Abstract
Two consecutive trials were conducted to investigate the effects of glucosinolates (GLS) in rapeseed cake (RSC) on nitrogen (N) metabolism and urine nitrous oxide (N2O) emissions in steers. In trial 1, 8 steers and 4 levels of RSC, i.e. 0%, 2.7%, 5.4% and 8.0% dry matter (DM) (0, 6.0, 12.1, 18.1 μmol GLS/g DM) were allocated in a replicated 4 × 4 Latin square. In trial 2, the static incubation technique was used for measuring the N2O emissions of the urine samples collected from trial 1. The results of trial 1 indicated that dietary inclusion of RSC decreased the digested N and increased the fecal N excretion (P < 0.01), whereas it did not affect the urinary N excretion, total N excretion and N retention (P > 0.10). Dietary inclusion of RSC decreased the urinary excretion of urea while it increased allantoin, total purine derivatives, the predicted rumen microbial N flow and thiocyanate (SCN) (P < 0.05). Dietary inclusion of RSC did not affect the plasma triiodothyronine and thyroxine while it down-regulated the plasma relative concentrations of 4-aminohippuric acid, 3α,7α-dihydroxycoprostanic acid, phosphatidylserine (14:0/16:0), 6β-hydroxyprogesterone, pyrrhoxanthinol, tatridin B, mandelonitrile rutinoside, taraxacoside (P < 0.05), and up-regulated hypoglycin B, neuromedin N (1-4), dhurrin, 5-deoxykievitone (P < 0.01). The results of trial 2 indicated that dietary RSC increased the steer urine N2O–N fluxes, the ratio of N2O–N to N application and the estimated steer urine N2O–N emissions (P < 0.01). A close correlation was found between the estimated steer urine N2O–N emissions and the output of urinary SCN (P < 0.001). In conclusion, dietary RSC increased the fecal N excretion, whereas it did not affect the urinary N excretion and the N retention rate in steers. Dietary RSC increased rather than decreased the urine N2O–N emissions even though it decreased the urinary excretion of urea. The SCN excreted in urine could be the major factor in increasing the urine N2O–N emissions. Whether other metabolites excreted into urine from RSC have an impact on the urine N2O–N emissions in steers needs to be investigated in the future.
Keywords: Cattle, Glucosinolates, Nitrogen metabolism, Nitrous oxide, Rapeseed cake
1. Introduction
Nitrous oxide (N2O) is a potent greenhouse gas with a global warming potential about 296 times greater than carbon dioxide (CO2) (WMO , 2017). Livestock farming results in approximately 53% of the anthropogenic N2O emissions, which are equal to 39.3% of the total agricultural N2O emissions (FAO, 2015). The N2O emissions from livestock are considerably emitted from the excreta deposition, particularly the urine from grazing animals (Di and Cameron, 2002).
N2O is produced from the excreta of animals during storage and fertilization in the processes of nitrogen (N) transformation by microorganisms (Clough et al., 2020). Four pathways including nitrifier nitrification, nitrifier denitrification, denitrification, and nitrification coupled denitrification by denitrifiers are identified for N2O formation in soil (Zhu et al., 2013). Nitrification is the metabolic pathway by which microorganisms oxidize ammonium (NH4+) to produce nitrate (NO3-) with several intermediate products including hydroxylamine and nitrite (NO2-), while denitrification is the pathway by which microorganisms reduce NO3- to nitrogen gas (N2) with several intermediate products including NO2-, nitric oxide (NO) and N2O (Levy-Booth et al., 2014). The N2O emissions from urine are generally higher than emissions from feces in cattle because urea accounts for the major part of urinary N (Dijkstra et al., 2013) which could be easily degraded into NH4+-N and used as the precursor of N2O in soil (Whitehead et al., 1989).
Approaches to mitigate N2O emission from animal excreta include decreasing the total N excretion, regulating the fecal N-to-urinary N ratio, and supplementing tannins and tannic acids to rations (Zhao, 2017; Zhao et al., 2020; Zhou et al., 2019). A recent method to mitigate the N2O emissions from the urine patches of grazing ruminants is to use dicyandiamide (DCD) which is an exogenous nitrification inhibitor (NI). It was reported that DCD loading within urine patches at 10 to 30 kg DCD/ha reduced the N2O emission factor (EF3, % N applied) by 34% to 64% (Minet et al., 2018). However, it is difficult to achieve a synchronism between the urinary N loading and the DCD application (Clough et al., 2020) because DCD is easily degraded in soil (McGeough et al., 2016). Supplementing DCD in the ration of dairy cows effectively reduced the urine N2O–N emissions because part of DCD was excreted into urine (Minet et al., 2018). However, about 1% of orally administered DCD to lactating dairy cows was recovered in milk (Welten et al., 2016), suggesting the potential negative effects of feeding DCD on milk quality and human health. Thus, it is necessary to find efficient endogenous NI that could be excreted into ruminant urine.
It was reported that the N2O emission from the urine of sheep fed fresh forage rape (Brassica napus L.) was much lower than fed fresh perennial ryegrass (Lolium perenne L.) (EF3 0.11% vs. 0.27%) and it was inferred that the effect could be caused by glucosinolates (GLS) in forage rape (Luo et al., 2015). In a laboratory static incubation, it was found that adding different types of isothiocyanates (ITC) from GLS hydrolysis decreased the N2O emissions from the soil applied with urea to different extents, while adding phenethyl ITC reduced the N2O emission by 51% (Balvert et al., 2017), indicating direct addition of ITC effectively inhibited the soil microbes such as NH4+-oxidizing bacteria (Bending and Lincoln, 2000; Aires et al., 2009). These results suggested that the metabolites excreted in urine from brassica forages had inhibitive effects on the urine N2O emissions in ruminants. However, Hoogendoorn et al. (2016) reported that the EF3 of N2O from the urine of sheep grazing on forage rape (Brassica napus L.) was higher than on the perennial ryegrass/white clover pasture (L. perenne L./Trifolium repens). Balvert (2018) also reported that the total N2O emissions of urine from cattle fed kale (Brassica oleracea L.) were higher than fed the perennial ryegrass/white clover pasture (L. perenne L./T. repens). The reasons for the contradictory results are unclear and the effects of Brassica forages on N2O emissions are inconclusive.
Rapeseed cake (RSC), which is the by-product of rapeseeds after oil extraction, is an important protein supplement for livestock and also contains high levels of GLS. The objectives of this study were to investigate the effects of dietary inclusion of RSC containing high GLS on the N metabolism and urinary nitrogenous components in steers, to clarify if the metabolites of GLS in RSC could be excreted into steer urine, to explore the effects of the metabolites on the urine N2O emissions, and to elucidate the mechanisms of the effects of GLS and metabolites on the urinary composition and animal health using plasma metabolome.
2. Materials and methods
2.1. Ethics statement
The use of animals and experimental procedures was approved by the Animal Care and Use Committee of China Agricultural University, Beijing, China (No. 20130611–1).
2.2. Animal experiment (trial 1)
Eight Simmental steers (initial body weight [BW] 219 ± 14 kg aged at 10 months) were used as the experimental animals. Four levels of RSC, i.e. 0%, 2.7%, 5.4% and 8.0% of dry matter (DM) (GLS contents: 0, 6.0, 12.1, 18.1 μmol/g DM) were included in the rations (Table 1) as experimental treatments, respectively. The animals and the treatments were randomly assigned in a replicated 4 × 4 Latin square design. The dietary crude protein (CP) and net energy for maintenance and fattening (NEmf) were 1.2 times the maintenance requirements for the steers (Feng, 2000). Each animal was supplied with 3.5 kg DM of total mixed ration (TMR) daily, which was about 90% of the ad libitum DM intake. No orts from the animals were left during the experiment. The animals were kept in individual pens. The daily TMR was divided into 2 equal portions and fed to each animal at 07:00 and 16:00, respectively. The animals had free access to fresh drinking water. Each experimental period included 15 d for adaptation and 5 d for sampling.
Table 1.
Ingredients and nutritional composition of the experimental rations (DM basis).
| Item | Dietary levels of RSC, % DM |
|||
|---|---|---|---|---|
| 0 | 2.7 | 5.4 | 8.0 | |
| Ingredients, % | ||||
| Corn | 27.50 | 28.30 | 28.90 | 29.15 |
| Corn gluten meal | 5.60 | 3.73 | 2.00 | 0.10 |
| Soybean meal | 5.35 | 4.32 | 3.20 | 2.20 |
| RSC | 0.00 | 2.65 | 5.35 | 8.00 |
| Sodium chloride | 1.00 | 1.00 | 1.00 | 1.00 |
| Sodium bicarbonate | 1.00 | 1.00 | 1.00 | 1.00 |
| Premix1 | 2.00 | 2.00 | 2.00 | 2.00 |
| Corn silage | 57.55 | 57.00 | 56.55 | 56.55 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutritional composition | ||||
| NEmf2, MJ/kg | 5.42 | 5.50 | 5.48 | 5.48 |
| OM, % | 89.52 | 89.42 | 89.41 | 89.68 |
| CP, % | 11.96 | 12.06 | 12.04 | 11.93 |
| RDP3, % CP | 64.53 | 63.52 | 61.42 | 58.18 |
| RUP3, % CP | 35.47 | 36.48 | 38.58 | 41.82 |
| ADIP, % CP | 7.85 | 8.45 | 9.11 | 10.09 |
| Neutral detergent fiber, % | 44.85 | 44.14 | 44.52 | 44.91 |
| Acid detergent fiber, % | 25.54 | 25.49 | 25.99 | 26.46 |
| Ether extract, % | 1.49 | 1.80 | 1.82 | 2.05 |
| GLS and metabolites, mmol/g | ||||
| GLS | 0.00 | 5.99 | 12.10 | 18.09 |
| SCN | 0.00 | 0.01 | 0.02 | 0.03 |
| Total | 0.00 | 6.00 | 12.12 | 18.12 |
DM = dry matter; RSC = rapeseed cake; NEmf = net energy for maintenance and fattening; OM = organic matter; CP = crude protein; RDP = rumen degradable protein; RUP = rumen undegradable protein; ADIP = acid detergent insoluble protein; GLS = glucosinolates; SCN = thiocyanate.
