Abstract
Myocarditis is a nonischemic inflammatory disease of the myocardium that can be triggered by a multitude of events, including viral infection and toxins. Recently, there has been heightened interest in myocarditis given its association with COVID-19 vaccination. Timely identification of myocarditis can affect patient management and prognosis. Therefore, it is crucial for radiologists and cardiac imagers to understand the role of cardiac imaging to establish a diagnosis and inform treatment decisions. Cardiac MRI is the most important noninvasive imaging modality for evaluation of myocarditis, with typical findings of focal or diffuse myocardial edema and myocardial damage, including presence of late gadolinium enhancement. There are currently limited data available to indicate that the pattern of myocardial injury following COVID-19 vaccination is similar to other causes of myocarditis, although the severity of disease may be relatively mild. A description of the role of imaging and typical imaging features will be reviewed here, with a focus on emerging data in the setting of myocarditis after COVID-19 vaccination.
Keywords: MRI, Heart, Inflammation
© RSNA, 2021
Keywords: MRI, Heart, Inflammation
Summary
Radiologists and other cardiac imagers should understand the role of cardiac imaging and typical features in the setting of myocarditis, including after COVID-19 vaccination, given that timely identification can affect patient treatment and prognosis.
Essentials
■ Cardiac MRI is the most important noninvasive cardiac imaging modality for the evaluation of myocarditis with typical imaging findings including myocardial edema and late gadolinium enhancement.
■ Myocarditis can be triggered by a multitude of events, although recent attention has focused on the association with COVID-19 vaccination, particularly in younger males.
■ Limited data to date indicate that the pattern of myocardial injury following COVID-19 vaccination is similar to other causes of myocarditis; the disease severity may be relatively mild, although outcome data are lacking, and further study is needed.
Introduction
Myocardial inflammation is an important cause of myocardial injury, which often results from the host immune response, and can be triggered by infection, autoimmune diseases, ischemic injury, or toxins. Myocarditis is a more specific term, defined as a nonischemic inflammatory disease of the myocardium. Traditionally, diagnosis has relied on histologic evaluation of the myocardium showing inflammation and myocyte damage (1). Recently, there has been heightened awareness of myocarditis due to reports of myocardial inflammation after COVID-19 illness and vaccination. Cardiac MRI plays an important role in the assessment of suspected myocarditis, as timely identification can affect patient management and prognosis (2). The aim of this review is to provide an overview of the role of cardiac MRI and typical findings in patients with nonischemic myocardial inflammation, with a focus on emerging data in the setting of acute myocarditis after COVID-19 vaccination.
Incidence and Pathophysiology of Myocarditis
The incidence of myocarditis is difficult to establish, as clinical symptoms are nonspecific, including chest pain and shortness of breath, and endomyocardial biopsy is not frequently performed for definitive diagnosis. Approximately one-third of patients presenting with acute coronary syndrome without substantial coronary artery disease are ultimately diagnosed with acute myocarditis (3).
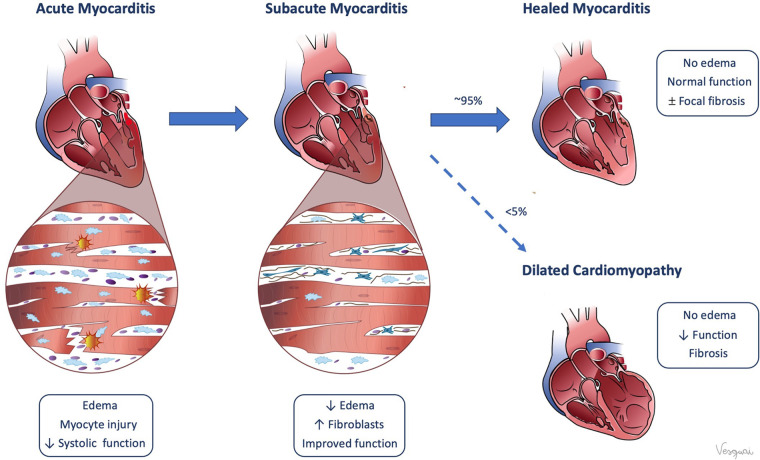
Even across diverse causes (Table 1), myocarditis is ultimately driven by an immune response directed at cardiomyocytes. In acute myocarditis, the initial trigger is either direct myocardial injury or immune dysregulation that induces inflammation by activating an innate or adaptive immune response. Myocardial injury can manifest across a spectrum of clinical severity—from subclinical disease, to myocarditis with preserved cardiac function, to more severe cases that result in reduced systolic or diastolic function, arrhythmia, and rarely hemodynamic collapse and cardiogenic shock. In most patients, the immune response is self-limited and downregulates with clearance of the initial trigger. However, depending on the degree of myocardial injury, patients may have residual myocardial dysfunction and fibrosis. In a minority of patients, the inflammatory response can persist or recur, leading to chronic myocarditis. Most patients recover completely after acute myocarditis, but a small proportion, estimated at less than 5%, will progress to dilated cardiomyopathy due to myocardial remodeling (Fig 1) (4).
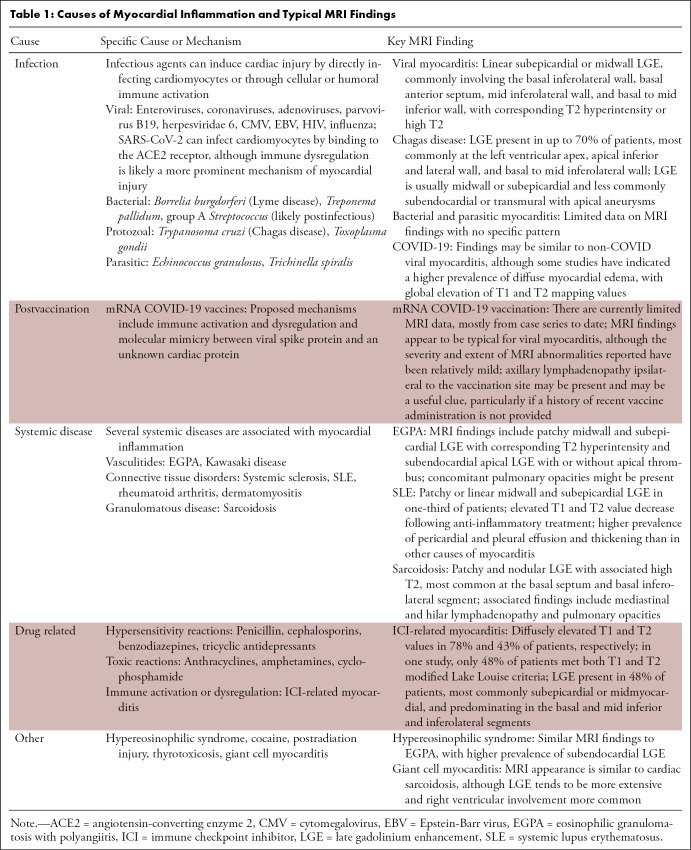
Table 1:
Causes of Myocardial Inflammation and Typical MRI Findings
Figure 1:
Pathophysiology of myocarditis. (Reprinted, with permission, from Valentina Sanchez Tijmes).
