Abstract
Microbial communities perform emergent activities that are essentially different from those carried by their individual members. The gut microbiome and its metabolites have a significant impact on the host, contributing to homeostasis or disease. Food molecules shape this community, being fermented through cross-feeding interactions of metabolites such as lactate, acetate, and amino acids, or products derived from macromolecule degradation. Mathematical and experimental approaches have been applied to understand and predict the interactions between microorganisms in complex communities such as the gut microbiota. Rational and mechanistic understanding of microbial interactions is essential to exploit their metabolic activities and identify keystone taxa and metabolites. The latter could be used in turn to modulate or replicate the metabolic behavior of the community in different contexts. This review aims to highlight recent experimental and modeling approaches for studying cross-feeding interactions within the gut microbiome. We focus on short-chain fatty acid production and fiber fermentation, which are fundamental processes in human health and disease. Special attention is paid to modeling approaches, particularly kinetic and genome-scale stoichiometric models of metabolism, to integrate experimental data under different diet and health conditions. Finally, we discuss limitations and challenges for the broad application of these modeling approaches and their experimental verification for improving our understanding of the mechanisms of microbial interactions.
Keywords: Cross-feeding, Microbial interactions, Ecological modeling, Genome-scale metabolic model
1. Introduction
The gut microbiota harbors hundreds of different microbial species that actively metabolize dietary and host-derived substrates, with an evident influence on host health [1], [2], [3]. Next-generation sequencing has greatly increased our understanding of the composition of the microbiota. 16S rRNA and metagenomic studies have allowed scientists to evaluate the structure of this community in terms of relative abundance of species (richness), intra and inter-diversity, and metabolic functions [4]. Advances have been made to understand the assembly process in the infant gut microbiota [5], characterized by a succession of microorganisms, the wide diversity in microbiome compositions in the adult and elderly, the impact of antibiotics on this community, and its resilience [6], [7]. These advances have contributed to new hypotheses or validations of the role of the gut microbiota in disease.
Unfortunately, this static view does not take into account all the interactions that shape the composition of a microbial community so complex such as the microbiota. With hundreds of gut microorganisms inhabiting (or transiting) this ecosystem, active at disparate rates, present at different abundances and colonizing different niches, the extent of microbial interactions is enormous and a challenging task to analyze or quantify. At least 30,000 interactions could be counted at a specific time in the gut microbiota [8]. Conversely, current experimental set-ups to study microbial interactions have analyzed only small synthetic consortia up to 25 gut species [9], [10]. There is evidently a gap in our understanding of microbial interactions, which has raised the interest in top-down systems biology approaches to simplify this tremendous complexity.
Microbial interactions vary in their specificity and cost resulting in different ecological outcomes [11]. Microbe-microbe interactions in the gut include quorum-sensing [12], a process little studied in gut microbes. Genomic analysis of gut microbes suggest the intestine is dominated by interference competition [13], with a high abundance of genes producing antimicrobials such as bacteriocins and Type 6 Secretion Systems (T6SS) [14]. The human host has several ways to modulate its microbiome, such as immunoglobulin A coating, secretions, or antimicrobial peptides [15], [16]. The forces influencing microbiome profiles are largely unknown. However, physiological factors such as intestinal oxygen concentration, peristaltic movements, and innate immunity effectors are evident [17]. Moreover, microbes are not homogeneously distributed in the intestine, displaying a concentration gradient, and they usually prefer a luminal lifestyle or residing near the mucus layer [18].
Diet is one of the main factors dictating which species will be dominant in the microbiota. Major bacterial groups and patterns regarding the fermentation of complex dietary polysaccharides have been described [19]. These include microbes able to access complex glycans and other species that can capture degradation products (monosaccharides, amino acids) or fermentation byproducts. Then cross-feeding takes place, which can be understood as the metabolic exchange between microorganisms. The exchange of short-chain fatty acids (SCFAs) such as acetate, and organic acids such as lactate and succinate, is a common metabolic interaction [20], [21]. Other molecules such as amino acids, vitamins, or branched SCFAs also participate in metabolic cross-feeding [22].
While cross-feeding interactions are at the core of the gut microbiome network [8], [9], [23], there is a lack of quantitative studies revealing their actual prevalence in the gut. Among other approaches, kinetic models based on ordinary differential equations (ODE) have been used to quantify the extent of interactions and influence of certain gut microbes, among others [24]. An approach of preference are genome-scale metabolic models (GSMMs). These are mathematical structures derived from genome-scale network reconstructions [25], described by all the reactions associated with metabolic genes in the organism's genome. GSMMs are complemented with experimental data about cellular composition, growth capabilities, and consumption/production profiles to represent the metabolic phenotype [26]. By using appropriate optimization procedures [27], [28], cellular growth and the exchange rate of metabolites can be quantified, which can be later validated using co-culture studies [29].
The cross-talk between top-down and bottom-up systems biology approaches, modeling, and experimental approaches is currently being used to provide a rationale for complex diseases. They have been applied to determine the alteration of cross-feeding patterns in inflammatory bowel disease (IBD) [30] and Parkinson's disease (PD) [31], [32] and to design therapeutic approaches towards health improvement through microbiota modulation [9]. Ultimately, through the integration of increasingly broader and deeper omics datasets, modeling approaches enable the exploration of explanatory hypotheses with varying degrees of complexity that can be later verified, significantly reducing the amount of experimentation, and yielding novel insights about the interaction mechanisms. However, there are several challenges and opportunities in this field. This review summarizes recent advances in the ecology and modeling approaches to study cross-feeding in the gut microbiota.
2. Experimental studies of cross-feeding
The study of cross-feeding interactions has been supported by improvements in microbial isolation techniques and deposition in culture banks [33]. Most gut microbes are highly oxygen-sensitive and present unpredictable metabolic requirements. One example is KLE1738, a gut isolate that solely uses Bacteroides-derived GABA as a carbon and energy source [34]. Cross-feeding experimental set-ups often use a catalog of potentially representative gut microbes that should be simple to culture, traceable, and representative or relevant to their ecosystem [35]. Current experimental models to study cross-feeding include microwell assays, batch fermentations with a small number of microorganisms, and fecal inocula in bioreactors [35]. Advanced systems such as SHIME (Simulator of Human Intestinal Microbial Ecosystem) provide global information and general deductions regarding dietary alterations [36]. Continuous bioreactors have also been studied, with the advantage of reaching steady-state and inflow and outflow rates similar to human digestion [37]. Advances in multi-layer microfluidics in vitro systems have enabled satisfactory mimicking microbial processes along the gastrointestinal tract [38], [39], [40], [41]. There are still limitations with the accurate simulation of host-related responses, e.g., continuous removal of cross-feeding metabolites, enzyme and acid secretions, and immune surveillance. However, current systems have shown promise for a representative simulation of human-microbe interactions under relevant conditions [41].
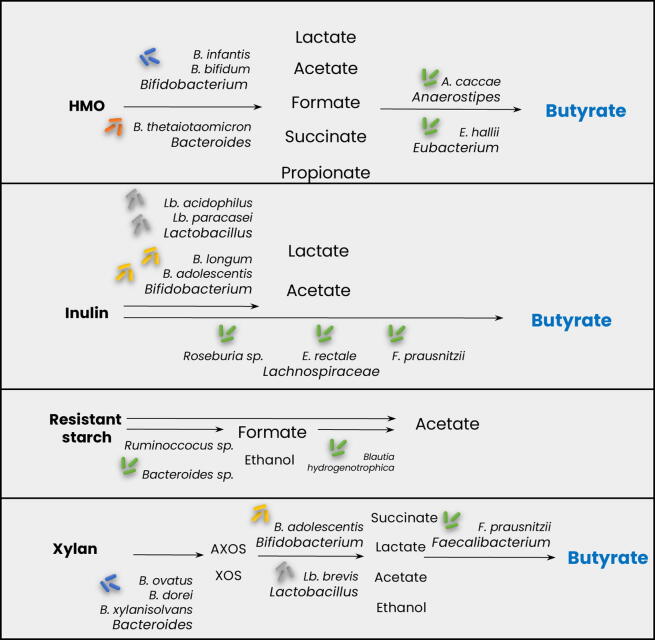
Metabolic interactions in the gut microbiota usually involve a complex carbohydrate and an SCFA, although amino acids have also been shown to participate [42], [43]. For example, human milk oligosaccharides (HMOs) promote cross-feeding of glycan degradation products between Bifidobacterium species, thereby promoting the colonization of butyrate-producing bacteria in the newborn (Fig. 1) [5]. Host-derived molecules, such as mucin glycans and bile-salts, can also support metabolic exchanges [44], [45], [46], [22], [47], [48]. Butyrate production derived from microbial fermentation networks balances pH, provides immune system stimulation and reduces the risk of infections [2]. Bacteroides spp. are among the most studied bacteria for their broad preference for dietary glycans such as xylan, pectins, and starch [49]. Their degradation process usually results in cross-feeding interactions. Inulin is a prebiotic, a fructose polymer of up to 60 molecules. Several members of the gut microbiota have the machinery for its consumption, including Bifidobacterium, Roseburia, Bacteroides, and Lactobacillus species [50]. Inulin-derived fructans, as well as SCFAs derived from inulin fermentation, are well known for promoting the growth of butyrate-producing bacteria such as Faecalibacterium prausnitzii [51]. This stimulation is mediated by acetate and lactate. Other important cross-feeding currencies are formate [52] and H2 [53], which could be used by methanogenic gut archaea (Blautia hydrogenotrophica). Notably, there are indications of complementary functions as evidenced by pathway parts (e.g., related to nitrogen metabolism) expressed by different human gut bacteria [54]. Fig. 1 provides examples of cross-feeding interactions in the gut microbiota.
