Abstract
Burn survivors experience myriad associated symptoms such as pain, pruritus, fatigue, impaired motor strength, post-traumatic stress, depression, anxiety, and sleep disturbance. Many of these symptoms are common and remain chronic, despite current standard of care. One potential novel intervention to target these post burn symptoms is transcranial direct current stimulation (tDCS). tDCS is a non-invasive brain stimulation (NIBS) technique that modulates neural excitability of a specific target or neural network. The aim of this work is to review the neural circuits of the aforementioned clinical sequelae associated with burn injuries and to provide a scientific rationale for specific NIBS targets that can potentially treat these conditions. We ran a systematic review, following the PRISMA statement, of tDCS effects on burn symptoms. Only three studies matched our criteria. One was a feasibility study assessing cortical plasticity in chronic neuropathic pain following burn injury, one looked at the effects of tDCS to reduce pain anxiety during burn wound care, and one assessed the effects of tDCS to manage pain and pruritus in burn survivors. Current literature on NIBS in burn remains limited, only a few trials have been conducted. Based on our review and results in other populations suffering from similar symptoms as patients with burn injuries, three main areas were selected: the prefrontal region, the parietal area and the motor cortex. Based on the importance of the prefrontal cortex in the emotional component of pain and its implication in various psychosocial symptoms, targeting this region may represent the most promising target. Our review of the neural circuitry involved in post burn symptoms and suggested targeted areas for stimulation provide a spring board for future study initiatives.
Keywords: Non-invasive brain stimulation, Transcranial direct current, stimulation, Pain, Pruritus, Psychosocial disorders
1. Introduction
Burn survivors experience myriad associated symptoms such as pain, pruritus, fatigue, impaired motor strength, post-traumatic stress, depression, anxiety, and sleep disturbance [1]. Many of these symptoms are common and remain chronic, despite current standard of care. Given these persistent symptoms, there is a need for novel targeted interventions. Burn injuries often have lasting ramifications on survivor physical and mental health. In regards to mental health, symptoms of post-traumatic stress, depression, and anxiety have been observed persisting after injury, creating lasting psychosocial consequences with impact on social functioning and disability [2]. About a third of burn survivors report moderate to severe psychological or social difficulties [3,4] which dramatically impact return to work, social integration, and consequently quality of life [5]. Similarly, symptoms of pain and pruritus cause equally concerning sequelae for burn patients, affecting sleep [6], daily activities [7], and quality of life [8]. The prevalence and chronicity of these post burn symptoms therefore underscores the need to identify effective therapies to ameliorate these symptoms and ensuing effects. Treatment of these chronic symptoms would thus have an impact on many patients’ quality of life.
One potential novel intervention to target these post burn symptoms is low intensity transcranial electrical stimulation (tES) [9]. tES is a non-invasive brain stimulation (NIBS) technique that modulates neural excitability of a specific target or neural network. Most studies used transcranial direct current stimulation (tDCS), which modulates cortical excitability through the application of a weak electrical current in the form of direct current brain polarization [10]. Another technique is transcranial alternating current stimulation (tACS), which is similar to tDCS but it oscillates a sinusoidal current at a specific frequency to interact with the brain’s natural cortical oscillations [11]. In the current literature, tES has been shown to reduce pain, improve motor function, enhance cognitive abilities and treat depression in various populations. However, there is a paucity of literature in regards to tES use on post-burn sequelae.
The aim of this work is to review the neural circuits of the aforementioned clinical sequelae associated with burn injuries and to provide a scientific rationale for specific non-invasive brain stimulation targets that potentially treat these conditions. This review focuses on the adult population only as the issues associated with growth and development of the brain in the pediatric population are extremely complex. We review the mechanisms in which tES modulates these neural circuits and their related behaviors in various patient populations as well as the current state of the science on tDCS to improve burn related symptoms. Given that the current literature is limited in the burn population, this review aims to provide a model-driven approach for potential neural targets to treat burn associated symptoms that will open areas of future inquiry in the field of burn care.
2. Search methodology
We searched on PubMed using the following search terms: “burn” and tDCS “transcranial direct current stimulation” or “neuromodulation” or “non-invasive brain stimulation”.
We included studies investigating the effects of tDCS on burn related symptoms (i.e., pain, pruritus, psychosocial disorders, sleep disturbance, fatigue and impaired motor strength) in burn survivors. We included case-reports, open-label studies and randomized clinical trials. We excluded studies not written in English or French, reviews or opinion papers, conference abstracts, and those not using tES to treat burn related symptoms in burn survivors.
We followed the PRISMA statement to evaluate the articles we found and reported the results.
3. Results
31 studies were found; only three matched our inclusion criteria (see flowchart) [12–14].
Two studies have investigated the effects of tDCS on pain when applied over the motor cortex (M1). A first pilot study including 4 patients with burn injury and chronic pruritus showed that a single session of active anodal tDCS over the primary motor cortex induced a decrease in cortical excitability (i.e., decreased alpha activity in the occipital area and low beta activity in the frontal area) [12]. Another study using similar parameters (anodal tDCS over M1) evaluated the effects of 10 sessions of tDCS on pain and itch levels in 31 burn survivors [14] but active tDCS did not influence pain or itch levels. Finally, one randomized control trial on 60 patients tested the effects of a single session of cathodal (or sham) tDCS over the sensory cortex aiming to reduce self-reported pain and anxiety [13]. Pain and anxiety scores were significantly lower in the active tDCS group compared with the sham group.
3.1. Burn symptom 1: pain
Pain management after burn injury is critical since it may have an important impact on burn recovery and quality of life [15,16]. Almost half of burn survivors still present with pain years after the incident [17]. Further, two-thirds of survivors report that pain interferes with their rehabilitation, and about half report that pain interferes with their daily lives [17], thus underscoring the significant impact of pain on patients’ quality of life. Pain is therefore a critical element to consider at the acute, rehabilitative, and chronic phases of burn recovery. In the acute stage, following burn injury, some nerve endings are undamaged, resulting in the experience of significant pain at the site of the injury. Conversely, if the nerve ending is entirely destroyed, excised or significantly damaged, the injured area is insensate, and does not experience pain. Burn survivors may also suffer from neuropathic-like pain, which can become chronic [18]. Chronic pain conditions are characterized by several maladaptive neural changes, such as central sensitization [19].
Acute pain perception begins with activation of peripheral nociceptors and via the spinothalamic tract that reaches the thalamus and the somatosensory cortex. In acute pain, processes are in place to prevent tissue damage. However, this pain may become chronic when maladaptive neural changes occur, including central sensitization [20]. The neural mechanisms of central sensitization in chronic neuropathic conditions are only partially understood [21,22]. Both acute and chronic pain processes are multidimensional, comprising of nociceptive, cognitive, emotional, and affective components, each of these relating to specific brain structures [23]. Cortical and subcortical brain areas have been investigated in previous studies and associated with a common formation called the pain matrix [23,24]. This large neural network consists of the primary and secondary somatosensory cortex, the prefrontal lobe, anterior cingulate cortex, amygdala, insula, and the thalamus. Besides the somatosensory areas activated during pain, neuroimaging studies have also demonstrated that the dorsolateral prefrontal cortex, thalamus and medial prefrontal cortex play a crucial role in pain modulation as well [25,26]. In addition, the anterior cingulate cortex and the insula, part of the limbic system, are also important for the emotional aspects of pain [27] (Fig. 1).
