Abstract
Background
Gatipotuzumab is a humanized monoclonal antibody recognizing the carbohydrate-induced epitope of the tumor-associated mucin-1 (TA-MUC1). This study aimed to evaluate the efficacy and safety of switch maintenance therapy with gatipotuzumab in patients with TA-MUC1-positive recurrent ovarian, fallopian tube, or primary high-grade serous peritoneal cancer.
Patients and methods
In this double-blind, randomized, placebo-controlled, phase II trial, patients with at least stable disease (SD) following chemotherapy were randomized 2:1 to receive intravenous gatipotuzumab (500 mg followed by 1700 mg 1 week later) or placebo every 3 weeks until tumor progression or unacceptable toxicity occurred. Stratification factors were the number of prior chemotherapy lines (2 versus 3-5), response versus SD after the most recent chemotherapy, and progression-free survival (PFS) <6 versus 6-12 months following the prior therapy. Primary endpoint was PFS according to modified immune-related RECIST 1.1 response criteria. Secondary endpoints were PFS at 6 months, safety, overall response rate, CA-125 progression, overall survival, quality of life, and pharmacokinetics.
Results
Overall, 216 patients were randomized to gatipotuzumab (n = 151) or placebo (n = 65). Median PFS with gatipotuzumab was 3.5 months as compared with 3.5 months with placebo (hazard ratio 0.96, 95% confidence interval 0.69-1.33, P = 0.80). No advantage for gatipotuzumab over placebo was seen in the secondary efficacy endpoints or in any stratified subgroups. Gatipotuzumab was well tolerated, with mild to moderate infusion-related reactions being the most common adverse events.
Conclusions
Gatipotuzumab switch maintenance therapy does not improve outcome in TA-MUC1-positive ovarian cancer patients.
Trial registration
ClinicalTrials.govNCT01899599; https://clinicaltrials.gov/ct2/show/NCT01899599
Key words: gatipotuzumab, palliative care, TA-MUC 1, ovarian cancer, ADCC
Highlights
-
•
Evaluation of the efficacy and safety of a switch maintenance therapy with gatipotuzumab.
-
•
Patients with TA-MUC1-positive recurrent ovarian, fallopian tube, or primary high-grade serous peritoneal cancer evaluated.
-
•
Gatipotuzumab is a humanized monoclonal antibody recognizing the carbohydrate-induced tumor-associated epitope mucin-1.
-
•
Gatipotuzumab was well tolerated, with mild to moderate infusion-related reactions.
-
•
Gatipotuzumab switch maintenance therapy does not improve outcome in TA-MUC1-positive ovarian cancer patients.
Introduction
Relapse after standard chemotherapy occurs in ∼70% patients with ovarian, fallopian tube, or peritoneal cancer. A response to further chemotherapy commonly occurs, but the duration is relatively short and other strategies are needed to prolong progression-free survival (PFS).1 The approaches that have been studied include additional treatments during and after chemotherapy, or switch maintenance treatment after chemotherapy. The antiangiogenic drugs bevacizumab and cediranib have been used with chemotherapy and as maintenance and both have been shown to prolong PFS in recurrent ovarian cancer.2,3 Switch maintenance with drugs inhibiting epidermal growth factor receptor (EGFR), angiogenic tyrosine kinase inhibitors, or the Hedgehog pathway have either failed to produce a delay in PFS or have done so insufficiently to be made available for the clinic.4, 5, 6, 7 However, this strategy has been successful using poly (ADP-ribose) polymerase (PARP) inhibitors, and maintenance therapy with these drugs is now considered a standard treatment for recurrent platinum-sensitive ovarian cancers.8, 9, 10, 11 Studies examining monoclonal antibodies as maintenance therapy have so far been unsuccessful. A study with oregomovab, a monoclonal antibody targeting CA-125, was performed in the first-line setting, but showed no improvement in PFS.12 An alternative approach is to target the tumor-associated mucin-1 (TA-MUC1), a novel carbohydrate-induced conformational epitope overexpressed on the surface of tumor cells only.13 Gatipotuzumab (formerly known as PankoMab-GEX) is a glyco-engineered, humanized IgG1 monoclonal antibody which in contrast to other anti-MUC1 antibodies binds TA-MUC1 specifically and with high affinity.14 Tumor-specific uptake of gatipotuzumab has been shown in human cancer cell lines and studies in animal models suggest that activation of the immune system can occur to induce antibody-dependent cellular cytotoxicity (ADCC) against TA-MUC1-expressing tumor cells. This effect may be more pronounced in patients with specific polymorphisms of the Fcγ receptors (FcγR) of the effector immune cells. Phagocytosis, apoptosis induction, and proliferation inhibition may also contribute to the antitumor activity demonstrated by gatipotuzumab.
