Abstract
Introduction and importance
Metastatic ovarian cancer of breast carcinoma is rare and accounts for only 0.68%–2% of all ovarian tumors, the majority of which are diagnosed incidentally during follow-up or therapeutic oophorectomy. Risk-reducing salpingo-oophorectomy (RRSO) is a highly recommended prophylactic surgery associated with a significant decrease in ovarian cancer risk in both BRCA1 and BRCA2 mutation carriers, and in those with and without prior breast cancer.
Case presentation
We present two cases who presented with a lump in the right axilla and left breast respectively and were subsequently diagnosed as invasive mammary carcinoma but later, diagnosed with metastatic ovarian carcinoma with breast primary after RRSO. The patients were treated with cycles of chemo-radiation.
Discussion
Invasive lobular carcinoma, larger tumor size, advanced breast cancer increases the risk of developing ovarian metastases. Thorough macroscopic, microscopic, immunohistochemical, and molecular tests are considered the cornerstone in the diagnosis of metastatic breast cancer to the ovaries. Bilateral salpingo-oophorectomy, a risk-reducing surgery for the BRCA gene mutation, has been shown to reduce the risk of death from ovarian cancer.
Conclusions
Metastatic ovarian cancer in breast cancer is a rare possibility among patients undergoing RRSO for breast cancer. The importance of continued surveillance of ovaries rather than the delayed diagnosis of ovarian metastasis for patients with newly diagnosed breast cancer cannot be less emphasized.
Keywords: Case report, Metastasis, Ovarian cancer, Breast cancer, Risk-reducing salpingo-oophorectomy
Highlights
-
•
Metastatic ovarian cancer of carcinoma of the breast is rare.
-
•
Risk-reducing salpingo-oophorectomy decreases ovarian cancer risk in breast cancer.
-
•
Regular follow-up of breast cancer cases with gynecological examination is necessary.
1. Introduction
Ovarian metastasis of breast carcinoma is uncommon. A study in the Netherlands with 2648 patients aged <41 years and diagnosed with primary invasive breast cancer (BC) who underwent a unilateral/bilateral oophorectomy as a prophylactic or therapeutic purpose, showed that only 63 patients (2.4%) had histologically proven ovarian metastasis [1]. Similarly, another study with 10,944 new cases of ovarian cancer showed that only 75 cases (0.68%) were diagnosed as metastatic breast cancer [2,3].
In the majority of cases, the patients are asymptomatic and diagnosis is made incidentally during follow-up breast cancer examination or therapeutic oophorectomy/castration surgery [4].
Among patients with BRCA1 and BRCA2 mutations, risk-reducing salpingo-oophorectomy (RRSO) can significantly reduce the risk of ovarian cancer from 85% to 95% and is thus strongly advised once childbearing is complete [5]. In addition, patients with prior breast cancer have a significant decrease in ovarian cancer after RRSO [6]. In addition to decreasing this risk, RRSO also helps to detect occult early-stage ovarian or fallopian tube cancer (2–10% of cases) along with metastatic breast cancer if any (1–2%), after the procedure [7].
Herein, we report two case reports of diagnosed invasive carcinoma of the breast in whom ovarian metastasis was found after RRSO along with a review of the literature. The cases have been reported in line with SCARE criteria [8].
2. Presentation of case
2.1. Case 1
A 37-year P1L1, regularly menstruating, presented with a complaint of a single, progressive lump over the right axilla for a year. There was no complaint of pain, overlying skin changes, change in lump size during menstruation and nipple discharge, and no complaint of the contralateral breast. She had no family/personal history of breast and ovarian cancer.
She presented to our center a diagnosed case of invasive mammary carcinoma with liver and bone metastasis. She had received seven cycles of cyclophosphamide-based neoadjuvant chemotherapy.
On follow-up CT scan, liver lesions were static. Additionally, lungs and bone metastasis were detected. MRI of the breast revealed locally aggressive primary neoplasm of the right breast with skin involvement. The bone scan also showed bony metastasis in C7/D1, D4-5. In addition, her CA-15.3 was raised to 52.8 U/ml. No lesions were detected in the ovaries on the CT scan and the USG of the abdomen and pelvis.
