression of Sox3 in the developing XX gonads resulted in the complete sex reversal of XX females to males in mice (Mus musculus) (71), and loss-of-function of sox3 caused sex reversal of XY males in the Indian rice fish (Oryzias dancena) (72). The expression of sox3 also gradually increased during the protogynous sex change (female-to-male) of the hermaphroditic fish (Epinephelus coioides) (73). This gene is essential in the up-regulation of downstream key-related genes for testicular differentiation, gsdf in the Indian rice fish (72) and sox9 in mice (71). Here, we also detected that up-regulation of sox3 appeared chronologically before the up-regulation of sox9a suggesting similar mechanisms. Sox3 was strongly hypomethylated at HT in both males and females. These methylation levels were very close (<200 bp) from the TSS and within the first exon. The methylation and gene expression data together suggest that HT could affect sox3 expression at the flexion stage through DNA hypomethylation-mediated unlocking of gene expression and continuing this state to adults through mitosis. The hypomethylation levels found in this gene at 1-y-old gonads and the higher levels of expression of this gene at HT found at earlier stages match the standard negative correlation between DNA methylation and gene expression (61). It is highly difficult to assess whether the observed methylation changes are the cause or the consequence. However, DNA methylation is known to contribute to the acquisition and maintenance of cell identity, making it possible that changes in DNA methylation during sex differentiation contribute to stabilize the gene expression program of each sex. Regarding the exact function of sox9a and sox3, only a proper experiment involving the knockout of these genes would allow to fully understand their role in temperature-induced masculinization.
Regarding other epigenetic mechanisms, genes from the “histone modifications” GO term were always differentially expressed in all our analyses, confirming their implication in the sex-specific response to temperature (74). However, the specific up-regulation of DNA methyltransferases, which are key in the regulation of DNA methylation, was never detected. This was unexpected owing to the previous demonstration of specific methylation of promoters of both cyp19a1 and dmrt1 in males and females of European sea bass, respectively (9, 36). Indeed, epigenetic reprogramming mediated by changes in sexually dimorphic DNA methylation has been suggested to be a key mechanism in the determination of sexual fate in sexually isogenic species (75, 76). Furthermore, we found that key Jumonji family (Jmj) gene jmjd1c, involved in histone demethylation at the H3K9 site, was up-regulated in male gonads compared to female gonads. Additionally, jmjd1c expression was highly impacted by temperature in individuals in the middle of the eGST distribution, with up-regulation in the gonads of HT-exposed individuals and down-regulation in those exposed to LT. Interestingly, this signal was still detectable in gonads of LT fish even after the LT treatment had ended (e.g., fish from the LT treatment were at 21 °C from day 60). It has been hypothesized that temperature may reset epigenetic marks thus redirecting sexual fate (1), supporting our observed results. In certain reptilian species, some specific and related histone demethylases (JMJD3, JARID2, and KDM6B) were shown to be crucial in the shaping of the gonadal phenotype during temperature-induced sex determination (10, 77). Furthermore, although the results presented here are not sufficient to infer the functionality of genes bcor, wiz, and auts2, this study suggests that these genes could be involved in the transduction of temperature signals influencing sexual fate in teleosts, which warrants further examination.
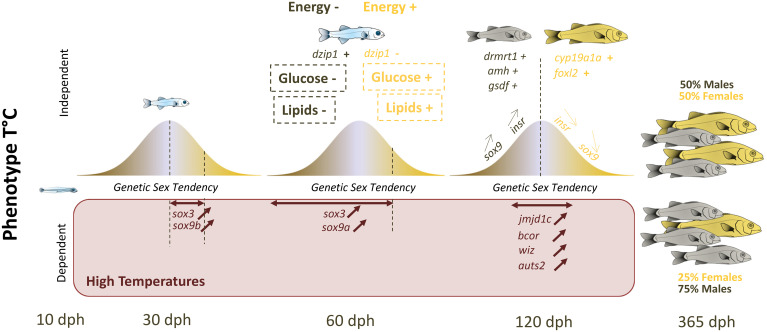
To conclude, we found evidence that sex reversal rather than genotype-specific mortality was the cause of some mismatches between the sex predicted by the eGST and the actual phenotypic sex. We did not find any evidence that stress-axis activation was involved in masculinization. Rather, our data support the involvement of conserved sex-related pathways, epigenetic and energetic processes as the key to understanding temperature-induced masculinization in the European sea bass. We propose a model where the GST of individuals drives the specific expression of genes involved in lipid and glucose metabolism independently of temperature (Fig. 6). In that scheme, individuals presenting higher energy content (transduced by higher transcript levels of genes involved in lipids and glucose production) would become females and those with lower energy would become males (Fig. 6). This may explain the early sexually dimorphic differences in growth usually observed in European sea bass and why domestication (and selection for growth) leads to an increased proportion of females (78). Once the sex is fixed, a classical cascade of genes involved in sex differentiation is activated, starting by sox9a. However, if fish are exposed very early to high temperatures, this first triggers an up-regulation of sox3 that is then followed by an up-regulation of sox9a, determining the sex of individuals in the middle of the eGST distribution (Fig. 6). This signal is then maintained, likely thanks to epigenetic processes (DNA methylation and histone modifications, e.g., through jmjd1c), which leads to individuals with intermediate eGST values developing as males.