Fig. 1 –
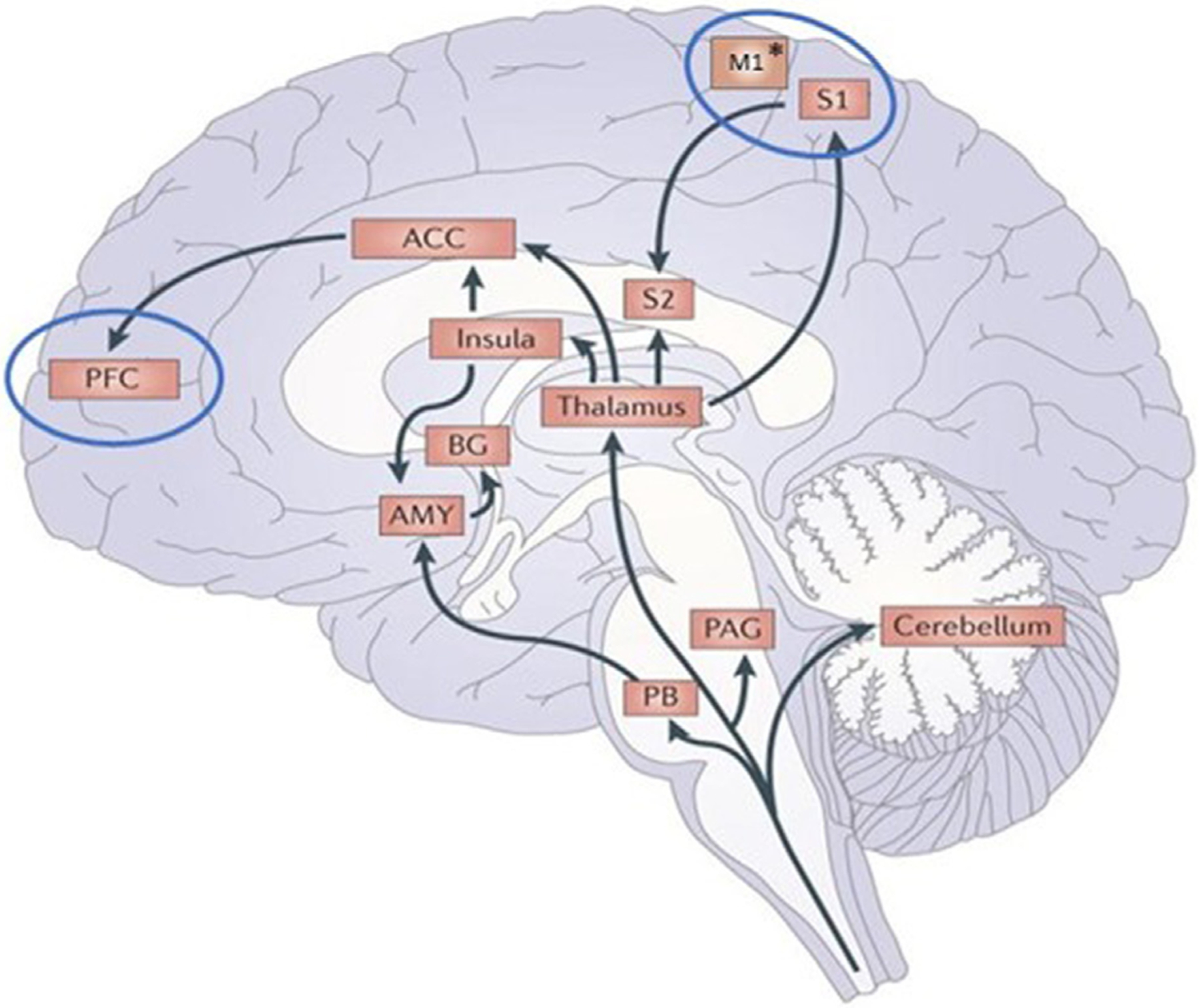
Afferent pain pathways. Nociception information from the spinal cord reach the thalamus, parabrachial nucleus (PB), and the peridaqueducal grey nucleus (PAG). From the thalamus, nociception information is projected to the insula, anterior cingulate cortex (ACC), primary somatosensory cortex (S1), and secondary somatosensory cortex (S2). From the ACC, the prefrontal cortex (PFC) will also be activated. Whereas from the PB, information will reach the amygdala (AMY) and is then projected to the basal ganglia (BG). Cortical targets to modulate pain perception via non-invasive brain stimulation are enclosed within the blue circles. M1 represent the primary motor cortex, preferably selected as a target. *the stimulated region overlaps M1 and S1. Adapted from [27].
tDCS applied over the sensorimotor cortex may help in normalizing pain processes and managing neuropathic pain symptoms. Many studies have demonstrated the analgesic effects of tDCS applied over the somatosensory cortex, as well as over the prefrontal cortex to reduce pain level in various conditions, such as fibromyalgia, osteoarthritis or chronic visceral pain (for a review see [28]). In healthy subjects, tDCS has been shown to modulate the pain threshold and conditioned pain modulation [29], a marker of the endogenous pain inhibitory system, which is altered in patients with neuropathic pain [30]. Given that burn injury may result in the development of chronic neuropathic pain due to central neural changes associated with sensory deafferentation, tDCS applied over the sensorimotor cortex may induce a decrease in maladaptive plasticity and therefore reduce pain in patients with burn injuries. The rationale for motor cortex stimulation is to enhance thalamocortical connectivity and thus compensate for some of the lost sensory afference that is observed with severe burn injury [31]. In fact, the goal is to enhance activity of primary motor cortex with anodal tDCS.
In the current literature of burn injury only two studies have investigated the effects of tDCS on pain when applied over the motor cortex (M1). A first pilot study including 4 patients with burn injury and chronic pruritus showed that a single session of active anodal tDCS over the primary motor cortex induced a decrease in cortical excitability (i.e., decreased alpha activity in the occipital area and low beta activity in the frontal area) [12]. Another study using similar parameters (anodal tDCS over M1) evaluated the effects of 10 sessions of tDCS on pain and itch levels in 31 patients [14]. However, in this study, active tDCS failed to reduce pain or itch intensities at the end of the 10 sessions and at follow-up. The main reason is likely because the subjects in such study had more pruritus than pain [14].
A third study tested the effect of tDCS on pain and anxiety during burn wound care in acute patients [13]. The authors found that a single cathodal tDCS session applied over the sensory cortex induced a significant reduction of about 10% of anxiety related to pain, compared to the sham group.
Given the limited literature on this topic, further studies are merited to investigate the potential therapeutic benefits of this modality on post-burn pain. We also suggest that such studies should include subjects with truly neuropathic pain as the main component of pain.
3.2. Burn symptom 2: pruritus
Pruritus is a commonly reported symptom among burn survivors affecting as many as 87% of survivors 3 months post-injury and still 67% at 24 months post-injury [32]. Factors found to be predictors of itch include deep dermal injury, post-traumatic stress symptoms, female gender, total burn surface area >40%, and injuries requiring >3 weeks to heal [32]. Further, post-burn pruritus has been demonstrated to significantly impact survivor quality of life, affecting sleep, activities of daily living, and psychosocial health [6]. As many as 94% of survivors with chronic pruritus in a cross-sectional study described it as unbearable and as many as 86% of survivors with acute itch described it as unbearable as well [6].
The primary mechanism of itch involves mediation of the A-delta and C-nociceptors [33] in the top layers of the skin [34] with activation of the primary somatosensory cortex, located in the post-central sulcus and the secondary somatosensory cortex, located in the upper lateral sulcus [35]. Once a pruritogenic agent instigates the itch mechanism, unmyelinated C fibers activate and relay the sensation of itch to the brain [33,36]. In addition, it is hypothesized that peripheral sensitization decreases the activation threshold and thus increases the activity of itch-related receptors and nerve fibers [36]. Central sensitization, however, occurs in the spinal cord and brain, and causes non-pruritic stimuli to be presented instead as itch, thus increasing and exacerbating the symptomology of itch [36]. Neuropathic itch, however, may result from increased peripheral firing or impaired central inhibition of neurons involved in the itch neural pathway [37] which may be related to a compensatory mechanism. Patients with chronic itch have been noted with developed changes in the excitability and organization in their brains [34].
Despite the frequency of reported pruritus among survivors, the modulation of this symptom with tDCS is not well investigated in literature thus far. It is hypothesized that tDCS can reduce symptoms of pruritus by modulating pathways of neuronal firing and affecting neural excitability [38,39]. The neural pathway of itch has been noted to be closely associated with that of pain, specifically with overlap in pain-processing networks [34]. Correspondingly, tDCS modulates pain processing pathways including the periacqueductal gray area, with evidence of itch relief [34,35]. However, differences in the two pathways remain. While closely related, chronic pain seemingly involves more extensive changes in neuroplasticity compared to chronic itch, thus resulting in decreased receptiveness to tDCS stimulation compared to itch [34]. In addition, brain areas involved in pruritus involve activation of the thalamus, somatosensory cortex, parietal cortex, motor areas (primary, supplementary, premotor cortex). However, in differentiation to pain, the secondary somatosensory cortex is not activated with itch symptoms [40].
In a study on healthy subjects and histamine induced itch sensation, bi-hemispheric tDCS has been more effective than uni-hemispheric tDCS for symptom alleviation [41]. In a case report by Knotkova et al. (2013), a patient with chronic pruritus was administered 20minutes of tDCS for 5 days with resultant reduction in pruritus for 3 months [34].
