Abstract
This review identifies some key concepts of muscle regeneration, viewed from perspectives of classical and modern research. Early insights noted the pattern and sequence of regeneration across species was similar, regardless of the type of injury, and differed from epimorphic limb regeneration. While potential benefits of exercise for tissue repair was debated, regeneration was not presumed to deliver functional restoration, especially after ischemia–reperfusion injury; muscle could develop fibrosis and ectopic bone and fat. Standard protocols and tools were identified as necessary for tracking injury and outcomes. Current concepts vastly extend early insights. Myogenic regeneration occurs within the environment of muscle tissue. Intercellular cross-talk generates an interactive system of cellular networks that with the extracellular matrix and local, regional, and systemic influences, forms the larger gestalt of the satellite cell niche. Regenerative potential and adaptive plasticity are overlain by epigenetically regionalized responsiveness and contributions by myogenic, endothelial, and fibroadipogenic progenitors and inflammatory and metabolic processes. Muscle architecture is a living portrait of functional regulatory hierarchies, while cellular dynamics, physical activity, and muscle–tendon–bone biomechanics arbitrate regeneration. The scope of ongoing research—from molecules and exosomes to morphology and physiology—reveals compelling new concepts in muscle regeneration that will guide future discoveries for use in application to fitness, rehabilitation, and disease prevention and treatment.
Keywords: Satellite cell, Myogenesis, Fibro-adipogenic precursors, Inflammation, Extracellular matrix, Migration, Exercise
Foundational literature
The basis of any scientific exploration lies in the richness of observation and experimentation conveyed by researchers, including their respectful discussion of previous and concurrent work. Generations of emerging scientists, finding a research niche, contributing new concepts, and augmenting earlier ideas as their experience and resources grow, further decorate the process of providing this review. The field of research on muscle regeneration is remarkably broad in its diversity of approaches and prospects, though nowhere near as remarkable as the myriad processes that are integrated to restore function and enable adaptation. So, a toast to skeletal muscle and all its fascinatingly interactive components, and to the ingeniously creative use of tools and concepts to push the frontiers of understanding muscle regeneration and how it can shape life.
Muscle and physical activity
Early observations, including potential influences of weather (barometric pressure, temperature) on strength (manual grip strength) by Fischer (1947), and the sociopolitical context of rehabilitation after World Wars I and II described by Boynton (1947), illustrate the broad scope of many early ideas about recovery and tissue repair. Few reports are specific to what we now understand as muscle-tissue regeneration, although the perspectives illustrate the way concepts can shape understanding and either extend or squelch curiosity-driven research.
In 1900, for instance, a pathologist (and assistant medical officer for the Royal Exchange Assurance Company) noted that exercise should not be abused (i.e., over-indulged), or undertaken over the level of normal daily activity, as it led to many negative consequences. Lister (1900) cautioned against “violent efforts” on summer holidays and offered now-quaint commentary on the consequence of over-exertion in the non-athletic. He also described that incidence of such injury is likely to increase with the “growing demands made on energies, knowledge, and intelligence in the working months by the increasingly-rapid inter-communications and severe competition of advancing civilization.” The comment that over-exertion was much less frequent among women as they did household chores gives the paper a historical context.
In 1922, Rowlands (1922) suggested for the first time, that activity might accelerate wound healing. While not specific to muscle regeneration per se, Rowlands questioned the prescription of prolonged bedrest after surgery—typical accompaniment to hospitalization after many procedures well into the 1980s. Rowlands was “not aware of any delay in wound healing due to getting up early and taking gentle exercise…[since] gentle movements of wounded soft parts do not hinder but help the process of healing.” In concluding his conference paper, Rowlands called for a more thorough assessment of the influence of activity in post-operative recovery, to prevent adhesions and muscle shortening that appear after prolonged inactivity. He politely recommended that “taking gradually increasing exercise, just short of fatigue” would be useful and provide interesting “mental diversion” during recovery.
Assessing muscle function
By 1949, Newman (1949) recognized “the importance of an accurate evaluation of a disability”, since “muscle strength, to a great extent, governs the amount of function of a part.” While this might seem obvious now, many early measurement devices were not standardized and their use was not subject to guidelines for positioning a patient or calibration that could be reproduced over time. Use of the tools was not standardized across investigators or clinical staff, to assure consistent application, and thus jeopardized assessment of function. In 1949, Woodard (1949) noted that muscle injury in athletes differed from that incurred by non-athletes, and thus required particular therapy including gentle muscle activity rather than rest, for optimal repair.
Brewerton and Darcus (1956) prefaced a 1956 review paper on methods of increasing muscle strength by stating that there little new information on muscle function had accrued since 1900. He cited Siebert (writing in German in Z. Klin. Med., 1928) for recognizing that an increase in muscle size is not achieved by increasing the number of muscle fibers. (This is the current understanding of growth by fiber hypertrophy rather than a hyperplasia of fibers that occurs in indeterminate growers such as sturgeon (Hiebert and Anderson 2020). Darcus noted that “one of the few well-established facts is that the performance of muscles can be improved by systematic voluntary exercise.” He then discussed the lack of consensus on how that could happen or the changes in muscle structure and function that happen during strengthening, despite “the determination of the workers in this field” in developing training routines and equipment. He also noted that researchers were still looking for evidence that the muscle capillary network increases with training and adaptation.
The neuromuscular system and adaptation
Studying the neuromuscular system, Darcus distinguished changes to muscle (from force generation) from those to the nervous system (related to making best use of available force generation). By 1923, it was already established that reinnervating axons could regenerate through nerve stumps from early parabiotic-pair experiments (Morpugo 1923). However, Darcus was not sure that the normal process of muscle hypertrophy was different from that in muscle atrophied by denervation or inactivity. Further, it was not established how much fiber hypertrophy actually contributed to increases in human muscle strength, since findings from animal experimentation were not directly applicable to rehabilitation in people. Darcus recognized that training-induced increases in strength implied the existence of important neuromuscular adaptations in skill, recruitment of motor neurons and motor units, and sensory (afferent) inflow to the central nervous system from muscle spindle contractions and from other tissues. However, he did not know whether the exercise by patients recovering from injury should be allowed to induce muscle fatigue, and cautioned against inducing “subjectively unpleasant” sensations that produced residual stiffness or soreness. In short, Darcus considered neuromuscular adaptations to be more important in the outcome of training than structural and biochemical changes in muscle. Neuromuscular adaptation, later termed muscle plasticity, was reviewed more recently (Pette 2001).
By 1949, Denny-Brown had published some of his classical work on electromyography and motor unit recruitment (Denny-Brown 1949); others had reported the importance of afferent information from muscle, tendons, and muscle spindles in regulating contractile force. Darcus noted that neuromuscular impulses facilitated synaptic transmission at nerve–muscle junctions (NMJs) and all along the reflex arc, and with sufficient repetition, might reduce resistance to signal transmission from stimulus to contraction.
Writing in the context of a raging polio epidemic, Darcus and his contemporaries would have had many daily reminders that compensatory movements during rehabilitation were critical to restoring overall function. Relevant to the current review, Darcus cited a 1943 paper by Griffiths (1943) that “the ultimate recovery of function may be delayed by inhibiting the normal habit” of a muscle. This further emphasized that exercise or at least muscle activity is important to functional recovery. Griffiths distinguished passive movement and anti-gravity assistance from active movement during recovery to restore muscle power after injury or surgery, and noted effective venous return was dependent on muscle contraction, without discussing myogenic repair itself.
The following year, Eccles (1944) concluded from animal physiology experiments that disuse atrophy is best countered by exercise in which muscle shortens during contraction; he noted that strong isometric contractions can maintain muscle condition reasonably well. Eccles also determined that the effectiveness of muscle stimulation to maintain muscle mass depended on more than changes in muscle length since the outcome of stimulation differed between extensor and flexor muscles. A 1957 address to the British Medical Society (Anonymous 1957) referenced Darcus’ work on neuromuscular adaptation, and noted a lack of consensus on how best to tailor resistance-exercise protocols to specifically build endurance, speed, strength, or coordination.
Modeling muscle
The cultivation of chopped muscle in a medium of embryo extract, Tyrode’s solution, and plasma, using a “lying drop” technique was described by Popogeff and Murray in 1956 (1956). Use of that culturing method quickly revealed that precursor cell dormancy (muscle satellite cells) was longer in normal than in dystrophic human muscle, and demonstrated myotube growth or hypertrophy in culture (Geiger and Garvin 1957). The advent of tissue-culture methodologies opened the door to many research questions.
Studying muscle regeneration
As early as 1947, Diaz-Guerrero and Thomson reported (Diaz-Guerrero et al. 1947b) that the restoration of function after denervating gastrocnemius muscle (using a crush injury of the tibial nerve) was slower in hypothyroid than hyperthyroid rats. As well, they reported that denervation atrophy occurred more quickly in hyper- than hypothyroid animals. These findings were based on initial studies of muscle strength without denervation. The force of muscle contraction produced after indirect stimulation (i.e., stimulation to an innervating nerve) was lower, and fatigue greater in hyperthyroid than normal animals; hypothyroid animals showed no change in strength and had only a slightly increased fatigue of muscles tested with the nerve-stimulation protocol (Diaz-Guerrero et al. 1947a). In that exercise and activity provide voluntary (nerve-dependent) generation of muscle contractile force, these early studies established the important impact of the metabolic state on nerve–muscle interactions, atrophy, and neuromuscular repair.
Studitsky conducted early experiments focussed on muscle regeneration from 1952 to 1964, He compared regeneration in young and old animals (often rats), and transplanted crushed or minced muscle into an emptied muscle bed. He also documented the progressive return of function during muscle recovery from damage. Unfortunately, the intriguing titles in Russian are not accompanied by translations (Carlson 1968). His book chapter in English in 1963 (Studitsky 1963) describes the fascinating process of myogenesis in cultures of muscle explants. Fortunately, Carlson visited and worked in Studitsky’s lab, and was able to bring to broad attention to those experiments in later reports in English. By 1968, Carlson confirmed that use of minced-muscle in modeling cellular processes provides major opportunities for observing the chronology of a synchronized process of muscle regeneration (Carlson 1968).
Notably, by 1945, the surgical literature had identified the features of muscle regeneration induced by loss of vascular supply, including the “myotube stage” discussed by Le Gros Clark and Blomfield (1945) and took note of previous studies of contractures after traumatic wartime injuries or vascular occlusion (Brooks 1922). Brooks confirmed findings of an earlier paper by Rowlands (1905) that identified the serious sequelae of muscle damage from ischemia–reperfusion injury. Church cited even earlier work by Waldeyer (1865) and Volkman (1893) that described regeneration processes that involved “continuous sprouting from viable fibres… and discontinuous budding from surviving fibres of single cells… that ultimately give rise to new fibers” (Church et al. 1966). These were early observations that identified the role of activated satellite cells in myogenic repair, although the cells were not named as such, or identified in their quiescent state, for years to come.
Carlson’s impressive monograph in 1973, The Regeneration of Minced Muscles (Carlson 1972), detailed the histological progression of muscle tissue repair. In the epilogue, Carlson notes seven “potentially… most profitable directions for future research,” including how damaged muscle survives, the source of muscle progenitor cells, muscle interactions (with vessels, nerves, and endocrine signals), the functional outcome of muscle regenerates, and the possibility that transplanting muscle minces or cells could be used to treat muscle damage or disease. Carlson identified problems in repairing muscle damage that involved excessive deposition of connective tissue and skeletal nodules; those sequelae were often related to poor vascularization at a particular stage of myogenic regeneration.
Muscle histology
Our current knowledge of the muscle regeneration process was founded on the rich descriptions of histological features of normal or pathological muscle repair under various conditions reported by many investigators, typically using mammalian models (e.g., Le Gros Clark and Blomfield 1945; Murray and Kodicek 1949). The often-voluminous descriptive text in these reports would never be accepted for publication in current journals, it clearly provides important details. Early histologists thoroughly explored the chemistry of tissue staining and learned about muscle metabolism. This was followed within a few decades by enzyme histochemistry (Snow 1973) and an early method of staining muscle satellite cells in a particular state (Ontell 1974). Sometimes descriptions were “persuaded” by presumptions about the process under investigation. For example, a description of fiber fragmentation (Murray and Kodicek 1949) refers in error, to two structures shown in figures that are actually muscle spindle fibers and “cuffs” of myoblasts and myotubes within endomysial sheaths of fiber remnants. Since such observations, confounded by interpretation or assumption, can linger in the literature and our brains, their influence on understanding may persist longer than new evidence. There is real merit to digging back with a skeptical lens, through classical reports to explore the basis of our current etymologies and insights. Often, though, early descriptions are valuable, and serve as a caution when we consider an observation is “the first report” on a topic.
Muscle satellite cells
Histology studies were quickly augmented by major advances in development of electron microscopy techniques in the 1950s. Techniques for ultrathin sections and electron-dense stains led to Mauro’s report (1961) of a presumptive muscle precursor cell, termed the satellite cell due to the position of those cells on muscle fibers. The same cells were the subject of speculation by Church et al. (1966), Shafiq et al. (1967), and Carlson (1968) who suggested they may become activated into myoblast precursors for muscle formation. By 1966 (Church et al. 1966), satellite cells were known to survive muscle damage and serve as reserve cells that transform into myoblasts that proliferate and form new fibers.
The 1969 monograph on Regeneration of Striated muscle, and Myogenesis (Mauro et al. 1970) illustrates the intense discussions during an international conference at the Institute for Muscle Disease in New York on the time-course of regeneration in amphibians and rodent muscle. Attendees at the conference also discussed the myoblast cell cycle, myoblast fusion into myotubes in vivo and in vitro, and the ultrastructural morphology of the satellite cell. In 1968, Carlson showed that muscle fibers regenerate with guidance from the internal structure of connective tissue in a muscle, such that new fibers are aligned to the direction of tension in the muscle regenerated from minced fragments, and that early muscle regeneration occurs independent of nerve supply (Carlson 1968).
In 1975, Bischoff (1975) reported use of a method of culturing single muscle fibers to examine the population of inactive satellite cells in residence on isolated fibers. In addition, he used a direct method, autoradiography to track the entry of satellite cells into the cell cycle, by their uptake of tritiated-thymidine into new strands of DNA during mitosis. Although immune detection methods are much faster, autoradiography is still a valuable technique, especially in combination with other methods of tracking gene expression and the histology of regenerative processes (Anderson et al. 1996, 1998a; McIntosh et al. 1998). By the 1980s, research had identified differences between muscle development in the embryo and muscle regeneration in adults (e.g., Hansen-Smith and Carlson 1979; Carlson and Faulkner 1983) and many details of the time-course of muscle regeneration (e.g., Aloisi 1970; Reznik 1970; Shafiq 1970; Hall-Craggs 1980; Maxwell et al. 1984; Schultz and Jaryszak 1985; Ontell 1986; Grounds 1987; Grounds and McGeachie 1987; McGeachie and Grounds 1987; Brooks and Faulkner 1988; Carlson and Faulkner 1989).
Muscle fiber types
In 1968, McComas and Thomas (1968) reported electrophysiology studies that implicated the existence of slow and fast muscles in humans. In 1976, Khan confirmed the likelihood that slow-twitch muscle had the same histochemical pH-dependent staining pattern as type-I oxidative fibers (Khan 1976b) and had identified fast-oxidative-glycolytic fibers (type-IIA) (Khan 1976a). By the end of the 1980s, there was a burgeoning literature describing fiber-typing studies that compared different muscles in a huge range of species, and during development, adaptation, aging, regeneration, reinnervation, metabolic shifts, and disease (McComas and Thomas 1968; Tomonaga 1977; Haggmark et al. 1981; Haggmark et al. 1986; Staron and Pette 1986, 1987a, b; Anderson et al. 1988; Coulton et al. 1988; Jakobsson et al. 1988; Termin et al. 1989; Dusterhoft et al. 1990; Klitgaard et al. 1990; LaFramboise et al. 1990; Parry and Wilkinson 1990; Goldspink, 1991; Coggan et al. 1992; Welle et al. 1993).
The basics of myogenic regeneration
Myogenic regeneration begins with damage to fibers that degenerate and are removed by phagocytosis. Damage also activates satellite cells which proliferate as spindle-shaped myoblasts that cycle, fuse and form new syncytial fibers, initially with central nuclei. Myotubes subsequently elongate toward attachment sites at tendons and on bone. Bone also responds to the presence or loss of pull from muscle (Anderson et al. 1993). Tissue repair in a muscle belly can be highly successful, comprising restoration of many types of tissue: myogenic, vascular (initially capillaries), nervous (initially Wallerian degeneration of axons and then elongation of growth cones through any surviving nerve stumps, or toward NMJs on remnant tubes of endomysial basal lamina tubes (previously surrounding fibers), and connective tissues (extracellular matrix (ECM) including endomysium, perimysium, epimysium, and tendon connections to fibers). Regenerated muscle fibers are typically aligned to the original orientation of fibers.
Given the architecture of skeletal muscle tissue and the wide range of injuries and diseases that provoke muscle to regenerate, it is important to remember that a reproducible approach to sampling the regeneration time-course requires attention to the location and type of injury in the tissue and the time elapsed since injury. Regeneration is typically localized around an area of injury, with some extension into the surrounding undamaged tissue due to the migration of myoblasts along fiber and endomysial remnants in the ECM (McIntosh et al. 1994). Injury can be very focal, more broadly segmental across a short portion of the fiber length as in muscular dystrophy, or widespread as in avascular necrosis modelled by denervation–devascularization injury (Anderson 1991; Mechalchuk and Bressler 1992; Lefaucheur and Sébille 1995). Thus, the extent and location of initial tissue damage, whether it is synchronous or sporadic and recurrent, and its homogeneity in a muscle belly should determine the timing and location of any approach to investigating the time-course or outcome of regeneration. It is useful to remember that delayed regeneration is not necessarily defective.
Early concepts
-
i.
Myogenic regeneration follows a similar pattern and sequence, albeit with different timing, across species and many types of damage; the pattern differs from the epimorphic regeneration of limbs in urodele amphibians.
-
ii.
Muscle tissue regeneration can be very successful but does not always restore function.
-
iii.
Large areas of damage often leave a deep fibrotic scar.
-
iv.
Cells in skeletal muscle can form bone, adipose, and fibrous connective tissues.
-
v.
Regeneration from ischemia–reperfusion injury has a less functional outcome than after anoxia.
-
vi.
Assessing muscle function to track regeneration requires standardized protocols and tools.
The current context
Myogenic regeneration is the subject of many excellent reports and reviews (Charge and Rudnicki 2004; Ciciliot and Schiaffino 2010; Carosio et al. 2011; Tidball 2011; Musaro 2014; Dumont et al. 2015a; Domingues-Faria et al. 2016; Qaisar et al. 2016; Joanisse et al. 2017; Le Moal et al. 2017; Zammit 2017; Cornelison 2018; Li et al. 2018; Wosczyna and Rando 2018; Chen and Shan 2019; Chazaud 2020; Forcina et al. 2020), and there are many more related to the benefit of activity or exercise, the impact of aging, differential injury-dependent regenerative responses, roles of microRNAs in regulating the stem cell niche, and the responsiveness of satellite cells. Although selected topics in the overall process will be highlighted, this review is primarily focussed on a high-level synthesis rather than a comprehensive overview of the wealth of important or essential factors involved in orchestrating effective myogenic regeneration and muscle tissue repair.
Injury
A diverse range of injuries leads to muscle regeneration, and some damage to fibers is needed to prompt the onset of the muscle regeneration cascade; this is a truly beautiful example of tissue homeostasis in action (Musaro 2014; Musarò 2020). The impact of trauma changes the environment of all types of proximate and surrounding cells and the acellular matrix, and cells respond in marvelously coordinated fashion toward resolving an injury and restoring muscle function. However, the extent to which muscle-tissue damage is followed by regeneration and functionality is highly dependent on many variables. There are considerations about the initial insult, such as whether it is an acute or chronic injury or a single or repetitive injury; the volume of tissue injury and its distribution (widespread or focal) are also important. The age of the individual or animal are notable features as well, since age affects the number of satellite cells, the state of satellite cells (activation, senescence, stemness and self-renewal capacity, and exhaustion), and their responsiveness to muscle injury. Age also affects the composition and character of the ECM, the vascular supply, innervation, insertion points on bone, and any accrual of prior injury, scarring or disease conditions. The nature and configuration of the ECM (fibrotic or not) are additional determinants to the ultimate recovery of function, as is the possible involvement of denervation that may accompany the injury to fibers.