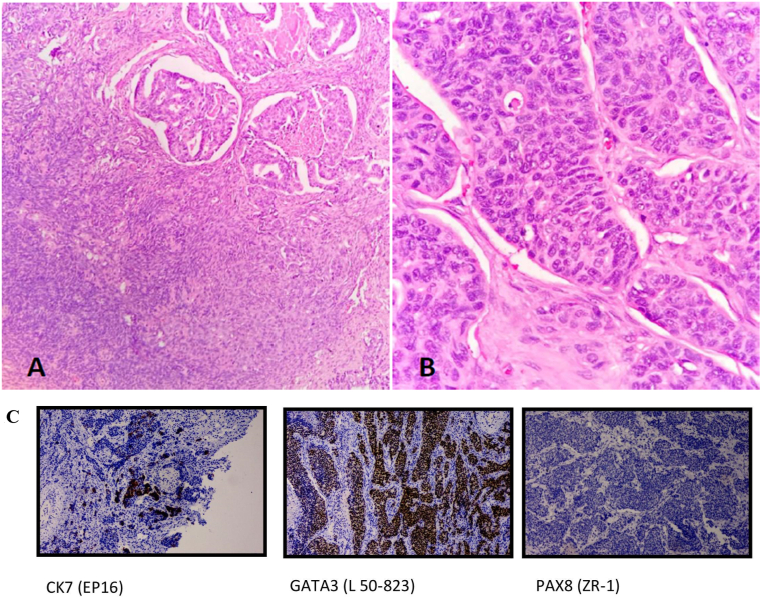
After metastatic work-up, the patient underwent a right-modified radical mastectomy (MRM) along with bilateral RRSO by the team of breast oncosurgeon and gynecologists owing to the hormone status (ER+, PR+) of the breast tumor. Histopathological examination of the excised specimen from the right breast showed invasive carcinoma of no special type and the examination of the left ovary revealed a metastatic breast carcinoma with ER/PR+. Immunohistochemistry studies showed ovarian tumor cells positive for CK7, GATA3, mammoglobin, ER and PR while negative for CK20, WT1, and PAX8 (Fig. 1).
Fig. 1.
A) Section shows tumor cells infiltrating the ovarian parenchyma. B) The tumor shows pleomorphism and has enlarged nuclei, irregular nuclear contours, visible nucleoli, and a scant to moderate amount of cytoplasm. Mitosis noted. (Case 1)
Fig. 1C: Immunohistochemistry analysis from the ovary showing tumor cells positive for CK7, GATA3, and negative for PAX8. (Case 1).
However, gross and microscopic examination of the right ovary was unremarkable. Because of ER and PR positive status, the patient was prescribed letrozole and zoledronic acid four weekly.
She received 10 cycles of radiation therapy on the lower cervical spine with margin (C5-D2) over two weeks because of severe back pain due to bone metastasis. Currently, the patient is on letrozole, Palbociclib, and zoledronic acid and is on regular follow-up.
2.2. Case two
A 33-year P1L1, regularly menstruating, presented with a gradually increasing painless lump in the left breast for three weeks. There was no history of any skin changes, ipsilateral axillary lumps, contralateral breast complaints or family history of breast/ovarian malignancy.
On examination, an approximately 3 cm × 3 cm non-tender, hard palpable lump in the left lower quadrant of the breast was present which was fixed to the overlying skin and had well-defined margins. Ultrasound of the left breast showed an ill-defined lobulated hypoechoic mass and the right breast was normal. Mammography showed irregular hypoechoic subcutaneous lesion at 4–5 o'clock zone B/C of the left lower outer quadrant measuring 2.2cm × 1.8cm. Multiple enlarged lymph nodes were noted in the left axilla. No significant enlarged lymph nodes were noted in the left supra/infraclavicular region or internal mammary chain. Fine needle aspiration cytology of the breast mass was suggestive of invasive ductal carcinoma of the breast.
The patient underwent a left MRM. Histopathological examination showed invasive carcinoma of no special type, Grade II, pT2N2a. She received irradiation on the left chest wall after the completion of three cycles of chemotherapy. Evaluation after radiation therapy showed complete remission and the patient was under regular follow-up with complete remission for three years with hormonal therapy.