In the setting of burn injury related itch, a study noted that active tDCS over M1 was ineffective in treating itch reported by burn injury patients while the sham condition tDCS effectively decreased symptoms two weeks after stimulation [14]. tDCS increases the sensory threshold, as shown in healthy subjects [29] and may thus decrease the response to peripheral sensory afference of itch, leading to such effects [14]. In fact, we hypothesized in that study that tDCS for chronic pain would be different than tDCS for chronic pruritus. In the latter case, an inhibitory tDCS may be effective. Currently, no other studies have investigated the use of tDCS in alleviating post burn pruritus, or in other populations of patients. Therefore, given the lack of literature in regards to burn related itch and tDCS, further investigation is merited on the topic.
3.3. Burn symptom 3: psychosocial disorders
Psychosocial disorders encompass, among others, anxiety, depression and post-traumatic stress disorder (PTSD). Anxiety refers to feelings of excessive fear, worry, and unease caused by external or internal potential threats [42] lasting greater than 6 months [43]. Depression is characterized by low mood often in concordance with low self-esteem, loss of interest in normally enjoyable activities, and low energy for at least two weeks [44]. Finally, PTSD is defined as a mental disorder caused or triggered by exposure to either death, serious injury, or sexual violence [43]. Symptoms may include disturbing thoughts, feelings, or dreams related to the events, mental or physical distress to trauma-related cues, and attempts to avoid trauma-related cues [45].
Following a burn injury, rates of depression are high, reported up to 10–23% at 1 year post-injury [46]. Some factors, such as severity of pain, have been shown to be a predictor of suicidal ideation [47]. Therefore, pain management may help manage depression and reduction of suicidal ideation. Similarly, the prevalence of PTSD in burn survivors can be as high as 40% at 6 months and up to 45% at 12 months post-injury [2]. Main symptoms reported by survivors include sleep disturbances, recollections of the injury and avoidance of thoughts or feelings associated with the burn and distress when reminded of the burn.
Studies have reported high rates of PTSD in burn survivors ranging from 20% to 69% [48–50]. Main symptoms comprise of sleep disturbances, recollections of the injury and avoidance of thoughts or feelings associated with the burn and distress when reminded of the burn [51]. The high incidence and severity of PTSD have been associated with extensive post-burn scarring, female gender, large burn surface area, pre-traumatic depressive behaviors, low psychological resilience, and inadequate social support [49].
The prefrontal area, similar as for other psychosocial dysregulation pathologies, plays a critical role. Indeed, the neural correlates of these psychiatric disorders, neuroimaging and lesion studies have identified the medial prefrontal cortex (mPFC) as one of the main structures involved [52–54]. More specifically, PTSD is thought to be linked to a dysregulated neurocircuit that mainly involves the amygdala, prefrontal regions, and hippocampus. A recent meta-analysis of transcranial magnetic stimulation (TMS) on the mPFC in psychiatric disorders confirms the involvement of this key structure in the management of such symptoms [55].
tDCS use in management of depressive symptoms has been widely studied. Regarding the most efficient target, tDCS studies focusing on the prefrontal region have shown to reduce depression symptoms in multiple studies [56]. The most well-known trial is the ELECT non-inferiority trial published in 2017 [57] in which the anode and cathode were placed on the left and right dorsolateral prefrontal cortexes of participants for a total of 22 sessions. This study demonstrated the non-inferiority of tDCS as compared to escitalopram and that both escitalopram and tDCS were superior to placebo in reducing depression [57].
From a therapeutic perspective, it is hypothesized that tDCS treatment over the prefrontal areas may help management of executive control of fear responses and thus, reduce PTSD symptoms. In this context, tDCS applied over the prefrontal area has been investigated for various psychiatric disorders including reduction of PTSD symptoms with promising results in veterans with PTSD [58,59]. Besides PTSD, tDCS has also been demonstrated to reduce stress levels when applied over the prefrontal region (i.e., right medial-prefrontal cortex [60]). An increase in cerebral blood flow was observed in the right medial-prefrontal cortex and in the amygdala after 20 minutes of anodal tDCS. These results demonstrated that application of tDCS over the prefrontal cortex may reduce stress and PTSD symptoms in patients with burn injuries.
For depression and PTSD not related to a burn injury (e.g., in veterans), the prefrontal area may be the best region to target for treatment. No clinical trial, or open-label studies have tested the effects of prefrontal tDCS on these symptoms in patients with burn injuries. Following a protocol looking at the effect of M1-tDCS on pain and itch level, the injury’s psychosocial impact was also measured (see supplementary material). The data demonstrates that tDCS, when applied over M1 did not induce a significant improvement on impact of event, depression, anxiety, or sleep. It may be hypothesized that, if pain and/or itch levels would have been reduced following M1-tDCS [14], an impact on the associated psychiatric symptoms may have been observed via an indirect pathway. However, as neither improvement of pain nor itch was observed in this study, it may explain why no reduction of depression, PTSD or anxiety was found and targeting the prefrontal area may induce stronger effects as for other populations.
Indeed, tDCS applied over the prefrontal region has been shown to reduce depression [61] and PTSD symptoms [62] in patients suffering from these pathologies.
3.4. Burn symptom 4: sleep disturbance
Sleep disturbances encompass disorders of initiating and maintaining sleep, excessive somnolence, sleep–wake schedule perturbation, and any dysfunctions associated with sleep, sleep stages, or partial arousals [63]. Sleep disturbance can be due to traumatic experience, psychiatric disorders or neurological diseases. Though sleep affects a significant proportion of the population, little is known about the exact mechanisms of these symptoms given the heterogeneous nature of the disorders [64].
Among the burn survivor population, sleep disturbance may be related to pain, itch or behavioral health conditions. The proportion of patients with sleep disturbance following a burn injury is as high as 74%. Most frequently reported problems include nighttime awakenings, daytime napping, nighttime pain and difficulty with sleep onset [65]. Poor sleep quality has been shown to be associated with high levels of pain and analgesic intake during the day and a myriad of other symptoms [66].
Some recent studies have shown the positive effects of tDCS on sleep to promote vigilance and sleepiness [67]. Specifically, prefrontal tDCS applied during a wake period may improve the quality of subsequent sleep [68,69] in two different conditions, post-polio syndrome [68] and euthymic bipolarism [69]. tDCS may also improve sleep efficiency in patients with fibromyalgia [70]. Another study tested the effects of prefrontal stimulation applied during stage 2 sleep. In this study, tACS (transcranial alternating current stimulation) at 0.75Hz in insomniac patients was used. Prefrontal tACS may thus ameliorate sleep quality and decrease the number of wake times after sleep onset [71]. These behavioral findings were coupled with electrophysiological changes as an increase of stage 3 duration and a decrease of stage 1 duration were also observed.
Based on the current literature on non-invasive brain stimulation to manage sleep disturbance, the prefrontal area has been shown to be an effective target both when stimulated during wakefulness and during sleep. Unfortunately, there are no current studies in burn outcomes evaluating the effects of tDCS on sleep quality. As mentioned previously, M1-tDCS applied in patients with burn injuries did not lead to sleep quality improvement. As sleep disturbance is linked to pain and itch, it may also explain why M1-tDCS did not promote sleep. Therefore, targeting the prefrontal region may induce more promising effects.
3.5. Burn symptom 5: fatigue
Fatigue is a common symptom reported after burn injury, with many patient reports of persistence even at 24 months post-injury [72]. Symptoms of fatigue may contribute negatively to a survivor’s recovery from hindering their ability to fully participate in rehabilitation exercises to affecting injury healing [73]. Larger burn size was found to be associated with symptoms of fatigue [72]. Further, post-injury fatigue has been demonstrated to impact survivors’ post-injury quality of life as well as work-related disability [74]. Given the persistent implications of fatigue on quality of life, it is therefore imperative that future research targets strategies to alleviate this post-burn sequela.
Fatigue is oftentimes subjective and is defined clinically by an increased sense of effort in the initiation and maintenance of both physical and cognitive activities [75]. Fatigue may be further categorized as myopathic or subjective fatigue. Symptoms of myopathic fatigue are due to muscle weakness resulting from decreased muscle force output [75]. This type of fatigue is common among patients with myopathic disorders, neuromuscular junction disorders, and peripheral nerve disorders. Conversely, symptoms of subjective, or cognitive, fatigue are due to lesions in pathways implicated in arousal and attention, the basal ganglia, and the reticular and limbic systems [75]. This type of fatigue is more commonly noted in peripheral, autonomic, and central nervous system disorders [75].
