Abstract
Rationale
The characteristics of patients with respiratory complaints and/or lung radiologic abnormalities after hospitalisation for coronavirus disease 2019 (COVID-19) are unknown. The objectives were to determine their characteristics and the relationships between dyspnoea, radiologic abnormalities and functional impairment.
Methods
In the COMEBAC (Consultation Multi-Expertise de Bicêtre Après COVID-19) cohort study, 478 hospital survivors were evaluated by telephone 4 months after hospital discharge, and 177 who had been hospitalised in an intensive care unit (ICU) or presented relevant symptoms underwent an ambulatory evaluation. New-onset dyspnoea and cough were evaluated, and the results of pulmonary function tests and high-resolution computed tomography of the chest were collected.
Results
Among the 478 patients, 78 (16.3%) reported new-onset dyspnoea, and 23 (4.8%) new-onset cough. The patients with new-onset dyspnoea were younger (56.1±12.3 versus 61.9±16.6 years), had more severe COVID-19 (ICU admission 56.4% versus 24.5%) and more frequent pulmonary embolism (18.0% versus 6.8%) (all p≤0.001) than patients without dyspnoea. Among the patients reassessed at the ambulatory care visit, the prevalence of fibrotic lung lesions was 19.3%, with extent <25% in 97% of the patients. The patients with fibrotic lesions were older (61±11 versus 56±14 years, p=0.03), more frequently managed in an ICU (87.9 versus 47.4%, p<0.001), had lower total lung capacity (74.1±13.7 versus 84.9±14.8% pred, p<0.001) and diffusing capacity of the lung for carbon monoxide (DLCO) (73.3±17.9 versus 89.7±22.8% pred, p<0.001). The combination of new-onset dyspnoea, fibrotic lesions and DLCO <70% pred was observed in eight out of 478 patients.
Conclusions
New-onset dyspnoea and mild fibrotic lesions were frequent at 4 months, but the association of new-onset dyspnoea, fibrotic lesions and low DLCO was rare.
Short abstract
New-onset dyspnoea is a frequent complaint 4 months after #COVID19 and is generally multifactorial, and the combination of new-onset dyspnoea, fibrotic lesions and DLCO <70% pred is rarely observed https://bit.ly/3q4hyyM
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has provoked an ongoing global pandemic of coronavirus disease 2019 (COVID-19), which has affected more than 240 million individuals to date [1]. There are multiple respiratory symptoms associated with COVID-19, ranging from mild upper respiratory tract symptoms to severe acute respiratory distress syndrome [2–5]. There is also growing evidence that some patients have long-term effects of COVID-19 that can affect multiple organ systems. These effects have been grouped as “post-acute COVID-19 syndrome”, defined by persistent symptoms and/or delayed or long-term complications of SARS-CoV-2 infection beyond 4 weeks from the onset of symptoms [6]. As part of post-acute COVID-19 syndrome, the persistence of respiratory symptoms seems to be common, affecting 15–81% of patients [7–13]. However, the characteristics of patients with persistent or residual respiratory complaints after hospitalisation for COVID-19 remain poorly described and understood. Recently, the Consultation Multi-Expertise de Bicêtre Après COVID-19 (COMEBAC) cohort study reported the outcomes of 478 patients 4 months after hospitalisation for COVID-19 [14]. Half of the patients reported at least one symptom that did not exist before the disease. High-resolution computed tomography (HRCT) of the chest frequently revealed persistent lung abnormalities, including fibrotic lung lesions, in a minority of patients [14].
The aims of this study were to determine: 1) the prevalence of persistent respiratory symptoms or residual respiratory complaints after hospitalisation for COVID-19; 2) the characteristics of patients with persistent respiratory symptoms; 3) the prevalence of fibrotic lung lesions; 4) the characteristics of patients with fibrotic lung lesions; and 5) the relationships between respiratory complaints, respiratory functional impairment and radiologic abnormalities 4 months after COVID-19 in the COMEBAC study cohort.
Materials and methods
Patients
The COMEBAC cohort study (NCT04704388) prospectively included adult patients admitted to the Bicêtre Hospital (Paris-Saclay University hospitals – Assistance Publique – Hôpitaux de Paris) for COVID-19 during the first wave of the pandemic in France [14]. There were two levels of enrolment in the study.
First, patients who met the following inclusion and exclusion criteria were screened for a telephone consultation. The inclusion criteria were as follows: survival 4 months after hospital discharge or after intensive care unit (ICU) discharge for patients who had been admitted to an ICU, age older than 18 years, hospitalisation for greater than 24 h primarily because of COVID-19, and diagnosis of SARS-CoV-2 infection by reverse transcriptase PCR (RT-PCR), by typical HRCT of the chest associated with clinical features, or by both. The exclusion criteria were as follows: death within 4 months after discharge, persistent hospitalisation, end-stage cancer, dementia, nosocomial COVID-19 infection, and incidental positive SARS-CoV-2 RT-PCR result during a hospital stay for a different medical indication.
Second, all the ICU patients and those who were symptomatic at the telephone consultation were invited for further evaluation in the ambulatory care setting. Symptomatic patients were defined as those reporting symptoms (except for anosmia) at the telephone interview, those with persistent creatinine-level elevation, and those with persistent abnormalities on a lung computed tomography (CT) scan conducted after hospitalisation (including any residual ground-glass opacities, bronchial or bronchioloalveolar abnormalities, lung consolidations, or interstitial thickening). “New-onset dyspnoea or cough” was defined as the presence of symptoms that did not exist before COVID-19 or as the worsening of pre-existing symptoms.
The telephone consultation was made 3–4 months after hospital discharge by a medical officer with a questionnaire focused on the general medical condition and symptoms (supplemental methods). The characteristics of the patients who were hospitalised for acute COVID-19 were extracted from electronic health records. The patients provided informed consent after ICU hospitalisation or at the beginning of the telephone consultation and before the ambulatory care setting. The Ethics Committee of the French Intensive Care Society (CE20-56) approved this study.
Respiratory assessment during the ambulatory care visit
Respiratory assessment
The functional impact of dyspnoea was evaluated using the modified Medical Research Council (mMRC) scale (Table E1). A non-encouraged 6-min walk test (6MWT) was performed according to current recommendations [15]. The patients completed standard pulmonary function tests (PFTs) with spirometry, whole-body plethysmography and single-breath diffusing lung capacity for carbon monoxide (DLCO) according to the European Respiratory Society/American Thoracic Society guidelines [16]. Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), total lung capacity (TLC) and DLCO are expressed as the percentages of predicted values (% pred) using the Global Lung Function Initiative 2012 [17] and European Community for Coal and Steel 1993 equations [18, 19]. The Nijmegen questionnaire (Table E2) was given, and the patients were considered to have “functional respiratory complaints” when the Nijmegen questionnaire score was >22/64 [20].
HRCT of the chest
HRCT of the chest was performed in patients assessed at the ambulatory care visit. Two radiologists (OM and SS) who were blinded to the clinical evaluation reviewed the HRCT images and reached a consensus regarding any disagreements. The presence and extension of consolidations, ground-glass opacities, crazy paving, reticulations, fibrosis and emphysema were assessed. The diagnosis of fibrotic lung lesions was based on the presence of traction bronchiectasis or on the association of interface signs with reticulations.
Cardiac assessment
All the patients who were admitted to the ICU, those who developed pulmonary embolism during hospitalisation, and those with cardiac symptoms on examination at the outpatient clinic were evaluated with transthoracic echocardiography.
Statistical analysis
Study data were collected and managed with Research Electronic Data Capture tools hosted at Assistance Publique Hôpitaux de Paris (AP-HP). Analysis was performed with the R statistical package version 4.0.1 (R Foundation for Statistical Computing). We report continuous variables as either the mean±sd or median (interquartile range, IQR) as appropriate and categorical variables as the number and frequency (percentage of group). Comparisons between patients with and without new-onset dyspnoea and patients with and without fibrotic lesions in lungs were performed using the t-test for normally distributed quantitative variables and the Mann–Whitney test for non-normally distributed quantitative variables. Pearson's chi-squared test or Fisher's exact test, as appropriate, was used to compare discrete variables between two groups. Differences were considered significant when the p-value was less than 0.05. We performed multivariate analysis for new-onset dyspnoea among the population who had the telephone consultation and for lung fibrotic lesions among the population who came to the ambulatory care visit. For the multivariate analysis, we focused on variables that in the univariate analysis had a p-value <0.1 and were clinically important and not collinear (consensus among investigators).