In a first-in-human study, patients with advanced solid tumors were treated with 3 weekly (q3w) intravenous doses of gatipotuzumab from 1 to 2200 mg or weekly doses (qw) from 300 to 700 mg.15 At all doses, gatipotuzumab was well tolerated and exhibited linear pharmacokinetics (PK) with a mean half-life of 189 ± 66 h and 108 ± 28 h during the q3w and qw regimen, respectively, regardless of the dose. The most common drug-related adverse events (AEs) were infusion-related reactions (IRRs) with an incidence of ∼50% during the first infusion. In the ovarian cancer subgroup, 1 patient had a complete response (CR) and 7/20 (35%) patients had stable disease (SD) in the intent-to-treat population. Based on the observed safety and PK profile, a q3w treatment schedule with 1700 mg gatipotuzumab was deemed most appropriate for further clinical testing of the drug in ovarian cancer. To mitigate the risk of IRRs, a starting dose of 500 mg gatipotuzumab was proposed to be given 1 week before starting the q3w cycles with 1700 mg. The purpose of the current phase II study was to evaluate the efficacy and safety of maintenance therapy with gatipotuzumab versus placebo after at least two lines of chemotherapy in patients with recurrent ovarian carcinoma.
Material and methods
Patients
Eligible patients had (i) recurrent epithelial primary ovarian, fallopian tube, or primary peritoneal cancer with high-grade serous histology, immunohistologically confirmed to be TA-MUC1-positive with an immuno-reactive score ≥3; (ii) received two to five lines of chemotherapy (excluding neoadjuvant lines) prior to the start of maintenance treatment; (iii) at least SD following their most recent line of chemotherapy (regardless of the prior response); (iv) progressed within the first 12 months after the chemotherapy line immediately before the study treatment; and (v) signed prior written informed consent. An experienced pathologist assessed TA-MUC1 staining based on the predominant staining intensity observed in the tumor tissue, ranging from no (category 0), over weak (category 1), and moderate (category 2) to strong (category 3) staining intensity. Randomization had to take place within the first 6 weeks since the last chemotherapy dose.
Study design
In this multicenter, double-blind, randomized, placebo-controlled, two-arm, phase II study, eligible patients were randomized at a 2:1 ratio to receive maintenance therapy with either intravenous gatipotuzumab or placebo. Central randomization was stratified by (i) the duration of PFS after the penultimate chemotherapy (≤6 versus >6 to ≤12 months); (ii) the type of response to the chemotherapy the patient had just received: CR, partial response (PR) versus SD; and (iii) the number of preceding chemotherapy lines (2 versus 3-5). Randomized patients received a starting dose of either gatipotuzumab (500 mg) or matching placebo on day 1 of the study, gatipotuzumab (1700 mg) or placebo on day 8, and then a maintenance dose q3w of either gatipotuzumab (1700 mg) or placebo until either disease progression according to modified immune-related RECIST (irRECIST) version 1.1 or unacceptable toxicity occurred. Tumors were assessed at screening, 8, 14, and 20 weeks after randomization, thereafter every 8 weeks during the first year, and every 12 weeks from the second year of study treatment. Patients who discontinued for reasons other than disease progression stayed on the regular assessment schedule until disease progression. When the study drug was discontinued, patients returned for a safety follow-up visit 28 (+3) days after the last infusion. After study termination, all patients who were still on gatipotuzumab treatment were offered to continue treatment in accordance with country regulations. National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0 were employed to grade the severity of AEs. The study was compliant with the Declaration of Helsinki, ICH regulations of Good Clinical Practice, and all applicable legislation in participating countries. It was registered on clinicaltrials.gov (NCT01899599).
Treatment
The gatipotuzumab/placebo regimen was diluted with saline to a total volume of 500 ml and administered intravenously over a 3-h period. Paracetamol and an H1/H2 antagonist were given prior to the first two infusions; steroids prior to the first infusion only. Thereafter, infusions were given without premedication unless an IRR was observed, in which case premedication without steroids was continued or resumed. In case of IRRs of grade ≤2, the duration of the infusion was to be extended or interrupted and restarted at a slower rate; in case of severe or life-threatening (grade 3 or 4) toxicity, the infusion was stopped and the study drug was discontinued. After the first and second infusion, the patients were monitored for signs of allergic or toxic reactions in an emergency care setting over 2 h or longer if an IRR required treatment.