Cognitive fatigue, differing from myopathic fatigue, is observed in most acute and chronic inflammatory diseases [76]. In chronic inflammatory disorders, fatigue has been found to be correlated with high observed levels of inflammatory cytokines such as IL-6, IL-1, and TNF [77]. It is hypothesized that the increased levels of these markers signal the central nervous system to subsequently respond and generate the feeling of fatigue. Similarly, in burn injury, inflammation markers of IL-6, TNF- α, and IL-1 β are also released, contributing to the stress response and thus likely to the symptoms of fatigue as well [78].
tDCS has been previously investigated for treatment of fatigue, specifically, in multiple sclerosis (MS). In the treatment of multiple sclerosis, tDCS modulates the postulated disrupted cortico-subcortical loop [79]. In addition, in the disease process of multiple sclerosis, neural cell axons become demyelinated due to a combination of inflammation, demyelination, and oxidative stress [80]. tDCS is also thought to improve the activation and migration of neural stem cells and therefore promote axonal regeneration [79,81]. By improving conduction though axonal regeneration of the demyelinated neural cell axons [80], tDCS may further alleviate symptoms of fatigue [79,80]. Given the implications of inflammation and fatigue, tDCS may work to modulate this post burn fatigue through similar mechanisms as with fatigue observed in MS. Further, while no studies have investigated the use of tDCS on post-burn fatigue, previous studies have noted the use of tDCS in subjective cognitive fatigue. tDCS has been demonstrated to have a positive effect on patients with mild-moderate cognitive fatigue compared to patients with severe cognitive fatigue with parietal stimulation [82]. In addition, tDCS stimulation, applied over the parietal and frontal area, was able to improve fatigue-related reaction time while performing cognitive tasks [82,83].
Currently, there has not been literature demonstrating the use of tDCS in treating symptoms of fatigue after burn injury. However, given the efficacy of tDCS in improving multiple sclerosis related fatigue, tDCS applied in the parietal and frontal area may be a likely target for future investigation with promising results. Given the safety profile and ease of implementation, tDCS may be a potential favorable treatment option to alleviate fatigue [38,84], especially given pharmacologic limitations in treatment that patients may encounter [79].
3.6. Burn symptom 6: impaired motor strength
Muscular weakness is commonly reported after burn injury whether due to muscle wasting or due to the increased catabolism of skeletal muscle, with resultant loss of body mass, experienced after injury [85,86]. Critical illness polyneuropathy, a diffuse neuropathy that may occur with severe burn injuries, may be another source of weakness, causing extremity flaccid weakness due to axonal damage of the motor neurons [87]. The degeneration of sensory and motor neuron nerves leads to resultant skeletal muscle degeneration [88] and subsequent symptoms of motor weakness. Among the burn population, this type of weakness occurs anywhere between 2% and 29% of survivors [89,90]. Predictors of impaired motor strength, or muscular weakness, include initial myostatin serum concentration levels and greater total burn surface area [91]. In order to generate fine distal movements, activation of the primary motor cortex is required [92]. When one side of the motor cortex becomes impaired, increased transcallosal inhibition from the unaffected motor cortex to the affected cortex disrupts the primary motor cortex. The resultant decrease in cortical excitability thereby impairs muscle strength [92–94].
tDCS has been shown to be efficacious in improving motor function and strength in variable disorders through activation of motor areas and enhancement of action potentials for movement execution when applied over M1 [95]. Anodal tDCS increases motor learning by decreasing GABAergic activity in the motor cortex and subsequently increasing functional connectivity of the motor network [96]. Various studies have demonstrated positive effects of tDCS stimulation on movement and strength improvement. In a study by Kim et al, anodal tDCS applied to the affected hemisphere of patients with subacute stroke was found to improve function of the affected hand [97]. Similarly, in a study of children with spastic hemiparetic cerebral palsy, anodal M1 tDCS was noted to improve upper limb movements of these patients as quantified through decreased total movement duration time, decreased returning movement time, and overall reduced movement execution time [95]. Further, M1 tDCS may also improve the maximum force of knee extension in patients with chronic subcortical stroke [98], an effect suggested to be resultant of tDCS induced corticospinal excitability over the lower limb primary motor cortex [39,99,100]. Finally, several studies have shown that motor cortex stimulation does enhance exercise performance, including muscle strength and also physical endurance [101–104].
Though other therapies such as strength training [86] have been demonstrated to be efficacious improving muscular strength post burn injury, the role of tDCS in affecting this symptom has not been investigated. However, given the positive outcomes of tDCS applied over the motor cortex observed in other disorders, future studies may be focused on this area of stimulation.
4. Discussion
There is evidence in other fields that suggest that tES may be a successful treatment for common post burn symptomology. Although there is a lack of data on the effects of tES in the burn population, given the frequency of chronic symptoms there is a need for novel interventions. This paper reviews prior literature to develop an anatomical map of potential areas of brain stimulation to treat burn symptoms. Many burn symptoms share well described neurophysiology seen in other populations and literature examining the utility of tES treatments.
Based on these observations we can draw three main conclusions: (1) The prefrontal cortex may be a better target than the motor area to reduce both pain and psychosocial symptoms related symptoms. (2) tES applied over the parietal cortex may help with fatigue and improve vigilance, though data is limited even in other conditions. (3) tES applied over M1 could be useful to help with muscle weakness in the subpopulation of patients with burn suffering from motor disorders.
tES applied over the prefrontal area has been shown to improve cognitive symptoms, such as attention and memory, in various pathologies [e.g., 105,106], as well as to help with psychosocial symptoms of PTSD or anxiety and sleep disturbance [e.g., 107,108]. In addition, the prefrontal region may also play a role in the emotional reaction to pain. In the past decade, many neuroimaging and neurophysiological studies have demonstrated the critical role of the DLPFC not only in pain processes [109] but also in fatigue [79], depression [110], or attention [111], including the attentional circuit dedicated to noxious stimuli [112]. Therefore, modulating the prefrontal cortex via non-invasive brain stimulation could also help manage acute and neuropathic pain in patients with burn injury. Similar to management of fibromyalgia or neuropathic pain in patients with multiple sclerosis [113], tES over the prefrontal area did show promising analgesic effects. Similar montage should also be tested in patients with burn injury. This approach appears more promising as this population of patients often suffers from depression and fatigue, symptoms involved by the prefrontal area.
tES applied over the parietal cortex may be efficacious in reducing fatigue, as previously investigated, such as in multiple sclerosis. In addition, the parietal cortex is also involved in itch regulation as demonstrated by neuroimaging studies. tES effects on fatigue may be linked to an improvement of vigilance level, as neuroanatomically, vigilance depends on a network involving the brainstem, the thalamus, and the frontal and parietal cortices [114].
tES applied over the motor cortex may still be useful to help rehabilitation, especially when patients remain bedridden for an extended period of time and suffer from ensuing muscle weakness. Patients with extensive burn injuries often stay in intensive care units before beginning rehabilitation therapy. During this time, muscle atrophy can occur, consequently slowing down functional improvement. In this scenario, applying tES over the motor cortex during rehabilitation may speed up the functional recovery. However, based on our previous data, M1 tES, especially using tDCS, does not seem to induce an analgesic effect or to reduce itch sensation in patients with burn injury [14]. It does not seem to have an effect on psychosocial symptoms, while stimulating the prefrontal cortex seems to have a more straightforward approach to improve such factors (Fig. 2).