The most common trigger for myocarditis in developed countries is viral infection (5,6). Although traditional serologic studies, viral cultures, and molecular techniques can be used to identify viral pathogens in the setting of myocarditis, these techniques lack both sensitivity and specificity (7). Myocardial injury is also associated with COVID-19 illness, with elevated troponin levels in more than 60% of hospitalized patients (8). Although SARS-CoV-2 can infect cardiomyocytes by binding to the angiotensin-converting enzyme 2, indirect myocardial inflammation due to immune dysregulation may be a more prominent mechanism of myocardial injury (8).
Noninfectious causes of myocardial inflammation include autoimmune and immune-mediated disorders such as vasculitides, connective tissue disorders such as systemic lupus erythematosus, and granulomatous diseases such as giant cell myocarditis. Several drugs and medications are associated with myocarditis, including amphetamines and immune checkpoint inhibitors. Myocarditis is an uncommon adverse event after immunization (9). However, there is emerging evidence that COVID-19 vaccination is associated with myocarditis in a minority of patients.
Diagnosis
Establishing a diagnosis of acute myocarditis is important, as timely recognition can impact patient management and outcomes (2). Myocarditis is an important cause of sudden cardiac death in young adults, accounting for up to 12% of sudden cardiac death cases, according to postmortem analysis (10). Due to increased risk of sudden cardiac death, particularly when performing exercise, avoidance of competitive sports is typically recommended for at least 3 months in patients with acute myocarditis (11).
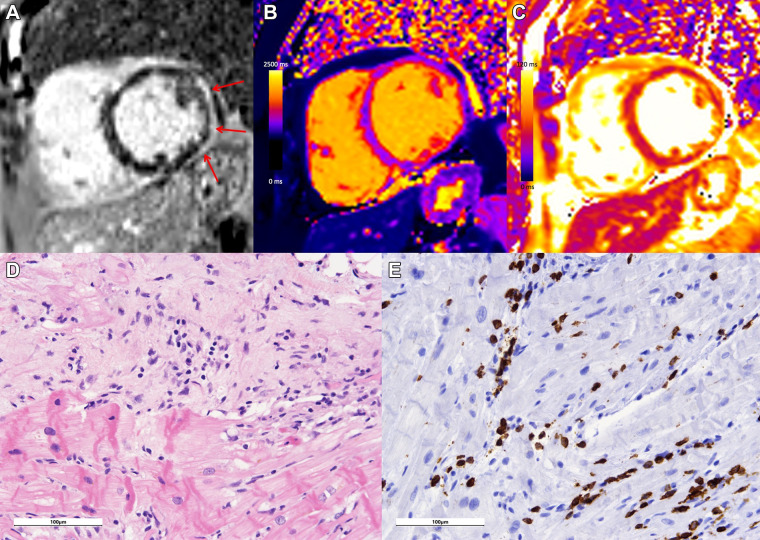
Endomyocardial biopsy is still considered the reference standard for definitive diagnosis of myocarditis; however, it is not frequently performed due to the invasive nature of the procedure and associated risks, as well as low sensitivity compared with cardiac explant at autopsy (12). Endomyocardial biopsy is usually only indicated if there is clinical evidence that the results will have a meaningful effect on therapeutic decisions (13). When endomyocardial biopsy is performed, the Dallas criteria are commonly used, which require histologic evidence of inflammatory infiltrates within the myocardium associated with myocyte damage and/or necrosis of nonischemic origin for definitive diagnosis (Fig 2) (7). Newer proposed criteria rely on immunohistochemical techniques, which may be more sensitive (14).
Figure 2:
Case example in a 68-year-old woman with lymphocytic myocarditis related to immune checkpoint inhibitor therapy. Cardiac MRI performed at 1.5 T demonstrates extensive subepicardial late gadolinium enhancement at (A) the basal to mid anterior, anterior lateral, inferior lateral, and inferior wall (red arrows) with (B) corresponding high regional native T1 (1280 msec) and (C) high regional T2 (69 msec) on short-axis images, in keeping with myocardial edema and damage. (D) Histologic images from endomyocardial biopsy demonstrate inflammation, including an active (dense inflammation) and healing (looser mixed inflammation, expanded matrix) component, with myocyte damage evident as myocytolytic change, vacuolization, and atrophy on hematoxylin-eosin stain. (E) At CD3 immunohistochemistry, a substantial portion of the inflammatory population was CD3 positive, consistent with a T-cell–mediated (lymphocytic) active myocarditis. Both histologic images were acquired with a Leica DM2500 microscope with a 20× objective and an OMAX A35180U3 camera. Images were acquired with ToupView software; no further adjustments were made. Scale bars (100 µm) are as shown.
In clinical practice, diagnostic criteria for suspected myocarditis that are based on expert consensus are more commonly employed. Acute myocarditis is considered clinically suspected if at least one clinical criterion and at least one diagnostic criterion are met (15). Clinical criteria include acute chest pain, new onset dyspnea, palpitations, unexplained arrhythmia symptoms, syncope, aborted sudden cardiac death, and unexplained cardiogenic shock. Diagnostic criteria include electrocardiographic, Holter monitor, or stress test abnormalities; elevated troponin levels; functional and structural abnormalities at cardiac imaging; and typical tissue characterization features of edema and/or late gadolinium enhancement (LGE) at cardiac MRI. Cardiac MRI can be used to meet either of the latter two criteria, highlighting the important role of imaging for diagnosis in acute myocarditis (15). Imaging findings can also be useful in identifying or excluding other potential diagnoses that may have a similar clinical presentation, including acute coronary syndrome or stress-induced cardiomyopathy. In some circumstances, imaging findings may suggest a specific potential cause for myocardial injury, although there is substantial overlap in imaging findings between different causes of myocarditis.
Imaging Myocardial Inflammation
The American Heart Association recommends testing for patients with signs consistent with myocarditis, using one or more cardiac imaging techniques, such as echocardiography or cardiac MRI (16).
Echocardiography
Echocardiography is often the first imaging modality used in patients with suspected myocarditis, as it is widely available and allows for relatively rapid assessment of cardiac size and function. Typical findings, including increased myocardial wall thickness and echogenicity, impaired global systolic function and strain, regional wall motion abnormalities, and ventricular dilatation, are relatively nonspecific (17). However, echocardiography provides important prognostic information, as increased left ventricular (LV) size and impaired function are predictors of poor outcomes (18).
CT Imaging
Coronary CT angiography is a noninvasive imaging modality that may be useful in excluding obstructive coronary artery disease in patients presenting with acute chest pain and elevated troponin levels, due to its high negative predictive value. Late iodine enhancement may be useful in evaluating myocardial damage, particularly in patients with a contraindication to MRI, although there are limited data specifically in acute myocarditis (19).
PET Imaging
Fluorodeoxyglucose PET is well established in the evaluation of active myocardial inflammation in the setting of cardiac sarcoidosis. Limited data available demonstrate that fluorodeoxyglucose PET can also identify inflammation in the setting of acute myocarditis (20). PET is typically performed in conjunction with CT for anatomic localization, although more recently combined PET/MRI scanners have become available, which could provide complementary information from both modalities in patients with myocarditis (21).