Endpoints
The primary efficacy endpoint was PFS as assessed by the investigator using modified irRECIST version 1.1 criteria or death due to any cause. Tumor assessments were based on computed tomography scans or magnetic resonance imaging at scheduled visits and if there were signs of clinical progression, the same imaging method was used consistently for any individual patient. Secondary efficacy endpoints were (i) PFS according to blinded central review (based on both irRECIST and RECIST, version 1.1); (ii) PFS according to Gynecologic Cancer Intergroup (GCIG) criteria, based on central laboratory data; (iii) PFS rate at 6 months, as assessed by investigator and central review; (iv) objective response rate [i.e. best overall response (BOR) = CR or PR] and clinical benefit rate (CBR; i.e. BOR = CR, PR, or SD), as assessed by both investigator and central review; (v) overall survival (OS), that is, the time from randomization until death due to any cause; and (vi) quality of life (QoL) scores as assessed by European Oncology Research Trials Committee (EORTC) QoL questionnaires QLQ-C30 and QLQ-OV28 (according to availability of validated translations). In addition, the relationship of stratification factors to activity parameters and TA-MUC1 immunohistochemistry score in the tumor tissue samples, soluble TA-MUC1 serum levels, and FcγRIIa and FcγRIIIa status; stratification factors; CA-125 and human epididymis protein 4 (HE4), were explored using a Cox-proportional hazard model.
Safety endpoints were the incidence and severity of AEs and IRRs coded according to the Medical Dictionary for Regulatory Activities (MedDRA, Version 16.0) and analyzed by system organ class and preferred term. Further safety endpoints were the incidence of anti-drug antibodies, laboratory measurements, vital signs, electrocardiogram readings, and physical examinations.
For the determination of PK, gatipotuzumab serum concentrations were measured immediately before and at the end of each infusion from the first until the seventh infusion (day 1 of cycles 0 and 6, respectively) and at 28 days after the last infusion in all gatipotuzumab-treated patients. Additional samples were taken in a PK subgroup of 20 gatipotuzumab-treated patients at 5 h, 24 h, 7 days, and 14 days after the start of the second (cycle 1) and fifth infusion (cycle 4) to characterize both these infusions by noncompartmental analysis.
Statistical analysis
It was assumed that the control arm would have a median PFS of 4 months. The trial was designed to detect a prolongation to 6 months with gatipotuzumab and accounted for drop-outs occurring at exponential distribution with a drop-out rate of 10% after 24 months of observation. Thus a sample size of 210 patients, randomized at a 2:1 ratio (i.e. 140 to gatipotuzumab and 70 to placebo), was required and the analysis was planned after 180 PFS events, providing a power of 82% at an overall one-sided significance level of 0.05, considered to be adequate in an exploratory phase II setting.
The main results of efficacy (including PFS and OS) were estimated in the intent-to-treat population (i.e. all randomized patients with signed informed consent). The final analysis was performed 15 months after randomization of the last patient, at which point the study was terminated. For efficacy, a stratified log-rank test was used to test the null hypothesis of equal treatment effects. PFS was calculated using the Kaplan–Meier method. The Cochran–Mantel–Haenszel test stratified by the same factors as for the primary endpoint was performed to evaluate the effects of treatment on the CBR. Because of the expected low number of patients with objective response, nonstratified Fisher's tests were applied to objective response rate variables. Exact 95% confidence intervals (CIs) were calculated for the rates and their treatment differences. The analysis of QoL and exploratory analyses of response were performed using Cox regression models and proportional hazard models. For safety, incidences of treatment emergent AEs (TEAEs) were calculated for preferred terms and system organ classes by treatment group. On each analysis level, a patient was counted only once. A PK profile and a noncompartmental PK analysis was performed based on the PK sample concentrations measured in the PK subgroup of 20 patients.
Results
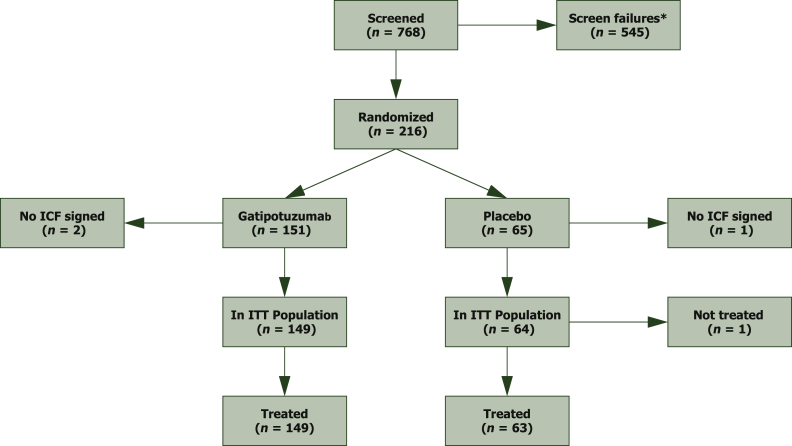
From November 2013 to October 2016, 768 patients were screened, 216 randomized, and 213 eventually treated with study drug, 149 with gatipotuzumab and 64 with placebo (Figure 1). After a median follow-up of 13.2 months, the study was sufficiently powered to detect differences in PFS but not in OS. Patients in both treatment arms did not show any relevant difference in demographic or disease characteristics at baseline (Tables 1 and 2 and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100311).