Fig. 6.
Summary of the polygenic sex determination system of the European sea bass. In the Top panel (temperature-independent sexual phenotype), all fish have their sexual genotype eGST within a Gaussian-like distribution, with those that present a male tendency (maroon) in the left extreme and those that present a female tendency (yellow) in the right extreme. The genes involved in lipid and glucose (in dashed square) regulation are correlated to the eGST, which likely explains the difference in energy content between future males and future females at the “all fins” stage (around 60 dph). This “energetic” information likely drives sex determination, starting with the overexpression of sox9a. At 120 dph, the gonad is undergoing molecular sex differentiation involving classical sex pathways. Those in the middle of the distribution can still change sex. Now, if European sea bass are kept at high temperature, this results in the overexpression of sox3 and sox9b for individuals with a relatively “low” female eGST at 30 dph and that is conserved at 60 dph, followed by an increase in the expression of sox9a. This sexual phenotype is then likely maintained thanks to the overexpression of key genes involved in histone modification at high, but not low, temperatures for individuals in the middle of the eGST distribution. Following these processes, 25% of the population is then likely masculinized at HT. The double-headed horizontal brown arrows depict individuals with a specific eGST for which we observe increased gene expression at high temperature.
From an adaptive and evolutionary point of view, the conditions favoring the emergence of ESD over GSD have been extensively discussed (28, 79). But species where both strategies coexist, with additive effects, provide new challenges for evolutionary scientists. Understanding how external factors can override genetic information, affecting an essential trait such as phenotypic sex is indeed of major importance in a global warming context. Furthermore, the insights provided by this study on European sea bass can help to illuminate other systems where temperature, stress, or other environmental cues can override a GSD system, as reported in vertebrates, from fish to mammals (80). The present study, therefore, helps pave the way for our understanding of such mechanisms; the genetic architecture likely provides information linked to the regulation of energy and growth, constituting a strong example in support of the hypothesis linking metabolism and sex determination (81). Any environmental cues that might affect this relationship (e.g., temperature influencing metabolism) will trigger a specific expression of a cascade of genes that will, in turn, affect the phenotypic sex of sensitive genotypes.
Materials and Methods
SI Appendix provides a detailed description of the materials and methods used in this study.
Fish Production and Rearing.
The fish population used was the result of a mating design including eight males and one female from a West Mediterranean Sea strain of European sea bass, performed by artificial fertilization (March 22, 2017). Fertilized eggs were incubated at 14 °C until 48 h postfertilization. Eggs were then evenly dispatched in six tanks of 500 L each, and the temperature was gradually increased from 14 °C to 16 °C within 1 d. Following hatching, fish density was of 50 larvae per liter. Larvae were then exposed to 21 °C (HT) in triplicates or kept at 16 °C (LT) in triplicates, as described in ref. 82. HT treatment consisted in gradually increasing temperature to reach 21 °C, from 3 dph to 8 dph. From 10 dph onwards, fish were fed Artemia nauplii for 40 d, then weaned on a commercial sea bass diet (Pro Start and Pro Wean, BioMar). For the LT treatment, the temperature was also increased from day 59 (1 °C/day) to day 64, to reach 21 °C. Fish were reared at the Ifremer Plateforme Expérimentale d'Aquaculture (Palavas-les-Flots, France), accredited to use and breed laboratory animals (no. C341926), and the project was approved by the Animal Care Committee no. 36 under project authorization no. APAFIS 19676.
Sampling.
Fish were evenly sampled in the triplicates of each temperature treatment, at four different developmental stages. Since fish growth is favored at HT, the development of fish generally greatly differs between thermal treatments. Hence, to enable data comparison between individuals kept at different temperatures, the first three samplings were carried out at the same sum of degree-day (base 10 °C, DD10 °C), a procedure previously used to allow standardized measurement of growth in fishes (83). Thus, larvae were not collected at the same date but at the same DD10 °C: 77 DD10 °C, 242 DD10 °C, and 550 DD10 °C (reference SI Appendix, Table S4 for details regarding stage, age, and size). The fourth and last sampling of juveniles, aiming at obtaining developing gonads, was, however, based on size rather than age, since growth difference between treatments declined with age (SI Appendix, Fig. S1 and Table S4). For this reason, gonads were sampled on juveniles at 117 dph (HT) or 124 dph (LT). At the end of the experiment, after 1 y (at 390 dph), all fish were euthanized using benzocaine (150 mg/L), measured, weighed, and sexed by in situ gonad examination. All samples were genotyped with an SNP chip (see Genotyping), but only subsamples of those performed at 242 DD10 °C, 550 DD10 °C, and 117 to 124 dph were used for molecular analysis (SI Appendix, Table S4).