Cardiac MRI
Cardiac MRI is the most important noninvasive cardiac imaging modality for the diagnosis, follow-up, and risk stratification of patients with nonischemic myocardial inflammation, with unparalleled ability to characterize myocardial tissue. According to the 2021 American Heart Association/American College of Cardiology/American Society of Echocardiography/American College of Chest Physicians/Society for Academic Emergency Medicine/Society of Cardiovascular Computed Tomography/Society for Cardiovascular Magnetic Resonance Guideline for the Evaluation and Diagnosis of Chest Pain, cardiac MRI is useful in distinguishing myocarditis from other causes of acute chest pain in patients with myocardial injury who have nonobstructive coronary arteries at anatomic testing. Cardiac MRI is also useful in patients with suspected myocarditis or myopericarditis if there is diagnostic uncertainty or to determine the presence and extent of myocardial or pericardial inflammation and fibrosis (22).
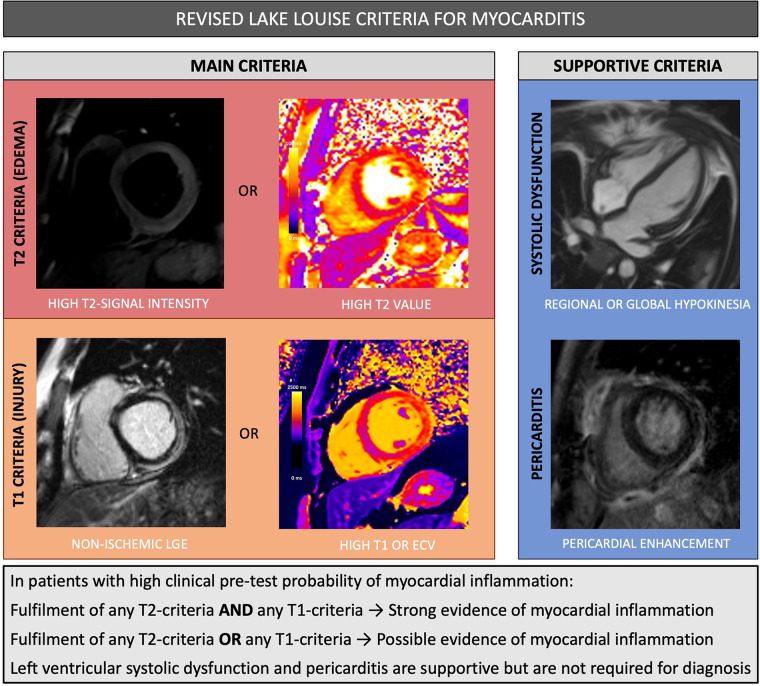
Updated Lake Louise Criteria
MRI findings of myocardial inflammation are commonly assessed using expert consensus guidelines, the Lake Louise criteria (LLC), initially published in 2009. These criteria were broadly used in clinical practice, although evaluation was limited due to subjectivity in qualitative assessment and moderate diagnostic sensitivity (23). The LLC were revised in 2018 to incorporate parametric mapping, which allows for quantitative assessment of regional and global myocardial T1 and T2 relaxation times and extracellular volume (ECV) (24). In comparison to the original LLC, the revised criteria have significantly higher sensitivity (88% vs 73%) while maintaining very high specificity (96%) (25). According to the revised criteria, cardiac MRI provides strong evidence of acute myocardial inflammation in patients with high clinical pretest probability if at least one criterion in each of the following two categories is positive: a T2-based marker of myocardial edema and a T1-based marker of myocardial damage (Fig 3). The presence of only one marker may still support the diagnosis of myocardial inflammation in the appropriate clinical context, although with lower specificity. Importantly, these criteria were intended to be applied in patients with clinically suspected myocardial inflammation and not applied broadly as a screening test for myocardial injury in asymptomatic patients.
Figure 3:
Summary of revised Lake Louise criteria for myocarditis. ECV =extracellular volume, LGE = late gadolinium enhancement.
T2-based Criteria for Myocardial Edema
Tissue edema is a hallmark of inflammation that is often focal in the setting of myocarditis, although diffuse edema can also be identified (26). T2-based criteria for myocardial edema include regional high T2 signal intensity, global T2 signal intensity ratio equal to or greater than 2.0 on T2-weighted images, or regional or global increase of myocardial T2 relaxation time.
Assessment of myocardial edema at cardiac MRI was previously reliant on T2-weighted imaging, which has high diagnostic accuracy for focal edema, although image quality can be degraded by artifact and signal inhomogeneity, limiting reproducibility (27). T2 mapping allows for direct quantification of T2 relaxation times and is particularly useful for ruling out active inflammation given its very high sensitivity (89%) (28). High T2 signal is specific for increased tissue water and therefore can discriminate between active and healed myocarditis (29).
T1-based Criteria for Myocardial Injury
If myocardial inflammation is severe enough, it can result in myocardial injury and necrosis, ultimately leading to fibrosis. T1 criteria for myocardial injury include LGE in a nonischemic pattern or regional or global increase of myocardial native T1 or ECV values.
LGE imaging remains one of the most important MRI techniques in the setting of suspected myocarditis, given that the presence of myocardial damage is a characteristic feature of myocarditis. Gadolinium-based contrast agents are retained within injured and necrotic tissue, resulting in hyperintensity at T1-weighted inversion-recovery imaging. The pattern of LGE in patients with myocarditis is most commonly subepicardial or midwall and often in a linear configuration. On the other hand, the pattern of LGE in the setting of ischemic myocardial injury is subendocardial to transmural and corresponds to a coronary artery territory. The most common location for LGE in viral myocarditis is the basal inferolateral wall. Other segments that are frequently involved include the basal anterior septum, mid inferolateral wall, and basal to mid inferior wall. Transmural enhancement and more diffuse LGE have been described, particularly in severe cases of fulminant and giant cell myocarditis.
LGE is present both in the setting of acute inflammation (with myocyte necrosis and hyperemia) and in the setting of fibrosis (due to expansion of the extracellular space) and therefore cannot reliably differentiate between acute and healed myocarditis (6,24). Over time, the extent of LGE usually decreases as inflammation resolves and scar contracts. T1 and ECV are elevated in the setting of interstitial and replacement myocardial fibrosis. Native T1 is a composite measurement reflecting signal from both the intracellular (mainly myocytes) and extracellular (mainly interstitial) myocardial compartments, while ECV is an estimate of the proportion of the extracellular space only. These parametric mapping techniques may have incremental diagnostic and prognostic value beyond LGE, particularly in the setting of diffuse inflammation, given the ability for direct quantification of myocardial tissue changes.
T1 and ECV are also elevated in the setting of myocardial edema, although unlike elevated T2, these changes are not specific for acute inflammation (29). Given the complementary information provided by T1 and T2 mapping, it is useful to interpret these values together. For example, in a patient with suspected myocarditis, corresponding elevated T2, T1, and ECV values indicate a high likelihood of myocardial edema, while elevated T1 and ECV in the setting of normal T2 suggest the presence of fibrosis or infiltration without acute inflammation (Fig 4).