Fig. 1.
Schematic representation of cross-feeding interactions in the gut microbiota, starting from different dietary sources and leading to butyrate production. HMOs: human milk oligosaccharides; AXOS: arabinoxylanooligosaccharides; XOS: xyloligosaccharides.
3. Metabolic interactions and ecology based-modeling
Metabolic cross-feeding can be understood as the exchange of metabolites between microorganisms. It differs from true cooperation in that the production of metabolites does not represent a fitness cost for the producer, i.e., they are byproducts or the result of macromolecule degradation, and therefore their production does not need additional energy [55]. Cooperation is associated with more dependent interactions, where one microorganism invests part of its energy in synthesizing a metabolite that is necessary for another, concomitantly receiving a benefit for this production. An interesting example of true cooperation is presented by Bacteroides ovatus, a gut commensal that degrades several polysaccharides such as inulin, amylopectin, and xylan [56]. Inulin degradation by this microorganism requires two putative extracellular xylanases (BACOVA_04502/04503). Mutant analyses revealed that these enzymes do not provide a direct benefit for B. ovatus in vivo. The surface degradation of polysaccharides sometimes results in the extensive release of degradation products for other microorganisms, for instance, Bacteroides vulgatus. The expression of these enzymes represents a cost for the producer B. ovatus [56]. Interestingly, mutant analyses also showed that B. ovatus receives a benefit in a more complex microbiota mediated by these enzymes. However, most Bacteroides species do not engage in cooperative metabolic interactions and maximize intracellular glycan degradation instead [57], [58]. A similar observation is found in the infant gut microbiota, where two glycan degradation strategies have been characterized between Bifidobacterium longum subsp. infantis and Bifidobacterium bifidum [59]. These interspecies interactions could be classified as true cooperation involving the production of costly proteins that benefit the community. Some studies predict cooperation to be unstable in steady-state and probably not dominant in complex ecosystems [4]. However, this view has been challenged by other studies [55], [60], [61].
Exploitative competition is characterized by limited resources that reduce the growth of two or more microorganisms. Exploitative competition and metabolic commensalism were predicted as the most prevalent interactions among 773 gut microbes [62]. Competition for cross-feeding substrates such as monosaccharides, oligosaccharides, and SCFAs is common in the gut microbiota, even for taxonomically unrelated microorganisms [62]. Competition usually results in two possible outcomes: niche partitioning or competitive exclusion [63].
A dense cross-feeding network is predicted in the gut microbiome [62]. Exchanged metabolites derive from the fermentation of complex dietary carbohydrates, proteins, or host secretions. Other released metabolites are fermentation end-products that report no cost for the producer, but a clear benefit for the user. Anaerostipes caccae, a prominent butyrate-producing bacterium in the gut microbiota, presents a product yield five-fold higher in butyrate from lactate, a cross-feeding metabolite, compared to glucose [9], [64]. Sometimes this metabolic interaction could be bidirectional (both microorganisms receiving a benefit), or unidirectional instead [21]. Indirect benefits for the release of cross-feeding metabolites could be the removal of potentially toxic byproducts or continuing microbial fermentation.
While cross-feeding interactions are widespread in the gut microbiota, in vivo they are limited by distance [65]. Four Lactococcus lactis strains with different metabolic capabilities were cultured in a 3D system allowing unidirectional cross-feeding (a galactose consumer-glucose producer, a lactose consumer, a glucose consumer, and a competitive glucose consumer). All strains were embedded in agarose beads to monitor glucose exchange by flow cytometry [66]. Combining the experimental results with mathematical modeling of reaction–diffusion processes, a maximum of 15 µm between beads was established as the limit for cross-feeding in an aqueous medium. This study highlights the importance of spatial limitations in metabolite diffusion in cross-feeding. It would be interesting to contrast these findings with co-localization data in biofilms of microcolonies in the gut microbiota. These communities are exposed to other influences such as shear forces, oxygen gradients, and host-derived antimicrobials.
In a more complex set up, intestinal contractions have been shown to influence microbiota composition through mixing [67]. The minigut is a fluid channel mimicking the gut including important variables such as flow rate and mixing. Simple diffusion models were able to capture the microbial behavior in time in this system [67]. Interestingly, the gut microbiota is dependent on feeding/fasting regimes and the host's circadian rhythm [68]. Therefore, the gut microbiota shows an oscillatory nature in its composition with diurnal cycles [69], [70]. Interestingly, jet-lag induces microbiome dysbiosis in mice, characterized by glucose resistance [71]. Microbial metabolites such as butyrate also show daily variations [72]. Some studies have proposed mathematical models to understand the influence of time and space in the gut microbiota [37], [73], [74].
How do cross-feeding interactions emerge? McNally combined an in silico reductive gene-loss model with GSMMs between two generalist E. coli strains and identified that metabolic interdependencies commonly appear as evolved cross-feeding interactions [75]. Some strains could not develop the ability to cross-fed on certain metabolites being released (missed opportunities). From thousands of trajectories, 35% resulted in a commensal community, 10% collapsed, and 51% were independent. Metabolic interdependencies were found between tyrosine and thymidine. Formate and tyrosine were the metabolites that created the largest dependency from the other partner, while acetate and succinate most usually never created interdependency. In the long term, metabolic cross-feeding permits the co-occurrence of species in certain communities by the emergence of metabolically interdependent groups [76]. This co-occurrence is reinforced by gene loss as an adaptation where one microorganism obtains all its carbon and energy sources from the enzymatic action of others [63].
Are some gut species essential, or in contrast functionally replaceable, in terms of ecosystem stability? The gut microbiome is characterized by functional redundancy [77], supporting stability and resilience to particular interventions. The keystone species concept derives from the ecological theory of Paine [78], which are species so essential for the stability of an ecosystem that their loss or removal results in a collapse of the community. A synthetic microbiome with 14 species, including one butyrate producer, was recently cultured in batch bioreactors, with inulin as a dietary source. Experiments were repeated leaving one species out at a time, to study the impact of individual deletions [10]. All combinations followed similar trajectories in terms of their biomass production and inulin substrate consumption. The community however did not collapse in any of the deletions [10]. SCFAs profiles were however different in each deletion experiment. For example, deletion of Bacteroides dorei incremented acetate amounts, and deletions of Ruminococcus gnavus and Bacteroides vulgatus resulted in higher butyrate concentrations. An increment in a SCFA suggested that the deleted species contributed negatively to SCFA production. Consequently, only three species contributed significantly to butyrate production: E. coli, B. dorei, and L. symbiosum. Besides, negative interactions between bacterial pairs were also identified: E. coli absence allowed a higher growth of Bifidobacterium adolescentis, and the deletion of B. dorei and L. symbiosum resulted in a community dominated by Lactobacillus plantarum [10].
Another recurrent question when analyzing cross-feeding interactions is whether they are organized in modules or form higher-level hierarchies. Functional studies indicate a multi-level trophic organization in the microbiome, with at least three and up to 10 functional groups [19]. Using ecology-based modeling, Wang et al. predicted four iterative, trophic levels of metabolite production and consumption in the gut microbiota [79]. Their analysis started with a manually-curated metabolic reconstruction of microbial interactions and nutrient dependencies [46], containing 567 gut microbes and 235 metabolites consumed and secreted by these microorganisms. Combined with metagenomics data, the number of substrates converted into metabolites feeding other microbes potentially being carried to lower trophic levels was determined. A part of these substrates was also converted into bacterial biomass, and certain byproducts are used by the next trophic level [79]. Their approach also includes intestinal nutrient intake and byproduct release. Intra and inter-person diversity were shown to be unevenly distributed across four trophic levels. As expected, the diversity of metabolites was higher in upper trophic groups and narrower in the lower groups. One conclusion obtained from this analysis was that genus-level competition could explain taxonomic diversity of the microbiome [79].
This ecological platform has been recently combined with machine-learning methods to predict novel, unexplored metabolic interactions in the gut microbiome. The gut cross-feeding predictor (GutCP) builds from the multi-level trophic organization and predicts a theoretical metabolome from metagenomics data and known consumption links [8]. The GutCP algorithm improved the original network, reducing the error between prediction and actual data by adding new consumption links. Undiscovered interactions that result in more accurate estimations would possibly be true cross-feeding interactions. The main features of ecological as well as other modeling approaches are shown in Fig. 2.
Fig. 2.