Figure 1.
CONSORT chart.
ICF, informed consent form; ITT, intent to treat.
Table 1.
Patient and disease characteristics at baseline
| Characteristics | Gatipotuzumab (n = 149) | Placebo (n = 64) | ||
|---|---|---|---|---|
| Age (years), mean (standard deviation) | 61.2 (9.55) | 60.3 (10.49) | ||
| Body mass index (kg/m2), mean (standard deviation) | 27.1 (5.42) | 26.4 (5.27) | ||
| ECOG status, n (%) | ||||
| 0 | 81 (54.4) | 30 (46.9) | ||
| 1 | 68 (45.6) | 34 (53.1) | ||
| Recurrent cancer type, n (%) | ||||
| Epithelial ovarian | 145 (97.3) | 63 (98.4) | ||
| Fallopian tube | 2 (1.3) | 1 (1.6) | ||
| Primary peritoneal | 2 (1.3) | 0 | ||
| FIGO stage, n (%), at | Diagnosis | Baseline | Diagnosis | Baseline |
| Missing | 5 (3.4) | 19 (12.8) | 3 (4.7) | 7 (10.9) |
| I | 7 (4.7) | 2 (1.3) | 1 (1.6) | 0 |
| II | 8 (5.4) | 7 (4.7) | 9 (14) | 5 (7.8) |
| III | 98 (66) | 70 (47) | 38 (59.4) | 30 (47) |
| IV | 31 (20.8) | 51 (34.2) | 13 (20.3) | 22 (34.4) |
| Time since (month) | ||||
| Initial diagnosis, mean (standard deviation) | 41.1 (27.06) | 40.5 (23.51) | ||
| Most recent relapse, mean (standard deviation) | 8.0 (7.22) | 7.9 (2.83) | ||
ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics.
Table 2.
Preceding lines of therapy
| Therapy | Gatipotuzumab (n = 149), n (%) | Placebo (n = 64), n (%) | Total, n (%) |
|---|---|---|---|
| Chemotherapy | 149 (100.0) | 64 (100.0) | 213 (100.0) |
| Hormone therapy | 4 (2.7) | 0 | 4 (1.9) |
| Radiotherapy | 8 (5.4) | 2 (3.1) | 10 (4.7) |
| Other | 20 (13.4) | 12 (18.8) | 32 (15.0) |
| Surgery | 145 (97.3) | 63 (98.4) | 208 (97.7) |
| Best overall response to any chemotherapy | |||
| Complete response | 102 (68.5) | 32 (50.0) | 134 (62.9) |
| Partial response | 40 (26.8) | 19 (29.7) | 59 (27.7) |
| Stable disease | 7 (4.7) | 12 (18.8) | 19 (8.9) |
| Not assessable | 0 (0.0) | 1 (1.6) | 1 (0.5) |
Efficacy
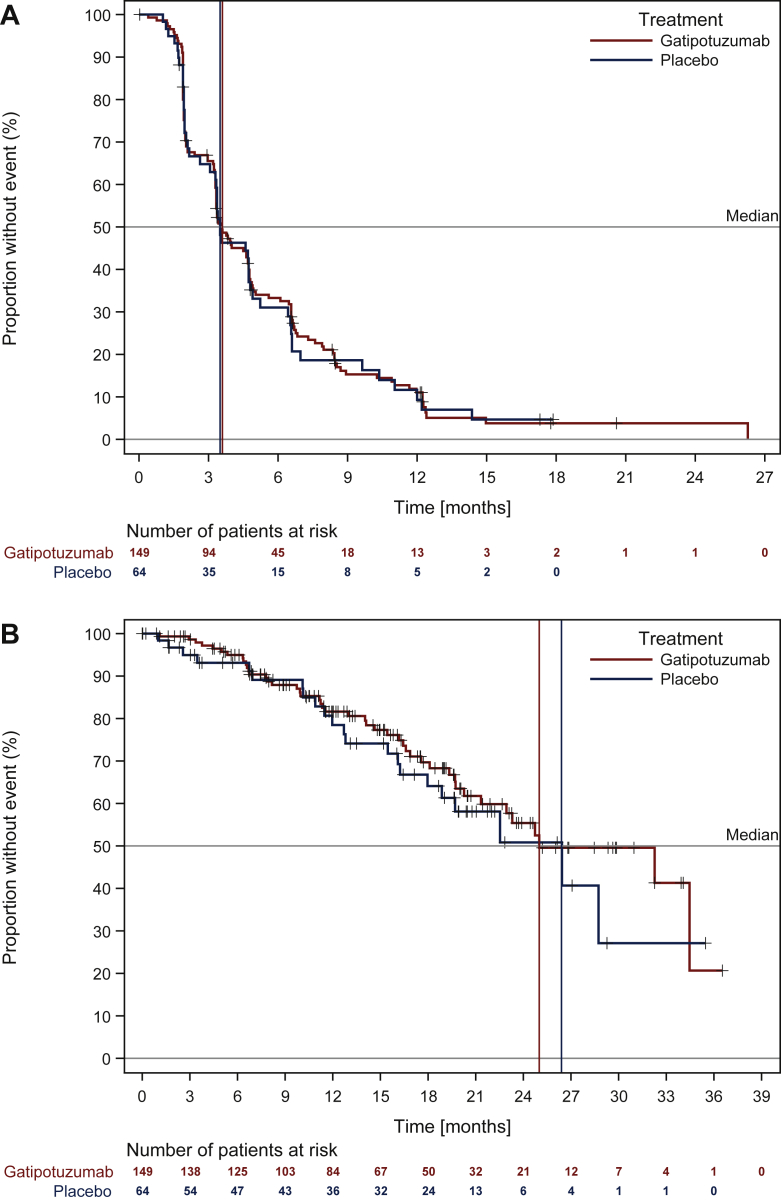
There was no significant difference in PFS in patients treated with gatipotuzumab versus those treated with placebo, when assessed by the investigator [median (95% confidence interval (CI) 3.5 (3.3-4.6) versus 3.5 (3.0-4.7) months, P = 0.42; hazard ratio (HR) 0.96 (95% CI 0.69-1.33), P = 0.80; Figure 2A] or by independent central review [median (95% CI) 3.2 (2.0-3.3) versus 3.2 (2.0-3.5) months; P = 0.94]. No differences were seen in the 6-month PFS rate (33.3% versus 31%) in analyses using RECIST [central review: median (95% CI) 3.3 (2.0-3.5) versus 3.2 (1.9-4.2), P = 0.75] or GCIG criteria [median (95% CI) 6.7 (5.5-10.8) versus 5.1 (3.8-11.8) months, P = 0.24] instead of irRECIST criteria, or in any of the stratification subgroups. No differences occurred in any of the secondary endpoints.