Results
Characteristics of the patients with persistent respiratory symptoms
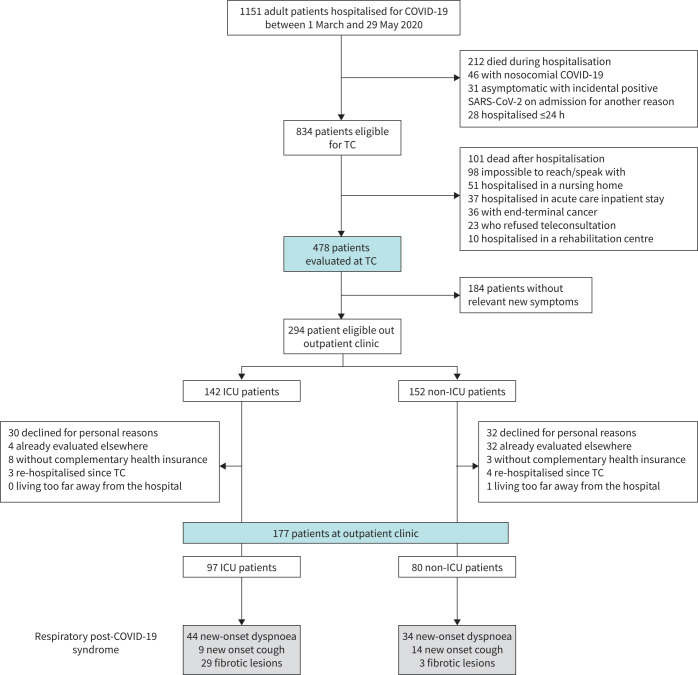
The flowchart of the study is presented in figure 1. Of the 478 patients evaluated by telephone, COVID-19 was diagnosed with RT-PCR in 415 patients (86.8%) and by an association of typical clinical signs and HRCT of the chest in 63 patients (13.2%). To ensure accurate diagnosis, a serological test was performed in the 177 patients who were evaluated at the outpatient clinic, and 172 of 177 patients (97.2%) tested positive. During the telephone consultation, 78 patients among 478 reported new-onset dyspnoea, and 23 reported new-onset cough, corresponding to a minimal prevalence of new-onset dyspnoea and cough of 16.3% and 4.8%, respectively. Compared to patients without new-onset dyspnoea, the patients with new-onset dyspnoea at the telephone consultation were younger (56.1±12.3 versus 61.9±16.6 years, p=0.001), but there was no difference in the body mass index or the frequencies of diabetes and hypertension (table 1); these patients also experienced more severe initial episodes of COVID-19, with a longer duration of hospital stay (13 (7–23) versus 8 (4–14) days, p<0.001) and more frequent admission to the ICU (56.4 versus 24.5%, p<0.001). They also more frequently exhibited pulmonary embolism in the acute phase (18.0 versus 6.8%, p<0.001). In the multivariate analysis, only ICU hospitalisation and an episode of pulmonary embolism were significatively associated with new-onset dyspnoea (Table E3).
FIGURE 1.
Flow chart of the study. COVID-19: coronavirus disease 2019; ICU: intensive care unit; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; TC: telephone consultation.
TABLE 1.
Baseline and hospitalisation characteristics of patients who were evaluated by telephone 4 months after hospital discharge according to the presence of new-onset dyspnoea
| Available data | All patients (478) | Patients with new-onset dyspnoea (78) | Patients without new-onset dyspnoea (400) | p-value | |
| Demographic data | |||||
| Age, years | 478 | 61.0±16.1 | 56.1±12.3 | 61.9±16.6 | 0.001 |
| Women | 478 | 201 (42.1%) | 30 (38.5%) | 171 (42.8%) | 0.56 |
| Body mass index, kg·m–2 | 351 | 28.8±5.6 | 29.0±5.1 | 28.8±5.8 | 0.69 |
| Smoking | |||||
| No (<5 pack-years) | 452 | 343 (75.9%) | 60 (81.1%) | 283 (74.9%) | |
| Former (≥5 pack-years) | 452 | 83 (18.4%) | 11 (14.9%) | 72 (19.0%) | 0.63 |
| Active | 452 | 26 (5.8%) | 3 (4.1%) | 23 (6.1%) | |
| Pre-COVID-19 comorbidities | |||||
| Respiratory disease | |||||
|
478 | 17 (3.6%) | 2 (2.6%) | 15 (3.8%) | 1 |
|
478 | 75 (15.7%) | 12 (15.4%) | 63 (15.8%) | 1 |
| Hypertension | 478 | 225 (47.1%) | 30 (38.5%) | 195 (48.8%) | 0.12 |
| Chronic heart disease | 478 | 77 (16.1%) | 4 (5.1%) | 73 (18.2%) | 0.007 |
| Diabetes | 478 | 128 (26.8%) | 24 (30.8%) | 104 (26.0%) | 0.47 |
| Chronic kidney disease | 478 | 51 (10.7%) | 2 (2.6%) | 49 (12.2%) | 0.02 |
| Declared psychiatric disorder | 478 | 42 (8.8%) | 5 (6.4%) | 37 (9.3%) | 0.55 |
| Neurodegenerative disorder | 478 | 34 (7.1%) | 0 (0%) | 34 (8.5%) | 0.02 |
| Alcohol misuse | 450 | 21 (4.7%) | 3 (4.1%) | 18 (4.8%) | 1 |
| Active cancer | 478 | 18 (3.8%) | 2 (2.6%) | 16 (4.0%) | 0.75 |
| Other immunosuppression | 478 | 18 (3.8%) | 2 (2.6%) | 16 (4.0%) | 0.75 |
| Long-term dialysis | 478 | 17 (3.6%) | 0 (0%) | 17 (4.3%) | 0.09 |
| HIV infection | 478 | 12 (2.5%) | 1 (1.3%) | 11 (2.8%) | 0.7 |
| Solid organ transplantation | 478 | 9 (1.9%) | 1 (1.3%) | 8 (2.0%) | 1 |
| Liver disease | 478 | 7 (1.5%) | 2 (2.6%) | 5 (1.3%) | 0.32 |
| Pregnancy | 478 | 5 (1.1%) | 0 (0%) | 5 (1.3%) | 1 |
| Hospitalisation characteristics | |||||
| Total duration of hospitalisation, days | 478 | 9 (4–15) | 13 (7–23) | 8 (4–14) | <0.001 |
| Hospitalisation in the ICU | 478 | 142 (29.7%) | 44 (56.4%) | 98 (24.5%) | <0.001 |
| Duration of ICU stay, days | 141 | 9 (4–19) | 9 (4–21) | 9 (4–19) | 0.73 |
| High flow oxygen | 142 | 62 (43.7%) | 20 (45.5%) | 42 (42.9%) | 0.92 |
| Intubation during hospitalisation | 142 | 73 (51.4%) | 25 (56.8%) | 48 (49.0%) | 0.50 |
| Duration of intubation, days | 73 | 18 (11–32) | 24 (12–38) | 16 (11–27) | 0.21 |
| Pulmonary embolism | 430 | 39 (9.1%) | 14 (18.0%) | 25 (6.8%) | <0.001 |
| Active anticoagulation (at the full therapeutic dose) | 478 | 75 (15.7%) | 30 (38.5%) | 45 (11.2%) | <0.001 |
| Specific treatments during hospitalisation | |||||
| Azithromycin | 478 | 120 (25.1%) | 28 (35.9%) | 92 (23.0%) | 0.02 |
| Anti-IL-6 | 478 | 37 (7.7%) | 12 (15.4%) | 25 (6.2%) | 0.01 |
| Hydroxychloroquine | 478 | 32 (6.7%) | 9 (11.5%) | 23 (5.8%) | 0.10 |
| Corticosteroids | 478 | 24 (5.0%) | 1 (1.3%) | 2 (5.8%) | 0.15 |
| Lopinavir/ritonavir | 478 | 16 (3.4%) | 6 (7.7%) | 10 (2.5%) | 0.03 |
| Anti-IL-1 | 478 | 11 (2.3%) | 3 (3.9%) | 8 (2.0%) | 0.40 |
| Remdesivir | 478 | 5 (1.1%) | 1 (1.3%) | 4 (1.0%) | 0.59 |
Values are expressed as the median (interquartile range), mean±sd, or number and frequency. The p-values refer to a comparison between patients with and without new-onset dyspnoea. COPD: chronic obstructive pulmonary disease; COVID-19: coronavirus disease 2019; ICU: intensive care unit; IL: interleukin.