Genotyping.
We genotyped three generations of European sea bass using the Thermofisher DlabChip European sea bass array of 57,000 SNP markers (39). Generation 0 includes the parents of the eight sires. Generation 1 corresponds to the parents (the dam and the eight sires). Generation 2 includes the 2,030 offspring, composed of the larvae sampled at 77 DD10 °C (n = 192), at 242 DD10 °C (n = 300), and at 550 DD10 °C (n = 280), the juveniles collected at 117 dph (HT, n = 70) and 124 dph (LT, n = 70), and fish sexed at 390 dph (n = 1,118). Fish were sampled at random at each stage, and the number of fish per family was known a posteriori following genotyping and parentage assignment (SI Appendix, Table S4). Complete details of the genotyping analysis can be found in SI Appendix, Supplementary Materials and Methods.
Heritability, eGST Prediction, and Genome-Wide Association Scan.
We predicted the sex using a liability threshold model, which has been used in a large variety of settings, often with diseases (29, 84) but also for sex determination (28, 32). With this model, the binary phenotype is the realization of an underlying continuous phenotype, the liability trait, here called phenotypic sex tendency (PST). When PST exceeds a given threshold, animals differentiate as females, while they differentiate as males when PST remains below the threshold (SI Appendix, Fig. S4). The PST is itself the addition of a genetic and an environmental sex tendency. In a polygenic sex determination system, the PST is considered a quantitative trait, influenced by many genes with small effects plus environmental effects (28). The GST, which cannot be measured directly, is the genetic part of this PST, positive for individuals more likely to develop as females in a neutral environment and negative for individuals more likely to develop as males (SI Appendix, Fig. S4). Variance components and heritability were estimated for PST, the underlying liability of the binary sex, with a threshold model using THRGIBBS1F90 (85). Complete details for the establishment of eGST, QTL presence, and heritability assessment can be found in SI Appendix, Supplementary Materials and Methods.
Transcriptomics.
The transcriptomic approach aimed to detect genes whose expression would be correlated to the eGST. Since we expected to have fewer females at HT versus LT, we collected more individuals in the HT than in the LT treatment to ensure having a sufficient number of females to analyze. Overall, 70 larvae, 40 from the HT group and 30 from the LT group, were randomly collected at the “flexion” (242 DD10 °C) and the “all fins” stages (550 DD10 °C), euthanized (benzocaine 150 mg/L), and snap frozen in liquid nitrogen. At these stages, the RNA extraction was performed on entire individuals because of the impossibility to neatly dissect the gonads of such small individuals. This was justified from two points of view: 1) it has been previously shown that the chance of underestimating gene expression of sex-related genes using body trunks was negligible in sea bass (86) and 2) we aimed to have a whole picture of the physiological processes associated with temperature sex determination (TSD). At the juvenile stage (117 to 124 dpf), the gonads of 35 euthanized (benzocaine 150 mg/L) individuals from each temperature treatment were collected and snap frozen in liquid nitrogen. At this stage, the biggest individuals were collected to ensure the sampling/collection of enough gonadal tissue for transcriptomic analysis. All samples were stored at −80 °C until RNA extraction. Complete details for RNA extraction, RNA-Seq, and RNA-Seq data analysis can be found in SI Appendix, Supplementary Materials and Methods. The raw data of RNA-seq can be downloaded from ref. 87.
Histology.
At the juvenile stage, 20 gonads (10 HT and 10 LT) were fixed in Bouin’s fluid for 6 to 8 h, rinsed in clear water for 1 h, and stocked in a 70% alcohol solution. Each gonad was stained with eosin and then placed in agarose (to improve detection) before being dehydrated and embedded in paraffin. Sections of 5- to 6-mm thickness were stained with Trichrome de Masson, Haematoxylin Groat, Fuschine Ponceau, and Aniline blue using an automated device.
Elemental Analysis.