Representation of computational biology approaches to interrogate metabolic interactions in the gut microbiota. A: Species deletions simulate and study the impact of the absence of one species in a community, while combinations of literature-based metabolic reconstructions of the gut microbiota and machine learning allow evaluating the hierarchy in metabolic interactions. B: gLV-based models evaluate the impact of one microorganism in another's growth, usually by estimating an interaction coefficient α. Its combination with Bayesian methods has been used to design microbiome consortia. C: ODE-based models simulate mechanistic processes as equations and require extensive parameterization. D: GSMMs combined with community design algorithms enable the identification of cross-feeding metabolites in simple and large networks. The main features and requirements of each approach are highlighted in the blue and orange boxes, respectively.
4. ODE-based mechanistic models of microbial communities
Computational modeling approaches of microbial interactions are at the forefront of microbial community research [80], [81], [82]. They are helpful to identify interactions and mechanisms that are likely to occur, reducing the burden of extensive experimentation (Fig. 2). They could be classified as static or dynamic models, depending on if they explicitly include information on how the components of a community interact over time. Dynamic modeling approaches describe how a microbial community and its components change over time, enabling the study of variations in the properties included in the model. The first classification of dynamic models is based on the level of detail, from the coarseness of the population-level strain composition of a consortium to detailed models of all metabolic reactions in each cell. A second classification uses the type of algorithm employed to simulate the model. Two of the main types are deterministic models based on differential equations and stochastic models whose behavior depends on randomly selected events. A third criterion serves to classify what is represented in the model. Metabolic models represent the metabolism of the bacteria in the community, often at a molecular level, while in ecological models, strains or species interact, reproduce and change their numbers and behaviors at what can be called cellular level [8], [83].
Models based on Ordinary Differential Equations (ODEs) employ a set of mathematical functions to represent microbial communities at different levels of detail. These deterministic mathematical functions describe the changes in quantity or concentration of different species in time. One of the simplest microbial community ODE-based models is the generalized Lotka-Volterra equation (gLV) [84]. Using a small number of parameters such as growth rate and abundances in single and co-culture, it evaluates the effect of one microorganism on the growth rate of another. Therefore, it is a suitable model for assessing pairwise interactions and determining whether they are positive (cooperation) or negative (competition). Using a synthetic microbiome of 12 species, their pairwise interactions were experimentally determined in vitro using the gLV model [85]. Determined parameters were useful to infer interspecies interactions in the whole consortium. The model was validated with time-resolved measurements of the full consortium and single species deletions [85]. This and other in vitro studies show that pairwise interactions appear to be sufficient to predict higher-order interactions and drive community dynamics, at least in the gut microbiota [11], [85], [86].
A similar framework was recently applied to design high-butyrate microbiome consortia based on microbial interactions [9]. A two-stage modeling approach was followed: the gLV model representing community dynamics, and a Bayesian inference approach determined parameter uncertainties. Using a 25-species synthetic microbiome, paired co-cultures produced a wide range of butyrate concentrations (0–50 mM). However, complete or one species-deleted consortia showed narrower concentrations. Interestingly, deletion of Anaerostipes caccae dropped butyrate concentration to zero, and deletion of Desulfovibrio piger, a H2S producing microbe, significantly increased butyrate concentration. It was later shown that butyrate production by A. caccae is highly sensitive to H2S and pH. This modeling framework identified how much one single species contributed to growth and butyrate production in the synthetic consortium [9].
Other approaches use ODE-based models where the equations aim to predict microbial interactions based on mechanistic knowledge. For example, in Pinto et al. [87], four equations with 17 parameters were employed to build an ODE system that simulates microbial abundance, substrate consumption, and production of SCFA in a four infant gut species consortium. This approach used modified Monod expressions [88] to model cell growth under various conditions including product inhibition and cooperation. Adding more bacteria to the simulation means the number of equations and parameters increases, giving rise to the so-called combinatorial expression problem when it comes to fixing the values of the parameters that reproduce known experimental data [89]. For relatively small communities with few different types of microorganisms, this problem is manageable with current computational infrastructures and time-resolved measurements.
5. Genome-scale stoichiometric models of metabolism
5.1. Genome-scale network reconstructions
Genome-scale network reconstructions (GENREs) encompass the entire collection of enzyme-catalyzed reactions encoded by genes of known function found in an organism's genome [90]. As such, they describe its metabolic potential. Starting from the annotation of the organism's genome, Gene-Protein-Reaction (GPR) relationships are identified, which link the genotype with the metabolic phenotype. Detailed protocols have been developed to standardize and ensure high quality for the reconstruction [90]. Several computational platforms and tools have been developed to leverage the current genomic information and accelerate the reconstruction of these networks for single and multiple organisms in a (semi)automatic fashion [91], [92], [93]. These top-down tools integrate functional gene annotation – either performed by these or external tools – using databases like KEGG [94] and BioCyc [95]. Potential gaps in the network, i.e., missing reactions due to unidentified metabolic enzymes, can be resolved using appropriate gap-filling tools [96], [97]. There are also bottom-up tools for GENRE construction that employ high-quality template metabolic network(s) for the reconstruction process [97], [98], [99]. These tools typically yield higher quality reconstructions, although their performance ultimately depends on the initial network template(s). CarveMe is an automatic tool for the development of metabolic models for microbial communities [98]. It has demonstrated high efficiency and performance for generating a community network of 74 members of the human gut microbiota, and the assembly of a comprehensive GENREs database with over 5,500 bacterial metabolic reconstructions [98]. More recently, novel reconstruction pipelines tailored to use metagenomic data have been proposed for building community reaction networks. Metage2Metabo [100] has shown to yield meaningful community networks of the human gut microbiota capable of respectively enabling community-level flux balance computations and identifying keystone species in terms of metabolic cooperation [100].
The continuous and rapid reconstruction of GENREs of greater quality has led to the emergence of various reconstruction catalogs. AGORA is a comprehensive resource for community modeling, particularly human gut microbiota. It contains over 773 literature-based GENREs for various microbial species [62]. An extension to this resource with over 7200 GENREs of members of the human microbiome, called AGORA2, has been recently developed, and it is under review at the time of preparing this article [101], [102]. GENREs from AGORA have been employed to simulate bile acids metabolism in the human gut [103], identify microbial gut alterations associated with Parkinson's disease [31], characterize host-microbe symbiosis in the mammalian gut [104] and co-metabolism in the human intestine [105]. NJC19 [106] is another reconstruction resource that contains human and mouse microbiome data (6 mouse and human cell types and 883 microbial species) that can be leveraged to construct more comprehensive models of human molecular physiology [107].
5.2. Genome-scale metabolic models and constraint-based methods
GSMMs encode the information contained in GENREs into a mathematical structure – the stoichiometric matrix –, which contains the mass balances for each intracellular metabolite and enables flux calculations. Provided that sufficient external substrate sources (i.e., exchange reactions) and a growth equation are defined and included in this matrix, a feasible flux distribution or metabolic phenotype can be computed. It assumes no accumulation of intracellular metabolites (i.e., steady-state) and requires solving the resulting system of linear equations. As this system is almost always undetermined, i.e., there are infinite solutions that can explain the set of observed exchange reactions, particular flux solutions are computed using optimization or constraint-based modeling (CBM) methods [108]. A popular tool for CBM is the COBRA Toolbox (COnstraint-Based Reconstruction and Analysis), which includes a diversity of computational methods for genome-scale metabolic modeling and reconstruction and analysis [28].
CBM methods require the formulation of an objective function and definition of capacity constraints (reaction bounds) to compute a feasible steady-state flux solution. A typical objective function is the maximization of cellular growth as a proxy for biological fitness. However, other alternatives have also been proposed when the latter objective does not yield accurate predictions [109]. This method is known as Flux Balance Analysis (FBA), and it has been successfully applied in numerous studies for modeling interactions among multiple cells or organisms [110], understanding human physiology [43], [111], disease [112], and studying evolutionary processes [113]. Another commonly used FBA-derived method is Flux Variability Analysis (FVA) [114], which enables the calculation of alternative optima under the assumption of FBA (sub)optimality. FBA-based methods have also been extended for modeling dynamic processes. For instance, dynamic FBA (d-FBA) [115] describes the accumulation of biomass and external metabolite concentrations by coupling the evolution of a set of ODEs for the latter species – parameterized with kinetic expressions (e.g., Michaelis-Menten) – with the iterative solution of FBA problems at each time step. As in FBA, no net accumulation of intracellular metabolites is enforced. Importantly, d-FBA enables the description of the evolution of cellular growth with the corresponding changes in metabolic states and can be naturally extended to model communities.