In all, 177 patients who still had symptoms and/or had been admitted to the ICU during the acute phase were reassessed at the outpatient clinic after a median time of 125 (107–144]) days (table 2). As reported in table 1 of the COMEBAC cohort study article [14], patients reassessed at the ambulatory care visit were comparable to patients who had only a telephone consultation, except for a more severe initial COVID-19 with more hospitalisations in ICU. Among these patients, 78 (44.1%) had new-onset dyspnoea. The mMRC score was higher in the patients with new-onset dyspnoea than in those without, although 28.2% of the patients with new-onset dyspnoea were classified as mMRC 0, as they declared new-onset dyspnoea only for strenuous exercise. 23 patients with new-onset dyspnoea (29.5%) had a Nijmegen questionnaire score of >22 and were considered to have “functional respiratory complaints”. Fibrotic lesions on HRCT were present in 18 (23.1%) patients with new-onset dyspnoea. Among the patients assessed at the ambulatory care visit, those with new-onset dyspnoea more often had new-onset cough (19.2 versus 8.1%, p=0.04) and a lower FVC (85.6±16.3 versus 92.1±16.0% pred, p =0.02) and TLC (80.0±15.2 versus 85.1±15.0% pred, p=0.04) than those without new-onset dyspnoea. No difference was observed in DLCO (85.6±23.7 versus 87.7±22.1% pred, p=0.57) (table 2). Echocardiography was performed in 40 patients with new-onset dyspnoea and revealed a mild decrease in left ventricular systolic function (ejection fraction 40–49%) in six (15%) patients, no signs of pulmonary hypertension and no significant difference compared with patients without new-onset dyspnoea (table 2). Among the 177 patients reassessed at the outpatient clinic, 23 (13.0%) had new-onset cough. The majority of these 23 patients (60.9%) had been hospitalised in the ward for COVID-19 and 78.3% did not show fibrotic lesions on HRCT.
TABLE 2.
Characteristics of patients evaluated at the ambulatory care visit according to the presence of new-onset dyspnoea
| Available data | All (177) | Patients with new-onset dyspnoea (78) | Patients without new-onset dyspnoea (99) | p-value | |
| Time from hospital discharge to the outpatient clinic, days | 177 | 125 (107–144) | 118 (105–140) | 126 (108–146) | 0.28 |
| Assessment at the ambulatory care visit | |||||
| mMRC scale score for dyspnoea | 177 | <0.0001 | |||
|
87 (49.2%) | 22 (28.2%) | 65 (65.7%) | ||
|
76 (42.9%) | 48 (61.5%) | 28 (28.3%) | ||
|
14 (7.9%) | 8 (10.3%) | 6 (6.0%) | ||
| New-onset cough | 177 | 23 (13.0%) | 15 (19.2%) | 8 (8.1%) | 0.04 |
| 6-min walk distance, m | 161 | 462 (380–507) | 450 (377–495) | 474 (384–516) | 0.35 |
| Abnormal HRCT of the chest | 171 | 108 (63.2%) | 47 (61.0%) | 61 (64.9%) | 0.72 |
| Reticulations | 171 | 91 (53.2%) | 41 (53.2%) | 50 (53.2%) | 1 |
| Persistent ground-glass opacities | 171 | 72 (42.1%) | 36 (46.8%) | 36 (38.3%) | 0.30 |
| Fibrotic lesions | 171 | 33 (19.3%) | 18 (23.1%) | 15 (16.0%) | 0.28 |
| Pulmonary function tests | |||||
| FEV1, % pred | 157 | 90.8±17.8 | 87.8±16.5 | 93.3±18.5 | 0.06 |
| FEV1/VC, % pred | 157 | 82.1±7.4 | 82.3±6.9 | 82.0±7.9 | 0.77 |
| VC, % pred | 152 | 89.1±16.4 | 85.6±16.3 | 92.1±16.0 | 0.02 |
| TLC, % pred | 149 | 82.8±15.3 | 80.0±15.2 | 85.1±15.0 | 0.04 |
| DLCO, % pred | 152 | 86.7±22.7 | 85.6±23.7 | 87.7±22.1 | 0.57 |
| DLCO<70% | 152 | 33 (21.7%) | 17 (24.6%) | 16 (19.3%) | 0.55 |
| Nijmegen score>22 | 168 | 36 (21.4%) | 23 (29.5%) | 13 (14.1%) | 0.02 |
| LVEF≤50% on echocardiography | 83 | 10 (12.0%) | 6 (15.0%) | 4 (9.3%) | 0.50 |
Values are expressed as the median (interquartile range), mean±sd, or number and frequency. The p-values refer to a comparison between patients with and without new-onset dyspnoea.
DLCO: diffusing capacity of the lungs for carbon monoxide; FEV1: forced expiratory volume in the first second of expiration; HRCT: high-resolution computed tomography; LVEF: left ventricular ejection fraction; mMRC: modified Medical Research Council; VC: vital capacity.
Pulmonary function tests
As shown in the table 2, the pulmonary volumes (FVC, TLC, FEV1) were normal in the majority of the 177 patients assessed at the ambulatory care visit but DLCO was decreased in 22% of the patients.
Echocardiography results
Among the 177 patients assessed at the ambulatory care visit, an echocardiography was performed in 83 patients and 12% had a decreased left ventricular ejection fraction but none had echocardiographic signs of pulmonary hypertension.
Radiologic characteristics on HRCT of the chest