Elemental analysis was performed to estimate the energy content of fish with a known eGe. Elemental composition was determined using a microVario Elemental analyzer (Elementar), allowing to obtain the percentage of carbon, hydrogen, nitrogen, sulfur, and oxygen (CHNSO) per milligram of dry weight. In total, 70 sampled fish were weighted and measured before being euthanized (benzocaine 150 mg/L) and kept at −20 °C. Then, fish at each stage (“flexion,” “all fins,” and juveniles) were lyophilized simultaneously for 24 h. Dried fish were then individually homogenized using ball mills. About 1 mg was used for CHNS analyses. Another 1 mg of the same samples was used for oxygen analysis. The analysis was performed by the laboratory of Physical measures (https://lmp.edu.umontpellier.fr/elem) of Montpellier (certified Association Française de Normalisation, Iso 9001). Individual elemental composition was then transformed to a relative energetic content (cal ⋅ mg−1 dry weight) using the Given (88) formula, as recommended by the analyzer program:
where C, H, O, and S refer to the percentages of carbon, hydrogen, oxygen, and sulfur in a sample. It was then divided by 4.184 to obtain the energetic content in joules ⋅ mg−1.
RRBS Library Preparation and Analysis.
Genomic DNA was isolated from ∼25 mg frozen gonadal tissue from the 1-y-old fish with the Qiagen Blood and Cell Culture kit ( catalog no. 13323). Genome-wide profiling of DNA methylation levels was performed by RRBS using the Premium RRBS Kit (catalog no. C02030033; Diagenode) according to the manufacturer’s instructions. Complete details for RRBS sequencing and preliminary analysis can be found in SI Appendix, Supplementary Materials and Methods.
Data Analysis.
Final sex ratio was analyzed using a binomial mixed model, with “tank” added as a random factor and “initial temperature treatment” as a fixed factor. For the three transcriptomes datasets of fish at the “flexion” stage, “all fins” stage, and juvenile stage, we adopted a traditional approach based on group comparison, where differentially expressed genes are detected using the Bioconductor (89) package DESeq2 version 1.18.1 (90) in R (91) and data normalized using the default method of DESeq2. Regarding the 10 transcriptomes at the “flexion” stage, group comparisons were performed between LT (n = 5) and HT (n = 5) fish with a positive but not extreme eGST (i.e., 0 < eGST < 0.5). Regarding the 68 transcriptomes at the “all fins” stage, individuals were grouped based on their eGST, within each initial temperature treatment (note that they were collected at the same temperature [T, °C]). Those at each extreme of the eGST distribution were considered as extremes genetic males (eM, eGST < −0.5) or extreme genetic females (eF, eGST > 0.5), respectively, and those in the middle of the distribution were considered neutrals males (nM, −0.5 < eGST < 0) or neutrals females (nF, 0 < eGST < 0.5). Group comparisons between temperature treatments with DESeq2 were thus performed between eight groups, eM-HT (n = 6), nM-HT (n = 10), nF-HT (n = 12), eF-HT (n = 11), eM-LT (n = 8), nM-LT (n = 6), nF-LT (n = 13), and eF-LT (n = 3). For juveniles’ gonads, we considered only two groups, those with an eGST > 0 (genetic females, n = 26) and those with an eGST < 0 (genetic males, n = 17) to detect differentially expressed genes according to the genetic sex. For all comparisons, genes with an adjusted P value less than 5% (according to the false discovery rate method from Benjamini-Hochberg) were declared differentially expressed.
For the two transcriptomic datasets with the highest number of samples (43, 68), we also considered another approach using our linear predictor, the eGST, as a fixed value in linear models. Using normalized (DESeq2) and filtered data (keeping those with more than 30 reads in average), we ran a loop in R (R version 4.0.4) to detect all genes that are significantly correlated to the eGST in a linear and a quadratic model where the temperature treatment (HT or LT) is added as an interaction term with the eGST (T × eGST). For the linear model, we considered only the genes without significant interaction with temperature. Once detected, we also ran a linear model with a quasibinomial distribution (link = Logit) for those interesting genes displaying a dichotomic pattern of expression. For the quadratic model, we considered either the genes with a significant correlation with eGST plus a significant quadratic term for the interaction (I(eGST2) × T) or those where the quadratic term (I(eGST2)) plus the interaction between the temperature treatment and the quadratic term (I(eGST2) × T) are significant. One outlier (LT) was removed since it was at the extreme for eGST with unusual intermediate values for all sex-related genes, though keeping this outlier resulted in similar outcomes for those genes (SI Appendix, Fig. S11). Linear models were also used to assess the effect of eGST on energy content (joules ⋅ mg-1) and the size of the fishes.