Provided per kilogram DM of ration: 54 mg Zn (ZnSO4); 70 mg Fe (FeSO4); 38 mg Mn (MnSO4); 12.8 mg Cu (CuSO4); 1.2 mg I (KI); 0.17 mg Se (Na2SeO3); 0.6 mg Co (CoCl2); 2.31 mg vitamin A; 75 μg vitamin D3.
NEmf was calculated according to Feng (2000).
The contents of RDP and RUP were calculated according to NRC (2001) based on the nitrogenous fractions of feeds analyzed using the methods of Licitra et al. (1996).
The feces from each animal were totally collected daily using a plastic bucket during the sampling period. The daily output of the feces from each animal was recorded, and 2% of the total was sampled and mixed with 20 mL H2SO4 (10%, vol/vol) per 100 g of feces to keep the pH < 3.0 to prevent the N loss. The total urine from each animal was also collected using a rubber funnel connected by polyvinyl chloride pipe to a plastic barrel surrounded by ice packs. The daily urine output was recorded and homogenized, and 1% of the total urine from each steer was sampled and mixed with 10 mL H2SO4 (10%, vol/vol) per 70 mL urine to keep the pH < 3.0 to prevent the N loss, while another urine sample was taken without adding H2SO4. The feeds were also sampled daily during the sampling periods. All the samples were stored at −20 °C for later analysis. A blood sample was taken from the jugular vein of each animal 3 h after feeding in the morning on the last day of each experimental period. The blood samples were centrifuged at 2,000 × g for 15 min to obtain the plasma samples which were then stored at −80 °C for later analysis.
2.3. Static incubation experiment (trial 2)
The static incubation technique (Bao et al., 2018) was used to determine the N2O emissions from the urine samples collected in trial 1. The soil for static incubation of steer urine (sand 50.4% dry weight [DW], clay 4.0% DW, and silt 45.6% DW; pH-KCl 7.57; 0.91 g N/kg soil DW; water content 15.9% [wt/wt]) was collected from a crop field at the depth of 0 to 20 cm in Shandong province, China (37°13′12″N, 118°1′12″E). The soil was screened through a sieve with 2 mm in pore size.
Glass jars which were 14 cm in length, 8.5 cm in internal diameter, and 560 mL in scaled volume were used as the vessels for static incubation. Each jar was packed with 274.8 g of soil DW, 5 cm in height, and 1.16 g soil DW/cm3 in bulk density. Approximately 320 mL headspace of air was left over the soil in each jar. The soil moisture content was adjusted to 19.7% (wt/wt) by adding distilled water. The jars were covered by parafilm (Bemis Company, Inc.) with 4 mini holes to allow the exchange of air and pre-incubated at 25 ± 0.1 °C in a biochemical incubator (SHP-250, Shanghai Peiyin Instrument Co., Ltd., China) for 3 d-stabilization. The soil moisture content was kept at 19.7% by adding distilled water during pre-incubation. Then 10 mL urine sample without adding H2SO4 was added to the surface of the soil in each jar at different points to yield the water-filled pore space (WFPS) at 60%. Then the jars were incubated in a biochemical incubator at 25 ± 0.1 °C (SHP-250, Shanghai Peiyin Instrument Co., Ltd., China). During incubation, the WFPS of the soil was kept at 60% by adding distilled water based on the weight loss of each jar every 3 d. Three jars were used as the replicates for each urine sample to determine the daily N2O emission during 15 d-incubation, while 10 jars for each urine sample were used as the replicates for soil sampling, of which 2 jars were used for soil sampling every 3 d. Three jars containing soil without adding urine but 10 mL distilled water were used as the blanks.
Gas sampling was conducted using the methods of Sanchez-Martín et al. (2017) and Kool et al. (2006) with slight modifications. At 09:00 on d 2 after urine application, the parafilm on each jar was removed and a mini fan was used to blow into it for 15 min. Then, each jar was covered by a rubber stopper fitted with a glass tube and a valve. Immediately, 20 mL gas in each jar was sampled using a syringe. A second 20 mL gas sample was collected after 20 min. Since a regression relationship between the N2O–N fluxes and the 2 successive sampling time points (0 and 20 min) was found to be linear (R2 > 0.99) (Bao et al., 2018), 2 gas samples taken at t0 min and t20 min were used for predicting the daily N2O–N emissions. At d 0, 3, 6, 9, 12 of incubation, 2 jars of each urine sample were used for soil sampling. At the end of incubation, the last 3 jars of each urine sample used for determining the daily N2O–N emissions were also used for soil sampling. All the soil samples were stored at −20 °C.
2.4. Chemical analysis
2.4.1. Feeds, feces and urine
The feces and the corn silage were lyophilized using a LGJ-12 freeze dryer (Bejing Songyuan Huaxing Technology Development Co., Ltd, Beijing, China). The fecal samples were milled using a mortar with a pestle and the daily fecal samples from each animal during each experimental period were composited as one sample. The freeze-dried corn silage and other feeds were ground and screened through a sieve with a pore size of 1 mm.
The DM in feeds and feces was determined in an oven at 105 °C using the AOAC method No. 934.01 (2012). The crude ash and ether extract (EE) of feeds were determined using AOAC (2012) methods No. 942.05 and 920.39, respectively. The organic matter (OM) content was calculated by DM minus crude ash. The neutral detergent fiber (aNDF) and acid detergent fiber (ADF) of feeds inclusive of residual ash were analyzed on an Ankom A200i Fiber Analyzer (Ankom Technology Corp., USA) using the methods of Van Soest et al. (1991). The rumen degradable protein (RDP) and rumen undegradable protein (RUP) of feeds were predicted according to NRC (2001) based on the nitrogenous fractions of feeds (PA, PB1, PB2, PB3, PC) analyzed using the methods of Licitra et al. (1996). The total N of feeds, feces and urine was analyzed using the Kjeldahl procedures according to AOAC (2012) method no.988.05. The CP content of feeds was calculated by the total N content multiplied by 6.25.
Urinary urea and creatinine were analyzed by colorimetric methods using commercial kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) on an ultraviolet spectrophotometer (UV-1801, Beijing Beifen Ruili Analytical Instrument Co., Ltd, Beijing, China). The urinary hippuric acid was analyzed according to China Hygienic Standard (WS/T 52-1996a). The urinary purine derivatives (PD) including uric acid and allantoin were analyzed according to the methods of Chen and Gomes (1992). The rumen microbial N flow was predicted based on the urinary total PD using the method of Chen and Ørskov (2004). The urinary thiocyanate (SCN) was analyzed according to China Hygenic Standard (WS/T 39-1996b). The urinary goitrin and ITC were analyzed using the methods of Thomke et al. (1998) and Matthäus and Fiebig (1996), respectively. The GLS in RSC was extracted using the method of Tholen et al. (1989) and analyzed using the method of Wathelet et al. (1988) with slight modification.
2.4.2. Analysis of plasma biochemical indices
The plasma total protein (TP), albumin (ALB), total cholesterol (TC), triglyceride, glucose and urea were analyzed on a biochemical analyzer (A7, Beijing Shining Sun Technology Co., Ltd, Beijing, China) using commercial kits (BioSino Bio-Technology and Science Inc., Beijing, China). The plasma total antioxidant capacity (T-AOC) was analyzed using commercial kit (HY-60021, Beijing Sino-UK Institute of Biological Technology, Beijing, China) on a biochemical analyzer (A7, Beijing Shining Sun Technology Co., Ltd, Beijing, China). Considering metabolites of GLS could cause the goiter of animals (Halkier and Gershenzon, 2006), the plasma triiodothyronine (T3) and thyroxine (T4) were analyzed using enzyme-linked immunosorbent assay (ELISA) kits (BioSino Bio-Technology and Science Inc., Beijing, China) on a microplate reader (DR-200BS, Wuxi Hiwell Diatek Instruments Co., Ltd, Jiangsu, China). The plasma SCN was analyzed according to China Hygiene Standard (WS/T 39-1996b). The plasma goitrin and ITC were analyzed using the methods of Thomke et al. (1998) and Matthäus and Fiebig (1996), respectively.
2.4.3. Analysis of plasma metabolome
The extraction of plasma metabolites from the animals fed 0 and 8.0% RSC rations for LC/MS analysis was according to the method of Wang et al. (2019). Briefly, 100 μL of each plasma sample was mixed with 300 μL of the extracted solution (acetonitrile:methanol = 1:1, vol/vol) and 20 μL of the internal standard (DL-o-chlorophenylalanine). The mixture was homogenized on a high throughput tissue crusher (Wonbio-96c, Shanghai Wanbo Biotechnology Co., Ltd, Shanghai, China) at 50 Hz for 6 min, followed by vortex treatment for 30 s and ultrasonic treatment at 40 kHz for 30 min at 5 °C. The mixture was centrifuged at 13,000 × g for 15 min at 4 °C and the supernatant was used for LC/MS analysis.
The LC/MS analysis was performed using a UPLC-TripleTOF system (AB Sciex, Framingham, MA, USA) with a Waters Acquity HSS T3 column (100 mm × 2.1 mm i.d., 1.8 μm, Waters, Milford, MA, USA) preheated to 40 °C. The volume of each sample for injection was 10 μL. The mobile phase consisted of 0.1% (vol/vol) formic acid in water (phase A) and 0.1% (vol/vol) formic acid in a mixture of acetonitrile and isopropanol (1:1, vol/vol) (phase B) at a flow rate of 0.40 mL/min. The elution gradient was used as: 0 to 3.0 min, 5% to 20% phase B; 3.0 to 9.0 min, 20% to 95% phase B; 9.0 to 13.0 min, 95% phase B; 13.0 to 13.1 min, 95% to 5% phase B; 13.1 to 16 min, 5% phase B. The parameters of the electron spray ionization (ESI) source were set according to Liu et al. (2019). The quality control (QC) samples were prepared by mixing all extracted plasma samples and were injected at regular intervals (every 5 samples).