The modeling capabilities of FBA-based methods are ideal for modeling complex communities where metabolic parameters are scarce. Applications include modeling microbe-microbe and host-microbe interactions such as metabolic cross-feeding (metabolite exchange between species) and competition (uptake of common substrates). For example, FBA shows how fructoselysine is a key cross-feeding metabolite in dual gastrointestinal infections [116]. Nevertheless, FBA-based methods for modeling microbial communities require special considerations, and it has not been until recently that robust methods have emerged. Challenges related to the accurate description of flux exchanges between taxa and the selection of a biologically relevant objective function must be addressed. One of the earliest CBM methods for modeling community growth was OptCom, which was later followed by its dynamic extension d-OptCom [27], based on the same assumptions of d-FBA. These methods use a multi-level multiobjective optimization approach to optimize the total community biomass while maximizing individual-specific growth rates. Inspection of the directionalities of the metabolic exchange profile enables the determination of possible interactions (e.g., syntrophy, cross-talk, commensalism, and competition). Community FBA (cFBA) [117] and SteadyCom [118] are more recent methods where the community growth rate is maximized, which is the same for all the community members under the assumption of a steady-state balanced growth. As a byproduct of this calculation, the microbial composition that supports this optimal growth can also be found. For instance, the application of SteadyCom recapitulated the known dominance of the bacterial phyla Bacteroides and Firmicutes in the gut microbiota under the average American diet [118]. Notably, SteadyCom can be readily combined with other CBM methods like FVA to determine the abundance and metabolic flexibility of a community under suboptimal conditions. The aforementioned methods assume perfect mixing in the culture, i.e., there are no concentration gradients. This assumption may not be reasonable in some scenarios, for example, in some regions of the human gut [119]. Furthermore, it may hinder the analysis of emergent metabolic interactions due to spatial distribution and concentration gradients [25], [120], [121].
5.3. Probing microbe-diet-host interactions in health and disease using GSMMs
A key indicator of a healthy gut microbiota health is its composition, which remains fairly stable over long periods even under short-term perturbations [122]. Alterations in the human gut microbiota are collectively referred to as dysbiosis and can be typically linked to certain health conditions such as metabolic, gastrointestinal, and mental disorders [123], [124], [125]. A recent study illustrated the predictive power of GSMMs and CBM methods for probing the metabolic behavior of the microbiota of healthy and Parkinson's disease patients [31]. By constraining the relative abundance of the microbiota members derived from 16S rRNA sequencing data of fecal samples, personalized community models were evaluated for their production potential of 129 metabolites under a simulated average European diet. FBA showed that nine metabolites were differentially affected by Parkinson's disease, including methionine and cysteinylglycine. These results were later verified in a different study where four microbial reactions involved in homoserine metabolism (precursor of methionine) were identified to be altered in Parkinson's disease patients [32]. In a recent study, GSMMs were used to build and interrogate personalized brain region models of diseased Alzheimer's and healthy patients [112]. Integration of large transcriptomic datasets and targeted metabolomics data into the GSMMs suggested that some of the bile acids found in brain samples from Alzheimer's subjects originate in the gut microbiota and are then transported to the brain [112].
Another application of GSMMs for probing the consequences of dysbiosis comes from Crohn's disease. The latter condition is characterized by altered blood and fecal metabolome and gut microbiota composition [126]. By using metagenomics data and fecal metabolites from a group of control and Crohn's disease patients, personalized microbiota models were built and analyzed for significant differences in the predicted metabolite secretion profile in the feces and the species contribution to each metabolite under an average European diet [30]. Simulation results pointed to reduced diversity in the number of secreted metabolites due to the reduced microbial diversity in Crohn's disease patients. Proteobacteria were found to increase the potential for amino acids synthesis, which was in line with experimental observations [30].
GSMMs have also been employed to understand the interactions present in a healthy gut microbiome. A collection of metabolic models was built from metagenomic samples from individuals fed on a Western diet [127], [128], to simulate cross-feeding interactions potentially involved in SCFA production in the gut microbiota. Analysis of possible metabolic interactions revealed competition as the most common interaction, agreeing with previous reports [129]. A closer examination of the microbiota behavior also suggested potential niche partitioning; either a group of bacteria consumes fibers and starches (e.g., inulin, xylan, and pectin), or another consumes branched-chain amino acids [127]. In another study, a tool called CASINO [42] was developed to quantify the changes induced in the human gut microbiota following a dietary intervention in obese and overweight individuals. Again, 16S rRNA data was employed to personalized the metabolic models [43] and predict metabolic production profiles in plasma and feces. Amino acids and SCFA levels were correctly predicted after the dietary change. These predictions were underpinned by acetate cross-feeding between B. thetaiotaomicron, R. bromii, and B. adolescentis to E. rectale and F. prausnitzii, which in turn supported butyrate production by the latter [43].
6. Other approaches for community modeling
Other dynamic modeling approaches such as stochastic modeling [130], [131] or agent-based modeling [132], [133] have been applied to interrogate microbial interactions. They use experimental time series of microbiome compositions. Unfortunately, data on microbial interactions at a large scale is usually scarce, and this information is indispensable for this type of modeling [134]. Other approaches help to understand the composition and functions of different communities but do not simulate their dynamical properties. This type of static modeling relies on a wide array of algorithms and computational methods to determine and study the interaction between different organisms, even if most of the time they only evidence the existence of a relationship, not its type. For example, using Bayesian modeling, it is possible to determine the structure/topology of the gene regulatory network of several bacteria living together in the same environment [135]. Other approaches use machine learning algorithms, for example, to determine a network of cross-feeding interactions [136]; classification of host-disease phenotypes from metagenomic data [137], [138]; or which bacteria are part of the normal community in certain gut regions [139]. Machine learning has been recently combined with GSMMs, expanding the potential of both approaches [140]. An inherent difficulty of machine learning is that this approach requires relatively large amounts of data to parameterize the algorithm and estimate its performance [141], thus reducing the applicability of machine learning in this field.
Finally, co-occurrence networks could provide important information regarding microbial interactions [142]. They rely on the identification of the co-occurrence of two or more species in multiple samples. In this way, if two species appear in the same sample and are absent in others, they are related. This approach also requires numerous experiments that help determine the species found in a sample, taking advantage of extensive collections available in databases such as IMG/M [143] or datasets like the Human Microbiome Project [HMP] [144]. Co-occurrence has been applied to different conditions, for example, to identify relationships between different taxa [145], predict microbial relationships within and between body sites [142], microbial communities linked to colorectal tumors [146], obesity, and diabetes type 2 [147], inflammatory bowel disease [148], and asthma [149].
7. Challenges and outlook
Microbial interactions stand out at the inner core of the composition of the human gut microbiota. Their study is hampered by the inherent complexity of this community in terms of functional diversity and forces shaping these cross-talks. For this task, experimental and modeling approaches have shown relevant patterns and highlighted how microbial interactions influence host health and disease.
A significant experimental gap in this field is how little we know regarding the functions and metabolic potential of gut microbes. Their high numbers, differences in spatial and time distributions as well as the large diversity of species, sets a high bar for their modeling. The metabolism of only a few gut species is well known, but for most gut microbes, only general aspects are understood. Moreover, sometimes only one amino acid different is necessary for a gut microbe to inactivate a drug [150]. Besides culturing techniques and microbial collections, high-throughput experimental studies for pairwise interactions are highly demanded, as well as reliable gut simulation platforms closely simulating intestinal processes.
Another experimental gap is how to reduce the breach between synthetic in vitro studies and the actual relevance of microbial interactions in the gut. It is difficult to comprehensively quantify the extent of microbial interactions in the gut or how changes in metabolic interactions contribute to disease, such as IBDs. Besides, it is unclear if the extent of experimentally validated cross-feeding interactions is biologically meaningful. Importantly, cross-feeding is only one of many microbial interactions that could be found in the gut microbiota. The host or other competitive or cooperative interactions would challenge in vitro results. In any case, experimental studies of cross-feeding have been instrumental in deriving general conclusions on which metabolites, groups of microorganisms, or pathways are involved in this process. These validations are the foundations of several modeling approaches, and several of them might not have been prevalent in vivo.
Modeling approaches can significantly help reduce the hypothesis space and offer testable experiments to validate potential metabolic interactions in the human gut. Amongst the various modeling approaches employed for this purpose, we have highlighted ecological, mechanistic ODE-based (kinetic), and genome-scale stoichiometric modeling as valuable tools for probing the interaction space. While ecological and kinetic models are relatively simple to interpret, they are often limited by the amount of data required for their construction (i.e., identifying the relevant model structure) and parameter fitting [151]. It is expected that increasing accessibility to next-generation sequencing, especially metagenomics, contribute positively to this gap. In contrast to ecological and kinetic models, GSMMs are mathematical structures that can be readily reconstructed using high-throughput sequencing data fed to various computational pipelines [91], [92], [93]. These model structures are often referred to as “parameter-free”, meaning they do not require to fit parameters but rely on the imposition of constraints derived from the data. The larger the amount of data available, the more reduced the solution space, leading to tighter predictions. While expression, protein, and fluxomic data can be readily used as constraints [109], the use of metabolomic data is not so direct. However, recent approaches have shown promise (see for example, MAMBO [152]). More importantly, GSMMs describe the metabolic potential of the cell and thus, offer unparallel capabilities for understanding the mechanisms of interaction in great detail. The latter helps explain the surge in the GSMMs use to analyze the gut microbiota [110]. Still, reconstruction of accurate GENREs remains a challenging task, as our knowledge of the diversity in metabolic functions is limited to well-studied organisms. Consequently, the metabolic capabilities of GSMMs are inevitably restricted, which raises questions about the relevance and overall validity of GSMMs for less studied organisms – particularly prevalent in the gut microbiome [153]. Overall, careful consideration and use of GSMMs has to be exercised when modeling the gut microbiota. Recent tools like MEMOTE [154] can help users better understand the quality and coverage of GSMMs.