The timing by which inflammation is resolved has a major impact on functional recovery, as is the timing of vascular regeneration to ensure blood supply for debris removal and support regenerating tissue. Adiposity within muscle fascicles and the level of physical activity prior to an injury also play into the timing and extent of recovery, along with stresses of metabolic disease, transient systemic conditions, and/or the secondary impact of genetic, biochemical, developmental or epigenetic modifications to cells or molecules that mediate the outcome of regeneration, as reviewed elsewhere (Urso 2013; Mok et al. 2017; Robinson and Dilworth 2018; Mitchell et al. 2019; Long et al. 2021; Machado et al. 2021; Vechetti et al. 2021; Wen et al. 2021).
The discovery of muscle regulatory factor genes (MRFs) was a remarkable turning point in our understanding of the embryological development of muscle (Esteves de Lima and Relaix 2021); the four MRFs are re-expressed during the post-natal myogenic process of muscle regeneration. Expression of the first two MRF genes, MyoD and Myf5 by satellite cells increases after satellite cell activation. MyoD and Myf5 regulate the commitment of daughter myoblasts into the muscle lineage and myoblast proliferation, and induce expression of myogenin. Myogenin and MRF4 are expressed soon after under appropriate conditions, and induce differentiation (Bentzinger et al. 2012; Knappe et al. 2015; Zammit 2017). Pax7 and Pax3 function in advance of MRFs in postnatal myogenesis (Buckingham and Rigby 2014; Buckingham and Relaix 2015).
Satellite cell activation, stemness, and motility
Initial damage induces degeneration of fibers and at the same time, activates muscle satellite cells, the stem cells that possess the all-important dual capability of self-renewing the stem cell compartment and producing committed myogenic cells. Satellite cells activation and cell cycle entry are regulated through Notch Wnt-signaling pathways (Conboy and Rando 2002; Conboy et al. 2003; Dhawan and Rando 2005; Luo et al. 2005; Le Grand and Rudnicki 2007; Srivastava et al. 2010; von Maltzahn et al. 2012; Subramaniam et al. 2013). It is well established that muscle satellite cells are a heterogeneous population (Tierney and Sacco 2016) that is regulated to maintain quiescence (Subramaniam et al. 2013; Arora et al. 2017; van Velthoven et al. 2017; Purohit and Dhawan 2019; Puri et al. 2021). Satellite cells are rapidly activated in response to damage, mechanical stretching and nitric oxide release, exercise and physical activity (Anderson 2000; Anderson and Pilipowicz 2002; Wozniak et al. 2003; Wozniak and Anderson 2007, 2009; Wang et al. 2009). Such regulation is affected by aging (Brack et al. 2005, 2007; Brack and Rando 2007; Collins et al. 2007; Joanisse et al. 2017; Snijders and Parise 2017; Hwang and Brack 2018; Tierney et al. 2018, 2019; Kimmel et al. 2020).
Recent study of the capacity for stem-cell renewal—by way of CD34 expression modulated through insulin-like growth factor-1 (IGF1)-mediated Akt activation that reduces CD34 expression by inhibiting FoxO—introduced even further complexity in satellite cell heterogeneity. Experiments identified a state of “primed stemness” (cells with myogenic commitment) and more prevalent “genuine stemness” in muscle of younger animals (García-Prat et al. 2020), expanding on earlier studies of CD34-mediated satellite cell motility (Alfaro et al. 2011).
Although the proportion of the satellite-cell population with genuine-stem capability declines with age (Day et al. 2010; García-Prat et al. 2020) and satellite cells become increasingly refractory to stretch-induced activation with age (Leiter and Anderson 2010), those cells retain the capability of responding to damage and producing effective repair in old animals (Smythe et al. 2008; Shavlakadze et al. 2010; Lee et al. 2013; Domingues-Faria et al. 2016; Snijders and Parise 2017; Franco et al. 2019; García-Prat et al. 2020; Kimmel et al. 2020; Yamakawa et al. 2020; Delsmann et al. 2021). Thus, satellite cells clearly respond to mechanical stimulation through exercise (Wozniak et al. 2003; Gomes et al. 2004; Tidball 2005; Tatsumi and Allen 2008; Wozniak and Anderson 2009; Leiter and Anderson 2010; Tatsumi 2010; Hara et al. 2012; Zhang and Anderson 2014; Li et al. 2018; Eliazer et al. 2019); that and the regenerative response to injury are muscle-specific and age-dependent (Leiter and Anderson 2010; Gigliotti et al. 2015, 2016).
The timing of satellite cell activation is important in muscle tissue regeneration. Premature activation before injury, established using treatment with a nitric oxide donor, can accelerate myogenesis following a myotoxic or crush injury, whereas the restoration of NMJs on newly formed myotubes is disrupted (Daneshvar et al. 2020). This reminds us that the desired outcome of regeneration, strength and voluntary control, results from coordinated nerve–muscle interactions, and that myogenesis is only one, albeit an important aspect of muscle-tissue regeneration. Interestingly, experiments with nitric oxide-donor treatments in mice also improve regeneration and reduce exercise-induced damage in dystrophic mice (Archer et al. 2006; Wang et al. 2009; Mizunoya et al. 2011), attenuate disuse atrophy after hindlimb suspension (Anderson et al. 2017b), enable exercise-induced hypertrophy in atrophic muscle of old mice (Leiter et al. 2012). While many mouse studies highlight the exciting potential for treating human Duchenne dystrophy via nitric oxide donors (Sciorati et al. 2006, 2010, 2011, 2013; Brunelli et al. 2007; De Palma and Clementi 2012), simply increasing its bioavailability by inhibiting phosphodiesterase can be detrimental (Timpani et al. 2020). Further investigations of applications to safely deliver nitric oxide to replace the normal functional release during muscle activity and mechanical stretch (Wozniak and Anderson 2007, 2009) in a dosing regimen that avoids excessive oxidative stress could promote muscle growth and regeneration, and benefit people with dystrophy, disuse atrophy, and sarcopenia (Alrushaid et al. 2018). Satellite cells are essential for myogenesis, hypertrophy during maturation, and regeneration. By contrast, muscle hypertrophy in adults can occur without satellite cell contributions to myofibers. However, that hypertrophy ultimately restricts growth, vascular arborization, strength, and remodelling of collagen, which affect longer term plasticity and adaptability (McCarthy et al. 2011; Jackson et al. 2012; Fry et al. 2014, 2015; Englund et al. 2020, 2021). The elegant experimentation demonstrated that the satellite cell population affects expression by myonuclei inside fibers, possibly through their production of extracellular vesicles. To quote a perspective on the paper, “in essence, satellite cells are more than just myonuclei in waiting, capable of affecting muscle health in a fusion-independent fashion”(Hawke 2020).
Migratory movements by myoblasts and others cells are implicated as determinants of the success of muscle regeneration (Siegel et al. 2009, 2011; Alfaro et al. 2011; Stark et al. 2011; Kowalski et al. 2017). Myoblast movement is itself, mediated from the supporting substrate containing fibronectin, which regulates Wnt7a signaling and myoblast proliferation (Bentzinger et al. 2013, 2014). The research platform of microfluidics technology with applications to tissue engineering, is a powerful tool in studying migration. Microfluidics experiments provide major advances over earlier approaches to the real-time visualization of cellular physiology under exquisitely controlled conditions (Roveimiab et al. 2019) and disease modeling (Wang et al. 2021). For example, our recent microfluidics study explored the mechanisms by which the niche substrate has significant impact on responses by myoblasts (Roveimiab et al. 2020). Myoblast interactions with the substrate composition (collagens, fibronectin, laminin, etc.) as well as the extent and nature of connective tissue elements in the substrate, affect myoblast migration by the complex process of haptotaxis. Very recent experiments with inhibitors (Roveimiab, Lin and Anderson, unpublished) suggest a hierarchy of disruptions to different aspects of adhesion, speed and direction of movement, and cell–cell fusion result from interference in the fibronectin-integrin signaling pathway, affect the alignment of one myoblast and its juxtaposition toward the nucleus of a differentiated myotube. The movements of individual cells play a significant role in the outcome of myogenesis during muscle-tissue regeneration.
In addition, soluble elements in the niche also provide input to guide myoblast movements including the speed and direction of migration along isolated fibers (Siegel et al. 2009, 2011) or within a microfluidic device (Roveimiab et al. 2020). In particular, hepatocyte growth factor (HGF), also called scatter factor, binds to the “motogenic” c-met receptor expressed by both activated and quiescent satellite cells (Hartmann et al. 1992; Sonnenberg et al. 1993; Cornelison and Wold 1997; Dietrich et al. 1999; Webster and Fan 2013). HGF delivers a potent signal for positive chemotaxis and mobilizes populations of myoblasts (Roveimiab et al. 2020). Traction enabled by a haptotaxis substrate interacts with the configuration of the chemokine signal provided by the concentration or a directional gradient of HGF (Roveimiab et al. 2020). Even more complex is the signaling among different cell types including leukocytes (Panci and Chazaud 2021) and other inflammatory cells (Sakaguchi et al. 2014; Chen et al. 2015; Ferreira et al. 2015; Saini et al. 2016) in the environment of muscle precursors that are mobilized by injury.
Recently Hox10 expression and epigenetic methylation events at the Hox-A locus were found to mediate the topographical distribution of differential responsiveness by adult muscle stem cells (Yoshioka et al. 2021). This paper recalled earlier reports on cranial muscles that present continuous “remodeling” through satellite cell turnover and fusion into undamaged muscle fibers above levels seen in limb muscle fibers (e.g., McLoon and Wirtschafter 2002, 2003; McLoon et al. 2004). In these cases, there was higher Hox-A hypermethylation and Hox-A and Hox-C cluster gene expression, both robustly sustained after transplantation and regeneration by engrafted satellite cells. Loss of Hoxa10 expression by postnatal muscle reduced the capacity for regeneration, indicating that satellite cells display a fascinating “positional memory” based on developmental topography (including distinction between limb muscles and those innervated by cranial nerves). This memory-of-source feature of regeneration did not affect Pax7 or MyoD expression, the size of the satellite cell population or its ability to become activated. However, with Hoxa10 inactivation, satellite cells resident on fibers cultured ex vivo, were not as good at migration—an essential process in myogenic repair; they also had impaired proliferation without displaying apoptosis. Rather, satellite cell-specific disruption of Hoxa10 affected genes important in chromosomal segregation and spindle formation during mitosis, and in limb muscle, induced changes that led to the formation of micronuclei and chromosomal bridges without affecting myogenic differentiation or self-renewal capacity. Human satellite cells in culture mirrored the position-dependent distinctions in cell-cycling, gene expression, and the impact of HOXA10 knockdown by short interference RNA (siRNA) found in the transgenic mouse experiments. This report opens a new avenue toward understanding regional and muscle-specific differences in regeneration capacity and plasticity of limb muscles toward adaptation.
Two ideas: that the potential of muscle for regeneration and plasticity is regionalized, and that stem-cell function is influenced by epigenetic acetylation of Pax7 (Sincennes et al. 2021), together bring new implications to considering epigenetic modifications in responses to exercise, training, aging, hormones. Such ideas also raise to mind the regional patterning of genetic neuromuscular diseases such as the family of muscular dystrophies involving disruptions of the dystrophin-associated cytoskeleton (Sunada and Campbell 1995; Cohn and Campbell 2000; Durbeej and Campbell 2002). Epigenetic modifications to post-mitotic muscle fibers, recently identified to involve long non-coding RNA molecules, also affect adaptive responses to stress (El Said et al. 2021). The full-length influences of RNA sequences will likely be revealed to have further impact on skeletal muscle adaptation and repair.
Inflammation
Inflammatory cells are resident (as macrophages) in muscle tissue and attracted to the site of injury. They promote sarcolemmal repair, fiber growth, and regeneration (Cheung and Tidball 2003; Tidball and Wehling-Henricks 2007) in addition to removing damaged tissue following muscle injury. Macrophages resident within muscle tissue are highly plastic in establishing the stem cell niche and interact with satellite and other cells (Ratnayake et al. 2021). Experiments using a beautifully designed system to follow individual macrophages in real time in living zebrafish (Montandon et al. 2021) revealed that there are early interactions of muscle-resident macrophages with satellite cells through a macrophage-secreted compound, nicotinamide phosphoribosyl transferase (NAMPT). NAMPT in turn, acts via the CCR5 chemokine receptor and establishes a short-lived niche that activates satellite cells; the same signaling contributes to regulate the pace and timing of myogenic regeneration (Ratnayake et al. 2021).
Muscle stem cell interactions with pro-inflammatory macrophages are thus very broad, and as macrophages rapidly shift to become anti-inflammatory, clues for both myogenic and non-myogenic processes are compiled over time through interactions of immune cells including macrophages with muscle stem cells (Chazaud 2020). Recent literature highlights the notion that macrophage activity determines whether repair is regenerative or fibrotic (Moyer and Wagner 2011; Muñoz-Cánoves and Serrano 2015; Juban et al. 2018; Yang and Hu 2018) through considerable cross-talk with muscle cells (Mann et al. 2011; Muñoz-Cánoves et al. 2013; Yang and Hu 2018). Thus, there is a balance between inflammation and myogenic repair in regenerating muscle: the inflammatory response to damage will ideally maximize the prospects for an effective regenerative response while avoiding a large catabolic response (Urso 2013).
The timing of macrophage transition from pro- to anti-inflammatory—required to trigger the resolution of inflammation during muscle regeneration—is critical, since early and late transition are both detrimental for recovery (Chazaud 2020). Signals that resolve inflammation begin when prostaglandins and other proinflammatory signals are inactivated or blocked; inflammatory cells are then removed from the damaged tissue by reverse migration, drainage and cell apoptosis, and apoptotic neutrophils (to minimize secondary necrosis) are cleared from the tissue. The transition to an anti-inflammatory state is facilitated by macrophage plasticity (Sugimoto et al. 2019).
Notably, muscle regeneration is impaired in autoimmune myopathies that result from inflammatory cell activation by antibodies against signal-recognition protein or HMG-CoA reductase; the impairment, a defect in myoblast fusion and fiber formation, was associated with deficient levels of IL-4 and IL-13 (Arouche-Delaperche et al. 2017). Thus, inflammation, its resolution and its etiology including direct muscle infection by viruses such as Zika, arboviruses, and SARS-CoV-2 (COVID-19) (Disser et al. 2020; Filippone et al. 2020; Legros et al. 2020; Paliwal et al. 2020), are important during regeneration.
Fibro-adipogenic precursors
Fibro-adipogenic precursors (FAPs) in muscle are derived from neural crest; since the first report in 2010, FAPs are now understood to play key roles in configuring lineage determination and the outcome of proliferation by mesenchymal cells—both essential in regeneration of muscle (Joe et al. 2010; Uezumi et al. 2010; Theret et al. 2021) and other tissues (Natarajan et al. 2010; Lemos et al. 2012; Paylor et al. 2014; Giuliani et al. 2021). FAPs are non-myogenic and give rise to fibroblasts and adipocytes, depending on signals such as from the innate immune system that regulates eosinophil and anti-inflammatory macrophage activity soon after tissue damage. Interleukin signaling, specifically IL-4 from eosinophils, promotes FAP proliferation and restricts the progression of FAPs to adipocytes. Notably, FAPs also mediate the removal of damaged, necrotic tissues after injury or in culture, and stimulate myogenic differentiation and MRF-gene expression by muscle precursors (Heredia et al. 2013).
Recently, a subset of FAPs that express Gli1 and Hh (hedgehog) proliferates rapidly after muscle injury and was identified as being responsible for restricting the development of adipose tissue during muscle regeneration. The role of Gli1-positive FAPs was emphasized by genetic ablation of Gli1-expressing cells, which impaired muscle regeneration through a transient expansion that produces paracrine signaling factors such as IL-6 and IGF-1 that act on satellite cells (Yao et al. 2021). That fibrosis—another essential aspect of muscle regeneration—reduces satellite cell proliferation and delays fiber growth during the regenerative response when FAP proliferation is inhibited (Fiore et al. 2016). Essentially, although the influences of FAPs during muscle repair may be short-lived, they make major contributions to the overall “tuning” of different molecular players regulating myogenic and other stem cells (Biferali et al. 2019; Forcina et al. 2020). Interactions of FAPs with fibroblast growth factor 2 (FGF2) through via miRNA-29a signaling promotes formation of adipose tissue in muscle during aging (Mathes et al. 2021).
Fascinatingly, fibroblasts were recently shown to fuse to skeletal muscle, a feature that enables the transfer of fibroblast-specific mRNAs that facilitate the transition of the fiber toward its tendinous attachment sites (Yaseen et al. 2021). That report solved a puzzle from earlier electron microscopy observations of muscle regeneration (Anderson 1991).
Very recently, direct isolation of FAPs, muscle stem cells and macrophages from human skeletal muscle biopsies, using a combination of fluorescence-activated cell sorting and mRNA sequencing at the single-cell level, was found to preserve the phenotypic behavior and expression of those cells (Jensen et al. 2021). The transcriptomics approach should prove exceedingly useful in translating discoveries of FAP contributions to muscle regeneration, and age-related changes in muscle tissue.
Extracellular matrix
The ECM plays a major role in muscle health, development, disease, and regeneration (Goetsch et al. 2003; Csapo et al. 2020; Forcina et al. 2020). Dynamic ECM interactions with every type of cell in the tissue (Cisternas et al. 2014; Dunn et al. 2018, 2019; Marcinczyk et al. 2019; Patel et al. 2019) occur through enzymatic degradation and remodelling by proteinases, including matrix metalloproteinases (Chen and Li 2009; Lu et al. 2011). The ECM also serves as a reservoir for growth factors, including HGF and IGF-1, with which matrix metalloproteinases can interact to promote regeneration (Kok and Barton 2021). ECM actually promotes muscle regeneration (Kuraitis et al. 2012), in part by haptotaxis-induced myoblast migration (Roveimiab et al. 2020). And, ECM proteins mediate adhesion or attachment of muscle stem cells, meaning the ECM mediates satellite cell responses to mechanical activity and tissue perturbation (Li et al. 2018; Moyle et al. 2020).
The ECM forms a complex three-dimensional meshwork of proteins that surrounds myogenic and vascular cells, fibroblasts, and nerves within muscle tissue; that meshwork is synthesized and then modified by epigenetic and post-translational modifications with important implications in disease pathophysiology (Theocharis et al. 2016) and stem cell function (Sincennes et al. 2021). Proteins such as proteoglycan, integrin, collagen, fibronectin, hyaluronan, elastin, tenascin, and others, form a vast reference “library” that participates in any aspect of cell physiology, including regeneration. Realignment, damage, and remodelling of the ECM during regeneration also affect function and the outcome of regeneration, partly through cell attachments to the ECM through growth factor and other ligands it displays for binding with receptor-expressing cells. Such binding thus enables the internal actin cytoskeleton of those cells to be polarized according to their residency and migration within the interstitial space (Li and Gundersen 2008).
Our understanding of the signaling interplay between myogenic cells (and other cells) with components of the ECM and basement membrane adherent to cells (Pozzi et al. 2017) through receptors, proteolysis, and unmasking of cryptic sites in ECM proteins (Clause and Barker 2013; Brown et al. 2015; Barker and Engler 2017; Yeh et al. 2021) has shown major recent advances. These new ideas have brought biomechanics, tissue engineering, and nano-scale three-dimensional scaffold production into the realm of therapeutics for muscle regeneration (Turner and Badylak 2012; Choi et al. 2018; Dunn et al. 2019; Marcinczyk et al. 2019; Patel et al. 2019; Baiguera et al. 2020; Gilbert-Honick and Grayson 2020; Mihaly et al. 2021), promising prospects for implantable volumes of muscle tissue that will promote the ingrowth of vessels and nerves (Laumonier and Menetrey 2016; Gilbert-Honick and Grayson 2020). Interstitial fibroblasts between muscle fibers, produce many components of the extracellular matrix (ECM) and wrap around collagen cables (Gillies and Lieber 2011; Gillies et al. 2017).