2.4.4. Processing of metabolome data
The LC/MS raw data were generated by Waters MassLynx Software (version 4.1, Waters Corporation, Milford, MA, USA). The Progenesis QI (version 2.3, Nonlinear Dynamics, Newcastle, UK) was used to process the raw data for peak detection and alignment. The human metabolome database (HMDB, http://www.hmdb.ca/) and the Metlin database (http://metlin.scripps.edu/) were used to identify the metabolites. The multivariate statistical analyses including the principal component analysis (PCA) and the orthogonal partial least squares discrimination analysis (OPLS-DA) were performed using the ropls package (version 1.6.2, R software) from Bioconductor on Majorbio Cloud Platform (https://cloud.majorbio.com). The differential metabolites between treatments were identified based on the variable importance (VIP) from the OPLS-DA analysis and the student's t-test (VIP >1 and P < 0.05). The fold-change (FC) value of each metabolite was calculated by comparing the means of the peak area, and the log2FC value was used to indicate the specific variable quantity in the comparison.
2.4.5. Analysis of N2O in gas and NO3--N and NH4+-N in soil
The N2O concentration of the gas samples was analyzed according to the method of Bao et al. (2018) on a gas chromatograph (3420A, Beijing Beifen Tianpu Instrument Technology Co. Ltd, Beijing, China) equipped with an electron capture detector (ECD), 2 Porapak Q columns (2 m in length, 3 mm in internal diameter, Beijing Beifen Tianpu Instrument Technology Co. Ltd, Beijing, China). The NO3--N and NH4+-N of soil samples were analyzed according to China National Standard (GB/T 32737-2016) and China National Environmental Protection Standard (HJ 634-2012) on a spectrophotometer (K5500, Beijing Kaiao Technology Development Co., Ltd, Beijing, China), respectively.
2.5. Calculations
The rumen microbial N flow was predicted according to the method of Chen and Ørskov (2004) based on the total urinary PD output including uric acid and allantoin:
where Y refers to the total urinary PD (mmol/d); X, the absorbed PD (mmol/d); BW0.75, the metabolic body weight of steers (kg); 0.85, the recovery rate of absorbed purines as PD in urine; 0.147, the endogenous PD excretion (mmol/kg BW0.75 per d).
where the unit for microbial N flow is g/d; X refers to the absorbed PD (mmol/d); 70, the N content of purines (mg N/mmol); 0.116, the ratio of purine N/total N in mixed rumen microbes; 0.83, the digestibility of microbial purines.
The daily N2O–N flux was calculated according to the method of Watanabe et al. (1997):
where F refers to the N2O–N flux (μg N2O–N/kg soil DW per d); 273, the Kelvin temperature; T, the incubation temperature (°C); 28, the mass of N in N2O (g/mol); 1,440, the total minutes of 1 d (min); V, the volume of each jar over soil (L); dc/dt, the average changing rate of N2O concentration with time (nL/L per min); 22.4, the molar volume of gas at 273 K (L/mol); W, the DW of soil in each jar (kg).
The 15 d-cumulative N2O–N fluxes (FCumulative) of 10-mL urine sample was calculated as:
where F1, F2, … F15 (μg N2O–N/kg soil DW per d) refer to the N2O–N emissions of d 1, 2, … 15, respectively and the unit of FCumulative is μg N2O–N/kg soil DW.
The estimated steer urine N2O–N emissions (E, μg) was calculated as:
where FCumulative refers to the 15 d-cumulative N2O–N flux of 10 mL urine sample applied to soil, μg N2O–N/kg soil DW; U, the daily urine volume of each steer, mL; W, the DW of soil in each jar, kg.
The ratio of N2O–N to applied urine-N was calculated as:
| N2O–N to applied urine-N ratio = (Urine sample N2O–N emissions – Blank N2O–N emissions)/Urine sample-N × 100% , |
where the unit for N2O–N emissions and urinary sample-N is micrograms.
2.6. Statistical analysis
The data were subjected for statistical analysis using PROC MIXED procedure of SAS (version 9.4; SAS Inst. Inc., Cary, NC) using the following model:
| Yijk = μ + Ti + Pj + Ck + eijk , |
where Yijk is the dependent variable, μ is the overall mean, Ti is the ith effect of ration, Pj is the jth effect of period, Ck is the kth random effect of animal, and eijk is the residual experimental error. The statistical model included the random effect of animals (n = 8) and periods (n = 4), and fixed effects of rations (n = 4) for some variables. Linear and quadratic contrasts were used to test the effect of increasing levels of RSC. Multiple linear regression analysis between the estimated steer urine N2O–N emissions and the outputs of urinary constituents was performed using the stats package of R software (version 3.3.3). The fitted curve equation for all values of the cumulative N2O–N in each treatment was analyzed by the basicTrendline package of R software (version 3.3.3). The data were declared significant when P < 0.05 and trends were indicated at 0.05 < P < 0.10. All values were presented as least-squares means ± standard errors of means (SEM).
3. Results
3.1. Animal experiment (trial 1)
3.1.1. N utilization and liveweight gain
Dietary inclusion with RSC decreased the digested N (P = 0.003) and increased the fecal N excretion (P < 0.001) in a linear manner and did not affect the urinary N excretion (P = 0.452), resulting in a linear increase in the ratio of fecal N to urinary N (P = 0.035). Dietary inclusion with RSC did not affect the total N excretion, N retention, N retention rate and daily liveweight in steers (P > 0.10, Table 2).
Table 2.
Effects of feeding RSC containing high GLS on the N metabolism in steers (g/d).
| Item | Dietary levels of RSC, % DM |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2.7 | 5.4 | 8.0 | Ration | Linear | Quadratic | ||
| Daily liveweight gain, kg | 0.28 | 0.33 | 0.32 | 0.36 | 0.04 | 0.513 | 0.176 | 0.877 |
| Ingested N | 66.67 | 67.25 | 67.57 | 66.94 | – | – | – | – |
| Digested N | 38.77a | 38.20a | 38.49a | 36.46b | 1.12 | 0.007 | 0.003 | 0.099 |
| N excretion | ||||||||
| Fecal N | 27.90a | 29.05a | 29.08a | 30.48b | 1.12 | 0.005 | <0.001 | 0.772 |
| Urinary N | 22.82 | 23.34 | 22.20 | 22.33 | 1.18 | 0.688 | 0.452 | 0.803 |
| Fecal N to urinary N ratio | 1.24 | 1.26 | 1.34 | 1.37 | 0.07 | 0.178 | 0.035 | 0.891 |
| Total N excretion | 50.72 | 52.39 | 51.28 | 52.80 | 1.80 | 0.234 | 0.158 | 0.927 |
| N retention | 15.95 | 14.87 | 16.29 | 14.14 | 1.80 | 0.221 | 0.264 | 0.493 |
| N retention rate1, % | 23.92 | 22.11 | 24.11 | 21.12 | 2.69 | 0.236 | 0.234 | 0.614 |
RSC = rapeseed cake; GLS = glucosinolates; N = nitrogen; LW = liveweight.
a,b Means in the same row with different superscripts differ (P < 0.05, n = 8).
N retention rate = N retention/ingested N × 100.
3.1.2. Plasma indices
Dietary inclusion with RSC did not affect the plasma concentrations of T3 and T4 (P > 0.10, Table 3), and the TP, ALB, urea, glucose, TC, triglyceride, and T-AOC (P > 0.10). Dietary inclusion with RSC linearly increased the plasma concentration of SCN (P < 0.001) while goitrin and ITC were undetectable at the limits of 1 μg/L and 7 mg/L plasma, respectively.
Table 3.
Effects of feeding RSC containing high GLS on the plasma biochemical parameters in steers.
| Item | Dietary levels of RSC, % DM |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2.7 | 5.4 | 8.0 | Ration | Linear | Quadratic | ||
| Nutrients | ||||||||
| TP, g/L | 54.24 | 52.85 | 52.93 | 51.78 | 3.93 | 0.819 | 0.382 | 0.948 |
| ALB, g/L | 16.98 | 16.78 | 17.22 | 16.42 | 0.662 | 0.659 | 0.554 | 0.515 |
| Urea, mmol/L | 1.75 | 1.78 | 1.83 | 1.75 | 0.172 | 0.947 | 0.930 | 0.631 |
| Glucose, mmol/L | 3.61 | 3.53 | 3.45 | 3.60 | 0.178 | 0.707 | 0.839 | 0.301 |
| TC, mmol/L | 1.25 | 1.36 | 1.47 | 1.39 | 0.162 | 0.265 | 0.155 | 0.246 |
| Triglyceride, mmol/L | 0.731 | 0.671 | 0.735 | 0.657 | 0.048 | 0.403 | 0.389 | 0.825 |
| Hormones | ||||||||
| T3, ng/mL | 1.10 | 1.01 | 1.06 | 1.23 | 0.11 | 0.592 | 0.399 | 0.268 |
| T4, ng/mL | 53.18 | 52.85 | 54.44 | 55.72 | 2.68 | 0.721 | 0.302 | 0.678 |
| Antioxidant | ||||||||
| T-AOC, U/mL | 11.54 | 11.48 | 13.84 | 13.83 | 1.68 | 0.353 | 0.120 | 0.985 |
| GLS metabolites | ||||||||
| SCN, μmol/L | 0.00a | 24.48b | 61.72c | 87.07d | 4.83 | <0.001 | <0.001 | 0.919 |
| Goitrin, μmol/L | ND | ND | ND | ND | – | – | – | – |
| ITC, μmol/L | ND | ND | ND | ND | – | – | – | – |
RSC = rapeseed cake; GLS = glucosinolates; TP = total protein; ALB = albumin; TC = total cholesterol; T3 = triiodothyronine; T4 = thyroxine; T-AOC = total antioxidant capacity; SCN = thiocyanate; ND = undetectable; ITC = isothiocyanate.
a-d Means in the same row with different superscripts differ (P < 0.05, n = 8).