Figure 2.
Efficacy of switch maintenance treatment with gatipotuzumab versus placebo.
Kaplan–Meier plots of (A) PFS based on irRECIST criteria (investigator assessment) and (B) OS. irRECIST, immune-related RECIST; OS, overall survival; PFS, progression-free survival.
At the time of the analysis, a low number of OS events was observed (67/213 patients) and there was no difference between gatipotuzumab and placebo-treated patients in median OS (24.6 versus 26.1 months; Figure 2B) or 6-month OS rate (95.0% versus 93.1%). An OS analysis by stratification factors revealed a higher risk of death in platinum-resistant versus intermediate-sensitive patients [HR 2.10 (95% CI 1.28-3.43) Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100311].
The median time to deterioration of QoL by ≥10 points on the EORTC-QLQ-C30 scale was also in favor of gatipotuzumab, although the difference from placebo again was not significant [median (95% CI) 9.9 (6.6-11.7) months versus 11.8 (5.8-n.d.), P = 0.33]. Functional scales on the EORTC-QLQ-OV28 scale did not show any significant difference between treatments.
Pharmacodynamic and prognostic factors
In a Cox proportional hazard model, the covariates TA-MUC1 immunohistochemistry score, TA-MUC1-positive cells, the FcγRIIa and FcγRIIIa status, the baseline body weight, body mass index, surface area, and the type of chemotherapy preceding study entry had no significant impact on PFS, CBR, or OS. By contrast, using the baseline serum levels of TA-MUC1 (greater or less than the median 64.7 U/ml: HR 1.36 (95% CI 1.00-1.85), P = 0.05], CA-125 (>3× upper limit of normal versus ≤upper limit of normal: HR 1.70 95% CI 1.16-2.47, P = 0.02), and HE4 [greater or less than the median 98 pmol/l: HR 1.74 (95% CI 1.27-2.39), P = 0.01] turned out to be of prognostic value for a decreased PFS (as assessed by the investigator based on irRECIST criteria) and CBR.
Pharmacokinetics
PK parameters were comparable after the first and multiple dose administrations (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100311). Based on the matching half-lives (t1/2) and areas under the concentration-time curve (AUCinf and AUC0-τ, respectively) after cycles 1 and 4, with >5 half-lives in-between both measurements, steady-state conditions after cycle 4 can be assumed. Cox regression models did not reveal any influence of either Cmin or Cmax gatipotuzumab levels measured before and after cycle 2 and 5 on PFS or CBR. No obvious difference in serum gatipotuzumab levels were observed between patients with and without clinical benefit.