Figure 4:
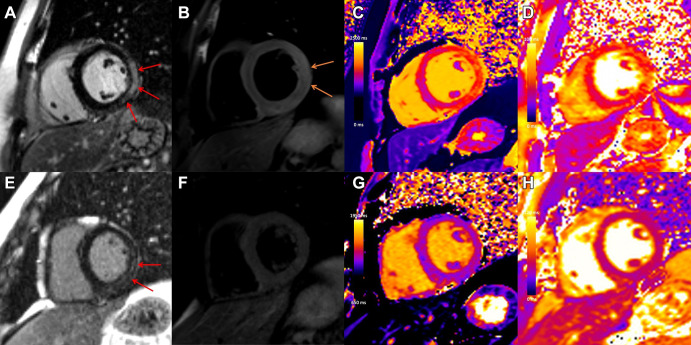
Case example in a 31-year-old man with viral myocarditis. Initial cardiac MRI performed at 3 T within 1 week of symptom onset demonstrates (A) subepicardial to nearly transmural late gadolinium enhancement (LGE) at the basal to mid anterior lateral, inferior lateral, and inferior wall (red arrows) with (B) corresponding high T2 signal, in keeping with edema (orange arrows), (C) high regional native T1 (1480 msec), and (D) high regional native T2 (56 msec) on short-axis images. Images from follow-up cardiac MRI performed at 1.5 T 5 months later demonstrate contraction of subepicardial LGE at the basal to mid inferior lateral and inferior wall (E, red arrows) with (G) corresponding high regional native T1 suggestive of fibrosis (1305 msec) and (F) resolution of edema with no corresponding high T2 signal and (H) normalization of T2 mapping values (46 msec).
LV Dysfunction
In more severe cases of myocarditis, regional wall motion abnormalities and systolic LV dysfunction can be identified at MRI. Systolic LV dysfunction (either regional or global) is a supportive criterion for myocarditis but is not required to make the diagnosis according to the revised LLC. After an acute episode of myocarditis, global systolic function often improves rapidly and, in most cases, returns to normal. Systolic dysfunction is typically more severe in fulminant myocarditis, and despite frequent improvement in the acute phase, LV function remains lower on average compared with nonfulminant cases at long-term follow-up (30). Myocardial strain quantification may increase the sensitivity for subtle wall motion abnormalities but has not been routinely implemented in clinical practice to date (24).
Pericardial Inflammation
Findings of pericardial inflammation are also considered to be supportive for the diagnosis of myocarditis, including pericardial enhancement, high T1 or T2 mapping values, or the presence of a pericardial effusion. When present, concomitant pericarditis is most commonly observed involving the pericardium adjacent to areas of inflamed myocardium, although it can also be diffuse.
Adverse Risk Markers at MRI
LGE is a strong, independent predictor of cardiac and all-cause mortality in patients with myocarditis (31). The risk of major adverse cardiovascular events increases by approximately 79% for every 10% increase in quantitative LGE extent (32). Of note, the presence of LGE with concomitant T2 hyperintensity is associated with better prognosis compared with isolated LGE without T2 hyperintensity. This is most likely due to the fact that LGE without associated edema typically reflects fibrosis, which is irreversible, while LGE in the context of T2 hyperintensity confers the possibility of at least partial recovery as edema improves over time (33). Other important adverse prognostic MRI markers include global systolic dysfunction (LV ejection fraction < 40%) and higher T1 and ECV (32,34). In patients with acute myocarditis with evidence of myocardial edema and/or LV dysfunction, follow-up cardiac MRI may be considered 3 to 6 months after the baseline study to assess for functional recovery and the possibility of residual scarring.
Cardiac MRI Protocol and Postprocessing
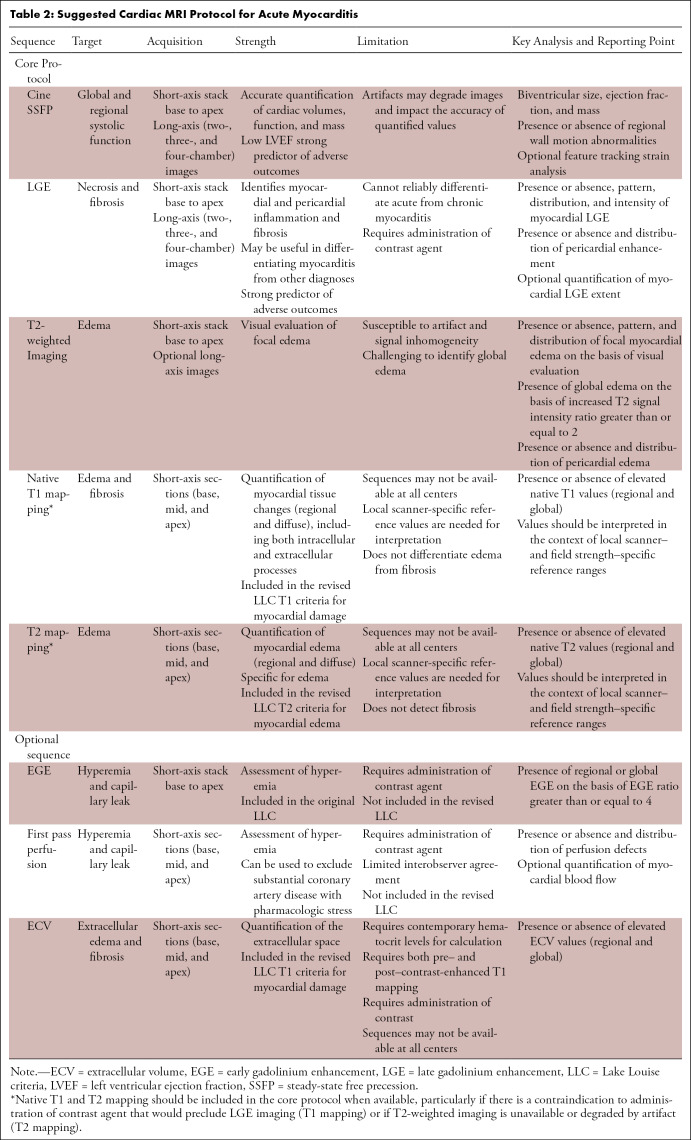
In the setting of suspected myocardial inflammation, the MRI protocol should include short- and long-axis cine sequences for assessment of ventricular volumes and function, T2-based imaging (black blood T2-weighted imaging and/or T2 parametric maps), and T1-based imaging (LGE and/or pre– and post–contrast-enhancement T1 mapping) (Table 2).
Table 2:
Suggested Cardiac MRI Protocol for Acute Myocarditis
One important consideration with respect to the evaluation of parametric maps is that values vary substantially on the basis of technical and patient-specific factors, including field strength. T2 values are higher at 1.5 T compared with 3 T, while T1 values are substantially higher at 3 T compared with 1.5 T. Therefore, mapping values should be compared with local reference ranges (35). Maps should be assessed visually as well as quantitatively, including global assessment of diffuse tissue changes along with focal evaluation in myocardial segments that are visually abnormal or demonstrate regional wall motion abnormalities.