Excessive deposits of connective tissue are detrimental to muscle function in muscular dystrophy, as suggested over 40 years ago (Duance et al. 1980); that impact is now well established in physiological experiments, computational modeling (Mann et al. 2011; Martin et al. 2016; Virgilio et al. 2021), and the clinical literature (Best et al. 2013; Lieber and Ward 2013; Teixeira and Duarte 2016). For example, the negative functional impact of extensive fibrosis and collagen deposition in skeletal and cardiac muscle of dystrophic mdx mice was demonstrated by functional gains after an anti-fibrosis treatment that reduced expression of collagens I and III. Treatment attenuated dystrophic damage due to the tissue stiffness that affects myofibers (Huebner et al. 2008), the satellite cell niche (Moyle et al. 2020), satellite cell proliferation, and myogenesis (Teixeira and Duarte 2016).
Vascular tissue
The supply and configuration of blood vessels around, along, and sometimes within muscle fibers is critical to regeneration and maintenance of muscle. Plasma carries potent systemic factors, and proteins including hormones, and cytokines (some anabolic and/or catabolic) (Conboy et al. 2005; Brack et al. 2007; Cornish et al. 2020a) while perfusion removes waste products and conveys red blood cells. Vascular architecture itself regenerates differently among various models of injury by freezing or toxicity (Hardy et al. 2016). Muscle satellite cells in particular, survive the loss of vascular supply and local ischemia, and will actively recruit endothelial cells to their niche during muscle regeneration (Collins and Kardon 2018). Notably, the impact of many circulating factors including metabolic regulators and signaling ligands conveyed via vascular supply, will depend on revascularization in regeneration.
Cell–cell cross-talk
Clearly there is cross-talk—a “social network”—among cells and between cells and the abiotic environment of degenerating and regenerating muscle tissue (Wosczyna and Rando 2018; Biferali et al. 2019; Scognamiglio et al. 2020). Drawing a parallel between populations of organisms and populations of cells in muscle tissue [similar to cancerous cells (Somarelli 2021)], such a network could be considered a “cellular ecosystem,” since both populations are influenced by their environment (as in ecology, the interactions of organisms to each other and their surroundings), during adaptation and regeneration. There is very keen interest in what drives the outcome, which cell or combination of cells coordinates the many possible responses by other cells, and a strong push to identify the signaling networks that integrate cellular, tissue, and systemic processes. For example, research on the dystrophin cytoskeleton connected to ideas of mechanically induced satellite cell activation by transients of nitric oxide (NO) gas (Wozniak et al. 2003, 2005; Wozniak and Anderson 2005, 2007, 2009). Other research on activity-induced angiogenesis (McAllister et al. 2008; Alfaro et al. 2011) and changes in NO concentration during muscle disuse, aging or dystrophin deficiency (Anderson 2000; Anderson and Pilipowicz 2002; Tatsumi et al. 2002; Anderson and Wozniak 2004; Wozniak and Anderson 2005; Leiter et al. 2012; Janke et al. 2013; Allen et al. 2016; Anderson et al. 2017b; Rogers et al. 2017) are related. The important structural role of dystrophin in the dystroglycan complex, and its anchorage of neuronal NO synthase are considered to “orchestrate” the epigenetic profile of muscle cells and affect adipogenesis through miRNA regulatory pathways during differentiation and regeneration (Marrone and Shcherbata 2011).
Redox control mechanisms also contribute to the process and outcome of muscle regeneration (Le Moal et al. 2017) through NO mediation of initial inflammation, myogenic repair, and the FAP-mediated processes of fibrosis and adipogenesis (Filippin et al. 2009, 2011a, b). Potential therapeutics to manipulate NO concentration or the NO-cGMP signaling pathway that could promote muscle regeneration or growth, attenuate muscular dystrophy, or prevent muscle-disuse atrophy were broadly explored (Archer et al. 2006; Pisconti et al. 2006; Tatsumi et al. 2006, 2009b; Betters et al. 2008a, b; Yamada et al. 2008; Heydemann and McNally 2009; Song et al. 2009; Wang et al. 2009, 2018; Wozniak and Anderson 2009; Li et al. 2010; Tatsumi 2010; Villanueva and Giulivi 2010; Filippin et al. 2011a, b; Mizunoya et al. 2011; Janke et al. 2013; Bonafè et al. 2015; Gigliotti et al. 2015; Aguiar et al. 2017; Anderson et al. 2017b). Mechanically mediated cellular interactions and the potency of NO signaling to satellite cells and surrounding blood vessels must be placed in context with the overarching genetic regulation of satellite cell activation and quiescence by Wnts, Notch, quiescence-specific gene-signaling networks, and epigenetics.
Satellite cells recruit endothelial cells and also promote angiogenesis by secreting vascular endothelial growth factor (VEGF); in turn, endothelial cells promote satellite cell expansion (Latroche et al. 2015). Satellite cells also pattern the architecture of nearby capillaries through VEGF-A signaling, and endothelial cells produce the Notch ligand Dll4 that helps sustain the stemness of satellite cells close to vessels by maintaining their quiescence (Verma et al. 2018). The proximate, even intimate, juxtaposition of two cell types, in this case endothelial and muscle satellite cells, has potent meaning for interpretation of regenerative signaling, just as the proximity of satellite cells on the fiber sarcolemma has for mechanical signal transduction in satellite cell activation (Anderson 2000; Wozniak et al. 2003; Wozniak and Anderson 2009) and satellite cell influences on myonuclei and fibroblasts (Englund et al. 2021). Satellite cell interactions with macrophages include receiving macrophage-derived HGF which mediates secretion of semaphorin3A (Sema3A) by myoblasts (Yamada et al. 2010; Do et al. 2011; Sato et al. 2013; Sakaguchi et al. 2014). In turn, this influences many cells involved in muscle regeneration (Anderson et al. 2017a), and the development of slow fibers through neuropilinA–plexinA3, myogenin, and Mef2D signaling to slow myosin expression (Tatsumi et al. 2017). Importantly, Sema3A is implicated in synchronizing motor neurite elongation during the re-establishment of NMJs (Tatsumi et al. 2009a; Sato et al. 2013).
It is interesting to consider that while FAPs contribute to regulating satellite cell activity, satellite cells also configure the responsiveness of fibroblasts in the niche around fibers during hypertrophy (Fry et al. 2014). This cross-talk among cells during muscle regeneration thus has a systemic context (Pillon et al. 2013; Xie et al. 2013; Ferreira et al. 2015; Anderson et al. 2017a; Argiles et al. 2016; Belizário et al. 2016; Domingues-Faria et al. 2016; Gorski and Price 2016; Collins and Kardon 2018; Yang and Hu 2018; Chazaud 2020; Daneshvar et al. 2020), and that context means that cell-level responses at the local area niche (Mashinchian et al. 2018) integrate all the interactions among satellite cells, fibers, macrophages, terminal Schwann cells (possibly via Sema3A), the ECM (Huijbregts et al. 2001), and circulating factors such as cytokines, growth factors, and many other molecules.
Nerve–muscle interaction
The fascinating intricacies of nerve–muscle interaction, reviewed elsewhere (Delbono 2011; Shi et al. 2012; Blaauw et al. 2013; Witzemann et al. 2013; English et al. 2014; Tintignac et al. 2015; Gordon and English 2016; Gordon and Borschel 2017; Cornish et al. 2018; Macefield and Knellwolf 2018; Lepore et al. 2019; Rudolf et al. 2019; Swenarchuk 2019; Gordon 2020), underlie the essence of voluntary muscle function after regeneration, and depend on the extent and location of initial and secondary damage to fibers. Terminal Schwann cells (TSCs) bridge the synaptic cleft between axon terminals and the specialized sarcolemma at the NMJ (Barik et al. 2016), help maintain synaptic structure (Reddy et al. 2003; Feng et al. 2005; Feng and Ko 2008), and also contribute to the satellite cell niche. Recent experiments from our lab further implicate satellite-cell derived Sema3A secretion in mediating interactions of satellite cells with terminal Schwann cells during muscle regeneration and reinnervation processes (Daneshvar et al. 2020) (and Daneshvar, Matsuyoshi, Tatsumi, and Anderson, unpublished).
Physical activity and exercise
Physical activity and exercise induce muscle plasticity and adaptive responses in the absence of injury. During activity, fibers release myokines (cytokines) that exert autocrine, paracrine and systemic effects on muscle fibers (anabolic and catabolic) and satellite cells (Bugera et al. 2018; Cornish et al. 2018, 2020a, b). Those actions are integrated into the network of signals released from inflammatory and other cells (Febbraio and Pedersen 2005, 2020; Pedersen and Febbraio 2008, 2012) including bone and adipose tissues (Kirk et al. 2020). Regeneration of muscle after injury is significantly enhanced by exercise especially in synergy with injection of muscle stem cells (Contreras-Muñoz et al. 2021). Physical activity also modulates the immune response, releasing growth factors that promote angiogenesis (Gregory et al. 1995; Brutsaert et al. 2002; Faria et al. 2008; Aurora et al. 2014), and muscle contractile activity induced by electrical stimulation promotes nerve repair by upregulating neurotrophins and brain-derived neurotrophic factor (Gordon and English 2016).
Interestingly, optimal fiber hypertrophy requires satellite cell proliferation and accrued contributions of their daughter cells toward fusion, fiber hypertrophy is possible through myonuclear domain expansion without satellite cell contributions, and satellite cells are not essential for fiber-type adaptations to life-long physical activity (Egan and Zierath 2013; Qaisar et al. 2016; Englund et al. 2020). One form of severe muscle atrophy in humans is associated with admission to an intensive care unit (ICU) and is exceedingly resistant to treatment; it is attributed to the critical illness behind admission. A recent review distinguished ICU-acquired weakness by the predominant loss of myosin that affects both type-I (slow-twitch) and type-II (fast-twitch) fibers, and suggested resistance exercise during restricted blood flow (BFR) as a potential treatment (Lad et al. 2020). Metformin, a drug compound used to increase glucose transport into muscle and reduce blood glucose, reduces hypertrophy and transcriptional responses of skeletal muscle to resistance exercise training (Walton et al. 2019; Kulkarni et al. 2020). Interestingly, recent findings on a trial of BFR resistance-exercise training suggested that changes in systemic concentrations of a subset of myokines show differential responses based on age and resistance training, in association with increases in muscle strength and quality (Cordingly, Anderson, and Cornish, submitted).
A very recent paper demonstrated that exercise training in adult mice has differential impact on DNA methylation in muscle and non-muscle interstitial cells; detraining and also retraining demonstrated further differential responses, implicating “epigenetic memory” of prior adaptation that may help account for well-established differential response to exercise among muscles (Lavin et al. 2021; Sharples 2021; Wen et al. 2021). Of note, that even non-muscle cells respond to exercise training highlights the system-wide impact of physical activity.
Metabolism
Systemic and muscle-tissue metabolism, including variations due to nutritional status, metabolic diseases (e.g., cachexia), and non-neuromuscular conditions such as diabetes and non-alcoholic fatty liver, affect the outcome of muscle regeneration—so many features of muscle growth, power, strength, endurance, and plasticity are reliant on anabolic processes. Anything that impacts protein synthesis, turnover, and remodelling—including responses to systemic stress—will reduce the capacity for timely regeneration (i.e., the speed of tissue repair) if not its effectiveness. The issue of protein turnover is especially important in interpreting results, given that use of transgenic technologies to conditionally alter the expression of particular genes in a cell or tissue of interest at a particular age, are typically validated by testing the level of mRNA or protein expression from tissue, without identifying the half-life of the targeted protein or synthetic rate after tamoxifen administration ends.
A recently reported atlas of single cells and nuclei in damaged muscle and liver revealed a common stress–response profile or signature is shared by many types of cells in both tissues, and other tissues as found in published datasets. In both liver and muscle stem cells, quiescent prior to tissue damage, ERK1/2 expression was essential prior to the onset of Notch-regulated myogenesis (Machado et al. 2021). With parallel stress-response expression patterns in muscle and liver, it is not surprising to find that muscle is receptive to systemic physiological stressors (or that cell isolation itself is a stressor). The observation that metabolism itself makes system-wide interconnections across many types of cells including muscle stem cells. Each cell type has its own internal metabolic character, responsiveness and regulatory patterns mediated in its own distinctive niche; that idea forms a window into understanding tissue physiology (Chen et al. 2019; Purohit and Dhawan 2019).
A recent exciting report demonstrated systemic signaling through release of exosomal vesicles from myoblasts. Those vesicles are now known to mediate muscle growth (Murach et al. 2021; Vechetti et al. 2021) in the absence of satellite cell cycling. The notion of non-cellular, circulating and/or local influences on growth, cell–cell signaling, and likely regeneration in muscle, extend findings of systemic influences on regenerative capacity between old and younger animals in earlier parabiosis experiments (Conboy et al. 2005; Brack et al. 2007).
Myogenesis vs. muscle-tissue regeneration
Muscle regeneration is a “team effort”; an individual cell lineage acting on its own, cannot establish a fully functional muscle. The effective outcome of muscle regeneration therefore, depends on all the cellular players, the timing of damage and inflammation, muscle-specific architecture, and the physical use and activity of a muscle. The exquisite genetic, biochemical, physiological, and epigenetic regulation of the timing of satellite cell activation and changes in satellite cell transitions to and from quiescence with aging (Addicks et al. 2019; Kimmel et al. 2020; Sincennes et al. 2021) have major implications for muscle regeneration capacity. As well, the distribution of fibrosis, vascular compromise and oxygenation, inflammation, and muscle anatomy before injury will affect the whole gamut of myogenic processes that ensue, from a particular mode of injury in each muscle to the outcome of satellite cell cycling and myotube maturation (Csete et al. 2001). Again, fiber type plays a role, since shear forces on the sarcolemma are higher in rapidly contracting type-II fibers and make them more susceptible to exercise-related damage. Different species and taxa also present fascinating distinctions across many features of muscle development, satellite cell distribution and regulation, muscle use, and evolutionary modifications based on life-history that may affect regeneration capacity, as recently explored in a variety of fish species (Zhang and Anderson 2014; Knappe et al. 2015; Gurevich et al. 2016; Ratnayake and Currie 2017; Anderson et al. 2019; Pourghadamyari et al. 2019; Tingle et al. 2019; Christian and Benian 2020; Hiebert and Anderson 2020; Montandon et al. 2021). Although autophagy may decrease with aging, inducing an increase in autophagy can rescue the effectiveness of regeneration by muscles in old animals (Park et al. 2019; You and Chen 2021; You et al. 2021).
The balance between stem capability and myogenic commitment by proliferating myoblasts is highly dependent on the cytoskeleton of the underlying muscle fiber. The dystrophin-associated protein complex (DAPC) distributes neuronal NO synthase (NOS-Iµ) within the dividing cell; that distribution, in turn, shapes the polarity of asymmetric division by muscle stem cells and normally ensures stem cell renewal (Dumont et al. 2015a, b; Chang et al. 2018; Feige et al. 2018; Addicks et al. 2019). Loss of dystrophin from the cytoskeletal complex has serious impact in dystrophic muscle, and leads to exhaustion of the stem cells (and self-renewal capacity) during the ongoing regenerative response to dystrophic damage. The mechanistic fine-tuning that retains or restores and sustains the viability of stem cell pool (Wang et al. 2019) is an essential concept for undertaking future research; that viability encapsulates what otherwise may appear as a stochastic or randomized process that determines the fate of daughter cells—that was the basis of a fascinating discussion at conferences in the late 1990s, and the puzzle took decades of focussed research to resolve. The process by which epidermal growth factor rescues the function of dystrophic satellite cells and builds the stem-cell potential of their progeny (Wang et al. 2019) is only one of many details that now guides the search for therapies to promote regeneration.
The regulation of regenerative-muscle-cell lineages follows the pattern of networks mediated by muscle regulatory genes during development (Hernández-Hernández et al. 2017). The discovery of MyoD and other MRF genes in the mid-1980s (Zammit 2017) and the impact of their deficient expression (Megeney et al. 1996; Anderson et al. 1998b; McIntosh et al. 1998; Huijbregts et al. 2001) have since revealed many key aspects of muscle regeneration (Lassar 2017). The identification of non-coding micro RNAs that help regulate development (Mok et al. 2017) and regeneration (Drummond et al. 2008; Safdar et al. 2009; Aoi et al. 2010; Guller and Russell 2010; Nielsen et al. 2010; Wessner et al. 2010; Cheung et al. 2012; Sharma et al. 2014; Diniz and Wang 2016) adds additional layers of complexity to muscle responses to injury and disease. Further, the notion that exosomal vesicles convey important intercellular signals vociferously demands new approaches for testing hypotheses, including those used to characterize exocrine regulation (Mitchell et al. 2019).
A systems-biology approach incorporates the complexities of stem cell function (Wosczyna and Rando 2018) and myogenic regeneration that proceeds in synchrony with inflammation and its resolution (Csete and Doyle 2002; Vodovotz et al. 2008), exosome signaling, re-innervation processes (Daneshvar et al. 2020), vascular perfusion, metabolism, and exercise (Henriksen et al. 2012; Bugera et al. 2018; Cornish et al. 2020a). Future research will reveal key intersections of the different processes that regulate muscle regeneration.
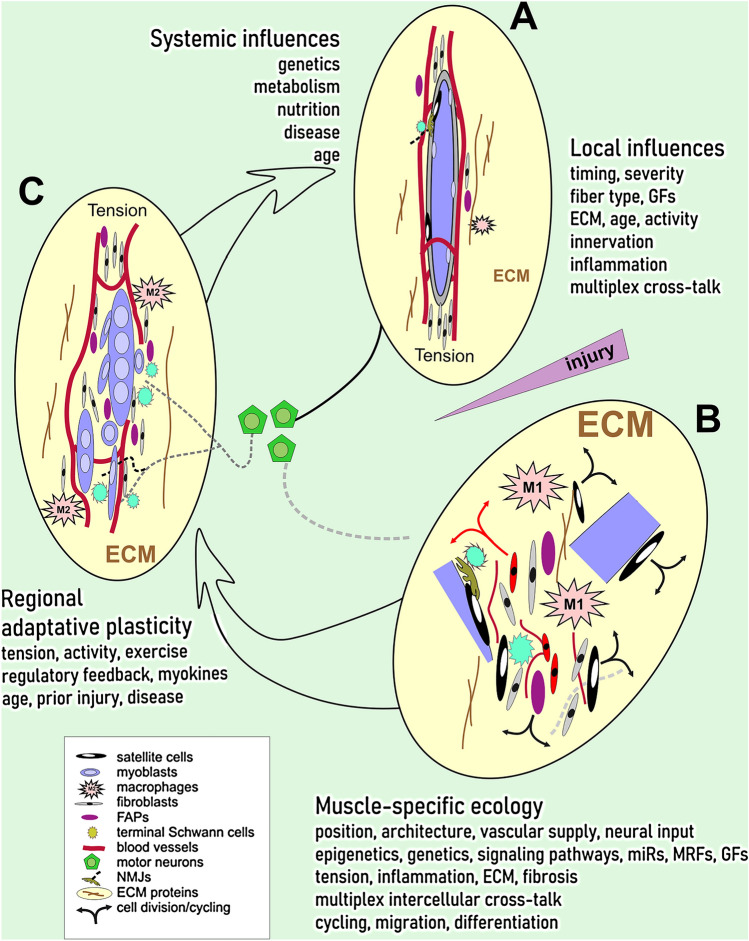
Notably, the mechanisms and actions of at least 25 gene products considered essential for myoblast fusion into the multinucleated syncytium known as a muscle fiber are not known (Deng et al. 2017). There is so much more to learn about myoblast interaction with, and migration through the ECM, and many physical properties of the cellular and acellular components of the niche around myoblasts as they fuse (Roveimiab et al. 2020). Figure 1 provides an overview of local influences, muscle-specific environment, regional adaptive plasticity, and systemic influences on muscle regeneration from a generic injury.
Fig. 1.