For all transcriptomic analyses, the GO dataset of the mouse was used as it gives a more complete overview of the genes involved in each pathway. We adopted two strategies: 1) with a priori, where GOs were selected based on our hypotheses (SI Appendix, Supplementary Materials and Methods); and 2) without a priori, where GOs were automatically detected from a functional enrichment analysis. For this second approach, we used a functional enrichment analysis. The clusterProfiler version 3.16.1 (92), an R package, was used to analyze function profiles of genes to identify major biological functions of genes. GO terms were predicted based on differentially expressed genes (from the group comparisons and linear models), including biological process and cellular component categories. Enrichment analysis was performed and a P value <0.05 was considered to indicate a statistically significant difference. PCA was performed using gene expression levels and illustrated using the ggplot2 package. The same package was used for representing raw values of genes for linear, quadratic regressions and selected genes from group comparisons. The “lme4” package was used for mixed models. Heatmaps of standardized expressions (mean subtraction followed SD division) were created using the pheatmap package. Methylkit and Genomic Ranges version 1.44.0 (93) packages were used to filter percent methylation data for the target regions: gene body ± 2,000 bp. DMCs were defined as cytosines with a methylation difference of ≥15% between temperature treatments and a significance threshold of q-value <0.01.
Supplementary Material
Acknowledgments
The study was supported by the European Maritime and Fisheries Fund (3S, Seabass Sex and Stress, Grant No. 4320175237) allocated to B.G. Production of the fish benefited from AQUAEXCEL2020 Transnational access grant “Transsexbass” to F.P. and M.V. Research at the F.P. laboratory was supported by Spanish Ministry of Science Grant No. PID2019-108888RB-I00. We thank Pierre Lopez (MARine Biodiversity, Exploitation and Conservation [MARBEC]) for kindly drawing fish at the different stages of development, Thomas Régnier for providing background on CHNSO analysis, Sandrine Skiba for discussion of the results, and Mako Pegart for samplings.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2112660118/-/DCSupplemental.
Data Availability
RNA-Seq and .xls tables data have been deposited in a publicly accessible database: SEXTAN (https://sextant.ifremer.fr/record/5cb1485f-385e-46e9-9cc1-4b4d77802183/). All other study data are included in the article and/or supporting information.
References
- 1.Capel B., Vertebrate sex determination: Evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 18, 675–689 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Charnier M., Action of temperature on the sex ratio in the Agama agama (Agamidae, Lacertilia) embryo. C. R. Seances Soc. Biol. Fil. 160, 620–622 (1966). [PubMed] [Google Scholar]
- 3.Conover D. O., Kynard B. E., Environmental sex determination: Interaction of temperature and genotype in a fish. Science 213, 577–579 (1981). [DOI] [PubMed] [Google Scholar]
- 4.Devlin R. H., Nagahama Y., Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 208, 191–364 (2002). [Google Scholar]
- 5.Geffroy B., Bardonnet A., Sex differentiation and sex determination in eels: Consequences for management. Fish Fish. 17, 375–398 (2016). [Google Scholar]
- 6.Ospina-Álvarez N., Piferrer F., Temperature-dependent sex determination in fish revisited: Prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS One 3, e2837 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Römer U., Beisenherz W., Environmental determination of sex in Apistogrammai (Cichlidae) and two other freshwater fishes (Teleostei). J. Fish Biol. 48, 714–725 (1996). [Google Scholar]
- 8.Hattori R. S., Castañeda-Cortés D. C., Arias Padilla L. F., Strobl-Mazzulla P. H., Fernandino J. I., Activation of stress response axis as a key process in environment-induced sex plasticity in fish. Cell. Mol. Life Sci., 77 4223–4236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piferrer F., et al. , The model of the conserved epigenetic regulation of sex. Front. Genet. 10, 857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deveson I. W., et al. , Differential intron retention in Jumonji chromatin modifier genes is implicated in reptile temperature-dependent sex determination. Sci. Adv. 3, e1700731 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georges A., Holleley C. E., How does temperature determine sex? Science 360, 601–602 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Geffroy B., Douhard M., The adaptive sex in stressful environments. Trends Ecol. Evol. 34, 628–640 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Geffroy B., Wedekind C., Effects of global warming on sex ratios in fishes. J. Fish Biol. 97, 596–606 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Baroiller J. F., D’Cotta H., Bezault E., Wessels S., Hoerstgen-Schwark G., Tilapia sex determination: Where temperature and genetics meet. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 153, 30–38 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Tabata K., Reduction of female proportion in lower growing fish separtated from normal and feminized seedlings of hirame Paralichthys olivaceus. Fish. Sci. 61, 199–201 (1995). [Google Scholar]