3.1.3. Plasma metabolome indices
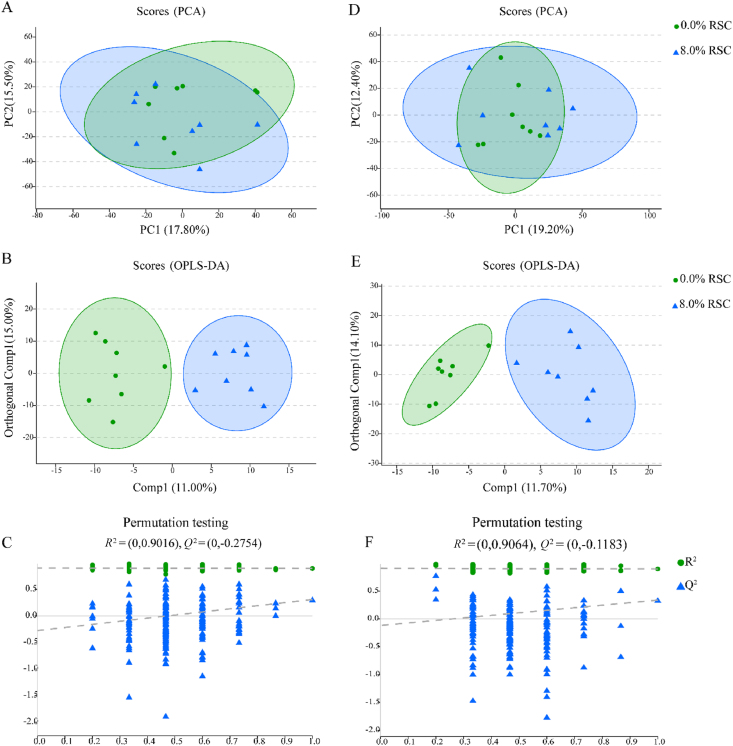
The LC/MS detected 687 valid peaks that were unique and non-overlapping in plasma samples. After quality control, 578 compounds were obtained from the peaks. No obvious separation of the PCA plot was detected between treatments of adding RSC at 0 and 8.0% DM (Fig. 1). Clear separation of the plasma metabolites was found between treatments of adding RSC at 0 and 8.0% DM, as shown in the OPLS-DA score plots (Fig. 1). The R2Y intercepts (0.90 and 0.91 > 0.50) and the Q2 intercepts (−0.28 and −0.12 < 0.00) of the permutation test in Fig. 1 showed that the model had no over-fitting, and it was effective and satisfactory to identify the plasma differential metabolites based on the VIP values of OPLS-DA analysis.
Fig. 1.
Plot of principal component analysis (PCA) (A), orthogonal partial least squares discrimination analysis (OPLS-DA) score (B), OPLS-DA permutation (C) in the positive model, and plot of PCA (D), OPLS-DA score (E), OPLS-DA permutation (F) in the negative model of plasma samples corresponding to the comparison between the steers fed with 0% and 8.0% rapeseed cake (RSC) (dry matter basis). PC = principal component; Comp = component.
Combined with the statistical analysis and the VIP values obtained from the OPLS-DA analysis, 12 different metabolites that differentiate between treatments of adding RSC at 0 and 8.0% DM were identified (P < 0.05, FC > 1.20 or < 0.83, VIP >1, Table 4). Compared with 0 RSC, adding RSC at 8.0% DM downregulated the plasma relative concentrations of 4-aminohippuric acid, 3α,7α-dihydroxycoprostanic acid, phosphatidylserine (14:0/16:0), 6β-hydroxyprogesterone, pyrrhoxanthinol, tatridin B, mandelonitrile rutinoside and taraxacoside (P < 0.05), and upregulated the plasma hypoglycin B, neuromedin N (1-4), dhurrin and 5-deoxykievitone (P < 0.05).
Table 4.
Differential plasma metabolites in steers fed with 8.0% versus 0 RSC (DM basis) using a VIP threshold of 1 (fold change > 1.20 or < 0.83).
| Chemical taxonomy |
Metabolite name | VIP | Log2FC | P-value | |
|---|---|---|---|---|---|
| Super class | Sub class | ||||
| Downregulated | |||||
| Benzenoids | Benzoic acids and derivatives | 4-aminohippuric acid | 1.35 | −0.33 | 0.017 |
| Lipids and lipid-like molecules | Bile acids, alcohols and derivatives | 3α,7α-dihydroxycoprostanic acid | 3.61 | −0.39 | 0.002 |
| Lipids and lipid-like molecules | Glycerophosphoserines | Phosphatidylserine (14:0/16:0) | 3.96 | −0.58 | 0.001 |
| Lipids and lipid-like molecules | Pregnane steroids | 6β-hydroxyprogesterone | 2.77 | −0.36 | 0.012 |
| Lipids and lipid-like molecules | Terpene lactones | Pyrrhoxanthinol | 2.27 | −0.46 | 0.028 |
| Lipids and lipid-like molecules | Terpene lactones | Tatridin B | 2.60 | −0.55 | 0.043 |
| Organic oxygen compounds | Carbohydrates and carbohydrate conjugates | Mandelonitrile rutinoside | 2.40 | −0.50 | 0.042 |
| Organic oxygen compounds | Carbohydrates and carbohydrate conjugates | Taraxacoside | 2.46 | −0.30 | 0.033 |
| Upregulated | |||||
| Organic acids and derivatives | Amino acids, peptides, and analogues | Hypoglycin B | 4.83 | 1.24 | <0.001 |
| Organic acids and derivatives | Amino acids, peptides, and analogues | Neuromedin N (1-4) | 5.97 | 1.92 | <0.001 |
| Organic oxygen compounds | Carbohydrates and carbohydrate conjugates | Dhurrin | 2.85 | 0.39 | <0.001 |
| Phenylpropanoids and polyketides | Isoflavans | 5-deoxykievitone | 4.37 | 0.84 | <0.001 |
RSC = rapeseed cake; DM = dry matter; VIP = variable importance; FC = fold-change.
3.1.4. Urinary nitrogenous components and GLS metabolites
Dietary inclusion with RSC linearly decreased the daily urine volume (P = 0.046), the urinary urea excretion (P = 0.001) and the urea-N-to-urinary N ratio (P = 0.004), linearly increased the urinary excretions of allantoin (P = 0.023) and the total urinary PD (P = 0.027), the ratios of allantoin-N-to-urinary N (P = 0.043) and the PD-N-to-urinary N (P = 0.048), and the predicted rumen microbial N flow (P = 0.028), whereas it did not affect the urinary excretions of hippuric acid, creatinine and uric acid (P > 0.10). Dietary inclusion with RSC tended to increase the urinary N concentration (P = 0.072).
Dietary inclusion with RSC linearly increased both the urinary concentration and the excretion of SCN (P < 0.001) (Table 5) whereas other metabolites of GLS including goitrin and ITC were undetectable in urine at the limits of 1 μg/L and 7 mg/L, respectively.
Table 5.
Effects of feeding RSC containing high GLS on the urinary components in steers.
| Item | Dietary levels of RSC, % DM |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2.7 | 5.4 | 8.0 | Ration | Linear | Quadratic | ||
| UN, g/L | 2.48 | 2.39 | 2.91 | 2.77 | 0.34 | 0.101 | 0.072 | 0.882 |
| Urea excretion, mmol/d | 573.19a | 576.68a | 498.12b | 472.45b | 35.92 | 0.006 | 0.001 | 0.507 |
| Urea-N-to-UN ratio, % | 70.30a | 68.85a | 63.18ab | 59.22b | 2.92 | 0.027 | 0.004 | 0.627 |
| Hippuric acid excretion, mmol/d | 7.39 | 8.48 | 8.11 | 7.05 | 1.36 | 0.474 | 0.670 | 0.138 |
| Hippuric acid-N-to-UN ratio, % | 2.61 | 2.83 | 2.88 | 2.45 | 0.47 | 0.599 | 0.698 | 0.201 |
| Creatinine excretion, mmol/d | 55.57 | 56.47 | 54.95 | 56.84 | 2.50 | 0.846 | 0.763 | 0.765 |
| Creatinine-N-to-UN ratio, % | 10.31 | 10.27 | 10.47 | 10.74 | 0.42 | 0.730 | 0.307 | 0.632 |
| Allantoin excretion, mmol/d | 18.11 | 22.59 | 28.51 | 27.35 | 6.90 | 0.072 | 0.023 | 0.304 |
| Allantoin-N-to-UN ratio, % | 4.59 | 5.44 | 7.40 | 6.68 | 1.98 | 0.105 | 0.043 | 0.355 |
| Uric acid excretion, mmol/d | 3.90 | 3.64 | 4.02 | 3.69 | 0.62 | 0.882 | 0.898 | 0.929 |
| Uric acid-N-to-UN ratio, % | 0.94 | 0.86 | 1.03 | 0.92 | 0.15 | 0.629 | 0.805 | 0.907 |
| Total PD excretion, mmol/d | 22.01 | 26.23 | 32.53 | 30.68 | 7.32 | 0.081 | 0.027 | 0.315 |
| Total PD-N-to-UN ratio, % | 5.53 | 6.30 | 8.42 | 7.60 | 1.93 | 0.106 | 0.048 | 0.367 |
| Rumen microbial N flow, g/d | 11.38 | 14.98 | 20.32 | 18.71 | 6.36 | 0.084 | 0.028 | 0.314 |
| SCN, μmol/L | 0.00a | 12.59a | 111.03b | 262.76c | 24.83 | <0.001 | <0.001 | 0.005 |
| SCN excretion, mmol/d | 0.00a | 0.13a | 1.08b | 2.56c | 0.33 | <0.001 | <0.001 | 0.014 |
| Goitrin, μmol/L | ND | ND | ND | ND | – | – | – | – |
| ITC, μmol/L | ND | ND | ND | ND | – | – | – | – |
RSC = rapeseed cake; GLS = glucosinoltaes; N = nitrogen; UN = urinary N; PD = purine derivatives; SCN = thiocyanate; ND = undetectable; ITC = isothiocyanate.
a-c Means in the same row with different superscripts differ (P < 0.05, n = 8).