Fig. 2 –
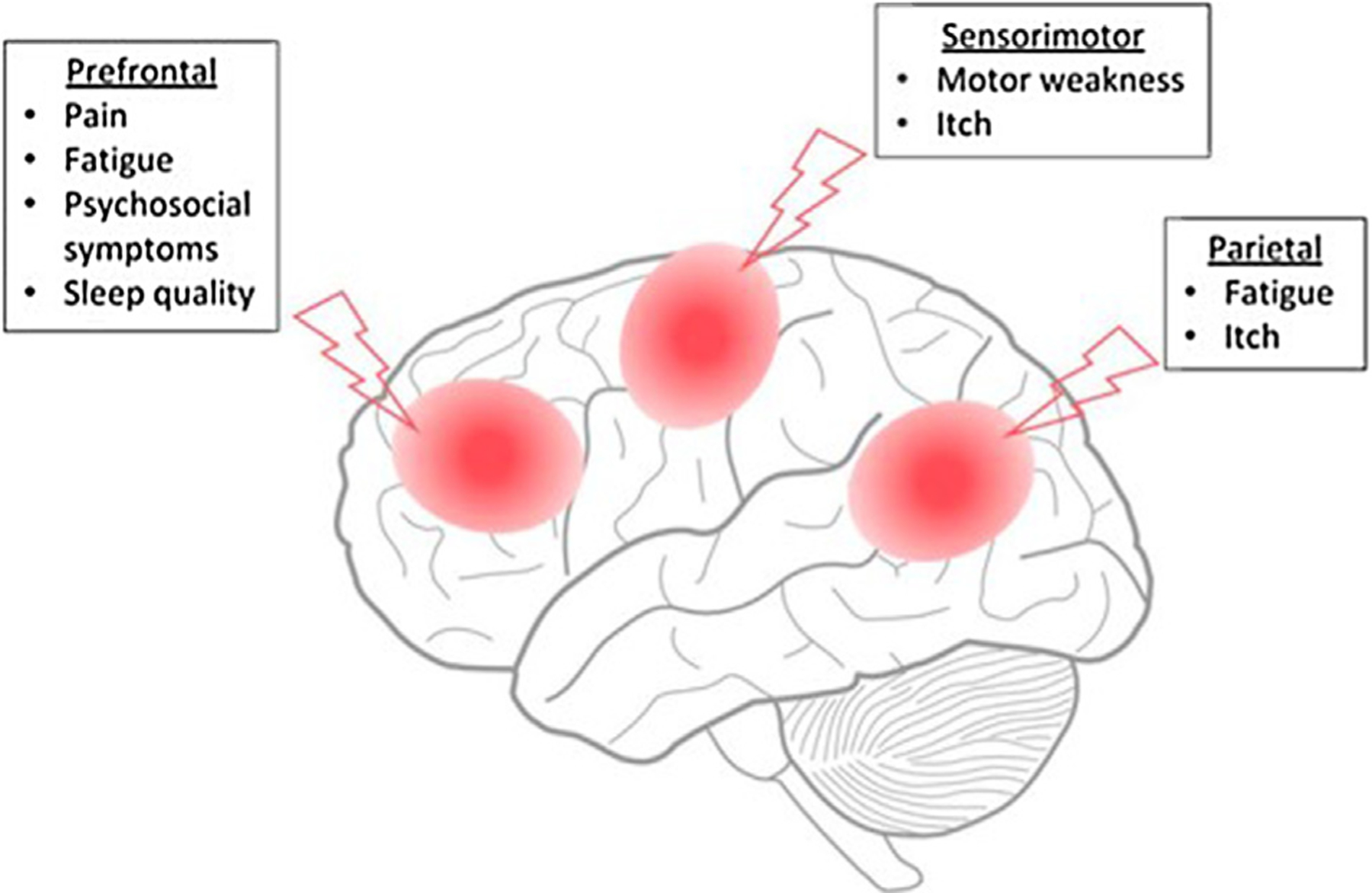
Main suggested cortical regions (red circles) for target treatment. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Techniques such as tDCS, tACS or other NIBS tools have several advantages as well as some limitations. In our opinion, especially in the burn population, one of the main advantages is the non-pharmacological nature of tES. While opioids remain the cornerstone of pain management in the burn population [115]; given the risk of addiction, alternatives to pharmacological treatment are necessitated. tES represents a safe, inexpensive, and well tolerated modality as compared to other treatment strategies [116]. Mechanistically, NIBS techniques have the potential to modulate specific brain areas depending on the underlying symptoms to treat, via long-term potential and depression-like plasticity mechanisms [117–119]. However, currentlythistechniquetypicallyinvolvesadministrationby trained staff under clinical supervision, requiring the administration of tES to take place in research facilities or hospitals. In addition, dosing is an important parameter as the duration of the effects is thought to be linked to the duration of tES application. Repeated sessions may have cumulative effects leading to long-lasting clinically relevant effects, as shown in previous studies evaluating the effects of tDCS in psychiatric [120], motor [121], and pain conditions [122]. Recently, some studies have utilized home-based tDCS devices with promising results and, most importantly, with no side-effects [123–125]. Thus, home-based supervised sessions, as recommended by Charvetandcolleagues[126], couldbeanalternativetofacilitate tDCS and tACS implementation and reduce attrition rates as often observed in clinical trials of long duration [127], as well as to promote long-lasting tES-related effects. Besides the number of sessions, the intensity of stimulation is another parameter to take into account. Recent evidence has shown that 4mA tES is safe and can potentially induce stronger neurophysiological effects, and thus stronger behavioral effects too [128,129]. However, a direct link between clinical effects and higher intensities of stimulation still needs to be proven.
5. Conclusion
Non-invasive brain stimulation (NIBS) techniques represent a valuable alternative to pharmacologic interventions in the management of chronic neuropathic pain, psychiatric morbidities and sleep disturbance. However, current literature on NIBS in burn for treating these and other associated symptoms remains limited and future trials are merited to investigate the efficacy of this approach. Specifically, home-based supervised devices may be utilized in order to limit attrition rates as observed in previous trials [127]. Our review of the neural circuitry involved in post burn symptoms and suggested targeted areas for stimulation provides a spring board for future study initiatives. Given the significant impact of these symptoms on survivors’ lives, definition of these target areas allow for focused studies of treatment of these symptoms and eventual improved quality of life. Based on the importance of the prefrontal cortex in the emotional component of pain and its implication in various psychosocial symptoms, this region may represent the most promising treatment target.
Supplementary Material
Acknowledgments
Dr. Aurore Thibaut is a FNRS is a post-doctoral research fellow. The contents of this manuscript were developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research, NIDILRR grant numbers 90DP0035, 90DPBU0001, and 90DP0055. NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this manuscript do not necessarily represent the policy of NIDILRR, ACL, HHS, and you should not assume endorsement by the Federal Government.
Funding
The contents of this manuscript were developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant number 90DP0035). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this manuscript do not necessarily represent the policy of NIDILRR, ACL, HHS, and you should not assume endorsement by the Federal Government.
Abbreviations:
- M1
primary motor cortex
- NIBS
non-invasive brain stimulation
- PTSD
post-traumatic stress disorder
- tDCS
transcranial direct current stimulation
- tACS
transcranial alternating current stimulation
Footnotes
Conflict of interest
The authors report no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.burns.2020.06.005.