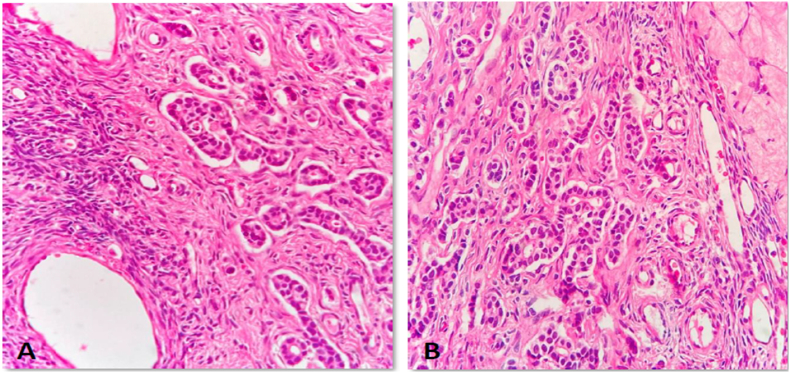
However, three years after remission, during regular evaluation, MRI whole body showed minimal pleural effusion with multiple pleural-based nodules with foci of restricted diffusion in left pleural space, suspicious of metastasis, and bilateral small ovarian cyst with a small hemorrhagic cyst in left ovary. Biopsy of pleural mass revealed metastatic poorly differentiated carcinoma. CA-15.3 was elevated to 98.8 U/mL. In the view of relapsed breast carcinoma, she was planned for goserelin, oral palbociclib, and letrozole. After 6 months of chemotherapy, the patient underwent laparoscopic bilateral salpingo-oophorectomy for persistent complex ovarian cyst (Fig. 2). Histopathology of the left ovary showed metastatic ovarian carcinoma with breast primary (Fig. 3). As per the consensus of the expert panel, a second-line chemotherapy fulvestrant was started and the patient is under regular follow-up.
Fig. 2.
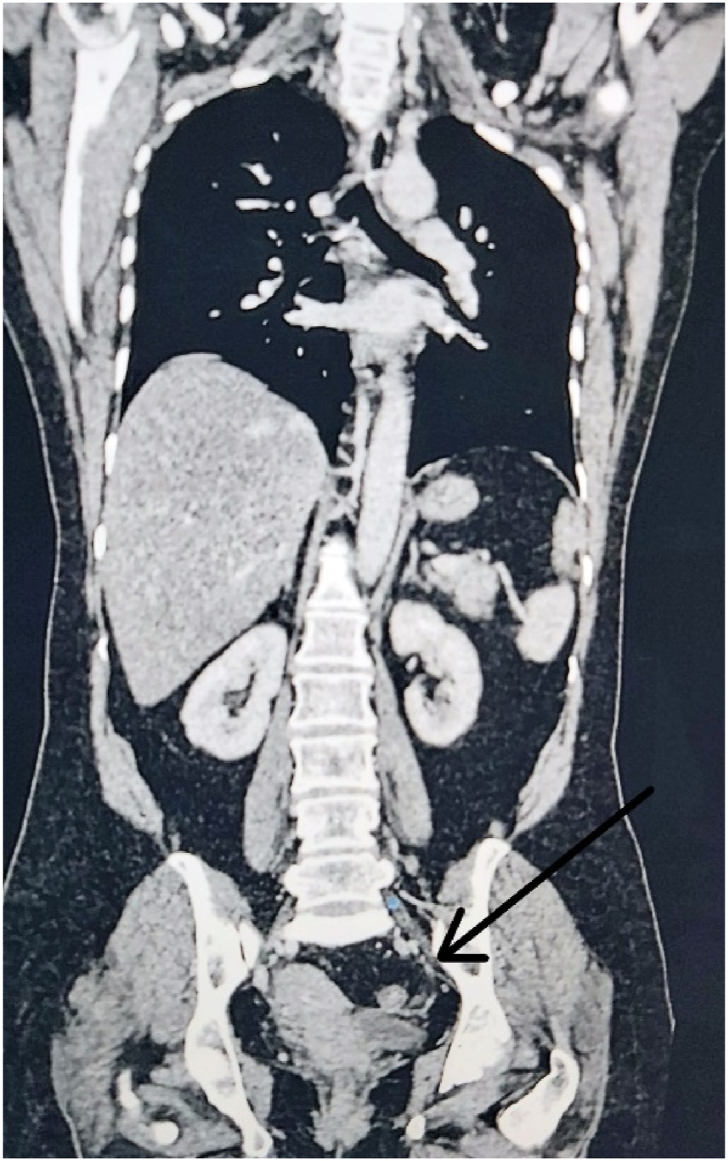
CT scan (coronal section) shows left ovarian cyst with internal septations. (Case 2).
Fig. 3.
A & B) Section from the ovary shows features of metastatic carcinoma, likely from the breast. These tumors show atypical cells infiltrating the ovarian parenchyma. They are arranged in tubules and clusters. (Case 2).