3.2. Static incubation experiment (trial 2)
3.2.1. Urine N2O–N fluxes
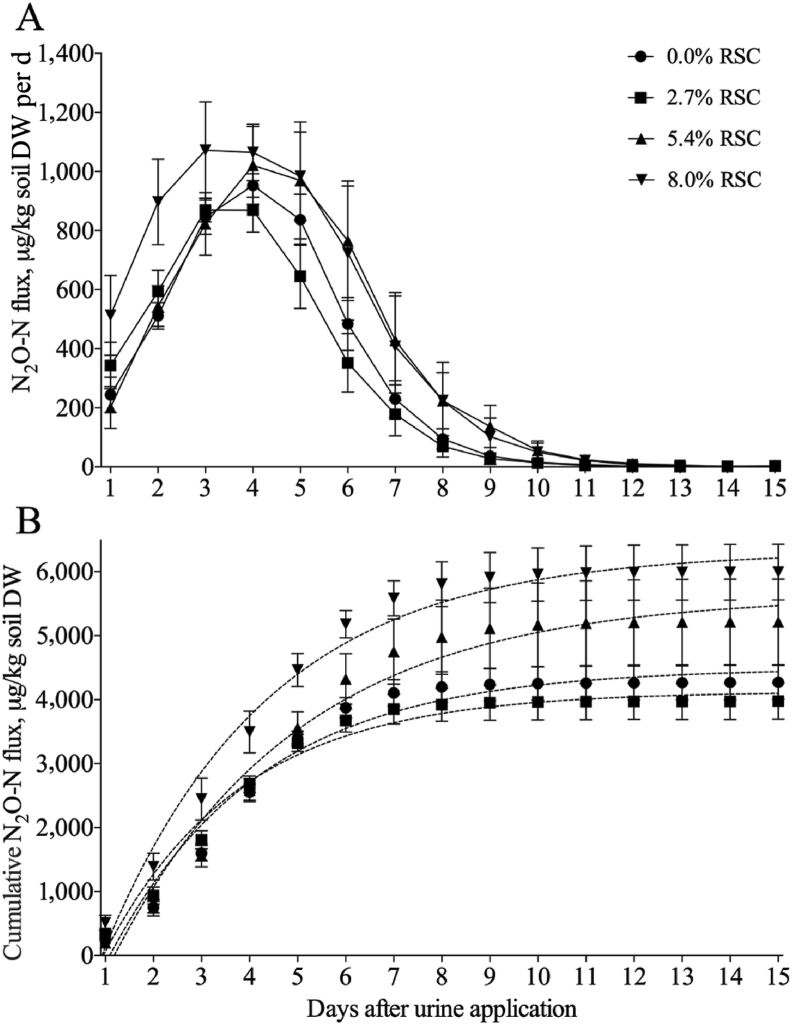
The urine N2O–N fluxes increased from d 1 to 4 and decreased after d 4 during incubation (Fig. 2A). The peak N2O–N fluxes were observed on d 3 or 4 and came to zero after d 13 during incubation. The cumulative N2O–N fluxes increased from d 1 to 9 and nearly reached the maximum levels after d 9 (Fig. 2B). Close exponential correlations were found between the cumulative N2O–N flux (Y) and the incubation days (X) (Fig. 2B). Results in Table 6 showed that dietary inclusion with RSC linearly increased the 15 d-urine sample N2O–N flux, the ratio of N2O–N to N application, and the estimated steer urine N2O–N emissions (P < 0.001).
Fig. 2.
Dynamic changes in (A) the daily and (B) the cumulative N2O–N fluxes of soil applied with the urine samples from steers fed RSC (dry matter basis). The fitted curves of the cumulative N2O–N flux were: Y = 4,492 – 6,356e−0.318X (R2 = 0.83, 0 RSC, P < 0.001), Y = 4,128 – 5,786e−0.353X (R2 = 0.77, 2.7% RSC, P < 0.001), Y = 5,627 – 7,646e−0.259X (R2 = 0.60, 5.4% RSC, P < 0.001), Y = 6,317 – 8,293e−0.293X (R2 = 0.78, 8.0% RSC, P < 0.001). Dashed lines represented the fitted curves of treatments. Error bars denoted the least squares mean of the cumulative N2O–N fluxes on each day. Vertical bars denoted the standard errors of the mean (n = 8). RSC = rapeseed cake; DW = dry weight.
Table 6.
Effects of feeding RSC containing high GLS on the urine N2O emissions in steers.
| Item | Dietary levels of RSC, % DM |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2.7 | 5.4 | 8.0 | Ration | Linear | Quadratic | ||
| N2O–N flux, mg/kg soil DW | 4.27ab | 3.98a | 5.22bc | 6.32c | 0.45 | 0.002 | <0.001 | 0.091 |
| N2O–N to N application ratio, % | 4.58a | 4.79a | 5.28a | 6.72b | 0.51 | 0.001 | <0.001 | 0.110 |
| Urine volume, L/d | 9.87 | 10.17 | 8.95 | 8.81 | 1.23 | 0.119 | 0.046 | 0.623 |
| Estimated N2O–N emissions, mg | 1,049.0a | 1,102.8a | 1,142.3a | 1,491.4b | 112.1 | <0.001 | <0.001 | 0.026 |
RSC = rapeseed cake; GLS = glucosinoltaes; DM = dry matter; N2O = nitrous oxide; N = nitrogen; DW = dry weight.
a-c Means in the same row with different superscripts differ (P < 0.05, n = 8).
3.2.2. NH4+-N and NO3--N in soil
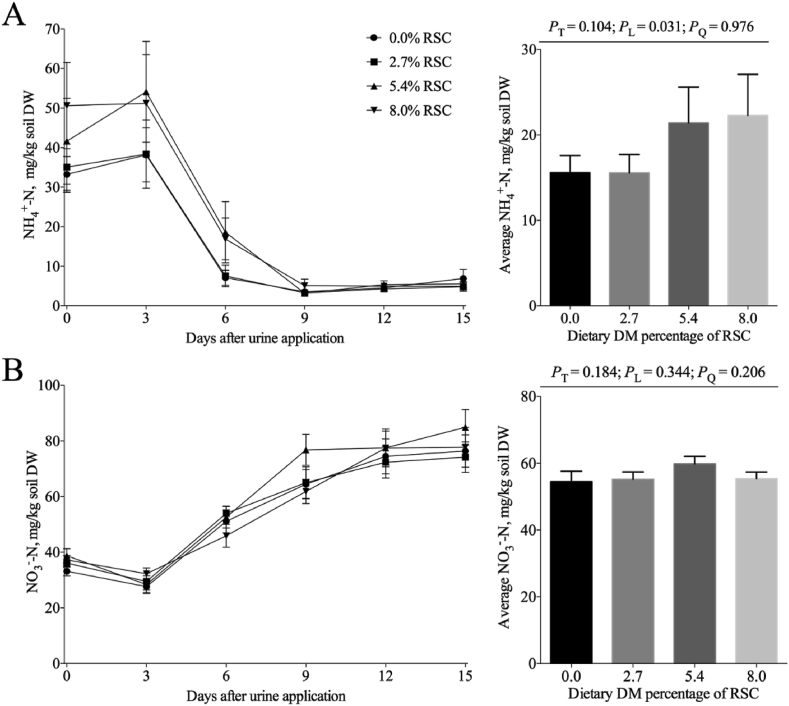
Dietary inclusion with RSC tended to linearly increase the soil concentrations of NH4+-N on d 0 (P = 0.056) and 6 (P = 0.051, Fig. 3) and linearly increased the average soil concentrations of NH4+-N (P = 0.031), while it did not affect the soil concentrations of NO3--N and the average soil concentrations of NO3--N (P > 0.10, Fig. 3).
Fig. 3.
NH4+-N (A) and NO3--N (B) concentrations of the soil applied with the urine samples from steers fed RSC (dry matter basis). Vertical bars indicated the standard errors of means for sampling times. PT: P value of treatments; PL: linear P value; PQ: quadratic P value. RSC = rapeseed cake; DW = dry weight.
3.2.3. Correlations between N2O–N emission and urinary constituents
The estimated steer urine N2O–N emission was positively correlated with the daily urinary excretions of urea-N (P = 0.045) and SCN (P < 0.001), and negatively correlated with the urinary excretion of creatinine-N (P = 0.041, Table 7) whereas no correlations were found between the estimated steer urine N2O–N emission and the urinary excretions of hippuric acid-N, uric acid-N or allantoin-N (P > 0.10). A multiple linear correlation was found between the estimated steer urine N2O–N emission and the urinary excretions of urea-N, hippuric acid-N, creatinine-N, uric acid-N, allantoin-N and SCN (R2 = 0.617, P < 0.001, Table 7).
Table 7.
Multiple linear regression between the estimated urine N2O–N emissions and the urinary excretions of different components in steers (g/d).
| Item | Coefficient estimate | P-value |
|---|---|---|
| Urea-N | 0.04 | 0.045 |
| Hippuric acid-N | −0.03 | 0.882 |
| Creatinine-N | −0.34 | 0.041 |
| Uric acid-N | 0.03 | 0.960 |
| Allantoin-N | −0.07 | 0.255 |
| SCN | 3.22 | <0.001 |
| Intercept | 1.32 | 0.002 |
| Fitting degree of multiple linear equation: R2 = 0.617, P-value < 0.001 | ||
N = nitrogen; SCN = thiocyanate.