Among the 177 patients assessed at the ambulatory care visit, HRCT of the chest was performed in 171 (96.6%). One or more abnormalities related to COVID-19 were observed in 108 patients (63.2%). The most frequent abnormalities were reticulations (53.2%) and ground-glass opacities (42.1%). 33 patients (19.3%) demonstrated fibrotic lesions (table 3). The extent of lesions was limited to <10% of parenchymal involvement in the majority of the patients with ground-glass opacities (69.4%), consolidations (80.0%) and fibrotic lesions (51.5%). The extent of fibrotic lesions was <25% in 97% of the patients (table 3).
TABLE 3.
Lung abnormalities on HRCT at the ambulatory care visit (n=171)
| Ground-glass opacities | |
| Ground-glass opacities, n (%) | 72 (42.1%) |
| Extent of ground-glass opacities | |
| 0% | 98 (57.3%) |
| 1–10% | 50 (29.2%) |
| 11–25% | 19 (11.1%) |
| 26–50% | 3 (1.8%) |
| Consolidations | |
| Consolidations n (%) | 10 (5.9%) |
| Extent of consolidations | |
| 0% | 160 (93.6%) |
| 1–10% | 8 (4.7%) |
| 11–25% | 2 (1.2%) |
| Reticulations and crazy paving | |
| Reticulations, n (%) | 91 (53.2%) |
| Crazy paving, n (%) | 2 (1.2%) |
| Fibrotic lesions | |
| Fibrotic lesions, n (%) | 33 (19.3%) |
| Extent of fibrotic lesions | |
| 0% | 138 (80.7) |
| 1–10% | 17 (9.9%) |
| 11–25% | 13 (7.6%) |
| 26–50% | 2 (1.2%) |
| Other abnormalities | |
| Emphysema, n (%) | 11 (6.4%) |
| Pleural effusion, n (%) | 3 (1.8%) |
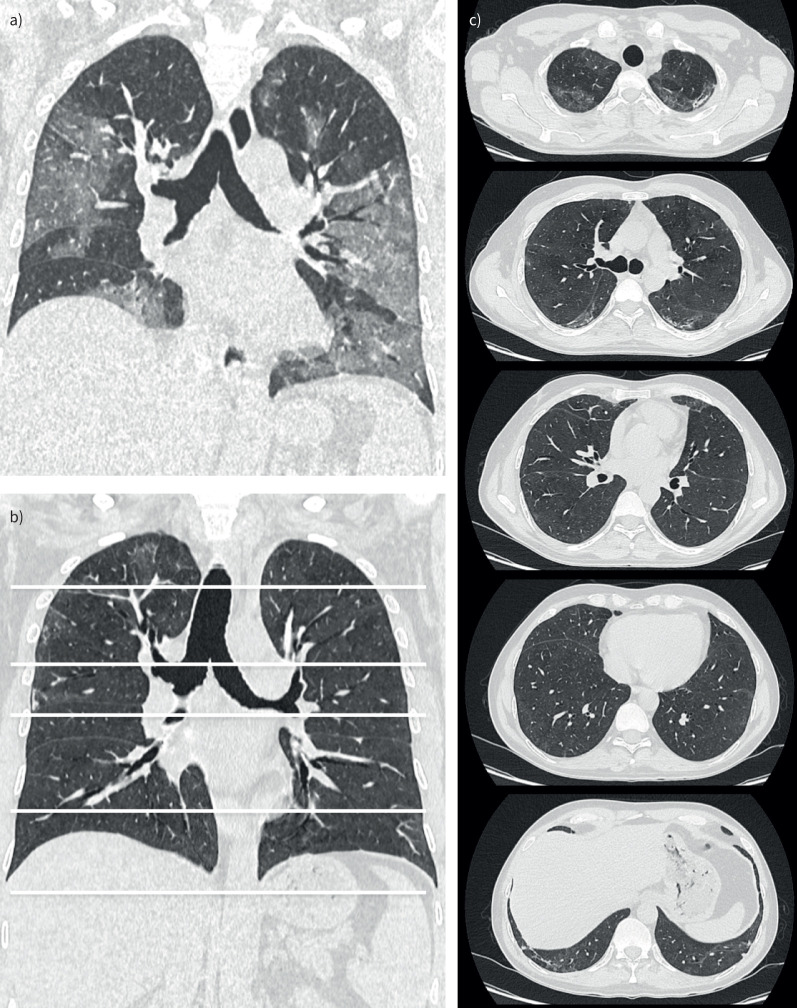
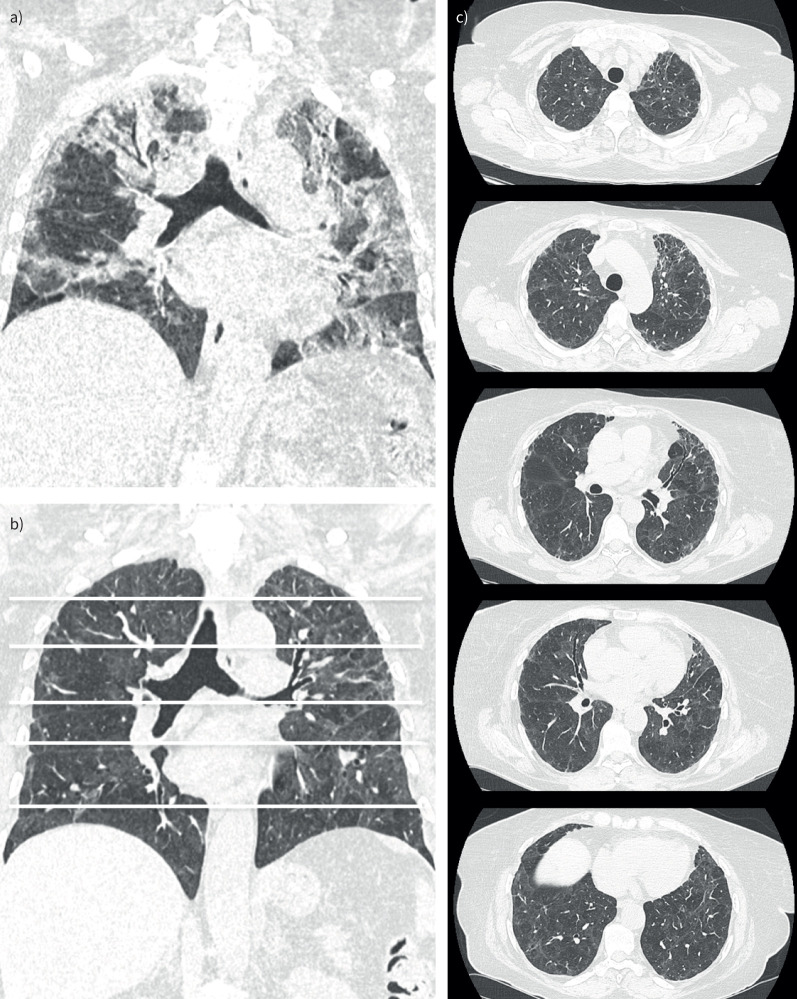
There was no significant difference in radiologic abnormalities (type of lesion and extension) between the patients with and without new-onset dyspnoea (table 2). A typical HRCT image of the chest in a patient with mild fibrotic lesions (<10% parenchymal involvement) is shown in figure 2, and that of a patient with severe fibrotic lesions (>50% parenchymal involvement) is shown in figure 3, compared with that for acute COVID-19.
FIGURE 2.
High-resolution computed tomography (HRCT) image of the chest in a patient with mild fibrotic lung lesions 4 months after hospitalisation for coronavirus disease 2019 (COVID-19) compared with that during acute COVID-19. Coronal a) multiplanar reconstruction of an HRCT image of the chest during acute COVID-19 with extensive bilateral ground-glass opacities. Coronal b) multiplanar reconstructions and axial sections c) of an HRCT image of the chest from the same patient showing mild fibrotic lung lesions at 4 months, demonstrating small traction bronchiectasis close to the marginal fibrotic sequelae with a sub-pleural predominance.
FIGURE 3.
High-resolution computed tomography (HRCT) image of the chest in a patient with severe fibrotic lung lesions 4 months after hospitalisation for coronavirus disease 2019 (COVID-19) compared with that during acute COVID-19. Coronal a) multiplanar reconstruction of an HRCT image of the chest during acute COVID-19 with extensive bilateral ground-glass opacities and consolidations. Coronal b) multiplanar reconstructions and axial sections c) of an HRCT image of the chest from the same patient showing severe fibrotic lung lesions at 4 months, demonstrating diffuse traction bronchiectasis and association with ground-glass opacities.
Characteristics of patients with fibrotic lesions on HRCT at 4 months
Of the patients with fibrotic lesions, 18 (54.5%) and 5 (15.1%) had new-onset dyspnoea and cough, respectively, which was not significantly different from patients without fibrotic lesions (58 (42.0%) and 17 (12.3%), respectively, all p>0.05) (table 4). Compared to patients without fibrotic lesions on HRCT, the patients with fibrotic lesions were older (61.2±10.9 versus 56.3±13.6 years, p =0.03). There was no significant difference in the sex ratio, BMI, comorbidities or smoking status (table 4). On the other hand, the patients with fibrotic lesions experienced significantly more severe episodes of COVID-19, with a longer duration of hospital stay (27 (15–44) versus 11 (5–17) days, p<0.001), more frequent admission to the ICU (87.9 versus 47.4%, p<0.001), a longer duration of mechanical ventilation (28 (16–43) versus 18 (10–25) days, p=0.03) and more frequently had acute pulmonary embolism (39.4 versus 11.6%, p<0.001). Associated with the higher frequency of hospitalisation in the ICU, patients with fibrotic lesions also had more often received anti-interleukin (IL) 6 (36.4% versus 10.2%, p=0.001) and anticoagulants at the therapeutic dose (45.5 versus 24.8%, p=0.03). Of note, at this period, very few patients (with and without fibrotic lesions) were treated with corticosteroids (9% and 3%, respectively).
TABLE 4.