A critical challenge for modeling the host-gut interactions is the temporal and spatial resolution required to identify critical cross-feeding interactions. The spatial distribution of the gut microbiota is highly heterogeneous as its diversity. Different types of Inflammatory Bowel Diseases (IBDs) can be promoted by an abnormal spatial distribution of the microbial gut members due to diet and inherent host responses, among others [155]. Scenarios resembling some of these conditions can be modeled with GSMMs [25], although they require more sophisticated computational methods that are more far more difficult to implement [120], [156], [157]. More recently, the COMETS framework has been released as an open-source package enabling the temporal and spatial simulation of microbial consortia using GSMMs in different environments [158]. As more data under experimentally relevant conditions becomes available (e.g., using microfluidics in vitro systems [41]), we foresee that modeling tools capturing temporal and spatial variations in microbial consortia will become more relevant for contextualizing cross-feeding in the human gut.
CRediT authorship contribution statement
Pedro Saa: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition. Arles Urrutia: Writing – original draft. Claudia Silva-Andrade: Writing – original draft. Alberto J. Martín: Writing – original draft, Funding acquisition. Daniel Garrido: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded in part by ANID grants Fondecyt Regular 1190074 (DG), Fondecyt Regular 1181089 (AJM), FONDEQUIP EQM190070, Fondecyt Iniciacion 11190871 (PS), PUC School of Engineering SeedFund 2020, and PUC Postdoctoral School of Engineering scholarship.
References
- 1.Nayfach S., Shi Z.J., Seshadri R., Pollard K.S., Kyrpides N.C. New insights from uncultivated genomes of the global human gut microbiome. Nature. 2019;568(7753):505–510. doi: 10.1038/s41586-019-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasaly N., de Vos P., Hermoso M.A. Impact of bacterial metabolites on gut barrier function and host immunity: A focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol. 2021;12:1807. doi: 10.3389/fimmu.2021.658354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cronin P., Joyce S.A., O’Toole P.W., O’Connor E.M. Dietary fibre modulates the gut microbiota. Nutrients. 2021;13(5):1655. doi: 10.3390/nu13051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyte K.Z., Schluter J., Foster K.R. The ecology of the microbiome: Networks, competition, and stability. Science. 2015;350(6261):663–666. doi: 10.1126/science.aad2602. [DOI] [PubMed] [Google Scholar]
- 5.Tsukuda N., Yahagi K., Hara T., Watanabe Y., Matsumoto H., Mori H., et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 2021;15(9):2574–2590. doi: 10.1038/s41396-021-00937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aires J. First 1000 days of life: Consequences of antibiotics on gut microbiota. Front Microbiol. 2021;12:681427. doi: 10.3389/fmicb.2021.681427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer F., Anderson J.M., Bharti R., Raes J., Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15(10):630–638. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 8.Goyal A., Wang T., Dubinkina V., Maslov S. Ecology-guided prediction of cross-feeding interactions in the human gut microbiome. Nat Commun. 2021;12:1335. doi: 10.1038/s41467-021-21586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark R.L., Connors B.M., Stevenson D.M., Hromada S.E., Hamilton J.J., Amador-Noguez D., et al. Design of synthetic human gut microbiome assembly and butyrate production. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-22938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez N., Garrido D., Rawls J.F. Species deletions from microbiome consortia reveal key metabolic interactions between gut microbes. MSystems. 2019;4(4) doi: 10.1128/mSystems.00185-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacheco A.R., Segrè D. A multidimensional perspective on microbial interactions. FEMS Microbiol Lett. 2019;366 doi: 10.1093/femsle/fnz125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coquant G., Grill J.-P., Seksik P. Impact of N-Acyl-homoserine lactones, quorum sensing molecules, on gut immunity. Front Immunol. 2020;11:1827. doi: 10.3389/fimmu.2020.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding M., Yang B.o., Ross R.P., Stanton C., Zhao J., Zhang H., et al. Crosstalk between sIgA-coated bacteria in infant gut and early-life health. Trends Microbiol. 2021;29(8):725–735. doi: 10.1016/j.tim.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 14.García-Bayona L., Coyne M.J., Comstock L.E., Blokesch M. Mobile Type VI secretion system loci of the gut Bacteroidales display extensive intra-ecosystem transfer, multi-species spread and geographical clustering. PLOS Genet. 2021;17(4):e1009541. doi: 10.1371/journal.pgen.1009541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong T., Fu J., Shi L., Chen X., Zong X. Antimicrobial peptides in gut health: A review. Front Nutr. 2021;8:711. doi: 10.3389/fnut.2021.751010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huus K.E., Petersen C., Finlay B.B. Diversity and dynamism of IgA−microbiota interactions. Nat Rev Immunol. 2021;21(8):514–525. doi: 10.1038/s41577-021-00506-1. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14(1):20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klymiuk I., Singer G., Castellani C., Trajanoski S., Obermüller B., Till H. Characterization of the luminal and mucosa-associated microbiome along the gastrointestinal tract: results from surgically treated preterm infants and a murine model. Nutr. 2021;13(3):1030. doi: 10.3390/nu13031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kettle H., Louis P., Holtrop G., Duncan S.H., Flint H.J. Modelling the emergent dynamics and major metabolites of the human colonic microbiota. Environ Microbiol. 2015;17(5):1615–1630. doi: 10.1111/1462-2920.12599. [DOI] [PubMed] [Google Scholar]
- 20.Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 21.D'Souza G., Shitut S., Preussger D., Yousif G., Waschina S., Kost C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat Prod Rep. 2018;35(5):455–488. doi: 10.1039/C8NP00009C. [DOI] [PubMed] [Google Scholar]
- 22.Belzer C., Chia L.W., Aalvink S., Chamlagain B., Piironen V., Knol J., et al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B 12 production by intestinal symbionts. mBio. 2017;8(5) doi: 10.1128/mBio.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung J., Kim S., Cabatbat J.J.T., Jang S., Jin Y.-S., Jung G.Y., et al. Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat Commun. 2017;8(1) doi: 10.1038/ncomms15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saa P.A., Nielsen L.K. Formulation, construction and analysis of kinetic models of metabolism: A review of modelling frameworks. Biotechnol Adv. 2017;35(8):981–1003. doi: 10.1016/j.biotechadv.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Altamirano Á., Saa P.A., Garrido D. Inferring composition and function of the human gut microbiome in time and space: A review of genome-scale metabolic modelling tools. Comput Struct Biotechnol J. 2020;18:3897–3904. doi: 10.1016/j.csbj.2020.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis N.E., Nagarajan H., Palsson B.O. Constraining the metabolic genotype-phenotype relationship using a phylogeny of in silico methods. Nat Rev Microbiol. 2012;10(4):291–305. doi: 10.1038/nrmicro2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zomorrodi A.R., Maranas C.D., Rao C.V. A multi-level optimization framework for the metabolic modeling and analysis of microbial communities. PLoS Comput Biol. 2012;8(2):e1002363. doi: 10.1371/journal.pcbi.1002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heirendt L., Arreckx S., Pfau T., Mendoza S.N., Richelle A., Heinken A., et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox vol 3.0. Nat Protoc. 2019;14(3):639–702. doi: 10.1038/s41596-018-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zomorrodi A.R., Islam M.M., Maranas C.D. D-OptCom: dynamic multi-level and multi-objective metabolic modeling of microbial communities. ACS Synth Biol. 2014;3(4):247–257. doi: 10.1021/sb4001307. [DOI] [PubMed] [Google Scholar]
- 30.Heinken A., Hertel J., Thiele I. Metabolic modelling reveals broad changes in gut microbial metabolism in inflammatory bowel disease patients with dysbiosis. Npj Syst Biol Appl. 2021;7:19. doi: 10.1038/s41540-021-00178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldini F., Hertel J., Sandt E., Thinnes C.C., Neuberger-Castillo L., Pavelka L., et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020;18(1) doi: 10.1186/s12915-020-00775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hertel J., Harms A.C., Heinken A., Baldini F., Thinnes C.C., Glaab E., et al. Integrated analyses of microbiome and longitudinal metabolome data reveal microbial-host interactions on sulfur metabolism in Parkinson’s Disease. Cell Rep. 2019;29(7):1767–1777.e8. doi: 10.1016/j.celrep.2019.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renwick S., Ganobis C.M., Elder R.A., Gianetto-Hill C., Higgins G., Robinson A.V., et al. Culturing human gut microbiomes in the laboratory. Annu Rev Microbiol. 2021;75(1):49–69. doi: 10.1146/annurev-micro-031021-084116. [DOI] [PubMed] [Google Scholar]