Skeletal muscle regeneration in context of local, regional, and systemic influences. A Under normal conditions (top right), muscle fibers with adjacent muscle satellite cells are innervated at neuromuscular junctions (NMJs) and situated in an active environment of multiplex cross-talk with components of the extracellular matrix (ECM), fibro-adipogenic precursors (FAPs), resident macrophages, fibroblasts, terminal Schwann cells, and endothelial cells lining blood vessels. The tissue complex is under tension from muscle insertions. B Soon after a disruptive injury (pointed wedge at the right), cells and fiber fragments (bottom right) are dispersed in the ECM, inflammatory (M1) macrophages infiltrate, satellite cells are activated, and many types of cells proliferate. Axons from spinal cord motor neurons (centre) begin to undergo Wallerian degeneration. Cellular cross-talk is fully engaged at cellular and molecular levels. Muscle-specific ecology related to 3-dimensional position and architecture (formed through development), the vascular and nerve supplies, and influences of genetics and epigenetics, engages signaling pathways through microRNAs, muscle regulatory genes, growth factors, exosomal vesicles, and their interplay with tension, inflammatory processes, ECM composition and fibrosis, with impact on cell cycling, migration, and differentiation behavior. C Regional adaptive plasticity shapes the regenerating muscle tissue (left) through a balance of tension, activity, and exercise with metabolic regulatory feedback loops (through endocrine, exocrine, and myokine pathways), age, prior injury, and extant disease. These influences act on myoblasts, fibroblasts, FAPs, endothelial and anti-inflammatory (M2) macrophages, as neurites begin to reconnect with elongating myotubes at nascent NMJs. Systemic influences of other tissues on the regenerating muscle, including genetics, metabolism, nutrition, disease and age, all contribute to the maturation of muscle 3-dimensional structure, stiffness, function, and adaptive responsiveness. Legend to symbols appears in the box (bottom left)
Current key concepts of muscle regeneration
Myogenic regeneration occurs within the cellular ecosystem of muscle tissue. The ecosystem includes signals and responses by satellite cells, inflammatory and vascular cells, fibro-adipogenic precursors and derivatives, the ECM, and nervous tissue. Cells and their interactions within muscle tissue during myogenesis are further embedded in systemic physiology, physical activity, and aging. Evaluating the integrative physiology of this “ecosystem” with an open-ended systems-biology approach will become an increasingly essential, counterpart to the exciting and more focussed research that targets the single-cell behavior or molecular interactions.
Regenerative potential and the adaptive plasticity of skeletal muscle are overlain by regionalized epigenetic modifications implicated in responses to exercise, aging, hormones, circulating factors, and disease. The extent and timing of regeneration are muscle-specific and interrelated with the vascular architecture and the use of that muscle.
Cells “talk” and socialize amongst themselves and with the ECM while they respond to systemic influences. Precisely how this happens is exquisitely complex, as tantalizingly illustrated by ongoing discovery of processes influenced via nanotubes, exosomal vesicles, gaseous transmission, and systemic factors. New hypotheses now must account for the hierarchy by which responses of one cell induce and direct subsequent responses by other cells, including fibers. This systemic, intercellular cross-talk during muscle regeneration forms the gestalt at the satellite cell niche, the focal point of muscle regeneration. However, we should not forget that satellite cells also influence the postmitotic nuclei inside fibers, and can optimize long-term adaptation and muscle responses to exercise.
Cells move; all types of cells in muscle, will move, and their migration processes are essential to regeneration. Migration behavior is highly dynamic, both biomechanically and in time. Myoblasts build and change their own operational niche and leave exosome and nano-scale “clues” to other cells along their trajectory, with major implications to fiber formation and health, as well as regeneration. Anticipating cell movement necessitates innovative experiments to visualize and track functional molecular cascades – these methods are real-time “lenses” to help interpret observations by accounting for motility.
Physical activity and concomitant signaling are high-impact arbiters of the outcome of regeneration. As in muscle hypertrophy, such signals to and from satellite (and other) cells in muscle, are mediated by integrated influences of metabolism, myokines, angiogenesis, innervation, and the configuration and composition of the ECM.
The intricate architecture of skeletal muscle, from the level of gross morphology in classical anatomy to its structure at ultrastructural, spectroscopic, and nanoscale resolutions, displays the functional hierarchy of a highly evolved muscle physiology. The complexity of muscle regeneration in three dimensions over time cannot be fully modeled by deconstructing the tissue processes in culture.
Research tools
Astute experimental design is critical to the clarity of research findings. The variables we measure, the research tools we use, the independent animal controls or human participants, and the specificity of our hypotheses—the questions we ask—are all part of anticipating research outcomes.
The tools for tracking the outcome of muscle regeneration have evolved tremendously since the early development of standardized instrumentation. Even a new, simple way to isolating primary satellite cells using ice-cold incubation, can advance research on regeneration (Benedetti et al. 2021). Recent discovery of MYC-dependent satellite cell function, revealed through CRISPR/Cas9 editing of the MyoD locus in young growing muscle, opened new potential for understanding how genome topology mediates activation of satellite cells (He et al. 2021). Exciting new avenues for conditional, cell-specific genetic manipulation will provide highly refined, wonderful insights into processes that change with gain- or loss-of-function by particular cells at particular junctures in regeneration, disease, and activity [e.g., (Wen et al. 2021)].
Multivariate readouts of muscle and single-cell transcriptomics, physiology and metabolism; systemic metabolism and biochemical interactions; and muscle structure coupled with functional genomics, cell-lineage tracing, and single-cell real-time tracking are all powerful tools, often accessible through collaboration. In effect, data readouts from studies on single fibers, dispersed cell cultures and co-cultures, and in vivo muscle tissue, coupled with diagnostics and treatment outcomes in a clinical setting can now be re-integrated by systems approaches (Owens et al. 2015). My lab ventured a short distance in that direction in our investigations of potential denervation and preserved satellite-cell responsiveness in muscle after rotator-cuff injury (Gigliotti et al. 2015, 2016, 2017), aiming toward eventual clinical trials to promote regeneration (Gigliotti et al. 2016).
Gaps
There are still major gaps in our knowledge of muscle regeneration related to the impact and type of physical activity, with all the local and systemic mediators released by exercise (Bugera et al. 2018; Cornish et al. 2018, 2020a, b). Physical activity likely influences the resolution of inflammation, where the timing is critical; myotube alignment during regeneration; and the role of the ECM and ECM-bound ligands in guiding cells during movement. Myoblast migration (Roveimiab et al. 2020) and the mechanisms of axonal targeting to neuromuscular junctions through synchronized myogenesis and nerve–muscle connectivity during regeneration (Daneshvar et al. 2020) can be closely scrutinized by microfluidics applications, now used for high-throughput screening for mutations (Markin et al. 2021) and biological assays (Grant et al. 2018).
Newly formed fibers will continue to grow by hypertrophy and nuclear accretion as part of adaptations to activity or compensation for disease, but the epigenetic “finesse” on the outcome of regeneration (Giordani and Puri 2013) remains to be mapped. As well, the mitotic clock that shortens telomere length with repetitive cell cycling of precursor cells during regeneration from disease (Decary et al. 2000; Renault et al. 2000; Cooper et al. 2003) plays a role that is still contentious. We have yet to understand observations that in athletes, minimum terminal restriction fragment length is reduced only in proportion to the degree of stress during distance running and power lifting (Mouly et al. 2005; Kadi and Ponsot 2010).
Conclusions
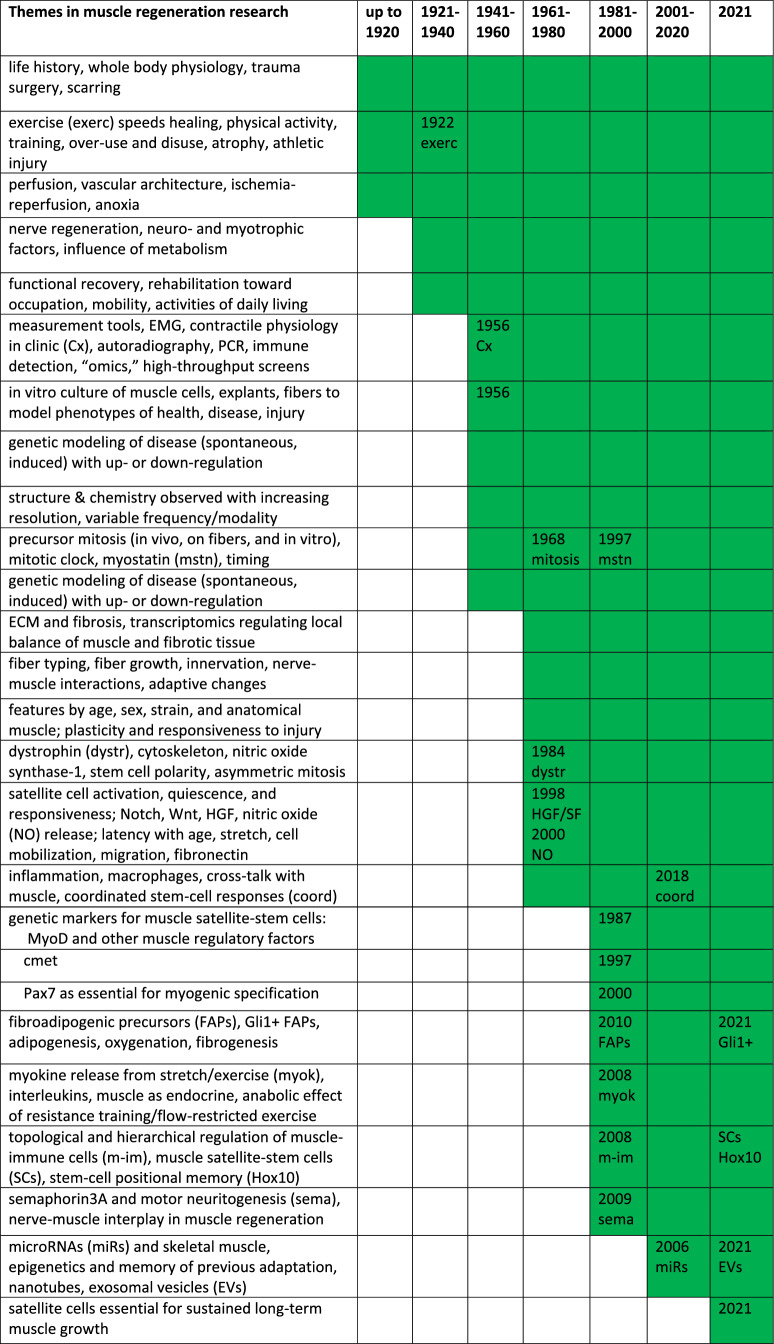
Overall, the philosophy of research on muscle regeneration, if it could be characterized as a philosophy, is ultimately directed toward improving the outcome, however, remotely feasible. Indeed, functional recovery is the primary hallmark of muscle regeneration (Forcina et al. 2020). In considering how key concepts in this field of muscle regeneration have changed in the past 100 years (Table 1) and how methodologies and technologies have advanced, we find that even classical ideas, misinterpretations and assumptions (revealed by our current knowledge), and speculation and discussion by experts, all contribute to re-imagining the horizons of possibility.
Table 1.
Side by side compilation of early and current concepts of muscle regeneration, as presented in the review
| Early concepts | Current concepts |
|---|---|
| Cells in skeletal muscle can form bone, adipose, and connective tissues | Contributions by muscle satellite-stem cells, fibroadipogenic precursors, immune cells, endothelial precursors, and innervation occur within an “ecosystem” of systemic physiology, physical activity, and aging |
| Regeneration of muscle does not always restore function | Muscle-specific overlay of regionalized epigenetic influences and vascular architecture |
| large Injuries often leave a scar | Cell–cell cross-talk via exosomal vesicles, nanotube connectivity, gaseous transmission, systemic and local secretions, including satellite cell influences on myonuclei |
| Similar pattern across types of injury with variable timing | Cells are dynamic in time and through biomechanical influences on molecular signaling pathways |
| often Less successful after ischemia–reperfusion injury than after anoxia | Physical activity arbitrates the outcome, mediated by metabolism, myokines, angiogenesis, innervation, and the extracellular matrix |
| Tracking functional repair requires standardized protocols and tools | 3-Dimensional skeletal muscle architecture over a broad range of resolutions, is an important readout of a highly evolved hierarchy of function |
The stepwise pattern of tissue changes during muscle regeneration is well established, involving inflammatory cells (M1 and M2), vasculature, satellite cells, fibers, FAPs, extracellular matrix, nerves, and myelinating and terminal Schwann cells. Each of type of cell both produces and receives multiple signals within their occupied niche at any given time. While the niche locations of satellite cells and terminal Schwann are particularly identifiable, every cell occupies and tailors its niche—a niche that presents signaling ligands and receptors, and stores signaling molecules produced elsewhere. And every cell responds or very likely will respond to metabolic and mechanical or activity-based signals, from local to systemic.
All those signals change in a time-dependent manner during regeneration; each type of cell and structure “recognizes” the signals and compiles or integrates them in a way that directs a particular response or action. That integration parallels the way in which the electrical output of a neuron is compiled from excitatory and inhibitory input at dendritic spines, soma, and axon in a complex summative and time-dependent mechanism. In muscle, systemic and localized signaling combine to impact the manner of such responses. That understanding enhances our ability to dissect and describe the behaviour of a given cell population in a region of regenerating muscle tissue.
Notably, the population-level behaviour of any type of cell will be displayed as an average response that accounts for the level of population heterogeneity. Single-cell approaches are astute in displaying responses based on gene and protein expression, morphological interactions, and extant physiology at a single time point; vital and video-capture imaging help capture those responses. We use many models of muscle regeneration, imposing particular injuries or metabolic and disease conditions to test new ideas, and have found great success in revealing how those cells interact. Over the decades of inquiry, those many models of muscle damage were explored with increasing ability to resolve patterns. Technologies have developed through application of highly specific molecular, cellular, and imaging tools that can observe and manipulate individual cells or a cell population. And objective observations and data can be analyzed with powerful computational and statistical approaches of genomics, genetics, computational biology, and time-lapse imaging. Ongoing research will probe ever-further into the mechanisms regulating cell behaviour and interactions.
While science rapidly embraces new methods, classical methods are still valuable, as is a review of historical literature. Understanding the trends in thinking can gives insights that refine an experiment or modify an approach. Reading old monographs is also fascinating as it helps us feel the energy of previous scientists as they reached toward their own technological limits to pose questions and open doors, ever hopeful they too, might advance the concepts of muscle regeneration.
The goal of this review was not to characterize the burgeoning literature on molecular genetics, epigenetics, and cell signaling by all types of cells involved in muscle regeneration. Nor was it to review the exquisite combinations of modern technology such as CRISPR in intraosseous chimeric cell therapy to promote systemic muscle regeneration and prevent disease progression, now supporting huge strides in systemic treatment of diseases such as Duchenne muscular dystrophy (Siemionow et al. 2019, 2021). Rather, the review aimed to identify distinctions between current concepts in muscle regeneration and earlier historical ideas, and trace the general timeline of research advances (Table 2). Our conceptualization of a process shapes the way we approach its exploration, just as the model of injury we select for an experiment will shape the nature of findings and variables that are available in studying the outcome of a perturbation or potential therapy we might superimpose (Hardy et al. 2016; Tatsumi et al. 2017).
Table 2.
Rough timeline of topical themes and approaches in research on muscle regeneration
It is the accessible scope of activities or responses made by a single cell, a cell population, or a mixed collection of all the cells within a tissue, that provides the basis of our clinical and research practices. Concepts—how we think about each level of organization—form the foundational map of our understanding. Alas, our concepts also form the basis of our assumptions, since we use that map to speculate on the unknown, choose a direction, and anticipate the next horizon. It’s exciting to learn new concepts in our own and other fields, since those ideas bring, even force, a paradigm shift in perspectives and redirect our attention. Our focus shifts to the next question, whether new or refreshed from classical literature by modern technologies, insights, and skepticism.
The impact of intricate molecular-genetic signaling networks—a critical foundation in understanding cell behaviour in muscle regeneration and therapeutics—must be interpreted in context of the bigger picture of structure–function relationships and systemic influences (Swaggart and McNally 2014). Ongoing discoveries highlight that every part of the muscle structure is important in some fashion. That notion provides important clues to us as researchers, about the functional context and functionality of muscle during regeneration. The beautiful three-dimensional architecture of skeletal muscle has evolved to optimize the functions of all cells in normal muscle; recognizing how that architecture integrates with physiology is critical to advancing our understanding of muscle regeneration. Deciphering the interplay of layered mechanisms of damage in traumatic muscle injury, for example, by high-energy ballistic damage from firearms (Moriscot et al. 2021), will advance our thinking about rehabilitation approaches that promote regeneration and function.
Research on muscle regeneration intersects at many levels, with ideas gleaned from other research domains. Even apparently simple concepts can spark creativity and drive new hypotheses directed to pose the why? and how? and what happens then? questions at the core of research. Skeletal muscle, the engine of our voluntary activity, is critical to health, both physical and mental; one can only hope that the remarkable capability of muscle tissue to regenerate through myriad dynamic inter-cellular interactions and signaling processes, continues to capture the imagination of future generations of researchers. Key concepts in skeletal muscle regeneration will evolve as does our understanding of the processes influencing cellular and molecular interactions.
Acknowledgements
Many collaborators, colleagues and students have made this work possible.
Abbreviations
- cGMP
Cyclic guanosine monophosphate
- ECM
Extracellular matrix
- FAP
Fibro-adipogenic progenitor
- HGF
Hepatocyte growth factor
- Hh
Hedgehog
- ICU
Intensive care unit
- IGF-1
Insulin-like growth factor 1
- IL
Interleukin
- mRNA
Messenger RNA
- miRNA
MicroRNA
- MRF
Muscular regulatory factor
- NAMPT
Nicotinamide phosphoribosyl transferase
- NMJ
Neuromuscular junction
- NO
Nitric oxide
- NOS-Iµ
Neuronal nitric oxide synthase
- Sema3A
Semaphorin3A
- siRNA
Short interference RNA
- TSC
Terminal Schwann cell
- VEGF
Vascular endothelial growth factor
Author contributions
JA is the sole author; after the invitation to write this review, the author conducted all research for the literature review, drafted and edited the manuscript, and composed the figure.
Funding
The author is grateful for research funding from the Natural Science and Engineering Research Council of Canada (NSERC-RG-PIN-3833-2015).