Safety
Overall, 128 patients (85.9%) in the gatipotuzumab group and 50 patients (79.4%) in the placebo group reported 919 and 355 TEAEs, respectively. Of these, 33 AEs were serious, all but 1 were unrelated to the study drugs. TEAEs of grade 3 were reported for 19.5% of gatipotuzumab-treated patients compared with 14.3% of placebo-treated patients and were most frequently reported as pleural effusion, abdominal pain, and dyspnea. Incidence of serious adverse events was slightly lower in the gatipotuzumab-group (9.4% versus 12.7%) with the majority being unrelated to study drug. Three patients (1.4%) in the placebo group died from progressive pleural effusion, death in sleep, and hepatic failure, which were all not or unlikely to be related to study drugs. The only serious adverse event reported in more than two patients were device-related infections in one (1.6%) placebo-treated and two (1.3%) gatipotuzumab-treated patients.
TEAEs not classified as IRRs were reported with similar frequencies in both treatment groups [gatipotuzumab: 117 patients (78.5%) experienced 736 events; placebo: 49 patients (77.8%) experienced 296 events]. IRRs overall (40.3% versus 20.6%) and those related to study drug (38.9% versus 19.0%) were reported more frequently in gatipotuzumab-treated patients, with pruritus (9.4% versus 0.0%), erythema (5.4% versus 0%), rash (6.7% versus 0%), and chills (4.0% versus 6.3%) being the most common. Most IRRs were of grade 1 and none reached grade 4 or 5. Only one grade 3 IRR of severe cough was reported in a gatipotuzumab patient, who recovered without sequelae the same day.
Study-drug related AEs requiring treatment interruption, infusion rate reduction, or additional medication were more common with gatipotuzumab than with placebo, whereas those leading to study drug discontinuation occurred in both groups at similar frequencies (Table 3). There were generally more study drug-related AEs in the gatipotuzumab group, in particular pyrexia, musculoskeletal, vascular, and eye disorders, mainly of mild intensity, and all patients recovered. Drug-related AEs were also more severe, with those of grade 3 (vomiting, cough, hypocalcemia, neutropenia, and leukopenia) reported in five (3.4%) gatipotuzumab-treated patients only.
Table 3.
Treatment-related AEs, given as number of treated patients (%) and number of events
| Gatipotuzumab (n = 149) | Placebo (n = 63) | |
|---|---|---|
| Treatment-related AE | 97 (65.1), 403 | 33 (52.4), 158 |
| Infusion-related reactions | 58 (38.9), 178 | 12 (19.0), 57 |
| Serious AE | 1 (0.7), 1 | 0 (0.0), 0 |
| Significant AE, that is, requiring | ||
| Premature discontinuation | 3 (2.0), 10 | 1 (1.6), 1 |
| Drug interruption or reduction | 23 (15.4), 45 | 3 (4.8), 3 |
| Additional medication | 53 (35.6), 132 | 15 (23.8), 29 |
| By severity | ||
| Grade 1: Mild | 49 (32.9), 310 | 18 (28.6), 130 |
| Grade 2: Moderate | 42 (28.2), 83 | 15 (23.8), 28 |
| Grade 3: Severe | 5 (3.4), 5 | 0 (0.0) 0 |
| By SOC and PT (with incidence ≥5.0%) | ||
| General disorders | 46 (30.9), 104 | 14 (22.2), 20 |
| Fatigue | 12 (8.1), 15 | 4 (6.3), 4 |
| Asthenia | 10 (6.7), 13 | 5 (7.9), 6 |
| Pyrexia | 14 (9.4), 36 | 1 (1.6), 1 |
| Chills | 8 (5.4), 13 | 4 (6.3), 4 |
| Skin and subcutaneous tissue disorders | 48 (32.2), 85 | 11 (17.5), 15 |
| Pruritus | 21 (14.1), 28 | 3 (4.8), 3 |
| Erythema | 13 (8.7), 18 | 4 (6.3), 6 |
| Rash | 10 (6.7), 14 | 1 (1.6), 1 |
| Gastrointestinal disorders | 32 (21.5), 58 | 13 (20.6), 24 |
| Nausea | 14 (9.4), 28 | 4 (6.3), 6 |
| Vomiting | 7 (4.7), 8 | 4 (6.3), 10 |
| Nervous system disorders | 27 (18.1), 39 | 10 (15.9), 41 |
| Headache | 12 (8.1), 17 | 5 (7.9), 5 |
| Musculoskeletal, connective tissue disorders | 19 (12.8), 30 | 3 (4.8), 18 |
| Respiratory, thoracic, mediastinal disorders | 14 (9.4), 23 | 6 (9.5), 8 |
| Vascular disorders | 12 (8.1), 13 | 2 (3.2), 3 |
| Eye disorders | 10 (6.7), 11 | 2 (3.2), 2 |
AE, adverse event; PT, preferred term; SOC, system organ class.
Clinically significant changes in safety laboratory, vital signs, or electrocardiogram readings were not reported in any of the groups.