3. Discussion
Ovarian metastasis as compared to primary ovarian tumors is difficult to diagnose, manage and has a worse prognosis [9]. History of breast cancer, ovarian cancer in the family, BRCA1/2 gene mutation, advanced breast cancer, and premenopausal state which tend to activate cancer cells increase the risk of metastatic ovarian cancer [10,11]. Both of our patients were premenopausal with no family history of malignancy but metastatic lesions were present in the first case. The risk of ovarian metastasis in breast cancer depends on the morphological subtypes of breast cancer. Though, ovarian metastasis is often seen in patients with invasive ductal carcinoma owing to a higher frequency of invasive ductal carcinoma. However, the metastatic tendency of invasive lobular carcinoma to the ovaries is three times greater [4]. On the contrary, Peters et al. found that there are no significant differences in histological subtype between the cases and matched controls among young breast cancer patients [1]. Additionally, larger tumor size (i.e. >5 cm), the presence of inflammatory breast cancer, and the presence of other organ-system metastasis are associated with an increased risk of developing ovarian metastasis [1,12]. Both of our cases had invasive ductal carcinoma with a tumor size of less than 5cm. However, the first patient had metastasis to the liver and bone at the time of presentation and thus RRSO was performed as a palliative procedure.
Ovarian metastasis of breast cancer is usually bilateral, and often seen in premenopausal hormone receptor-positive young women [13]. However, metastatic and early primary ovarian carcinoma cannot be differentiated based on laterality [14]. Interestingly our case had a unilateral finding of metastasis in the left ovary. No clinically detectable lesion suggestive of malignancy was present in our case and a prophylactic bilateral salpingo-oophorectomy was done for persistent complex cyst in the second case. However, both the cases of breast cancer were ER and PR positive.
As it is difficult to diagnose ovarian metastasis from breast cancer, a high level of suspicion along with macroscopic, microscopic, immunohistochemical, and molecular tests are the gold standard in the diagnosis of metastatic breast cancer to the ovaries [15]. Positive mammaglobin aids in determining breast as the origin for metastasis [14].
The most important step in the management of ovarian metastasis from breast cancer is surgery as it also helps in further diagnosis and staging. For a suspicious ovarian mass in breast cancer patients, a simple laparoscopic oophorectomy can help pathological diagnosis, metastatic tumor removal, and therapeutic castration [4]. However, high-risk women should be offered prophylactic oophorectomy and the need to possibly undergo a staging procedure if indicated should be well explained to those patients [16]. Additional systemic therapies (chemotherapy, endocrine therapy, and anti-HER2 therapy) should be considered if there are other organ metastasis [4].
As ovarian metastasis of breast cancer often has a poor prognosis, patients should be carefully monitored after treatment of primary breast cancer including a proper gynecological assessment. At present, women with a family history of breast cancer and ovarian cancer with high suspicion should be offered appropriate genetic testing and counseling for BRCA1/2 mutations. Though RRSO offers an enormous benefit in preventing primary ovarian cancer among women with BRCA mutation, Genetic screening for BRCA1/2 mutation is highly expensive and not easily available in developing countries like Nepal. We had advised both of the patients to undergo the mutation analysis but they refused owing to the costliness of the test. Hence, we need to rely on meticulous history, clinical examination, and investigations such as sonography and tumor markers. However, we do counsel all patients on germline tests as standard practice and on hereditary breast/ovarian cancer.
4. Conclusion
Ovarian metastasis in breast cancer is rare. RRSO done as prophylactic surgery reduces ovarian cancer risk in patients with primary breast cancer. Regular follow-up of women with breast cancer is necessary; clinical assessment should not neglect gynecological examination. Finally, we emphasize the importance of continued surveillance of ovaries for patients with newly diagnosed breast cancer.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
This research work did not receive any kind of funding.
Ethical approval
Not Applicable.
Consent
Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Prafulla Shakya- Study concept, data collection and analysis and surgical treatment of the patient. Anu Shakya- Study concept, data collection and analysis and surgical treatment of the patient. Suraj Shrestha- Study design, data analysis and writing the paper. Sanjeev Kharel- Study design, data analysis and writing the paper. Rubina Maharajan- Study design, data analysis and writing the paper. Aagon Krishna Shrestha-data collection and analysis and surgical treatment of the patient.
Registration of research studies
Name of the registry:
Unique Identifying number or registration ID:
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Dr. Prafulla Shakya, Email: Prafulla_shakya@hotmail.com.
Declaration of competing interest
None to declare.
Acknowledgment
None.