For highest diagnostic performance, MRI should ideally be performed in the acute phase. Cardiac MRI markers of myocardial inflammation typically demonstrate rapid and continuous improvement during the first few weeks after the onset of symptoms (36). The sensitivity for detection of myocardial edema in particular is much lower if patients are imaged weeks after the initial clinical presentation. Establishing a diagnosis of nonacute myocarditis is particularly challenging, as findings are often nonspecific.
Cardiac MRI in Specific Causes of Myocarditis
Cardiac MRI findings demonstrate substantial overlap between different causes of myocarditis, and therefore, it is imperative that clinical features are taken into consideration. Clinical and cardiac MRI findings in specific causes of nonischemic myocardial inflammation are summarized in Table 1. Given the recent focus on the role of cardiac imaging in patients with COVID-19 and in patients with suspected myocarditis after COVID-19 vaccination, specific cardiac MRI findings in these settings are highlighted below.
COVID-19
Several cardiac MRI studies have evaluated myocardial damage in patients who recovered from COVID-19, although estimates of myocardial abnormalities have ranged widely, likely reflecting differences in patient populations, including baseline cardiac risk factors and the severity of COVID-19 illness, as well as the timing of imaging after the initial infection. A recent study found that T1 and T2 values were more commonly diffusely elevated in patients recently recovered from COVID-19 compared with patients with non–COVID-19 myocarditis (37). However, other studies have reported more focal MRI abnormalities typical of non-COVID myocarditis in patients who have recovered from COVID-19, including subepicardial LGE (Fig 5) (38). Data regarding MRI findings in COVID-19–related myocardial injury continue to evolve, with multiple large studies currently underway.
Figure 5:
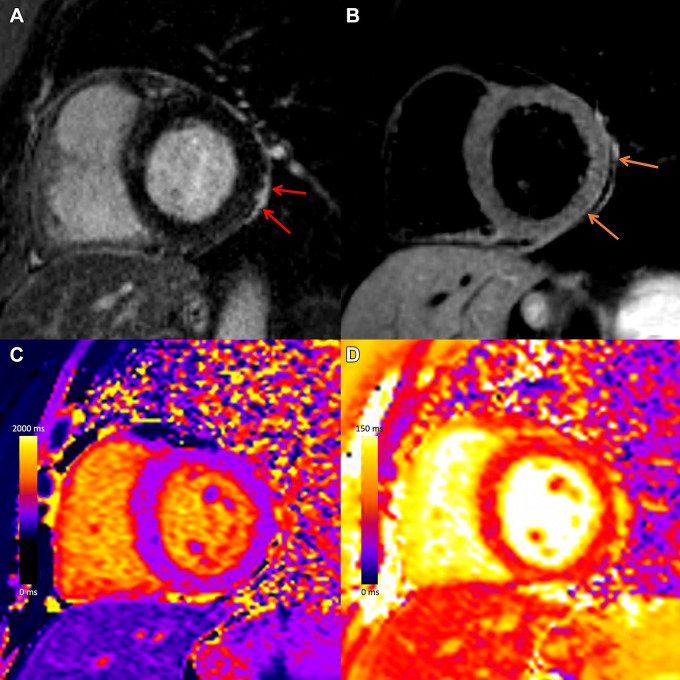
Myocardial injury and pericarditis following COVID-19. Case example in a 57-year-old woman with COVID-19 who presented with chest pain after having elevated troponin levels. Cardiac MRI performed at 1.5 T 4 weeks after polymerase chain reaction–confirmed diagnosis of SARS-CoV-2 infection demonstrates subepicardial late gadolinium enhancement at the (A) basal inferior lateral wall with adjacent pericardial enhancement (red arrows), with (B) corresponding high T2 signal (orange arrows), and (C) high regional native T1 (1236 msec) and (D) high regional native T2 (67 msec) on short-axis images, in keeping with myopericarditis.
Myocarditis after COVID-19 Vaccination
Myocarditis has been reported in a minority of people following administration of mRNA-based COVID-19 vaccines, including mRNA-1273 (Moderna) and BNT162b2 mRNA (Pfizer-BioNTech), with symptom onset typically within a few days of vaccination (median, 2–3 days). Myocarditis is three to five times more frequent after the second dose compared with the first, although patients with prior history of COVID-19 are at higher risk after the first dose. The U.S. Vaccine Adverse Event Reporting System (VAERS) received 1903 reports of myopericarditis among people who received at least one dose of a COVID-19 vaccine as of August 18, 2021 (9), in the context of nearly 360 million total doses administrated. As of June 2021, there were approximately 40.6 cases of myocarditis reported per million second doses administrated to males aged 12–29 years and 2.4 cases reported per million second doses in men aged 30 years or older (39); for females, reported rates were 4.2 and 1.0 per million second doses for the same categories, respectively. Importantly, VAERS relies on passive reporting, and the data cannot be used to determine whether a vaccine is causally related to an adverse event. Data from the largest integrated health care organization in Israel indicate that vaccination with BNT162b2 mRNA vaccine is associated with an excess risk of myocarditis (risk ratio 3.2 and risk difference 2.7 events per 100 000 persons when compared with age- and risk-matched controls). However, the risk of myocarditis following SARS-CoV-2 infection was much higher (risk ratio 18.3 and risk difference 11.0 events per 100 000 persons) (40).