REFERENCES
- [1].Schneider JC, Spires MC. Burn rehabilitation. In: Frontera WR, editor. DeLisa’s phys med rehabil 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. p. 1125–50. [Google Scholar]
- [2].Corry N, Klick B, Fauerbach J. Posttraumatic stress disorder and pain impact functioning and disability after major burn injury. J Burn Care Res 2010. [DOI] [PubMed] [Google Scholar]
- [3].Faber AW, Klasen HJ, Sauer EW, Vuister FM. Psychological and social problems in burn patients after discharge. Scand J Plast Reconstr Surg Hand Surg 1987, doi: 10.3109/02844318709086468. [DOI] [PubMed] [Google Scholar]
- [4].Blakeney PE, Rosenberg L, Rosenberg M, Faber AW. Psychosocial care of persons with severe burns. Burns 2008, doi: 10.1016/j.burns.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [5].Ohrtman EA, Shapiro GD, Simko LC, Dore E, Slavin MD, Saret C, et al. Social interactions and social activities after burn injury: a life impact burn recovery evaluation (LIBRE) study. J. Burn Care Res 2018, doi: 10.1093/jbcr/iry038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Parnell LKS, Nedelec B, Rachelska G, Lasalle L. Assessment of pruritus characteristics and impact on burn survivors. J Burn Care Res 2012, doi: 10.1097/BCR.0b013e318239d206. [DOI] [PubMed] [Google Scholar]
- [7].Bell L, McAdams T, Morgan R, Parshley P, Pike R, Riggs P, et al. Pruritus in burns: a descriptive study. J Burn Care Rehabil 1988;9:305–8. [PubMed] [Google Scholar]
- [8].Malenfant A, Forget R, Papillon J, Amsel R, Frigon JY, Choinière M. Prevalence and characteristics of chronic sensory problems in burn patients. Pain 1996, doi: 10.1016/0304-3959(96)03154-5. [DOI] [PubMed] [Google Scholar]
- [9].Bikson M, Esmaeilpour Z, Adair D, Kronberg G, Tyler WJ, Antal A, et al. Transcranial electrical stimulation nomenclature. Brain Stimul 2019, doi: 10.1016/j.brs.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Paulus W. Transcranial direct current stimulation (tDCS). Suppl Clin Neurophysiol 2003;56:249–54. [DOI] [PubMed] [Google Scholar]
- [11].Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul 2008, doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [12].Portilla A, Bravo G, Miraval F, Villamar M, Schneider J, Ryan C, et al. A feasibility study assessing cortical plasticity in chronic neuropathic pain following burn injury. J Burn Care Res 2013;34:e48–52, doi: 10.1097/BCR.0b013e3182700675. [DOI] [PubMed] [Google Scholar]
- [13].Hosseini Amiri M, Tavousi SH, Mazlom SR, Manzari ZS. Effect of transcranial direct current stimulation on pain anxiety during burn wound care. Burns 2016;42:872–6, doi: 10.1016/j.burns.2016.01.006. [DOI] [PubMed] [Google Scholar]
- [14].Thibaut A, Ohrtman EA, Morales-Quezada L, Simko LC, Ryan CM, Zafonte R, et al. Distinct behavioral response of primary motor cortex stimulation in itch and pain after burn injury. Neurosci Lett 2019;690:89–94, doi: 10.1016/j.neulet.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ryan CM, Lee A, Kazis LE, Schneider JC, Shapiro GD, Sheridan RL, et al. Recovery trajectories after burn injury in young adults: does burn size matter? J Burn Care Res 2015;36:118–29, doi: 10.1097/BCR.0000000000000214. [DOI] [PubMed] [Google Scholar]
- [16].Gauffin E, Öster C, Sjöberg F, Gerdin B, Ekselius L. Health-related quality of life (EQ-5D) early after injury predicts long-term pain after burn. Burns 2016, doi: 10.1016/j.burns.2016.05.016. [DOI] [PubMed] [Google Scholar]
- [17].Dauber A, Osgood PF, Breslau AJ, Vernon HL, Carr DB. Chronic persistent pain after severe burns: a survey of 358 burn survivors. Pain Med 2002;3:6–17, doi: 10.1046/j.1526-4637.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- [18].Schneider JC, Harris NL, Shami A, El Sheridan RL, Schulz JT, Bilodeau ML, et al. A descriptive review of neuropathic-like pain after burn injury. J Burn Care Res 2006;27:524–8, doi: 10.1097/01.BCR.0000226019.76946.5D. [DOI] [PubMed] [Google Scholar]
- [19].Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol 2009;194:31–74, doi: 10.1007/978-3-540-79090-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 2017. [DOI] [PubMed] [Google Scholar]
- [21].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926, doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Woolf CJ. Central Sensitization: implications for the diagnosis and treatment of pain. Pain 2012;152:1–31, doi: 10.1016/j.pain.2010.09.030.Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maeoka H, Matsuo A, Hiyamizu M, Morioka S, Ando H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci Lett 2012;512:12–6, doi: 10.1016/j.neulet.2012.01.037. [DOI] [PubMed] [Google Scholar]
- [24].Rêgo GG, Lapenta OM, Marques LM, Costa TL, Leite J, Carvalho S, et al. Hemispheric dorsolateral prefrontal cortex lateralization in the regulation of empathy for pain. Neurosci Lett 2015;594:12–6, doi: 10.1016/j.neulet.2015.03.042. [DOI] [PubMed] [Google Scholar]
- [25].Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003, doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- [26].Boggio PS, Zaghi S, Fregni F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS). Neuropsychologia 2009;47:212–7, doi: 10.1016/j.neuropsychologia.2008.07.022. [DOI] [PubMed] [Google Scholar]
- [27].Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013, doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lefaucheur J. Cortical neurostimulation for neuropathic pain: state of the art and perspectives. Pain 2016;157 Suppl:S81–9, doi: 10.1097/j.pain.0000000000000401. [DOI] [PubMed] [Google Scholar]
- [29].Reidler JS, Mendonca ME, Santana MB, Wang X, Lenkinski R, Motta AF, et al. Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. J Pain 2012;13:450–8, doi: 10.1016/j.jpain.2012.01.005. [DOI] [PubMed] [Google Scholar]
- [30].Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC. Reliability of conditioned pain modulation: a systematic review. Pain 2016, doi: 10.1097/j.pain.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Castillo Saavedra L, Mendonca M, Fregni F. Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Med Hypotheses 2014;83:332–6, doi: 10.1016/j.mehy.2014.06.007. [DOI] [PubMed] [Google Scholar]
- [32].Van Loey NEE, Bremer M, Faber AW, Middelkoop E, Nieuwenhuis MK. Itching following burns: epidemiology and predictors. Br J Dermatol 2008;158:95–100, 10.1111/j.1365-2133.2007.08278.x. [DOI] [PubMed] [Google Scholar]
- [33].Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci 1997;17:8003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Knotkova H, Portenoy RK, Cruciani RA. Transcranial direct current stimulation (tDCS) relieved itching in a patient with chronic neuropathic pain. Clin J Pain 2013;29:621–2, doi: 10.1097/AJP.0b013e318261329. [DOI] [PubMed] [Google Scholar]
- [35].Mochizuki H, Schut C, Nattkemper LA, Yosipovitch G. Brain mechanism of itch in atopic dermatitis and its possible alteration through non-invasive treatments. Allergol Int 2017;66:14–21, doi: 10.1016/j.alit.2016.08.013. [DOI] [PubMed] [Google Scholar]
- [36].Dhand A, Aminoff MJ. The neurology of itch. Brain 2014;137:313–22, doi: 10.1093/brain/awt158. [DOI] [PubMed] [Google Scholar]
- [37].Steinhoff M, Schmelz M, Szabó IL, Oaklander AL. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol 2018;17:709–20, doi: 10.1016/S1474-4422(18)30217-5. [DOI] [PubMed] [Google Scholar]
- [38].Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul 2012;5:175–95, doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527 Pt(3):633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Papoiu ADP, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage 2012;59:3611–23, doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nakagawa K, Mochizuki H, Koyama S, Tanaka S, Sadato N, Kakigi R. A transcranial direct current stimulation over the sensorimotor cortex modulates the itch sensation induced by histamine. Clin Neurophysiol 2016;127:827–32, doi: 10.1016/j.clinph.2015.07.003. [DOI] [PubMed] [Google Scholar]
- [42].Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 2013, doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mittal VA, Walker EF. Diagnostic and statistical manual of mental disorders. Psychiatry Res 2011, doi: 10.1016/j.psychres.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].NIMH. Depression 2016. [Google Scholar]
- [45].American Psychiatric Association. Diagnostic and statistical manual of mental disorder fouth edition (DSM-IV), doi: 10.1073/pnas.0703993104. [DOI]
- [46].Tedstone JE, Tarrier N, Faragher EB. An investigation of the factors associated with an increased risk of psychological morbidity in burn injured patients. Burns 1998, doi: 10.1016/S0305-4179(98)00049-7. [DOI] [PubMed] [Google Scholar]
- [47].Edwards RR, Magyar-Russell G, Thombs B, Smith MT, Holavanahalli RK, Patterson DR, et al. Acute pain at discharge from hospitalization is a prospective predictor of long-term suicidal ideation after burn injury. Arch Phys Med Rehabil 2007, doi: 10.1016/j.apmr.2007.05.031. [DOI] [PubMed] [Google Scholar]