- 16.Santi S., et al. , Thermosensitivity of the sex differentiation process in the African catfish, Clarias gariepinus: Determination of the thermosensitive period. Aquaculture 455, 73–80 (2016). [Google Scholar]
- 17.Hattori R. S., et al. , The duplicated Y-specific amhy gene is conserved and linked to maleness in silversides of the genus Odontesthes. Genes (Basel) 10, 679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitano T., Hayashi Y., Shiraishi E., Kamei Y., Estrogen rescues masculinization of genetically female medaka by exposure to cortisol or high temperature. Mol. Reprod. Dev. 79, 719–726 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Valdivia K., et al. , High temperature increases the masculinization rate of the all-female (XX) rainbow trout “Mal” population. PLoS One 9, e113355 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H., et al. , Temperature-control-induced masculinization in tiger puffer Takifugu rubripes. J. Oceanol. Limnol. 37, 1125–1135 (2019). [Google Scholar]
- 21.Hattori R. S., et al. , Cortisol-induced masculinization: Does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination? PLoS One 4, e6548 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi Y., et al. , High temperature causes masculinization of genetically female medaka by elevation of cortisol. Mol. Reprod. Dev. 77, 679–686 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Kamiya T., et al. , A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 8, e1002798 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi K., Hattori R. S., Strüssmann C. A., Yokota M., Yamamoto Y., Phenotypic/genotypic sex mismatches and temperature-dependent sex determination in a wild population of an Old World atherinid, the cobaltcap silverside Hypoatherina tsurugae. Mol. Ecol. 29, 2349–2358 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Yano A., et al. , The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol. Appl. 6, 486–496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baroiller J. F., Chourrout D., Fostier A., Jalabert B., Temperature and sex chromosomes govern sex ratios of the mouthbrooding Cichlid fish Oreochromis niloticus. J. Exp. Zool. 273, 216–223 (1995). [Google Scholar]
- 27.Wang X., et al. , High temperature causes masculinization of genetically female olive flounder (Paralichthys olivaceus) accompanied by primordial germ cell proliferation detention. Aquaculture 479, 808–816 (2017). [Google Scholar]
- 28.Bulmer M. G., Bull J. J., Models of polygenic sex determination and sex ratio control. Evolution 36, 13–26 (1982). [DOI] [PubMed] [Google Scholar]
- 29.Hujoel M. L. A., Gazal S., Loh P.-R., Patterson N., Price A. L., Liability threshold modeling of case-control status and family history of disease increases association power. Nat. Genet. 52, 541–547 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piferrer F., Blázquez M., Navarro L., González A., Genetic, endocrine, and environmental components of sex determination and differentiation in the European sea bass (Dicentrarchus labrax L.). Gen. Comp. Endocrinol. 142, 102–110 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Saillant E., et al. , Temperature effects and genotype-temperature interactions on sex determination in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool. 292, 494–505 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Vandeputte M., Dupont-Nivet M., Chavanne H., Chatain B., A polygenic hypothesis for sex determination in the European sea bass Dicentrarchus labrax. Genetics 176, 1049–1057 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandeputte M., et al. , Low temperature has opposite effects on sex determination in a marine fish at the larval/postlarval and juvenile stages. Ecol. Evol. 10, 13825–13835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Díaz N., Ribas L., Piferrer F., The relationship between growth and sex differentiation in the European sea bass (Dicentrarchus labrax). Aquaculture 408–409, 191–202 (2013). [Google Scholar]
- 35.Díaz N., Piferrer F., Lasting effects of early exposure to temperature on the gonadal transcriptome at the time of sex differentiation in the European sea bass, a fish with mixed genetic and environmental sex determination. BMC Genomics 16, 679 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro-Martín L., et al. , DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 7, e1002447 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandeputte M., et al. , Low temperature has opposite effects on sex determination in a marine fish at the larval/postlarval and juvenile stages. Ecol. Evol. 10, 13825–13835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anastasiadi D., Vandeputte M., Sánchez-Baizán N., Allal F., Piferrer F., Dynamic epimarks in sex-related genes predict gonad phenotype in the European sea bass, a fish with mixed genetic and environmental sex determination. Epigenetics 13, 988–1011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griot R., et al. , Genome-wide association studies for resistance to viral nervous necrosis in three populations of European sea bass (Dicentrarchus labrax) using a novel 57k SNP array DlabChip. Aquaculture 530, 735930 (2021). [Google Scholar]
- 40.Meuwissen T. H., Hayes B. J., Goddard M. E., Prediction of total genetic value using genome-wide dense marker maps. Genetics 157, 1819–1829 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roblin C., Bruslé J., Ontogenèse gonadique et différenciation sexuelle du loup Dicentrarchus labrax, en conditions d’élevage. Reprod. Nutr. Dev. 23, 115–127 (1983). [PubMed] [Google Scholar]