- 34.Strandwitz P., Kim K.H., Terekhova D., Liu J.K., Sharma A., Levering J., et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bengtsson-Palme J. Microbial model communities: To understand complexity, harness the power of simplicity. Comput Struct Biotechnol J. 2020;18:3987–4001. doi: 10.1016/j.csbj.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van den Abbeele P., Belzer C., Goossens M., Kleerebezem M., De Vos W.M., Thas O., et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7(5):949–961. doi: 10.1038/ismej.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medina D.A., Pinto F., Ortuzar M.V., Garrido D. Simulation and modeling of dietary changes in the infant gut microbiome. FEMS Microbiol Ecol. 2018;94 doi: 10.1093/femsec/fiy140. [DOI] [PubMed] [Google Scholar]
- 38.Kim H.J., Huh D., Hamilton G., Ingber D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–2174. doi: 10.1039/C2LC40074J. [DOI] [PubMed] [Google Scholar]
- 39.Molly K., Vande Woestyne M., Verstraete W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl Microbiol Biotechnol. 1993;39(2):254–258. doi: 10.1007/BF00228615. [DOI] [PubMed] [Google Scholar]
- 40.Marzorati M., Vanhoecke B., De Ryck T., Sadaghian Sadabad M., Pinheiro I., Possemiers S., et al. The HMITM module: a new tool to study the Host-Microbiota Interaction in the human gastrointestinal tract in vitro. BMC Microbiol. 2014;14:133. doi: 10.1186/1471-2180-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah P., Fritz J.V., Glaab E., Desai M.S., Greenhalgh K., Frachet A., et al. A microfluidics-based in vitro model of the gastrointestinal human–microbe interface. Nat Commun. 2016;7(1) doi: 10.1038/ncomms11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoaie S., Ghaffari P., Kovatcheva-Datchary P., Mardinoglu A., Sen P., Pujos-Guillot E., et al. Quantifying Diet-Induced Metabolic Changes of the Human Gut Microbiome. Cell Metab. 2015;22(2):320–331. doi: 10.1016/j.cmet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Shoaie S., Karlsson F., Mardinoglu A., Nookaew I., Bordel S., Nielsen J. Understanding the interactions between bacteria in the human gut through metabolic modeling. Sci Rep. 2013;3(1) doi: 10.1038/srep02532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egan M., O’Connell Motherway M., Kilcoyne M., Kane M., Joshi L., Ventura M., et al. Cross-feeding by Bifidobacterium breve UCC2003 during co-cultivation with Bifidobacterium bifidum PRL2010 in a mucin-based medium. BMC Microbiol. 2014;14(1) doi: 10.1186/s12866-014-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunesova V., Lacroix C., Schwab C. Mucin cross-feeding of infant Bifidobacteria and Eubacterium hallii. Microb Ecol. 2018;75(1):228–238. doi: 10.1007/s00248-017-1037-4. [DOI] [PubMed] [Google Scholar]
- 47.van Best N., Rolle-Kampczyk U., Schaap F.G., Basic M., Olde Damink S.W.M., Bleich A., et al. Bile acids drive the newborn’s gut microbiota maturation. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-17183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holert J., Yücel O., Suvekbala V., Kulić Ž., Möller H., Philipp B. Evidence of distinct pathways for bacterial degradation of the steroid compound cholate suggests the potential for metabolic interactions by interspecies cross-feeding. Environ Microbiol. 2014;16:1424–1440. doi: 10.1111/1462-2920.12407. [DOI] [PubMed] [Google Scholar]
- 49.Shetty S.A., Hugenholtz F., Lahti L., Smidt H., de Vos W.M. Intestinal microbiome landscaping: Insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol Rev. 2017;41:182–199. doi: 10.1093/femsre/fuw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivière A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laverde Gomez J.A., Mukhopadhya I., Duncan S.H., Louis P., Shaw S., Collie-Duguid E., et al. Formate cross-feeding and cooperative metabolic interactions revealed by transcriptomics in co-cultures of acetogenic and amylolytic human colonic bacteria. Environ Microbiol. 2019;21(1):259–271. doi: 10.1111/1462-2920.14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith N.W., Shorten P.R., Altermann E., Roy N.C., McNabb W.C. Examination of hydrogen cross-feeders using a colonic microbiota model. BMC Bioinf. 2021;22:3. doi: 10.1186/s12859-020-03923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ravcheev D.A., Thiele I. Systematic genomic analysis reveals the complementary aerobic and anaerobic respiration capacities of the human gut microbiota. Front Microbiol. 2014;5:674. doi: 10.3389/fmicb.2014.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavaliere M., Feng S., Soyer O.S., Jiménez J.I. Cooperation in microbial communities and their biotechnological applications. Environ Microbiol. 2017;19:2949–2963. doi: 10.1111/1462-2920.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rakoff-Nahoum S., Foster K.R., Comstock L.E. The evolution of cooperation within the gut microbiota. Nature. 2016;533(7602):255–259. doi: 10.1038/nature17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogowski A., Briggs J.A., Mortimer J.C., Tryfona T., Terrapon N., Lowe E.C., et al. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun. 2015;6(1) doi: 10.1038/ncomms8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuskin F., Lowe E.C., Temple M.J., Zhu Y., Cameron E.A., Pudlo N.A., et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 2015;517(7533):165–169. doi: 10.1038/nature13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomson P., Medina D.A., Garrido D. Human milk oligosaccharides and infant gut bifidobacteria: Molecular strategies for their utilization. Food Microbiol. 2018;75:37–46. doi: 10.1016/j.fm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Cremer J., Melbinger A., Wienand K., Henriquez T., Jung H., Frey E. Cooperation in microbial populations: theory and experimental model systems. J Mol Biol. 2019;431:4599–4644. doi: 10.1016/j.jmb.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 61.Damore J.A., Gore J. Understanding microbial cooperation. J Theor Biol. 2012;299:31–41. doi: 10.1016/j.jtbi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magnúsdóttir S., Heinken A., Kutt L., Ravcheev D.A., Bauer E., Noronha A., et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat Biotechnol. 2017;35(1):81–89. doi: 10.1038/nbt.3703. [DOI] [PubMed] [Google Scholar]
- 63.Ghoul M., Mitri S. The ecology and evolution of microbial competition. Trends Microbiol. 2016;24:833–845. doi: 10.1016/j.tim.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Duncan S.H., Louis P., Flint H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70(10):5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dal Co A., van Vliet S., Kiviet D.J., Schlegel S., Ackermann M. Short-range interactions govern the dynamics and functions of microbial communities. Nat Ecol Evol. 2020;4(3):366–375. doi: 10.1038/s41559-019-1080-2. [DOI] [PubMed] [Google Scholar]
- 66.van Tatenhove-Pel R.J., Rijavec T., Lapanje A., van Swam I., Zwering E., Hernandez-Valdes J.A., et al. Microbial competition reduces metabolic interaction distances to the low µm-range. ISME J. 2021;15(3):688–701. doi: 10.1038/s41396-020-00806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cremer J., Segota I., Yang C.-y., Arnoldini M., Sauls J.T., Zhang Z., et al. Effect of flow and peristaltic mixing on bacterial growth in a gut-like channel. Proc Natl Acad Sci USA. 2016;113(41):11414–11419. doi: 10.1073/pnas.1601306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaczmarek J.L., Thompson S.V., Holscher H.D. Complex interactions of circadian rhythms, eating behaviors, and the gastrointestinal microbiota and their potential impact on health. Nutr Rev. 2017;75:673–682. doi: 10.1093/nutrit/nux036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matenchuk B.A., Mandhane P.J., Kozyrskyj A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53 doi: 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- 70.Zarrinpar A., Chaix A., Yooseph S., Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thaiss C., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A., et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 72.Leone V., Gibbons S., Martinez K., Hutchison A., Huang E., Cham C., et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17(5):681–689. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geng J., Ji B., Li G., López-Isunza F., Nielsen J. CODY enables quantitatively spatiotemporal predictions on in vivo gut microbial variability induced by diet intervention. Proc Natl Acad Sci. 2021;118(13) doi: 10.1073/pnas.2019336118. e2019336118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silverman J.D., Durand H.K., Bloom R.J., Mukherjee S., David L.A. Dynamic linear models guide design and analysis of microbiota studies within artificial human guts. Microbiome. 2018;6:202. doi: 10.1186/s40168-018-0584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McNally C.P., Borenstein E. Metabolic model-based analysis of the emergence of bacterial cross-feeding via extensive gene loss. BMC Syst Biol. 2018;12:69. doi: 10.1186/s12918-018-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelezniak A., Andrejev S., Ponomarova O., Mende D.R., Bork P., Patil K.R. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc Natl Acad Sci USA. 2015;112(20):6449–6454. doi: 10.1073/pnas.1421834112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian L., Wang X.-W., Wu A.-K., Fan Y., Friedman J., Dahlin A., et al. Deciphering functional redundancy in the human microbiome. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-19940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paine R.T. A note on trophic complexity and community stability. Am Nat. 1969;103(929):91–93. doi: 10.1086/282586. [DOI] [Google Scholar]