Baseline and hospitalisation characteristics of patients who were evaluated at ambulatory care visits according to the presence of fibrotic lesions in lungs
| Available data | All (171) | Patients with fibrotic lesions (33) | Patients without fibrotic lesions (138) | p-value | |
| Demographic data | |||||
| Age, years | 171 | 57.3±13.2 | 61.2±10.9 | 56.3±13.6 | 0.03 |
| Women | 171 | 65 (38.2%) | 3 (9.1%) | 56 (40.9%) | 0.21 |
| Body mass index, kg·m–2 | 159 | 29.1±5.4 | 28.2±4.9 | 29.4±5.5 | 0.24 |
| Smoking | |||||
| No (<5 pack-years) | 162 | 125 (77.2%) | 22 (71.0%) | 103 (78.6%) | |
| Former (≥5 pack-years) | 162 | 24 (14.8%) | 5 (16.1%) | 19 (14.5%) | 0.46 |
| Active | 162 | 13 (8.0%) | 4 (12.9%) | 9 (6.9%) | |
| Pre-COVID-19 comorbidities | |||||
| Respiratory disease | |||||
|
170 | 5 (2.9%) | 1 (3.0%) | 4 (2.9%) | 1 |
|
170 | 30 (17.6%) | 5 (15.2%) | 25 (18.2%) | 0.87 |
| Hypertension | 170 | 74 (43.5%) | 12 (36.4%) | 62 (45.3%) | 0.47 |
| Chronic heart disease | 170 | 14 (8.2%) | 3 (9.1%) | 11 (8.0%) | 0.74 |
| Diabetes | 170 | 51 (30.0%) | 7 (21.2%) | 44 (32.1%) | 0.31 |
| Chronic kidney disease | 170 | 16 (9.4%) | 1 (3.0%) | 15 (10.9%) | 0.32 |
| Declared psychiatric disorder | 170 | 10 (5.9%) | 5 (15.2%) | 5 (3.7%) | 0.03 |
| Neurodegenerative disorder | 170 | 2 (1.2%) | 0 (0%) | 2 (1.5%) | 1 |
| Alcohol misuse | 161 | 8 (5.0%) | 1 (3.2%) | 7 (5.4%) | 1 |
| Active cancer | 170 | 3 (1.8%) | 1 (3.0%) | 2 (1.5%) | 0.48 |
| Other immunosuppression | 170 | 7 (4.1%) | 1 (3.0%) | 6 (4.4%) | 1.0 |
| Long-term dialysis | 170 | 6 (3.5%) | 0 (0%) | 6 (4.4%) | 0.60 |
| HIV infection | 170 | 2 (1.2%) | 0 (0%) | 2 (1.5%) | 1 |
| Solid organ transplantation | 170 | 4 (2.3%) | 0 (0%) | 4 (2.9%) | 1 |
| Liver disease | 170 | 5 (2.9%) | 0 (0%) | 5 (3.7%) | 0.58 |
| Pregnancy | 170 | 1 (0.6%) | 0 (0%) | 1 (0.7%) | 1 |
| Hospitalisation characteristics | |||||
| Total duration of hospitalisation, days | 170 | 13 (6–25) | 27 (15–44) | 11 (5–17) | <0.001 |
| Hospitalisation in the ICU | 170 | 94 (55.3%) | 39 (87.9%) | 65 (47.4%) | <0.001 |
| Duration of ICU stay, days | 170 | 9 (4–22) | 22 (5–33) | 8 (3–14) | 0.006 |
| High flow oxygen | 170 | 44 (46.8%) | 18 (62.1%) | 26 (40%) | 0.08 |
| Intubation during hospitalisation | 170 | 49 (52.1%) | 18 (62.1%) | 31 (47.7%) | 0.29 |
| Duration of intubation, days | 170 | 20 (12–34) | 28 (16–43) | 18 (10–25) | 0.03 |
| Pulmonary embolism | 171 | 29 (17.0%) | 13 (39.4%) | 16 (11.6%) | <0.001 |
| Active anticoagulation (at the full therapeutic dose) | 170 | 49 (28.8%) | 15 (45.5%) | 34 (24.8%) | 0.03 |
| Specific treatments during hospitalisation | |||||
| Azithromycin | 170 | 53 (31.2%) | 12 (36.4%) | 41 (29.9%) | 0.61 |
| Anti-IL-6 | 170 | 26 (15.3%) | 12 (36.4%) | 14 (10.2%) | 0.001 |
| Hydroxychloroquine | 170 | 18 (10.6%) | 5 (15.2%) | 13 (9.5%) | 0.35 |
| Corticosteroids | 170 | 7 (4.1%) | 3 (9.1%) | 4 (2.9%) | 0.13 |
| Lopinavir/ritonavir | 170 | 7 (4.1%) | 2 (6.1%) | 5 (3.7%) | 0.62 |
| Anti-IL-1 | 170 | 8 (4.7%) | 3 (9.1%) | 5 (3.7%) | 0.19 |
| Remdesivir | 170 | 3 (1.8%) | 0 (0%) | 3 (2.2%) | 1 |
Values are expressed as the median (interquartile range), mean±sd, or number and frequency. The p-values refer to a comparison between patients with and without fibrotic lesions.
COPD: chronic obstructive pulmonary disease; COVID-19: coronavirus disease 2019; ICU: intensive care unit; IL: interleukin.
TABLE 5.
Characteristics of patients evaluated at the ambulatory care visit according to the presence of fibrotic lesions in lungs
| Available data | All (171) | Patients with fibrotic lesions (33) | Patients without fibrotic lesions (138) | p-value | ||
| Time from hospital discharge to the outpatient clinic, days | 171 | 122 (106–143) | 109 (94–125) | 127 (109–146) | 0.004 | |
| Assessment at the ambulatory care visit | ||||||
| New-onset dyspnoea | 171 | 76 (44.4%) | 18 (54.5%) | 58 (42.0%) | 0.28 | |
| mMRC scale score for dyspnoea | 171 | 0.65 | ||||
|
83 (48.5%) | 15 (45.5%) | 68 (49.3%) | |||
|
74 (43.3%) | 14 (42.4%) | 60 (43.5%) | |||
|
14 (8.2%) | 4 (12.1%) | 10 (7.2%) | |||
| New-onset cough | 171 | 22 (13.3%) | 5 (15.1%) | 17 (12.3%) | 0.77 | |
| 6-min walk distance, m | 155 | 459 (378–504) | 486 (401–510) | 454 (375–498) | 0.24 | |
| Abnormal HRCT of the chest | 171 | 108 (63.5%) | 33 (100%) | 75 (54.5%) | <0.001 | |
| Reticulations | 171 | 91 (53.5%) | 31 (93.9%) | 60 (43.5%) | <0.001 | |
| Persistent ground-glass opacities | 171 | 72 (42.1%) | 22 (66.6%) | 50 (36.2%) | 0.03 | |
| Pulmonary function tests | ||||||
| FEV1, % pred | 151 | 90.9±18.0 | 86.2±20.0 | 92.1±17.3 | 0.14 | |
| FEV1/VC, % | 151 | 82.0±7.5 | 82.3±6.3 | 82.0±7.8 | 0.82 | |
| VC, % pred | 146 | 89.2±16.3 | 80.6±20.0 | 91.5±14.4 | 0.007 | |
| TLC, % pred | 143 | 82.6±15.2 | 74.1±13.7 | 84.9±14.8 | <0.001 | |
| DLCO, % pred | 146 | 86.5±22.8 | 73.3±17.9 | 89.7±22.8 | <0.001 | |
| DLCO<70% | 146 | 32 (21.9%) | 12 (41.4%) | 20 (17.1%) | 0.01 | |
| Nijmegen score>22 | 162 | 35 (21.6%) | 2 (6.3%) | 33 (25.4%) | 0.03 | |
| LVEF≤50% on echocardiography | 80 | 10 (12.5%) | 5 (19.2%) | 5 (9.3%) | 0.28 | |
Values are expressed as the median (interquartile range), mean±sd, or number and frequency. The p-values refer to a comparison between patients with and without fibrotic lesions.
DLCO: diffusing capacity of the lungs for carbon monoxide; FEV1: forced expiratory volume in the first second of expiration; HRCT: high-resolution computed tomography; LVEF: left ventricular ejection fraction; mMRC: modified Medical Research Council; VC: vital capacity.
No difference in the mMRC score or 6MWT distance was observed. Patients with fibrotic lesions had a significantly lower FVC (80.6±20.0 versus 91.5±14.4% pred, p=0.007), TLC (74.1±13.7 versus 84.9±14.8% pred, p<0.001) and DLCO (73.3±17.9 versus 89.7±22.8% pred, p<0.001). The proportion of patients with DLCO under 70% pred was also higher among those with fibrotic lesions (41.4% versus 17.1%, p=0.01). In the multivariate analysis, only hospitalisation in the ICU and an episode of pulmonary embolism were significantly associated with fibrotic lung lesions (Table E4).
The presence of new-onset dyspnoea, fibrotic lesions and decreased DLCO under 70% pred was rare, as it was observed in only eight patients (4.5% of the population assessed at the ambulatory care visit and 1.6% of the whole population) (figure 4). When we compared patients with fibrotic lesions according to the presence of new-onset dyspnoea, the only differences were lower levels of FEV1 (79.3 versus 94.6% pred, p=0.04), FVC (73.9 versus 88.7% pred, p=0.04) and TLC (68.6 versus 81.3% pred, p=0.01) in patients with new-onset dyspnoea (Tables E5 and E6).
FIGURE 4.
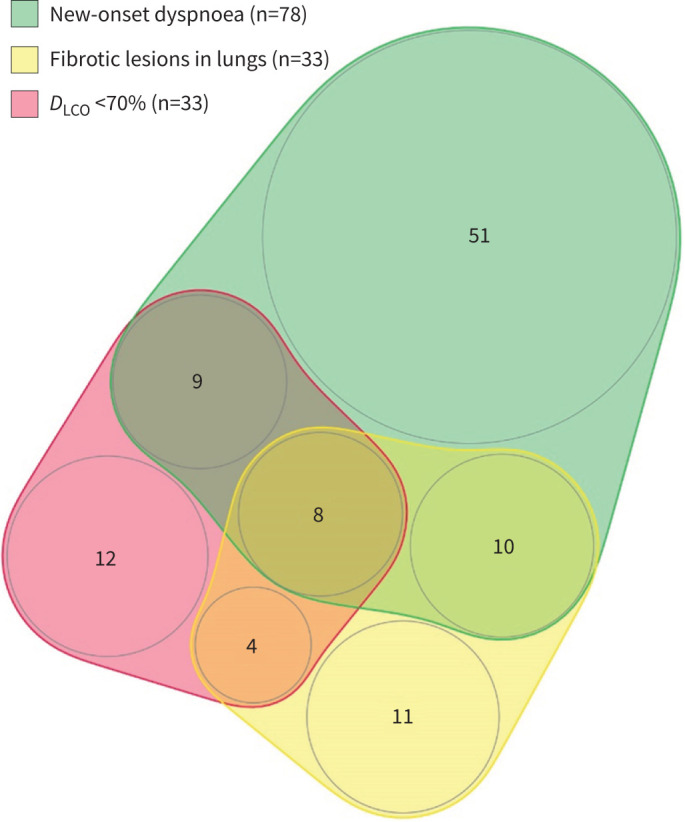
Distribution of patients evaluated at ambulatory care visits according to new-onset dyspnoea, fibrotic lung lesions on high-resolution computed tomography and decreased DLCO <70%. DLCO: diffusing capacity of the lung for carbon monoxide.