4. Discussion
4.1. Effects of RSC containing high GLS on N excretion and urinary nitrogenous compounds in steers
The RSC used in the present study was from rapeseeds after oil extraction by hot-pressing. Therefore, the RUP content of the rations increased with the percentages of RSC and the RUP flowing to the hindgut should have been increased. The results of the animal experiment indicated that the fecal N excretion increased linearly with the increasing levels of RSC in the rations, resulting in lower CP digestibility. The results suggested that the RUP was not well digested. There are 2 reasons for the results. One is that the CP in RSC could have been over-protected by hot-pressing, as indicated by the increased dietary acid detergent insoluble protein (ADIP) while another is that GLS and metabolites negatively impacted the CP digestion. The results were in agreement with Rinne et al. (2015) who reported that dietary RSC decreased the CP digestibility in dairy cows and Dillard et al. (2018) who reported that dietary GLS decreased the CP digestibility in in vitro rumen fermentation.
N intake is a major driver of urinary N excretion in ruminants (Dong et al., 2014). The results of the present study showed that dietary inclusion with different levels of RSC in the animal experiment did not alter the urinary N excretion in steers. The reason for the results could be that the CP and NEmf contents of the rations and the N intakes of the steers among different groups were similar. The results were in agreement with Rinne et al. (2015) who reported that replacing soybean expeller (1.3 kg DM/d) by rapeseed expeller (1.8 kg DM/d) in the ration of dairy cows yielded similar levels of dietary metabolizable protein and metabolizable energy and did not affect the urinary N excretion.
Since dietary inclusion with different levels of RSC increased the fecal N excretion and did not affect the urinary N excretion, the fecal N-to-urinary N ratio was increased linearly. However, dietary inclusion with different levels of RSC did not affect the total N excretion and the N retention. The reason for the results could be that the variability in urinary N is higher than that of fecal N and the higher variability could be resulted from the effects of some metabolites originated from RSC. The results were in accordance with the unchanged daily liveweight gains among different treatments. The results indicated that dietary inclusion with RSC up to 8.0% DM did not depress the N utilization in steers even though RSC contained high level of GLS.
Urea is the major nitrogenous compound in the urine of cattle and urea-N accounts for about 52.1-93.5% of urinary N (Dijkstra et al., 2013). The urinary urea was from the blood synthesized in the liver by using ammonia-N (NH3–N) absorbed from the rumen. Our previous results showed that dietary inclusion with hot-pressed RSC decreased the ruminal concentration of NH3–N (Gao et al., 2021). Hence, dietary inclusion with RSC should have reduced the amount of NH3–N absorbed from the rumen, resulting in less urea synthesis in the liver and less urinary excretion of urea in steers. Since dietary inclusion with RSC did not affect the total urinary N excretion, the urea-N-to-urinary N ratio was decreased. The results were in agreement with Maxin et al. (2013) and Broderick et al. (2015) who reported that replacing soybean meal by rapeseed meal in the ration of dairy cows decreased the urinary excretion of urea and the urea-N-to-urinary N ratio. The results of the present study also showed that dietary inclusion with RSC increased the urinary excretions of allantoin and total PD and the predicted rumen microbial N flow. The results indicated that dietary inclusion with RSC improved the efficiency of rumen microbial N synthesis. Since the rumen microbial N synthesis is affected by many dietary factors, the exact mechanism for the results needs to be investigated in further research.
4.2. Effects of RSC on plasma indices and metabolome
Many studies indicated that feeding monogastric animals with RSC containing high GLS was harmful to thyroid function (Bourdon and Aumaitre, 1990). Ruminants are more tolerant to GLS than monogastric animals. However, feeding ruminants with high dietary GLS for long terms also negatively affects their health (Tripathi et al., 2001) since the metabolites of GLS such as goitrin and SCN can cause the goiter in animals (Halkier and Gershenzon, 2006). Results of the present study showed that dietary inclusion with RSC up to 8.0% DM did not affect the plasma concentrations of T3 and T4. The results are in agreement with Veselý et al. (2009) and Trøan et al. (2018), who reported that dietary inclusion with extruded RSC at 11.8% DM and heat-treated RSC at 8.4% DM, respectively, did not affect the plasma thyroid hormones in dairy cows. The results of present study indicated that the dietary content of GLS and metabolites up to 18.12 μmol/g DM did not affect the thyroid function of steers even though the plasma concentration of SCN was increased up to 87.07 μmol/L. It should be noted that each experimental period in the present study was only 20 d. Therefore, longer term feeding experiments are needed to be carried out to clarify the results.
The plasma metabolome analysis showed that dietary inclusion with RSC at 8.0% DM containing high GLS upregulated the plasma relative concentration of dhurrin, which was a type of cyanogenic glucoside similar to GLS. The results suggested that RSC not only contained GLS but also other secondary metabolites which could be transformed into dhurrin in metabolism, possibly because the CYP79 homologues in GLS-producing plants are able to convert amino acids (AA) into cyanogenic glucosides and GLS (Bak et al., 1998). Dhurrin could be readily hydrolyzed into hydrogen cyanide which is toxic and reduces the apparent nutrient digestibility in livestock (Etuk et al., 2012). The plasma dhurrin could possibly be derived from some antinutritional factors in RSC which could be one of the reasons for decreasing the digested N.
The plasma metabolome analysis also indicated that dietary inclusion with RSC at 8.0% DM decreased the plasma relative concentration of 4-aminohippuric acid (an acyl glycine), and increased hypoglycin B (an AA chemically related to lysine) compared with the treatment of 0 RSC. The impact could have resulted from the differences in the AA composition between RSC and soybean meal (NRC, 2001) and the dietray GLS. However, the exact mechanisms were unclear. The 4-aminohippuric acid is a non-toxic organic anion. It is unable to bind plasma protein or moves across the erythrocyte membranes (Rodríguez-Romero et al., 2015). Hypoglycin A could be converted into hypoglycin B by γ-glutamyl transpeptidase. The hypoglycin A and its metabolites can interfere with gluconeogenesis, which could result in a rapid depletion of hepatic glycogen (Gordon, 2015), suggesting that dietary inclusion with RSC could possibly depress the energy utilization in steers.
Dietary inclusion with RSC at 8.0% DM downregulated the plasma relative concentration of phosphatidylserine (14:0/16:0) in steers. The reason for the results could be that soybean meal contains phosphatidylserine (Sur et al., 2010) whereas RSC does not contain the compound. Phosphatidylserine functions as a constitutive component of membrane anionic domains that bind and thereby activate cytosolic proteins involved in neuronal signaling (Kim et al., 2000).
4.3. Effects of RSC containing high GLS on urine N2O emissions
The nitrogenous compounds in the urine of cattle mainly include urea, uric acid, creatinine, allantoin, hippuric acid etc., of which urea accounts for the major part of the compounds. Urea can be easily degraded into NH3 by microbial urease in soil and NH3 can be transformed into NH4+ (Whitehead et al., 1989) which is used as the major precursor for N2O formation during nitrification and denitrification in soil (Clough et al., 2020). Thus, urea in steer urine is the major N source for N2O formation. The positive correlation between the estimated steer urine N2O–N emission and the urinary urea-N in the present study confirmed the effect of urea on increasing the N2O–N emission. Since dietary inclusion with RSC decreased the urinary excretion of urea, it could be expected the urine N2O emission could be reduced. However, the results of the present study showed that dietary inclusion with RSC increased the urine N2O–N emissions even though the urinary excretion of urea was decreased. The results suggested that other urinary components should have increased the urine N2O–N emissions.
Gardiner et al. (2018) reported that non-urea nitrogenous compounds including allantoin, creatinine, creatine, and uric acid in cattle urine did not affect the EF3 of N2O. Hence, although dietary inclusion with RSC linearly increased the urinary excretions of allantoin and total PD, these compounds should have not affected the urine N2O–N emissions in the present study. It could be inferred that the GLS metabolites excreted in urine greatly affected the N2O–N emissions.
GLS are the specific compounds in brassica forages (Clarke, 2010) and can be hydrolyzed into SCN, goitrin and ITC (Oliviero et al., 2018). It was reported that the GLS in RSC was hydrolyzed into SCN in the rumen of steers whereas goitrin and ITC were undetectable (Gao et al., 2021). The results of the present study showed that dietary inclusion with RSC containing high GLS increased both the plasma and the urinary concentrations of SCN in steers. The results suggested that the SCN which was hydrolyzed from GLS in the rumen of steers, had been absorbed into the blood and partly excreted into the urine.
Snyder et al. (2010) reported that the SCN released from Brassicaceae Sinapis alba seed meal could be an important factor to inhibit the nitrification in soil. Kim et al. (2008) also reported that SCN (200 mg/L) slightly inhibited the nitrification in sludge. However, the actual effects of SCN on the nitrification in soil are unclear. Direct addition of ITC (i.e. 2-propenyl-ITC and phenylethyl ITC) also showed the impact on inhibiting the nitrification process and subsequently reducing the N2O emissions from soil applied with urine (Bending and Lincoln, 2000; Balvert et al., 2017). Since ITC as well as goitrin in steer urine were extremely low and undetectable in the present study, the effect of GLS metabolites on urine N2O formation should have come from SCN.
Majak (1992) reported that the disappearance rate of ITC was about 50 times that of SCN in in vitro culture of bovine ruminal fluid. Bheemreddy and Jeffery (2007) reported that most ITC degraded from GLS existed in the conjugates of ITC and N-acetylcysteine in rat urine. The results could explain the reason that the urinary ITC was undectable in steers fed RSC. The results suggested that the urine N2O emission can not be reduced by increasing the urinary excretion of ITC in steers.