- 42.Ødegård J., Meuwissen T. H., Estimation of heritability from limited family data using genome-wide identity-by-descent sharing. Genet. Sel. Evol. 44, 16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribas L., et al. , Characterization of the European sea bass (Dicentrarchus labrax) gonadal transcriptome during sexual development. Mar. Biotechnol. (NY) 21, 359–373 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Faggion S., Vandeputte M., Chatain B., Gagnaire P.-A., Allal F., Population-specific variations of the genetic architecture of sex determination in wild European sea bass Dicentrarchus labrax L. Heredity 122, 612–621 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palaiokostas C., et al. , A new SNP-based vision of the genetics of sex determination in European sea bass (Dicentrarchus labrax). Genet. Sel. Evol. 47, 68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bláquez M., Zanuy S., Carillo M., Piferrer F., Effects of rearing temperature on sex differentiation in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool. 281, 207–216 (1998). [Google Scholar]
- 47.Pavlidis M., et al. , Evidence of temperature-dependent sex determination in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool. 287, 225–232 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Navarro-Martín L., Blázquez M., Viñas J., Joly S., Piferrer F., Balancing the effects of rearing at low temperature during early development on sex ratios, growth and maturation in the European sea bass (Dicentrarchus labrax).: Limitations and opportunities for the production of highly female-biased stocks. Aquaculture 296, 347–358 (2009). [Google Scholar]
- 49.Alper C. A., Awdeh Z., Incomplete penetrance of MHC susceptibility genes: Prospective analysis of polygenic MHC-determined traits. Tissue Antigens 56, 199–206 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Cooper D. N., Krawczak M., Polychronakos C., Tyler-Smith C., Kehrer-Sawatzki H., Where genotype is not predictive of phenotype: Towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum. Genet. 132, 1077–1130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saillant E., et al. , Sexual differentiation and juvenile intersexuality in the European sea bass (Dicentrarchus labrax). J. Zool. (Lond.) 260, 53–63 (2003). [Google Scholar]
- 52.Geffroy B., Guiguen Y., Fostier A., Bardonnet A., New insights regarding gonad development in European eel: Evidence for a direct ovarian differentiation. Fish Physiol. Biochem. 39, 1129–1140 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Kent J., Wheatley S. C., Andrews J. E., Sinclair A. H., Koopman P., A male-specific role for SOX9 in vertebrate sex determination. Development 122, 2813–2822 (1996). [DOI] [PubMed] [Google Scholar]
- 54.Nef S., et al. , Testis determination requires insulin receptor family function in mice. Nature 426, 291–295 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Stévant I., Nef S., Genetic control of gonadal sex determination and development. Trends Genet. 35, 346–358 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Blázquez M., Navarro-Martín L., Piferrer F., Expression profiles of sex differentiation-related genes during ontogenesis in the European sea bass acclimated to two different temperatures. J. Exp. Zoolog. B Mol. Dev. Evol. 312, 686–700 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Moreno-Mendoza N., Harley V. R., Merchant-Larios H., Temperature regulates SOX9 expression in cultured gonads of Lepidochelys olivacea, a species with temperature sex determination. Dev. Biol. 229, 319–326 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Radhakrishnan S., Literman R., Neuwald J., Severin A., Valenzuela N., Transcriptomic responses to environmental temperature by turtles with temperature-dependent and genotypic sex determination assessed by RNAseq inform the genetic architecture of embryonic gonadal development. PLoS One 12, e0172044 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shoemaker C., Ramsey M., Queen J., Crews D., Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Dev. Dyn. 236, 1055–1063 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Sun D., et al. , Sox9-related signaling controls zebrafish juvenile ovary-testis transformation. Cell Death Dis. 4, e930 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore L. D., Le T., Fan G., DNA methylation and its basic function. Neuropsychopharmacology 38, 23–38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anastasiadi D., Esteve-Codina A., Piferrer F., Consistent inverse correlation between DNA methylation of the first intron and gene expression across tissues and species. Epigenetics Chromatin 11, 37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urs S., et al. , Sprouty1 is a critical regulatory switch of mesenchymal stem cell lineage allocation. FASEB J. 24, 3264–3273 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urs S., Henderson T., Le P., Rosen C. J., Liaw L., Tissue-specific expression of Sprouty1 in mice protects against high-fat diet-induced fat accumulation, bone loss and metabolic dysfunction. Br. J. Nutr. 108, 1025–1033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prada P. O., et al. , EGFR tyrosine kinase inhibitor (PD153035) improves glucose tolerance and insulin action in high-fat diet-fed mice. Diabetes 58, 2910–2919 