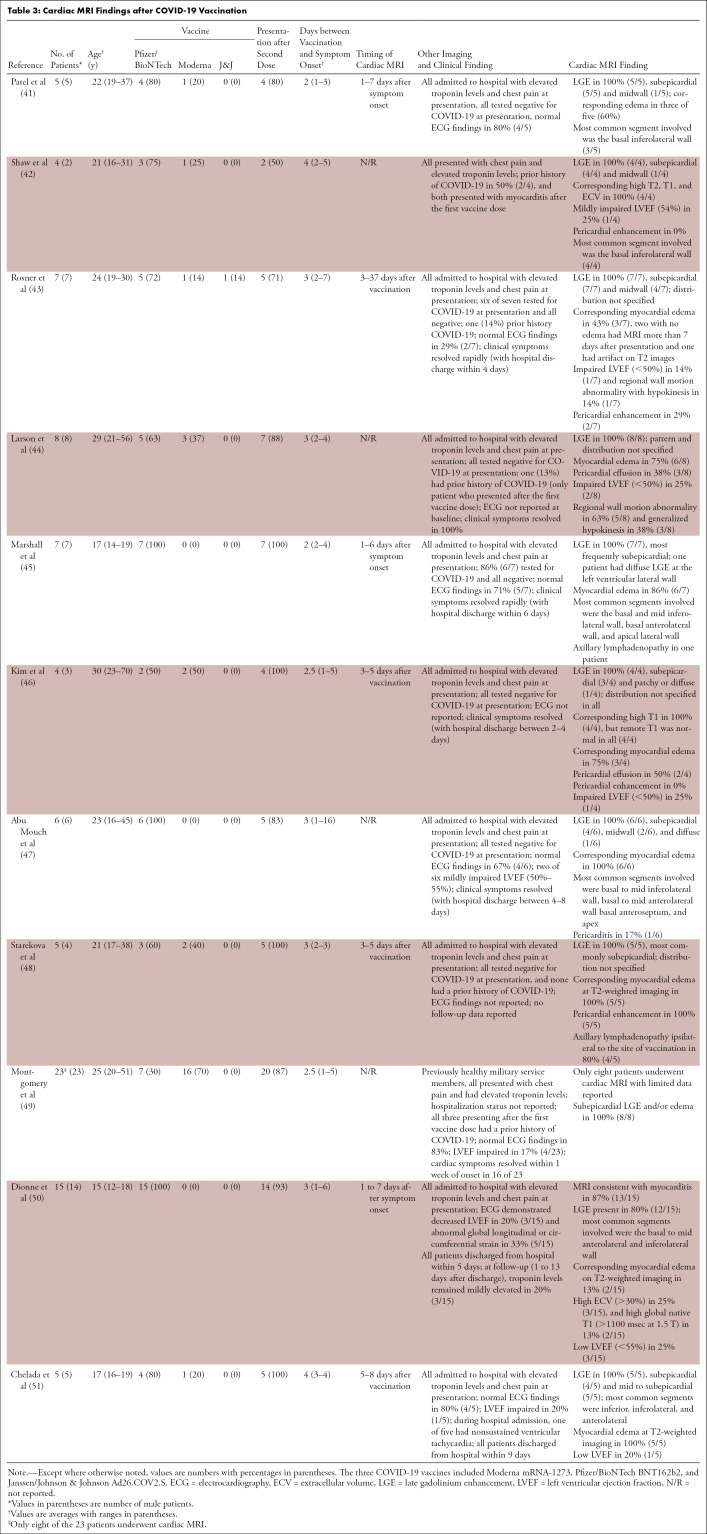
Given the relatively short time frame with which COVID-19 vaccines have been administered, data regarding the prevalence and pattern of abnormalities at cardiac MRI following vaccination are still emerging. There are only a few published case series describing cardiac MRI findings after COVID-19 vaccination to date, summarized in Table 3. The largest MRI case series of vaccine-associated myocarditis includes 15 patients (range, four to 15 patients). Of note, almost all patients who underwent MRI in the context of myocarditis following COVID-19 vaccination included in case series to date have been hospitalized. It is possible that these patients reflect the more severe end of the spectrum of vaccine-associated myocardial changes due to reporting bias. Typical cardiac MRI findings reported to date in patients with myocarditis following COVID-19 vaccination are similar to findings in nonvaccine myocarditis, including subepicardial LGE with a predilection for the basal inferolateral wall along with corresponding myocardial edema (Fig 6) (41–51). Other findings include pericardial enhancement and axillary lymphadenopathy ipsilateral to the vaccine administration site (52). When reported, impaired LV ejection fraction (<50%–55%) was identified in 14%–25% of patients.
Table 3:
Cardiac MRI Findings after COVID-19 Vaccination
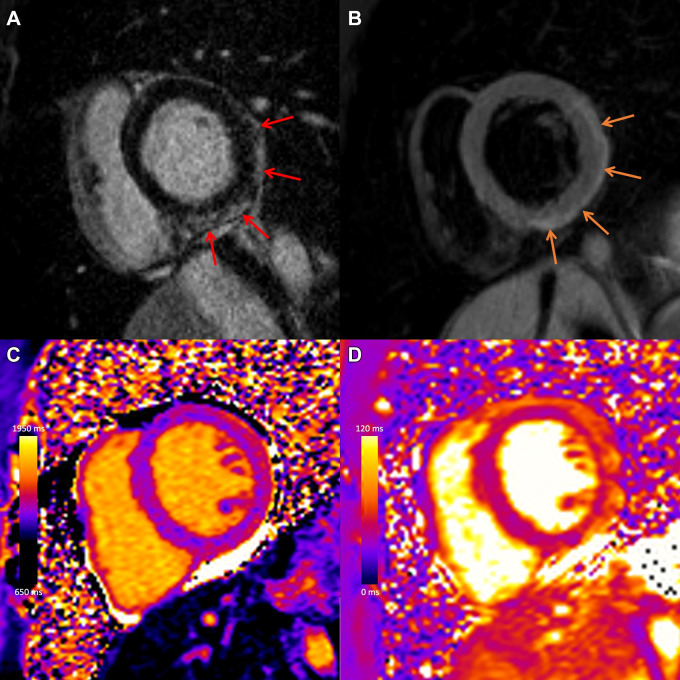
Figure 6:
COVID-19 vaccine–associated myocarditis. Case example in a 27-year-old man with myocarditis 3 days following COVID-19 vaccine administration. Images from cardiac MRI performed at 1.5 T demonstrate subepicardial late gadolinium enhancement at the (A) basal to mid anterior lateral, inferior lateral, and inferior wall (red arrows), with (B) corresponding high T2 signal (orange arrows), (C) high regional native T1 (1173 msec), and (D) high regional native T2 (59 msec) on short-axis images.
Differentiating vaccine-associated myocarditis from other causes of myocardial injury at cardiac MRI may be a challenge, as the pattern of findings is similar, and there are no longitudinal imaging studies to suggest how long abnormalities persist. However, accurate diagnosis is important, as this could impact patient treatment; current recommendations indicate that individuals who develop myocarditis or pericarditis after a dose of an mRNA vaccine defer receiving a subsequent dose until additional data are available (53). Clinical history, including the timing of symptom onset in relation to vaccine administration, is highly relevant. In patients with signs or symptoms suggestive of myocarditis following vaccination, cardiac MRI should ideally be performed as soon as possible after the onset of symptoms to maximize the likelihood of detecting myocardial edema, which would suggest an acute process (36). If MRI is performed several weeks to months after symptom onset and no T2 abnormality is identified, it is difficult to attribute myocardial tissue changes to a specific cause. This may be a particular challenge in symptomatic patients who have received an mRNA vaccine and have a prior history of COVID-19. Importantly, there are no data to suggest a role for routine imaging or screening of asymptomatic individuals after COVID-19 vaccination in the absence of signs or symptoms suggestive of myocarditis.
In most reported cases of myocarditis following COVID-19 vaccination, the clinical course has been favorable, with rapid resolution of symptoms and corresponding decreases in troponin levels over short-term follow-up, suggesting that patients might have a good long-term prognosis. Given that the risk of myocardial injury and other severe outcomes after COVID-19 is higher, current data are supportive of continued COVID-19 immunization on the basis of the balance of risks and benefits (54). Larger studies with longer-term follow-up are required to evaluate long-term outcomes, to directly compare imaging findings after COVID-19 vaccination to other causes of myocarditis, to assess longitudinal MRI changes after clinical recovery, and to determine the risk associated with subsequent vaccine administration in patients with a prior history of myocarditis.