- 79.Wang T., Goyal A., Dubinkina V., Maslov S., Tikhonov M. Evidence for a multi-level trophic organization of the human gut microbiome. PLOS Comput Biol. 2019;15(12):e1007524. doi: 10.1371/journal.pcbi.1007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antonella S., Wanjiku M.F., Oliver E., George O. A diverse community to study communities: integration of experiments and mathematical models to study microbial consortia. J Bacteriol. 2021;199:e00865–e916. doi: 10.1128/JB.00865-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bosi E., Bacci G., Mengoni A., Fondi M. Perspectives and challenges in microbial communities metabolic modeling. Front Genet. 2017;8:88. doi: 10.3389/fgene.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Garcia O., Lear G., Singhal N. Metabolic network modeling of microbial interactions in natural and engineered environmental systems. Front Microbiol. 2016;7:673. doi: 10.3389/fmicb.2016.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santibáñez R., Garrido D., Martin A.J.M. A tool for statistical and multi-objective calibration of Rule-based models. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-51546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qian Y., Lan F., Venturelli O.S. Towards a deeper understanding of microbial communities: integrating experimental data with dynamic models. Curr Opin Microbiol. 2021;62:84–92. doi: 10.1016/j.mib.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Venturelli O.S., Carr A.V., Fisher G., Hsu R.H., Lau R., Bowen B.P., et al. Deciphering microbial interactions in synthetic human gut microbiome communities. Mol Syst Biol. 2018;14(6) doi: 10.15252/msb:20178157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedman J., Higgins L.M., Gore J. Community structure follows simple assembly rules in microbial microcosms. Nat Ecol Evol. 2017;1:109. doi: 10.1038/s41559-017-0109. [DOI] [PubMed] [Google Scholar]
- 87.Pinto F., Medina D.A., Pérez-Correa J.R., Garrido D. Modeling metabolic interactions in a consortium of the infant gut microbiome. Front Microbiol. 2017;8:1–12. doi: 10.3389/fmicb.2017.02507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sacher J., Saa P., Cárcamo M., López J., Gelmi C.A., Pérez-Correa R. Improved calibration of a solid substrate fermentation model. Electron J Biotechnol. 2011;14:7. [Google Scholar]
- 89.Julien-Laferrière A., Bulteau L., Parrot D., Marchetti-Spaccamela A., Stougie L., Vinga S., et al. A combinatorial algorithm for microbial consortia synthetic design. Sci Rep. 2016;6(1) doi: 10.1038/srep29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thiele I., Palsson B.Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat Protoc. 2010;5(1):93–121. doi: 10.1038/nprot.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henry C.S., DeJongh M., Best A.A., Frybarger P.M., Linsay B., Stevens R.L. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat Biotechnol. 2010;28(9):977–982. doi: 10.1038/nbt.1672. [DOI] [PubMed] [Google Scholar]
- 92.Wang H., Marcišauskas S., Sánchez B.J., Domenzain I., Hermansson D., Agren R., et al. RAVEN 2.0: A versatile toolbox for metabolic network reconstruction and a case study on Streptomyces coelicolor. PLOS Comput Biol. 2018;14(10):e1006541. doi: 10.1371/journal.pcbi.1006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dias O., Rocha M., Ferreira E.C., Rocha I. Reconstructing genome-scale metabolic models with merlin. Nucleic Acids Res. 2015;43:3899–3910. doi: 10.1093/nar/gkv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caspi R., Billington R., Ferrer L., Foerster H., Fulcher C.A., Keseler I.M., et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016;44(D1):D471–D480. doi: 10.1093/nar/gkv1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thiele I., Vlassis N., Fleming R.M. fastGapFill: efficient gap filling in metabolic networks. Bioinformatics. 2014;30:2529–2531. doi: 10.1093/bioinformatics/btu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zimmermann J., Kaleta C., Waschina S. gapseq: informed prediction of bacterial metabolic pathways and reconstruction of accurate metabolic models. Genome Biol. 2021;22:81. doi: 10.1186/s13059-021-02295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Machado D., Andrejev S., Tramontano M., Patil K.R. Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic Acids Res. 2018;46:7542–7553. doi: 10.1093/nar/gky537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aite M., Chevallier M., Frioux C., Trottier C., Got J., Cortés M.P., et al. Traceability, reproducibility and wiki-exploration for “à-la-carte” reconstructions of genome-scale metabolic models. PLOS Comput Biol. 2018;14(5):e1006146. doi: 10.1371/journal.pcbi.1006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Belcour A., Frioux C., Aite M., Bretaudeau A., Hildebrand F., Siegel A. Metage2Metabo, microbiota-scale metabolic complementarity for the identification of key species. Elife. 2020;9 doi: 10.7554/eLife.61968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heinken A., Acharya G., Ravcheev D.A., Hertel J., Nyga M., Okpala O.E., et al. AGORA2: Large scale reconstruction of the microbiome highlights wide-spread drug-metabolising capacities. BioRxiv. 2020 doi: 10.1101/2020.11.09.375451. [DOI] [Google Scholar]
- 102.Heinken A., Magnúsdóttir S., Fleming R.M.T., Thiele I., Vitek O. DEMETER: efficient simultaneous curation of genome-scale reconstructions guided by experimental data and refined gene annotations. Bioinformatics. 2021;37(21):3974–3975. doi: 10.1093/bioinformatics/btab622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heinken A., Ravcheev D.A., Baldini F., Heirendt L., Fleming R.M.T., Thiele I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome. 2019;7:75. doi: 10.1186/s40168-019-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heinken A., Sahoo S., Fleming R.M.T., Thiele I. Systems-level characterization of a host-microbe metabolic symbiosis in the mammalian gut. Gut Microbes. 2013;4(1):28–40. doi: 10.4161/gmic.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heinken A., Thiele I. Systematic prediction of health-relevant human-microbial co-metabolism through a computational framework. Gut Microbes. 2015;6(2):120–130. doi: 10.1080/19490976.2015.1023494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim R., Cabatbat J.J.T., Martin T.L.P., Kim H., Kim S., Sung J., et al. Large-scale metabolic interaction network of the mouse and human gut microbiota. Sci Data. 2020;7(1) doi: 10.1038/s41597-020-0516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sen P., Orešič M. Metabolic Modeling of Human Gut Microbiota on a Genome Scale: An Overview. Metabolites. 2019;9:22. doi: 10.3390/metabo9020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Orth J.D., Thiele I., Palsson B.Ø. What is flux balance analysis? Nat Biotechnol. 2010;28(3):245–248. doi: 10.1038/nbt.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Price N.D., Reed J.L., Palsson B.Ø. Genome-scale models of microbial cells: evaluating the consequences of constraints. Nat Rev Microbiol. 2004;2(11):886–897. doi: 10.1038/nrmicro1023. [DOI] [PubMed] [Google Scholar]
- 110.Gu C., Kim G.B., Kim W.J., Kim H.U., Lee S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019;20:121. doi: 10.1186/s13059-019-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thiele I., Sahoo S., Heinken A., Hertel J., Heirendt L., Aurich M.K., et al. Personalized whole-body models integrate metabolism, physiology, and the gut microbiome. Mol Syst Biol. 2020;16 doi: 10.15252/msb.20198982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baloni P., Funk C.C., Yan J., Yurkovich J.T., Kueider-Paisley A., Nho K., et al. Metabolic network analysis reveals altered bile acid synthesis and metabolism in Alzheimeŕs Disease. Cell Reports Med. 2020;1(8):100138. doi: 10.1016/j.xcrm.2020.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pacheco A.R., Moel M., Segrè D. Costless metabolic secretions as drivers of interspecies interactions in microbial ecosystems. Nat Commun. 2019;10:103. doi: 10.1038/s41467-018-07946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mahadevan R., Schilling C.H. The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab Eng. 2003;5(4):264–276. doi: 10.1016/j.ymben.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 115.Mahadevan R., Edwards J.S., Doyle F.J. Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys J. 2002;83(3):1331–1340. doi: 10.1016/S0006-3495(02)73903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abdel-Haleem A.M., Ravikumar V., Ji B., Mineta K., Gao X., Nielsen J., et al. Integrated Metabolic Modeling, Culturing, and Transcriptomics Explain Enhanced Virulence of Vibrio cholerae during Coinfection with Enterotoxigenic Escherichia coli. MSystems. 2020;5(5) doi: 10.1128/mSystems.00491-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khandelwal R.A., Olivier B.G., Röling W.F.M., Teusink B., Bruggeman F.J., Vera J. Community Flux Balance Analysis for Microbial Consortia at Balanced Growth. PLoS ONE. 2013;8(5):e64567. doi: 10.1371/journal.pone.0064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chan S.H.J., Simons M.N., Maranas C.D., Price N.D. Predicting microbial abundances while ensuring community stability. PLoS Comput Biol. 2017;13(5):e1005539. doi: 10.1371/journal.pcbi.1005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan S.H.J., Friedman E.S., Wu G.D., Maranas C.D. Predicting the Longitudinally and Radially Varying Gut Microbiota Composition using Multi-Scale Microbial Metabolic Modeling. Processes. 2019;7 doi: 10.3390/pr7070394. [DOI] [Google Scholar]