- [48].Macleod R, Shepherd L, Thompson AR. Posttraumatic stress symptomatology and appearance distress following burn injury: an interpretative phenomenological analysis. Heal Psychol 2016, doi: 10.1037/hea0000391. [DOI] [PubMed] [Google Scholar]
- [49].Waqas A, Raza N, Zahid T, Rehman A, Hamid T, Hanif A, et al. Predictors of post-traumatic stress disorder among burn patients in Pakistan: the role of reconstructive surgery in post-burn psychosocial adjustment. Burns 2018, doi: 10.1016/j.burns.2017.09.012. [DOI] [PubMed] [Google Scholar]
- [50].Ashfaq A, Lashari UG, Saleem S, Naveed S, Tariq H, Waqas A. Exploring symptoms of post-traumatic stress disorders and perceived social support among patients with burn injury. Cureus 2018, doi: 10.7759/cureus.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yabanoglu H, Yagmurdur MC, Taskintuna N, Karakayali H. Early period psychiatric disorders following burn trauma and the importance of surgical factors in the etiology. Turkish J Trauma Emerg Surg 2012, doi: 10.5505/tjtes.2012.98511. [DOI] [PubMed] [Google Scholar]
- [52].Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA 2010, doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Poletti S, Radaelli D, Cucchi M, Ricci L, Vai B, Smeraldi E, et al. Neural correlates of anxiety sensitivity in panic disorder: a functional magnetic resonance imaging study. Psychiatry Res Neuroimaging 2015, doi: 10.1016/j.pscychresns.2015.05.013. [DOI] [PubMed] [Google Scholar]
- [54].Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry 2005, doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- [55].Vieira L, Marques D, Cantilino A. Transcranial magnetic stimulation of the medial prefrontal cortex for psychiatric disorders: a systematic review. Braz J Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shiozawa P, Fregni F, Benseñor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol 2014;17:1443–52, doi: 10.1017/S1461145714000418. [DOI] [PubMed] [Google Scholar]
- [57].Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, Fernandes RA, et al. Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med 2017, doi: 10.1056/nejmoa1612999. [DOI] [PubMed] [Google Scholar]
- [58].van’t Wout M, Longo SM, Reddy MK, Philip NS, Bowker MT, Greenberg BD. Transcranial direct current stimulation may modulate extinction memory in posttraumatic stress disorder. Brain Behav 2017, doi: 10.1002/brb3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Saunders N, Downham R, Turman B, Kropotov J, Clark R, Yumash R, et al. Working memory training with tDCS improves behavioral and neurophysiological symptoms in pilot group with post-traumatic stress disorder (PTSD) and with poor working memory. Neurocase 2015, doi: 10.1080/13554794.2014.890727. [DOI] [PubMed] [Google Scholar]
- [60].Antal A, Fischer T, Saiote C, Miller R, Chaieb L, Wang DJJ, et al. Transcranial electrical stimulation modifies the neuronal response to psychosocial stress exposure. Hum Brain Mapp 2014, doi: 10.1002/hbm.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Palm U, Hasan A, Strube W, Padberg F. tDCS for the treatment of depression: a comprehensive review. Eur Arch Psychiatry Clin Neurosci 2016;1–14, doi: 10.1007/s00406-016-0674-9. [DOI] [PubMed] [Google Scholar]
- [62].D’Urso G, Mantovani A, Patti S, Toscano E, De Bartolomeis A. Transcranial direct current stimulation in obsessive–compulsive disorder, posttraumatic stress disorder, and anxiety disorders. J ECT 2018, doi: 10.1097/YCT.0000000000000538. [DOI] [PubMed] [Google Scholar]
- [63].Kruse JA. Clinical methods: the history, physical, and laboratory examinations. J Am Med Assoc 2011, doi: 10.1001/jama.1990.03450210108045. [DOI] [PubMed] [Google Scholar]
- [64].Lee AF, Ryan CM, Schneider JC, Kazis LE, Li NC, Rose M, et al. Quantifying risk factors for long-term sleep problems after burn injury in young adults. J Burn Care Res 2017, doi: 10.1097/BCR.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Boeve SA, Aaron LA, Martin-Herz SP, Peterson A, Cain V, Heimbach DM, et al. Sleep disturbance after burn injury. J Burn Care Rehabil 2002, doi: 10.1097/00004630-200201000-00007. [DOI] [PubMed] [Google Scholar]
- [66].Raymond I, Ancoli-Israel S, Choinière M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med 2004, doi: 10.1016/j.sleep.2004.07.007. [DOI] [PubMed] [Google Scholar]
- [67].Annarumma L, D’Atri A, Alfonsi V, De Gennaro L. The efficacy of transcranial current stimulation techniques to modulate resting-state EEG, to affect vigilance and to promote sleepiness. Brain Sci 2018, doi: 10.3390/brainsci8070137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Acler M, Bocci T, Valenti D, Turri M, Priori A, Bertolasi L. Transcranial Direct Current Stimulation (tDCS) for sleep disturbances and fatigue in patients with post-polio syndrome. Restor Neurol Neurosci 2013, doi: 10.3233/RNN-130321. [DOI] [PubMed] [Google Scholar]
- [69].Minichino A, Bersani FS, Bernabei L, Spagnoli F, Vergnani L, Corrado A, et al. Prefronto–cerebellar transcranial direct current stimulation improves visuospatial memory, executive functions, and neurological soft signs in patients with euthymic bipolar disorder. Neuropsychiatr Dis Treat 2015, doi: 10.2147/NDT.S79108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Roizenblatt S, Fregni F, Gimenez R, Wetzel T, Rigonatti SP, Tufik S, et al. Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham-controlled study. Pain Pr 2007;7:297–306, doi: 10.1111/j.1533-2500.2007.00152.x. [DOI] [PubMed] [Google Scholar]
- [71].Saebipour MR, Joghataei MT, Yoonessi A, Sadeghniiat-Haghighi K, Khalighinejad N, Khademi S. Slow oscillating transcranial direct current stimulation during sleep has a sleep-stabilizing effect in chronic insomnia: a pilot study. J Sleep Res 2015, doi: 10.1111/jsr.12301. [DOI] [PubMed] [Google Scholar]
- [72].Simko LC, Espinoza LF, McMullen K, Herndon DN, Suman O, Fauerbach JA, et al. Fatigue following burn injury: a burn model system National Database Study. J Burn Care Res 2018;39:450–6, doi: 10.1097/BCR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Toh C, Li M, Finlay V, Jackson T, Burrows S, Wood FM, et al. The Brief Fatigue Inventory is reliable and valid for the burn patient cohort. Burns 2015, doi: 10.1016/j.burns.2014.11.014. [DOI] [PubMed] [Google Scholar]
- [74].Gabbe BJ, Cleland H, Watterson D, Schrale R, McRae S, Taggart S, et al. Predictors of moderate to severe fatigue 12 months following admission to hospital for burn: results from the Burns Registry of Australia and New Zealand (BRANZ) Long Term Outcomes project. Burns 2016, doi: 10.1016/j.burns.2016.08.036. [DOI] [PubMed] [Google Scholar]
- [75].Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet (London, England) 2004;363:978–88, doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- [76].Louati K, Berenbaum F. Fatigue in chronic inflammation – a link to pain pathways. Arthritis Res Ther 2015, doi: 10.1186/s13075-015-0784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bower JE. Cancer-related fatigue – mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014, doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Auger C, Samadi O, Jeschke MG. The biochemical alterations underlying post-burn hypermetabolism. Biochim Biophys Acta Mol Basis Dis 2017, doi: 10.1016/j.bbadis.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chalah MA, Riachi N, Ahdab R, Créange A, Lefaucheur J-P, Ayache SS. Fatigue in multiple sclerosis: neural correlates and the role of non-invasive brain stimulation. Front Cell Neurosci 2015;9:460, doi: 10.3389/fncel.2015.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lu C, Wei Y, Hu R, Wang Y, Li K, Li X. Transcranial direct current stimulation ameliorates behavioral deficits and reduces oxidative stress in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neuromodulation Technol Neural Interface 2015;18:442–7, doi: 10.1111/ner.12302. [DOI] [PubMed] [Google Scholar]
- [81].Keuters MH, Aswendt M, Tennstaedt A, Wiedermann D, Pikhovych A, Rotthues S, et al. Transcranial direct current stimulation promotes the mobility of engrafted NSCs in the rat brain. NMR Biomed 2015;28:231–9, doi: 10.1002/nbm.3244. [DOI] [PubMed] [Google Scholar]
- [82].Hanken K, Bosse M, Möhrke K, Eling P, Kastrup A, Antal A, et al. Counteracting fatigue in multiple sclerosis with right parietal anodal transcranial direct current stimulation. Front Neurol 2016;7:154, doi: 10.3389/fneur.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fiene M, Rufener KS, Kuehne M, Matzke M, Heinze H-J, Zaehle T. Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. J Neurol 2018;265:607–17, doi: 10.1007/s00415-018-8754-6. [DOI] [PubMed] [Google Scholar]