Hoogendoorn et al. (2016) reported that the EF3 of N2O from the urine of sheep grazing on forage rape (Brassica napus L.) was higher than on the perennial ryegrass/white clover pasture (L. perenne L./T. repens). Balvert (2018) also reported that the total N2O emissions of the urine from cattle fed kale (Brassica oleracea L.) were higher than fed perennial ryegrass/white clover pasture (L. perenne L./T. repens). The results of the present study showed that the N2O–N emissions from the urine of steers fed with different levels of RSC linearly increased rather than decreased. A close positive correlation was found between the estimated N2O–N emission and the urinary SCN excretion in the present study. The results were in agreement with Hoogendoorn et al. (2016) and Balvert (2018) and suggested that the SCN excreted in the urine of steers fed with RSC had a positive effect on the urine N2O–N emissions. However, Luo et al. (2015) reported that the N2O from the urine of sheep fed fresh forage rape (Brassica napus L.) was much lower than fed fresh perennial ryegrass (L. perenne L.) (EF3 0.11% vs. 0.27%). The inconsistency in the results of different experiments could have resulted from the differences in urinary components of the animals in different experiments.
It was reported that the ammonia-oxidizing bacteria (AOB) was able to reduce NO2- and increase the N2O production through the nitrifier denitrification in soil (Wrage et al., 2001). In the aerobic reactor of wastewater, SCN was found to be able to increase the population of AOB (Kim et al., 2011). The results of the present study showed that SCN was the only metabolite of GLS excreted into the urine of the steers. This suggests that the urinary SCN could have increased the steer urine N2O–N emissions through enhancing the population of AOB in the soil. The results also showed that dietary inclusion with RSC increased the concentration of NH4+-N in the soil. However, it did not alter the concentration of NO3--N in the soil, suggesting that the conversion rate from NH4+-N to NO3--N was decreased in the soil applied with steer urine and more NH4+-N was converted into N2O–N.
Bell (1984) reviewed that the anti-nutritional factors in rapeseeds include GLS, erucic acid, sinapine, tannins and phytate. Leung et al. (1979) reported that the tannins in rapeseeds were condensed tannins which were only found in rapeseed hulls but not in rapeseed meats. Therefore, the metabolites of rapeseed tannins could hardly be excreted in steer urine and impact the urine N2O formation in the present study. However, it is unclear if the metabolites of erucic acid, sinapine, and phytate in RSC could be excreted in steer urine and affected the steer urine N2O emissions. This needs to be investigated in the future.
5. Conclusions
Dietary inclusion with RSC containing high GLS decreased the CP digestibility, but did not affect the urinary N excretion or the N retention rate in steers. GLS metabolite SCN was found in the plasma and the urine of steers fed RSC containing high GLS. Feeding steers RSC containing high GLS increased rather than decreased the urine N2O emissions although it decreased the urinary excretion of urea. The SCN excreted in urine could be the major factor increasing the urine N2O–N emissions. Other metabolites in RSC could possibly be excreted into the urine and consequently affect the urine N2O emissions in steers. Field trials are necessary to clarify the effects of SCN hydrolyzed from the GLS of RSC on steer urine N2O–N emissions.
Author contributions
Jian Gao: conducted the research, analyzed the data and drafted the manuscript; Bingbing Cheng, Yanfeng Sun and Yuchao Zhao: conducted part of the research; Guangyong Zhao: designed and supervised the research and revised the manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was supported by National Natural Science Foundation of China (grant no. 31772626).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Aires A., Mota V.R., Saavedra M.J., Rosa E.A.S., Bennett R.N. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J Appl Microbiol. 2009;106:2086–2095. doi: 10.1111/j.1365-2672.2009.04180.x. [DOI] [PubMed] [Google Scholar]
- AOAC . 19th ed. AOAC; Arlington, VA: 2012. Official methods of analysis, association of official analytical chemists. [Google Scholar]
- Balvert S. The University of Waikato; 2018. Can naturally occurring glucosinolate related compounds from brassica crops act as biological nitrification inhibitors and reduce nitrous oxide emissions? [Doctor degree thesis dissertation] [Google Scholar]
- Balvert S.F., Luo J., Schipper L.A. Do glucosinolate hydrolysis products reduce nitrous oxide emissions from urine affected soil? Sci Total Environ. 2017;603:370–380. doi: 10.1016/j.scitotenv.2017.06.089. [DOI] [PubMed] [Google Scholar]
- Bak S., Nielsen H.L., Halkier B.A. The presence of CYP79 homologues in glucosinolate-producing plants shows evolutionary conservation of the enzymes in the conversion of amino acid to aldoxime in the biosynthesis of cyanogenic glucosides and glucosinolates. Plant Mol Biol. 1998;38:725–734. doi: 10.1023/a:1006064202774. [DOI] [PubMed] [Google Scholar]
- Bao Y., Zhou K., Zhao G. Nitrous oxide emissions from the urine of beef cattle as regulated by dietary crude protein and gallic acid. J Anim Sci. 2018;96:3699–3711. doi: 10.1093/jas/sky252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. Nutrients and toxicants in rapeseed meal: a review. J Anim Sci. 1984;58:996–1010. doi: 10.2527/jas1984.584996x. [DOI] [PubMed] [Google Scholar]
- Bending G.D., Lincoln S.D. Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products. Soil Biol Biochem. 2000;32:1261–1269. [Google Scholar]
- Bheemreddy R.M., Jeffery E.H. The metabolic fate of purified glucoraphanin in F344 rats. J Agric Food Chem. 2007;55:2861–2866. doi: 10.1021/jf0633544. [DOI] [PubMed] [Google Scholar]
- Bourdon D., Aumaitre A. Low-glucosinolate rapeseeds and rapeseed meals: effect of technological treatments on chemical composition, digestible energy content and feeding value for growing pigs. Anim Feed Sci Technol. 1990;30:175–191. [Google Scholar]
- Broderick G.A., Faciola A.P., Armentano L.E. Replacing dietary soybean meal with canola meal improves production and efficiency of lactating dairy cows. J Dairy Sci. 2015;98:5672–5687. doi: 10.3168/jds.2015-9563. [DOI] [PubMed] [Google Scholar]
- Chen X.B., Gomes M.J. Rowett Research Institute; Aberdeen: 1992. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivatives-an overview of the technical details. [Google Scholar]
- Chen X.B., Ørskov E.R. In: Estimation of microbial protein supply in ruminant using urinary purine derivatives. Makkar H.P.S., Chen X.B., editors. Kluwer Academic Publishers; The Netherlands: 2004. Research on urinary excretion of purine derivatives in ruminants: past, present and future. [Google Scholar]
- China Hygienic Standard . Standards Press of China; Beijing: 1996. Urine—determination of hippuric acid—spectrophotometric method No. WS/T 52-1996. [Google Scholar]
- China Hygienic Standard . Standards Press of China; Beijing: 1996. Urine—determination of thiocyanate—pyridine-barbituric acid spectrophotometric method No. WS/T 39-1996. [Google Scholar]
- China National Environmental Protection . Standards Press of China; Beijing: 2012. Standard. Soil-Determination of ammonuim, nitrite and nitrate by extraction with potassium chloride solution-spectrophotometric method No. HJ 634-2012. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2016. Determination of nitrate nitrogen in soil-Ultraviolet spectrophotometry method No. GB/T 32737-2016. [Google Scholar]
- Clarke D.B. Glucosinolates, structures and analysis in food. Anal Methods-UK. 2010;2:310–325. [Google Scholar]
- Clough T.J., Cardenas L.M., Friedl J., Wolf B. Nitrous oxide emissions from ruminant urine: science and mitigation for intensively managed perennial pastures. Curr Opin in Env Sust. 2020;47:1–7. [Google Scholar]
- Di H., Cameron K. The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in a simulated grazed and irrigated grassland. Soil Use Manag. 2002;18:395–403. [Google Scholar]
- Dijkstra J., Oenema O., Van Groenigen J.W., Spek J.W., Van Vuuren A.M., Bannink A. Diet effects on urine composition of cattle and N2O emissions. Animal. 2013;7:292–302. doi: 10.1017/S1751731113000578. [DOI] [PubMed] [Google Scholar]
- Dillard S.L., Roca-Fernández A.I., Rubano M.D., Elkin K.R., Soder K.J. Enteric methane production and ruminal fermentation of forage brassica diets fed in continuous culture. J Anim Sci. 2018;96:1362–1374. doi: 10.1093/jas/sky030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R., Zhao G., Chai L., Beauchemin K.A. Prediction of urinary and fecal nitrogen excretion by beef cattle. J Anim Sci. 2014;92:4669–4681. doi: 10.2527/jas.2014-8000. [DOI] [PubMed] [Google Scholar]
- Etuk E.B., Okeudo N.J., Esonu B.O., Udedibie A.B.I. Anti-nutritional factors in sorghum: chemistry, mode of action and effects on livestock and poultry. Online J Anim Feed Res. 2012;2:113–119. [Google Scholar]
- FAO (Food and Agriculture Organisation) FAOSTAT; 2015. Online statistical database: emissions-Agriculture.http://faostat3.fao.org/faostat-gateway/go/to/home/ [Google Scholar]
- Feng Y. China Agricultural University Press; Beijing: 2000. The nutrient requirements and feeding standards of beef cattle. [Google Scholar]