Discussion
This study investigated the respiratory complications of post-acute COVID-19 syndrome at 4 months in a well-characterised population to define the characteristics of patients with new-onset dyspnoea and the relationships between respiratory symptoms, radiologic abnormalities and functional impairment. New-onset dyspnoea and cough were identified in 16.3% and 4.8% of the COMEBAC population, respectively. The mechanisms identified as possibly related to dyspnoea were multifactorial, with frequent “functional respiratory complaints”. Fibrotic lung lesions were often limited and were more frequently observed in patients with the most severe forms of initial COVID-19. Fibrotic lesions had limited consequences on the functional status and were not systematically associated with persistent respiratory symptoms.
This study confirms that new-onset dyspnoea is not rare 4 months after hospitalisation for COVID-19, as it affected at least 16.3% of patients who were discharged alive. This result is in accordance with previous studies in which patients were assessed between 1 and 12 months after COVID-19 and that reported a prevalence of persistent dyspnoea ranging from 15 to 81% after hospitalisation [12, 21–25] and approximately 12% in non-hospitalised patients with mild COVID-19 [26]. A recent meta-analysis on 15244 hospitalised during COVID-19 and 9011 non-hospitalised patients found a prevalence of dyspnoea at 3 months after COVID-19 of 33.3% in hospitalised patients and of 19.1% in non-hospitalised patients [27]. Telephone interviews seem to be an effective approach to detect residual respiratory symptoms requiring complementary investigations at ambulatory care visits. Indeed, with more than 240 million people infected with COVID-19 worldwide [1], the percentage of patients with new-onset dyspnoea after infection (16%) could have a major impact on public health programmes, potentially affecting nearly 40 million people worldwide.
Previous data on SARS-CoV and Middle East respiratory syndrome-coronavirus (MERS-CoV), which are responsible for epidemics of severe acute respiratory syndrome, showed that approximately 8–30% of patients developed fibrotic lesions on chest CT within 3 months after discharge [28, 29]. Because SARS-CoV-2 shares many similarities with SARS-CoV and MERS-CoV, with the frequent occurrence of severe pneumoniae or acute respiratory distress syndrome (ARDS), it was feared that the SARS-CoV-2 epidemic could be followed by a significant number of patients with respiratory sequelae leading to serious functional consequences [30]. This study demonstrated that the mechanisms of post-COVID-19 dyspnoea are rather multifactorial and cannot be related only to parenchymal sequelae. In particular, some patients with new-onset dyspnoea had a Nijmegen questionnaire score greater than 22, suggesting “functional respiratory complaints”, while others had fibrotic lesions with lower respiratory volumes on pulmonary function tests. Indeed, despite generally normal PFT results in the whole population, the patients with new-onset dyspnea had lower FCV and TLC, suggesting a possible role for lung sequalae in new-onset dyspnea. It has been suggested that dyspnoea could also be induced by cardiovascular dysfunction or muscular deconditioning independent of respiratory sequelae [9, 13, 31, 32]. However, in our study, left ventricular systolic dysfunction was not overrepresented in patients with new-onset dyspnoea, suggesting that left ventricular systolic dysfunction pre-existed in this at-risk population. The role of thromboembolic events in residual dyspnoea after COVID-19 remains unclear. In the studied population, pulmonary embolism during acute infection was more frequently observed in patients with new-onset dyspnoea, and this difference remained in multivariate analysis and could suggest the role of pulmonary embolism in residual dyspnoea; however, none of these patients had signs of persistent pulmonary hypertension on echocardiography.
In this cohort, patients with fibrotic lesions experienced significantly more severe episodes of COVID-19, with more frequent hospitalisation in the ICU and a longer duration of intubation. At 4 months, ground-glass opacities were frequently observed (>40%). Even in transient lesions, the long-term evolution of these abnormalities remains an unresolved issue. By contrast, fibrotic lesions were rare, as previously described [33], and usually had limited extension and no functional impact. The precise characterisation and evolving nature (irreversible, progressive or potentially regressive) of these lesions are matters of debate. Fibrotic lesions seem to be generally in the same areas as acute lesions as seen in figures 2 and 3. van Gassel et al. [11] reported signs of reticulation, including course fibrous bands either with or without obvious parenchymal distortion, bronchiectasis, and bronchiolectasis, in almost 67% of 95 mechanically ventilated survivors of COVID-19 3 months after hospital discharge, and fibrotic lesions could also have a rapid onset in patients who never required mechanical ventilation [34]. COVID-19 patients with ARDS and diffuse alveolar damage can progress to the fibrosing pattern as seen on post mortem analysis [35] even if traction bronchiectasis does not always correlate with the histologic fibrosis pattern [36]. However, histological data on surviving patients with radiological signs of fibrotic lesions in lungs are lacking. It has been suggested that the signs of fibrosis may represent areas of consolidation as in organising pneumonia, which could reverse [37]. This hypothesis is reinforced by studies showing an improvement in residual interstitial lesions, including fibrotic lesions, after corticosteroid therapy or spontaneously [38, 39]. Fibrotic lung lesions were also more frequently associated with episodes of pulmonary embolism during COVID-19, and this difference was still present in multivariate analysis. This could suggest the presence of parenchymal sequelae of pulmonary embolism, such as pulmonary infarcts, intertwined with fibrosing lesions, but there was no evidence of typical pulmonary infarcts on the HRCT images. Even though patients with fibrotic lesions had significantly lower respiratory volumes and DLCO, functional impairment was usually mild and was not associated with a poor impact on the mMRC scale. Indeed, the presence of new-onset dyspnoea, fibrotic lesions and decreased DLCO <70% was found in only 1.6% of the whole population. While other studies have reported that lung radiologic abnormalities are correlated with poor pulmonary function and lung diffusion disorder [8, 10, 40], no study has demonstrated a clear association with dyspnoea or limited effort capacity [7–11, 25, 41]. In accordance with that, in a recent study, while there was an improvement in lung function and DLCO between 3 and 6 months after COVID-19, there was no improvement in dyspnoea and quality of life [42].
Interestingly, 13.0% of the patients investigated at outpatient clinics and 4.8% of the whole population had new-onset cough. This finding is in agreement with studies showing that cough can persist for weeks or months after SARS-CoV-2 infection with a prevalence in a recent meta-analysis of 10.4% in hospitalised patients and 6.7% in non-hospitalised patients [27, 43]. Cough should therefore be included in the respiratory complaints after hospitalisation for COVID-19 and does not seem to be associated with lung sequelae, as cough appeared to be similarly distributed in patients with or without lung fibrosis.
Even if long-term studies are still needed to determine whether respiratory symptoms and radiologic lesions could resolve or worsen over time, the first 1-year follow-up studies after COVID-19 have recently been published and allow us to better understand the evolution of respiratory symptoms and sequelae of COVID-19 at a distance from the acute episode. Wu et al. [12] were the first to show that among 83 patients reassessed 1 year after severe COVID-19 who did not require mechanical ventilation, dyspnoea scores and exercise capacity improved over time but that a subgroup had persistent physiological and radiographic changes. In a recent study comparing symptoms and respiratory assessment between 6 and 12 months after COVID-19, it was shown on the contrary that dyspnoea score slightly worsen between 6 and 12 months and that there was no improvement in DLCO while TLC and lung imaging abnormality gradually recovered [44]. As some studies have shown improvement in both FVC and DLCO and in lung imaging abnormality from 6 months after COVID-19 [42, 45], the precise evolution of respiratory symptoms and of functional and radiological lung damage remains to be described and specified in long-term prospective follow-up studies.
This prospective study has some limitations. First, there was a selection bias for the comparison of the results of PFTs and lung CT scans between patients with and without new-onset dyspnoea, given that patients who were evaluated at ambulatory care visits were selected on the basis of the initial severity of the episode (ICU stay) or the presence of persistent symptoms. This bias was alleviated by comparing the characteristics of patients with and without new-onset dyspnoea among the entire cohort who was consulted via telephone. Second, of the 177 patients reassessed at the ambulatory care visit, five had negative SARS-CoV-2 serologic tests, and we cannot rule out that some patients included in the study did not in fact have COVID-19 initially. Moreover, the design of this study did not allow us to assess the prevalence of respiratory symptoms in outpatients. Additionally, this study was conducted during the first wave of the pandemic and, at that time, the use of corticosteroids and anti-IL6 was limited. We cannot evaluate the impact of anti-inflammatory treatments and new variants on the occurrence of persistent or residual respiratory complaints after hospitalisation for COVID-19.