Declarations
Conflict of interest
The author has no relevant financial or non-financial interests to disclose that are relevant to the content of this article. The review was researched and written by the author, alone, after invitation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Addicks GC, Brun CE, Sincennes MC, Saber J, Porter CJ, Francis Stewart A, Ernst P, Rudnicki MA. MLL1 is required for PAX7 expression and satellite cell self-renewal in mice. Nat Commun. 2019;10:4256. doi: 10.1038/s41467-019-12086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar AF, Vechetti-Junior IJ, Souza RW, Piedade WP, Pacagnelli FL, Leopoldo AS, Casonatto J, Pai MD. Nitric oxide synthase inhibition impairs muscle regrowth following immobilization. Nitric Oxide. 2017;69:22–27. doi: 10.1016/j.niox.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Alfaro LA, Dick SA, Siegel AL, Anonuevo AS, McNagny KM, Megeney LA, Cornelison DD, Rossi FM. CD34 promotes satellite cell motility and entry into proliferation to facilitate efficient skeletal muscle regeneration. Stem Cells. 2011;29:2030–2041. doi: 10.1002/stem.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Whitehead NP, Froehner SC. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev. 2016;96:253–305. doi: 10.1152/physrev.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi M. Patterns of muscle regeneration. In: Mauro A, Shafiq SA, Milhorat AT, editors. Regneration of striated muscle, and myogenesis. Amsterdam: Excerpta Medica; 1970. pp. 180–193. [Google Scholar]
- Alrushaid S, Davies NM, Anderson JE, Le T, Yáñez JA, Maayah ZH, El-Kadi AOS, Rachid O, Sayre CL, Löbenberg R, Burczynski FJ. Pharmaceutical characterization of MyoNovin, a novel skeletal muscle regenerator: in silico, in vitro and in vivo studies. J Pharm Pharm Sci. 2018;21:29683. doi: 10.18433/J3MS8H. [DOI] [PubMed] [Google Scholar]
- Anderson JE. Dystrophic changes in mdx muscle regenerating from denervation and devascularization. Muscle Nerve. 1991;14:268–279. doi: 10.1002/mus.880140311. [DOI] [PubMed] [Google Scholar]
- Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Pilipowicz O. Activation of muscle satellite cells in single-fiber cultures. Nitric Oxide. 2002;7:36–41. doi: 10.1016/s1089-8603(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Wozniak AC. Satellite cell activation on fibers: modeling events in vivo—an invited review. Can J Physiol Pharmacol. 2004;82:300–310. doi: 10.1139/y04-020. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Bressler BH, Ovalle WK. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil. 1988;9:499–515. doi: 10.1007/BF01738755. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Lentz DL, Johnson RB. Recovery from disuse osteopenia coincident to restoration of muscle strength in mdx mice. Bone. 1993;14:625–634. doi: 10.1016/8756-3282(93)90084-n. [DOI] [PubMed] [Google Scholar]
- Anderson JE, McIntosh LM, Poettcker R. Deflazacort but not prednisone improves both muscle repair and fiber growth in diaphragm and limb muscle in vivo in the mdx dystrophic mouse. Muscle Nerve. 1996;19:1576–1585. doi: 10.1002/(SICI)1097-4598(199612)19:12<1576::AID-MUS7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Garrett K, Moor A, McIntosh L, Penner K. Dystrophy and myogenesis in mdx diaphragm muscle. Muscle Nerve. 1998;21:1153–1165. doi: 10.1002/(sici)1097-4598(199809)21:9<1153::aid-mus6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Anderson JE, McIntosh LM, Moor AN, Yablonka-Reuveni Z. Levels of MyoD protein expression following injury of mdx and normal limb muscle are modified by thyroid hormone. J Histochem Cytochem. 1998;46:59–67. doi: 10.1177/002215549804600108. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Do MQ, Daneshvar N, Suzuki T, Dort J, Mizunoya W, Tatsumi R. The role of semaphorin3A in myogenic regeneration and the formation of functional neuromuscular junctions on new fibres. Biol Rev Camb Philos Soc. 2017;92:1389–1405. doi: 10.1111/brv.12286. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Zhu A, Mizuno TM. Nitric oxide treatment attenuates muscle atrophy during hind limb suspension in mice. Free Radic Biol Med. 2017;115:458–470. doi: 10.1016/j.freeradbiomed.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Cunha A, Docker MF. Novel "omega muscle units" in superficial body-wall myotomes during metamorphosis in the northern brook lamprey (Ichthyomyzon fossor) Can J Zool. 2019;97:1218–1224. [Google Scholar]
- Anonymous Increasing muscle strength. Br Med J. 1957;2:150–151. [PMC free article] [PubMed] [Google Scholar]
- Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ichikawa H, Yoshikawa T. The microRNA miR-696 regulates PGC-1{alpha} in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab. 2010;298:E799–E806. doi: 10.1152/ajpendo.00448.2009. [DOI] [PubMed] [Google Scholar]
- Archer JD, Vargas CC, Anderson JE. Persistent and improved functional gain in mdx dystrophic mice after treatment with L-arginine and deflazacort. FASEB J. 2006;20:738–740. doi: 10.1096/fj.05-4821fje. [DOI] [PubMed] [Google Scholar]
- Argiles JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Manas L. Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc. 2016;17:789–796. doi: 10.1016/j.jamda.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Arora R, Rumman M, Venugopal N, Gala H, Dhawan J. Mimicking muscle stem cell quiescence in culture: methods for synchronization in reversible arrest. Methods Mol Biol (clifton, NJ) 2017;1556:283–302. doi: 10.1007/978-1-4939-6771-1_15. [DOI] [PubMed] [Google Scholar]
- Arouche-Delaperche L, Allenbach Y, Amelin D, Preusse C, Mouly V, Mauhin W, Tchoupou GD, Drouot L, Boyer O, Stenzel W, Butler-Browne G, Benveniste O. Pathogenic role of anti-signal recognition protein and anti-3-Hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: Myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann Neurol. 2017;81:538–548. doi: 10.1002/ana.24902. [DOI] [PubMed] [Google Scholar]
- Aurora A, Garg K, Corona BT, Walters TJ. Physical rehabilitation improves muscle function following volumetric muscle loss injury. BMC Sports Sci Med Rehabil. 2014;6:41. doi: 10.1186/2052-1847-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiguera S, Del Gaudio C, Di Nardo P, Manzari V, Carotenuto F, Teodori L. 3D printing decellularized extracellular matrix to design biomimetic scaffolds for skeletal muscle tissue engineering. Biomed Res Int. 2020;2020:2689701. doi: 10.1155/2020/2689701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik A, Li L, Sathyamurthy A, Xiong WC, Mei L. Schwann cells in neuromuscular junction formation and maintenance. J Neurosci. 2016;36:9770–9781. doi: 10.1523/JNEUROSCI.0174-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TH, Engler AJ. The provisional matrix: setting the stage for tissue repair outcomes. Matrix Biol. 2017;60–61:1–4. doi: 10.1016/j.matbio.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizário JE, Fontes-Oliveira CC, Borges JP, Kashiabara JA, Vannier E. Skeletal muscle wasting and renewal: a pivotal role of myokine IL-6. Springerplus. 2016;5:619. doi: 10.1186/s40064-016-2197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A, Cera G, De Meo D, Villani C, Bouche M, Lozanoska-Ochser B. A novel approach for the isolation and long-term expansion of pure satellite cells based on ice-cold treatment. Skelet Muscle. 2021;11:7. doi: 10.1186/s13395-021-00261-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, von Maltzahn J, Dumont NA, Stark DA, Wang YX, Nhan K, Frenette J, Cornelison DD, Rudnicki MA. Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength. J Cell Biol. 2014;205:97–111. doi: 10.1083/jcb.201310035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012;4:a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best TM, Gharaibeh B, Huard J. Stem cells, angiogenesis and muscle healing: a potential role in massage therapies? Br J Sports Med. 2013;47:556–560. doi: 10.1136/bjsports-2012-091685. [DOI] [PubMed] [Google Scholar]
- Betters JL, Lira VA, Soltow QA, Drenning JA, Criswell DS. Supplemental nitric oxide augments satellite cell activity on cultured myofibers from aged mice. Exp Gerontol. 2008;43:1094–1101. doi: 10.1016/j.exger.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Betters JL, Long JH, Howe KS, Braith RW, Soltow QA, Lira VA, Criswell DS. Nitric oxide reverses prednisolone-induced inactivation of muscle satellite cells. Muscle Nerve. 2008;37:203–209. doi: 10.1002/mus.20915. [DOI] [PubMed] [Google Scholar]
- Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front Physiol. 2019;10:1074. doi: 10.3389/fphys.2019.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat Rec. 1975;182:215–235. doi: 10.1002/ar.1091820207. [DOI] [PubMed] [Google Scholar]
- Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr Physiol. 2013;3:1645–1687. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- Bonafè F, Guarnieri C, Muscari C. Nitric oxide regulates multiple functions and fate of adult progenitor and stem cells. J Physiol Biochem. 2015;71:141–153. doi: 10.1007/s13105-014-0373-9. [DOI] [PubMed] [Google Scholar]
- Boynton BL. Trends in training in physical medicine. Arch Phys Med Rehabil. 1947;28:301–303. [PubMed] [Google Scholar]
- Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci. 2005;118:4813–4821. doi: 10.1242/jcs.02602. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 2007;3:226–237. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- Brewerton DA, Darcus HD. Discussion on an evaluation of the methods of increasing muscle strength. Proc R Soc Med. 1956;49:999–1008. [PMC free article] [PubMed] [Google Scholar]
- Brooks B. Pathological changes in muscle as a result of disturbances of circulation. An experimental study of Volkmann's ischemic paralysis. Arch Surg. 1922;5:188–216. [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Dysart MM, Clarke KC, Stabenfeldt SE, Barker TH. Integrin α3β1 binding to fibronectin is dependent on the ninth type III repeat. J Biol Chem. 2015;290:25534–25547. doi: 10.1074/jbc.M115.656702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli S, Sciorati C, D'Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, Ongini E, Cossu G, Clementi E. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci USA. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert TD, Gavin TP, Fu Z, Breen EC, Tang K, Mathieu-Costello O, Wagner PD. Regional differences in expression of VEGF mRNA in rat gastrocnemius following 1 hr exercise or electrical stimulation. BMC Physiol. 2002;2:8. doi: 10.1186/1472-6793-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Relaix F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin Cell Dev Biol. 2015;44:115–125. doi: 10.1016/j.semcdb.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Rigby PW. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell. 2014;28:225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Bugera EM, Duhamel TA, Peeler JD, Cornish SM. The systemic myokine response of decorin, interleukin-6 (IL-6) and interleukin-15 (IL-15) to an acute bout of blood flow restricted exercise. Eur J Appl Physiol. 2018;118:2679–2686. doi: 10.1007/s00421-018-3995-8. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Regeneration of the completely excised gastrocnemius muscle in the frog and rat from minced muscle fragments. J Morphol. 1968;125:447–471. doi: 10.1002/jmor.1051250405. [DOI] [PubMed] [Google Scholar]
- Carlson BM. The regeneration of minced muscles. Mongr Dev Biol. 1972;4:1–128. [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. The regeneration of skeletal muscle fibers following injury: a review. Med Sci Sports Exerc. 1983;15:187–198. [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J PhysiOL. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- Carosio S, Berardinelli MG, Aucello M, Musaro A. Impact of ageing on muscle cell regeneration. Ageing Res Rev. 2011;10:35–42. doi: 10.1016/j.arr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Chang NC, Sincennes MC, Chevalier FP, Brun CE, Lacaria M, Segalés J, Muñoz-Cánoves P, Ming H, Rudnicki MA. The dystrophin glycoprotein complex regulates the epigenetic activation of muscle stem cell commitment. Cell Stem Cell. 2018;22:755–768.e756. doi: 10.1016/j.stem.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chazaud B. Inflammation and skeletal muscle regeneration: leave it to the macrophages! Trends Immunol. 2020;41:481–492. doi: 10.1016/j.it.2020.04.006. [DOI] [PubMed] [Google Scholar]
- Chen YC, Allen SG, Ingram PN, Buckanovich R, Merajver SD, Yoon E. Single-cell migration chip for chemotaxis-based microfluidic selection of heterogeneous cell populations. Sci Rep. 2015;5:1–13. doi: 10.1038/srep09980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr. 2009;3:337–341. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Shan T. The role of satellite and other functional cell types in muscle repair and regeneration. J Muscle Res Cell Motil. 2019;40:1–8. doi: 10.1007/s10974-019-09511-3. [DOI] [PubMed] [Google Scholar]
- Chen F, Zhou J, Li Y, Zhao Y, Yuan J, Cao Y, Wang L, Zhang Z, Zhang B, Wang CC, Cheung TH, Wu Z, Wong CC, Sun H, Wang H. YY1 regulates skeletal muscle regeneration through controlling metabolic reprogramming of satellite cells. Embo J. 2019;38:e99727. doi: 10.15252/embj.201899727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EV, Tidball JG. Administration of the non-steroidal anti-inflammatory drug ibuprofen increases macrophage concentrations but reduces necrosis during modified muscle use. Inflamm Res. 2003;52:170–176. doi: 10.1007/s000110300068. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Park SJ, Yi HG, Lee H, Kim DS, Cho DW. Muscle-derived extracellular matrix on sinusoidal wavy surfaces synergistically promotes myogenic differentiation and maturation. J Mater Chem B. 2018;6:5530–5539. doi: 10.1039/c8tb01475b. [DOI] [PubMed] [Google Scholar]
- Christian CJ, Benian GM. Animal models of sarcopenia. Aging Cell. 2020;19:e13223. doi: 10.1111/acel.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JCT, Noronha RFX, Allbrook DB. Satellite cells and skeletal muscle regeneration. Br J Surg. 1966;53:638–642. [Google Scholar]
- Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des. 2010;16:906–914. doi: 10.2174/138161210790883453. [DOI] [PubMed] [Google Scholar]
- Cisternas P, Henriquez JP, Brandan E, Inestrosa NC. Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol. 2014;49:574–589. doi: 10.1007/s12035-013-8540-5. [DOI] [PubMed] [Google Scholar]
- Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013;24:830–833. doi: 10.1016/j.copbio.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Collins BC, Kardon G. Won't you be my neighbor? Muscle stem cells recruit endothelial cells to their niche. Cell Stem Cell. 2018;23:455–456. doi: 10.1016/j.stem.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Perez RA, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Contreras-Muñoz P, Torrella JR, Venegas V, Serres X, Vidal L, Vila I, Lahtinen I, Viscor G, Martínez-Ibáñez V, Peiró JL, Järvinen TAH, Rodas G, Marotta M. Muscle precursor cells enhance functional muscle recovery and show synergistic effects with postinjury treadmill exercise in a muscle injury model in rats. Am J Sports Med. 2021;49:1073–1085. doi: 10.1177/0363546521989235. [DOI] [PubMed] [Google Scholar]
- Cooper RN, Thiesson D, Furling D, Di Santo JP, Butler-Browne GS, Mouly V. Extended amplification in vitro and replicative senescence: key factors implicated in the success of human myoblast transplantation. Hum Gene Ther. 2003;14:1169–1179. doi: 10.1089/104303403322168000. [DOI] [PubMed] [Google Scholar]
- Cornelison D. "Known Unknowns": current questions in muscle satellite cell biology. Curr Top Dev Biol. 2018;126:205–233. doi: 10.1016/bs.ctdb.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cornish SM, Bugera EM, Duhamel TA, Peeler JD, Anderson JE. A focused review of myokines as a potential contributor to muscle hypertrophy from resistance-based exercise. Eur J Appl Physiol. 2020;120:941–959. doi: 10.1007/s00421-020-04337-1. [DOI] [PubMed] [Google Scholar]
- Cornish SM, Chase JE, Bugera EM, Giesbrecht GG. Systemic IL-6 and myoglobin response to three different resistance exercise intensities in older men. J Aging Phys Act. 2018;26:451–456. doi: 10.1123/japa.2017-0167. [DOI] [PubMed] [Google Scholar]
- Cornish SM, Chilibeck PD, Candow DG. Potential importance of immune system response to exercise on aging muscle and bone. Curr Osteoporos Rep. 2020;18:350–356. doi: 10.1007/s11914-020-00596-1. [DOI] [PubMed] [Google Scholar]
- Coulton GR, Morgan JE, Partridge TA, Sloper JC. The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol. 1988;14:53–70. doi: 10.1111/j.1365-2990.1988.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Csapo R, Gumpenberger M, Wessner B. Skeletal muscle extracellular matrix—what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol. 2020;11:253. doi: 10.3389/fphys.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csete ME, Doyle JC. Reverse engineering of biological complexity. Science. 2002;295:1664–1669. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]
- Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- Daneshvar N, Tatsumi R, Peeler J, Anderson JE. Premature satellite cell activation before injury accelerates myogenesis and disrupts neuromuscular junction maturation in regenerating muscle. Am J Physiol Cell Physiol. 2020;319:C116–C128. doi: 10.1152/ajpcell.00121.2020. [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340:330–343. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10:113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr Aging Sci. 2011;4:248–259. doi: 10.2174/1874609811104030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsmann MM, Sturznickel J, Amling M, Ueblacker P, Rolvien T. Musculoskeletal laboratory diagnostics in competitive sport. Der Orthopade. 2021;50:700–712. doi: 10.1007/s00132-021-04072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Azevedo M, Baylies M. Acting on identity: Myoblast fusion and the formation of the syncytial muscle fiber. Semin Cell Dev Biol. 2017;72:45–55. doi: 10.1016/j.semcdb.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D. Interpretation of the electromyogram. Arch Neurol Psychiatry. 1949;61:99–128. doi: 10.1001/archneurpsyc.1949.02310080003001. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Diaz-Guerrero R, Thomson JD, Hines HM. Effect of hypothyroidism and hyperthyroidism on mammalian skeletal muscle. Proc Soc Exp Biol Med Soc Exp Biol Med (new York, NY) 1947;66:95–96. doi: 10.3181/00379727-66-15993. [DOI] [PubMed] [Google Scholar]
- Diaz-Guerrero R, Thomson JD, Hines HM. Effect of thymectomy, hyperthyroidism and hypothyroidism on neuromuscular atrophy and regeneration. Am J Physiol. 1947;151:91–95. doi: 10.1152/ajplegacy.1947.151.1.91. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126:1621–1629. doi: 10.1242/dev.126.8.1621. [DOI] [PubMed] [Google Scholar]
- Diniz GP, Wang DZ. Regulation of skeletal muscle by microRNAs. Compr Physiol. 2016;6:1279–1294. doi: 10.1002/cphy.c150041. [DOI] [PubMed] [Google Scholar]
- Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, Toresdahl BG, Rodeo SA, Casey EK, Mendias CL. Musculoskeletal consequences of COVID-19. J Bone Jt Surg Am. 2020;102:1197–1204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MK, Sato Y, Shimizu N, Suzuki T, Shono J, Mizunoya W, Nakamura M, Ikeuchi Y, Anderson JE, Tatsumi R. Growth factor regulation of neural chemorepellent Sema3A expression in satellite cell cultures. Am J Physiol Cell Physiol. 2011;301:C1270–C1279. doi: 10.1152/ajpcell.00257.2011. [DOI] [PubMed] [Google Scholar]
- Domingues-Faria C, Vasson MP, Goncalves-Mendes N, Boirie Y, Walrand S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res Rev. 2016;26:22–36. doi: 10.1016/j.arr.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, McCarthy JJ, Fry CS, Esser KA, Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab. 2008;295:E1333–E1340. doi: 10.1152/ajpendo.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duance VC, Stephens HR, Dunn M, Bailey AJ, Dubowitz V. A role for collagen in the pathogenesis of muscular dystrophy? Nature. 1980;284:470–472. doi: 10.1038/284470a0. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5:1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, Rudnicki MA. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med. 2015;21:1455–1463. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A, Marcinczyk M, Talovic M, Patel K, Haas G, Garg K. Role of stem cells and extracellular matrix in the regeneration of skeletal muscle. In: Sakuma PK, editor. Muscle cell and tissue —current status of research field. London: InTechOpen; 2018. [Google Scholar]