Contributor Information
Prafulla Shakya, Email: prafulla_shakya@hotmail.com.
Anu Bajracharya, Email: dr.anubajracharya42@gmail.com.
Suraj Shrestha, Email: multisurazz@gmail.com.
Sanjeev Kharel, Email: kharel_sanjeev@iom.edu.np.
Rubina Maharjan, Email: dr.rubina.maharjan@gmail.com.
Aagon Krishna Shrestha, Email: aaga555_shrestha@hotmail.com.
References
- 1.Peters I.T.A., van Zwet E.W., Smit V.T.H.B.M., et al. Prevalence and risk factors of ovarian metastases in breast cancer patients < 41 Years of age in The Netherlands: a nationwide retrospective cohort study. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skírnisdóttir I., Garmo H., Holmberg L. Non-genital tract metastases to the ovaries presented as ovarian tumors in Sweden 1990-2003: occurrence, origin and survival compared to ovarian cancer. Gynecol. Oncol. 2007;105(1):166–171. doi: 10.1016/j.ygyno.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Bigorie V., Morice P., Duvillard P., et al. Ovarian metastases from breast cancer: report of 29 cases. Cancer. 2010;116(4):799–804. doi: 10.1002/cncr.24807. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Tian W., Zhou Y., Zhang X., Deng Y. Ovarian metastasis from breast cancer in three Chinese females: three case reports. Medicine. 2019;98(17) doi: 10.1097/MD.0000000000015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchetti C., De Felice F., Palaia I., et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Wom. Health. 2014;14:150. doi: 10.1186/s12905-014-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domchek S.M., Friebel T.M., Singer C.F., et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. J. Am. Med. Assoc. 2010;304(9):967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callahan M.J., Crum C.P., Medeiros F., et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol. 2007;25(25):3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Simpkins F., Zahurak M., Armstrong D., Grumbine F., Bristow R. Ovarian malignancy in breast cancer patients with an adnexal mass. Obstet. Gynecol. 2005;105(3):507–513. doi: 10.1097/01.AOG.0000154162.51442.14. [DOI] [PubMed] [Google Scholar]
- 10.Bergfeldt K., Rydh B., Granath F., et al. Risk of ovarian cancer in breast-cancer patients with a family history of breast or ovarian cancer: a population-based cohort study. Lancet. 2002;360(9337):891–894. doi: 10.1016/S0140-6736(02)11023-3. [DOI] [PubMed] [Google Scholar]
- 11.Ternovoĭ S.K., Matkhev S., Meladze N.V., Solopova A.E., Abduraimov A.B., Les’ko K.A. [Mammographic screening with the assessment of risk factors and incidence of BRCA-associated breast cancer in the Republic of India] Vestn Rentgenol Radiol. 2012;5:16–21. https://www.ncbi.nlm.nih.gov/pubmed/23516880 [PubMed] [Google Scholar]
- 12.Feng Y., Zhao X., Lv S., Xiong B., Li Y., Zhang M. Long disease-free survival (48 months) in a breast cancer patient with ovarian and pelvic metastasis—a case report. Transl. Cancer Res. 2020;9(12):7669–7675. doi: 10.21037/tcr-20-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pimentel C., Becquet M., Lavoue V., Henno S., Leveque J., Ouldamer L. Ovarian metastases from breast cancer: a series of 28 cases. Anticancer Res. 2016;36(8):4195–4200. https://www.ncbi.nlm.nih.gov/pubmed/27466531 [PubMed] [Google Scholar]
- 14.Rabban J.T., Barnes M., Chen L.-M., Powell C.B., Crawford B., Zaloudek C.J. Ovarian pathology in risk-reducing salpingo-oophorectomies from women with BRCA mutations, emphasizing the differential diagnosis of occult primary and metastatic carcinoma. Am. J. Surg. Pathol. 2009;33(8):1125–1136. doi: 10.1097/PAS.0b013e31819e986a. [DOI] [PubMed] [Google Scholar]
- 15.Kubeček O., Laco J., Špaček J., et al. The pathogenesis, diagnosis, and management of metastatic tumors to the ovary: a comprehensive review. Clin. Exp. Metastasis. 2017;34(5):295–307. doi: 10.1007/s10585-017-9856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leeper K., Garcia R., Swisher E., Goff B., Greer B., Paley P. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol. Oncol. 2002;87(1):52–56. doi: 10.1006/gyno.2002.6779. [DOI] [PubMed] [Google Scholar]