In conclusion, persistent respiratory symptoms, especially new-onset dyspnoea and cough, are not rare 4 months after hospitalisation for COVID-19. New-onset dyspnoea was rarely associated with severe fibrotic lesions, and the association between new-onset dyspnoea, fibrotic lesions and low DLCO was rare. There was no difference in echocardiographic results according to the presence of a new-onset dyspnoea either. Radiologically persistent lesions were mainly associated with the initial severity of COVID-19 but had mild functional consequences. Therefore, new-onset dyspnoea is the direct consequence of neither fibrotic lesions nor cardiologic sequalae but may be a multifactorial consequence of lung sequalae, vascular sequalae of pulmonary embolism, dysfunctional breathing, muscular deconditioning and probably other unknown causes, and the importance of each of these causes may be different in each patient. Due to the large number of COVID-19 patients worldwide, the long-term respiratory complications of COVID-19 could lead to the major use of health resources. Physicians should be aware of this condition and of the mechanisms that could lead to persistent dyspnoea in these patients to propose individual management adapted to each condition. Further long-term studies are needed to determine the evolution of respiratory symptoms and radiologic lesions over time.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00479-2021.supplement (288.8KB, pdf)
Acknowledgments
The authors thank the patients, their families, and all the healthcare professionals and administrative staff from Bicêtre Hospital for their outstanding support.
Footnotes
Provenance: Submitted article, peer reviewed.
COMEBAC Study Group: Luc Morin (AP-HP, Service de réanimation pédiatrique et médecine néonatale, Université Paris-Saclay, Hôpital de Bicêtre, DMU 3 Santé de l'enfant et de l'adolescent, Le Kremlin-Bicêtre, France), Laurent Savale (AP-HP, Service de pneumologie et soins intensifs respiratoires, Université Paris-Saclay, Hôpital de Bicêtre, DMU 5 Thorinno, Inserm UMR_S999, Le Kremlin-Bicêtre, France), Tài Pham (AP-HP, Service de médecine intensive-réanimation, Université Paris-Saclay, hôpital de Bicêtre, DMU 4 CORREVE Maladies du cœur et des vaisseaux, Inserm UMR_S999, Le Kremlin-Bicêtre, France), Romain Colle (AP-HP, Service de psychiatrie, Université Paris-Saclay, Hôpital de Bicêtre, DMU 11, équipe MOODS, INSERM U1178, CESP (Centre de Recherche en Epidémiologie et Santé des Populations), Le Kremlin-Bicêtre, France), Samy Figueiredo (AP-HP, Service de réanimation chirurgicale, Université Paris-Saclay, Hôpital de Bicêtre, DMU 12 Anesthésie, réanimation, douleur, Le Kremlin-Bicêtre, France), Anatole Harrois (AP-HP, Service de réanimation chirurgicale, Université Paris-Saclay, Hôpital de Bicêtre, DMU 12 Anesthésie, réanimation, douleur, Le Kremlin-Bicêtre, France), Matthieu Gasnier (AP-HP, Service de psychiatrie, Université Paris-Saclay, Hôpital de Bicêtre, DMU 11, équipe MOODS, INSERM U1178, CESP (Centre de Recherche en Epidémiologie et Santé des Populations), Le Kremlin-Bicêtre, France), Anne-Lise Lecoq (AP-HP, Université Paris-Saclay, DMU 13 Santé publique, Information médicale, Appui à la recherche clinique, INSERM U1018, CESP (Centre de Recherche en Epidémiologie et Santé des Populations)), Olivier Meyrignac (AP-HP, Service de radiologie diagnostique et interventionnelle, Université Paris-Saclay, Hôpital de Bicêtre, DMU 14 Smart Imaging, BioMaps, Le Kremlin-Bicêtre, France), Nicolas Noel (AP-HP, Service de médecine interne et immunologie clinique, Université Paris-Saclay, Hôpital de Bicêtre, DMU 7 Endocrinologie-immunités-inflammations-cancer-urgences, Le Kremlin-Bicêtre, France), Elodie Baudry (AP-HP, Service de gériatrie aiguë, Université Paris-Saclay, Hôpital de Bicêtre, DMU 1 Médecine territoire gériatrie, Le Kremlin-Bicêtre, France), Antoine Beurnier (AP-HP, Service de physiologie et d'explorations fonctionnelles respiratoires, Université Paris-Saclay, Hôpital de Bicêtre, DMU 5 Thorinno, Inserm UMR_S999, Le Kremlin-Bicêtre, France), Walid Choucha (AP-HP, Service de psychiatrie, Université Paris-Saclay, Hôpital de Bicêtre, DMU 11, équipe MOODS, INSERM U1178, CESP (Centre de Recherche en Epidémiologie et Santé des Populations), Le Kremlin-Bicêtre, France), Emmanuelle Corruble (AP-HP, Service de psychiatrie, Université Paris-Saclay, Hôpital de Bicêtre, DMU 11, équipe MOODS, INSERM U1178, CESP (Centre de Recherche en Epidémiologie et Santé des Populations), Le Kremlin-Bicêtre, France), Laurent Dortet (AP-HP, Service de microbiologie, Université Paris-Saclay, Hôpital de Bicêtre, DMU 15 Biologie-Génétique-PUI, INSERM 1193, Le Kremlin-Bicêtre, France), Isabelle Hardy-Leger (AP-HP, Service de médecine interne et immunologie clinique, Université Paris-Saclay, Hôpital de Bicêtre, DMU 7 Endocrinologie-immunités-inflammations-cancer-urgences, Le Kremlin-Bicêtre, France), François Radiguer (AP-HP, Service de réanimation chirurgicale, Université Paris-Saclay, Hôpital de Bicêtre, DMU 12 Anesthésie, réanimation, douleur, Le Kremlin-Bicêtre, France), Sabine Sportouch (AP-HP, Service de médecine intensive-réanimation, Université Paris-Saclay, hôpital de Bicêtre, DMU 4 CORREVE Maladies du cœur et des vaisseaux, Inserm UMR_S999, Le Kremlin-Bicêtre, France), Christiane Verny (AP-HP, Service de gériatrie aiguë, Université Paris-Saclay, Hôpital de Bicêtre, DMU 1 Médecine territoire gériatrie, Le Kremlin-Bicêtre, France), Benjamin Wyplosz (AP-HP, Service des maladies infectieuses et tropicales, Université Paris-Saclay, Hôpital de Bicêtre, DMU 7 Endocrinologie-immunités-inflammations-cancer-urgences, INSERM U1018, CESP (Centre de Recherche en Epidémiologie et Santé des Populations), Le Kremlin-Bicêtre, France), Mohamad Zaidan (AP-HP, Service de néphrologie transplantation, Université Paris-Saclay, Hôpital de Bicêtre, DMU 4 CORREVE Maladies du cœur et des vaisseaux, Le Kremlin-Bicêtre, France), Laurent Becquemont (AP-HP, Université Paris-Saclay, AP-HP, Centre de recherche Clinique Paris-Saclay, DMU 13 Santé publique, Information médicale, Appui à la recherche clinique, INSERM U1018, CESP (Centre de Recherche en Epidémiologie et Santé des Populations)), David Montani (AP-HP, Service de pneumologie et soins intensifs respiratoires, Université Paris-Saclay, Hôpital de Bicêtre, DMU 5 Thorinno, Inserm UMR_S999, Le Kremlin-Bicêtre, France) and Xavier Monnet (AP-HP, Service de médecine intensive-réanimation, Université Paris-Saclay, DMU 4 CORREVE Maladies du cœur et des vaisseaux, Inserm UMR_S999, Le Kremlin-Bicêtre, France).
This article has an editorial commentary: https://doi.org/10.1183/23120541.00709-2021
Author contributions: E-M. Jutant, O. Meyrignac, A. Beurnier, X. Jaïs, T. Pham, L. Morin, A. Boucly, S. Bulifon, S. Figueiredo, A. Harrois, M. Jevnikar, N. Noël, J. Pichon, A. Roche, A. Seferian, S. Soliman, J. Duranteau, L. Becquemont, X. Monnet, O. Sitbon, M-F. Bellin, M. Humbert, L. Savale, D. Montani and the COMEBAC Study Group contributed substantially to the study design, data analysis and interpretation, and read and approved the manuscript. O. Meyrignac and S. Soliman reviewed the HRCT images. T. Pham performed the statistical analysis. E-M. Jutant, L. Savale and D. Montani wrote the manuscript.