- Dunn A, Talovic M, Patel K, Patel A, Marcinczyk M, Garg K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J Orthop Res. 2019;37:1246–1262. doi: 10.1002/jor.24212. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Dusterhoft S, Yablonka-Reuveni Z, Pette D. Characterization of myosin isoforms in satellite cell cultures from adult rat diaphragm, soleus and tibialis anterior muscles. Differentiation. 1990;45:185–191. doi: 10.1111/j.1432-0436.1990.tb00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC. Investigations on muscle atrophies arising from disuse and tenotomy. J Physiol. 1944;103:253–266. doi: 10.1113/jphysiol.1944.sp004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Eliazer S, Muncie JM, Christensen J, Sun X, D'Urso RS, Weaver VM, Brack AS. Wnt4 from the niche controls the mechano-properties and quiescent state of muscle stem cells. Cell Stem Cell. 2019;25:654–665.e654. doi: 10.1016/j.stem.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Ward PJ. Exercise, neurotrophins, and axon regeneration in the PNS. Physiology (bethesda) 2014;29:437–445. doi: 10.1152/physiol.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund DA, Figueiredo VC, Dungan CM, Murach KA, Peck BD, Petrosino JM, Brightwell CR, Dupont AM, Neal AC, Fry CS, Accornero F, McCarthy JJ, Peterson CA. Satellite cell depletion disrupts transcriptional coordination and muscle adaptation to exercise. Function (Oxf) 2021;2:zqaa033. doi: 10.1093/function/zqaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund DA, Murach KA, Dungan CM, Figueiredo VC, Vechetti IJ, Jr, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Depletion of resident muscle stem cells negatively impacts running volume, physical function, and muscle fiber hypertrophy in response to lifelong physical activity. Am J Physiol Cell Physiol. 2020;318:C1178–C1188. doi: 10.1152/ajpcell.00090.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves de Lima J, Relaix F. Master regulators of skeletal muscle lineage development and pluripotent stem cells differentiation. Cell Regener (London, England) 2021;10:31. doi: 10.1186/s13619-021-00093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria FE, Ferrari RJ, Distefano G, Ducatti AC, Soares KF, Montebelo MI, Minamoto VB. The onset and duration of mobilization affect the regeneration in the rat muscle. Histol Histopathol. 2008;23:565–571. doi: 10.14670/HH-23.565. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Who would have thought - myokines two decades on. Nat Rev Endocrinol. 2020;16:619–620. doi: 10.1038/s41574-020-00408-7. [DOI] [PubMed] [Google Scholar]
- Feige P, Brun CE, Ritso M, Rudnicki MA. Orienting Muscle Stem Cells for Regeneration in Homeostasis, Aging, and Disease. Cell Stem Cell. 2018;23:653–664. doi: 10.1016/j.stem.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Ko CP. The role of glial cells in the formation and maintenance of the neuromuscular junction. Ann N Y Acad Sci. 2008;1132:19–28. doi: 10.1196/annals.1405.016. [DOI] [PubMed] [Google Scholar]
- Feng Z, Koirala S, Ko CP. Synapse-glia interactions at the vertebrate neuromuscular junction. Neurosci. 2005;11:503–513. doi: 10.1177/1073858405277409. [DOI] [PubMed] [Google Scholar]
- Ferreira MM, Dewi RE, Heilshorn SC. Microfluidic analysis of extracellular matrix-bFGF crosstalk on primary human myoblast chemoproliferation, chemokinesis, and chemotaxis. Integr Biol. 2015;7:569–579. doi: 10.1039/c5ib00060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippin LI, Cuevas MJ, Lima E, Marroni NP, Gonzalez-Gallego J, Xavier RM. Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide. 2011;24:43–49. doi: 10.1016/j.niox.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Filippin LI, Cuevas MJ, Lima E, Marroni NP, Gonzalez-Gallego J, Xavier RM. The role of nitric oxide during healing of trauma to the skeletal muscle. Inflamm Res. 2011;60:347–356. doi: 10.1007/s00011-010-0277-2. [DOI] [PubMed] [Google Scholar]
- Filippin LI, Moreira AJ, Marroni NP, Xavier RM. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide. 2009;21:157–163. doi: 10.1016/j.niox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Filippone C, Legros V, Jeannin P, Choumet V, Butler-Browne G, Zoladek J, Mouly V, Gessain A, Ceccaldi PE. Arboviruses and muscle disorders: from disease to cell biology. Viruses. 2020;12:616. doi: 10.3390/v12060616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore D, Judson RN, Low M, Lee S, Zhang E, Hopkins C, Xu P, Lenzi A, Rossi FM, Lemos DR. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res. 2016;17:161–169. doi: 10.1016/j.scr.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Fischer E. Muscle strength and the weather. Arch Phys Med Rehabil. 1947;28:295–300. [PubMed] [Google Scholar]
- Forcina L, Cosentino M, Musaro A. Mechanisms regulating muscle regeneration: Insights into the interrelated and time-dependent phases of tissue healing. Cells Tissues Organs. 2020;9:1297–11325. doi: 10.3390/cells9051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco I, Fernandez-Gonzalo R, Vrtačnik P, Lundberg TR, Eriksson M, Gustafsson T. Healthy skeletal muscle aging: The role of satellite cells, somatic mutations and exercise. Int Rev Cell Mol Biol. 2019;346:157–200. doi: 10.1016/bs.ircmb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28:1654–1665. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Prat L, Perdiguero E, Alonso-Martín S, Dell'Orso S, Ravichandran S, Brooks SR, Juan AH, Campanario S, Jiang K, Hong X, Ortet L, Ruiz-Bonilla V, Flández M, Moiseeva V, Rebollo E, Jardí M, Sun HW, Musarò A, Sandri M, Del Sol A, Sartorelli V, Muñoz-Cánoves P. FoxO maintains a genuine muscle stem-cell quiescent state until geriatric age. Nat Cell Biol. 2020;22:1307–1318. doi: 10.1038/s41556-020-00593-7. [DOI] [PubMed] [Google Scholar]
- Geiger RS, Garvin JS. Pattern of regeneration of muscle from progressive muscular dystrophy patients cultivated in vitro as compared to normal human skeletal muscle. J Neuropathol Exp Neurol. 1957;16:523–543. [PubMed] [Google Scholar]
- Gigliotti D, Leiter JR, MacDonald PB, Peeler J, Anderson JE. Altered satellite cell responsiveness and denervation implicated in progression of rotator-cuff injury. PLoS One. 2016;11:e0162494. doi: 10.1371/journal.pone.0162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti D, Leiter JR, Macek B, Davidson MJ, MacDonald PB, Anderson JE. Atrophy, inducible satellite cell activation, and possible denervation of supraspinatus muscle in injured human rotator-cuff muscle. Am J Physiol Cell Physiol. 2015;309:C383–C391. doi: 10.1152/ajpcell.00143.2015. [DOI] [PubMed] [Google Scholar]
- Gigliotti D, Xu MC, Davidson MJ, Macdonald PB, Leiter JRS, Anderson JE. Fibrosis, low vascularity, and fewer slow fibers after rotator-cuff injury. Muscle Nerve. 2017;55:715–726. doi: 10.1002/mus.25388. [DOI] [PubMed] [Google Scholar]
- Gilbert-Honick J, Grayson W. Vascularized and innervated skeletal muscle tissue engineering. Adv Healthc Mater. 2020;9:e1900626. doi: 10.1002/adhm.201900626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies AR, Chapman MA, Bushong EA, Deerinck TJ, Ellisman MH, Lieber RL. High resolution three-dimensional reconstruction of fibrotic skeletal muscle extracellular matrix. J Physiol. 2017;595:1159–1171. doi: 10.1113/JP273376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–331. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani L, Puri PL. Epigenetic control of skeletal muscle regeneration: Integrating genetic determinants and environmental changes. FEBS J. 2013;280:4014–4025. doi: 10.1111/febs.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani G, Rosin, M, Reggio A (2021) Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease. FEBS J [DOI] [PubMed]
- Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genom. 2003;14:261–271. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- Goldspink DF. Exercise-related changes in protein turnover in mammalian striated muscle. J Exp Biol. 1991;160(127–48):127–148. doi: 10.1242/jeb.160.1.127. [DOI] [PubMed] [Google Scholar]
- Gomes AR, Coutinho EL, Franca CN, Polonio J, Salvini TF. Effect of one stretch a week applied to the immobilized soleus muscle on rat muscle fiber morphology. Braz J Med Biol Res. 2004;37:1473–1480. doi: 10.1590/s0100-879x2004001000005. [DOI] [PubMed] [Google Scholar]
- Gordon T. peripheral nerve regeneration and muscle reinnervation. Int J Mol Sci. 2020;21:8652. doi: 10.3390/ijms21228652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Borschel GH. The use of the rat as a model for studying peripheral nerve regeneration and sprouting after complete and partial nerve injuries. Exp Neurol. 2017;287:331–347. doi: 10.1016/j.expneurol.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. 2016;43:336–350. doi: 10.1111/ejn.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JP, Price JL. Bone muscle crosstalk targets muscle regeneration pathway regulated by core circadian transcriptional repressors DEC1 and DEC2. BoneKEy Rep. 2016;5:850. doi: 10.1038/bonekey.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Rudnicki M. Satellite and stem cells in muscle growth and repair. Development. 2007;134:3953–3957. doi: 10.1242/dev.005934. [DOI] [PubMed] [Google Scholar]
- Grant J, Goudarzi SH, Mrksich M. High-throughput enzyme kinetics with 3D microfluidics and imaging SAMDI mass spectrometry. Anal Chem. 2018;90:13096–13103. doi: 10.1021/acs.analchem.8b04391. [DOI] [PubMed] [Google Scholar]
- Gregory TM, Heckmann RA, Francis RS. The effect of exercise on the presence of leukocytes, erythrocytes and collagen fibers in skeletal muscle after contusion. J Manip Physiol Ther. 1995;18:72–78. [PubMed] [Google Scholar]
- Griffiths HE. Principles of occupational therapy in the treatment of the injured. Postgrad Med J. 1943;19:2–7. doi: 10.1136/pgmj.19.206.2-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros Clark WE, Blomfield LB. The efficiency of intramuscular anastomoses, with observations on the regeneration of devascularized muscle. J Anat. 1945;79:15. [PMC free article] [PubMed] [Google Scholar]
- Grounds MD. Phagocytosis of necrotic muscle in muscle isografts is influenced by the strain, age, and sex of host mice. J Pathol. 1987;153:71–82. doi: 10.1002/path.1711530110. [DOI] [PubMed] [Google Scholar]
- Grounds MD, McGeachie JK. A model of myogenesis in vivo, derived from detailed autoradiographic studies of regenerating skeletal muscle, challenges the concept of quantal mitosis. Cell Tissue Res. 1987;250:563–569. doi: 10.1007/BF00218947. [DOI] [PubMed] [Google Scholar]
- Guller I, Russell AP. MicroRNAs in skeletal muscle: their role and regulation in development, disease and function. J Physiol. 2010;588:4075–4087. doi: 10.1113/jphysiol.2010.194175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich DB, Nguyen PD, Siegel AL, Ehrlich OV, Sonntag C, Phan JM, Berger S, Ratnayake D, Hersey L, Berger J, Verkade H, Hall TE, Currie PD. Asymmetric division of clonal muscle stem cells coordinates muscle regeneration in vivo. Science. 2016;353:aad9969. doi: 10.1126/science.aad9969. [DOI] [PubMed] [Google Scholar]
- Haggmark T, Eriksson E, Jansson E. Muscle fiber type changes in human skeletal muscle after injuries and immobilization. Orthopedics. 1986;9:181–185. doi: 10.3928/0147-7447-19860201-08. [DOI] [PubMed] [Google Scholar]
- Haggmark T, Jansson E, Eriksson E. Fiber type area and metabolic potential of the thigh muscle in man after knee surgery and immobilization. Int J Sports Med. 1981;2:12–17. doi: 10.1055/s-2008-1034577. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs EC. Early ultrastructural changes in skeletal muscle exposed to the local anaesthetic bupivacaine (Marcaine) Br J Exp Pathol. 1980;61:139–149. [PMC free article] [PubMed] [Google Scholar]
- Hansen-Smith FM, Carlson BM. Cellular responses to free grafting of the extensor digitorum longus muscle of the rat. J Neurol Sci. 1979;41:149–173. doi: 10.1016/0022-510x(79)90035-2. [DOI] [PubMed] [Google Scholar]
- Hara M, Tabata K, Suzuki T, Do MK, Mizunoya W, Nakamura M, Nishimura S, Tabata S, Ikeuchi Y, Sunagawa K, Anderson JE, Allen RE, Tatsumi R. Calcium influx through a possible coupling of cation channels impacts skeletal muscle satellite cell activation in response to mechanical stretch. Am J Physiol Cell Physiol. 2012;302:C1741–C1750. doi: 10.1152/ajpcell.00068.2012. [DOI] [PubMed] [Google Scholar]
- Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon JM, Tajbakhsh S, Rocheteau P, Chrétien F. Comparative study of injury models for studying muscle regeneration in mice. PLoS ONE. 2016;11:e0147198. doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G, Naldini L, Weidner KM, Sachs M, Vigna E, Comoglio PM, Birchmeier W. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but not mitogenesis. Proc Natl Acad Sci USA. 1992;89:11574–11578. doi: 10.1073/pnas.89.23.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ. Expanding roles for muscle satellite cells in exercise-induced hypertrophy. Function. 2020;2:zqaa040. doi: 10.1093/function/zqaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Ding Y, Zhao Y, So KK, Peng XL, Li Y, Yuan J, He Z, Chen X, Sun H, Wang H. CRISPR/Cas9/AAV9-mediated in vivo editing identifies MYC regulation of 3D genome in skeletal muscle stem cell. Stem Cell Rep. 2021;16:2442–2458. doi: 10.1016/j.stemcr.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T, Green C, Pedersen BK. Myokines in myogenesis and health. Recent Pat Biotechnol. 2012;6:167–171. doi: 10.2174/1872208311206030167. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle Regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Hernández JM, García-González EG, Brun CE, Rudnicki MA. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol. 2017;72:10–18. doi: 10.1016/j.semcdb.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydemann A, McNally E. NO more muscle fatigue. J Clin Invest. 2009;119:448–450. doi: 10.1172/JCI38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert A, Anderson JE. Satellite cell division and fiber hypertrophy alternate with new fiber formation during indeterminate muscle growth in juvenile lake sturgeon (Acipenser fulvescens) Can J Zool. 2020;98:449–459. [Google Scholar]
- Huebner KD, Jassal DS, Halevy O, Pines M, Anderson JE. Functional resolution of fibrosis in mdx mouse dystrophic heart and skeletal muscle by halofuginone. Am J Physiol Heart Circ Physiol. 2008;294:H1550–H1561. doi: 10.1152/ajpheart.01253.2007. [DOI] [PubMed] [Google Scholar]
- Huijbregts J, White JD, Grounds MD. The absence of MyoD in regenerating skeletal muscle affects the expression pattern of basement membrane, interstitial matrix and integrin molecules that is consistent with delayed myotube formation. Acta Histochem. 2001;103:379–396. doi: 10.1078/0065-1281-00607. [DOI] [PubMed] [Google Scholar]
- Hwang AB, Brack AS. Muscle stem cells and aging. Curr Top Dev Biol. 2018;126:299–322. doi: 10.1016/bs.ctdb.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol. 2012;303:C854–861. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson F, Borg K, Edstrom L, Grimby L. Use of motor units in relation to muscle fiber type and size in man. Muscle Nerve. 1988;11:1211–1218. doi: 10.1002/mus.880111205. [DOI] [PubMed] [Google Scholar]
- Janke A, Upadhaya R, Snow WM, Anderson JE. A new look at cytoskeletal NOS-1 and ƒ-dystroglycan changes in developing muscle and brain in control and mdx dystrophic mice. Dev Dyn. 2013;242:1369–1381. doi: 10.1002/dvdy.24031. [DOI] [PubMed] [Google Scholar]
- Jensen JB, Møller AB, Just J, Mose M, de Paoli FV, Billeskov TB, Fred RG, Pers TH, Pedersen SB, Petersen KK, Bjerre M, Farup J, Jessen N. Isolation and characterization of muscle stem cells, fibro-adipogenic progenitors, and macrophages from human skeletal muscle biopsies. Am J Physiol Cell Physiol. 2021;321:C257–c268. doi: 10.1152/ajpcell.00127.2021. [DOI] [PubMed] [Google Scholar]
- Joanisse S, Nederveen JP, Snijders T, McKay BR, Parise G. Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology. 2017;63:91–100. doi: 10.1159/000450922. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juban G, Saclier M, Yacoub-Youssef H, Gondin J, Mounier R, Chazaud B. TGF-B1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep. 2018;25:2163–2176. doi: 10.1016/j.celrep.2018.10.077. [DOI] [PubMed] [Google Scholar]
- Kadi F, Ponsot E. The biology of satellite cells and telomeres in human skeletal muscle: effects of aging and physical activity. Scand J Med Sci Sports. 2010;20:39–48. doi: 10.1111/j.1600-0838.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- Khan MA. Histochemical and ultrastructural characteristics of a new muscle fibre type in avian striated muscle. Histochem J. 1976;11:321–335. doi: 10.1007/BF01005031. [DOI] [PubMed] [Google Scholar]
- Khan MA. Histochemical characteristics of vertebrate striated muscle: a review. Prog Histochem Cytochem. 1976;8:1–48. doi: 10.1016/s0079-6336(76)80005-8. [DOI] [PubMed] [Google Scholar]
- Kimmel JC, Hwang AB, Scaramozza A, Marshall WF, Brack AS. Aging induces aberrant state transition kinetics in murine muscle stem cells. Development. 2020;147:dev183855. doi: 10.1242/dev.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk B, Feehan J, Lombardi G, Duque G. Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep. 2020;18:388–400. doi: 10.1007/s11914-020-00599-y. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140:41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- Knappe S, Zammit PS, Knight RD. A population of Pax7-expressing muscle progenitor cells show differential responses to muscle injury dependent on developmental stage and injury extent. Front Aging Neurosci. 2015;7:161. doi: 10.3389/fnagi.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok HJ, Barton ER. Actions and interactions of IGF-I and MMPs during muscle regeneration. Semin Cell Dev Biol. 2021;119:11–22. doi: 10.1016/j.semcdb.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski K, Kołodziejczyk A, Sikorska M, Płaczkiewicz J, Cichosz P, Kowalewska M, Stremińska W, Jańczyk-Ilach K, Koblowska M, Fogtman A, Iwanicka-Nowicka R, Ciemerych MA, Brzoska E. Stem cells migration during skeletal muscle regeneration - the role of Sdf-1/Cxcr4 and Sdf-1/Cxcr7 axis. Cell Adh Migr. 2017;11:384–398. doi: 10.1080/19336918.2016.1227911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AS, Peck BD, Walton RG, Kern PA, Mar JC, Windham ST, Bamman MM, Barzilai N, Peterson CA. Metformin alters skeletal muscle transcriptome adaptations to resistance training in older adults. Aging (Albany NY) 2020;12:19852–19866. doi: 10.18632/aging.104096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraitis D, Ebadi D, Zhang P, Rizzuto E, Vulesevic B, Padavan DT, Al Madhoun A, McEwan KA, Sofrenovic T, Nicholson K, Whitman SC, Mesana TG, Skerjanc IS, Musarò A, Ruel M, Suuronen EJ. Injected matrix stimulates myogenesis and regeneration of mouse skeletal muscle after ischaemic injury. Eur Cells Mater. 2012;24:175–195. doi: 10.22203/ecm.v024a13. [DOI] [PubMed] [Google Scholar]
- LaFramboise WA, Daood MJ, Guthrie RD, Butler-Browne GS, Whalen RG, Ontell M. Myosin isoforms in neonatal rat extensor digitorum longus, diaphragm, and soleus muscles. Am J Physiol. 1990;259:L116–L122. doi: 10.1152/ajplung.1990.259.2.L116. [DOI] [PubMed] [Google Scholar]
- Lad H, Saumur TM, Herridge MS, dos Santos CC, Mathur S, Batt J, Gilbert PM. Intensive care unit-acquired weakness: not just another muscle atrophying condition. Int J Mol Sci. 2020;21:7840. doi: 10.3390/ijms21217840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar AB. Finding MyoD and lessons learned along the way. Semin Cell Dev Biol. 2017;72:3–9. doi: 10.1016/j.semcdb.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latroche C, Gitiaux C, Chretien F, Desguerre I, Mounier R, Chazaud B. Skeletal muscle microvasculature: a highly dynamic lifeline. Physiology (bethesda) 2015;30:417–427. doi: 10.1152/physiol.00026.2015. [DOI] [PubMed] [Google Scholar]