- 120.Harcombe W., Riehl W., Dukovski I., Granger B., Betts A., Lang A., et al. Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep. 2014;7(4):1104–1115. doi: 10.1016/j.celrep.2014.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bauer E., Zimmermann J., Baldini F., Thiele I., Kaleta C., Maranas C.D. BacArena: Individual-based metabolic modeling of heterogeneous microbes in complex communities. PLOS Comput Biol. 2017;13(5):e1005544. doi: 10.1371/journal.pcbi.1005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G., Gasbarrini A., et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kunasegaran T., Balasubramaniam V.R.M.T., Arasoo V.J.T., Palanisamy U.D., Ramadas A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biol. 2021;10(10):1027. doi: 10.3390/biology10101027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sultan S., El-Mowafy M., Elgaml A., Ahmed T.A.E., Hassan H., Mottawea W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front Physiol. 2021;12:1489. doi: 10.3389/fphys.2021.715506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clapp M., Aurora N., Herrera L., Bhatia M., Wilen E., Wakefield S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin Pract. 2017;7(4):131–136. doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nishida A., Inoue R., Inatomi O., Bamba S., Naito Y., Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11(1):1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 127.Diener C., Gibbons S.M., Resendis-Antonio O., Chia N. MICOM: Metagenome-Scale Modeling To Infer Metabolic Interactions in the Gut Microbiota. MSystems. 2020;5(1) doi: 10.1128/mSystems.00606-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu F.B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 129.Foster K., Bell T. Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol. 2012;22(19):1845–1850. doi: 10.1016/j.cub.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 130.Descheemaeker L., de Buyl S. Stochastic logistic models reproduce experimental time series of microbial communities. Elife. 2020;9 doi: 10.7554/eLife.55650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vega N.M., Gore J., Cadwell K. Stochastic assembly produces heterogeneous communities in the Caenorhabditis elegans intestine. PLOS Biol. 2017;15(3):e2000633. doi: 10.1371/journal.pbio.2000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shashkova T., Popenko A., Tyakht A., Peskov K., Kosinsky Y., Bogolubsky L., et al. Agent Based Modeling of Human Gut Microbiome Interactions and Perturbations. PLoS ONE. 2016;11(2):e0148386. doi: 10.1371/journal.pone.0148386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin C., Culver J., Weston B., Underhill E., Gorky J., Dhurjati P., et al. GutLogo: Agent-based modeling framework to investigate spatial and temporal dynamics in the gut microbiome. PLoS ONE. 2018;13(11):e0207072. doi: 10.1371/journal.pone.0207072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ibrahim M., Raajaraam L., Raman K. Modelling microbial communities: Harnessing consortia for biotechnological applications. Comput Struct Biotechnol J. 2021;19:3892–3907. doi: 10.1016/j.csbj.2021.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buetti-Dinh A., Herold M., Christel S., El Hajjami M., Delogu F., Ilie O., et al. Reverse engineering directed gene regulatory networks from transcriptomics and proteomics data of biomining bacterial communities with approximate Bayesian computation and steady-state signalling simulations. BMC Bioinf. 2020;21(1) doi: 10.1186/s12859-019-3337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pfeiffer T., Sanchez-Valdenebro I., Nuno J., Montero F., Schuster S. METATOOL: For studying metabolic networks. Bioinformatics. 1999;15(3):251–257. doi: 10.1093/bioinformatics/15.3.251. [DOI] [PubMed] [Google Scholar]
- 137.Arabameri A., Asemani D., Teymourpour P. Detection of colorectal carcinoma based on microbiota analysis using generalized regression neural networks and nonlinear feature selection. IEEE/ACM Trans Comput Biol Bioinforma. 2020;17:547–557. doi: 10.1109/TCBB.2018.2870124. [DOI] [PubMed] [Google Scholar]
- 138.Fukui H., Nishida A., Matsuda S., Kira F., Watanabe S., Kuriyama M., et al. Usefulness of machine learning-based gut microbiome analysis for identifying patients with irritable bowels syndrome. J Clin Med. 2020;9(8):2403. doi: 10.3390/jcm9082403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flynn K.J., Ruffin M.T., Turgeon D.K., Schloss P.D. Spatial variation of the native colon microbiota in healthy adults. Cancer Prev Res. 2018;11(7):393–402. doi: 10.1158/1940-6207.CAPR-17-0370. [DOI] [PubMed] [Google Scholar]
- 140.Zampieri G., Vijayakumar S., Yaneske E., Angione C., Nielsen J. Machine and deep learning meet genome-scale metabolic modeling. PLOS Comput Biol. 2019;15(7):e1007084. doi: 10.1371/journal.pcbi.1007084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Walsh I., Fishman D., Garcia-Gasulla D., Titma T., Pollastri G., Capriotti E., et al. DOME: recommendations for supervised machine learning validation in biology. Nat Methods. 2021;18(10):1122–1127. doi: 10.1038/s41592-021-01205-4. [DOI] [PubMed] [Google Scholar]
- 142.Faust K., Sathirapongsasuti J.F., Izard J., Segata N., Gevers D., Raes J., et al. Microbial Co-occurrence Relationships in the Human Microbiome. PLOS Comput Biol. 2012;8(7):e1002606. doi: 10.1371/journal.pcbi.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen I.-M., Chu K., Palaniappan K., Ratner A., Huang J., Huntemann M., et al. The IMG/M data management and analysis system vol 6.0: new tools and advanced capabilities. Nucleic Acids Res. 2021;49(D1):D751–D763. doi: 10.1093/nar/gkaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Methé B.A., Nelson K.E., Pop M., Creasy H.H., Giglio M.G., Huttenhower C., et al. A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vanderhaeghen S., Lacroix C., Schwab C., Margolles A. Methanogen communities in stools of humans of different age and health status and co-occurrence with bacteria. FEMS Microbiol Lett. 2015;362(13):fnv092. doi: 10.1093/femsle/fnv092. [DOI] [PubMed] [Google Scholar]
- 146.Warren R.L., Freeman D.J., Pleasance S., Watson P., Moore R.A., Cochrane K., et al. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1(1) doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thingholm L.B., Rühlemann M.C., Koch M., Fuqua B., Laucke G., Boehm R., et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe. 2019;26(2):252–264.e10. doi: 10.1016/j.chom.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baldassano S.N., Bassett D.S. Topological distortion and reorganized modular structure of gut microbial co-occurrence networks in inflammatory bowel disease. Sci Rep. 2016;6:26087. doi: 10.1038/srep26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuenzig M.E., Bishay K., Leigh R., Kaplan G.G., Benchimol E.I., Team CSRR Co-occurrence of asthma and the inflammatory bowel diseases: A systematic review and meta-analysis. Clin Transl Gastroenterol. 2018:9. doi: 10.1038/s41424-018-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maini Rekdal V., Bess E.N., Bisanz J.E., Turnbaugh P.J., Balskus E.P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364(6445) doi: 10.1126/science:aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ghannam R.B., Techtmann S.M. Machine learning applications in microbial ecology, human microbiome studies, and environmental monitoring. Comput Struct Biotechnol J. 2021;19:1092–1107. doi: 10.1016/j.csbj.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garza D.R., van Verk M.C., Huynen M.A., Dutilh B.E. Towards predicting the environmental metabolome from metagenomics with a mechanistic model. Nat Microbiol. 2018;3(4):456–460. doi: 10.1038/s41564-018-0124-8. [DOI] [PubMed] [Google Scholar]
- 153.Lugli G.A., Duranti S., Albert K., Mancabelli L., Napoli S., Viappiani A., et al. Unveiling genomic diversity among members of the species bifidobacterium pseudolongum, a widely distributed gut commensal of the animal kingdom. Appl Environ Microbiol. 2019;85(8) doi: 10.1128/AEM.03065-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lieven C., Beber M.E., Olivier B.G., Bergmann F.T., Ataman M., Babaei P., et al. MEMOTE for standardized genome-scale metabolic model testing. Nat Biotechnol. 2020;38(3):272–276. doi: 10.1038/s41587-020-0446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 156.Gardner J.J., Hodge B.-M., Boyle N.R. Multiscale Multiobjective Systems Analysis (MiMoSA): an advanced metabolic modeling framework for complex systems. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-53188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Borer B., Ataman M., Hatzimanikatis V., Or D., Reed J.L. Modeling metabolic networks of individual bacterial agents in heterogeneous and dynamic soil habitats (IndiMeSH) PLOS Comput Biol. 2019;15(6):e1007127. doi: 10.1371/journal.pcbi.1007127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dukovski I., Bajić D., Chacón J.M., Quintin M., Vila J.C.C., Sulheim S., et al. A metabolic modeling platform for the computation of microbial ecosystems in time and space (COMETS) Nat Protoc. 2021;16(11):5030–5082. doi: 10.1038/s41596-021-00593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]