Conflict of interest: E-M. Jutant has nothing to disclose.
Conflict of interest: O. Meyrignac has nothing to disclose.
Conflict of interest: A. Beurnier has nothing to disclose.
Conflict of interest: X. Jaïs has nothing to disclose.
Conflict of interest: T. Pham has nothing to disclose.
Conflict of interest: L. Morin has nothing to disclose.
Conflict of interest: A. Boucly has nothing to disclose.
Conflict of interest: S. Bulifon has nothing to disclose.
Conflict of interest: S. Figueiredo has nothing to disclose.
Conflict of interest: A. Harrois has nothing to disclose.
Conflict of interest: M. Jevnikar has nothing to disclose.
Conflict of interest: N. Noël has nothing to disclose.
Conflict of interest: J. Pichon has nothing to disclose.
Conflict of interest: A. Roche has nothing to disclose.
Conflict of interest: A. Seferian has nothing to disclose.
Conflict of interest: S. Soliman has nothing to disclose.
Conflict of interest: J. Duranteau has nothing to disclose.
Conflict of interest: L. Becquemont has nothing to disclose.
Conflict of interest: X. Monnet has nothing to disclose.
Conflict of interest: O. Sitbon has nothing to disclose.
Conflict of interest: M-F. Bellin has nothing to disclose.
Conflict of interest: M. Humbert has nothing to disclose.
Conflict of interest: L. Savale has nothing to disclose.
Conflict of interest: D. Montani has nothing to disclose.
References
- 1.WHO Coronavirus (COVID-19) Dashboard . https://covid19.who.int/
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W-H, Guan W-J, Li C-C, et al. . Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur Respir J 2020; 55: 2000562. doi: 10.1183/13993003.00562-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, et al. . Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y, Tu L, Zhu P, et al. . Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med 2020; 201: 1372–1379. doi: 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalbandian A, Sehgal K, Gupta A, et al. . Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerum TV, Aaløkken TM, Brønstad E, et al. . Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur Respir J 2020; 57: 2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González J, Benítez ID, Carmona P, et al. . Pulmonary function and radiological features in survivors of critical COVID-19: a 3-month prospective cohort. Chest 2021; 160: 187–198. doi: 10.1016/j.chest.2021.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellan M, Soddu D, Balbo PE, et al. . Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open 2021; 4: e2036142. doi: 10.1001/jamanetworkopen.2020.36142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Huang L, Wang Y, et al. . 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Gassel RJJ, Bels JLM, Raafs A, et al. . High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med 2021; 203: 371–374. doi: 10.1164/rccm.202010-3823LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Liu X, Zhou Y, et al. . 3-month, 6–month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med 2021; 9: 747–754. doi: 10.1016/S2213-2600(21)00174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdallah SJ, Voduc N, Corrales-Medina VF, et al. . Pulmonary function and functional capacity four months after COVID-19. Ann Am Thorac Soc 2021; 18: 1912–1917. doi: 10.1513/AnnalsATS.202012-1489RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Writing Committee for the COMEBAC Study Group , Morin L, Savale L, et al. . Four-month clinical status of a cohort of patients after hospitalisation for COVID-19. JAMA 2021; 325: 1525–1534. doi: 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland AE, Spruit MA, Troosters T, et al. . An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 16.Graham BL, Brusasco V, Burgos F, et al. . ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49: 1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotes JE, Chinn DJ, Quanjer PH, et al. . Standardisation of the measurement of transfer factor (diffusing capacity). Report working party standardisation of lung function tests. Eur Respir J Suppl 1993; 16: 41–52. doi: 10.1183/09041950.041s1693 [DOI] [PubMed] [Google Scholar]
- 19.Quanjer PH, Tammeling GJ, Cotes JE, et al. . Lung volumes and forced ventilatory flows. Report working party standardisation of lung function tests. Eur Respir J Suppl 1993; 16: 5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 20.van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res 1985; 29: 199–206. doi: 10.1016/0022-3999(85)90042-x [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y-M, Shang Y-M, Song W-B, et al. . Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25: 100463. doi: 10.1016/j.eclinm.2020.100463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Lorenzo R, Conte C, Lanzani C, et al. . Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS ONE 2020; 15: e0239570. doi: 10.1371/journal.pone.0239570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. . Persistence of symptoms and quality of life at 35 days after hospitalisation for COVID-19 infection. PLoS ONE 2020; 15: e0243882. doi: 10.1371/journal.pone.0243882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosn J, Piroth L, Epaulard O, et al. . Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalisation: results from a large prospective cohort. Clin Microbiol Infect 2021; 27: 1041.E1–1041.E4doi: 10.1016/j.cmi.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armange L, Bénézit F, Picard L, et al. . Prevalence and characteristics of persistent symptoms after non-severe COVID-19: a prospective cohort study. Eur J Clin Microbiol Infect Dis 2021; 40: 2421–2425. doi: 10.1007/s10096-021-04261-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augustin M, Schommers P, Stecher M, et al. . Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021; 6: 100122. doi: 10.1016/j.lanepe.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. . Prevalence of post-COVID-19 symptoms in hospitalised and non-hospitalised COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med 2021; 92: 55–70. doi: 10.1016/j.ejim.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie L, Liu Y, Xiao Y, et al. . Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest 2005; 127: 2119–2124. doi: 10.1378/chest.127.6.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das KM, Lee EY, Singh R, et al. . Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging 2017; 27: 342–349. doi: 10.4103/ijri.IJRI_469_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spagnolo P, Balestro E, Aliberti S, et al. . Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med 2020; 8: 750–752. doi: 10.1016/S2213-2600(20)30222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debeaumont D, Boujibar F, Ferrand-Devouge E, et al. . Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalisation – a pilot study. Phys Ther 2021; 101: pzab099. doi: 10.1093/ptj/pzab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohr A, Dannerbeck L, Lange TJ, et al. . Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidiscip Respir Med 2021; 16: 732. doi: 10.4081/mrm.2021.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guler SA, Ebner L, Aubry-Beigelman C, et al. . Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021; 57: 2003690. doi: 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Combet M, Pavot A, Savale L, et al. . Rapid onset honeycombing fibrosis in spontaneously breathing patient with COVID-19. Eur Respir J 2020; 56: 2001808. doi: 10.1183/13993003.01808-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Wu J, Wang S, et al. . Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology 2021; 78: 542–555. doi: 10.1111/his.14249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kianzad A, Meijboom LJ, Nossent EJ, et al. . COVID-19: histopathological correlates of imaging patterns on chest computed tomography. Respirology 2021; 26: 869–877. doi: 10.1111/resp.14101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vijayakumar B, Shah PL. Is fibrosis really fibrosis? Am J Respir Crit Care Med 2021; 203: 1440–1442. doi: 10.1164/rccm.202102-0334LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myall KJ, Mukherjee B, Castanheira AM, et al. . Persistent post-COVID-19 inflammatory interstitial lung disease: an observational study of corticosteroid treatment. Ann Am Thorac Soc 2021; 18: 799–806. doi: 10.1513/AnnalsATS.202008-1002OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou J-N, Sun L, Wang B-R, et al. . The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT. PLoS ONE 2021; 16: e0248957. doi: 10.1371/journal.pone.0248957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frija-Masson J, Debray M-P, Boussouar S, et al. . Residual ground glass opacities three months after Covid-19 pneumonia correlate to alteration of respiratory function: The post Covid M3 study. Respir Med 2021; 184: 106435. doi: 10.1016/j.rmed.2021.106435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGroder CF, Zhang D, Choudhury MA, et al. . Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021; 76: 1242–1245. doi: 10.1136/thoraxjnl-2021-217031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah AS, Ryu MH, Hague CJ, et al. . Changes in pulmonary function and patient-reported outcomes during COVID-19 recovery: a longitudinal, prospective cohort study. ERJ Open Res 2021; 7: 00243-2021. doi: 10.1183/23120541.00243-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernández-de-Las-Peñas C, Guijarro C, Plaza-Canteli S, et al. . Prevalence of post-COVID-19 cough one year after SARS-CoV-2 infection: a multicenter study. Lung 2021; 199: 249–253. doi: 10.1007/s00408-021-00450-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L, Yao Q, Gu X, et al. . 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398: 747–758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hellemons ME, Huijts S, Bek L, et al. . Persistent health problems beyond pulmonary recovery up to 6 months after hospitalization for SARS-CoV-2; A longitudinal study of respiratory, physical and psychological outcomes. Ann Am Thorac Soc 2021. in press [ 10.1513/AnnalsATS.202103-340OC 10.1513/AnnalsATS.202103-340OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00479-2021.supplement (288.8KB, pdf)