- Laumonier T, Menetrey J. Muscle injuries and strategies for improving their repair. J Exp Orthop. 2016;3:15. doi: 10.1186/s40634-016-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin KM, Bell MB, McAdam JS, Peck BD, Walton RG, Windham ST, Tuggle SC, Long DE, Kern PA, Peterson CA, Bamman MM. Muscle transcriptional networks linked to resistance exercise training hypertrophic response heterogeneity. Physiol Genomics. 2021;53:206–221. doi: 10.1152/physiolgenomics.00154.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Anderson JE, Joya JE, Head SI, Pather N, Kee AJ, Gunning PW, Hardeman EC. Aged skeletal muscle retains the ability to fully regenerate functional architecture. BioArchitecture. 2013;3:25–37. doi: 10.4161/bioa.24966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Sébille A. The cellular events of injured muscle regeneration depend on the nature of the injury. Neuromuscul Disord. 1995;5:501–509. doi: 10.1016/0960-8966(95)00012-c. [DOI] [PubMed] [Google Scholar]
- Legros V, Jeannin P, Burlaud-Gaillard J, Chaze T, Gianetto QG, Butler-Browne G, Mouly V, Zoladek J, Afonso PV, Gonzàlez MN, Matondo M, Riederer I, Roingeard P, Gessain A, Choumet V, Ceccaldi PE. Differentiation-dependent susceptibility of human muscle cells to Zika virus infection. PLoS Negl Trop Dis. 2020;14:e0002282. doi: 10.1371/journal.pntd.0008282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter JR, Anderson JE. Satellite cells are increasingly refractory to activation by nitric oxide and stretch in aged mouse-muscle cultures. Int J Biochem Cell Biol. 2010;42:132–136. doi: 10.1016/j.biocel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Leiter JR, Upadhaya R, Anderson JE. Nitric oxide and voluntary exercise together promote quadriceps hypertrophy and increase vascular density in female 18-mo-old mice. Am J Physiol Cell Physiol. 2012;302:C1306–C1315. doi: 10.1152/ajpcell.00305.2011. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Paylor B, Chang C, Sampaio A, Underhill TM, Rossi FM. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem Cells. 2012;30:1152–1162. doi: 10.1002/stem.1082. [DOI] [PubMed] [Google Scholar]
- Lepore E, Casola I, Dobrowolny G, Musarò A. Neuromuscular junction as an entity of nerve-muscle communication. Cells. 2019;8:906. doi: 10.3390/cells8080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Bareja A, Judge L, Yue Y, Lai Y, Fairclough R, Davies KE, Chamberlain JS, Duan D. Sarcolemmal nNOS anchoring reveals a qualitative difference between dystrophin and utrophin. J Cell Sci. 2010;123:2008–2013. doi: 10.1242/jcs.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9:860–873. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- Li EW, McKee-Muir OC, Gilbert PM. Cellular biomechanics in skeletal muscle regeneration. Curr Top Dev Biol. 2018;126:125–176. doi: 10.1016/bs.ctdb.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol. 2013;305:C241–252. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister TD. The abuse of exercise. Hospital (Lond 1886) 1900;28:201. [PMC free article] [PubMed] [Google Scholar]
- Long DE, Peck BD, Tuggle SC, Villasante Tezanos AG, Windham ST, Bamman MM, Kern PA, Peterson CA, Walton RG. Associations of muscle lipid content with physical function and resistance training outcomes in older adults: altered responses with metformin. Geroscience. 2021;43:629–644. doi: 10.1007/s11357-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol. 2005;16:612–622. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Knellwolf TP. Functional properties of human muscle spindles. J Neurophysiol. 2018;120:452–467. doi: 10.1152/jn.00071.2018. [DOI] [PubMed] [Google Scholar]
- Machado L, Geara P, Camps J, Dos Santos M, Teixeira-Clerc F, Van Herck J, Varet H, Legendre R, Pawlotsky J-M, Sampaolesi M, Voet T, Maire P, Relaix F, Mourikis P. Tissue damage induces a conserved stress response that initiates quiescent muscle stem cell activation. Cell Stem Cell. 2021;28:1125–1135.e1127. doi: 10.1016/j.stem.2021.01.017. [DOI] [PubMed] [Google Scholar]
- von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinczyk M, Dunn A, Haas G, Madsen J, Scheidt R, Patel K, Talovic M, Garg K. The effect of laminin-111 hydrogels on muscle regeneration in a murine model of injury. Tissue Eng Part A. 2019;25:1001–1012. doi: 10.1089/ten.tea.2018.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin CJ, Mokhtari DA, Sunden F, Appel MJ, Akiva E, Longwell SA, Sabatti C, Herschlag D, Fordyce PM. Revealing enzyme functional architecture via high-throughput microfluidic enzyme kinetics. Science. 2021;373:eabf8761. doi: 10.1126/science.abf8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone AK, Shcherbata HR. Dystrophin orchestrates the epigenetic profile of muscle cells via miRNAs. Front Genet. 2011;2:64. doi: 10.3389/fgene.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KS, Virgilio KM, Peirce SM, Blemker SS. Computational modeling of muscle regeneration and adaptation to advance muscle tissue regeneration strategies. Cells Tissues Organs. 2016;202:250–266. doi: 10.1159/000443635. [DOI] [PubMed] [Google Scholar]
- Mashinchian O, Pisconti A, Le Moal E, Bentzinger CF. The muscle stem cell niche in health and disease. Curr Top Dev Biol. 2018;126:23–65. doi: 10.1016/bs.ctdb.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Mathes S, Fahrner A, Ghoshdastider U, Rüdiger HA, Leunig M, Wolfrum C, Krützfeldt J (2021) FGF-2-dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc Natl Acad Sci USA 118 [DOI] [PMC free article] [PubMed]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A, Shafiq SA, Milhorat AT. Regeneration of striated muscle, and myogenesis. Amsterdam: Ekcerpta Medica; 1970. [Google Scholar]
- Maxwell LC, Faulkner JA, White TP, Hansen-Smith FM. Growth of regenerating skeletal muscle fibers in cats. Anat Rec. 1984;209:153–163. doi: 10.1002/ar.1092090203. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nut Metab Physiol Appl Nutr Metab. 2008;33:173–178. doi: 10.1139/H07-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas AJ, Thomas HC. Fast and slow twitch muscles in man. J Neurol Sci. 1968;7:301–307. doi: 10.1016/0022-510x(68)90150-0. [DOI] [PubMed] [Google Scholar]
- McGeachie JK, Grounds MD. Initiation and duration of muscle precursor replication after mild and severe injury to skeletal muscle of mice. An autoradiographic study. Cell Tissue Res. 1987;248:125–130. doi: 10.1007/BF01239972. [DOI] [PubMed] [Google Scholar]
- McIntosh LM, Garrett KL, Megeney L, Rudnicki MA, Anderson JE. Regeneration and myogenic cell proliferation correlate with taurine levels in dystrophin- and MyoD-deficient muscles. Anat Rec. 1998;252:311–324. doi: 10.1002/(SICI)1097-0185(199810)252:2<311::AID-AR17>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McIntosh LM, Pernitsky AN, Anderson JE. The effects of altered metabolism (hypothyroidism) on muscle repair in the mdx dystrophic mouse. Muscle Nerve. 1994;17:444–453. doi: 10.1002/mus.880170413. [DOI] [PubMed] [Google Scholar]
- McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004;29:707–715. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoon LK, Wirtschafter J. Activated satellite cells are present in uninjured extraocular muscles of mature mice. Trans Am Ophthalmol Soc. 2002;100:119–123. [PMC free article] [PubMed] [Google Scholar]
- McLoon LK, Wirtschafter J. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol vis Sci. 2003;44:1927–1932. doi: 10.1167/iovs.02-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechalchuk CL, Bressler BH. Contractility of mdx skeletal muscle after denervation and devascularization. Muscle Nerve. 1992;15:310–317. doi: 10.1002/mus.880150309. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Mihaly E, Altamirano DE, Tuffaha S, Grayson W. Engineering skeletal muscle: Building complexity to achieve functionality. Semin Cell Dev Biol. 2021;119:61–69. doi: 10.1016/j.semcdb.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R, Mellows B, Sheard J, Antonioli M, Kretz O, Chambers D, Zeuner MT, Tomkins JE, Denecke B, Musante L, Joch B, Debacq-Chainiaux F, Holthofer H, Ray S, Huber TB, Dengjel J, De Coppi P, Widera D, Patel K. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res Ther. 2019;10:116. doi: 10.1186/s13287-019-1213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunoya W, Upadhaya R, Burczynski FJ, Wang G, Anderson JE. Nitric oxide donors improve prednisone effects on muscular dystrophy in the mdx mouse diaphragm. Am J Physiol Cell Physiol. 2011;300:C1065–C1077. doi: 10.1152/ajpcell.00482.2010. [DOI] [PubMed] [Google Scholar]
- Le Moal E, Pialoux V, Juban G, Groussard C, Zouhal H, Chazaud B, Mounier R. Redox control of skeletal muscle regeneration. Antioxid Redox Signal. 2017;27:276–310. doi: 10.1089/ars.2016.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok GF, Lozano-Velasco E, Munsterberg A. microRNAs in skeletal muscle development. Semin Cell Dev Biol. 2017;2017:67–76. doi: 10.1016/j.semcdb.2017.10.032. [DOI] [PubMed] [Google Scholar]
- Montandon M, Currie PD, Ruparelia AA (2021) Examining muscle regeneration in zebrafish models of muscle disease. J Vis Exp JoVE. 10.3791/62071 [DOI] [PubMed]
- Moriscot A, Miyabara EH, Langeani B, Belli A, Egginton S, Bowen TS. Firearms-related skeletal muscle trauma: pathophysiology and novel approaches for regeneration. NPJ Regen Med. 2021;6:17. doi: 10.1038/s41536-021-00127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpugo B. Nerve regeneration from one into the other of two rats united in Siamese pairs. J Physiol. 1923;58:98–100. doi: 10.1113/jphysiol.1923.sp002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly V, Aamiri A, Bigot A, Cooper RN, Di DS, Furling D, Gidaro T, Jacquemin V, Mamchaoui K, Negroni E, Perie S, Renault V, Silva-Barbosa SD, Butler-Browne GS. The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand. 2005;184:3–15. doi: 10.1111/j.1365-201X.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- Moyer AL, Wagner KR. Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol. 2011;23:568–573. doi: 10.1097/BOR.0b013e32834bac92. [DOI] [PubMed] [Google Scholar]
- Moyle LA, Cheng RY, Liu H, Davoudi S, Ferreira SA, Nissar AA, Sun Y, Gentleman E, Simmons CA, Gilbert PM. Three-dimensional niche stiffness synergizes with Wnt7a to modulate the extent of satellite cell symmetric self-renewal divisions. Mol Biol Cell. 2020;31:1703–1713. doi: 10.1091/mbc.E20-01-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach KA, Peck BD, Policastro RA, Vechetti IJ, Van Pelt DW, Dungan CM, Denes LT, Fu X, Brightwell CR, Zentner GE, Dupont-Versteegden EE, Richards CI, Smith JJ, Fry CS, McCarthy JJ, Peterson CA. Early satellite cell communication creates a permissive environment for long-term muscle growth. iScience. 2021;24:102372. doi: 10.1016/j.isci.2021.102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PDF, Kodicek E. Bones, Muscles and Vitamin C. I. The effect of a partial deficiency of vitamin C on the repair of bone and muscle in guinea-pigs. J Anat. 1949;83:158–174. [PubMed] [Google Scholar]
- Musaro A. The basis of muscle regeneration. Adv Biol. 2014;2014:1–16. [Google Scholar]
- Musarò A (2020) Muscle homeostasis and regeneration: from molecular mechanisms to therapeutic opportunities. Cells 9 [DOI] [PMC free article] [PubMed]
- Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280:4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Cánoves P, Serrano AL. Macrophages decide between regeneration and fibrosis in muscle. Trends Endocrinol Metab. 2015;26:449–450. doi: 10.1016/j.tem.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Natarajan A, Lemos DR, Rossi FM. Fibro/adipogenic progenitors: a double-edged sword in skeletal muscle regeneration. Cell Cycle. 2010;9:2045–2046. doi: 10.4161/cc.9.11.11854. [DOI] [PubMed] [Google Scholar]
- Newman LB. A new device for measuring muscle strength—the myometer. Arch Phys Med Rehabil. 1949;30:234–237. [PubMed] [Google Scholar]
- Nielsen S, Scheele C, Yfanti C, Akerstrom T, Nielsen AR, Pedersen BK, Laye M. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol. 2010;588:4029–4037. doi: 10.1113/jphysiol.2010.189860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontell M. Muscle satellite cells: a validated technique for light microscopic identification and a quantitative study of changes in their population following denervation. Anat Rec. 1974;178:211–227. doi: 10.1002/ar.1091780206. [DOI] [PubMed] [Google Scholar]
- Ontell M. Morphological aspects of muscle fiber regeneration. Fed Proc. 1986;45:1461–1465. [PubMed] [Google Scholar]
- Owens DJ, Sharples AP, Polydorou I, Alwan N, Donovan T, Tang J, Fraser WD, Cooper RG, Morton JP, Stewart C, Close GL. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am J Physiol Endocrinol Metab. 2015;309:E1019–1031. doi: 10.1152/ajpendo.00375.2015. [DOI] [PubMed] [Google Scholar]
- Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID-19. Neurol Sci. 2020;41:3039–3056. doi: 10.1007/s10072-020-04708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma C, Clementi E. Nitric oxide in myogenesis and therapeutic muscle repair. Mol Neurobiol. 2012;46:682–692. doi: 10.1007/s12035-012-8311-8. [DOI] [PubMed] [Google Scholar]
- Panci G, Chazaud B. Inflammation during post-injury skeletal muscle regeneration. Semin Cell Dev Biol. 2021;119:32–38. doi: 10.1016/j.semcdb.2021.05.031. [DOI] [PubMed] [Google Scholar]
- Park SS, Seo YK, Kwon KS. Sarcopenia targeting with autophagy mechanism by exercise. BMB Rep. 2019;52:64–69. doi: 10.5483/BMBRep.2019.52.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DJ, Wilkinson RS. The effect of reinnervation on the distribution of muscle fibre types in the tibialis anterior muscle of the mouse. Can J Appl Physiol Revue Can Physiol Appl. 1990;68:596–602. doi: 10.1139/y90-086. [DOI] [PubMed] [Google Scholar]
- Patel KH, Dunn AJ, Talovic M, Haas GJ, Marcinczyk M, Elmashhady H, Kalaf EG, Sell SA, Garg K. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed Mater (Bristol, England) 2019;14:035010. doi: 10.1088/1748-605X/ab0b06. [DOI] [PubMed] [Google Scholar]
- Paylor B, Joe AW, Rossi FM, Lemos DR. In vivo characterization of neural crest-derived fibro/adipogenic progenitor cells as a likely cellular substrate for craniofacial fibrofatty infiltrating disorders. Biochem Biophys Res Commun. 2014;451:148–151. doi: 10.1016/j.bbrc.2014.07.089. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Pette D. Historical Perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol (1985) 2001;90:1119–1124. doi: 10.1152/jappl.2001.90.3.1119. [DOI] [PubMed] [Google Scholar]
- Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab. 2013;304:E453–465. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- Pisconti A, Brunelli S, Di Padova M, De Palma C, Deponti D, Baesso S, Sartorelli V, Cossu G, Clementi E. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol. 2006;172:233–244. doi: 10.1083/jcb.200507083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Popogeff IA, Murray MR. Form and behavior of adult mammalian skeletal muscle in vitro. Anat Rec. 1956;95:321. doi: 10.1002/ar.1090950308. [DOI] [PubMed] [Google Scholar]
- Pourghadamyari H, Rezaei M, Ipakchi-Azimi A, Eisa-Beygi S, Basiri M, Tahamtani Y, Baharvand H. Establishing a new animal model for muscle regeneration studies. Mol Biol Res Commun. 2019;8:171–179. doi: 10.22099/mbrc.2019.34611.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol. 2017;57–58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri D, Swamy CVB, Dhawan J, Mishra RK. Comparative nuclear matrix proteome analysis of skeletal muscle cells in different cellular states. Cell Biol Int. 2021;45:580–598. doi: 10.1002/cbin.11499. [DOI] [PubMed] [Google Scholar]
- Purohit G, Dhawan J. Adult muscle stem cells: exploring the links between systemic and cellular metabolism. Front Cell Dev Biol. 2019;7:312. doi: 10.3389/fcell.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaisar R, Bhaskaran S, Van Remmen H. Muscle fiber type diversification during exercise and regeneration. Free Radic Biol Med. 2016;98:56–67. doi: 10.1016/j.freeradbiomed.2016.03.025. [DOI] [PubMed] [Google Scholar]
- Ratnayake D, Currie PD. Stem cell dynamics in muscle regeneration: insights from live imaging in different animal models. BioEssays. 2017;39:1700011. doi: 10.1002/bies.201700011. [DOI] [PubMed] [Google Scholar]
- Ratnayake D, Nguyen PD, Rossello FJ, Wimmer VC, Tan JL, Galvis LA, Julier Z, Wood AJ, Boudier T, Isiaku AI, Berger S, Oorschot V, Sonntag C, Rogers KL, Marcelle C, Lieschke GJ, Martino MM, Bakkers J, Currie PD. Macrophages provide a transient muscle stem cell niche via NAMPT secretion. Nature. 2021;591:281–287. doi: 10.1038/s41586-021-03199-7. [DOI] [PubMed] [Google Scholar]
- Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron. 2003;40:563–580. doi: 10.1016/s0896-6273(03)00682-2. [DOI] [PubMed] [Google Scholar]
- Renault V, Piron-Hamelin G, Forestier C, DiDonna S, Decary S, Hentati F, Saillant G, Butler-Browne GS, Mouly V. Skeletal muscle regeneration and the mitotic clock. Exp Gerontol. 2000;35:711–719. doi: 10.1016/s0531-5565(00)00151-0. [DOI] [PubMed] [Google Scholar]
- Reznik M. Satellie cells, myoblasts, and skeletal muscle regeneration. In: Mauro A, Shafiq SA, Milhorat AT, editors. Regneration of striated muscle, and myogenesis. Amsterdam: Excerpta Medica; 1970. pp. 133–156. [Google Scholar]
- Robinson DCL, Dilworth FJ. Epigenetic regulation of adult myogenesis. Curr Top Dev Biol. 2018;126:235–284. doi: 10.1016/bs.ctdb.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Rogers GD, Thistlethwaite JE, Anderson ES, Abrandt Dahlgren M, Grymonpre RE, Moran M, Samarasekera DD. International consensus statement on the assessment of interprofessional learning outcomes. Med Teach. 2017;39:347–359. doi: 10.1080/0142159X.2017.1270441. [DOI] [PubMed] [Google Scholar]
- Roveimiab Z, Lin F, Anderson JE. Emerging development of microfluidics-based approaches to improve studies of muscle cell migration. Tissue Eng Part B Rev. 2019;25:30–45. doi: 10.1089/ten.TEB.2018.0181. [DOI] [PubMed] [Google Scholar]
- Roveimiab Z, Lin F, Anderson JE. Traction and attraction: haptotaxis substrates collagen and fibronectin interact with chemotaxis by HGF to regulate myoblast migration in a microfluidic device. Am J Physiol Cell Physiol. 2020;319:C75–c92. doi: 10.1152/ajpcell.00417.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands RP. A case of Volkmann's contracture treated by shortening the radius and ulna. Lancet. 1905;166:1168–1171. [Google Scholar]
- Rowlands RP. The value of freedom and exercise after operations. Br Med J. 1922;1:52–53. doi: 10.1136/bmj.1.3185.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Khan MM, Witzemann V. Motor endplate-anatomical, functional, and molecular concepts in the historical perspective. Cells. 2019;8:387. doi: 10.3390/cells8050387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, Abadi A, Akhtar M, Hettinga BP, Tarnopolsky MA. miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS One. 2009;4:e5610. doi: 10.1371/journal.pone.0005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Said NH, Della Valle F, Liu P, Paytuví-Gallart A, Adroub S, Gimenez J, Orlando V. Malat-1-PRC2-EZH1 interaction supports adaptive oxidative stress dependent epigenome remodeling in skeletal myotubes. Cell Death Dis. 2021;12:850. doi: 10.1038/s41419-021-04082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini J, McPhee JS, Al-Dabbagh S, Stewart CE, Al-Shanti N. Regenerative function of immune system: Modulation of muscle stem cells. Ageing Res Rev. 2016;27:67–76. doi: 10.1016/j.arr.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Shono J, Suzuki T, Sawano S, Anderson JE, Do MK, Ohtsubo H, Mizunoya W, Sato Y, Nakamura M, Furuse M, Yamada K, Ikeuchi Y, Tatsumi R. Implication of anti-inflammatory macrophages in regenerative moto-neuritogenesis: promotion of myoblast migration and neural chemorepellent semaphorin 3A expression in injured muscle. Int J Biochem Cell Biol. 2014;54:272–285. doi: 10.1016/j.biocel.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Sato Y, Do MK, Suzuki T, Ohtsubo H, Mizunoya W, Nakamura M, Furuse M, Ikeuchi Y, Tatsumi R. Satellite cells produce neural chemorepellent semaphorin 3A upon muscle injury. Anim Sci J. 2013;84:185–189. doi: 10.1111/asj.12014. [DOI] [PubMed] [Google Scholar]