- [84].Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul 2008;1:206–23, doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- [85].Porter C, Tompkins RG, Finnerty CC, Sidossis LS, Suman OE, Herndon DN. The metabolic stress response to burn trauma: current understanding and therapies. Lancet (London, England) 2016;388:1417–26, doi: 10.1016/S0140-6736(16)31469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Grisbrook TL, Elliott CM, Edgar DW, Wallman KE, Wood FM, Reid SL. Burn-injured adults with long term functional impairments demonstrate the same response to resistance training as uninjured controls. Burns 2013, doi: 10.1016/j.burns.2012.09.005. [DOI] [PubMed] [Google Scholar]
- [87].Van Mook WNKa. Hulsewe-Evers RPMG. Critical illness polyneuropathy. Curr Opin Crit Care 2002, doi: 10.1055/s-0031-1275805. [DOI] [PubMed] [Google Scholar]
- [88].Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar A, et al. Critical illness polyneuropathy: Risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med 2001, doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- [89].Marquez S, Turley JJE, Peters WJ. Neuropathy in burn patients. Brain 1993, doi: 10.1093/brain/116.2.471. [DOI] [PubMed] [Google Scholar]
- [90].Helm PA, Ralph Johnson E, McIntosh Carlton A. Peripheral neurological problems in the acute burn patient. Burns 1977, doi: 10.1016/0305-4179(77)90022-5. [DOI] [Google Scholar]
- [91].Wallner C, Wagner JM, Dittfeld S, Drysch M, Lehnhardt M, Behr B. Myostatin serum concentration as an indicator for deviated muscle metabolism in severe burn injuries. Scand J Surg 2018, doi: 10.1177/1457496918812230. [DOI] [PubMed] [Google Scholar]
- [92].Rabadi MH, Aston CE. Effect of transcranial direct current stimulation on severely affected arm-hand motor function in patients after an acute ischemic stroke: a pilot randomized control trial. Am J Phys Med Rehabil 2017;96:S178–84, doi: 10.1097/PHM.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 2002;125:1896–907. [DOI] [PubMed] [Google Scholar]
- [94].Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 2004;55:400–9, doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- [95].Moura RCF, Santos C, Collange Grecco L, Albertini G, Cimolin V, Galli M, et al. Effects of a single session of transcranial direct current stimulation on upper limb movements in children with cerebral palsy: a randomized, sham-controlled study. Dev Neurorehabil 2017;20:368–75, doi: 10.1080/17518423.2017.1282050. [DOI] [PubMed] [Google Scholar]
- [96].Stagg CJ, Bachtiar V, Amadi U, Gudberg CA, Ilie AS, Sampaio-Baptista C, et al. Local GABA concentration is related to network-level resting functional connectivity. Elife 2014;3, doi: 10.7554/eLife.01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kim DY, Ohn SH, Yang EJ, Park C-I, Jung KJ. Enhancing motor performance by anodal transcranial direct current stimulation in subacute stroke patients. Am J Phys Med Rehabil 2009;88:829–36, doi: 10.1097/PHM.0b013e3181b811e3. [DOI] [PubMed] [Google Scholar]
- [98].Tanaka S, Takeda K, Otaka Y, Kita K, Osu R, Honda M, et al. Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabil Neural Repair 2011;25:565–9, doi: 10.1177/1545968311402091. [DOI] [PubMed] [Google Scholar]
- [99].Jeffery DT, Norton JA, Roy FD, Gorassini MA. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res 2007;182:281–7, doi: 10.1007/s00221-007-1093-y. [DOI] [PubMed] [Google Scholar]
- [100].Madhavan S, Stinear JW. Focal and bi-directional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul 2010;3:42, doi: 10.1016/j.brs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Vitor-Costa M, Okuno NM, Bortolotti H, Bertollo M, Boggio PS, Fregni F, et al. Improving cycling performance: transcranial direct current stimulation increases time to exhaustion in cycling. PLOS ONE 2015, doi: 10.1371/journal.pone.0144916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Cabral ME, Baltar A, Borba R, Galvão S, Santos L, Fregni F, et al. Transcranial direct current stimulation: before, during, or after motor training? Neuroreport 2015;26:618–22, doi: 10.1097/WNR.0000000000000397. [DOI] [PubMed] [Google Scholar]
- [103].Okano AH, Fontes EB, Montenegro RA, De Tarso Veras Farinatti P, Cyrino ES, Li LM, et al. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br J Sports Med 2015, doi: 10.1136/bjsports-2012-091658. [DOI] [PubMed] [Google Scholar]
- [104].Angius L, Pageaux B, Hopker J, Marcora SM, Mauger AR. Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neuroscience 2016, doi: 10.1016/j.neuroscience.2016.10.028. [DOI] [PubMed] [Google Scholar]
- [105].Rushby JA, De Blasio FM, Logan JA, Wearne T, Kornfeld E, Wilson EJ, et al. tDCS effects on task-related activation and working memory performance in traumatic brain injury: a within group randomized controlled trial. Neuropsychol Rehabil 2020, doi: 10.1080/09602011.2020.1733620. [DOI] [PubMed] [Google Scholar]
- [106].Sreeraj VS, Bose A, Chhabra H, Shivakumar V, Agarwal SM, Narayanaswamy JC, et al. Working memory performance with online-tDCS in schizophrenia: a randomized, double-blinded, sham-controlled, partial cross-over proof-of-concept study. Asian J Psychiatr 2020, doi: 10.1016/j.ajp.2020.101946. [DOI] [PubMed] [Google Scholar]
- [107].Frase L, Selhausen P, Krone L, Tsodor S, Jahn F, Feige B, et al. Differential effects of bifrontal tDCS on arousal and sleep duration in insomnia patients and healthy controls. Brain Stimul 2019, doi: 10.1016/j.brs.2019.01.001. [DOI] [PubMed] [Google Scholar]
- [108].Ironside M, Browning M, Ansari TL, Harvey CJ, Sekyi-Djan MN, Bishop SJ, et al. Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: a randomized clinical trial. JAMA Psychiatry 2019, doi: 10.1001/jamapsychiatry.2018.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079–91. [DOI] [PubMed] [Google Scholar]
- [110].Gobbi C, Rocca MA, Riccitelli G, Pagani E, Messina R, Preziosa P, et al. Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult Scler 2014, doi: 10.1177/1352458513493684. [DOI] [PubMed] [Google Scholar]
- [111].Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci 2012, doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Legrain V, Van Damme S, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain 2009, doi: 10.1016/j.pain.2009.03.020. [DOI] [PubMed] [Google Scholar]
- [113].Ayache SS, Palm U, Chalah MA, Al-Ani T, Brigno A, Abdellaoui M, et al. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci 2016;10:1–12, doi: 10.3389/fnins.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 2001;14:S76–84, doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- [115].James DL, Jowza M. Principles of burn pain management. Clin Plast Surg 2017, doi: 10.1016/j.cps.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [116].Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol 2017, doi: 10.1016/j.clinph.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul 2017, doi: 10.1016/j.brs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- [118].Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul 2016;10:51–8, doi: 10.1016/j.brs.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011;17:37–53, doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- [120].Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013;70:383–91, doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- [121].Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci 2007;25:123–9. [PubMed] [Google Scholar]
- [122].Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, Brandao R, Coutinho L, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain 2016;17:14–26, doi: 10.1016/j.jpain.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Martens G, Lejeune N, O’Brien AT, Fregni F, Martial C, Wannez S, et al. Randomized controlled trial of home-based 4-week tDCS in chronic minimally conscious state. Brain Stimul 2018, doi: 10.1016/j.brs.2018.04.021. [DOI] [PubMed] [Google Scholar]
- [124].Kasschau M, Reisner J, Sherman K, Bikson M, Datta A, Charvet LE. Transcranial direct current stimulation is feasible for remotely supervised home delivery in multiple sclerosis. Neuromodulation Technol Neural Interface 2016;19:824–31, doi: 10.1111/ner.12430. [DOI] [PubMed] [Google Scholar]
- [125].Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016, doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Charvet LE, Kasschau M, Datta A, Knotkova H, Michael C, Alonzo A, Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols 2015;9:1–13, doi: 10.3389/fnsys.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ohrtman EA, Zaninotto AL, Carvalho S, Shie VL, Leite J, Ianni CR, et al. Longitudinal clinical trial recruitment and retention challenges in the burn population: lessons learned from a trial examining a novel intervention for chronic neuropathic symptoms. J Burn Care Res 2019, doi: 10.1093/jbcr/irz084. [DOI] [PubMed] [Google Scholar]
- [128].Khadka N, Borges H, Paneri B, Kaufman T, Nassis E, Zannou AL, et al. Adaptive current tDCS up to 4 mA. Brain Stimul 2019;1–11, doi: 10.1016/j.brs.2019.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Borges H, Khadka N, Boateng A, Paneri B, Nassis E, Shin Y, et al. Tolerability of up to 4 mA tDCS using adaptive stimulation. Brain Stimul 2017, doi: 10.1016/j.brs.2017.04.044. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