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Koledova Z., et al. , SPRY1 regulates mammary epithelial morphogenesis by modulating EGFR-dependent stromal paracrine signaling and ECM remodeling. Proc. Natl. Acad. Sci. U.S.A. 113, E5731–E5740 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marguet D., et al. , Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc. Natl. Acad. Sci. U.S.A. 97, 6874–6879 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faggion S., et al. , Sex dimorphism in European sea bass (Dicentrarchus labrax L.): New insights into sex-related growth patterns during very early life stages. PLoS One 16, e0239791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herpin A., Schartl M., Plasticity of gene-regulatory networks controlling sex determination: Of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 16, 1260–1274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myosho T., Takehana Y., Hamaguchi S., Sakaizumi M., Turnover of sex chromosomes in celebensis group medaka fishes. G3 (Bethesda) 5, 2685–2691 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutton E., et al. , Identification of SOX3 as an XX male sex reversal gene in mice and humans. J. Clin. Invest. 121, 328–341 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takehana Y., et al. , Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 5, 4157 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Yao B., Zhou L., Wang Y., Xia W., Gui J.-F., Differential expression and dynamic changes of SOX3 during gametogenesis and sex reversal in protogynous hermaphroditic fish. J. Exp. Zool. A Ecol. Genet. Physiol. 307, 207–219 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Piferrer F., Epigenetics of sex determination and gonadogenesis. Dev. Dyn. 242, 360–370 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Gemmell N. J., Todd E. V., Goikoetxea A., Ortega-Recalde O., Hore T. A., “Natural sex change in fish” in Current Topics in Developmental Biology, Sex Determination in Vertebrates, Capel B., Ed. (Academic Press, 2019), pp. 71–117. [DOI] [PubMed] [Google Scholar]
- 76.Tsakogiannis A., et al. , The gene toolkit implicated in functional sex in sparidae hermaphrodites: Inferences from comparative transcriptomics. Front. Genet. 9, 749 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ge C., et al. , The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 360, 645–648 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Geffroy B., et al. , Parental selection for growth and early-life low stocking density increase the female-to-male ratio in European sea bass. Sci. Rep. 11, 13620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Charnov E. L., Bull J., When is sex environmentally determined? Nature 266, 828–830 (1977). [DOI] [PubMed] [Google Scholar]
- 80.Piferrer F., Anastasiadi D., Do the offspring of sex reversals have higher sensitivity to environmental perturbations? Sex Dev. 15, 134–147 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Mittwoch U., The elusive action of sex-determining genes: Mitochondria to the rescue? J. Theor. Biol. 228, 359–365 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Goikoetxea A., et al. , Genetic pathways underpinning hormonal stress responses in fish exposed to short- and long-term warm ocean temperatures. Ecol. Indic. 120, 106937 (2021). [Google Scholar]
- 83.Chezik K. A., Lester N. P., Venturelli P. A., Fish growth and degree-days I: Selecting a base temperature for a within-population study. Can. J. Fish. Aquat. Sci. 71, 47–55 (2014). [Google Scholar]
- 84.Weissbrod O., Lippert C., Geiger D., Heckerman D., Accurate liability estimation improves power in ascertained case-control studies. Nat. Methods 12, 332–334 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Tsuruta S., Misztal I., “THRGIBBS1F90 for estimation of variance components with threshold and linear models” in Proceedings of the 8th World Congress on Genetics Applied to Livestock Production (Instituto Prociência, 2006), pp. 27–31.
- 86.Blázquez M., González A., Papadaki M., Mylonas C., Piferrer F., Sex-related changes in estrogen receptors and aromatase gene expression and enzymatic activity during early development and sex differentiation in the European sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol. 158, 95–101 (2008). [DOI] [PubMed] [Google Scholar]
- 87.B. Geffroy, RNA-Seq juvéniles de bar: Projet 3S (Seabass, Sex and Stress). Sextant. https://doi.org/10.12770/5cb1485f-385e-46e9-9cc1-4b4d77802183. Deposited 9 March 2018. [Google Scholar]
- 88.P. H. Given, D. Weldon, J. H. Zoeller, Calculation of calorific values of coals from ultimate analyses: theoretical basis and geochemical implications. Fuel 65, 849–854 (1986). [Google Scholar]
- 89.Gentleman R. C., et al. , Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.R Core Team, R: A Language and Environment for Statistical Computing (R Found. Stat. Comput, Vienna, Austria, 2021). [Google Scholar]
- 92.Yu G., Wang L.-G., Han Y., He Q.-Y., clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawrence M., et al. , Software for computing and annotating genomic ranges. PLOS Comput. Biol. 9, e1003118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq and .xls tables data have been deposited in a publicly accessible database: SEXTAN (https://sextant.ifremer.fr/record/5cb1485f-385e-46e9-9cc1-4b4d77802183/). All other study data are included in the article and/or supporting information.