- Schultz E, Jaryszak DL. Effects of skeletal muscle regeneration on the proliferation potential of satellite cells. Mech Ageing Dev. 1985;30:63–72. doi: 10.1016/0047-6374(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Sciorati C, Buono R, Azzoni E, Casati S, Ciuffreda P, D'Angelo G, Cattaneo D, Brunelli S, Clementi E. Co-administration of ibuprofen and nitric oxide is an effective experimental therapy for muscular dystrophy, with immediate applicability to humans. Br J Pharmacol. 2010;160:1550–1560. doi: 10.1111/j.1476-5381.2010.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorati C, Galvez BG, Brunelli S, Tagliafico E, Ferrari S, Cossu G, Clementi E. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J Cell Sci. 2006;119:5114–5123. doi: 10.1242/jcs.03300. [DOI] [PubMed] [Google Scholar]
- Sciorati C, Miglietta D, Buono R, Pisa V, Cattaneo D, Azzoni E, Brunelli S, Clementi E. A dual acting compound releasing nitric oxide (NO) and ibuprofen, NCX 320, shows significant therapeutic effects in a mouse model of muscular dystrophy. Pharmacol Res. 2011;64:210–217. doi: 10.1016/j.phrs.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorati C, Staszewsky L, Zambelli V, Russo I, Salio M, Novelli D, Di Grigoli G, Moresco RM, Clementi E, Latini R. Ibuprofen plus isosorbide dinitrate treatment in the mdx mice ameliorates dystrophic heart structure. Pharmacol Res. 2013;73:35–43. doi: 10.1016/j.phrs.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Scognamiglio C, Soloperto A, Ruocco G, Cidonio G. Bioprinting stem cells: building physiological tissues one cell at a time. Am J Physiol Cell Physiol. 2020;319:C465–c480. doi: 10.1152/ajpcell.00124.2020. [DOI] [PubMed] [Google Scholar]
- Shafiq SA, Gorycki MA, Milhorat AT. An electron microscopic study of regeneration and satellite cells in human muscle. Neurology. 1967;17:567–574. doi: 10.1212/wnl.17.6.567. [DOI] [PubMed] [Google Scholar]
- Shafiq SA. Satellite cells and fiber nuclei in muscle regeneration. In: Mauro A, Shafiq SA, Milhorat AT, editors. Regneration of striated muscle, and myogenesis. Amsterdam: Excerpta Medica; 1970. pp. 122–132. [Google Scholar]
- Sharma M, Juvvuna PK, Kukreti H, McFarlane C. Mega roles of microRNAs in regulation of skeletal muscle health and disease. Front Physiol. 2014;5:239. doi: 10.3389/fphys.2014.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples AP. Skeletal muscle possesses an epigenetic memory of exercise: role of nucleus type-specific DNA methylation. Function. 2021;2:zqab047. doi: 10.1093/function/zqab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavlakadze T, McGeachie J, Grounds MD. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology. 2010;11:363–376. doi: 10.1007/s10522-009-9260-0. [DOI] [PubMed] [Google Scholar]
- Shi L, Fu AK, Ip NY. Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends Neurosci. 2012;35:441–453. doi: 10.1016/j.tins.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DD. 3D timelapse analysis of muscle satellite cell motility. Stem Cells. 2009;27:2527–2538. doi: 10.1002/stem.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AL, Kuhlmann PK, Cornelison DD. Muscle satellite cell proliferation and association: new insights from myofiber time-lapse imaging. Skelet Muscle. 2011;1:7. doi: 10.1186/2044-5040-1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemionow M, Langa P, Harasymczuk M, Cwykiel J, Sielewicz M, Smieszek J, Heydemann A. Human dystrophin expressing chimeric (DEC) cell therapy ameliorates cardiac, respiratory, and skeletal muscle's function in Duchenne muscular dystrophy. Stem Cells Transl Med. 2021;10:1406–1418. doi: 10.1002/sctm.21-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemionow M, Malik M, Langa P, Cwykiel J, Brodowska S, Heydemann A. Cardiac protection after systemic transplant of dystrophin expressing chimeric (DEC) cells to the mdx mouse model of Duchenne muscular dystrophy. Stem Cell Rev Rep. 2019;15:827–841. doi: 10.1007/s12015-019-09916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincennes MC, Brun CE, Lin AYT, Rosembert T, Datzkiw D, Saber J, Ming H, Kawabe YI, Rudnicki MA. Acetylation of PAX7 controls muscle stem cell self-renewal and differentiation potential in mice. Nat Commun. 2021;12:3253. doi: 10.1038/s41467-021-23577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe GM, Shavlakadze T, Roberts P, Davies MJ, McGeachie JK, Grounds MD. Age influences the early events of skeletal muscle regeneration: studies of whole muscle grafts transplanted between young (8 weeks) and old (13–21 months) mice. Exp Gerontol. 2008;43:550–562. doi: 10.1016/j.exger.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Snijders T, Parise G. Role of muscle stem cells in sarcopenia. Curr Opin Clin Nutr Metab Care. 2017;20:186–190. doi: 10.1097/MCO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- Snow MH. Metabolic activity during the degenerative and early regenerative stages on skeletal muscle. Anat Rec. 1973;176:185–204. doi: 10.1002/ar.1091760207. [DOI] [PubMed] [Google Scholar]
- Somarelli JA (2021) The hallmarks of cancer as ecologically driven phenotypes. Front Ecol Evol 9 [DOI] [PMC free article] [PubMed]
- Song W, Kwak HB, Kim JH, Lawler JM. Exercise training modulates the nitric oxide synthase profile in skeletal muscle from old rats. J Gerontol A Biol Sci Med Sci. 2009;64:540–549. doi: 10.1093/gerona/glp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Mishra RK, Dhawan J. Regulation of cellular chromatin state: insights from quiescence and differentiation. Organogenesis. 2010;6:37–47. doi: 10.4161/org.6.1.11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DA, Karvas RM, Siegel AL, Cornelison DD. Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development. 2011;138:5279–5289. doi: 10.1242/dev.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron RS, Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry. 1986;86:19–23. doi: 10.1007/BF00492341. [DOI] [PubMed] [Google Scholar]
- Staron RS, Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit Tibialis anterior muscle. Biochem J. 1987;243:695–699. doi: 10.1042/bj2430695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron RS, Pette D. Nonuniform myosin expression along single fibers of chronically stimulated and contralateral rabbit tibialis anterior muscles. Pflug Arch. 1987;409:67–73. doi: 10.1007/BF00584751. [DOI] [PubMed] [Google Scholar]
- Studitsky AM. Dynamics of the development of myogenic tissue under conditions of explantation and transplantation. In: Rose GG, editor. Cinemicrography in cell biology. New York: Academic Press; 1963. pp. 171–200. [Google Scholar]
- Subramaniam S, Sreenivas P, Cheedipudi S, Reddy VR, Shashidhara LS, Chilukoti RK, Mylavarapu M, Dhawan J. Distinct transcriptional networks in quiescent myoblasts: a role for Wnt signaling in reversible vs. irreversible arrest. PLoS One. 2013;8:e65097. doi: 10.1371/journal.pone.0065097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto MA, Vago JP, Perretti M, Teixeira MM. Mediators of the resolution of the inflammatory response. Trends Immunol. 2019;40:212–227. doi: 10.1016/j.it.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Sunada Y, Campbell KP. Dystrophin-glycoprotein complex: molecular organization and critical roles in skeletal muscle. Curr Opin Neurol. 1995;8:379–384. [PubMed] [Google Scholar]
- Swaggart KA, McNally EM. Modifiers of heart and muscle function: where genetics meets physiology. Exp Physiol. 2014;99:621–626. doi: 10.1113/expphysiol.2013.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenarchuk LE. Nerve, Muscle, and Synaptogenesis. Cells. 2019;8:1448. doi: 10.3390/cells8111448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R. Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim Sci J. 2010;81:11–20. doi: 10.1111/j.1740-0929.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Allen RE. Mechano-biology of resident myogenic stem cells: Molecular mechanism of stretch-induced activation of satellite cells. Anim Sci J. 2008;79:279–290. doi: 10.1111/j.1740-0929.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell. 2002;13:2909–2918. doi: 10.1091/mbc.E02-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:C1487–C1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, Suzuki T, Yamada M, Rhoads RP, Jr, Ikeuchi Y, Allen RE. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol. 2009;297:C238–C252. doi: 10.1152/ajpcell.00161.2009. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Suzuki T, Do MQ, Ohya Y, Anderson JE, Shibata A, Kawaguchi M, Ohya S, Ohtsubo H, Mizunoya W, Sawano S, Komiya Y, Ichitsubo R, Ojima K, Nishimatsu SI, Nohno T, Ohsawa Y, Sunada Y, Nakamura M, Furuse M, Ikeuchi Y, Nishimura T, Yagi T, Allen RE. Slow-myofiber commitment by semaphorin 3A secreted from myogenic stem Cells. Stem Cells. 2017;35:1815–1834. doi: 10.1002/stem.2639. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Wuollet AL, Tabata K, Nishimura S, Tabata S, Mizunoya W, Ikeuchi Y, Allen RE. A role for calcium-calmodulin in regulating nitric oxide production during skeletal muscle satellite cell activation. Am J Physiol Cell Physiol. 2009;296:C922–C929. doi: 10.1152/ajpcell.00471.2008. [DOI] [PubMed] [Google Scholar]
- Teixeira E, Duarte JA. Skeletal muscle loading changes its regenerative capacity. Sports Med (auckland, NZ) 2016;46:783–792. doi: 10.1007/s40279-015-0462-0. [DOI] [PubMed] [Google Scholar]
- Termin A, Staron RS, Pette D. Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry. 1989;92:453–457. doi: 10.1007/BF00524756. [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Theret M, Rossi FMV, Contreras O. Evolving roles of muscle-resident fibro-adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Front Physiol. 2021;12:673404. doi: 10.3389/fphys.2021.673404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol. 2005;98:1900–1908. doi: 10.1152/japplphysiol.01178.2004. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol. 2011;1:2029–2062. doi: 10.1002/cphy.c100092. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MT, Sacco A. Satellite cell heterogeneity in skeletal muscle homeostasis. Trends Cell Biol. 2016;26:434–444. doi: 10.1016/j.tcb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MT, Stec MJ, Rulands S, Simons BD, Sacco A. Muscle stem cells exhibit distinct clonal dynamics in response to tissue repair and homeostatic aging. Cell Stem Cell. 2018;22:119–127.e113. doi: 10.1016/j.stem.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MT, Stec MJ, Sacco A. Assessing muscle stem cell clonal complexity during aging. Methods Mol Biol (clifton, NJ) 2019;2045:1–11. doi: 10.1007/7651_2018_139. [DOI] [PubMed] [Google Scholar]
- Timpani CA, Mamchaoui K, Butler-Browne G, Rybalka E. Nitric oxide (NO) and duchenne muscular dystrophy: no way to go? Antioxidants (basel, Switzerland) 2020;9:1268. doi: 10.3390/antiox9121268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle CF, Magnuson B, Zhao Y, Heisel CJ, Kish PE, Kahana A. Paradoxical changes underscore epigenetic reprogramming during adult Zebrafish extraocular muscle regeneration. Invest Ophthalmol vis Sci. 2019;60:4991–4999. doi: 10.1167/iovs.19-27556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintignac LA, Brenner HR, Rüegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev. 2015;95:809–852. doi: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- Tomonaga M. Histochemical and ultrastructural changes in senile human skeletal muscle. J Am Geriatr Soc. 1977;25:125–131. doi: 10.1111/j.1532-5415.1977.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759–774. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Urso ML. Anti-inflammatory interventions and skeletal muscle injury: benefit or detriment? J Appl Physiol (1985) 2013;115:920–928. doi: 10.1152/japplphysiol.00036.2013. [DOI] [PubMed] [Google Scholar]
- Vechetti IJ, Jr., Peck BD, Wen Y, Walton RG, Valentino TR, Alimov AP, Dungan CM, Van Pelt DW, von Walden F, Alkner B, Peterson CA, McCarthy JJ. Mechanical overload-induced muscle-derived extracellular vesicles promote adipose tissue lipolysis. FASEB J. 2021;35:e21644. doi: 10.1096/fj.202100242R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velthoven CTJ, de Morree A, Egner IM, Brett JO, Rando TA. Transcriptional profiling of quiescent muscle stem cells in vivo. Cell Rep. 2017;21:1994–2004. doi: 10.1016/j.celrep.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B, McLoon LK, Asakura A. Muscle satellite cell cross-talk with a vascular niche maintains quiescence via VEGF and notch signaling. Cell Stem Cell. 2018;23:530–543.e539. doi: 10.1016/j.stem.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva C, Giulivi C. Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic Biol Med. 2010;49:307–316. doi: 10.1016/j.freeradbiomed.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgilio KM, Jones BK, Miller EY, Ghajar-Rahimi E, Martin KS, Peirce SM, Blemker SS. Computational models provide insight into in vivo studies and reveal the complex role of fibrosis in mdx muscle regeneration. Ann Biomed Eng. 2021;49:536–547. doi: 10.1007/s10439-020-02566-1. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y, Csete M, Bartels J, Chang S, An G. Translational systems biology of inflammation. PLoS Comput Biol. 2008;4:e1000014. doi: 10.1371/journal.pcbi.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann R. Beitr Path Anat. 1893;12:233. [Google Scholar]
- Waldeyer W. Virchows Arch Path Anat Physiol. 1865;34:473. [Google Scholar]
- Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, Bush HM, Villasante Tezanos AG, McGwin G, Windham ST, Ovalle F, Bamman MM, Kern PA, Peterson CA. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial. Aging Cell. 2019;18:e13039. doi: 10.1111/acel.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Burczynski FJ, Hasinoff BB, Zhang K, Lu Q, Anderson JE. Development of a nitric oxide-releasing analogue of the muscle relaxant guaifenesin for skeletal muscle satellite cell myogenesis. Mol Pharm. 2009;6:895–904. doi: 10.1021/mp800226z. [DOI] [PubMed] [Google Scholar]
- Wang YX, Feige P, Brun CE, Hekmatnejad B, Dumont NA, Renaud JM, Faulkes S, Guindon DE, Rudnicki MA. EGFR-aurka signaling rescues polarity and regeneration defects in dystrophin-deficient muscle stem cells by increasing asymmetric divisions. Cell Stem Cell. 2019;24:419–432.e416. doi: 10.1016/j.stem.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang G, Wang H, Chen Q, Burczynski FJ. Review of recent patents and developments in skeletal muscle regeneration. Recent Pat Drug Deliv Formul. 2018;12:238–251. doi: 10.2174/1872211313666190102102005. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou CJ, Khodabukus A, Tran S, Han SO, Carlson AL, Madden L, Kishnani PS, Koeberl DD, Bursac N. Three-dimensional tissue-engineered human skeletal muscle model of Pompe disease. Commun Biol. 2021;4:524. doi: 10.1038/s42003-021-02059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MT, Fan CM. c-MET regulates myoblast motility and myocyte fusion during adult skeletal muscle regeneration. PLoS One. 2013;8:e81757. doi: 10.1371/journal.pone.0081757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol. 1993;264:E693–E698. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]
- Wen Y, Dungan CM, Mobley CB, Valentino T, Von Walden F, Murach KA. Nucleus type-specific DNA methylomics reveals epigenetic “memory” of prior adaptation in skeletal muscle. Function. 2021;2:zqab038. doi: 10.1093/function/zqab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessner B, Gryadunov-Masutti L, Tschan H, Bachl N, Roth E. Is there a role for microRNAs in exercise immunology? A synopsis of current literature and future developments. Exerc Immunol Rev. 2010;16:22–39. [PubMed] [Google Scholar]
- Witzemann V, Chevessier F, Pacifici PG, Yampolsky P. The neuromuscular junction: selective remodeling of synaptic regulators at the nerve/muscle interface. Mech Dev. 2013;130:402–411. doi: 10.1016/j.mod.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Woodard CR. Recent athletic injuries and their treatment. Physiotherapy. 1949;35:105–108. [PubMed] [Google Scholar]
- Wosczyna MN, Rando TA. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev Cell. 2018;46:135–143. doi: 10.1016/j.devcel.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak AC, Anderson JE. Single-fiber isolation and maintenance of satellite cell quiescence. Biochem Cell Biol. 2005;83:674–676. doi: 10.1139/o05-046. [DOI] [PubMed] [Google Scholar]
- Wozniak AC, Anderson JE. Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn. 2007;236:240–250. doi: 10.1002/dvdy.21012. [DOI] [PubMed] [Google Scholar]
- Wozniak AC, Anderson JE. The dynamics of the nitric oxide release-transient from stretched muscle cells. Int J Biochem Cell Biol. 2009;41:625–631. doi: 10.1016/j.biocel.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Wozniak AC, Kong J, Bock E, Pilipowicz O, Anderson JE. Signaling satellite-cell activation in skeletal muscle: Markers, models, stretch, and potential alternate pathways. Muscle Nerve. 2005;31:283–300. doi: 10.1002/mus.20263. [DOI] [PubMed] [Google Scholar]
- Wozniak AC, Pilipowicz O, Yablonka-Reuveni Z, Greenway S, Craven S, Scott E, Anderson JE. C-met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem. 2003;51:1437–1445. doi: 10.1177/002215540305101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Karaca G, Swiderska-Syn M, Michelotti GA, Kruger L, Chen Y, Premont RT, Choi SS, Diehl AM. Cross-talk between notch and hedgehog regulates hepatic stellate cell fate. Hepatology. 2013;58:1801–1813. doi: 10.1002/hep.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Sankoda Y, Tatsumi R, Mizunoya W, Ikeuchi Y, Sunagawa K, Allen RE. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol. 2008;40:2183–2191. doi: 10.1016/j.biocel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tatsumi R, Yamanouchi K, Hosoyama T, Shiratsuchi S, Sato A, Mizunoya W, Ikeuchi Y, Furuse M, Allen RE. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol. 2010;298:C465–C476. doi: 10.1152/ajpcell.00449.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H, Kusumoto D, Hashimoto H, Yuasa S. Stem cell aging in skeletal muscle regeneration and disease. Int J Mol Sci. 2020;21:1830. doi: 10.3390/ijms21051830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Hu P. Hierarchical signaling transduction of the immune and muscle cell crosstalk in muscle regeneration. Cell Immunol. 2018;326:2–7. doi: 10.1016/j.cellimm.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Yao L, Tichy ED, Zhong L, Mohanty S, Wang L, Ai E, Yang S, Mourkioti F, Qin L. Gli1 defines a subset of fibro-adipogenic progenitors that promote skeletal muscle regeneration with less fat accumulation. J Bone Miner Res. 2021;36:1159–1173. doi: 10.1002/jbmr.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen W, Kraft-Sheleg O, Zaffryar-Eilot S, Melamed S, Sun C, Millay DP, Hasson P. Fibroblast fusion to the muscle fiber regulates myotendinous junction formation. Nat Commun. 2021;12:3852. doi: 10.1038/s41467-021-24159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CR, Bingham GC, Shetty J, Hu P, Barker TH. Decellularized extracellular matrix (ECM) as a model to study fibrotic ECM mechanobiology. Methods Mol Biol (clifton, NJ) 2021;99:237–261. doi: 10.1007/978-1-0716-1382-5_18. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Nagahisa H, Miura F, Araki H, Kamei Y, Kitajima Y, Seko D, Nogami J, Tsuchiya Y, Okazaki N, Yonekura A, Ohba S, Sumita Y, Chiba K, Ito K, Asahina I, Ogawa Y, Ito T, Ohkawa Y, Ono Y. Hoxa10 mediates positional memory to govern stem cell function in adult skeletal muscle. Sci Adv. 2021;7:eabd7924. doi: 10.1126/sciadv.abd7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Chen J. Autophagy-dependent regulation of skeletal muscle regeneration and strength by a RHOGEF. Autophagy. 2021;17:1044–1045. doi: 10.1080/15548627.2021.1886721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Singh N, Reyes-Ordonez A, Khanna N, Bao Z, Zhao H, Chen J. ARHGEF3 regulates skeletal muscle regeneration and strength through autophagy. Cell Rep. 2021;34:108594. doi: 10.1016/j.celrep.2020.108594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Zhang H, Anderson JE. Satellite cell activation and populations on single muscle-fiber cultures from adult zebrafish (Danio rerio) J Exp Biol. 2014;217:1910–1917. doi: 10.1242/jeb.102210. [DOI] [PubMed] [Google Scholar]