Discussion
In the current trial, we evaluated another drug target and a new mechanism of action, that is, the activation of the immune system to induce ADCC against TA-MUC1-expressing tumor cells. The MUC1 target had already been employed in an earlier phase III trial in which local radiotherapy with a single intraperitoneal dose of 25 mg of a yttrium-90-labeled murine HMFG1 monoclonal antibody (90Y-muHMFG1) in addition to standard treatment was tested versus standard treatment alone.16 Although the study failed to demonstrate a PFS or OS benefit of the add-on treatment, it significantly increased anti-MUC1 IgG serum levels, which beyond a certain threshold were associated with prolonged PFS and OS.17 Accordingly, immunotherapy against MUC1 was hypothesized to be a treatment approach that warrants further investigation in ovarian cancer patients. In line with this, in the ovarian cancer subgroup of the first-in-human study of gatipotuzumab, 1 patient achieved a CR and 7/20 (35%) patients had SD as best response.
However, based on the results of our placebo-controlled study, single-agent gatipotuzumab did not confirm a benefit by RECIST response and was not effective in prolonging PFS as switch maintenance therapy of patients with recurrent serous epithelial ovarian, fallopian tube, or high-grade primary peritoneal carcinoma. Furthermore, no immune-related responses were observed using IrRECIST. No advantage for gatipotuzumab over placebo was seen in any secondary efficacy endpoints or in any patient subgroup, including those with FcγR polymorphisms expected to benefit the most from an agent potentiating ADCC. Our study did not reveal new safety signals and confirmed good tolerability, safety, and PK profile already demonstrated in the first-in-human study.15 It also provided PK at steady state which was reached after cycle 4 and still supports the q3w dosing schedule. It is possible that the single-agent use in a maintenance setting was not the most suitable for showing activity, as most antibodies used today against solid tumors are rarely given as monotherapy. Thus, glyco-engineered antibodies combined with chemotherapies or immunotherapies may have a synergistic effect at least in a subgroup of patients identified by specific markers. Conversely, it is also possible that the potentiated ADCC observed in vitro with glyco-engineered antibodies in general, and more specifically with gatipotuzumab will not translate into clinically meaningful survival improvements when used in more complex clinical conditions.18
In conclusion, while gatipotuzumab alone proved not to be an efficacious maintenance therapy of recurrent ovarian cancer, its high specificity may still be beneficial in combination with other treatment options and these are currently being explored in further clinical studies. A recently completed phase I study combining gatipotuzumab and anti-EGFR antibodies has shown promising preliminary activity.19 Moreover, its tumor targeting capabilities may most efficiently be combined with the cancer-killing abilities of cytotoxic drugs giving rise to the development of an antibody–drug conjugate allowing for the discrimination between tumor and healthy tissue and for sparing the latter.
Acknowledgments
Funding
The study was sponsored and funded by Glycotope GmBH.
Disclosure
JL reports institutional research grant from AstraZeneca and MSD/Merck; serves on the steering group and advisory board of Pfizer; serves on the advisory board and provides lectures for AstraZeneca, Artios Pharma; GSK, MSD, Clovis Oncology, Eisai, and Neopharm; is a VBL Therapeutics Chair; is on the IDMC for Regeneron; is the Vice President of ESGO; and an editor of the ESMO Gynaecological Clinical Practice Guidelines; FR reports honoraria from GSK and MSD; is on the speakers bureau for GSK and MSD; reports travel, accommodations, expenses paid by GSK, MSD, and PharmaMar. UDG reports consultant fees from Janssen, Astellas Pharma, Sanofi, Bayer, Pfizer, BMS, Novartis, Ipsen, and Merck. JAA reports honoraria from Astellas Pharma and Pfizer; consulting or advisory role for Pfizer, Astellas Pharma, Janssen-Cilag, MSD, Bristol-Myers Squibb, EUSA Pharma, Merck, and AstraZeneca; and research funding from Bristol-Myers Squibb (institution); MRM reports grants from GSK, Pfizer, AstraZeneca, and Clovis; CC is on the advisory boards of Boehringer Ingelheim, Novartis, Pfizer, and Merck; reports lecture fees from AstraZeneca, BMS, Bayer, Ipsen, Astellas, MSD, and Sandoz; reports travel grants from Alvogen, Merck, and Boehringer Ingelheim; and reports educational grants from AstraZeneca. ACH reports grants or contracts from PharmaMar, AstraZeneca, Lilly, Eisai, and Tesaro/GSK; reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Roche, PharmaMar, AstraZeneca, Lilly, Tesaro/GSK, and Deciphera; reports support for attending meetings and/or travel from PharmaMar, Roche, Lilly, GSK/Tesaro, and Merck; and reports participation on a data safety