Conclusion
Cardiac MRI is an important imaging modality in patients with suspected myocardial inflammation and myocarditis, allowing for noninvasive assessment of myocardial edema and injury, and identification of potentially treatable underlying causes of inflammation to guide management and improve patient outcomes. Cardiac MRI may be particularly useful in patients presenting with signs and symptoms suggestive of myocarditis after COVID-19 vaccine administration, although further study is needed.
P.T. supported by a Canada Research Chair in Cardio-oncology. J.A.U. supported by a Department of Medicine, University of Toronto Merit Award and receives support from Ontario Ministry of Colleges and Universities Early Researcher Award (ER15-11-037).
Disclosures of conflicts of interest: F.S.T. No relevant relationships. P.T. No relevant relationships. J.A.U. Grants or contracts from AstraZeneca, Amgen, Bayer, Boehringer Ingelheim-Lilly, Janssen; consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim-Lilly, Janssen, Merck, Novartis, and Sanofi; honoraria for lectures from Merck and Sanofi. M.A.S. No relevant relationships. K.H. Payment or honoraria for lectures, presentations, or educational events from Sanofi, Amicus, and Medscape; member of Radiology: Cardiothoracic Imaging editorial board.
Abbreviations:
- ECV
- extracellular volume
- LGE
- late gadolinium enhancement
- LLC
- Lake Louise criteria
- LV
- left ventricular
- VAERS
- U.S. Vaccine Adverse Event Reporting System
References
- 1. Aretz HT , Billingham ME , Edwards WD , et al . Myocarditis. A histopathologic definition and classification . Am J Cardiovasc Pathol 1987. ; 1 ( 1 ): 3 – 14 . [PubMed] [Google Scholar]
- 2. Heymans S , Eriksson U , Lehtonen J , Cooper LT Jr . The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy . J Am Coll Cardiol 2016. ; 68 ( 21 ): 2348 – 2364 . [DOI] [PubMed] [Google Scholar]
- 3. Tornvall P , Gerbaud E , Behaghel A , et al . Myocarditis or “true” infarction by cardiac magnetic resonance in patients with a clinical diagnosis of myocardial infarction without obstructive coronary disease: A meta-analysis of individual patient data . Atherosclerosis 2015. ; 241 ( 1 ): 87 – 91 . [DOI] [PubMed] [Google Scholar]
- 4. Ammirati E , Cipriani M , Moro C , et al . Clinical Presentation and Outcome in a Contemporary Cohort of Patients With Acute Myocarditis: Multicenter Lombardy Registry . Circulation 2018. ; 138 ( 11 ): 1088 – 1099 . [DOI] [PubMed] [Google Scholar]
- 5. Kühl U , Pauschinger M , Noutsias M , et al . High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction . Circulation 2005. ; 111 ( 7 ): 887 – 893 . [DOI] [PubMed] [Google Scholar]
- 6. Tschöpe C , Ammirati E , Bozkurt B , et al . Myocarditis and inflammatory cardiomyopathy: current evidence and future directions . Nat Rev Cardiol 2021. ; 18 ( 3 ): 169 – 193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baughman KL . Diagnosis of myocarditis: death of Dallas criteria . Circulation 2006. ; 113 ( 4 ): 593 – 595 . [DOI] [PubMed] [Google Scholar]
- 8. Giustino G , Croft LB , Stefanini GG , et al . Characterization of Myocardial Injury in Patients With COVID-19 . J Am Coll Cardiol 2020. ; 76 ( 18 ): 2043 – 2055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su JR , McNeil MM , Welsh KJ , et al . Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018 . Vaccine 2021. ; 39 ( 5 ): 839 – 845 . [DOI] [PubMed] [Google Scholar]
- 10. Fabre A , Sheppard MN . Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death . Heart 2006. ; 92 ( 3 ): 316 – 320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maron BJ , Udelson JE , Bonow RO , et al . Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement From the American Heart Association and American College of Cardiology . Circulation 2015. ; 132 ( 22 ): e273 – e280 . [DOI] [PubMed] [Google Scholar]
- 12. Chow LH , Radio SJ , Sears TD , McManus BM . Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis . J Am Coll Cardiol 1989. ; 14 ( 4 ): 915 – 920 . [DOI] [PubMed] [Google Scholar]
- 13. Cooper LT , Baughman KL , Feldman AM , et al . The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology . Circulation 2007. ; 116 ( 19 ): 2216 – 2233 . [DOI] [PubMed] [Google Scholar]
- 14. Fronczek J , van de Goot FRW , Krijnen PAJ , van der Wal AC , Niessen HWM . Diagnosing Lymphocytic Myocarditis in Adult Autopsies Combining the Dallas Criteria with Immunohistochemical Stainings . J Forensics Res 2016. ; 7 ( 2 ): 1000325 . [Google Scholar]
- 15. Caforio AL , Pankuweit S , Arbustini E , et al . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Eur Heart J 2013. ; 34 ( 33 ): 2636 – 2648 , 2648a – 2648d . [DOI] [PubMed] [Google Scholar]
- 16. Kociol RD , Cooper LT , Fang JC , et al . Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association . Circulation 2020. ; 141 ( 6 ): e69 – e92 . [DOI] [PubMed] [Google Scholar]
- 17. Chinali M , Franceschini A , Ciancarella P , et al . Echocardiographic two-dimensional speckle tracking identifies acute regional myocardial edema and sub-acute fibrosis in pediatric focal myocarditis with normal ejection fraction: comparison with cardiac magnetic resonance . Sci Rep 2020. ; 10 ( 1 ): 11321 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kindermann I , Kindermann M , Kandolf R , et al . Predictors of outcome in patients with suspected myocarditis . Circulation 2008. ; 118 ( 6 ): 639 – 648 . [DOI] [PubMed] [Google Scholar]
- 19. Chang S , Han K , Youn JC , et al . Utility of Dual-Energy CT-based Monochromatic Imaging in the Assessment of Myocardial Delayed Enhancement in Patients with Cardiomyopathy . Radiology 2018. ; 287 ( 2 ): 442 – 451 . [DOI] [PubMed] [Google Scholar]
- 20. Tanimura M , Dohi K , Imanaka-Yoshida K , et al . Fulminant Myocarditis With Prolonged Active Lymphocytic Infiltration After Hemodynamic Recovery . Int Heart J 2017. ; 58 ( 2 ): 294 – 297 . [DOI] [PubMed] [Google Scholar]
- 21. Hanneman K , Kadoch M , Guo HH , et al . Initial Experience With Simultaneous 18F-FDG PET/MRI in the Evaluation of Cardiac Sarcoidosis and Myocarditis . Clin Nucl Med 2017. ; 42 ( 7 ): e328 – e334 . [DOI] [PubMed] [Google Scholar]
- 22. Gulati M , Levy PD , Mukherjee D , et al . 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines . Circulation 2021. . 10.1161/CIR.0000000000001030. Published online October 28, 2021. [DOI] [PubMed] [Google Scholar]
- 23. Pan JA , Lee YJ , Salerno M . Diagnostic Performance of Extracellular Volume, Native T1, and T2 Mapping Versus Lake Louise Criteria by Cardiac Magnetic Resonance for Detection of Acute Myocarditis: A Meta-Analysis . Circ Cardiovasc Imaging 2018. ; 11 ( 7 ): e007598 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferreira VM , Schulz-Menger J , Holmvang G , et al . Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations . J Am Coll Cardiol 2018. ; 72 ( 24 ): 3158 – 3176 . [DOI] [PubMed] [Google Scholar]