- Gao J., Sun Y., Bao Y., Zhou K., Kong D., Zhao G. Effects of different levels of rapeseed cake containing high glucosinolates in steer ration on rumen fermentation, nutrient digestibility and the rumen microbial community. Br J Nutr. 2021;125:266–274. doi: 10.1017/S0007114520002767. [DOI] [PubMed] [Google Scholar]
- Gardiner C.A., Clough T.J., Cameron K.C., Di H.J., Edwards G.R., De Klein C.A. Assessing the impact of non-urea ruminant urine nitrogen compounds on urine patch nitrous oxide emissions. J Environ Qual. 2018;47:812–819. doi: 10.2134/jeq2018.03.0112. [DOI] [PubMed] [Google Scholar]
- Gordon A. In: Food safety and quality systems in developing countries. Gordon A., editor. Academic Press; New York: 2015. Biochemistry of hypoglycin and toxic hypoglycemic syndrome; pp. 47–61. [Google Scholar]
- Halkier B.A., Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn C.J., Luo J., Lloyd-West C.M., Devantier B.P., Lindsey S.B., Sun S., et al. Nitrous oxide emission factors for urine from sheep and cattle fed forage rape (Brassica napus L.) or perennial ryegrass/white clover pasture (Lolium perenne L./Trifolium repens) Agric Ecosyst Environ. 2016;227:11–23. [Google Scholar]
- Kim H.Y., Akbar M., Lau A., Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3): role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–35223. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- Kim Y.M., Park D., Lee D.S., Park J.M. Inhibitory effects of toxic compounds on nitrification process for cokes wastewater treatment. J Hazard Mater. 2008;152:915–921. doi: 10.1016/j.jhazmat.2007.07.065. [DOI] [PubMed] [Google Scholar]
- Kim Y.M., Cho H.U., Lee D.S., Park C., Park D., Park J.M. Response of nitrifying bacterial communities to the increased thiocyanate concentration in pre-denitrification process. Bioresour Technol. 2011;102:913–922. doi: 10.1016/j.biortech.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Kool D.M., Hoffland E., Hummelink E.W.J., Van Groenigen J.W. Increased hippuric acid content of urine can reduce soil N2O fluxes. Soil Biol Biochem. 2006;38:1021–1027. [Google Scholar]
- Leung J., Fenton T., Mueller M., Clandinin O. Condensed tannins of rapeseed meal. J Food Sci. 1979;44:1313–1317. [Google Scholar]
- Levy-Booth D.J., Prescott C.E., Grayston S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol Biochem. 2014;75:11–25. [Google Scholar]
- Licitra G.T., Hernandez M., Van Soest P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim Feed Sci Technol. 1996;57:347–358. [Google Scholar]
- Liu C., Wu H., Liu S., Chai S., Meng Q., Zhou Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front Microbiol. 2019;10:1116. doi: 10.3389/fmicb.2019.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Sun X.Z., Pacheco D., Ledgard S.F., Lindsey S.B., Hoogendoorn C.J., et al. Nitrous oxide emission factors for urine and dung from sheep fed either fresh forage rape (Brassica napus L.) or fresh perennial ryegrass (Lolium perenne L.) Animal. 2015;9:534–543. doi: 10.1017/S1751731114002742. [DOI] [PubMed] [Google Scholar]
- Majak W. Stability of allylthiocyanate and allylisothiocyanate in bovine ruminal fluid. Toxicol Lett. 1992;63:75–78. doi: 10.1016/0378-4274(92)90109-w. [DOI] [PubMed] [Google Scholar]
- Matthäus B., Fiebig H.J. Simultaneous determination of isothiocyanates, indoles, and oxazolidinethiones in myrosinase digests of rapeseeds and rapeseed meal by HPLC. J Agric Food Chem. 1996;44:3894–3899. [Google Scholar]
- Maxin G., Ouellet D.R., Lapierre H. Ruminal degradability of dry matter, crude protein, and amino acids in soybean meal, canola meal, corn, and wheat dried distillers grains. J Dairy Sci. 2013;96:5151–5160. doi: 10.3168/jds.2012-6392. [DOI] [PubMed] [Google Scholar]
- McGeough K., Watson C., Müller C., Laughlin R., Chadwick D. Evidence that the efficacy of the nitrification inhibitor dicyandiamide (DCD) is affected by soil properties in UK soils. Soil Biol Biochem. 2016;94:222–232. [Google Scholar]
- Minet E., Ledgard S., Grant J., Murphy J., Krol D., Lanigan G., et al. Feeding dicyandiamide (DCD) to cattle: an effective method to reduce N2O emissions from urine patches in a heavy-textured soil under temperate climatic conditions. Sci Total Environ. 2018;615:1319–1331. doi: 10.1016/j.scitotenv.2017.09.313. [DOI] [PubMed] [Google Scholar]
- NRC . 7th ed. National Academy of Sciences; Washington DC: 2001. Nutrient requirements of dairy cattle. [Google Scholar]
- Oliviero T., Verkerk R., Dekker M. Isothiocyanates from Brassica vegetables—effects of processing, vooking, mastication, and digestion. Mol Nutr Food Res. 2018;62:1701069. doi: 10.1002/mnfr.201701069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne M., Kuoppala K., Ahvenjärvi S., Vanhatalo A. Dairy cow responses to graded levels of rapeseed and soya bean expeller supplementation on a red clover/grass silage-based diet. Animal. 2015;9:1958–1969. doi: 10.1017/S1751731115001263. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Romero V., González-Villalva K.I., Reyes J.L., Franco-Bourland R.E., Guízar-Sahagún G., et al. A novel, simple and inexpensive procedure for the simultaneous determination of iopamidol and p-aminohippuric acid for renal function assessment from plasma samples in awake rats. J Pharmaceut Biomed Anal. 2015;107:196–203. doi: 10.1016/j.jpba.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Sanchez-Martín L., Beccaccia A., De Blas C., Sanz-Cobena A., García-Rebollar P., Estellés F., et al. Diet management to effectively abate N2O emissions from surface applied pig slurry. Agric Ecosyst Environ. 2017;239:1–11. [Google Scholar]
- Snyder A.J., Johnson-Maynard J.L., Morra M.J. Nitrogen mineralization in soil incubated with 15N-labeled Brassicaceae seed meals. Appl Soil Ecol. 2010;46:73–80. [Google Scholar]
- Sur B.J., Lee B., Yeom M., Han J.J., Choi H.D., Lee H., et al. Inhibitory effect of phosphatidylserine on atopy-like dermatitis in NC/Nga mice. Food Sci Biotechnol. 2010;19:1513–1518. [Google Scholar]
- Tholen J.T., Shen S., Truscott R.J.W. The thymol method for glucosinolate determination. J Sci Food Agric. 1989;49:157–165. [Google Scholar]
- Thomke S., Pettersson H., Neil M., Håkansson J. Skeletal muscle goitrin concentration and organ weights in growing pigs fed diets containing rapeseed meal. Anim Feed Sci Technol. 1998;73:207–215. [Google Scholar]
- Tripathi M.K., Agrawal I.S., Sharma S.D., Mishra D.P. Effect of untreated, HCl treated or copper and iodine supplemented high glucosinolate mustard (Brassica juncea) meal on nutrient utilization, liver enzymes, thyroid hormones and growth of calves. Anim Feed Sci Technol. 2001;92:73–85. [Google Scholar]
- Trøan G., Pihlava J.M., Brandt-kjelsen A., Salbu B., Prestløkken E. Heat-treated rapeseed expeller press cake with extremely low glucosinolate content reduce transfer of iodine to cow milk. Anim Feed Sci Technol. 2018;239:66–73. [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Veselý A., Křížová L., Třináctý J., Hadrová S., Navrátilová M., Herzig I., et al. Changes in fatty acid profile and iodine content in milk as influenced by the inclusion of extruded rapeseed cake in the diet of dairy cows. Czech J Anim Sci. 2009;54:201–209. [Google Scholar]
- Watanabe T., Osada T., Yoh M., Tsuruta H. N2O and NO emissions from grassland soils after the application of cattle and swine excreta. Nutrient Cycl Agroecosyst. 1997;49:35–39. [Google Scholar]
- Wathelet J.P., Wagstaffe P.J., Biston R., Marlier M., Severin M. Rapeseed reference materials for glucosinolate analysis. Fresenius Z Anal Chem. 1988;332:689–693. [Google Scholar]
- Wang B., Ma M.P., Diao Q.Y., Tu Y. Saponin-induced shifts in the rumen microbiome and metabolome of young cattle. Front Microbiol. 2019;10:356. doi: 10.3389/fmicb.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welten B., Ledgard S., Balvert S., Kear M., Dexter M. Effects of oral administration of dicyandiamide to lactating dairy cows on residues in milk and the efficacy of delivery via a supplementary feed source. Agric Ecosyst Environ. 2016;217:111–118. [Google Scholar]
- WMO (World Metrorological Organization) 2017. WMO Greenhouse gas bulletin.https://public.wmo.int/en/resources/library/wmo-greenhouse-gas-bulletin [Google Scholar]
- Whitehead D.C., Lockyer D.R., Raistrick N. Volatilization of ammonia from urea applied to soil: influence of hippuric acid and other constituents of livestock urine. Soil Biol Biochem. 1989;21:803–808. [Google Scholar]
- Wrage N., Velthof G.L., Van Beusichem M.L., Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem. 2001;33:1723–1732. [Google Scholar]
- Zhao G.Y. Modulation of protein metabolism to mitigate nitrous oxide (N2O) emission from excreta of livestock. Curr Protein Pept Sci. 2017;18:525–531. doi: 10.2174/1389203717666160627080423. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Rahman M.S., Zhao G.Y., Bao Y., Zhou K. Dietary supplementation of rumen-protected methionine decreases the nitrous oxide emissions of urine of beef cattle through decreasing urinary excretions of nitrogen and urea. J Sci Food Agric. 2020;100:1797–1805. doi: 10.1002/jsfa.10217. [DOI] [PubMed] [Google Scholar]
- Zhou K., Bao Y., Zhao G.Y. Effects of dietary crude protein and tannic acid on nitrogen excretion, urinary nitrogenous composition and urine nitrous oxide emissions in beef cattle. J Anim Physiol Anim Nutr. 2019;103:1675–1683. doi: 10.1111/jpn.13186. [DOI] [PubMed] [Google Scholar]
- Zhu X., Burger M., Doane T.A., Horwath W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci USA. 2013;110:6328–6333. doi: 10.1073/pnas.1219993110. [DOI] [PMC free article] [PubMed] [Google Scholar]