monitoring board or advisory board of PharmaMar, Roche, Merk, Lilly, Eisai, and Karyopharm; NC reports advisory and consultancy role for Roche, PharmaMar, AstraZeneca, MSD/Merk, Clovis Oncology, Tesaro, GSK, Novartis, Pfizer, Takeda, BIOCAD, Immunogen, Mersana, Eisai, and OncXerna MH reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from GSK and Clovis Oncology; reports participation on a data safety monitoring board or advisory board of Amgen, GSK, and Clovis Oncology; reports receipt of equipment, materials, drugs, medical writing, gifts, or other services from Clovis Oncology, BMS, and Merck educational grants for clinical trials. AJ is employee at Premier Research, the CRO conducting the study. IA-F, AZ, and HB are employees or consultants at Glycotope, the sponsor of the study. In addition, HB owns stock or stock option of Glycotope. JS reports grants or contracts from Roche, GSK, AstraZeneca, Clovis, MSD, Merck, and Eisai; and participation in advisory boards at Roche, GSK, AstraZeneca, Clovis, MSD, Merck, Eisai as well as a Congress Leadership President at NOGGO. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Buechel M., Herzog T.J., Westin S.N., et al. Treatment of patients with recurrent epithelial ovarian cancer for whom platinum is still an option. Ann Oncol. 2019;30(5):721–735. doi: 10.1093/annonc/mdz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghajanian C., Goff B., Nycum L.R., et al. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2015;139(1):10–16. doi: 10.1016/j.ygyno.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledermann J.A., Embleton A.C., Raja F., et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387(10023):1066–1074. doi: 10.1016/S0140-6736(15)01167-8. [DOI] [PubMed] [Google Scholar]
- 4.Vergote I.B., Jimeno A., Joly F., et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J Clin Oncol. 2014;32(4):320–326. doi: 10.1200/JCO.2013.50.5669. [DOI] [PubMed] [Google Scholar]
- 5.Herzog T.J., Scambia G., Kim B.G., et al. A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. Gynecol Oncol. 2013;130(1):25–30. doi: 10.1016/j.ygyno.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Ledermann J.A., Hackshaw A., Kaye S., et al. Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. J Clin Oncol. 2011;29(28):3798–3804. doi: 10.1200/JCO.2010.33.5208. [DOI] [PubMed] [Google Scholar]
- 7.Kaye S.B., Fehrenbacher L., Holloway R., et al. A phase II, randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clin Cancer Res. 2012;18(23):6509–6518. doi: 10.1158/1078-0432.CCR-12-1796. [DOI] [PubMed] [Google Scholar]
- 8.Ledermann J., Harter P., Gourley C., et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 9.Mirza M.R., Monk B.J., Herrstedt J., et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 10.Pujade-Lauraine E., Ledermann J.A., Selle F., et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 11.Coleman R.L., Oza A.M., Lorusso D., et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berek J., Taylor P., McGuire W., et al. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27(3):418–425. doi: 10.1200/JCO.2008.17.8400. [DOI] [PubMed] [Google Scholar]
- 13.Deng J., Wang L., Chen H., et al. The role of tumour-associated MUC1 in epithelial ovarian cancer metastasis and progression. Cancer Metastasis Rev. 2013;32(3-4):535–551. doi: 10.1007/s10555-013-9423-y. [DOI] [PubMed] [Google Scholar]
- 14.Danielczyk A., Stahn R., Faulstich D., et al. PankoMab: a potent new generation anti-tumour MUC1 antibody. Cancer Immunol Immunother. 2006;55(11):1337–1347. doi: 10.1007/s00262-006-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiedler W., DeDosso S., Cresta S., et al. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur J Cancer. 2016;63:55–63. doi: 10.1016/j.ejca.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Verheijen R.H., Massuger L.F., Benigno B.B., et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol. 2006;24(4):571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 17.Oei A.L., Moreno M., Verheijen R.H., et al. Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. Int J Cancer. 2008;123(8):1848–1853. doi: 10.1002/ijc.23725. [DOI] [PubMed] [Google Scholar]
- 18.Bridgewater J., Cervantes A., Markman B., et al. Efficacy and safety analysis of imgatuzumab (GA201), a novel dual-acting monoclonal antibody designed to enhance antibody-dependent cellular cytotoxicity, in combination with FOLFIRI compared to cetuximab plus FOLFIRI in second-line KRAS exon 2 wild type or with FOLFIRI alone in mutated metastatic colorectal cancer. J Clin Oncol. 2015;33:669. [Google Scholar]
- 19.Macchini M., Garralda E., Fiedler W., et al. Results from the primary analysis of a 30 patient extension of the GATTO study, a phase Ib study combining the anti-MUC1 gatipotuzumab (GAT) with the anti-EGFR tomuzotuximab (TO) or panitumumab in patients with refractory solid tumors. Eur J Cancer. 2020;138(S2):S5–S6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.