- 25. Luetkens JA , Faron A , Isaak A , et al . Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort . Radiol Cardiothorac Imaging 2019. ; 1 ( 3 ): e190010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thavendiranathan P , Walls M , Giri S , et al . Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping . Circ Cardiovasc Imaging 2012. ; 5 ( 1 ): 102 – 110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srichai MB , Lim RP , Lath N , Babb J , Axel L , Kim D . Diagnostic performance of T2-weighted CMR for evaluation of acute myocardial injury . J Cardiovasc Magn Reson 2012. ; 14 ( Supplement 1 ): O62 . [DOI] [PubMed] [Google Scholar]
- 28. von Knobelsdorff-Brenkenhoff F , Schüler J , Dogangüzel S , et al . Detection and Monitoring of Acute Myocarditis Applying Quantitative Cardiovascular Magnetic Resonance . Circ Cardiovasc Imaging 2017. ; 10 ( 2 ): e005242 . [DOI] [PubMed] [Google Scholar]
- 29. Galán-Arriola C , Lobo M , Vílchez-Tschischke JP , et al . Serial Magnetic Resonance Imaging to Identify Early Stages of Anthracycline-Induced Cardiotoxicity . J Am Coll Cardiol 2019. ; 73 ( 7 ): 779 – 791 . [DOI] [PubMed] [Google Scholar]
- 30. Ammirati E , Cipriani M , Lilliu M , et al . Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis . Circulation 2017. ; 136 ( 6 ): 529 – 545 . [DOI] [PubMed] [Google Scholar]
- 31. Grün S , Schumm J , Greulich S , et al . Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery . J Am Coll Cardiol 2012. ; 59 ( 18 ): 1604 – 1615 . [DOI] [PubMed] [Google Scholar]
- 32. Gräni C , Eichhorn C , Bière L , et al . Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis . J Am Coll Cardiol 2017. ; 70 ( 16 ): 1964 – 1976 [Published correction appears in J Am Coll Cardiol 2017;70(21):2736.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aquaro GD , Ghebru Habtemicael Y , Camastra G , et al . Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients With Acute Myocarditis . J Am Coll Cardiol 2019. ; 74 ( 20 ): 2439 – 2448 . [DOI] [PubMed] [Google Scholar]
- 34. Thavendiranathan P , Zhang L , Zafar A , et al . Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients With Immune Checkpoint Inhibitor-Associated Myocarditis . J Am Coll Cardiol 2021. ; 77 ( 12 ): 1503 – 1516 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Messroghli DR , Moon JC , Ferreira VM , et al . Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) . J Cardiovasc Magn Reson 2017. ; 19 ( 1 ): 75 [Published correction appears in J Cardiovasc Magn Reson 2018;20(1):9.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luetkens JA , Homsi R , Dabir D , et al . Comprehensive Cardiac Magnetic Resonance for Short-Term Follow-Up in Acute Myocarditis . J Am Heart Assoc 2016. ; 5 ( 7 ): e003603 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luetkens JA , Isaak A , Öztürk C , et al . Cardiac MRI in Suspected Acute COVID-19 Myocarditis . Radiol Cardiothorac Imaging 2021. ; 3 ( 2 ): e200628 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Starekova J , Bluemke DA , Bradham WS , et al . Evaluation for Myocarditis in Competitive Student Athletes Recovering From Coronavirus Disease 2019 With Cardiac Magnetic Resonance Imaging . JAMA Cardiol 2021. ; 6 ( 8 ): 945 – 950 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gargano JW , Wallace M , Hadler SC , et al . Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021 . MMWR Morb Mortal Wkly Rep 2021. ; 70 ( 27 ): 977 – 982 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barda N , Dagan N , Ben-Shlomo Y , et al . Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting . N Engl J Med 2021. ; 385 ( 12 ): 1078 – 1090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patel YR , Louis DW , Atalay M , Agarwal S , Shah NR . Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID-19 vaccination: a case series . J Cardiovasc Magn Reson 2021. ; 23 ( 1 ): 101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shaw KE , Cavalcante JL , Han BK , Gössl M . Possible Association Between COVID-19 Vaccine and Myocarditis: Clinical and CMR Findings . JACC Cardiovasc Imaging 2021. ; 14 ( 9 ): 1856 – 1861 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosner CM , Genovese L , Tehrani BN , et al . Myocarditis Temporally Associated With COVID-19 Vaccination . Circulation 2021. ; 144 ( 6 ): 502 – 505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Larson KF , Ammirati E , Adler ED , et al . Myocarditis After BNT162b2 and mRNA-1273 Vaccination . Circulation 2021. ; 144 ( 6 ): 506 – 508 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marshall M , Ferguson ID , Lewis P , et al . Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination . Pediatrics 2021. ; 148 ( 3 ): e2021052478 . [DOI] [PubMed] [Google Scholar]
- 46. Kim HW , Jenista ER , Wendell DC , et al . Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination . JAMA Cardiol 2021. ; 6 ( 10 ): 1196 – 1201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abu Mouch S , Roguin A , Hellou E , et al . Myocarditis following COVID-19 mRNA vaccination . Vaccine 2021. ; 39 ( 29 ): 3790 – 3793 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Starekova J , Bluemke DA , Bradham WS , Grist TM , Schiebler ML , Reeder SB . Myocarditis Associated with mRNA COVID-19 Vaccination . Radiology 2021. ; 301 ( 2 ): E409 – E411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Montgomery J , Ryan M , Engler R , et al . Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military . JAMA Cardiol 2021. ; 6 ( 10 ): 1202 – 1206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dionne A , Sperotto F , Chamberlain S , et al . Association of Myocarditis With BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children . JAMA Cardiol 2021. . 10.1001/jamacardio.2021.3471. Published online August 10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chelala L , Jeudy J , Hossain R , Rosenthal G , Pietris N , White C . Cardiac MRI Findings of Myocarditis After COVID-19 mRNA Vaccination in Adolescents . AJR Am J Roentgenol 2021. . 10.2214/AJR.21.26853. Published online October 27, 2021. [DOI] [PubMed] [Google Scholar]
- 52. Hanneman K , Iwanochko RM , Thavendiranathan P . Evolution of Lymphadenopathy at PET/MRI after COVID-19 Vaccination . Radiology 2021. ; 299 ( 3 ): E282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luk A , Clarke B , Dahdah N , et al . Myocarditis and Pericarditis After COVID-19 mRNA Vaccination: Practical Considerations for Care Providers . Can J Cardiol 2021S0828-282X ( 21 ) 00624 – 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shay DK , Shimabukuro TT , DeStefano F . Myocarditis Occurring After Immunization With mRNA-Based COVID-19 Vaccines . JAMA Cardiol 2021. ; 6 ( 10 ): 1115 – 1117 . [DOI] [PubMed] [Google Scholar]